Among hepatitis B (HB) surface antigen–negative/human immunodeficiency virus–infected pregnant women in Thailand, 14% had isolated antibody to HB core antigen (anti-HBc); of whom 24% had occult HB virus (HBV) infection with low HBV DNA levels. None transmitted HBV to their infants.
Keywords: HIV-1–infected pregnant women, isolated anti-HBc, occult HBV infection, perinatal transmission
Abstract
Background. Prevalence and risk factors for isolated antibody to hepatitis B core antigen (anti-HBc) and occult hepatitis B virus (HBV) infection are not well known in human immunodeficiency virus type 1 (HIV-1)–infected pregnant women. It is unclear if women with occult infections are at risk of transmitting HBV to their infants.
Methods. HIV-1–infected and HBV surface antigen (HBsAg)–negative pregnant women were tested for antibody to HBsAg (anti-HBs) and anti-HBc using enzyme immunoassay. Women with isolated anti-HBc were assessed for occult HBV infection, defined as HBV DNA levels >15 IU/mL, using the Abbott RealTime HBV DNA assay. Infants born to women with isolated anti-HBc and detectable HBV DNA were tested at 4 months of age for HBV DNA. Logistic regression analysis was used to identify factors associated with isolated anti-HBc and occult HBV infection.
Results. Among 1812 HIV-infected pregnant women, 1682 were HBsAg negative. Fourteen percent (95% confidence interval [CI], 12%–15%) of HBsAg-negative women had an isolated anti-HBc that was independently associated with low CD4 count, age >35 years, birth in northern Thailand, and positive anti–hepatitis C virus serology. Occult HBV infection was identified in 24% (95% CI, 18%–30%) of women with isolated anti-HBc, representing 2.6% (95% CI, 1.9%–3.5%) of HIV-1–infected pregnant women, and was inversely associated with HIV RNA levels. None of the women with isolated anti-HBc and occult HBV infection transmitted HBV to their infants.
Conclusions. HIV-1–infected pregnant women with isolated anti-HBc and occult HBV infection have very low HBV DNA levels and are thus at very low risk to transmit HBV to their infants.
Over the past decade, individuals with isolated antibody to hepatitis B core antigen (anti-HBc) serology (ie, anti-HBc positive in the absence of detectable hepatitis B surface antigen [HBsAg] and hepatitis B surface antibody [anti-HBs]) have drawn much attention to their management because they are potentially infectious. Indeed, transmission of hepatitis B virus (HBV) from isolated anti-HBc individuals has been reported following sexual contact [1], blood transfusion [2], organ transplantation [3], or during the perinatal period [4]. Furthermore, immunosuppressed patients with isolated anti-HBc can reactivate HBV replication following therapies or infectious diseases [5]. The clinical significance of this serology pattern remains unclear. Although the majority of individuals with this serology pattern present with normal liver enzyme levels and no sign of liver disease, it has been observed in patients presenting with cirrhosis and hepatocellular carcinoma [6, 7] and has been associated with a significantly shorter survival in patients infected with human immunodeficiency virus (HIV) as compared to those with anti-HBs antibody [8]. Moreover, the Multicenter AIDS Cohort Study reported a 3.6-fold increased risk of non-AIDS-related deaths in HIV-infected men with isolated anti-HBc compared to those without HBV infection [9]. The prevalence of isolated anti-HBc varies according to that of chronic HBV infection in the population; the rate among blood donors ranges between 1% and 4% [1, 10] in countries with low chronic HBV infection prevalence and between 1%–21% in high chronic HBV infection prevalence countries [11–13]. Higher isolated anti-HBc prevalence, ranging between 17% and 81%, has been found among HIV-infected [14–18] or hepatitis C virus (HCV)–infected individuals [19] and injection drug users [18, 20]. Individuals with isolated anti-HBc represent a heterogeneous population that comprises individuals who had completely cleared HBV infection but lost their anti-HBs antibody and those with occult HBV infection defined by the presence of HBV DNA in their serum and/or liver without detectable HBsAg, irrespective of other HBV serological markers [21]. The latter may present higher risk of transmission and liver disease progression.
Among HIV-infected patients, prevalence of occult HBV infection ranges between 0% and 89%, with most studies reporting HBV DNA values of <1000 IU/mL [22], and is much higher among individuals with isolated anti-HBc [23]. However, there are still very limited data on occult HBV infection in HIV-infected pregnant women and its impact on HBV transmission to their infants.
In this study, we assessed the prevalence and risk factors for isolated anti-HBc and occult HBV infection among a large number of HIV type 1 (HIV-1)–infected pregnant women in Thailand and evaluated the risk of HBV transmission to their infants.
MATERIALS AND METHODS
Study Population
The study population was derived from HIV-1–infected pregnant women who participated between 2001 and 2003 in a clinical trial conducted in Thailand that investigated the efficacy of zidovudine plus single-dose nevirapine to prevent mother-to-child transmission of HIV-1 (PHPT-2 [ClinicalTrials.gov identifier: NCT00398684]) [24]. Demographic, clinical, and biological data were collected before enrollment and during the study. Maternal and infant blood samples were collected at entry and during study and plasma/sera were stored frozen. Only HBsAg-negative women were included in this study, which was approved by the Ethics Committee of the Faculty of Associated Medical Sciences, Chiang Mai University.
Analysis of HBV Infection Markers
Women were screened for HBsAg using an enzyme immunoassay of 250 pg/mL sensitivity (DiaSorin ETI-MAK-2, Salluggia, Italy). HBsAg-negative women were tested for anti-HBc (Monolisa anti-HBc PLUS) and anti-HBs (Monolisa anti-HBs PLUS, Bio-Rad Laboratories, Marnes La Coquette, France). Women positive for both anti-HBc and anti-HBs antibodies were considered as having resolved HBV infection; those with only anti-HBc were considered as having acquired HBV infection; those positive for anti-HBs only were considered as having received hepatitis B vaccine; and those negative for both anti-HBc and anti-HBs antibodies were considered as having not acquired HBV infection.
Women with isolated anti-HBc had HBV DNA quantified using the Abbott RealTime HBV DNA assay (Abbott France, Rungis, France; lower limit of detection of 15 IU/mL or 1.18 log10 IU/mL) and HBsAg verified using an HBsAg test kit of 50 pg/mL sensitivity (Monolisa HBsAg Ultra, Bio-Rad Laboratories) and able to detect up to 30 additional mutations on HBsAg proteins.
Infants born to women with isolated anti-HBc and detectable HBV DNA were tested at 4 months of age for HBV DNA using the Abbott RealTime HBV DNA assay. Children were followed up until 12 months of age.
HBV Sequencing
HBV sequencing was performed for women with detectable HBV DNA. HBV DNA was extracted from women's plasma using the automatic sample extraction system (Abbott M2000sp, Rungis, France). Ten microliters of HBV DNA extract was used as the template for nested polymerase chain reaction (PCR). Published primers were used to amplify HBV surface/polymerase region (nucleotide position 251 to 1058) [25]. Amplicons were sequenced using the BigDye Terminator Mix V. 1.1 (Applied Biosystems, Foster City, California) and the ABI PRISM 3100 Genetic Analyzer, and sequencing data were analyzed using the software Bioedit (version 7.0.9.0).
Statistical Analysis
Characteristics of women including age at enrollment, region of birth, prior pregnancies, alanine aminotransferase (ALT) level, white blood cells, lymphocytes, CD4+ and CD8+ T-cell counts, and the presence of antibodies against syphilis and hepatitis C virus were described using number and percentage for categorical data and median with interquartile range (IQR) for continuous data. Univariate analyses were performed using logistic regression analysis to identify risk factors for having isolated anti-HBc or occult HBV infection. Continuous variables were transformed into categorical variables using common cutoff values. For multivariate analysis, all factors with a P value <.20 identified by univariate analysis were then introduced into the forward stepwise logistic regression model, to investigate independent risk factors associated with isolated anti-HBc serology or occult HBV infection. All data analyses were performed using Stata version 10.1 software (StataCorp, College Station, Texas). Differences were considered statistically significant if the P value was ≤.05.
RESULTS
Characteristics of Study Population
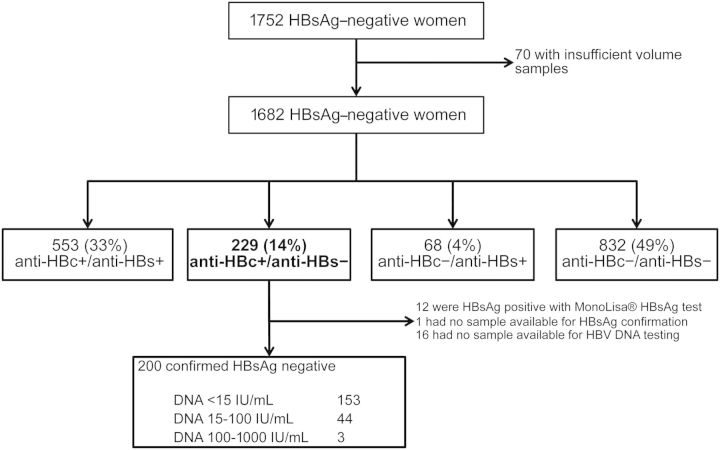
Of 1812 HIV-1–infected pregnant women, 1752 were found to be HBsAg-negative, of whom 1682 (96.0%) had sufficient samples to be included in this study (Figure 1). Baseline characteristics of HBsAg-negative women are described in Table 1. At enrollment, the median age was 26 years, median ALT level was normal, median HIV RNA load was 4.03 log10 copies/mL, and median CD4+ and CD8+ T-cell counts were 378 cells/µL and 915 cells/µL, respectively. One percent of women were anti–syphilis antibody positive and 5% were anti-HCV positive (Table 1). Women who were not included in this study because of insufficient samples had similar baseline characteristics: age of enrollment, ALT level, CD4+ and CD8+ T-cell counts, and HIV RNA load (data not shown).
Figure 1.
Overall study diagram. Abbreviations: anti-HBc, antibody to hepatitis B core antigen; anti-HBs, antibody to hepatitis B surface antigen; HBV, hepatitis B virus; HBsAg, hepatitis B surface antigen.
Table 1.
Characteristics of HIV-1–Infected Pregnant Women Negative for Hepatitis B Surface Antigen
| Characteristic | No. | Median (IQR) or No. (%) |
|---|---|---|
| Age at enrollment, y | 1682 | 25.9 (22.7–29.7) |
| Region of birth | 1682 | |
| Central | 373 (22) | |
| Eastern | 256 (15) | |
| Northern | 348 (21) | |
| Northeastern | 553 (33) | |
| Southern | 76 (5) | |
| Western | 76 (5) | |
| Prior pregnancy | 1678 | 1041 (62) |
| SGPT or ALT, IU/L | 1638 | 15 (10–24) |
| WBC count, cells/μL | 1652 | 8615 (7300–10 160) |
| Absolute lymphocyte count, cells/μL | 1650 | 1805 (1430–2250) |
| Absolute CD4 count, cells/μL | 1671 | 378 (245–531) |
| Absolute CD8 count, cells/μL | 1634 | 915 (700–1193) |
| HIV RNA load, log10 copies/mL | 1660 | 4.03 (3.37–4.65) |
| Anti-syphilis antibody positive | 1649 | 17 (1) |
| Anti-HCV antibody positive | 1659 | 75 (5) |
Abbreviations: ALT, alanine aminotransferase; HCV, hepatitis C virus; HIV, human immunodeficiency virus; IQR, interquartile range; SGPT, serum glutamic pyruvic transaminas; WBC, white blood cell.
HBV Serology Profile Among HBsAg-Negative, HIV-1–Infected Pregnant Women
Of 1682 HBsAg-negative women, 553 (33%; 95% CI, 31%–35%) had markers of resolved HBV infection (anti-HBc and anti-HBs positive), 229 (14%; 95% CI, 12%–15%) had marker of exposure to HBV (isolated anti-HBc), 68 (4%; 95% CI, 3%–5%) had marker of hepatitis B vaccine (anti-HBs positive only), and 832 (49%; 95% CI, 47%–52%) had no markers of exposure to HBV (anti-HBc and anti-HBs negative).
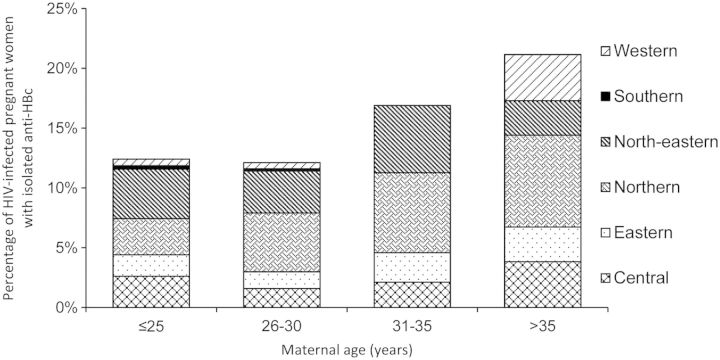
The prevalence of isolated anti-HBc antibodies differed according to the region of birth; the highest rate, 22%, was found in women born in the northern region of Thailand, whereas the lowest rate, 4%, was found in the southern region (Table 2). The rate of isolated anti-HBc in HIV-1–infected pregnant women increased with their age (Figure 2).
Table 2.
Hepatitis B Virus Serological Status According to Region of Birth of Hepatitis B Surface Antigen–Negative Women
| Status | Central | Eastern | Northern | Northeastern | Southern | Western | Total |
|---|---|---|---|---|---|---|---|
| Anti-HBc+/anti-HBs+ | 128 (34) | 74 (29) | 148 (43) | 161 (29) | 20 (26) | 22 (29) | 553 (33) |
| Anti-HBc+/anti-HBs− | 38 (10) | 31 (12) | 77 (22) | 69 (12) | 3 (4) | 11 (14) | 229 (14) |
| Anti-HBc−/anti-HBs+ | 26 (7) | 10 (5) | 16 (5) | 10 (2) | 1 (1) | 5 (7) | 68 (4) |
| Anti-HBc−/anti-HBs− | 181 (49) | 141 (55) | 107 (31) | 313 (57) | 52 (68) | 38 (50) | 832 (49) |
Abbreviations: anti-HBc,antibody to hepatitis B core antigen; anti-HBs, antibody to hepatitis B surface antigen.
Figure 2.
Age-related distribution of patients with isolated antibody to hepatitis B core antigen. Abbreviations: anti-HBc, antibody to hepatitis B core antigen; HIV, human immunodeficiency virus.
Factors Associated With Isolated Anti-HBc
Among all parameters analyzed, univariate analysis showed that age >35 years, birth in northern region, white blood cell count <7500 cells/µL, lymphocyte count <1000 cells/µL, CD4+ T-cell count <350 cells/µL, and HCV infection were significantly associated with isolated anti-HBc serology in HIV-1–infected pregnant women (Table 3). After adjustment on all significant parameters, factors independently associated with isolated anti-HBc were age >35 years (adjusted odds ratio [AOR], 1.8; P = .029); birth in northern region (AOR, 1.8; P< .001); absolute CD4+ cell count <350 cells/µL (AOR, 1.5; P = .02) and, much more significant, CD4+ cell count <200 cells/µL (AOR, 2.8; P < .001); and exposure to HCV (AOR, 2.6; P = .001) (Table 3).
Table 3.
Factors Associated With Isolated Antibody to Hepatitis B Core Antigen in HIV-1–Infected Pregnant Women
| Parameter | Category | No. | Isolated Anti-HBc (%) | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Valuea | OR (95% CI) | P Valueb | ||||
| Age at enrollment, y | ≤25 | 725 | 90 (12) | 1.0 | |||
| 26–30 | 569 | 69 (12) | 1.0 (0.7–1.4) | .88 | |||
| 31–35 | 284 | 48 (17) | 1.4 (1.0–2.1) | .06 | |||
| >35 | 104 | 22 (21) | 1.9 (1.1–3.2) | .016 | 1.8 (1.1–2.9) | .029 | |
| Region of birth | Central | 373 | 38 (10) | 1.0 | |||
| Eastern | 256 | 31 (12) | 1.2 (.7–2.0) | .45 | |||
| Northern | 348 | 77 (22) | 2.5 (1.6–3.8) | <.001 | 1.8 (1.3–2.5) | <.001 | |
| Northeastern | 553 | 69 (12) | 1.3 (.8–1.9) | .29 | |||
| Southern | 76 | 3 (4) | .4 (.1–1.2) | .10 | .4 (.1–1.1) | NS | |
| Western | 76 | 11 (14) | 1.5 (.7–3.1) | .28 | |||
| Prior pregnancy | No | 637 | 83 (13) | 1.0 | |||
| Yes | 1041 | 145 (14) | 1.1 (.8–1.4) | .60 | |||
| ALT, IU/L | ≤30 | 1437 | 190 (13) | 1.0 | |||
| 31–60 | 168 | 25 (15) | 1.1 (.7–1.8) | .55 | |||
| >60 | 33 | 7 (21) | 1.8 (.8–4.1) | .19 | |||
| WBC count, cells/μL | >10 000 | 440 | 44 (10) | 1.0 | |||
| 7501–10 000 | 723 | 91 (13) | 1.3 (.9–1.9) | .18 | |||
| 5001–7500 | 442 | 78 (18) | 1.9 (1.3–2.9) | .001 | |||
| ≤5000 | 47 | 10 (21) | 2.4 (1.1–5.2) | .02 | |||
| Absolute lymphocyte count, cells/μL | >2000 | 624 | 74 (12) | 1.0 | |||
| 1501–2000 | 533 | 63 (12) | 1.0 (.7–1.4) | .98 | |||
| 1001–1500 | 349 | 52 (15) | 1.3 (.9–1.9) | .18 | |||
| ≤1000 | 144 | 33 (23) | 2.2 (1.4–3.5) | .001 | |||
| Absolute CD4+ T cell count, cells/μL | >500 | 489 | 44 (9) | 1.0 | |||
| 351–500 | 423 | 43 (10) | 1.1 (.7–1.8) | .55 | |||
| 201–350 | 446 | 65 (15) | 1.7 (1.1–2.6) | .009 | 1.5 (1.1–2.2) | .02 | |
| ≤200 | 313 | 76 (24) | 3.2 (2.2–4.9) | <.001 | 2.8 (2.0–4.0) | <.001 | |
| Absolute CD8+ T-cell count, cells/μL | >1500 | 179 | 21 (12) | 1.0 | |||
| 1001–1500 | 489 | 58 (12) | 1.0 (.6–1.7) | .96 | |||
| 501–1000 | 835 | 119 (14) | 1.3 (.8–2.1) | .38 | |||
| ≤500 | 131 | 24 (18) | 1.7 (.9–3.2) | .11 | |||
| HIV RNA load, log10 copies/μL | Undetectable | 42 | 5 (12) | 1.0 | |||
| 1.18–3.00 | 216 | 23 (11) | .9 (.3–2.5) | .81 | |||
| 3.01–4.00 | 552 | 75 (14) | 1.2 (.4–3.1) | .76 | |||
| 4.01–5.00 | 659 | 85 (13) | 1.1 (.4–2.9) | .85 | |||
| >5.00 | 191 | 38 (20) | 1.8 (.7–5.0) | .23 | |||
| Anti-syphilis antibody | No | 1632 | 220 (13) | 1.0 | |||
| Yes | 17 | 2 (12) | .9 (.2–3.8) | .84 | |||
| Anti-HCV antibody | No | 1584 | 202 (13) | 1.0 | |||
| Yes | 75 | 23 (31) | 3.0 (1.8–5.1) | <.001 | 2.6 (1.5–4.3) | .001 | |
Boldface text indicates significant values (≤.05).
Abbreviations: ALT, alanine aminotransferase; anti-HBc, antibody to hepatitis B core antigen; CI, confidence interval; HCV, hepatitis C virus; HIV-1, human immunodeficiency virus type 1; NS, not significant; OR, odds ratio; WBC, white blood cell.
a Logistic regression analysis was used.
b Multivariate logistic regression analysis was used.
Prevalence of Occult HBV Infection Among HIV-1–Infected Pregnant Women With Isolated Anti-HBc
We first verified the absence of HBsAg in all women with isolated anti-HBc using a different HBsAg test kit. Of 228 women with available samples, 12 (5%) tested positive for HBsAg with the new test kit. Samples of all but 1 woman showed a low signal-to-cutoff ratio, ranging from 1.02 to 2.79 (median, 1.4 [IQR, 1.1–2.0]). Women with discrepant HBsAg results were then excluded from further analysis.
Among all 216 HIV-1–infected pregnant women with confirmed isolated anti-HBc serology, 200 had a sample available for HBV DNA quantification. All 200 women had HBV DNA <1000 IU/mL; 153 had HBV DNA below the limit of detection (15 IU/mL), 44 had HBV DNA level between 15–100 IU/mL, and 3 had HBV DNA between 101 and 1000 IU/mL. The prevalence of occult HBV infection among women with isolated anti-HBc was thus 23.5% (47/200; 95% CI, 18%–30%). Of 47 women with detectable HBV DNA (>15 IU/mL), 2 had successful HBV sequencing: one (16 IU/mL HBV DNA level) had sS117I, sT118K, and sR160K mutations (GenBank accession number: JX402002), and the other (117 IU/mL HBV DNA) had no S gene mutation (GenBank accession number: JX402003).
Factors Associated With Occult HBV Infection in HIV-1–Infected Pregnant Women With Isolated Anti-HBc Serology
Among all parameters analyzed, only HIV RNA level was inversely associated with occult HBV infection in HIV-1–infected pregnant women having isolated anti-HBc serological pattern (AOR, 0.2; P = .013; Table 4).
Table 4.
Factors Associated With Occult Hepatitis B Virus Infection in HIV-1–Infected Pregnant Women With Isolated Antibody to Hepatitis B Core Antigen
| Parameters | Category | No. | Occult HBV Infection (%) | Univariate Analysis |
Multivariate Analysis |
||
|---|---|---|---|---|---|---|---|
| OR (95% CI) | P Valuea | OR (95% CI) | P Valueb | ||||
| Age at enrollment, y | ≤25 | 83 | 20 (24) | 1 | |||
| 26–30 | 57 | 12 (21) | .8 (.4–1.9) | .67 | |||
| 31–35 | 39 | 8 (21) | .8 (.3–2.1) | .66 | |||
| >35 | 21 | 7 (33) | 1.6 (.6–4.4) | .39 | |||
| Region of birth | Central | 29 | 9 (31) | 1 | |||
| Eastern | 27 | 4 (14) | .4 (.1–1.4) | .16 | |||
| Northern | 69 | 18 (26) | .8 (.3–2.0) | .62 | |||
| Northeastern | 62 | 16 (26) | .8 (.3–2.0) | .60 | |||
| Southern | 3 | 0 (0) | … | … | |||
| Western | 10 | 0 (0) | … | … | |||
| Previous pregnancy | No | 75 | 16 (21) | 1 | |||
| Yes | 124 | 31 (25) | 1.2 (.6–2.4) | .56 | |||
| ALT, IU/L | ≤30 | 165 | 42 (25) | 1 | |||
| 31–60 | 22 | 2 (9) | .3 (.1–1.3) | .11 | |||
| >60 | 6 | 2 (33) | 1.5 (.3–8.3) | .67 | |||
| WBC count, cells/μL | >10 000 | 36 | 7 (19) | 1 | |||
| 7501–10 000 | 80 | 17 (21) | 1.1 (.4–3.0) | .82 | |||
| 5001–7500 | 70 | 20 (29) | 1.7 (.6–4.4) | .31 | |||
| ≤5000 | 8 | 2 (25) | 1.4 (.2–8.4) | .73 | |||
| Absolute lymphocyte count, cells/μL | >2000 | 62 | 13 (21) | 1 | |||
| 1501–2000 | 55 | 16 (29) | 1.5 (.7–3.6) | .31 | |||
| 1001–1500 | 45 | 13 (29) | 1.5 (.6–3.7) | .35 | |||
| ≤1000 | 31 | 4 (13) | .6 (.2–1.9) | .35 | |||
| Absolute CD4+ T-cell count, cells/μL | >500 | 40 | 10 (25) | 1 | |||
| 351–500 | 37 | 8 (22) | .8 (.3–2.4) | .73 | |||
| 201–350 | 54 | 15 (28) | 1.2 (.5–2.9) | .76 | |||
| ≤200 | 68 | 14 (21) | .8 (.3–2.0) | .60 | |||
| Absolute CD8+ T-cell count, cells/μL | >1500 | 18 | 7 (39) | 1 | |||
| 1001–1500 | 51 | 9 (18) | .3 (.1–1.0) | .07 | |||
| 501–1000 | 104 | 25 (24) | .5 (.2–1.4) | .19 | |||
| ≤500 | 22 | 5 (23) | .5 (.1–1.8) | .27 | |||
| HIV RNA load, log10 copies/μL | ≤ 3.00 | 24 | 10 (42) | 1 | |||
| 3.01–4.00 | 68 | 18 (26) | .5 (.2–1.3) | .17 | .5 (.2–1.3) | .17 | |
| 4.01–5.00 | 71 | 15 (21) | .4 (.1–1.0) | .05 | .4 (.1–1.0) | .05 | |
| > 5.00 | 34 | 4 (12) | .2 (.05–.7) | .013 | .2 (.05–.7) | .013 | |
| Anti-syphilis antibody | No | 191 | 45 (24) | 1 | |||
| Yes | 2 | 1 (50) | 3.2 (.2–53) | .41 | |||
| Anti-HCV antibody | No | 174 | 39 (22) | 1 | |||
| Yes | 22 | 8 (36) | 2.0 (.8–5.1) | .15 | |||
Boldface text indicates significant values (≤.05).
Abbreviations: ALT, alanine aminotransferase; CI, confidence interval; HBV, hepatitis B virus; HCV, hepatitis C virus; HIV-1, human immunodeficiency virus type 1; OR, odds ratio; WBC, white blood cell.
a Logistic regression analysis was used.
b Multivariate logistic regression analysis was used.
Assessment of HBV Infection in Infants Born to Mothers With Occult HBV Infection
We assessed HBV infection in infants born to 47 mothers with detectable HBV DNA (>15 IU/mL) at enrollment. No HBV DNA was detected in any of their infants at 4 months of age.
DISCUSSION
This is the first detailed analysis of HBV serologic markers among a large number of HIV-1–pregnant women. We have analyzed 3 variables associated with isolated anti-HBc profile and occult HBV infection: prevalence, risk factors, and impact on perinatal transmission of HBV. Consistent with data from regions where vertical transmission of HBV has significant contribution, about half of HIV-1–infected pregnant women in our study showed HBV exposure markers. Fourteen percent had isolated anti-HBc. This rate is close to that observed among HIV-1–infected adults in Bangkok (20%) [20], northern areas (13%; S. Thongsawat, unpublished data), and other countries with high prevalence of chronic HBV infection [11–13].
About half of HIV-1–infected pregnant women had no HBV serological markers, indicating they are an HBV-susceptible population. Because immunization with HBV vaccine is strongly recommended for all HIV-infected individuals without immunity to HBV [26], our finding highlights the need for testing of all HIV-infected patients in order to vaccinate uninfected people who are not immune.
We identified several independent risk factors for isolated anti-HBc serological status in HIV-1–infected pregnant women: low CD4 count, age >35 years, and HCV infection. These factors have also been found in other populations, both HIV-infected [8, 22, 27] and HIV-uninfected [28]. Severe immunocompromised status, CD4 T-cell count <100 cells/µL, has been associated with loss of anti-HBs and development of isolated anti-HBc in HIV-positive patients [22]. The effect of age may be related to the progressive loss of anti-HBs producing capacity over time after resolution of HBV infection, or insufficient level of anti-HBs production [29]. We also found that being born in the northern region of Thailand was independently associated with isolated anti-HBc. Other studies have reported higher prevalence of HBsAg positivity in the northern region of Thailand as compared to the southern region [30–32], which may explain the rates of isolated anti-HBc observed in our study. Our results are also consistent with other studies describing HCV infection as a main factor for isolated anti-HBc in both HIV-infected and -uninfected populations [1, 14, 17, 19, 20, 33], possibly as a result of the direct interference of HCV core protein on the synthesis of HBsAg [34, 35].
A wide range (0%–89%) of occult HBV infection has been reported in HIV-infected patients with isolated anti-HBc [22]. The heterogeneity of study populations and the usage of different sensitivity and specificity of HBV DNA assays may account for these discrepancies. In this study, we used a highly sensitive commercial technique to detect HBV DNA and were thus able to detect HBV DNA in 24% (47 of 200) of HIV-1–infected pregnant women with isolated anti-HBc serological profile. These rates of occult HBV infection are within the range found in isolated anti-HBc blood donors (4%–24%) of high-HBV-endemic areas such as India, Taiwan, Japan, and Sardinia [36]. When considering the whole population of HIV-1–infected pregnant women, the prevalence of isolated anti-HBc and occult HBV infection was 2.6% (47/1783; 95% CI, 1.9%–3.5%).
One intriguing observation was the inverse association between the detection of HBV DNA and HIV RNA concentrations. Unlike Lo Re et al [37] who found more frequently occult HBV infection in patients with HIV RNA >1000 copies/mL (17% in patients with a high HIV RNA level vs 4.6% in those with a low HIV RNA level, n = 179), we observed a higher rate of occult HBV infection in patients (n = 200) with low HIV RNA concentrations than in those with high HIV RNA level (42% vs 21%; P = .04). Possible explanations to this could be that 73% of patients in Lo Re et al's study were on highly active antiretroviral treatment, whereas in our study all women were naive to antiretroviral treatment. Further studies are needed to understand this discrepancy.
The clinical relevance of isolated anti-HBc and impact of low levels of HBV DNA in HIV-pregnant women with isolated anti-HBc are not well known. Walz et al recently reported that 7 of 105 infants born to women with isolated anti-HBc were infected with HBV, but none of the infants were positive for both HBsAg and HBV DNA. Interestingly, only 1 woman was HBV DNA positive [38]. In our study, the level of HBV DNA was <1000 IU/mL in 47 women with occult HBV infection, and none transmitted HBV to their infants.
Our study has several limitations. First, we quantified HBV DNA at only 1 time point, which may be insufficient as HBV DNA levels can fluctuate over time, depending on the phase of infection and host immune responses. However, pregnant women have been shown to have stable HBV DNA levels, with slight increases during late pregnancy [39], likely due to their relative immune-suppressed state. In our study, HBV DNA was measured in pregnant women during the last trimester. Our results are thus in favor of a low level of HBV replication in women with isolated anti-HBc. Second, infant HBV infection status was determined using HBV DNA PCR at 4 months of age. Because children in the original study (prevention of mother-to-child transmission of HIV-1) were only followed up until 12 months of age, it was not possible to reliably rule out a perinatal exposure to HBV based on anti-HBc testing. Indeed, anti-HBc immunoglobulin G, a marker of exposure to HBV, is passively transmitted through placenta to the fetus and can persist in children several months before being completely cleared at 24 months of age [40].
In conclusion, our study shows that the prevalence of HIV-1–infected pregnant women presenting with isolated anti-HBc/occult HBV infection was low (2.6%) and that women with isolated anti-HBc and occult HBV infection have very low HBV DNA levels and are thus at very low risk to transmit HBV to their infants.
Notes
Acknowledgments. We are grateful to the women who participated in this study and the medical teams involved in their care. We thank Satawat Thongsawat (Chiang Mai University) for advice on the discussion; Paporn Mongkolwat, Ampika Kaewbundit, Duangthida Saeng-ai, and Kankanitta Pongmorn for laboratory assistance; Sanuphong Chailoet for providing clinical data; and Kathleen A Culhane-Pera and Timothy Cressey for their review and useful comments.
PHPT-2 study co-investigators. (Numbers of women included in the analysis are given in parentheses.) Rayong Hospital (138): W. Suwankornsakul, S. Weerawatgoompa-Lorenz, S. Ariyadej, W. Karnchanamayul, P. Dumrongkitchaiporn; Hat Yai Hospital (84): S. Lamlertkittikul, T. Jarupanich, B. Warachit, T. Borkird; Samutsakhon Hospital (81): T. Sukhumanant, P. Thanasiri; Nakhonpathom Hospital (79): V. Chalermpolprapa, S. Bunjongpak; Samutprakarn Hospital (79): P. Sabsanong, C. Sriwacharakarn; Bhumibol Adulyadej Hospital (76): S. Prommas, P. Layangool, K. Kengsakul; Banglamung Hospital (61): J. Ithisuknanth, S. Sirithadthamrong, K. Chumpakdee; Chiangrai Prachanukroh Hospital (60): P. Wattanaporn, J. Achalapong, R. Hansudewechakul; Queen Sirikit Hospital (58): T. Hinjiranandana, P. Waithayakul; Prapokklao Hospital (57): C. Veerakul, P. Yuthavisuthi, C. Ngampiyaskul; Somdej Prapinklao Hospital (54): S. Suphanich, N. Kamonpakorn; Health Promotion Center Region 10 (53): S. Sangsawang, W. Pattanaporn, W. Jitphiankha, V. Sittipiyasakul; Chonburi Hospital (53): N. Chotivanich, S. Hongsiriwon; Buddhachinaraj Hospital (50): W. Wannapira, W. Ardong; Pranangklao Hospital (48): S. Pipatnakulchai, S. Wanwaisart, S. Watanayothin; Klaeng Hospital (46): S. Techapalokul, S. Hotrawarikarn; Bhuddasothorn Hospital (44): A. Kanjanasing, R. Kaewsonthi, R. Kwanchaipanich; Chiang Kham Hospital (36): C. Putiyanun, P. Jittamala; Ratchaburi Hospital (36): T. Chonladarat, O. Bamroongshawkaseme, P. Malitong; Kalasin Hospital (35): B. Suwannachat, S. Srirojana; Lamphun Hospital (34): W. Matanasarawut, K. Pagdi, P. Wannarit, R. Somsamai, S. Khunpradit; Regional Health Promotion Centre 6 (32): N. Winiyakul, S. Hanpinitsak, N. Pramukkul; Nong Khai Hospital (31): N. Puarattana-aroonkorn, S. Potchalongsin; Mahasarakam Hospital (31): S. Nakhapongse, W. Worachet, P. Sitsirat, S. Tonmat, K. Kovitanggoon; Roi-et Hospital (31): W. Atthakorn; Phaholpolpayuhasaena Hospital (30): Y. Srivarasat, P. Attavinijtrakarn; Khon Kaen Hospital (29): J. Ratanakosol, W. Chandrakachorn; Mae Chan Hospital (28): S. Buranabanjasatean; Health Promotion Hospital Regional Center I (28): W. Laphikanont, S. Sirinontakan; Mae Sai Hospital (26): S. Nanta, T. Meephian; Nakornping Hospital (26): A. Limtrakul, V. Gomuthbutra, S. Kanjanavanit, P. Krittigamas; Srinagarind Hospital (25): C. Sakondhavat, R. Sripanidkulchai; Phan Hospital (24): S. Jungpichanvanich; Phayao Provincial Hospital (23): S. Bhakeecheep, U. Sriminipun, S. Techakulviroj; Nopparat Rajathanee Hospital (23): T. Chanpoo, S. Surawongsin, S. Ruangsirinusorn, P. Suntarattiwong; Kranuan Crown Prince Hospital (17): R. Thongdej, S. Benchakhanta; Prajaksilapakom Army Hospital (16): W. Srichandraphan.
Financial support. This work was supported by the Agence Nationale de Recherches sur le Sida et les hépatites virales (grant number ANRS 12-179); the National Institute of Child Health and Human Development (R01 HD 39615); the National Institutes of Health, Fogarty International Research Collaboration Award (R03 TW01346); the Thailand International Development Cooperation Agency; the Global Fund to fight AIDS, TB and Malaria (GFATM PRDDC-H-N-008/SSF); the Franco-Thai cooperation program in Higher Education and Research; the Faculty of Associated Medical Sciences, Chiang Mai University; the French Ministry of Foreign Affairs; and the Institut de Recherche pour le Développement (IRD), France. W. K. has received scholarships from the IRD and the Franco-Thai cooperation program in higher education and research.
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Grob P, Jilg W, Bornhak H, et al. Serological pattern “anti-HBc alone”: report on a workshop. J Med Virol. 2000;62:450–5. doi: 10.1002/1096-9071(200012)62:4<450::aid-jmv9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.Hoofnagle JH, Seeff LB, Bales ZB, Zimmerman HJ. Type B hepatitis after transfusion with blood containing antibody to hepatitis B core antigen. N Engl J Med. 1978;298:1379–83. doi: 10.1056/NEJM197806222982502. [DOI] [PubMed] [Google Scholar]
- 3.De Feo TM, Poli F, Mozzi F, Moretti MP, Scalamogna M. Risk of transmission of hepatitis B virus from anti-HBC positive cadaveric organ donors: a collaborative study. Transplant Proc. 2005;37:1238–9. doi: 10.1016/j.transproceed.2004.12.041. [DOI] [PubMed] [Google Scholar]
- 4.Descos B, Scotto J, Fayol V, et al. Anti-HBc screening for the prevention of perinatal transmission of hepatitis B virus in France. Infection. 1987;15:434–9. doi: 10.1007/BF01647225. [DOI] [PubMed] [Google Scholar]
- 5.Chamorro AJ, Casado JL, Bellido D, Moreno S. Reactivation of hepatitis B in an HIV-infected patient with antibodies against hepatitis B core antigen as the only serological marker. Eur J Clin Microbiol Infect Dis. 2005;24:492–4. doi: 10.1007/s10096-005-1355-1. [DOI] [PubMed] [Google Scholar]
- 6.Jain M, Chakravarti A, Kar P. Clinical significance of isolated anti-HBc positivity in cases of chronic liver disease in New Delhi, India. J Glob Infect Dis. 2009;1:29–32. doi: 10.4103/0974-777X.52978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ikeda K, Kobayashi M, Someya T, et al. Occult hepatitis B virus infection increases hepatocellular carcinogenesis by eight times in patients with non-B, non-C liver cirrhosis: a cohort study. J Viral Hepat. 2009;16:437–43. doi: 10.1111/j.1365-2893.2009.01085.x. [DOI] [PubMed] [Google Scholar]
- 8.Sheng WH, Kao JH, Chen PJ, et al. Evolution of hepatitis B serological markers in HIV-infected patients receiving highly active antiretroviral therapy. Clin Infect Dis. 2007;45:1221–9. doi: 10.1086/522173. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann CJ, Seaberg EC, Young S, et al. Hepatitis B and long-term HIV outcomes in coinfected HAART recipients. AIDS. 2009;23:1881–9. doi: 10.1097/QAD.0b013e32832e463a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hennig H, Puchta I, Luhm J, Schlenke P, Goerg S, Kirchner H. Frequency and load of hepatitis B virus DNA in first-time blood donors with antibodies to hepatitis B core antigen. Blood. 2002;100:2637–41. doi: 10.1182/blood-2002-03-0798. [DOI] [PubMed] [Google Scholar]
- 11.Asim M, Ali R, Khan LA, Husain SA, Singla R, Kar P. Significance of anti-HBc screening of blood donors and its association with occult hepatitis B virus infection: implications for blood transfusion. Indian J Med Res. 2010;132:312–7. [PubMed] [Google Scholar]
- 12.Thedja MD, Roni M, Harahap AR, Siregar NC, Ie SI, Muljono DH. Occult hepatitis B in blood donors in Indonesia: altered antigenicity of the hepatitis B virus surface protein. Hepatol Int. 2010;4:608–14. doi: 10.1007/s12072-010-9203-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seo DH, Whang DH, Song EY, Kim HS, Park Q. Prevalence of antibodies to hepatitis B core antigen and occult hepatitis B virus infections in Korean blood donors. Transfusion. 2011;51:1840–6. doi: 10.1111/j.1537-2995.2010.03056.x. [DOI] [PubMed] [Google Scholar]
- 14.Gandhi RT, Wurcel A, Lee H, et al. Isolated antibody to hepatitis B core antigen in human immunodeficiency virus type-1–infected individuals. Clin Infect Dis. 2003;36:1602–5. doi: 10.1086/375084. [DOI] [PubMed] [Google Scholar]
- 15.Neau D, Winnock M, Galperine T, et al. Isolated antibodies against the core antigen of hepatitis B virus in HIV-infected patients. HIV Med. 2004;5:171–3. doi: 10.1111/j.1468-1293.2004.00206.x. [DOI] [PubMed] [Google Scholar]
- 16.Liang SH, Chen TJ, Lee SS, et al. Risk factors of isolated antibody against core antigen of hepatitis B virus: association with HIV infection and age but not hepatitis C virus infection. J Acquir Immune Defic Syndr. 2010;54:122–8. doi: 10.1097/QAI.0b013e3181daafd5. [DOI] [PubMed] [Google Scholar]
- 17.Gandhi RT, Wurcel A, McGovern B, et al. Low prevalence of ongoing hepatitis B viremia in HIV-positive individuals with isolated antibody to hepatitis B core antigen. J Acquir Immune Defic Syndr. 2003;34:439–41. doi: 10.1097/00126334-200312010-00013. [DOI] [PubMed] [Google Scholar]
- 18.French AL, Operskalski E, Peters M, et al. Isolated hepatitis B core antibody is associated with HIV and ongoing but not resolved hepatitis C virus infection in a cohort of US women. J Infect Dis. 2007;195:1437–42. doi: 10.1086/515578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cacciola I, Pollicino T, Squadrito G, Cerenzia G, Orlando ME, Raimondo G. Occult hepatitis B virus infection in patients with chronic hepatitis C liver disease. N Engl J Med. 1999;341:22–6. doi: 10.1056/NEJM199907013410104. [DOI] [PubMed] [Google Scholar]
- 20.Jongjirawisan Y, Ungulkraiwit P, Sungkanuparph S. Isolated antibody to hepatitis B core antigen in HIV-1 infected patients and a pilot study of vaccination to determine the anamnestic response. J Med Assoc Thai. 2006;89:2028–34. [PubMed] [Google Scholar]
- 21.Allain JP. Occult hepatitis B virus infection: implications in transfusion. Vox Sang. 2004;86:83–91. doi: 10.1111/j.0042-9007.2004.00406.x. [DOI] [PubMed] [Google Scholar]
- 22.Sun HY, Lee HC, Liu CE, et al. Factors associated with isolated anti-hepatitis B core antibody in HIV-positive patients: impact of compromised immunity. J Viral Hepat. 2010;17:578–87. doi: 10.1111/j.1365-2893.2009.01212.x. [DOI] [PubMed] [Google Scholar]
- 23.Filippini P, Coppola N, Pisapia R, et al. Impact of occult hepatitis B virus infection in HIV patients naive for antiretroviral therapy. AIDS. 2006;20:1253–60. doi: 10.1097/01.aids.0000232232.41877.2a. [DOI] [PubMed] [Google Scholar]
- 24.Lallemant M, Jourdain G, Le Coeur S, et al. Single-dose perinatal nevirapine plus standard zidovudine to prevent mother-to-child transmission of HIV-1 in Thailand. N Engl J Med. 2004;351:217–28. doi: 10.1056/NEJMoa033500. [DOI] [PubMed] [Google Scholar]
- 25.Villeneuve JP, Durantel D, Durantel S, et al. Selection of a hepatitis B virus strain resistant to adefovir in a liver transplantation patient. J Hepatol. 2003;39:1085–9. doi: 10.1016/j.jhep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 26.Kaplan JE, Benson C, Holmes KH, Brooks JT, Pau A, Masur H. Guidelines for prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: recommendations from CDC, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. MMWR Recomm Rep. 2009;58:1–207. quiz CE201–204. [PubMed] [Google Scholar]
- 27.Laukamm-Josten U, Muller O, Bienzle U, Feldmeier H, Uy A, Guggenmoos-Holzmann I. Decline of naturally acquired antibodies to hepatitis B surface antigen in HIV-1 infected homosexual men. AIDS. 1988;2:400–1. doi: 10.1097/00002030-198810000-00014. [DOI] [PubMed] [Google Scholar]
- 28.Manzini P, Girotto M, Borsotti R, et al. Italian blood donors with anti-HBc and occult hepatitis B virus infection. Haematologica. 2007;92:1664–70. doi: 10.3324/haematol.11224. [DOI] [PubMed] [Google Scholar]
- 29.Ponde RA, Cardoso DD, Ferro MO. The underlying mechanisms for the ‘anti-HBc alone’ serological profile. Arch Virol. 2010;155:149–58. doi: 10.1007/s00705-009-0559-6. [DOI] [PubMed] [Google Scholar]
- 30.Poovorawan Y, Theamboonlers A, Vimolket T, et al. Impact of hepatitis B immunisation as part of the EPI. Vaccine. 2000;19:943–9. doi: 10.1016/s0264-410x(00)00216-4. [DOI] [PubMed] [Google Scholar]
- 31.Louisirirotchanakul S, Myint KS, Srimee B, et al. The prevalence of viral hepatitis among the Hmong people of northern Thailand. Southeast Asian J Trop Med Public Health. 2002;33:837–44. [PubMed] [Google Scholar]
- 32.Pradutkanchana S, Nasongkla K, Pradutkanchana J, Heembai U. A ten-year trend of the prevalence of hepatitis B surface antigen in pregnant women at Songklanagarind hospital. J Infect Dis Antimicrob Agents. 2005;22:111–4. [Google Scholar]
- 33.Berger A, Doerr HW, Rabenau HF, Weber B. High frequency of HCV infection in individuals with isolated antibody to hepatitis B core antigen. Intervirology. 2000;43:71–6. doi: 10.1159/000025026. [DOI] [PubMed] [Google Scholar]
- 34.Chen SY, Kao CF, Chen CM, et al. Mechanisms for inhibition of hepatitis B virus gene expression and replication by hepatitis C virus core protein. J Biol Chem. 2003;278:591–607. doi: 10.1074/jbc.M204241200. [DOI] [PubMed] [Google Scholar]
- 35.Schuttler CG, Fiedler N, Schmidt K, Repp R, Gerlich WH, Schaefer S. Suppression of hepatitis B virus enhancer 1 and 2 by hepatitis C virus core protein. J Hepatol. 2002;37:855–62. doi: 10.1016/s0168-8278(02)00296-9. [DOI] [PubMed] [Google Scholar]
- 36.Allain JP. Occult hepatitis B virus infection. Transfus Clin Biol. 2004;11:18–25. doi: 10.1016/j.tracli.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Lo Re V, 3rd, Frank I, Gross R, et al. Prevalence, risk factors, and outcomes for occult hepatitis B virus infection among HIV-infected patients. J Acquir Immune Defic Syndr. 2007;44:315–20. doi: 10.1097/QAI.0b013e31802ea499. [DOI] [PubMed] [Google Scholar]
- 38.Walz A, Wirth S, Hucke J, Gerner P. Vertical transmission of hepatitis B virus (HBV) from mothers negative for HBV surface antigen and positive for antibody to HBV core antigen. J Infect Dis. 2009;200:1227–31. doi: 10.1086/605698. [DOI] [PubMed] [Google Scholar]
- 39.Soderstrom A, Norkrans G, Lindh M. Hepatitis B virus DNA during pregnancy and post partum: aspects on vertical transmission. Scand J Infect Dis. 2003;35:814–9. doi: 10.1080/00365540310016547. [DOI] [PubMed] [Google Scholar]
- 40.Wang JS, Chen H, Zhu QR. Transformation of hepatitis B serologic markers in babies born to hepatitis B surface antigen positive mothers. World J Gastroenterol. 2005;11:3582–5. doi: 10.3748/wjg.v11.i23.3582. [DOI] [PMC free article] [PubMed] [Google Scholar]