Abstract
Background
Bone marrow-derived endothelial progenitor cells (EPCs) are critical for metastatic progression. This study explores the effect of tetrathiomolybdate (TM), an anti-angiogenic copper chelator, on EPCs in patients at high risk for breast cancer recurrence.
Patients and methods
This phase 2 study enrolled breast cancer patients with stage 3 and stage 4 without evidence of disease (NED), and stage 2 if triple-negative. TM 100 mg orally was administered to maintain ceruloplasmin <17 mg/dl for 2 years or until relapse. The primary end point was change in EPCs.
Results
Forty patients (28 stage 2/3, 12 stage 4 NED) were enrolled. Seventy-five percent patients achieved the copper depletion target by 1 month. Ninety-one percent of triple-negative patients copper-depleted compared with 41% luminal subtypes. In copper-depleted patients only, there was a significant reduction in EPCs/ml by 27 (P = 0.04). Six patients relapsed while on study, of which only one patient had EPCs maintained below baseline. The 10-month relapse-free survival was 85.0% (95% CI 74.6%–96.8%). Only grade 3/4 toxicity was hematologic: neutropenia (3.1% of cycles), febrile neutropenia (0.2%), and anemia (0.2%).
Conclusions
TM is safe and appears to maintain EPCs below baseline in copper-depleted patients. TM may promote tumor dormancy and ultimately prevent relapse.
Keywords: breast cancer, endothelial progenitor cells, tetrathiomolybdate
introduction
Despite improvements in adjuvant therapy of breast cancer over the past two decades, there is significant risk of relapse in a high-risk subset of patients. Although the definition of high risk has been evolving, it still includes patients with stage 3 breast cancer and those with stage 4 with no evidence of disease (NED). The risk of relapse in stage 3 patients is 50%–75% over 5 years, and patients with stage 4 breast cancer will inevitably recur even when temporarily rendered disease-free by surgery, radiation, or chemotherapy [1]. Reflecting the increasing importance of biology over stage, now included in the definition is the triple-negative subset [lack of expression of estrogen receptor (ER)/progesterone receptor (PR), nor overexpression of human epidermal growth factor 2 (HER2)]. These patients have a poor prognosis in earlier stages and represent a disproportionately increased percentage with metastatic disease [2–4].
The tumor microenvironment and its components, including stromal fibroblasts and endothelial and inflammatory cells, play a major role in the establishment, progression, and metastatic dissemination of cancer [5–11]. Using preclinical models of breast cancer that metastasize to the lungs, the premetastatic niche is comprised of recruited bone marrow-derived cells, including endothelial progenitor cells (EPCs; CD45dim, CD133+, VEGFR2+), that modulate the angiogenic switch for the progression of avascularized micrometastases to vascularized macrometastases. VEGFR1+ hematopoietic progenitor cells (HPCs) and CD11b+ myeloid progenitor cells establish the premetastatic niche and recruit EPCs, among other cells, to activate this angiogenic switch [12, 13]. EPC deficiency results in impaired macrometastatic formation as a result of severe angiogenesis inhibition [9]. We extended these analyses to breast cancer patients, in which significant increases in EPCs and HPCs were observed immediately before overt relapse, suggesting that these cells comprise a critical component for the propagation of macrometastases [14].
While there are many important components of angiogenesis, copper is emerging as essential through experiments that demonstrate decreased endothelial cell proliferation, blood vessel formation, and tumor growth with copper depletion [15–18]. Copper is a required cofactor in the expression, activation, and secretion of key activators of angiogenesis through multiple mechanisms including NF-κB and HIF-1 alpha. Copper is a key component of enzymes, including superoxide dismutase-1, vascular adhesion protein-1, and lysyl oxidase, implicated in the priming of the tumor microenvironment [19–22]. Copper may also play a role in migration and invasion as perinuclear copper is translocated to the leading edge of endothelial cell projections during angiogenesis and is transported across the cell membrane [23].
Tetrathiomolybdate (TM), an oral copper chelator developed for the treatment of Wilson's disease, blocks angiogenesis through the inactivation of copper chaperones and decreased incorporation of copper into copper-containing enzymes [24]. Copper levels needed for physiologic functions are lower than those favored by tumor angiogenesis; therefore, copper must be depleted to a therapeutic window. This is achieved by measuring serum ceruloplasmin (Cp), a major extracellular copper transporter, used as a surrogate marker of copper availability [25]. In non-human primates, copper depletion has been shown to decrease peripheral circulation of EPCs [26]. In an HER2/neu breast cancer mouse model, TM was studied as a chemo-preventive agent and it delayed tumor development by >200 days, suggesting that TM maintained these micrometastatic tumors in a dormant-like state [20, 27]. Phase I/II studies of TM in advanced malignancies demonstrated safety and promising efficacy, particularly in patients with minimal residual disease [28–30].
Encouraged by these data, we investigated TM as a drug to promote tumor dormancy in breast cancer patients with NED but at high risk of relapse. We hypothesized that targeting the tumor microenvironment via copper depletion prevents relapse by disrupting the EPC-mediated angiogenic switch required for the progression of micro- to macrometastasis. We further hypothesized that TM might promote tumor dormancy, as reflected by a decrease in circulating EPCs. We report here the results for the first 12 months of copper depletion.
methods
study design
This phase II, open-label, single-arm study enrolled patients on an Institutional Review Board approved trial (NCT00195091, 0903-882, 0309006307) at Weill Cornell Medical College–Iris Cantor Breast Cancer Center. Written informed consent was obtained before undergoing any study-specific procedures in accordance with the Declaration of Helsinki.
study objectives
The primary objective was to assess the change in the number of EPCs in patients treated with TM. Secondary objectives were to evaluate safety, relapse-free survival (RFS), number of HPCs, and levels of plasma angiogenic factors and cytokines.
patients
Female patients were eligible for inclusion in the study if they met the following criteria: at least 18 years of age; histologically confirmed stage 3, stage 4 with NED, or stage 2 triple-negative breast cancer; lack of radiographic, biochemical, or physical evidence of recurrent breast cancer; >6 weeks from previous chemotherapy, biologic therapy, surgery, or radiation; ECOG performance status 0–1; and adequate organ function.
Patients were stratified by molecular subtype according to immunohistochemical marker profile. Stage 2 triple-negative breast cancer patients were included because their estimated risk of relapse is equivalent to stage 3 hormone-receptor-positive patients [31]. Only concurrent hormonal therapy was permitted. All HER2-positive patients were required to have completed 1 year of standard trastuzumab therapy. Physical examination, laboratory studies, and imaging studies [computerized tomography of chest, abdomen, and pelvis (CT c/a/p) and bone scan or positron emission tomography (PET)/CT scan] were required <4 weeks before enrollment.
treatment
TM was administered to outpatient in two phases, induction and maintenance.
induction
TM 180 mg daily in four divided doses until Cp levels decreased to a target range of 5–16 mg/dl. Twenty-eight days of TM comprised one cycle. Cp levels were tested every 2 weeks for the first 4 weeks, then weekly until target Cp was reached. When Cp was within target, patients were switched to maintenance.
maintenance
TM 100 mg was taken daily in divided doses. Doses were reduced in 20 mg increments to minimize toxicity and/or increased in 20 mg increments every 2 weeks to maintain Cp target. Patients were taken off study due to relapse or unacceptable toxicity. Patients brought completed medication logs. The duration of the trial was 2 years.
Clinical grade TM was purchased in bulk from Sigma-Aldrich Chemical Company (Milwaukee, WI) under IND 71380. TM was stored under argon and stability testing was routinely carried out [32]. Research pharmacists dispensed TM in gelatin capsules and maintained an inventory, using the NCI Drug Accountability Record Form.
clinical and radiographic assessments
Patients were seen monthly for physical examination and laboratory studies including complete blood count, complete metabolic panel, tumor markers, and research studies. Imaging of investigator's choice, CT c/a/p or PET/CT, was done every 6 months, using RECIST.
safety
The National Cancer Institute – Common Toxicity Criteria for Adverse Events (CTCAE), version 3.0, were used for adverse event reporting. In the event of grade 3/4 toxicity, dosing was held until recovery. Treatment was resumed at investigator's discretion at 50% of the previous dose. If recovery did not occur within 2 weeks, the patient was removed from study. In the event of grade 2 toxicity, the dose of TM was held until recovery and a new cycle could be initiated at 100%. If grade 2 toxicity recurred, dosing was held until recovery and the next cycle was resumed at 50%.
hemangiogenic progenitor cells and angiogenic factors
Ten to 20 ml of venous blood was collected in EDTA-containing tubes, and peripheral blood mononuclear cells were isolated by Ficoll density-gradient centrifugation within 12 h. EPCs were defined as CD45dim, CD133+, VEGFR2+, and HPCs as CD34+, CD45+, VEGFR1+. Flow cytometry was carried out as previously described [14]. Plasma SDF-1 was detected by enzyme-linked immunosorbent assay (R & D Systems, Minneapolis, MN) and c-kit, VEGFR2, and matrix metalloproteinases (MMP)-1, -3, and -9 by multiplex assays (Meso Scale Discovery, Gaithersburg, MD) as per manufacturer's protocol.
statistical analysis
Descriptive statistics for demographic and angiogenic variables were calculated for all patients. Incidence of adverse events and their associated 95% confidence intervals were estimated using standard methods for proportions. RFS was evaluated using survival analysis techniques. Baseline Cp and EPC values were compared with subsequent time points by the Wilcoxon signed-rank or Mann–Whitney, as appropriate. To assess the association between Cp and EPC over time, three independent mixed effects linear models with subject as a random effect were used to account for the correlation between observations on the same subject. A sample size of 35 achieves a 90% power to detect a difference of 0.5 between EPC/ml at baseline and at last time point, with an estimated standard deviation of 1.1 and two-sided alpha level of 0.05. All analyses were carried out in R: A Language and Environment for Statistical Computing, R Development Core Team, Vienna, Austria, 2011.
results
patient characteristics
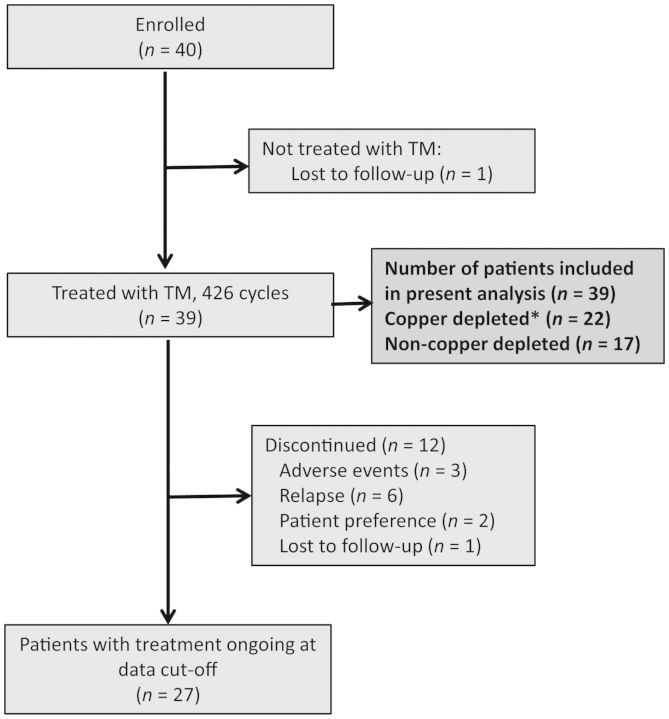
Between 1 June 2007 and 30 June 2010, 40 patients were enrolled (Figure 1). One patient withdrew consent after enrollment and did not ingest TM. A total of 426 cycles were administered to 39 patients in their first 12 months of therapy (mean 10.7 cycles/patient). Twenty-seven patients remain currently on study. Reasons for discontinuation include relapse (six), toxicity (three), patient preference (two), and loss to follow up (one). Baseline characteristics are shown in Table 1. The median age was 50 years (range 29–66). The majority of patients had a very high risk of relapse (i.e. exceeding 60% relapse risk at 10 years), including any subtype stage 4 NED (30.0%), stage 3 triple-negative (12.5%), and stage 3 HER2-positive (17.5%). Twenty-six (65.0%) patients were receiving endocrine therapy while on trial. The median time between completing last treatment and initiating TM was 4.6 months.
Figure 1.
Schema of breast cancer patients on trial. The asterick indicates that copper depletion is defined by a ceruloplasmin < 17 mg/dl.
Table 1.
Baseline patient demographics and clinical characteristics in the intent-to-treat population
| Characteristic | Results (N = 40) |
|---|---|
| Median age, years (range) | 50 (29–66) |
| Race/ethnicity (%) | |
| White | 32 (80) |
| Black | 0 |
| Hispanic | 5 (12.5) |
| Asian/Pacific | 2 (5) |
| Other | 1 (2.5) |
| ECOG performance status (%) | |
| 0 | 36 (90) |
| 1 | 4 (10) |
| AJCC stage | |
| Stage 2, N (%) | 2 (5) |
| Stage 3, N (%) | 26 (65) |
| Stage 4 NED, N (%) | 12 (30) |
| Primary tumor characteristics | |
| Median tumor size, cm (range) | 3.5 (1.2–7) |
| Median number of positive lymph nodes, N (range) | 9 (0–42) |
| Prior metastatic sites in stage 4 NED patients, N | |
| Chest wall | 7 |
| Liver | 3 |
| Brain | 1 |
| Bone only | 1 |
| Axilla | 1 |
| Peritoneum | 1 |
| Molecular subtype, N (%) | |
| Luminal A (ER+/HER2−/Ki67 < 14%) | 11 (27.5) |
| Luminal B (ER+/HER2−/Ki67 ≥ 14%) | 11 (27.5) |
| Luminal-HER2 (ER+ and/or PR+/HER2+) | 4 (10.0) |
| HER2-enriched (ER+/PR+/HER2+) | 3 (7.5) |
| Triple-negative (ER−/PR−/HER2−) | 11 (27.5) |
| Triple-negative patients by stage (N = 11) | |
| Stage 2, N (%) | 2 (18.1) |
| Stage 3, N (%) | 5 (45.4) |
| Stage 4 NED, N (%) | 4 (36.3) |
| Prior antitumor therapy for primary breast cancer, N (%) | |
| Anthracycline and/or taxane-based | 26 (15.0) |
| Trastuzumab | 7 (17.5) |
| High-dose chemo with stem cell support | 2 (5.0) |
| Prior antitumor therapy for metastasis, N (%) | |
| Local treatment (surgery and/or radiation) | 7 (17.5) |
| Hormone therapy | 4 (10) |
| Anthracycline | 1 (2.5) |
| Taxane | 1 (2.5) |
| Capecitabine | 2 (5.0) |
ECOG, Eastern Cooperative Oncology Group; NED, no evidence of disease; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor 2; AJCC, American Joint Committee on Cancer.
effect of TM on copper levels
TM effectively decreased copper levels in a majority of patients
The ITT population consisted of 40 patients. The mean baseline Cp level was 29.7 mg/dl (range 20–47), which decreased to a mean Cp level of 14.2 mg/dl (7–26) while on treatment, P < 0.0001 (Figure 2A). Seventy-five percent of patients copper-depleted by month 1, with the target for copper depletion defined as an average Cp <17 mg/dl. Patients receiving TM for at least 4 weeks decreased their Cp level by 41.9% (range −0.10 to 75.0) from baseline. The mean baseline Cp of copper-depleted patients and non-copper-depleted patients was not significantly different (28.1 versus 31.4 mg/dl; P = 0.19). The mean Cp on treatment of copper-depleted patients and non-copper-depleted patients was 14.5 and 22.1 mg/dl, respectively, P < 0.0001 (Figure 2B). Copper-depleted patients spent a mean of 78% of time (range 55–100%) within target Cp levels during the study. Those on tamoxifen (N = 10) had higher baseline Cp levels of 35.9 mg/dl (range 29–47) compared with 27.8 (22–36) on aromatase inhibitors (N = 13). Mixed effects linear models of Cp over time showed a significant association with type of hormone therapy (P = 0.01), concomitant proton pump inhibitor (PPI) (P = 0.01), and dosage of TM (P < 0.05). Cp levels did not significantly correlate with age, body mass index, or stage.
Figure 2.
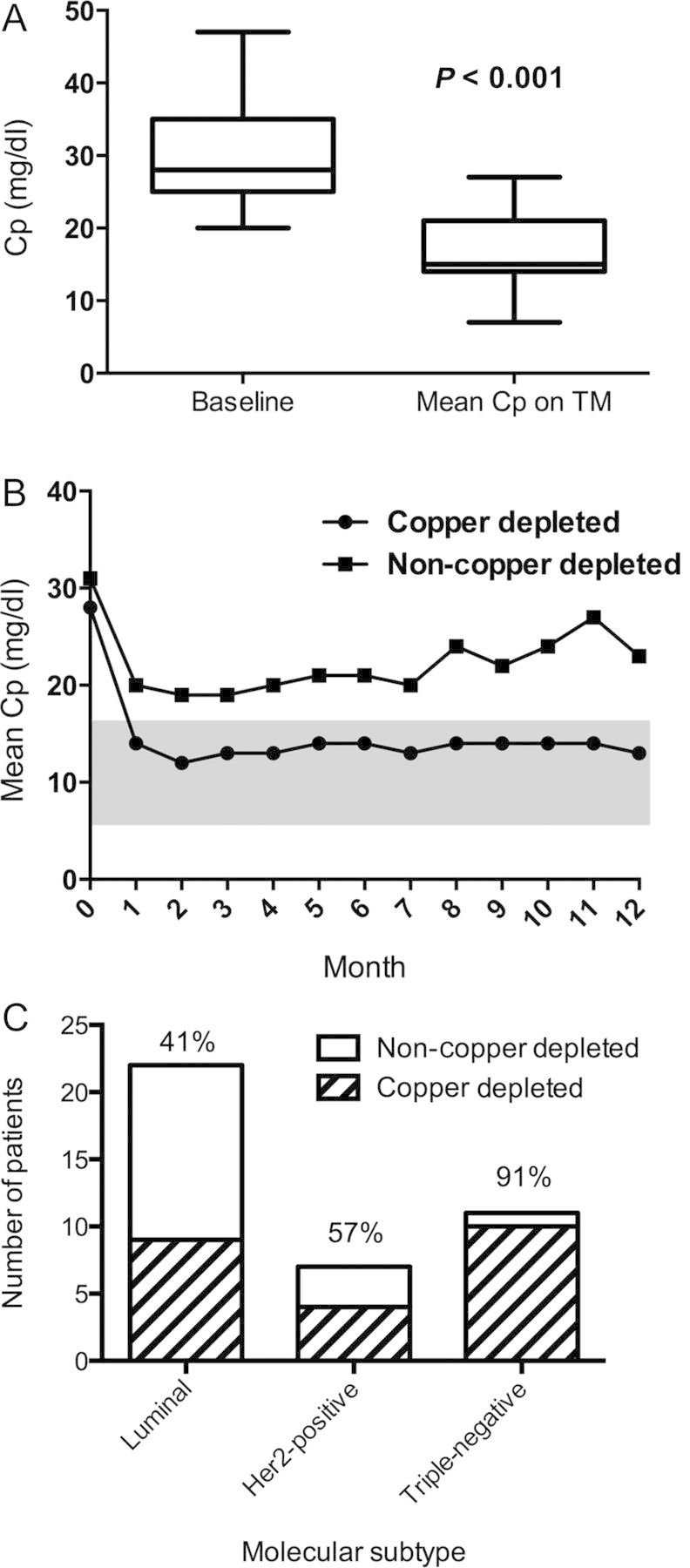
(A) The mean ceruloplasmin (Cp) level significantly decreases in all patients from baseline of 29.7–14.2 mg/dl while on treatment with tetrathiomolybdate (TM). The horizontal line indicates mean; box, standard deviation; whiskers, minimum to maximum values. (B) Mean Cp remains suppressed over time only in copper-depleted patients. The shaded area indicates target for copper depletion. (C) More triple-negative breast cancer patients achieve copper depletion than other molecular subtypes. The percentage of patients copper-depleted is noted above the bar.
triple-negative breast cancer patients copper-deplete most effectively
Patients with triple-negative disease had a lower Cp at baseline (mean 25.9 mg/dl) compared with patients with luminal subtypes (31.7). Ninety-one percent of triple-negative patients copper-depleted compared with luminal (36–45%) and HER2-positive subtypes (50%–67%), though the test for interaction was not significant (Figure 2C).
effect of TM on circulating bone marrow-derived progenitor cells
reduction of EPCs observed only in copper-depleted patients
The mean baseline number of EPCs in the ITT population was 39.8 cells/ml (range 0–207), which decreased over time to 36.3 (0–179) at 1 year of TM therapy (P = 0.52). In copper-depleted patients, mean EPCs/ml decreased from baseline to last dose by 27 (P = 0.04, Figure 3A). In patients who did not achieve the copper depletion target, mean EPCs/ml increased by 61 (P = 0.95). There was no significant difference between the baseline values of copper-depleted and non-copper-depleted subgroups. High-risk subtypes (triple-negative, HER2-positive, and stage 4 NED) had higher mean EPC/ml levels at baseline (45.6, range 0–207) compared with the luminal stage 3 patients (27.7, range 0–114). A decrease in Cp from baseline corresponded to a decrease in EPCs from baseline in triple-negative patients more so than the other molecular subtypes (Figure 3B). Use of non-tamoxifen hormone therapy (P = 0.01), PPI (P = 0.001), Cp levels (P = 0.04), and dosage of TM (P = 0.01) were statistically significant in explaining the decrease of EPCs over time when a multivariable model was used. Additionally, there was a significant interaction between the PPI group and time (P = 0.007), reflecting a decreasing monthly average trend of EPCs in the patients taking PPI. The interaction between Cp and dosage of TM is significantly greater than zero (P = 0.03), suggesting that mean EPCs increased in patients with rising Cp levels. EPC levels did not significantly correlate with age, body mass index, stage, or molecular subtype.
Figure 3.
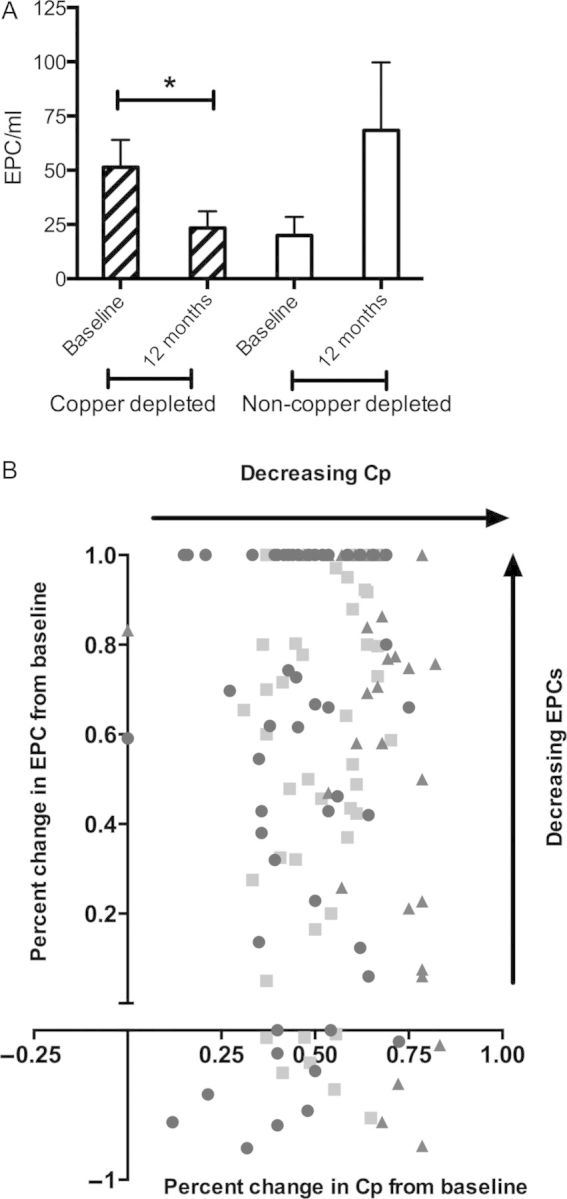
(A) Endothelial progenitor cells (EPCs) significantly decrease from baseline only in copper-depleted patients. The bar indicates mean with standard error of measurement; *P < 0.05. (B) Decrease in ceruloplasmin (Cp) corresponds to a decrease in EPCs in majority of copper-depleted patients and an exploratory analysis by molecular subtype. Square indicates luminal; circle, triple-negative; triangle, human epidermal growth factor 2 (HER2)-positive.
lack of effect of copper depletion on HPCs
The mean baseline HPC/ml for all patients was 2590 (range 66.7–19 300); it was 2170 (range 195–10 700; non-significant) after 12 months of therapy. HPCs did not significantly correlate with copper depletion status.
circulating markers of angiogenesis
There were no significant effects on circulating angiogenic markers, including VEGFR2, ckit, SDF1, MMP-1, -3, or -9. The mean SDF1 decreased only in copper-depleted patients at 12 months, and increased in patients before relapse (supplementary Figure S1, available at Annals of Oncology online). Similarly, mean MMP-1 and -3 increased before relapse (supplementary Figure S2, available at Annals of Oncology online).
toxicity
Overall TM was well tolerated (Table 2).
Table 2.
Summary of incidence of drug-related adverse events in the intent-to-treat population, comprising 426 cycles. N indicates the number of cycles affected by the adverse event.
| Adverse event | Grade 1, N (%) | Grade 2, N (%) | Grade 3, N (%) | Grade 4, N (%) |
|---|---|---|---|---|
| Hematologic | ||||
| Anemia | 43 (10.1) | 7 (1.6) | 1 (0.2) | 0 |
| Neutropenia | 39 (9.2) | 28 (6.6) | 6 (1.4) | 7 (1.6) |
| Febrile neutropenia | 1 (0.2) | 0 | ||
| Leukopenia | 36 (8.5) | 29 (6.8) | 5 (1.2) | 0 |
| Thrombocytopenia | 7 (1.6) | 0 | 0 | 0 |
| Gastrointestinal | ||||
| Sulfur burps | 79 (18.5) | 0 | 0 | 0 |
| Nausea | 3 (0.7) | 0 | 0 | 0 |
| Vomiting | 2 (0.5) | 0 | 0 | 0 |
| Diarrhea | 5 (1.2) | 1 (0.2) | 0 | 0 |
| Constipation | 2 (0.5) | 0 | 0 | 0 |
| Abdominal pain | 5 (1.2) | 0 | 0 | 0 |
| General | ||||
| Fatigue | 29 (6.8) | 0 | 0 | 0 |
| Neurologic | ||||
| Dizziness | 0 | 1 (0.2) | 0 | 0 |
| Neuropathy | 6 (1.4) | 0 | 0 | 0 |
hematologic toxicity
Sixty-seven (15.7%) cycles were complicated by grade 1/2 neutropenia in 23 (59.0%) patients and 13 (3.1%) cycles by grade 3/4 neutropenia in 9 (23.1%) patients. One patient required hospital admission for neutropenic fever and was taken off study. Fifty (11.7%) cycles were complicated by grade 1/2 anemia in 14 (35.9%) patients. Only one (0.2%) cycle was affected by grade 3 anemia in a patient later diagnosed with B12 deficiency. No patients required growth factor support.
non-hematologic toxicity
There was no grade 3 or 4 non-hematologic toxicity. One patient with grade 2 diarrhea, likely due to lactose used as a filler in the TM pills, left the study. Sulfurous eructations resolved with initiation of PPI therapy in >90% of patients.
clinical outcomes
Twenty-seven patients remain relapse-free on study. Six patients (15%) recurred during their first 12 months on TM; three with stage 3 disease (one triple-negative) relapsed after 2, 3, and 10 months and three with stage 4 NED (one triple-negative, one HER2-positive, one luminal) relapsed after 1, 10, and 10 months of therapy. Of these relapsed patients, Cp decreased to target in four patients but EPCs were maintained below baseline in only one patient. RFS curves are shown in Figure 4.
Figure 4.
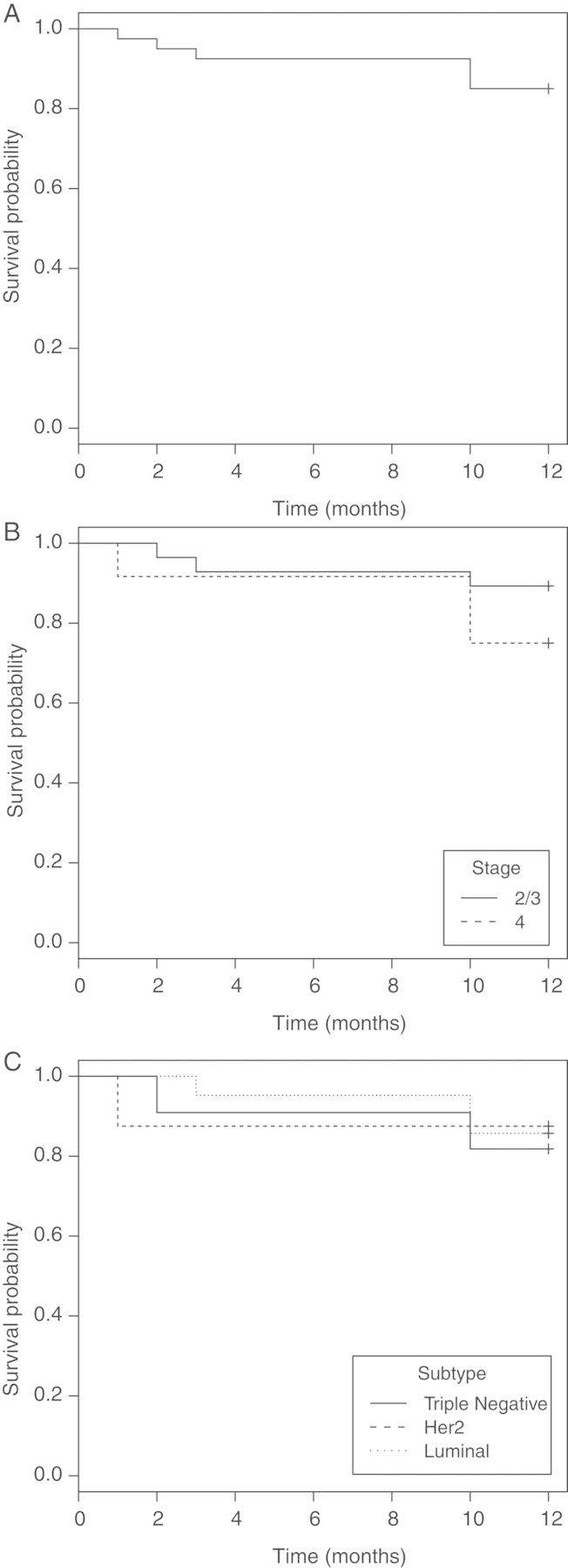
Relapse-free survival (RFS) in (A) all patients, (B) by stage [stage 2/3 versus stage 4 without evidence of disease (NED)], and (C) by molecular subtype (luminal subtypes versus human epidermal growth factor 2 (HER2)-positive versus triple-negative). The 10-month RFS in all patients was 85.0% (95% CI 74.6%–96.8%). The 10-month RFS was lower in stage 4 NED patients compared with stage 2 and 3 patients (75.0% versus 89.3%), and in triple-negative patients compared with luminal patients (81.8% versus 85.7%).
discussion
Understanding the process of breast cancer metastases is critical to eradicating tumor progression. We have conducted a series of translational studies demonstrating that bone marrow-derived hematopoietic progenitors are a critical component of this process. This is the first human trial utilizing a copper depletion strategy to modulate EPCs, an essential component of the microenvironment, in breast cancer patients with an extraordinarily high-risk of relapse from occult residual disease. Since tumor progression may be dependent on critical copper-dependent processes, we hypothesized that copper depletion could prevent an overt relapse by the inhibition of the EPC-mediated angiogenic switch.
In this study, we demonstrated that TM effectively and rapidly depletes copper levels in the majority of patients without untoward effects. In fact, we have safely copper-depleted a proportion of patients for >65 months on an extension study. Concomitant administration of a PPI significantly correlated with copper depletion and may be considered an adjunct to TM treatment. We observed a significant, sustained reduction in EPCs with copper depletion. EPCs did not significantly change in patients unable to achieve or maintain the Cp target. Taken together, copper depletion may inhibit the production, release, or mobilization of EPCs from the bone marrow, leading to a suppressed angiogenic switch and maintained tumor dormancy. Moreover, these findings suggest that TM may have direct effects on the tumor microenvironment at the level of the bone marrow niche, the sanctuary site for EPCs. Though HPCs did not significantly change in patients receiving TM, our previous studies showed that HPCs predicted relapse and progression of disease, suggesting that these cells may also be important in the metastatic cascade, but upstream of the processes was affected by copper depletion [14].
As per subgroup analysis, triple-negative patients copper-depleted more effectively compared with luminal patients. Though the test for interaction was not significant, this study was not powered to show these differential effects. Angiogenesis-related genes are frequently overexpressed in triple-negative tumors [33], and antiangiogenic agents may be more effective in this subset. Intriguingly, several studies evaluating bevacizumab, a monoclonal antibody targeting VEGF, suggested greater efficacy in triple-negative patients [34, 35].
To gain a better understanding of the targets of TM, we measured circulating markers of angiogenesis. SDF1 and its receptor CXCR4, involved in angiogenesis and metastatic progression, may have a role in EPC recruitment [36, 37]. In breast cancer patients, expression of SDF1 has been inversely correlated with survival [38]. In our study, SDF1 decreased with copper depletion but increased before relapse. Likewise, MMPs increased in patients before relapse. MMPs regulate tumor growth and invasion [36] and provide a permissive bone marrow niche to facilitate mobilization of progenitor cells [39]. Our observations suggest that SDF1 and MMPs may have important roles in metastatic progression.
Though a randomized clinical trial is necessary to assess survival, efficacy measures were promising. Of 11 triple-negative patients on study, only 2 relapsed. One patient did not adequately copper-deplete despite incremental dose increases. The other patient progressed within 2 months of TM therapy, suggesting that active neoangiogenesis was occurring at the time of enrollment, which could not be halted due to delayed effects of copper depletion. We are cautiously optimistic about the low incidence of relapse and have extended the study to 6 years in selected patients. Two stage-4 NED triple-negative patients remain disease-free at 65 and 49 months on TM therapy, which is encouraging given the dismal median survival of 9 months in metastatic triple-negative patients [2].
In summary, we have conducted the first phase-II study employing copper chelation in high-risk breast cancer patients with minimal residual disease. As the evidence accumulates in favor of copper depletion to prevent relapse, a large, randomized, multicenter trial enriched with triple-negative and stage 4 NED patients may be of utility.
funding
We greatly appreciate the generous support from the following: Anne Moore Breast Cancer Research Fund, Stephen and Madeline Anbinder Foundation, Rozaliya Kosmandel Research Fund, Susan G. Komen for the Cure, New York Community Trust, Cancer Research and Treatment Fund, and Berman Fund. SMHR was partially supported by the grant from the Clinical Translational Science Center (CTSC) (UL1-RR024996) [no other grant numbers].
disclosure
The authors have declared no conflicts of interest.
Supplementary Material
acknowledgements
We would like to thank Dr Sofia Merajver and Dr George Brewer, whose guidance was critical in many of the practical issues with initiating this clinical trial, and the Weill Cornell Translational Core Laboratory for processing the samples.
references
- 1.O'Shaughnessy J, Vukelja S, Moiseyenko V, et al. Results of a large, phase III trial of Xeloda/taxotere combination therapy versus taxotere monotherapy in patients with metastatic breast cancer. Breast Cancer Res Treat. 2000;53 abstract 381. [Google Scholar]
- 2.Dent R, Trudeau M, Pritchard KI, et al. Triple-negative breast cancer: clinical features and patterns of recurrence. Clin Cancer Res. 2007;13:4429–4434. doi: 10.1158/1078-0432.CCR-06-3045. [DOI] [PubMed] [Google Scholar]
- 3.Hernandez-Aya LF, Chavez-Macgregor M, Lei X, et al. Nodal status and clinical outcomes in a large cohort of patients with triple-negative breast cancer. J Clin Oncol. 2011;29:2628–2634. doi: 10.1200/JCO.2010.32.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wo JY, Chen K, Neville BA, et al. Effect of very small tumor size on cancer-specific mortality in node-positive breast cancer. J Clin Oncol. 2011;29:2619–2627. doi: 10.1200/JCO.2010.29.5907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Folkman J. Angiogenesis in cancer, vascular, rheumatoid and other disease. Nat Med. 1995;1:27–31. doi: 10.1038/nm0195-27. [DOI] [PubMed] [Google Scholar]
- 6.Gao D, Nolan DJ, Mellick AS, et al. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 7.Weidner N, Semple J, Welch W. Tumor angiogenesis and metastasis-correlation in invasive breast cancer. N Engl J Med. 1991;324:1–8. doi: 10.1056/NEJM199101033240101. [DOI] [PubMed] [Google Scholar]
- 8.Iruela-Arispe M, Dvorak H. Angiogenesis: a dynamic balance of stimulators and inhibitors. Thromb Hemostasis. 1997;78:672–677. [PubMed] [Google Scholar]
- 9.Gao D, Nolan D, McDonnell K, et al. Bone marrow-derived endothelial progenitor cells contribute to the angiogenic switch in tumor growth and metastatic progression. Biochim Biophys Acta. 2009;1796:33–40. doi: 10.1016/j.bbcan.2009.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Joyce JA, Pollard JW. Microenvironmental regulation of metastasis. Nat Rev Cancer. 2009;9:239–252. doi: 10.1038/nrc2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pitteri SJ, Kelly-Spratt KS, Gurley KE, et al. Tumor microenvironment-derived proteins dominate the plasma proteome response during breast cancer induction and progression. Cancer Res. 2011;71:5090–5100. doi: 10.1158/0008-5472.CAN-11-0568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–827. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erler JT, Bennewith KL, Cox TR, et al. Hypoxia-induced lysyl oxidase is a critical mediator of bone marrow cell recruitment to form the premetastatic niche. Cancer Cell. 2009;15:35–44. doi: 10.1016/j.ccr.2008.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jain S, Ward MM, O'Loughlin J, et al. Incremental increase in VEGFR1 hematopoietic progenitor cells and VEGFR2 endothelial progenitor cells predicts relapse and lack of tumor response in breast cancer patients. Breast Cancer Res Treat. 2012;132:235–242. doi: 10.1007/s10549-011-1906-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Badet J, Soncin F, Guitton J, et al. Specific binding of angiogenin to calf pulmonary artery endothelial cells. Proc Natl Acad Sci USA. 1989;86:8427–8431. doi: 10.1073/pnas.86.21.8427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brem S, Zagzag D, Tsanaclis A, et al. Inhibition of angiogenesis and tumor growth in the brain: suppression of endothelial cell turnover by penicillamine and depletion of copper, an angiogenic cofactor. Am J Pathol. 1990;137:1121–1142. [PMC free article] [PubMed] [Google Scholar]
- 17.Juarez JC, Betancourt OJ, Pirie-Shepherd SR, et al. Copper binding by tetrathiomolybdate attenuates angiogenesis and tumor cell proliferation through the inhibition of superoxide dismutase. Clin Cancer Res. 2006;12:4974–4982. doi: 10.1158/1078-0432.CCR-06-0171. [DOI] [PubMed] [Google Scholar]
- 18.Hassouneh B, Islam M, Nagel T, et al. Tetrathiomolybdate promotes tumor necrosis and prevents distant metastases by suppressing angiogenesis in head and neck cancer. Mol Cancer Ther. 2007;6:1039–1045. doi: 10.1158/1535-7163.MCT-06-0524. [DOI] [PubMed] [Google Scholar]
- 19.Lowndes SA, Harris AL. The role of copper in tumour angiogenesis. J Mammary Gland Biol Neoplasia. 2005;10:299–310. doi: 10.1007/s10911-006-9003-7. [DOI] [PubMed] [Google Scholar]
- 20.Pan Q, Kleer CG, van Golen KL, et al. Copper deficiency induced by tetrathiomolybdate suppresses tumor growth and angiogenesis. Cancer Res. 2002;62:4854–4859. [PubMed] [Google Scholar]
- 21.Marttila-Ichihara F, Auvinen K, Elima K, et al. Vascular adhesion protein-1 enhances tumor growth by supporting recruitment of Gr-1+CD11b+ myeloid cells into tumors. Cancer Res. 2009;69:7875–7883. doi: 10.1158/0008-5472.CAN-09-1205. [DOI] [PubMed] [Google Scholar]
- 22.Erler JT, Bennewith KL, Nicolau M, et al. Lysyl oxidase is essential for hypoxia-induced metastasis. Nature. 2006;440:1222–1226. doi: 10.1038/nature04695. [DOI] [PubMed] [Google Scholar]
- 23.Finney L, Mandava S, Ursos L, et al. X-ray fluorescence microscopy reveals large-scale relocalization and extracellular translocation of cellular copper during angiogenesis. Proc Natl Acad Sci USA. 2007;104:2247–2252. doi: 10.1073/pnas.0607238104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alvarez HM, Xue Y, Robinson CD, et al. Tetrathiomolybdate inhibits copper trafficking proteins through metal cluster formation. Science. 2010;327:331–334. doi: 10.1126/science.1179907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gartner EM, Griffith KA, Pan Q, et al. A pilot trial of the anti-angiogenic copper lowering agent tetrathiomolybdate in combination with irinotecan, 5-flurouracil, and leucovorin for metastatic colorectal cancer. Invest New Drugs. 2009;27:159–165. doi: 10.1007/s10637-008-9165-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Donate F, Juarez JC, Burnett ME, et al. Identification of biomarkers for the antiangiogenic and antitumour activity of the superoxide dismutase 1 (SOD1) inhibitor tetrathiomolybdate (ATN-224) Br J Cancer. 2008;98:776–783. doi: 10.1038/sj.bjc.6604226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pan Q, Rosenthal DT, Bao L, et al. Antiangiogenic tetrathiomolybdate protects against Her2/neu-induced breast carcinoma by hypoplastic remodeling of the mammary gland. Clin Cancer Res. 2009;15:7441–7446. doi: 10.1158/1078-0432.CCR-09-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brewer G, Dick D, Grover K, et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: phase I study. Clin Cancer Res. 2000;6:1–11. [PubMed] [Google Scholar]
- 29.Redman BG, Esper P, Pan Q, et al. Phase II trial of tetrathiomolybdate in patients with advanced kidney cancer. Clin Cancer Res. 2003;9:1666–1672. [PubMed] [Google Scholar]
- 30.Pass HI, Brewer GJ, Dick R, et al. A phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: final results. Ann Thorac Surg. 2008;86:383–389. doi: 10.1016/j.athoracsur.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 31. Adjuvant! Online [Internet] New York: Association of Cancer Online Resources, Inc.; c2000–01 [updated 16 May 2002; cited 1 May 2012] Available from http://www.adjuvantonline.com .
- 32.Brewer G, Dick R, Yuxbasiyan-Gurkin V, et al. Initial therapy of patients with Wilson's disease with tetrathiomolybdate. Arch Neurol. 1991;48:42–47. doi: 10.1001/archneur.1991.00530130050019. [DOI] [PubMed] [Google Scholar]
- 33.Rody A, Karn T, Liedtke C, et al. A clinically relevant gene signature in triple-negative and basal-like breast cancer. Breast Cancer Res. 2011;13:R97. doi: 10.1186/bcr3035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Minckwitz G, Eidtmann H, Rezai M, et al. Neoadjuvant chemotherapy and bevacizumab for HER2-negative breast cancer. N Engl J Med. 2012;366:299–309. doi: 10.1056/NEJMoa1111065. [DOI] [PubMed] [Google Scholar]
- 35.Miller K, Wang M, Gralow J, et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N Engl J Med. 2007;357:2666–2676. doi: 10.1056/NEJMoa072113. [DOI] [PubMed] [Google Scholar]
- 36.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 37.Moore MA, Hattori K, Heissig B, et al. Mobilization of endothelial and hematopoietic stem and progenitor cells by adenovector-mediated elevation of serum levels of SDF-1, VEGF, and angiopoietin-1. Ann NY Acad Sci. 2001;938:36–47. doi: 10.1111/j.1749-6632.2001.tb03572.x. [DOI] [PubMed] [Google Scholar]
- 38.Kang H, Watkins G, Parr C, et al. Stromal cell derived factor-1: its influence on invasiveness and migration of breast cancer cells in vitro, and its association with prognosis and survival in human breast cancer. Breast Cancer Res. 2005;7:402–410. doi: 10.1186/bcr1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rafii S, Heissig B, Hattori K. Efficient mobilization and recruitment of marrow-derived endothelial and hematopoietic stem cells by adenoviral vectors expressing angiogenic factors. Gene Ther. 2002;9:631–641. doi: 10.1038/sj.gt.3301723. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.