Editor’s Highlight: Seth and colleagues examined a two-hit model for nonalcoholic steatohepatitis, showing that bromodichloromethane interacts with a high fat diet to induce liver inflammation and metabolic alterations in a CYP2E1-dependent manner. Moreover, bromodichloromethane dramatically affected the satiety-inducing leptin pathway, which appears to be mechanistically involved in the metabolic pathogenesis. In addition to characterizing the phenomenon, this study highlights the potential that genetic polymorphisms and diet may play in determining susceptibility to the metabolic outcomes of common environmental contaminants. The overall metabolic disturbances are likely to be more complex and systemic than described in the present article and future research in this area will be exceedingly valuable to understanding the impact of environmental contaminants on public health. — Matthew Campen
Key Words: bromodichloromethane, hepatocyte, lipid peroxidation, fibrosis, Glut-1, glycolysis, gluconeogenesis, PPAR-γ, SREBP-1c, tumor necrosis factor, ob/ob mice.
Abstract
Obesity is associated with strong risks of development of chronic inflammatory liver disease and metabolic syndrome following a second hit. This study tests the hypothesis that free radical metabolism of low chronic exposure to bromodichloromethane (BDCM), a disinfection byproduct of drinking water, causes nonalcoholic steatohepatitis (NASH), mediated by cytochrome P450 isoform CYP2E1 and adipokine leptin. Using diet-induced obese mice (DIO), mice deficient in CYP2E1, and mice with spontaneous knockout of the leptin gene, we show that BDCM caused increased lipid peroxidation and increased tyrosine nitration in DIO mice, events dependent on reductive metabolism by CYP2E1. DIO mice, exposed to BDCM, exhibited increased hepatic leptin levels and higher levels of proinflammatory gene expression and Kupffer cell activation. Obese mice exposed to BDCM also showed profound hepatic necrosis, Mallory body formation, collagen deposition, and higher alpha smooth muscle actin expression, events that are hallmarks of NASH. The absence of CYP2E1 gene in mice that were fed with a high-fat diet did not show NASH symptoms and were also protected from hepatic metabolic alterations in Glut-1, Glut-4, phosphofructokinase and phosphoenolpyruvate carboxykinase gene expressions (involved in carbohydrate metabolism), and UCP-1, PGC-1α, SREBP-1c, and PPAR-γ genes (involved in hepatic fat metabolism). Mice lacking the leptin gene were significantly protected from both NASH and metabolic alterations following BDCM exposure, suggesting that higher levels of leptin induction by BDCM in the liver contribute to the development of NASH and metabolic alterations in obesity. These results provide novel insights into BDCM-induced NASH and hepatic metabolic reprogramming and show the regulation of obesity-linked susceptibility to NASH by environmental factors, CYP2E1, and leptin.
Obesity is associated with a low inflammatory condition where there is steatosis and insulin resistance (Johnson et al., 2012; Rius et al., 2012). Simple steatosis can progress to more severe inflammatory condition of steatohepatitis, cirrhosis, and hepatocellular carcinoma following a “second hit” or “multiple hits” (Day and James, 1998; Farrell et al., 2012; Tilg and Moschen, 2010). The conceptual “two hit” or “multihit” paradigm can come from the environmental factors, reactive oxygen species, or a host of other conditions that can exacerbate the low lying inflammation in obesity (Jou et al., 2008; Torres et al., 2012). There is, however, no clear evidence that the environment contributes in progression of nonalcoholic steatohepatitis (NASH). The proinflammatory nature of obesity is marked by increased release of cytokines and adipokine leptin, the levels of which are increased in circulation following leptin resistance (Munzberg, 2010; Parola and Marra, 2011). Our research has indicated that free radical metabolism of carbon tetrachloride, a known hepatotoxin, increases hepatic leptin levels over and above the amounts found in obesity (Chatterjee et al., 2012a). Fibrosis of liver, which is a feature of full-blown NASH, causes a significant reprogramming of the hepatic carbohydrate and lipid metabolism, in order to accommodate the changed metabolic microenvironment of the obese liver (Chen et al., 2012). Because obesity is highly correlated with increased inflammatory liver disease incidences, we explored the possibility that environmental exposure of disinfection byproduct (DBP) bromodichloromethane (BDCM) might act as a second hit to exacerbate development of NASH. Chlorine-based disinfectants have played a critical role in protecting drinking water supply from waterborne infectious diseases for nearly a century (Wigle, 1998). BDCM is part of a wide range of chemicals known as trihalomethanes, which are formed after chlorine is added to the water for disinfection (Lilly et al., 1997b). BDCM exposure can happen through the oral route, inhalation, and skin contact, with reports suggesting the maximum exposure through ingestion and by skin contact (Silva et al., 2012; Torti et al., 2001). It is metabolized by the CYP450 enzymes primarily in the microsomes of hepatocytes (Allis and Zhao, 2002; Lilly et al., 1997a). The toxic effects of BDCM are mediated by the free radical metabolism of this compound by CYP2E1, an isoform of cytochrome P450, forming highly toxic hydroxynonenal adducts causing hepatocellular necrosis (Das et al., 2013; Tomasi et al., 1985).
The ability of BDCM to cause hepatocellular free radical generation in doses above 3.0mmol/kg might not be relevant to clinical hepatology and public health because the EPA permissible limits are far lower than is reported in literature (National Toxicology Program, 1987). Further lower doses of BDCM have been found to exert little or no effect in normal adult rats (National Toxicology Program, 1987). We hypothesized that chronic exposure to BDCM at doses that are lower than those that cause hepatocellular necrosis might exacerbate inflammatory liver disease in susceptible obese mice. The study reported here utilizes a chronic systemic route of exposure to explore the molecular mechanisms of disease progression because BDCM toxicity has been reported following exposure via dermal, oral, and ip routes in rodents.
This study, which uses diet-induced obese (DIO) mice model that does not progress to NASH by high-fat diet alone, investigates the BDCM-induced oxidative stress and leptin’s role in development of NASH. It also examines reprogramming of carbohydrate and lipid metabolism by BDCM exposure. The results reported in this study show for the first time that low chronic exposure to BDCM, a contaminant of chlorinated drinking water, causes NASH by the direct involvement of CYP2E1 and leptin in obese mice.
MATERIALS AND METHODS
Obese mice.
Pathogen-free, custom DIO adult male mice with a C57BL6/J background (Jackson Laboratories, Bar Harbor, ME) were used as models of diet-induced obesity. They were fed with a high-fat diet (60% kcal) (protein: 26.2 gm%, 20 kcal%; carbohydrate: 26.3 gm%, 20 kcal%; and fat: 34.9 gm%, 60 kcal%) from 6 to 16 weeks. The animals were housed one in each cage before any experimental use. Mice that contained the disrupted ob gene (leptin knockout [KO], spontaneous KO of leptin gene) (B6.V-Lep(/J) (Jackson Laboratories) and disrupted CYP2E1 gene (CYP2E1 KO or CYP2E1 gene-deficient mice) (129/Sv-Cyp2e1tm1Gonz/J) (Jackson Laboratories) were fed with a high-fat diet and treated identically to DIO mice. Mice had ad libitum access to food and water and were housed in a temperature-controlled room at 23°C–24°C with a 12-h light/dark cycle. All animals including transgenic mice were treated in strict accordance with the NIH Guide for the Humane Care and Use of Laboratory Animals, and the experiments were approved by the institutional review board both at NIEHS and the University of South Carolina at Columbia.
Induction of liver injury in obese mice.
DIO mice or high-fat-diet-fed gene-specific KO mice at 16 weeks were administered BDCM (1.0mmol/kg, diluted in olive oil) through the ip route, two doses per week for 4 weeks. DIO mice treated with olive oil (diluent of BDCM) were used as control. After completion of the treatment, mice of all study groups were sacrificed for liver tissue, blood, and serum for the further experiments.
Clinical analysis of NASH in obese mice.
Histological assessment, assessment of NASH by calculating the histological activity index (HAI), hydroxyproline assay, picro sirius red staining for fibrosis, and serum aspartate aminotransferase (AST) and alanine aminotransferase (ALT) measurements were carried out to ascertain the symptoms of NASH (Supplementary methods).
ELISA.
Immunoreactivity for nitrotyrosine was detected in liver homogenates using standard ELISA.
Immunohistochemistry.
Formalin-fixed, paraffin-embedded liver tissue from all the mice groups were cut into 5-µm-thick tissue sections. Each section was deparaffinized using standard protocol and used for staining (Supplementary methods).
Molecular techniques.
Hepatic mRNA expression was examined by quantitative real-time PCR analysis. Western blot analysis of proteins was carried out for assessing protein levels in tissue samples (Supplementary methods).
RESULTS
Chronic BDCM Exposure-Induced Hepatic Oxidative Stress in Obesity Is Mediated by CYP2E1
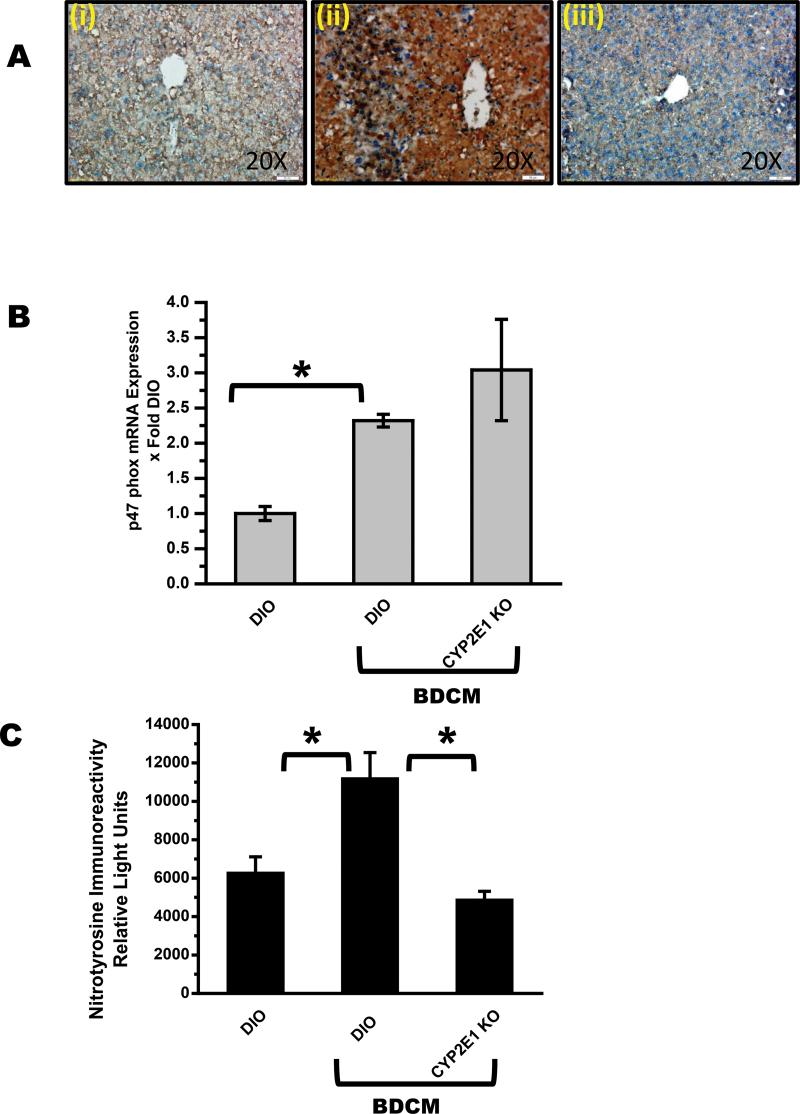
To determine the effects of chronic BDCM exposure in high-fat-diet-fed mice in generation of free radicals following metabolism by CYP2E1, lipid peroxidation was analyzed. Results showed that there was an increase in lipid peroxidation as indicated by increased immunoreactivity of 4-hydroxynonenal, a stable adduct of peroxidation of lipids in DIO + BDCM group (Fig. 1A ii) compared with DIO (Fig. 1A i) group or in mice that are deficient in CYP2E1 but fed with a high-fat diet (CYP2E1 KO) (Fig. 1A iii). BDCM exposure also significantly increased p47 phox expression, a cytosolic subunit of the NADPH oxidase, compared with DIO-only mice, (p < 0.05) (Fig. 1B). CYP2E1 KO mice had no significant difference in p47 phox mRNA expression compared with DIO + BDCM group, suggesting an absence of the role of CYP2E1 in p47 phox expression (Fig. 1B). 3-Nitrotyrosine immunoreactivity (nitrosative stress) as measured by ELISA was significantly elevated in DIO + BDCM group compared with DIO-only group (p < 0.05)(Fig. 1C). CYP2E1 KO mice that were fed with high-fat diet had a significant decrease in 3-nitrotyrosine immunoreactivity compared with DIO + BDCM group (p < 0.05), suggesting the clear role of CYP2E1 in formation of tyrosine radicals and nitration of tyrosine residues in the BDCM-exposed group (Fig. 1C). Further it also showed that BDCM metabolism by CYP2E1 is central to free radical stress in the obese liver.
Fig. 1.
Chronic BDCM exposure in DIO mice generates CYP2E1-mediated oxidative stress. (A) Immunohistochemistry of mouse liver slices depicting 4-hydroxynonenal immunoreactivity (lipid peroxidation) in DIO, DIO + BDCM, and CYP2E1 KO + BDCM-treated mice (n = 3). ×20 image. (B) Quantitative real-time PCR (qRTPCR) analysis of liver p47 phox mRNA expression of DIO, DIO + BDCM, and CYP2E1 KO + BDCM mice. Y-axis represents fold of mRNA expression when normalized against DIO-only group, n = 3. (C) ELISA of liver homogenate, nitrotyrosine immunoreactivity in DIO, DIO + BDCM and CYP2E1 KO + BDCM groups. Y-axis represents the chemiluminescent units as an index of nitrotyrosine immunoreactivity, n = 5. *p < 0.05 is considered statistically significant.
Chronic BDCM Exposure and CYP2E1-Mediated Hepatic Free Radical Stress Exacerbate Inflammation and Leptin Release
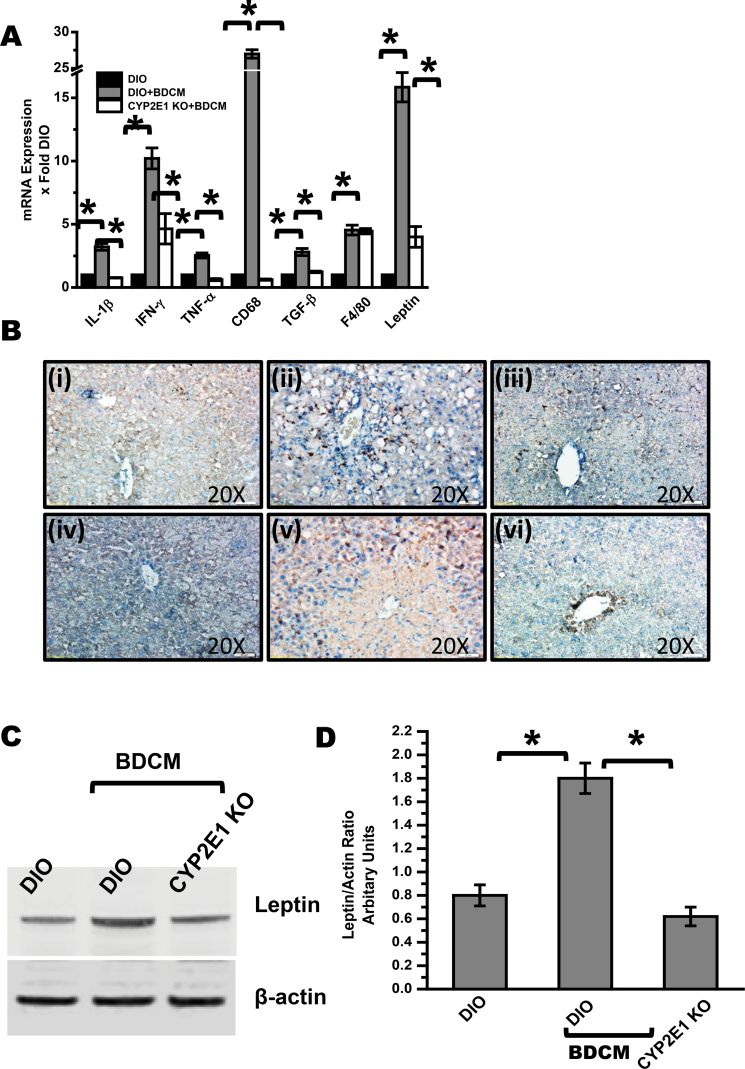
To investigate the role of BDCM exposure and its free radical metabolism in exacerbating the inflammatory response in obesity, hepatic mRNA expression profiles of interleukin (IL)-1β, interferon-γ (IFN-γ), and tumor necrosis factor (TNF)-α were analyzed. Results indicated that there was a significant increase in the expression profiles of IL-1β, IFN-γ, and TNF-α in DIO + BDCM group compared with DIO-only group (p < 0.05) (Fig. 2A). Mice deficient in CYP2E1 had a significant decrease in the expressions of these proinflammatory genes (p < 0.05) (Fig. 2A). DIO + BDCM group had a significant increase in the expression of CD68, a Kupffer cell activation marker, which plays a significant role in development and progression of NASH (p < 0.05) compared with DIO-only group (Fig. 2A). CYP2E1 KO mice fed with a high-fat diet had a significant decrease in the expression of CD68 mRNA expression, indicating that CYP2E1 plays a prominent role in the Kupffer cell activation (p < 0.05) (Fig. 2A). Though a significant increase in macrophage marker F4/80 was observed in DIO + BDCM group compared with DIO-only group, no such difference was observed in absence of CYP2E1 gene (Fig. 2A). DIO + BDCM group had a significant elevation in the expression of transforming growth factor (TGF)-β, a TH3 cytokine known for its role in fibrosis, compared with DIO-only group (p < 0.05) (Fig. 2A). The CYP2E1 KO mice had a significant decrease in the TGF-β expression compared with DIO + BDCM group, suggesting the direct role of CYP2E1 and the free radical metabolism of BDCM in the expression patterns of this cytokine (p < 0.05) (Fig. 2A). Results also showed that hepatic leptin expression was significantly increased in DIO + BDCM group compared with DIO-only group (p < 0.05) (Fig. 2A). Deletion of the CYP2E1 gene in obese mice (CYP2E1 KO) decreased expression of leptin compared with expression in DIO + BDCM group (p < 0.05) (Fig. 2A). Immunolocalization of CD68, a Kupffer cell activation marker, was increased in hepatic sinusoids of DIO + BDCM group (Fig. 2B ii) compared with DIO-only group (Fig. 2B i). CYP2E1 KO obese mice had a decreased immunolocalization of CD68 compared with DIO + BDCM group (Fig. 2B iii). TGF-β levels were also decreased in CYP2E1 KO mice compared with DIO + BDCM group, suggesting a direct role of CYP2E1 free radical metabolism in TGF-β levels in obese liver (Fig. 2B iv–vi). Similar to our observations in the mRNA expressions of adipokine leptin, leptin protein levels were significantly increased in obese mice that were exposed to BDCM compared with those in DIO-only mice (p < 0.05) (Figs. 2C and D). CYP2E1 KO mice had a significantly decreased leptin protein levels compared with DIO + BDCM group, suggesting a strong correlation with BDCM exposure, its free radical metabolism by CYP2E1, and hepatic leptin production (p < 0.05) (Figs. 2C and D).
Fig. 2.
BDCM exposure in obese mice causes hepatic inflammation, Kupffer cell activation, TGF-β expression, and increased leptin release. (A) qRTPCR analysis of liver IL1-β, IFN-γ, TNF-α, CD68 (Kupffer cell activation marker), TGF-β, Pan macrophage marker F4/80, and leptin mRNA expression of DIO, DIO + BDCM, and CYP2E1 KO + BDCM mice. Y-axis represents fold of mRNA expression when compared against DIO-only group, n = 4. (B) Immunohistochemistry of liver slices of DIO, DIO + BDCM, and CYP2E1 KO + BDCM for CD68 (panels i–iii) and TGF-β (panels iv–vi) showing ×20 images. (C) Western blot analysis of adipokine leptin from liver homogenates of DIO, DIO + BDCM, and CYP2E1 KO + BDCM groups. (D) Immunoreactive band analysis of leptin normalized against beta actin. Y-axis depicts the leptin/actin ratio from DIO, DIO + BDCM, and CYP2E1 KO + BDCM groups (n = 3). *p < 0.05 is considered statistically significant.
Chronic BDCM Exposure Causes NASH and Is CYP2E1 Dependent
To study the role of BDCM exposure and free radical generation in development of full-blown NASH, experiments were designed to analyze the fibrotic and histopathological indices in high-fat-diet-fed mice. Results showed that there was a significant increase in mRNA expressions of collagen-1α-1 (COL-1α-1) and α-smooth muscle actin (SMA) in DIO + BDCM group compared with DIO-only group (p < 0.05) (Fig. 3A). High-fat-diet-fed CYP2E1 KO mice had a significant decrease in COL-1α-1 and α-SMA expression (p < 0.05), suggesting a role of the free radical metabolism following BDCM exposure in the expression of these genes. Analysis of the hydroxyproline content of the liver revealed a significant increase in the DIO + BDCM group compared with the DIO-only group (p < 0.05) (Fig. 3B). CYP2E1 KO mice had a significant decrease in hydroxyproline content compared with DIO + BDCM group (p < 0.05) (Fig. 3B). Immunolocalization of α-SMA, a stellate cell proliferation marker, was increased in DIO + BDCM group compared with DIO-only group, suggesting a marked increase in fibrosis and stellate cell proliferation, a common event in NASH progression (Fig. 3C). α-SMA immunoreactivity decreased in CYP2E1 KO mice compared with DIO + BDCM group (Supplementary fig. 1). Serum levels of ALT and AST were significantly higher in DIO + BDCM group compared with DIO-only group, whereas CYP2E1 KO mice had a significant decrease in the levels of these enzymes (p < 0.05) (Fig. 3D). Further evaluations of NASH progression were carried out by assessing the histopathological profiles of BDCM-exposed liver tissue (Fig. 3E). Histopathological evaluations were analyzed by Histological Activity Score (HAI) (Supplementary table 1). Liver histology showed marked increases in hepatocyte necrosis, leukocyte infiltration, and ballooning degeneration in DIO + BDCM group (Fig. 3E ii) compared with DIO-only group (Fig. 3E i). CYP2E1 KO mice showed marked decrease in necrosis, ballooning degeneration, and infiltration of leukocytes compared with the BDCM-treated group. HAI scores showed similar results in inflammation and fibrosis, with a score that was significantly higher in DIO + BDCM group compared with DIO-only or CYP2E1 KO groups (Supplementary table 1). Picro sirius red staining of DIO + BDCM mouse liver showed increased micro- and macrovesicular fibrosis compared with both DIO mouse liver and CYP2E1 KO mouse liver (Fig. 3F).
Fig. 3.
BDCM exposure causes hepatic fibrosis and stellate cell proliferation, features of full-blown NASH. (A) qRTPCR analysis of mRNA expression of COL-1-α-1 and α-SMA from liver homogenates of DIO, DIO + BDCM, and CYP2E1 KO + BDCM mice (n = 4). (B) Total hydroxyproline content in liver homogenate, an index of collagen deposition in DIO, DIO + BDCM, and CYP2E1 KO + BDCM groups (n = 3). (C) Immunoreactivity of α-SMA in liver tissue sections of DIO and DIO + BDCM groups. Panel (i) represents mouse liver section from DIO group and panel (ii) represents mouse liver sections from DIO + BDCM group. Images depict ×20 magnification of field size. (D) Serum levels of ALT and AST from DIO, DIO + BDCM, and CYP2E1 KO + BDCM group expressed in international Units/liter. (E) Hematoxylin and eosin staining of liver slices from DIO (panel i), DIO + BDCM (panel ii), and CYP2E1 KO mice (panel iii). Arrows show necrosis in areas of Zone 3 (perivenular region). (F) Picro sirius red staining of liver slices from DIO (panel i), DIO + BDCM (panel ii), and CYP2E1 KO + BDCM (panel iii). Arrows show macro- and microvesicular fibrosis as depicted by red staining. *p < 0.05 is considered statistically significant.
BDCM Exposure and Resulting Reductive Free Radical Metabolism of CYP2E1 Modulate Hepatic Glucose and Fat Metabolism
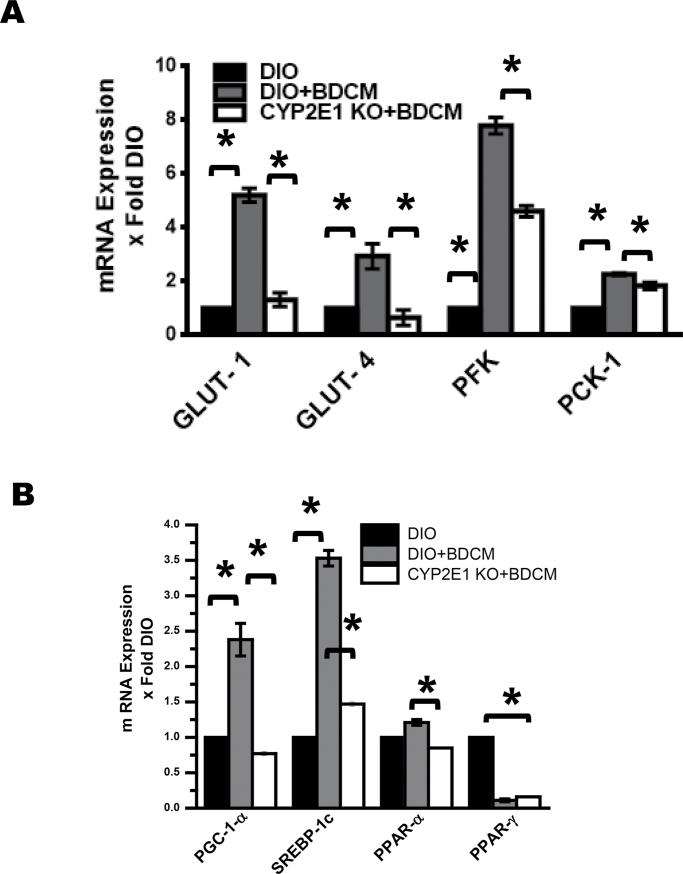
To determine whether the exposure to BDCM and its corresponding free radical metabolism altered carbohydrate and lipid metabolism, mRNA expressions of glucose transporter genes Glut-1 and Glut-4 followed by phosphofructokinase (PFK) and phosphoenolpyruvate carboxykinase (PCK-1) genes were studied. Results showed that Glut-1 and Glut-4 mRNA expressions were significantly increased in DIO + BDCM group compared with DIO-only group (p < 0.05) (Fig. 4A), whereas its expression decreased significantly in the CYP2E1 KO mice (p < 0.05) (Fig. 4A). There was a significant increase in the mRNA expression of key glycolysis regulatory enzyme PFK and gluconeogenesis enzyme PCK-1 in the DIO + BDCM group compared with the DIO-only group (p < 0.05) (Fig. 4A), whereas the CYP2E1 mice fed with high-fat diet had significantly decreased expressions (p < 0.05) (Fig. 4A). Next we examined whether BDCM exposure in obese mice significantly modulated lipogenesis and lipid metabolism. Results showed that mRNA expressions of lipogenic genes PGC-1α and SREBP-1c were significantly increased in DIO + BDCM group compared with DIO-only group, whereas the mRNA expression profiles of PPAR-α were unchanged (p < 0.05) (Fig. 4B). The expression of these genes was significantly decreased in CYP2E1 KO mice compared with DIO + BDCM group (p < 0.05)(Fig. 4B). Interestingly, the mRNA expression profiles of PPAR-γ were significantly downregulated in DIO + BDCM group compared with DIO-only group (p < 0.05) (Fig. 4B), suggesting that BDCM exposure might have a significant role in PPAR-γ downregulation by a different mechanism that is not dependent on the presence of CYP2E1 or its free radical metabolism (Fig. 4B).
Fig. 4.
BDCM exposure and CYP2E1-dependent modulation of hepatic glucose and fat metabolism. (A) Liver mRNA expression of glucose transporter (Glut-1, Glut-4), glycolytic enzyme (PFK), and gluconeogenic enzyme (PCK-1) in DIO, DIO + BDCM, and CYP2E1 KO + BDCM mice (n = 3). (B) Liver mRNA expression of lipid metabolism master regulator, PGC-1α, lipogenic gene SREBP-1c, and regulators of lipid metabolism, PPAR-α and PPAR-γ in DIO, DIO + BDCM, and CYP2E1 KO + BDCM mice (n = 3). *p < 0.05 is considered statistically significant.
Increased Hepatic Leptin Following BDCM Exposure Modulates Inflammation and Kupffer Cell Activation
To study the role of BDCM exposure–induced higher leptin levels in the progression of NASH in obesity, ob/ob mice that are a spontaneous KO of leptin were used. Results showed that mRNA expressions of proinflammatory cytokine IFN-γ and CD68, a Kupffer cell activation marker, were significantly decreased in the leptin KO mice compared with the DIO + BDCM (wild type) mice (p < 0.05) (Fig. 5A). Interestingly, there was a significant change in the expression profiles of another proinflammatory cytokine TNF-α in leptin KO mice compared with DIO + BDCM group (Fig. 5A). Liver hydroxyproline level, which is a strong indicator of collagen levels in the liver, was decreased significantly in leptin KO mice compared with DIO + BDCM (wild type) group, suggesting less collagen deposition in these mice (p < 0.05) (Fig. 5B). This was also reflected in the immunohistochemistry analysis of CD68 and α-SMA (Fig. 5C). Kupffer cell activation marker CD68 (Fig. 5C i and ii) and α-SMA (Fig. 5C iii and iv) immunoreactivity was decreased in liver sections of mice drawn from leptin KO mice compared to wild-type mice that were fed with a high-fat diet and exposed to BDCM.
Fig. 5.
Leptin controls BDCM-induced NASH development in obesity. (A) qRTPCR analysis of mRNA expression of IFN-γ, TNF-α, and Kupffer cell marker CD68 in DIO + BDCM and Leptin KO + BDCM groups. Y-axis represents fold change compared with DIO-only controls (n = 4). (B) Hydroxyproline content in DIO + BDCM and Leptin KO + BDCM groups. Y-axis represents fold change (n = 4). (C) Immunohistochemistry of Kupffer cell marker CD68 and stellate cell proliferation marker α-SMA in DIO + BDCM and Leptin KO + BDCM groups. Panels (i and ii) represent CD68, and panels (iii and iv) represent α-SMA in ×20 magnification. *p < 0.05 is considered statistically significant.
Increased Hepatic Leptin Following BDCM Exposure Alters Glucose and Fat Metabolism and Augments the Progression of NASH
Next we analyzed whether the presence or absence of leptin significantly altered the expression of glycolytic and gluconeogenic enzymes in the course of NASH progression following BDCM exposure. Results showed that the mRNA expression of glucose transporter Glut-1 and the glycolytic enzyme PFK was significantly decreased in leptin KO mice compared with wild-type mice fed with a high-fat diet and coexposed to BDCM (p < 0.05) (Fig. 6A). The expressions of gluconeogenic enzyme PCK-1 were unchanged (Fig. 6A). Lipid metabolism enzymes like PGC-1α and SREBP-1c were significantly downregulated in leptin KO mice compared with wild-type mice that were fed with high-fat diet and exposed to BDCM (p < 0.05) (Fig. 6B). Interestingly the mRNA expression of PPAR-γ was significantly increased in leptin KO mice compared with wild-type mice coexposed to high-fat diet and BDCM (p < 0.05) (Fig. 6B). Fibrosis as assessed by picro sirius red staining decreased in leptin KO mice (Fig. 6C ii) compared with high-fat-diet-fed and BDCM-exposed mice (Fig. 6C i). Hematoxylin and eosin staining of liver section of leptin KO mice had decreased inflammation and hepatocyte necrosis (Fig. 6D ii). There was also decreased serum ALT and AST levels (p < 0.05) (Fig. 6E) in leptin KO mice compared with wild-type mice that were fed with high-fat diet and coexposed to BDCM (Figs. 6D i and E).
Fig. 6.
Leptin controls BDCM-induced alterations in glycolysis, gluconeogenesis, and lipid metabolism, thus aiding in development of liver injury in NASH. (A) mRNA expressions of liver Glut-1, PFK, and PCK-1 in DIO + BDCM and Leptin KO +BDCM groups. (B) mRNA expressions of liver PGC-1α, SREBP-1c, and PPAR-γ in DIO + BDCM and Leptin KO + BDCM groups. (C) Picro sirius red staining showing micro- and macrovesicular fibrosis (arrow) in DIO + BDCM (panel i) and Leptin KO + BDCM (panel ii) groups. (D) Hematoxylin and eosin staining showing hepatocellular necrosis (arrow) in DIO + BDCM (panel i) and Leptin KO + BDCM (panel ii) groups. (E) Serum ALT and AST enzyme levels in DIO + BDCM and Leptin KO + BDCM groups (n = 4). *p < 0.05 is considered statistically significant.
DISCUSSION
Here in this study, we report the development of NASH in obese mice following exposure to moderately lower dose of BDCM, a DBP of drinking water. Results also show that the free radical metabolism of BDCM, via CYP2E1 produces lipid peroxidation and the ensuing oxidative stress, exacerbates inflammation and adipokine leptin release (Figs. 1–3). The weekly doses of BDCM that were continued for 4 weeks were higher than the EPA permissible annualized average, but the study for the first time highlights the development of metabolic syndrome in obesity, albeit in rodent models. The disease progression from steatosis (fatty liver) to NASH was dependent on the free radical metabolism and adipokine leptin, whose release was a consequence of the free radical metabolism of BDCM (Figs. 5 and 6). Further the exposure to BDCM and the oxidative stress altered glucose transport, glycolytic pathway, gluconeogenesis, and skewed lipid metabolism toward lipogenesis (Fig. 4). The results also assume significance in clinical hepatology research because there is a direct correlation between obesity and development of chronic liver diseases like NASH as supported by a Center for Disease Control and Prevention report (Ogden et al., 2007). Our results find a correlation between the environmental toxicant BDCM and development of NASH in obesity; like toxicity-associated steatohepatitis, BDCM-exposed NASH is clinically relevant, even more to general population worldwide owing to wide access of chlorinated drinking water (Cave et al., 2010; Driedger et al., 2002). As a consequence, our results indicate that the levels of drinking water contaminant like BDCM should be strictly monitored to avoid any health hazards, though the studies reported here are restricted to rodent models only. Our results also for the first time show the molecular mechanisms by which the metabolism of BDCM causes generation of toxic free radical metabolites and exacerbates the inflammatory process in obesity, thus augmenting the development of a full-blown NASH. BDCM has been shown to be metabolized by CYP2E1 through a free radical mechanism, generating dihalomethyl radicals (Tomasi et al., 1985). Using immune-spin trapping, previous research reports from this group have shown that BDCM produces protein radical adducts (Das et al., 2013). Protein radical adducts from BDCM metabolism were also detected in the chronic dosing regimen (Supplementary fig. 2).
Prolonged exposure to BDCM in a mouse model of high-fat-diet-induced obesity showed hydroxynonenal adduct formation and nitrosative stress in Zone 3 of the liver and tyrosine nitration, events that were dependent on the CYP2E1 protein (Fig. 1). BDCM-exposed high-fat-diet-fed mice liver also showed increase in the p47 phox gene expression, an important component of the NADPH oxidase, but was independent of CYP2E1 (Fig. 1B). This expression profile might be due to the Kupffer cell activation process (increased CD68 expression; Fig. 2A), which can be triggered by the downstream actions of P2X7 receptor activation, as reported by Chatterjee et al.(2012b), in a similar model of liver injury with CCl4 exposure.
The metabolism of BDCM by CYP2E1 was responsible for a significant increase in the exacerbation of the inflammatory response in obese mice. Inflammation is key to progression of steatosis to steatohepatitis in obesity (Copaci et al., 2006; Jou et al., 2008; Farrell et al., 2012). The role of proinflammatory cytokines like TNF-α, IL-1β, IFN-γ, and TH3 cytokine TGF-β in NASH progression is well documented (Copaci et al., 2006; Larter and Farrell, 2006). Kupffer cell activation, which is marked by increased NADPH oxidase activity, MCP-1 release, peroxynitrite formation, increased MHC Class II expression and TNF-α release plays a significant role in NASH pathogenesis (Chatterjee et al., 2012a; Tosello-Trampont et al., 2012; Wang et al., 2009). The increased expression of CD68 (> 20-fold), a Kupffer cell activation marker, in BDCM-exposed mice compared with only DIO mice confirm the inflammatory surge and activation of Kupffer cells in the obese liver following BDCM exposure (Fig. 2A). NASH development is characterized by high degree of fibrosis and increased deposition of collagen, increased hepatic stellate cell proliferation, and increased hydroxyproline content (Syn et al., 2011). Liver biopsies reveal ballooning degeneration of hepatocytes, Mallory body formation, and infiltration of leukocytes (Diehl, 1999; Farrell et al., 2012). Our results showed significant expression of COL-1α-1 and α-SMA in the liver that was dependent on the presence of the CYP2E1 protein, suggesting the metabolism of BDCM by CYP2E1 as an important factor in the expression of the fibrotic indicator collagen and hepatic stellate cell proliferation marker, α-SMA (Figs. 3A and C) (Abdelmegeed et al., 2012). The fibrosis was also reflected by increased hydroxyproline content and intense staining of picro sirius red in liver tissues of DIO + BDCM group (Figs. 3B and F). Histopathology and analysis of liver ALT and AST levels were also indicative of liver injury and NASH development, both of which were dependent on the CYP2E1 presence, showing the importance of the free radical metabolism of BDCM by CYP2E1 as a primary cause of disease progression (Abdelmegeed et al., 2012) (Figs. 3D and E).
Obesity-linked nonalcoholic fatty liver disease is associated with insulin and leptin resistance (Bugianesi et al., 2004; Choudhury and Sanyal, 2005; Könner and Bruning, 2012). Defects in insulin receptor activation, which is a consequence of obesity, result in insulin resistance and secondary hyperinsulinemia (Choudhury and Sanyal, 2005). Insulin resistance as a consequence of obesity sets the stage for severe metabolic alterations in the liver due to predominance of de novo lipogenesis and stalling of beta oxidation of fatty acids (Choudhury and Sanyal, 2005). Defects in insulin signaling lead to alteration of Glut-4-induced glucose transport and increase in gluconeogenesis and glycogenolysis (Choudhury and Sanyal, 2005; Leguisamo et al., 2012). The DIO mice model (C57BL/6J) showed prominent diabetes with fasting blood glucose levels of greater than 240mg/dl and blood insulin levels of greater than 150 µU/ml (Surwit et al., 1988). We explored whether BDCM-induced NASH and especially the free radical-mediated oxidative stress produced metabolic reprogramming in the liver of DIO mice. Our results showed that there was an increase in Glut-4 and Glut-1 levels in the liver (Fig. 4A). These increases assume significance in fibrosis because stellate cell proliferation requires increased glucose availability (Tang and Chen, 2010). Further there was a significant increase in the glycolytic enzyme PFK and gluconeogenic enzyme PCK-1 (Fig. 4A). The BDCM exposure to DIO mice not only induced NASH but also led to metabolic alterations that might cause reprogramming of the key glycolytic enzymes that furthered de novo lipogenesis and increased inflammation. Similar to alterations in the enzymes of carbohydrate metabolism, a principal regulator of de novo lipogenesis (SREBP-1c) was increased significantly in DIO mice following exposure to BDCM (Fig. 4B). Further the transcription factor PPAR-α (slight increase, but not statistically significant) and transcriptional coactivator PGC-1α, master regulators of lipid metabolism in the liver, increased albeit moderately following BDCM exposure. On the other hand, there was a significant downregulation of PPAR-γ, the effect being dependent on the presence of the CYP2E1 gene (Fig. 4B). Decreased expression of PPAR-γ is associated with NASH because PPAR-γ KO mice develop severe steatohepatitis (Wu et al., 2010). Our observation of a decreased expression of this gene linked to the CYP2E1 reductive metabolism established a new paradigm of oxidative stress and metabolic reprogramming in NASH.
Another important aspect of obesity and the progression of NASH are linked to the development of leptin resistance, resulting in high circulatory levels of leptin, primarily due to the defect in leptin signaling (Kamada et al., 2008; Könner and Bruning, 2012; Morris and Rui, 2009; Munzberg, 2010). BDCM coexposure in obesity significantly increased hepatic leptin levels over the already high levels that existed in DIO mice (Figs. 2A, C, and D). Absence of leptin (ob/ob or Leptin KO mice) protected mice from NASH development by BDCM coexposure and restored the expression of metabolic regulators in both glucose and lipid genesis pathways to DIO levels (Figs. 5 and 6). These results are significant because leptin levels were increased following BDCM exposure, thus explaining the direct role of BDCM in augmenting the development of NASH from simple steatosis. This also explains the role of BDCM-induced free radical generation as a probable second hit that helped in progression of the disease.
In summary, our results clearly showed for the first time that chlorinated drinking water that contains moderate levels of DBPs, when consumed over time, can be a potential cause for development of NASH in obesity models of mice. Future studies in rodent models and epidemiological studies in humans, in areas where BDCM levels are higher than EPA permissible limits, might shed more important light about the possible link of DBPs of drinking water and development of metabolic syndrome and/or NASH. Our results show that NASH development and progression in experimental models of obesity can develop, at least in part, from the daily random exposure to low and tolerable levels of DBPs. Human susceptibility to NASH from environmental factors can be a possibility given the wide variance in susceptibility in individuals following CYP2E1 polymorphisms (Daly, 2012; Ginsberg et al., 2009; Jimenez-Lopez and Cederbaum, 2005; Neafsey et al., 2009). Because BDCM is metabolized primarily by CYP2E1, individuals with CYP2E1 polymorphisms might respond to lower doses of BDCM, thus making them candidates for NASH progression. Though it is speculative at this point, polymorphisms of other proteins associated with BDCM metabolism may also enhance pathogenicity even at very low levels of exposure. The data presented here stress the involvement of the environmental conditions in progression of NASH and its comorbidities and provide novel insight into the risk factors associated with NASH progression and the potential global health impact of this significant environmental toxicant.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
This work has been supported by National Institutes of Health Pathway to Independence Award (4R00ES019875-02 to S.C.), National Institutes of Health (R01-DK053792 to A.M.D.).
Supplementary Material
ACKNOWLEDGMENTS
The authors gratefully acknowledge the technical services of Benny Davidson at the IRF, University of South Carolina, School of Medicine and Jeoffrey Hurlburt and Ralph Wilson at NIEHS. We also thank the Instrumentation resource facility (IRF) at the University of South Carolina School of Medicine for equipment usage and consulting services. The authors declare no conflict of interest.
REFERENCES
- Abdelmegeed M. A., Banerjee A., Yoo S. H., Jang S., Gonzalez F. J., Song B. J. (2012). Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J. Hepatol. 57, 860–866 doi:10.1016/j.jhep.2012.05.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allis J. W., Zhao G. (2002). Quantitative evaluation of bromodichloromethane metabolism by recombinant rat and human cytochrome P450s. Chem. Biol. Interact. 140, 137–153 [DOI] [PubMed] [Google Scholar]
- Bugianesi E., Zannoni C., Vanni E., Marzocchi R., Marchesini G. (2004). Non-alcoholic fatty liver and insulin resistance: A cause-effect relationship? Dig. Liver Dis. 36, 165–173 doi:10.1016/j.dld.2003.12.008 [DOI] [PubMed] [Google Scholar]
- Cave M., Falkner K. C., Ray M., Joshi-Barve S., Brock G., Khan R., Bon Homme M., McClain C. J. (2010). Toxicant-associated steatohepatitis in vinyl chloride workers. Hepatology. 51, 474–481 doi:10.1002/hep.23321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Ganini D., Tokar E. J., Kumar A., Das S., Corbett J., Kadiiska M., Waalkes M., Diehl A. M., Mason R. P. (2012a). Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental nonalcoholic steatohepatitis. J. Hepatol. 58 778–784 doi:10.1016/ j.jhep.2012.11.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Rana R., Corbett J., Kadiiska M. B., Goldstein J., Mason R. P. (2012b). P2X7 receptor-NADPH oxidase axis mediates protein radical formation and Kupffer cell activation in carbon tetrachloride-mediated steatohepatitis in obese mice. Free Radic. Biol. Med. 52, 1666–1679 doi:10.1016/j.freeradbiomed.2012.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Choi S. S., Michelotti G. A., Chan I. S., Swiderska-Syn M., Karaca G. F., Xie G., Moylan C. A., Garibaldi F., Premont R., et al. (2012). Hedgehog controls hepatic stellate cell fate by regulating metabolism. Gastroenterology. 143, 1319–1329.e11 doi:10.1053/j.gastro.2012.07.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhury J., Sanyal A. J. (2005). Insulin resistance in NASH. Front. Biosci. 10, 1520–1533 [DOI] [PubMed] [Google Scholar]
- Copaci I., Micu L., Voiculescu M. (2006). The role of cytokines in non-alcoholic steatohepatitis. A review. J. Gastrointestin. Liver Dis. 15, 363–373 [PubMed] [Google Scholar]
- Daly A. K. (2012). Genetic polymorphisms affecting drug metabolism: Recent advances and clinical aspects. Adv. Pharmacol. 63, 137–167 doi:10.1016/b978-0-12-398339-8.00004-5 [DOI] [PubMed] [Google Scholar]
- Das S., Kumar A., Seth R. K., Tokar E. J., Kadiiska M. B., Waalkes M. P., Mason R. P., Chatterjee S. (2013). Proinflammatory adipokine leptin mediates disinfection byproduct bromodichloromethane-induced early steatohepatitic injury in obesity. Toxicol. Appl. Pharmacol. 269, 297–306 doi:10.1016/j.taap.2013.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day C. P., James O. F. (1998). Steatohepatitis: A tale of two “hits”? Gastroenterology. 114, 842–845 [DOI] [PubMed] [Google Scholar]
- Diehl A. M. (1999). Nonalcoholic steatohepatitis. Semin. Liver Dis. 19, 221–229 doi:10.1055/s-2007-1007111 [DOI] [PubMed] [Google Scholar]
- Driedger S. M., Eyles J., Elliott S. D., Cole D. C. (2002). Constructing scientific authorities: Issue framing of chlorinated disinfection byproducts in public health. Risk Anal. 22, 789–802 [DOI] [PubMed] [Google Scholar]
- Farrell G. C., van Rooyen D., Gan L., Chitturi S. (2012). NASH is an inflammatory disorder: Pathogenic, prognostic and therapeutic implications. Gut Liver. 6, 149–171 doi:10.5009/gnl.2012.6.2.149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginsberg G., Smolenski S., Neafsey P., Hattis D., Walker K., Guyton K. Z., Johns D. O., Sonawane B. (2009). The influence of genetic polymorphisms on population variability in six xenobiotic-metabolizing enzymes. J. Toxicol. Environ. Health B Crit. Rev. 12, 307–333 doi:10.1080/ 10937400903158318 [DOI] [PubMed] [Google Scholar]
- Jimenez-Lopez J. M., Cederbaum A. I. (2005). CYP2E1-dependent oxidative stress and toxicity: Role in ethanol-induced liver injury. Expert Opin. Drug Metab. Toxicol. 1, 671–685 doi:10.1517/17425255.1.4.671 [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Milner J. J., Makowski L. (2012). The inflammation highway: Metabolism accelerates inflammatory traffic in obesity. Immunol. Rev. 249, 218–238 doi:10.1111/j.1600-065X.2012.01151.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jou J., Choi S. S., Diehl A. M. (2008). Mechanisms of disease progression in nonalcoholic fatty liver disease. Semin. Liver Dis. 28, 370–379 doi:10.1055/s-0028-1091981 [DOI] [PubMed] [Google Scholar]
- Kamada Y., Takehara T., Hayashi N. (2008). Adipocytokines and liver disease. J. Gastroenterol. 43, 811–822 doi:10.1007/s00535-008-2213-6 [DOI] [PubMed] [Google Scholar]
- Könner A. C., Brüning J. C. (2012). Selective insulin and leptin resistance in metabolic disorders. Cell Metab. 16, 144–152 doi:10.1016/j.cmet.2012.07.004 [DOI] [PubMed] [Google Scholar]
- Larter C. Z., Farrell G. C. (2006). Insulin resistance, adiponectin, cytokines in NASH: Which is the best target to treat? J. Hepatol. 44, 253–261 doi:10.1016/j.jhep.2005.11.030 [DOI] [PubMed] [Google Scholar]
- Leguisamo N. M., Lehnen A. M., Machado U. F., Okamoto M. M., Markoski M. M., Pinto G. H., Schaan B. D. (2012). GLUT4 content decreases along with insulin resistance and high levels of inflammatory markers in rats with metabolic syndrome. Cardiovasc. Diabetol. 11, 100 doi:10.1186/1475-2840-11-100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lilly P. D., Andersen M. E., Ross T. M., Pegram R. A. (1997a). Physiologically based estimation of in vivo rates of bromodichloromethane metabolism. Toxicology. 124, 141–152 [DOI] [PubMed] [Google Scholar]
- Lilly P. D., Ross T. M., Pegram R. A. (1997b). Trihalomethane comparative toxicity: Acute renal and hepatic toxicity of chloroform and bromodichloromethane following aqueous gavage. Fundam. Appl. Toxicol. 40, 101–110 [DOI] [PubMed] [Google Scholar]
- Morris D. L., Rui L. (2009). Recent advances in understanding leptin signaling and leptin resistance. Am. J. Physiol. Endocrinol. Metab. 297, E1247–E1259 doi:10.1152/ajpendo.00274.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munzberg H. (2010). Leptin-signaling pathways and leptin resistance. Forum Nutr. 63, 123–132 doi:10.1159/000264400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Toxicology Program (1987). NTP toxicology and carcinogenesis studies of bromodichloromethane (CAS No. 75-27-4) in F344/N rats and B6C3F1 mice (Gavage Studies). Natl. Toxicol. Program Tech. Rep. Ser. 321, 1–182 [PubMed] [Google Scholar]
- Neafsey P., Ginsberg G., Hattis D., Johns D. O., Guyton K. Z., Sonawane B. (2009). Genetic polymorphism in CYP2E1: Population distribution of CYP2E1 activity. J. Toxicol. Environ. Health. B. Crit. Rev. 12, 362–388 doi:10.1080/10937400903158359 [DOI] [PubMed] [Google Scholar]
- Ogden C. L., Yanovski S. Z., Carroll M. D., Flegal K. M. (2007). The epidemiology of obesity. Gastroenterology. 132, 2087–2102 doi:10.1053/j.gastro.2007.03.052 [DOI] [PubMed] [Google Scholar]
- Parola M., Marra F. (2011). Adipokines and redox signaling: Impact on fatty liver disease. Antioxid. Redox Signal. 15, 461–483 doi:10.1089/ars.2010.3848 [DOI] [PubMed] [Google Scholar]
- Rius B., López-Vicario C., González-Périz A., Morán-Salvador E., García-Alonso V., Clária J., Titos E. (2012). Resolution of inflammation in obesity-induced liver disease. Front. Immunol. 3, 257 doi:10.3389/fimmu.2012.00257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Z. I., Rebelo M. H., Silva M. M., Alves A. M., Cabral Mda. C., Almeida A. C., Aguiar F. R., de Oliveira A. L., Nogueira A. C., Pinhal H. R., et al. (2012). Trihalomethanes in Lisbon indoor swimming pools: Occurrence, determining factors, and health risk classification. J. Toxicol. Environ. Health. A. 75, 878–892 doi:10.1080/15287394.2012.690706 [DOI] [PubMed] [Google Scholar]
- Surwit R. S., Kuhn C. M., Cochrane C., McCubbin J. A., Feinglos M. N. (1988). Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 37, 1163–1167 [DOI] [PubMed] [Google Scholar]
- Syn W. K., Choi S. S., Liaskou E., Karaca G. F., Agboola K. M., Oo Y. H., Mi Z., Pereira T. A., Zdanowicz M., Malladi P., et al. (2011). Osteopontin is induced by hedgehog pathway activation and promotes fibrosis progression in nonalcoholic steatohepatitis. Hepatology. 53, 106–115 doi:10.1002/hep.23998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Chen A. (2010). Curcumin prevents leptin raising glucose levels in hepatic stellate cells by blocking translocation of glucose transporter-4 and increasing glucokinase. Br. J. Pharmacol. 161, 1137–1149 doi:10.1111/j.1476-5381.2010.00956.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilg H., Moschen A. R. (2010). Evolution of inflammation in nonalcoholic fatty liver disease: The multiple parallel hits hypothesis. Hepatology. 52, 1836–1846 doi:10.1002/hep.24001 [DOI] [PubMed] [Google Scholar]
- Tomasi A., Albano E., Biasi F., Slater T. F., Vannini V., Dianzani M. U. (1985). Activation of chloroform and related trihalomethanes to free radical intermediates in isolated hepatocytes and in the rat in vivo as detected by the ESR-spin trapping technique. Chem. Biol. Interact. 55, 303–316 [DOI] [PubMed] [Google Scholar]
- Torres D. M., Williams C. D., Harrison S. A. (2012). Features, diagnosis, and treatment of nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 10, 837–858 doi:10.1016/j.cgh.2012.03.011 [DOI] [PubMed] [Google Scholar]
- Torti V. R., Cobb A. J., Everitt J. I., Marshall M. W., Boorman G. A., Butterworth B. E. (2001). Nephrotoxicity and hepatotoxicity induced by inhaled bromodichloromethane in wild-type and p53-heterozygous mice. Toxicol. Sci. 64, 269–280 [DOI] [PubMed] [Google Scholar]
- Tosello-Trampont A. C., Landes S. G., Nguyen V., Novobrantseva T. I., Hahn Y. S. (2012). Kuppfer cells trigger nonalcoholic steatohepatitis development in diet-induced mouse model through tumor necrosis factor-α production. J. Biol. Chem. 287, 40161–40172 doi:10.1074/jbc.M112.417014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Leclercq I., Brymora J. M., Xu N., Ramezani-Moghadam M., London R. M., Brigstock D., George J. (2009). Kupffer cells mediate leptin-induced liver fibrosis. Gastroenterology. 137, 713–723 doi:10.1053/j.gastro.2009.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigle D. T. (1998). Safe drinking water: A public health challenge. Chronic Dis. Can. 19, 103–107 [PubMed] [Google Scholar]
- Wu C. W., Chu E. S., Lam C. N., Cheng A. S., Lee C. W., Wong V. W., Sung J. J., Yu J. (2010). PPARgamma is essential for protection against nonalcoholic steatohepatitis. Gene Ther. 17, 790–798 doi:10.1038/gt.2010.41 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.