Abstract
Inhaled pollutants induce the release of vasoactive factors into the systemic circulation, but little information is available regarding the nature of these factors or their receptors. The pattern recognition receptor CD36 interacts with many damage-related circulating molecules, leading to activation of endothelial cells and promoting vascular inflammation; therefore, we hypothesized that CD36 plays a pivotal role in mediating cross talk between inhaled ozone (O3)-induced circulating factors and systemic vascular dysfunction. O3 exposure (1 ppm × 4h) induced lung inflammation in wild-type (WT) mice, which was absent in the CD36 deficient (CD36−/−) mice. Acetylcholine (ACh)-evoked vasorelaxation was impaired in isolated aortas from O3-exposed WT mice but not in vessels from CD36−/− mice. To delineate whether vascular impairments were caused by lung inflammation or CD36-mediated generation of circulating factors, naïve aortas were treated with diluted serum from control or O3-exposed WT mice, which recapitulated the impairments of vasorelaxation observed after inhalation exposures. Aortas from CD36−/− mice were insensitive to the effects of O3-induced circulating factors, with robust vasorelaxation responses in the presence of serum from O3-exposed WT mice. Lung inflammation was not a requirement for production of circulating vasoactive factors, as serum from O3-exposed CD36−/− mice could inhibit vasorelaxation in naïve WT aortas. These results suggest that O3 inhalation induces the release of circulating bioactive factors capable of impairing vasorelaxation to ACh via a CD36-dependent signaling mechanism. Although lung inflammatory and systemic vascular effects were both dependent on CD36, the presence of circulating factors appears to be independent of CD36 and inflammatory responses.
Key Words: ozone, CD36, vascular, inflammation, vasorelaxation, air pollution, endothelial, pattern recognition receptor, scavenger receptor.
Air pollution remains a serious problem in urban regions throughout the world. Globally, an estimated 800,000 people every year die prematurely due to air pollution (Brook, 2008), mostly from cardiovascular disease (CVD). Ground-level ozone (O3), considered one of the most important air pollutants, may cause the premature deaths of over 3000 people each year in the United States, alone (WHO, 2002) and costs the economy billions of dollars in medical care and productivity losses (Hubbell et al., 2005). Ground-level O3 is formed when pollutants emitted by cars, and other sources undergo chemical and photochemical reactions in the atmosphere (Cole and Freeman, 2009). As a result, mortality and morbidity rates associated with O3 exposure are expected to increase as temperatures continue to rise with global warming, adding urgency to the need for action to limit the damaging effects of air pollution. Uncertainty remains concerning the mechanism(s) underlying systemic responses to inhaled pollutants, negatively impacting our ability to manage vulnerable populations and establish precise, effective regulatory policies.
There is growing evidence suggesting that inhaled pollutants evoke a systemic inflammatory response that ultimately results in endothelial injury and dysfunction—two key features of CVD. Using a variety of air pollutants, including O3, research in our laboratory and others have documented adverse effects on systemic vascular reactivity both in animals and, to a more limited extent, in humans (Brook et al., 2002; Channell et al., 2012; Cherng et al., 2011; Chuang et al., 2009; Lund et al., 2011). Recent controlled human exposure studies with diesel emissions and nitrogen dioxide (NO2) note definitive transference of toxicity to the circulation, with the evident induction of as yet unknown factors that have the potential to induce adhesion molecules and cytokine release from primary human coronary artery endothelial cells (Channell et al., 2012).
Given its reactivity, O3 cannot be directly transferred into the circulation but instead reacts with the surfactant to generate a mix of secondary and tertiary reactants (Frampton et al., 1999; Kafoury et al., 1999; Postlethwait et al., 1998; Pryor et al., 1995). These reactants have the potential to modify lipids or produce protein adducts, capable of binding as epitopes to pattern recognition receptors (PRRs), such as lectin-like oxidized low-density lipoprotein receptor-1, Toll-like receptor 4 (TLR4), and cluster of differentiation receptor 36 (CD36) (Garantziotis et al., 2010; Kampfrath et al., 2011; Kumano-Kuramochi et al., 2012; Li et al., 2011). Although lipid peroxidation products present in the lung lining fluid following exposure to particulate matter (PM) have been reported to mediate systemic cellular inflammatory responses through TLR4 (Kampfrath et al., 2011), CD36 has not been well studied with respect to air pollution. CD36, a class B scavenger receptor, recognizes many ligands and is widely expressed on the surface of multiple cell types, including macrophages and endothelial cells (Febbraio et al., 2001; Sawada et al., 2012). CD36 has been strongly implicated in pathological conditions associated with air pollution, including atherosclerosis and inflammation (Febbraio et al., 2001; Kuda et al., 2011; Silverstein and Febbraio, 2009). Increased expression of CD36 in the vasculature was correlated positively with increased entry of monocytes into atherosclerotic plaques following diesel exhaust (DE) exposure (Bai et al., 2011). Currently, this is the only reported role of CD36 in relation to air pollution exposure, and no studies to date have investigated potential links between CD36, vascular dysfunction, and O3 exposure.
We hypothesized that the CD36 PRR contributes to systemic vascular impairment downstream of O3 inhalation. To test this hypothesis, we developed an innovative methodology for assessing potential cumulative effects of circulating mediators on vascular function, in both CD36 knockout (CD36−/−) and wild-type (WT) mice. In this study, we demonstrate that O3 inhalation induces the generation of circulating bioactive factors, leading to impaired vascular responses to ACh via a CD36-dependent signaling mechanism.
MATERIALS AND METHODS
Animals.
Female C57BL/6 WT mice (Harlan Laboratories) and CD36−/− mice aged 8–10 weeks were used in the study. CD36−/− mice, generated on C57BL/6 background, as previously described (Febbraio et al., 1997), were kindly provided by Dr Maria Febbraio (University of Alberta, Edmonton, Canada). Upon arrival, mice were housed four per cage under controlled environmental conditions (21°C ± 2°C; 12-h light/dark cycle) with access to tap water and standard chow ad libitum (Harlan). The mice were allowed to acclimate for at least 1 week prior to the start of experimentation. All procedures were approved by the Institutional Animal Care and Use Committee at the University of New Mexico. Mice were euthanized with an overdose of anesthesia (isoflurane; concentration 5%) or by exsanguination via cardiac puncture while under anesthesia (isoflurane; concentration 1.5–2%).
O3 exposures.
O3 was generated using an OREC silent arc discharge O3 generator (Osmonics, Phoenix, AZ). The O3 concentration was continuously monitored using a photometric O3 analyzer (TG-501; GrayWolf, Shelton, CT), and temperature was maintained at 21°C ± 2°C. Concentrations of carbon monoxide and nitrogen oxides (NOx) were unaltered. The mice were randomly assigned a group and were exposed to either filtered air (FA) or 1 ppm O3 for 4h. During exposures, the mice were singly housed within a sealed chamber (Biospherics) without bedding. Food, but not water, was withheld during the 4-h exposure period to preclude ingestion of ozonation products. Mice were euthanized for tissue collections 24h after exposure.
Collection and analysis of bronchoalveolar lavage fluid.
Bronchoalveolar lavage (BAL) fluid was collected for assessment of lung inflammatory responses of mice following inhalation. Following euthanasia, the lungs were lavaged three times with 0.8ml of sterile saline. BAL fluid was centrifuged at 1800 × g for 5min. The supernatant from the first lavage was stored at −80°C until required for biochemical determination. Total protein content was assessed with a bicinchoninic acid assay kit (Pierce, Rockford, IL). The cell pellets from all lavages were resuspended in 0.8ml of physiological saline and combined. Total cell numbers were determined and 10,000 cells were centrifuged onto cytospin slides for differential staining as described (Robertson et al., 2012).
Ex vivo vascular function using myography.
Rings from the thoracic and abdominal aorta were isolated from mice 24h after exposure, and cleaned of connective tissue. Segments of aorta (2–3mm length) were mounted in a 4-chamber myograph system (610M; Danish Myo Technology A/S, Aarhus, Denmark). Vessels were submerged in physiological salt solution (composition in millimolar: 119.0 NaCl, 25.0 NaHCO3, 5.5 glucose, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 0.027 EDTA, 2.5 CaCl2) bubbled at 37°C with 21%O2-5%CO2-balance N2 and left to equilibrate at 2 mN of tension for ~30min. Tension was gradually applied over 10min to an optimal passive tension of 10 mN. Preliminary experiments showed that this tension produced optimal contraction and relaxation responses. Data from force transducers were processed by a MacLab/4e A-Dl converter displayed through LabChart software (AD Instruments).
Vessel viability was confirmed by a contractile response on addition of high potassium physiological salt solution (KPSS in millimolar: 64.9 NaCl, 25.0 NaHCO3, 5.5 glucose, 58.9 KCl, 1.2 MgSO4, 1.2 KH2PO4, 0.027 EDTA, 2.5 CaCl2), repeated twice. Cumulative concentration-response curves were constructed for the Thromboxane A2 analogue, U-46619 (Sigma-Aldrich). Following preconstriction with the negative logarithm concentration of U-46619 eliciting 50% of the maximum response to KPSS (58.9nM KCl; EC50), cumulative concentration-response curves were generated with the endothelium-dependent vasorelaxant acetylcholine (ACh; 10−9–10−5M; Sigma-Aldrich). In some experiments, the vessel lumen was rubbed with a strand of moose main to disrupt the endothelium. Successful removal of vascular endothelium was confirmed by the failure of ACh to relax preconstricted aortic rings, before a cumulative concentration-response curve to the NO donor spermine NONOate was generated (10−9–10−5M; Cayman Chemicals).
Effect of serum on the responses of the aorta from naïve WT and CD36−/− mice to ACh.
Aortic rings isolated from naïve WT and CD36−/− mice were mounted in a myograph and challenged twice with KPSS as described above. After a 30-min equilibration period, vessels were incubated with 2.5% serum from WT or CD36−/− mice following exposure to FA or O3. Because addition of serum induced contraction of aortic rings, cumulative concentration-response curves to ACh (10−9–10−5M) were performed after responses to serum had stabilized.
Statistical analysis.
Data are expressed as the mean ± SEM. Contractile responses are expressed as a percentage of the maximal contraction induced by KPSS (58.9nM KCl). U-46619 EC50 values were calculated with a nonlinear regression analysis. All vasorelaxation responses are expressed as percentage of the precontraction to EC50 U-46619 or expressed as a percentage of the response to 2.5% serum, with 100% representing basal tension. Statistical comparisons were performed by two-way ANOVA using the Bonferroni’s post hoc test, unless otherwise stated. Statistical analyses were performed using GraphPad Prism software (V5.0; GraphPad Software Inc.). p < 0.05 was considered to be statistically significant.
RESULTS
Loss of CD36 Blunts Lung Inflammatory Cell Influx Following O3 Exposure
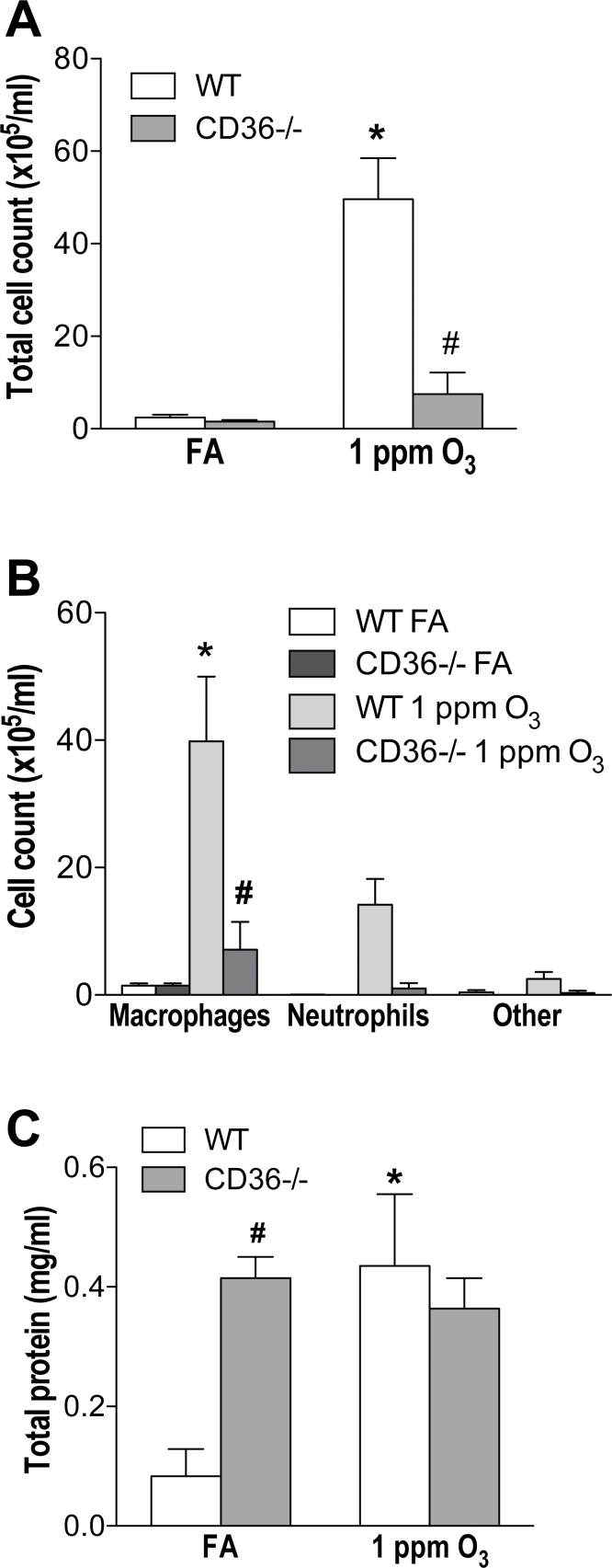
Consistent with previous findings (Fakhrzadeh et al., 2004; Johnston et al., 2005; Savov et al., 2004), O3 inhalation increased total BAL fluid cell count in WT mice compared with WT FA-exposed mice (p < 0.05 compared with WT FA-exposed mice; Fig. 1A). The increase in cell count was mostly macrophages (39.8±10.1×105 cells/ml vs. 1.5±0.3×105 cells/ml in WT FA-exposed mice; Fig. 1B). In contrast, the CD36−/− mice did not display this O3-induced cell influx in the lungs (p < 0.05 compared with O3-exposed WT mice; Fig. 1A). No significant differences in total and differential cell counts were observed in the CD36−/− mice exposed to FA or O3 (Figs. 1A and B). As well as cell influx, the total protein concentration in the BAL fluid was measured. The protein concentration of BAL fluid increased significantly in WT mice 24h after O3 exposure (p < 0.05 compared with WT FA-exposed mice; Fig. 1C). BAL protein levels in the CD36−/− mice did not change significantly between those exposed to FA or O3 (Fig. 1C); however, baseline protein levels were approximately threefold higher in FA-exposed CD36−/− mice compared with WT (Fig. 1C).
FIG. 1.
CD36−/− mice are partially protected from O3-induced lung inflammation. Female CD36−/− mice or WT animals were exposed to either FA or O3 (1 ppm) for 4h. (A) Total cell count, (B) differential cell count, and (C) total protein were analyzed in the BAL fluid 24-h postexposure. Columns represent mean ± SEM (n = 4–6). *p < 0.05 versus WT FA exposed; #p < 0.05 versus WT O3 exposed; two-way ANOVA followed by Bonferroni’s post hoc test.
Circulating total white blood cell (WBC) counts and differentials were compared among groups. Total WBC cell counts and the relative proportion of different types of WBCs did not change in WT mice exposed to FA compared with those exposed to O3 (Supplementary figs. Ia and b). CD36−/− mice had significantly lower levels of total WBCs than WT mice, but there was no change in the proportion of individual types of WBCs (Supplementary fig. Ib). Additionally, there were no statistically significant differences in WBC counts between CD36−/− mice exposed to O3 compared with those exposed to FA (Supplementary figs. Ia and b). RBC and platelet counts did not differ between strains or across treatment groups (data not shown).
Loss of CD36 Prevents Vascular Endothelial Dysfunction Following Exposure to O3
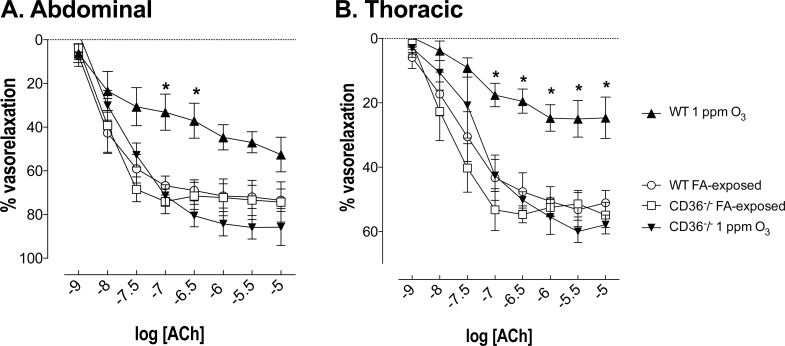
There was no change in basal tone of thoracic and abdominal aortic rings among the experimental groups (Supplementary table I). Responses to U-46619 were not influenced by O3 exposure in both the endothelium-intact thoracic (p = 0.0543) and abdominal (p = 0.1153) aortas (Supplementary figs. IIa and b; EC50 values are presented in Table 1). ACh-induced relaxations were assessed ex vivo in U-46619-precontracted thoracic and abdominal aorta rings from mice 24h after exposure. In the thoracic aorta from FA-exposed WT mice, ACh (10−9–10−5M) relaxed U-46619-preconstricted thoracic aortas, reaching a maximum of 53.2±5.3% (Fig. 2A). The ACh-induced relaxation was significantly attenuated in WT mice exposed to O3. The maximum relaxation due to ACh reduced by ~50% in thoracic aortas from WT mice exposed to O3 compared with their respective FA-exposed control littermates (p < 0.05; Fig. 2A). In addition, this impaired relaxing effect to ACh was also observed in abdominal aorta (Fig. 2B). Similar to what has been shown before (Pagano et al., 1999), ACh-induced relaxation was stronger in abdominal aortas from FA-exposed WT mice than in thoracic aorta (Figs. 2A and B). The ability of ACh to cause relaxation of the aorta is mainly attributed to nitric oxide (NO) released from the endothelium, diffusing into the underlying vascular smooth muscle. Relaxation responses to spermine NONOate in endothelium-denuded aortas were generated to investigate whether the impaired ACh-mediated vascular relaxation following O3 exposure is due to reduced release of NO from the endothelium or because of a reduced sensitivity of vascular smooth muscle cells in response to NO. There was actually an increased sensitivity of aortas from O3-exposed mice to spermine NONOate (Supplementary fig. III), thus providing addition support for an effect of O3 to impair endothelial release of NO.
TABLE 1.
–log EC50 Values of Concentration-Response Curves Performed for U-46619 in Aortic Rings From WT and CD36−/− Mice
| –log EC50 [M] | ||
|---|---|---|
| Abdominal aorta | Thoracic aorta | |
| WT | ||
| FA | 8.3±0.3 | 8.2±0.2 |
| 1 ppm O3 | 8.0±0.2 | 8.2±0.1 |
| CD36−/− | ||
| FA | 8.1±0.1 | 8.1±0.2 |
| 1 ppm O3 | 7.8±0.2 | 8.0±0.2 |
Note. EC50, concentration for half-maximal contraction (obtained using nonlinear regression); unit, millimolar (mM). Values are mean ± SEM (n = 4–8).
FIG. 2.
Loss of the CD36 receptor protects against the impaired ACh-mediated endothelium-dependent relaxation in isolated (A) thoracic aorta and (B) abdominal aorta of O3-exposed WT mice. Relaxation responses to ACh were reduced in thoracic and abdominal aorta rings from rats exposed to O3 24h prior, an effect prevented in the CD36−/− mice. Data are expressed as mean ± SEM (n = 4–5). *p < 0.05 versus WT FA exposed; two-way ANOVA followed by Bonferroni’s post hoc test.
We next investigated whether O3 pollution impairs endothelial function through activation of the CD36 receptor. Relaxation in response to ACh in both the thoracic and abdominal aortas did not differ between WT and CD36−/− mice exposed to FA (Figs. 2A and B). However, CD36−/− mice were protected against the O3-induced impairments of ACh-dependent vasorelaxation in aortic rings (Figs. 2A and B). Whether this protection was due to a role for vascular CD36 or the abrogation of lung inflammation remained unclear from these in vivo inhalation studies, necessitating the development of ex vivo homologous serum assays.
CD36 Mediates Vascular Endothelium Dysfunction Induced by Circulating Factors Following Exposure to Inhaled O3
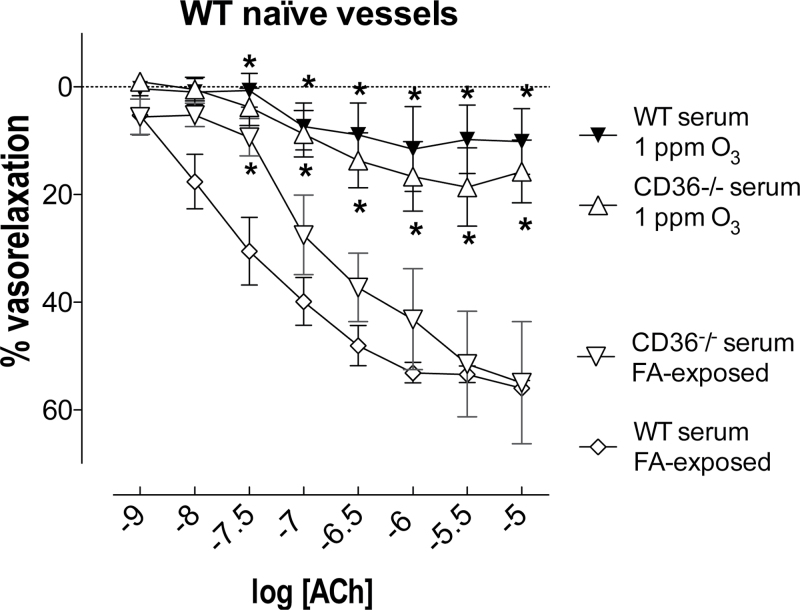
To determine whether changes in the serum after O3 exposure were a contributor to impaired vascular function, relaxation responses were determined in thoracic aortas from naïve (unexposed) WT mice following incubation with 2.5% serum samples obtained from WT mice 24h following exposure to FA or O3. There were no differences in basal tone or constriction to serum between groups (Supplementary table II). Serum samples obtained from WT mice after FA exposure did not modify relaxation to ACh in the aorta (Fig. 3). For serum collected from WT mice previously exposed to O3, relaxation in response to ACh was impaired in the aorta of WT naïve mice (Fig. 3). Maximum relaxation due to ACh was reduced by ~85% in aortas from WT naïve mice incubated with serum from WT mice previously exposed to O3 compared with aortas from a FA-exposed WT mouse (p < 0.05; Fig. 3).
FIG. 3.
O3 exposure induces the secretion of bioactive circulating factors, leading to impaired vascular responses to ACh, independently of CD36 function or pulmonary inflammation. A mixture of 2.5% serum obtained from WT and CD36−/− mice 24h after 1 ppm O3 or FA exposure diminished vasorelaxation to ACh in thoracic aortas from naïve (unexposed) WT mice. Data are expressed as mean ± SEM (n = 3–5). *p < 0.05 versus WT serum FA exposed; two-way ANOVA followed by Bonferroni’s post hoc test.
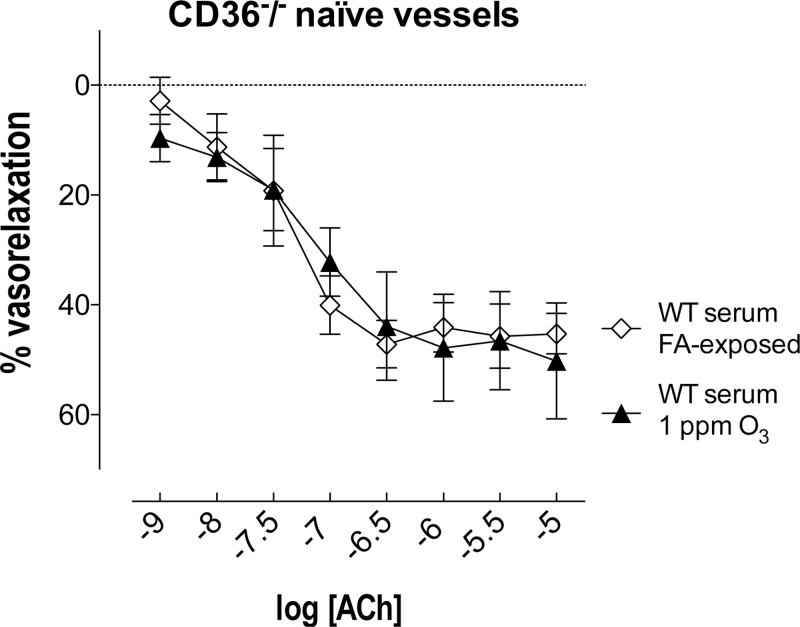
By contrast, serum obtained from WT mice 24h after O3 exposure had no effect on the relaxation response to any concentration of ACh in thoracic aorta of naïve CD36−/− mice (Fig. 4). To investigate whether the O3-induced circulating factors capable of impairing vascular responses to ACh in WT mice were independent of O3-induced lung inflammation, relaxation responses were determined in vessels from naïve WT mice following incubation with 2.5% serum samples obtained from CD36−/− mice 24h following exposure to FA or O3. As before, there were no differences in basal tone or constriction to serum in the thoracic aorta of WT naïve mice (Supplementary table II). Serum samples obtained from CD36−/− mice after FA exposure did not modify relaxation to ACh in the thoracic aorta of naïve WT mice (Fig. 3). For serum collected from the O3-exposed CD36−/−, relaxation in response to ACh was impaired in the thoracic aorta of WT naïve mice (Fig. 3). Notably, the constrictor effect of serum collected from CD36−/− mice previously exposed to O3 was greater on thoracic aortas of naïve WT mice compared with naïve CD36−/− mice (Supplementary table II).
FIG. 4.
Vascular CD36 mediates impairments in ACh-mediated vasorelaxation caused by O3-induced circulating factors. Serum (2.5%) obtained from WT mice 24h after O3 exposure had no effect on thoracic aortas of CD36−/− naïve mice. Data are expressed as mean ± SEM (n = 3–5). No significant differences were found between groups (two-way ANOVA).
DISCUSSION
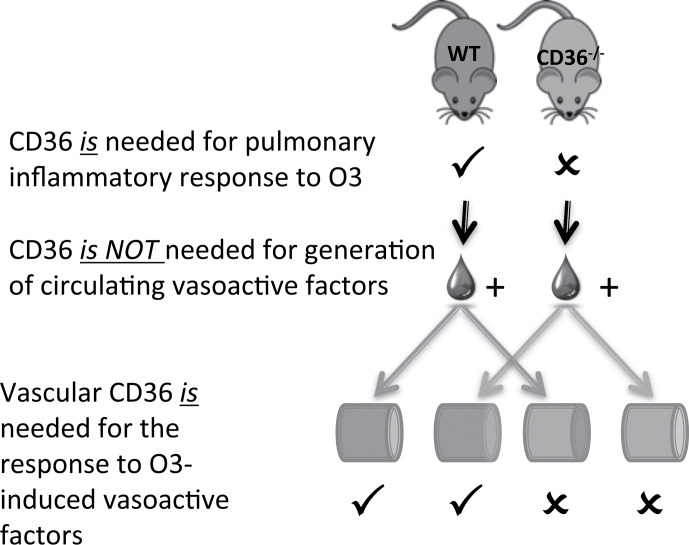
In this study, we demonstrate that O3 inhalation induces circulating vasoactive factors, which can directly impair vasorelaxation to ACh. Moreover, as illustrated in Figure 5, the ex vivo homologous serum study design enables us to conclude that the CD36 PRR can influence (1) pulmonary inflammatory response to inhaled O3 and (2) the vasorelaxation impairments downstream of inhaled O3, and these responses are independent of one another. Furthermore, the generation of circulating vasoactive factors is independent of CD36, as serum from both the WT and CD36−/− models impaired ACh-mediated relaxation in WT vessels. Our study contributes support to the emerging view that changes in the serum/plasma composition caused by inhaled pollutants facilitate signals in the circulation that can drive vascular inflammatory responses (Channell et al., 2012; Lund et al., 2011).
FIG. 5.
Summary of homologous serum assay results, highlighting a role for CD36 in mediating (1) the pulmonary inflammatory response to O3 and (2) the impaired vasorelaxation due to O3-induced circulating factors. However, the production of such circulating factors was independent of CD36.
Consistent with earlier experimental studies (Fakhrzadeh et al., 2004; Johnston et al., 2005; Savov et al., 2004), O3 exposure induced lung inflammation in vivo reflected by inflammatory cell influx and increased total protein in BAL fluid. We further show that this O3-induced cell influx was absent in CD36−/− mice. These data strongly suggest that the CD36 receptor plays a role in the inflammatory response to O3 exposure. Although studies have shown that CD36 stimulation mediates key downstream inflammatory signaling pathways (Kuda et al., 2011; Silverstein and Febbraio, 2009), this is the first report on the effects of CD36 on O3-induced lung inflammation. Unexpectedly, we observed a threefold increase in total protein in the BAL fluid from FA-exposed CD36−/− mice compared with their respective WT control. One may speculate that this could be due to the role of CD36, which is expressed on alveolar macrophages (AMs) (Smith et al., 2007), mediating the phagocytosis of (among other things) anionic phospholipids (Rigotti et al., 1995; Tait and Smith, 1999), fatty acids (Febbraio et al., 2001), and albumin (Baines et al., 2012). AM phagocytosis is essential for the maintenance of lung structure and function. Consequently, it is possible that a reduced capacity of the AMs to clear the lung of inhaled debris is contributing to the increased BAL total protein in mice deficient in CD36. The parallel information obtained between presumed function of CD36 on lung macrophages and on systemic endothelial cells in the present study may provide clues as to the circulating factors produced after O3 inhalation. Although previously termed “inflammatory spillover,” we propose that damage-associated molecular patterns formed by the cleavage of a number of lung phospholipids, proteins, or glycoproteins could, in fact, be a “preinflammatory” message that affects a vascular endothelial response. This concept is analogous to findings of hyaluronan fragment-dependent hyperreactivity that was differentiated from inflammation in a similar model (Li et al., 2010, 2011).
Because the lung inflammatory response was a clear complicating factor in determining the role of vascular CD36, we implemented the homologous serum experiments, treating naïve vessels with serum from control and exposed mice. The results of this experiment revealed two distinct conclusions (1) that circulating components induced by O3 exposure decreased the vasorelaxation caused by ACh, independent of lung inflammation and (2) that the circulating components act through vascular CD36. Vascular CD36 expression has not been thoroughly studied with respect to air pollution but was found to be closely associated with vascular oxidative stress in response to inhaled DE emissions (Bai et al., 2011). Previously, we reported a possible role of reactive oxygen species (ROS) in mediating the DE-induced vascular toxicity (Cherng et al., 2011). Acute exposure to DE resulted in increased vascular oxidative stress in rats, and pretreatment, in vitro, with a free radical scavenger could reverse the diminished NO-dependent vasorelaxation in coronary arteries (Cherng et al., 2011). There is also evidence that DE inhalation may activate a feed-forward mechanism, involving uncoupling of endothelial NO synthase (eNOS), capable of not only impairing NOS-dependent relaxation pathways but those dependent on cyclooxygenase, as well (Cherng et al., 2011). This mechanism may thus contribute to the impaired vasorelaxation following exposure to O3. However, the involvement of CD36 suggests that somewhat different mechanisms may be involved in the vascular response to O3. CD36 has been shown to modulate the activity of eNOS via phosphorylation and displacement of the enzyme from the basolateral membrane (Shaul, 2002), and this may be an important avenue for future research. eNOS uncoupling and ROS generation may only partly explain the endothelial dysfunction routinely observed in humans exposed to lower concentrations of pollutants (Barath et al., 2010; Mills et al., 2005). The contribution that pollution-induced circulating factors participate in the relocation and subsequent inhibition of eNOS activity has not yet been explored.
In this study, we have used a novel method to demonstrate a pivotal role of CD36 in mediating the vascular effects induced by the cumulative impact of O3 exposure in the circulation. The method described in this article is capable of assessing potential cumulative effects of circulating mediators on vascular function and is both anatomically more sophisticated and translationally coherent than comparable methods involving direct application of airborne pollutants to vessels or cultured vascular cells. Although the present application was limited to O3, this ex vivo approach for the evaluation of the vasorelaxant effect of homologous serum samples is clearly applicable to other environmental pollutants. There are, however, some caveats with our overall approach that should be mentioned. The use of a high O3 concentration was necessary to offset the well-recognized species insensitivity to this reactive air pollutant. Rodents are obligatory nose breathers and exhibit roughly one third of the sensitivity to O3 compared with primates (Brown et al., 2005; Harkema et al., 2006). It is important to keep in mind, however, that O3 levels higher than that used in this study have been reported in some metropolitan areas on hot humid days (Alonso et al., 2002; Bytnerowicz et al., 2002a ,b). Nevertheless, exposure of humans to O3 at levels typically encountered on the ground, O3 does not induce such clear pulmonary inflammation as we have observed in the present study, and it is difficult to entirely rule out this response as a contributor to the systemic vascular effects. However, recent studies utilizing levels below the national ambient air quality standard revealed small but significant lung function decrements without clear evidence of pulmonary inflammation (Kim et al., 2011). The formation of aldehydes in the airways has been demonstrated in humans exposed to only 0.12 ppm (Frampton et al., 1999). Additionally, Brook et al. (2002) noted significant narrowing of brachial artery diameter in healthy subjects exposed to 0.12 ppm O3 and 150 μg/m3 concentrated PM. Notably, levels of inhaled NO2 below what has been previously reported to cause lung inflammation did induce changes in human plasma that led to inflammatory activation of cultured endothelial cells (Channell et al., 2012); thus in human exposures, it may be possible to oxidatively modify serum components at much lower levels of O3.
To conclude, a role of CD36 as a mediator of vascular impairments following O3 inhalation has been demonstrated using a straightforward homologous serum technique and global CD36 knockout model. The homologous serum methods further substantiate recent findings in which plasma obtained from human subjects following exposure to NO2, a pollutant with similar toxic properties as O3, was able to induce expression of inflammatory adhesion molecules in treated human coronary artery endothelial cells (Channell et al., 2012). CD36 additionally has an important role in regulating inflammatory responses in the lung and, quite possibly, clearance of aged proteins from the airways. Far from any consideration of CD36 as a singularly responsible receptor that explains the association between O3 levels and cardiovascular morbidity, we believe these studies highlight a wide realm of possible interactions between chemically modified blood components and receptors of the systemic endothelium.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (MJC ES014639, PRH AI090917); Environmental Protection Agency (RD-83479601-0, STAR R83399001).
Supplementary Material
ACKNOWLEDGMENTS
We thank Dr Terry Wu and Quiteria Sanchez for their help with the Coulter Counter. The authors have no competing interests to declare.
REFERENCES
- Alonso R., Bytnerowicz A., Arbaugh M. (2002). Vertical distribution of ozone and nitrogenous pollutants in an air quality class I area, the San Gorgonio wilderness, southern California. ScientificWorldJournal 2, 10–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai N., Kido T., Suzuki H., Yang G., Kavanagh T. J., Kaufman J. D., Rosenfeld M. E., van Breemen C., Eeden S. F. (2011). Changes in atherosclerotic plaques induced by inhalation of diesel exhaust. Atherosclerosis 216, 299–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baines R. J., Chana R. S., Hall M., Febbraio M., Kennedy D., Brunskill N. J. (2012). CD36 mediates proximal tubular binding and uptake of albumin and is upregulated in proteinuric nephropathies. Am. J. Physiol. Renal Physiol. 303, F1006–F1014 [DOI] [PubMed] [Google Scholar]
- Barath S., Mills N. L., Lundbäck M., Törnqvist H., Lucking A. J., Langrish J. P., Söderberg S., Boman C., Westerholm R., Löndahl J., et al. (2010). Impaired vascular function after exposure to diesel exhaust generated at urban transient running conditions. Part. Fibre Toxicol. 7, 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook R. D. (2008). Cardiovascular effects of air pollution. Clin. Sci. (Lond). 115, 175–187 [DOI] [PubMed] [Google Scholar]
- Brook R. D., Brook J. R., Urch B., Vincent R., Rajagopalan S., Silverman F. (2002). Inhalation of fine particulate air pollution and ozone causes acute arterial vasoconstriction in healthy adults. Circulation 105, 1534–1536 [DOI] [PubMed] [Google Scholar]
- Brown J. S., Wilson W. E., Grant L. D. (2005). Dosimetric comparisons of particle deposition and retention in rats and humans. Inhal. Toxicol. 17, 355–385 [DOI] [PubMed] [Google Scholar]
- Bytnerowicz A., Parker D. R., Padgett P. E. (2002a). Vertical distribution of ozone and nitric acid vapor on the Mammoth Mountain, eastern Sierra Nevada, California. ScientificWorldJournal 2, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bytnerowicz A., Tausz M., Alonso R., Jones D., Johnson R., Grulke N. (2002b). Summer-time distribution of air pollutants in Sequoia National Park, California. Environ. Pollut. 118, 187–203 [DOI] [PubMed] [Google Scholar]
- Channell M. M., Paffett M. L., Devlin R. B., Madden M. C., Campen M. J. (2012). Circulating factors induce coronary endothelial cell activation following exposure to inhaled diesel exhaust and nitrogen dioxide in humans: Evidence from a novel translational in vitro model. Toxicol. Sci. 127, 179–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherng T. W., Paffett M. L., Jackson-Weaver O., Campen M. J., Walker B. R., Kanagy N. L. (2011). Mechanisms of diesel-induced endothelial nitric oxide synthase dysfunction in coronary arterioles. Environ. Health Perspect. 119, 98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang G. C., Yang Z., Westbrook D. G., Pompilius M., Ballinger C. A., White C. R., Krzywanski D. M., Postlethwait E. M., Ballinger S. W. (2009). Pulmonary ozone exposure induces vascular dysfunction, mitochondrial damage, and atherogenesis. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L209–L216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole M. P., Freeman B. A. (2009). Promotion of cardiovascular disease by exposure to the air pollutant ozone. Am. J. Physiol. Lung Cell. Mol. Physiol. 297, L205–L208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhrzadeh L., Laskin J. D., Gardner C. R., Laskin D. L. (2004). Superoxide dismutase-overexpressing mice are resistant to ozone-induced tissue injury and increases in nitric oxide and tumor necrosis factor-alpha. Am. J. Respir. Cell Mol. Biol. 30, 280–287 [DOI] [PubMed] [Google Scholar]
- Febbraio M., Hajjar D. P., Silverstein R. L. (2001). CD36: A class B scavenger receptor involved in angiogenesis, atherosclerosis, inflammation, and lipid metabolism. J. Clin. Invest. 108, 785–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febbraio M., Abumrad N. A., Hajjar D. P., Sharma K., Cheng W., Pearce S. F., Silverstein R. L. (1999). A null mutation in murine CD36 reveals an important role in fatty acid and kipoprotein metabolism. J. Biol. Chem. 274, 19055–19062 [DOI] [PubMed] [Google Scholar]
- Frampton M. W., Pryor W. A., Cueto R., Cox C., Morrow P. E., Utell M. J. (1999). Ozone exposure increases aldehydes in epithelial lining fluid in human lung. Am. J. Respir. Crit. Care Med. 159,(4 Pt 1)1134–1137 [DOI] [PubMed] [Google Scholar]
- Garantziotis S., Li Z., Potts E. N., Lindsey J. Y., Stober V. P., Polosukhin V. V., Blackwell T. S., Schwartz D. A., Foster W. M., Hollingsworth J. W. (2010). TLR4 is necessary for hyaluronan-mediated airway hyperresponsiveness after ozone inhalation. Am. J. Respir. Crit. Care Med. 181, 666–675 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Harkema J. R., Carey S. A., Wagner J. G. (2006). The nose revisited: A brief review of the comparative structure, function, and toxicologic pathology of the nasal epithelium. Toxicol. Pathol. 34, 252–269 [DOI] [PubMed] [Google Scholar]
- Hubbell B. J., Hallberg A., McCubbin D. R., Post E. (2005). Health-related benefits of attaining the 8-hr ozone standard. Environ. Health Perspect. 113, 73–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. A., Schwartzman I. N., Flynt L., Shore S. A. (2005). Role of interleukin-6 in murine airway responses to ozone. Am. J. Physiol. Lung Cell. Mol. Physiol. 288, L390–L397 [DOI] [PubMed] [Google Scholar]
- Kafoury R. M., Pryor W. A., Squadrito G. L., Salgo M. G., Zou X., Friedman M. (1999). Induction of inflammatory mediators in human airway epithelial cells by lipid ozonation products. Am. J. Respir. Crit. Care Med. 160, 1934–1942 [DOI] [PubMed] [Google Scholar]
- Kampfrath T., Maiseyeu A., Ying Z., Shah Z., Deiuliis J. A., Xu X., Kherada N., Brook R. D., Reddy K. M., Padture N. P., et al. (2011). Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ. Res. 108, 716–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C. S., Alexis N. E., Rappold A. G., Kehrl H., Hazucha M. J., Lay J. C., Schmitt M. T., Case M., Devlin R. B., Peden D. B., et al. (2011). Lung function and inflammatory responses in healthy young adults exposed to 0.06 ppm ozone for 6.6 hours. Am. J. Respir. Crit. Care Med. 183, 1215–1221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuda O., Jenkins C. M., Skinner J. R., Moon S. H., Su X., Gross R. W., Abumrad N. A. (2011). CD36 protein is involved in store-operated calcium flux, phospholipase A2 activation, and production of prostaglandin E2. J. Biol. Chem. 286, 17785–17795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumano-Kuramochi M., Shimozu Y., Wakita C., Ohnishi-Kameyama M., Shibata T., Matsunaga S., Takano-Ishikawa Y., Watanabe J., Goto M., Xie Q., et al. (2012). Identification of 4-hydroxy-2-nonenal-histidine adducts that serve as ligands for human lectin-like oxidized LDL receptor-1. Biochem. J. 442, 171–180 [DOI] [PubMed] [Google Scholar]
- Li Z., Potts E. N., Piantadosi C. A., Foster W. M., Hollingsworth J. W. (2010). Hyaluronan fragments contribute to the ozone-primed immune response to lipopolysaccharide. J. Immunol. 185, 6891–6898 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Li Z., Potts-Kant E. N., Garantziotis S., Foster W. M., Hollingsworth J. W. (2011). Hyaluronan signaling during ozone-induced lung injury requires TLR4, MyD88, and TIRAP. PLoS One 6, e27137 [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- Lund A. K., Lucero J., Harman M., Madden M. C., McDonald J. D., Seagrave J. C., Campen M. J. (2011). The oxidized low-density lipoprotein receptor mediates vascular effects of inhaled vehicle emissions. Am. J. Respir. Crit. Care Med. 184, 82–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills N. L., Törnqvist H., Robinson S. D., Gonzalez M., Darnley K., MacNee W., Boon N. A., Donaldson K., Blomberg A., Sandstrom T., et al. (2005). Diesel exhaust inhalation causes vascular dysfunction and impaired endogenous fibrinolysis. Circulation 112, 3930–3936 [DOI] [PubMed] [Google Scholar]
- Pagano P. J., Griswold M. C., Najibi S., Marklund S. L., Cohen R. A. (1999). Resistance of endothelium-dependent relaxation to elevation of O(-)(2) levels in rabbit carotid artery. Am. J. Physiol. 277(5 Pt 2),H2109–H2114 [DOI] [PubMed] [Google Scholar]
- Postlethwait E. M., Cueto R., Velsor L. W., Pryor W. A. (1998). O3-induced formation of bioactive lipids: Estimated surface concentrations and lining layer effects. Am. J. Physiol. 274(6 Pt 1),L1006–L1016 [DOI] [PubMed] [Google Scholar]
- Pryor W. A., Squadrito G. L., Friedman M. (1995). A new mechanism for the toxicity of ozone. Toxicol. Lett. 82–83, 287–293 [DOI] [PubMed] [Google Scholar]
- Rigotti A., Acton S. L., Krieger M. (1995). The class B scavenger receptors SR-BI and CD36 are receptors for anionic phospholipids. J. Biol. Chem. 270, 16221–16224 [DOI] [PubMed] [Google Scholar]
- Robertson S., Gray G. A., Duffin R., McLean S. G., Shaw C. A., Hadoke P. W., Newby D. E., Miller M. R. (2012). Diesel exhaust particulate induces pulmonary and systemic inflammation in rats without impairing endothelial function ex vivo or in vivo. Part. Fibre Toxicol. 9, 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savov J. D., Whitehead G. S., Wang J., Liao G., Usuka J., Peltz G., Foster W. M., Schwartz D. A. (2004). Ozone-induced acute pulmonary injury in inbred mouse strains. Am. J. Respir. Cell Mol. Biol. 31, 69–77 [DOI] [PubMed] [Google Scholar]
- Sawada H., Saito Y., Noguchi N. (2012). Enhanced CD36 expression changes the role of Nrf2 activation from anti-atherogenic to pro-atherogenic in apoE-deficient mice. Atherosclerosis 225, 83–90 [DOI] [PubMed] [Google Scholar]
- Shaul P. W. (2002). Regulation of endothelial nitric oxide synthase: Location, location, location. Annu. Rev. Physiol. 64, 749–774 [DOI] [PubMed] [Google Scholar]
- Silverstein R. L., Febbraio M. (2009). CD36, a scavenger receptor involved in immunity, metabolism, angiogenesis, and behavior. Sci. Signal. 2, re3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Standiford T. J., Reddy R. C. (2007). PPARs in alveolar macrophage biology. PPAR Res. 2007, 23812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait J. F., Smith C. (1999). Phosphatidylserine receptors: Role of CD36 in binding of anionic phospholipid vesicles to monocytic cells. J. Biol. Chem. 274, 3048–3054 [DOI] [PubMed] [Google Scholar]
- The World Health Report 2002—Reducing Risks, Promoting Healthy Life. Available at: http://www.who.int/whr/2002/en/ Accessed May 23, 2013. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.