Abstract
Arsenic in drinking water promotes a number of diseases that may stem from dysfunctional adipose lipid and glucose metabolism. Arsenic inhibits adipocyte differentiation and promotes insulin resistance; however, little is known of the impacts of and mechanisms for arsenic effects on adipose lipid storage and lipolysis. Based on our earlier studies of arsenic-signaling mechanisms for vascular remodeling and inhibition of adipogenesis, we investigated the hypothesis that arsenic acts through specific adipocyte G-protein-coupled receptors (GPCRs) to promote lipolysis and decrease lipid storage. We first demonstrated that 5-week exposure of mice to 100 μg/l of arsenic in drinking water stimulated epididymal adipocyte hypertrophy, reduced the adipose tissue expression of perilipin (PLIN1, a lipid droplet coat protein), and increased perivascular ectopic fat deposition in skeletal muscle. Incubating adipocytes, differentiated from adipose-derived human mesenchymal stem cell, with arsenic stimulated lipolysis and decreased both Nile Red positive lipid droplets and PLIN1 expression. Arsenic-stimulated lipolysis was not associated with increased cAMP levels. However, preincubation of adipocytes with the Gi inhibitor, Pertussis toxin, attenuated As(III)-stimulated lipolysis and lipid droplet loss. Antagonizing Gi-coupled endothelin-1 type A and B receptors (EDNRA/EDNRB) also attenuated arsenic effects, but antagonizing other adipose Gi-coupled receptors that regulate fat metabolism was ineffective. The endothelin receptors have different roles in arsenic responses because only EDNRA inhibition prevented arsenic-stimulated lipolysis, but antagonists to either receptor protected lipid droplets and PLIN1 expression. These data support a role for specific GPCRs in arsenic signaling for aberrant lipid storage and metabolism that may contribute to the pathogenesis of metabolic disease caused by environmental arsenic exposures.
Key Words: arsenic, endothelin-1, adipose, adipocyte, G-protein-coupled receptor, lipid storage, lipolysis.
Arsenic (As(III)) is a ubiquitous naturally occurring metalloid found in drinking water that poses health risks to more than 2% of the world population. Chronic As(III) exposure increases the risk of a number of cancers and chronic noncancer diseases, including cardiovascular, pulmonary, and metabolic diseases (Abhyankar et al., 2012; Hughes et al., 2011; Maull et al., 2012; Moon et al., 2012; Parvez et al., 2010). Even low to moderate As(III) exposures may increase the risk of cardiovascular disease (Chen et al., 2011; Moon et al., 2012) and potentially enhance insulin resistance in metabolic disease (Gribble et al., 2012; Maull et al., 2012). The etiology and pathogenic mechanisms of As(III)-promoted metabolic dysfunction and toxicity remain undefined but may relate to impacts on adipose tissue remodeling and function.
Adipose tissue was believed to be an inert tissue but is now recognized as a critical endocrine organ that is essential for control of energy metabolism, insulin sensitivity, and appropriate lipid storage. It is a dynamic tissue responsive to and responsible for hormonal, inflammatory, and metabolic interactions (both homeostatic and pathogenic) with other organs (Turer et al., 2012). Paradoxically, both excess adipose tissue in obesity and loss of adipose tissue in lipodystrophies contribute to metabolic diseases and pathogenic consequences of ectopic lipid storage in nonadipose tissues (Gustafson et al., 2007; Turer et al., 2012; Vigouroux et al., 2011). Hypertrophic expansion of adipose tissue resulting from excess lipid storage suppresses adipose regeneration and causes dysfunctional ectopic storage of lipid in liver, heart, and skeletal muscle (Gustafson et al., 2007; Turer et al., 2012). Conversely, lack or loss of adipose tissue and the ability of adipose tissue to store lipid result in metabolic and oxidative stress, as well as ectopic lipid storage and systemic inflammation (Gustafson et al., 2007). Although the severity of metabolic disease caused by disrupted lipid storage varies, it is evident that factors that chronically impair proper lipid metabolism and storage greatly enhance risk for cardiovascular, liver, impaired skeletal muscle composition and metabolism, and development of diabetes (Gustafson et al., 2007; Turer et al., 2012; Vigouroux et al., 2011).
A significant number of human lipodystrophies have genetic origins with mutations found in genes coding for enzymes regulating lipid storage or lipolysis, as well as proteins that regulate lipid droplet formation and maintenance (Vigouroux et al., 2011). Lipid storage and lipolysis are highly regulated by lipases, fatty acid-binding proteins, and proteins coating the lipid droplets, such as perilipin (PLIN1) (Bézaire et al., 2009; Kolditz and Langin, 2010). The canonical pathway for physiological lipolysis involves Gs-protein-coupled receptor-mediated (e.g., β-adrenergic stimulation) increase of intracellular cAMP levels that stimulate protein kinase A (PKA) phosphorylation of hormone-sensitive lipase and PLIN1. This signaling allows activated lipase access to cleave stored lipid droplet triglycerides (Kolditz and Langin, 2010; Soeder et al., 1999). In addition, PLIN1 expression regulates lipid droplet size and basal lipolytic rate. Lipolysis increases as PLIN1 expression decreases, as seen in a number of pathologic conditions and following chronic TNF-α-stimulated wasting (Bézaire et al., 2009; Kolditz and Langin, 2010). PLIN1 loss raises basal lipolysis but impairs stimulated lipolysis.
Autocrine, paracrine, hormonal, and possibly environmental factors impact the critical lipid storage balance. Insulin is the major hormonal factor promoting both lipid storage and glucose homeostasis. However, hypertrophic expansion of mature adipocytes, inflammation, and tissue injury increases levels of ligands for a number of G-protein-coupled receptors (GPCRs) whose activation disrupts insulin signaling, inhibits adipogenesis, and promotes lipolysis (Bhattacharya and Ullrich, 2006; Eriksson et al., 2009; Janke et al., 2002; Mogi et al., 2006; Tomono et al., 2008; van Harmelen et al., 2008). Obese tissues and mature adipocytes paradoxically increase lipolysis and suppress adipose regeneration in part by increasing paracrine action of the secreted peptides, endothelin-1, and angiotensin II (Eriksson et al., 2009; Janke et al., 2002; Juan et al., 2006; Tomono et al., 2008; van Harmelen et al., 2008). These peptides are ligands for Gi- and Gq-coupled GPCRs (EDNRA/EDNRB and AGTR1/AGTR2, respectively) that, when stimulated, regulate adipocyte differentiation and adipose tissue maintenance (Elbaz et al., 2000; Iwai et al., 2009; Janke et al., 2002; Tomono et al., 2008; van Harmelen et al., 2008). In addition, stimulation of AGRT2 may transinactivate insulin signaling (Elbaz et al., 2000; Iwai et al., 2009) and chronic stimulation of EDNRA decreases insulin sensitivity by decreasing expression of insulin receptor and insulin receptor substrates (van Harmelen et al., 2008). Endothelin-stimulated lipolysis is seen predominantly in visceral fat and may be mediated by EDNRB (van Harmelen et al., 2008). Chronic elevation of endothelin in obese tissue, however, may also promote lipolysis through EDNRA (Eriksson et al., 2009). Additional GPCRs that regulate adipocyte function and lipid storage include the sphingosine-1-phosphate receptors (S1PR1/S1PR2) (He et al., 2010; Nincheri et al., 2009). S1PR1 was also shown to mediate As(III)-stimulated oxidant production and remodeling of vascular endothelial cells (Straub et al., 2009).
As(III) promotes metabolic dysfunction, metabolic-associated diseases, and impairs insulin responsiveness (Chen et al., 2011; Gribble et al., 2012; Maull et al., 2012; Moon et al., 2012; Paul et al., 2008). As(III) inhibits differentiation of stem cells into adipocytes (Cheng et al., 2011; Klei et al., 2013; Wauson et al., 2002) and disrupts insulin signaling that stimulates glucose uptake (Paul et al., 2008; Walton et al., 2004). The impact of As(III) on lipid storage and lipolysis has not been investigated nor have the potential mechanisms that underlie these pathogenic metabolic actions. We recently found that Pertussis toxin (Ptx)-sensitive and endothelin-1 GPCRs (EDNRA/EDNRB) mediate a significant portion of the antiadipogenic effect of As(III) (Klei et al., 2013). Therefore, we investigated the hypothesis that As(III) activates specific GPCR signaling pathways in adipocytes to stimulate lipolysis and reduce lipid storage capacity. The following studies provide support for this hypothesis and elucidate the roles of EDNRA and EDNRB signaling in mediating the effects of arsenic on adipocyte lipid storage and metabolism.
MATERIALS AND METHODS
In vivo mouse exposure.
To investigate pathogenic effects of As(III) on adipose metabolism, groups of eight 5- to 6-week-old male C57BL/6 Tac mice (Taconic Farms, Hudson, NY) were exposed for 5 weeks to 0 or 100 μg/l sodium arsenite (ThermoFisher, Pittsburgh, PA) in their drinking water. This exposure is representative of a moderate human drinking water arsenic exposure lasting 2–3 years. Fresh As(III) solutions were provided three times per week to maintain effective concentrations of As(III). At the end of the exposure period, the mice were euthanized with CO2, and epididymal fat and tibialis anterior muscle were collected for histological examination and measurement of protein expression. Epididymal fat was isolated because it is one of the largest visceral adipose depots in rodents, and change in visceral fat is a risk factor for humans to develop cardiovascular and metabolic diseases. Skeletal muscle composition was evaluated to determine whether As(III) exposure causes lipid redistribution and pathogenic ectopic lipid deposition. All mouse experiments were performed in agreement with institutional guidelines for animal safety and welfare and under the supervision of the University of Pittsburgh, Department of Laboratory Animal Research.
Cell culture.
Adipose tissue-derived primary human mesenchymal stem cells (hMSCs) from young female donors (Lifeline Cell Technology, Frederick, MD; passages 4–8) were grown to confluence in StemLife MSC Medium. The hMSCs used in these studies were from two separate lots derived from different donors. As(III) responses were comparable in cells from both donors, as well as in hMSCs derived from a male donor that were not used in these experiments. The experimental paradigm is shown in Figure 1. At confluence, cells were seeded onto glass coverslips (immunofluorescence) or into 12-well plates (protein or RNA extraction). Differentiation was initiated by change to AdipoLife DfKt-1 adipogenesis medium for 48h. The cells were then maintained in AdipoLife Maintenance Medium, which was changed every 3 days until cells were fully differentiated (day 9). On days 9, 10, and 11, 0 or 1μM As(III) was added to provide 72-, 48-, and 24-h exposures, respectively, and determine the time course for arsenic effects in lipid storage and metabolism, as well as gene or protein expression. All cell cultures were harvested 12 days after differentiation.
Fig. 1.
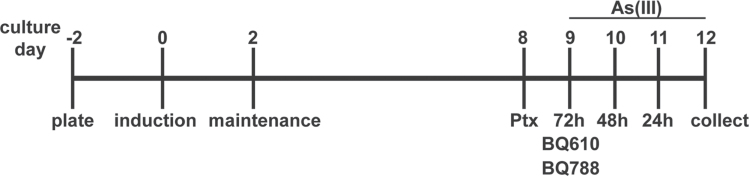
Experimental design for cell culture experiments. The scheme shows the time line for hMSC culture and differentiation, followed by exposure to arsenic in the absence and presence of inhibitors. Controls were differentiated cells that received no treatments for the 12-day postinduction.
Inhibitor treatments.
On culture day 9, cells were treated with BQ610 or BQ788 (competitive antagonists of EDNRA and EDNRB, respectively; Enzo-Life Sciences, Farmingdale, NY) 30min before adding As(III). Other inhibitors added 30min before As(III) included VPC23019 (S1PR1/3 competitive antagonist: Avanti Polar Lipids, Inc., Alabaster, AL) or L158,809 (AGTR1 competitive antagonist: a kind gift from Merck Research Laboratories). Ptx (Sigma-Aldrich, St Louis MO) was added to the cells the night of culture day 8 before medium change and As(III) addition on day 9.
Protein isolation and Western analysis.
Epididymal fat tissue was homogenized in modified RIPA buffer (50mM Tris-HCl, pH 7.6, 150mM NaCl, 1mM EDTA, 10mM NaF, 1% Triton X-100, 0.1% SDS, and supplemented with protease inhibitors and sodium orthovanadate) and then extracted by rotation at 4°C for 2h. The samples were centrifuged at 13,000 × g and the supernatant collected for protein determination. For cultured cells, whole cell lysates were prepared with Tris SDS Lysis Buffer as described (Klei et al., 2013). Protein concentrations in all samples were determined using a modified Bradford assay (Pierce Coomassie Plus Reagent, ThermoFisher). Equal amounts of protein were resolved by SDS-polyacrylamide gel electrophoresis and probed by Western analysis, as previously described (Klei et al., 2013). Antibodies used included anti-PLIN1 (rabbit mAb no. 9349, Cell Signaling Technology, Danvers, MA) and anti-β-actin (mouse monoclonal, Sigma-Aldrich). Reacted bands were detected by horseradish peroxidase-conjugated secondary antibodies and enhanced chemiluminescence substrates (Perkin Elmer, Boston, MA). PLIN1 band densities were quantified using Image J software v1.38x (National Institutes of Health, Bethesda, MD) and normalized to the band density of β-actin in the same sample.
Lipolysis assay.
Cell culture medium was collected from each experiment, centrifuged at 13,000 × g, and supernatant collected and stored at −80°C until assayed for glycerol content. Glycerol levels were measured using a colorimetric enzyme immunoassay (Glycerol Cell-Based Assay Kit, Cayman Chemical Company, Ann Arbor, MI) according to the manufacturer’s instructions. All measurements were performed in duplicate samples from each culture and quantified against a standard curve.
Microscopy and quantitative imaging.
Histological analysis of adipose tissue was performed on hematoxylin and eosin (H&E)-stained thin sections of 2.5% paraformaldehyde-fixed, paraffin-embedded tissues with images captured at ×20 magnification using brightfield illumination on an inverted Nikon Diaphot microscope. Skeletal muscle analysis was performed with frozen, fixed section of tibialis muscles that were stained with H&E. Confocal microscopy with quantitative fluorescent imaging of cells grown on glass coverslips was used to measure lipid droplet formation (Nile Red staining) and PLIN1 protein expression, as described (Klei et al., 2013). Four fields of cells from each coverslip were imaged at ×40 using an Olympus Fluoview 1000 Confocal Microscope. The percentage of thresholded pixel density per unit area for Nile Red or PLIN1 staining was calculated using MetaMorph (Molecular Devices, Downingtown, PA) software and normalized to the percent thresholded pixel density for DAPI staining. Mean values were calculated for fluorescence on four coverslips of cells from two separate experiments and averaged to provide values for each treatment group.
Quantitative reverse transcription-PCR.
Total RNA was harvested from cells using Trizol (Life Technologies, Grand Island, NY) and analyzed for messenger RNA levels of adiponectin (ADIPOQ), PLIN1, and the housekeeping gene RPL13A, as previously described (Klei et al., 2013). Gene transcripts were quantified using standard curves for the respective cDNA products. Target transcript levels were normalized to RPL13A transcript levels to determine the picogram of normalized products per milliliter of reaction.
Statistics.
Standard unpaired t-tests were used to test significance between control and single treatment groups. One-way ANOVA was used to identify significant differences (p < 0.05) between multiple treatment groups and controls. The degree of significance between groups was compared using Bonferroni’s or Newman-Keuls post hoc tests. All statistics were performed using GraphPad Prism, v 5.02 software (GraphPad Software, San Diego, CA). Data are presented as means ± SEM of quantified values or fold control.
RESULTS
As(III) Decreases Adipocyte Number and PLIN1 Expression In Vivo
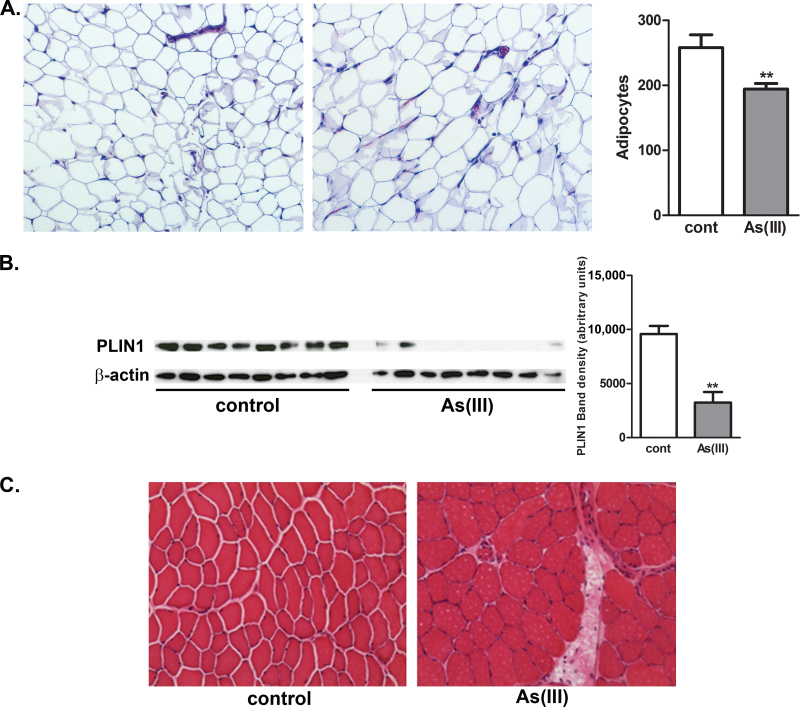
To investigate the impact of As(III) exposure on adipose composition, lipid storage, and lipid redistribution, mice were exposed for 5 weeks to 100 μg/l As(III) in their drinking water before determining adipocyte size and number, as well as expression of Plin1. Histological analysis of epididymal fat indicated adipocyte hypertrophy in the As(III)-exposed mice, relative to control (Fig. 2A). This was confirmed by demonstrating that the number of adipocytes per ×10 microscopic field was decreased in As(III)-exposed mice, relative to controls. The adipose expression of PLIN1, measured with Western analysis, was inhibited by As(III) exposure (Fig. 2B) indicating that despite the larger size of the adipocytes, there was a functional deficit in lipid storage capacity. Consistent with the change in adipose tissue lipid storage, histological examination of tibialis anterior muscle demonstrated ectopic lipid deposition with pronounced perivascular fat droplets (Fig. 2C).
Fig. 2.
As(III) effects on adipose fat storage, fat droplet coat protein expression, and ectopic fat storage in skeletal muscle. Male mice were exposed to 0 (control) or 100 μg/l As(III) in drinking water for 5 weeks. At the end of exposure, epididymal fat and tibialis anterior muscle were collected for histological analysis (H&E stain of paraformaldehyde-fixed, paraffin-embedded, or cryopreserved thin sections, respectively) and Western analysis of PLIN1 expression. (A) Morphology of epididymal fat in control and As(III)-exposed mice showing relative size of adipocytes captured at ×10 magnification. The graph presents mean ± SEM number of adipocytes measured in three separate microscopic fields from eight mice in each group (B). The immunoblots show expression of PLIN1 and β-actin in the epididymal adipose tissues with protein in each lane isolated from individual mice. The graph presents mean ± SEM of the relative band density of PLIN1 normalized to β-actin (** represents significance at p < 0.01 as determined by t-test). (C) Cross-sections of tibialis anterior muscle compare morphology of muscle fibers and perivascular fat droplet deposition (×20 magnification). The images represent muscle images taken from eight mice in each group.
As(III) Stimulates Adipocyte Lipolysis and Reduces PLIN1-Coated Lipid Droplets
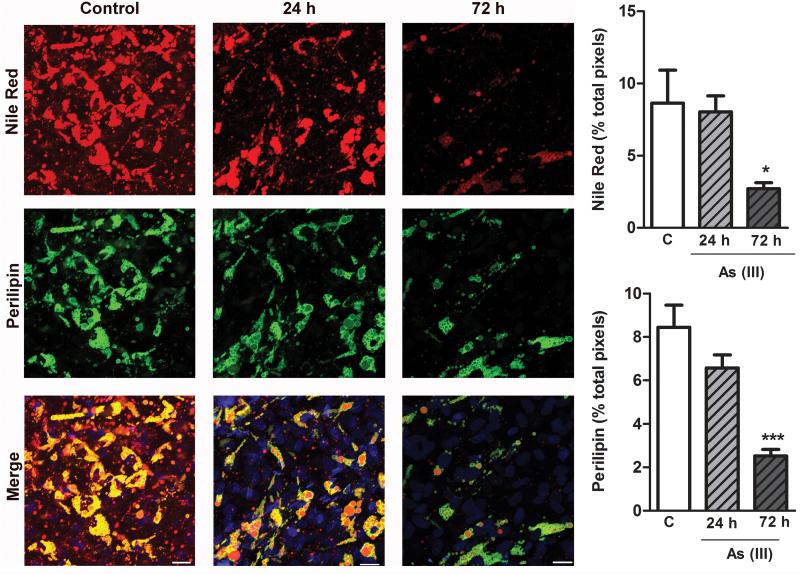
The effects of As(III) on lipid mobilization was investigated in a model of cultured human adipocytes differentiated from primary adipose-derived hMSC. The cells were treated with As(III) in the late stages of differentiation when the cells contained pronounced PLIN1 and Nile Red positive lipid droplets. Within 24h of exposure, As(III) maximally increased lipolysis, as measured by release of glycerol from the exposed cells relative to control cells (Fig. 3). The increased lipolysis was followed by a progressive loss of lipid droplets (Nile Red staining), and PLIN1 coating the lipid droplets that was significant by 72h of exposure (Fig. 4). More detailed Western analysis demonstrated significant loss of PLIN1 by 48h (Fig. 5) and this loss of protein paralleled a loss of ~74% of PLIN1 transcript. There was also a decrease of ~72% of ADIPOQ transcript, a marker for mature adipocytes (Fig. 5). This indicated a programmatic transcriptional change in the adipocytes rather than a selective effect of arsenic on PLIN1 expression. Dose-response comparisons demonstrated that the threshold for these effects of As(III) on adipocytes was between 0.1 and 0.2μM and exposures to greater than 2.5μM caused toxicity (data not shown).
Fig. 3.
As(III) increases lipolysis in adipocytes. Adipocytes differentiated from hMSC were incubated with or without 1μM As(III) for 72h, and medium above the cells was sampled at the indicated times for glycerol content as a measure of lipolysis (control was sampled at 72h). Released glycerol was measured using a colorimetric assay, as described in Materials and Methods section, and data are presented as mean ± SEM glycerol released in three separate cultures and from two independent experiments (each sample assayed in duplicate). Data were analyzed for significance by ANOVA and Newman-Keuls post hoc test for differences between groups (**p < 0.01 from control).
Fig. 4.
As(III) causes progressive loss of adipocyte PLIN1-coated lipid droplets. Adipocytes were differentiated and grown on glass coverslips and then treated with 1μM As(III) for the indicated times. The cells were fixed and stained for neutral lipid droplets (Nile Red), PLIN1 (green), and nuclei (DAPI blue) content. Images of four fields per cover slip were captured at ×40 (scale bars = 50 μm) and the thresholded fluorescence quantified and averaged. The data in the graphs present mean ± SEM percentage of positive pixels normalized to DAPI staining in four separate cultures representative of two separate experiments. Data were analyzed by ANOVA and Newman-Keuls post hoc test for differences between groups (*p < 0.05 and ***p < 0.001 from control).
Fig. 5.
As(III) inhibits PLIN1 expression. Adipocytes were cultured in the absence or presence of 1μM As(III) for 24, 48, or 72h before extraction or protein or RNA. (A and B) Total protein extracts were probed by Western analysis for PLIN1 and β-actin. (B) The graph presents mean ± SEM fold band densities relative to control for PLIN1 immunoblots normalized to β-actin from three separate cultures in two independent experiments. (C and D) Total RNA was probed for PLIN1, ADIPOQ, or RPL13A transcript levels by qRT-PCR. The data are presented as mean ± SEM of the pg/ml of PCR product normalized to the housekeeping gene RPL13. Data were analyzed by one-way ANOVA and Bonferroni’s post hoc test (*p < 0.05 and **p < 0.01 relative to untreated cells).
GPCR in As(III)-Stimulated Lipolysis and Loss of PLIN1-Coated Droplets
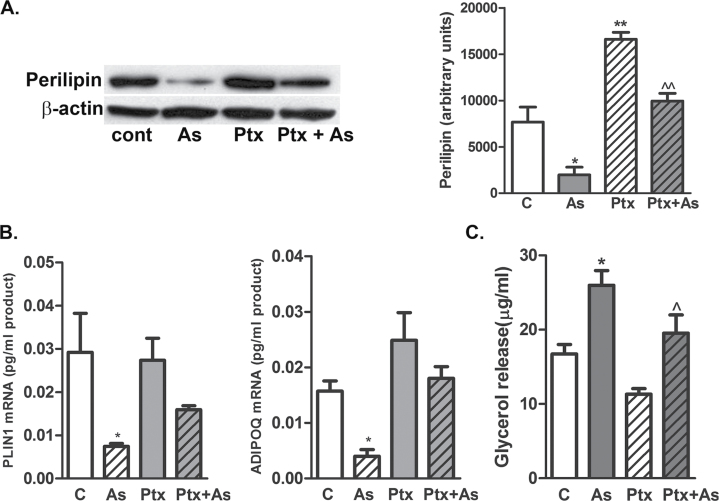
Physiologic lipolysis is stimulated through Gs-coupled GPCR signaling and increased AMP levels and PKA activity (Juan et al., 2006; Vigouroux et al., 2011) or Gi/Gq GPCR-mediated inhibition of insulin-stimulated lipid storage (Mogi et al., 2006; van Harmelen et al., 2008; Vigouroux et al., 2011). We recently demonstrated that a major portion of As(III)-inhibited hMSC differentiation into adipocytes was mediated by Ptx-sensitive GPCRs (Klei et al., 2013). To test whether similar receptors mediated As(III) effects on adipocytes, differentiated cells were incubated with Ptx overnight before incubation with As(III) for 72h. Ptx treatment completely prevented As(III)-stimulated loss of lipid loss droplets (Supplementary fig. 1) and 50 (Western analysis, Fig. 6A) to 87% (quantitative immunofluorescence, Supplementary fig. 1) loss of PLIN1 protein expression. Similarly, Ptx attenuated As(III)-induced repression of PLIN1 and ADIPOQ transcript levels (Fig. 6B). Functionally, Ptx treatment attenuated As(III)-stimulated lipolysis (Fig. 6C). In contrast to stimulating lipolysis through Gi-coupled signaling, As(III) exposure of 30min to 4h or for 24h (data not shown) did not stimulate Gs-coupled signaling for increased intracellular cAMP levels relative to isoproterenol, a positive control for Gs-coupled receptor activation (Supplementary fig. 2).
Fig. 6.
Effect of Ptx on As(III)-exposed adipocytes. Ptx (1μM) was added to the indicated groups of differentiated adipocytes for 24h before 1μM As(III) was added. After 72h, medium was collected for glycerol measurements, and the cells were harvested for protein and RNA isolation. (A) Total proteins from cell lysates were probed by Western analysis for PLIN1 and β-actin levels, and the graph presents densitometric analysis from six cultures in each group (mean ± SEM of fold difference from untreated cells [control]). (B) Total RNA was assayed by qRT-PCR for PLIN1, ADIPOQ, or RPL13A transcript levels. Data are mean ± SEM of at least two independent experiments. (C) Released glycerol was quantified and data are presented as mean ± SEM μg of glycerol released per ml of medium in three separate cultures and from two independent experiments (each sample assayed in duplicate). Data were analyzed by one-way ANOVA and Bonferroni’s post hoc test for significance (*p < 0.05, **p < 0.01).
As(III) Stimulates Endothelin-1 Receptors to Cause Adipocyte Dysfunction
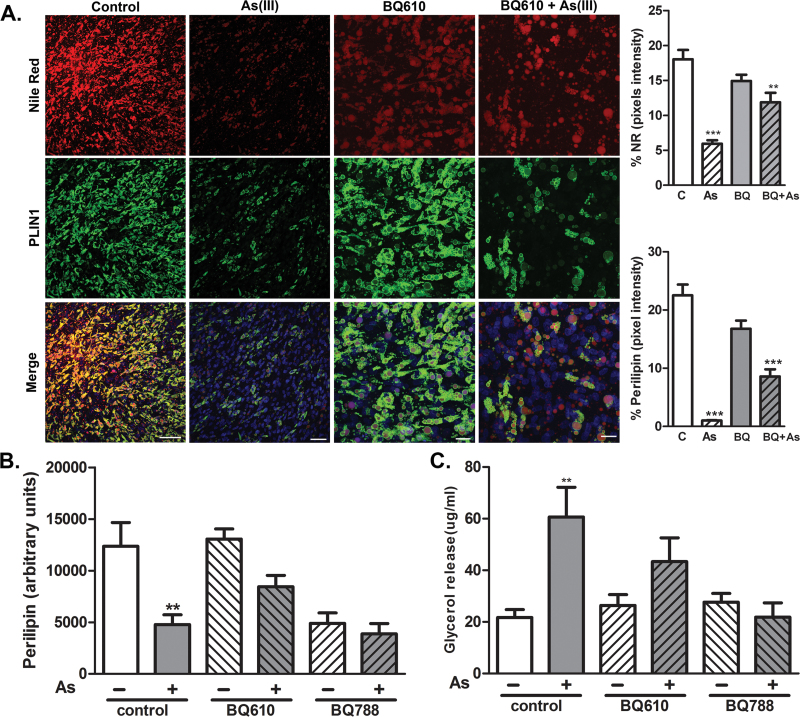
Ptx selectively adenosine diphosphate-ribosylates Gi proteins to block Gi-coupled receptor signaling. There are at least three types of Gi-linked receptors that affect adipocyte lipolysis, including S1PR1/3, AGTR1, and EDNRA/EDNRB (Bhattacharya and Ullrich, 2006; Hashimoto et al., 2009; Tomono et al., 2008; van Harmelen et al., 2008). To identify which receptors mediate the As(III) effects, we pretreated adipocytes with specific antagonists before and during 3-day incubations in the presence or absence of As(III). As seen in Figures 7A and 7B, antagonizing EDNRA with BQ610 attenuated As(III)-induced loss of lipid droplets and PLIN1 expression. In contrast, antagonizing EDNRB with BQ788 reduced basal PLIN1 expression and there was no further reduction by As(III) (Fig. 7B). Blocking either EDNRA or EDNRB prevented As(III)-stimulated lipolysis (Fig. 7C). Antagonizing S1PR1 or AGTR1 with VPC23019 or L-158,809, respectively, did not prevent As(III) effects on lipolysis (data not shown) or PLIN1 expression (Supplementary fig. 3).
Fig. 7.
Endothelin-1 receptors mediate As(III)-stimulated adipocyte dysfunction. Adipocytes grown on coverslips (A) or in 12-well culture plates (B and C) were incubated for 30min in the absence or presence of ENDRA antagonist BQ610 (1μM) or ENDRB antagonist BQ788 (1μM), and then incubated with 1μM As(III) for 72h. (A) Coverslips were processed for imaging of triglycerides with Nile Red, PLIN1 (green), and nuclei (blue). Images of four fields per cover slip were captured at ×40 (scale bars = 50 μm) and the thresholded fluorescence quantified and averaged. The data in the graphs present mean ± SEM percentage of positive pixels normalized to DAPI staining in three separate cultures representative of two separate experiments. In the experiment in well plates, culture medium was collected and total protein extracts prepared from cell lysates. (B) Protein extracts were analyzed by Western analysis with antibodies to PLIN1 or β-actin. Data represents mean ± SEM of band density of extracts from three independent cultures in two separate experiments. (C) Released glycerol was quantified and data are presented as mean ± SEM of glycerol (μg/ml medium) released in three separate cultures and from two independent experiments (each sample assayed in duplicate). Data were analyzed by one-way ANOVA with Newman-Keuls post hoc test (**p < 0.01, ***p < 0.001) versus control.
DISCUSSION
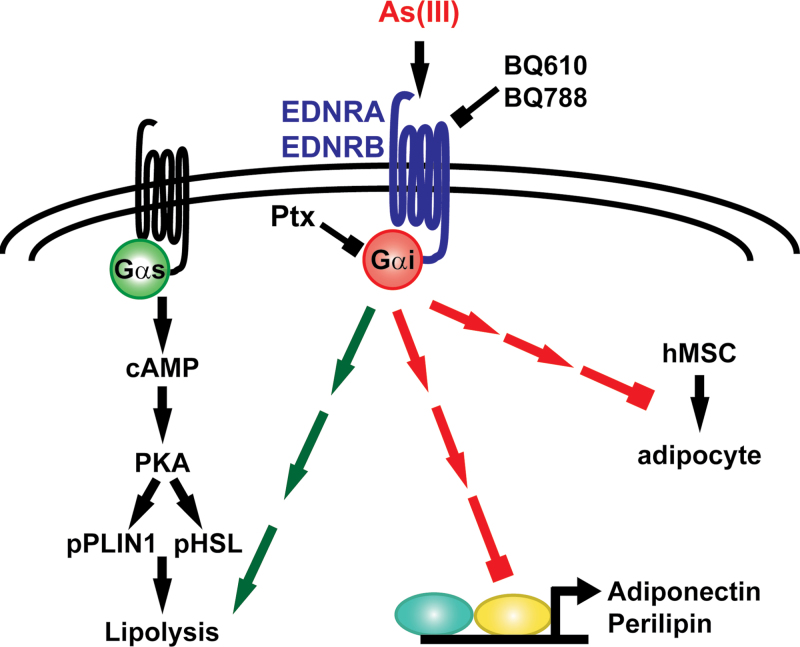
The data presented support the hypothesis that As(III)-stimulated lipid mobilization and inhibited lipid storage is mediated or initiated in part by activating Gi-coupled receptor signaling. Stimulating Ptx-inhibitable receptors accounted for almost all of As(III)-induced loss of lipid droplets and a majority of As(III)-stimulated lipolysis and decreased expression of lipid coat protein. The inhibitor studies suggest that endothelin GPCRs mediate a majority of the Ptx-inhibitable responses; however, it is evident that other nonidentified receptors and signaling pathways contribute to As(III) inhibition of normal adipose tissue metabolism and fat distribution. The hypothetical signaling scheme for As(III) effects on adipogenesis and adipocyte function is shown in Figure 8.
Fig. 8.
Signaling scheme for endothen-1 receptor mediates effects of arsenic on adipocytes. As(III) stimulates endothelin receptors (EDNRA/EDNRB) to increase lipolysis and decrease expression of lipid droplet coat protein, PLIN1. In addition, the scheme includes the signaling for As(III) inhibition of differentiation of hMSCs to adipocytes (Klei et al., 2013). The broken arrows indicate the multiple steps in the signaling cascade between receptor activation and lipolysis or effects on transcription or differentiation.
Imbalances in fat storage, either excess energy storage in obesity or fat loss in lipodystrophies, result in metabolic disorders with impaired regulation of glucose and lipid metabolism in both adipose and nonadipose tissues (Gustafson et al., 2007; Ouwens et al., 2010; Sell et al., 2006; Vigouroux et al., 2011). As(III) exposure is associated with increased metabolic disease risk or an inability to control metabolic syndromes (Chen et al., 2011; Gribble et al., 2012; Maull et al., 2012; Moon et al., 2012; Paul et al., 2008). In the current study, As(III) caused remodeling of epididymal adipose tissue with enhanced adipocyte size and ectopic lipid deposition in muscle. As(III) also inhibited expression of PLIN1 in vivo and in cultured cells, which would increase lipolysis and reduce the adipocyte capacity to store lipid. Increased lipolysis following decreased PLIN1 expression is seen in a number of pathologic conditions (Bézaire et al., 2009; Kolditz and Langin, 2010). Decreased PLIN1 expression raises the basal rate of lipolysis but impairs stimulated lipolysis (Bézaire et al., 2009; Vigouroux et al., 2011). This PLIN1-dependent shift in basal lipolysis may explain the apparent new steady state of lipolysis seen in the As(III)-exposed adipocytes (Fig. 3).
The pattern of ectopic intramyocellular lipid storage seen in Figure 2C is observed in both obese individuals and patients with lipodystrophic. The abundance of intramyocellular fat may be an earlier sign of insulin resistance and impaired metabolism than free fatty acids in serum (Gustafson et al., 2007; Sell et al., 2006; Vigouroux et al., 2011). Redistribution of adipose lipids to perivascular fat accumulation occurs in the pathogenesis of cardiomyopathies, coronary artery disease, and atherosclerosis (Ouwens et al., 2010; Turer et al., 2012). Thus, remodeling of adipose tissue and redistribution of lipid deposition may combine with As(III) inhibition of adipogenesis (Klei et al., 2013) to support a novel mechanism for the pathogenesis of As(III)-induced metabolic and cardiovascular diseases.
There are several GPCR-mediated mechanisms that regulate adipogenesis, lipolysis, lipid metabolism, and lipid storage. As(III) did not increase intracellular levels of cAMP indicating that it does not activate the Gs-coupled physiological pathway for stimulating lipolysis for energy production. However, all of the effects of arsenic on adipocyte lipid metabolism and storage measured in these studies were attenuated by Ptx treatment. Depending on the endpoint measured and assay used in the measurements, Ptx ribosylation of Gi proteins prevented 50–87% of As(III) effects. This is consistent with our previous observations of Ptx attenuation of As(III)-inhibited adipogenesis (Klei et al., 2013). It is evident, however, that stimulating Gi-protein-coupled signaling is not responsible for all of As(III) actions on the adipocytes, and it is likely that other signaling pathways contribute to the full impact of As(III) on adipocytes and adipose tissues. This separation of pathways is similar to angiogenic and remodeling As(III) effects on endothelial cells being mediated by activation of S1PR1 receptors that is independent of As(III)-induced stress responses (Straub et al., 2009).
Suppression of adipogenesis and adipose tissue maintenance by Ptx-sensitive Gi-coupled receptors is well established (Hauner et al., 1994; Janke et al., 2002; Shinohara et al., 1992; Tomono et al., 2008; van Harmelen et al., 2008). Gi-coupled receptors, such as AGTR1 (Elbaz et al., 2000; Janke et al., 2002) or EDNRA/EDNRB (Eriksson et al., 2009; van Harmelen et al., 2008) oppose increases in cAMP and block insulin signaling that suppresses lipolysis. As with receptor-mediated As(III) inhibition of hMSC differentiation into adipocytes (Klei et al., 2013), endothelin-1 receptor activation accounted for most, but not all of Ptx protection from As(III)-stimulated lipolysis, loss of lipid droplets, and PLIN1 repression. Endothelin-1 produced and released by endothelial cells in the adipose tissue vasculature stimulates lipolysis and inhibits insulin-stimulated lipid storage (Eriksson et al., 2009; Juan et al., 2007; van Harmelen et al., 2008). Endothelin-1 release and action increases more than 2.5-fold in obese humans, but its impact on lipolysis and adipose remodeling is greater in visceral fat, relative to subcutaneous fat (van Harmelen et al., 2008). This is consistent with our observation that As(III) acting through endothelin receptors decreases PLIN1 expression and possibly lipid storage in epididymal fat but increased ectopic fat deposition in muscle. However, even though no role was found for other major Gi-coupled receptors that regulate adipose function, such as AGRT1 and SIPR1, there are clearly other unidentified Gi-coupled/Ptx-inhibitable receptors that mediate As(III) effects.
The subtype of endothelin receptor that regulates endothelin-1-stimulated lipolysis and insulin resistance is not clear with reports of the receptors acting differentially in vivo and in different cell culture models. In human adipocytes differentiated from progenitor cells isolated from visceral but not subcutaneous fat, endothelin-1 induced the expression of EDNRB and stimulated the receptor to cause a sustained loss on insulin receptor and its downstream repression of lipolysis (van Harmelen et al., 2008). In contrast, EDNRA was found responsible for endothelin-1-stimulated lipolysis and repression of ADIPOQ expression in freshly isolated primary human adipocytes and in differentiated mouse 3T3-Li adipocytes, respectively (Eriksson et al., 2009; Juan et al., 2007). In our model of human adipocytes differentiated from hMSC isolated from visceral fat, we found that EDNRA mediates As(III)-stimulated lipolysis, loss of lipid droplets, and repression of PLIN1 expression. Antagonizing EDNRB inhibited PLIN1 expression to such an extent that further inhibition by As(III) was not observed. However, antagonizing EDNRB was equally as effective as antagonizing EDNRA in preventing As(III)-induced lipolysis without affecting basal lipolysis (Fig. 6). This suggests that the As(III)-stimulated Gi-mediated pathways for lipolysis and transcriptional repression are separate. It is important to note that EDNRA and EDNRB signaling is often cooperative with the formation of heterodimeric signaling complexes (Watts, 2010; Zuccarello et al., 1999), suggesting that As(III) may be acting through a complex of EDNR to repress PLIN1 expression. In addition, we found that siRNA knockdown of either receptor affected As(III) effects on the expression of the other receptor in differentiating adipocytes (Klei et al., 2013). This adds to the complexity of dissecting the roles of the individual receptors in As(III)-stimulated effects.
In conclusion, the data support a pathogenic pathway for As(III)-stimulated adipose remodeling and redistribution of adipose deposition that is substantially mediated by selective Gi-coupled receptor stimulation. As we found previously, Ptx-inhibition of all Gi-receptor signaling was more effective than antagonizing any individual Gi receptor in protecting against As(III) effects and that the type of Gi-receptor-mediating As(III) effects are tissue specific (Klei et al., 2013; Straub et al., 2009). Blocking the endothelin-1 receptors attenuated As(III) lipolytic actions, but the decreased efficacy relative to Ptx suggests that additional Gi proteins might mediate portions of the As(III) effects. These selective receptor-mediated effects may contribute to the molecular pathogenesis of As(III)-promoted adipose tissue remodeling and insulin resistance observed in As(III)-induced metabolic and cardiovascular diseases.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Support for this research was provided by grants R01ES013781 and R01ES013781-S1 (A.B.) from the National Institute of Environmental Health Sciences, National Institutes of Health, and the Pennsylvania Department of Health/Health Research Program, SAP no.: 4100061184 (F.A., A.B.). Additional support was provided by a MaryAnne Stock Student Research Award (DYG) from the Allegheny & Eric Regional Chapter of the Society of Toxicology.
Supplementary Material
REFERENCES
- Abhyankar L. N., Jones M. R., Guallar E., Navas-Acien A. (2012). Arsenic exposure and hypertension: A systematic review. Environ. Health Perspect. 120, 494–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bézaire V., Mairal A., Anesia R., Lefort C., Langin D. (2009). Chronic TNFalpha and cAMP pre-treatment of human adipocytes alter HSL, ATGL and perilipin to regulate basal and stimulated lipolysis. FEBS Lett. 583, 3045–3049 [DOI] [PubMed] [Google Scholar]
- Bhattacharya I., Ullrich A. (2006). Endothelin-1 inhibits adipogenesis: Role of phosphorylation of Akt and ERK1/2. FEBS Lett. 580, 5765–5771 [DOI] [PubMed] [Google Scholar]
- Chen Y., Graziano J. H., Parvez F., Liu M., Slavkovich V., Kalra T., Argos M., Islam T., Ahmed A., Rakibuz-Zaman M, et al. (2011). Arsenic exposure from drinking water and mortality from cardiovascular disease in Bangladesh: Prospective cohort study. BMJ. 342, d2431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng H., Qiu L., Zhang H., Cheng M., Li W., Zhao X., Liu K., Lei L., Ma J. (2011). Arsenic trioxide promotes senescence and regulates the balance of adipogenic and osteogenic differentiation in human mesenchymal stem cells. Acta Biochim. Biophys. Sin. (Shanghai). 43, 204–209 [DOI] [PubMed] [Google Scholar]
- Elbaz N., Bedecs K., Masson M., Sutren M., Strosberg A. D., Nahmias C. (2000). Functional trans-inactivation of insulin receptor kinase by growth-inhibitory angiotensin II AT2 receptor. Mol. Endocrinol. 14, 795–804 [DOI] [PubMed] [Google Scholar]
- Eriksson A. K., van Harmelen V., Stenson B. M., Aström G., Wåhlén K., Laurencikiene J., Rydén M. (2009). Endothelin-1 stimulates human adipocyte lipolysis through the ET A receptor. Int. J. Obes. (Lond). 33, 67–74 [DOI] [PubMed] [Google Scholar]
- Gribble M. O., Howard B. V., Umans J. G., Shara N. M., Francesconi K. A., Goessler W., Crainiceanu C. M., Silbergeld E. K., Guallar E., Navas-Acien A. (2012). Arsenic exposure, diabetes prevalence, and diabetes control in the Strong Heart Study. Am. J. Epidemiol. 176, 865–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson B., Hammarstedt A., Andersson C. X., Smith U. (2007). Inflamed adipose tissue: A culprit underlying the metabolic syndrome and atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 27, 2276–2283 [DOI] [PubMed] [Google Scholar]
- Hashimoto T., Igarashi J., Kosaka H. (2009). Sphingosine kinase is induced in mouse 3T3-L1 cells and promotes adipogenesis. J. Lipid Res. 50, 602–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauner H., Petruschke T., Gries F. A. (1994). Endothelin-1 inhibits the adipose differentiation of cultured human adipocyte precursor cells. Metabolism. 43, 227–232 [DOI] [PubMed] [Google Scholar]
- He X., H’ng S. C., Leong D. T., Hutmacher D. W., Melendez A. J. (2010). Sphingosine-1-phosphate mediates proliferation maintaining the multipotency of human adult bone marrow and adipose tissue-derived stem cells. J. Mol. Cell Biol. 2, 199–208 [DOI] [PubMed] [Google Scholar]
- Hughes M. F., Beck B. D., Chen Y., Lewis A. S., Thomas D. J. (2011). Arsenic exposure and toxicology: A historical perspective. Toxicol. Sci. 123, 305–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwai M., Tomono Y., Inaba S., Kanno H., Senba I., Mogi M., Horiuchi M. (2009). AT2 receptor deficiency attenuates adipocyte differentiation and decreases adipocyte number in atherosclerotic mice. Am. J. Hypertens. 22, 784–791 [DOI] [PubMed] [Google Scholar]
- Janke J., Engeli S., Gorzelniak K., Luft F. C., Sharma A. M. (2002). Mature adipocytes inhibit in vitro differentiation of human preadipocytes via angiotensin type 1 receptors. Diabetes. 51, 1699–1707 [DOI] [PubMed] [Google Scholar]
- Juan C. C., Chang L. W., Huang S. W., Chang C. L., Lee C. Y., Chien Y., Hsu Y. P., Ho P. H., Chen Y. C., Ho L. T. (2006). Effect of endothelin-1 on lipolysis in rat adipocytes. Obesity (Silver Spring). 14, 398–404 [DOI] [PubMed] [Google Scholar]
- Juan C. C., Chuang T. Y., Chang C. L., Huang S. W., Ho L. T. (2007). Endothelin-1 regulates adiponectin gene expression and secretion in 3T3-L1 adipocytes via distinct signaling pathways. Endocrinology. 148, 1835–1842 [DOI] [PubMed] [Google Scholar]
- Klei L. R., Garciafigueroa D. Y., Barchowsky A. (2013). Arsenic activates endothelin-1 Gi protein-coupled receptor signaling to inhibit stem cell differentiation in adipogenesis. Toxicol. Sci. 131, 512–520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolditz C. I., Langin D. (2010). Adipose tissue lipolysis. Curr. Opin. Clin. Nutr. Metab. Care. 13, 377–381 [DOI] [PubMed] [Google Scholar]
- Maull E. A., Ahsan H., Edwards J., Longnecker M. P., Navas-Acien A., Pi J., Silbergeld E. K., Styblo M., Tseng C. H., Thayer K. A, et al. (2012). Evaluation of the association between arsenic and diabetes: A National Toxicology Program workshop review. Environ. Health Perspect. 120, 1658–1670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogi M., Iwai M., Horiuchi M. (2006). Emerging concept of adipogenesis regulation by the renin-angiotensin system. Hypertension. 48, 1020–1022 [DOI] [PubMed] [Google Scholar]
- Moon K., Guallar E., Navas-Acien A. (2012). Arsenic exposure and cardiovascular disease: An updated systematic review. Curr. Atheroscler. Rep. 14, 542–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nincheri P., Luciani P., Squecco R., Donati C., Bernacchioni C., Borgognoni L., Luciani G., Benvenuti S., Francini F., Bruni P. (2009). Sphingosine 1-phosphate induces differentiation of adipose tissue-derived mesenchymal stem cells towards smooth muscle cells. Cell. Mol. Life Sci. 66, 1741–1754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouwens D. M., Sell H., Greulich S., Eckel J. (2010). The role of epicardial and perivascular adipose tissue in the pathophysiology of cardiovascular disease. J. Cell. Mol. Med. 14, 2223–2234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parvez F., Chen Y., Brandt-Rauf P. W., Slavkovich V., Islam T., Ahmed A., Argos M., Hassan R., Yunus M., Haque S. E, et al. (2010). A prospective study of respiratory symptoms associated with chronic arsenic exposure in Bangladesh: Findings from the Health Effects of Arsenic Longitudinal Study (HEALS). Thorax. 65, 528–533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D. S., Devesa V., Hernandez-Zavala A., Adair B. M., Walton F. S., Drobnâ Z., Thomas D. J., Styblo M. (2008). Environmental arsenic as a disruptor of insulin signaling. Met. Ions Biol. Med. 10, 1–7 [PMC free article] [PubMed] [Google Scholar]
- Sell H., Dietze-Schroeder D., Eckel J. (2006). The adipocyte-myocyte axis in insulin resistance. Trends Endocrinol. Metab. 17, 416–422 [DOI] [PubMed] [Google Scholar]
- Shinohara O., Murata Y., Shimizu M. (1992). Enhancement of differentiation of cultured adipogenic cells (TA1) by pertussis toxin. Biochem. Cell Biol. 70, 650–655 [DOI] [PubMed] [Google Scholar]
- Soeder K. J., Snedden S. K., Cao W., Della Rocca G. J., Daniel K. W., Luttrell L. M., Collins S. (1999). The beta3-adrenergic receptor activates mitogen-activated protein kinase in adipocytes through a Gi-dependent mechanism. J. Biol. Chem. 274, 12017–12022 [DOI] [PubMed] [Google Scholar]
- Straub A. C., Klei L. R., Stolz D. B., Barchowsky A. (2009). Arsenic requires sphingosine-1-phosphate type 1 receptors to induce angiogenic genes and endothelial cell remodeling. Am. J. Pathol. 174, 1949–1958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomono Y., Iwai M., Inaba S., Mogi M., Horiuchi M. (2008). Blockade of AT1 receptor improves adipocyte differentiation in atherosclerotic and diabetic models. Am. J. Hypertens. 21, 206–212 [DOI] [PubMed] [Google Scholar]
- Turer A. T., Hill J. A., Elmquist J. K., Scherer P. E. (2012). Adipose tissue biology and cardiomyopathy: Translational implications. Circ. Res. 111, 1565–1577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Harmelen V., Eriksson A., Aström G., Wåhlén K., Näslund E., Karpe F., Frayn K., Olsson T., Andersson J., Rydén M, et al. (2008). Vascular peptide endothelin-1 links fat accumulation with alterations of visceral adipocyte lipolysis. Diabetes. 57, 378–386 [DOI] [PubMed] [Google Scholar]
- Vigouroux C., Caron-Debarle M., Le Dour C., Magré J., Capeau J. (2011). Molecular mechanisms of human lipodystrophies: From adipocyte lipid droplet to oxidative stress and lipotoxicity. Int. J. Biochem. Cell Biol. 43, 862–876 [DOI] [PubMed] [Google Scholar]
- Walton F. S., Harmon A. W., Paul D. S., Drobná Z., Patel Y. M., Styblo M. (2004). Inhibition of insulin-dependent glucose uptake by trivalent arsenicals: Possible mechanism of arsenic-induced diabetes. Toxicol. Appl. Pharmacol. 198, 424–433 [DOI] [PubMed] [Google Scholar]
- Watts S. W. (2010). Endothelin receptors: What’s new and what do we need to know? Am. J. Physiol. Regul. Integr. Comp. Physiol. 298, R254–R260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wauson E. M., Langan A. S., Vorce R. L. (2002). Sodium arsenite inhibits and reverses expression of adipogenic and fat cell-specific genes during in vitro adipogenesis. Toxicol. Sci. 65, 211–219 [DOI] [PubMed] [Google Scholar]
- Zuccarello M., Boccaletti R., Rapoport R. M. (1999). Does blockade of endothelinB1-receptor activation increase endothelinB2/endothelinA receptor-mediated constriction in the rabbit basilar artery? J. Cardiovasc. Pharmacol. 33, 679–684 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.