Abstract
Hydroxyurea, an antineoplastic drug, is a model teratogen. The administration of hydroxyurea to CD1 mice on gestation day 9 induces oxidative stress, increasing the formation of 4-hydroxy-2-nonenal adducts to redox-sensitive proteins such as glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the caudal region of the embryo. GAPDH catalytic activity is reduced, and its translocation into the nucleus is increased. Because the nuclear translocation of GAPDH is associated with oxidative stress–induced cell death, we hypothesized that this translocation plays a role in mediating the teratogenicity of hydroxyurea. Deprenyl (also known as selegiline), a drug used as a neuroprotectant in Parkinson’s disease, inhibits the nuclear translocation of GAPDH. Hence, timed pregnant CD1 mice were treated with deprenyl (10mg/kg) on gestation day 9 followed by the administration of hydroxyurea (400 or 600mg/kg). Deprenyl treatment significantly decreased the hydroxyurea-induced nuclear translocation of GAPDH in the caudal lumbosacral somites. Deprenyl enhanced hydroxyurea-mediated caudal malformations, inducing specifically limb reduction, digit anomalies, tail defects, and lumbosacral vertebral abnormalities. Deprenyl did not augment the hydroxyurea-induced inhibition of glycolysis or alter the ratio of oxidized to reduced glutathione. However, it did dramatically increase cleaved caspase-3 in embryos. These data suggest that nuclear GAPDH plays an important, region-specific, role in teratogen-exposed embryos. Deprenyl exacerbated the developmental outcome of hydroxyurea exposure by a mechanism that is independent of oxidative stress. Although the administration of deprenyl alone did not affect pregnancy outcome, this drug may have adverse consequences when combined with exposures that increase the risk of malformations.
Key Words: teratogen, oxidative stress, 4-hydroxy-2-nonenal, glyceraldehyde-3-phosphate dehydrogenase, nuclear translocation, glutathione, apoptosis, cell death, caspase-3.
The risk of developmental abnormalities in humans is increased by specific maternal conditions and exposure to a number of drugs and environmental chemicals (Schardein, 2000). Although some of these conditions or exposures have distinct targets and etiologies, it has been suggested that oxidative stress may represent a common effector pathway in mediating their developmental toxicity (Ornoy, 2007; Wells et al., 2005). Oxidative stress is produced when reactive oxygen species are generated in amounts that exceed the antioxidant capability of the organism. Organogenesis stage embryos are particularly susceptible to oxidative stress because of their limited capacity to detoxify reactive oxygen species (Schlisser et al., 2010; Yan and Hales, 2005). Exposure to a variety of teratogenic substances, including hydroxyurea, induces oxidative stress in the embryo (DeSesso et al., 1994; Wells et al., 2005).
Hydroxyurea, a potent teratogen in all species tested to date, inactivates ribonucleotide reductase and inhibits DNA synthesis (Kovacic, 2011). In addition to genotoxic stress, hydroxyurea treatment induces oxidative stress. Treatment with hydroxyurea during organogenesis induces malformations (DeSesso et al., 2000; Yan and Hales, 2005). Exposure to hydroxyurea on gestation day 9 (GD 9) in the mouse results predominantly in caudal malformations of the hindlimbs, the lumbar, sacral, and caudal vertebrae, and the tail (Yan and Hales, 2005). It is thought that the rapid production of hydrogen peroxide and hydroxyl radicals observed after hydroxyurea treatment contributes to its teratogenicity (DeSesso et al., 2000). Hydroxyurea-induced oxidative stress increases lipid peroxidation, the radical-initiated degradation of ω-6-polyunsaturated fatty acids found abundantly in mammalian cells (Esterbauer et al., 1991). Previous studies have shown that 4-hydroxy-2-nonenal (4-HNE), a major product of lipid peroxidation, accumulates in the embryo in malformation-sensitive regions (Yan and Hales, 2006). This 4-HNE forms adducts with proteins, several of which are involved in glycolysis; these include glyceraldehyde-3-phosphate dehydrogenase (GAPDH), aldolase A, and glutamate oxaloacetate transaminase (Schlisser et al., 2010). GAPDH metabolic activity and lactate concentrations are decreased significantly in embryos exposed to hydroxyurea. Furthermore, hydroxyurea exposure increases the translocation of GAPDH into the nucleus. The role of nuclear GAPDH as a key mediator of the action of teratogens that induce oxidative stress is not known.
Diverse functions have been attributed to nuclear GAPDH, ranging from a role in mediating cell death (Sen et al., 2008) to the maintenance of genome integrity (Azam et al., 2008). Indeed, nuclear GAPDH participates in apoptosis, cell cycle regulation, and DNA repair. Oxidative modifications to GAPDH, such as S-nitrosylation, increase binding to Siah1, a nuclear localization signal-containing protein, and mediate its nuclear translocation (Ortiz-Ortiz et al., 2010). Nuclear S-nitrosylated GAPDH may also be important in cell signaling pathways because it transnitrosylates other nuclear proteins (Kornberg et al., 2010). Modifications to GAPDH may determine its targets within the nucleus. Nuclear GAPDH is acetylated by the p300/CREB-binding complex (CBP); this interaction, in turn, stimulates the catalytic activity of p300/CBP (Sen et al., 2008). Consequently, downstream targets of p300/CBP, such as p53, are activated, resulting in cell death (Sen et al., 2008; Tristan et al., 2011). In addition, nuclear GAPDH upregulates the expression of proapoptotic proteins in the nucleus (Tristan et al., 2011). GAPDH is also modified by PARP-1 catalyzed ADP-ribosylation in response to oxidative stress, inhibiting its interaction with proteins involved in DNA repair and cell cycle regulation, such as uracil-DNA glycosylase, apurinic/apyrimidinic endonuclease 1, and the cyclin B-cdk1 regulatory protein, SET (Azam et al., 2008; Carujo et al., 2006; Meyer-Siegler et al., 1991). Oxidative stress–induced GAPDH nucleocytoplasmic shuffling and modifications are associated with cancer and neurodegenerative diseases, such as Alzheimer’s and Parkinson’s diseases.
Deprenyl (also known as selegiline, Eldepryl) (R-N-methyl-N-(1-phenylpropan-2-yl) prop-2-yn-1-amine), a selective monoamine oxidase (MAO)-B inhibitor, has been used therapeutically in Parkinson’s disease; deprenyl potentiates the effects of dopamine and slows down neuronal degradation (Magyar and Szende, 2004). During organogenesis and fetal stages of development, MAO-B expression is low (Nicotra et al., 2004), only increasing to adult levels postnatally (Diez and Maderdrut, 1977; Nicotra et al., 2004). Furthermore, MAO-B deficient transgenic mice showed no overt abnormalities or altered levels of dopamine, norepinephrine, or serotonin in the cerebral cortex, substantia nigra, or hippocampus (Nicotra et al., 2004). Thus, it is unlikely that any effects of deprenyl in the organogenesis stage embryo are mediated by its effects on MAO-B activity. However, studies on the developmental toxicology of deprenyl in animals showed no teratogenic effects during gestation although decreases in fetal body weight (at the highest dose tested in the rat study, 36mg/kg orally) and in resorptions and postimplantation loss (at the highest dose tested in the rabbit study, 50mg/kg orally) were noted (Human prescription drug label, Somerset Pharmaceuticals, 2009). Deprenyl binds to GAPDH and prevents its interaction with Siah1, an E3-ubiquitin ligase, which translocates GAPDH to the nucleus (Hara and Snyder, 2006).
We hypothesize that the nuclear translocation of modified GAPDH induced by teratogens that trigger oxidative stress plays a role in mediating their development toxicity. To test this hypothesis, we have determined the consequences of inhibiting the GAPDH nuclear translocation induced by hydroxyurea by coadministering deprenyl. We show that, contrary to our expectations, deprenyl treatment enhanced the teratogenicity of hydroxyurea.
MATERIALS AND METHODS
Animal experiments were approved by McGill University under protocol number 1825 and were conducted in accordance with the guidelines outlined in the Canadian Council on Animal Care Guide to the Care and Use of Experimental Animals. Timed pregnant CD1 mice, mated between 8:00 a.m. and 10:00 a.m. (GD 0), were purchased from Charles River Canada Ltd (St Constant, QC, Canada) and housed in the McIntyre Animal Resource Centre (McGill University, Montreal, QC, Canada). On GD 9, mice were randomly divided into one of six treatment groups. Dams were treated at 8:00 a.m. with (-)-deprenyl (10mg/kg, Sigma-Aldrich Canada Ltd, Oakville, ON, Canada) or vehicle (saline) by ip injection. One hour later, at 9:00 a.m., mice were treated with hydroxyurea (400 or 600mg/kg, Sigma-Aldrich Canada Ltd) or vehicle, also by ip injection. Dams were euthanized by CO2 overdose and cervical dislocation on GD 9 at 12:00 p.m. or on GD 18 at 9:00 a.m.
On GD 9, the uteri were removed and embryos were explanted into Hank’s balanced salt solution (Life Technologies Inc., Burlington, ON, Canada). Embryos collected on GD 9 were subjected to protein extraction for Western blot analysis of cleaved caspase-3 and 4-HNE immunoreactivity; alternatively, embryo homogenates were processed for the determination of lactate concentrations. Whole GD 9 embryos were fixed and prepared for immunofluorescence analysis of nuclear GAPDH. Entire litters were used for each experiment.
On GD 18, the uteri were removed and the numbers of resorption sites were recorded (8–10 litters/treatment group). Fetuses were weighed and examined for external malformations or stored in 95% ethanol and stained with alcian blue (cartilage) and alizarin red S (bone) for the assessment of skeletal malformations, as described previously (Yan and Hales, 2005).
Immunofluorescence.
Dams were treated with deprenyl or vehicle and 600mg/kg hydroxyurea or vehicle on GD 9, as described above. Three hours after treatment with hydroxyurea or vehicle, embryos were explanted and immersed in 4% paraformaldehyde for 4h at 4°C. Embryos were then dehydrated in ethanol, embedded in paraffin, and serially sectioned (5 μm sections) along the sagittal plane. Tissues were deparaffinized and rehydrated with PBS for 5min each and then incubated for 30min in blocking solution (0.1% bovine serum albumin, 0.1% Triton X-100, and PBS). Blocking serum was gently tipped off the slides and then GAPDH primary antibody (1:100; Cat no. ab36840, Abcam Inc., MA) diluted in blocking serum was added for an overnight incubation at 4°C in a humidified chamber. Sections were rinsed thrice for 5min in PBS and then incubated with secondary goat fluorescein 488 anti-rabbit IgG (H+L) (1:200; Cat no. F1-1000, Vector Laboratories, Burlington, Ontario, Canada) diluted in blocking serum for 1h at room temperature. After washing thrice for 5min in PBS, sections were mounted with VectaShield, with 4′-6-diamidino-2-phenylindole (DAPI) for nuclear staining (Vector Laboratories, Inc.). As a negative control, only the secondary antibody was added. Slides were then stored at 4°C and visualized with confocal microscopy within 2 days.
Confocal microscopy and quantitative analysis.
A Zeiss LSM 510 Axiovert 100M confocal microscope with a Plan-Apochromat x63/1.4 oil digital image correlation objective was used to visualize the green fluorescence in GAPDH-immunostained sagittal sections of embryos. Optimal settings for laser scanning fluorescence imaging were determined experimentally for both GAPDH antibody and DAPI. Z-stack images of three independent vehicle-treated and 600mg/kg hydroxyurea-treated embryos were acquired, as previously described (Schlisser et al., 2010); quantitative analysis was done with IMARIS Software (Bitplane AG, Zurich, Switzerland). 3D isosurfaces of vehicle- and 600mg/kg hydroxyurea-treated embryos were generated. Only the lumbosacral somites were analyzed in this study because they were identified previously as a predominant site of 4-HNE protein adducts and nuclear GAPDH translocation (Schlisser et al., 2010; Yan and Hales, 2006).
Western blot analysis of cleaved caspase-3 and 4-HNE-protein adducts.
Protein concentrations were determined using the Bio-Rad Bradford protein assay (Bio-Rad Laboratories, Hercules, CA). For cleaved caspase-3 analysis and 4-HNE protein adduct determination, proteins (20 μg) from embryos from each treatment group were resolved by 10% SDS-polyacrylamide gel electrophoresis (PAGE) and then transferred onto equilibrated polyvinylidene difluoride membranes (Amersham Biosciences, Buckinghamshire, UK) by electroblotting. Membranes were blocked using 10% nonfat milk and then probed for primary antibodies against cleaved caspase-3 (1:1000; rabbit polyclonal IgG, Cell Signaling Technology, Inc., Danvers, MA, Catalog no. 9661L), 4-HNE-protein adducts (1:500; mouse monoclonal IgG, Oxis International Inc., Beverly Hills, CA, Catalog no. 24327), GAPDH (1:1000; rabbit polyclonal IgG, Abcam, Inc., Catalog no. ab36840), or β-actin (1:500; donkey polyclonal IgG, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, Catalog no. sc-2056) overnight at 4°C. Membranes were incubated with horseradish peroxidase–conjugated secondary antibodies for cleaved caspase-3, GAPDH, 4-HNE, and β-actin (1:10,000; GE Healthcare, Buckinghamshire, UK) for 2h at room temperature, and proteins were detected by enhanced chemiluminescence (Amersham Biosciences). Protein bands were quantified by densitometric analysis using a ChemiImager 400 Imaging system (Alpha Innotech, San Leandro, CA); the peak area represented the intensity of the signal.
Assessment of skeletal malformations.
Ethanol-fixed GD 18 fetuses were immersed in a water bath (70°C) for 7 s. The fetuses were skinned, eviscerated, and placed in 95% ethanol overnight. The ethanol was decanted and replaced with alcian blue solution (15mg alcian blue; 80ml 95% ethanol; 20ml glacial acetic acid) for 24h. This solution was then replaced with 95% ethanol. After 24h, the ethanol was substituted with alizarin red S solution (25mg/l alizarin red S in 1% potassium hydroxide) for 24h. The dye was drained and replaced with 0.5% potassium hydroxide for 24h. The skeletons were placed in a 2:2:1 solution (2 parts 70% ethanol:2 parts glycerin:1 part benzyl alcohol). After 24h, stained skeletons were placed in 1:1 solution (1 part 70% ethanol:1 part glycerin) for subsequent analysis of skeletal malformations, as previously described (Yan and Hales, 2005).
Lactate assay.
GD 9 embryos were pooled from vehicle- and drug-treated dams; there were four samples for each group with each sample representing two litters. Samples were homogenized in PBS, flash frozen, and stored at −80°C. Lactate concentration was determined with the use of a Lactate Assay Kit (Biovision Research Products, Mountain View, CA). Ingredients, including lactate assay buffer, lactate probe (in anhydrous dimethyl sulfoxide), lactate enzyme mix, and L(+)-lactate standard (100 nmol/μl), were added to the homogenized samples. Lactate concentrations were determined by measuring the change in absorbance at 570nm. Lactate contents (nmol) were determined from the standard curve and adjusted for protein content, assessed using the Bio-Rad Bradford protein assay.
Glutathione determinations.
On GD 9, embryos were explanted into Hank’s balanced salt solution, flash frozen in liquid nitrogen, and stored at −80°C. Samples were defrosted and homogenized with 5-sulfosalicylic acid (5%, wt/vol). Both total (glutathione [GSH] + glutathione disulfide [GSSG]) and oxidized (GSSG) glutathione were assayed using the Microplate Assay for GSH and GSSG from Oxford Biomedical Research (Cedarlane Laboratories Ltd, Burlington, ON, Canada, Product no. GT40). Briefly, the reaction of GSH with Ellman’s reagent (5,5′-dithiobis-2-nitrobenzoic acid) gives rise to a product that is quantified spectrophotometrically at 412nm (SPECTRAmax PLUS 384, Molecular Devices, Sunnyvale, CA). A pyridine derivative is used as a thiol-scavenging reagent to assess the relative amounts of GSH and GSSG. Oxidative stress is represented as the ratio of GSSG to GSH.
Statistical analyses.
Statistical analyses were done by chi-square and two-way ANOVA, as appropriate, followed by a post hoc Bonferroni’s correction for multiple comparisons using the GraphPad Prism computer program. The α priori level of significance was p < 0.05.
RESULTS
Deprenyl Inhibited the Hydroxyurea-Induced Nuclear Translocation of GAPDH in the Lumbosacral Somites
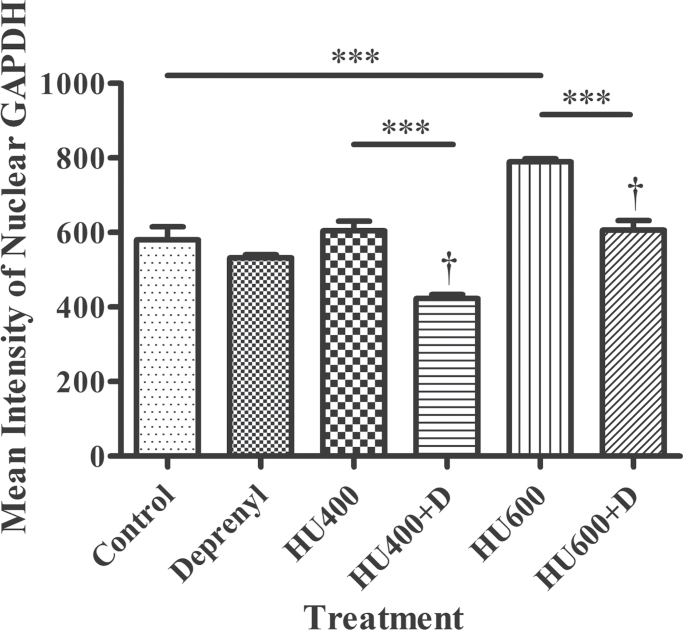
Immunofluorescence confocal microscopy and IMARIS 3D image analysis were done to quantify nuclear GAPDH. Treatment with deprenyl alone did not affect the amount of GAPDH reactivity in cell nuclei in the lumbosacral somites (Fig. 1). Although treatment with 400mg/kg hydroxyurea did not increase the nuclear GAPDH content, exposure to 600mg/kg hydroxyurea did significantly increase GAPDH nuclear translocation compared with control. Deprenyl cotreatment significantly reduced nuclear GAPDH content relative to the respective groups treated with hydroxyurea alone (Fig. 1). Thus, cotreatment with deprenyl did inhibit the nuclear translocation of GAPDH induced by hydroxyurea in organogenesis stage embryos.
Fig. 1.
Analysis of the confocal images of GAPDH immunofluorescence in the lumbosacral regions of GD 9 embryos using IMARIS. The immunofluorescence intensity of isolated nuclear GAPDH is represented here. HU 400, 400mg/kg hydroxyurea; HU600, 600mg/kg hydroxyurea; D, deprenyl. Two-way ANOVA and a post hoc Bonferroni correction were done. Asterisks (***) denote a statistically significant difference (p < 0.001). † denotes a significant difference between the hydroxyurea-treated groups in the absence and presence of deprenyl (p < 0.05).
Western blot analysis of 4-HNE-tagged proteins revealed that 4-HNE immunoreactivity was found predominantly in the same molecular weight range as GAPDH immunoreactivity (Supplementary fig. S1A). Exposure to deprenyl alone, hydroxyurea alone, or the combination did not significantly increase 4-HNE immunoreactivity in this molecular weight range although there was a tendency toward an increase in embryos exposed to high-dose hydroxyurea (600mg/kg) (Supplementary fig. S1B). Thus, the decrease in nuclear GAPDH in embryos exposed to deprenyl and hydroxyurea was not a consequence of an overall effect on 4-HNE-tagged GAPDH.
Effects of Deprenyl and Hydroxyurea on Pregnancy Outcome
Timed pregnant females were treated with deprenyl or vehicle prior to the administration of hydroxyurea to determine the effects of deprenyl coadministration on the teratogenicity of hydroxyurea. Treatment with deprenyl in the presence or absence of hydroxyurea did not affect the numbers of implantation sites per litter (Table 1). Although treatment with 400mg/kg hydroxyurea did not significantly affect the incidence of resorptions or the number of viable fetuses per litter, treatment with the high-dose hydroxyurea (600mg/kg) increased the number of resorptions and decreased the number of live fetuses per litter. Cotreatment with deprenyl did not alter these measures of pregnancy outcome. Treatment with deprenyl alone did result in a statistically significant but small (3%) decrease in mean fetal weights per litter. Hydroxyurea treatment produced a dose-dependent decrease in mean fetal weights per litter (Table 1). Treatment with deprenyl and 400mg/kg hydroxyurea resulted in a significant reduction in fetal weights per litter; no further reduction was observed in the litters exposed to deprenyl and high-dose hydroxyurea.
Table 1.
Cesarean Section Observations for Dams Treated With Hydroxyurea and/or Deprenyl on GD 9
| Saline | Deprenyl | HU (400mg/kg) | HU (400mg/kg) + deprenyl | HU (600mg/kg) | HU (600mg/kg) + deprenyl | |
|---|---|---|---|---|---|---|
| Number of dams | 9 | 9 | 10 | 8 | 9 | 9 |
| Implantation sites | 12.0±2.8 | 10.8±4.5 | 11.9±1.9 | 12.6±2.3 | 12.6±2.3 | 12.9±1.4 |
| Late resorptions | 0.3±0.5 | 1.2±2.0 | 1.9±1.8 | 1.8±1.6 | 6.1±3.4*** | 4.1±3.6* |
| Viable fetuses | 11.7±2.9 | 10.1±4.1 | 10.1±2.9 | 11.0±1.6 | 6.1±5.6* | 7.6±5.1 |
| Fetal weights (g) | 1.36±0.05 | 1.32±0.1* | 1.27±0.11* | 1.16±0.14*† | 1.05±0.07* | 1.06±0.07* |
Note. The data are presented as mean/litter values ± SEM. Two-way ANOVA and a post hoc Bonferroni correction were used to determine significance.
Asterisks (*) and (***) denote a statistically significant difference (p < 0.05) and (p < 0.0001), respectively, from controls; † denotes a significant difference between the HU-treated groups in the absence or presence of deprenyl (p < 0.05).
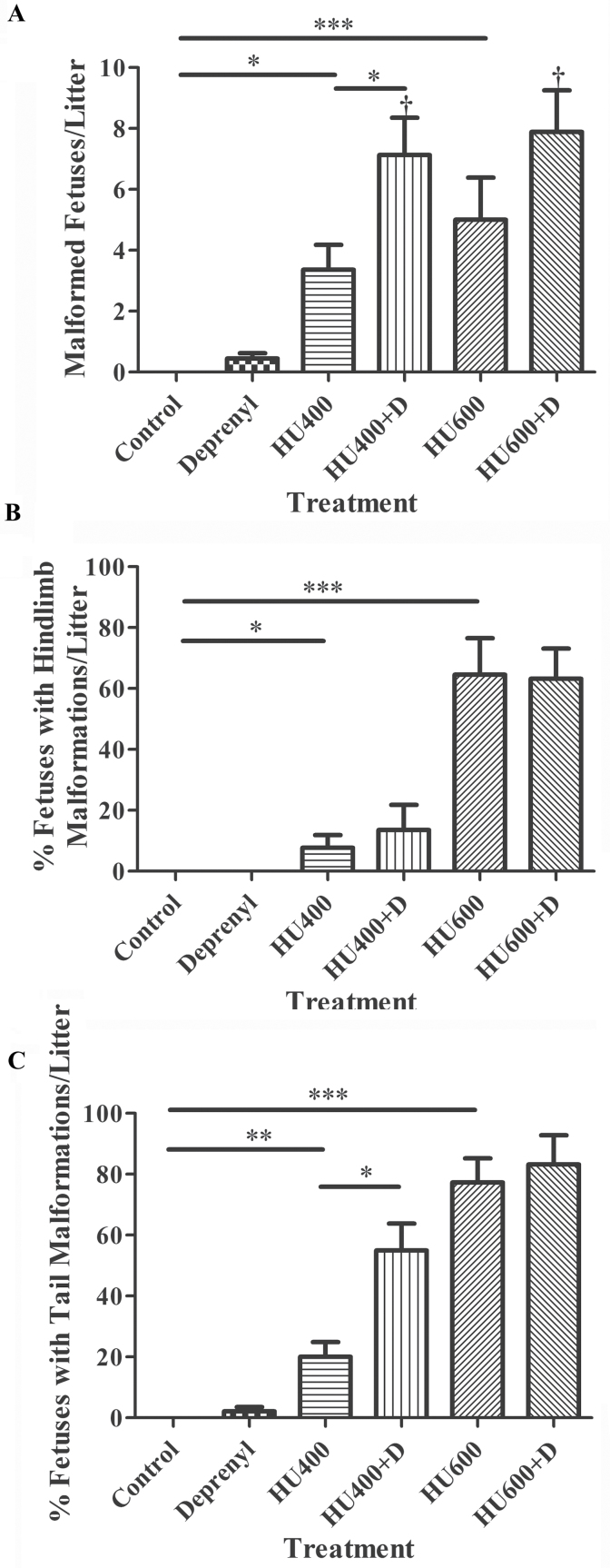
The administration of deprenyl alone did not significantly increase the mean number of malformed fetuses per litter (Fig. 2A) although one fetus with curly tail (1 of 91) was observed in this treatment group. Exposure to hydroxyurea (400 or 600mg/kg) increased the mean number of malformed fetuses per litter in a dose-dependent manner. Treatment with deprenyl in combination with hydroxyurea significantly increased the mean number of malformed fetuses per litter in both the 400 and 600mg/kg hydroxyurea treatment groups compared with those treated with hydroxyurea alone. Although some forelimb defects were observed in fetuses exposed to 400mg/kg hydroxyurea (4.5%), 600mg/kg hydroxyurea (9.7%), and 600mg/kg hydroxyurea plus deprenyl (6.5%) (data not shown), the majority of the external malformations observed were in the caudal region. A spectrum of hindlimb defects was observed in fetuses exposed to 400 or 600mg/kg hydroxyurea; these included agenesis, truncation of hindlimbs, showing complete absence of the phalanges and carpals, aplasia/hypoplasia of the first digit or of more than one digit, syndactyly, ectrodactyly, and polydactyly. Deprenyl coadministration did not significantly enhance the hindlimb malformations induced by 400 or 600mg/kg hydroxyurea (Fig. 2B). However, deprenyl did significantly increase the tail malformations observed in litters exposed to 400mg/kg hydroxyurea (Fig. 2C). Deprenyl coadministration did not result in a further increase in the already high incidence of tail malformations induced by exposure to 600mg/kg hydroxyurea.
Fig. 2.
The incidence of external malformations in fetuses from the litters of dams treated with saline, deprenyl, and/or hydroxyurea (HU) on GD 9. (A) The mean number of malformed fetuses per litter. (B) Fetuses presenting strictly with a hindlimb malformation were counted and are represented as a percentage of the live fetuses without a malformation. (C) Fetuses presenting strictly with a curly/hypoplastic tail were counted and are represented as a percentage of the live fetuses without a malformation. Numbers represent means ± standard errors of the mean. The statistical analysis carried out was a two-way ANOVA followed by Dunnett’s test. Asterisks (*), (**), and (***) denote a statistically significant difference (p < 0.05), (p < 0.01), and (p < 0.001). † denotes a significant difference between the hydroxyurea-treated groups in the presence or absence of deprenyl (p < 0.05).
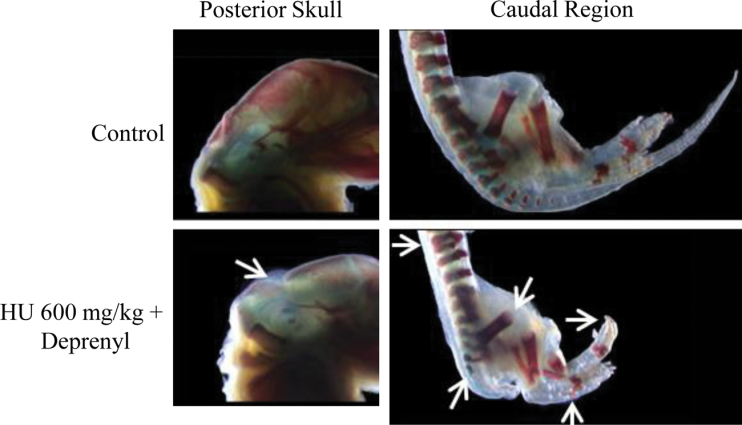
The skeletal assessment of fetuses from the litters exposed to hydroxyurea revealed a significant increase in lumbosacral vertebral, hindlimb, and tail defects, as anticipated from the external malformations that were observed (Table 2); examples of these defects are shown in Figure 3. Control and deprenyl-treated fetuses appeared normal (Fig. 3). Fetuses exposed to 400mg/kg hydroxyurea had shortened or kinked tails and digit hypoplasia. Fetuses exposed to 400mg/kg hydroxyurea and deprenyl were generally more severely malformed than those treated with hydroxyurea alone; these fetuses tended to be smaller, with numerous vertebral column defects (fused, misaligned, and partial ossification of the vertebrae), missing lumbar and sacral vertebrae, tail aplasia, shortening of hindlimb long bones (tibia hypoplasia, femur hypoplasia), digit hypoplasia, neural tube defects, craniofacial defects, partial ossification of ribs and sternum, and forelimb digit hypoplasia. Similar malformations were observed in the 600mg/kg hydroxyurea-exposed fetuses; these included fused and partial ossification of the thoracic vertebrae, partial ossification of the sacral vertebrae, hindlimb long bone malformations (femur hypoplasia, bent fibula), and hindlimb digit hypoplasia. Fetuses exposed to deprenyl and 600mg/kg hydroxyurea displayed a variety of vertebral column defects (fused and misaligned vertebrae, partial ossification of the centra), partial ossification of the lumbar vertebrae, missing sacral vertebrae, tail aplasia, shortening of the hindlimbs (tibia and femur hypoplasia), hindlimb digit hypoplasia, shortening of the forelimbs (hypoplasia of the radius and ulna), partial ossification of the supraoccipital bone, and partial ossification of ribs and sternum (Fig. 3).
Table 2.
Fetal Axial Skeletal Defects
| Saline | Deprenyl | HU (400mg/kg) | HU (400mg/kg) + deprenyl | HU (600mg/kg) | HU (600mg/kg) + deprenyl | |
|---|---|---|---|---|---|---|
| Fetuses (litters) | 32(9) | 19(9) | 20(5) | 16(8) | 15(9) | 21(8) |
| Abnormal supraoccipital | — | — | 1(1) | — | — | 3(3) |
| Abnormal thoracic vertebrae | — | — | 1(8) | — | — | — |
| Abnormal sternebrae | — | — | — | 1(1) | — | 1(1) |
| Forked ribs | — | — | — | 1(1) | — | — |
| Abnormal forelimbs | — | — | — | 2(1) | 2(2) | 3(3) |
| Abnormal lumbosacral vertebrae | 2(2) | 2(1) | 8(6)*** | 15(8)***,† | ||
| Abnormal hindlimbs | — | — | 2(2) | 2(2) | 5(5)** | 13(8)***,† |
| Amelia | — | — | — | — | — | 1(1) |
| Femur hypoplasia | — | — | 1(1) | — | 1(1) | — |
| Tibial aplasia/hypoplasia | — | — | 1(1) | — | 1(1) | — |
| Fibula bent | — | — | — | — | 1(1) | — |
| Digital aplasia/hypoplasia | — | — | 2(2) | 2(2) | 5(5)** | 13(8)***,† |
| Tail aplasia/hypoplasia | — | 2(2) | 8(5)*** | 8(8)*** | 13(9)*** | 18(8)*** |
Note. HU, hydroxyurea. The data are presented as number of fetuses (litters). Statistical analysis was done with two-way ANOVA and Dunnett’s post-hoc test.
Asterisks (**) and (***) denote a statistically significant difference (p < 0.01) and (p < 0.0001), respectively, compared with controls. † denotes a significant difference between the hydroxyurea-treated groups in the presence or absence of deprenyl (p < 0.05).
Fig. 3.
Illustrations of some of the skeletal defects observed in GD 18 fetuses after exposure to saline (control) or deprenyl and 600mg/kg hydroxyurea. Bones appear red (alizarin red S); cartilage appears blue (alcian blue). Malformations are indicated by arrows and described in the text.
A quantitative analysis of the predominant lumbosacral vertebral, hindlimb, and tail defects observed after treatment with hydroxyurea in the absence or presence of deprenyl is presented in Table 2. Exposure to saline or deprenyl had either no effect or a minimal effect (mild shortening of the tail with deprenyl alone) on skeletal development (Table 2). Fetuses from litters exposed to hydroxyurea alone had lumbosacral vertebral malformations (400mg/kg: 10.0%; 600mg/kg: 53.3%), hindlimb malformations (400mg/kg: 10.0%; 600mg/kg: 33.3%), and tail aplasia (400mg/kg: 40.0%; 600mg/kg: 86.7%). The incidence of lumbosacral vertebral and hindlimb malformations was significantly increased in the group exposed to 600mg/kg hydroxyurea and deprenyl in comparison to those treated with 600mg/kg hydroxyurea alone (Table 2).
Effects of Deprenyl and Hydroxyurea on Glycolysis
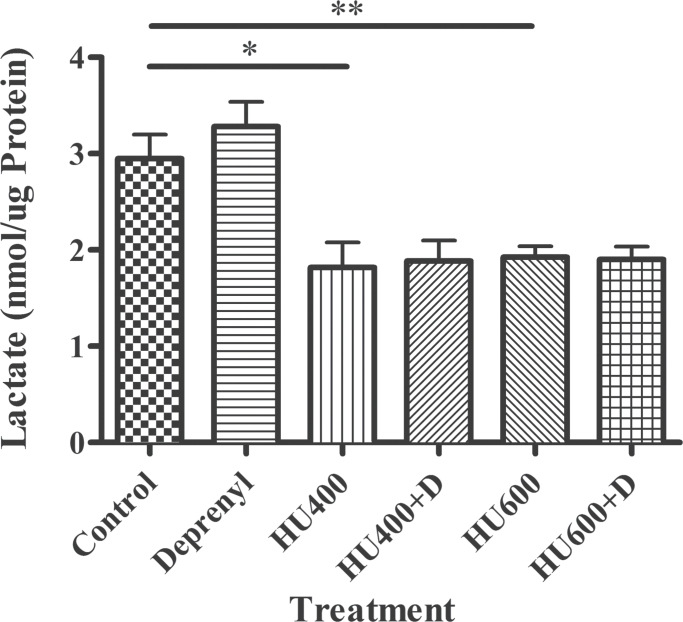
Deprenyl had no effect on glycolysis as assessed by lactate production (Fig. 4). As anticipated, the exposure of organogenesis stage embryos to hydroxyurea decreased lactate production, indicating that glycolysis was inhibited. Deprenyl coadministration did not affect this hydroxyurea-induced decrease in lactate production (Fig. 4).
Fig. 4.
Lactate production in GD 9 embryos exposed to saline (control), deprenyl (D), and/or hydroxyurea (HU) at 400 or 600mg/kg. The data represent means ± SEM. (n = 6 separate experiments, with 8–15 embryos per sample). Two-way ANOVA and a Dunnett’s post hoc were done. Asterisks (*) and (**) denote a statistically significant difference (p < 0.05) and (p < 0.01) compared with controls.
Effects of Deprenyl and Hydroxyurea on Glutathione Homeostasis
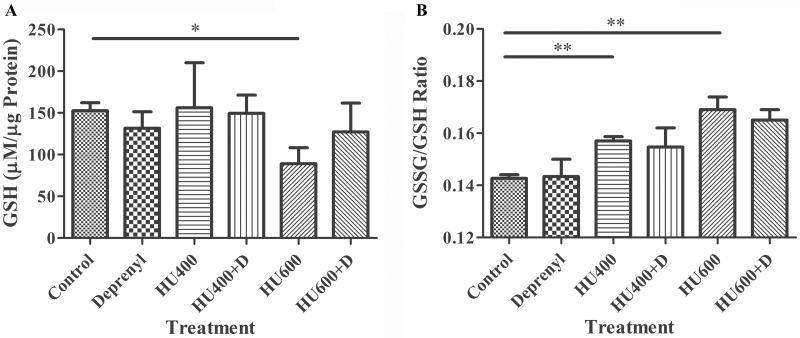
To assess the effects of deprenyl and hydroxyurea on the redox status of the embryos, we measured reduced (GSH) and oxidized (GSSG) glutathione in embryos collected 3h after hydroxyurea treatment (Fig. 5). There were no significant differences in GSH content in embryos treated with deprenyl alone or with 400mg/kg hydroxyurea in the absence or presence of deprenyl compared with control (Fig. 5A). Exposure to 600mg/kg hydroxyurea resulted in a significant depletion of embryonic GSH; the GSH content of embryos in the group exposed to deprenyl in combination with this dose of hydroxyurea was not different from control.
Fig. 5.
Glutathione status of GD 9 embryos treated with saline (control), deprenyl, and/or hydroxyurea (HU) in the absence or presence of deprenyl. (A) GSH concentrations. (B) GSSG/GSH ratios. The bars indicate the means ± SEM (n = 3 separate experiments, with 8–15 embryos per sample). Two-way ANOVA and a post hoc Bonferroni or Dunnett’s test were done. Asterisks (*) and (**) denote a statistically significant difference (p < 0.05) and (p < 0.01) compared with controls.
The ratio of GSSG/GSH was computed as a measure of oxidative stress (Fig. 5B). Deprenyl alone did not affect the GSSG/GSH ratio. Treatment with either 400 or 600mg/kg hydroxyurea significantly increased the GSSG/GSH ratio, indicative of oxidative stress in the hydroxyurea-exposed embryos. The presence of deprenyl in combination with 400 or 600mg/kg hydroxyurea did not significantly affect the GSSG/GSH ratio (Fig. 5B).
Effects of Deprenyl and Hydroxyurea on Apoptosis
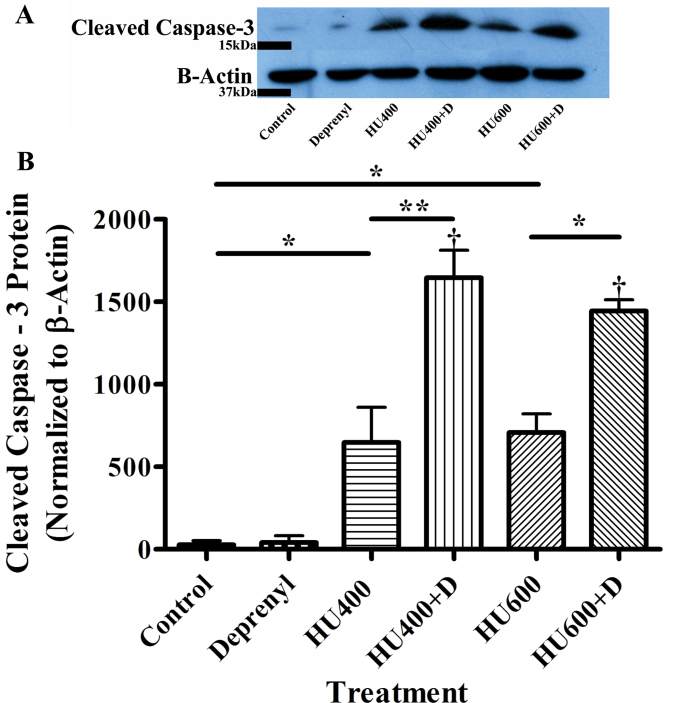
Because the nuclear translocation of GAPDH is associated with a cell death cascade of events, we examined the effects of exposure to hydroxyurea in the absence or presence of deprenyl on cleaved caspase-3 protein, a widely used marker of apoptotic cell death. Deprenyl alone did not increase the amount of cleaved caspase-3 observed in embryos (Fig. 6). Exposure to hydroxyurea (400 or 600mg/kg) produced a dramatic increase in caspase-3 cleavage. Deprenyl, in combination with either 400 or 600mg/kg hydroxyurea, significantly amplified this response, suggesting that apoptosis was dramatically enhanced in the embryos exposed to deprenyl and hydroxyurea.
Fig. 6.
Western blot analysis of the effects of treatment with saline (control), deprenyl, and/or hydroxyurea (HU, 400 or 600mg/kg) on cleaved caspase-3 in GD 9 embryos. (A) Western blots depict cleaved caspase-3(~17kDa), with β-actin as a loading control. (B) Quantification of Western blots. The data represent means ± SEM (n = 4 separate experiments, with 8–15 embryos per sample). Two-way ANOVA and a post- hoc Bonferroni correction were done. Asterisks (*) and (**) denote a statistically significant difference (p < 0.05) and (p < 0.01), respectively. † denotes a significant difference between the hydroxyurea-treated groups in the presence or absence of deprenyl (p < 0.05).
DISCUSSION
The underlying mechanisms by which the maternal conditions and environmental exposures that induce oxidative stress act as developmental toxicants are not known. Using hydroxyurea as a model oxidative stress–inducing teratogen, we reported previously that teratogenic doses enhance the formation of 4-HNE adducts of GAPDH and the nuclear translocation of GAPDH (Schlisser et al., 2010). GAPDH has emerged to be an important sensor of oxidative and genotoxic stress (Tristan et al., 2011). Changes in GAPDH expression, glycolytic activity, nuclear accumulation, and apparent molecular size and conformation have been observed in a number of experimental paradigms (Tristan et al., 2011). In this study, deprenyl was used as a tool to test the hypothesis that the nuclear translocation of GAPDH plays a role in mediating the teratogenicity of hydroxyurea. Here, we demonstrate that deprenyl coadministration inhibited the nuclear translocation of GAPDH and increased the caudal malformations induced by hydroxyurea in embryos.
In the organogenesis stage mouse embryo, GD 9 is a time of high susceptibility to insult. Interestingly, deprenyl coadministration increased the incidence of the malformations induced by hydroxyurea in the more caudal structures, i.e., the lumbosacral vertebrae and hindlimbs (Table 2). The somites that subsequently differentiate to form the axial skeleton, the muscles of the trunk, and the limbs are formed from segmentation of the paraxial mesoderm in a cranial caudal sequence. Two distinct mechanisms, replication stress and oxidative stress, have been suggested for the teratogenicity of hydroxyurea (DeSesso et al., 2000). Previous studies have shown that there is a DNA damage response, as assessed by the formation of γ-H2AX foci, in hydroxyurea-exposed embryos at 3h in all tissues examined, including the somites; at this time, significant increases in p38 MAPK signalling were not observed in the somites (Banh and Hales, 2013). This differential response is intriguing because the p38 family of kinases mediates responses to a wide variety of signals, including both DNA damage and oxidative stress.
Enhancement of the teratogenicity of hydroxyurea by deprenyl administration appears counterintuitive based on much of the literature. Deprenyl is used clinically to attenuate symptoms in early stage Parkinson’s disease, as an adjunct therapy with carbidopa/levodopa, and for depression. Initially, it was thought that the action of deprenyl as a neuroprotectant was dependent on the inhibition of MAO-B, thus reducing the production of reactive oxygen species that accompanies the metabolism of dopamine (Olanov, 1993). We now know that other mechanisms are also important because there are effects of deprenyl even in the absence of MAO-B (Le et al., 1997; Tatton et al., 1996). Furthermore, it is now clear that deprenyl does not only have protective effects. In vitro exposure to high concentrations of deprenyl has been reported to enhance the apoptosis induced by the 1-methyl-4-phenylpyridium ion (MPP+) in a dopaminergic cell line (Le et al., 1997) and by dexamethasone in thymocytes (Szende et al., 2001). Deprenyl has been reported to act as an antioxidant by activating Nrf2 nuclear translocation and thus upregulating the expression of antioxidant enzymes (Xiao et al., 2011). However, deprenyl exposure did not enhance the oxidative stress induced by hydroxyurea because it did not affect the glutathione status of the embryos, as indicated by the GSSG/GSH ratios.
The nuclear translocation of GAPDH plays a role in the apoptotic cascade under various pathological conditions, including Parkinson’s disease (Hara and Snyder, 2006; Hara et al., 2006; Thangima Zannat et al., 2011). Deprenyl inhibits the S-nitrosylation of GAPDH that triggers binding to Siah1, leading to its nuclear translocation (Sen et al., 2008). Inhibition of the nuclear translocation of GAPDH by deprenyl was linked to an inhibition of apoptosis in neuronal cells (Carlile et al., 2000) and in retinal Műller cells (Kusner et al., 2004). However, using a rat mesencephalic cell line, Ortiz-Ortiz et al. (2010) reported that even though preincubation with deprenyl did inhibit the nuclear translocation of GAPDH, it failed to prevent cell death triggered by paraquat. Here, we demonstrate that deprenyl augmented cell death, as assessed by caspase-3 cleavage, in hydroxyurea-exposed embryos. The mechanism by which the inhibition of GAPDH nuclear translocation triggers apoptosis is not known. One possibility is that deprenyl also inhibits the S-nitrosylation of caspase-3. Caspase-3 S-nitrosylation decreases its catalytic activity and is associated with a decrease in apoptosis in fibroblasts (Jiang et al., 2009). S-nitrosylated (SNO-) GAPDH has been reported to physiologically transnitrosylate target nuclear proteins, including histone deacetylases and DNA-activated protein kinase (Kornberg et al., 2010). To the best of our knowledge, this function has not been reported for cytoplasmic GAPDH. Hydroxyurea exposure may trigger transnitrosylation signaling because it is converted to nitric oxide (NO) in vivo and thus may serve as a source of NO (Huang et al., 2006).
Because inhibition of the hydroxyurea-induced nuclear translocation of GAPDH by deprenyl was not accompanied by an effect on either glycolysis or glutathione homeostasis, it seems likely deprenyl is affecting the function of nuclear GAPDH. There is strong evidence that nuclear GAPDH plays an important role in the regulation of transcription, DNA repair, and the cell cycle (Azam et al., 2008; Carujo et al., 2006; Zheng et al., 2003). Because hydroxyurea inactivates ribonucleotide reductase, exposure leads to DNA replication stress and damage response signalling, so a role in modifying DNA repair mechanisms may be highly relevant. Elucidation of the underlying mechanisms and specific binding partners of GAPDH in the nucleus will be critical to our understanding of this response.
The enhanced developmental toxicity of hydroxyurea by deprenyl provides novel insight into its pharmacological effects. Although deprenyl is deemed to be an important neuroprotectant drug, caution should be exercised in pregnant women who may be at risk due to their exposure to maternal conditions and agents that increase oxidative stress.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
Canadian Institutes of Health Research (FRN 57867). B.F.H. is the recipient of a James McGill Professorship.
Supplementary Material
REFERENCES
- Azam S., Jouvet N., Jilani A., Vongsamphanh R., Yang X., Yang S., Ramotar D. (2008). Human glyceraldehyde-3-phosphate dehydrogenase plays a direct role in reactivating oxidized forms of the DNA repair enzyme APE1. J. Biol. Chem. 283, 30632–30641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banh S., Hales B. F. (2013). Hydroxyurea exposure triggers tissue-specific activation of p38 mitogen-activated protein kinase signaling and the DNA damage response in organogenesis-stage mouse embryos. Toxicol. Sci. 133 298–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlile G. W., Chalmers-Redman R. M., Tatton N. A., Pong A., Borden K. E., Tatton W. G. (2000). Reduced apoptosis after nerve growth factor and serum withdrawal: Conversion of tetrameric glyceraldehyde-3-phosphate dehydrogenase to a dimer. Mol. Pharmacol. 57, 2–12. [PubMed] [Google Scholar]
- Carujo S., Estanyol J. M., Ejarque A., Agell N., Bachs O., Pujol M. J. (2006). Glyceraldehyde 3-phosphate dehydrogenase is a SET-binding protein and regulates cyclin B-cdk1 activity. Oncogene 25, 4033–4042. [DOI] [PubMed] [Google Scholar]
- DeSesso J. M., Jacobson C. F., Scialli A. R., Goeringer G. C. (2000). Hydroxylamine moiety of developmental toxicants is associated with early cell death: A structure-activity analysis. Teratology 62, 346–355. [DOI] [PubMed] [Google Scholar]
- DeSesso J. M., Scialli A. R., Goeringer G. C. (1994). D-mannitol, a specific hydroxyl free radical scavenger, reduces the developmental toxicity of hydroxyurea in rabbits. Teratology 49, 248–259. [DOI] [PubMed] [Google Scholar]
- Diez J. A., Maderdrut J. L. (1977). Development of multiple forms of mouse brain monoamine oxidase in vivo and in vitro. Brain Res. 128, 187–192. [DOI] [PubMed] [Google Scholar]
- Esterbauer H., Schaur R. J., Zollner H. (1991). Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic. Biol. Med. 11, 81–128. [DOI] [PubMed] [Google Scholar]
- Hara M. R., Snyder S. H. (2006). Nitric oxide-GAPDH-Siah: A novel cell death cascade. Cell. Mol. Neurobiol. 26, 527–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara M. R., Thomas B., Cascio M. B., Bae B. I., Hester L. D., Dawson V. L., Dawson T. M., Sawa A., Snyder S. H. (2006). Neuroprotection by pharmacologic blockade of the GAPDH death cascade. Proc. Natl. Acad. Sci. U.S.A. 103, 3887–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J., Yakubu M., Kim-Shapiro D. B., King S. B. (2006). Rat liver-mediated metabolism of hydroxyurea to nitric oxide. Free Radic. Biol. Med. 40, 1675–1681. [DOI] [PubMed] [Google Scholar]
- Human prescription drug label; Eldepryl (selegiline hydrochloride) capsule. Somerset Pharmaceuticals Inc. 2009.
- Jiang Z. L., Fletcher N. M., Diamond M. P., Abu-Soud H. M., Saed G. M. (2009). S-nitrosylation of caspase-3 is the mechanism by which adhesion fibroblasts manifest lower apoptosis. Wound Repair Regen. 17, 224–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg M. D., Sen N., Hara M. R., Juluri K. R., Nguyen J. V., Snowman A. M., Law L., Hester L. D., Snyder S. H. (2010). GAPDH mediates nitrosylation of nuclear proteins. Nat. Cell Biol. 12, 1094–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacic P. (2011). Hydroxyurea (therapeutics and mechanism): Metabolism, carbamoyl nitroso, nitroxyl, radicals, cell signaling and clinical applications. Med. Hypotheses. 76, 24–31. [DOI] [PubMed] [Google Scholar]
- Kusner L. L., Sarthy V. P., Mohr S. (2004). Nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase: A role in high glucose-induced apoptosis in retinal Müller cells. Invest. Ophthalmol. Vis. Sci. 45, 1553–1561. [PubMed] [Google Scholar]
- Le W., Jankovic J., Xie W., Kong R., Appel S. H. (1997). (-)-Deprenyl protection of 1-methyl-4 phenylpyridium ion (MPP+)-induced apoptosis independent of MAO-B inhibition. Neurosci. Lett. 224, 197–200. [DOI] [PubMed] [Google Scholar]
- Magyar K., Szende B. (2004). (-)-Deprenyl, a selective MAO-B inhibitor, with apoptotic and anti-apoptotic properties. Neurotoxicology 25, 233–242. [DOI] [PubMed] [Google Scholar]
- Meyer-Siegler K., Mauro D. J., Seal G., Wurzer J., deRiel J. K., Sirover M. A. (1991). A human nuclear uracil DNA glycosylase is the 37-kDa subunit of glyceraldehyde-3-phosphate dehydrogenase. Proc. Natl. Acad. Sci. U.S.A. 88, 8460–8464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotra A., Pierucci F., Parvez H., Senatori O. (2004). Monoamine oxidase expression during development and aging. Neurotoxicology 25, 155–165. [DOI] [PubMed] [Google Scholar]
- Olanow C. W. (1993). A rationale for monoamine oxidase inhibition as neuroprotective therapy for Parkinson’s disease. Mov. Disord. 8(Suppl. 1), S1–S7. [DOI] [PubMed] [Google Scholar]
- Ornoy A. (2007). Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reprod. Toxicol. 24, 31–41. [DOI] [PubMed] [Google Scholar]
- Ortiz-Ortiz M. A., Morán J. M., Ruiz-Mesa L. M., Bravo-San Pedro J. M., Fuentes J. M. (2010). Paraquat exposure induces nuclear translocation of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and the activation of the nitric oxide-GAPDH-Siah cell death cascade. Toxicol. Sci. 116, 614–622. [DOI] [PubMed] [Google Scholar]
- Sen N., Hara M. R., Kornberg M. D., Cascio M. B., Bae B. I., Shahani N., Thomas B., Dawson T. M., Dawson V. L., Snyder S. H, et al. (2008). Nitric oxide-induced nuclear GAPDH activates p300/CBP and mediates apoptosis. Nat. Cell Biol. 10, 866–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schardein J. L. (2000). Chemically Induced Birth Defects, 3rd ed., pp. 566–574. Marcel Dekker, Inc, New York, NY. [Google Scholar]
- Schlisser A. E., Yan J., Hales B. F. (2010). Teratogen-induced oxidative stress targets glyceraldehyde-3-phosphate dehydrogenase in the organogenesis stage mouse embryo. Toxicol. Sci. 118, 686–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szende B., Bökönyi G., Bocsi J., Kéri G., Timár F., Magyar K. (2001). Anti-apoptotic and apoptotic action of (-)-deprenyl and its metabolites. J. Neural Transm. 108, 25–33. [DOI] [PubMed] [Google Scholar]
- Tatton W. G., Wadia J. S., Ju W. Y., Chalmers-Redman R. M., Tatton N. A. (1996). (-)-Deprenyl reduces neuronal apoptosis and facilitates neuronal outgrowth by altering protein synthesis without inhibiting monoamine oxidase. J. Neural Transm. Suppl. 48, 45–59. [DOI] [PubMed] [Google Scholar]
- Thangima Zannat M., Bhattacharjee R. B., Bag J. (2011). In the absence of cellular poly (A) binding protein, the glycolytic enzyme GAPDH translocated to the cell nucleus and activated the GAPDH mediated apoptotic pathway by enhancing acetylation and serine 46 phosphorylation of p53. Biochem. Biophys. Res. Commun. 409, 171–176. [DOI] [PubMed] [Google Scholar]
- Tristan C., Shahani N., Sedlak T. W., Sawa A. (2011). The diverse functions of GAPDH: Views from different subcellular compartments. Cell. Signal. 23, 317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells P. G., Bhuller Y., Chen C. S., Jeng W., Kasapinovic S., Kennedy J. C., Kim P. M., Laposa R. R., McCallum G. P., Nicol C. J, et al. (2005). Molecular and biochemical mechanisms in teratogenesis involving reactive oxygen species. Toxicol. Appl. Pharmacol. 207(2 Suppl)354–366. [DOI] [PubMed] [Google Scholar]
- Xiao H., Lv F., Xu W., Zhang L., Jing P., Cao X. (2011). Deprenyl prevents MPP(+)-induced oxidative damage in PC12 cells by the upregulation of Nrf2-mediated NQO1 expression through the activation of PI3K/Akt and Erk. Toxicology 290, 286–294. [DOI] [PubMed] [Google Scholar]
- Yan J., Hales B. F. (2005). Activator protein-1 (AP-1) DNA binding activity is induced by hydroxyurea in organogenesis stage mouse embryos. Toxicol. Sci. 85, 1013–1023. [DOI] [PubMed] [Google Scholar]
- Yan J., Hales B. F. (2006). Depletion of glutathione induces 4-hydroxynonenal protein adducts and hydroxyurea teratogenicity in the organogenesis stage mouse embryo. J. Pharmacol. Exp. Ther. 319, 613–621. [DOI] [PubMed] [Google Scholar]
- Zheng L., Roeder R. G., Luo Y. (2003). S phase activation of the histone H2B promoter by OCA-S, a coactivator complex that contains GAPDH as a key component. Cell 114, 255–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.