Abstract
1,2,5,6,9,10-Hexabromocyclododecane (HBCD) is a high production volume cycloaliphatic used as an additive flame retardant primarily in polystyrene foam building materials. HBCD mixtures contain three major stereoisomers, alpha (α), beta (β), and gamma (γ), at a typical ratio of 1.2:0.6:8.2. The toxicokinetic properties of the α and γ isomers differ. For instance, α-HBCD has greater bioavailability and potential for accumulation in mice than γ-HBCD. The present study reports comparative kinetics data for β-HBCD needed to support toxicological evaluations of HBCD mixtures. Results indicated that a single oral dose of 3mg/kg of [14C]-labeled β-HBCD was absorbed rapidly (≥ 85% total dose) in the female C57BL/6 mouse. The C max for β-HBCD-derived radioactivity in tissues, except adipose, was observed 3h following gavage. Approximately 90% of the administered dose was excreted in urine and feces within 24h, primarily as β-HBCD-derived metabolites. A portion of the dose (circa 9%) was excreted in feces as γ-HBCD. Oral administration of 30 or 100mg/kg of β-HBCD resulted initially in slower rates of [14C] elimination; however, cumulative excretion data were similar across the dosing range 4 days postdosing. Residual concentrations of [14C] in tissues were highest in adipose and liver. β-HBCD-derived radioactivity accumulated in most tissues following four consecutive daily oral doses of 3mg/kg. The extent of metabolism and excretion of β-HBCD in female C57BL/6 mice was similar to that for γ-HBCD. The potential for accumulation of β-HBCD-derived material in most tissues appeared to be less than for α-HBCD.
Key Words: hexabromocyclododecane, toxicokinetics, mouse, brominated flame retardant, disposition, risk assessment, persistent organic pollutant.
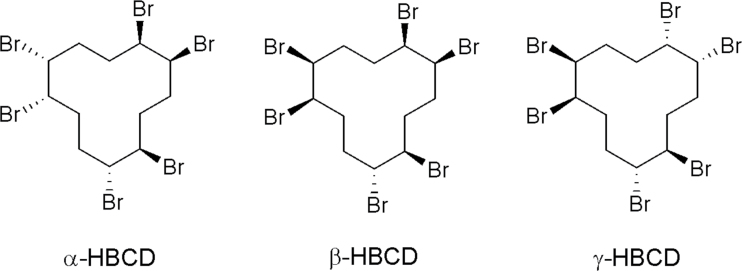
1,2,5,6,9,10-Hexabromocyclododecane (HBCD) is a high production volume cycloaliphatic used as an additive flame retardant in polystyrene foam building materials and in some textile and electronic products (Marvin et al., 2011). Commercial HBCD mixtures are composed primarily of three enantiomer pairs designated as alpha (α)-, beta (β)-, and gamma (γ)-HBCD (Heeb et al., 2005; Fig. 1). As a consequence of high usage, physiochemical properties, and potential for long-range transport, these isomers are detected in the global environment, including Arctic regions and have been observed in wildlife and humans (Brandsma et al., 2009; Covaci et al., 2006; Darnerud et al., 2011; de Wit et al., 2010; Klosterhaus et al., 2012; Toms et al., 2012). The chemical is considered to be persistent, bioaccumulative, and toxic and will be reviewed by the Stockholm Convention for recommended elimination of production and use (Marvin et al., 2011; Stockholm Convention Web site).
Fig. 1.
Structures of the three major isomers in HBCD commercial flame retardants. Each stereoisomer consists of an enantiomer pair.
Humans may be at greatest exposure risk to HBCD adsorbed to indoor dust or present in food products (Schecter et al., 2012; Stapleton et al., 2008; Törnkvist et al., 2011). A risk assessment conducted by the European Commission (2008) estimated consumer exposure to HBCD from these sources to be in the low ng/kg/day range and calculated a no observed adverse effect level of 22.9mg/kg/day from a repeat-dose study conducted by van der Ven et al. (2006) in rats. The HBCD isomeric mixture is a developmental neurotoxicant and endocrine disruptor in rodents (Ema et al., 2008; Eriksson et al., 2006; van der Ven et al., 2006, 2009). Disruption of thyroid homeostasis is a purported mechanism of toxicity, supported by observations of effects on thyroid weight, follicle size, and serum thyroid-stimulating hormone in rats, and T4 levels in rats, trout, and captive kestrels exposed to HBCD (Ema et al., 2008; Marteinson et al., 2011; Palace et al., 2010; van der Ven et al., 2006, 2009). In rats, HBCD isomers have been shown to induce CYP2B and CYP3A, a phenobarbital-type induction associated with depletion of thyroid hormone in rodents (Germer et al., 2006). Exposure to HBCD has resulted in cytotoxicity in neuronal and hepatic cells and hyperthyroidal, estrogenic, antiandrogenic, and antiprogestagenic activity in breast and ovarian cancer cell lines and/or other in vitro assays (Al-Mousa and Michelangeli, 2012; Dorosh et al., 2010; Hamers et al., 2006; Ibhazehiebo et al., 2011; Park et al., 2011; Zhang et al., 2008). The toxicity of individual HBCD isomers has not been characterized in a mammalian model.
Covaci et al. (2006) reported that α, β, and γ, comprised 10–13%, 1–12%, and 75–89%, respectively, of commercial grade HBCD mixtures. γ-HBCD is most often found at the greatest concentration in sediment, soil, and sludge samples because of its relative abundance in mixtures (Covaci et al., 2006; Klosterhaus et al., 2012). Conversely, α-HBCD has been detected at a higher concentration than γ-HBCD in lipid of aquatic invertebrates, marine mammals, fish, birds, and humans (Abdallah and Harrad, 2011; Covaci et al., 2006; Klosterhaus et al., 2012; Xia et al., 2011; Zegers et al., 2005). Exceptions have been noted, with higher levels of γ-HBCD observed in some human (milk, adipose tissue) and fish (muscle) samples (Johnson-Restrepo et al., 2008; Toms et al., 2012). Recent evidence indicates that the in vivo fates of individual isomers play a substantial role in their differential profiles in abiotic and biotic matrices. Studies conducted by Szabo et al. (2010, 2011) demonstrated that oral doses of [14C]-labeled α-HBCD were less metabolized and eliminated, and retained at much higher concentrations in tissues of female C57BL/6 mice over time than similar [14C]-labeled doses of γ-HBCD. γ-HBCD has been shown to isomerize thermally to α-HBCD (Heeb et al., 2008), and in vivo isomerization of γ-HBCD to α- and β-HBCD was observed by Szabo et al. (2010, 2011). In contrast, isomerization of α-HBCD was not detected in female mice used in those studies.
Exposure to β-HBCD may involve direct contact in the environment and/or in vivo formation from γ-HBCD. β-HBCD was detected in brain, liver, adipose, and feces of mice receiving oral doses of γ-HBCD (Szabo et al., 2010). Characterization of the in vivo fate of HBCD isomers is critical to assessing exposure risk to the mixture. However, disposition data for β-HBCD are lacking, and the toxicological implications of exposure are unknown. Therefore, the objective of the present study was to determine the in vivo fate of β-HBCD in the mouse model used in previous toxicokinetic studies of α- and γ-HBCD. These data will support toxicological evaluations and risk assessment of HBCD isomeric mixtures.
MATERIALS AND METHODS
Chemicals and dosing solutions.
[14C]-Labeled and nonlabeled β-HBCD used in these experiments were prepared by Drs Heldur Hakk and Janice Huwe of Agricultural Research Service/United States Department of Agriculture, Fargo, ND, as described by Szabo et al. (2010). Briefly, [14C]-labeled β-HBCD was obtained by separation of a mixture of [14C] β- and γ-HBCD obtained from American Radiochemicals Corporation (St Louis, MO). The specific activity and radiochemical purity were 2 mCi/mmol and > 99%, respectively. The identity and purity (> 99%) of nonlabeled β-HBCD were determined using liquid chromatography-tandem mass spectrometry, by comparison with an authentic standard of β-HBCD obtained from Wellington (Guelph, Ontario, Canada). Dosing solutions were formulated to administer ~10 µCi/kg/mouse and contained amounts of nonlabeled β-HBCD for delivery of doses up to 100mg/kg. Doses were chosen to match those used in previous toxicokinetic studies of α- and γ-HBCD (Szabo et al., 2010, 2011). β-HBCD dissolved in toluene was added to a corn oil vehicle for oral administration. The toluene was removed from the dosing solution via evaporation under a steady stream of N2. For iv administration, β-HBCD in toluene was added to one part Cremophor EL (Sigma-Aldrich, St Louis, MO). The toluene was evaporated with N2, and one part ethanol and three parts water were added to complete the dosing solution. Dose volumes were 2 and 5ml/kg for iv-injected and gavaged mice, respectively.
Animals and treatment.
All procedures involving animals were approved by the Institutional Care and Use Committee of the National Institute of Environmental Health Sciences. Female C57BL/6 mice, obtained from Charles River (Raleigh, NC), were ~8 weeks old and 20g at time of dosing. Dose response was investigated in mice (n = 4/treatment group) receiving single oral administration of 3, 30, or 100mg/kg of β-HBCD. All oral doses were administered using a no. 20 ball-tipped stainless steel feeding needle. Following gavage, the mice were housed individually in metabolism cages for daily collection of urine and feces for 4 days postdosing. Distribution to and clearance of [14C] from tissues over time were investigated in treatment groups (n = 4) receiving 3mg/kg following termination at time points of 1, 3, 8, and 24h postdosing. Urine and feces were collected from the 8- and 24-h groups. Accumulation of [14C] over time was investigated in a group of animals receiving four consecutive daily doses of 3mg/kg by gavage. These mice were euthanized 24h after the last dose. Mice (n = 4) receiving 3mg/kg by tail vein injection were euthanized 24h postdosing to provide comparative data for assessing the extent of absorption in gavaged mice.
Two of the iv-treated mice were placed in cages allowing for the collection of [14C] in expired air, either as volatile organic compounds (VOC) or 14CO2, using the method described by Sanders et al. (2000). Food (NIH no. 31) and water were available for ad libitum consumption by all mice used in this study. Euthanasia in all experiments was by CO2 asphyxiation. Blood was collected by cardiac puncture at time of death. Liver, muscle, adipose tissue (from the abdominal cavity), brain, lung, skin (ear site), and kidney were collected at necropsy. Blood, skin, muscle, and adipose tissue were estimated to be 8, 12, 35, and 8%, respectively, of body weight as previously reported for female C57BL/6 mice of similar age by Szabo et al. (2010). All other tissues were weighed gravimetrically. These values were used to calculate % total dose in tissues following necropsy.
Analysis of [14C] content in biological samples.
The amount of β-HBCD-derived radioactivity in urine was determined by pipetting triple aliquots (10 µl) of each sample directly into vials containing Ecolume (MP Biomedicals, Solon, OH) for analysis in a liquid scintillation counter (LSC) (Beckman Coulter, Brea, CA). The [14C] values obtained from triple aliquots (50 µl each) of cage rinsates were added to those obtained for urine at each time point. The [14C] content in tissue and feces samples was determined by oxidizing triple aliquots (≤ 100mg; wet weight, except feces) of these samples in a biological sample oxidizer (Perkin Elmer, Waltham, MA) prior to LSC analysis. Feces were air-dried, weighed, and ground to a fine powder before combustion.
Analytical high-performance liquid chromatography methods and sample preparation.
The relative amounts of β-HBCD and its metabolites in urine and tissue and feces extracts were determined using high-performance liquid chromatography (HPLC). The HPLC analysis was based on a method described previously (Yu et al., 2008). The system consisted of a Waters (Milford, MA) 1525 binary pump coupled to a Waters 2487 dual absorbance detector and a IN/US β-RAM model 3 radiochemical detector (LabLogic, Brandon, FL). Samples were prepared as described below and separated using an Agilent (Santa Clara, CA) Eclipse Plus C18 column (3.5 μm, 4.6×150mm) with a Zorbax Reliance cartridge guard column. Mobile phases consisted of (A): 0.1% trifluoroacetic acid (TFA) in water and (B): methanol/water (80/20, vol/vol). A gradient of 80% A, reduced to 10% A in 2min, held at 10% A for 33min, then returned to initial conditions over 5min was used. The flow rate throughout the run was 300 μl/min. For radiochemical analyses, Inflow ES scintillation cocktail (LabLogic) was used at a 3:1 ratio based on the manufacturer’s recommendation. Breeze (v. 3.30, Waters) and Laura-Lite (v. 3.3.10.49, LabLogic) software packages were used for instrument control and data analyses. Prior to HPLC analysis, urine samples were filtered by centrifugation using nonsterile Ultrafree MC filter units (0.22 μm; Millipore, Billerica, MA) and an Eppendorf 5415 D centrifuge (Eppendorf, Hauppauge, NY) at 12,700rpm for 15min. Aliquots (~250mg) of air-dried fecal or tissue (wet weight) samples were placed in Ultrafree MC cartridges, rehydrated with 400 μl water (containing 0.1% TFA), and centrifuged as described. This process was repeated twice more, with the filtrates transferred and pooled. Each retentate was then extracted with 95% ethanol (3×400 μl), refiltered, and the filtrates transferred and pooled. Aqueous and organic extracts, maintained separately, were concentrated using a Savant SPD1010 SpeedVac (ThermoScientific, Waltham, MA) without heating, and aliquots were injected onto the HPLC.
Statistical analysis and calculation of half-lives of [14C] in tissues.
The data were subjected to statistical analysis using one-way ANOVA followed by the Tukey-Kramer test for pairwise comparisons (JMP Statistical Software, SAS Institute Inc., Cary, NC). Values were considered to be significantly different at p < 0.05. Noncompartmental pharmacokinetic data analysis was performed using the means of the tissue data collected from mice receiving 3mg/kg by gavage (PK Solutions 2.0, Summit Research, Montrose, CO).
RESULTS
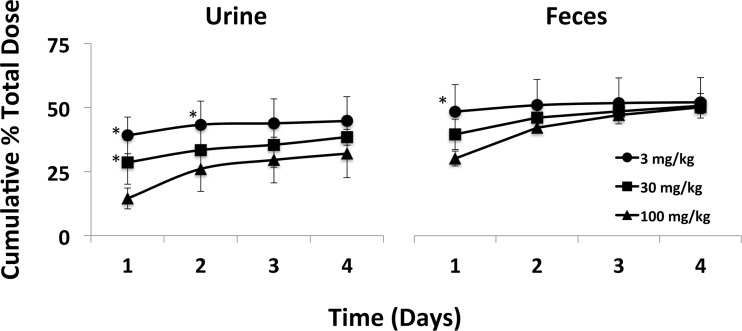
Approximately 90% of the lowest orally administered dose (3mg/kg) of β-HBCD was excreted in urine and feces within 24h of treatment (Fig. 2). The majority of the remaining dose was excreted within 48h, although measurable amounts of [14C] remained in tissues up to 4 days postdosing (Fig. 3 and Table 1). Administration of higher doses (30 and 100mg/kg) resulted in less excretion of β-HBCD-derived radioactivity in urine and feces 24h after gavage (Fig. 2). However, there were no statistically significant differences in the cumulative 4-day excretion of [14C] over the dose range. No overt signs of toxicity were observed in any mice following dosing. However, loss of up to 25% total body weight was noted at the 4-day terminal time point in the mice receiving a single dose of 100mg/kg. Necropsy results indicated large decreases in the amount of adipose tissue in the body cavity relative to mice treated with lower doses (data not shown). It was uncertain if these mice consumed less food than others because daily food consumption was not measured in this study. Similar weight loss was not reported in mice receiving comparable doses of either α- or γ-HBCD (Szabo et al., 2010, 2011).
Fig. 2.
Excretion of β-HBCD-derived radioactivity over time following single doses of 3, 30, or 100mg/kg by gavage. Values are the mean ± SD of four mice. *The value is significantly higher than that for the 100mg/kg group (p < 0.05).
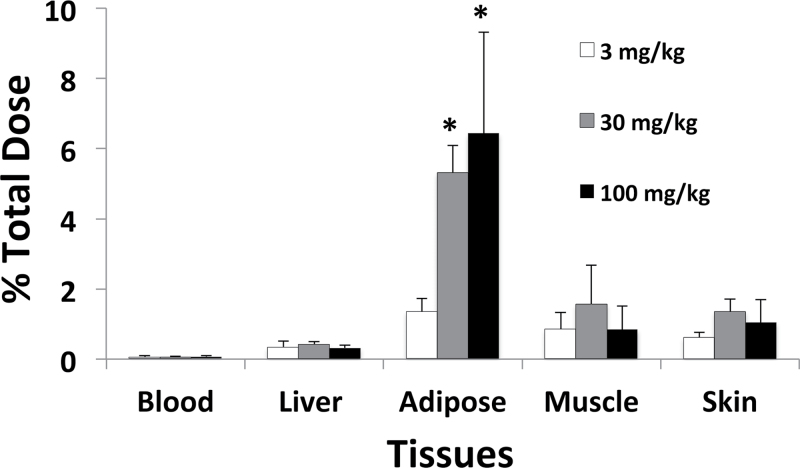
Fig. 3.
β-HBCD-derived radioactivity remaining in tissues 4 days following single doses of 3, 30, or 100mg/kg by gavage. Values are the mean ± SD for three to four mice. *The value is significantly higher than that for the 3mg/kg group (p < 0.05).
Table 1.
Amount and Half-Lives of β-HBCD-Derived Radioactivity in Tissues of Female Mice Receiving 3mg/kg by Gavage
| Single dose | Four daily doses | Alpha t1/2 | Beta t1/2 | |||||
|---|---|---|---|---|---|---|---|---|
| Tissue | 1ha | 3 h | 8 h | 24 h | 96 h | 24 h | Days | Days |
| Blood | 148±39b(0.34±0.1)c | 206±29(0.54±0.08) | 125±36(0.31±0.09) | 55±19(0.13±0.04) | 20±12(0.05±0.04) | 120±10(0.08±0.01) | 0.2 | 2.1 |
| Liver | 1632±288(2.21±0.78) | 4462±246(6.78±0.40) | 1638±245(2.46±0.47) | 692±54(1.07±0.22) | 147±37(0.33±0.18) | 1077±46(0.46±0.03) | 0.1 | 1.3 |
| Muscle | 110±93(1.12±0.98) | 443±191(5.01±2.18) | 130±32(1.39±0.35) | 110±79(1.15±0.75) | 74±43(0.85±0.48) | 163±82(0.44±0.21) | 0.04 | 5.3 |
| Adipose | 543±317(1.26±0.78) | 1585±825(4.08±2.07) | 2345±847(5.07±2.11) | 1136±131(2.79±0.45) | 498±148(1.36±0.38) | 3126±1187(2.14±0.51) | NDd | 2.5 |
| Brain | 72±33(0.04±0.02) | 228±23(0.15±0.01) | 94±23(0.06±0.01) | 7±3(0.01±0.00) | 8±6(> 0.01) | 41±9(0.01±0.00) | 0.02 | 0.2 |
| Lung | 320±91(0.07±0.02) | 581±103(0.14±0.01) | 374±174(0.09±0.04) | 170±67(0.03±0.01) | 44±28(0.01±0.01) | 431±110(0.09±0.13) | 0.2 | 1.5 |
| Skin | 153±95(0.53±0.35) | 315±178(1.22±0.68) | 249±62(0.91±0.23) | 202±37(0.74±0.13) | 150±42(0.61±0.16) | 464±86(0.44±0.10) | 0.1 | 7.0 |
| Kidney | 646±172(0.20±0.05) | 1222±226(0.41±0.08) | 694±187(0.21±0.06) | 151±47(0.05±0.02) | 55±23(0.02±0.01) | 373±40(0.03±0.00) | 0.2 | 2.1 |
Note. aTerminal time point in hours after receiving a single dose or the last of four daily doses.
bEach value represents the mean and SD of 4 mice/treatment group expressed as ng [14C]-equivalents/gram of tissue.
cEach value in parenthesis represents the data expressed as % of the administered dose.
dCould not be determined.
β-HBCD-derived radioactivity was rapidly distributed to all assayed tissues following a single oral dose of 3mg/kg, with the observed C max for most tissues at the 3-h time point (Table 1). The highest concentration of [14C] over time was detected in liver. This amounted to ~7% of the total dose at the observed C max. Adipose tissue was also a major target, containing the highest concentration of [14C] at the 8-h time point. The peak concentration of [14C] in adipose tissue appeared to be less than that in liver; however, [14C] was more persistent over time in adipose tissue, resulting in approximately threefold higher levels of [14C] at 4 days postdosing than in liver. The brain contained the least amount of [14C] of the assayed tissues over time. The tissue:blood ratio for [14C] in the brain was near unity at the common C max, and the concentration of [14C] was at or near background by 24h postdosing. The concentration of [14C] in the blood over time was low compared with that of liver and adipose tissue and was within twofold of background at the 4-day time point.
The amount of [14C] in adipose tissue 24h after a single dose of β-HBCD indicated potential for accumulation of β-HBCD-derived material in lipid upon repeated exposure (Table 1). Further, the elimination half-life of [14C] exceeded 24h in all assayed tissues, except brain. The repeat-dose experiment demonstrated a higher concentration of [14C] in all assayed tissues, except muscle, 24h following the last of four daily doses than following a single dose. The cumulative amount of [14C] recovered in urine of repeat-dose mice increased slightly over time (29±4% total dose after 1 day vs. 35±5% after 4 days), whereas the amount excreted in feces declined from 59±12% total dose after 1 day to 51±5% after 4 days. However, the differences in the means were not statistically significant. No differences were detected in the metabolic profiles of [14C] in excreta over time (data not shown).
The % total dose remaining in the assayed tissues 4 days following oral administration of single doses of 3, 30, and 100mg/kg is shown in Figure 3. Less than 1% total dose was present in the blood at this time point. The % total dose remaining in brain, kidney, and lung was less than observed for blood; therefore, those values are not shown. The amount of [14C] in blood, liver, muscle, skin, brain, kidney, and lung was generally proportional to dose. However, proportionally more [14C] was present in the adipose tissue of the 30 and 100mg/kg treatment groups than observed for adipose tissue from the low-dose group.
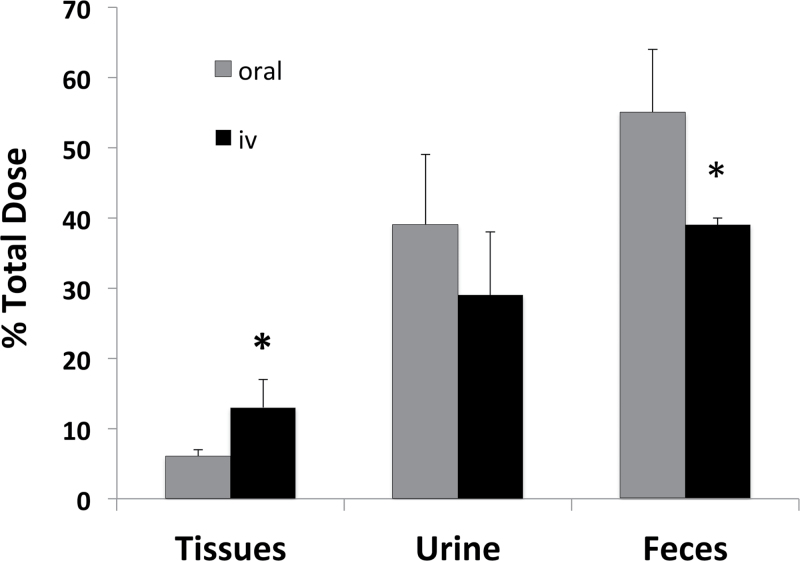
Mice receiving 3mg/kg of β-HBCD by single iv injection excreted significantly less [14C] in feces than mice receiving a similar dose by gavage (Fig. 4). Although less [14C] was recovered in urine of iv-injected mice, the means were not significantly different. Conversely, iv-injected mice retained a significantly higher concentration of [14C] in tissues, specifically muscle and skin (not shown), than did gavaged mice. Trace amounts of [14C] were excreted in expired air as VOC (0.24% total dose) and 14CO2 (0.87% total dose) from iv-treated mice.
Fig. 4.
β-HBCD-derived radioactivity remaining in tissues and cumulatively excreted 24h following oral and iv administration of 3mg/kg. Values are for the mean ± SD of four mice. *The value is significantly different from the corresponding value (p < 0.05).
β-HBCD was extensively metabolized following oral or iv administration to mice. Following oral administration of 3mg/kg, the [14C] in urine was recovered in two early eluting radiolabeled peaks from the HPLC system (combined and listed as hydrophilic metabolites in Table 2). Neither of the radiolabeled peaks in urine coeluted with an authentic standard of β-HBCD in the system (R t = 30.9min). The retention times of these peaks (7 and 9min) were similar to that of two radiolabeled peaks present in the aqueous extracts of feces from β-HBCD-treated mice. The organic extracts of feces contained two [14C]-labeled peaks coeluting with β- and γ-HBCD standards. The amount of β-HBCD and γ-HBCD excreted in feces each represented ~8–9% of the total dose. Approximately 30% of the [14C] in the feces was nonextractable and was assumed to represent β-HBCD-derived metabolites. Trace amounts (< 0.3% of the total dose) of α- and γ-HBCD were detected in extracts of adipose tissue and liver of β-HBCD-treated mice. The [14C] profile in urine and feces samples from mice receiving 100mg/kg was similar to that of lower dose mice (data not shown).
Table 2.
Profile and Amount of [14C] Contained in Urine or Feces/Tissue Extracts of Mice Receiving 3mg/kg β-HBCD by Gavagea
| Sample | Hydrophilic metabolites | α-HBCD | β-HBCD | γ-HBCD | Nonextractable |
|---|---|---|---|---|---|
| Urine (0–8h) | 25 (100)b | NDc | ND | ND | — |
| Urine (0–24h) | 39 (100) | ND | ND | ND | — |
| Feces (0–24h)d | 22 (39) | ND | 8 (14) | 9 (16) | 17 (30) |
| Adipose (8h)d | < 0.1 (0.3) | 0.1 (3) | 3 (92) | 0.1 (3) | < 0.1 (1.4) |
| Liver (3h)d | 4 (66) | < 0.1 (0.2) | < 0.1 (0.3) | < 0.1 (0.2) | 2 (33) |
Note. aData are derived from HPLC chromatograms of representative samples pooled from all mice in the specified treatment group.
bValues are “% of the administered dose (% of the total [14C] in the assayed sample).”
cNot detected.
dValues are from combined aqueous and organic extracts.
DISCUSSION
β-HBCD was rapidly absorbed from the gastrointestinal tract of female C57BL/6 mice. The extent of absorption was estimated to be ≥ 85% of the total dose, based on comparison of oral- and iv-derived excretion data and the amount of unmetabolized HBCD in extracts of feces from gavaged mice. A comparison of the present data with those obtained by Szabo et al. (2010, 2011) demonstrated that the extent of absorption, specific target tissues, and excretion routes were similar for the three major HBCD stereoisomers in C57BL/6 female mice. However, differences in the observed C max and T max of [14C] in tissues over time were evident among α-, β-, and γ-HBCD-treated mice receiving a comparable dose (3mg/kg). For instance, comparisons of [14C] in major tissues demonstrated that liver contained the highest mean concentration following oral administration of the three stereoisomers; however, the T max for the tissue varied from 1 to 3h and the C max varied up to 21-fold between the isomers. The differences in tissue disposition were greatest between α-HBCD-treated mice and mice dosed with the other stereoisomers. For example, the C max for [14C] in adipose, liver, and blood of α-HBCD-treated mice was 8-, 11-, and 200-fold higher than mice treated with a comparable dose of β-HBCD. On the other hand, the maximum concentrations of [14C] in liver, blood, and other assayed tissues (except adipose) were within twofold of each other in β- and γ- HBCD-treated mice, with the higher amounts (except in skin) associated with β-HBCD treatment. Adipose tissue from β-HBCD-treated mice contained 24-fold greater amounts at the peak concentration than did adipose tissue from γ-HBCD-treated mice. These observations indicate that α-HBCD has greater bioavailability than either β- or γ-HBCD and has greater potential to accumulate in tissues following repeat exposure than the two other major HBCD isomers. However, this conclusion does not preclude accumulation of β-HBCD-derived material in tissues following chronic exposure, at least at a concentration of 3mg/kg in mice, as shown in the present study.
In a study conducted by Zegers et al. (2005), rapid metabolism was observed for β- and γ-HBCD, but not α-HBCD, when incubated with male Wistar rat liver microsomes. These results suggested that observed accumulation of α-HBCD in tissues of harbor porpoise and dolphins in European seas was driven largely by differences in the extent of metabolism of the three stereoisomers. In the present study, the elimination half-lives of [14C] in assayed tissues from β-HBCD-treated mice were significantly shorter than for α-HBCD, but similar to those for γ-HBCD as calculated by Szabo et al. (2010, 2011). Furthermore, HPLC analysis demonstrated that the extent of in vivo metabolism of β-HBCD was similar to that of γ-HBCD, not α-HBCD. The series of kinetic studies of the three major HBCD stereoisomers conducted in this laboratory confirmed that the extent of metabolism plays a primary role in the pattern of accumulation in tissues of mammalian species exposed to the HBCD stereoisomers.
In the present study, [14C] β-HBCD was excreted in urine of mice solely as metabolic product, as were the other two stereoisomers in the studies reported by Szabo et al. (2010, 2011). Metabolites of each of the HBCD stereoisomers were also excreted in feces of treated mice. Hakk et al. (2012) identified α- and γ-HBCD-derived metabolites from the Szabo et al. (2010, 2011) studies, showing that α-HBCD was monohydroxylated, debrominated to a tri- or tetrabrominated species, and conjugated with glutathione. Metabolism of γ-HBCD was more complex, with detection of hexa-, tetra-, and tribrominated carboxylic acid moieties, as well as metabolites arising from hydroxylation and glutathione conjugation. In the present study, all of the [14C] excreted in urine and most of the [14C] excreted in feces of mice consisted of metabolites of β-HBCD. Although these metabolites were not identified, they are assumed to arise through similar biotransformation pathways utilized by α- and γ-HBCD. Thus, they probably represent hydroxylation, debromination, and conjugation products of parent β-HBCD.
Enrichment of α-HBCD in biota may also involve enzymatic isomerization of other HBCD stereoisomers to α-HBCD. γ-HBCD has been shown to convert to α- and β-HBCD both thermally and in vivo (Heeb et al., 2008; Köppen et al., 2008; Szabo et al., 2010). On the other hand, thermal conversion of α-HBCD to other isomers is less favored (Heeb et al., 2008; Köppen et al., 2008), and there was no evidence for isomerization of α-HBCD in mice (Szabo et al., 2011). In the present work, a measurable portion of β-HBCD was converted to γ-HBCD and excreted in feces. It is uncertain if this conversion occurred as the result of microflora activity in the gut or metabolic activity in tissues.
In conclusion, the results from the present study demonstrated that β-HBCD was well absorbed, distributed to tissues, and rapidly excreted in urine and feces following oral administration to female mice. Administration of higher doses resulted in apparent saturation of kinetics as indicated by lower initial [14C] excretion rates, as well as an indication of toxicity (weight loss) at the highest dose. β-HBCD was extensively metabolized, with evidence of in vivo isomerization to γ-HBCD. Comparison of β-HBCD disposition to that reported for the other major isomers (Szabo et al., 2010, 2011) indicated that β- and γ-HBCD were more extensively metabolized and had less potential to accumulate in tissues of female C57BL/6 mice than α-HBCD.
FUNDING
This research was conducted by employees of the National Institutes of Health and was funded by the Intramural Research Program of the National Cancer Institute.
ACKNOWLEDGMENTS
The authors thank Ms Sherry Coulter and Mr Abdella Sadik for excellent technical assistance.
REFERENCES
- Abdallah M. A., Harrad S. (2011). Tetrabromobisphenol-A, hexabro- mocyclododecane and its degradation products in UK human milk: Relationship to external exposure. Environ. Int. 37, 443–448 [DOI] [PubMed] [Google Scholar]
- Al-Mousa F., Michelangeli F. (2012). Some commonly used brominated flame retardants cause Ca2+-ATPase inhibition, beta-amyloid peptide release and apoptosis in SH-SY5Y neuronal cells. PLoS One. 7, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandsma S. H., van der Ven L. T., De Boer J., Leonards P. E. (2009). Identification of hydroxylated metabolites of hexabromocyclododecane in wildlife and 28-days exposed Wistar rats. Environ. Sci. Technol. 43, 6058–6063 [DOI] [PubMed] [Google Scholar]
- Covaci A., Gerecke A. C., Law R. J., Voorspoels S., Kohler M., Heeb N. V., Leslie H., Allchin C. R., De Boer J. (2006). Hexabromocyclododecanes (HBCDs) in the environment and humans: A review. Environ. Sci. Technol. 40, 3679–3688 [DOI] [PubMed] [Google Scholar]
- Darnerud P. O., Aune M., Larsson L., Lignell S., Mutshatshi T., Okonkwo J., Botha B., Agyei N. (2011). Levels of brominated flame retardants and other pesistent organic pollutants in breast milk samples from Limpopo Province, South Africa. Sci. Total Environ. 409, 4048–4053 [DOI] [PubMed] [Google Scholar]
- de Wit C. A., Herzke D., Vorkamp K. (2010). Brominated flame retardants in the Arctic environment–trends and new candidates. Sci. Total Environ. 408, 2885–2918 [DOI] [PubMed] [Google Scholar]
- Dorosh A., Dĕd L., Elzeinová F., Pěknicová J. (2010). Assessing oestrogenic effects of brominated flame retadants hexabromcyclododecane and tetrabromobisphenol A on MCF-7 cells. Folia Biol. (Praha). 56, 35–39 [PubMed] [Google Scholar]
- Ema M., Fujii S., Hirata-Koizumi M., Matsumoto M. (2008). Two-generation reproductive toxicity study of the flame retardant hexabromocyclododecane in rats. Reprod. Toxicol. 25, 335–351 [DOI] [PubMed] [Google Scholar]
- Eriksson P., Fischer C., Wallin M., Jakobsson E., Fredriksson A. (2006). Impaired behaviour, learning and memory, in adult mice neonatally exposed to hexabromocyclododecane (HBCDD). Environ. Toxicol. Pharmacol. 21, 317–322 [DOI] [PubMed] [Google Scholar]
- European Commission (2008). Risk Assessment. Hexabromocyclododecane. CAS No.: 25637-99-4. EINECS No.: 247-148-4. Final Report 2008 Available at: http://esis.jrc.ec.europa.eu/doc/risk_assessment/REPORT/hbcddreport044.pdf Accessed April 15, 2013
- Germer S., Piersma A. H., van der Ven L., Kamyschnikow A., Fery Y., Schmitz H. J., Schrenk D. (2006). Subacute effects of the brominated flame retardants hexabromocyclododecane and tetrabromobisphenol A on hepatic cytochrome P450 levels in rats. Toxicology. 218, 229–236 [DOI] [PubMed] [Google Scholar]
- Hakk H., Szabo D. T., Huwe J., Diliberto J., Birnbaum L. S. (2012). Novel and distinct metabolites identified following a single oral dose of α- or γ-hexabromocyclododecane in mice. Environ. Sci. Technol. 46, 13494–13503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamers T., Kamstra J. H., Sonneveld E., Murk A. J., Kester M. H., Andersson P. L., Legler J., Brouwer A. (2006). In vitro profiling of the endocrine-disrupting potency of brominated flame retardants. Toxicol. Sci. 92, 157–173 [DOI] [PubMed] [Google Scholar]
- Heeb N. V., Schweizer W. B., Kohler M., Gerecke A. C. (2005). Structure elucidation of hexabromocyclododecanes–a class of compounds with a complex stereochemistry. Chemosphere. 61, 65–73 [DOI] [PubMed] [Google Scholar]
- Heeb N. V., Schweizer W. B., Mattrel P., Haag R., Gerecke A. C., Schmid P., Zennegg M., Vonmont H. (2008). Regio- and stereoselective isomerization of hexabromocyclododecanes (HBCDs): Kinetics and mechanism of gamma- to alpha-HBCD isomerization. Chemosphere. 73, 1201–1210 [DOI] [PubMed] [Google Scholar]
- Ibhazehiebo K., Iwasaki T., Shimokawa N., Koibuchi N. (2011). 1,2,5,6,9,10-αHexabromocyclododecane (HBCD) impairs thyroid hormone-induced dendrite arborization of Purkinje cells and suppresses thyroid hormone receptor-mediated transcription. Cerebellum. 10, 22–31 [DOI] [PubMed] [Google Scholar]
- Johnson-Restrepo B., Adams D. H., Kannan K. (2008). Tetrabromobisphenol A (TBBPA) and hexabromocyclododecanes (HBCDs) in tissues of humans, dolphins, and sharks from the United States. Chemosphere. 70, 1935–1944 [DOI] [PubMed] [Google Scholar]
- Klosterhaus S. L., Stapleton H. M., La Guardia M. J., Greig D. J. (2012). Brominated and chlorinated flame retardants in San Francisco Bay sediments and wildlife. Environ. Int. 47, 56–65 [DOI] [PubMed] [Google Scholar]
- Köppen R., Becker R., Jung C., Nehls I. (2008). On the thermally induced isomerisation of hexabromocyclododecane stereoisomers. Chemosphere. 71, 656–662 [DOI] [PubMed] [Google Scholar]
- Marteinson S. C., Kimmins S., Letcher R. J., Palace V. P., Bird D. M., Ritchie I. J., Fernie K. J. (2011). Diet exposure to technical hexabromocyclododecane (HBCD) affects testes and circulating testosterone and thyroxine levels in American kestrels (Falco sparverius). Environ. Res. 111, 1116–1123 [DOI] [PubMed] [Google Scholar]
- Marvin C. H., Tomy G. T., Armitage J. M., Arnot J. A., McCarty L., Covaci A., Palace V. (2011). Hexabromocyclododecane: Current understanding of chemistry, environmental fate and toxicology and implications for global management. Environ. Sci. Technol. 45, 8613–8623 [DOI] [PubMed] [Google Scholar]
- Palace V., Park B., Pleskach K., Gemmill B., Tomy G. (2010). Altered thyroxine metabolism in rainbow trout (Oncorhynchus mykiss) exposed to hexabromocyclododecane (HBCD). Chemosphere. 80, 165–169 [DOI] [PubMed] [Google Scholar]
- Park M. A., Hwang K. A., Lee H. R., Yi B. R., Jeung E. B., Choi K. C. (2011). Cell growth of BG-1 ovarian cancer cells is promoted by di-n-butyl phthalate and hexabromocyclododecane via upregulation of the cyclin D and cyclin-dependent kinase-4 genes. Mol. Med. Rep. 5, 761–766 [DOI] [PubMed] [Google Scholar]
- Sanders J. M., Chen L. J., Burka L. T., Matthews H. B. (2000). Metabolism and disposition of luminol in the rat. Xenobiotica. 30, 263–272 [DOI] [PubMed] [Google Scholar]
- Schecter A., Szabo D. T., Miller J., Gent T. L., Malik-Bass N., Petersen M., Paepke O., Colacino J. A., Hynan L. S., Harris T. R., et al. (2012). Hexabromocyclododecane (HBCD) stereoisomers in U.S. food from Dallas, Texas. Environ. Health Perspect. 120, 1260–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapleton H. M., Allen J. G., Kelly S. M., Konstantinov A., Klosterhaus S., Watkins D., McClean M. D., Webster T. F. (2008). Alternate and new brominated flame retardants detected in U.S. house dust. Environ. Sci. Technol. 42, 6910–6916 [DOI] [PubMed] [Google Scholar]
- Stockholm Convention Web site Available at: http://chm.pops.int Accessed January 16, 2013
- Szabo D. T., Diliberto J. J., Hakk H., Huwe J. K., Birnbaum L. S. (2010). Toxicokinetics of the flame retardant hexabromocyclododecane gamma: Effect of dose, timing, route, repeated exposure, and metabolism. Toxicol. Sci. 117, 282–293 [DOI] [PubMed] [Google Scholar]
- Szabo D. T., Diliberto J. J., Hakk H., Huwe J. K., Birnbaum L. S. (2011). Toxicokinetics of the flame retardant hexabromocyclododecane alpha: Effect of dose, timing, route, repeated exposure, and metabolism. Toxicol. Sci. 121, 234–244 [DOI] [PubMed] [Google Scholar]
- Toms L. M., Guerra P., Eljarrat E., Barceló D., Harden F. A., Hobson P., Sjodin A., Ryan E., Mueller J. F. (2012). Brominated flame retardants in the Australian population: 1993-2009. Chemosphere. 89, 398–403 [DOI] [PubMed] [Google Scholar]
- Törnkvist A., Glynn A., Aune M., Darnerud P. O., Ankarberg E. H. (2011). PCDD/F, PCB, PBDE, HBCD and chlorinated pesticides in a Swedish market basket from 2005–levels and dietary intake estimations. Chemosphere. 83, 193–199 [DOI] [PubMed] [Google Scholar]
- van der Ven L. T., van de Kuil T., Leonards P. E., Slob W., Lilienthal H., Litens S., Herlin M., Håkansson H., Cantón R. F., van den Berg M., et al. (2009). Endocrine effects of hexabromocyclododecane (HBCD) in a one-generation reproduction study in Wistar rats. Toxicol. Lett. 185, 51–62 [DOI] [PubMed] [Google Scholar]
- van der Ven L. T., Verhoef A., van de Kuil T., Slob W., Leonards P. E., Visser T. J., Hamers T., Herlin M., Håkansson H., Olausson H., et al. (2006). A 28-day oral dose toxicity study enhanced to detect endocrine effects of hexabromocyclododecane in Wistar rats. Toxicol. Sci. 94, 281–292 [DOI] [PubMed] [Google Scholar]
- Xia C., Lam J. C., Wu X., Sun L., Xie Z., Lam P. K. (2011). Hexabromocyclododecanes (HBCDs) in marine fishes along the Chinese coastline. Chemosphere. 82, 1662–1668 [DOI] [PubMed] [Google Scholar]
- Yu Z., Peng P., Sheng G., Fu J. (2008). Determination of hexabromocyclododecane diastereoisomers in air and soil by liquid chromatography-electrospray tandem mass spectrometry. J. Chromatogr. A. 1190, 74–79 [DOI] [PubMed] [Google Scholar]
- Zegers B. N., Mets A., Van Bommel R., Minkenberg C., Hamers T., Kamstra J. H., Pierce G. J., Boon J. P. (2005). Levels of hexabromocyclododecane in harbor porpoises and common dolphins from western European seas, with evidence for stereoisomer-specific biotransformation by cytochrome p450. Environ. Sci. Technol. 39, 2095–2100 [DOI] [PubMed] [Google Scholar]
- Zhang X., Yang F., Xu C., Liu W., Wen S., Xu Y. (2008). Cytotoxicity evaluation of three pairs of hexabromocyclododecane (HBCD) enantiomers on Hep G2 cell. Toxicol. In Vitro. 22, 1520–1527 [DOI] [PubMed] [Google Scholar]