Abstract
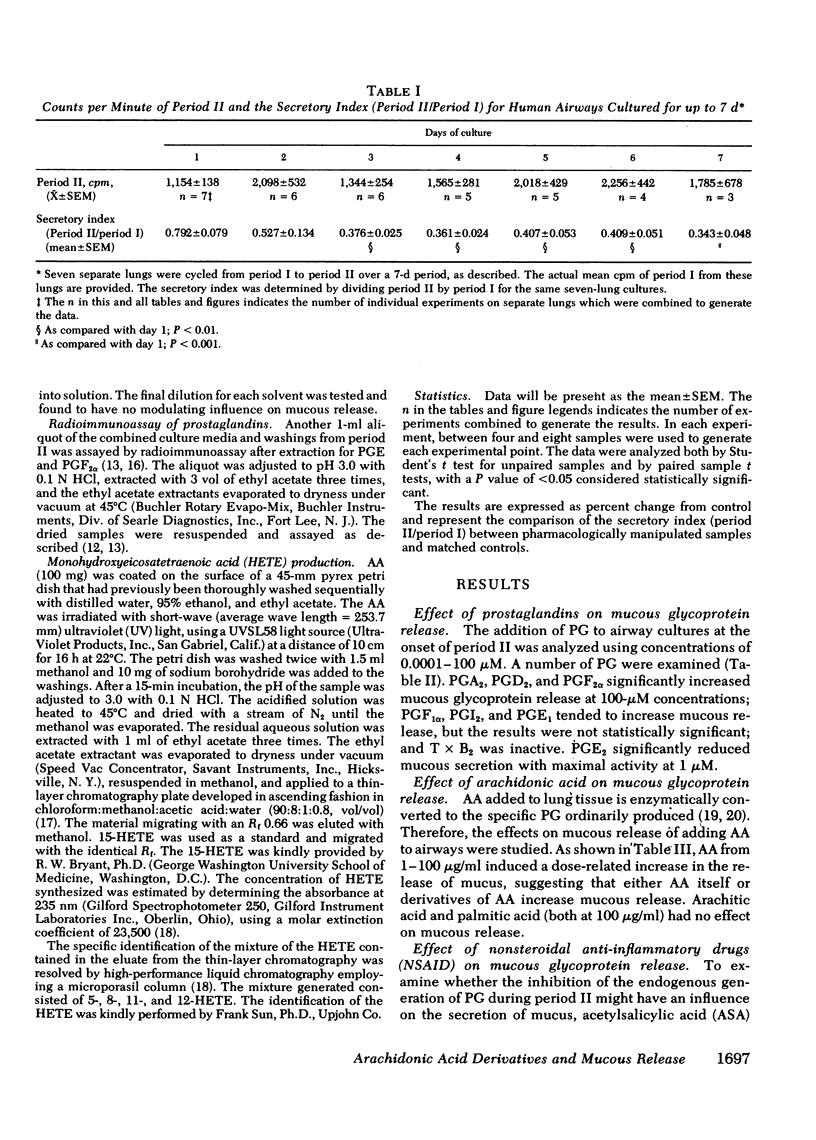
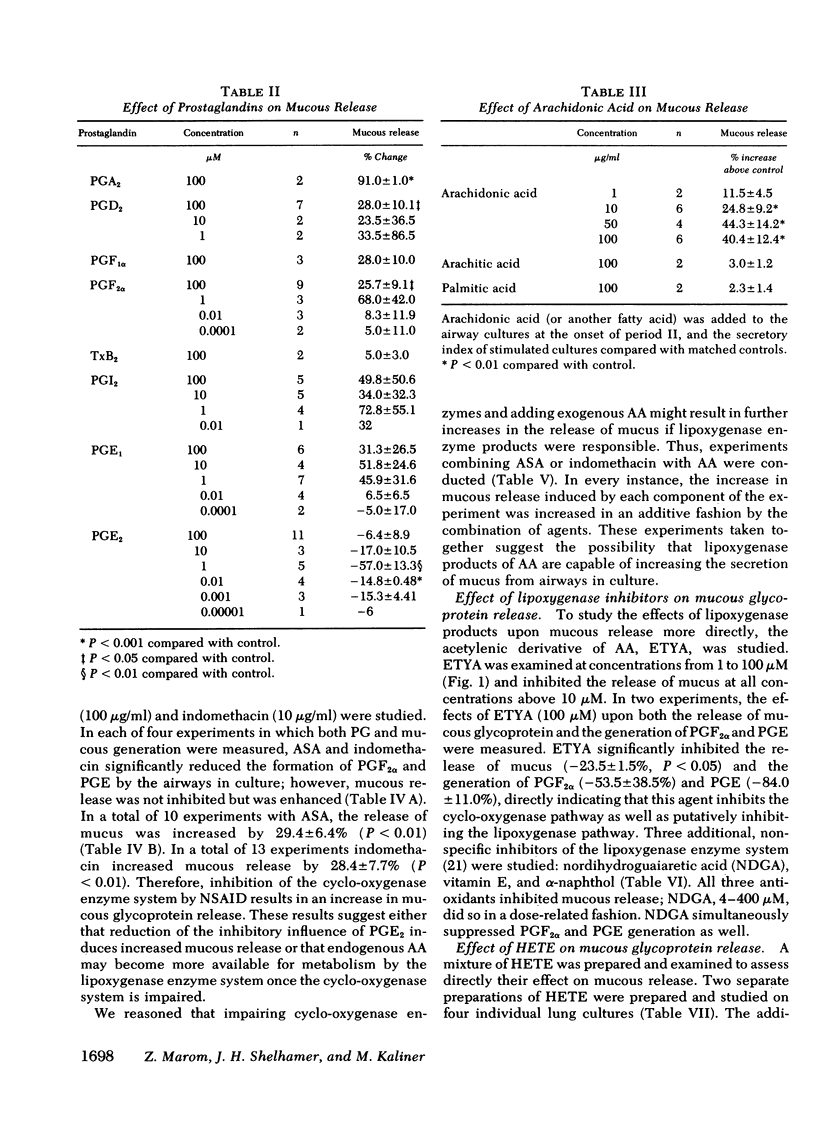
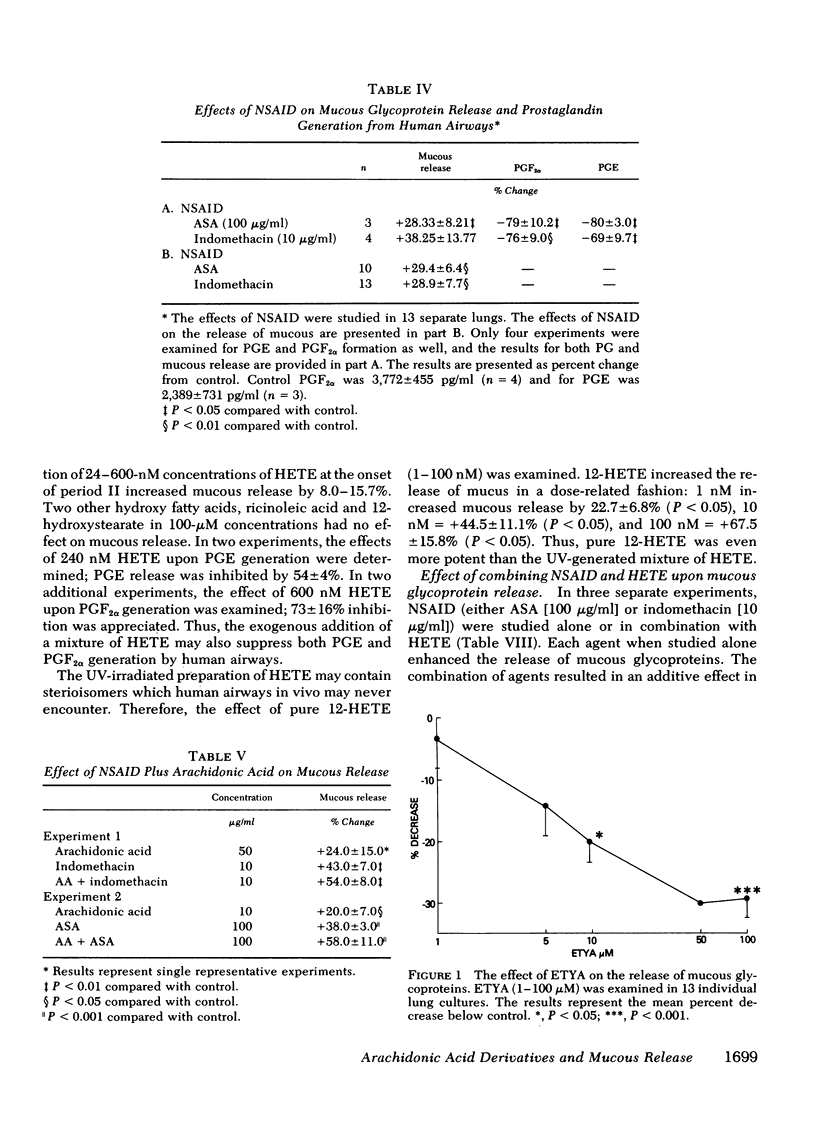
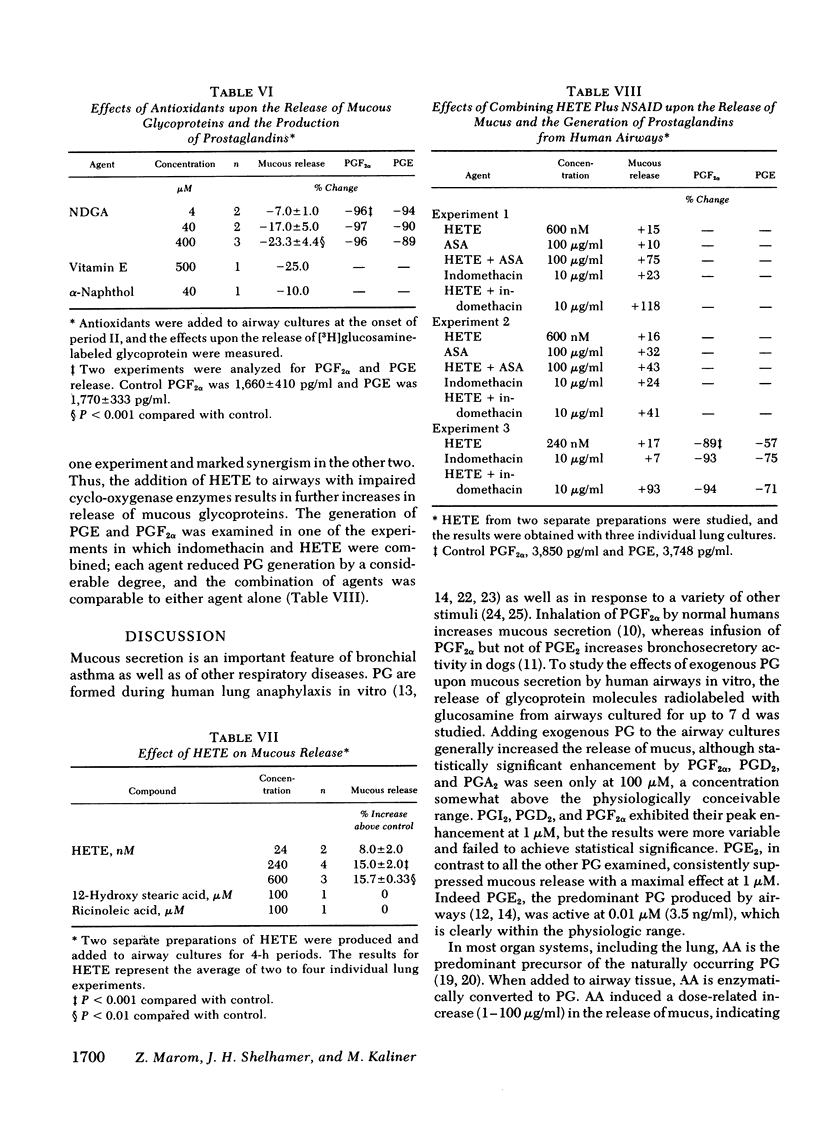
Human lung explants maintained in culture for 7 d incorporate [3H]glucosamine into mucous glycoproteins. Ethanol-precipitable, glucosamine-labeled mucous secretion was measured, and the effects of different pharmacologic agents upon this secretion were investigated. Anaphylaxed human lung generates prostaglandin (PG) synthesis and increased mucous release. Arachidonic acid (AA), PGA2, PGD2, and PGF2α significantly increased mucous glycoprotein release, whereas PGE2 significantly reduced release. Evidence which suggests that lipoxygenase products of AA augment mucous release includes the following: (a) Nonsteroidal anti-inflammatory drugs (NSAID: acetylsalicylic acid and indomethacin) increase mucous release while preventing prostaglandin formation. (b) The increase in mucous release induced by AA or NSAID is additive once the agents are combined. (c) Several nonspecific lipoxygenase inhibitors (eicosa-5,8,11,14-tetraynoic acid; vitamin E; nordihydroguaiaretic acid; and α-naphthol) inhibit mucous release. Three additional lines of evidence directly indicate that monohydroxyeicosatetraenoic acid (HETE) causes increased mucous release: (a) the addition of a mixture of synthetic HETE (24-600 nM) increases mucous release; (b) pure 12-HETE (1-100 nM) also increases mucous release; (c) mucous release is increased synergistically by the combination of HETE and NSIAD.
These data taken together demonstrate that HETE are capable of increasing mucous release and that conditions which may influence HETE production alter mucous release. Thus, although not directly demonstrating HETE production by human airways, the data strongly suggest that lipoxygenase products of AA in airways may profoundly influence mucous release; and it seems possible that lipoxygenase inhibitors may have a role in treating bronchorrhea.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adkinson N. F., Jr, Newball H. H., Findlay S., Adams K., Lichtenstein L. M. Anaphylactic release of prostaglandins from human lung in vitro. Am Rev Respir Dis. 1980 Jun;121(6):911–920. doi: 10.1164/arrd.1980.121.6.911. [DOI] [PubMed] [Google Scholar]
- Ahern D. G., Downing D. T. Inhibition of prostaglandin biosynthesis by eicosa-5,8,11,14-tetraynoic acid. Biochim Biophys Acta. 1970 Sep 8;210(3):456–461. doi: 10.1016/0005-2760(70)90042-1. [DOI] [PubMed] [Google Scholar]
- Anggård E., Samuelsson B. Biosynthesis of prostaglandins from arachidonic acid in guinea pig lung. Prostaglandins and related factors. 38. J Biol Chem. 1965 Sep;240(9):3518–3521. [PubMed] [Google Scholar]
- BROCKLEHURST W. E. The release of histamine and formation of a slow-reacting substance (SRS-A) during anaphylactic shock. J Physiol. 1960 Jun;151:416–435. doi: 10.1113/jphysiol.1960.sp006449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey J. M., Bryant R. W., Feinmark S. J., Makheja A. N. Differential separation of thromboxanes from prostaglandins by one and two-dimensional thin layer chromatography. Prostaglandins. 1977 Mar;13(3):479–492. doi: 10.1016/0090-6980(77)90026-0. [DOI] [PubMed] [Google Scholar]
- Barrett L. A., McDowell E. M., Frank A. L., Harris C. C., Trump B. F. Long-term organ culture of human bronchial epithelium. Cancer Res. 1976 Mar;36(3):1003–1010. [PubMed] [Google Scholar]
- Chakrin L. W., Baker A. P., Spicer S. S., Wardell J. R., Jr, De Sanctis N., Dries C. Synthesis and secretion of macromolecules by canine trachea. Am Rev Respir Dis. 1972 Mar;105(3):368–381. doi: 10.1164/arrd.1972.105.3.368. [DOI] [PubMed] [Google Scholar]
- Downing D. T., Ahern D. G., Bachta M. Enzyme inhibition by acetylenic compounds. Biochem Biophys Res Commun. 1970 Jul 13;40(1):218–223. doi: 10.1016/0006-291x(70)91069-7. [DOI] [PubMed] [Google Scholar]
- Fanburg B. L. Prostaglandins and the lung. Am Rev Respir Dis. 1973 Sep;108(3):482–489. doi: 10.1164/arrd.1973.108.3.482. [DOI] [PubMed] [Google Scholar]
- Ferreira S. H., Vane J. R. Prostaglandins: their disappearance from and release into the circulation. Nature. 1967 Dec 2;216(5118):868–873. doi: 10.1038/216868a0. [DOI] [PubMed] [Google Scholar]
- Flower R. J. Drugs which inhibit prostaglandin biosynthesis. Pharmacol Rev. 1974 Mar;26(1):33–67. [PubMed] [Google Scholar]
- Goetz J. M., Sprecher H., Cornwell D. G., Panganamala R. V. Inhibition of prostaglandin biosynthesis by triynoic acids. Prostaglandins. 1976 Aug;12(2):187–192. doi: 10.1016/0090-6980(76)90113-1. [DOI] [PubMed] [Google Scholar]
- Goetzl E. J., Brash A. R., Tauber A. I., Oates J. A., Hubbard W. C. Modulation of human neutrophil function by monohydroxy-eicosatetraenoic acids. Immunology. 1980 Apr;39(4):491–501. [PMC free article] [PubMed] [Google Scholar]
- Goetzl E. J., Sun F. F. Generation of unique mono-hydroxy-eicosatetraenoic acids from arachidonic acid by human neutrophils. J Exp Med. 1979 Aug 1;150(2):406–411. doi: 10.1084/jem.150.2.406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. On the metabolism of prostaglandins E 1 and E 2 in man. J Biol Chem. 1971 Nov 25;246(22):6713–6721. [PubMed] [Google Scholar]
- Hyman A. L., Spannhake E. W., Kadowitz P. J. Prostaglandins and the lung. Am Rev Respir Dis. 1978 Jan;117(1):111–136. doi: 10.1164/arrd.1978.117.1.111. [DOI] [PubMed] [Google Scholar]
- Jaffe B. M., Smith J. W., Newton W. T., Parker C. W. Radioimmunoassay for prostaglandins. Science. 1971 Feb 5;171(3970):494–496. doi: 10.1126/science.171.3970.494. [DOI] [PubMed] [Google Scholar]
- Lopez-Vidriero M. T., Das I., Smith A. P., Picot R., Reid L. Bronchial secretion from normal human airways after inhalation of prostaglandin F2alpha, acetylcholine, histamine, and citric acid. Thorax. 1977 Dec;32(6):734–739. doi: 10.1136/thx.32.6.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyrick B., Reid L. In vitro incorporation of (3H)threonine and (3H)glucose by the mucous and serous cells of the human bronchial submucosal gland. A quantitative electron microscope study. J Cell Biol. 1975 Nov;67(2PT1):320–344. doi: 10.1083/jcb.67.2.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy R. C., Hammarström S., Samuelsson B. Leukotriene C: a slow-reacting substance from murine mastocytoma cells. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4275–4279. doi: 10.1073/pnas.76.9.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugteren D. H. Arachidonate lipoxygenase in blood platelets. Biochim Biophys Acta. 1975 Feb 20;380(2):299–307. doi: 10.1016/0005-2760(75)90016-8. [DOI] [PubMed] [Google Scholar]
- Orange R. P., Murphy R. C., Karnovsky M. L., Austen K. F. The physicochemical characteristics and purification of slow-reacting substance of anaphylaxis. J Immunol. 1973 Mar;110(3):760–770. [PubMed] [Google Scholar]
- Panganamala R. V., Miller J. S., Gwebu E. T., Sharma H. M., Cornwell D. G. Differential inhibitory effects of vitamin E and other antioxidants on prostaglandin synthetase, platelet aggregation and lipoxidase. Prostaglandins. 1977 Aug;14(2):261–271. doi: 10.1016/0090-6980(77)90171-x. [DOI] [PubMed] [Google Scholar]
- Parker C. W., Huber M. M., Hoffman M. K., Falkenhein S. F. Characterization of the two major species of slow reacting substance from rat basophilic leukemia cells as glutathionyl thioethers of eicosatetraenoic acids oxygenated at the 5 position. Evidence that peroxy groups are present and important for spasmogenic activity. Prostaglandins. 1979 Nov;18(5):673–686. doi: 10.1016/0090-6980(79)90088-1. [DOI] [PubMed] [Google Scholar]
- Parker C. W. Prostaglandins and slow-reacting substance. J Allergy Clin Immunol. 1979 Jan;63(1):1–14. doi: 10.1016/0091-6749(79)90155-6. [DOI] [PubMed] [Google Scholar]
- Piper P. J., Walker J. L. The release of spasmogenic substances from human chopped lung tissue and its inhibition. Br J Pharmacol. 1973 Feb;47(2):291–304. doi: 10.1111/j.1476-5381.1973.tb08327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platshon L. F., Kaliner M. The effects of the immunologic release of histamine upon human lung cyclic nucleotide levels and prostaglandin generation. J Clin Invest. 1978 Dec;62(6):1113–1121. doi: 10.1172/JCI109230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuelsson B., Granström E., Green K., Hamberg M., Hammarström S. Prostaglandins. Annu Rev Biochem. 1975;44:669–695. doi: 10.1146/annurev.bi.44.070175.003321. [DOI] [PubMed] [Google Scholar]
- Shelhamer J. H., Marom Z., Kaliner M. Immunologic and neuropharmacologic stimulation of mucous glycoprotein release from human airways in vitro. J Clin Invest. 1980 Dec;66(6):1400–1408. doi: 10.1172/JCI109993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soin J. S., Wagner H. N., Jr, Thomashaw D., Brown T. C. Increased sensitivity of regional measurements in early detection of narcotic lung disease. Chest. 1975 Mar;67(3):325–330. doi: 10.1378/chest.67.3.325. [DOI] [PubMed] [Google Scholar]
- Steel L., Platshon L., Kaliner M. Prostaglandin generation by human and guinea pig lung tissue: comparison of parenchymal and airway responses. J Allergy Clin Immunol. 1979 Oct;64(4):287–293. doi: 10.1016/0091-6749(79)90146-5. [DOI] [PubMed] [Google Scholar]
- Stenson W. F., Parker C. W. Monohydroxyeicosatetraenoic acids (HETEs) induce degranulation of human neutrophils. J Immunol. 1980 May;124(5):2100–2104. [PubMed] [Google Scholar]
- Strandberg K., Mathé A. A., Yen S. S. Release of histamine and formation of prostaglandins in human lung tissue and rat mast cells. Int Arch Allergy Appl Immunol. 1977;53(6):520–529. doi: 10.1159/000231794. [DOI] [PubMed] [Google Scholar]
- Sturgess J., Reid L. An organ culture study of the effect of drugs on the secretory activity of the human bronchial submucosal gland. Clin Sci. 1972 Oct;43(4):533–543. doi: 10.1042/cs0430533. [DOI] [PubMed] [Google Scholar]
- Sullivan T. J., Parker C. W. Possible role of arachidonic acid and its metabolites in mediator release from rat mast cells. J Immunol. 1979 Feb;122(2):431–436. [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Yamatake Y., Yanaura S. New method for evaluating bronchomotor and bronchosecretory activities: Effects of prostaglandins and antigen. Jpn J Pharmacol. 1978 Jun;28(3):391–402. doi: 10.1254/jjp.28.391. [DOI] [PubMed] [Google Scholar]



