Abstract
Gold nanoparticles have received wide interest in disease diagnosis and therapy, but one of the important issues is their toxicological effects in vivo. Sex differences in the toxicity of gold nanoparticles are not clear. In this work, body weight, organ weight, hematology, and biochemistry were used to evaluate sex differences in immune response and liver and kidney damage. Pathology was used to observe the general toxicity of reproductive organs. The immune response was influenced significantly in female mice, with obvious changes in spleen and thymus index. Hematology results showed that male mice treated with 22.5 nm gold nanoparticles received more significant infection and inflammation than female mice. Meanwhile, the biochemistry results showed that 4.4 and 22.5 nm gold nanoparticles caused more significant liver damage in male mice than female mice, while 22.5, 29.3, and 36.1 nm gold nanoparticles caused more significant kidney damage in female mice than male mice. No significant toxicological response was found in the reproductive system for female or male mice. It was found that gold nanoparticles caused more serious liver toxicity and infection in male mice than female mice. These findings indicated that sex differences may be one of the important elements for in vivo toxicity of gold nanoparticles.
Keywords: sex differences, toxicity, gold nanoparticles
Introduction
More and more attention has been focused on gold-based nanomaterials in diverse biomedical applications, such as biosensors,1 optical imaging,2,3 photothermal therapy,4,5 and drug delivery,6 due to their unique surface chemistry and tunable optical properties.7–13 Recently, gold nanoparticles (NPs) have also been conceived as contrast agents of X-ray imaging for cancer therapy, because of their enhancement effects in photoelectronic absorption and secondary electron generation.14–17 Compared with other novel therapy methods, radiation therapy is closely related to clinical application which means that it is necessary to accept more rigorous topological examination and toxicological observation for gold nanostructures.18 Thus, it is of interest to investigate the topological response in detail, which will benefit both nanopharmacology and nanotoxicology.
In vivo biocompatibilities of gold NPs have been widely investigated in recent years.19–21 It has been demonstrated that the toxicity of gold-based nanomaterials is closely related to surface chemistry,22–24 physical dimension,25–30 dose,31 and administration routes.31 In particular, surface chemistry plays an important role in the toxicity of gold NPs. Naked gold NPs are not stable in the physiological environment, and can form 100 nm aggregators in blood by interaction between NPs and proteins.16,32 These hybrid aggregators can be absorbed rapidly by the reticuloendothelial system, and arrive at the liver and spleen.33,34 Polyethylene glycol (PEG) coating can reduce the surface charge (zeta potential), and thus improve blood stability.34 Compared with naked gold NPs, PEG-coated gold NPs can present relatively monodisperse distribution in the physiological environment. Meanwhile, PEG-modified NPs present long-term blood circulation and long blood half-life. It was found that PEG-coated gold NPs and nanorods at 10 mg/kg could increase the duration of blood circulation and tumor distribution.35 In spite of these benefits, the toxicity response of PEG-coated gold NPs in tail-vein injection 10–100 mg/kg still caused liver and kidney injuries and abnormal gene expression in liver and spleen owing to particle accumulation.36 Thus, it is generally accepted that long-term accumulation of gold NPs will inevitably produce side effects in vivo. Besides, size is another important element for in vivo toxicity of NPs. For instance, 5 and 30 nm particles could lead to an increase of biochemical indicators, but 10 and 60 nm particles caused relatively slight damage in the liver.16,27,29,30,37 Additionally, typical 13.5 nm PEG-coated NPs preferred to accumulate in the liver and spleen, but NPs larger than 100 nm could be cleared partially by excretion.29
Apart from these elements, sex is an easily overlooked factor that also attracts our interest. Indeed, it has been demonstrated that sex differences are an important aspect in pharmacokinetic and pharmacodynamics research.38 An obvious significant sex difference is physiological change during the menstrual cycle, as well as changes in plasma protein and hormonal levels.39 These elements can cause possible effects on interactions between blood plasma and NPs, and thus induce different physiological responses during blood circulation for males and females. Therefore, sex differences result in possible toxicological differences, eg, reproductive system and liver injury. In the field of nanotoxicology, it has been demonstrated that sex differences can lead to significant differences in the toxicity of Ag NPs. It was found that exposure to more than 300 mg of 60 nm Ag NPs in Sprague Dawley rats may lead to liver damage.40,41 Meanwhile, it has been found that Ag NPs at a low dose (<10 mg/kg) were safe for biomedical application and had no side effects, but high dosages (>20 mg/kg) were toxic. These results inspired us to investigate sex difference-related toxicity.
In our previous work, we investigated size-dependent, administration route-dependent toxicity of gold NPs. Especially, it was found that glutathione-protected Au nanoclusters are low toxic and metabolizable.16,17,24,29,31 In the present study, we investigated sex differences in in vivo toxicity of four kinds of gold NPs. The widely used PEG coating was employed for the NPs. In the experiment, we observed sex differences in immune responses and liver and kidney injuries, based on the evaluation of body weights, organ indexes, hematology, and biochemistry. Histopathology changes were investigated to show the general toxicity of reproductive organs.
Materials and methods
Fabrication
The gold NPs were synthesized following the classical method devised by Turkevich et al.42 Chloroauric acid (HAuCl4 4H2O) solution (10 mL, 0.1%) was brought to a boil, then 1.3, 0.8, and 0.5 mL of 1% preheated sodium citrate solution were added respectively to the boiling solution. The reduction reaction was completed after 5 minutes, and the solution was then boiled for a further 30 minutes and left at room temperature. The spherical gold NPs of about 22.5, 29.3, and 36.1 nm size could be synthesized by this method. However, gold NPs of 4.4 nm size should be obtained by NaBH4. Subsequently, PEG2000-sulfhydryl (SH) (1 mg; Yare Biotech, Shanghai, People’s Republic of China) was added to the NP solutions and stirred for 1 hour to synthesize the PEG-coated gold NPs. Subsequently, excess PEG2000-SH molecules could be removed by ultrafiltration using 10 kDa molecular weight cutoff filters and washed several times with pure water.
Physical properties of materials
Optical absorption spectra in the wavelength range of 200–850 nm were measured with a DU800 Spectrophotometer (Beckman Coulter, Brea, CA, USA) in a 5 mL glass cuvette with 2.5 nm slit width. The zeta (ζ) potential of the gold NPs was determined with the Nano ZS Zetasizer particle analyzer (Malvern Instruments, Malvern, UK). Data were acquired in the phase-analysis light-scattering mode at 25°C, and sample solutions were prepared by diluting gold NPs into 10 mM phosphate-buffered saline solution (pH 7.4). For the transmission electron microscope (TEM) analysis, NPs were observed using a Hitachi (Tokyo, Japan) HF-2000 field-emission high-resolution TEM operating at 200 kV.
Animal administration
Animals were purchased, maintained, and handled with protocols approved by the Institute of Radiation Medicine, Chinese Academy of Medical Sciences. The C57 mice (30 female and male) were obtained from the institute’s laboratories at 11 weeks of age and were housed in a 12-hour/12-hour light/dark cycle, and were given food and water ad libitum. Thirty female (or 30 male) mice were randomly divided into five groups (six in each group): control group, and four experimental groups for treatment with differently sized PEG-coated gold NPs. The mice received an intraperitoneal injection of approximately 200 μL of gold NP solution at a dose of 4000 μg/kg, based on the previously identified toxic dose in vivo.16,29 The mice were weighed and assessed for behavior after injection every 2 days for 28 days.
Hematology, biochemistry, and sample collection
After 28 days of treatment, all mice were killed, and blood was collected for blood chemistry, biochemistry, and organ studies. Using a standard blood-collection technique, we drew 200 μL blood from the saphenous vein into a potassium ethylenediaminetetraacetic acid-collection tube for hematology analysis. Mice were killed using isoflurane anesthetic and angiocatheter exsanguination with phosphate-buffered saline. During necropsy, liver, kidneys, spleen, heart, lung, thymus, uterus, ovaries, and testis were collected and weighed. One female and male mouse from each group was fixed with 10% buffered formalin following phosphate-buffered saline exsanguination. To examine the immune response explicitly, spleen and thymus indexes (Sx) can be defined as:
Major organs from those mice were processed routinely into paraffin, and stained with hematoxylin and eosin. Pathology was examined using a digital microscope.
Results and discussion
TEM and spectrophotometer
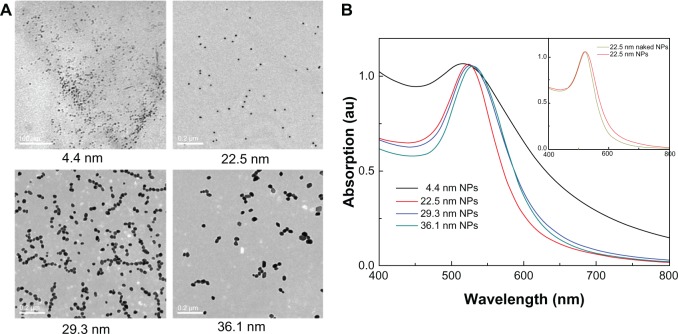
The TEM images and optical absorption of gold NPs can be seen in Figure 1. The average sizes of gold NPs were about 4.4, 22.5, 29.3, and 36.1 nm (Figure 1A), which is close to that of previous work.29 Significant optical absorption peaks, namely surface plasmon resonance (SPR), can be seen for all NPs in Figure 1B. The absorption center is located at 516, 522, 527, and 529 nm, corresponding to 4.4, 22.5, 29.3, and 36.1 nm gold NPs, respectively. The strong absorption peaks can be interpreted by the interaction between excitation light and metal NPs. The SPR can be reformulated by Mie’s classic theory.43 With increase in particle size, the SPR will shift to long-wavelength regions, which is consistent with the reported results.44 The PEG2000 coating is not macromolecules, thus it is not expected that the SPR will shift obviously. Actually, we found that PEG2000 only made a very tiny contribution to SPR shift. For example, the SPR of 22.5 nm naked gold NPs is at 519 nm, while the SPR of 22.5 nm PEG-coated gold NPs is located at 522 nm, which can be seen in Figure 1B. Besides, the surface chemical activity can be modified by adding the PEG coating. The ζ-potentials of 4.4, 22.5, 29.3, and 36.1 nm naked gold particles were −28.1, −16.23, −8.7, and −8.27 mV, respectively, due to citric acid coating and negative charge on the surface. The PEG-SH coating induced decreases of ζ-potentials to −6.01, −1.92, −1.89, and −0.98 mV, respectively. So the PEG-SH coating can decrease ζ-potentials and improve the monodispersity of gold NPs.
Figure 1.
(A) Transmission electron microscopy images of 4.4, 22.5, 29.3, and 36.1 nm nanoparticles (NPs). (B) Optical absorption of the polyethylene glycol-coated gold NPs.
Body weight and organ weights
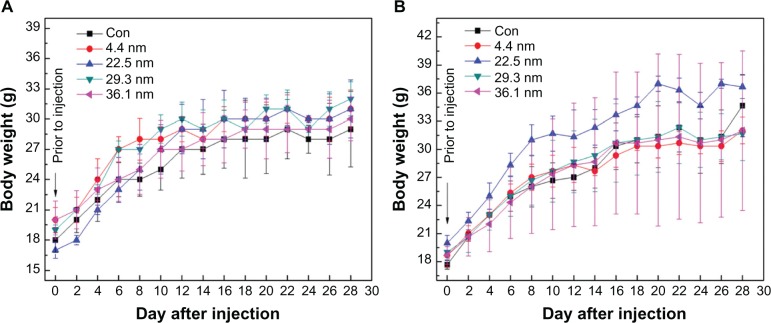
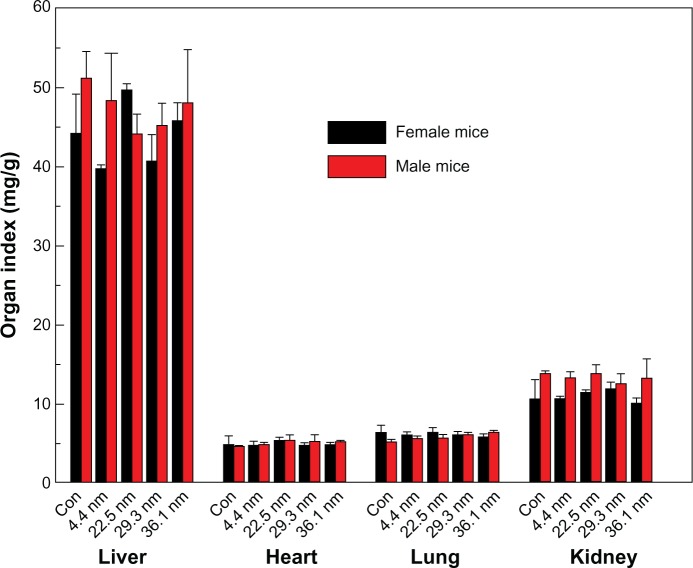
Figure 2 shows the variation in body weight of mice treated with gold NPs of different sizes. Figure 2A and B present the body weights of female and male mice over 28 days, respectively. The body weight of the mice was measured every 2 days. Each point represents the mean ± standard deviation of six mice. Data were analyzed using analysis of variance with SPSS 13.0 (IBM, Armonk, NY, USA), and the differences between the different groups and control group for each organ were not significant (P >0.05). Average body weight of control female mice was 29 g, while body weights of 4.4, 22.5, 29.3, and 36.1 nm NP-treated mice were about 31, 31, 32, and 30 g, respectively, at 28 days. Similar results can be found in male mice. It can be seen in Figure 2A that the body weight of mice treated with 22.5 nm gold NPs significantly changed, which also can be found in Figure 2B. Other-size NPs, such as 4.4, 29.3, and 36.1 nm, exhibited very small deviations. Generally, it was found that the standard deviation of male mice was larger than that of female mice. Furthermore, no abnormal clinical signs or behaviors were detected in the control or treated groups. Figure 3 gives the liver, heart, lung, and kidney indexes of mice treated with gold NPs of different sizes. It can be found that the weights of the heart, liver, spleen, lung, and kidneys showed no obvious variation at a dose of 4000 μg/kg. It is necessary to investigate the organ indexes for the spleen and thymus further.
Figure 2.
Body-weight changes in female mice (A) and male mice (B) for the 4.4, 22.5, 29.3, and 36.1 nm gold nanoparticles at a dose of 4000 μg/kg. The body weight of the treated mice was measured every 2 days. Each point represents the mean ± standard deviation of six mice. Data were analyzed using analysis of variance with SPSS 13.0, and the differences between the different groups and control (Con) group for each organ were not significant (P >0.05).
Figure 3.
Liver, heart, lung, and kidney indexes of female and male mice. Bars represent mean ± standard deviation. Data were analyzed using analysis of variance with SPSS 13.0, and the differences between the different groups and control (Con) group for each organ were not significant (P >0.05).
Immune response and pathology
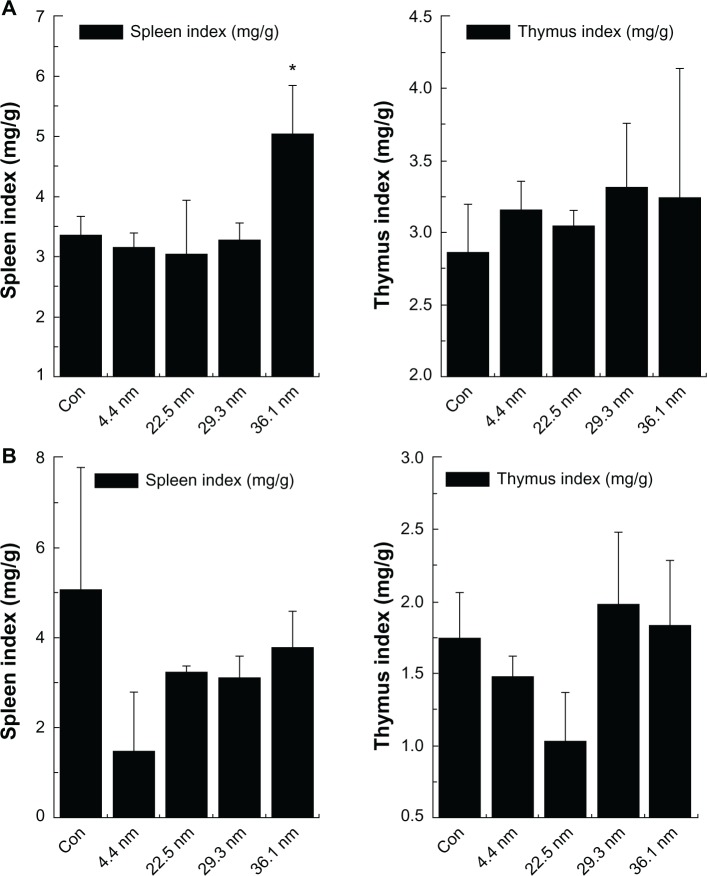
Figure 4 shows the immune response of mice treated by gold NPs based on spleen and thymus index. We calculate the spleen index and thymus index of female mice (Figure 4A) and male mice (Figure 4B) to examine immune system damage induced by the PEG-coated gold NPs in mice. For the female mice, the average values of thymus and spleen index in the control group were 2.7 and 3.3, respectively. In the NP groups, 4.4, 22.5, and 29.3 nm NPs did not induce significant changes, but 36.1 nm gold NP-treated female mice presented a sharp increase in spleen index. Generally speaking, the increase in spleen index indicates the immune response of mice, which has been widely reported elsewhere.45 For the treated male mice, the average values of thymus and spleen index in the control group were 1.7 and 5.1, respectively. The spleen index of 4.4, 22.5, 29.3, and 36.1 nm gold NP-treated male mice changed to 1.5, 3.3, 3.1, and 3.8, respectively. It can be seen that the spleen index of the male mice treated with 4.4 nm gold NPs decreased. For the treated male mice, the spleen and thymus index showed no significant changes (Figure 4B). As a contrast, for the female mice, the organ indexes of gold NP-treated groups had significant statistical differences when compared with the control group.
Figure 4.
Spleen index and thymus index of female mice (A) and male mice (B). Bars represent mean ± standard deviation. Data were analyzed using analysis of variance with SPSS 13.0, and the differences between the different groups and control (Con) group for each organ were not significant (P >0.05). * Represent significant differences from the control.
The indexes of spleen and thymus are important indicators for the immune system of mice.45 Changes in spleen and thymus index always indicate that the organism is assaulted by enthetic invasion, which will cause potential toxicological response. Besides, increases in other organ indexes infer that the organism is being protected, with the toxicity responses released. It is known that PEG-coated NPs prefer to accumulate in the spleen. In spite of no obvious statistical difference, the PEG-coated 36.1 nm NPs still caused a change in spleen index in female mice. These changes impelled us to explore more findings through hematology and biochemistry.
Hematology and biochemistry
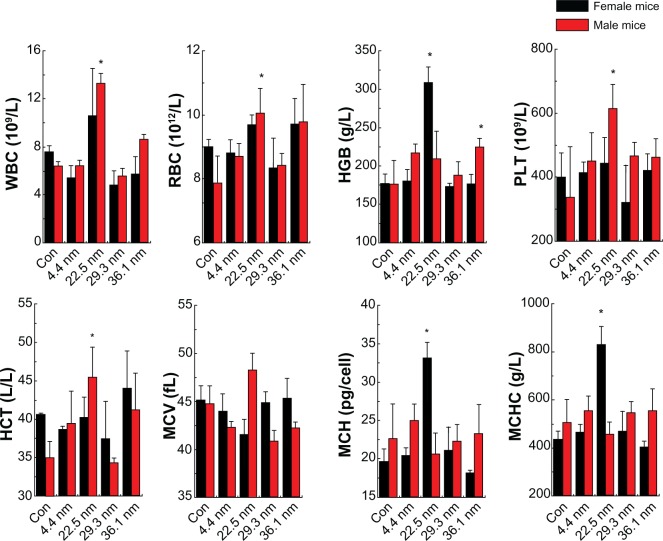
When NPs are intravenously administered, the first physiological system they interact with in vivo is the blood and blood components. Then they may induce inflammatory responses, which will increase or decrease the activity of the immune system, and alteration of hematology factors, such as white blood cells. Therefore, understanding the properties of hemocompatibility is an important step during the initial characterization process of NPs. Figure 5 shows the hematology analysis of female and male mice treated by differently sized NPs. We selected standard hematology markers for analysis, such as white blood cells (WBCs), red blood cells (RBCs), hematocrit (HCT), mean corpuscular volume (MCV), hemoglobin (HGB), platelets (PLTs), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). For the female mice, two important indicators, the WBC and RBC, did not show significant changes. The HGB of the mice treated with 22.5 nm gold NPs was as high as 309 g/l, while that of the control group was just 177 g/l. Meanwhile, the MCH and MCHC of the mice treated with 22.5 nm gold NPs also increased significantly. The MCH sharply increased from 20 to 33, and the MCHC rose from 420 g/l to 850 g/l. Other parameters such as PLT and HCT showed no significant difference. As expected, for the male mice, the results were different from the female mice. The WBC of the mice treated with 22.5 and 36.1 nm gold NPs showed significant statistical differences when compared with the control group. WBC increased to 3×109/L for 22.5 nm NP-treated mice, and 8.2 g/l for 36.1 nm NP-treated mice. Meanwhile, RBC, PLT, and HCT of the mice treated with 22.5 nm increased significantly. In particular, RBC significantly increased from 3×1012/L. Other parameters showed no significant differences. It is clearly shown that sex difference is notable for toxicological response.
Figure 5.
Hematology results of the nanoparticles, including the hematology results of the female and male mice. These results show means and standard deviation of white blood cells (WBC), red blood cells (RBC), hemoglobin (HGB), platelets (PLT), hematocrit (HCT), mean corpuscular volume (MCV), mean corpuscular hemoglobin (MCH), and mean corpuscular hemoglobin concentration (MCHC). Bars represent means ± standard deviation. Data were analyzed by Student’s t-test, and stars represent significant differences from the control (Con) group (P <0.05). * Represent significant differences from the control.
As is well known, WBC and RBC are the most important indicators for in vivo response, which are directly related to infection and inflammation. Male mice treated with 22.5 nm particles showed notable variations in WBC and RBC, which suggests that serious infections may have occurred. Meanwhile, the increase in WBC seen in male mice treated with 36.1 nm NPs may also have been connected to infections. In contrast, the gold NPs did not induce significant increases of RBC and WBC for female mice, which indicates that gold NPs cause more significant infections for male mice than female mice. The HGB, MCH, and MCHC of 22.5 nm NP-treated female mice and PLT and HCT of 22.5 nm NP-treated male mice showed significant differences when compared with the control, but WBC and RBC reach a stable level. Differently sized NPs can induce significant difference for in vivo toxicity, but generally male mice may suffer more serious toxicity than female mice. Besides, for size-dependent effects, it is clear that 22.5 nm gold NP-treated mice presented with significant infections, as shown by increased WBC and RBC levels. As a contrast, 4.4 and 29.3 nm gold NP-treated mice did not show any significant infections.
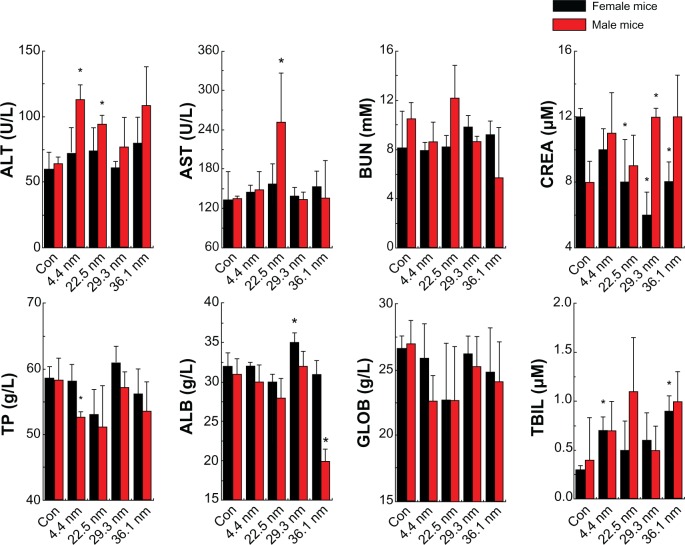
After the NPs leave the bloodstream, they will arrive at the liver and kidneys. Therefore, it is necessary to clarify/investigate whether or not the NPs can induce toxicity for these organs. By measuring a variety of biochemical factors in the blood serum, it is possible to assess liver-function failure, hepatocellular injury, and cholestasis. To confirm these results, we investigated the blood biochemistry index. We present the biochemistry results of the NPs in Figure 6, comprising alanine transaminase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN), creatinine (CREA), total protein (TP), albumin (ALB), globulin (GLOB), and total bilirubin (TBIL). For the female mice, in Figure 6 it is observed that the CREA of the mice treated with 22.5, 29.3, and 36.1 nm NPs significantly decreased to 8 μM, 5.8 μM, and 8 μM, respectively. Meanwhile, the ALB level of the mice treated with 29.3 nm NPs increased significantly from 32 g/L to 35 g/L. The TBIL level of mice treated with 4.4 and 36.1 nm NPs showed significant differences when compared with the control group. For the male mice, the ALT of the mice treated with 4.4, 22.5 and 36.1 nm NPs increased significantly to 115 U/L, 93 U/L, and 105 U/L, respectively. Meanwhile, the AST of the mice treated with 22.5 nm significantly increased from 130 U/L to 250 U/L. The CREA of the mice treated with 29.3 nm NPs also showed significant statistical differences, increasing from 8 μM to 12 μM, while the TP of the 4.4 nm group and ALB of the 36.1 nm group both decreased. These results clearly show significant liver and kidney toxicity for all these NPs.
Figure 6.
Biochemistry results of the nanoparticles, including the biochemistry results of the female and male mice. These results show means and standard deviations of alanine transaminase (ALT), aspartate transaminase (AST), blood urea nitrogen (BUN), creatinine (CREA), total protein (TP), albumin (ALB), globulin (GLOB), and total bilirubin (TBIL). Bars represent means ± standard deviation. Data were analyzed by Student’s t-test, and stars represent significant differences from the control (Con) group (P <0.05). * Represent significant differences from the control.
In blood biochemistry, ALT and AST are dominant indicators for liver toxicity. The former is specific for cellular damage in the liver, and the latter is nonspecific. Reduction of liver function and cholestasis can be assessed by the substances produced by liver, including ALB, GLOB, TP, and TBIL. The significant increase of ALT and AST found in mice probably indicates obvious liver toxicity. In our work, the NP-treated female mice did not show any increase of ALT and AST, but NP-treated male mice showed significant increase of ALT and AST, which clearly shows that male mice suffer more serious liver toxicity than female mice. Meanwhile, CREA is a crucial marker for kidney function. In the results, a sharp decrease of CREA can be found in female mice, which indicates their kidneys may have been damaged. It has been shown that large particles prefer to aggregate in the liver, while small particles can be partially metabolized by renal clearance. Thus, NPs accumulation in the kidneys can induce significant kidney damage. Meanwhile, the PEG-coated gold NPs can form 100 nm hybrid materials, which will gradually accumulate in the liver by blood circulation.16 For sex differences in toxicity, this is interesting. Many hormones have similar chemistry elements. Meanwhile, the amino group is a relatively active group and can absorb gold NPs through the surface by Au-NH2 covalent binding. Thus, it is most probably the fact that hormones from female mice can delay interaction between gold NPs and blood, and that gold NPs can be partially cleared by the kidneys, reducing liver damage. In contrast, change of hormone level for male mice is not significant, thus inducing the formation of larger NPs in the blood and finally accumulating in the liver, causing liver damage. All things considered, male mice will suffer more serious liver toxicity than female mice when treated with gold NPs.
Also, it is important to consider size-dependent effects. It can be seen that 4.4 nm gold NPs showed minimal liver and kidney toxicity, and no indicators showed any significant variations, except for AST, TBIL, and TP. The 22.5 nm gold NPs, however, caused significant change of ALT, AST, and CREA, indicating both liver and kidney damage. The 29.3 and 36.1 nm gold NP-treated mice showed significant kidney and slight liver toxicity. Many investigations27,29,30 about size-dependent toxicity have shown that small NPs prefer to accumulate in the kidneys and large NPs in the liver, but the actual behavior of NPs in blood is complex. Our work again confirms that PEG-coated NPs with sizes of 10–30 nm are still not sufficiently safe to use.
Finally, we investigated sex differences in reproductive system toxicity of gold NPs. The reproductive system is also an important indicator for sex differences. To investigate sex differences in toxicity, histological assessment of uterus, ovaries, and testis were performed to determine whether the gold NPs can cause tissue damage. It can be seen from Figure 7 that there were no apparent histopathological abnormalities related to those animals treated with gold NPs. The pathology result provides macroscopic and visual evidence of toxicity. It shows that the PEG-coated gold NPs did not bring about significant reproductive toxicity in mice. Sex differences have not induced the significant difference in reproductive organs.
Figure 7.
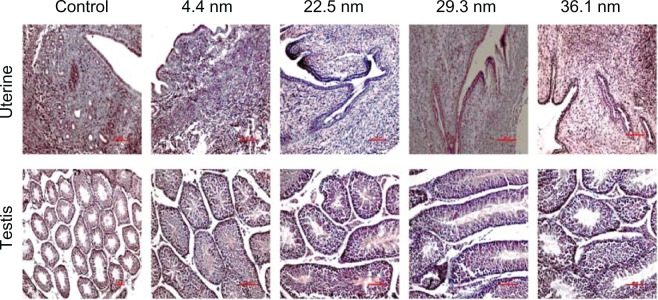
Pathological results from the uterus of female mice and testis of male mice after 28 days. Appreciable pathological changes were not found in the organs.
These results suggest that further metabolism of gold-based materials must be investigated. In vivo toxicity and biodistribution studies show that PEG-coated gold NPs are different from naked gold NPs. We found in previous experiments that 12.1 nm PEG-coated gold NPs had more efficient diffusion and absorption for tumors, so they were more efficient for targeting tumors.16 Nevertheless, naked gold NPs were strongly associated with essential blood proteins (such as albumin, fibrinogen, γ-globulin, histone, and insulin), and the degree of binding between NPs and proteins was increased with increasing size.46,47 We speculate that pharmacokinetics of PEG-coated NPs is closely related to the sex of mice. Further, these interactions, such as enhanced binding and conformational change of the proteins, may influence the sex differences in toxicity. Thus, further study is necessary to understand mechanisms of sex differences in toxicity of gold NPs. Meanwhile, it is not clear that these NPs in the liver or kidney will cause abnormal gene expression for male and female mice. Changes in key indicators such as ALT and AST are still not clear after 6 months. Therefore, there is still much room for subsequent work.
Conclusion
In summary, we carried out a study about sex differences in toxicity of gold NP-treated mice. Gold NP treatment of mice did not induce significant change in body weight. The gold NPs, however, caused significant increases in spleen and thymus indexes, which represented the activation of immune response, for female mice but not male mice. Further hematology results showed 22.5 and 36.1 nm gold NPs caused obvious increases of WBC and RBC in male mice, indicating infection occurred, but this was not significant in female mice. In further analysis, it was found that male mice also suffered significant liver toxicity, with ALT and AST significantly increased, and except for that, obvious kidney toxicity could be found in female mice. For size effects, 4.4 nm gold NPs presented low liver toxicity and minimal infection, but 22.5, 29.3, and 36.1 nm gold NPs showed significant liver and kidney toxicity, among which the toxicity of 22.5 nm gold NPs was the highest. Also, these NPs did not cause appreciable toxicity in reproductive systems 28 days after injection. Generally, male mice presented significant liver toxicity, and female mice presented high kidney toxicity. These findings will give us insights to sex differences in nanotoxicology.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant 81000668), the Natural Science Foundation of Tianjin (grant 13JCQNJC13500), the Subject Development Foundation of the Institute of Radiation Medicine, CAMS (grants SF1207 and SZ1336), and the Foundation of Union New Star, CAMS. Prof Sun would like to thank the National Natural Science Foundation of China (grant 30970867) for its support.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Elghanian R, Storhoff JJ, Mucic RC, Letsinger RL, Mirkin CA. Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles. Science. 1997;277:1078–1081. doi: 10.1126/science.277.5329.1078. [DOI] [PubMed] [Google Scholar]
- 2.Sokolov K, Follen M, Aaron J, et al. Real-time vital optical imaging of precancer using anti-epidermal growth factor receptor antibodies conjugated to gold nanoparticles. Cancer Res. 2003;63:1999–2004. [PubMed] [Google Scholar]
- 3.Murphy CJ, Gole AM, Stone JW, et al. Gold nanoparticles in biology: beyond toxicity to cellular imaging. Acc Chem Res. 2008;41:1721–1730. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 4.Huang X, El-Sayed IH, Qian W, El-Sayed MA. Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods. J Am Chem Soc. 2006;128:2115–2120. doi: 10.1021/ja057254a. [DOI] [PubMed] [Google Scholar]
- 5.Day ES, Bickford LR, Slater JH, Riggall NS, Drezek RA, West JL. Antibody-conjugated gold-gold sulfide nanoparticles as multifunctional agents for imaging and therapy of breast cancer. Int J Nanomedicine. 2010;5:445–454. doi: 10.2147/ijn.s10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ghosh P, Han G, De M, Kim CK, Rotello VM. Gold nanoparticles in delivery applications. Adv Drug Deliv Rev. 2008;60:1307–1315. doi: 10.1016/j.addr.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 7.Giljohann DA, Seferos DS, Daniel WL, Massich MD, Patel PC, Mirkin CA. Gold nanoparticles for biology and medicine. Angew Chem Int Ed Engl. 2010;49:3280–3294. doi: 10.1002/anie.200904359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eustis S, El-Sayed MA. Why gold nanoparticles are more precious than pretty gold: noble metal surface plasmon resonance and its enhancement of the radiative and nonradiative properties of nanocrystals of different shapes. Chem Soc Rev. 2006;35:209–217. doi: 10.1039/b514191e. [DOI] [PubMed] [Google Scholar]
- 9.Boisselier E, Astruc D. Gold nanoparticles in nanomedicine: preparations, imaging, diagnostics, therapies and toxicity. Chem Soc Rev. 2009;38:1759–1782. doi: 10.1039/b806051g. [DOI] [PubMed] [Google Scholar]
- 10.Hu M, Chen J, Li ZY, et al. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem Soc Rev. 2006;35:1084–1094. doi: 10.1039/b517615h. [DOI] [PubMed] [Google Scholar]
- 11.Wang S, Huang P, Nie L, et al. Single continuous wave laser induced photodynamic/plasmonic photothermal therapy using photosensitizer – functionalized gold nanostars. Adv Mater. 2013 Feb 13; doi: 10.1002/adma.201204623. Epub. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luo Z, Yuan X, Yu Y, et al. From the aggregation-induced emission of Au(I)-thiolate complexes to ultrabright Au(0)@Au(I)-thiolate core-shell nanoclusters. J Am Chem Soc. 2012;134:16662–16670. doi: 10.1021/ja306199p. [DOI] [PubMed] [Google Scholar]
- 13.Xie J, Zheng Y, Ying JY. Protein-directed synthesis of highly fluorescent gold nanoclusters. J Am Chem Soc. 2009;131:888–889. doi: 10.1021/ja806804u. [DOI] [PubMed] [Google Scholar]
- 14.Chithrani DB, Jelveh S, Jalali F, et al. Gold nanoparticles as radiation sensitizers in cancer therapy. Radiat Res. 2010;173:719–728. doi: 10.1667/RR1984.1. [DOI] [PubMed] [Google Scholar]
- 15.Hainfield JF, Slatkin DN, Smilowitz HM. The use of gold nanoparticles to enhance radiotherapy in mice. Phys Med Biol. 2004;49:N309–N315. doi: 10.1088/0031-9155/49/18/n03. [DOI] [PubMed] [Google Scholar]
- 16.Zhang XD, Wu D, Shen X, et al. Size-dependent radiosensitization of PEG-coated gold nanoparticles for cancer radiation therapy. Biomaterials. 2012;33:6408–6419. doi: 10.1016/j.biomaterials.2012.05.047. [DOI] [PubMed] [Google Scholar]
- 17.Zhang XD, Guo ML, Wu HY, et al. Irradiation stability and cytotoxicity of gold nanoparticles for radiotherapy. Int J Nanomedicine. 2009;4:165–173. doi: 10.2147/ijn.s6723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hauck TS, Anderson RE, Fischer HC, Newbigging S, Chan WC. In vivo quantum-dot toxicity assessment. Small. 2009;6:138–144. doi: 10.1002/smll.200900626. [DOI] [PubMed] [Google Scholar]
- 19.Connor EE, Mwamuka J, Gole A, Murphy CJ, Wyatt MD. Gold nanoparticles are taken up by human cells but do not cause acute cytotoxicity. Small. 2005;1:325–327. doi: 10.1002/smll.200400093. [DOI] [PubMed] [Google Scholar]
- 20.Chen H, Dorrigan A, Saad S, Hare DJ, Cortie MB, Valenzuela SM. In vivo study of spherical gold nanoparticles: inflammatory effects and distribution in mice. PLoS One. 2013;8:e58208. doi: 10.1371/journal.pone.0058208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Söderstjerna E, Johansson F, Klefbohm B, Englund Johansson U. Gold-and silver nanoparticles affect the growth characteristics of human embryonic neural precursor cells. PLoS One. 2013;8:e58211. doi: 10.1371/journal.pone.0058211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goodman CM, McCusker CD, Yilmaz T, Rotello VM. Toxicity of gold nanoparticles functionalized with cationic and anionic side chains. Bioconjug Chem. 2004;15:897–900. doi: 10.1021/bc049951i. [DOI] [PubMed] [Google Scholar]
- 23.Hauck TS, Ghazani AA, Chan WC. Assessing the effect of surface chemistry on gold nanorod uptake, toxicity, and gene expression in mammalian cells. Small. 2007;4:153–159. doi: 10.1002/smll.200700217. [DOI] [PubMed] [Google Scholar]
- 24.Zhang XD, Wu D, Shen X, Liu PX, Fan FY, Fan SJ. In vivo renal clearance, biodistribution, toxicity of gold nanoclusters. Biomaterials. 2012;33:4628–4638. doi: 10.1016/j.biomaterials.2012.03.020. [DOI] [PubMed] [Google Scholar]
- 25.Pan Y, Neuss S, Leifert A, et al. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–1949. doi: 10.1002/smll.200700378. [DOI] [PubMed] [Google Scholar]
- 26.Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 27.De Jong WH, Hagens WI, Krystek P, Burger MC, Sips AJ, Geertsma RE. Particle size-dependent organ distribution of gold nanoparticles after intravenous administration. Biomaterials. 2008;29:1912–1919. doi: 10.1016/j.biomaterials.2007.12.037. [DOI] [PubMed] [Google Scholar]
- 28.Cho WS, Cho M, Jeong J, et al. Acute toxicity and pharmacokinetics of 13 nm-sized PEG-coated gold nanoparticles. Toxicol Appl Pharmacol. 2009;236:16–24. doi: 10.1016/j.taap.2008.12.023. [DOI] [PubMed] [Google Scholar]
- 29.Zhang XD, Wu D, Shen X, et al. Size-dependent in vivo toxicity of PEG-coated gold nanoparticles. Int J Nanomedicine. 2011;6:2071–2081. doi: 10.2147/IJN.S21657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cho WS, Cho M, Jeong J, et al. Size-dependent tissue kinetics of PEG-coated gold nanoparticles. Toxicol Appl Pharmacol. 2010;245:116–123. doi: 10.1016/j.taap.2010.02.013. [DOI] [PubMed] [Google Scholar]
- 31.Zhang XD, Wu HY, Wu D, et al. Toxicologic effects of gold nanoparticles in vivo by different administration routes. Int J Nanomedicine. 2010;5:771–781. doi: 10.2147/IJN.S8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Niidome T, Yamagata M, Okamoto Y, et al. PEG-modified gold nanorods with a stealth character for in vivo applications. J Control Release. 2006;114:343–347. doi: 10.1016/j.jconrel.2006.06.017. [DOI] [PubMed] [Google Scholar]
- 33.Zhang G, Yang Z, Lu W, et al. Influence of anchoring ligands and particle size on the colloidal stability and in vivo biodistribution of polyethylene glycol-coated gold nanoparticles in tumor-xenografted mice. Biomaterials. 2009;30:1928–1936. doi: 10.1016/j.biomaterials.2008.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lipka J, Semmler-Behnke M, Sperling RA, et al. Biodistribution of PEG-modified gold nanoparticles following intratracheal instillation and intravenous injection. Biomaterials. 2010;31:6574–6581. doi: 10.1016/j.biomaterials.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 35.von Maltzahn G, Park J-H, Agrawal A, et al. Computationally guided photothermal tumor therapy using long-circulating gold nanorod antennas. Cancer Res. 2009;69:3892–3900. doi: 10.1158/0008-5472.CAN-08-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xiao K, Li Y, Luo J, et al. The effect of surface charge on in vivo biodistribution of PEG-oligocholic acid based micellar nanoparticles. Biomaterials. 2011;32:3435–3446. doi: 10.1016/j.biomaterials.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balasubramanian SK, Jittiwat J, Manikandan J, Ong CN, Yu LE, Ong WY. Biodistribution of gold nanoparticles and gene expression changes in the liver and spleen after intravenous administration in rats. Biomaterials. 2010;31:2034–2042. doi: 10.1016/j.biomaterials.2009.11.079. [DOI] [PubMed] [Google Scholar]
- 38.Beierle I, Meibohm B, Derendorf H. Gender differences in pharmacokinetics and pharmacodynamics. Int J Clin Pharmacol Ther. 1999;37:529–547. [PubMed] [Google Scholar]
- 39.Gleiter C, Gundert-Remy U. Gender differences in pharmacokinetics. Eur J Drug Metab Pharmacokinet. 1996;21:123–128. doi: 10.1007/BF03190260. [DOI] [PubMed] [Google Scholar]
- 40.Kim WY, Kim J, Park JD, Ryu HY, Yu IJ. Histological study of gender differences in accumulation of silver nanoparticles in kidneys of Fischer 344 rats. J Toxicol Environ Health A. 2009;72:1279–1284. doi: 10.1080/15287390903212287. [DOI] [PubMed] [Google Scholar]
- 41.Kim YS, Kim JS, Cho HS, et al. Twenty-eight-day oral toxicity, genotoxicity, and gender-related tissue distribution of silver nanoparticles in Sprague-Dawley rats. Inhal Toxicol. 2008;20:575–583. doi: 10.1080/08958370701874663. [DOI] [PubMed] [Google Scholar]
- 42.Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75. [Google Scholar]
- 43.Mie G. A contribution to the optics of turbid media, especially colloidal metallic suspensions. 1908;2:377–445. [Google Scholar]
- 44.Wu D, Zhang XD, Liu PX, Zhang LA, Fan FY, Guo ML. Gold nanostructure: fabrication, surface modification, targeting imaging, and enhanced radiotherapy. Curr Nanosci. 2011;7:110–118. [Google Scholar]
- 45.Chen Z, Meng H, Xing G, et al. Acute toxicological effects of copper nanoparticles in vivo. Toxicol Lett. 2006;163:109–120. doi: 10.1016/j.toxlet.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 46.Lacerda SHDP, Park JJ, Meuse C, et al. Interaction of gold nanoparticles with common human blood proteins. ACS Nano. 2009;4:365–379. doi: 10.1021/nn9011187. [DOI] [PubMed] [Google Scholar]
- 47.Dobrovolskaia MA, Patri AK, Zheng J, et al. Interaction of colloidal gold nanoparticles with human blood: effects on particle size and analysis of plasma protein binding profiles. Nanomedicine. 2009;5:106–117. doi: 10.1016/j.nano.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]