Abstract
Effects of varenicline (Champix), a nicotinic partial agonist, were evaluated on subjective effects of nicotine (drug discrimination), motivation for nicotine taking (progressive-ratio schedule of intravenous nicotine self-administration) and reinstatement (cue-induced reinstatement of previously extinguished nicotine-seeking behavior). Effects on motor performance were assessed in rats trained to discriminate nicotine (0.4 mg/kg) from saline under a fixed-ratio (FR10) schedule of food delivery and in rats trained to respond for food under a progressive-ratio schedule. At short pretreatment times (5–40 min), varenicline produced full or high levels of partial generalization to nicotine’s discriminative-stimulus effects and disrupted responding for food, while there were low levels of partial generalization and no disruption of responding for food at 2- or 4-hour pretreatment times. Varenicline (1 and 3 mg/kg, 2-hour pretreatment time) enhanced discrimination of low doses of nicotine and to a small extent decreased discrimination of the training dose of nicotine. It also dose-dependently decreased nicotine-taking behavior, but had no effect on food-taking behavior under progressive-ratio schedules. Finally, varenicline significantly reduced the ability of a nicotine-associated cue to reinstate extinguished nicotine-seeking behavior. The ability of varenicline to reduce both nicotine-taking and nicotine-seeking behavior can contribute to its relatively high efficacy in treating human smokers.
Keywords: Varenicline, Nicotine, Progressive ratio schedule, Drug discrimination, Cue-induced reinstatement
INTRODUCTION
Smoking is currently responsible for the death of one in ten adults’ worldwide (about 5 million deaths each year). Half the people that smoke today -that is about 650 million people- will eventually be killed by tobacco (Tobacco Advisory Group of the Royal College of Physicians, 2000) and this morbidity and mortality can be mostly avoided if subjects stop smoking (Department of Health and Human Services, 1990). Several pharmacological treatments (nicotine replacement, bupropion and varenicline) have demonstrated their efficacy as treatments for smoking cessation (Le Foll and George, 2007). However, despite treatment a majority of subjects will relapse and there is the need to develop novel and more effective medications. The availability of drugs that have demonstrated their utility in humans allows testing the predictive value of the animal models that are used (Lerman et al., 2007). There is a strong need to test those drugs in the various animals used in this field to validate the preclinical approaches (Lerman et al., 2007).
Varenicline is a novel medication that is available for the treatment of smokers. (Cahill et al., 2007). It is a α4β2 subtype nicotinic receptor partial agonist that has actions on other nicotinic receptor subunits (Rollema et al., 2007). Varenicline appears more effective than nicotine-replacement therapy and bupropion to help smokers quit smoking (Cahill et al., 2007). The effects of varenicline have been evaluated in various animal models. First, it has been reported that varenicline administered 15 min prior to the session significantly decreased nicotine self-administration under a 5-response fixed-ratio (FR5) schedule of reinforcement in rats (Rollema et al., 2007). In rats trained to self-administer nicotine under a progressive-ratio (PR) schedule, varenicline maintained lower break-points than nicotine when it was tested by substitution (Rollema et al., 2007). In rats trained to discriminate nicotine from saline, varenicline produced full generalization in one study when administered 5 min before the session (Rollema et al., 2007) and partial generalization in another study when administered 25 min before the session (LeSage et al., 2009). Varenicline has been reported to be effective in blocking nicotine-induced reinstatement of drug-seeking behavior in rats when administered 15 min prior to the session (O’Connor et al., 2010). Although varenicline also reduced cue-reactivity in human smokers (Franklin et al., In Press), it was not effective in reducing cue-induced reinstatement of drug-seeking behavior in rats (O’Connor et al., 2010). However, since this study used a short pretreatment time (15 min), the agonist properties of varenicline may have interfered with its ability to reduce cue-induced reinstatement. In a more recent study, varenicline tended to decrease cue-induced reinstatement of nicotine-seeking behavior when administered at a 30 min pretreatment time, although effects did not reach statistical significance (Wouda et al., In Press).
To further investigate this issue, we first assessed the influence of pretreatment time on FR responding for food, the ability of varenicline to generalize to a nicotine cue and its effects of nicotine dose-response curve in a drug-discrimination paradigm in rats. We then evaluated the effects of varenicline on nicotine self-administration maintained under a PR schedule and the effects of varenicline on cue-induced reinstatement of nicotine-seeking behavior. Control experiments were performed in rats trained to respond for food.
MATERIALS AND METHODS
Animals
Male Long Evans rats (Charles River, Lachine, PQ, Canada) for the food and nicotine self-administration and progressive-ratio experiments were experimentally naive at the start of the study. They initially weighed 250 to 275 g. Rats were individually housed in a temperature- and humidity-controlled room on a 12-h reverse light/dark cycle (lights off from 07:00 to 19:00 h). Experiments were conducted during the dark phase. Prior to any experimental manipulation, animals were given a minimum of seven days to habituate to the colony room, during which they were weighed and handled.
For the nicotine discrimination study, male Sprague Dawley rats (Charles River, Wilmington, MA, USA) initially weighing 250 to 275 g were used. They were housed individually in a temperature- and humidity-controlled room on a regular 12-h light/dark cycle (lights on from 07.00 to 19:00 h). Experiments were conducted during the light phase. For all experiments, water was available ad libitum and a diet restriction was maintained throughout the studies (~20g/day).
All the experimental procedures described in this report were carried out in compliance with the guidelines of the Canadian Council on Animal Care and corresponding NIH guidelines, and were reviewed and approved by the institutional Animal Care Committee. All efforts were made to minimize animal suffering, and to reduce the number of animals required. Use of a repeated-measures design contributed to the latter.
Techniques for initial training and surgery were similar to those previously reported (Corrigall and Coen, 1989; Corrigall et al., 2001). Animals were trained to press a lever on a schedule in which each press resulted in the delivery of a 45 mg food pellet (continuous reinforcement, CRF, no associated cues). Once trained, each animal was surgically prepared with a chronic intravenous catheter implanted in the jugular vein; the catheters exited between the scapulae. Surgery was performed under anesthesia induced by xylazine (10 mg/kg), given intraperitoneally (IP) and ketamine hydrochloride (75 mg/kg, IP). Incision sites were infiltrated with the local anesthetic bupivacaine (0.125%). Buprenorphine was given for post-operative analgesia (0.01 mg/kg), given subcutaneously (SC), and a single dose of penicillin (30,000 units, IM) was administered before surgical procedures. Animals were allowed to recover for a 1-week period before drug SA sessions were begun.
Drugs
(−)Nicotine hydrogen tartrate (Sigma-Aldrich, St Louis, Mo., USA) was dissolved in saline, the pH was adjusted to 7.0 (±0.2), and the solution was filtered through a 0.22 mm syringe filter (Fisher Scientific, Pittsburgh, Pa., USA) for sterilization purposes. All nicotine doses are reported as free base concentrations. Nicotine was administered intravenously (IV) in a volume of 100 μl/kg/injection or SC in a volume of 1 ml/kg. Varenicline (gift from Pfizer, USA) was diluted in saline, the pH was adjusted to 7.0 (±0.2) and was administered IP in a volume of 1 ml/kg body weight.
Acquisition of the nicotine or food self-administration behavior
Self-administration sessions were carried out in experimental chambers equipped with two levers (Med Associates, St. Albans, Vt., USA). Session start was signaled by the illumination of a house-light; extinction of this light indicated the time-out (TO) period. Rapid delivery of the self-administered drug (approximately 1-s delivery time) was achieved with Med Associates Model PHM-104 pumps. Unit doses were 100 μl/kg; volume adjustments were used to accommodate inter-animal or between-session differences in body weight. Responding on one of the levers resulted in drug delivery when schedule requirements were met, while responding on the other lever was recorded but did not produce any change of lights or drug infusion (active levers are counterbalanced). Self-administration sessions occurred mostly 5 days a week.
In this study, rats acquired nicotine self-administration under a FR schedule of reinforcement and the unit dose was 30 μg/kg per infusion of nicotine base. Session duration was 60 min, and the TO period (turning off of the house light and illumination of a cue light above the active lever) following each infusion was 1 min. During the first five days of acquisition, each lever press during the time-in period resulted in the delivery of an infusion (FR-1), then the response requirement was increased to FR2 for 3 days and then to the final value of FR-5 (i.e., animals were required to make five lever presses for each drug infusion) for 5 days.
The apparatus, the stimuli associated with food delivery and the schedule of the acquisition were exactly the same as described above. The rats received a food pellet (45 mg precision pellets, BioServ) instead of a nicotine injection.
Testing under the progressive-ratio (PR) schedule
After training under FR5, the animals were switched to a PR schedule where the response requirement increased with each successive injection or food pellet delivery. The response requirement progression was based on the formula 5e(0.25*[inj. number + 3]))−5, with the first two values replaced by 5 and 10 (modified from (Roberts, 1992)). Thus, the response requirements for successive injections were 5, 10, 17, 24, 32, 42, 56, 73, 95, 124, 161, 208, etc. The break point (BP) was defined as the highest ratio completed prior to the first 30 min period without a response on the active lever in both nicotine- and food-SA.
Sessions under the PR schedule lasted a maximum of 4 h. The animals were allowed 10–15 days depending upon time of stabilization of nicotine or food self administration on the PR schedule before testing with an IP injection of varenicline or vehicle. A counterbalanced within-subject design was employed for the testing. The baseline condition represented a vehicle (saline) injection given 2 h prior to session start, as was done for varenicline injections. Rats in the nicotine self-administration experiments were also tested in a session where saline was substituted for nicotine (a saline-substitution test conducted after all doses of varenicline were tested on the PR schedule). Testing for catheter patency was conducted at multiple time points throughout the PR schedule portion of the experiment and subjects analyzed in the results section exclude rats with non-patent catheters.
Cue-induced reinstatement of nicotine seeking
After the rats were tested for the effects of varenicline on nicotine self-administration (PR), an additional few nicotine self-administration sessions were conducted without any treatment and the self-administration behavior was then extinguished. During the extinction phase rats were placed into the self-administration chamber with the houselight illuminated and responses on the active or inactive lever were recorded, but had no consequences. The criterion for extinction was less than 20 active lever presses per 1-h session over two consecutive days. After stable extinction, these rats were tested for the effects of varenicline (0.3 – 3 mg/kg) on cue-induced reinstatement of nicotine-seeking behavior in a counterbalanced within-subject design. Reinstatement tests were conducted under conditions identical to those of self-administration sessions, except that 1) a single presentation of the cues (light above the active lever on and house-light off for 1 min) was delivered response-independently immediately at the start of the session and 2) responses on the active lever (on an FR5 schedule) resulted in contingent presentation of the cues) without nicotine availability (no injections). Responses on the inactive lever were recorded but had no programmed consequence. The testing sessions lasted 1 h.
Nicotine discrimination procedure
Rats acquired food-maintained behavior as described previously (Justinova et al., 2009; Le Foll et al., 2005; Le Foll et al., 2008). Under a discrete-trial schedule of food-pellet delivery, they learned to respond on one lever after an injection of a training dose of 0.4 mg/kg nicotine and on the other lever after an injection of 1 ml/kg of saline vehicle (n= 10). Injections of nicotine or saline were given subcutaneously 10 min before the start of the session. At the start of the session, a white house light was turned on and in its presence the rats were required to make ten consecutive responses (FR 10 schedule of food delivery) on the lever appropriate to the pre-session treatment. The completion of ten consecutive responses on the correct lever produced the delivery of a 45 mg food pellet and initiated a 45-s time-out period during which lever-press responses had no programmed consequences and the chamber was dark. Responses on the incorrect lever had no programmed consequences other than to reset the FR requirement on the correct lever. After each time-out period, the house light was again turned on and the next trial began. Each session ended after the completion of 20 FR trials or after 30 min elapsed, whichever occurred first. Discrimination-training sessions were conducted 5 days per week under a double alternation schedule (i.e. DDSSDDSS etc., D = drug; S = saline). Training continued until there were eight consecutive sessions during which rats completed at least 90% of their responses during the session on the correct lever and no more than four responses occurred on the incorrect lever during the first trial. Test sessions with other doses and other drugs were then initiated.
During the test sessions, a range of doses of varenicline were substituted for the training dose of nicotine. The influence of different pretreatment time was evaluated at the following pretreatment times: 5, 10, 20, 40, 120, and 240 min before the session. Varenicline, given 120 min before the session, was also administered together with nicotine (given 10 min before the session). Test sessions were identical to training sessions, with the exception that both levers were active and ten consecutive responses on either one of the two levers resulted in the delivery of a food pellet. Switching responses from one lever to the other lever reset the FR requirement to 10. In a test phase, a single alternation schedule was introduced and test sessions were usually conducted on Tuesdays and Fridays. Thus, a 2-week sequence starting on Monday was: DTSDTSTDST (T = test). In this way, test sessions occurred with equal probability after saline and drug sessions. Test sessions were conducted only if the criterion of 90% accuracy and not more than 4 incorrect responses during the first trial was maintained in two preceding training sessions.
For the drug-discrimination studies, two independent measures of behavior were collected: a measure of discrimination performance expressed as the percentage of nicotine-associated responses and a measure of motor performance expressed as response rate. The percentage of nicotine-associated responses during each session (training or test) reflected the percentage of the number of responses emitted on the nicotine-associated lever relative to the total number of responses emitted on both levers during a session. The percentage of nicotine-associated responses was individually calculated for each rat and then expressed as a group mean (±SEM). Nicotine-associated lever selection data were excluded from analysis if a rat emitted fewer than ten responses during the test session. Full generalization to the nicotine cue was defined as a percentage of responding on the nicotine-associated lever of 80% or higher. No generalization to the nicotine cue was defined as a percentage of responding on the nicotine-associated lever of 20% or lower. Partial generalization to the nicotine cue was defined as a percentage of responding on the nicotine-associated lever ranging from >20% to <80%. Response rates (responses/s) during each session were calculated by dividing the total number of responses emitted on both levers during a session by the total session length. Response rates were individually calculated for each rat and then expressed as group means (±SEM)
Data analysis
For drug-discrimination studies, results were subjected to two way ANOVAs followed by LSD post-hoc tests. For drug self-administration studies, repeated measures ANOVAs, followed when appropriate by post hoc Dunnett’s tests for comparisons with the baseline (BL) condition (the BL value was the mean of the values the day before each test session with an injection with the appropriate vehicle) for self-administration studies under the PR schedule of reinforcement; and by post hoc Newman-Keuls tests for multiple comparisons for studies on reinstatement of nicotine-seeking behavior. Changes were considered significant when p<0.05.
RESULTS
Influence of pretreatment time on response rates and ability of varenicline to generalize to the nicotine cue
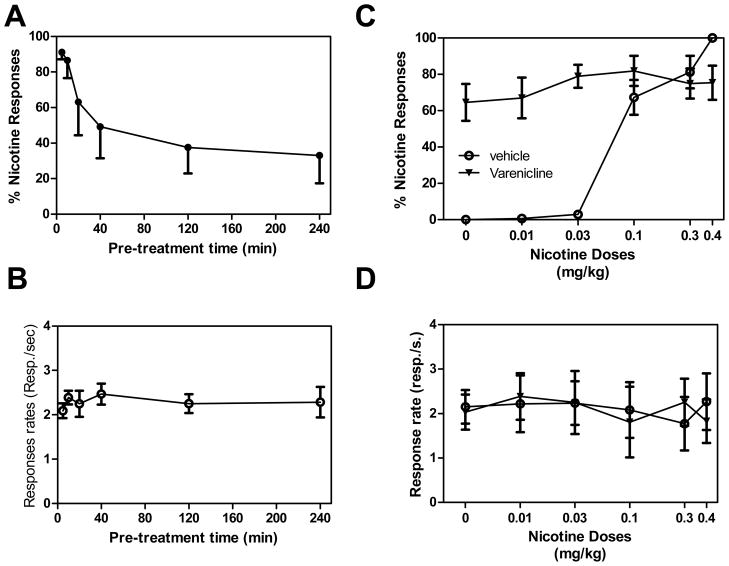
Two-way ANOVA performed on the response-rates of the rats indicated that there was a significant effect of varenicline dose (F1,88=68.5, P<0.0001), a significant effect of pretreatment time (F5,88=7.5, P<0.0001) and a significant interaction between varenicline dose and pretreatment time (F5,88=7.3, P<0.0001; see Fig. 1 b). Post-hoc analysis indicated that there was no disruption of response rates in rats receiving 1 mg/kg varenicline (all P>0.38). In contrast, 3 mg/kg had dramatic effects on the ability of the rats to respond. Rats receiving varenicline 5 and 10 min (P<0.0001 for both) and 20 min (P<0.01) before the session had a significant disruption of responding as compared to rats receiving varenicline 120 or 240 min before the sessions.
Figure 1. Significant impact of pretreatment time on ability of varenicline to generalize to nicotine cue and effect on nicotine discrimination.
A,B Effects of pretreatment time on ability of 1 and 3 mg/kg varenicline to generalize to the nicotine cue. Varenicline was given alone in rats previously trained to discriminate 0.4 mg/kg nicotine from saline. Varenicline, at 1 mg/kg, produced full generalization to the 0.4 mg/kg nicotine cue at short pretreatment times, but this effect decreased at longer pretreatment times (A). There was no effect of pretreatment time on response rates at the 1 mg/kg dose of varenicline, but the 3 mg/kg dose markedly depressed responding at shorter pretreatment times (B). C,D: 1 and 3 mg/kg varenicline administered 2 h before the session potentiated the discriminative stimulus effects of a low dose of nicotine (C), while having no effect on response rates of the rats (D). The percentage of responses on the lever associated with nicotine administration is shown as a function of dose during test sessions (upper panels), and response rates are expressed as responses per second averaged over the session (bottom panels). Data are means ± SEM (*P<0.05).
Two-way ANOVA performed on the percentage of lever presses performed on the nicotine-associated lever indicated no significant effect of varenicline dose (F1,88=0.003, P=0.9), a significant effect of pretreatment time (F5,88=3.7, P<0.01) and no significant interaction between varenicline dose and pretreatment time (F5,88=0.8, P=0.6). Rats receiving 1 mg/kg varenicline 5 min prior to the session had full generalization, whereas rats administered at 10, 20, 40, 120 and 240 min before the test session displayed partial generalization (see Fig. 1 a). The ability of 1 mg/kg varenicline to generalize for nicotine cue was significantly lowest in rats receiving varenicline 120 min (P=0.007) and 240 min (P=0.003) before the session as compared to 5 min pretreatment time. The ability of 3 mg/kg varenicline to generalize for nicotine cue was significantly lower in rats receiving varenicline 240 min (P=0.02) before the session as compared to 5 min pretreatment time.
Effect of varenicline administered 2 h before the session on nicotine discrimination
Varenicline administered in combination with various doses of nicotine or alone did not significantly modify the rate of responding by the rats when administered 120 min before the session. Two-WAY ANOVAs performed on response rates indicated that there was no significant effect of varenicline (F2,152=1.7, P=0.18), no significant effect of nicotine dose (F5,152=0.6, P=0.7) and no significant interaction between varenicline and nicotine (F10,152=0.7, P=0.7; Fig. 1 d).
Varenicline given 120 min before the session, followed by various doses of nicotine given immediately prior to the session, significantly modified nicotine discrimination performance of the rats. Two-WAY ANOVAs performed on percentage of responses on the nicotine lever indicated a significant effect of varenicline (F2,152=12.9, P<0.0001), a significant effect of nicotine dose (F5,152=8.8, P<0.0001) and a significant interaction between varenicline and nicotine (F10,152=4.4, P<0.0001)(Fig. 1 c). Post-hoc analysis indicated that in rats pretreated with 1 mg/kg or 3 mg/kg varenicline, followed by administration of 0.1 – 0.4 mg/kg doses of nicotine there was no significant change in discrimination performance, compared to pretreatment with vehicle (all P>0.55 and P>0.26 for 1 and 3 mg/kg varenicline, respectively). Although, the discrimination of the training dose of nicotine (0.4 mg/kg) seemed to be antagonized to a small extent by varenicline pretreatment, but the effect did not reach statistical significance.. Discrimination performance with lower nicotine dose 0.03 mg/kg was enhanced after pretreatment with 1 and 3 mg/kg varenicline, compared to vehicle treated animals (all P<0.01), but this was not the case with the lowest dose of nicotine (0.01 mg/kg), where the effect of varenicline pretreatment was only additive.
Effects of varenicline (0.3, 1 and 3 mg/kg IP, 2-h pretreatment time) on motivation for nicotine assessed by the progressive-ratio schedule
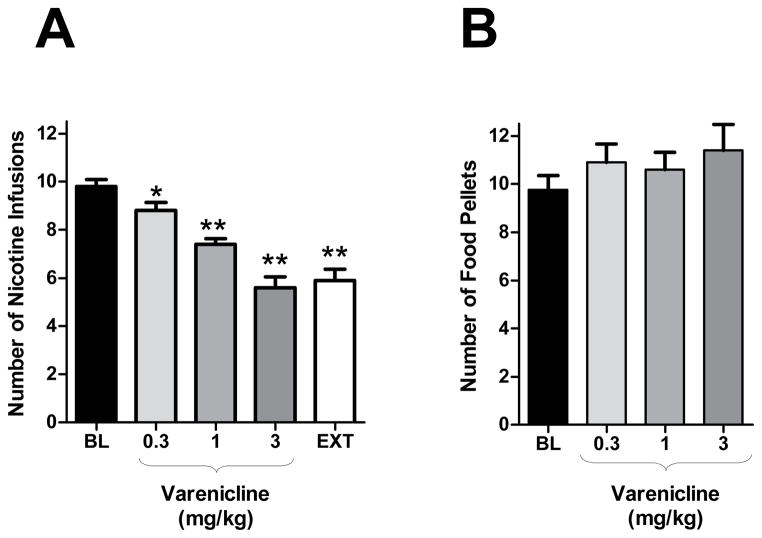
The repeated measure ANOVA performed on the number of nicotine injections received by the rats (n = 10) showed a main effect of treatment (F4,36 = 44.2, p<0.0001). Pair wise comparisons with the baseline (BL) level indicated that pretreatment with 0.3 mg/kg varenicline (2-h pretreatment time) slightly, but significantly, decreased motivation for nicotine (P=0.0459) and that higher doses of varenicline had much stronger effects (P<0.0001). There was no significant difference between number of injections in rats receiving 3 mg/kg varenicline + nicotine or in the rats for which nicotine was substituted by saline (p>0.05) (Fig. 2 a).
Figure 2. Effects of varenicline on nicotine-taking and food-taking behaviour under the progressive-ratio (PR) schedule.
Effects of pretreatment with varenicline (0 – 3 mg/kg, IP, 120 min before the session) on nicotine (A, n = 10) or food (B, n = 10) self-administration under PR schedule. Varenicline produced a dose dependent decrease in the number of self-administered nicotine infusions (A) but not in the number of self-administered food pellets (B). Data is expressed as means (± SEM) of the number of nicotine infusions or food pellets earned during the sessions. *P<0.05, ***P<0.01 vs. vehicle pretreatment (Dunnett’s tests after significant ANOVA for repeated measures)
The repeated measures ANOVA performed on the inactive lever responding showed a main effect of treatment (F4,36 = 9.1, p<0.0001). The means±SEM for BL, 0.3, 1.0, 3.0 mg/kg and saline substitution were 22.34±4.284, 22.42±5.086, 26.45±7.901 16.58±3.938 and 61.80±7.402, respectively. Multiple comparisons showed that the saline substitution resulted in significantly higher inactive lever responding compared to all other treatments (p<0.0001). However, no dose of varenicline produced any significant difference in inactive lever responding when compared with BL.
Effects of varenicline (0.3, 1 and 3 mg/kg IP, 2-h pretreatment time) on motivation for food assessed by the progressive-ratio schedule
The repeated measure ANOVA performed on the number of food pellets that the rats (n = 10) received following varenicline or vehicle administration (2 hr pretreatment time) showed no main effect of treatment (F3,27 = 1.7, p>0.05) (Fig. 2 b).
The repeated measures ANOVA performed on inactive lever responding showed no effect of treatment (F4,27 = 0.71, p>0.05). The means±SEM for BL, 0.3, 1.0, and 3.0 mg/kg were 19.10± 4.621, 27.90± 8.410, 58.80± 39.34, 25.30± 8.204..
Effects of varenicline (0.3, 1 and 3 mg/kg IP, 120 min pretreatment time) on cue-induced reinstatement of nicotine seeking behavior
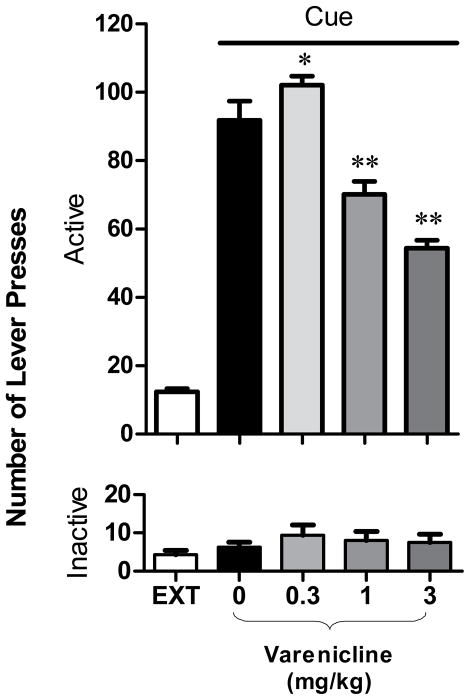
The ANOVAs performed on active and inactive lever presses of rats (n = 8) indicated a main effect of lever type (F1,56=770, p<0.0001), of varenicline (F4,56=94.3, p<0.0001) and a significant interaction between lever type and varenicline treatment (F4,56=79.8, p<0.0001; Fig. 3). The post-hoc analysis showed that cue presentation elicited a significant increase of the active (p=0.0002), but not the inactive (p=0.6) lever presses, under the vehicle condition in comparison to extinction responding. Pretreatment with 0.3 mg/kg varenicline significantly increased the cue-induced reinstatement (p=0.008) as compared with vehicle, whereas pretreatment with 1 and 3 mg/kg varenicline significantly decreased the reinstatement (both p=0.0001). In addition, no significant changes in responding on the inactive lever was observed under all conditions (all p>0.6; mean number of inactive lever presses ± SEM under the various testing conditions fluctuated between 6.3 ± 1.3 and 9.4 ± 2.7).
Figure 3. Effects of varenicline on reinstatement of nicotine-seeking behavior induced by presentation of nicotine-associated cues.
Effects of pretreatment with varenicline (0.3 – 3 mg/kg, IP, 2 h before the session) on cue-induced reinstatement of nicotine-seeking (n = 8). A significant reinstatement of nicotine-seeking was found by cues alone (P<0.01, Newman-Keuls tests for multiple comparions after significant ANOVA for repeated measures). A low 0.3 mg/kg dose of varenicline significantly increased, whereas higher 1 and 3 mg/kg doses of varenicline significantly decreased cue-induced reinstatement (* p<0.001, ** p<0.0001).
DISCUSSION
This is the first study to evaluate the impact of pretreatment time on discriminative-stimulus effects induced by varenicline; the first to evaluate the effect of varenicline on motivation to respond for nicotine under a progressive-ratio schedule of reinforcement; and the first to report a significant decrease in the ability of nicotine-associated cues to induce nicotine-seeking behavior following varenicline administration.
In the present study, varenicline produced full or high levels of partial generalization to the nicotine cue when administered at a very short pretreatment times (5, 10, and 20 min). At later time-points, varenicline produced lower levels of partial generalization. This data is consistent with previous findings by Rollema et al. (2007) where a pretreatment time of 5 min was used. Interestingly, another study reporting a lesser ability of varenicline to generalize to nicotine was performed using a longer pretreatment time of 25 min (LeSage et al., 2009). However, since those two studies did not directly evaluate the impact of pretreatment time, our study is the first to reveal that this aspect is important.
In the present experiments, nicotine (30μg/kg/infusion) supported self-administration under a progressive-ratio schedule in Long-Evans rats at levels comparable to previously reported studies (Donny et al., 1999; Forget et al., 2010a; Forget et al., 2010b). Consistent with previous findings obtained using FR schedules (George et al., 2011; O’Connor et al., 2010), varenicline in the present study dose-dependently reduced the ability of nicotine to maintain self-administration behavior. Interestingly, the effect of varenicline was of the same magnitude as the effect of substituting saline for nicotine, indicating that the reinforcing efficacy of nicotine was fully reversed by varenicline. Moreover, in our study, varenicline did not affect responding for food under the same schedule of reinforcement. Some investigators have reported that varenicline, administered 15 min prior to the session, can disrupt responding for food (O’Connor et al., 2010), while others have reported that varenicline increases responding for food or sucrose when administered either 15 or 30 min prior to the session (Rollema et al., 2007; Wouda et al., In Press). Our study indicates that pretreatment time is a critical factor that can influence the effects of varenicline on rates of responding (see Fig. 1). Other factors could have some influence under specific conditions, for example, use of naïve animals, different route of administration, etc. Also, it should be noted that Sprague-Dawley rats were used for the drug-discrimination experiments and Long Evans rats for the food and nicotine self-administration experiments, respectively. Although, using those two strains allowed us to be consistent with our previously published work, we cannot exclude the possibility that the pretreatment-time effect may not be similar in the two rodent species. In addition, further parametric studies varying the unit dose of varenicline and of nicotine on different addictive models could be of value to clarify the interactions between nicotine and varenicline. Those parametric studies could explain why low doses of varenicline increased cue-induced nicotine seeking, while higher doses of varenicline decreased cue-induced nicotine seeking.
The major finding of the present experiments is that varenicline significantly reduces cue-induced reinstatement of nicotine seeking (Fig. 3). These findings are consistent with previous studies in humans indicating that varenicline can reduce the effects of cue presentation on brain activity in human subjects (Franklin et al., In Press). In contrast, no significant effects of varenicline on cue-induced reinstatement have previously been reported in rats, though a trend toward significant attenuation was recently reported when varenicline was administered 30 min prior to cue-induced reinstatement (Wouda et al., In Press). We believe that the long 2-h pretreatment time used here could explain the different effects found in our present study and previous studies.
One limitation of the current study is the use of only one drug. Since varenicline has potentially other targets than alpha4beta2 nicotinic receptors, we cannot directly implicate a particular nicotinic subunit in these effects (Rollema et al., 2007). However, this was not the goal of the present experiments, which were performed to explore the effects of an established treatment compound, varenicline, in animal models that will be used to screen for future medications for treatment of nicotine dependence. It would be of value to expand the present findings to other models such as intracranial self-stimulation and withdrawal, which could reflect other aspects of the addiction cycle (Lerman et al., 2007). Some of the biochemical results reported with varenicline indicates that varenicline can produce both agonist and antagonistic effects depending on the way it is administered (Rollema et al., 2009). Here, varenicline administered without nicotine produced partial generalization to the nicotine cue in the drug-discrimination paradigm, suggesting some agonistic effects. In contrast, when administered in the drug-reinstatement paradigm (in the absence of nicotine), varenicline reduced nicotine-seeking. This effect could be mediated through the agonistic properties of varenicline, although we did not perform experiments to explore this hypothesis directly. When administered in combination with nicotine, varenicline decreased the motivation to self-administer nicotine under the progressive-ratio nicotine self-administration paradigm and displayed a partial agonist profile in the drug-discrimination paradigm, where it enhanced discrimination of low doses of nicotine and, to a small extent, attenuated discrimination of the training dose of nicotine (although this was not statistically significant). These findings suggest that the antagonistic properties of varenicline can be demonstrated under certain conditions.
In conclusion, the present study explored time-course of effects of varenicline pretreatment and showed that the pretreatment time significantly affected generalization of varenicline to a nicotine cue and, as well, as affected rates of responding for food in a drug-discrimination setting. Thus, varenicline pretreatment time should be considered an important variable under different experimental conditions. Moreover, the present findings support and extend previous findings that varenicline is able to act on several aspects of nicotine dependence. Animals pretreated with varenicline displayed decreased motivation for nicotine as assessed by a progressive-ratio drug self-administration procedure. In addition, varenicline caused the presentation of nicotine-associated stimuli to be less effective at reinstating nicotine-seeking behavior. These results support the use of nicotine-taking and nicotine-seeking paradigms as screens for evaluating future medications for nicotine dependence treatment.
Acknowledgments
The varenicline utilized in this study was a gift from Pfizer. This research was supported in part by the Intramural Research Program of the National Institute on Drug Abuse, NIH, DHHS, Baltimore, Maryland, USA. We thank Dr. Zuzana Justinova for her help with the revision of this manuscript.
Footnotes
STATEMENT OF INTEREST
Dr Bernard Le Foll has received speaking fees and research grants from Pfizer.
References
- Cahill K, Stead LF, Lancaster T. Nicotine receptor partial agonists for smoking cessation. Cochrane Database of Systematic Reviews. 2007:CD006103. doi: 10.1002/14651858.CD006103.pub2. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology (Berlin) 1989;99:473–478. doi: 10.1007/BF00589894. [DOI] [PubMed] [Google Scholar]
- Corrigall WA, Coen KM, Zhang J, Adamson KL. GABA mechanisms in the pedunculopontine tegmental nucleus influence particular aspects of nicotine self-administration selectively in the rat. Psychopharmacology (Berlin) 2001;158:190–197. doi: 10.1007/s002130100869. [DOI] [PubMed] [Google Scholar]
- Department of Health and Human Services. DHHS publication n 90–8416. Washington, D.C: Government Printing Office; 1990. The health benefits of smoking cessation: a report of the Surgeon General. [Google Scholar]
- Donny EC, Caggiula AR, Mielke MM, Booth S, et al. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology (Berl) 1999;147:135–142. doi: 10.1007/s002130051153. [DOI] [PubMed] [Google Scholar]
- Forget B, Pushparaj A, Le Foll B. Granular insular cortex inactivation as a novel therapeutic strategy for nicotine addiction. Biological Psychiatry. 2010a;68:265–271. doi: 10.1016/j.biopsych.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Forget B, Wertheim C, Mascia P, Pushparaj A, et al. Noradrenergic alpha1 receptors as a novel target for the treatment of nicotine addiction. Neuropsychopharmacology. 2010b;35:1751–1760. doi: 10.1038/npp.2010.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin T, Wang Z, Suh JJ, Hazan R, et al. Effects of Varenicline on Smoking Cue-Triggered Neural and Craving Responses. Archives of General Psychiatry. doi: 10.1001/archgenpsychiatry.2010.190. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George O, Lloyd A, Carroll FI, Damaj MI, et al. Varenicline blocks nicotine intake in rats with extended access to nicotine self-administration. Psychopharmacology (Berlin) 2011;213:715–722. doi: 10.1007/s00213-010-2024-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justinova Z, Ferré S, Barnes C, Wertheim CE, et al. Effects of chronic caffeine exposure on the involvement of adenosine A1 and A2A receptors in the discriminative-stimulus effects of nicotine, methamphetamine and cocaine in rats. Psychopharmacology (Berlin) 2009;203:355–367. doi: 10.1007/s00213-008-1270-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, George TP. Treatment of tobacco dependence: integrating recent progress into practice. Canadian Medical Association Journal. 2007;177:1373–1380. doi: 10.1503/cmaj.070627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Foll B, Sokoloff P, Stark H, Goldberg SR. Dopamine D3 ligands block nicotine-induced conditioned place preferences through a mechanism that does not involve discriminative-stimulus or antidepressant-like effects. Neuropsychopharmacology. 2005;30:720–730. doi: 10.1038/sj.npp.1300622. [DOI] [PubMed] [Google Scholar]
- Le Foll B, Wertheim CE, Goldberg SR. Effects of baclofen on conditioned rewarding and discriminative stimulus effects of nicotine in rats. Neuroscience Letters. 2008;443:236–240. doi: 10.1016/j.neulet.2008.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerman C, Lesage MG, Perkins KA, SOMS, et al. Translational research in medication development for nicotine dependence. Nature Reviews Drug Discovery. 2007;6:746–762. doi: 10.1038/nrd2361. [DOI] [PubMed] [Google Scholar]
- LeSage MG, Shelley D, Ross JT, Carroll FI, et al. Effects of the nicotinic receptor partial agonists varenicline and cytisine on the discriminative stimulus effects of nicotine in rats. Pharmacology, Biochemistry, and Behavior. 2009;91:461–467. doi: 10.1016/j.pbb.2008.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor EC, Parker D, Rollema H, Mead AN. The alpha4beta2 nicotinic acetylcholine-receptor partial agonist varenicline inhibits both nicotine self-administration following repeated dosing and reinstatement of nicotine seeking in rats. Psychopharmacology (Berlin) 2010;208:365–376. doi: 10.1007/s00213-009-1739-5. [DOI] [PubMed] [Google Scholar]
- Roberts DS. Self-administration of stimulants and serotonergic systems. NIDA Research Monograph. 1992;119:136–140. [PubMed] [Google Scholar]
- Rollema H, Chambers LK, Coe JW, Glowa J, et al. Pharmacological profile of the alpha4beta2 nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- Rollema H, Hajos M, Seymour PA, Kozak R, et al. Preclinical pharmacology of the alpha4beta2 nAChR partial agonist varenicline related to effects on reward, mood and cognition. Biochemical Pharmacology. 2009;78:813–824. doi: 10.1016/j.bcp.2009.05.033. [DOI] [PubMed] [Google Scholar]
- Tobacco Advisory Group of the Royal College of Physicians. Nicotine addiction in Britain: A report of the Tobacco Advisory Group of the Royal College of Physicians. London: Royal College of Physicians of London; 2000. [Google Scholar]
- Wouda JA, Riga D, De Vries W, Stegeman M, et al. Varenicline attenuates cue-induced relapse to alcohol, but not nicotine seeking, while reducing inhibitory response control. Psychopharmacology (Berlin) doi: 10.1007/s00213-011-2213-8. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]