Abstract
Targeting co-stimulatory molecules to modulate the immune response has been shown to have useful therapeutic effects for autoimmune diseases. Among the co-stimulatory molecules, CD2 and CD58 are very important in the early stages of generation of an immune response. Our goal was to utilize CD2-derived peptides to modulate protein-protein interactions between CD2 and CD58, thereby modulating the immune response. Several peptides were designed based on the structure of the CD58 binding domain of CD2 protein. Among the CD2-derived peptides, peptide 6 from the F and C β-strand region of CD2 protein exhibited inhibition of cell-cell adhesion in the nanomolar concentration range. Peptide 6 was evaluated for its ability to bind to CD58 in Caco-2 cells and to CD48 in T cells from rodents. A molecular model was proposed for binding a peptide to CD58 and CD48 using docking studies. Furthermore, in vivo studies were carried out to evaluate the therapeutic ability of the peptide to modulate the immune response in the collagen-induced arthritis (CIA) mouse model. In vivo studies indicated that peptide 6 was able to suppress the progression of CIA. Evaluation of the antigenicity of peptides in CIA and transgenic animal models indicated that this peptide is not immunogenic.
Keywords: CD2, CD58, collagen-induced arthritis, cyclic peptide, docking, immune response, protein-protein interaction
Introduction
Many biological therapeutic agents that have gained momentum for treating autoimmune diseases are focused on targeting T cell-mediated immune response. In the T cell-mediated immune response process, co-stimulatory molecules are very attractive targets for treating inflammatory and autoimmune diseases. Different strategies have been developed to block co-stimulatory signals. These include the use of antibodies, recombinant proteins, peptides, and small molecules. Among the co-stimulatory molecules, CD2-CD58 is very important in the early stages of immune response. To generate an immune response, T-cell receptors (TCR) engage with the antigenic peptide: major histocompatibility complex (pMHC) on the surface of antigen-presenting cells (APC), constituting the first signal (Signal 1) (1). The co-stimulatory signal (Signal-2) is delivered by cell adhesion molecules, including CD2-CD58, LFA-1-ICAM-1 (CD11a-CD18-CD54), and CD28-B7 (CD28-CD80) (2–6). Co-stimulatory/adhesion molecules have important roles in the tight adhesion of T cells to APC. Studies related to kinetics of interaction of TCR-pMHC indicated that the affinity of TCR for pMHC is on the order of 10−4–10−6 M (2). In the presence of TCR-pMHC interaction (Signal-1), blocking of one or more of the pairs of co-stimulatory signals will result in prevention of the primary immune response (7, 8). CD2 is a transmembrane protein in T cells that binds to its ligand CD58 on APC in humans and on CD48 in rodents. Protein-protein interaction between CD2 and CD58 helps to enhance T cell-APC adhesion and thus promotes T-cell activation. In addition to its adhesion function, CD2 has also been suggested to be involved in signaling (2–4). Blocking of the CD2-CD58 interaction leads to immunosuppression in both model systems and humans, indicating its importance for the cellular immune response. Alefacept, a recombinant human CD58-Ig fusion protein that effectively binds to CD2 and prevents its interaction with CD58 expressed on APC, has been shown to be efficacious in the treatment of moderate-to-severe chronic plaque psoriasis in adult patients with psoriatic arthritis (PsA) (6, 9, 10) .
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disease characterized by infiltration by T cells and inflammation of the synovium (11, 12). A recent study has shown an association between the high expression of CD2/CD58 molecules and RA (13). CD58 expression is known to be upregulated in inflammatory lesions; this leads to targeted recruitment of T cells into inflammatory sites (11, 12, 14, 15). CD58 is widely distributed among cell types of the synovial microenvironment and provides numerous cell types with which lymphocytes can interact via CD2 molecule. It is proposed that the onset of autoimmunity is associated with CD58 upregulation and ligation of CD2 on dendritic cells; subsequent autocrine release of IL-1β increases the release of IL-12 and, in turn, activates T cells (16). CD2-CD58 interactions facilitate T cell-APC contact at close proximity. These interactions result in induction of IFN-γ and subsequent regulation of HLA-DR, ICAM-1, and B-7 molecules on APC, resulting in amplification of the signal required for generation of an immune response. Furthermore, CD2 is densely expressed on memory T cells, making it an important target for understanding the immune response during transplantation(5, 8, 9, 17–19) and in autoimmune diseases.
Our goal was to utilize CD2-derived peptides to modify protein-protein interaction between CD2 and CD58 and modulate the immune response. The peptides were designed based on the structural epitope of the CD58 binding domain of CD2 protein. We have used peptides from the CD2 protein based on the structural epitope of the CD58 binding domain of CD2 for modulating immune response (20–25). In our earlier work, we have shown that the peptides designed from CD2 protein were able to inhibit cell adhesion between Caco-2 cells and T cells at low nanomolar concentration (26). In this paper, we describe the in vivo activity of a peptide (peptide 6) in frequently studied mouse model of rheumatoid arthritis, collagen-induced arthritis (CIA). In vivo studies suggested that a conformationally constrained peptide designed from CD2 was able to suppress the progression of CIA in a therapeutic protocol in DBA/1 mice. We further evaluated the ability of peptide 6 to modulate antigen-specific immune response in a human RA-relevant animal model by utilizing CIA-susceptible HLA-class II transgenic mice. Our in vitro data shows that this peptide binds to CD58-bearing Caco-2 cells as well as to CD48 (homologous to CD58 in humans) from rodents. A molecular model is proposed for binding peptide 6 to CD58 and CD48 using docking studies. Modulation of the interaction between CD2-CD58 could cause suppression of cellular and humoral immune responses, leading to a therapeutic effect that is clinically significant for autoimmune diseases such as RA.
Experimental Methods
Peptides
Synthesis of the control peptide (Table 1) was carried out by solid-phase peptide synthesis using a peptide synthesizer (Tribute, Protein Technologies, Inc. Tucson, AZ) at Louisiana State University (LSU) peptide synthesis facility. Cyclic peptide 6 and fluorescently labeled peptide were custom synthesized by New England Peptide LLC (Gardner, MA, USA). The purity of the products was analyzed by HPLC, electrospray mass spectrometry (ESI-MS), and high resolution mass spectrometry (HR-MS). Analysis of the peptides for purity by HPLC indicated >90% pure peptides and correct molecular ion.
Table 1.
Structures of peptide P6 and control peptide. For comparison, similar sequences from mouse and rat CD2 are also shown.
| Code | Sequence | Origin |
|---|---|---|
| P6 | Cyclo (1,10)S1I2Y3D4(D)P5P6D7D8I9K10 | Human |
| P61 | Cyclo(1,10) M1V2Y3G4 (D) P5P6D7E8V9R10 | Mouse |
| P62 | Cyclo(1,10) T1V2Y3S4 (D) P5P6D7E8V9R10 | Rat |
| Control | S1A2V3K4A5G6K7I8T9K10D11I12 | Human |
(D)P5 refers to D amino acid proline
Cell lines/cells
The human colon adenocarcinoma cell line (Caco-2) and the T-leukemia Jurkat cell line were obtained from the American Type Culture Collection (Rockville, MD, USA). Caco-2 cells were maintained in minimum essential medium-α containing 20% FBS, 1% nonessential amino acids, 1 mM Na-pyruvate, 1% L-glutamine, and 100 mg/L of penicillin/streptomycin. T cells were maintained in RPMI1640 (Gibco/BRL, Bethesda, MD) supplemented with 10% FBS, 2 mM L-glutamine, 100 mg/L of penicillin/streptomycin, and 5 mg of bovine insulin in 500 mL medium.
Competitive binding studies
The competitive binding of peptide 6 with FITC-labeled antibody CD58 was evaluated using flow cytometry. Caco-2 cells were suspended in PBS at a density of 1 × 106 cells/100 µL in 1.5 mL Eppendorf tubes. 50 µL of FITC-labeled anti-CD58 was added and used as a positive control. In another set of Eppendorf tubes, Caco-2 cells were added with 50 µL of FITC-labeled anti-CD58, 100 µL of peptide 6 at different concentrations was added and incubated for 30 min at 4 °C. After the incubation, the cells were spun down at 720 × g at room temperature for 10 min and resuspended in 4–5 mL of PBS. The solution was then transferred into flow cytometry tubes on ice and covered with aluminum foil. The samples were analyzed using a BD FACS flow cytometer. Fluorescence from antibody/peptide was detected using excitation λ at 485 nm and emission λ at 528 nm. The shift in the number of cells with or without FITC was observed. The negative control was Caco-2 cells without FITC-anti-CD58. Caco-2 cells simultaneously treated with the peptide at various concentrations and with FITC-labeled anti-CD58 were analyzed using flow cytometry. Competitive binding was also analyzed by fluorescence plate reader assay. Approximately 1 × 104 Caco-2 cells were coated on 96-well plates. After the cells achieved confluence, they were incubated with FITC-AbCD58 (pre-diluted, 20 µL) and/or peptide 6 for 1 h. After washing three times, medium was added and fluorescence was read using a microplate reader at excitation λ 485 nm and emission λ 528 nm. Data were obtained from triplicate experiments. Fluorescence from the blank was subtracted for the final representation.
Binding of fluorescently-labeled peptide 6 to Caco-2 cells bearing CD58 in the presence of unlabeled anti-CD58
Peptide 6 was labeled with fluorescent 5-carboxyfluorescein (5FAM), and its competitive binding was evaluated with anti-CD58 on Caco-2 cells expressing CD58 protein using a BD FACS flow cytometer. Experimental details were similar to those explained above. Cells were first incubated with unlabeled antibody and then with fluorescently labeled peptide 6.
Peptides from CD2 bind to mouse cells bearing CD48
Rodents express CD48, a homolog of CD58 (27). To evaluate the binding of peptide 6 to CD48, T cells from the spleens of DBA/1 mice (Harlan Laboratories, Indianapolis, IN, USA) were utilized. The spleen of a mouse was harvested and crushed for single cell suspension. Cells were centrifuged, the supernatant portion was discarded, and T cells were isolated from the spleen using established procedures (28). The expression level of CD48 on mouse T cells was evaluated using FITC-anti-CD48. The competitive binding of peptide 6 with FITC-labeled antibody CD48 was evaluated using flow cytometry. T cells were suspended in PBS at a density of 1 × 106 cells/100 µL in 1.5 mL along with 50 µL of FITC-labeled anti-CD48 and used as a control. In another set of experiments, isolated T cells were incubated with 50 µL of FITC-labeled anti-CD48 for 30 min at 4 °C, after which different concentrations of peptide P6 (50, 100, 200 µM) were added followed by an incubation period of 30 min at 4 °C. After the incubations, the cells were spun down at 720 × g at room temperature for 10 min and resuspended in 4–5 mL of PBS. The samples were analyzed using a BD FACS flowcytometer.
Docking
Docking of peptide 6 to CD58/CD48 protein was performed using AUTODOCK software(29) . Crystal structures for CD58 (PDBID: 1QA9) (30) and CD48 (PDB ID: 2PTT)(31) were obtained from the Protein Databank (32). Solvent molecules were removed and polar hydrogen atoms were added to the structure. A grid box with dimensions of 128×128×128 Å3 was used for calculations covering residues 22–32 and the CD2 binding surface of CD58 protein. In earlier publications we described the three-dimensional structure of peptide 6 using NMR and molecular dynamics simulations (26). From the family of NMR structures, a representative structure of peptide 6 was chosen for docking calculations. The structure of peptide 6 was saved as a mol2 file and converted to pdbqt files in Autodock using Autodock tools. Peptide 6 was made flexible for docking calculations using the autotorsions option in the Autodock tools software. Docking calculations were performed in two stages. First, a trial was carried out with 10 runs and 250,000 energy evaluations. In the second stage, 100 runs with 10 million evaluations were carried out using a Lamarkian genetic algorithm for docking. Docking calculations were performed on a Linux cluster on high-performance supercomputers at LSU Baton Rouge via Louisiana Optical Network Initiative (LONI). Docked structures were listed in increasing order of energy, and low energy clusters were used as the most probable binding models. Docking calculations were repeated three times by changing the total number of runs and keeping all other docking parameters constant. Structures from low energy docking were displayed and analyzed using PyMol software (Schrodinger LLC, Portland OR).
In vivo studies
Female DBA/1 mice were obtained from Harlan Labs for in vivo studies. Mice were divided into seven groups with eight mice in each group (control, arthritic mice, arthritic mice treated with control peptide, arthritic mice treated with peptide 6 at concentrations of 1 mg/kg, 0.5 mg/kg and 0.25 mg/kg.), and vehicle control (PBS). Arthritis was induced in mice by intradermal injection of type II collagen formulation (CII) emulsified in Freund’s complete adjuvant (CFA) following published procedures (33–35). One hundred microliters of the emulsion containing 3 mg/mL of CII was injected intradermally at the base of the tail for the first immunization. 21 days post immunization, a booster injection of CII emulsified with incomplete Freund’s adjuvant (IFA) was given (33, 34). Peptide 6 and control peptide were dissolved in PBS and injected by intravenous route in the tail vein beginning on day 22. Peptide 6 was administered on alternate days for a total of 5 injections. The dosage of peptide 6 was 0.25, 0.5 and 1 mg/kg in 100 µL total volume of PBS. Similar injections were done with control peptide (Table 1) at 1 mg/kg dose. All mice were monitored beginning on day 21 for onset and severity of arthritis. Clinical signs of CIA were assessed by two different methods: 1) scoring of visual appearance of the mouse limb and 2) histopathology of the joints. Wrist and ankle joints were inspected on alternate days beginning on the 21st day post-immunization. The visual appearance of the limbs was graded on a scale of 0–4 (33, 34- 37) where 0 = no arthritis, 1 = paws with swelling of 1 joint (wrist/ankle or digit), 2 = swelling of 2 joints or more, 3 = swelling of all joints, and 4 = ankylosed joints. The scores were determined for all 4 paws. A maximal score of 4 can be reached per paw, resulting in a maximum total score of 16 per mouse. Statistical differences among the groups for disease onset and severity were determined compared to the control peptide by Mann Whitney Test and one-way Anova analysis using GraphPad Prism (La Jolla, CA). For analysis, data from 40 to 46 days were compared. P values <0.05 were considered significant. Analysis was also done within the treatment groups to compare the effect of different doses of peptide 6. The mice were sacrificed on day 57. Histopathology for disease severity was done on hind limbs in 4 mice from each group as published (37). Hind limbs were fixed in 10% formalin and decalcified in EDTA for 60 days. Decalcification was verified by adding oxalic acid and observing the precipitate. Sections were stained with haematoxylin and eosin (H & E). At the end of the experiment, sera from all mice were collected and used for collagen antibody analysis. In vivo experiments were repeated twice. Data shown are for one set of experiments.
T-cell proliferation assay
DBA-1 mice were immunized with 100 µg of native type II collagen emulsified with Complete Freund’s adjuvant 12 days prior to the T-cell proliferation assay. Naïve mice without type II collagen injection were used to generate APCs. Spleen cells isolated from the immunized and control mice (mice without administration of collagen) were suspended in Dulbecco’s Minimum Essential medium (DMEM) supplemented with 1% homologus normal serum, penicillin-streptomycin, and 2-mercaptoethanol. A prewashed nylon-wool column was used to specifically filter T cells. Cells were washed 3 times with DMEM and then loaded onto the column inserted in a 10 mL syringe, which was then incubated for 30 min in a CO2 incubator at 37 °C. The unbound cells were eluted with 20 mL DMEM and then centrifuged at 116 × g for 8 min. Approximately 4 × 106 cells were collected and suspended in DMEM. Spleens of normal mice were collected in a similar manner. 0.1 mL (4 × 106 cells) enriched T-cells, 0.1 mL (1 × 107 cells) of APC, and 10–20 µL of 1 mg/mL arthrogen-CIA T-cell grade type II collagen (Chondrex, Inc. Redmond, WA) were seeded in 96-well flat bottom plates and incubated at 37 °C for 3 days in a CO2 incubator. After 3 days, peptide 6 was added at 1 and 0.25 µM concentrations. Inhibition studies were done by adding anti-CD4 and anti CD8-antibodies (100 µM) to culture wells. Concanavalin (Con A) (20 µg/mL) was used as a positive control while medium containing only cells was used as a negative control. Plates were incubated for 45 min in a CO2 incubator at 37°C. This was followed by the final step of reading the fluorescence using a CellTiter-Glo® (CTG) assay with a plate reader. Similar experiments were performed using control peptide (Table 1) (36, 38). Proliferation was represented as relative fluorescence intensity and compared with controls.
The immunogenicity of peptide 6 was also determined using human leukocyte antigen (HLA) transgenic mice expressing the HLA-DQA1*0301, DQB1*0302 (DQ8) gene but lacking all four chains of mouse endogenous class II molecules, DQ8.AE° mice. Generation of HLA-DQ8 mice has been described previously (39, 40). HLA-DQ8 mice are susceptible to develop collagen-induced arthritis. Mice were immunized with 200 µM of peptide 6 and a control CII-derived peptide (200 µg) known to be non-immunogenic in DQ8 mice, as published (41). Ten days post-immunization, lymph node cells (LNCs) were harvested and cultured in vitro. LNCs (1 × 106) were cultured in HEPES-buffered RPMI 1640 containing 5% heat-inactivated horse serum and antibiotics streptomycin and penicillin in 96-well flat bottom tissue culture plates. Cells were challenged by adding 100 µL of RPMI medium (negative control), Con A (20 µg/mL, positive control) and varying concentrations of peptide 6 and control peptide. The cells were incubated for 48 h at 37 °C. During the last 18 h, the cells were pulsed with 3H-thymidine (1 µCi/well). At the end of the assay, the cells were harvested using a plate harvester, and incorporated radioactivity was determined using an automated counter (Microbeta, Perkin Elmer Wallac). The results are depicted as delta cpm.
Measurement of anti-collagen Ab levels
At the termination of the study, blood samples were collected through tail vein puncture from all the mice. The serum was separated by centrifugation at 824×g and was used for determining anti-collagen Ab titers in various groups of mice. Immulon II plates ( Dynex technologies, VA) were coated with bovine type II collagen (1 µg/mL, 0.1 mL/well) in carbonate/bicarbonate buffer and kept overnight at 4 °C. The following day the plates were washed with PBS containing 0.05% Tween-20 and blocked with 1% BSA for 30 minutes. Serum samples were diluted 50-fold in 1% BSA followed by threefold serial dilutions. 50 µL of the diluted samples were added into the well in triplicate and incubated for 2 h at room temperature followed by washing with PBS containing 0.05% Tween-20. Bound anti-collagen Abs were determined using HRP-labeled goat anti-mouse IgG (H+L chain specific, Southern Biotechnology Associates, Birmingham AL, USA) at 100 µL/well (1:5000) and incubated for 2 h at room temperature. The plates were washed again with PBS containing 0.05% Tween-20, and tetramethyl benzidine (TMB) (Kirkegaard and Perry Labs, Gaithersburg, MD, USA) (100 µL/well) was added to each well. Color was allowed to develop for 15 min before stopping the reaction with 1 M sulfuric acid. Absorbance was read at 450 nm with background subtraction at 630 nm (42). Readings were taken in triplicate and the experiment was repeated twice.
Results
Peptide 6 competitively binds to Caco-2 cells bearing CD58 in the presence of FITC-labeled anti-CD58
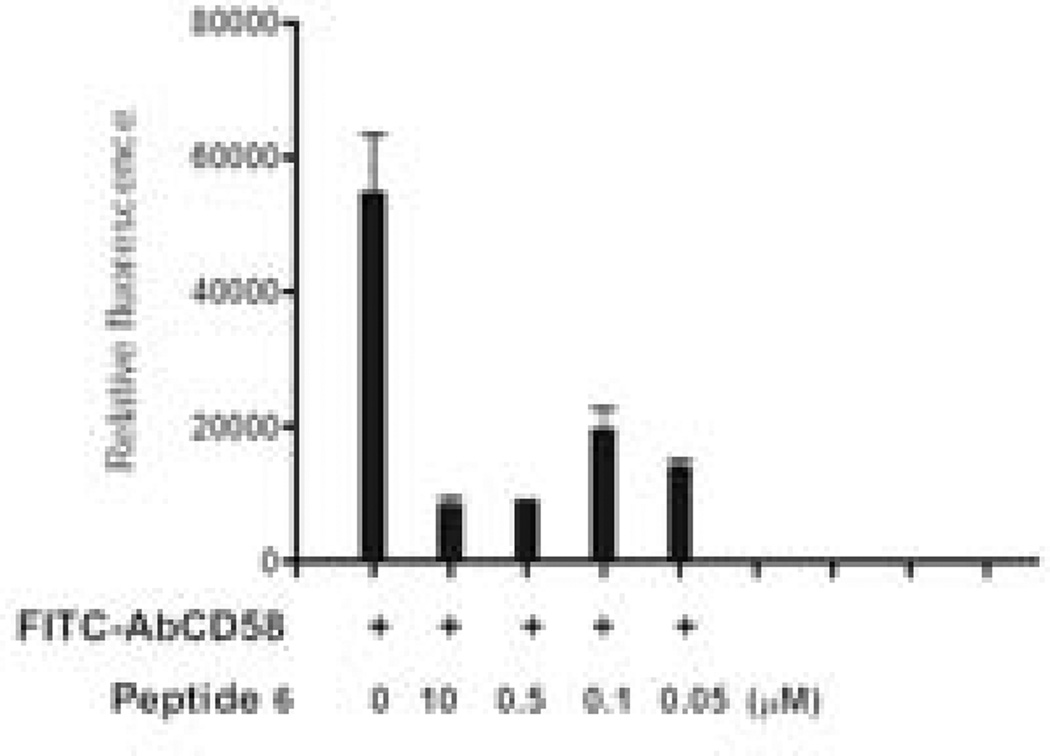
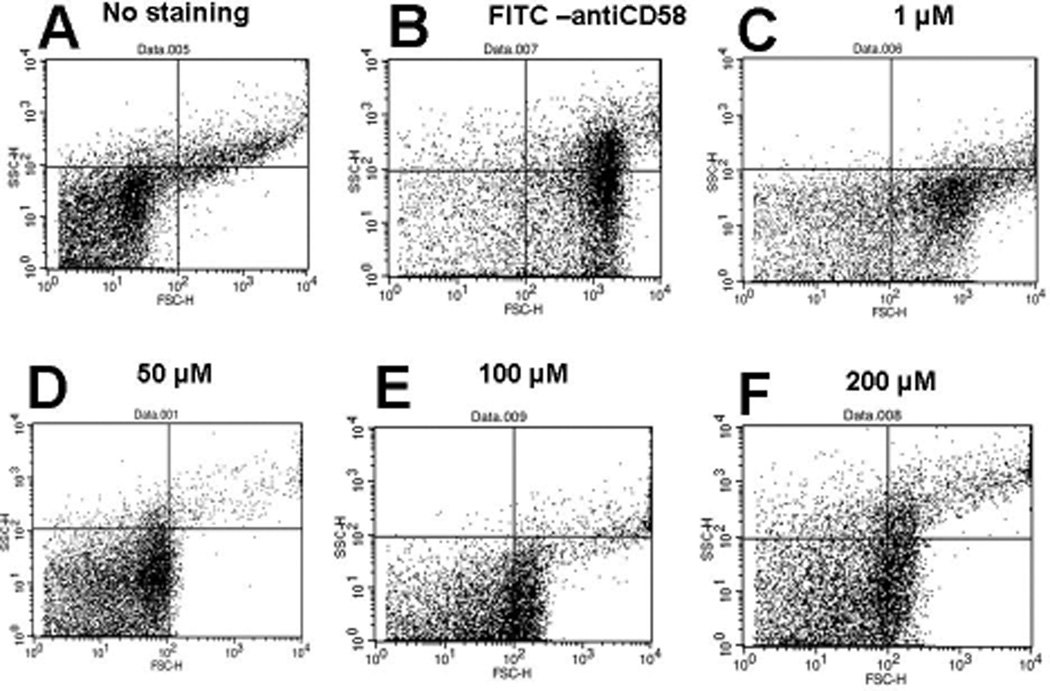
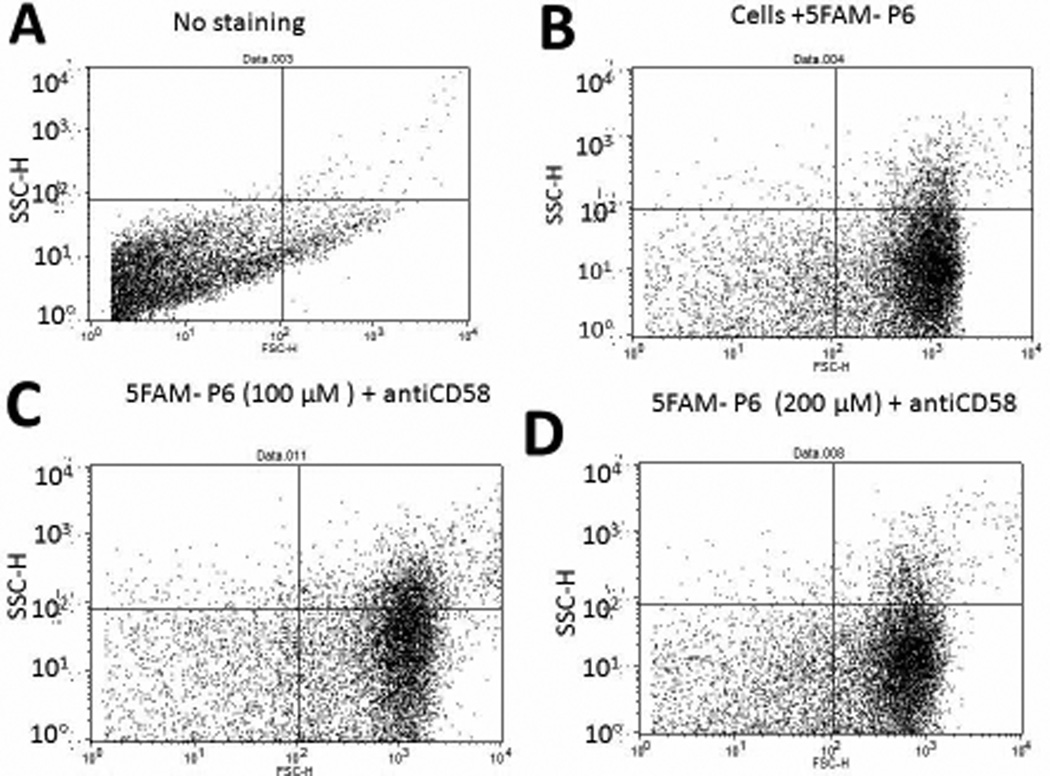
Peptide 6 was designed from the CD58 binding region of CD2 protein and, hence, was presumed to bind to CD58. To evaluate the binding of peptide 6 to CD58, Caco-2 cells that express CD58 were used in a competitive binding study with FITC-anti-CD58 using a fluorescence assay and flow cytometry. Expression of CD58 protein on Caco-2 cells was verified by binding of FITC-anti-CD58. For fluorescence assays using a plate reader, peptide 6 was incubated at various concentrations (0.05 to 10 µM) in the presence of FITC-anti-CD58 and relative fluorescence was measured. In the presence of peptide 6, the fluorescence intensity of FITC-anti-CD58 was decreased drastically even at 0.05 µM concentration of the peptide, suggesting competitive binding of peptide 6 to CD58 protein on Caco-2 cells (Figure 1). Flow cytometry analysis indicated that, as concentration of the peptide was increased from 1 to 100 µM, there was a shift of cells that were labeled with FITC-anti-CD58; this suggests the competitive binding of peptide 6 to CD58. Nearly 60% antibody binding inhibition was observed at 100 µM concentration of the peptide (Figure 2). Since antibody is known to bind to the adhesion domain of CD58, the results clearly indicate that the peptide binds to the CD2 binding region of CD58. To further evaluate whether fluorescently-labeled peptide can bind to CD58 in the presence of anti-CD58, unlabeled anti-CD58 was used. Unlabeled anti-CD58 and 5FAM-labeled peptide 6 were incubated with Caco-2 cells and analyzed by flow cytometry. There was no change in 5FAM-peptide 6 stained cells when anti-CD58 was added (Figure 3), suggesting that 5FAM-labeled peptide 6 competes with antibody binding to CD58 and confirming the results described above.
Figure 1.
Competitive binding of peptide 6 with FITC-labeled antibody to CD58 protein (anti-CD58 is known to bind to adhesion domain of CD58) on Caco-2 cells expressing CD58 protein. Inhibition of antibody binding to Caco-2 cells at different concentrations of peptide 6 is shown. Relative fluorescence intensity was represented. Approximately 1 × 104 Caco-2 cells/well were coated on 96-well plates. After cells achieved confluence, they were incubated with FITC-AbCD58 (+ signs, pre-diluted, 20 µL) and/or peptide 6 for 1 h. After washing three times, medium was added and fluorescence was read using a microplate reader at excitation λ 485 nm and emission λ 528 nm. Fluorescence from the blank was subtracted for representation. Data are from triplicate experiments.
Figure 2.
Competitive binding of peptide 6 with FITC-labeled antibody to Caco-2 cells expressing CD58 monitored by flow cytometry (BD FACS Calibur). 10,000 cells were counted using green fluorescence. The figure represents forward and side scatter representation of Caco-2 cells. A) Caco-2 cells without peptide or antibody. More than 85% of the unstained cells are observed in the lower left quadrant. B) Cells with FITC-AbCD58. There was a forward shift and more than 82% of stained cells were observed in the lower right quadrant. C, D, E, and F) Cells with peptide 6 and FITC-AbCD58 at peptide concentrations of 1, 50, 100, and 200 µM, respectively. Notice that in the presence of peptide 6, more Caco-2 cells without antibody labeling (cell population shifted to left compared to B) were observed, suggesting the inhibition of FITC-AbCD58 to Caco-2 cells.
Figure 3.
Competitive binding of fluorescently labeled peptide 6 (5FAM-peptide 6) with anti-CD58 to Caco-2 cells expressing CD58 monitored by flow-cytometry. 10,000 cells were counted using green fluorescence. The figure represents forward and side scatter representation of Caco-2 cells. A) Caco-2 cells without peptide or antibody. B) Cells with 5FAM peptide 6, 100 µM. C) Cells with 5FAM-peptide 6, 100 µM, and anti-CD58. More than 75% of the cells were stained with peptide 6. D) Cells with 5FAM peptide 6, 200 µM, and anti-CD58. More than 80% of the cells were stained with peptide 6.
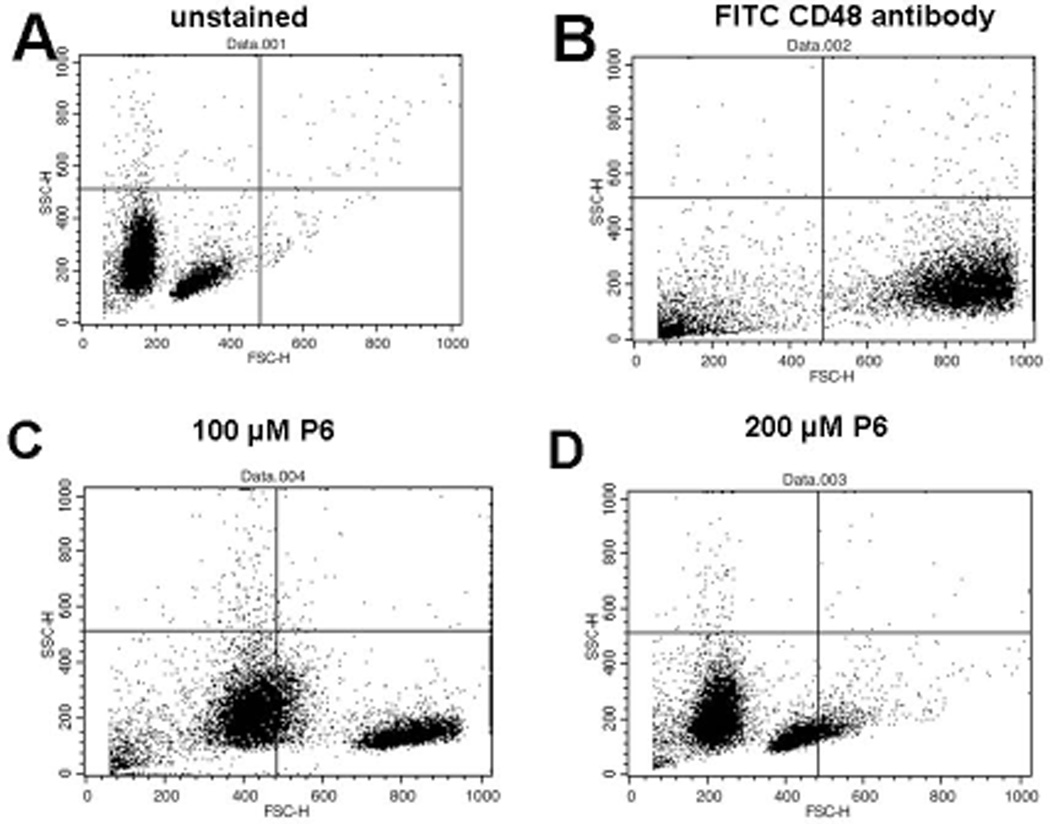
Peptide 6 binds to mouse cells bearing CD48
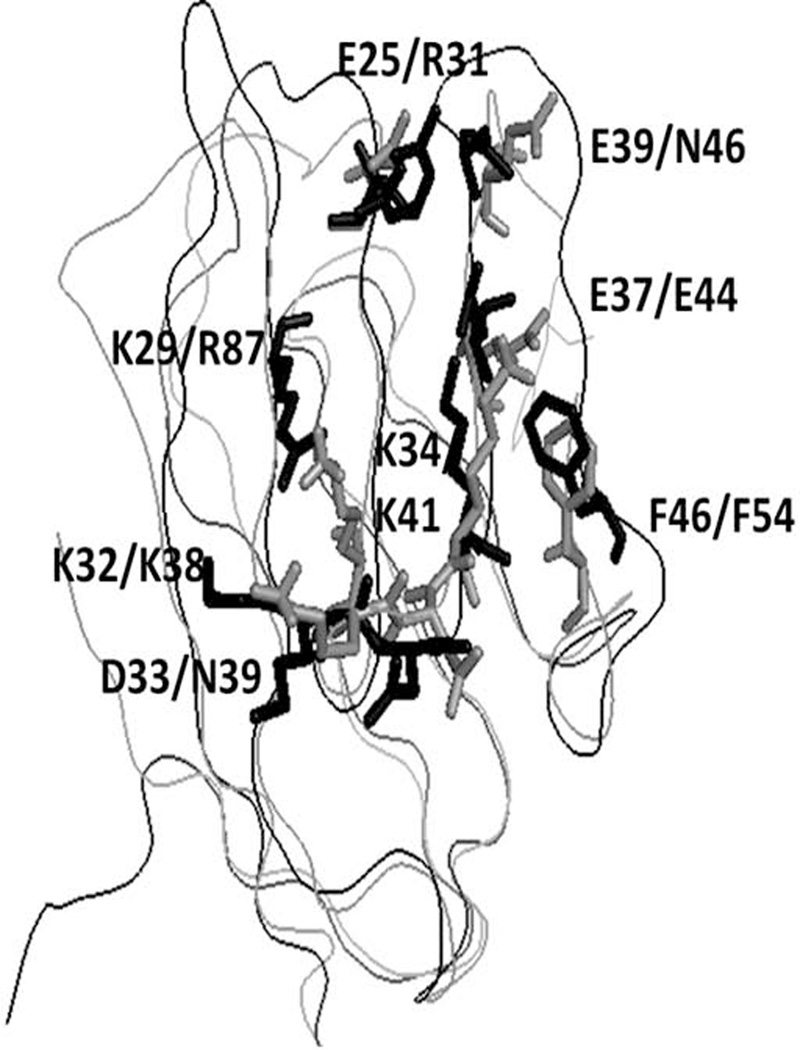
In rodents, the CD2 binds to its ligand CD48, a homolog of CD58, to generate an immune response (27). CD48 has high degree of homology with CD58 and a similar 3-D structure (31, 43). The adhesion domains of CD48 and CD58 proteins overlap with backbone rmsd of 1.8 Å (Figure 4). Our objective was to evaluate the binding of peptide 6 to CD48 on T cells derived from mice. T cells were isolated from mouse spleen and CD48 expression and its binding of peptide 6 was analyzed by flow cytometry. When peptide 6 was incubated with T cells from mice at various concentrations in the presence of FITC-anti-CD48, there was a shift in the cell population compared to that of FITC-anti-CD48 stained cells in the absence of peptide 6. At 100 µM of peptide 6, nearly 50% antibody binding inhibition was observed, and at 200 µM of peptide 6, nearly 80% inhibition of antibody binding was observed; this suggests that peptide derived from human CD2 binds to CD48 on cells derived from mice (Figure 5).
Figure 4.
Comparison of crystal structures of CD58 and CD48. CD58 is shown as gray and CD48 shown as black. Amino acids from CD58 that are shown to be important in binding to CD2 using mutagenesis are shown as sticks with labels. For comparison, amino acids of CD48 are also shown. Single letter codes for amino acids; first label refers to CD58 and second label refers to CD48.
Figure 5.
T cells were collected from mice spleen and treated with FITC-labelled anti- CD48 along with peptide 6 at various concentrations to evaluate the competitive binding. A) unstained T-cells were observed in the lower left quadrant. B) Cells+ FITC-labelled anti-CD48; nearly 80% cells shifted into the lower right quadrant. C) Cells+ FITC-labelled anti-CD48+ peptide 6 100 µM. More than 50% cells were found to be unstained in the lower left quadrant. D) Cells+ FITC-labelled anti-CD48+ peptide 6 200 µM; more than 90% of cells in the lower left quadrant were found to be unstained.
A model for binding of peptide 6 to CD58 and CD48
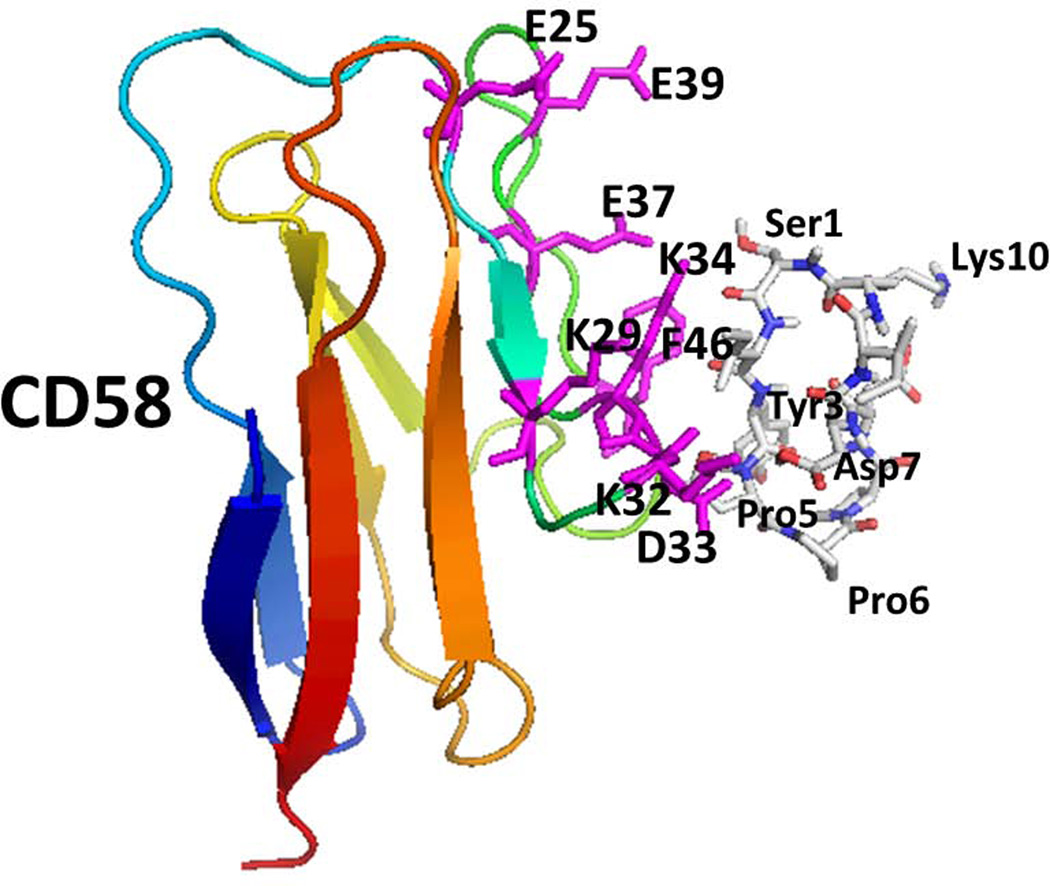
From the antibody binding inhibition studies using peptide 6, it is clear that the peptide from CD2 protein binds to human CD58 as well as to mouse CD48. We generated a model of peptide 6 and protein CD58/CD48 interaction, by docking studies. Low energy docked structures were analyzed for possible binding sites of peptide to the protein. In the case of CD58 and peptide 6 docking, the lowest energy structure had interaction energy of –2.9 kcal/mol. Structures within 2 kcal/mol of this lowest energy were used for analysis as possible binding modes. The low energy docked structures formed three clusters on the CD58 protein surface. All three clusters were near the CD2 binding surface of the CD58 protein. One of the three modes of binding of peptide 6 is represented in Figure 6A. The binding surface involved the amino acid residues Phe46, Asp33, Lys34, Lys29, and Lys30 from CD58 that are important in binding to CD2 protein. Peptide 6 formed two hydrogen bonds with CD58, Asp 7 of the peptide with Lys34 backbone carbonyl and amide hydrogen. There was a hydrophobic interaction between Tyr3 of the peptide and Phe46 of CD58 protein.
Figure 6.
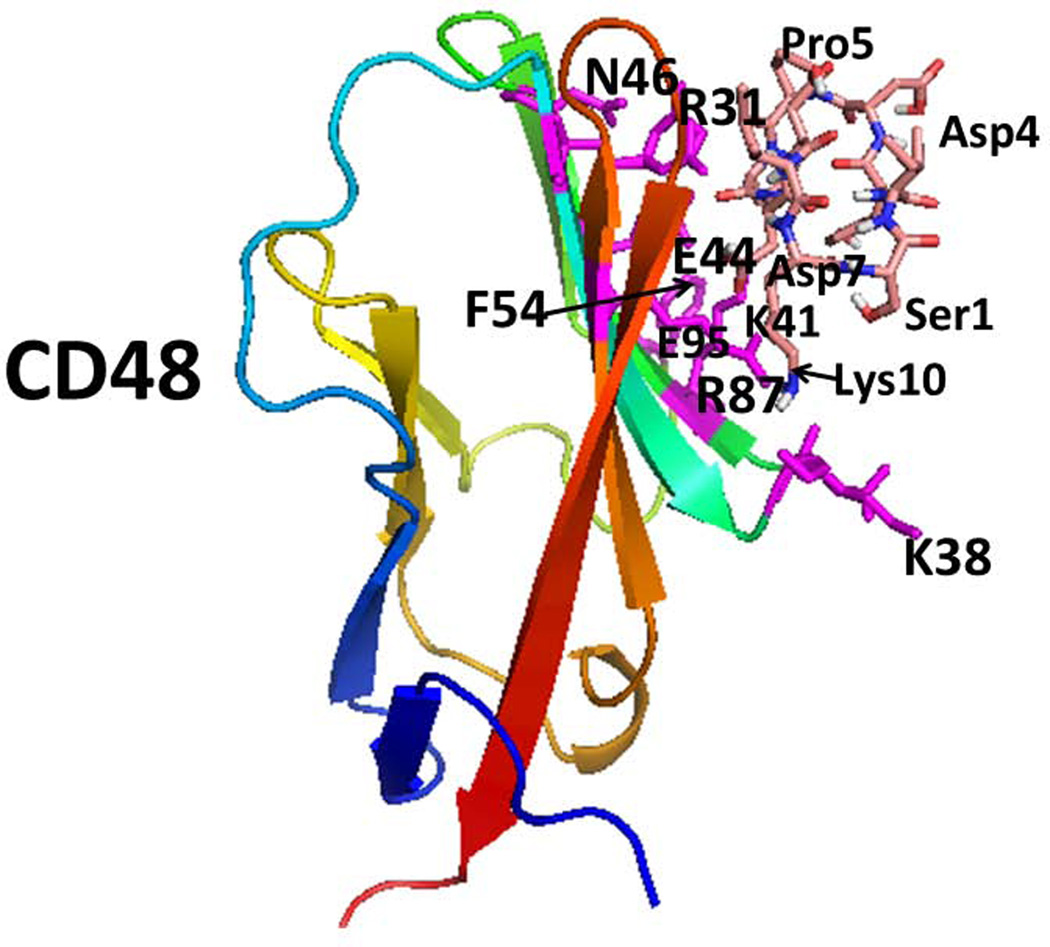
Proposed model for binding of peptide 6 on CD58 and CD48 using docking studies. A) Low energy docked structure of peptide 6 binding to adhesion domain of CD58. Amino acid residues that are shown to be important in binding to CD2 on CD58 are shown as sticks (magenta). Peptide 6 peptide is shown as sticks. B) Low energy docked structure of Peptide 6 binding to adhesion domain of CD48. Residues that are important in binding to CD2 were compared with CD2-CD58 structure. Similar residues in CD48 are represented as sticks (magenta). Amino acids from the protein are labeled with single letter codes and those from the peptide are labeled with three letter codes for clarity.
Binding of peptide 6 to CD48 showed a low energy of −2.8 kcal/mol. A representative docked structure is shown in Figure 6B. Peptide 6 was docked near the amino acid residues, Arg87, Lys41, Phe54, Glu44, Arg31, and Asn46 on the CD48 protein surface. These residues are in the CD2 binding region of CD48. Peptide 6 exhibited three hydrogen bonding interactions with CD48, between Lys10 of peptide 6 to Glu95 of CD48, Asp7 of peptide 6 to Lys41 and Arg87 of CD48, and peptide 6 to Arg31 of CD48. There was a hydrophobic interaction between peptide 6 of Tyr3 to Phe54 and Lys 31 of the side chain of CD48. Although peptide 6 docking studies indicated binding of peptide 6 to CD58 and CD48 proteins, the docking mode was different in CD58 and CD48.
Peptide 6 does not exhibit any immunogenicity in mice
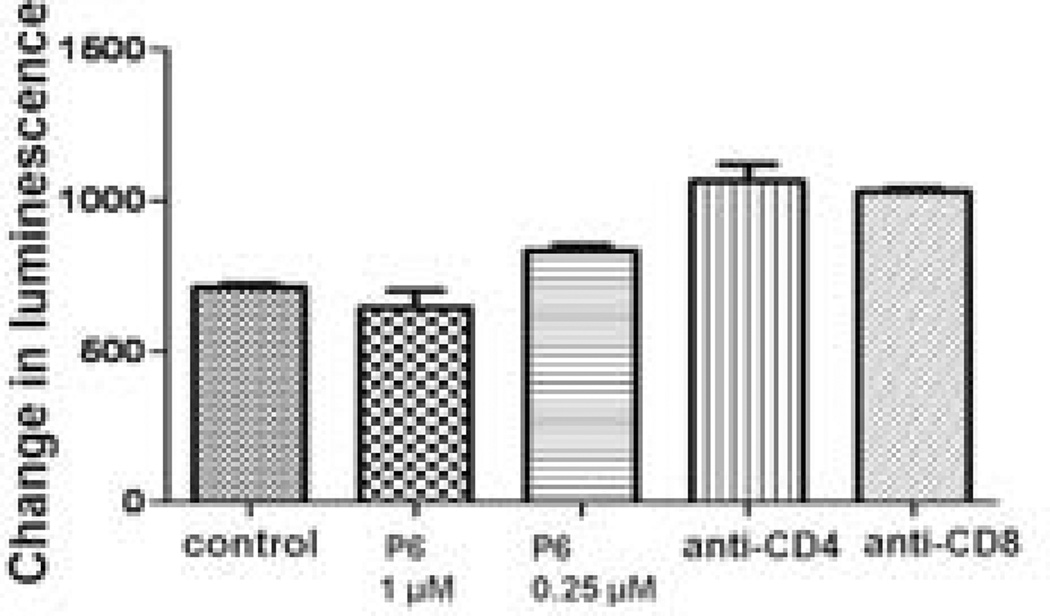
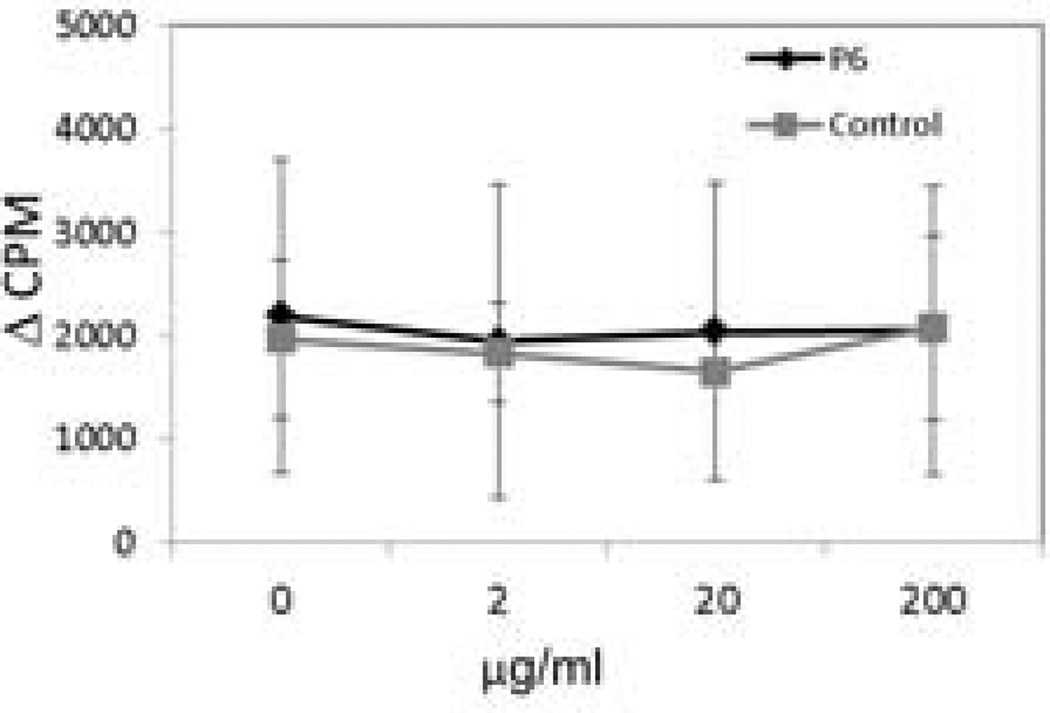
To investigate if peptide 6 is immunogenic and generates immune response upon administration [36, 39], mice were primed with peptide 6. The immunogenicity of peptide 6 was tested by measuring the response of the lymph node cells (LNCs) and splenic cells, isolated from the primed mice, to the peptide in vitro. Varying doses of peptide 6 did not generate any measurable T-cell response in splenic cells. Proliferation of T cells challenged with peptide 6 at 1 and 0.25 µM was comparable to that of the control and anti-CD4- and anti-CD8-treated T cells, suggesting that peptide 6 does not produce any immune response and, hence, is not immunogenic in the mouse model (Figure 7). Culturing splenic cells with ConA resulted in significant proliferation, suggesting that the cells and culture conditions functioned properly (not shown).
Figure 7.
Cells from spleen of mice primed with peptide 6 challenged with peptide 6 for antigenicity. The results suggest that peptide 6 is not immunogenic. Concanavalin A was used as a positive control. The signal from ConA was 40 times higher than that of control.
Next, we determined whether peptide 6 is immunogenic to cells expressing HLA genes associated with susceptibility to RA. For this assay, HLA transgenic mice carrying a RA-susceptible gene, HLA-DQ8, but lacking endogenous class II molecules, AEoDQ8, were used. Lymph node cells were isolated from primed mice and cultured in vitro in the presence or absence of varying concentrations of peptide 6 or a control peptide (Figure 8). The control peptide was a type II collagen-derived peptide known to be unresponsive in DQ8 mice. There was no significant difference in the T cell response when challenged in vitro by the control peptide or peptide 6, suggesting that peptide 6 is not immunogenic.
Figure 8.
Immunogenicity of peptide 6 in HLA transgenic mice expressing human HLA-DQA1*0301, DQB1*0302 (DQ8) gene but lacking all four chains of mouse endogenous class II molecules. LNCs (1 × 106) from transgenic mice were cultured. Con A (20 µg/mL, positive control) and varying concentrations of peptide 6 and control peptide. The cells were incubated for 48 h at 37 °C. During the last 18 h, the cells were pulsed with 3H-thymidine (1 µCi/well). At the end of the assay, the cells were harvested using a plate harvester, and incorporated radioactivity was determined using an automated counter (Microbeta, Perkin Elmer Wallac). The results are depicted as delta cpm. There was no significant difference in response when challenged in vitro by the control peptide or peptide 6, suggesting that peptide 6 is not immunogenic.
Peptide 6 suppresses CIA in DBA/1 mice
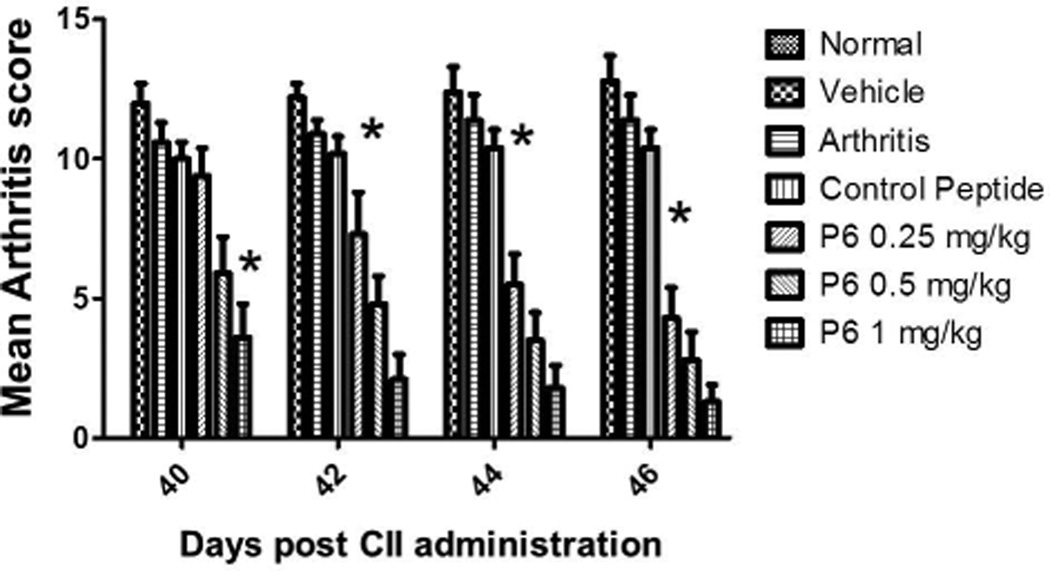
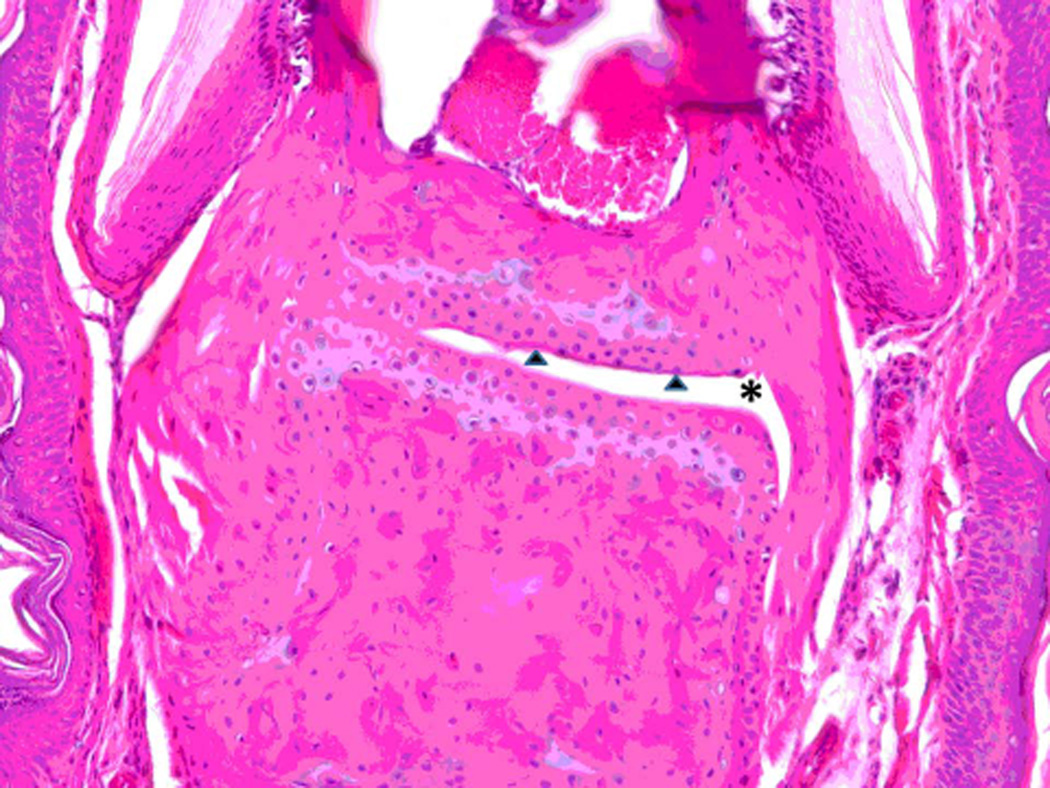
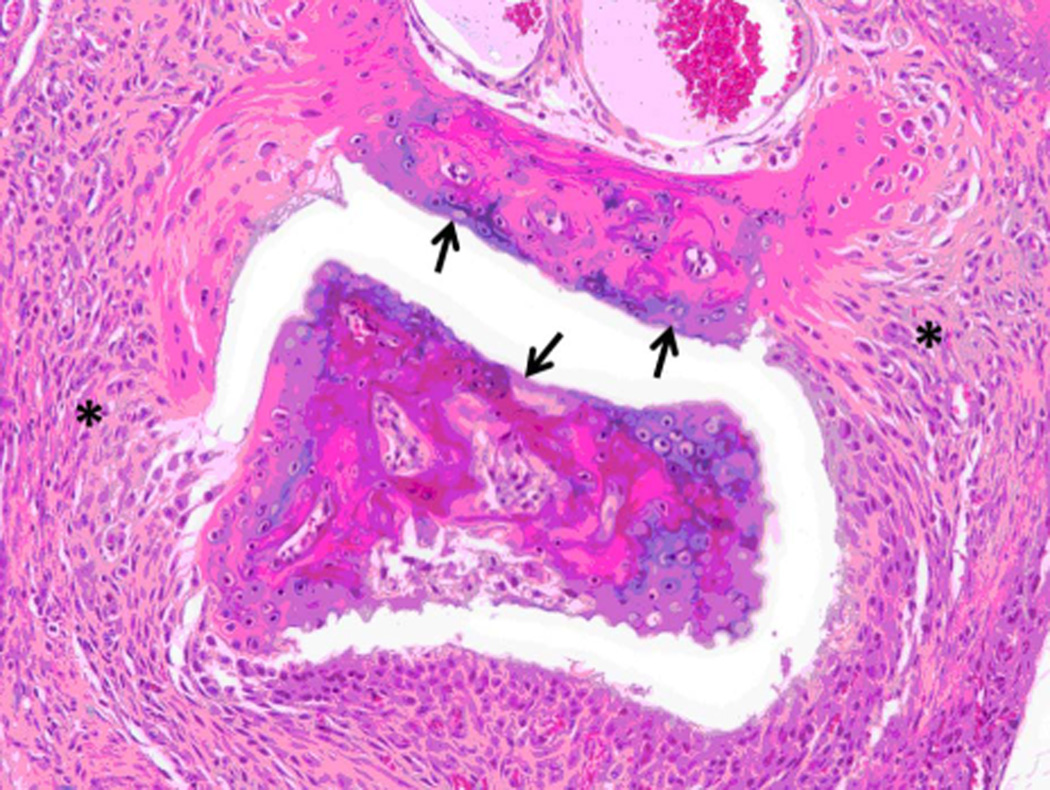
A preclinical animal model of collagen-induced arthritis (CIA) in DBA/1 mice was used to evaluate the ability of peptide 6 to suppress arthritis in a therapeutic protocol. Peptide P6 was administered via i.v injection starting on day 22 after inducing arthritis and then 5 doses on alternate days. The disease progression was evaluated by visual appearance of the limbs in mice and was scored blindly. The mean arthritis score was decreased significantly in peptide 6-treated mice compared to control peptide-treated and untreated mice (Figure 9). Among the different doses of peptide 6 used for treatment, 1 mg/kg produced a significant reduction in arthritis score on days 40 to 46. There was a significant difference between the control peptide and peptide 6 at different concentrations as indicated by one-way Anova non-parametric analysis (P = 0.0058). To determine the statistical significance and variability within the treatment groups, a Mann Whitney test was performed using a 95% confidence interval. There was a significant difference between treatment groups with 1 mg/kg and 0.25 mg/kg (P = 0.028). A Mann Whitney test between control peptide and peptide 6 at 1 mg/kg indicated significant differences on days 40–46 (P = 0.029). Because the mice did not show acute clinical signs of illness (i.e. anorexia, hunched posture, ruffled fur, and/or lethargy) during dosing or prior to the onset of observed clinical arthritis or any early mortality, these observations suggest minimal, if any, direct toxicity of the peptide. Histopathology of paws showed no abnormality in control animals while arthritic mice with severe disease showed erosion of cartilage and bone resorption (Figure 10 A–D) (44). There was infiltration of neutrophils, mixed mononuclear inflammatory cells, and fibroblasts (Figure 10 B,C,D). Animals treated with 0.5 mg/kg peptide 6 showed clear joint space with normal cartilage interface. Connective tissue surrounding joints was very minimally infiltrated with mixed inflammatory cells (Figure 10E), suggesting that peptide 6 suppresses the progression of arthritis in the CIA model.
Figure 9.
Suppression of arthritis by peptide 6 in collagen-induced arthritis. The bar diagram depicts suppression of CIA 40 days post-immunization. Mice treated with peptide 6 at concentrations of 0.25, 0.5 and 1 mg/kg showed statistically significant differences in arthritis severity (* P<0.05) from days 42–46 compared to the control peptide. Scoring was done according to the published procedure (0 to 4) as described in the text for all four limbs with a maximum score of 16. Plots depict mean scores in each group. Only arthritic mice were used for severity index. Values represent the mean ± SEM.
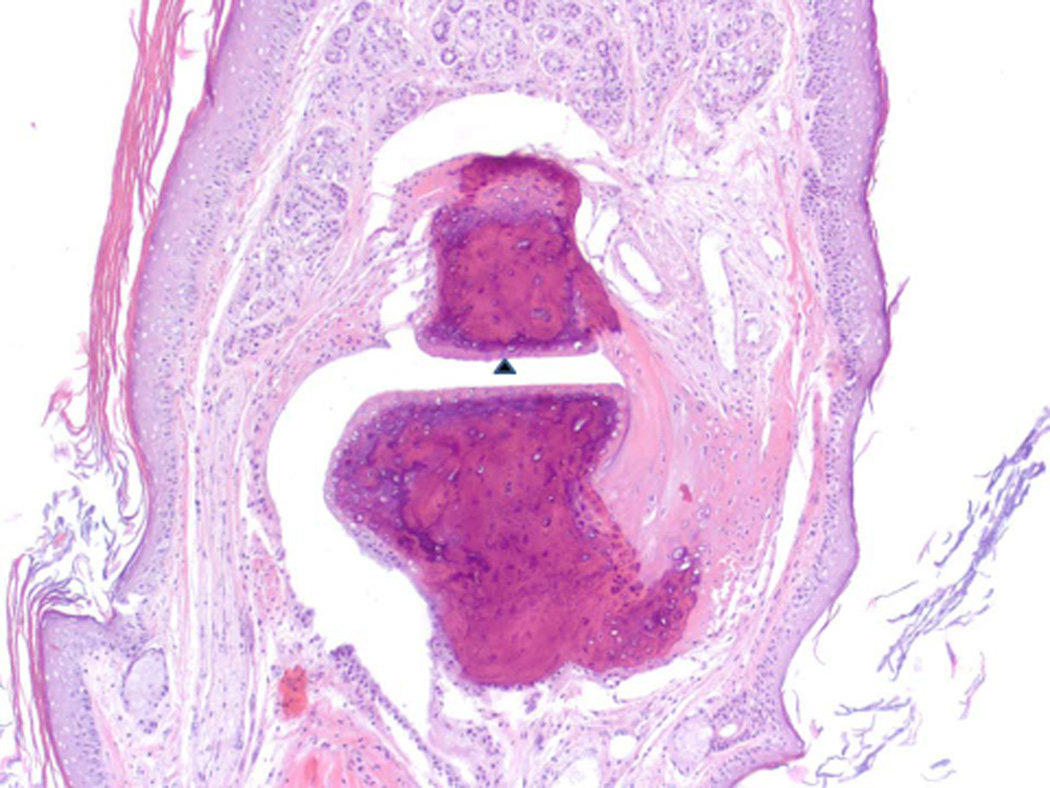
Figure 10.
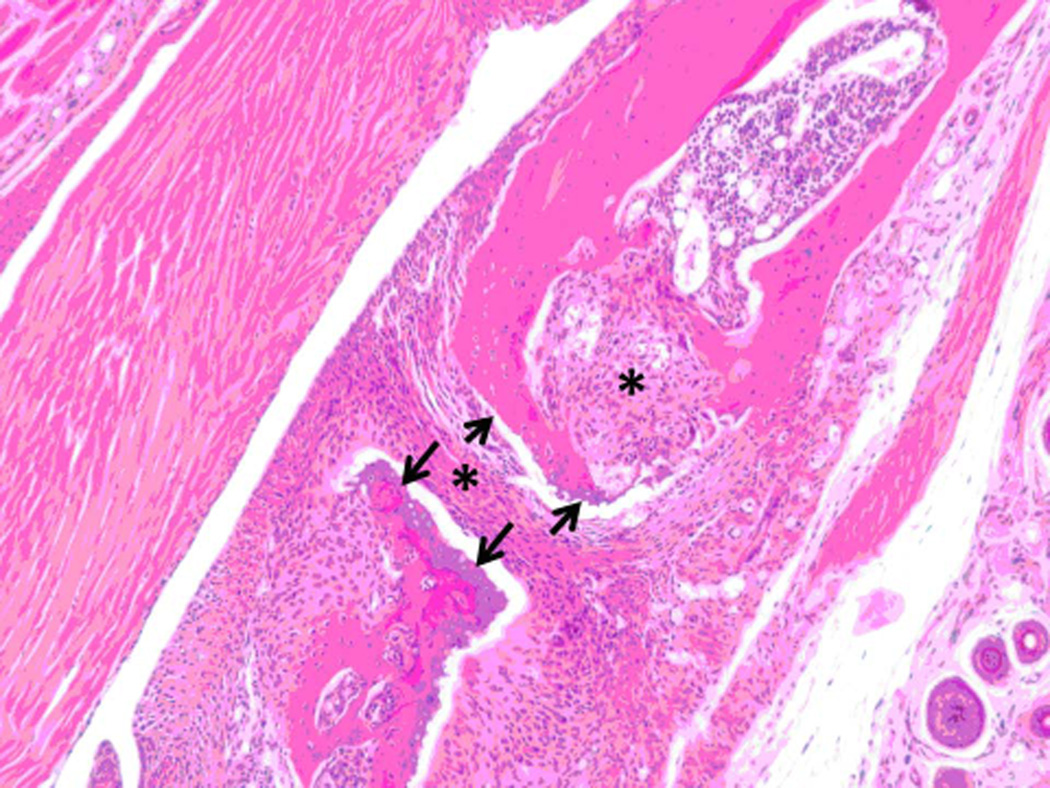
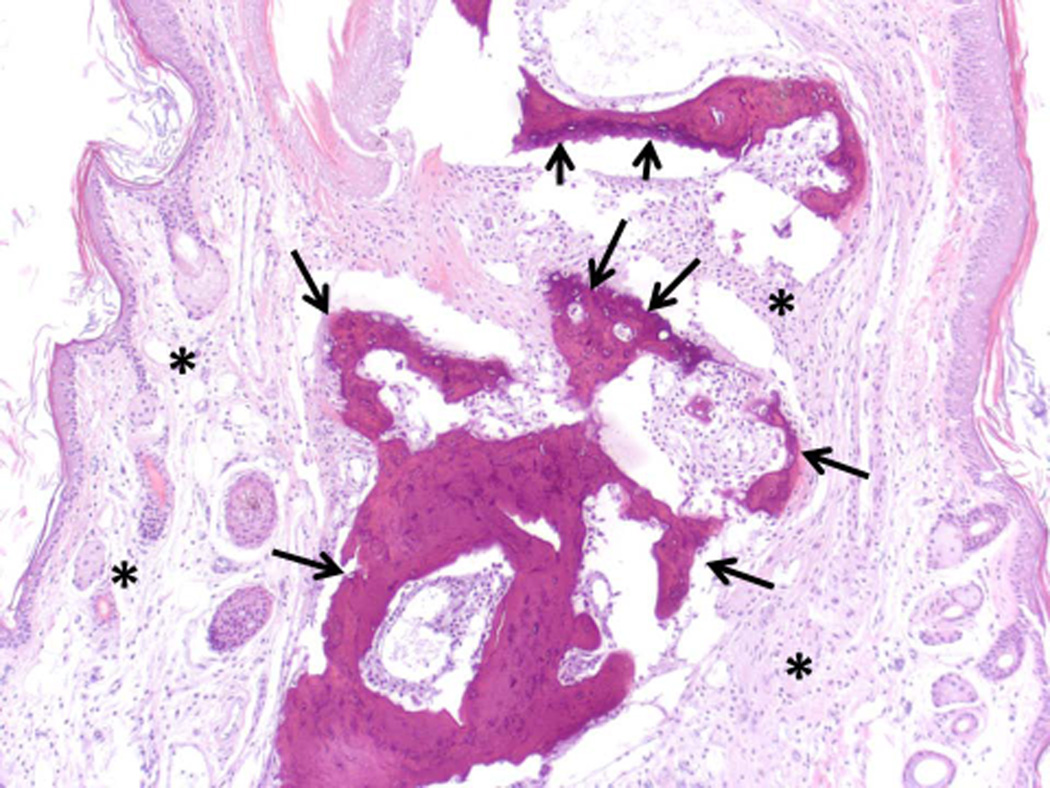
Histopathology analysis of sample from paws from normal, arthritic, and treated DBA/1 mice. Sections of paws from 6 mice were chosen, and representative sections were chosen for final analysis. A) Normal phalangeal joints of hind foot. Cartilaginous surfaces are intact and smooth (arrow heads) and joint space is clear (*). H & E staining. Original magnification 200X. B) Arthritis hind paw with severe (grade 4) arthritis of phalangeal joint. Joint is swollen. Marked cartilaginous erosion of joint surface and pannus circumscribing the phalanges. H & E staining. Original magnification 200X.
C) and D) Arthritis hind paw with severe (grade 4) erosion of cartilage and bone resorption (arrows) of phalanges. The connective tissue (*) of the dermis, hypodermis, and peristeum is mildly edematous and infiltrated with neutrophils, mixed mononuclear inflammatory cells, and fibroblasts. H & E staining. Original magnification 100X. E) Hind paw of mouse treated with peptide 6 (0.5 mg/kg). Joint space is clear (*) with normal cartilage interface. Connective tissue surrounding joint is only very minimally infiltrated with mixed inflammatory cells. H & E staining. Original magnification 100X.
P6 inhibited the production of anti-collagen Abs
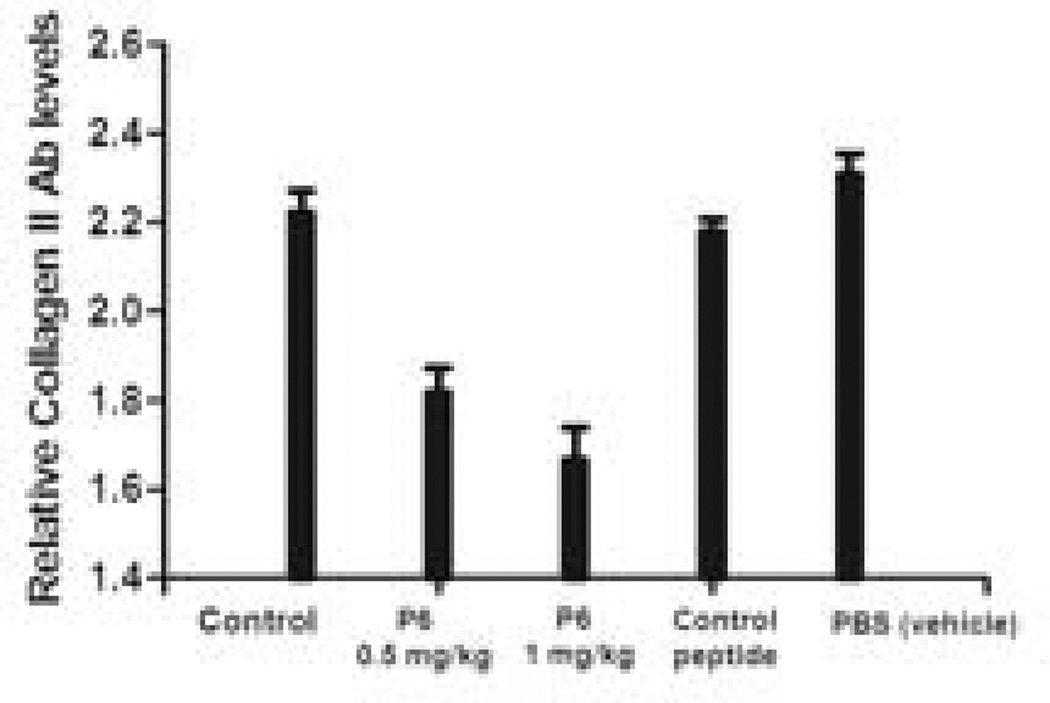
A role of autoreactive T cells and B cells is implicated in CIA. The autoimmune response to CII can be evaluated by measuring the quantity of the CII-specific antibody in the sera of the mice (33). To determine whether peptide 6 can inhibit the production of anti CII Abs, serum samples were analyzed at the termination of the study in seven different groups of mice—control group without any treatment, arthritic, treatment group (three groups), vehicle control (PBS), and control peptide. Animals that were administered peptide 6 at 0.5 and 1 mg/kg showed a dose-dependent reduction in the anti-CII antibodies in sera. (Figure 11), suggesting that blocking interaction between CD2-CD58/CD48 was able to inhibit humoral immune response in mice.
Figure 11.
Reduction of circulating anti-CII Ab titer in serum of mice with CIA. N = 6. Peptide 6 was injected i.v at 0.5 mg/kg and 1 mg/kg. Relative levels of anti-CII Ab titer were observed with peptide 6-treated mice compared to control peptide and control groups with no treatment. Values represent the mean ± SEM. Statistical analysis indicated that p < 0.05 for peptide 6 treated mice compared to control and control peptide-treated mice.
Discussion
Cell adhesion/co-stimulatory molecules have been targeted for treatment of autoimmune diseases (6, 19, 45, 46). Among these co-stimulatory molecules, LFA-1, CD80, CD28, and CD2, as well as CD58 have been shown to be important in autoimmune diseases such as RA (47, 48). Recently, molecules that target the EGFR kinase domain have shown therapeutic effects for RA (49, 50). However, there are more than 500 kinases that have similar ATP binding sites, making EGFR a difficult target for the design of specific molecules. Our approach was to use peptides to target the CD2-CD58 interaction. In the present study we have demonstrated that a peptide, peptide 6, derived from the adhesion domain of CD2, binds to human CD58 as well as to mouse CD48, and inhibits cell adhesion interaction in the lower nanomolar concentration range. The adhesion domain of CD2 has important residues in F and C strands that participate in hydrogen bonding and hydrophobic interaction with CD58 protein to stabilize protein-protein interactions for cell adhesion (51). Peptide 6 has Tyr and and Asp residues placed in a β-hairpin structure that resembles F and C strands of CD2 protein. Antibody binding inhibition assay results clearly suggest that peptide 6 binds to CD58 expressed on Caco-2 cells. Competitive binding studies showed that peptide 6 can inhibit anti-CD58 binding to Caco-2 cells, even in the nanomolar concentration range. Competitive binding studies suggested that although antibody inhibition was relatively low at a concentration of 0.1 and 0.05 µM peptide compared to 10 and 0.5 µM, there was no clear evidence of dose-dependent response for antibody binding inhibition of peptide 6 (Figure 1). Since antibody to CD58 binds with high specificity at nanomolar concentration and complete inhibition of FITC-antiCD58 is difficult to achieve with peptides, we believe that the observed results did not show concentration-dependent dose response. Since the antibody binds to the adhesion domain of CD58 and peptides block the antibody binding, we suggest that peptides from CD2 bind to CD58 and block the CD2-CD58 interaction.
In rodents, the CD2 binding partner homologous to CD58 is CD48 (27). It has been postulated that CD48 and CD58 have the same evolutionary origin. In mice, CD2 binds to its ligand CD48 to generate an immune response. CD48 has high degree of homology with CD58 and a similar 3-D structure (31, 43). The adhesion domains of the two proteins overlap with backbone rmsd of 1.8 Å. Mutagenesis studies have indicated the important residues in CD58 (51). The crystal structures of CD58 and CD48 (Figure 4) suggest that the amino acids that are important in binding to CD2 are similar in both of the structures. Sequence comparison of human CD2 peptide 6 and rat and mouse CD2 (Table 1) indicates that rat, mouse, and human CD2 have similar residues in the F and C strands of the protein that are important in binding to CD58. A sequence comparison of the F and C strands of CD58 and CD48 suggests that human, mouse, and rat CD2 contain Tyr, an important amino acid, and two Asp or Glu residues in the binding region (51). Competitive antibody binding inhibition assays showed that peptide 6 binds to CD48-expressing T cells from mice. Taken together, these data suggest that peptides derived from human CD2 can bind to CD48 and modulate immune response in mice. To provide a model for the interaction of peptide 6 with CD58 and CD48, docking studies were carried out. The proposed model of docking suggested that peptide 6 binds to the CD58 and CD48 adhesion domains. However, the binding mode is slightly different in these proteins. In CD58, the binding seems to be in the lower part of the β-sheets near Lys34 and Phe46, and in CD48 the binding occurs around the upper part of the β-sheets above Lys41 and near Glu44 and Arg31. The difference observed in binding for CD48 and CD58 could be due to the mode of binding and the structure used for analysis based on docking score. For analysis of docking, we used the lowest energy docked structure to represent the possible mode of binding (Figure 6A & B). However, some structures that were docked in the binding site on CD58 similar to peptide 6 on CD48 had somewhat higher energy (2 kcal/mol energy difference compared to that of the lowest energy docked structures). Hence, for the final representation, they were not included. Alternatively, the difference in binding site observed for peptide 6 in the case of CD48 and CD58 may be due to the limitation of the docking methods used in the software.
Our ultimate aim is to demonstrate that peptides derived from CD2 can be used as therapeutic agents against autoimmune diseases. Rheumatoid arthritis is an autoimmune disease characterized by inflammation of synovial-lined joints that are infiltrated with T cells and upregulation of CD2 and CD58. Collagen-induced arthritis is a well-documented and widely used as a model for evaluating the efficacy of compounds for treatment of RA (44) . Administration of peptide 6 to arthritis-induced mice at doses 0.25, 0.5 and 1 mg/kg suppressed the progression of arthritis, indicating therapeutic ability of peptide 6 to modulate the immune response in in vivo studies. Mice were treated on alternate days because daily dosing can cause scarring of the tail vein that can take at least a day to heal. In an earlier study(26), we reported that peptide 6 inhibits cell adhesion in the nanomolar concentration range with an IC50 value of 6 nM. Based on in vitro data and previously reported studies by other research groups(42, 52, 53), we evaluated the effect of peptide 6 at concentrations of 0.25, 0.5 and 1 mg/kg dose in a therapeutic protocol. Arthritis symptoms started appear on days 18 to 25, and the severity reached a maximum around day 36. The arthritis score observed visually and by histopathology confirmed the therapeutic effect of peptide 6. The suppression of arthritis became apparent after day 36, since the treatment was started after the development of arthritis, and was significant for days 40–46 (Figure 9). The major reason for this delayed effect can be explained as follows: the peptide blocks T-cell response so that no further T- and B-cell activation occurs; however, ongoing inflammation subsided 10 days post-treatment with peptide 6. After the administration of peptide 6, the anti-collagen level decreased, leading to suppression of CIA. These observations of are similar to those observed in other animal models of arthritis (42, 52, 53). These studies were performed in a therapeutic protocol with clinical relevance to human RA; we believe that an earlier intervention should also suppress arthritis. Based on thees studies, the authors also conclude that concentration of peptide 6 must be increased (2 to 5 mg/kg) to observe any immediate effects of the peptide on CIA. Such studies will be carried out in the future using a transgenic mouse model for relevance to human disease. Suppression of arthritis was also associated with a reduction in anti-CII antibody levels. Although several adhesion or co-stimulatory molecules are involved in the immune response (54), the data from the antibody binding assay and the docking model suggest that peptide 6 modulates the immune response mediated by CD2-CD48 interactions in the mouse model.
Peptides and peptide vaccines have been developed to generate the immune response (55) and, thereby, generate antibodies against a particular cell surface molecule to suppress the response. However, in our studies, we have directly targeted CD58 protein with peptides to inhibit protein-protein interaction between CD2-CD58/CD48. Immunogenecity studies on peptide 6 indicated that this peptide is not immunogenic. Hence, the inhibition and immunomodulation by peptide via CD2-CD58/CD48 interaction is produced directly by CD2 peptides and may not be due to antibodies generated against any of these proteins. Thus, our studies demonstrate that protein-protein interactions between CD2-CD58/CD48 could be modulated by peptides in vitro and in vivo, and that these peptides could be therapeutically useful for treating autoimmune diseases.
Conclusions and future directions
Conformationally constrained cyclic peptides designed from the CD58 binding region of CD2 protein bind to CD58-bearing Caco-2 cells. We have also shown that peptides from CD2 bind to rodent CD48, which is homologous to CD58 in humans. A molecular model is proposed for binding a peptide to CD58 and CD48 using docking studies. Modeling studies suggested that peptides from CD2 bind to the adhesion domain of CD58/CD48. Furthermore, in vivo therapeutic studies suggested that peptide 6 was able suppress the progression of CIA in an animal model. Evaluation of the antigenicity of peptides in CIA and a transgenic animal model indicated that they are not immunogenic. These studies clearly suggest that peptides from co-stimulatory molecules, in particular, CD2, can be used to modulate immune responses and are helpful in designing therapeutic agents for autoimmune diseases. Future studies will be focused on the stability and formulation of CD2 peptides for in vivo administration. Chemical stability as well as the stability of the peptide in mouse serum will be evaluated.
Acknowledgments
This research was supported by Louisiana Board of Regents Grant LEQSF(2009–12)-RD-A-23(SJ). The authors thank the Mass Spectrometry Facility, Department of Chemistry, Louisiana State University, Baton Rouge, LA, for high resolution mass spectra of compounds. Docking studies were conducted using HPC resources at LSU Baton Rouge via Louisiana Optical Network Initiative (LONI). V.T. is supported by NIH Grant AI075262.
References
- 1.Bretscher PA. A two-step, two-signal model for the primary activation of precursor helper T cells. Proc Natl Acad Sci U S A. 1999;96:185–190. doi: 10.1073/pnas.96.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Merwe PA, Davis SJ. Molecular interactions mediating T cell antigen recognition. Annu Rev Immunol. 2003;21:659–684. doi: 10.1146/annurev.immunol.21.120601.141036. [DOI] [PubMed] [Google Scholar]
- 3.Dustin ML, Olszowy MW, Holdorf AD, Li J, Bromley S, Desai N, et al. A novel adaptor protein orchestrates receptor patterning and cytoskeletal polarity in T-cell contacts. Cell. 1998;94:667–677. doi: 10.1016/s0092-8674(00)81608-6. [DOI] [PubMed] [Google Scholar]
- 4.Davis SJ, Ikemizu S, Evans EJ, Fugger L, Bakker TR, van der Merwe PA. The nature of molecular recognition by T cells. Nat Immunol. 2003;4:217–224. doi: 10.1038/ni0303-217. [DOI] [PubMed] [Google Scholar]
- 5.Ellis CN, Krueger GG. Treatment of chronic plaque psoriasis by selective targeting of memory effector T lymphocytes. N Engl J Med. 2001;345:248–255. doi: 10.1056/NEJM200107263450403. [DOI] [PubMed] [Google Scholar]
- 6.Chamian F, Lowes MA, Lin SL, Lee E, Kikuchi T, Gilleaudeau P, et al. Alefacept reduces infiltrating T cells, activated dendritic cells, and inflammatory genes in psoriasis vulgaris. Proc Natl Acad Sci U S A. 2005;102:2075–2080. doi: 10.1073/pnas.0409569102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strand V. New therapies in development for autoimmune diseases: their rationale for combination treatment. Springer Semin Immunopathol. 2001;23:43–61. doi: 10.1007/s002810100071. [DOI] [PubMed] [Google Scholar]
- 8.Anderson ME, Siahaan TJ. Targeting ICAM-1/LFA-1 interaction for controlling autoimmune diseases: designing peptide and small molecule inhibitors. Peptides. 2003;24:487–501. doi: 10.1016/s0196-9781(03)00083-4. [DOI] [PubMed] [Google Scholar]
- 9.Webber A, Hirose R, Vincenti F. Novel strategies in immunosuppression: issues in perspective. Transplantation. 2011;91:1057–1064. doi: 10.1097/TP.0b013e3182145306. [DOI] [PubMed] [Google Scholar]
- 10.Mrowietz U. Treatment targeted to cell surface epitopes. Clin Exp Dermatol. 2002;27:591–596. doi: 10.1046/j.1365-2230.2002.01171.x. [DOI] [PubMed] [Google Scholar]
- 11.Hale LP, Martin ME, McCollum DE, Nunley JA, Springer TA, Singer KH, et al. Immunohistologic analysis of the distribution of cell adhesion molecules within the inflammatory synovial microenvironment. Arthritis Rheum. 1989;32:22–30. doi: 10.1002/anr.1780320105. [DOI] [PubMed] [Google Scholar]
- 12.Kraan MC, van Kuijk AW, Dinant HJ, Goedkoop AY, Smeets TJ, de Rie MA, et al. Alefacept treatment in psoriatic arthritis: reduction of the effector T cell population in peripheral blood and synovial tissue is associated with improvement of clinical signs of arthritis. Arthritis Rheum. 2002;46:2776–2784. doi: 10.1002/art.10543. [DOI] [PubMed] [Google Scholar]
- 13.Raychaudhuri S, Thomson BP, Remmers EF, Eyre S, Hinks A, Guiducci C, et al. Genetic variants at CD28, PRDM1 and CD2/CD58 are associated with rheumatoid arthritis risk. Nat Genet. 2009;41:1313–1318. doi: 10.1038/ng.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bierer BE, Burakoff SJ. T cell adhesion molecules. Faseb J. 1988;2:2584–2590. doi: 10.1096/fasebj.2.10.2838364. [DOI] [PubMed] [Google Scholar]
- 15.Hoffmann JC, Bayer B, Zeidler H. Characterization of a soluble form of CD58 in synovial fluid of patients with rheumatoid arthritis (RA) Clin Exp Immunol. 1996;104:460–466. doi: 10.1046/j.1365-2249.1996.41749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cemerski S, Shaw A. Immune synapses in T-cell activation. Curr Opin Immunol. 2006;18:298–304. doi: 10.1016/j.coi.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Snanoudj R, Zuber J, Legendre C. Co-stimulation blockade as a new strategy in kidney transplantation: benefits and limits. Drugs. 2010;70:2121–2131. doi: 10.2165/11538140-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 18.Liossis SN, Sfikakis PP. Costimulation blockade in the treatment of rheumatic diseases. BioDrugs. 2004;18:95–102. doi: 10.2165/00063030-200418020-00003. [DOI] [PubMed] [Google Scholar]
- 19.Vincenti F, Kirk AD. What's next in the pipeline. Am J Transplant. 2008;8:1972–1981. doi: 10.1111/j.1600-6143.2008.02403.x. [DOI] [PubMed] [Google Scholar]
- 20.Satyanarayanajois SD, Buyuktimkin B, Gokhale A, Ronald S, Siahaan TJ, Latendresse JR. A peptide from the beta-strand region of CD2 protein that inhibits cell adhesion and suppresses arthritis in a mouse model. Chem Biol Drug Des. 2010;76:234–244. doi: 10.1111/j.1747-0285.2010.01001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu J, Li C, Ke S, Satyanarayanajois SD. Structure-based rational design of beta-hairpin peptides from discontinuous epitopes of cluster of differentiation 2 (CD2) protein to modulate cell adhesion interaction. J Med Chem. 2007;50:4038–4047. doi: 10.1021/jm0700868. [DOI] [PubMed] [Google Scholar]
- 22.Liu J, Chow VT, Jois SD. A novel, rapid and sensitive heterotypic cell adhesion assay for CD2-CD58 interaction, and its application for testing inhibitory peptides. J Immunol Methods. 2004;291:39–49. doi: 10.1016/j.jim.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 23.Giddu S, Subramanian V, Yoon HS, Satyanarayanajois SD. Design of beta-hairpin peptides for modulation of cell adhesion by beta-turn constraint. J Med Chem. 2009;52:726–736. doi: 10.1021/jm8008212. [DOI] [PubMed] [Google Scholar]
- 24.Li C, Satyanarayanajois SD. Structure-function studies of peptides for cell adhesion inhibition: identification of key residues by alanine mutation and peptide-truncation approach. Peptides. 2007;28:1498–1508. doi: 10.1016/j.peptides.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 25.Jining L, Makagiansar I, Yusuf-Makagiansar H, Chow VT, Siahaan TJ, Jois SD. Design, structure and biological activity of beta-turn peptides of CD2 protein for inhibition of T-cell adhesion. Eur J Biochem. 2004;271:2873–2886. doi: 10.1111/j.1432-1033.2004.04198.x. [DOI] [PubMed] [Google Scholar]
- 26.Gokhale A, Weldeghiorghis TK, Taneja V, Satyanarayanajois SD. Conformationally constrained peptides from CD2 to modulate protein-protein interactions between CD2 and CD58. J Med Chem. 2011;54:5307–5319. doi: 10.1021/jm200004e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ianelli CJ, Edson CM, Thorley-Lawson DA. A ligand for human CD48 on epithelial cells. J Immunol. 1997;159:3910–3920. [PubMed] [Google Scholar]
- 28.Corradin G, Etlinger HM, Chiller JM. Lymphocyte specificity to protein antigens. I. Characterization of the antigen-induced in vitro T cell-dependent proliferative response with lymph node cells from primed mice. J Immunol. 1977;119:1048–1053. [PubMed] [Google Scholar]
- 29.Morris GM, Huey R, Lindstrom W, Sanner MF, Belew RK, Goodsell DS, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang JH, Smolyar A, Tan K, Liu JH, Kim M, Sun ZY, et al. Structure of a heterophilic adhesion complex between the human CD2 and CD58 (LFA-3) counterreceptors. Cell. 1999;97:791–803. doi: 10.1016/s0092-8674(00)80790-4. [DOI] [PubMed] [Google Scholar]
- 31.Velikovsky CA, Deng L, Chlewicki LK, Fernandez MM, Kumar V, Mariuzza RA. Structure of natural killer receptor 2B4 bound to CD48 reveals basis for heterophilic recognition in signaling lymphocyte activation molecule family. Immunity. 2007;27:572–584. doi: 10.1016/j.immuni.2007.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, et al. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brand DD, Latham KA, Rosloniec EF. Collagen-induced arthritis. Nat Protoc. 2007;2:1269–1275. doi: 10.1038/nprot.2007.173. [DOI] [PubMed] [Google Scholar]
- 34.Williams RO. Collagen-induced arthritis as a model for rheumatoid arthritis. Methods Mol Med. 2004;98:207–216. doi: 10.1385/1-59259-771-8:207. [DOI] [PubMed] [Google Scholar]
- 35.Taneja V, Behrens M, Mangalam A, Griffiths MM, Luthra HS, David CS. New humanized HLA-DR4-transgenic mice that mimic the sex bias of rheumatoid arthritis. Arthritis Rheum. 2007;56:69–78. doi: 10.1002/art.22213. [DOI] [PubMed] [Google Scholar]
- 36.Vaucher Rde A, Velho Gewehr Cde C, Correa AP, Sant’Anna V, Ferreira J, Brandelli A. Evaluation of the immunogenicity and in vivo toxicity of the antimicrobial peptide P34. Int J Pharm. 2011;421:94–98. doi: 10.1016/j.ijpharm.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 37.Khachigian LM. Collagen antibody-induced arthritis. Nat Protoc. 2006;1:2512–2516. doi: 10.1038/nprot.2006.393. [DOI] [PubMed] [Google Scholar]
- 38.Morgan ME, Flierman R, van Duivenvoorde LM, Witteveen HJ, van Ewijk W, van Laar JM, et al. Effective treatment of collagen-induced arthritis by adoptive transfer of CD25+ regulatory T cells. Arthritis Rheum. 2005;52:2212–2221. doi: 10.1002/art.21195. [DOI] [PubMed] [Google Scholar]
- 39.Taneja V, Taneja N, Paisansinsup T, Behrens M, Griffiths M, Luthra H, et al. CD4 and CD8 T cells in susceptibility/protection to collagen-induced arthritis in HLA-DQ8-transgenic mice: implications for rheumatoid arthritis. J Immunol. 2002;168:5867–5875. doi: 10.4049/jimmunol.168.11.5867. [DOI] [PubMed] [Google Scholar]
- 40.Behrens M, Trejo T, Luthra H, Griffiths M, David CS, Taneja V. Mechanism by which HLA-DR4 regulates sex-bias of arthritis in humanized mice. Journal of autoimmunity. 2010;35:1–9. doi: 10.1016/j.jaut.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taneja V, Taneja N, Behrens M, Griffiths MM, Luthra HS, David CS. Requirement for CD28 may not be absolute for collagen-induced arthritis: study with HLA-DQ8 transgenic mice. J Immunol. 2005;174:1118–1125. doi: 10.4049/jimmunol.174.2.1118. [DOI] [PubMed] [Google Scholar]
- 42.Suchard SJ, Stetsko DK, Davis PM, Skala S, Potin D, Launay M, et al. An LFA-1 (alphaLbeta2) small-molecule antagonist reduces inflammation and joint destruction in murine models of arthritis. J Immunol. 2010;184:3917–3926. doi: 10.4049/jimmunol.0901095. [DOI] [PubMed] [Google Scholar]
- 43.Clarkson NG, Brown MH. Inhibition and activation by CD244 depends on CD2 and phospholipase C-gamma1. J Biol Chem. 2009;284:24725–24734. doi: 10.1074/jbc.M109.028209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kannan K, Ortmann RA, Kimpel D. Animal models of rheumatoid arthritis and their relevance to human disease. Pathophysiology. 2005;12:167–181. doi: 10.1016/j.pathophys.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 45.Bissonnette R, Langley RG, Papp K, Matheson R, Toth D, Hultquist M, et al. Humanized anti-CD2 monoclonal antibody treatment of plaque psoriasis: efficacy and pharmacodynamic results of two randomized, double-blind, placebo-controlled studies of intravenous and subcutaneous siplizumab. Arch Dermatol Res. 2009;301:429–442. doi: 10.1007/s00403-009-0961-7. [DOI] [PubMed] [Google Scholar]
- 46.Goeb V, Buch MH, Vital EM, Emery P. Costimulation blockade in rheumatic diseases: where we are? Curr Opin Rheumatol. 2009;21:244–250. doi: 10.1097/BOR.0b013e328329a401. [DOI] [PubMed] [Google Scholar]
- 47.Beier KC, Kallinich T, Hamelmann E. T-cell co-stimulatory molecules: novel targets for the treatment of allergic airway disease. Eur Respir J. 2007;30:383–390. doi: 10.1183/09031936.00094406. [DOI] [PubMed] [Google Scholar]
- 48.Ford ML, Larsen CP. Translating costimulation blockade to the clinic: lessons learned from three pathways. Immunol Rev. 2009;229:294–306. doi: 10.1111/j.1600-065X.2009.00776.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Flanagan ME, Blumenkopf TA, Brissette WH, Brown MF, Casavant JM, Shang-Poa C, et al. Discovery of CP-690,550: a potent and selective Janus kinase (JAK) inhibitor for the treatment of autoimmune diseases and organ transplant rejection. J Med Chem. 2010;53:8468–8484. doi: 10.1021/jm1004286. [DOI] [PubMed] [Google Scholar]
- 50.Swanson CD, Akama-Garren EH, Stein EA, Petralia JD, Ruiz PJ, Edalati A, et al. Inhibition of epidermal growth factor receptor tyrosine kinase ameliorates collagen-induced arthritis. J Immunol. 2012;188:3513–3521. doi: 10.4049/jimmunol.1102693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim M, Sun ZY, Byron O, Campbell G, Wagner G, Wang J, et al. Molecular dissection of the CD2-CD58 counter-receptor interface identifies CD2 Tyr86 and CD58 Lys34 residues as the functional "hot spot". J Mol Biol. 2001;312:711–720. doi: 10.1006/jmbi.2001.4980. [DOI] [PubMed] [Google Scholar]
- 52.Bender AT, Spyvee M, Satoh T, Gershman B, Teceno T, Burgess L, et al. Evaluation of a candidate anti-arthritic drug using the mouse collagen antibody induced arthritis model and clinically relevant biomarkers. Am J Transl Res. 2013;5:92–102. [PMC free article] [PubMed] [Google Scholar]
- 53.Williams RO. Collagen-induced arthritis in mice: a major role for tumor necrosis factor-alpha. Methods Mol Biol. 2007;361:265–284. doi: 10.1385/1-59745-208-4:265. [DOI] [PubMed] [Google Scholar]
- 54.Kallinich T, Beier KC, Gelfand EW, Kroczek RA, Hamelmann E. Co-stimulatory molecules as potential targets for therapeutic intervention in allergic airway disease. Clin Exp Allergy. 2005;35:1521–1534. doi: 10.1111/j.1365-2222.2005.02369.x. [DOI] [PubMed] [Google Scholar]
- 55.Kaumaya PT, Foy KC. Peptide vaccines and targeting HER and VEGF proteins may offer a potentially new paradigm in cancer immunotherapy. Future Oncol. 2012;8:961–987. doi: 10.2217/fon.12.95. [DOI] [PMC free article] [PubMed] [Google Scholar]