Abstract
Murine T cells exposed to rapamycin maintain flexibility towards Th1/Tc1 differentiation, thereby indicating that rapamycin promotion of regulatory T cells (Tregs) is conditional. The degree to which rapamycin might inhibit human Th1/Tc1 differentiation has not been evaluated. In the presence of rapamycin, T cell costimulation and polarization with IL-12 or IFNα permitted human CD4+ and CD8+ T cell differentiation towards a Th1/Tc1 phenotype; activation of STAT1 and STAT4 pathways essential for Th1/Tc1 polarity was preserved during mTOR blockade but instead abrogated by PI3 kinase inhibition. Such rapamycin-resistant human Th1/Tc1 cells: (1) were generated through autophagy (increased LC3BII expression; phenotype reversion by autophagy inhibition via 3-MA or siRNA for Beclin 1); (2) expressed anti-apoptotic bcl-2 family members (reduced Bax, Bak; increased phospho-Bad); (3) maintained mitochondrial membrane potentials; and (4) displayed reduced apoptosis. In vivo, type I polarized and rapamycin-resistant human T cells caused increased xenogeneic graft-versus-host disease (x-GVHD). Murine recipients of rapamycin-resistant human Th1/Tc1 cells had: (1) persistent T cell engraftment; (2) increased T cell cytokine and cytolytic effector function; and (3) T cell infiltration of skin, gut and liver. Rapamycin therefore does not impair human T cell capacity for type I differentiation. Rather, rapamycin yields an anti-apoptotic Th1/Tc1 effector phenotype by promoting autophagy.
Keywords: Th1/Tc1, rapamycin, autophagy, apoptosis resistance, xenogeneic GVHD
Introduction
Rapamycin is an immunosuppressive drug that blocks cell surface signaling through inhibition of the kinase, mammalian target of rapamycin (mTOR).1 In previous studies, addition of rapamycin to human T cell cultures preferentially expanded regulatory T cells (Tregs),2 thereby indicating that rapamycin may operate at least in part through a Treg mechanism. However, in murine studies that utilized optimal type I and type II cytokine polarization conditions, we found that T cell differentiation towards both Th2/Tc2 and Th1/Tc1 polarity was preserved in rapamycin.3,4 Rapamycin is an agent that was found to induce T cell anergy even in the setting of co-stimulation;5 as such, our finding that rapamycin-resistant co-stimulated Th1/Tc1 cells mediated increased type I immunity in vivo was relatively unexpected.4 In further studies, we found that the increased in vivo efficacy of rapamycin-resistant T cells was associated with a multifaceted anti-apoptotic phenotype,6 thereby linking the processes of rapamycin- and apoptosis-resistance. As such, depending on the cytokine and co-stimulatory milieu, it appears that rapamycin may exert either an immune inhibition effect (induction of anergy or Tregs) or an immune stimulation effect (induction of effector cells of type I or type II polarity). Given the increasing utilization of mTOR inhibitors in the clinical transplantation setting7,8 and in the oncology setting for therapy of tumors with increased mTOR activation,9 an improved understanding of the role of rapamycin in human T cell modulation is essential. As such, we initiated experiments to address the role of rapamycin on human Th1/Tc1 cell differentiation, and to evaluate the possibility, analogous to our murine results, that rapamycin might induce the generation of anti-apoptotic human Th1/Tc1 cells with increased in vivo effector function.
In light of our murine results, we hypothesized that in the presence of type I polarizing cytokines such as IFNα or IL-12, human T cell differentiation into a Th1/Tc1 phenotype would be preserved. IFNα has previously been demonstrated to promote human T cell differentiation towards a type I cytokine phenotype;10,11 however, others have determined that IL-12 is superior relative to IFNα for promotion of human type I differentiation.12 We evaluated the role of IFNα or IL-12 on human T cell type I polarization through in vitro assays, and also through two in vivo models of xenogeneic graft-versus-host disease (GVHD). First, we developed an LPS-induced, TNFα mediated xenogeneic GVHD model to mimic established murine models of Th1-driven GVHD;13,14 second, we utilized a natural history model similar to other xenogeneic GVHD models described in the literature.15–17 Given the known role of Th1/Tc1 cells for GVHD induction,18 if rapamycin indeed preserved human Th1/Tc1 differentiation, we hypothesized that rapamycin-exposed human Th1/Tc1 cells would mediate robust xenogeneic GVHD.
In addition, we evaluated the effects of rapamycin on the apoptotic threshold of human Th1/Tc1 cells. In some contexts, rapamycin has been shown to promote apoptosis;19,20 however, rapamycin can also reduce apoptosis through upregulation of anti-apoptotic molecules such as bcl-x(L)21 or the Pim kinases.22,23 An improved understanding of the role of rapamycin on human Th1/Tc1 cell apoptosis is an important goal for the experimental clinical field of T cell immunotherapy because successful therapy is reliant on the capacity of ex vivo-generated populations to persist and mediate effector function in vivo after adoptive transfer. CD28-based co-stimulation represents one method of T cell manufacturing that has been extensively evaluated in clinical trials for therapy of HIV disease24 and cancer, including in the setting of autologous25 and allogeneic26,27 hematopoietic stem cell transplantation. Co-stimulation likely promotes T cell in vivo persistence in part because of CD28 upregulation of the anti-apoptotic molecule, bcl-x(L);28 indeed, gene-modified, co-stimulated T cells can persist for months to years in patients after adoptive transfer.29 Also, CD28 co-stimulation promotes a Th1/Tc1 (type I) cytokine profile30 that has in general been associated with enhanced T cell anti-viral and anti-cancer effects (reviewed in ref. 31). Given this background, we established the further goal of identifying whether rapamycin might yield an anti-apoptotic effect in human Th1/Tc1 cells distinct from that achieved with co-stimulation.
Results
IFNα and IL-12 similarly promote human Th1/Tc1 polarity during induction of rapamycin resistance
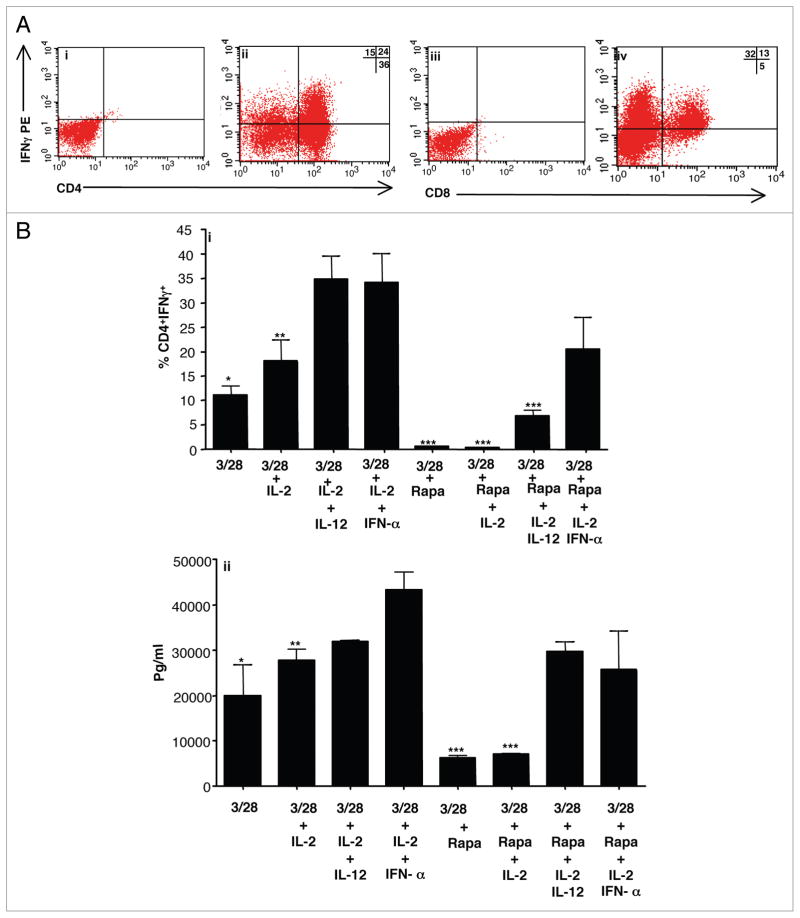
First, we evaluated the effect of IFNα and IL-12 on human T cell differentiation after co-stimulation and culture in rapamycin. In the presence of both rapamycin and IFNα, CD4+ and CD8+ T cells acquired capacity to produce IFNγ (representative data; Fig. 1A, parts i–iv). Relative to co-stimulation alone or co-stimulation with IL-2 addition, the further presence of either IL-12 or IFNα during induction of rapamycin-resistance significantly increased the frequency of CD4+ (Fig. 1B, part i) and CD8+ T cells (not shown) capable of IFNγ secretion. Typically, T cells expanded approximately 10-fold over 6 days in culture without rapamycin (“T1” condition) and only 1- to 2-fold with rapamycin (“T1.R” condition); the presence or absence of type I polarizing cytokines did not significantly influence T cell expansion (data not shown).
Figure 1.
IL-12 or IFNα promote human Th1/Tc1 polarization during induction of rapamycin resistance. Lymphocytes were isolated from normal donors and co-stimulated with anti-CD3, anti-CD28 with six-day culture. In addition to co-stimulation, cytokines were added as indicated: IL-2, IL-12, IFNα and rapamycin. (A) Representative intra-cellular (IC) flow cytometry data for T cells expanded in IL-2 + IFNα + rapamycin (i–iv). On day 6 of culture, T cells were restimulated and stained with surface CD4 (i and ii), surface CD8 (iii and iv), IC isotype control (i and iii), or IC IFNγ (ii and iv). (B) Frequency of CD4+IFNγ+ cells by IC flow cytometry for the specific culture conditions (part i; n = 3 replicates per condition). IFNγ secretion after day 6 repeat co-stimulation (part ii; Multiplex suspension array on culture supernatants, n = 3 per condition). *indicates that observed differences were statistically significant (p < 0.05) as compared to IFNα groups.
Further experiments were performed to evaluate the effect of IL-12 or IFNα either with or without rapamycin during co-stimulation. In the absence of rapamycin, the additional supplementation of IL-12 or IFNα did not increase co-stimulated T cell capacity for IFNγ secretion in a statistically significant manner (Fig. 1B, part ii). In marked contrast, in the presence of rapamycin, addition of IL-12 or IFNα to co-stimulation significantly increased the resultant capacity of T cells to secrete IFNγ (Fig. 1B, part ii). T cells exposed to both rapamycin and type I polarizing cytokines had a similar magnitude of IFNγ secretion relative to T cells expanded in the absence of rapamycin.
IFNα or IL-12 exposure in the presence of rapamycin promotes differentiation of Th1/Tc1 cells with a T central memory phenotype
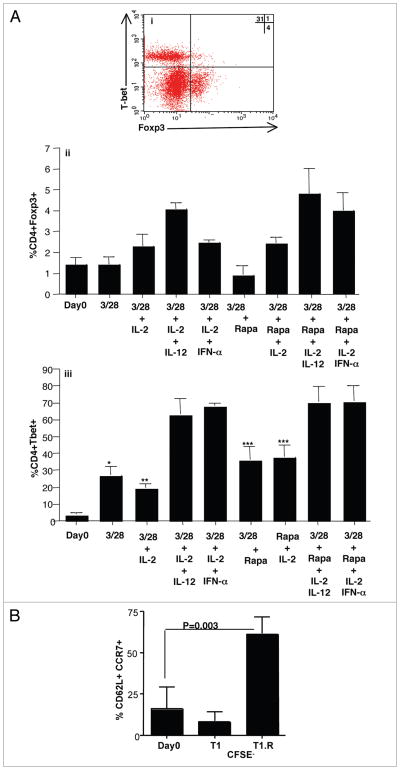
We further evaluated T cell subset differentiation status by flow cytometric expression of the Th1-type transcription factor T-bet relative to the Treg cell transcription factor Foxp3 (representative flow plot, Fig. 2Ai). Co-stimulation, when combined with IL-2 and a type I polarizing cytokine, modestly increased resultant CD4+ T cell expression of Foxp3, with slightly higher values obtained with rapamycin exposure (Fig. 2Aii; frequency of CD4+Foxp3+ cells: increased from median value of 1.5% at culture input to 4.8% after co-stimulation in the presence of IL-2, a type I polarizing cytokine, and rapamycin). In marked contrast, co-stimulation in the presence of either IL-12 or IFNα greatly increased CD4+ T cell expression of T-bet from a median value of 4.1% at culture input to 72.8% after culture (Fig. 2Aiii). This marked increase in T-bet expression was observed with or without rapamycin addition; of note, in the setting of co-stimulation without a type I polarizing cytokine, addition of rapamycin actually increased T-bet expression.
Figure 2.
IL-12 or IFNα in the presence of rapamycin promotes human Th1/Tc1 differentiation and a central memory phenotype. (A) Representative flow plot of T1.R cells evaluating IC expression of T-bet vs. Foxp3 (part i). Summation data evaluating frequency of CD4+Foxp3+ events and CD4+T-bet+ events for each culture condition (parts ii and iii, respectively; n = 3 per condition). (B) T central memory phenotype of cells, as defined by co-expression of CD62L and CCR7, was measured at day 0 using flow cytometry. The input T cells were CFSE-labeled in order to evaluate the effect of T cell proliferation on cell surface marker expression. “CFSE-” indicates that analysis was performed on T cells that had proliferated at least one cell division. *indicates that observed differences were statistically significant (p < 0.05) as compared to IFNα groups.
In previous studies that utilized murine T cells, our group4 and others32 found that mTOR inhibition increased expression of molecules associated with central memory differentiation that promote T cell homing, including CD62L and CCR7. We therefore evaluated T central memory surface marker expression in human lymphocytes prior to culture in rapamycin (Fig. 2B; percent of T cells expressing CD62L+CCR7+: 5.8%; median value, n = 5 donors). At day 6 of culture, T1.R cells and control T1 cells were assessed for expression of these molecules: relative to T1 cells, T1.R cells had increased expression of both CD62L (median values of 47% and 80%) and CCR7 (median values of 25% and 85%). In a separate experiment, starting T cells were CFSE-labeled to allow assessment of these T cell markers relative to the number of proliferative cycles under T1 or T1.R culture conditions. At day 6 of culture, T cells that had not divided (CSFE non-diluted) did not have an increase in CD62L+CCR7+ events relative to the starting T cell population (data not shown). In contrast, T cells in the T1.R condition that were proliferating, as defined by dilution of CSFE dye, had an increased frequency of CD62L+CCR7+ T cells relative to the frequency of CD62L+CCR7+ T cells observed in the proliferating fraction of T cells in the control T1 condition (Fig. 2B; median values of co-expressing T cells, 50% versus 8%, respectively; p = 0.03, n = 3 donors). Of note, in the T1.R condition, similar frequencies of co-expressing cells were observed independent of the number of T cell proliferative cycles (data not shown). In sum, these data indicate that the rapamycin-induced increase in T central memory markers required T cell proliferation; however, because the increase was observed in each proliferative cycle, the rapamycin effect was more consistent with de novo induction of the T central markers rather than a clonal outgrowth of a subpopulation of input T cells.
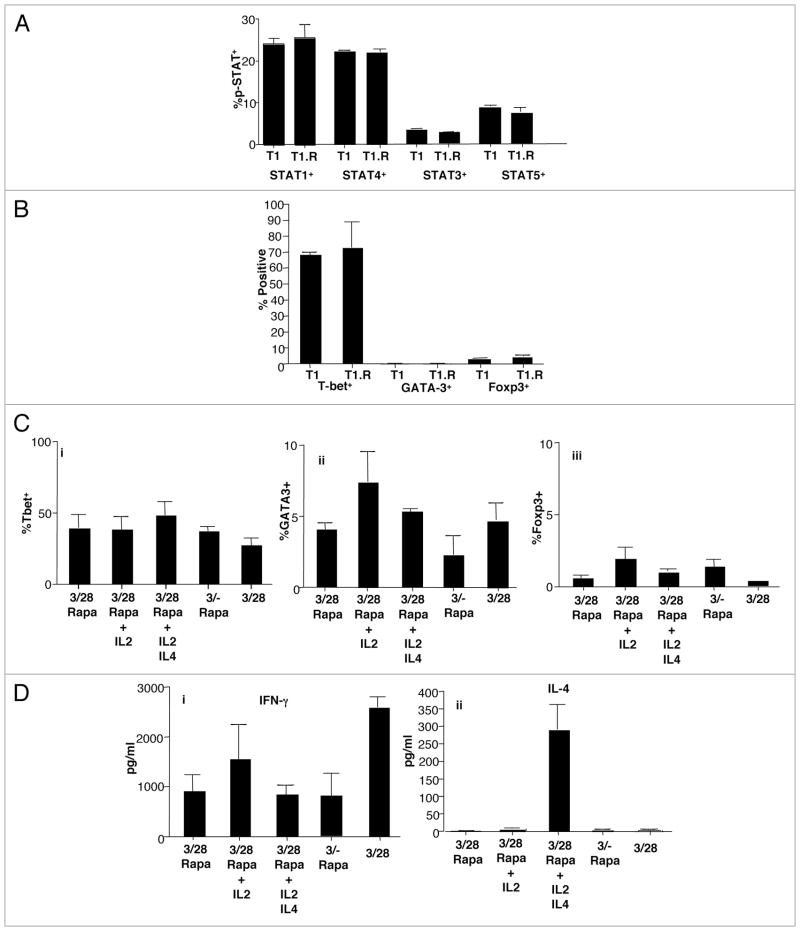
STAT activation, transcription factor expression, and stability of T1 differentiation
Co-stimulation and type I polarization through IFNα addition resulted in activation (phosphorylation) of the two signaling pathways associated with Th1/Tc1 differentiation, STAT1,33 and STAT4,10 (Fig. 3A); this pattern of STAT activation was observed for both T1 and T1.R cells. By comparison, there was a lower level of activation of the STAT3,34 and STAT5,35 pathways that are associated with Th17 and TREG cell differentiation, respectively. Activation of STAT1 and STAT4 in T1 and T1.R cells correlated with high-level expression of T-bet and minimal expression of either Foxp3 or the type II cytokine transcription factor GATA-3 (Fig. 3B). We next evaluated the stability of cytokine polarization by propagation of T1.R cells in culture conditions that might influence T cell differentiation, including high-dose IL-2, high-dose IL-4, lack of co-stimulation, or rapamycin removal. T1.R cells propagated under such conditions did not have greatly altered differentiation status because T-bet expression was maintained, and GATA-3 and Foxp3 expression were not significantly increased above control T1.R cell levels (Fig. 3C). In addition to stable transcription factor expression, T1.R cells propagated under such conditions maintained their capacity for IFNγ secretion (Fig. 3Di); however, exposure of T1.R cells to IL-4 primed for subsequent T1.R cell IL-4 secretion (Fig. 3Dii).
Figure 3.
IFNα induces STAT activation, stable transcription factor changes, and stable Th1/Tc1 polarization. Human T cells were stimulated for 6 days with anti-CD3 and anti-CD28 in media containing IL2 and IFNα either with rapamycin (T1.R) or without rapamycin (T1). (A) Control T1 and T1.R cells at day 6 were evaluated by IC flow for activation (phosphorylation) of STAT1, STAT4, STAT3 and STAT5 (% STAT+ cells; pooled results of n = 3 experiments). (B) Control T1 and T1.R cell expression of T-bet, GATA-3 and Foxp3 (% transcription factor+ cells; n = 3). (C) Day 6 T1.R cells were re-stimulated and maintained in the same culture conditions for an additional six days (first column), expanded in high-dose IL-2 (second column), expanded in high-dose IL-2 plus IL-4 (third column), expanded with lack of co-stimulation (fourth column), or expanded without rapamycin addition (fifth column). On day 12 of culture, the frequency of T cells expressing T-bet, GATA-3 and Foxp3 was determined (parts i–iii, respectively). (D) At day 12 of culture, T cells maintained in these five separate conditions were re-stimulated, and the resultant supernatants were evaluated for content of IFNγ (part i) and IL-4 (part ii).
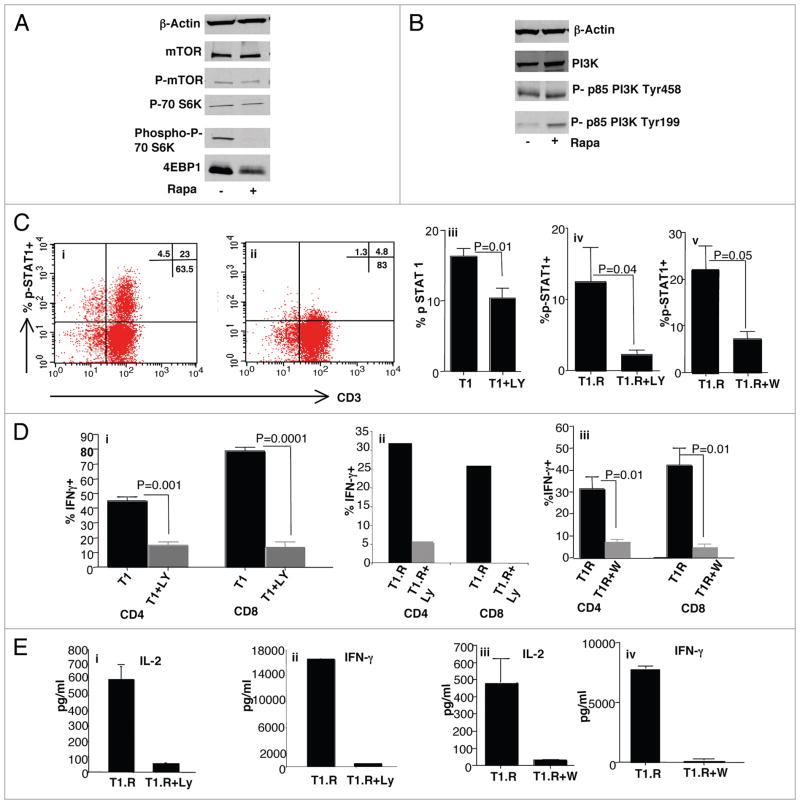
Type I polarization during induction of rapamycin resistance is PI3K-dependent
Experiments were performed to characterize the observed rapamycin resistance in primary human T cells and to further understand the mechanism of type I polarization during rapamycin exposure. First, human Th1/Tc1 cells generated in rapamycin differentiated in a relatively mTOR-independent manner because molecules downstream to mTOR such as the phosphorylated form of p70S6 kinase and 4-EBP1 were down-regulated (Fig. 4A). In light of a previous report that proliferation of rapamycin-resistant human CD8+ T cell clones occurred in a PI3K-dependent manner,21 we hypothesized that the type I polarization that we observed during rapamycin exposure might require intact PI3K signaling. To address this, we first confirmed that T1.R cells maintained PI3K expression in the active, phosphorylated state (Fig. 4B). Second, we evaluated T1.R cells for expression of STAT signaling molecules in the active, phosphorylated state; for this analysis, we focused on type I polarization through IFNα exposure because this cytokine is known to uniquely activate both STAT1 and STAT4 pathways.33 Indeed, both T1 and T1.R cells demonstrated a prompt capacity to phosphorylate STAT1 upon T cell activation in the presence of IFNα; such STAT1 activation was significantly abrogated by inclusion of the PI3K inhibitor, LY294002 [representative example, Fig. 4C (i and ii); summation of data, (iii and iv)]; similar inhibition of STAT1 activation was observed with an alternative PI3K inhibitor, wortmannin [100 μM; Fig. 4C (v)]. T1.R cells also had increased phosphorylation of STAT4, which also occurred in a PI3K-dependent manner (data not shown). Inhibition of PI3K during induction of rapamycin-resistance nearly completely abrogated the capacity of both CD4+ and CD8+ T cells to produce IFNγ [LY, IC flow data with T1, Fig. 4D (i), representative T1.R IC flow cytometry data, (ii); wortmannin, summation of n = 3 experiments, (iii)]. Furthermore, T1.R cell secretion of IL-2 and IFNγ was abrogated by PI3K inhibition [LY, Fig. 4E (i and ii); wortmannin, (iii and iv)]. Addition of wortmannin at significantly lower concentrations (ranging from 10 nM to 1 μM) did not inhibit T1.R cell cytokine production, thereby suggesting that more complete inhibition of PI3K was required to prevent polarization (data not shown). Addition of LY or wortmannin to the T1.R cell cultures did not reduce viability (data not shown).
Figure 4.
Th1/Tc1 polarization during induction of rapamycin resistance requires PI3 kinase. (A) Human Th1/Tc1 cells were generated by co-stimulation and six-day culture in IL-2 + IFNα either alone (“−”) or in the presence of rapamycin (“+”). The protein lysate was obtained and subjected to western blot analysis for the following molecules: β-Actin, mTOR/phospho-mTOR, p70S6 kinase/phospho-p70S6 kinase, and 4EBP1. A similar result was obtained in two additional experiments. (B) Protein lysates from human Th1/Tc1 cells (“−”) or T1.R cells (“+”) were also tested for expression of total PI3 kinase (“PI3K”) or two forms of phosphorylated PI3 kinase (“p85 Tyr458” and “p85 Tyr 199”). A similar result was obtained in two additional experiments. (C) Representative examples of STAT1 phosphorylation in T1.R cells by flow cytometry analysis after restimulation alone (part i) or with the PI3 kinase inhibitor, LY294002 (part ii); summation of results using PI3K inhibition by LY in T1 cells (“LY”; part iii, n = 3), T1.R cells (part iv, n = 3) or by wortmannin (“W”; part v, n = 3). (D) Evaluation of PI3K dependency of type I polarization of T1 cells using LY (part ii, n = 3), T1.R cells using LY (part i; representative example of three experiments); results are expressed as frequency of CD4+ or CD8+ T cells that express IFNγ by IC flow cytometry after re-stimulation. Similar PI3K-dependency was observed using wortmannin as inhibitor (part iii; summation data of n = 3 experiments). (E) PI3K-dependency of T1.R polarization was also evaluated by testing supernatants for IL-2 and IFNγ content (representative example with LY as inhibitor, (parts i and ii); summation of n = 3 experiments using wortmannin as inhibitor, parts iii and iv).
T1.R cells: resistance to apoptosis, bcl-2 family modulation
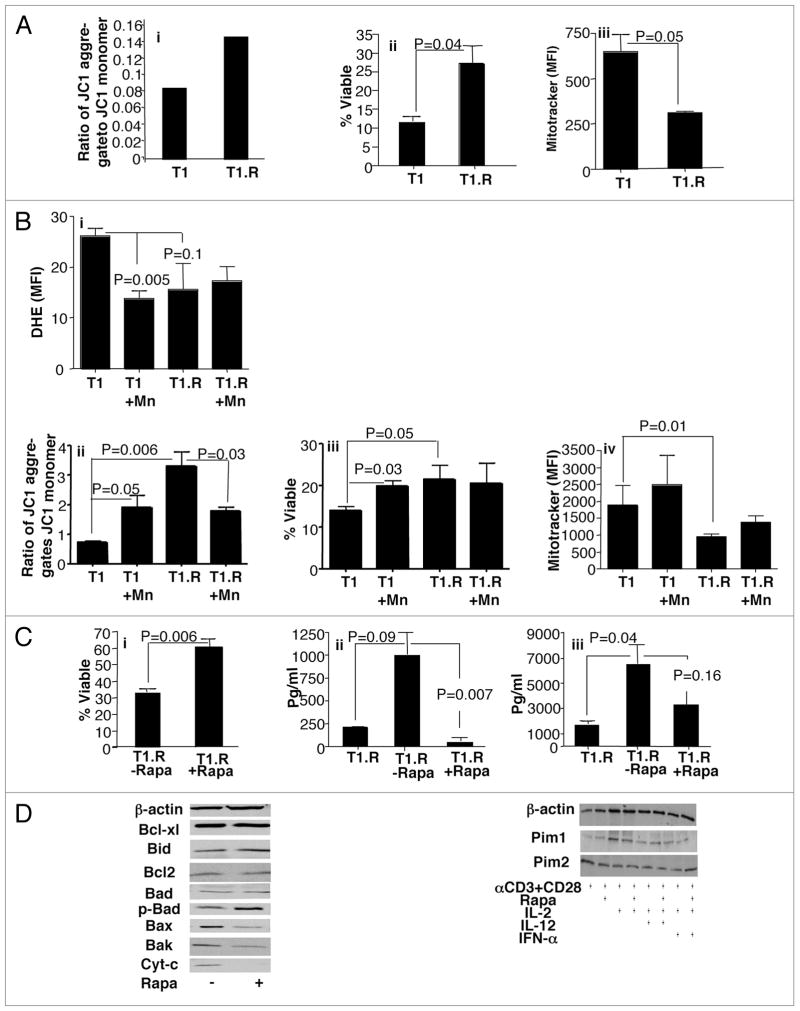
Previously, we found that rapamycin-resistant and polarized murine T cells expressed an anti-apoptotic phenotype.6 Given this information, we hypothesized that T1.R cells would have reduced apoptosis relative to control co-stimulated and polarized Th1/Tc1 cells generated without rapamycin. Upon T cell activation, control Th1/Tc1 cells displayed decreased mitochondrial membrane potential relative to T1.R cells [representative example, Fig. 5A; part i]. Maintenance of the mitochondrial membrane potential in T1.R cells predictably translated into a statistically significant increase in T cell viability (Fig. 5A; part ii). Furthermore, we observed decreased mitochondrial mass (as measured by mitotracker flow cytometry) in T1.R cells relative to control T1 cells (Fig. 4A; part iii). Finally, we evaluated the role of reactive oxygen species (ROS) in the T1.R cell phenotype through use of the ROS inhibitor, MnTBAP (Fig. 5B; parts i–iv). We found that inhibition of ROS resulted in the reversal of the apoptotic phenotype of T1.R cells; this was associated with an increase in T1.R cell mitochondrial mass. The enhanced cell viability in the T1.R condition was partially abrogated by removal of rapamycin from culture (Fig. 5C, part i). Removal of rapamycin also increased T1 cell effector function, as indicated by increased secretion of IL-2 and IFNγ [Fig. 5B (ii and iii)].
Figure 5.
Anti-apoptotic T1.R cells: bcl-2 family balance and role of autophagy. Human Th1/Tc1 cells were generated by co-stimulation, IFNα priming, and culture either without rapamycin (“T1”) or with rapamycin (“T1.R”) for six days. (A) Th1/Tc1 cells were subjected to repeat co-stimulation and evaluated for integrity of mitochondrial membrane potential by flow cytometry, as reflected by maintenance of a high ratio of JC1 aggregates relative to JC1 monomers (part i), representative result from one of three experiments. Percent viability of T1 and T1.R cells was determined by flow cytometry after staurosporine stimulation on day 6 of culture (part ii, data pooled from three independent experiments). Mitochondria mass was measured by flow cytometry using Mitotracker Green (part iii, n = 3). (B) ROS was measured using dihydroethidium (DHE) expression in T1 and T1.R cells. MnTBAP was using to block ROS in some cultures; subsequently, the effect on T cell mitochondrial membrane potential, viability and mitochondria mass were measured. ROS results in T1 and T1.R cells either with or without ROS inhibitor treatment are shown in part i (n = 3).
Mitochondrial membrane potential results are shown in part ii (n = 3). Total T cell viability is shown in (part iii) (n = 3), whereas total mitochondria mass is shown in part iv (n = 3). (C) T1.R cells were harvested on day 6, washed and re-seeded at 0.5 × 106/ml in T1 media either with rapamycin (“+”) or without rapamycin (“−”) for an additional 6 days. At day 12, T1 cells were challenged with staurosporine and the frequency of viable cells was determined (part i; n = 3 experiments). Cytokine secretion capacity was determined on the parent culture at day 6 of culture (T1.R) and at day 12 of culture by testing cytokine content of supernatants generated by repeat co-stimulation (part ii, IL-2 secretion; (part iii), IFNγ secretion). (C) Protein lysate was obtained from control T1 cells (“−”) or T1.R cells (“+”) and subjected to western blot analysis for analysis of bcl-2 family gene member expression (left), and pim kinase expression (right).
In previous murine studies,6 we found that the anti-apoptotic phenotype of rapamycin-resistant T cells was associated with a favorable balance of members of the bcl-2 gene family, specifically an increase in anti-apoptotic bcl-x(L) and a reduction in pro-apoptotic Bid. In the current experiments, we observed a novel pattern of bcl-2 gene family member expression in human T1.R cells: (1) increase in anti-apoptotic phospho-Bad; (2) reduction in pro-apoptotic effectors Bax and Bak; and (3) a resultant decrease in cytochrome c levels (Fig. 5D, left). In addition, human T1.R cells had preserved expression of pim-1 and pim-2 kinases, which confer rapamycin-resistance in murine T cells;23 addition of IL-12 or IFNα did not appear to independently contribute to the expression of the pim kinases (Fig. 5D, right).
Murine T1.R cells and Bcl-2 transgenic T1 cells: similar in vivo phenotype
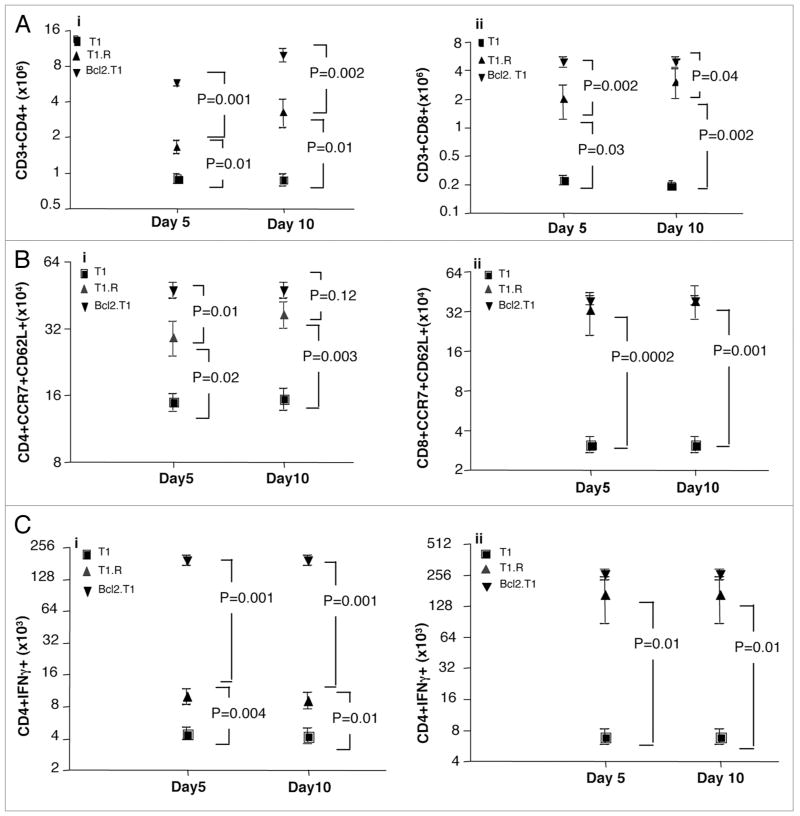
To further address the role of Bcl family genes in the rapamycin-resistant T cell phenotype, we utilized a murine fully allogeneic BMT model to compare the in vivo persistence of wild-type donor T1 cells, Bcl2-transgenic T1 cells, and wild-type T1.R cells. At days 5 and 10 post-BMT, T cell engraftment was increased in recipients of both T1.R cells and Bcl2-transgenic T1 cells relative to recipients of wild-type T1 cells (Fig. 6A, part i, absolute number of CD4+ T cells; part ii, absolute number of CD8+ T cells). Of note, absolute T cell numbers were higher in the transgenic T cell recipients relative to the numbers observed in T1.R cell recipients. Similarly, both T1.R and Bcl2-transgenic T1 cell recipients had an increase in the in vivo number of CD4+ and CD8+ T cells co-expressing the T central memory markers CD62L and CCR7 (Fig. 6B). Finally, both T1.R and Bcl2-transgenic T1 cell recipients had increased numbers of post-BMT CD4+ and CD8+ T cells capable of IFNγ secretion (Fig. 6C). In sum, these data indicate that T1.R cells and Bcl2-transgenic T1 cells have similarly increased in vivo persistence and effector function.
Figure 6.
T1.R cells and Bcl2-transgenic T1 cells: increased in vivo persistence. Murine T1, T1.R and Bcl2.Tg T1 cells were generated and adoptively transferred into lethally irradiated Balb/c mice. (A) At day 5 and day 10 after adoptive transfer, the absolute number of CD4+ Th1 cells (part i) and CD8+ Tc1 cells (part ii, n = 10 per group) were enumerated in the spleen; (B) The absolute number of T cells expressing the central memory markers CD62L and CCR7 was measured by flow cytometry, and the absolute number of post-BMT splenic CD4+ and CD8+ T cells of central memory phenotype were enumerated (parts i and ii, respectively); (C) Post-BMT splenocytes were stimulated for 2 hrs with PMA and ionomycin, which was then followed by a 2 hrs incubation with monensin and brefeldin A to identify cytokine production by intra-cellular flow cytometry. The total number of post-BMT splenic CD4+ and CD8+ T cells capable of IFNγ secretion was then enumerated (parts i and ii, respectively).
Acquisition of T cell rapamycin resistance requires autophagy
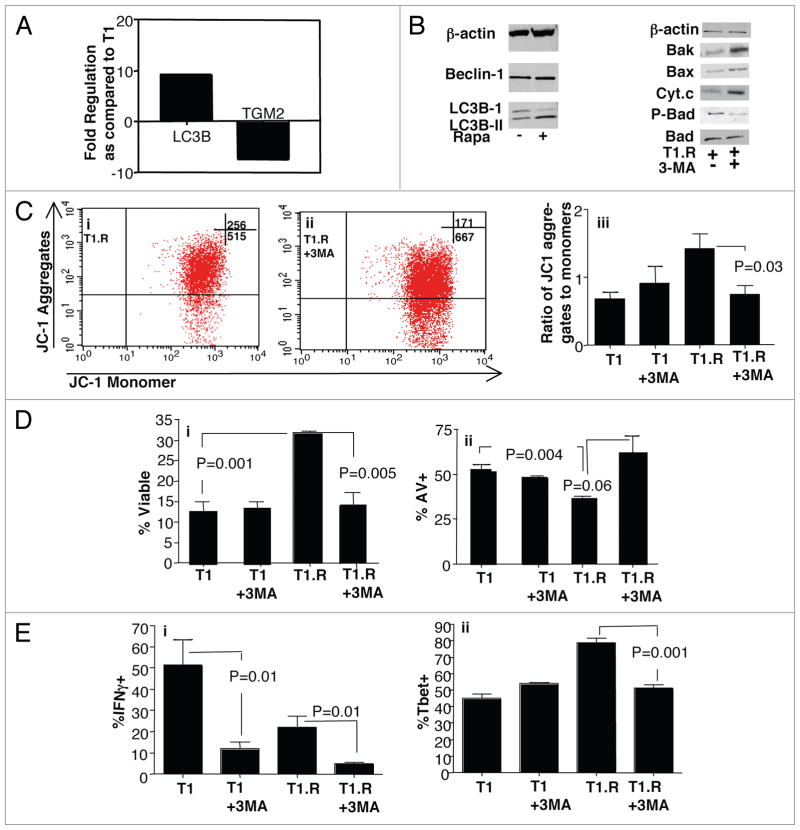
Rapamycin is known to induce autophagy,36 which reduces organelle mass to allow cell survival in nutrient deprived environments such as states of mTOR inhibition (reviewed in ref. 37). We therefore hypothesized that induction of rapamycin-resistance in human Th1/Tc1 cells would be dependent upon autophagy. First, we compared the mRNA expression of 84 autophagy-related genes in T1 and T1.R cells. Out of these 84 genes, only two genes were differentially expressed during induction of rapamycin-resistance. First, LC3B, which is a membrane-bound protein required for autophagosome formation,3,8 was overexpressed in T1.R cells (Fig. 7A; T1.R > T1, p = 0.04). And second, type II transglutaminase (TGM2), which is required for stabilization of apoptosis,39 was greatly underexpressed in T1.R cells (T1 > T1.R, p = 0.02).
Figure 7.
3-MA Modulates the T1.R Cell Anti-Apoptotic Phenotype. (A) RNA was isolated from control T1 and T1.R cells at day 4 of culture; cDNA was then prepared, and PCR array for autophagy gene expression was performed. Results are expressed as fold-increase or fold-decrease in T1.R cells relative to control T1 cells; results shown are the average values of four separate comparisons. (B) Expression of LC3B-II, a marker of autophagy, was also evaluated by western blotting at day 6 of culture (left); in addition, T1.R cells were subjected to treatment with the autophagy inhibitor 3-MA to assess modulation of bcl-2 family gene expression and cytochrome C expression (right). Data shown are representative of three independent experiments. (C) Control T1 or T1.R cells were generated with or without addition of the autophagy inhibitor, 3-MA, and evaluated for mitochondrial membrane stability by JC1 flow cytometry assay. Representative flow data are shown (parts i and ii); data were pooled across three experiments, with results shown in (part iii). (D) Frequency of T1 and T1.R cell viability and apoptosis were assessed by flow cytometry after staurosporine challenge with or without 3-MA addition (parts i and ii, respectively; n = 3 experiments). (E) Effect of 3-MA on polarization of T1 vs. T1.R cells was measured using IC flow cytometry of IFNg (part i, n = 3) and Tbet (part ii, n = 3).
These gene array results indicated that the T1.R cells may have been generated through an autophagocytic process and may manifest an anti-apoptotic phenotype. Further protein analysis was carried out to detect LC3B-II, which is a membrane-bound protein that is formed by conversion of cytosolic LC3B-1 and is required for autophagosome formation.38 Indeed, T1.R cells expressed increased LC3B-II protein and concomitantly had reduced expression of LC3B-I (Fig. 7B; left). To evaluate the functional significance of autophagy during T1.R cell generation, we performed experiments that incorporated an autophagy inhibitor, 3-MA.40 Inhibition of autophagy by 3-MA exposure during T1.R cell generation was associated with reversion to a pro-apoptotic bcl-2 family gene profile, including increased expression of Bak and Bax concomitant with reduced expression of phospho-Bad (Fig. 7B, right). Indeed, T1.R cells expanded in 3-MA had loss of mitochondrial membrane potential during apoptosis challenge [representative data, Fig. 7C (i and ii); summation data, (iii)] and a resultant loss of the T1.R cell anti-apoptotic phenotype by both viability assay [Fig. 7D (i)] and apoptosis assay [Fig. 7D (ii)]. Finally, 3-MA also reduced the capacity of T1 and T1.R cells to secrete IFNγ (Fig. 7E; part i) and reduced T1.R cell expression of the Th1 transcription factor, Tbet (Fig. 7E; part ii).
SiRNA knockdown of beclin 1 abrogates T1.R cell anti-apoptotic phenotype
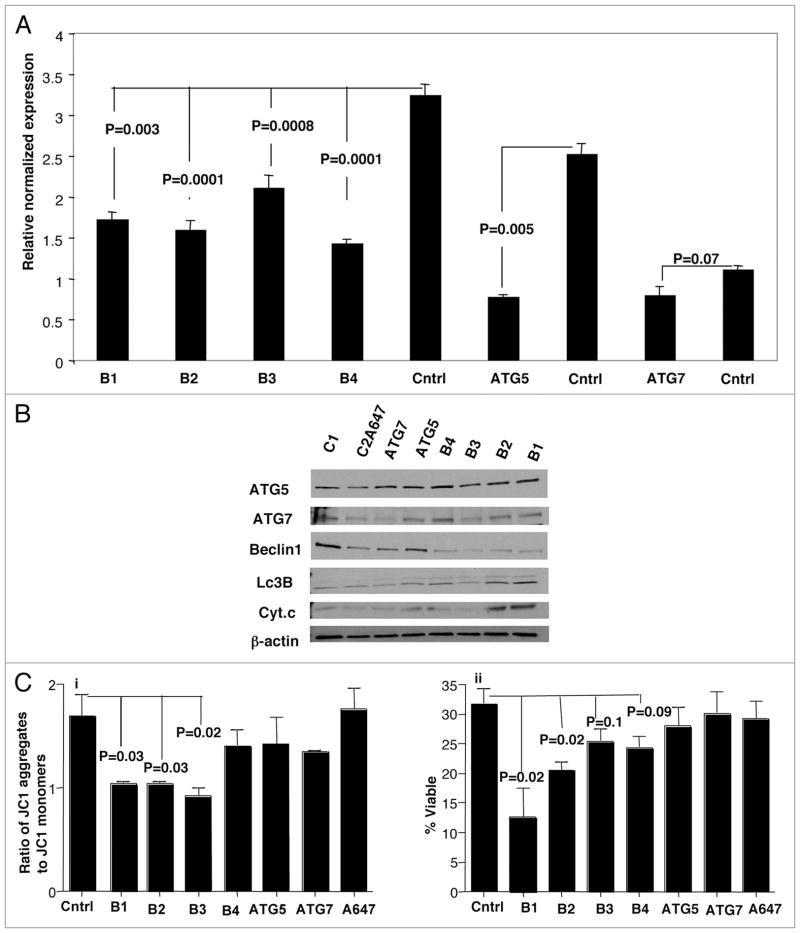
To further understand the role of autophagy in the anti-apoptotic phenotype of T1.R cells, we used siRNA to knockdown autophagy-related genes Beclin 1, ATG5 and ATG7. Molecule knockdown was confirmed by RT-PCR (Fig. 8A); such knockdown was associated with reduced protein expression by western blot analysis, particularly in the case of Beclin siRNA treatment (Fig. 8B). The observed reduction in Beclin 1 protein was associated with reversal of autophagy, as evidenced by the presence of both isoforms of LC3B protein and an increase in cytochrome c levels (Fig. 8B). Finally, functional analysis further confirmed that the anti-apoptotic phenotype was reversed: that is, Beclin inhibition resulted in a decrease in T1.R cell mitochondrial potential (Fig. 8C; part i) and a reduction in T1.R cell viability (Fig. 8C; part ii).
Figure 8.
SiRNA knockdown of autophagy-related genes reverse anti-apoptotic phenotype of T1.R cells. Human Th1/Tc1.R cells were cultured for 3 days. On day 3, cells were electroporated with Beclin 1 (B1, B2, B3 and B4), ATG5, ATG7, AllStars negative control and Alexa Flour 647 SiRNA. (A) On day 6, cells were harvested and mRNA knockdown was measured for by real time PCR. (B) Lysates were also obtained from the control and KO T cells and western blotting was performed for the indicated autophagy proteins and for LC3B. (C) SiRNA-treated T1.R cells were subjected to repeat co-stimulation with staurosporine and evaluated for integrity of mitochondrial membrane potential by flow cytometry (JC1 assay; part i) and viability (part ii).
Type I cytokine polarity augments co-stimulated human T cell capacity to induce lethal, TNFα mediated xenogeneic GVHD
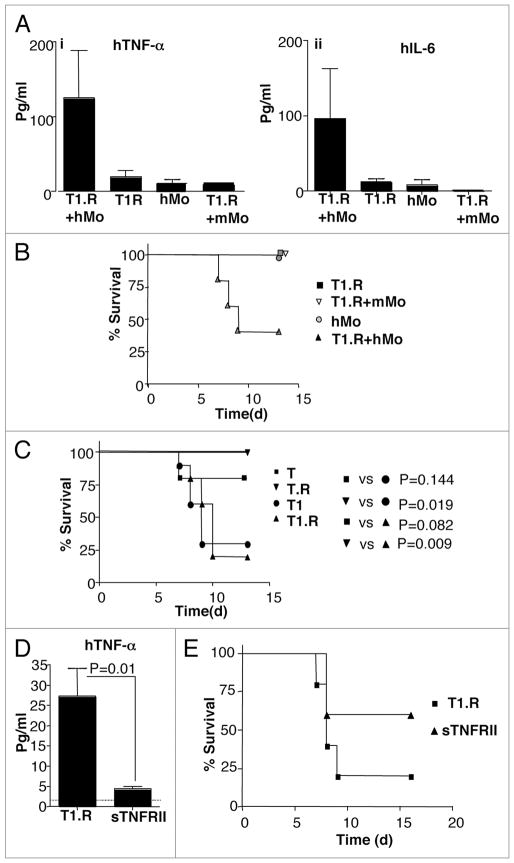
In sum, these in vitro experiments demonstrated that human Th1/Tc1 cells could be generated in rapamycin, and that such cells manifested an anti-apoptotic phenotype due to induction of autophagy. Next, we evaluated whether such human T1.R cells might promote a type I immune response in vivo after adoptive transfer. For this purpose, we developed a model of xenogeneic GVHD that incorporates post-transplant challenge with LPS to mirror established models of type I cytokine-driven murine GVHD.13,14 Such murine models are dependent upon cells of monocyte/macrophage lineage, which secrete pro-inflammatory cytokines such as TNFα upon in vivo LPS challenge and thereby reflect the ‘cytokine storm’ phase of GVHD.41 Given this biology, we hypothesized that co-infusion of human monocytes and T cells would be required to generate LPS-induced lethal x-GVHD. Indeed, only murine recipients of both human monocytes and T cells had significantly elevated serum TNFα and IL-6 after LPS challenge (Fig. 9A) and developed lethality after LPS challenge (Fig. 9B). Using this model, we next tested our hypothesis that human co-stimulated T cells that were polarized towards a Th1/Tc1 phenotype would mediate increased x-GVHD. Indeed, recipients of IFNα primed human T cells generated either with or without rapamycin had an increased incidence of lethal x-GVHD (Fig. 9C); across nine separate experiments, the incidence of lethality in recipients of IFNα polarized human Th1/Tc1 was 82% (58 deaths in 70 recipients). We next evaluated whether the increased TNFα secretion in recipients of type I polarized human T cells played a causative role in the observed lethality: indeed, recipients of type I polarized human Th1/Tc1 cells that were also treated with soluble TNFα receptor had greatly reduced post-LPS serum TNFα (Fig. 9D) and reduced x-GVHD lethality (Fig. 9E). In sum, these data demonstrate that human T1.R cells indeed mediated an in vivo immune response characteristic of type I immunity.
Figure 9.
Type I cytokine polarized co-stimulated human T cells mediate increased LPS-induced, TNFα mediated lethal xenogeneic GVHD. Human Th1/Tc1 cells were generated by co-stimulation ± type I cytokine priming with IFNα (1 M IU/ml) and ±rapamycin to generate four distinct human T cell populations denoted as “T” (no IFNα, no rapamycin), “T.R” (no IFNα, +rapamycin), “T1” (+IFNα, no rapamycin), and “T1.R” (+IFNα, +rapamycin). (A) A pilot experiment was performed to confirm the role of human monocytes for induction of cytokine-mediated GVHD. Rag2−/−γc−/− murine hosts received some combination of human T1.R cells, human monocytes (“hMo”), or murine monocytes (“mMo”), as indicated. On day 5 after adoptive transfer, mice received bacterial LPS; at 90 min post-LPS, serum was obtained and evaluated for human TNFα (part i) and IL-6 (part ii) content by Luminex® assay (results pooled from n = 5 recipients per cohort). (B) Cohorts of mice in this same experiment were also evaluated for post-LPS lethality (n = 5 per cohort). (C) In a separate experiment, Rag2−/−γc−/− murine hosts received human monocytes plus one of the four separate human T cell populations (T, T.R, T1 or T1.R), with subsequent assessment of post-LPS lethality (n = 10 subjects per cohort). (D) To evaluate whether lethality in this model was TNFα mediated, murine host received human monocytes and T1.R cells either alone (“T1.R”) or with in vivo injection of soluble TNF receptor type II (“T1.R + sTNFRII”). On day 5 post-transplant, LPS was injected, and the 90 min post-LPS serum was evaluated for content of human TNFα by Luminex® assay (results pooled from n = 5 recipients per cohort). (E) Cohorts of mice in the same experiment were also evaluated for post-LPS lethality (n = 10 recipients per cohort).
Rapamycin resistance enhances human Th1/Tc1 cell persistence in vivo
In murine studies, we found that RR-Th2 cells manifested an anti-apoptotic phenotype that translated into greatly increased in vivo persistence relative to control Th2 cells not generated in rapamycin.6 We therefore hypothesized that human T1.R cells, which expressed an anti-apoptotic phenotype, would also have increased persistence in vivo after adoptive transfer. To address this, we utilized a natural history model of x-GVHD that did not incorporate exogenous LPS challenge such that long-term engraftment of human Th1/Tc1 cells might be identified.
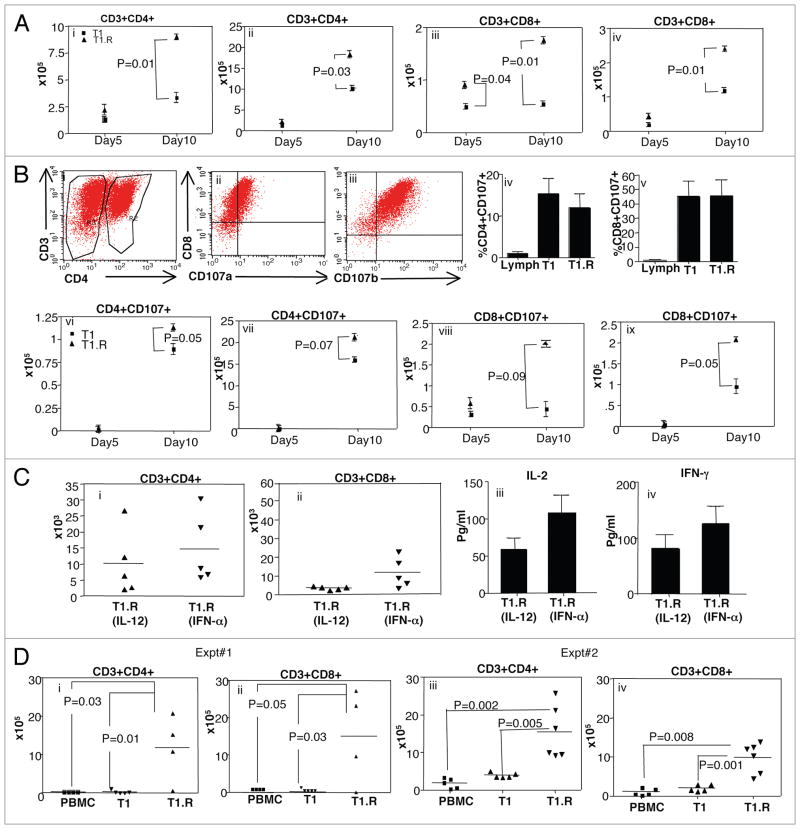
First, we performed a kinetic analysis to determine the in vivo number of T1 vs. T1.R cells present in the spleen and bone marrow at day 5 versus day 10 after adoptive transfer. At day 5 post-infusion, there were comparable numbers human CD4+ T cells in T1 and T1.R cell recipients in both the spleen and bone marrow; however, by day 10 post-infusion, there were significantly greater numbers of human CD4+ T cells in both the spleen and bone marrow in T1.R cell recipients [Fig. 10A (i and ii)]. With respect to in vivo numbers of human CD8+ T cells, recipients of the T1.R cell population had greater human T cell engraftment in the spleen at day 5 post-infusion and in the spleen and bone marrow at day 10 post-infusion [Fig. 10A (iii and iv)]. Second, we evaluated in a separate experiment whether the observed increase in human T cell engraftment in T1.R cell recipients was associated with activation of human T cell effector cytolytic function, as determined by expression of the degranulation marker, CD107.42 A representative example of this flow cytometry analysis is shown [Fig. 10B (i–iii)]; of note, at the time of culture completion just prior to adoptive transfer, T1 and T1.R cells had similar cytolytic activation by CD107 analysis [Fig. 10B (iv and v)]. However, in vivo, relative to recipients of control T1 cells, recipients of T1.R cells had either a statistically significant increase or a trend towards a statistically significant increase in the number of cytolytically active human CD4+ and CD8+ in the spleen and bone marrow at day 10 post-infusion [Fig. 10B (parts vi through ix)].
Figure 10.
T1.R cells have increased persistence in vivo. (A) T1 and T1.R cells were generated and adoptively transferred into immune-deficient murine hosts. At day 5 and day 10 after adoptive transfer, the absolute number of human CD4+ T cells were enumerated in the spleen (part i) and bone marrow (part ii); similarly, the absolute number of human CD8+ T cells were enumerated in the spleen (part iii) and bone marrow (part iv; n = 10 per group). (B) The cytolytic function of T1 and T1.R cells was evaluated by flow cytometric expression of the degranulation marker, CD107 (representative results, parts i–iii). Summation of results is shown in (parts iv and v) (n = 10 per group); “Lymph” indicates results using unmanipulated human T cells. After adoptive transfer the absolute number of CD4+CD107+ and CD8+CD107+ cells in spleen and bone marrow at day5 and day 10 post-infusion were enumerated (parts vi–ix). (C) Human T1.R cells were generated by either priming in IL-12 (“T1.R [IL-12]”) or IFNα (“T1.R[IFNα]”) and injected into Rag2−/−γc−/− murine hosts (n = 5 per cohort). (C) At day 30 post-transplant, the absolute number of human CD3+CD4+ T cells (part i) and CD3+CD8+ T cells (part ii) in the spleen was determined by flow cytometry and cell counting. Also at day 30, serum from transplant recipients was evaluated for IL-2 (part iii) and IFNγ (part iv) content by Luminex® assay. (D) In a separate transplant experiment, murine host received either unmanipulated peripheral blood mono-nuclear cells (“PBMC”), control Th1/Tc1 cells generated by co-stimulation in the presence of IL12 (“T1”), or RR-Th1/Tc1 cells generated by costimulation in the presence of both IL12 and rapamycin (“T1.R”). At day 48 post-transplant, the absolute number of human CD3+CD4+ T cells (part i) and CD3+CD8+ T cells (part ii) in the spleen was determined by flow cytometry and cell counting. (Parts iii and iv) show engraftment results from a second experiment.
Because we had determined that either IL-12 or IFNα enhanced type I polarization of co-stimulated RR-T cells, an additional experiment compared these two methodologies of polarization in terms of long-term engraftment. At day 30 after Th1/Tc1 cell transfer, murine recipients of IL-12 polarized or IFNα polarized human RR-Th1/Tc1 cells had similar absolute numbers of CD4+Th1 cells and CD8+Tc1 cells in vivo (Fig. 10C, parts i and ii); furthermore, T cells isolated from such recipients secreted similar levels of the type I cytokines IL-2 and IFNγ (Fig. 10C, parts iii and iv). A second experiment was performed to determine the effect of ex vivo-induced rapamycin resistance on long-term human Th1/Tc1 cell in vivo persistence; for this experiment, human T cells were co-stimulated in the presence of IL-12 either with or without rapamycin. An additional cohort of PBMC was added to compare the engraftment efficiency of T1.R cells with previously published xenogeneic GVHD models.15 Similar to the first experiment, in vivo persistence of RR-CD4+Th1 and RR-CD8+Tc1 cells was confirmed at day 48 post-infusion; in marked contrast, recipients of IL-12 polarized Th1/Tc1 cells not generated in rapamycin did not persist in vivo [Fig. 10D (i and ii)]. And finally, in a third experiment to evaluate long-term in vivo human T cell engraftment, mice that received T1.R cells polarized with IFNα had significantly greater human CD4+ and CD8+ T cell engraftment relative to recipients of control T1 cells or peripheral blood mononuclear cells [PBMC; Fig. 10D (iii and iv)].
T1.R cells infiltrate GVHD target tissue and cause x-GVHD without LPS challenge
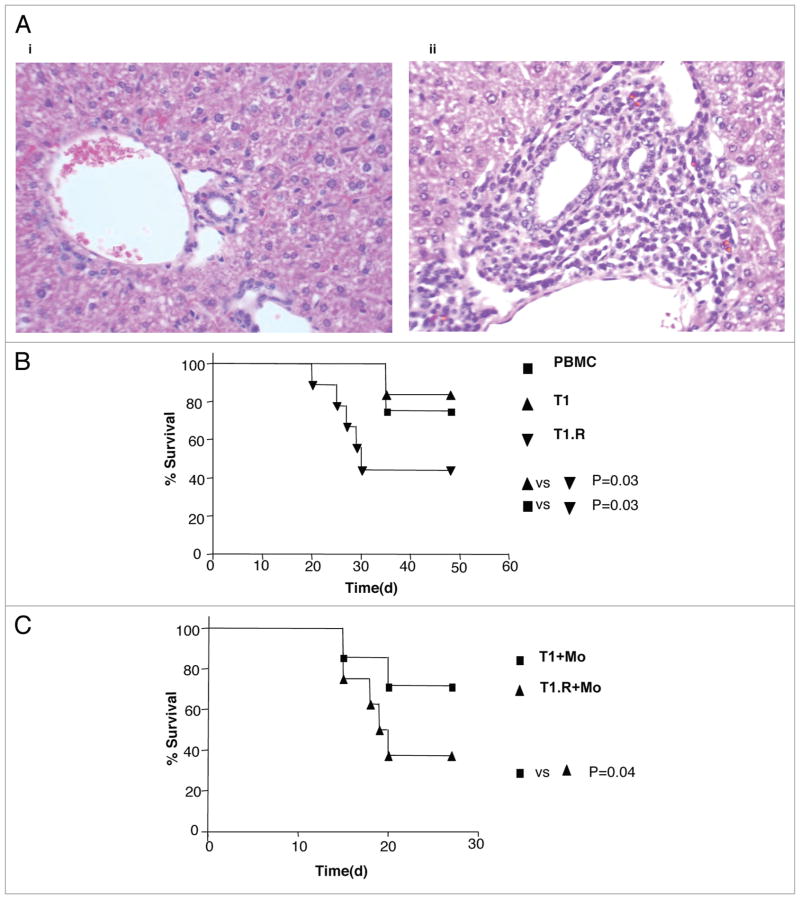
Further in vivo experiments were performed in the non-LPS model to assess human T cell infiltration into GVHD target tissue and to assess lethality in a natural history setting. Murine recipients of T1.R cells, but not control T1 cells, had human T cell infiltration of GVHD target tissues, including skin and intestine (not shown), and liver [Fig. 11A (i and ii)]. In the first natural history experiment, recipients of T1.R cells had an increased incidence of lethal x-GVHD relative to recipients of control T1 cells or PBMC (Fig. 11B). Similarly, in a second experiment, recipients of T1.R cells again had an increased incidence of lethal x-GVHD relative to recipients of control T1 cells (Fig. 11C).
Figure 11.
T1.R cells infiltrate target tissue and cause lethality in a natural history model of x-GVHD. (A) T1 and T1.R cells were adoptively transferred into Rag2−/−γc−/− murine hosts. At day 48, tissue infitration of human T cells were assessed in the liver. Recipients of T1 cells (part i) had no infiltration whereas murine recipients of T1.R had extensive human T cell infiltration (part ii). (B) Experiments were performed to assess the lethality of T1.R cells in a natural history model of x-GVHD. Rag2−/−γc−/− received either unmanipulated human PBMC (10 × 106/recipient), T1 cells (1 × 106/recipient) or T1.R cells (1 × 106/recipient) n = 10 recipients per cohort. (C) In a separate survival experiment, Rag2−/−γc−/− received human monocytes (3 × 106/recipient) along with either T1 cells (1 × 106/recipient) or T1.R cells (1 × 106/recipient).
Discussion
Inhibition of the mTOR pathway in T cells through exposure to the immunosuppressive agent rapamycin has generally resulted in an immune suppressive phenotype, including the induction of anergy,5 regulatory T cells,2,43,44 or a shift from type I to type II cytokine polarity.45 However, in murine studies, we determined that rapamycin, when combined with optimal conditions of co-stimulation and polarizing cytokines, yielded rapamycin-resistant Th1/Tc1 cells that mediated increased in vivo effects after adoptive transfer.4 In this study, we have extended these seemingly paradoxical results to human T cell biology. Specifically, we have determined that human T cells utilize PI3 kinase signaling to attain type I cytokine effector differentiation during mTOR blockade. Indeed, such rapamycin-resistant human Th1/Tc1 cells, through a process of autophagy, possess an anti-apoptotic phenotype that confers an ability to mediate increased type I immune reactions in vivo.
We conclude that ex vivo co-stimulation, which is utilized for adoptive T cell therapy approaches and is generally thought to promote a type I cytokine phenotype,30 can be further enhanced by the induction of rapamycin-resistance and the inclusion of type I polarizing cytokines. In the presence of rapamycin, either IFNα or IL-12 was required to achieve comparable levels of type I cytokine polarization as human T cells generated without rapamycin; by comparison, these cytokines did not dramatically increase type I polarization in the absence of rapamycin. As such, during the growth factor deprived state of mTOR inhibition, addition of polarization signals to CD28 signaling appears to be paramount for attaining type I differentiation. Of note, previous studies that associated ex vivo rapamycin with promotion of human Treg cell differentiation did not incorporate type I polarizing cytokines such as IFNα or IL-12.2,44 We found that polarizing cytokines bypass mTOR blockade by activating STAT molecules associated with type I polarizing cytokine receptors (STAT1 and STAT4) in a PI3 kinase-dependent manner; our results thereby assign a role for PI3 kinase in the function of human rapamycin-resistant T cells with respect to both cellular proliferation21 and now cytokine polarization. In sum, we conclude that rapamycin induction of anergy or regulatory T cell populations in humans is conditional, and can be overcome by signaling via polarizing cytokines.
Our results are also the first to indicate that ex vivo induction of rapamycin-resistance associates with enhanced in vivo persistence of human T cells after adoptive transfer. The enhanced persistence of rapamycin-resistant Th1/Tc1 cells was associated with in vivo human T cell activation in terms of upregulation of both cytokine secretion and cytolytic function, and was associated with increased xenogeneic GVHD in two different model systems. The human-into-murine transplantation data that we have generated thus parallels our recent findings in a murine model, whereby rapamycin-resistant T cells had increased persistence in vivo6 and increased efficacy for abrogation of stem cell allograft rejection.6,46 Based on our results, we project that the previously noted ability of ex vivo co-stimulation to yield prolonged T cell engraftment in clinical trials29 might be further enhanced by induction of rapamycin-resistance.
The human T1.R cells that we studied manifested an anti-apoptotic phenotype that likely contributed to the observed T cell in vivo persistence. These human results mirror our recent finding that the induction of rapamycin resistance in murine Th2/Tc2 cells or murine Th1 cells was associated with the concomitant induction of apoptosis resistance.6 The ability of rapamycin to facilitate human T cell apoptosis resistance on one hand seems paradoxical given the known role of mTOR inhibition to promote antigen-induced cell death;47 on the other hand, our results are consistent with findings that murine T cell states of rapamycin resistance are associated with an anti-apoptotic profile mediated by upregulation of the Pim-1 and Pim-2 kinases.22,48 This study is the first to show that human T1.R cells had preserved Pim-1 and Pim-2 expression, thereby suggesting that preservation of Pim signaling pathways during human T cell rapamycin resistance is not limited to regulatory T cell populations.49
We also determined that the T1.R cell population expressed a unique pattern of bcl-2 family gene expression. Rapamycin-induced alteration of bcl-2 family gene expression for induction of an anti-apoptotic state has been observed in various forms, including upregulation of bcl-2 in human tumor cells,50 upregulation of bcl-x(L) in human CD8+ T cells,21 or combined upregulation of bcl-x(L) and downregulation of pro-apoptotic Bim and Bid in murine Th2/Tc2 cells.6 In contrast to these previously described phenotypes, we have for the first time associated rapamycin-resistance with downregulation of Bax and Bak, which are the most distal apoptosis ‘executioner’ bcl-2 family members51 and essential for T cell regulation in vivo.52 We also found that T1.R cells had decreased cytochrome C content and enhanced stability of mitochondrial membrane polarization; these findings are consistent with the known role of bcl-2 family members for control of apoptosis through a mitochondrial site of action. As such, our data indicate that adoptive T cell therapy using ex vivo co-stimulation, which upregulates anti-apoptotic bcl-x(L),28 can be further enhanced by concomitant incorporation of ex vivo rapamycin, which downregulates the pro-apoptotic executioner molecules Bax and Bak.
Finally, our data indicate that autophagy may play a fundamental role in the induction of human T cell rapamycin resistance. Autophagy is a catabolic process that scavenges cellular organelles under conditions of stress, such as starvation, and can promote either programmed cell death or cell survival.53 In murine T cells, mTOR signaling inhibits autophagy whereas rapamycin inhibition of mTOR promotes autophagy.54 Given this information, we reasoned that the nutrient- and growth-factor deprived state of generating human Th1/Tc1 cells in rapamycin might promote autophagy as a means to preserve a component of T cell survival. Indeed, T1.R cells had an increased rate of conversion of LC3B-I to the LC3B-II form that is necessary for autophagosome formation. We further found that autophagy during induction of human rapamycin resistance was functionally relevant because autophagy inhibition by 3-MA reversed anti-apoptotic bcl-2 family member gene balance and reversed the anti-apoptotic phenotype of T1.R cells. Indeed, knockdown of the autophagy-related gene Beclin 1 reversed the anti-apoptotic phenotype of T1.R, as evidenced by a decrease in mitochondrial membrane potential and viability. As such, we conclude that mTOR blockade during human Th1/Tc1 cell generation induces autophagy, which promotes the survival of an anti-apoptotic T cell population that possesses the capacity to persist in vivo for prolonged intervals after adoptive transfer.
In conclusion, rapamycin induction of immunosuppressive T cell states can be bypassed by the provision of co-stimulation combined with polarizing cytokine signals, which operate through a PI3 kinase pathway. Rapamycin-resistant and type I cytokine polarized T cells are generated through a process of autophagy, which associates with an anti-apoptotic profile that dictates increased in vivo persistence and propagation of type I immunity in vivo. This demonstrable capacity to maintain type I immunity in the setting of rapamycin exposure may offer therapeutic opportunities in clinical settings of rapamycin therapy, and in particular, for adoptive T cell therapy efforts.
Materials and Methods
Mice
Female RAG2−/−γc−/− mice were obtained from (Taconic, 004111-MM-F) and utilized at 8–12 weeks of age. Experiments were performed according to a protocol approved by the NCI Animal Care and Use Committee. Mice were housed in a sterile facility and received sterile water and pellets. Bcl2.Tg mice were kindly provided by Dr. Alfred Singer of the Center for Cancer Research, NCI, NIH. In the xenogenic mice model, as previously reported,15 mice were injected with 0.1 ml chlodronate containing liposomes (Encapsula Nanoscience) for macrophage depletion and given low-dose irradiation (350 cGy).
Antibodies and reagents
X-VIVO 20 media was obtained from BioWhitaker (04-448Q) and AB serum was from Gem Cell (100–318). Anti-CD3, anti-CD28 coated tosyl-activated magnetic beads were manufactured as previously described.30 Rapamycin was from Wyeth (LBA005). Recombinant human (rh) IL-2, rhIL-12 and soluble rhTNFRII were from PeproTech (20002, 20012 and 500p168); rhIFNα was from Schering Plough (0085-0571-02). Bioplex cytokine kits for detection of IL-2, IFNγ, IL-6 and TNFα were from Bio-Rad (M50-00005L3). JC1 (M34152), 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (5(6)-CFDA, SE; CFSE)-mixed isomers (CFSE; C1157), mitotracker (M7514) and DHE (2310) was from Invitrogen. 3-methyladenine (3-MA), bacterial lipopolysacharide (LPS), wortmannin, and LY294002 (LY) were from Sigma (M9281, L2654, W1628 and L9908). The ROS inhibitor manganese (III) tetrakis (5, 10, 15, 20-benzoic acid) porphyrin (MnTBAP) was obtained from Calbiochem (475870). All antibodies were obtained from BD Biosciences unless otherwise stated.
Ex vivo culture of lymphocytes
Normal donor peripheral blood cells were collected by apheresis on an IRB-approved protocol. Lymphocytes and monocytes were isolated by elutriation (Elutra®; Gambro Systems; Lakewood, CO). Lymphocytes were activated by anti-CD3, anti-CD28 co-stimulation (bead:cell ratio, 3:1) as previously described30 and cultured in X-VIVO 20 with 5% heat-inactivated AB serum containing some combination of the following reagents: rapamycin (1 μM), rhIL-2 (20 IU/ml), rhIFN-α2b (1 million IU/ml), and rhIL-12 (20 ng/ml). All reagents were only added at day 0, except for IL-2, which was also added on days 2 and 4. Cultures were initiated at 1.5 × 106 cells/ml and harvested on day 6 for further in vitro or in vivo studies. Polarized T cells generated with and without rapamycin are referred to as “T1.R” and “T1” cells, respectively. For T central memory status experiments, lymphocytes were labeled with CFSE at day 0 and the diluted CFSE fraction was evaluated at day 6 for de novo induction of Tcm in T1.R conditions. For PI3-kinase inhibition experiments, LY294002 (50 μM) or wortmannin (100 μM) were added to the culture 30 min prior to IFNα addition. For autophagy inhibition experiments, 3-MA (5 μM) was added on day 4 of culture. In some experiments, T cells were harvested at day 6 of culture, washed and expanded with IL-2 plus rapamycin or IL-2 alone for an additional 6 days; at that time, T cell viability was assessed. In an experiment to determine the stability of T cell differentiation, T cells were harvested at day 6 of culture, washed and subjected to the following conditions: αCD3 stimulation alone (5 μg/ml; OKT3), αCD3 + αCD28 co-stimulation, co-stimulation plus high-dose IL-2 (100 IU/ml), or co-stimulation plus high-dose IL-2 plus high-dose rhIL4 (1,000 IU/ml); after an additional 6 days of culture, T cells were harvested and the T cell differentiation status was assessed.
Flow cytometry
T cells were washed with PBS supplemented with 0.1% BSA and 0.01% azide (FACS buffer) and stained using anti-: CD3 PB (558117, clone UCHT1), CD8 APC (558117, clone RPA-T8), CD4 Pe-Cy7 (557852, clone S3.5; Caltag), CCR7 PE (clone 150503; R&D Systems [R&D], FAB197P) and CD62L APC-cy7 (clone DREG-56; Biolegend; 304814). Mouse antibodies utilized were CD3 Apc-cy7 (557596), CD4 PB (558107), CD8 pecy5 (553034), CCR7 APC (120107; Biolegend) and CD62L FITC (553150). For intracellular (IC) flow cytometry, fixation and permeabilization buffer was utilized (eBioscience; 00-8222); four-color IC flow cytometry was performed with combinations of anti-: IFNγ PE (clone 4S.B3, 554552), CD3 PB, CD8 Pe-Cy5 (clone HIT8a, 555636) and CD4 APC-Cy7 (clone RPAT4, 557871). For murine T cell IC flow cytometry IFNγ PE (554412) was utilized. IC flow for transcription factors was performed with T-bet A647 (clone: 4B10; ebioscience, 51-5825-80), GATA-3 PE (clone: L50-823, 560074), Foxp3 (clone: 259D; Biolegend; 320208), CD4 APC-cy7 and CD8 PB. Viability was measured using Annexin V APC (550475) and 7AAD (559925) as per manufacturer’s instructions (BD Biosciences). Mitochondrial stability was measured by flow cytometry using the dye JC-1 (Invitrogen). Flow cytometry to assess phosphorylation status of STAT molecules was performed using BD phosphoflow kit containing BD lyse/fix buffer and perm buffer III (558050); STAT4 PE (clone 38; p-stat4, 558249), STAT1 A488 (clone 4a; p-stat1, 560191), STAT3 A647 (clone49; p-stat3, 557815) and STAT5 PB (clone47: p-stat5, 560311) antibodies were utilized. Briefly, cells were incubated for 15 min with IFNα, fixed for 15 min at 37°C with phospholyse buffer, and then fixed in perm buffer III (30 min, 4°). Cells were washed with FACS buffer and stained for STAT and surface markers, incubated at room temperature (20 min), and analyzed with FACSCalibur® and CellQuest® software (BD).
Detection of ROS in T1.R cells
The reactive oxygen species (ROS) in T cells was detected as previously described.55,56 The cells were resuspended in 1 ml of colorless DMEM containing 2.5 μM dihydroethidium (DHE). The cells were incubated at 37°C for 40 min, washed once with medium, resuspended in PBS containing 1% BSA, and then subjected to flow cytometric analysis. In ROS blocking experiments, the ROS inhibitor MnTBAP55–58 was added (100 μM) to cultures and then ROS measured on day 6 after addition.
Measurement of cytokine secretion
T cells were adjusted to 1 × 106 cells/ml and co-stimulated (bead to cell ratio, 3:1). Cell-free 24 hr supernatants were harvested and cytokines detected using Multiplex suspension array. Plasma samples were collected from mice at day 48 post-transplant (in x-GVHD natural history model) or 90 min following LPS injection (in x-GVHD TNFα mediated model). Cytokine content was determined using Luminex bead array.
Autophagy gene array
Total RNA was isolated from cultured cells (Qiagen; RNeasy mini kit). RNA was reverse transcribed using the RT2 first strand kit (SAbiosciences) and real time PCR was carried out using the RT2-real time SYBR master mix and RT2 profiler autophagy PCR array (PAHS 084); this array examines 84 genes that have been associated with autophagy. Analysis was performed using the Web-based PCR array analysis portal (SAbiosciences).
SiRNA knockdown of autophagy-related genes
SiRNA oligonucleotides for Beclin 1 (B1, S100055573; B2, S100055580, B3, S100055587; B4, S100055594), ATG 5 (S102655310), ATG 7 (S102655373) and AllStar Negative control SiRNA (1027281) were purchased from QIagen. Transfection of SiRNA was performed according to the manufacturer’s instructions (Amaxa). Transfected cells were transferred to complete culture media containing rapamycin and harvested for real time PCR, protein and functional assays at day 3 post transfection.
Protein determination by western blot analysis
Protein lysates were obtained at day 6 of T cell culture. Lysates were run on 10–20% SDS-PAGE gels and transferred onto nitrocellulose membrane. Membranes were blocked with 5% milk in TBST buffer (20 mmol/L Tris HCl, 500 mmol/L NaCl, and 0.01% Tweeen 20) and incubated overnight at 4°C with primary antibodies (Ab) in TBST containing either 5% milk or BSA. Immunoreactivity was detected by sequential incubation with HRP-conjugated secondary Ab and enzymatic chemiluminescence (Cell Signaling Technology; 7003). Primary Abs utilized were from Cell Signaling and included anti-: mTOR (2983), phospho-mTOR (2971; Ser2448), p70S6 kinase (9202), phospho-p70S6 kinase (9208; thr421/ser424), 4EBP1 (9452), PI3 kinase (3811), phospho-PI3 Kinase (4228; Tyr468/Tyr199), bcl-xl (2764), bid (2002), bcl-2 (2870), bad (9292), phospho-bad (9297; Ser112), Bax (2772), Bak (3814), cytochrome c (4280), pim-1 (2907), pim-2 (4723), Beclin 1 (3738), LC3B (2775) and β-actin (4967).
Murine BMT experiment
For the murine experiments, input T cells were either from wild-type B6 mice or Bcl2-transgenic mice. T cells were then expanded in T1 culture conditions to generate T1, T1.R, or Bcl2-transgenic T1 cells; ex vivo culture was for 6-days in the absence or presence of rapamycin (10 μM), and included anti-CD3, anti-CD28 co-stimulation in the presence of rhu IL-2 (20 IU/ml), rm IL-12 (20 ng/ml) and anti-IL-4 (clone 11B11). T cells were harvested at day 6, resuspended in HBSS and injected intravenously into lethally-irradiated host Balb/c mice; T1 cell dose was 5 × 106 cells per recipient. At days 5 and 10 post-transplant, splenocytes were harvested and evaluated by flow cytometry to quantify the absolute number of total CD4+ and CD8+ T cells and the absolute number of CD62L+CCR7+ CD4+ and CD8+ T cells per recipient. In addition, post-transplant T cells were evaluated by intracellular flow cytometry to quantify the absolute number of post-transplant CD4+ and CD8+ T cells capable of IFNγ secretion.
Xenogeneic GVHD models
Rag2−/−γc−/− mice were conditioned with chlodronate and radiation, and then injected with human Th1/Tc1 cells (i.v. by retro-orbital method as previously described17). Th1/Tc1 cell dose was either 1 or 10 × 106 cells/recipient, as indicated in figure legends. For the x-GVHD natural history model, mice were observed daily; in one such experiment, mice were euthanized at day 48 post-transplant to quantify human T cell engraftment in the spleen17 and evaluate histologic evidence of T cell infiltration in the skin, liver and intestine. In other experiments, mice were euthanized at days 5 and 10 after adoptive transfer to evaluate the in vivo kinetics of T1 and T1.R cell persistence in vivo and to determine the number of T1 and T1.R cells in vivo that possess cytotoxic potential, as determined by expression of CD107 (CD107a, clone: H4a3, 558661; CD107b, clone: H4B4, 555804).
For the TNFα mediated model of x-GVHD, mice were injected with both human T cells and human monocytes from the same donor (1 × 106 cells or 3 × 106 cells/recipient, as indicated in figure legends). On day 6 after human cell transfer, LPS was injected i.p (250 μg/mice) and plasma was obtained via retro-orbital puncture 90 min following LPS. Mice were then evaluated for clinical signs of cytokine storm (hunched posture, poor mobility, diarrhea); according to protocol, any pre-morbid mice were euthanized. In a separate experiment, an additional cohort received further treatment with soluble rhTNRII (25 μg injected i.p. one hour prior to LPS injection).
Statistical analysis
Flow cytometry and cytokine data were analyzed using student’s 2-tailed t tests. Comparison values of p < 0.05 were considered statistically significant. Survival was determined using Kaplan-Meyer’s test and Wilcoxon signed rank nonparametric test.
Acknowledgments
This work was supported by the Center for Cancer Research, National Cancer Institute, Intramural Research Program.
References
- 1.Sabers CJ, Martin MM, Brunn GJ, Williams JM, Dumont FJ, Wiederrecht G, et al. Isolation of a protein target of the FKBP12-rapamycin complex in mammalian cells. J Biol Chem. 1995;270:815–22. doi: 10.1074/jbc.270.2.815. [DOI] [PubMed] [Google Scholar]
- 2.Battaglia M, Stabilini A, Migliavacca B, Horejs-Hoeck J, Kaupper T, Roncarolo MG. Rapamycin promotes expansion of functional CD4+CD25+FOXP3+ regulatory T cells of both healthy subjects and type 1 diabetic patients. J Immunol. 2006;177:8338–47. doi: 10.4049/jimmunol.177.12.8338. [DOI] [PubMed] [Google Scholar]
- 3.Foley JE, Jung U, Miera A, Borenstein T, Mariotti J, Eckhaus M, et al. Ex vivo rapamycin generates donor Th2 cells that potently inhibit graft-versus-host disease and graft-versus-tumor effects via an IL-4-dependent mechanism. J Immunol. 2005;175:5732–43. doi: 10.4049/jimmunol.175.9.5732. [DOI] [PubMed] [Google Scholar]
- 4.Jung U, Foley JE, Erdmann AA, Toda Y, Borenstein T, Mariotti J, et al. Ex vivo rapamycin generates Th1/Tc1 or Th2/Tc2 Effector T cells with enhanced in vivo function and differential sensitivity to post-transplant rapamycin therapy. Biol Blood Marrow Transplant. 2006;12:905–18. doi: 10.1016/j.bbmt.2006.05.014. [DOI] [PubMed] [Google Scholar]
- 5.Powell JD, Lerner CG, Schwartz RH. Inhibition of cell cycle progression by rapamycin induces T cell clonal anergy even in the presence of costimulation. J Immunol. 1999;162:2775–84. [PubMed] [Google Scholar]
- 6.Mariotti J, Foley J, Jung U, Borenstein T, Kantardzic N, Han S, et al. Ex vivo rapamycin generates apoptosis-resistant donor th2 cells that persist in vivo and prevent hemopoietic stem cell graft rejection. J Immunol. 2008;180:89–105. doi: 10.4049/jimmunol.180.1.89. [DOI] [PubMed] [Google Scholar]
- 7.Antin JH, Kim HT, Cutler C, Ho VT, Lee SJ, Miklos DB, et al. Sirolimus, tacrolimus and low-dose methotrexate for graft-versus-host disease prophylaxis in mismatched related donor or unrelated donor transplantation. Blood. 2003;102:1601–5. doi: 10.1182/blood-2003-02-0489. [DOI] [PubMed] [Google Scholar]
- 8.Armand P, Gannamaneni S, Kim HT, Cutler CS, Ho VT, Koreth J, et al. Improved survival in lymphoma patients receiving sirolimus for graft-versus-host disease prophylaxis after allogeneic hematopoietic stem-cell transplantation with reduced-intensity conditioning. J Clin Oncol. 2008;26:5767–74. doi: 10.1200/JCO.2008.17.7279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meric-Bernstam F, Gonzalez-Angulo AM. Targeting the mTOR signaling network for cancer therapy. J Clin Oncol. 2009;27:2278–87. doi: 10.1200/JCO.2008.20.0766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rogge L, D’Ambrosio D, Biffi M, Penna G, Minetti LJ, Presky DH, et al. The role of Stat4 in species-specific regulation of Th cell development by type I IFNs. J Immunol. 1998;161:6567–74. [PubMed] [Google Scholar]
- 11.Tyler DR, Persky ME, Matthews LA, Chan S, Farrar JD. Pre-assembly of STAT4 with the human IFNalpha/beta receptor-2 subunit is mediated by the STAT4 N-domain. Mol Immunol. 2007;44:1864–72. doi: 10.1016/j.molimm.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ramos HJ, Davis AM, George TC, Farrar JD. IFNalpha is not sufficient to drive Th1 development due to lack of stable T-bet expression. J Immunol. 2007;179:3792–803. doi: 10.4049/jimmunol.179.6.3792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nestel FP, Price KS, Seemayer TA, Lapp WS. Macrophage priming and lipopolysaccharide-triggered release of tumor necrosis factor alpha during graft-versus-host disease. J Exp Med. 1992;175:405–13. doi: 10.1084/jem.175.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fowler DH, Kurasawa K, Husebekk A, Cohen PA, Gress RE. Cells of Th2 cytokine phenotype prevent LPS-induced lethality during murine graft-versus-host reaction. Regulation of cytokines and CD8+ lymphoid engraftment. J Immunol. 1994;152:1004–13. [PubMed] [Google Scholar]
- 15.van Rijn RS, Simonetti ER, Hagenbeek A, Hogenes MC, de Weger RA, Canningavan Dijk MR, et al. A new xenograft model for graft-versus-host disease by intravenous transfer of human peripheral blood mono-nuclear cells in RAG2−/− gammac−/− double-mutant mice. Blood. 2003;102:2522–31. doi: 10.1182/blood-2002-10-3241. [DOI] [PubMed] [Google Scholar]
- 16.Roychowdhury S, Blaser BW, Freud AG, Katz K, Bhatt D, Ferketich AK, et al. IL-15 but not IL-2 rapidly induces lethal xenogeneic graft-versus-host disease. Blood. 2005;106:2433–5. doi: 10.1182/blood-2005-04-1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nervi B, Rettig MP, Ritchey JK, Wang HL, Bauer G, Walker J, et al. Factors affecting human T cell engraftment, trafficking and associated xenogeneic graft-vs-host disease in NOD/SCID beta2mnull mice. Exp Hematol. 2007;35:1823–38. doi: 10.1016/j.exphem.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fowler DH, Breglio J, Nagel G, Eckhaus MA, Gress RE. Allospecific CD8+ Tc1 and Tc2 populations in graft-versus-leukemia effect and graft-versus-host disease. Journal of Immunology. 1996;157:4811–21. [PubMed] [Google Scholar]
- 19.Staruch MJ, Sigal NH, Dumont FJ. Differential effects of the immunosuppressive macrolides FK-506 and rapamycin on activation-induced T-cell apoptosis. Int J Immunopharmacol. 1991;13:677–85. doi: 10.1016/0192-0561(91)90180-f. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Li XC, Zheng XX, Wells AD, Turka LA, Strom TB. Blocking both signal 1 and signal 2 of T-cell activation prevents apoptosis of alloreactive T cells and induction of peripheral allograft tolerance. Nat Med. 1999;5:1298–302. doi: 10.1038/15256. [DOI] [PubMed] [Google Scholar]
- 21.Slavik JM, Lim DG, Burakoff SJ, Hafler DA. Rapamycin-resistant proliferation of CD8+ T cells correlates with p27kip1 downregulation and bcl-xL induction, and is prevented by an inhibitor of phosphoinositide 3-kinase activity. J Biol Chem. 2004;279:910–9. doi: 10.1074/jbc.M209733200. [DOI] [PubMed] [Google Scholar]
- 22.Fox CJ, Hammerman PS, Cinalli RM, Master SR, Chodosh LA, Thompson CB. The serine/threonine kinase Pim-2 is a transcriptionally regulated apoptotic inhibitor. Genes Dev. 2003;17:1841–54. doi: 10.1101/gad.1105003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fox CJ, Hammerman PS, Thompson CB. The Pim kinases control rapamycin-resistant T cell survival and activation. J Exp Med. 2005;201:259–66. doi: 10.1084/jem.20042020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levine BL, Bernstein WB, Aronson NE, Schlienger K, Cotte J, Perfetto S, et al. Adoptive transfer of costimulated CD4+ T cells induces expansion of peripheral T cells and decreased CCR5 expression in HIV infection. Nature Medicine. 2002;8:47–53. doi: 10.1038/nm0102-47. [DOI] [PubMed] [Google Scholar]
- 25.Laport GG, Levine BL, Stadtmauer EA, Schuster SJ, Luger SM, Grupp S, et al. Adoptive transfer of costimulated T cells induces lymphocytosis in patients with relapsed/refractory non-Hodgkin lymphoma following CD34+-selected hematopoietic cell transplantation. Blood. 2003;102:2004–13. doi: 10.1182/blood-2003-01-0095. [DOI] [PubMed] [Google Scholar]
- 26.Fowler DH, Odom J, Steinberg SM, Chow CK, Foley J, Kogan Y, et al. Phase I clinical trial of costimulated, IL-4 polarized donor CD4+ T cells as augmentation of allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2006;12:1150–60. doi: 10.1016/j.bbmt.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 27.Porter DL, Levine BL, Bunin N, Stadtmauer EA, Luger SM, Goldstein S, et al. A phase 1 trial of donor lymphocyte infusions expanded and activated ex vivo via CD3/CD28 costimulation. Blood. 2006;107:1325–31. doi: 10.1182/blood-2005-08-3373. [DOI] [PubMed] [Google Scholar]
- 28.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, et al. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3:87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 29.Mitsuyasu RT, Anton PA, Deeks SG, Scadden DT, Connick E, Downs MT, et al. Prolonged survival and tissue trafficking following adoptive transfer of CD4zeta gene-modified autologous CD4(+) and CD8(+) T cells in human immunodeficiency virus-infected subjects. Blood. 2000;96:785–93. [PubMed] [Google Scholar]
- 30.Levine BL, Bernstein WB, Connors M, Craighead N, Lindsten T, Thompson CB, et al. Effects of CD28 costimulation on long-term proliferation of CD4+ T cells in the absence of exogenous feeder cells. J Immunol. 1997;159:5921–30. [PubMed] [Google Scholar]
- 31.Fowler DH. Shared biology of GVHD and GVT effects: potential methods of separation. Crit Rev Oncol Hematol. 2006;57:225–44. doi: 10.1016/j.critrevonc.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Sinclair LV, Finlay D, Feijoo C, Cornish GH, Gray A, Ager A, et al. Phosphatidylinositol-3-OH kinase and nutrient-sensing mTOR pathways control T lymphocyte trafficking. Nat Immunol. 2008;9:513–21. doi: 10.1038/ni.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Matikainen S, Sareneva T, Ronni T, Lehtonen A, Koskinen PJ, Julkunen I. Interferon-alpha activates multiple STAT proteins and upregulates proliferation-associated IL-2Ralpha, c-myc and pim-1 genes in human T cells. Blood. 1999;93:1980–91. [PubMed] [Google Scholar]
- 34.Yang XO, Panopoulos AD, Nurieva R, Chang SH, Wang D, Watowich SS, et al. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J Biol Chem. 2007;282:9358–63. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 35.Antov A, Yang L, Vig M, Baltimore D, Van Parijs L. Essential role for STAT5 signaling in CD25+CD4+ regulatory T cell homeostasis and the maintenance of self-tolerance. J Immunol. 2003;171:3435–41. doi: 10.4049/jimmunol.171.7.3435. [DOI] [PubMed] [Google Scholar]
- 36.Blommaart EF, Luiken JJ, Blommaart PJ, van Woerkom GM, Meijer AJ. Phosphorylation of ribosomal protein S6 is inhibitory for autophagy in isolated rat hepatocytes. J Biol Chem. 1995;270:2320–6. doi: 10.1074/jbc.270.5.2320. [DOI] [PubMed] [Google Scholar]
- 37.Mizushima N, Levine B, Cuervo AM, Klionsky DJ. Autophagy fights disease through cellular self-digestion. Nature. 2008;451:1069–75. doi: 10.1038/nature06639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, et al. LC3, a mammalian homologue of yeast Apg8p, is localized in autophagosome membranes after processing. EMBO J. 2000;19:5720–8. doi: 10.1093/emboj/19.21.5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toth B, Garabuczi E, Sarang Z, Vereb G, Vamosi G, Aeschlimann D, et al. Transglutaminase 2 is needed for the formation of an efficient phagocyte portal in macrophages engulfing apoptotic cells. J Immunol. 2009;182:2084–92. doi: 10.4049/jimmunol.0803444. [DOI] [PubMed] [Google Scholar]
- 40.Seglen PO, Gordon PB. 3-Methyladenine: specific inhibitor of autophagic/lysosomal protein degradation in isolated rat hepatocytes. Proc Natl Acad Sci USA. 1982;79:1889–92. doi: 10.1073/pnas.79.6.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferrara JL, Abhyankar S, Gilliland DG. Cytokine storm of graft-versus-host disease: a critical effector role for interleukin-1. Transplant Proc. 1993;25:1216–7. [PubMed] [Google Scholar]
- 42.Betts MR, Brenchley JM, Price DA, De Rosa SC, Douek DC, Roederer M, et al. Sensitive and viable identification of antigen-specific CD8+ T cells by a flow cytometric assay for degranulation. J Immunol Methods. 2003;281:65–78. doi: 10.1016/s0022-1759(03)00265-5. [DOI] [PubMed] [Google Scholar]
- 43.Zeiser R, Leveson-Gower DB, Zambricki EA, Kambham N, Beilhack A, Loh J, et al. Differential impact of mammalian target of rapamycin inhibition on CD4+CD25+Foxp3+ regulatory T cells compared with conventional CD4+ T cells. Blood. 2008;111:453–62. doi: 10.1182/blood-2007-06-094482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Strauss L, Czystowska M, Szajnik M, Mandapathil M, Whiteside TL. Differential responses of human regulatory T cells (Treg) and effector T cells to rapamycin. PLoS One. 2009;4:5994. doi: 10.1371/journal.pone.0005994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Blazar BR, Taylor PA, Panoskaltsis-Mortari A, Vallera DA. Rapamycin inhibits the generation of graft-versus-host disease- and graft-versus-leukemia-causing T cells by interfering with the production of Th1 or Th1 cytotoxic cytokines. J Immunol. 1998;160:5355–65. [PubMed] [Google Scholar]
- 46.Mariotti J, Foley J, Ryan K, Buxhoeveden N, Kapoor V, Amarnath S, et al. Graft rejection as a Th1-type process amenable to regulation by donor Th2-type cells through an IL-4/STAT6 pathway. Blood. 2008 doi: 10.1182/blood-2008-05-154278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sandalova E, Wei CH, Masucci MG, Levitsky V. Regulation of expression of Bcl-2 protein family member Bim by T cell receptor triggering. Proc Natl Acad Sci USA. 2004;101:3011–6. doi: 10.1073/pnas.0400005101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hammerman PS, Fox CJ, Birnbaum MJ, Thompson CB. Pim and Akt oncogenes are independent regulators of hematopoietic cell growth and survival. Blood. 2005;105:4477–83. doi: 10.1182/blood-2004-09-3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basu S, Golovina T, Mikheeva T, June CH, Riley JL. Cutting edge: Foxp3-mediated induction of pim 2 allows human T regulatory cells to preferentially expand in rapamycin. J Immunol. 2008;180:5794–8. doi: 10.4049/jimmunol.180.9.5794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Calastretti A, Rancati F, Ceriani MC, Asnaghi L, Canti G, Nicolin A. Rapamycin increases the cellular concentration of the BCL-2 protein and exerts an anti-apoptotic effect. Eur J Cancer. 2001;37:2121–8. doi: 10.1016/s0959-8049(01)00256-8. [DOI] [PubMed] [Google Scholar]
- 51.Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–30. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rathmell JC, Lindsten T, Zong WX, Cinalli RM, Thompson CB. Deficiency in Bak and Bax perturbs thymic selection and lymphoid homeostasis. Nat Immunol. 2002;3:932–9. doi: 10.1038/ni834. [DOI] [PubMed] [Google Scholar]
- 53.Lum JJ, Bauer DE, Kong M, Harris MH, Li C, Lindsten T, et al. Growth factor regulation of autophagy and cell survival in the absence of apoptosis. Cell. 2005;120:237–48. doi: 10.1016/j.cell.2004.11.046. [DOI] [PubMed] [Google Scholar]
- 54.Li C, Capan E, Zhao Y, Zhao J, Stolz D, Watkins SC, et al. Autophagy is induced in CD4+ T cells and important for the growth factor-withdrawal cell death. J Immunol. 2006;177:5163–8. doi: 10.4049/jimmunol.177.8.5163. [DOI] [PubMed] [Google Scholar]
- 55.Chen W, Frank ME, Jin W, Wahl SM. TGFbeta released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity. 2001;14:715–25. doi: 10.1016/s1074-7613(01)00147-9. [DOI] [PubMed] [Google Scholar]
- 56.Amarnath S, Dong L, Li J, Wu Y, Chen W. Endogenous TGFβ Activation by Reactive Oxygen Species Is Key to Foxp3 Induction in TCR-Stimulated and HIV-1-Infected Human CD4+CD25− T Cells. Retrovirology. 2007;4:57. doi: 10.1186/1742-4690-4-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hildeman DA, Mitchell T, Teague TK, Hanson P, Day BJ, Kappler J, et al. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10:735–44. doi: 10.1016/s1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 58.Hildeman DA. Regulation of T-cell apoptosis by reactive oxygen species. Free Radic Biol Med. 2004;36:1496–504. doi: 10.1016/j.freeradbiomed.2004.03.023. [DOI] [PubMed] [Google Scholar]