Abstract
Rationale
Endothelial dysfunction is a characteristic feature of diabetes and obesity in animal models and humans. Deficits in nitric oxide production by endothelial nitric oxide synthase (eNOS) are associated with insulin resistance, which is exacerbated by high fat diet. Nevertheless, the metabolic effects of increasing eNOS levels have not been studied.
Objective
The current study was designed to test whether overexpression of eNOS would prevent diet-induced obesity and insulin resistance.
Methods and Results
In db/db mice and in high fat-fed wild-type (WT) C57BL/6J mice, the abundance of eNOS protein in adipose tissue was decreased without significant changes in eNOS levels in skeletal muscle or aorta. Mice overexpressing eNOS (eNOS-TG mice) were resistant to diet-induced obesity and hyperinsulinemia, although systemic glucose intolerance remained largely unaffected. In comparison with WT mice, high fat-fed eNOS-TG mice displayed a higher metabolic rate and attenuated hypertrophy of white adipocytes. Overexpression of eNOS did not affect food consumption or diet-induced changes in plasma cholesterol or leptin levels, yet plasma triglycerides and fatty acids were decreased. Metabolomic analysis of adipose tissue indicated that eNOS overexpression primarily affected amino acid and lipid metabolism; subpathway analysis suggested changes in fatty acid oxidation. In agreement with these findings, adipose tissue from eNOS-TG mice showed higher levels of PPAR-α and PPAR–γ gene expression, elevated abundance of mitochondrial proteins, and a higher rate of oxygen consumption.
Conclusions
These findings demonstrate that increased eNOS activity prevents the obesogenic effects of high fat diet without affecting systemic insulin resistance, in part, by stimulating metabolic activity in adipose tissue.
Keywords: Diabetes, mitochondria, metabolism, adipose tissue, cardiovascular disease, obesity, endothelial nitric oxide synthase
INTRODUCTION
Obesity and type 2 diabetes (T2D) have become major health challenges worldwide. Current data show that approximately 1.5 billion adults aged 20 years or older are overweight, and 10% are obese1. In the US, one-third of the population meets the criteria for metabolic syndrome2, 3. While lifestyle changes and lack of exercise are important risk factors for weight gain4, 5, excessive caloric intake appears to be one key factor fueling the epidemic of obesity. Poor dietary habits negatively affect a broad range of cardiovascular functions and promote the onset of T2D3.
Although it is currently believed that obesity results from excessive nutrient consumption6, 7, i.e., more calories are ingested than are utilized, recent evidence suggests that the balance between nutrient intake and energy expenditure is complex and is regulated by many inter-dependent mechanisms7. Several studies indicate that obesity and insulin resistance may be distinct sequelae of nutrient excess8. Hence, to stem the tide of the epidemics of T2D and obesity, it is important to understand the relationship between obesity and insulin resistance as well as the physiological processes that regulate their development.
Accumulating evidence suggests that the vascular endothelium regulates insulin action. In humans, states of obesity and insulin resistance are characterized by endothelial dysfunction, impaired vasodilation and insulin resistance9; and in rats, inhibition of endothelial nitric oxide synthase (eNOS) decreases insulin-stimulated uptake of glucose by skeletal muscle, suggesting that eNOS may be a key regulator of metabolic homeostasis. This role of eNOS is further corroborated by the observations that in mice deletion of the eNOS gene induces insulin resistance10, 11 and impairs fatty acid oxidation12. Nevertheless, the role of eNOS in regulating metabolic changes that contribute to obesity under conditions of nutrient excess is not well understood. In particular, it is unclear whether eNOS could prevent or attenuate diet-induced adiposity and insulin resistance.
To understand the metabolic role of eNOS, we studied the effects of high fat diet in mice overexpressing eNOS. Our hypothesis was that increasing eNOS levels mitigates the effects of high fat feeding by regulating adipose tissue metabolism. We found that eNOS-transgenic (eNOS-TG) mice were resistant to diet-induced weight gain, but not glucose intolerance. These findings reveal a new anti-obesogenic role of eNOS and its favorable influence on adipose tissue metabolism.
METHODS
Animal studies
The B6.BKS(D)-Leprdb/J (db/db) mice and C57BL/6J (wild-type; WT) mice were purchased from The Jackson Laboratory (Bar Harbor, ME). The eNOS-TG mice, which express bovine eNOS under the control of the preproendothelin-1 promoter13, were maintained on the C57BL/6J background. At 8 weeks of age, male mice were placed on either a 10% low fat diet (LFD; Research Diets, Inc., #D12450B) or a 60% high fat diet (HFD; Research Diets Inc., #D12492) and maintained for 6–15 additional weeks. Water and diet were provided ad libitum. Body weights were recorded weekly. During the 7th and 13th weeks of feeding, glucose and insulin tolerance tests were performed. Pyruvate tolerance tests were performed only after the 13th week of feeding; all other parameters were evaluated after euthanasia. All procedures were approved by the University of Louisville Institutional Animal Care and Use Committee.
Expression analyses
Tissue homogenates were prepared and used for Western blot protein expression analysis. For quantitative RT-PCR, RNA extracted from tissues was used to assess pgc1a, cytb6, gapdh, ppara, and pparg expression using commercially available primers (SABiosciences, Valencia, CA).
Glucose, insulin, and pyruvate tolerance tests
As described before14, glucose tolerance tests were performed following a 6 h fast by injection (i.p.) of D-glucose (1 mg/g) in sterile saline. Insulin tolerance tests were performed on nonfasted animals by i.p. injection of 1.5 U/kg Humulin R (Eli Lilly, Indianapolis, IN). After a 6 h fast, pyruvate tolerance tests were performed as described15.
Biochemical analyses
Plasma lipids, proteins, and metabolites were measured using a Cobas Mira Plus 5600 Autoanalyzer (Roche, Indianapolis, IN) or Luminex kits (Millipore, Billerica, MA, USA). Plasma levels of non-esterified free fatty acids and glycerol were measured by ELISA (Wako Chemicals, Richmond, VA and Cayman Chemical, Ann Arbor, MI, respectively). Nitrite and nitrate levels were measured as described16.
Adipocyte size measurements
Adipose tissue excised at the time of euthanasia was either snap-frozen at −80°C or fixed in 10% formalin (Leica), paraffin-embedded, and sectioned. The sections were stained in hematoxylin and eosin. Adipocyte cross-sectional area was measured using the Nikon Elements software. To assess relative mitochondrial abundance, the sections were stained with MitoID Red (Enzo Life Sciences, Farmingdale, NY). Crown-like structures and inflammatory cells indicative of adipose tissue inflammation were measured as described before17, 18.
Body composition and calorimetry
Body composition was measured by dual-energy X-ray absorptiometry using a mouse densitometer (PIXImus2; Lunar, Madison, WI). Whole body energy expenditure; respiratory exchange ratio; food consumption; and locomotion, ambulatory and fine movements were measured using a physiological/metabolic cage system (TSE PhenoMaster System, Bad Homberg, Germany).
Immunostaining of adipose tissue
Capillary density was quantified in paraffin-embedded sections using fluorescently labeled isolectin B4 as described19. Nitrotyrosine adducts were measured in paraffin-embedded tissues using anti-nitrotyrosine and goat-anti-rabbit IgG-Cy3 antibodies.
Adipose tissue bioenergetic measurements
The oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) of intact adipose tissue explants were measured using a Seahorse XF24 analyzer (Seahorse Bioscience, Billerica, MA). Briefly, freshly isolated epididymal adipose tissue was rinsed with unbuffered DMEM (Dulbecco’s modified Eagle’s medium, pH 7.4). The adipose tissue was cut into sections, and 10 mg were placed in each well of an XF 24 Islet Capture Microplate (Seahorse Bioscience, Billerica, MA). The tissue was then covered with a screen, which allows free perfusion while minimizing tissue movement. Unbuffered DMEM (500 μl) supplemented with 50 μM BSA-conjugated palmitic acid, 200 μM L-carnitine, and 2.5 mM D-glucose was then added to each well. At least two replicates from each animal were used for the assay, and each tissue section was examined to ensure absence of large vessels (which can skew oxygen consumption measurements). The plate was incubated at 37°C in a non-CO2 incubator for 1 h prior to extracellular flux analysis. After three baseline measurements, a mixture of antimycin A (10 μM) and rotenone (1 μM) was injected. Following injection, the OCR was closely monitored until the rates stabilized, and then the experiment was terminated.
Metabolomic analysis of adipose tissue
Epididymal adipose tissue was used for metabolic analysis. After tissue harvest, the metabolites were extracted in methanol and subjected to metabolic profiling by UHPLC/MS/MS and GC/MS20, 21. Details of sample preparation and data analysis are described in the Online Supplement.
Statistical analyses
Data are mean ± SEM. Multiple groups were compared using one-way or two-way ANOVA, followed by Bonferroni post-tests. Unpaired Student’s t test was used for direct comparisons. Statistical analysis of metabolic profiling is described in the online Supplement; p<0.05 was considered significant.
RESULTS
Nutrient excess alters eNOS abundance
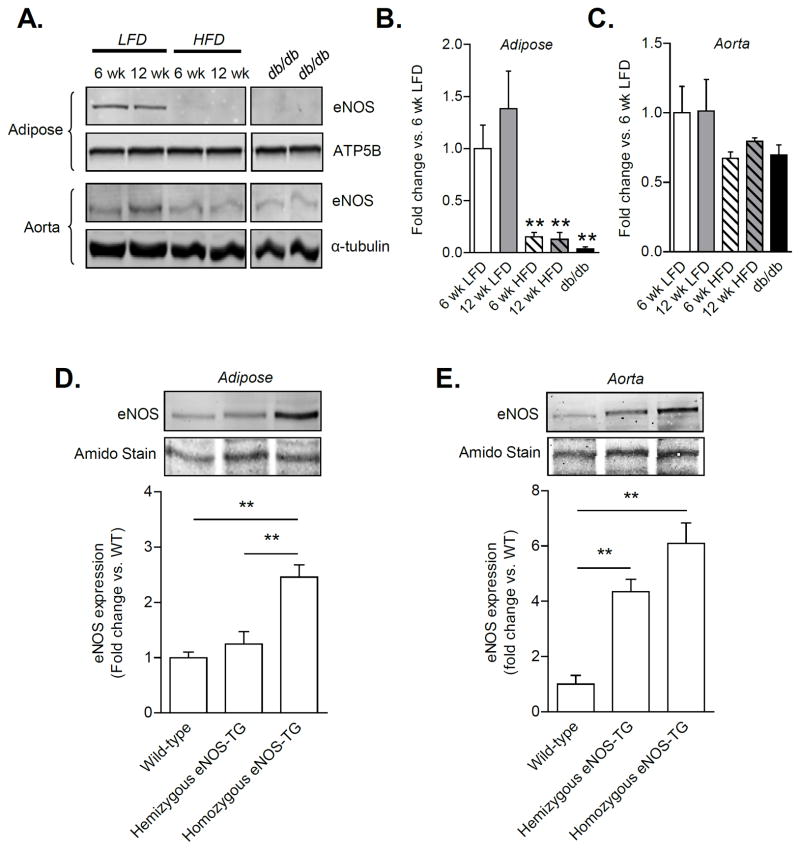
To study the effects of obesity and diabetes on eNOS protein levels, C57BL/6J mice were placed on a high fat diet22, and db/db mice were used as a model of T2D23. High fat feeding for 6 and 12 weeks resulted in a profound decrease in eNOS levels in adipose tissue (Fig. 1A,B), with no statistically significant changes in the aorta (Fig. 1A,C) or skeletal muscle (Online Fig. I-A,B). Similar changes were observed in 20 week old db/db mice, in which eNOS in the adipose tissue was undetectable despite a lack of change in eNOS levels in most other tissues. Interestingly, eNOS expression was increased in the hearts of db/db mice (Online Fig. I-A,C), which might be a compensatory change in response to an increase in NO demand. These data show that both obesity and diabetes result in tissue-specific changes in eNOS expression with a profound and selective decrease in eNOS levels in the adipose tissue. This decrease in eNOS in adipose tissue is consistent with previous reports in obese humans24, 25 and in mouse models of obesity26, indicating that the expansion of adipose tissue establishes a state of chronic eNOS deficiency.
Figure 1. Nutrient excess alters tissue eNOS levels.
Tissue levels of eNOS from mice fed a low fat (LFD) or high fat diet (HFD) for 6 or 12 weeks; age-matched db/db mice were included as an additional model of T2D: (A) Representative Western blots of eNOS from epididymal adipose tissue and aorta. (B,C) Quantification of eNOS expression from panel A. n = 3–4 per group;**p<0.01 vs. 6 week LFD. (D,E) Levels of eNOS in adipose tissue and aorta from wild-type (WT), littermate eNOS-TG hemizygous, and eNOS-TG homozygous mice. n = 3 per group; **p<0.01 vs. indicated groups.
Overexpression of eNOS prevents diet-induced obesity
To examine the role of eNOS, we used eNOS-TG mice13. Previous studies have shown that these mice reproduce in a Mendelian fashion, maintain normal growth characteristics, and are protected from numerous pathologies including myocardial27, hepatic28, lung29, and vascular injury30 as well as sepsis31. In comparison with WT mice, hemizygous mice showed a 4-fold increase in eNOS levels in the aorta, with no significant change in eNOS levels in the adipose tissue (Fig. 1D,E). In contrast, in homozygous mice there was a 2-fold increase in eNOS in the adipose tissue and a 6-fold increase in the aorta. The eNOS in TG animals localized exclusively with isolectin staining (Online Fig. II), indicating that the transgene was expressed only in the vasculature13, 32. Plasma from eNOS-TG mice showed increased L-citrulline and nitrite levels when compared with WT mice (Online Fig. III-A,C), and adipose tissue from eNOS-TG mice demonstrated an increase in L-citrulline (Online Fig. III-B). Due to high variability, there were no significant differences in nitrate or nitrite in adipose tissue (Online Fig. III-D) perhaps due to other confounding factors, such as nitrite/nitrate found in the diet or reduction of nitrite to NO.
When placed on a high fat diet for 6 weeks, the homozygous eNOS-TG mice gained 50% less weight than WT mice, and this effect persisted for 12 weeks (Fig. 2B,C). Food intake was not different between WT and eNOS-TG mice (Fig. 2D). A more modest resistance to weight gain was also observed in hemizygous eNOS-TG mice (Online Fig. I-D), perhaps due to lower adipose tissue eNOS levels in these mice compared with homozygous eNOS-TG mice. Hence, for all subsequent studies, only eNOS homozygous mice were used. Measurements of body composition by dual-energy X-ray absorptiometry (Dexascan) showed that after 6 weeks of high fat feeding, the body fat content was much lower in eNOS-TG mice than in non-transgenic mice (Fig. 2E,F). The transgenic mice maintained a higher percent of lean mass (Fig. 2G), although the tibia length in transgenic mice was only slightly smaller than in WT mice (Fig. 2H). These observations indicate that overexpression of eNOS decreases adiposity and prevents weight gain induced by high fat diet.
Figure 2. eNOS prevents diet-induced obesity.
Weight gain, adiposity, and indirect calorimetry measurements from WT and eNOS-TG mice fed a low fat (LFD) or high fat diet (HFD): (A) Body weights during 6 weeks of LF feeding, n = 22–26 per group; (B) body weights during 6 weeks of HF feeding, n = 26 per group; (C) summarized weight gain over the course of 6 weeks and 12 weeks of HF feeding, n = 28–29 per group for 6 week group, n = 4–7 per group for 12 week group; (D) food intake, n = 4 per group; (E) representative DexaScan images of mice fed a LFD or HFD for 6 weeks; (F) body fat percentage, n = 8–12 per group; (G) lean mass percentage, n = 8–12 per group; and (H) tibia length for mice fed a LFD or HFD for 6 weeks, n = 8–12 per group; (I) average oxygen consumption (VO2); (J) average carbon dioxide production (VCO2); (K) respiratory exchange ratio (RER); and (L) ambulatory counts. n = 4 per group; *p<0.05, **p<0.01, and ***p<0.001 vs. indicated groups; #p<0.05 vs. WT HFD.
eNOS overexpression increases whole body metabolism
To determine how eNOS overexpression affected whole-body metabolism, we measured oxygen consumption (VO2), carbon dioxide production (VCO2), and activity in high fat-fed WT and eNOS-TG mice over the course of a 12 h dark period and a 4.5 h light period. The fact that food intake was not different between WT and TG mice (Fig. 2D), indicates that the lean phenotype of eNOS-TG mice is not due to a decrease in food consumption. This view is reinforced by the observation that high fat feeding increased plasma cholesterol and leptin to similar levels in WT and eNOS-TG mice (Table 1). In comparison with WT mice, eNOS-TG mice showed higher mean VO2 and VCO2 rates throughout the dark and light periods (Fig. 2I,J), with no change in the respiratory exchange ratio (RER; Fig. 2K). Activity levels, assessed by horizontal activity count (beam breaks) showed similar patterns and levels of activity, and total ambulatory activity was not significantly different (Fig. 2L). Taken together, these observations suggest that on a high fat diet, eNOS-TG mice maintain a higher metabolic rate than WT mice. This increase in systemic metabolism, however, cannot be attributed to thyroid hormones, because plasma levels of triiodothyronine (T3) and thyroxine (T4) in WT and eNOS-TG mice were not significantly different (Online Fig. IV).
Table 1.
Parameters measured from plasma of low fat-fed and high fat-fed WT and eNOS-TG mice.
| Parameter | WT LFD | WT HFD | eNOS-TG LFD | eNOS-TG HFD |
|---|---|---|---|---|
| * Insulin (pg/ml) | 116.2±40.7 | 3010.6±537.3† | 480.8±95.4|| | 568.1±175.6# |
| Adiponectin (μg/ml) | 22.0±3.8 | 25.7±4.0 | 27.8±3.0 | 30.1±3.8 |
| Resistin (pg/ml) | 2449.8±156.1 | 7894.0±1155.0† | 3547.3±387.2|| | 6251.3±497.9§,@ |
| Leptin (pg/ml) | 209.0±38.3 | 5099.0±1265.0† | 997.4±203.4|| | 4874.4±783.1§,@ |
| Cholesterol (mg/dl) | 96.4±2.7 | 117.8±5.9† | 92.1±4.4|| | 124.0±6.8§,@ |
| Triglycerides (mg/dl) | 37.8±2.5 | 46.2±4.8 | 26.2±3.0|| | 23.8±2.8§,# |
| NEFA (mEq/L) | 0.39±0.05 | 0.38±0.06 | 0.19±0.04‡,|| | 0.16±0.02§,# |
| * Glycerol (mg/L) | 11.2±1.2 | 14.7±1.9 | 12.0±1.4 | 12.7±0.5 |
Wild-type (WT) and eNOS-TG mice were fed a low fat diet (LFD) or high fat diet (HFD) for 6 weeks. Plasma from the mice was used to measure the indicated parameters.
n = 6–7 mice per group; for all other parameters, the groups contained 13–14 mice per group.
WT LFD vs. WT HFD
WT LFD vs. eNOS-TG LFD
WT LFD vs. eNOS-TG HFD
WT HFD vs. eNOS-TG LFD
WT HFD vs. eNOS TG HFD
eNOS-TG LFD vs. eNOS-TG HFD
Effect of eNOS on diet-induced insulin resistance
Because we found that eNOS overexpression decreased diet-induced weight gain, we expected concurrent changes in insulin resistance. Indeed, we found that overexpression of eNOS completely prevented diet-induced hyperinsulinemia (Table 1), although plasma levels of adiponectin and resistin were not affected. This was associated with a remarkably lower HOMA-IR score (WT low fat, 1.45±0.65; WT high fat, 34.4±5.3, p<0.05 vs. WT low fat; TG low fat, 6.9±3.1; TG high fat, 8.2±2.9, p<0.05 vs. WT high fat). Moreover, even though 6 weeks of high fat feeding did not significantly increase triglycerides or plasma non-esterified free fatty acids (NEFA), both of these were decreased by 50% in the TG mice compared with WT mice (Table 1). We did not find significant differences in plasma glycerol between the groups (Table 1), suggesting that adipose tissue lipolysis was not affected. Other parameters measured in the plasma are shown in Online Table I. Collectively, these data indicate that overexpression of eNOS prevents high fat diet-induced hyperinsulinemia and decreases plasma triglycerides and fatty acids.
To examine how eNOS overexpression affects systemic glucose disposal, WT and eNOS-TG mice were placed on a high fat diet for 6 weeks, and GTT and ITT were performed. There was no significant difference in the basal blood glucose levels in non-fasted WT and eNOS-TG mice (Fig. 3A). After a fast of 6 h, the plasma glucose levels of both high fat-fed groups were significantly increased compared with the WT low fat-fed group. Fasting for 16 h resulted in near normalization of blood glucose in WT mice; however, glucose levels in the eNOS-TG mice remained slightly, but significantly, elevated (Fig. 3A). There were no significant differences in plasma HbA1c in any group (Fig. 3B). Measurements of insulin resistance by GTT indicated that, in both WT and eNOS-TG mice, high fat diet led to an increase in the GTT area-under-the-curve (AUC) indicating the onset of insulin resistance (Fig. 3C–E). The early increase in glucose levels in both the low fat- and high fat-fed groups showed a slight but significant increase in blood glucose in TG mice (Fig. 3C,D); but there was no significant differences when AUC was compared (Fig. 3E). Measurements of insulin tolerance (Fig. 3F–H) showed no significant difference in glucose disposition in any of the groups.
Figure 3. Effect of eNOS overexpression on indices of insulin resistance.
After 6 weeks of a low fat (LFD) or high fat diet (HFD), glucose tolerance and insulin sensitivity were examined in WT and eNOS-TG mice: (A) Non-fasting and fasting glucose levels; white bars, WT LFD; blue bars, eNOS-TG LFD; white hatched bars, WT HFD; blue hatched bars, eNOS-TG HFD; (B) HbA1c; (C–E) glucose tolerance tests; and (F–H) insulin tolerance tests. n = 14 per group; *p<0.05 vs WT LFD or otherwise indicated groups.
To test whether the effects of the transgene would manifest after prolonged feeding, we placed WT and eNOS-TG mice on high fat diet for 12 weeks and assessed insulin resistance. At completion of the feeding protocol, the GTT and ITT curves were superimposable suggesting that eNOS overexpression does not affect diet-induced insulin resistance even after prolonged nutrient excess (Online Fig. V-A–D). Although, plasma glucose levels in non-fasted and 6 h-fasted mice were not statistically different, a 16 h fast led to a greater decrease in blood glucose in WT compared with TG mice (Online Fig. V-E), indicating that the TG mice were more resistant to starvation-induced hypoglycemia, which could be due to increased gluconeogenesis in the liver. To test this, we performed pyruvate tolerance tests, which did not show remarkable differences between WT and TG mice (Online Fig. V-F,G), indicating that resistance to hypoglycemia in TG mice may not be due to increased hepatic production of glucose. Collectively, these data suggest that eNOS overexpression does not significantly affect diet-induced insulin resistance or glucose intolerance, but maintains glucose homeostasis during starvation.
Effect of eNOS on adipose tissue
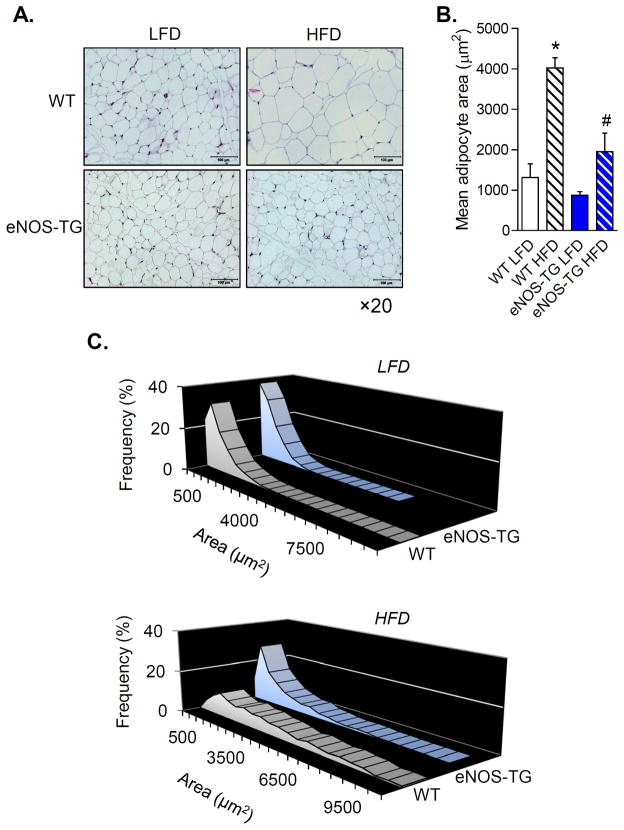
Given our observations that obesity and diabetes were associated with a selective decrease of eNOS levels in adipose tissue and that eNOS-TG mice were resistant to diet-induced weight gain, we measured changes in adipocyte area and size in epididymal fat pads. These measurements revealed that high fat diet induced adipocyte hypertrophy leading to a 3–4-fold increase in mean adipocyte area (Fig. 4A,B). Moreover, the high fat diet promoted size heterogeneity in WT, but not eNOS-TG mice (Fig. 4C), indicating that eNOS overexpression prevents diet-induced adipocyte hypertrophy and size dispersion.
Figure 4. eNOS overexpression decreases diet-induced adipocyte hypertrophy.
Adipocyte size measurements from WT and eNOS-TG mice fed a LFD or HFD for 6 weeks: (A) Representative hematoxylin and eosin-stained images of adipose tissue from the epididymal fat pad (×20 magnification; scale bar = 100 μm); (B) Mean adipocyte area; (C) Distribution of adipocyte sizes from mice fed a LFD (upper panel) and a HFD (lower panel). n = 5 per group, *p<0.05 vs. WT LFD; #p<0.05 vs. WT HFD.
In murine models of diet-induced obesity, adipocyte hypertrophy is associated with inflammation and accumulation of macrophages in adipose tissue33. This is commonly recognized by the presence of crown-like structures that appear between adipocytes18, 33, 34. In humans, obesity is similarly associated with adipose tissue inflammation, and weight loss interventions such as bariatric surgery improve endothelial function35, 36. Therefore, we examined adipose tissue inflammation in WT and eNOS-TG mice after 6 weeks of high fat diet. Analysis of adipose tissue showed no significant difference in the abundance of crown-like structures between WT and TG mice (Fig. 4A), and analysis of the adipose tissue stromal vascular fractions showed no difference in total F480+ cells or changes in macrophage subtypes (Online Fig. VI). These results are in accordance with studies showing that macrophage accumulation and insulin resistance occur only with prolonged high fat feeding (>10 weeks)22, 37 and suggest that the anti-hypertrophic effects of eNOS are not associated with significant changes in adipose tissue inflammation, but are likely to be related to favorable changes in metabolism that prevent lipid accumulation and adipocyte expansion.
Metabolic changes in adipose tissues of eNOS-overexpressing mice
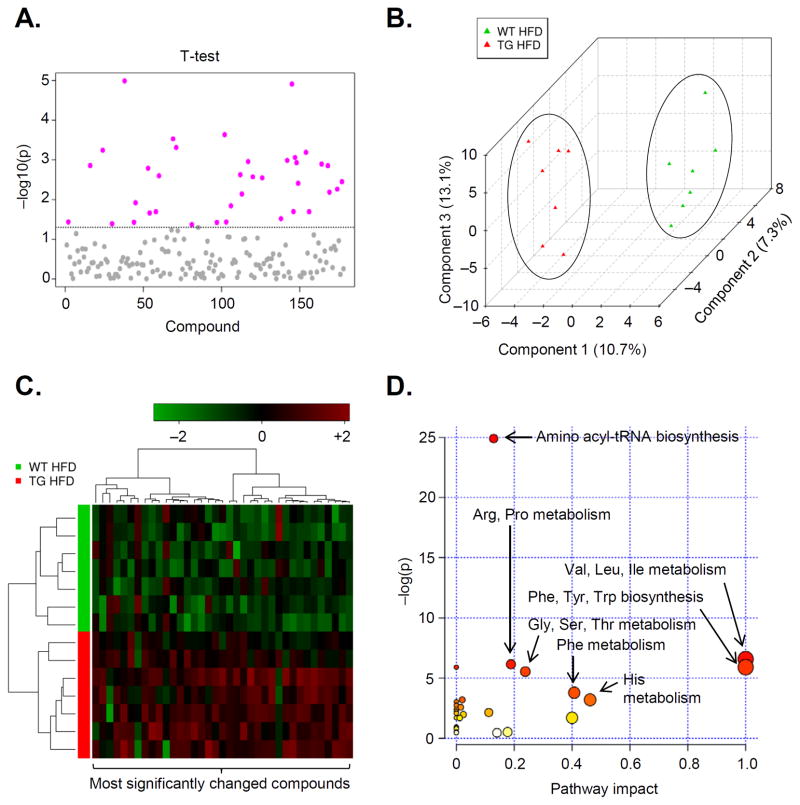
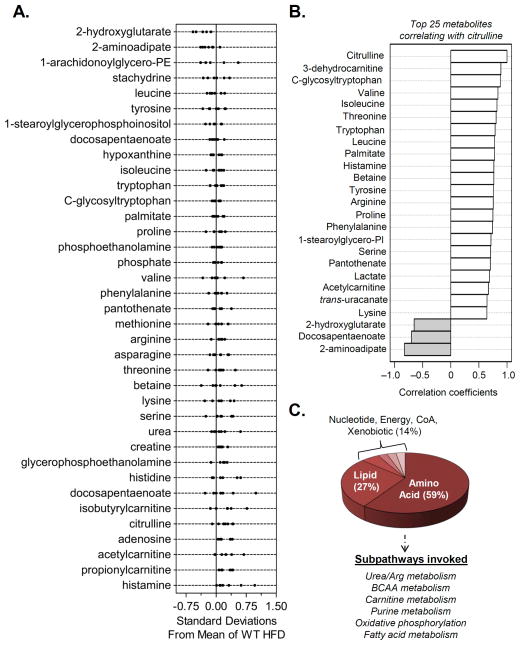
The lean phenotype of eNOS-TG mice and their resistance to diet-induced weight gain and adipocyte expansion clearly indicated to us that eNOS overexpression has a significant impact on adipocyte metabolism. Therefore, to assess this impact, we measured metabolite levels in epididymal adipose tissue of high fat-fed WT and eNOS-TG mice using UHPLC/MS/MS and GC/MS. Spectral data were identified, searched against a standard library, and quantified (see Online Supplement and Online Fig. VII for details). Internal standards, including injection standards, process standards, and alignment standards were used for quality control and to control for experimental and instrument variability. This analysis led to the identification of 192 metabolites of which 37 were significantly different between WT and eNOS-TG mice (Fig. 5A and Online Table II). Although intermediates in the glycolytic pathway and TCA cycle were not affected, there were significant increases in propionylcarnitine, acetylcarnitine, 3-dehydrocarnitine, and isobutyrylcarnitine, some of which have been shown to stimulate fatty acid oxidation38, 39. Higher levels of amino acids such as threonine, methionine, valine, isoleucine, and leucine were also observed in TG mice (Online Table II). Multivariate and cluster analyses showed that these changes were determining factors in the separation of the groups (Fig. 5B,C), and pathway impact analysis (Fig. 5D) suggested that the changes in amino acid synthesis and degradation may be important features regulating the lean phenotype of eNOS-TG mice. Plotting of the z-scores of metabolites from adipose tissues of eNOS-TG and WT mice showed increases in short-chain acylcarnitines as well as amino acids and their degradation products (Fig. 6A). Metabolites correlating with citrulline levels showed a similar pattern of metabolites (Fig. 6B). The adipose tissue metabolites in eNOS-TG mice shown to be significantly different from WT mice equated to differences in urea cycle/arginine metabolism, branched chain amino acid (BCAA) metabolism, carnitine metabolism, purine metabolism, oxidative phosphorylation, and fatty acid metabolism subpathways (Fig. 6C). Taken together, changes in metabolite levels in the adipose tissue indicated that overexpression of eNOS stimulates amino acid and fatty acid metabolism in adipose tissue.
Figure 5. Metabolomic analyses of adipose tissues from high fat-fed mice.
Metabolomic analyses of epididymal adipose tissue metabolites from WT and eNOS-TG mice fed HFD for 6 weeks: (A) Univariate analysis: t-tests of compounds from adipose tissues. All metabolites above the dotted line were found to be significantly different between WT and eNOS-TG mice (p<0.05). A table of these metabolites can be found in the data supplement (Online Table II); (B) Multivariate analysis: partial least squares-discriminant analysis (PLS-DA); (C) Hierarchial clustering: Heatmap and dendogram using the the most significantly different metabolites. (D) The significant metabolites were subjected to pathway impact analysis using Metaboanalyst MetPA and the Mus musculus pathway library. Fisher’s exact test was used for overrepresentation analysis, and relative betweenness centrality was used for pathway topology analysis. n = 14 animals: 7 WT HFD, 7 eNOS-TG HFD.
Figure 6. Overexpression of eNOS regulates intermediary metabolism in adipose tissue.
Metabolite analysis from adipose tissues of WT and eNOS-TG mice fed HFD for 6 weeks: (A) z-score plots of significantly changed metabolites; (B) Correlation analysis was assessed using the Spearman rank correlation test, and the metabolites that correlated with citrulline were then examined. (C) Super- and sub-pathway distribution of adipose tissue metabolites found to be significantly different between WT and eNOS-TG mice. n=14 animals: 7 WT HFD and 7 eNOS-TG HFD.
Adipose tissue mitochondria are increased in eNOS-TG mice
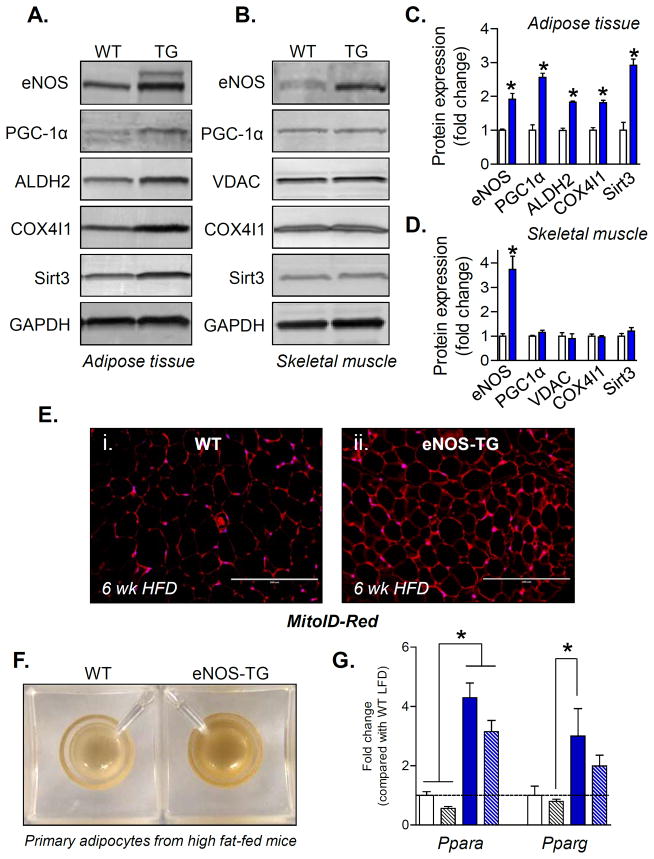
Favorable changes in BCAA and fatty acid metabolism are indicative of increased mitochondrial activity. Previous studies have shown that BCAA increases mitochondrial biogenesis and that this is attenuated in eNOS-null mice40. In addition, it has been reported that NO triggers mitochondrial biogenesis in adipocytes and that deletion of eNOS decreases mitochondrial content in adipose tissue41. Based on this evidence, we hypothesized that the change in BCAA and fatty acid metabolism in the adipose tissue of eNOS-TG mice may be related to greater mitochondrial content. Indeed, adipose tissue, but not skeletal muscle, from eNOS-TG mice showed significant increases in key mitochondrial proteins such as COX4I1 and ALDH2 (Fig. 7A,C). The increase in mitochondrial proteins in TG adipose tissue could be due to remodeling of the mitochondria or an increase in mitochondrial abundance. To distinguish between these possibilities, sections of adipose tissue were stained with a non-membrane potential-dependent mitochondrial stain, mitoID-Red. As shown in Fig. 7E, adipose tissue isolated from high-fat-fed eNOS-TG mice stained more strongly than WT mice, indicating that the adipose tissue mitochondrial content was higher in TG than WT mice. Indeed, adipocytes isolated from high fat-fed eNOS-TG mice were more brown in color than those isolated from WT mice (Fig. 7F), suggesting an increase in mitochondrial cytochromes. Indeed, in addition to increased abundance of COX4I1 (Fig. 7A,C), the expression of the mitochondrial gene cytochrome, cytochrome b6 (cytb6), was elevated 2-fold in eNOS-TG mice (cytb6:gapdh ratios, fold change: WT, 1.0±0.1; eNOS-TG, 2.0±0.3; n=4–7/group, p<0.05). That this increase in mitochondrial content may be due to increased biogenesis is supported by our observation that in comparison with WT mice, TG mice had higher adipose levels of PGC1α and Sirt3, as well as an increase in pparα and γ (Fig. 7G); factors that are important activators of mitochondrial biogenesis42–47.
Figure 7. Mitochondria are increased in the adipose tissue of eNOS-TG mice.
Measurements of mitochondria in epididymal adipose tissue and skeletal muscle from WT and eNOS-TG mice: (A) Representative Western blots of adipose tissue eNOS, PGC1α, ALDH2, COX4I1, and Sirt3; GAPDH was used as a loading control. (B) Representative Western blots of skeletal muscle eNOS, PGC1α, VDAC, COX4I1, and Sirt3. GAPDH was used as a loading control. (C) Quantification of protein expression from panel A. (D) Quantification of protein expression from panel B. n=3 per group; *p<0.05 vs. WT; White bars, WT; blue bars, TG. (E) Immunofluorescence images of adipose tissue sections from HF-fed WT (panel i) and eNOS-TG (panel ii) mice; the sections were stained with MitoID-Red as a qualitative index of mitochondrial mass. Scale bar=200 μM (F) Representative photomicrograph of adipocytes isolated from HF-fed WT and eNOS-TG mice (600,000 adipocytes per well). (G) mRNA analysis of Ppara and Pparg. White bars, WT LFD; blue bars, eNOS-TG LFD; white hatched bars, WT HFD; blue hatched bars, eNOS-TG HFD; n=6 per group; *p<0.05 vs. indicated groups.
Effect of eNOS on adipose tissue metabolic flux
To assess the functional implications of our observations, we measured oxygen consumption in adipose tissue explants using extracellular flux technology. As shown in Fig. 8B, adipose tissue from eNOS-TG mice showed a significantly higher oxygen consumption rate (OCR) compared with adipose tissue from WT mice. To determine the contribution of mitochondria to the OCR, we treated the explants with the electron transport chain inhibitors antimycin A and rotenone. The stabilized rate measured thereafter was used to calculate the mitochondria-derived OCR, which was 2-fold higher in eNOS-TG compared with WT adipose tissue (Fig. 8C). No statistically significant difference was observed in the extracellular acidification rate (ECAR), a surrogate index of glycolysis (Fig. 8D). Collectively, these observations corroborate our metabolic, biochemical, and anatomical measurements by demonstrating directly that the adipose tissue of eNOS-TG mice maintains a hypermetabolic state that could at least partially account for their increase in whole-body oxygen consumption and resistance to obesity.
Figure 8. eNOS overexpression increases adipose tissue mitochondrial energetics.
Extracellular flux (XF) analysis of adipose tissue explants from WT and eNOS-TG mice fed a HFD for 14 wks: (A) Representative photomicrographs of adipose tissue explants used for XF analysis; (B) Oxygen consumption rates (OCR) of adipose tissue explants: After three baseline measurements, antimycin A and rotenone (AA/Rot) were injected to identify the mitochondria-dependent OCR. (C) Mitochondrial OCR calculated from measurements in panel B. (D) Extracellular acidification rate (ECAR) measured from adipose explants; ECAR is a surrogate measure of glycolytic rate. n = 3–4 per group, *p<0.05 vs WT.
DISCUSSION
The major findings of this study are that high fat diet results in the downregulation of eNOS in adipose tissue and that overexpression of eNOS prevents diet-induced obesity. These findings support a causal role of eNOS in regulating obesity and whole-body metabolism. Our results suggest that the mechanism of this anti-obesogenic effect of eNOS is related to an increase in whole-body oxygen consumption associated with increased mitochondrial abundance and activity in the adipose tissue. Collectively, these observations support the notion that NO is an important regulator of adipocyte metabolism and thereby weight gain due to a high fat diet. While it has been shown before that deletion of eNOS gives rise to features of metabolic syndrome11, the rescue of the obese phenotype by increasing eNOS indicates that enhancing eNOS expression can overcome the metabolic changes caused by consumption of high fat diet.
Several lines of evidence gathered during this study support the view that the anti-obesogenic effects of eNOS are due to favorable changes in adipocyte metabolism. Although on the basis of current results we cannot rule out, or even fully assess all potential systemic effects, our observations that food consumption, activity, plasma levels of cholesterol, leptin and thyroid hormones were not different between WT and TG mice argue against a global, systemic change that could completely account for the lean phenotype of the TG mice. Both insulin resistance and obesity are complex phenotypes that are regulated by multiple interactions between several tissues, some or all of which might be affected in a manner not captured by our current analysis. Nevertheless, in regulating obesity, the adipose tissue appears to be a major target of eNOS. Our gene-dosage studies show that despite a 4-fold increase in eNOS in the aorta, diet-induced obesity was only marginally affected in eNOS hemizygous mice, in which there was no increase in eNOS in adipose tissue. Only in homozygous mice, in which eNOS was increased both in adipose tissue and aorta, did the anti-obesogenic effects of eNOS become apparent. This association of the lean phenotype with eNOS expression in adipose tissue supports the view that an increase in NO in adipose depots may be required for the manifestation of the anti-obesogenic effects of eNOS.
How does eNOS regulate adipose tissue metabolism? Our results suggest that eNOS supports both mitochondrial biogenesis and metabolic activity. Previous observations showing that β–oxidation is impaired in eNOS-null mice12 and that dietary supplementation with the NO precursor nitrite reverses features of metabolic syndrome in eNOS-null mice48 are supportive of this concept. Although AMP kinase (AMPK) has been shown to relate with NO levels49, 50, we did not find an increase in the phosphorylation state of AMPK in adipose tissue (Online Fig. VIII). However, we did find elevated levels of several metabolites such as BCAAs and short-chain acylcarnitines (e.g., acetylcarnitine, proprionylcarnitine) in the adipose tissue of TG mice that were indicative of high metabolic activity. Interestingly, oral supplementation with proprionylcarnitine reduces obesity and hyperinsulinemia in obese rats51, which at least partially recapitulates the phenotype of eNOS-TG mice. We also found in the adipose tissue of TG mice elevated levels of proteins such PGC1α and Sirt3 and increased expression of ppara and pparg that regulate mitochondrial activity, fatty acid oxidation, and biogenesis42–47, 52, 53. That the increase in these proteins was functionally significant is reflected by our observations that mitochondrial abundance and the rates of fatty acid oxidation were higher in the adipose tissue from eNOS-TG mice. On the basis of these observations, we propose that high levels of eNOS lead to an increase in mitochondrial biogenesis and stimulation of fatty acid oxidation. This establishes a state of heightened metabolism that attenuates the obesogenic effects of high fat consumption.
Although our results show that eNOS overexpression increases adipose tissue metabolism by increasing mitochondrial content and activity, metabolic activity could also be affected by eNOS-dependent changes in oxygen distribution. Hence, it is possible that adipocytes of eNOS-TG mice are better perfused than those of WT mice. Such an increase in tissue perfusion could be due to either regulatory effects on vascular tone54 and O2 consumption55 or an increase in angiogenesis56. Nevertheless, we found that capillary density was unaffected by eNOS overexpression, as isolectin B4 staining per adipocyte and VEGFR2 expression were similar between the groups (Online Fig. IX), suggesting that an increase in angiogenesis is unlikely to be a reason underlying the lean phenotype of eNOS-TG mice.
The metabolic role of eNOS, however, appears to be tissue-specific. We found that high fat feeding decreased eNOS in the adipose tissue but not in the heart or the skeletal muscle. Hence, we expected that overexpression of eNOS would ameliorate adipose tissue hypertrophy without affecting high fat-induced changes in other peripheral tissues. Data from eNOS-TG mice substantiated this expectation. These results showed that high fat-induced changes in glucose disposal were not different between WT and eNOS-TG mice indicating that whole body glucose metabolism, which is regulated primarily by glucose uptake by the skeletal muscle57, was not related to changes in eNOS levels. Nevertheless, the observation that despite their lean phenotype the TG mice develop insulin resistance is significant because a lean phenotype characterized by the browning of fat is usually associated with improved glucose tolerance58–61. It is likely that a decrease in eNOS is a critical event in adipose tissue but not skeletal muscle, and therefore elevated levels of eNOS in the adipose tissue prevent obesity without affecting systemic insulin resistance.
Results showing that overexpression of eNOS prevents obesity without affecting insulin resistance also suggest that the two symptoms of metabolic syndrome could be dissociated from one another. Similar segregation between obesity and insulin resistance has been described previously. For instance, it has been shown that overexpression of adiponectin completely rescues the diabetic phenotype of ob/ob mice while promoting morbid obesity62. Moreover, the observations that decreasing inflammation63–65 does not result in lower adiposity but improves insulin sensitivity, and that PPARγ agonists decrease insulin resistance but increase weight gain8 provide additional support to the concept that obesity and diabetes are disconnected and, in some cases, even conflicting events in the etiology of metabolic disease. However, it remains to be established how eNOS prevents hyperinsulinemia as well as impacts other processes that are associated with insulin resistance, such as inflammation. It is currently believed that, due to excessive adipocyte expansion, hypoxia and necrosis occur in adipose tissue, which in turn leads to the recruitment of inflammatory cells66, 67. The resultant low-grade chronic inflammation is proposed to establish a state of insulin resistance68, 69. However, the eNOS-TG mice develop the anti-obesogenic phenotype far before macrophage infiltration, inflammation, and insulin resistance in adipose tissue occur22, 37.
It is important to note that the eNOS-TG mice did not display a lipodystrophic phenotype. Lipodystrophy in humans and animal models generally results in severe hypertriglyceridemia, hyperinsulinemia, and insulin resistance70–72. The eNOS-TG mice, however, show decreased triglycerides and were protected from hyperinsulinemia despite developing diet-induced glucose intolerance. The prevention of hyperinsulinemia does not appear to be due to a pancreatic defect: baseline insulin levels were not significantly different from WT mice (Table 1), the glucose tolerance test showed a normal profile (Fig. 3 and Online Fig. V), and the pancreatic islets from eNOS-TG mice appeared unremarkable (Online Fig. X). These observations raise the interesting possibility that hyperinsulinemia in response to systemic insulin resistance may be in part regulated by the adipose tissue, although additional work is required to fully understand this relationship.
Additional investigations will also be required to assess how high fat diet affects eNOS activity and expression. Although it has been shown that eNOS levels are suppressed in high fat diet in part due to TNF–α–dependent mechanisms26, the effects of diet on eNOS protein and activity are less clear. The eNOS protein is subject to several post-translational modifications including phosphorylation73, O-GlyNAcylation74, S-glutathiolation75, and acylation76, 77. In addition, the enzyme could also be uncoupled and therefore generate superoxide instead of synthesizing NO. Interestingly, we found that while eNOS monomer abundance was maintained in eNOS-TG mice (Online Fig. XI), the phosphorylation of eNOS at Ser1177 and abundance of the eNOS dimer were significantly decreased in both WT and TG mice fed a high fat diet (Online Fig. XI). Although these changes in the eNOS-TG mice might be compensated by continually elevated levels of eNOS protein, as evidenced by persistently elevated citrulline levels (Online Fig. III-A,B), such changes in WT mice might result in a chronic state of NO deficiency. Moreover, uncoupling of the enzyme could lead to increased superoxide production and the formation of the toxic metabolite peroxynitrite. Indeed, we found increased nitrotyrosine formation in adipose tissue of high fat-fed mice (Online Fig. XII), although this was not significantly affected by eNOS overexpression. Hence, in future studies it will be important to identify the processes that regulate eNOS activity and how they might be involved in the development of diet-induced obesity and insulin resistance.
In conclusion, the present study shows that preventing eNOS depletion by forced expression of the eNOS transgene attenuates diet-induced obesity in mice, without ameliorating systemic insulin resistance. These findings reveal a novel anti-obesogenic role of eNOS and are consistent with the notion that eNOS prevents weight gain in high fat-fed mice by stimulating mitochondrial biogenesis and activity in adipose tissues. Further understanding of this role of eNOS could lead to the development of new therapeutic modalities for preventing obesity and weight gain in human populations.
Supplementary Material
Novelty and Significance.
What Is Known?
Obesity is positively and robustly associated with the risk of developing cardiovascular disease and diabetes.
Vascular dysfunction, and, in particular, deficits in endothelial-derived nitric oxide (NO) production and bioavailability, are associated with insulin resistance, adiposity, and deleterious changes in metabolism.
What New Information Does This Article Contribute?
The expression of endothelial NO synthase (eNOS) in adipose tissue is decreased in mouse models of obesity and type 2 diabetes.
Transgenic overexpression of eNOS in mice decreases circulating fatty acids and prevents obesity and hyperinsulinemia induced by a high fat diet.
Overexpression of eNOS prevents adipocyte hypertrophy, increases mitochondrial abundance and activity, and regulates branched chain amino acid metabolism.
Vascular dysfunction and decreased NO bioavailability are associated with metabolic syndrome; however, the therapeutic effects of increasing endogenous NO production have not been tested, and the effects of NO on metabolism are poorly understood. We show here that consumption of a high fat diet decreases the abundance of eNOS in adipose tissue and that increasing eNOS expression prevents diet-induced obesity. The anti-obesity effect of eNOS was associated with enhanced mitochondrial activity and significant changes in amino acid and lipid metabolism in adipose tissue. These findings suggest that eNOS prevents obesity by increasing the ability of adipocytes to burn excess fat. This novel regulation of adipocyte phenotype adds to our growing knowledge of the plasticity of adipose tissue and suggests that eNOS could regulate brown or “beige”-like gene programs. Thus, increasing NO availability or production may be an important therapeutic strategy for preventing obesity and its cardiovascular complications.
Acknowledgments
The authors wish to thank Chris Kevil (Louisiana State University) for providing the eNOS-TG mice as well as Dan Conklin, Don Mosley, and Emily Steinmetz for their help in animal handling and metabolic chamber studies.
SOURCES OF FUNDING
This work was supported in part by grants from the NIH (RR024489, HL55477, HL59378, and HL106173).
Non-standard Abbreviations
- AA
antimycin A
- ALDH2
aldehyde dehydrogenase 2
- ALT
alanine aminotransferase
- AMPK
AMP-activated protein kinase
- Arg
arginine
- AST
aspartate aminotransferase
- ATP5B,ATP
synthase beta subunit
- BCAA
branched chain amino acid
- BSA
bovine serum albumin
- BSTFA
bistrimethyl-silyl-triflouroacetamide
- CK
creatine kinase
- CoA
coenzyme A
- COX4I1
cytochrome c oxidase subunit 4 isoform 1
- CytB6
cytochrome B6
- DAPI
4′,6-diamidino-2-phenylindole
- Dexascan
dual-energy X-ray absorptiometry
- DMEM
Dulbecco’s modified Eagle’s medium
- ECAR
extracellular acidification rate
- ELISA
Enzyme-Linked Immunosorbent Assay
- eNOS
endothelial nitric oxide synthase
- eNOS-TG
endothelial nitric oxide synthase-transgenic
- ESI
electrospray ionization
- FDR
false discovery rate
- FT-ICR
Fourier transform ion cyclotron resonance
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GC
gas chromatography
- Gly
glycine
- GTT
glucose tolerance test
- HbA1c
hemoglobin A1c
- HDL
high-density lipoprotein
- HFD
high fat diet
- His
histidine
- HOMA-IR
homeostasis model of assessment-insulin resistance
- HPLC
high-performance liquid chromatography
- HRP
horseradish peroxidase
- IB
immunoblot
- IHC
immunohistochemistry
- Ile
isoleucine
- i.p
intraperitoneal
- ITT
insulin tolerance test
- LDH
lactate dehydrogenase
- LDL
low-density lipoprotein
- Leu
leucine
- LFD
low fat diet
- LIMS
Laboratory Information Management Systems
- LIT
linear ion-trap
- MS/MS
tandem mass spectrometry
- NEFA
non-esterified free fatty acids
- NO
nitric oxide
- OCR
oxygen consumption rate
- PBS
phosphate buffered saline
- PGC1α
peroxisome proliferator-activated receptor gamma coactivator 1 alpha
- Phe
phenylalanine
- Ppara
peroxisome proliferator-activated receptor alpha
- Pparg
peroxisome proliferator-activated receptor gamma
- Pro
proline
- PTT
pyruvate tolerance test
- QA/QC
quality assurance/quality control
- Qpcr
quantitative polymerase chain reaction
- RER
respiratory exchange ratio
- Rot
rotenone
- SAM
s-adenodyl methionine
- SDS-PAGE
sodium dodecyl sulfate-polyacrylamide gel electrophoresis
- Ser
serine
- Sirt3
sirtuin 3
- TCA cycle
the citric acid cycle
- TG
transgenic
- Thr
threonine
- TNF-α
tumor necrosis factor-alpha
- Trp
tryptophan
- Tyr
tyrosine
- T2D
type 2 diabetes
- T3
triiodothyronine
- T4
thyroxine
- UHPLC
ultra high-performance liquid chromatography
- Val
valine
- VCO2
carbon dioxide production
- VDAC
voltage-dependent anion channel
- VEGFR2
vascular endothelial growth factor receptor 2
- VO2
oxygen consumption
- WT
wild type
- XF
extracellular flux
Footnotes
DISCLOSURES
None.
References
- 1.Ahima RS. Digging deeper into obesity. J Clin Invest. 2011;121:2076–2079. doi: 10.1172/JCI58719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ervin RB. Prevalence of metabolic syndrome among adults 20 years of age and over, by sex, age, race and ethnicity, and body mass index: United states, 2003–2006. Natl Health Stat Report. 2009:1–7. [PubMed] [Google Scholar]
- 3.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2011 doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Williamson DF, Madans J, Anda RF, Kleinman JC, Kahn HS, Byers T. Recreational physical activity and ten-year weight change in a us national cohort. Int J Obes Relat Metab Disord. 1993;17:279–286. [PubMed] [Google Scholar]
- 5.Hu FB, Li TY, Colditz GA, Willett WC, Manson JE. Television watching and other sedentary behaviors in relation to risk of obesity and type 2 diabetes mellitus in women. JAMA. 2003;289:1785–1791. doi: 10.1001/jama.289.14.1785. [DOI] [PubMed] [Google Scholar]
- 6.Hill JO, Peters JC. Environmental contributions to the obesity epidemic. Science. 1998;280:1371–1374. doi: 10.1126/science.280.5368.1371. [DOI] [PubMed] [Google Scholar]
- 7.Gesta S, Tseng YH, Kahn CR. Developmental origin of fat: Tracking obesity to its source. Cell. 2007;131:242–256. doi: 10.1016/j.cell.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Lehrke M, Lazar MA. The many faces of ppargamma. Cell. 2005;123:993–999. doi: 10.1016/j.cell.2005.11.026. [DOI] [PubMed] [Google Scholar]
- 9.Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G, Baron AD. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J Clin Invest. 1996;97:2601–2610. doi: 10.1172/JCI118709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankar RR, Wu Y, Shen HQ, Zhu JS, Baron AD. Mice with gene disruption of both endothelial and neuronal nitric oxide synthase exhibit insulin resistance. Diabetes. 2000;49:684–687. doi: 10.2337/diabetes.49.5.684. [DOI] [PubMed] [Google Scholar]
- 11.Duplain H, Burcelin R, Sartori C, Cook S, Egli M, Lepori M, Vollenweider P, Pedrazzini T, Nicod P, Thorens B, Scherrer U. Insulin resistance, hyperlipidemia, and hypertension in mice lacking endothelial nitric oxide synthase. Circulation. 2001;104:342–345. doi: 10.1161/01.cir.104.3.342. [DOI] [PubMed] [Google Scholar]
- 12.Le Gouill E, Jimenez M, Binnert C, Jayet PY, Thalmann S, Nicod P, Scherrer U, Vollenweider P. Endothelial nitric oxide synthase (enos) knockout mice have defective mitochondrial beta-oxidation. Diabetes. 2007;56:2690–2696. doi: 10.2337/db06-1228. [DOI] [PubMed] [Google Scholar]
- 13.Ohashi Y, Kawashima S, Hirata K, Yamashita T, Ishida T, Inoue N, Sakoda T, Kurihara H, Yazaki Y, Yokoyama M. Hypotension and reduced nitric oxide-elicited vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. J Clin Invest. 1998;102:2061–2071. doi: 10.1172/JCI4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuinness OP, Ayala JE, Laughlin MR, Wasserman DH. Nih experiment in centralized mouse phenotyping: The vanderbilt experience and recommendations for evaluating glucose homeostasis in the mouse. Am J Physiol Endocrinol Metab. 2009;297:E849–855. doi: 10.1152/ajpendo.90996.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taniguchi CM, Kondo T, Sajan M, Luo J, Bronson R, Asano T, Farese R, Cantley LC, Kahn CR. Divergent regulation of hepatic glucose and lipid metabolism by phosphoinositide 3-kinase via akt and pkclambda/zeta. Cell Metab. 2006;3:343–353. doi: 10.1016/j.cmet.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 16.Kelpke SS, Chen B, Bradley KM, Teng X, Chumley P, Brandon A, Yancey B, Moore B, Head H, Viera L, Thompson JA, Crossman DK, Bray MS, Eckhoff DE, Agarwal A, Patel RP. Sodium nitrite protects against kidney injury induced by brain death and improves post-transplant function. Kidney Int. 2012 doi: 10.1038/ki.2012.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spite M, Hellmann J, Tang Y, Mathis SP, Kosuri M, Bhatnagar A, Jala VR, Haribabu B. Deficiency of the leukotriene b4 receptor, blt-1, protects against systemic insulin resistance in diet-induced obesity. J Immunol. 2011;187:1942–1949. doi: 10.4049/jimmunol.1100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellmann J, Tang Y, Kosuri M, Bhatnagar A, Spite M. Resolvin d1 decreases adipose tissue macrophage accumulation and improves insulin sensitivity in obese-diabetic mice. FASEB J. 2011;25:2399–2407. doi: 10.1096/fj.10-178657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue Y, Lim S, Brakenhielm E, Cao Y. Adipose angiogenesis: Quantitative methods to study microvessel growth, regression and remodeling in vivo. Nat Protoc. 2010;5:912–920. doi: 10.1038/nprot.2010.46. [DOI] [PubMed] [Google Scholar]
- 20.Sreekumar A, Poisson LM, Rajendiran TM, Khan AP, Cao Q, Yu J, Laxman B, Mehra R, Lonigro RJ, Li Y, Nyati MK, Ahsan A, Kalyana-Sundaram S, Han B, Cao X, Byun J, Omenn GS, Ghosh D, Pennathur S, Alexander DC, Berger A, Shuster JR, Wei JT, Varambally S, Beecher C, Chinnaiyan AM. Metabolomic profiles delineate potential role for sarcosine in prostate cancer progression. Nature. 2009;457:910–914. doi: 10.1038/nature07762. [DOI] [PMC free article] [PubMed] [Google Scholar] [Research Misconduct Found]
- 21.Macotela Y, Emanuelli B, Bang AM, Espinoza DO, Boucher J, Beebe K, Gall W, Kahn CR. Dietary leucine--an environmental modifier of insulin resistance acting on multiple levels of metabolism. PLoS One. 2011;6:e21187. doi: 10.1371/journal.pone.0021187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim F, Pham M, Maloney E, Rizzo NO, Morton GJ, Wisse BE, Kirk EA, Chait A, Schwartz MW. Vascular inflammation, insulin resistance, and reduced nitric oxide production precede the onset of peripheral insulin resistance. Arterioscler Thromb Vasc Biol. 2008;28:1982–1988. doi: 10.1161/ATVBAHA.108.169722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox JE, Powley TL. Development of obesity in diabetic mice pair-fed with lean siblings. J Comp Physiol Psychol. 1977;91:347–358. doi: 10.1037/h0077322. [DOI] [PubMed] [Google Scholar]
- 24.Georgescu A, Popov D, Constantin A, Nemecz M, Alexandru N, Cochior D, Tudor A. Dysfunction of human subcutaneous fat arterioles in obesity alone or obesity associated with type 2 diabetes. Clin Sci (Lond) 2011;120:463–472. doi: 10.1042/CS20100355. [DOI] [PubMed] [Google Scholar]
- 25.Perez-Matute P, Neville MJ, Tan GD, Frayn KN, Karpe F. Transcriptional control of human adipose tissue blood flow. Obesity (Silver Spring) 2009;17:681–688. doi: 10.1038/oby.2008.606. [DOI] [PubMed] [Google Scholar]
- 26.Valerio A, Cardile A, Cozzi V, Bracale R, Tedesco L, Pisconti A, Palomba L, Cantoni O, Clementi E, Moncada S, Carruba MO, Nisoli E. Tnf-alpha downregulates enos expression and mitochondrial biogenesis in fat and muscle of obese rodents. J Clin Invest. 2006;116:2791–2798. doi: 10.1172/JCI28570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jones SP, Greer JJ, Kakkar AK, Ware PD, Turnage RH, Hicks M, van Haperen R, de Crom R, Kawashima S, Yokoyama M, Lefer DJ. Endothelial nitric oxide synthase overexpression attenuates myocardial reperfusion injury. Am J Physiol Heart Circ Physiol. 2004;286:H276–282. doi: 10.1152/ajpheart.00129.2003. [DOI] [PubMed] [Google Scholar]
- 28.Duranski MR, Elrod JW, Calvert JW, Bryan NS, Feelisch M, Lefer DJ. Genetic overexpression of enos attenuates hepatic ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H2980–2986. doi: 10.1152/ajpheart.01173.2005. [DOI] [PubMed] [Google Scholar]
- 29.Takenaka K, Nishimura Y, Nishiuma T, Sakashita A, Yamashita T, Kobayashi K, Satouchi M, Ishida T, Kawashima S, Yokoyama M. Ventilator-induced lung injury is reduced in transgenic mice that overexpress endothelial nitric oxide synthase. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1078–1086. doi: 10.1152/ajplung.00239.2005. [DOI] [PubMed] [Google Scholar]
- 30.Kawashima S, Yamashita T, Ozaki M, Ohashi Y, Azumi H, Inoue N, Hirata K, Hayashi Y, Itoh H, Yokoyama M. Endothelial no synthase overexpression inhibits lesion formation in mouse model of vascular remodeling. Arterioscler Thromb Vasc Biol. 2001;21:201–207. doi: 10.1161/01.atv.21.2.201. [DOI] [PubMed] [Google Scholar]
- 31.Yamashita T, Kawashima S, Ohashi Y, Ozaki M, Ueyama T, Ishida T, Inoue N, Hirata K, Akita H, Yokoyama M. Resistance to endotoxin shock in transgenic mice overexpressing endothelial nitric oxide synthase. Circulation. 2000;101:931–937. doi: 10.1161/01.cir.101.8.931. [DOI] [PubMed] [Google Scholar]
- 32.Harats D, Kurihara H, Belloni P, Oakley H, Ziober A, Ackley D, Cain G, Kurihara Y, Lawn R, Sigal E. Targeting gene expression to the vascular wall in transgenic mice using the murine preproendothelin-1 promoter. J Clin Invest. 1995;95:1335–1344. doi: 10.1172/JCI117784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: Modulation by nutrients and inflammation. J Clin Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang H, Wang Y, Zhang J, Potter BJ, Sowers JR, Zhang C. Bariatric surgery reduces visceral adipose inflammation and improves endothelial function in type 2 diabetic mice. Arterioscler Thromb Vasc Biol. 2011;31:2063–2069. doi: 10.1161/ATVBAHA.111.225870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heusch G. Obesity and inflammatory vasculopathy: A surgical solution as ultima ratio? Arterioscler Thromb Vasc Biol. 2011;31:1953–1954. doi: 10.1161/ATVBAHA.111.232264. [DOI] [PubMed] [Google Scholar]
- 37.Nishimura S, Manabe I, Nagasaki M, Eto K, Yamashita H, Ohsugi M, Otsu M, Hara K, Ueki K, Sugiura S, Yoshimura K, Kadowaki T, Nagai R. Cd8+ effector t cells contribute to macrophage recruitment and adipose tissue inflammation in obesity. Nat Med. 2009;15:914–920. doi: 10.1038/nm.1964. [DOI] [PubMed] [Google Scholar]
- 38.Siliprandi N, Siliprandi D, Ciman M. Stimulation of oxidation of mitochondrial fatty acids and of acetate by acetylcarnitine. Biochem J. 1965;96:777–780. doi: 10.1042/bj0960777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sayed-Ahmed MM, Shouman SA, Rezk BM, Khalifa MH, Osman AM, El-Merzabani MM. Propionyl-l-carnitine as potential protective agent against adriamycin-induced impairment of fatty acid beta-oxidation in isolated heart mitochondria. Pharmacol Res. 2000;41:143–150. doi: 10.1006/phrs.1999.0583. [DOI] [PubMed] [Google Scholar]
- 40.D’Antona G, Ragni M, Cardile A, Tedesco L, Dossena M, Bruttini F, Caliaro F, Corsetti G, Bottinelli R, Carruba MO, Valerio A, Nisoli E. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010;12:362–372. doi: 10.1016/j.cmet.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 41.Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, Moncada S, Carruba MO. Calorie restriction promotes mitochondrial biogenesis by inducing the expression of enos. Science. 2005;310:314–317. doi: 10.1126/science.1117728. [DOI] [PubMed] [Google Scholar]
- 42.Wu Z, Puigserver P, Andersson U, Zhang C, Adelmant G, Mootha V, Troy A, Cinti S, Lowell B, Scarpulla RC, Spiegelman BM. Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator pgc-1. Cell. 1999;98:115–124. doi: 10.1016/S0092-8674(00)80611-X. [DOI] [PubMed] [Google Scholar]
- 43.Bogacka I, Xie H, Bray GA, Smith SR. Pioglitazone induces mitochondrial biogenesis in human subcutaneous adipose tissue in vivo. Diabetes. 2005;54:1392–1399. doi: 10.2337/diabetes.54.5.1392. [DOI] [PubMed] [Google Scholar]
- 44.Li P, Zhu Z, Lu Y, Granneman JG. Metabolic and cellular plasticity in white adipose tissue ii: Role of peroxisome proliferator-activated receptor-alpha. Am J Physiol Endocrinol Metab. 2005;289:E617–626. doi: 10.1152/ajpendo.00010.2005. [DOI] [PubMed] [Google Scholar]
- 45.Rong JX, Qiu Y, Hansen MK, Zhu L, Zhang V, Xie M, Okamoto Y, Mattie MD, Higashiyama H, Asano S, Strum JC, Ryan TE. Adipose mitochondrial biogenesis is suppressed in db/db and high-fat diet-fed mice and improved by rosiglitazone. Diabetes. 2007;56:1751–1760. doi: 10.2337/db06-1135. [DOI] [PubMed] [Google Scholar]
- 46.Koh YJ, Park BH, Park JH, Han J, Lee IK, Park JW, Koh GY. Activation of ppar gamma induces profound multilocularization of adipocytes in adult mouse white adipose tissues. Exp Mol Med. 2009;41:880–895. doi: 10.3858/emm.2009.41.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y. Sirtuin 3, a new target of pgc-1alpha, plays an important role in the suppression of ros and mitochondrial biogenesis. PLoS One. 2010;5:e11707. doi: 10.1371/journal.pone.0011707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Carlstrom M, Larsen FJ, Nystrom T, Hezel M, Borniquel S, Weitzberg E, Lundberg JO. Dietary inorganic nitrate reverses features of metabolic syndrome in endothelial nitric oxide synthase-deficient mice. Proc Natl Acad Sci U S A. 2010;107:17716–17720. doi: 10.1073/pnas.1008872107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, Jo H. Shear stress stimulates phosphorylation of endothelial nitric-oxide synthase at ser1179 by akt-independent mechanisms: Role of protein kinase a. J Biol Chem. 2002;277:3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- 50.Goirand F, Solar M, Athea Y, Viollet B, Mateo P, Fortin D, Leclerc J, Hoerter J, Ventura-Clapier R, Garnier A. Activation of amp kinase alpha1 subunit induces aortic vasorelaxation in mice. J Physiol. 2007;581:1163–1171. doi: 10.1113/jphysiol.2007.132589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mingorance C, Gonzalez del Pozo M, Dolores Herrera M, Alvarez de Sotomayor M. Oral supplementation of propionyl-l-carnitine reduces body weight and hyperinsulinaemia in obese zucker rats. Br J Nutr. 2009;102:1145–1153. doi: 10.1017/S0007114509389230. [DOI] [PubMed] [Google Scholar]
- 52.Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, Stevens RD, Li Y, Saha AK, Ruderman NB, Bain JR, Newgard CB, Farese RV, Jr, Alt FW, Kahn CR, Verdin E. Sirt3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 2010;464:121–125. doi: 10.1038/nature08778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, Shortreed MR, Prolla T, Markley JL, Smith LM, Zhao S, Guan KL, Denu JM. Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell. 2011;41:139–149. doi: 10.1016/j.molcel.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Moncada S, Higgs EA. The discovery of nitric oxide and its role in vascular biology. Br J Pharmacol. 2006;147 (Suppl 1):S193–201. doi: 10.1038/sj.bjp.0706458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Victor VM, Nunez C, D’Ocon P, Taylor CT, Esplugues JV, Moncada S. Regulation of oxygen distribution in tissues by endothelial nitric oxide. Circ Res. 2009;104:1178–1183. doi: 10.1161/CIRCRESAHA.109.197228. [DOI] [PubMed] [Google Scholar]
- 56.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–2578. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Petersen KF, Shulman GI. Pathogenesis of skeletal muscle insulin resistance in type 2 diabetes mellitus. Am J Cardiol. 2002;90:11G–18G. doi: 10.1016/s0002-9149(02)02554-7. [DOI] [PubMed] [Google Scholar]
- 58.Cederberg A, Gronning LM, Ahren B, Tasken K, Carlsson P, Enerback S. Foxc2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell. 2001;106:563–573. doi: 10.1016/s0092-8674(01)00474-3. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci U S A. 2009;106:19860–19865. doi: 10.1073/pnas.0906048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Polak P, Cybulski N, Feige JN, Auwerx J, Ruegg MA, Hall MN. Adipose-specific knockout of raptor results in lean mice with enhanced mitochondrial respiration. Cell Metab. 2008;8:399–410. doi: 10.1016/j.cmet.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 61.Seale P, Conroe HM, Estall J, Kajimura S, Frontini A, Ishibashi J, Cohen P, Cinti S, Spiegelman BM. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. doi: 10.1172/JCI44271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim JY, van de Wall E, Laplante M, Azzara A, Trujillo ME, Hofmann SM, Schraw T, Durand JL, Li H, Li G, Jelicks LA, Mehler MF, Hui DY, Deshaies Y, Shulman GI, Schwartz GJ, Scherer PE. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. J Clin Invest. 2007;117:2621–2637. doi: 10.1172/JCI31021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- 64.Patsouris D, Li PP, Thapar D, Chapman J, Olefsky JM, Neels JG. Ablation of cd11c-positive cells normalizes insulin sensitivity in obese insulin resistant animals. Cell Metab. 2008;8:301–309. doi: 10.1016/j.cmet.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. Mcp-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wood IS, de Heredia FP, Wang B, Trayhurn P. Cellular hypoxia and adipose tissue dysfunction in obesity. Proc Nutr Soc. 2009;68:370–377. doi: 10.1017/S0029665109990206. [DOI] [PubMed] [Google Scholar]
- 67.Ye J. Emerging role of adipose tissue hypoxia in obesity and insulin resistance. Int J Obes (Lond) 2009;33:54–66. doi: 10.1038/ijo.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, McDonnell M, Hess D, Joseph L, Gokce N. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lumeng CN, Deyoung SM, Saltiel AR. Macrophages block insulin action in adipocytes by altering expression of signaling and glucose transport proteins. Am J Physiol Endocrinol Metab. 2007;292:E166–174. doi: 10.1152/ajpendo.00284.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Garg A. Acquired and inherited lipodystrophies. N Engl J Med. 2004;350:1220–1234. doi: 10.1056/NEJMra025261. [DOI] [PubMed] [Google Scholar]
- 71.Cortes VA, Curtis DE, Sukumaran S, Shao X, Parameswara V, Rashid S, Smith AR, Ren J, Esser V, Hammer RE, Agarwal AK, Horton JD, Garg A. Molecular mechanisms of hepatic steatosis and insulin resistance in the agpat2-deficient mouse model of congenital generalized lipodystrophy. Cell Metab. 2009;9:165–176. doi: 10.1016/j.cmet.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cui X, Wang Y, Tang Y, Liu Y, Zhao L, Deng J, Xu G, Peng X, Ju S, Liu G, Yang H. Seipin ablation in mice results in severe generalized lipodystrophy. Hum Mol Genet. 2011;20:3022–3030. doi: 10.1093/hmg/ddr205. [DOI] [PubMed] [Google Scholar]
- 73.Fleming I, Busse R. Molecular mechanisms involved in the regulation of the endothelial nitric oxide synthase. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1–12. doi: 10.1152/ajpregu.00323.2002. [DOI] [PubMed] [Google Scholar]
- 74.Musicki B, Kramer MF, Becker RE, Burnett AL. Inactivation of phosphorylated endothelial nitric oxide synthase (ser-1177) by o-glcnac in diabetes-associated erectile dysfunction. Proc Natl Acad Sci U S A. 2005;102:11870–11875. doi: 10.1073/pnas.0502488102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen CA, Wang TY, Varadharaj S, Reyes LA, Hemann C, Talukder MA, Chen YR, Druhan LJ, Zweier JL. S-glutathionylation uncouples enos and regulates its cellular and vascular function. Nature. 2010;468:1115–1118. doi: 10.1038/nature09599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shaul PW, Smart EJ, Robinson LJ, German Z, Yuhanna IS, Ying Y, Anderson RG, Michel T. Acylation targets emdothelial nitric-oxide synthase to plasmalemmal caveolae. J Biol Chem. 1996;271:6518–6522. doi: 10.1074/jbc.271.11.6518. [DOI] [PubMed] [Google Scholar]
- 77.Garcia-Cardena G, Oh P, Liu J, Schnitzer JE, Sessa WC. Targeting of nitric oxide synthase to endothelial cell caveolae via palmitoylation: Implications for nitric oxide signaling. Proc Natl Acad Sci U S A. 1996;93:6448–6453. doi: 10.1073/pnas.93.13.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.