Abstract
Learning abstract rules is central to social and cognitive development. Across two experiments, we used Delayed Non-Matching to Sample tasks to characterize the longitudinal development and nature of rule-learning impairments in children with Autism Spectrum Disorder (ASD). Results showed that children with ASD consistently experienced more difficulty learning an abstract rule from a discrete physical reward than children with DD. Rule learning was facilitated by the provision of more concrete reinforcement, suggesting an underlying difficulty in forming conceptual connections. Learning abstract rules about social stimuli remained challenging through late childhood, indicating the importance of testing executive functions in both social and non-social contexts.
The ability to extract abstract rules from individual experiences is critical to a wide range of domains, including categorization (e.g., Martin & Caramazza, 1980), language (e.g., Marcus, Fernandes, & Johnson, 2007), and social behavior (e.g., Weston & Elliott, 1980). Individuals with autism spectrum disorder (ASD) show difficulty in the learning, application, and flexibility of abstract rules. This affects their ability to respond appropriately in social situations. For example, one study examined the ability of children with ASD to use emotional and self-presentational display rules like “respond positively even when you receive an unwanted gift” and found that they were able to use such rules less adequately than comparison groups (Barbaro & Dissanayake, 2007). Once one rule has been successfully mastered, individuals with ASD may have difficulty learning and applying a conflicting rule (e.g., Reed, Watts, & Truzoli, 2011). Rule violation may be particularly salient to individuals with ASD: for example, a functional neuroimaging study showed that children and adolescents with ASD show hyperactivation of the right insula and the dorsal prefrontal cortex when they observe a rule violation (Bolling et al., 2011). Taken together, findings suggest that difficulty learning and using rules may be common in individuals with ASD.
Rule learning deficits may be related to wider difficulties with executive functioning in ASD. Executive functioning refers to a suite of higher cognitive abilities dependent on the prefrontal cortex, a region that appears to develop atypically in ASD (e.g., Carper & Courchesne, 2005), and executive functioning impairments may relate to the development or expression of core symptoms of ASD (for review, Hill, 2004). Rule learning also involves the prefrontal cortex (e.g., Crone, Donohue, Honomichl, Wendelken, & Bunge, 2006). Rule learning deficits have been associated with symptoms of ASD: deficits in acquiring a contrasting rule are associated with more severe restrictive and repetitive behaviors (e.g., Reed et al., 2011). Initial rule learning has been concurrently related to level of joint attention skills (Dawson, Munson, et al., 2002), and predicted rate of growth in social and communicative adaptive skills over a 6-year period (Munson, Faja, Meltzoff, Abbott, & Dawson, 2008). Taken together, these observations suggest that rule-learning impairments may be related to the core neuropsychology of ASD.
Although impairments in rule learning in ASD have been observed across a number of studies, they are not necessarily specific to ASD. For example, using the same task Dawson and colleagues (1998) observed more severe impairments in rule-learning in 5-year-olds with ASD compared to age-matched children with Down syndrome (Dawson, Meltzoff, Osterling, & Rinaldi, 1998), whereas 3- to 4-year-old children with ASD performed at similar levels of impairment to a comparison group of children with idiopathic developmental delay (Dawson, Munson, et al., 2002). In another study, children with ASD showed more severe impairments than children with developmental delays on a spatial reversal task at age 5 years (McEvoy, Rogers, & Pennington, 1993) but not age 3.5 years (Griffith, Pennington, Wehner, & Rogers, 1999). The prefrontal cortex undergoes significant development over the preschool period (for review, Diamond, 2002), and disruption to this developmental trajectory in ASD (e.g., Carper & Courchesne, 2005) may drive the emergence of group differences in rule-learning ability. However, Loveland, Bachevalier, Pearson, and Lane (2008) did not observe differences in rule learning in adolescents with ASD relative to IQ-matched controls, and other studies have failed to find group differences in contrasting rule acquisition later in development (e.g., Edgin & Pennington, 2005), suggesting that there may be a complex relation between rule learning impairments and chronological age.
Understanding the development of rule-learning impairments in ASD requires longitudinal studies, because the heterogeneity in skills and functioning across ages and individuals makes cross-study comparisons challenging to interpret. This complexity may be compounded by the use of different strategies to select “matched” control groups. These include matching on chronological and verbal mental age (Dawson et al., 1998; Hughes et al., 1994), matching on chronological and composite mental age with a developmentally delayed comparison group (Dawson, Munson, et al., 2002), and matching the mental age of children with ASD to the chronological age of typically developing controls (e.g., Reed et al., 2011). Possibly, impairments in rule learning are restricted to a subset of individuals with ASD, or are shared by individuals with particular types of cognitive delay. Further characterization of how rule learning is related to core measures of functioning in children with ASD and comparison groups is required to determine the nature of rule-learning deficits in ASD.
It is important to identify why children with ASD might have difficulty in learning and applying rules. One possibility is disruption to the ability to learn from particular types of reward. Most rule-learning paradigms involve the provision of feedback, with more concrete and immediate rewards for younger children (e.g., access to a toy or food item) and more abstract rewards for older children and adults (e.g., accrual of points, a symbol indicating correctness), since the efficacy of rewards of different levels of abstraction changes over development. For example, for young typically developing infants highly contingent verbal praise is more effective in a rule-learning task than access to a desired toy, while for older infants these two reward types are equally effective (Diamond, Churchland, Cruess, & Kirkham, 1999). In typical development, children’s ability to wait for a larger reward improves over the preschool period (e.g., Thompson, Barresi, &Moore, 1997), suggesting a decreasing requirement for contiguity in reward provision. These developmental changes in the ability to benefit from more abstract and delayed rewards have been linked to the maturation of the prefrontal cortex (e.g., Diamond, 2006). If immaturity of the prefrontal cortex in ASD leads to difficulty learning from abstract rewards, the provision of concrete rewards might facilitate learning (Diamond, 2006). However, this prediction has not yet been tested.
The degree to which rule-learning impairments might differ across social and non-social domains is also unclear, since rule learning has typically been examined using non-social stimuli. For example, studies of the flexibility of rule use often employ simple drawings with different shapes and colors (e.g., Reed et al., 2011), and studies of abstract rule learning have employed non-nameable junk objects (e.g., Dawson et al., 1998; Loveland et al., 2008). However, a recent study suggests that the incorporation of social stimuli to an executive functioning task can increase its difficulty in typical development. Lagattuta, Sayfan, and Monsour (2011) created a social version of the day–night task, in which children must say “day” when they are shown a picture depicting night. This task measures the capacity to inhibit a prepotent response, which like rule-learning is thought to be dependent on the prefrontal cortex. Individuals from age 4- to adulthood found the happy–sad task more difficult than the day–night task, suggesting that the application of executive functions like inhibition in the social domain may differ from success in their application to non-social problems. If individuals with ASD find rule learning more difficult with social stimuli, we may underestimate the contribution of rule-learning impairments to the social symptoms of ASD by testing rule-learning solely in a non-social context.
The present study was designed to provide further insight into the longitudinal development of rule-learning abilities in ASD, relation to reward type, and whether impairments are more pronounced in a social context. Data were taken from a larger longitudinal study of children with ASD tested at three time points: 4 years (range 36– 52 months), 6 years (range 68–93 months), and 9 years (range 107–124 months) (e.g., Dawson, Munson, et al., 2002; Dawson et al., 2004; Munson et al., 2008). To assess rule-learning, we employed the Delayed Non-Matching to Sample (DNMS) task. In this procedure, individuals must learn through trial-and-error that the more novel of a pair of stimuli is associated with a reward. This requires the ability to remember the familiar stimulus and to distinguish it from the novel one, and to develop a rule that links novelty with reward. Since no verbal skills are required, the DNMS can be used from infancy to adulthood and with children of different abilities. This is particularly important since few investigations of rule learning have been included both lower and higher-functioning children. Further, the DNMS can be adapted to include both social and non-social stimuli to examine whether executive functioning deficits are greater in the social domain, and the nature of the reward provided for successful performance can be varied.
Selecting an appropriate comparison group is a critical parameter in examining the performance of children with ASD. In the current study, comparisons were primarily made with a group of children with developmental delay (DD), also tested longitudinally and matched as a group on chronological, verbal and non-verbal mental age when recruited at age 4 years. Groups remained matched on the same variables at subsequent assessment points. This comparison group enabled us to identify potential areas of “impairment” in ASD that could not solely be attributed to children’s degree of developmental delay or intellectual disability. For some analyses, additional comparisons were made with chronological age-matched typically developing (TD) children to clarify potential deficits that may be related to general developmental level.
EXPERIMENT 1
In the first experiment, we examined age-related changes in rule-learning, relations with cognitive and adaptive skill, and the role of reward type in shaping rule-learning difficulties in ASD. In an initial study using the DNMS task, Dawson and colleagues (1998) found that 5- to 7-year-old children with ASD perform worse than verbal mental age-matched children with typical development or developmental delay in learning the DNMS rule. In contrast, 4-year-old children with ASD performed similarly to mental age (MA)-matched controls on both rule learning and subsequent recognition memory (Dawson, Munson, et al., 2002). The authors suggest that greater degrees of rule-learning deficits relative to MA-matched comparison groups may emerge between early and middle childhood. To test this proposal, we examined DNMS performance at age 6 years in children initial tested at age 4 years (Dawson, Munson, et al., 2002; Analysis 1), and examined longitudinal change in performance between age 4 years and age 6 years (Analysis 2). We predicted that by age 6 years, children with ASD would perform worse in learning the DNMS rule than the MA-matched comparison groups as a result of reduced age-related improvement. We also examined whether heterogeneity in cognitive and adaptive skill is related to rule learning, predicting that rule-learning may be less impaired in children with ASD who have stronger cognitive skills.
Finally, we examined the role of reward type in rule learning. In a standard DNMS task, when children select the novel stimulus they uncover a reinforcing toy hidden in an underlying well. Finding the toy is both temporally and physically dissociated from touching the non-matching (correct) object, requiring children to form a conceptual connection between stimulus and reward. In a series of studies, Diamond and colleagues show that it is this stimulus–reward dissociation that is particularly challenging for typically developing 9- to 15-month-old infants (Diamond et al., 1999). Typically developing infants who do not learn the DNMS rule in a standard DNMS task can nonetheless learn the same rule when rewarded with verbal praise delivered at the moment of contact with the correct stimulus, or with a small reinforcing toy that is physically connected to the correct stimulus (Diamond et al., 1999). This effect is transient: by around 2 years of age, typically developing children have learned to extract abstract rules from dissociated rewards and approach ceiling levels of performance in the DNMS–Standard task (Diamond, Towle, & Boyer, 1994). Diamond (2006) proposes that in children with ASD, disrupted development of areas of the frontal cortex lead to difficulty in forming conceptual connections that is prolonged far beyond the infancy period. This leads to the prediction that children with ASD will find it easier to learn the DNMS rule if the reward does not involve the formation of a conceptual stimulus–reward connection. To test this proposal, at age 6 years (Analysis 3) we compared performance on a standard DNMS task (“DNMS–Standard”) with performance on the “DNMS–Praise” task, in which the reward was verbal praise that was delivered simultaneously with the child’s first touch to the correct stimulus (Diamond et al., 1999). We predicted that if children with ASD have difficulty learning a rule from a discrete physical reward because of its dissociation from the stimulus, they should perform better on the DNMS–Praise than the DNMS–Standard rule-learning trials; children with DD should show equally strong performance on the two versions. We did not test children with TD on the DNMS–Praise task because we anticipated ceiling levels of performance in the harder DNMS–Standard task (Diamond et al., 1994).
Methods
Participant groups
Three groups of children participated in the DNMS task(s) at age 6 years: (1) 69 children with ASD, enrolled at 4 years; (2) 39 children with Developmental Delay/DD (36 with idiopathic developmental delay and 3 with Down syndrome; 35 enrolled at 4 years, and 4 newly enrolled at 6 years); (3) 23 TD children with no family history of ASD (4 enrolled at 4 years and 19 newly enrolled at 6 years). At entry to the study, participants were recruited from local parent advocacy groups, public schools, the Department of Developmental Disabilities, clinical, hospitals, and the University of Washington Infant and Child Subject pool. Exclusionary criteria included significant sensory or motor impairment, major physical abnormalities, history of serious head injury, seizures, and/or neurological disease, and for the group with ASD or TD the presence of a neurological disorder of known etiology related to ASD (e.g., Fragile X).
Clinical assessment
Children were evaluated at age 4 years and age 6 years using the Vineland Adaptive Behaviors Scales–Expanded (VABS; Sparrow, Balla, & Cicchetti, 1984) and Mullen Scales of Early Development (Mullen, 1997) or the Differential Abilities Scale (DAS; Elliot, 1990). The VABS is a parent-report norm-referenced measure that yields standard scores in the domains of socialization, communication, motor skills and daily living. The Mullen and DAS are normed standardized observational measures of cognitive skills; both measures afford standard scores for verbal and non-verbal domains. ASD symptoms were evaluated using the Autism Diagnostic Interview–Revised (ADI–R; Lord, Rutter, & LeCouteur, 1994), the Autism Diagnostic Observation Schedule–Generic (ADOS–G; Lord, Rutter, DiLavore, & Risi, 1999), and clinical best estimate based on the Diagnostic and Statistical Manual of Mental Disorders (4th ed. [DSM-IV-TR]; American Psychiatric Association, 2000) criteria. At 4 and 6 years, the group with ASD met criteria for autism or autism spectrum disorder on the ADOS–G, were within two points of meeting criteria for autism on the ADI–R, and were clinically diagnosed with autistic disorder or pervasive developmental disorder not otherwise specified based on DSM-IV criteria. At 4 and 6 years, children with DD did not meet criteria for autism or autism spectrum disorder on the ADOS–G and ADI–R.
Matching
At 4 years, children with DD had standard scores ≤85 on at least three of four domains on the VABS and on the Mullen/DAS verbal and non-verbal domains. Many children did not continue to meet these cutoffs at 6 years; these children were not excluded from analyses because the cognitive level of the group with DD was not significantly different from that of the group with ASD at 6 years. At 4 and 6 years, children were included in the group with TD if they had standard scores over 85 on the Mullen/DAS, and over 85 on the VABS composite score and subdomain scores. The resulting groups did not significantly differ in chronological age, and the groups with ASD and DD did not significantly differ in verbal or nonverbal mental age at study entry or subsequent time-points. Table 1 presents demographic and descriptive data for children included in the final sample (ASD n = 64; DD n = 36; TD n = 23).
TABLE 1.
Demographic Information About the Three Diagnostic Groups; Mean (Standard Deviation) and Range Are Given
| 3–4 Years | ASD | DD | TD | |
|---|---|---|---|---|
| N (female) | 64 (12) | 36 (18) | 33(9) | |
| Age (months) | 43.5 (4.1) | 45.0 (5.7) | 44.4 (5.7) | n.s. |
| 36–52 | 33–57 | 26–55 | ||
| Mullen verbal DQ | 53.2 (24.6) | 60.7 (17.7) | 112.3 (11.6) | *ASD = DD < TD |
| 18–109 | 15–95 | 100–143 | ||
| Mullen nonverbal DQ | 64.5 (17.5) | 65.5 (17.7) | 103.0 (10.2) | *ASD = DD < TD |
| 34–104 | 27–95 | 89–128 | ||
| 5–7 Years | ASD | DD | TD | |
| N (female) | 64 (11) | 36 (14) | 23 (5) | |
| Age (months) | 75.1 (3.9) | 74.5 (3.2) | 76.6 (3.6) | n.s. |
| 68–93 | 69–84 | 72–82 | ||
| DAS verbal standard score | 70.3 (22.0) | 75.3 (18.7) | 110.8 (13.1) | *ASD = DD < TD |
| 50–127 | 50–107 | 92–133 | ||
| DAS non-verbal standard score | 73.6 (23.9) | 68.1 (21.0) | 107.9 (10.3) | *ASD = DD < TD |
| 43–124 | 43–105 | 88–132 | ||
| 9–11 Years | ASD | DD | ||
| N (female) | 37 (5) | 21 (9) | ||
| Age (months) | 111.8 (3.5) | 110.5 (3.5) | n.s. | |
| 107–124 | 105–122 | |||
| DAS verbal standard score | 75.8 (22.6) | 76.0 (19.3) | n.s. | |
| 50–127 | 43–105 | |||
| DAS non-verbal standard score | 82.4 (21.8) | 70.5 (22.0) | n.s. | |
| 43–124 | 43–105 | |||
Note. ASD = Autism Spectrum Disorder; TD = Typical Development; DD = Developmental Delay; DQ = Developmental Quotient (age-equivalent/chronological age).
Indicates significant effect of group p < .05.
Procedure
The general procedure for all DNMS tasks was based on the paradigm described by Diamond et al. (1999). In the sample phase of each trial, the child was shown a “sample” stimulus (a trial-unique 3D junk object) that covered a wooden well. When the child touched or displaced the sample stimulus, he/she was rewarded. In the DNMS–Standard task the reward was a small toy hidden in the well in addition to verbal reinforcement (“wow, you got it!”; “you found a dinosaur!”). In the DNMS–Praise task, if the child chose correctly the experimenter clapped emphatically and said “yay!” as soon as the child touched the object.
In the subsequent test phase the child was presented with the now-familiar (matching) sample stimulus and a novel (non-matching) stimulus, both covering identical wooden wells. If the child touched the non-matching stimulus first, he/she was again rewarded in the same manner as the sample phase. If the child touched the matching stimulus first, verbal feedback with neutral affect was provided and the child was not rewarded. The criterion for rule learning was five consecutive correct responses (maximum: 20 trials) presented with a 5-second sample-test delay; if criterion was met, a further 15 trials were presented with a 30-second delay.
All three diagnostic groups participated in the DNMS–Standard; only the groups with ASD and DD additionally participated in the DNMS–Praise. The order of presentation of the tasks (DNMS–Standard versus DNMS–Praise) was counterbalanced; tasks were administered a median of 9 days apart for the group with ASD and 7 days apart for the group with DD (t(63) = 1.7, p = .12).
Analysis
Trials were excluded from analysis if the child did not reach to one of the stimuli; performance on the task as a whole was excluded if the parent cued the child, there was experimenter error, or if the child was non-compliant with the task. Analysis variables included: (a) trials to criterion (Trials TC), the number of valid trials preceding the first of the five consecutive correct trials; (b) Percent correct on the 5-second delay trials (PC-5s delay); (c) Percent correct on the 30-second delay trials (PC-30s delay); (d) Proportion of children who met criterion. Of note, Trial TC and PC-30s delay can only be computed for children who met criterion.
Parametric variables (Trials TC, PC-5s delay, PC-30s delay) were analyzed separately with ANOVA, Bonferroni-corrected posthoc tests and follow-up tests for significant interactions; the proportion of children meeting criterion was analyzed with non-parametric chi-square and McNemar tests. Because of study design or invalidity of particular testing sessions, not all children in the wider sample contributed data to all analyses. For each analysis, multivariate ANOVAs on age, and verbal and nonverbal standard scores by group and inclusion status were used to assess whether subgroups were representative of the full cohort; discrepancies are noted.
Results
Group differences in rule-learning in the DNMS–Standard at 6 years
This analysis included children tested in the DNMS–Standard first (ASD n =36; DD n = 22; TD n = 22). PC-5s delay differed between the three diagnostic groups (ASD M = 70%, SD = 21; DD M = 80%, SD = 23; TD M = 85%, SD = 17, F(2,79) = 3.9, p = .02, ηp2= 0.09); the group with ASD performed significantly more poorly than the group with TD (p = .02) and the group with DD was intermediate (ps > .3). There were no significant group differences in Trials TC (F(2,60) = .6, p = .6). However, significantly fewer children in the group with ASD met criterion than in the group with TD (ASD 61%, TD 86% X2(1) = 4.2, p = .04, Φ = 0.27), and the group with DD again performed at an intermediate level (DD 77%; X2s < 1, ps > .5). PC-30s delay also differed between the three groups (ASD M = 80%, SD = 14; DD M = 77%, SD = 18; TD M = 92%, SD = 11; F(2,57) = 6.0, p< .01, ηp2= 0.18); the group with ASD performed significantly worse than the group with TD (p = .02), with the DD group intermediate (DD p = .06), and the groups with ASD and DD did not significantly differ (p = .8).
Finally, the groups differed in the impact of the post-criterion delay increase (F(2,55) = 3.4, p = .04, ηp2= 0.11): only the group with DD showed a significant decrease in performance between pre- and post-criterion trials (M = 90%, SD = 13; M = 77%, SD = 18; F(1,16) = 8.62, p = .01, ηp2= 0.35). Thus, children with ASD performed more poorly on the DNMS–Standard task than chronological age-matched controls over both short and long delays, but did not show worsening performance with increased delay. This is consistent with the notion that children with ASD have difficulty learning the rule, but not necessarily in remembering the familiar object. Performance was significantly disrupted by the increased delay for children with DD, a pattern consistent with a recognition memory impairment.
Multivariate analyses conducted with all children who provided valid data in the DNMS–Standard showed that children with ASD who met the criterion for having learned the rule were more advanced than those who did not meet criterion across verbal (met M = 76.6, SD = 22.2; Did not meet M = 61.9, SD = 2.1), and non-verbal (Met M = 82.2, SD = 22.7; Did not meet M = 60.0, SD = 2.7) standard scores and VABS socialization (Met M = 66.1, SD = 1.3; Did not meet M = 59.4, SD = 11.4) and communication (Met M = 68.4, SD = 17.6; Did not meet M = 55.7, SD = 19.3) standard scores (F(4,49) = 3.27, p = .02, ηp2= 0.2). Higher PC-5s delay and PC-30s delay also correlated with higher verbal (5s r(41) = .44, p = .004; 30s r(25) = .52, p = .006) and nonverbal (5s r(41) = .61, p < .001; 30s r(25) = .40, p = .05) cognitive scores and socialization (5s r(41) = .42, p = .006; 30s r(25) = .45, p = .025) and communication (5s r(41) = .44, p = .004; 30s r(25) = .43, p = .032) skills in the group with ASD. The only significant relations in the group with DD were between PC-30s delay and verbal (r(15) = .52, p = .045) and nonverbal (r(15) = .63, p = .012) cognitive skills. This provides further evidence that individual differences in rule-learning are related to key dimensions of cognitive and adaptive functioning in children with ASD (e.g., Munson et al., 2008), and suggests that the recognition memory component of the task may be most discriminative for children with DD.
Longitudinal development between age 4 years and age 6 years
Children included in this analysis provided valid data for the DNMS–Standard task tested first at 4 and 6 years (ASD n = 29, DD n = 16). A greater proportion of the group with DD met criterion at both time-points (69%) than the group with ASD (38%; X2(1) = 3.9, p = .048, Φ = 0.23). Further, across both time-points the group with DD outperformed the group with ASD on rule-learning trials (Trials TC ASD M = 1.68, SD = 2.45; DD M = 6.68, SD = 2.45; F(1,20) = 14.5, p = .001, ηp2= 0.42; PC-5s delay ASD M = 67%, SD = 16; DD M = 80%, SD = 16; F(1,43) = 7.4, p = .009, ηp2= 0.15). There was no evidence that performance on the rule-learning trials changed with age in either the groups with ASD or DD (Trials TC F(1,20) = 1.1, p = .31; PC-5s delay F(1,43) = .00, p = .99). The proportion of children who changed classification from pass to fail versus from fail to pass between 4 years and 6 years did not differ in either group (McNemar change test; ps > .9). However, performance on post-criterion memory trials improved between 4 and 6 years with no significant interaction with group (4 years M = 73%, SD = 12; 6 years M = 81%, SD = 1; F(1,20) = 6.0, p = .02, ηp2= 0.23).
Children with DD who met criterion at 4 years performed significantly better on 5-second delay trials at 6 years than children who did not (PC-5s delay: M = 85%, SD = .20; M = 51%, SD = .19, F(1,15) = 7.3, p = .017, ηp2= 0.34; Trials TC M = 15.0, M = 6.4, SD = 2.0, (F(1,11) = 16.07, p = .002, ηp2= 0.62). The group with ASD did not show this pattern (PC-5s delay: M = 69%, SD = .21; M = 66%, SD = .22, F(1,28) = .11, p = .74; Trials TC M = 9.82, SD = 5.0; M = 7.33, SD = 2.7, F(1,16) = 1.25, p = .28). Further, PC-30s delay was correlated at 4 years and 6 years only for the group with DD (DD: r(11) = .69, p = .02; ASD: r(11) = .4, p = .2). Thus, individual differences in performance were more stable over time for the group with DD.
In summary, the data did not indicate any overall improvement in performance between age 4 years and 6 years for either the group with ASD or DD. Although performance in the groups with ASD and DD did not significantly differ when performance was examined at a single time-point (age 6 years, Analysis 1), longitudinal analysis revealed that children with ASD performed more poorly than children with DD on the rule-learning trials. Interestingly, effect sizes showed that group differences were larger for speed of rule-learning (Trials TC) than for performance on the brief delay trials (PC-5s delay) in children who both did and did not learn the rule. Possibly, speed of rule learning is a metric less likely to be contaminated by memory difficulties that may impact performance in the DD group. Finally, individual differences in performance were more consistent over time in the group with DD than the group with ASD.
The role of reward: DNMS–Standard versus DNMS–Praise
Children included in this analysis were children who provided valid data in both the DNMS–Standard and DNMS–Praise tasks, administered in either order (ASD n = 42; DD n = 21). The groups of children tested with the task in different orders did not significantly differ in cognitive skills or symptom profiles (Fs< 1.5, ps > 0.2). Across groups, children who provided valid data in both the DNMS–Standard and DNMS–Praise were slightly younger (M = 74.1 m, SD = 4.0) than children who did not (M = 75.7 m, SD = 4.3; F(1,95) = 52.3, p = .03).
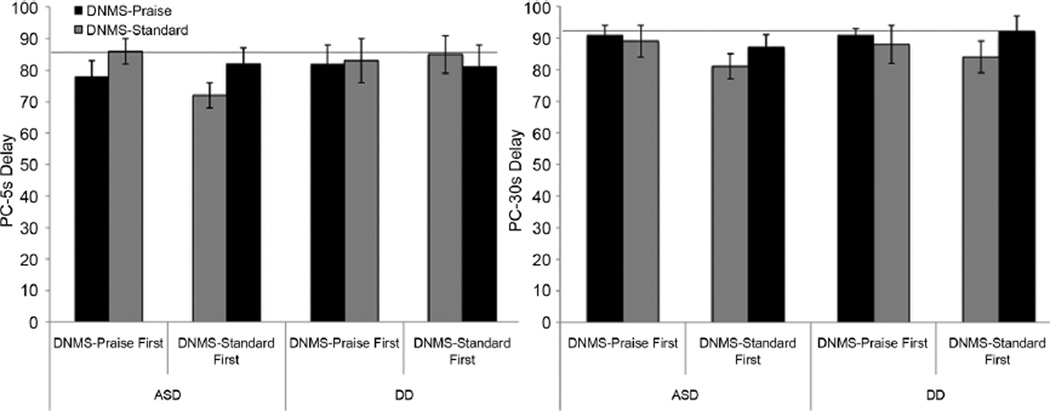
As seen in Figure 1, the groups with ASD and DD differed in how rule learning was impacted by task order and reward version. Order and task significantly impacted performance for the group with ASD (PC-5s delay F (1,40) = 6.3, p = .016, ηp2= 0.14). For children with ASD tested with DNMS–Standard then DNMS–Praise, performance on the rule-learning trials was better for DNMS–Praise than DNMS–Standard (PC-5s delay F(1,22) = 5.55, p = .028, ηp2= 0.20). In contrast, children with ASD tested with the DNMS–Praise first performed equally well on both tasks (PC-5s delay F(1,18) = 1.83, p = .19). Prior experience with the DNMS–Praise enabled children with ASD to perform better on 5-second delay trials in the DNMS–Standard than children who did not have prior experience with the DNMS–Praise (PC-5s delay F(1,41) = 5.7, p = .02, ηp2= 0.13), suggesting that children who had learned the rule in the DNMS–Praise task were subsequently able to apply it in the DNMS–Standard task. In contrast, performance on the DNMS–Praise was not affected by prior experience with the DNMS–Standard (PC-5s delay F(1,41) = .55, p = .46). Effects trended in the same direction for analyses with Trials TC, but failed to reach conventional significance. In contrast, performance in the group with DD did not differ by task or order on either metric (Fs < 3, ps > .1).
FIGURE 1.
Performance on the Delayed Non-Matching to Sample (DNMS)–Standard and DNMS–Praise tasks (Experiment 1) as a function of order of presentation and diagnostic group; bars show mean percent correct on the 5-sec delay trials (left) and post-criterion 30-sec delay trials (right). Error bars show +/−1 SE. The horizontal line indicates performance on the DNMS task by the chronological age-matched typically developing group (PC—5 sec delay: M = 85%, SE = 5%; PC—30 sec delay: M = 92%, SE = 2%).
Correct selection of the novel stimulus after a 30-second delay was more common on the DNMS–Praise than the DNMS–Standard with no order effect (PC-30s delay F(1,39) = 4.15, p = .05, ηp2= 0.096). This was significant in the group with ASD (F(1,25) = 6.98, p = .014, ηp2= 0.22), but not the group with DD (F(1,14) = 0.45, p = .51), suggesting that children with ASD continued to benefit from the contingent verbal praise when applying the rule over a longer delay.
In summary, children with ASD were better able to learn the DNMS rule when rewarded with highly contingent verbal praise than when rewarded with a discrete physical reward. These effects were only seen when children were tested in the DNMS–Standard task first, possibly because when the DNMS–Standard task was administered second children were helped by having previously learned the rule in the DNMS–Praise task. Reward type did not influence responding for children with DD. However, effect sizes were relatively small (ηp2=0.13–0.22), indicating that other factors accounted for the majority of variance in rule learning in the children with ASD.
Discussion
This longitudinal study examined rule-learning ability on the Delayed Non-Match to Sample task in children with ASD and DD over the late preschool to early elementary school period. Contrary to our prediction that deficits in rule-learning in children with ASD relative to children with DD would grow in early childhood, we found no evidence that rule-learning ability systematically declines or improves on this task for children with ASD between age 4 and 6 years. On average, however, greater performance stability across the two time-points was evident in the group with DD than the group with ASD, and we observed poorer rule-learning performance in the group with ASD relative to the group with DD when considering performance at multiple time-points. Poorer rule-learning skills may be an enduring problem for young children with ASD, and particularly for those children with low levels of cognitive and adaptive functioning. However, rewarding children with highly contingent verbal praise facilitated abstract and transferable rule-learning for children with ASD, suggesting potential avenues for intervention.
In addition to conducting a standard version of the DNMS task (DNMS–Standard) using objects as rewards for correct application of the rule, we conducted a novel version in which contingent praise was used as a reward (DNMS–Praise). This tested the prediction that children with ASD have difficulty forming associations between a stimulus and a discrete physical reward (Diamond, 2006). Supporting this hypothesis, at age 6 years children with ASD found it easier to learn the non-matching rule for the first time when rewarded with contingent praise than when rewarded with a physically discrete object. Further, children with ASD who were tested with the DNMS–Praise task first (allowing them to learn the non-matching rule) subsequently performed the DNMS–Standard task significantly better than children tested initially on the DNMS–Standard task. It is unlikely that heterogeneity within the group of children with ASD could account for significantly better performance in the children tested first with the DNMS–Praise condition. Groups tested in the different orders did not differ in performance on the DNMS–Praise or on cognitive skills and symptom levels, and performance of children tested first with the DNMS–Standard replicated findings with 6–7-year-old children tested in the DNMS–Standard task by Dawson et al. (1998), while the performance of children tested first with the DNMS–Praise condition differed. Rather, these observations support the contention that replacement of the dissociated physical reward with highly contingent verbal praise facilitated abstract and transferable rule-learning for children with ASD. This finding has potential implications for the types of rewards that might be most effective in teaching children with ASD.
Rule-learning ability bore significant relations with socialization and communication skills in the present dataset, core domains of impairment in ASD (see also Dawson, Munson, et al., 2002; Munson et al., 2008). If rule-learning impairments are related to social development because they make it difficult for individuals with ASD to learn rules about the social world, comparable or greater rule-learning impairments should be observable for social stimuli. We tested this possibility in Experiment 2.
EXPERIMENT 2: RULE-LEARNING IN THE SOCIAL DOMAIN
Impairments that are more pronounced in social than non-social contexts have been documented across a range of domains in ASD, including recognition memory (e.g., Blair, Frith, Smith, Abell, & Cipolotti, 2002), orienting (e.g., Dawson et al., 2004), and reward processing (e.g., Scott-Van Zeeland, Dapretto, Ghahremani, Poldrack, & Bookheimer, 2010). However, few studies have directly examined the application of executive function skills like rule learning in the social domain. To examine whether rule learning impairments may be more pronounced for social than non-social stimuli, we contrasted performance on two versions of the DNMS task administered in randomized order; one employing pictures of faces as the samples (DNMS–Face), and one employing pictures of objects (DNMS–Object). Faces were chosen because they have formed the “social” stimuli in a number of previous studies of social versus non-social processing in ASD (e.g., Blair et al., 2002; Scott-Van Zeeland et al., 2010). Children were tested at age 9 years, an age at which previous work would predict no significant impairment in rule learning when non-social stimuli are used (Loveland et al., 2008). We predicted that if children with ASD have increased difficulty in extracting rules from social stimuli, they should show comparable performance to the group with DD on the DNMS–Object, but poorer performance on the DNMS–Face.
Methods
Participant groups
Children with ASD or DD from Experiment 1 who continued to meet diagnostic criteria were invited to return at age 9 years. Children who provided valid data for both the DNMS–Face and DNMS–Object (see Table 1) included: (1) 37 children with ASD; (2) 21 children with DD (18 with idiopathic developmental delay and 3 with Down syndrome). Data were excluded for other children who did not complete both tasks (ASD n = 17; DD n = 14).
Protocol
The pre-criterion procedure for the DNMS–Face and DNMS–Object was identical to the DNMS–Standard, except that the samples were 2D trial-unique pictures of faces or pictures of objects, respectively. For the post-criterion protocol, we employed a “list-learning paradigm,” in which three unique samples were presented at 15-second intervals (e.g., A, B, C). Then, three test trials were administered at 15-second intervals (e.g., AD, BE, CF), resulting in a 45-second delay between sample and test. Five “lists” were learned for a total of 15 test trials. This manipulation was chosen to allow for increased delay without significantly increasing the total length of the protocol. The DNMS–Face and DNMS–Object were administered a median of 27 days apart for the group with ASD and 14 days apart for the group with DD (t(62) = 2.3, p = .02). Number of valid trials did not differ between groups for any analysis (Fs < 2, ps > .2).
Analysis
Very few children met criterion on both tasks, particularly when the DNMS–Face was administered first. Thus, performance on 5-second delay trials (PC-5s delay) was initially analyzed with repeated measures ANOVA with group (ASD, DD) and order (DNMS–Face first, DNMS–Object first) as between-subject variables and task as a within-subject variable (DNMS–Face, DNMS–Object). Analyses of Trials TC and post-criterion memory (PC-45s delay) were conducted on DNMS–Face and DNMS–Object data separately with between-subject variables of group (ASD, DD) and order (DNMS–Face first, DNMS–Object first). Follow-up analyses for all variables contrasted performance by task (DNMS–Face, DNMS–Object) and group (ASD, DD) for the first and second tasks in which children participated. We also used non-parametric analyses to explore pass rates in the two tasks. Within the groups with ASD and DD separately, we used correlations and multivariate analyses to explore relations between cognitive and adaptive skill and DNMS–Face and DNMS–Object performance.
Results
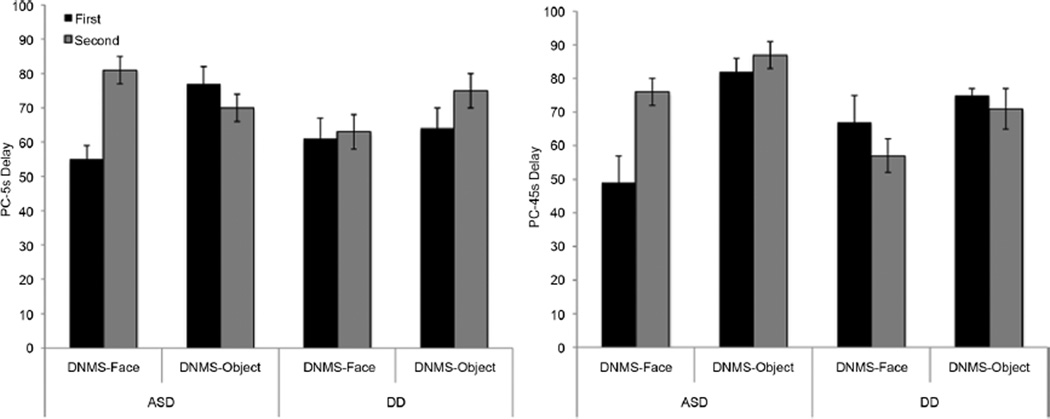
Rule learning was impacted by task order, stimulus type, and group (PC-5s delay; F(1,54) = 5.9, p = .02, ηp2= 0.1; see Figure 2). As seen in Figure 2, performance in the group with DD did not differ by order or stimulus type (F(18) = 1.59, p = .22), while these factors significantly impacted learning for children with ASD (F(35) = 5.78, p = .022, ηp2= 0.14). Children with ASD tested with the DNMS–Face then DNMS–Object demonstrated significantly poorer performance on the PC-5s delay in the DNMS–Face task than the DNMS–Object task (F(1,15) = 5.3, p = .04, ηp2= 0.26), and rates of meeting criterion were lower (44% vs. 63%; X2(1) = 4.06, p = .04, Φ = 0.33). Performance on 5s delay trials did not vary by task for children with ASD tested with DNMS–Object then DNMS–Face (F(1,20) = .51, p = .48; X2(1) = .02, p = .9). Children with ASD also performed significantly better on the 5-second DNMS–Face trials when preceded by the DNMS–Object than when tested on the DNMS–Face task first (PC-5s delay F(36) = 20.62, p < .001, ηp2= 0.37; Trials TC F(21) = 6.53, p = .019, ηp2= 0.25).
FIGURE 2.
Performance on the Delayed Non-Matching to Sample (DNMS)–Face and DNMS–Object tasks (Experiment 2) as a function of order of presentation and diagnostic group; bars show mean percent correct on the 5-sec delay trials (left) and post-criterion 45-sec delay trials (right). Error bars show +/−1 SE.
Similarly, both group (ASD, DD) and Order (DNMS–Face first, DNMS–Object first) affected performance on the post-criterion trials with a 45-second delay on the DNMS–Face task (F(1,32) = 7.0, p = .01, ηp2= 0.2) but not the DNMS–Object task (F(36)=0.41, p= .53; Figure 2, right panel). The group with ASD performed better on the 45-second delay trials in the DNMS–Face task when it was preceded by the DNMS–Object task than when administered first (PC-45s delay: F(1,22) = 1.0, p = .005, ηp2= 0.33). Order did not significantly affect PC-45s delay for the group with DD (F(1,10) = 1.0, p = .34).
In summary, children with ASD were better able to learn the DNMS rule when stimuli were objects rather than faces. These effects were only seen when children were tested in the DNMS–Face task first, possibly because when the DNMS–Face task was administered second children were helped by having previously learned the rule in the DNMS–Object task. Stimulus type did not influence responding for children with DD.
There were no significant differences in cognitive and adaptive scores between children who did and did not meet criterion in the DNMS–Face task (Fs < 3, ps > .08). Meeting criterion in the DNMS–Object was associated with higher DAS nonverbal standard scores (Didn’t meet M = 71.0, SD = 5.0; Met M = 91.0, SD = 4.0; F(1,52) = 9.3, p < .01, ηp2= 0.16). There were no significant associations between continuous metrics of performance and cognitive and adaptive measures for either task.
DISCUSSION
This experiment found poorer rule-learning ability for social (DNMS–Face) than non-social (DNMS–Object) stimuli in 9-year-old children with ASD but not DD. Unlike children with DD, children with ASD who received the DNMS–Face task first were less likely to meet criterion, met criterion more slowly, and performed more poorly on pre-criterion trials in the DNMS–Face task than the DNMS–Object task. DNMS–Face performance was better when it was preceded by the DNMS–Object task, suggesting that rule-learning difficulties were not driven by difficulty in recognizing the facial stimuli on individual trials. Together, these results support our prediction that children with ASD have more difficulty extracting a simple rule from social than non-social stimuli. This finding confirms that assessing executive functioning skills in solely non-social contexts may underestimate the degree to which impairments could impact social functioning in ASD.
Performance on the DNMS–Face and DNMS–Object tasks at 9 years was not associated with cognitive and adaptive skill, unlike performance on the DNMS–Standard rule-learning trials when children were tested at younger ages. Possibly, the smaller sample size limited our power to detect significant correlations. However, DNMS performance explained between 16% and 37% of the variance in cognitive and adaptive skills at the younger ages, suggesting moderate effect sizes. Alternatively, relations with individual differences in cognitive and adaptive level may be stronger when rule-learning is still an emerging skill. This is consistent with patterns in other developmental areas. For example, joint attention assessed during preschool but not middle school predicts language functioning in adolescence for individuals with ASD (Sigman & McGovern, 2005). Joint attention and simple rule learning may be foundation skills that are important in early cognitive development, but over time become less critical as the cognitive skills children are acquiring become more complex. Future studies testing rule-learning with other types of social stimuli may also be important. While facial identity provides key social information, face recognition represents a very small part of a complex network of social abilities. Possibly, stronger relations with social adaptive skills would be observed if children were asked to learn rules about other types of social information.
GENERAL DISCUSSION
The present study explored rule-learning in children with ASD and DD followed from age 3 to age 9. Three different aspect of rule learning were investigated: development, role of reward, and application to a social context. We found that from age 4 to 6 years children with ASD performed poorly relatively to those with developmental delay when learning a rule with a dissociated physical reward, but at age 6 years children were better able to learn the same rule when the reward was highly contingent verbal praise. No evidence of developmental change in measures of performance between age 4 and age 6 was observed. However, by age 9 years children with ASD had caught up with mental- and chronological-age matched peers in rule-learning from non-social stimuli. Nonetheless, children with ASD continued to show deficits at age 9 years when asked to extract a rule from social stimuli. This suggests that the integration of executive functioning with face processing skills may remain challenging for individuals with ASD, even once the constituent skills have been mastered.
Age-Related Change in Rule Learning
Although children with ASD found it difficult to learn from a dissociated reward at ages 4 and 6 years, by age 9 years the same group of children was just as likely to meet criteria in a nonsocial rule-learning task as children with DD. Since the abilities tapped by a standard version of the DNMS task asymptote around age 2 years in typical development (Diamond et al., 1994), equivalent performance in the groups with ASD or DD at age 9 years may reflect a “catching up” in this aspect of executive function. Indeed, this may explain the apparent contrast between rule-learning difficulties in the DNMS, and the apparent ease shown by individuals with ASD in learning the appropriate sorting rule in the first phase of the Wisconsin Card Sorting Task (WCST) since most of these studies have been conducted with individuals age 6 years and older (for review, Hill, 2004). “Catching up” has also been observed in other domains of executive function: Pellicano (2010) found a faster rate of improvement in planning skills in children with ASD tested longitudinally between 4 to 7 and 7 to 10 years than in typically developing children, who nonetheless outperformed the group with ASD at both time-points. Profiles of strength and weakness may significantly change over time for children with ASD, underlining the importance of considering developmental processes in any model of symptomology.
The present study found no evidence that executive functioning impairments emerged between the preschool and school-aged periods, as had been previously suggested (e.g., Dawson, Munson, et al., 2002). Since we found significant relations between performance on the rule-learning task and cognitive and adaptive skill in both age ranges, it is likely that cross-study differences in comparison group influence the likelihood of finding ASD-specific impairments in performance. The longitudinal approach taken in the present study also illustrates the importance of considering consistency over time when evaluating potential group differences on a neuropsychological task, since significant group differences between children with ASD and DD were only observed when examining longitudinal performance. This may be related to the greater variability in performance observed in the group with ASD. Since the executive functioning hypothesis of ASD has been challenged by repeated failures to identify deficits early in the development of the condition (for review, Hill, 2004), the present study underlines the need to revaluate this evidence with further longitudinal studies.
Learning From Reward in ASD
At age 7 years, children with ASD found it harder to learn a rule when the reward for correct performance was a toy hidden under the stimulus than when the reward was verbal praise. Notably, once children had learned the rule they were successfully able to apply it over longer delays, suggesting that the difficulty lies in learning rather than applying abstract rules. Diamond and colleagues (1999) have shown that the lack of physical connectedness between sample and reward in the standard version of the DNMS task is challenging for young typically developing infants, and suggest that dissociated rewards require children to understand the conceptual connection between two events. Diamond (2006) proposes that atypicalities in the periarcuate region in children with ASD may underlie both difficulty learning an abstract rule in the standard DNMS task and difficulty with processing the conceptual connections inherent in social interaction. Supporting the idea that conceptual connections are challenging for children with ASD, Biro and Russell (2001) found that children with ASD had greater difficulty learning to make a ball drop by placing a cup in front of the apparatus (a conceptual connection) than by depressing a lever fixed to the box (a physical connection). The present results corroborate the prediction that children with ASD would be able to learn abstract rules more readily if a more concrete association between stimulus and reward was provided (Diamond, 2006).
Difficulty with forming conceptual connections could play a role in social and communication impairments in ASD. In social interactions, conceptual connections must often be made between a child’s own behavior and the responses elicited in others. One particularly characteristic feature of ASD in early development is the use of another’s body as a tool (e.g., Lord et al., 1999); possibly, children are attempting to use a physical connection to guide another’s behavior because they have not yet learned the conceptual relation between asking and receiving help. Understanding language also requires the formation of conceptual connections between labels and their referents. Making these associations more concrete can help children with ASD communicate, as indicated by the relative success of communication systems that allow children to select photographs of common objects to convey their needs and wants (e.g., Flippin, Reszka, & Watson, 2010). In the initial phase of training, children are physically prompted to exchange the photograph of a desired item for the item itself, providing further physical scaffolding for learning. Testing the ability of children with ASD to form conceptual connections across a battery of tasks, and looking at the relation between scores and core symptoms of ASD, would provide one way to further explore the role of conceptual connections in the development of ASD.
While Diamond and colleagues (1999) have systematically rejected lack of physical and temporal proximity as alternative explanations for poor performance on the DNMS–Standard task in typically developing infants, the present study does not include all contrasts required to conclusively determine that physical connectedness is the key element for children with ASD. Verbal praise was also more temporally proximal to stimulus selection than was the physical reward, because the experimenter clapped and cheered as soon as the child touched the novel stimulus. To find the toy reward, the child had to displace the novel stimulus and then locate the toy in the well beneath. Although the role of temporal proximity in contingency learning in ASD has not been widely explored, there is evidence that temporal synchrony may be more salient to children with ASD than typically developing children (e.g., Ferrara & Hill, 1980). Unlike typically developing children, children with ASD prefer to watch a stimulus that is perfectly temporally synchronized with their movements over a stimulus that is highly but not perfectly synchronized (Gergely, Koos, & Watson, 2010). A diminished ability to benefit from rewards that are less temporally synchronous with behavior would particularly challenge learning during naturalistic social interactions, in which a social partner’s responses are not always perfectly temporally contingent with the child’s own actions. Since temporal contingency varies, examining whether increasing contingency can provide benefits for children with ASD would be an important direction for future research. Suggesting that this may be effective, children with ASD show increased social responsiveness after experiencing contingent imitation of their behavior by a parent or examiner (e.g., Dawson & Galpert, 1990).
Many authors have proposed that children with ASD have an altered perception of the reward value of social stimuli (e.g., Dawson, Webb, & McPartland, 2005), and the present results may appear inconsistent with reports of reduced inherent reward value of social stimuli in ASD (Scott-Van Zeeland et al., 2010). Possibly, social motivation is affected by the complexity of the social signal for children with ASD: the identical verbal praise provided after each correct response in the DNMS–Praise task may have been a clearer, more unambiguous signal to children than a symbolic smiling face plus text (e.g., Scott-Van Zeeland et al., 2010) or social signals that are embedded in the complex natural environment. Future work would be required to establish whether children with ASD were motivated by a social goal like receiving praise, or by the perfect contingency between touching the novel object and triggering an event in the environment that happened to be social in nature. Effect sizes were also relatively small, further indicating the need for replication of these findings. Nonetheless, the present findings do indicate that children with ASD can find it easier to learn from person-delivered feedback than a physical reward, challenging the idea that all forms of social feedback are challenging for children with ASD.
The Influence of Social Context on Rule Learning
Processing information from faces can be a weakness for children with ASD. For example, children with ASD show reduced neural responses to facial familiarity but not object familiarity (Dawson, Carver, et al., 2002), and experience greater difficulty in recognizing faces than objects (e.g., Blair et al., 2002). These difficulties are thought to arise either from initial atypicalities that include impairments in the fusiform gyrus or low-level perception, and/or disruption to the progressive specialization of the face processing system driven by a reduced interest in social stimuli (for review, Dawson et al., 2005). The present findings extend this work to show that even when individuals with ASD show evidence of intact face recognition relative to children matched on chronological and mental age, the extraction of a simple rule from facial stimuli can be impaired. Comparisons of rule-learning from face relative to object stimuli had moderate effect sizes (ηp2=0.2–0.37), suggesting it may be important to explore in future work.
Children with ASD show difficulty in using facial cues to guide their behavior across a range of other domains. For example, children with ASD have difficulty in following shifts in eye or head direction to identify a common referent (e.g., Dawson et al., 2004), and are less likely to modify their behavior in response to another person’s emotional expressions (e.g., Sigman, Kasari, Kwon, & Yirmiya, 1992). It has been argued that both gaze following (e.g., Triesch, Teuscher, Deak, & Carlson, 2006) and social referencing (Gewirtz & Pelaez-Nogueras, 1992) can be learned through the reliable pairing of facial cues (gaze direction or emotional expression) with positive or negative environmental consequences. The present results suggest that children with ASD have difficulty forming conceptual connections between facial cues and salient rewards, which may contribute to their difficulty in learning how to use facial cues to shape their behavior.
The present findings also indicate the importance of examining executive functions in both social and non-social contexts. Although pervasive, the role of executive functioning deficits in the development of ASD has been challenged because impairments do not appear to arise until later in development, may not be universal, and may also be seen in a range of other neurodevelopmental disorders (for review, Hill, 2004). However, the present results demonstrate that the focus on non-social stimuli may underestimate the degree to which executive functioning impairments could contribute to social symptoms of ASD. Very few studies have examined whether behavioral deficits are more pronounced when tested in the social domain, but recent evidence from neuroimaging studies suggests that this is an important area for future research. For example, Scott-Van Zeeland et al (2010) showed deficits in frontostriatal responses in children with ASD during an implicit learning task when children were rewarded with social but not monetary cues. Further, Dichter and Belger (2007) found that including face stimuli in a cognitive control paradigm led to marked hypoactivation in frontal regions in participants with ASD that was not observed when the stimuli were arrows. Although executive functioning has long been known to be reliant on the frontal lobes, it has become increasingly recognized that the connectivity between frontal regions and other areas is critical (for review, Carpenter, Just, & Reichle, 2000). Since applying executive functions to social and non-social stimuli likely thus relies on different connectivity networks, exploring how deficits in connectivity in ASD (for review, Minshew & Williams, 2007) are related to the application of executive functions in social contexts will be an important direction for future research.
Limitations
While longitudinal studies of children with ASD are central to building our understanding of the developmental course of the disorder, they present significant challenges. Neuropsychological procedures with few verbal requirements like the DNMS task provide a valuable source for assessments that can span wide age ranges, but still require modification to avoid ceiling and floor effects. Modifications made between time-points in the present study (such as using the DNMS–Object rather than the DNMS–Standard task for older children) retain dynamic range in the measure, but can reduce direct comparability across time-points. As well, because of the time constraints of longitudinal research with children with ASD, we could not explore as many manipulations of the DNMS procedure as in work with typically developing infants (e.g., Diamond et al., 1999), limiting our ability to draw similarly fine-grained conclusions about the mechanisms driving performance. Further, since typically developing children performed more poorly than would be expected based on previous work, replication of these findings is important.
ACKNOWLEDGMENT
Funding was provided by the NICHD sponsored Collaborative Programs of Excellence in Autism/CPEA (Dawson, P01HD34565); NICHD sponsored UW Autism Center of Excellence/ACE (Webb, Estes; P50HD055782); and Autism Speaks Postdoctoral Fellowship (Jones). Additional support was provided by collaborators, staff, and students associated with the UW, CPEA, and ACE.
We thank the families and children of the Early Development Study.
Footnotes
Publisher's Disclaimer: Full terms and conditions of use: http://www.tandfonline.com/page/terms-and-conditions
This article may be used for research, teaching, and private study purposes. Any substantial or systematic reproduction, redistribution, reselling, loan, sub-licensing, systematic supply, or distribution in any form to anyone is expressly forbidden.
The publisher does not give any warranty express or implied or make any representation that the contents will be complete or accurate or up to date. The accuracy of any instructions, formulae, and drug doses should be independently verified with primary sources. The publisher shall not be liable for any loss, actions, claims, proceedings, demand, or costs or damages whatsoever or howsoever caused arising directly or indirectly in connection with or arising out of the use of this material.
Contributor Information
E. J. H. Jones, Center on Child Health, Behavior and Development, Seattle Children’s Hospital, Seattle, Washington
S. J. Webb, Department of Psychiatry and Behavioral Sciences, University of Washington, Seattle, Washington, Center on Human Development & Disability, Seattle, Washington, Department of Psychology, University of Washington, Seattle, Washington, and Center on Child Health, Behavior and Development, Seattle Children’s Hospital, Seattle, Washington
A. Estes, Department of Speech and Hearing Sciences, University of Washington, Seattle, Washington, and Department of Psychology, University of Washington, Seattle, Washington
G. Dawson, Autism Speaks, New York, New York, Department of Psychology, University of Washington, Seattle, Washington, and Psychiatry Department, University of North Carolina, Chapel Hill, North Carolina
REFERENCES
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text rev. Washington, DC: Author; 2000. [Google Scholar]
- Barbaro J, Dissanayake C. A comparative study of the use and understanding of self-presentational display rules in children with high functioning autism and asperger’s disorder. Journal of Autism and Developmental Disorders. 2007;37:1235–1246. doi: 10.1007/s10803-006-0267-y. [DOI] [PubMed] [Google Scholar]
- Biro S, Russell J. The execution of arbitrary procedures by children with autism. Development and Psychopathology. 2001;13:97–110. doi: 10.1017/s0954579401001079. [DOI] [PubMed] [Google Scholar]
- Blair RJR, Frith U, Smith N, Abell F, Cipolotti L. Fractionation of visual memory: Agency detection and its impairment in autism. Neuropsychologia. 2002;40(1):108–118. doi: 10.1016/s0028-3932(01)00069-0. [DOI] [PubMed] [Google Scholar]
- Bolling DZ, Pitskel NB, Deen B, Crowley MJ, McPartland JC, Kaiser MD, Pelphrey K. Enhanced neural responses to rule violation in children with autism: A comparison to social exclusion. Developmental Cognitive Neuroscience. 2011 doi: 10.1016/j.dcn.2011.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter PA, Just MA, Reichle ED. Working memory and executive function: Evidence from neuroimaging. Current Opinion in Neurobiology. 2000;10:195–199. doi: 10.1016/s0959-4388(00)00074-x. [DOI] [PubMed] [Google Scholar]
- Carper RA, Courchesne E. Localized enlargement of the frontal cortex in early autism. Biological Psychiatry. 2005;57:126–133. doi: 10.1016/j.biopsych.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Crone EA, Donohue SE, Honomichl R, Wendelken C, Bunge SA. Brain regions mediating flexible rule use during development. The Journal of Neuroscience. 2006;25:11239–11247. doi: 10.1523/JNEUROSCI.2165-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Carver L, Meltzoff A, Panagiotides H, McPartland J, Webb S. Neural correlates of face and object recognition in young children with autism spectrum disorder, developmental delay, and typical development. Child Development. 2002;73:700–717. doi: 10.1111/1467-8624.00433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Galpert L. Mothers’ use of imitative play for facilitating social responsiveness and toy play in young autistic children. Development and Psychopathology. 1990;2(2):151–162. [Google Scholar]
- Dawson G, Meltzoff AN, Osterling J, Rinaldi J. Neuropsychological correlates of early symptoms of autism. Child Development. 1998;69:1276–1285. [PMC free article] [PubMed] [Google Scholar]
- Dawson G, Munson J, Estes A, Osterling J, McPartland J, Toth K, Abbott R. Neurocognitive function and joint attention ability in young children with autism spectrum disorder versus developmental delay. Child Development. 2002;73(2):345–358. doi: 10.1111/1467-8624.00411. [DOI] [PubMed] [Google Scholar]
- Dawson G, Toth K, Abbott R, Osterling J, Munson J, Estes A, Liaw J. Early social attention impairments in autism: Social orienting, joint attention, and attention to distress. Developmental Psychology. 2004;40(2):271–283. doi: 10.1037/0012-1649.40.2.271. [DOI] [PubMed] [Google Scholar]
- Dawson G, Webb S, McPartland J. Understanding the nature of face processing impairment in autism: Insights from behavioral and electrophysiological studies. Developmental Neuropsychology, Special Issue of Autism. 2005;27(3):403–424. doi: 10.1207/s15326942dn2703_6. [DOI] [PubMed] [Google Scholar]
- Diamond A. Normal development of prefrontal cortex from birth to young adulthood: Cognitive functions, anatomy and biochemistry. In: Stuss DT, Knight RT, editors. Principles of frontal lobe function. New York, NY: Oxford University Press; 2002. pp. 466–503. [Google Scholar]
- Diamond A. Bootstrapping conceptual deduction using physical connection: Rethinking frontal cortex. Trends in Cognitive Sciences. 2006;10(5):212–218. doi: 10.1016/j.tics.2006.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond A, Churchland A, Cruess L, Kirkham NZ. Early developments in the ability to understand the relation between stimulus and reward. Developmental Psychology. 1999;35(6):1507–1517. doi: 10.1037//0012-1649.35.6.1507. [DOI] [PubMed] [Google Scholar]
- Diamond A, Towle C, Boyer K. Young children’s performance on a task sensitive to the memory functions of the medial temporal-lobe in adults—The delayed-nonmatching-to-sample task—reveals problems that are due to non-memory-related task demands. Behavioral Neuroscience. 1994;108(4):659–668. doi: 10.1037//0735-7044.108.4.659. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Belger A. Social stimuli interfere with cognitive control in autism. Neuroimage. 2007;3:1219–1230. doi: 10.1016/j.neuroimage.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgin JO, Pennington BF. Spatial cognition in autism spectrum disorders: Superior, impaired, or just intact? Journal of Autism and Developmental Disorders. 2005;35:729–745. doi: 10.1007/s10803-005-0020-y. [DOI] [PubMed] [Google Scholar]
- Elliott CD. Differential ability scales (DAS) San Antonio, TX: The Psychological Corporation; 1990. [Google Scholar]
- Ferrara C, Hill SD. The responsiveness of autistic children to the predictability of social and nonsocial toys. Journal of Autism and Developmental Disorders. 1980;10(1):51–57. doi: 10.1007/BF02408432. [DOI] [PubMed] [Google Scholar]
- Flippin M, Reszka S, Watson LR. Effectiveness of the Picture Exchange Communication System (PECS) on communication and speech for children with autism spectrum disorders: A meta-analysis. American Journal of Speech-Language Pathology. 2010;19:178–195. doi: 10.1044/1058-0360(2010/09-0022). [DOI] [PubMed] [Google Scholar]
- Gergely G, Koós O, Watson JS. Contingency perception. In: Fuchs T, Sattel H, Henningsen P, editors. The embodied self: Dimensions, coherence and disorders. Stuttgart, Germany: Schattauer; 2010. pp. 141–169. [Google Scholar]
- Gewirtz JL, Pelaez-Nogueras M. B. F. Skinner’s legacy in human infant behavior and development. American Psychologist. 1992;47:1411–1422. doi: 10.1037//0003-066x.47.11.1411. [DOI] [PubMed] [Google Scholar]
- Griffith EM, Pennington BF, Wehner EA, Rogers SJ. Executive functions in young children with autism. Child Development. 1999;70:817–832. doi: 10.1111/1467-8624.00059. [DOI] [PubMed] [Google Scholar]
- Hill EL. Evaluating the theory of executive dysfunction in autism. Developmental Review. 2004;24:189–233. [Google Scholar]
- Lagatutta KH, Sayfan L, Monsour M. A new measure for assessing executive function across a wide age range: Children and adults find happy-sad more difficult than day-night. Developmental Science. 2011;14:481–489. doi: 10.1111/j.1467-7687.2010.00994.x. [DOI] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S. Autism diagnostic observation schedule-WPS (ADOS-WPS) Los Angeles, CA: Western Psychological Services; 1999. [Google Scholar]
- Lord C, Rutter M, Le Couteur A. Autism diagnostic interview–Revised. Journal of Autism and Developmental Disorders. 1994;24:659–685. doi: 10.1007/BF02172145. [DOI] [PubMed] [Google Scholar]
- Loveland KA, Bachevalier J, Pearson DA, Lane DM. Fronto-limbic functioning in children and adolescents with and without autism. Neuropsychologia. 2008;46(1):49–62. doi: 10.1016/j.neuropsychologia.2007.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus GF, Fernandes KJ, Johnson SP. Infant rule learning facilitated by speech. Psychological Science. 2007;18:387–391. doi: 10.1111/j.1467-9280.2007.01910.x. [DOI] [PubMed] [Google Scholar]
- Martin RC, Caramazza A. Classification in well-defined and ill-defined categories: Evidence for common processing strategies. Journal of Experimental Psychology: General. 1980;109:320–353. doi: 10.1037//0096-3445.109.3.320. [DOI] [PubMed] [Google Scholar]
- McEvoy R, Rogers SJ, Pennington BF. Executive function and social communication deficits in young autistic children. Journal of Child Psychology and Psychiatry. 1993;34:563–578. doi: 10.1111/j.1469-7610.1993.tb01036.x. [DOI] [PubMed] [Google Scholar]
- Minshew NJ, Williams DL. The new neurobiology of autism: Cortex, connectivity and neuronal organization. Archives of Neurology. 2007;64:945–950. doi: 10.1001/archneur.64.7.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen EM. Mullen scales of early learning. Los Angeles, CA: Western Psychological Services; 1997. [Google Scholar]
- Munson J, Faja S, Meltzoff A, Abbott R, Dawson G. Neurocognitive predictors of social and communicative developmental trajectories in preschoolers with autism spectrum disorders. Journal of the International Neuropsychological Society. 2008;14(06):956–966. doi: 10.1017/S1355617708081393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellicano E. The development of core cognitive skills in autism: A 3 Year prospective study. Child Development. 2010;81(5):1400–1416. doi: 10.1111/j.1467-8624.2010.01481.x. [DOI] [PubMed] [Google Scholar]
- Reed P, Watts H, Truzoli R. Flexibility in young people with autism spectrum disorders on a card sort task. Autism. 2011 doi: 10.1177/1362361311409599. [DOI] [PubMed] [Google Scholar]
- Scott-Van Zeeland AA, Dapretto M, Ghahremani DG, Poldrack RA, Bookheimer SY. Reward processing in autism. Autism Research. 2010;3(2):53–67. doi: 10.1002/aur.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigman M, McGovern CW. Improvement in cognitive and language skills from preschool to adolescence in autism. Journal of Autism and Developmental Disorders. 2005;35:15–23. doi: 10.1007/s10803-004-1027-5. [DOI] [PubMed] [Google Scholar]
- Sigman MD, Kasari C, Kwon J-H, Yirmiya N. Responses to the negative emotions of others by autistic, mentally retarded, and normal children. Child Development. 1992;63:796–807. [PubMed] [Google Scholar]
- Sparrow S, Balla D, Cicchetti D. Vineland adaptive behavior scales: Interview edition. Circle Pines, MN: American Guidance Service; 1984. [Google Scholar]
- Thompson C, Barresi J, Moore C. The development of future-oriented prudence and altruism in preschoolers. Cognitive Development. 1997;12:199–212. [Google Scholar]
- Triesch J, Teuscher C, Deak GO, Carlson E. Gaze following: Why (not) learn it? Developmental Science. 2006;9:125–157. doi: 10.1111/j.1467-7687.2006.00470.x. [DOI] [PubMed] [Google Scholar]
- Weston DR, Elliot T. Act-rule relations: Children’s concepts of social rules. Developmental Psychology. 1980;16:417–424. [Google Scholar]