Abstract
Background
Anemia is a common complication of chronic kidney disease (CKD) that negatively impacts the quality of life and is associated with numerous adverse outcomes. Excess levels of the iron regulatory hormone hepcidin are thought to contribute to anemia in CKD patients by decreasing iron availability from the diet and from body stores. Adenine treatment in rats has been proposed as an animal model of anemia of CKD with high hepcidin levels that mirrors the condition in human patients.
Methods
We developed a modified adenine-induced kidney disease model with a higher survival rate than previously reported models, while maintaining persistent kidney disease and anemia. We then tested whether the small molecule bone morphogenetic protein (BMP) inhibitor LDN-193189, which was previously shown to lower hepcidin levels in rodents, mobilized iron into the plasma and improved iron-restricted erythropoiesis in this model.
Results
Adenine-treated rats exhibited increased hepatic hepcidin mRNA, decreased serum iron, increased spleen iron content, low hemoglobin (Hb) and inappropriately low erythropoietin (EPO) levels relative to the degree of anemia. LDN-193189 administration to adenine-treated rats lowered hepatic hepcidin mRNA, mobilized stored iron into plasma and increased Hb content of reticulocytes.
Conclusions
Our data suggest that hepcidin lowering agents may provide a new therapeutic strategy to improve iron availability for erythropoiesis in CKD.
Keywords: anemia, chronic kidney disease, hepcidin, iron, mouse model
INTRODUCTION
A frequent complication of chronic kidney disease (CKD), anemia contributes to a reduced quality of life and increases the risk of blood transfusions [1]. Erythropoietin (EPO) deficiency and disordered iron homeostasis are key features of anemia of CKD, and erythropoiesis stimulating agents (ESAs) and iron are the cornerstones of therapy for this disease [1, 2]. While ESAs improve the quality of life and reduce transfusion requirements, they have not been demonstrated to improve other adverse outcomes associated with anemia of CKD, such as cardiovascular disease and mortality, in prospective randomized controlled trials [1]. In fact, recent clinical trials have raised important safety concerns about using ESAs, including an increased risk of death, cardiovascular events and stroke, particularly when ESAs are used in higher doses to target higher Hb levels, and in hyporesponsive patients [3–7].
CKD patients are prone to iron deficiency due to increased blood loss [8], increased iron utilization from ESA therapy [9] and poor dietary iron absorption [10]. Limited iron availability contributes to anemia and ESA hyporesponsiveness in CKD patients, and iron administration has been demonstrated to raise Hb levels and reduce ESA requirements [1, 11–22]. Consequently, iron status monitoring tests and iron administration to patients with low transferrin saturation and low ferritin are part of the mainstay of anemia management in CKD patients [1]. However, a common feature of anemia of CKD is functional iron deficiency or reticuloendothelial cell iron blockade, characterized by limited available circulating iron for efficient erythropoiesis despite adequate or elevated total body iron stores, often manifested as low serum transferrin saturation and elevated serum ferritin [23]. Guidelines for iron administration in these patients are limited by insufficient available data, and concerns over efficacy and toxicity [1], including the potential for secondary iron overload, oxidant-mediated tissue injury and/or infectious complications [24, 25].
Excess levels of the liver iron regulatory hormone hepcidin are hypothesized to cause the reticuloendothelial cell iron blockade and poor dietary iron absorption observed in anemic CKD patients [26, 27]. Hepcidin regulates systemic iron balance by downregulating the iron exporter ferroportin, effectively controlling intestinal iron absorption and iron release from body stores [28]. Hepcidin levels are elevated in CKD patients, presumably due to reduced kidney clearance and increased production by inflammatory signals [26, 29–31], with the interleukin-6 (IL-6)-signal transducer and activator of transcription 3 (STAT3) pathway being the most well-described inflammatory pathway involved in hepcidin regulation [26].
The bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway is central to hepcidin transcriptional regulation in the liver [32–37]. There appears to be a crosstalk between the BMP-SMAD and the IL-6-STAT3 pathways in hepcidin regulation, possibly at the level of the hepcidin promoter [34, 38]. Indeed, BMP inhibitors have been shown to inhibit inflammatory-mediated hepcidin expression both in vitro and in vivo [39–42].
Adenine treatment in rats has been proposed as an animal model of anemia of CKD with high hepcidin levels that mimics the clinical condition [43, 44]. However, prolonged adenine treatment causes a high mortality rate and the hematologic and iron phenotype of the adenine model has not been fully characterized.
Here, we generated a modified adenine model that improves survival while maintaining persistent kidney disease and anemia, and we characterized its hematologic profile, iron status and hepcidin expression. We then investigated whether the small molecule BMP inhibitor LDN-193189 inhibited hepcidin expression and mobilized stored iron into plasma to correct the reticuloendothelial cell iron blockade and anemia in this model.
METHODS
Animals
All animal protocols were approved by the Institutional Animal Care and Use Committee at the Massachusetts General Hospital (MGH) and used 8-week-old Wistar male rats (Charles River Labs).
For the original adenine model, rats received a control diet (Prolab 5P75 Isopro RMH 3000, 380 p.p.m. iron) or a similar diet supplemented with 0.75% adenine (Harlan Teklad) for 6 weeks. Tail vein phlebotomy (0.5–1 mL) was performed weekly, and rats were sacrificed at Weeks 4 and 6.
For the modified adenine model, rats were fed a 0.75% adenine diet for 3 weeks followed by a control diet for 5 weeks (modified adenine). Alternatively, rats were given a control diet throughout all 8 weeks (control). Phlebotomy was performed every 2 weeks, and rats were sacrificed at 1, 2, 4, 6 and 8 weeks.
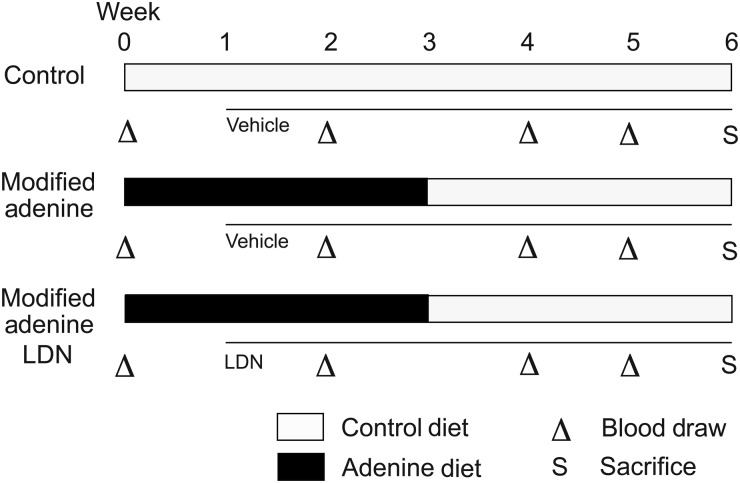
For LDN-193189 experiments, rats treated per the modified adenine model received either intraperitoneal (i.p.) injections of LDN-193189 (produced as described previously [45]) at 8 mg/kg in 20% 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich) in PBS (modified adenine LDN) or an equal volume of vehicle alone (modified adenine) daily for 5 weeks, starting 1 week after initiation of the adenine diet. A control group was fed a control diet for 6 weeks and treated with daily i.p. injections of vehicle starting at Week 1. Phlebotomy was performed every 1–2 weeks and rats were sacrificed 6 h after the last injection (see Figure 1).
FIGURE 1:
Schematic of LDN-193189 treatment strategy in a modified adenine-induced kidney disease model. Eight-week-old Wistar male rats were given either a control diet (open bars) for 6 weeks (control) or a 0.75% adenine supplemented diet (black bars) for 3 weeks followed by a control diet (open bars) for another 3 weeks. The adenine diet group was further treated with either a vehicle alone (modified adenine) or LDN-193189 at a dose of 8 mg/kg (modified adenine LDN), while the control group was treated with vehicle alone, starting at Week 1. Tail-vein blood draws (indicated by triangles) were performed at Weeks 0, 2, 4 and 5 for all groups. Animals were sacrificed (S) and tissues harvested for analysis at Week 6.
RNA extraction and quantitative real-time RT-PCR
Liver total RNA was isolated, and real-time quantification of hepcidin mRNA (Hamp) relative to the housekeeping hypoxanthine-guanine phosphoribosyltransferase mRNA (Hprt) was performed using two-step reverse-transcribed polymerase chain reaction (RT-PCR) with the ABI Prism 7900HT Sequence Detection system as previously described [46] utilizing primers summarized in Supplementary Table S1.
Western blot
Nuclear extracts were prepared and analyzed for phosphorylated-STAT3 relative to STAT3 and phosphorylated-SMAD1/5/8 relative to total SMAD1 protein expression by western blot as previously described [47], followed by chemiluminescence quantitation using ImageJ [48].
Hematologic analysis
Hematologic parameters were assessed in whole blood using the ADVIA 2120i and analyzed with multispecies software using rat settings at Children's Hospital Boston, or the Heska HemaTrue Veterinary Hematology Analyzer at the MGH Veterinary Clinical Pathology Laboratory.
Renal, liver function and electrolyte analyses
Serum samples were analyzed using a Heska DRI-CHEM veterinary chemistry analyzer at the MGH Veterinary Clinical Pathology Laboratory.
Serum iron measurements
Serum iron and unsaturated iron-binding capacity (UIBC) were measured by a colorimetric assay using the Iron/UIBC kit (Thermo Electron Corp), and transferrin saturation was calculated as previously described [39].
Tissue iron measurement
Quantitative measurement of nonheme iron was performed on spleen tissue as previously described [39].
ELISA
Serum EPO and IL6 concentrations were measured using mouse/rat EPO serum/plasma singleplex immunoassay (Meso Scale Discovery) and rat IL6 ELISA (Thermo Scientific) kits according to manufacturers' protocols.
Statistics
Data are expressed as mean + SEM. The Kaplan–Meier method was used to analyze survival rates with the Logrank test to determine statistical significance. Other data were evaluated by two-tailed Student's t-test or ANOVA with the Bonferroni post-hoc test for multiple comparisons using Prism (GraphPad). P < 0.05 was considered statistically significant.
RESULTS
A modified adenine model improves survival and maintains persistent kidney disease
We established an adenine-induced rat model of anemia of CKD [43, 44] in our laboratory to perform a more detailed characterization of the anemia phenotype, with the ultimate aim to test hepcidin-lowering agents as a new treatment strategy for disordered iron homeostasis and anemia of CKD. We found that a continuous 0.75% adenine diet leads to a very high mortality rate of 60% by 6 weeks (original adenine, Figure 2A). Although mortality was not mentioned by Hamada et al. [43, 44], a similarly high mortality rate was reported by other groups [49].
FIGURE 2:
Increased survival and maintenance of persistent kidney disease in the modified adenine model compared with the original adenine model. (A) Survival rates were measured at 1 to 2-week intervals for rats on a control diet for 8 weeks (control, CTL, dashed line), an adenine diet for 3 weeks followed by 5 weeks of a control diet (modified adenine, MA, solid line) or a continuous adenine diet for 6 weeks (original adenine, OA, dotted line). Sample sizes of each group at each time point are shown below the panels. The decrease in numbers at later time points is due to planned sacrifice of animals for tissue analysis and mortality. P < 0.01 for the original adenine group in comparison to the control group, P = not significant for the modified adenine group in comparison to the control group. (B and C) A subset of rats from panel A was analyzed by phlebotomy or at the time of sacrifice for serum CRE (B) and serum BUN (C). Sample sizes of each group at each time point are shown below the panels (control, CTL, open squares; modified adenine, MA, closed squares; original adenine, OA, closed triangles). ##P < 0.01 for the original adenine group in comparison to the control group at the same time point, **P < 0.01 and *P < 0.05 for the modified adenine group in comparison to the control group at the same time point.
Treatment with an adenine diet for 4 weeks followed by a normal diet was previously reported to maintain persistent kidney disease and anemia with improved survival to 81%, while an adenine diet for 2 weeks followed by a normal diet improved survival to 100%, but resulted in reversible kidney disease and only mild anemia [49]. To improve survival further, but maintain persistent kidney disease and more severe anemia, we tested the effects of a 0.75% adenine diet for 3 weeks followed by a control diet for 5 weeks (modified adenine).
Rats receiving the modified adenine diet greatly improved their survival rate to 98.6%, which was not statistically different from control rats on a normal diet (Figure 2A). Modified adenine rats exhibited a significant increase in serum creatinine (CRE) and blood urea nitrogen (BUN) that peaked at Week 3, and remained significantly increased 3 to 4-fold over the control group at Week 8, indicating persistent kidney disease with just 3 weeks of an adenine diet (Figure 2B and C). Modified adenine rats exhibited a small increase in serum potassium, but showed no other significant electrolyte imbalance or liver toxicity compared with the control group (Table 1).
Table 1.
Renal and liver biochemical parameters at 6 weeks in rats treated with a modified adenine diet or a control diet
| Units | Control (N = 4) | Modified adenine (N = 8) | |
|---|---|---|---|
| BUN | mg/dL | 21.70 + 1.8 | 66.36 + 19.45* |
| Creatinine | mg/dL | 0.25 + 0.10 | 0.94 + 0.29* |
| Phosphorus | mg/dL | 7.28 + 0.57 | 7.53 + 1.10 |
| Calcium | mg/dL | 9.83 + 0.92 | 11.13 + 0.18 |
| Total protein | g/dL | 5.10 + 0.47 | 4.98 + 0.21 |
| Albumin | g/dL | 3.40 + 0.24 | 3.48 + 0.17 |
| Globulin | g/dL | 1.70 + 0.27 | 1.50 + 0.11 |
| Glucose | mg/dL | 189.00 + 25.73 | 166.00 + 10.10 |
| Cholesterol | mg/dL | 63.25 + 9.64 | 94.13 + 12.96* |
| ALT (GPT) | U/L | 28.50 + 1.29 | 33.38 + 7.01 |
| ALP | U/L | 196.00 + 32.16 | 236.00 + 28.08 |
| GGT | U/L | 11.67 + 1.53 | 10.25 + 0.50 |
| Total bilirubin | mg/dL | 0.28 + 0.10 | 0.38 + 0.16 |
| Sodium | mEq/L | 134.50 + 6.45 | 141.50 + 2.39 |
| Potassium | mEq/L | 4.38 + 0.25 | 5.08 + 0.65* |
| Chloride | mEq/L | 95.25 + 4.92 | 101.88 + 3.09 |
| Na/K ratio | 30.75 + 1.50 | 28.38 + 4.07 |
*P < 0.01 compared with rats on a control diet.
A modified adenine model exhibits irreversible anemia, hypoferremia, reticuloendothelial cell iron blockade and hepcidin excess
Modified adenine rats developed anemia associated with kidney disease at Week 4 that persisted to Week 8 with Hb 9 g/dL and hematocrit 25% (Figure 3A and Supplementary Figure S1B). The decrease in Hb was due largely to a decrease in red blood cell (RBC) production as evidenced by a drastic reduction in reticulocytes at Week 1 (Figure 3B). The reticulocyte count remained decreased until Week 5, when it approached the level in control animals, but remained inappropriately low relative to the degree of anemia (Figure 3B). Adenine treatment also markedly decreased iron incorporation into reticulocytes from Weeks 1–3, as measured from the content of hemoglobin in reticulocytes (CHr) (Figure 3C). The mean corpuscular volume of reticulocytes (MCVr), mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) were also decreased between Weeks 1–4, consistent with iron restriction (Supplementary Figure S1C,D and F). In contrast to the decrease in RBCs, platelets and white blood cells were increased by the modified adenine diet (Supplementary Figure S1G–H), possibly an indication of inflammation and/or iron deficiency [50]. Thus, modified adenine rats developed a hypoproliferative, microcytic anemia within 4 weeks, characterized by a clear lack of iron incorporation into reticulocytes up to Week 3.
FIGURE 3:
Rats treated with the modified adenine diet develop increased hepatic hepcidin mRNA, splenic iron sequestration, decreased serum iron and transferrin saturation, decreased reticulocyte count, decreased Hb content of reticulocytes and persistent anemia compared with rats on a control diet. Rats treated with a modified adenine diet as described in Figure 1 (closed squares or black bars) or a control diet (open squares or white bars) were analyzed for (A) Hb, (B) reticulocyte count, (C) hemoglobin content of reticulocytes (CHr), (D) serum iron, (E) serum transferrin saturation (Tf Sat), (F) spleen iron content, (G) liver hepcidin mRNA (Hamp) relative to hypoxanthine-guanine phosphoribosyltransferase mRNA (Hprt) quantitated by real-time RT-PCR and (H and I) liver pSTAT3 relative to STAT3 protein analysis by western blot every 1–2 weeks either by phlebotomy or at the time of sacrifice. Blood analysis at Week 8 was performed on the Heska HemaTrue at MGH, which does not have the capacity to measure reticulocyte counts or CHr. For panels A–E, sample sizes for each group at each time point are shown below the panels (CTL, control; MA, modified adenine). For panels F–H, sample sizes for each group are 3–12. For panel I, representative blots of three animals per group were shown. For all panels, **P < 0.01, *P < 0.05 and ^P = 0.05 for the modified adenine group in comparison to the control group at the same time point.
Consistent with the decreased CHr, modified adenine rats exhibited reduced serum iron and transferrin saturation from Weeks 1 to 3 compared with control rats (Figure 3D and E). Modified adenine rats also exhibited iron retention in the spleen throughout the entire experimental period (Figure 3F). These data are consistent with a reticuloendothelial cell iron blockade. Interestingly, by Weeks 5 to 6, serum iron, transferrin saturation and CHr increased to surpass the level in control animals, suggestive of some improvement in iron availability after Week 3 in this model.
Hepcidin excess is thought to cause the functional iron deficiency and reticuloendothelial cell iron blockade seen in anemic CKD patients [26, 27, 29–31]. We, therefore, quantitated hepatic hepcidin (Hamp) mRNA in modified adenine compared with control rats. Hamp expression was increased by 3-fold within 1 week of adenine treatment and remained high until 4 weeks compared with control animals, but then decreased at Weeks 6–8 to or below the level of control animals (Figure 3G). The decrease in Hamp at later time points corresponded to increased iron availability for erythropoiesis, manifested by increased serum iron, transferrin saturation and CHr (Figure 3C–E). These data suggest that hepcidin excess may be at least in part responsible for the reticuloendothelial cell iron blockade and anemia of this modified adenine model, particularly in the early phase.
To understand the mechanism of the hepcidin increase in the modified adenine group, we quantitated liver phosphorylated-STAT3 (pSTAT3) and serum IL-6 levels by western blot and ELISA assay as a measure of inflammation, and we quantitated liver phosphorylated SMAD1/5/8 (pSMAD1/5/8) levels by western blot as a measure of BMP-SMAD signaling. Liver pSTAT3 levels were robustly increased at Weeks 1–4, but were not significantly changed at Week 8, in the modified adenine group compared with the control group (Figure 3H and I), mirroring hepcidin levels (Figure 3G). Serum IL-6 serum levels were below the level of detection for all samples (data not shown), as previously described by others [43]. Liver pSMAD1/5/8 levels were slightly but significantly increased in the modified adenine group at Week 2, but were not significantly different from the control group at other time points, and overall showed much more variability than pSTAT3 levels (Supplementary Figure S2).
LDN-193189 lowers hepcidin, mobilizes spleen iron stores, increases serum iron and increases iron incorporation into reticulocytes in a modified adenine model
Next, we investigated whether lowering hepcidin, by pharmacologic treatment with a small molecule BMP inhibitor LDN-193189 (LDN), would mobilize stored iron from reticuloendothelial macrophages, increase iron availability for erythropoiesis and ameliorate anemia in the modified adenine model (Figure 1). We treated rats starting at Week 1 of the adenine diet, when we observed increased hepcidin, reduced serum iron, spleen iron sequestration and reduced CHr suggestive of functional iron deficiency (Figure 3). We chose not to start treatment at a later time because although the anemia persisted, serum iron, CHr and hepcidin levels improved on their own by Weeks 4–6 (Figure 3).
After 5 weeks of LDN treatment, hepatic hepcidin expression was dramatically downregulated (modified adenine LDN) compared with vehicle-treated adenine rats (modified adenine) (Figure 4A). The effect of hepcidin inhibition was evident by Weeks 3 and 5, when serum iron and transferrin saturation were significantly increased in LDN-treated compared with vehicle-treated rats (Figure 4C and D). This surge in circulating iron was at least in part due to the release of stored iron from reticuloendothelial macrophages in the spleen, since LDN-treated rats had a significantly decreased spleen iron content compared with their vehicle-treated counterparts (Figure 4B). Indeed the spleen iron content in LDN-treated adenine rats at Week 6 was similar to the control diet group (Figure 3F). Importantly, this surge of iron availability correlated with an increase in CHr and MCVr at Week 5 (Figure 5A and B), suggesting that the iron was being efficiently incorporated into reticulocytes. The overall MCV and MCH were also significantly increased by Week 6 (Figure 5E and F). However, LDN treatment alone during this time course caused only a trend towards increased reticulocyte count, and did not correct Hb levels in the adenine rats (Figure 5C and D).
FIGURE 4:
LDN-193189 treatment of rats on a modified adenine diet decreases hepcidin expression and mobilizes stored iron into plasma. Rats receiving a modified adenine diet were treated with either vehicle alone (modified adenine, closed squares or black bars, n = 8) or LDN-193189 at a dose of 8mg/kg once daily (modified adenine LDN, gray diamonds or bars, n = 8) starting at Week 1 after initiation of the adenine diet until sacrifice. Mice were sacrificed at 6 weeks and analyzed for (A) hepcidin (Hamp) relative to Hprt mRNA by quantitative real-time RT-PCR and (B) spleen iron content. Blood collected via tail-vein phlebotomy at Weeks 0, 1, 3 and 5, and at the time of sacrifice at 6 weeks was analyzed for serum iron (C) and serum Tf sat (D). For all panels significant changes are shown as (**P < 0.01) for the modified adenine LDN group in comparison to the modified adenine group at the same time point.
FIGURE 5:
LDN-193189 treatment of rats on a modified adenine diet increases the reticulocyte hemoglobin content (CHr), reticulocyte MCVr, RBC MCV and MCH, but does not increase Hb levels. Blood from rats receiving a modified adenine diet treated with either vehicle alone (modified adenine, closed squares, n = 8) or LDN-193189 (modified adenine LDN, diamonds, n = 8) from Figure 4 was analyzed for (A) CHr, (B) MCVr, (C) reticulocyte count, (D) Hb, (E) MCV and (F) MCH. For all panels, **P < 0.01, *P < 0.05, ^P = 0.05 for the modified adenine LDN group in comparison to the modified adenine group at the same time point.
Adenine acutely decreases circulating erythropoietin to undetectable levels
To understand the contribution of EPO deficiency to the anemia of the modified adenine model, we measured serum EPO levels in rats maintained on a control diet compared with modified adenine diet rats in the absence and presence of LDN treatment. Serum EPO levels were below the limit of detection after 1 week of adenine treatment (Figure 6), directly corresponding to the precipitous drop in reticulocyte counts (Figure 3B). However, at Week 6, EPO levels in modified adenine rats with or without LDN treatment returned to a similar level as control rats, comparable to what has been reported in human CKD patients.
FIGURE 6:
Serum EPO levels were undetectable at Week 1 and unchanged from control animals at Week 6 of the modified adenine diet. Blood from rats receiving a control diet treated with vehicle alone (white bars, n = 6), or a modified adenine diet treated with either vehicle alone (modified adenine, black bars, n = 8) or LDN-193189 (modified adenine LDN, gray bars, n = 8) at Weeks 1 and 6 from Figure 4 were analyzed for serum EPO levels by ELISA. Significant changes are shown as **P < 0.01 in comparison to the control group at the same time point. EPO levels were not significantly changed in the modified adenine LDN group in comparison to the modified adenine group at Week 6 (P = 0.25).
DISCUSSION
Anemia of CKD is currently treated with ESAs and supplemental iron [1, 2]. However, existing therapies have been associated with increased morbidity and mortality in several clinical trials [3–7], not all patients respond adequately [1], and these therapies do not fully address the underlying pathophysiologic mechanisms of this disease [26, 27]. CKD patients, particularly hemodialysis patients, have excess levels of the iron regulatory hormone hepcidin [29–31], and hepcidin excess is proposed to contribute to the anemia of CKD by limiting iron availability for erythropoiesis [26, 27]. Here, we developed an animal model of anemia of CKD with hepcidin excess, and tested whether pharmacologic intervention to lower hepcidin expression corrected the functional iron deficiency that contributes to the anemia in this setting.
Dietary supplementation with adenine is thought to induce kidney disease in rats primarily due to the obstruction of renal tubules by 2,8-dihydroxyadenine crystals [49]. The adenine model is often used to study anemia of CKD because it better reflects the severe anemia seen in patients compared with the widely used 5/6 nephrectomy CKD model that exhibits a milder anemia without alterations in serum iron parameters [51–53]. Eight weeks of 0.75% adenine diet was also recently demonstrated to increase hepcidin levels in rats [43]. However, a full characterization of the hematologic and iron parameters in the adenine model has been limited [43, 49, 54], and the mortality rate of a continuous adenine diet is prohibitively high (60–70% at 6 weeks) (Figure 2A) [49]. We, therefore, developed a modified adenine model by giving a 0.75% adenine diet for 3 weeks followed by a normal diet. With this modification, we demonstrated a higher survival rate than other previously published models [43, 49, 54], while maintaining persistent kidney disease and anemia. Moreover, we demonstrated that adenine-treated rats have, at least transiently, increased hepatic hepcidin mRNA, decreased serum iron, increased spleen iron content, low Hb and inappropriately low EPO levels relative to the degree of anemia, reflective, to some extent, of the clinical condition in anemic CKD patients.
Our data provide the first proof-of-concept evidence that pharmacologic hepcidin lowering strategies can improve iron availability for erythropoiesis in an animal model of anemia of CKD. We showed here that the small molecule BMP inhibitor LDN-193189 lowered endogenous hepcidin production, mobilized stored iron, increased serum iron levels and increased iron incorporation into RBCs, as evidenced by increased CHr, MCVr, MCV and MCH. Previous studies in CKD patients suggest that iron deficiency can contribute to anemia and ESA hyporesponsiveness, and that iron therapy can raise Hb levels and reduce ESA requirements [1, 11–22]. By improving dietary iron absorption and iron mobilization from the patient's own body stores, hepcidin lowering strategies could provide an alternative and/or adjunctive therapy that minimizes the potential adverse consequences of high doses of ESAs and repeated i.v. iron administration. This would be particularly attractive for patients with low serum transferrin saturation and high serum ferritin, for whom current management guidelines are limited [1]. Future studies will be needed to determine whether there are any adverse consequences of inhibiting BMP signaling and/or hepcidin, such as increasing the risk of bacterial infection.
Despite an increase in serum iron and improvement in iron incorporation into RBCs, treatment with LDN-193189 alone did not prevent anemia progression in our model. This contrasts with studies in two other models of anemia of chronic disease without kidney disease, where treatment with LDN-193189 alone ameliorated anemia [40, 42]. In another anemia of the chronic disease model without kidney disease, a blocking hepcidin antibody alone did not prevent anemia development, but did improve anemia when co-administered with ESAs [55]. One explanation for the differences is that EPO deficiency may play a more prominent role in anemia of CKD compared with some other chronic diseases.
Indeed, we found that EPO levels dramatically fell and were undetectable at 1 week in the modified adenine model, corresponding to the precipitous drop in reticulocyte counts. It seems likely that the severe EPO deficiency of the early phase of this model was a limiting factor in the correction of anemia with LDN-193189 alone. This result is not reflective of EPO levels in anemic CKD patients, which are generally in the normal range or slightly increased, similar to what was seen in the later phase of the modified adenine model. Such ‘normal’ EPO levels in anemic CKD patients are suggested to be inappropriately low relative to the degree of anemia, since EPO levels are typically 10 to 100-fold higher in anemic patients without kidney disease [56–58]. Thus, relative EPO deficiency also likely contributed to the anemia in the later time points of the modified adenine model.
In addition to early severe EPO deficiency, another limitation of the modified adenine model is that hepcidin mRNA levels dropped and serum iron levels rose on their own after 4 weeks, thereby limiting the therapeutic window for hepcidin lowering agents. The mechanisms of hepcidin excess in CKD patients are thought to be due to reduced renal clearance and stimulation by inflammatory cytokines [26]. The robust increase in liver pSTAT3 levels in the early weeks of the modified adenine model is consistent with an inflammatory response as the primary driver of hepcidin excess. There was also a small increase in liver pSMAD1/5/8 levels at Week 2, which could be a minor contributing factor to the hepcidin excess. A similar increase in liver pSMAD1/5/8 levels has previously been reported in other animal models of anemia of inflammation [47]. We were unable to quantitate circulating bioactive hepcidin levels in this model because there is no readily available validated rat hepcidin assay. If reduced renal clearance were present, circulating bioactive hepcidin levels could be even higher than reflected by the hepatic hepcidin mRNA levels. Furthermore, hepcidin levels at Weeks 6–8 could still be inappropriately high relative to the degree of anemia, since anemia is a potent hepcidin suppressor [59]. Indeed, mice with a similar degree of anemia have ∼10-fold lower liver hepcidin mRNA levels compared with non-anemic mice [60]. However, relative hepcidin excess was unlikely a limiting factor in the anemia after 4 weeks, given the rise in serum iron above control levels, suggesting that circulating iron was available for erythropoiesis. The persistently elevated spleen iron content and anemia at later time points most likely reflects decreased iron utilization and decreased erythropoiesis as a result of relative EPO deficiency. It is possible that in the setting of ESA administration, iron restricted erythropoiesis would become evident due to relative hepcidin excess, as suggested by one previous study in the original adenine model [43], and hepcidin lowering agents could have an adjunctive role to ESA therapy in this setting.
Taken together, our data suggest that hepcidin excess and restricted iron availability are not limiting factors for the development of anemia in the modified adenine model, but that the limiting factor was likely EPO deficiency. Future studies will be needed to test whether co-administration of hepcidin-lowering agents such as LDN-193189 will be useful as an adjunctive therapy with ESAs to improve Hb levels while reducing ESA dose in models of anemia of CKD. Future studies will also be needed to develop animal models of anemia of CKD that more accurately reflect the characteristics of human patients with this disease to help further test alternative treatment strategies.
In summary, we have provided the first experimental evidence that lowering hepcidin can increase iron availability for incorporation into RBCs in an animal model of anemia of CKD. By correcting hepcidin excess to allow normal dietary iron absorption and mobilization of iron from body stores, such strategies could be anticipated to reduce the requirement for intravenous iron therapy and high-dose ESAs to improve anemia management in CKD patients.
SUPPLEMENTARY DATA
Supplementary data are available online at http://ndt.oxfordjournals.org.
ACKNOWLEDGEMENTS
C.C.S was supported by NIH NRSA Training grant T32 DK 7540-27. J.L.B was supported in part by NIH grant RO1-DK087727, the Satellite Dialysis Young Investigator Grant of the National Kidney Foundation, and Claflin Distinguished Scholar and Howard Goodman Fellowship Awards from the Massachusetts General Hospital. H.Y.L was supported by NIH grants RO1 DK-069533 and RO1 DK-071837.
CONFLICT OF INTEREST STATEMENT
C.C.S., J.L.B. and H.Y.L. have ownership interest in a startup company Ferrumax Pharmaceuticals, which has licensed technology from the Massachusetts General Hospital based on the work cited here and in prior publications. The results presented in this paper have not been published previously in whole or part, except in abstract format.
REFERENCES
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) Anemia Work Group. KDIGO Clinical Practice Guideline for Anemia in Chronic Kidney Disease. Kidney Int. 2012;2:279–335. doi:10.1038/kisup.2012.37. [Google Scholar]
- 2.Besarab A, Coyne DW. Iron supplementation to treat anemia in patients with chronic kidney disease. Nat Rev Nephrol. 2010;6:699–710. doi: 10.1038/nrneph.2010.139. doi:10.1038/nrneph.2010.139. [DOI] [PubMed] [Google Scholar]
- 3.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. doi:10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361:2019–2032. doi: 10.1056/NEJMoa0907845. doi:10.1056/NEJMoa0907845. [DOI] [PubMed] [Google Scholar]
- 5.Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590. doi: 10.1056/NEJM199808273390903. doi:10.1056/NEJM199808273390903. [DOI] [PubMed] [Google Scholar]
- 6.Szczech LA, Barnhart HX, Inrig JK, et al. Secondary analysis of the CHOIR trial epoetin-alpha dose and achieved hemoglobin outcomes. Kidney Int. 2008;74:791–798. doi: 10.1038/ki.2008.295. doi:10.1038/ki.2008.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon SD, Uno H, Lewis EF, et al. Erythropoietic response and outcomes in kidney disease and type 2 diabetes. N Engl J Med. 2010;363:1146–1155. doi: 10.1056/NEJMoa1005109. doi:10.1056/NEJMoa1005109. [DOI] [PubMed] [Google Scholar]
- 8.Eschbach JW, Cook JD, Scribner BH, et al. Iron balance in hemodialysis patients. Ann Intern Med. 1977;87:710–713. doi: 10.7326/0003-4819-87-6-710. [DOI] [PubMed] [Google Scholar]
- 9.Van Wyck DB. Iron deficiency in patients with dialysis-associated anemia during erythropoietin replacement therapy: strategies for assessment and management. Semin Nephrol. 1989;9:21–24. [PubMed] [Google Scholar]
- 10.Markowitz GS, Kahn GA, Feingold RE, et al. An evaluation of the effectiveness of oral iron therapy in hemodialysis patients receiving recombinant human erythropoietin. Clin Nephrol. 1997;48:34–40. [PubMed] [Google Scholar]
- 11.Fishbane S, Frei GL, Maesaka J. Reduction in recombinant human erythropoietin doses by the use of chronic intravenous iron supplementation. Am J Kidney Dis. 1995;26:41–46. doi: 10.1016/0272-6386(95)90151-5. doi:10.1016/0272-6386(95)90151-5. [DOI] [PubMed] [Google Scholar]
- 12.Sunder-Plassmann G, Horl WH. Importance of iron supply for erythropoietin therapy. Nephrol Dial Transplant. 1995;10:2070–2076. [PubMed] [Google Scholar]
- 13.DeVita MV, Frumkin D, Mittal S, et al. Targeting higher ferritin concentrations with intravenous iron dextran lowers erythropoietin requirement in hemodialysis patients. Clin Nephrol. 2003;60:335–340. doi: 10.5414/cnp60335. [DOI] [PubMed] [Google Scholar]
- 14.Coyne DW, Kapoian T, Suki W, et al. Ferric gluconate is highly efficacious in anemic hemodialysis patients with high serum ferritin and low transferrin saturation: results of the dialysis patients’ response to IV iron with elevated ferritin (DRIVE) study. J Am Soc Nephrol. 2007;18:975–984. doi: 10.1681/ASN.2006091034. doi:10.1681/ASN.2006091034. [DOI] [PubMed] [Google Scholar]
- 15.Kapoian T, O'Mara NB, Singh AK, et al. Ferric gluconate reduces epoetin requirements in hemodialysis patients with elevated ferritin. J Am Soc Nephrol. 2008;19:372–379. doi: 10.1681/ASN.2007050606. doi:10.1681/ASN.2007050606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mircescu G, Garneata L, Capusa C, et al. Intravenous iron supplementation for the treatment of anaemia in pre-dialyzed chronic renal failure patients. Nephrol Dial Transplant. 2006;21:120–124. doi: 10.1093/ndt/gfi087. doi:10.1093/ndt/gfi087. [DOI] [PubMed] [Google Scholar]
- 17.Silverberg DS, Iaina A, Peer G, et al. Intravenous iron supplementation for the treatment of the anemia of moderate to severe chronic renal failure patients not receiving dialysis. Am J Kidney Dis. 1996;27:234–238. doi: 10.1016/s0272-6386(96)90546-6. doi:10.1016/S0272-6386(96)90546-6. [DOI] [PubMed] [Google Scholar]
- 18.Chang CH, Chang CC, Chiang SS. Reduction in erythropoietin doses by the use of chronic intravenous iron supplementation in iron-replete hemodialysis patients. Clin Nephrol. 2002;57:136–141. doi: 10.5414/cnp57136. [DOI] [PubMed] [Google Scholar]
- 19.Macdougall IC, Tucker B, Thompson J, et al. A randomized controlled study of iron supplementation in patients treated with erythropoietin. Kidney Int. 1996;50:1694–1699. doi: 10.1038/ki.1996.487. doi:10.1038/ki.1996.487. [DOI] [PubMed] [Google Scholar]
- 20.Senger JM, Weiss RJ. Hematologic and erythropoietin responses to iron dextran in the hemodialysis environment. ANNA J. 1996;23:319–323. discussion 24–5. [PubMed] [Google Scholar]
- 21.Besarab A, Kaiser JW, Frinak S. A study of parenteral iron regimens in hemodialysis patients. Am J Kidney Dis. 1999;34:21–28. doi: 10.1016/s0272-6386(99)70103-4. doi:10.1016/S0272-6386(99)70103-4. [DOI] [PubMed] [Google Scholar]
- 22.Navarro JF, Teruel JL, Liano F, et al. Effectiveness of intravenous administration of Fe-gluconate-Na complex to maintain adequate body iron stores in hemodialysis patients. Am J Nephrol. 1996;16:268–272. doi: 10.1159/000169008. doi:10.1159/000169008. [DOI] [PubMed] [Google Scholar]
- 23.Weiss G, Goodnough LT. Anemia of chronic disease. N Engl J Med. 2005;352:1011–1023. doi: 10.1056/NEJMra041809. doi:10.1056/NEJMra041809. [DOI] [PubMed] [Google Scholar]
- 24.Daoud E, Nakhla E, Sharma R. Q: Is iron therapy for anemia harmful in the setting of infection? Cleve Clin J Med. 2011;78:168–170. doi: 10.3949/ccjm.78a.10156. doi:10.3949/ccjm.78a.10156. [DOI] [PubMed] [Google Scholar]
- 25.Drueke TB, Massy ZA. Intravenous iron: how much is too much? J Am Soc Nephrol. 2005;16:2833–2835. doi: 10.1681/ASN.2005080804. doi:10.1681/ASN.2005080804. [DOI] [PubMed] [Google Scholar]
- 26.Babitt JL, Lin HY. Molecular Mechanisms of Hepcidin Regulation: Implications for the Anemia of CKD. Am J Kidney Dis. 2010;55:726–741. doi: 10.1053/j.ajkd.2009.12.030. doi:10.1053/j.ajkd.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eleftheriadis T, Liakopoulos V, Antoniadi G, et al. The role of hepcidin in iron homeostasis and anemia in hemodialysis patients. Semin Dial. 2009;22:70–77. doi: 10.1111/j.1525-139X.2008.00532.x. [DOI] [PubMed] [Google Scholar]
- 28.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306:2090–2093. doi: 10.1126/science.1104742. doi:10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 29.Ganz T, Olbina G, Girelli D, et al. Immunoassay for human serum hepcidin. Blood. 2008;112:4292–4297. doi: 10.1182/blood-2008-02-139915. doi:10.1182/blood-2008-02-139915. [DOI] [PubMed] [Google Scholar]
- 30.Ashby DR, Gale DP, Busbridge M, et al. Plasma hepcidin levels are elevated but responsive to erythropoietin therapy in renal disease. Kidney Int. 2009;75:976–981. doi: 10.1038/ki.2009.21. doi:10.1038/ki.2009.21. [DOI] [PubMed] [Google Scholar]
- 31.Zaritsky J, Young B, Wang HJ, et al. Hepcidin—a potential novel biomarker for iron status in chronic kidney disease. Clin J Am Soc Nephrol. 2009;4:1051–1056. doi: 10.2215/CJN.05931108. doi:10.2215/CJN.05931108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Andriopoulos B, Jr, Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41:482–487. doi: 10.1038/ng.335. doi:10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meynard D, Kautz L, Darnaud V, et al. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41:478–481. doi: 10.1038/ng.320. doi:10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 34.Wang RH, Li C, Xu X, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2:399–409. doi: 10.1016/j.cmet.2005.10.010. doi:10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 35.Steinbicker AU, Bartnikas TB, Lohmeyer LK, et al. Perturbation of hepcidin expression by BMP type I receptor deletion induces iron overload in mice. Blood. 2011;118:4224–4230. doi: 10.1182/blood-2011-03-339952. doi:10.1182/blood-2011-03-339952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38:531–539. doi: 10.1038/ng1777. doi:10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 37.Papanikolaou G, Samuels ME, Ludwig EH, et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36:77–82. doi: 10.1038/ng1274. doi:10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 38.Verga Falzacappa MV, Casanovas G, Hentze MW, et al. A bone morphogenetic protein (BMP)-responsive element in the hepcidin promoter controls HFE2-mediated hepatic hepcidin expression and its response to IL-6 in cultured cells. J Mol Med. 2008;86:531–540. doi: 10.1007/s00109-008-0313-7. doi:10.1007/s00109-008-0313-7. [DOI] [PubMed] [Google Scholar]
- 39.Babitt JL, Huang FW, Xia Y, et al. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007;117:1933–1939. doi: 10.1172/JCI31342. doi:10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steinbicker AU, Sachidanandan C, Vonner AJ, et al. Inhibition of bone morphogenetic protein signaling attenuates anemia associated with inflammation. Blood. 2011;117:4915–4923. doi: 10.1182/blood-2010-10-313064. doi:10.1182/blood-2010-10-313064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4:33–41. doi: 10.1038/nchembio.2007.54. doi:10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Theurl I, Schroll A, Sonnweber T, et al. Pharmacologic inhibition of hepcidin expression reverses anemia of chronic inflammation in rats. Blood. 2011;118:4977–4984. doi: 10.1182/blood-2011-03-345066. doi:10.1182/blood-2011-03-345066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hamada Y, Kono TN, Moriguchi Y, et al. Alteration of mRNA expression of molecules related to iron metabolism in adenine-induced renal failure rats: a possible mechanism of iron deficiency in chronic kidney disease patients on treatment. Nephrol Dial Transplant. 2008;23:1886–1891. doi: 10.1093/ndt/gfm900. doi:10.1093/ndt/gfm900. [DOI] [PubMed] [Google Scholar]
- 44.Yokozawa T, Zheng PD, Oura H, et al. Animal model of adenine-induced chronic renal failure in rats. Nephron. 1986;44:230–234. doi: 10.1159/000183992. doi:10.1159/000183992. [DOI] [PubMed] [Google Scholar]
- 45.Cuny GD, Yu PB, Laha JK, et al. Structure-activity relationship study of bone morphogenetic protein (BMP) signaling inhibitors. Bioorg Med Chem Lett. 2008;18:4388–4392. doi: 10.1016/j.bmcl.2008.06.052. doi:10.1016/j.bmcl.2008.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meynard D, Vaja V, Sun CC, et al. Regulation of TMPRSS6 by BMP6 and iron in human cells and mice. Blood. 2011;118:747–756. doi: 10.1182/blood-2011-04-348698. doi:10.1182/blood-2011-04-348698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Theurl I, Schroll A, Nairz M, et al. Pathways for the regulation of hepcidin expression in anemia of chronic disease and iron deficiency anemia in vivo. Haematologica. 2011;96:1761–1769. doi: 10.3324/haematol.2011.048926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rasband WS. ImageJ. National Institutes of Health, Bethesda, Maryland, USA1997–2012; http://imagej.nih.gov/ij/
- 49.Okada H, Kaneko Y, Yawata T, et al. Reversibility of adenine-induced renal failure in rats. Clin Exp Nephrol. 1999;3:82–88. doi:10.1007/s101570050015. [Google Scholar]
- 50.Burkhard MJ, Brown DE, McGrath JP, et al. Evaluation of the erythroid regenerative response in two different models of experimentally induced iron deficiency anemia. Vet Clin Pathol. 2001;30:76–85. doi: 10.1111/j.1939-165x.2001.tb00262.x. [DOI] [PubMed] [Google Scholar]
- 51.Anderson S, Meyer TW, Rennke HG, et al. Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1985;76:612–619. doi: 10.1172/JCI112013. doi:10.1172/JCI112013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Garcia DL, Anderson S, Rennke HG, et al. Anemia lessens and its prevention with recombinant human erythropoietin worsens glomerular injury and hypertension in rats with reduced renal mass. Proc Natl Acad Sci U S A. 1988;85:6142–6146. doi: 10.1073/pnas.85.16.6142. doi:10.1073/pnas.85.16.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kawamura A, Higuchi M, Imai N, et al. Effect of purified recombinant human erythropoietin on anemia in rats with experimental renal failure induced by five-sixth nephrectomy. Biotherapy. 1990;2:77–85. doi: 10.1007/BF02172079. doi:10.1007/BF02172079. [DOI] [PubMed] [Google Scholar]
- 54.Ataka K, Maruyama H, Neichi T, et al. Effects of erythropoietin-gene electrotransfer in rats with adenine-induced renal failure. Am J Nephrol. 2003;23:315–323. doi: 10.1159/000072913. doi:10.1159/000072913. [DOI] [PubMed] [Google Scholar]
- 55.Sasu BJ, Cooke KS, Arvedson TL, et al. Anti-hepcidin antibody treatment modulates iron metabolism and is effective in a mouse model of inflammation-induced anemia. Blood. 2010;115:3616–3624. doi: 10.1182/blood-2009-09-245977. doi:10.1182/blood-2009-09-245977. [DOI] [PubMed] [Google Scholar]
- 56.Cotes PM. Immunoreactive erythropoietin in serum. I. Evidence for the validity of the assay method and the physiological relevance of estimates. Br J Haematol. 1982;50:427–438. doi: 10.1111/j.1365-2141.1982.tb01938.x. [DOI] [PubMed] [Google Scholar]
- 57.Garcia JF, Ebbe SN, Hollander L, et al. Radioimmunoassay of erythropoietin: circulating levels in normal and polycythemic human beings. J Lab Clin Med. 1982;99:624–635. [PubMed] [Google Scholar]
- 58.McGonigle RJ, Wallin JD, Shadduck RK, et al. Erythropoietin deficiency and inhibition of erythropoiesis in renal insufficiency. Kidney Int. 1984;25:437–444. doi: 10.1038/ki.1984.36. doi:10.1038/ki.1984.36. [DOI] [PubMed] [Google Scholar]
- 59.Muckenthaler MU. Fine tuning of hepcidin expression by positive and negative regulators. Cell Metab. 2008;8:1–3. doi: 10.1016/j.cmet.2008.06.009. doi:10.1016/j.cmet.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 60.Corradini E, Schmidt PJ, Meynard D, et al. BMP6 treatment compensates for the molecular defect and ameliorates hemochromatosis in Hfe knockout mice. Gastroenterology. 2010;139:1721–1729. doi: 10.1053/j.gastro.2010.07.044. doi:10.1053/j.gastro.2010.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.