Floral transition under drought conditions is accelerated by enabling ABA-dependent up-regulation of the florigen genes.
Abstract
Modulation of the transition to flowering plays an important role in the adaptation to drought. The drought-escape (DE) response allows plants to adaptively shorten their life cycle to make seeds before severe stress leads to death. However, the molecular basis of the DE response is unknown. A screen of different Arabidopsis (Arabidopsis thaliana) flowering time mutants under DE-triggering conditions revealed the central role of the flower-promoting gene GIGANTEA (GI) and the florigen genes FLOWERING LOCUS T (FT) and TWIN SISTER OF FT (TSF) in the DE response. Further screens showed that the phytohormone abscisic acid is required for the DE response, positively regulating flowering under long-day conditions. Drought stress promotes the transcriptional up-regulation of the florigens in an abscisic acid- and photoperiod-dependent manner, so that early flowering only occurs under long days. Along with the florigens, the floral integrator SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 is also up-regulated in a similar fashion and contributes to the activation of TSF. The DE response was recovered under short days in the absence of the floral repressor SHORT VEGETATIVE PHASE or in GI-overexpressing plants. Our data reveal a key role for GI in connecting photoperiodic cues and environmental stress independently from the central FT/TSF activator CONSTANS. This mechanism explains how environmental cues may act upon the florigen genes in a photoperiodically controlled manner, thus enabling plastic flowering responses.
The timing of the floral transition has significant consequences for the reproductive success of plants and consequently their adaptability to various environmental conditions. Plasticity in flowering time in response to changes in water availability has been documented in several plant species (Xu et al., 2005; Lafitte et al., 2006; Sherrard and Maherali, 2006; Franks et al., 2007; Franks, 2011; Ivey and Carr, 2012). As water scarcity results in a reduction of growing seasons, the drought-escape (DE) response defines the ability of plants to complete their life cycle before stress conditions lead to lethality (McKay et al., 2003; Verslues and Juenger, 2011). Thus, in natural environments, the onset of the DE response represents a key adaptive trait in triggering an acceleration of the floral transition and reproductive success (Franks, 2011). Despite its ecological significance, a DE response has not yet been ascribed to a mechanism of flowering gene regulation. Therefore, a key question is, what mechanism transduces a drought-derived signal into affecting the floral transition?
The floral transition is controlled by internal and external factors and occurs when the shoot apical meristem (SAM) receives appropriate signals and switches from producing vegetative leaves to producing flowers, fruits, and seeds (Bernier et al., 1993). The study of the model plant Arabidopsis (Arabidopsis thaliana) resulted in the definition of four major pathways involved in flowering time control: the photoperiodic, the vernalization, the autonomous, and the GA pathways (Amasino, 2010; Andrés and Coupland, 2012).
Flowering in annual Arabidopsis ecotypes is strongly promoted by long-day (LD) photoperiod conditions, typical of spring/early summertime. The photoperiodic pathway is characterized by three key components, whose regulation and activity is required for correct daylength measurement: GIGANTEA (GI), CONSTANS (CO), and FLOWERING LOCUS T (FT; Putterill et al., 1995; Fowler et al., 1999; Kardailsky et al., 1999; Kobayashi et al., 1999; Park et al., 1999). Mutations in any of these genes delay flowering under long days (LDs), with little effect under short-day (SD) conditions. Daylength duration is perceived in the leaves, where a systemic signal (known as the florigen) originates (Evans, 1971). During LDs, light promotes the interaction between GI and a family of light-sensing F-box ubiquitin ligases, which results in the degradation of a set of transcriptional repressors at the CO promoter (Imaizumi et al., 2005; Sawa et al., 2007; Fornara et al., 2009). LDs also promote the stabilization of the CO protein and the consequent activation of the florigen genes FT and TWIN SISTER OF FT (TSF) in the phloem companion cells (An et al., 2004; Valverde et al., 2004; Yamaguchi et al., 2005; Jang et al., 2009). However, the FT protein moves to the SAM, where it interacts with the bZIP transcription factor FLOWERING LOCUS D (FD) to orchestrate the floral transition (Abe et al., 2005; Wigge et al., 2005; Corbesier et al., 2007; Jaeger and Wigge, 2007; Mathieu et al., 2007). SUPPRESSOR OF OVEREXPRESSION OF CONSTANS1 (SOC1) encodes a MADS box transcription factor and represents an early target of the FT/FD complex in the SAM (Lee et al., 2000; Lee and Lee, 2010).
Mutations in the autonomous pathway cause a delay in flowering irrespective of the photoperiod. The autonomous pathway promotes flowering by down-regulating the floral repressor FLOWERING LOCUS C (FLC; Michaels and Amasino, 1999, 2001). The late-flowering phenotype of autonomous pathway mutants can be reverted by vernalization, which targets FLC chromatin by imposing a silenced epigenetic state (Kim et al., 2009). GAs play a key role in flowering, particularly under short days (SDs), since GA-deficient mutants do not flower under those conditions (Wilson et al., 1992).
In nature, plants are exposed to a variety of external cues with remarkable, yet contrasting, effects on flowering. For instance, warm temperatures (28°C) substantially accelerate flowering compared with cool temperatures (16°C) in Arabidopsis (Blázquez et al., 2003; Balasubramanian et al., 2006). Abiotic stresses such as UV-C exposure accelerate flowering (Martínez et al., 2004). Conversely, intermittent cold treatment and salt stress inhibit flowering (Achard et al., 2006; Seo et al., 2009). Recent data show the importance of nutrient availability and the opposing role of nitrate and phosphate on flowering (Kant et al., 2011). Thus, plants are able to discriminate the type of external “stress” and to integrate this information into the flowering network. A key goal in flowering studies, therefore, is to define the mechanistic basis underlying such integration and its physiological significance.
FT is a central node of floral integration, since its expression depends on multiple inputs (Pin and Nilsson, 2012). FT is mainly controlled in a photoperiodic manner. However, other external stimuli have been shown to directly converge at the FT promoter, including blue light and warm temperature (Liu et al., 2008b; Kumar et al., 2012). Besides being positively controlled, FT expression is further fine-tuned via modulation of the activity of several repressor complexes, including FLC/SHORT VEGETATIVE PHASE (SVP), TEMPRANILLO1, and SCHLAFMÜTZE/APETALA2-like (Castillejo and Pelaz, 2008; Li et al., 2008; Mathieu et al., 2009).
Warm temperature is arguably the best-characterized paradigm for stress-dependent FT up-regulation. However, warm ambient temperature triggers FT up-regulation both under SD and LD conditions (Kumar et al., 2012). Here, we propose a model for the interaction between photoperiod and drought stress, whereby photoperiod-activated GI enables the abscisic acid (ABA) and drought-mediated activation of FT/TSF and SOC1. Consequently, plants can maximize their fitness by coordinating stress responses according to seasonal cues.
RESULTS
Early Drought Stress Triggers the DE Response in Arabidopsis
To assess the presence of a DE response strategy in Arabidopsis and to define the genetic basis underlying this adaptive trait, we set up conditions to impose a persistent drought stress starting from early stages of development. Three-day-old seedlings were either watered daily to maintain a relative water soil content at 80% to 90% or not watered to allow soil moisture to decrease to 30% (Supplemental Fig. S1A). A bona-fide water stress condition was reached within 6 d after sowing, as confirmed by the increase in the ABA-dependent markers ABSCISIC ACID INSENSITIVE2 (ABI2) and RESPONSIVE TO ABA18 (RAB18; Lång and Palva, 1992; Nemhauser et al., 2006; Supplemental Fig. S1, B and C). These water deficiency conditions, maintained throughout the duration of the experiment, were nevertheless compatible with plant growth and survival and resulted in a robust early-flowering response. Compared with normal watering, drought-treated Columbia (Col-0) and Landsberg erecta (Ler) wild-type plants produced fewer vegetative leaves as well as an early bolting time, indicative of the DE response (Fig. 1, A–E). The early-flowering phenotype was reflected in the early up-regulation of the floral markers LEAFY and APETALA1 (Blázquez et al., 1997; Hempel et al., 1997) in plants undergoing drought stress compared with normal watering controls (Supplemental Fig. S1, D and E).
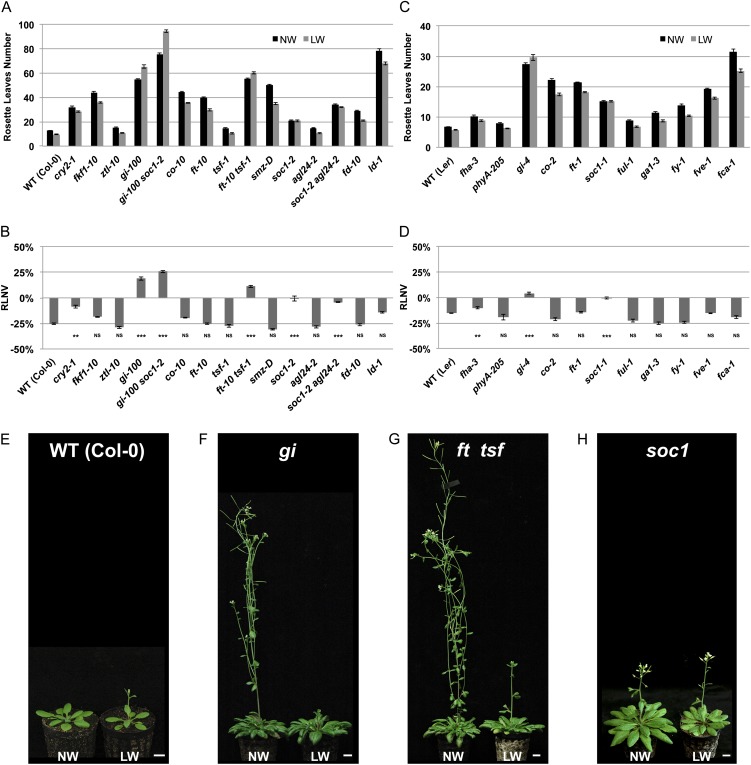
Figure 1.
The DE response requires components of the photoperiodic pathway. A and C, Rosette leaf mean numbers in wild-type (WT) Col-0 (A) and Ler (C) and flowering time mutants grown under LDs. Plants were subjected to normal-watering (NW; black bars) or low-watering (LW; gray bars) regimes. Error bars represent se (n = 15). B and D, Quantification of the DE response for each genotype detailed in A and C, respectively, expressed as relative leaf number variation (RLNV). Numbers indicate percentage variations in number of leaves in plants grown under the low-watering relative to the normal-watering condition. Error bars represent se. Student’s t test values are as follows: **P ≤ 0.01, ***P ≤ 0.001, NSP > 0.05, not significant. E to H, Images of representative plants of the indicated genotypes grown under LDs and subjected to normal-watering or low-watering regimes. Wild-type Col-0 plants are 3 weeks old (E), gi-2 plants are 12 weeks old (F), ft-10 tsf-1 plants are 16 weeks old (G), and soc1-2 plants are 8 weeks old (H). Bars = 1 cm. [See online article for color version of this figure.]
The DE Response Requires GI, FT/TSF, and SOC1
To determine whether the DE response observed in wild-type accessions was mediated by any of the known flowering time genes, we imposed DE-triggering conditions under LDs upon different late flowering time mutants that are representatives of all known floral pathways (Fig. 1, A–D).
Mutants in the autonomous pathway (lumnidependens [ld], fve, fy, and fca, which flower late irrespective of the photoperiod) and the GA pathway (ga1, impaired in GA production) produced a DE response relatively similar to the wild type, as they were consistently early flowering under DE-triggering conditions (Fig. 1, A–D). A complete absence of the DE response was observed in gi mutants both in the Col-0 (gi-100) and Ler (gi-4) backgrounds (Fig. 1, A–D and F). We confirmed the requirement for GI in triggering the DE response by analyzing independent alleles of gi (gi-1, gi-2, gi-5, and gi-6), ruling out an allele- or ecotype-specific effect (Supplemental Fig. S2, A and B). Furthermore, gi plants also displayed a significant delay in flowering time under a restricted watering regime, but this was more pronounced in the Col-0 background compared with Ler (Fig. 1, A–D and F; Supplemental Fig. S2, A and B).
Despite the known functional dependence of GI on light-sensing protein interactors such as FLAVIN-BINDING, KELCH REPEAT, F-BOX1 (FKF1) and ZEITLUPE (ZTL), responsible for GI-mediated CO activation and clock function, respectively (Imaizumi et al., 2005; Kim et al., 2007), no evident defects in the DE response were found in single fkf1 and ztl mutants (Fig. 1, A and B).
Interestingly, we found that mutants in the blue light photoreceptor CRYPTOCHROME2 (cry2-1 in Col-0 and fha-3 in Ler) were significantly impaired in their DE responses (Fig. 1, A–D). As CRY2 affects the photoperiodic pathway at different levels, including the promotion of GI protein stability (Yu et al., 2008; Zuo et al., 2011), this finding may support the central role of GI in mediating the DE response.
In accordance with GI being ultimately responsible for the photoperiodic activation of the florigen genes FT and TSF, ft tsf double mutants (but not their respective single mutants) lacked the DE response, largely mimicking the gi mutants (Fig. 1, A–D and G). Although these data point to a florigen-dependent mechanism for DE activation, this response does not appear to require the activity of CO, a transcriptional regulator of FT and TSF that acts downstream of GI in mediating the photoperiodic response. Also, no DE response defects were observed in phytochrome A mutants, which affect CO protein levels (Valverde et al., 2004) and thus are largely downstream of GI (Fig. 1, C and D).
GI-dependent but CO-independent pathways of FT activation have been described (Jung et al., 2007; Sawa and Kay, 2011). One such pathway involves the GI-dependent activation of microRNA172, resulting in the posttranscriptional gene silencing of the AP2-like genes (a class of FT transcriptional repressors; Yant et al., 2010). If this was the case, we would expect a reduction in the DE response in plants carrying an activation-tagged allele of the AP2-like gene SCHLAFMÜTZE (smz-D; Mathieu et al., 2009). However, smz-D plants exhibited an unaltered DE response, suggesting another mode of GI action (Fig. 1, A and B).
Despite the central role of the florigen proteins in mediating the DE response, no defects were found in fd, whose wild-type gene product represents a key FT interactor in the SAM (Fig. 1, A and B; Abe et al., 2005; Wigge et al., 2005). This could be due to FLOWERING LOCUS D PARALOG, mediating florigen signaling in the SAM redundantly with FD (Jaeger et al., 2013).
A strongly reduced DE response was present in soc1 plants (soc1-1 in Ler and soc1-2 in Col-0) but not in fruitfull (ful), both related MADS box-type transcription factors and downstream targets of FT in the SAM (Fig. 1, A–D and H; Gu et al., 1998; Samach et al., 2000). Previously, it was shown that mutations in AGAMOUS-LIKE24 (AGL24), a SOC1 interactor and regulator, aggravated the soc1 mutant flowering phenotype, suggesting partial redundancy between these two genes (Lee et al., 2008; Liu et al., 2008a). However, no DE response defects were apparent in agl24 single mutants, and soc1 agl24 were indistinguishable from soc1 mutants with respect to their DE responses (Fig. 1, A and B). Also, while gi soc1 double mutants were later flowering than gi, they were similar in their lack of DE, suggesting that GI and SOC1 were largely operating in the same pathway in the context of the DE response (Fig. 1, A and B). Taken together, our data reveal a cooption of GI, but not CO, to activate DE response in a florigen- and SOC1-dependent manner.
The Onset of the DE Response Is Photoperiod Dependent
We analyzed the DE phenotype of plants grown under SDs to test its photoperiod dependency. In contrast to LDs, wild-type plants (Ler or Col-0) did not generate the DE response under SDs (Fig. 2, , B, E, and G). Interestingly, SD-grown Col-0 wild-type plants (but not Ler) produced a significant delay in the floral transition under drought conditions compared with normal watering, reminiscent of that previously observed in gi or ft tsf mutants under LDs. Thus, the DE response appears to be dependent upon GI mediating LD photoperiodic cues, a finding that prompted us to test whether artificial ectopic expression of GI would be sufficient in restoring the DE response under SDs. 35S:GI (Ler) and 35S:HEMAGGLUTININ-GI (Col-0) recovered the DE response, supporting the photoperiod dependency model for DE activation (Fig. 2, A, B, and H).
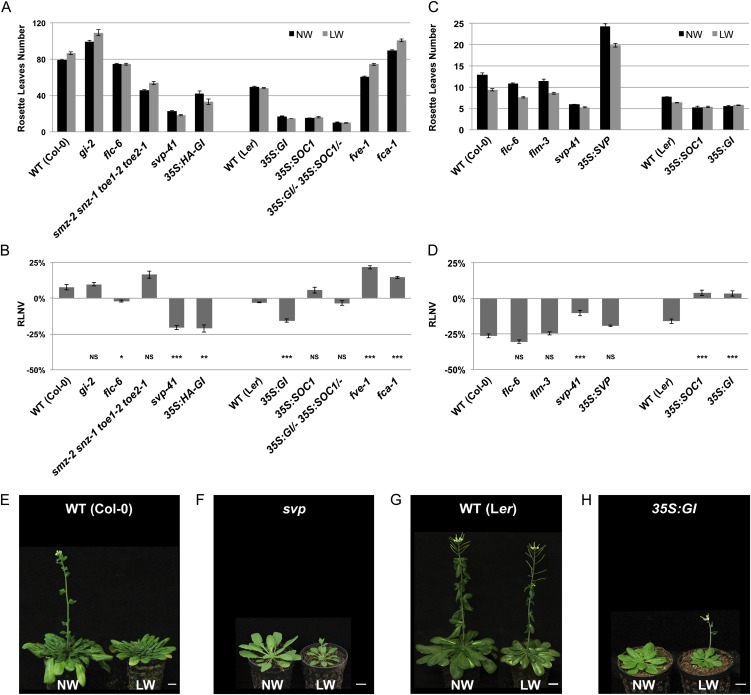
Figure 2.
The onset of the DE response is photoperiod dependent. A, Rosette leaf mean number of wild-type (WT) plants and flowering time mutants grown under SDs. Plants were subjected to normal-watering (NW; black bars) or low-watering (gray bars) regimes. Error bars represent se (n = 15). B, Quantification of the DE response for each genotype detailed in A expressed as relative leaf number variation (RLNV). Error bars represent se. Student’s t test values are as follows: *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001, NSP > 0.05, not significant. C, Rosette leaf mean number of wild-type plants and flowering time mutants grown under LDs. Plants were subjected to normal-watering (black bars) or low-watering (gray bars) regimes. Error bars represent se (n = 15). D, Quantification of the DE response for each genotype detailed in C expressed as relative leaf number variation. Error bars represent se. Student’s t test values are as follows: ***P ≤ 0.001, NSP > 0.05, not significant. E to H, Images of representative plants of the indicated genotypes grown under SDs and subjected to normal-watering or low-watering regimes. Wild-type Col-0 plants are 16 weeks old (E), svp-41 plants are 8 weeks old (F), wild-type Ler plants are 12 weeks old (G), and 35S:GI plants are 7 weeks old (H). Bars = 1 cm. [See online article for color version of this figure.]
Under LDs, 35S:GI and 35S:SOC1 plants did not display a DE response. This could be due to their early floral transition, occurring before the perception of any significant drought stress stimulus (Fig. 2D). 35S:SOC1 plants did not recover the DE response under SDs, exhibiting early flowering irrespective of the irrigation conditions (Fig. 2, A–C). Double hemizygous 35S:GI/− 35S:SOC1/− plants under SDs were earlier than their respective parental lines (Fig. 2, A and B) but did not produce the DE response, further indicating that SOC1 action is downstream of GI in the context of DE response activation. High levels of SOC1 may thus saturate the floral induction process independently of LDs, resulting in a lack of DE response. On the other hand, the partial reactivation of the photoperiodic response resulting from GI overexpression is sufficient to reinstall the DE response, even in the absence of favorable photoperiodic cues.
DE Response Recovery under SDs in svp Mutants
Drought stress can only promote flowering under LDs via a florigen-dependent mechanism. Therefore, we hypothesized that by relieving the repressive state at the promoter of the florigen genes, we could restore the DE response under SDs.
Several FT repressors have been characterized, namely the gene products FLC, FLOWERING LOCUS M (FLM), AP2-like (e.g. SMZ, SCHNARCHZAPFEN [SNZ], TARGET OF EAT1 [TOE1], and TOE2), and SVP (Yant et al., 2009). Under LDs, the effect of flc and flm mutations did not appear to alter the DE response (Fig. 2, C and D). In contrast, no significant DE response occurred in svp mutants, which exhibited an extremely early-flowering phenotype, independent of the irrigation regime (Fig. 2, C and D).
Under SDs, no DE was observed in flm or smz snz toe1 toe2 mutants (Fig. 2, A and B; Supplemental Fig. S3, A and B). As SMZ requires FLM to exert its repressive function on FT (Mathieu et al., 2009), these data indicate that the SMZ/FLM transcriptional repressor complex is not responsible for the lack of DE response under SDs. Rather, our results indicate an important role for the FLC/SVP complex in preventing the DE response under SDs. As expected, flc mutants were slightly earlier flowering under SDs compared with the wild type (Fig. 2, A and B; Supplemental Fig. S3, A and B). However, unlike the wild type, flc plants did not exhibit a floral delay when grown under drought conditions. Interestingly, svp plants were able to recover a strong DE response under SDs (Fig. 2, A, B, and F).
Although lacking the DE response, Ler wild-type plants did not exhibit a flowering delay under drought conditions when grown under SDs (Fig. 2, A, B, and G). The fact that the Ler ecotype carries a weaker allele of FLC compared with Col-0 (Lee et al., 1994), coupled with the lack of a floral delay in flc mutants (Col-0 background) under SDs, could account for this observation. In support of this hypothesis, fca and fve mutants (Ler; characterized by increased levels of FLC; Sheldon et al., 2000), produced a significant floral delay under drought conditions compared with normal watering (Fig. 2, A and B). Noticeably, compared with fca, fve plants exhibited a more pronounced floral delay, which correlates with the high levels of SVP being present in this particular genotype (Li et al., 2008).
Drought-induced changes in FLC/SVP transcript levels could account for such a floral delay. FLC transcript levels (but not SVP) were slightly but reproducibly increased under drought conditions in both LDs and SDs (Supplemental Fig. S4, A and B). However, such an increment in FLC transcript levels is unlikely to play a significant role under LDs, as fve, fy, ld, and fca plants did not exhibit obvious DE defects (Fig. 1, A–D). Also, plants ectopically expressing SVP (35S:SVP) under LDs did not exhibit DE defects (Fig. 2, C and D).
Taken together, these data indicate that SVP, likely in association with its interactor FLC, contributes to preventing the DE response upon drought conditions under SDs. Conversely, LD conditions overcome the FLC/SVP repression largely posttranscriptionally to enable the DE response.
The Phytohormone ABA Promotes the DE Response under LDs and Affects Flowering in a Photoperiod-Dependent Manner
The phytohormone ABA plays a pivotal role in orchestrating several drought responses, but its role in flowering time is poorly understood (Fujita et al., 2011). Mutants impaired in ABA biosynthesis, aba deficient1 and aba deficient2 (aba1-6 and aba2-4), flowered later than the wild type even under normal watering conditions, indicating a positive role for ABA in controlling the floral transition (Fig. 3, A and C). Despite being significantly later flowering than the wild type, aba2-4 plants were consistently earlier than aba1-6 (Student’s t test, P = 0.02), which could reflect the relative severity of this particular allele.
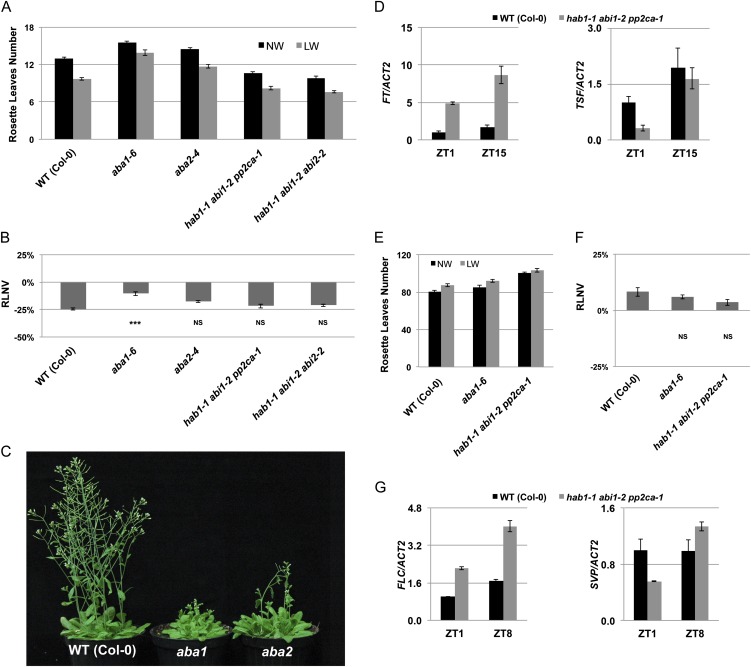
Figure 3.
ABA is required for the DE response by positively regulating flowering. A, Rosette leaf mean number of the wild type (WT) and ABA biosynthesis or signaling mutants grown under LDs. Plants were subjected to normal-watering (NW; black bars) or low-watering (LW; gray bars) regimes. Error bars represent se (n = 15). B, Quantification of the DE response for each genotype detailed in A expressed as relative leaf number variation (RLNV). Error bars represent se. Student’s t test values are as follows: ***P ≤ 0.001, NSP > 0.05, not significant. C, Images of ABA biosynthesis-deficient plants (aba1-6 and aba2-4) compared with the Col-0 wild type. Four-week-old plants grown under LDs are shown. D, Real-time quantitative PCR of FT and TSF transcripts in 11-d-old hab1-1 abi1-2 pp2ca-1 or wild-type (Col-0) seedlings. Plants were harvested at Zeitgeber times 1 and 15 (ZT1 and ZT15) in a 16-h-light/8-h-dark photoperiodic regime. Values represent fold change variations of FT and TSF transcript levels relative to Zeitgeber time 1 (arbitrarily set at 1 in Col-0). ACTIN2 expression (ACT2) was used for normalization; error bars represent sd of two technical replicates. A representative experiment of two biological replicates is shown. E, Rosette leaf mean number of the wild type and ABA biosynthesis or signaling mutants grown under SDs. Plants were subjected to normal-watering (black bars) or low-watering (gray bars) regimes. Error bars represent se (n = 15). F, Quantification of the DE response for each genotype detailed in E expressed as relative leaf number variation. Error bars represent se; Student’s t test values are as follows: NSP > 0.05, not significant. G, Real-time quantitative PCR of FLC and SVP transcripts in 3-week-old hab1-1 abi1-2 pp2ca-1 or wild-type (Col-0) seedlings. Plants were harvested at Zeitgeber times 1 and 8 (ZT1 and ZT8) in an 8-h-light/16-h-dark photoperiodic regime. Values represent fold change variations of FLC and SVP transcript levels relative to Zeitgeber time 1 (arbitrarily set at 1 in Col-0). ACT2 expression was used for normalization; error bars represent sd of two technical replicates. A representative experiment of two biological replicates is shown. [See online article for color version of this figure.]
Under drought stress conditions, aba1 mutant plants exhibited a reduced DE response compared with the wild type (Fig. 3, A and B). However, because of the residual DE response in aba1 mutants, other non-ABA-dependent pathways are likely to contribute to the early-flowering phenotype caused by drought. Alternatively, residual ABA production in these mutants (ethyl methanesulfonate generated, nucleotide substitution alleles, and unlikely to be null) was sufficient to generate the DE response. To distinguish between these possibilities, we analyzed an ABA1 transfer DNA (T-DNA) insertion line (Morris et al., 2006), which could represent a more severe allele. These aba1 mutants showed a late-flowering phenotype, similar to the aba1-6 allele, under normal watering conditions (Supplemental Fig. S5). However, unlike aba1-6 plants, they could not survive under drought stress conditions, thus precluding an evaluation of their DE response.
To further confirm such a positive role of ABA in flowering, we analyzed the phenotype of higher order mutants in the ABA negative regulator PROTEIN PHOSPHATASE2C (PP2C) gene family, known to result in hypersensitized ABA signaling (Rubio et al., 2009). Compared with the wild type, hypersensitive to aba1 (hab1-1), aba insensitive1 (abi1-2), abi2-2, and hab1-1 abi1-2 pp2ca-1 mutants were significantly earlier flowering, even under normal watering conditions (Fig. 3A). Under drought stress conditions, their DE response was relatively similar to the wild type, likely as a result of the combined contribution of increased ABA accumulation and increased sensitivity (Fig. 3B). In agreement with the floral promotive role of ABA under LDs, the early flowering of hab1-1 abi1-2 pp2ca-1 plants was accompanied by strongly increased FT (but not TSF) transcript accumulation (Fig. 3D).
We hypothesized that the constitutive activation of ABA signaling might overcome the lack of DE under SDs. However, hab1-1 abi1-2 pp2ca-1 plants were significantly later flowering compared with the wild type (producing more than 20 vegetative leaves) under normal watering regimes (Fig. 3E). FLC levels (but not SVP) were elevated in SD-grown hab1-1 abi1-2 pp2ca-1 compared with the wild type, which could contribute to the phenotype observed (Fig. 3G). In contrast, ABA biosynthesis-defective mutants (aba1-6) did not exhibit altered flowering time compared with the wild type (Fig. 3E). Under drought conditions, both ABA constitutive signaling and biosynthesis mutants generated a flowering delay, similar to the wild type (Fig. 3F).
Our results indicate that ABA acts as a positive regulator of flowering under LD conditions but suppresses flowering under noninductive SDs.
ABA Up-Regulates FT/TSF and SOC1 Expression in a Photoperiod-Dependent Manner
We sought to precisely monitor the expression of flowering genes in DE-defective genotypes. Normally irrigated or drought-stressed plants were grown under SDs and then shifted to LDs to allow the DE response. Upon LD shift in wild-type plants, FT and TSF transcripts levels strongly increased at dusk, coinciding with the first and second photoextension periods (Fig. 4A). Under drought conditions, FT and TSF up-regulation was dramatically increased compared with normally watered controls, especially during the second LD (Fig. 4A). Consistent with the DE response occurring in coincidence with LDs, no obvious FT or TSF transcript increases were detectable under SDs, irrespective of watering regime (Fig. 4, compare A with F). This was further confirmed by the lack of FT/TSF up-regulation in gi mutants despite the transfer to LDs (Fig. 4B). It is unlikely that the higher florigen transcript accumulation under drought stress derived from increased GI levels, as little variation in GI gene expression was apparent at any time point during the experiment, independent of the irrigation regimes (Supplemental Fig. S4C). Rather, the boost in FT and TSF expression was strongly ABA dependent, as it was nearly abolished in aba1-6 plants (Fig. 4C). Moreover, we found that aba1-6 had generally reduced photoperiod-dependent up-regulation of FT and TSF transcript levels compared with the wild type under normal watering conditions, especially upon the first photoextension period. Thus, ABA promotes flowering by contributing to florigen transcript accumulation and by potentiating florigen levels under drought conditions.
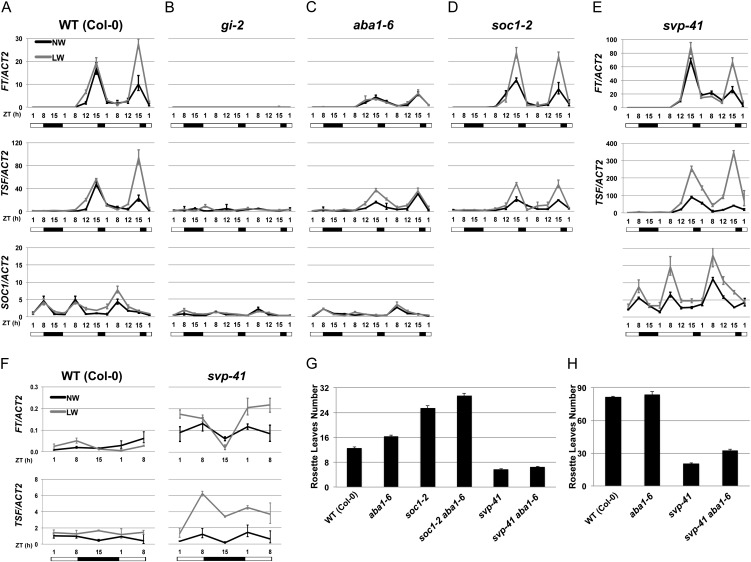
Figure 4.
ABA- and photoperiod-dependent up-regulation of FT, TSF, and SOC1 transcripts. A to E, Real-time quantitative PCR of FT, TSF, and SOC1 transcripts in 3-week-old wild-type (WT) Col-0 (A), gi-2 (B), aba1-6 (C), soc1-2 (D), and svp-41 (E) seedlings. Plants were subjected to normal-watering (NW; black lines) or low-watering (LW; gray lines) regimes and harvested at the indicated time points coinciding with the light phase (white bar) or in the dark (black bar) during an SD-to-LD shift. At each time point, values represent fold change variations of FT, TSF, and SOC1 transcript levels relative to Col-0 under normal watering. ACT2 expression was used for normalization; error bars represent sd of two technical replicates. A representative experiment of two biological replicates is shown. F, Closeup of the FT and TSF pattern of expression during the SD part of the experiment illustrated in A and E. G, Rosette leaf mean number of wild-type Col-0 and the indicated single and double mutants grown under LDs. Error bars represent se (n = 10–12). H, Same as G but grown under SDs. Error bars represent se (n = 10–12).
Upon a shift to LD conditions, SOC1 transcripts were also up-regulated in a drought-dependent manner in wild-type plants (Fig. 4A). Such up-regulation was abolished in gi mutants, suggesting that it was mediated by the photoperiod (Fig. 4B). We then established that SOC1 up-regulation under drought conditions required ABA and that ABA was also necessary for maintaining wild-type SOC1 transcript levels even under normal watering conditions (Fig. 4C). Thus, similar to the florigen genes, SOC1 is subjected to both ABA and photoperiod transcriptional control.
FT positively regulates SOC1 expression (Michaels et al., 2005; Yoo et al., 2005) and is responsible for SOC1 up-regulation in the SAM (Jang et al., 2009). Other floral integrators and FT targets are up-regulated in the SAM, namely FUL and AGL24, but these did not display a strong drought dependency in their expression (Supplemental Fig. S4, D and E). SOC1 is also expressed in leaves before the floral transition and could play a role in FT activation (Lee et al., 2000; Samach et al., 2000; Searle et al., 2006). The observed drought-dependent SOC1 up-regulation occurred very early after the LD shift; therefore, it is unlikely to reflect varying SOC1 levels in the SAM (Fig. 4A). In soc1 mutants grown under normal watering conditions, the expression levels of TSF (but not FT) were generally lower than in the wild type (Fig. 4D). Under drought conditions, soc1 mutants exhibited strongly reduced TSF up-regulation but no obvious change in FT expression. Thus, besides acting downstream of the florigen in the SAM, SOC1 also acts upstream of the TSF gene, possibly conveying an ABA-dependent signal. As observed previously, FT activation is independent of SOC1 (Searle et al., 2006) but still strongly ABA dependent. In support of this model of ABA independently acting on FT and SOC1, aba1 soc1 plants were later flowering than soc1 single mutants, indicating that ABA deficiency can delay flowering through pathways other than SOC1 (i.e. FT; Fig. 4G).
SVP has been shown to negatively regulate FT and SOC1 expression (Li et al., 2008; Jang et al., 2009). Because svp mutants recovered the DE response under SDs, we anticipated a photoperiod-independent up-regulation of the florigens and/or SOC1 upon drought conditions in the svp mutants. Compared with the wild type, the levels of FT were higher (up to 5-fold) in normally watered svp plants under the SD part of the experiment (Fig. 4, E and F). However, no strong FT up-regulation occurred at these time points upon drought conditions. Unlike FT, TSF levels did not greatly differ in svp mutants compared with the wild type under normal watering, but they were increased upon drought conditions (Fig. 4, E and F). However, this TSF up-regulation was relatively small if compared with the changes in TSF transcript levels occurring under LDs in wild-type plants (Fig. 4A). Under normal irrigation, SOC1 transcript levels were strongly increased in svp plants under SDs, resembling those observed in the wild type under LDs (Fig. 4, A and E). Strikingly, under drought conditions, the levels of SOC1 were further increased, implying that SVP normally prevents the drought-dependent activation of SOC1 under SDs (Fig. 4E). As expected, upon the shift to LDs, svp plants exhibited a dramatic SOC1 and florigen gene up-regulation compared with the wild type. Moreover such up-regulation was further boosted under drought conditions (Fig. 4E).
In summary, svp mutants recover the DE response under SDs, and this is reflected in SOC1 drought-dependent up-regulation, but not FT and only marginally TSF. To substantiate the involvement of ABA in mediating this drought-dependent signal in svp plants, we generated aba svp double mutants. Under LDs, these plants were slightly but significantly later flowering than svp single mutants (Student’s t test, P = 0.02; Fig. 4G). This could suggest that the contribution of ABA to flowering in the svp mutant background was additive and largely masked by the strong photoperiod-mediated activation of FT. However, under SDs, aba svp plants were much more late flowering than svp single mutants. This finding is consistent with the idea that, under SDs, the ABA-promotive role in flowering genes (e.g. SOC1) is normally impaired due to SVP repression (Fig. 4H).
DISCUSSION
Role of GI in the DE Response
In this work, we identified GI as a key component mediating the DE response in Arabidopsis. However, a key question emerges regarding what kind of signal GI transduces to activate the DE response. In the simplest scenario, GI mediates daylength, effectively enabling the superimposition of drought/ABA stimuli upon the FT/TSF promoters when daylength is favorable. The fact that DE is absent under SDs (phenocopying gi mutants under LDs) is in accord with this model. However, GI mediates different signaling pathways that could directly affect drought stress perception and/or responses, perhaps independently of its photoperiodic role. gi mutants were shown to be hypertolerant to oxidative stress, to be insensitive to salt-mediated floral delay, and to be primed for cold tolerance (Kurepa et al., 1998; Cao et al., 2005; Seo et al., 2009; Kim et al., 2013). In addition, gi mutants exhibit an enhanced starch accumulation, a relevant aspect to consider in the light of recent data highlighting the importance of starch metabolism and carbon signaling in flowering (Eimert et al., 1995; Wahl et al., 2013). However, the contribution of starch accumulation in ameliorating drought stress is currently poorly understood (Harb et al., 2010). Intriguingly, FT and EARLY FLOWERING3 (a target and an interactor of GI, respectively) have been recently involved in the control of guard cell activity (Kinoshita et al., 2011). Taken together, these observations may suggest a more complex model whereby GI mediates stress stimuli in concert and/or downstream of its photoperiodic role. Perhaps gi plants have a constitutive drought-tolerant phenotype (e.g. as a result of reduced FT expression in stomata), which alters their perception of drought stress. A future goal will be to investigate these possible mechanisms of GI action and to establish their relationship (if any) with the photoperiod.
Although we could not identify the exact role of GI action within the DE response, our expression data indicate that photoperiod-stimulated GI activity is essential for the up-regulation of FT/TSF gene expression under drought stress (Figs. 4, A and B, and 5). Therefore, we anticipate that the underlying mechanism will be different from other modes of environmental up-regulation of FT/TSF (e.g. warm ambient temperature), which can occur independently of photoperiodic cues (Balasubramanian et al., 2006; Kumar et al., 2012). The precise biochemical function of GI protein is still largely unknown, as it was found in association with different protein complexes, thus arguing against a single mode of action. GI activates flowering mainly through the CO-FT module, although it can also promote flowering independently of these genes (Kim et al., 2005; Mizoguchi et al., 2005; Jung et al., 2007; Sawa and Kay, 2011). GI has been shown to physically interact with different floral repressors, including SVP, FLC, and TEM, and to directly bind to the FT promoter, providing a CO-independent mode of FT activation (Sawa and Kay, 2011). Thus, under LDs, GI may promote the DE response by regulating chromatin accessibility and/or interfere with repressor activity at the florigen promoters to allow their ABA-dependent up-regulation (Fig. 5). Whether this model can be also applied to SOC1 activation is still unclear, as SOC1 up-regulation under drought conditions may largely derive from increased florigen levels. The observation that ft tsf double mutants are unable to trigger a DE response argues in favor of a florigen-dependent mechanism of SOC1 activation under drought conditions.
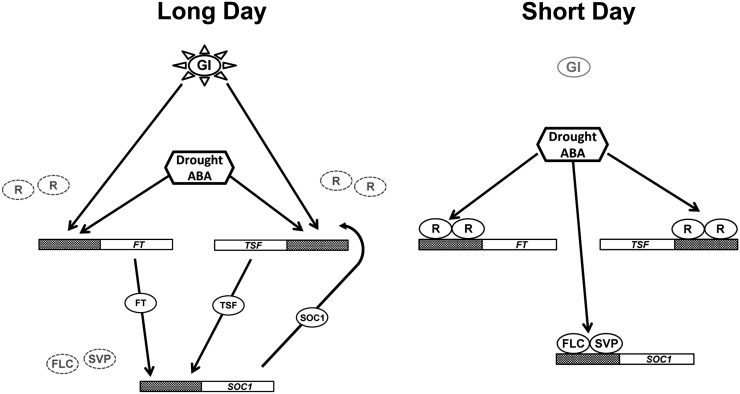
Figure 5.
Photoperiod dependency of the DE response in Arabidopsis. Drought stress, largely via ABA signaling, promotes the DE response under LDs but not SDs. Photoperiod-activated GI may relieve the transcriptional repression at the FT/TSF promoters, thus facilitating their ABA-dependent up-regulation. Increased florigen levels trigger SOC1 activation, which in turn contributes to TSF up-regulation. A floral delay occurs under SDs upon drought conditions. Drought and/or ABA may enhance the activity of different repressor complexes (e.g. SVP/FLC or other repressors [R]) through an unknown mechanism, thus interfering with the floral transition.
Our results highlight the importance of the SVP/FLC complex in preventing the DE response under SDs, but this was not reflected in the recovery of FT and TSF drought-dependent up-regulation (Fig. 2, A and B). This suggests the involvement of additional transcriptional repressors at the florigen promoter, hindering their ABA responsiveness (Fig. 5). Rather, the loss of SVP/FLC activity recovered the ABA-dependent SOC1 up-regulation (Fig. 5). Accordingly, the early-flowering phenotype of svp mutants was strongly attenuated under SDs in the svp aba1 double mutants, suggesting that SVP normally prevents ABA from positively activating SOC1 (Fig. 4H). An increase in SVP/FLC complex activity (as in fve or fca mutants) strongly delayed flowering under SDs and drought conditions without affecting the DE response under LDs (Figs. 1, C and D, and 2, A and B). Similarly 35S:SVP plants did not exhibit DE response defects under LDs. These observations indicate that under LDs, GI-enabled, ABA-dependent florigen gene up-regulation prevails over floral repression (Fig. 5).
SOC1 Potentiates the Drought-Dependent TSF Up-Regulation
soc1 plants displayed strongly attenuated drought-dependent TSF up-regulation (Fig. 4D). Thus, the DE nonresponsive phenotype of soc1 might derive from the combined effects of impaired TSF up-regulation and defective signaling downstream of FT. Beyond flowering time control, SOC1 is emerging as an important regulator of several developmental and stress responses. In conjunction with FUL, SOC1 controls meristem determinacy and cambial activity (Melzer et al., 2008). Furthermore, SOC1 orchestrates freezing tolerance responses by negatively regulating the C-REPEAT/DRE-BINDING FACTOR genes (Seo et al., 2009). A genome-wide survey of SOC1 binding sites revealed a significant enrichment in genes involved in the abiotic stress response process (Tao et al., 2012). The reduced TSF levels in soc1 mutants coupled with the fact that drought-mediated SOC1 up-regulation was strictly ABA dependent suggests a role for SOC1 in mediating part of the ABA-dependent transcriptional control over TSF. We speculate that SOC1 may also play a general role in coordinating other ABA-dependent responses.
The Phytohormone ABA Participates in the Floral Transition, But Its Effect Is Photoperiod Dependent
ABA levels increase upon water scarcity to orchestrate different drought responses (Leung and Giraudat, 1998; Nambara and Marion-Poll, 2005). However, ABA is regarded as a general inhibitor of flowering, as exogenous ABA applications delay flowering (Blazquez et al., 1998; Domagalska et al., 2010). Also, glucose insensitive1 (allelic to aba2) is early flowering compared with wild-type Wassilewskija (Cheng et al., 2002; Domagalska et al., 2010). However, plants overexpressing the ABA biosynthesis rate-limiting enzyme NCED3 did not exhibit a significantly altered flowering phenotype (Domagalska et al., 2010). Recent findings suggest a positive role for ABA in stress-induced flowering by promoting the nuclear tethering of the OXIDATIVE STRESS2 (OXS2) zinc-finger transcription factor, an activator of SOC1 (Blanvillain et al., 2011). The late-flowering phenotype we observed in independent ABA biosynthetic mutants (Col-0 background) coupled with their reduced DE response also indicates that endogenous ABA acts as a positive regulator of flowering under LDs. Supporting a positive role of ABA in flowering, constitutively activated ABA signaling mutants (e.g. hab1-1 abi1-2 pp2ca-1; Rubio et al., 2009) were early flowering under LDs. Also, the ectopic expression of the ABA-activated Snrk2.6/OPEN STOMATA1 (a positive ABA signaling regulator) has been reported to produce an early-flowering phenotype (Zheng et al., 2010).
Alongside these positive ABA effects on flowering (which could be explained in terms of patterns of SOC1 and florigen activation), our data reveal a negative role of drought and ABA under SDs. Compared with the wild type, hab1-1 abi1-2 pp2ca-1 exhibited a late-flowering phenotype under this photoperiod condition (Fig. 3A). Also, in wild-type plants, drought caused a floral delay compared with the normal watering control, and this was strongly dependent upon FLC/SVP complex activity (Fig. 2, A and C). However, FLC (but not SVP) transcript levels were only slightly up-regulated in wild-type plants upon drought conditions and in hab1-1 abi1-2 pp2ca-1 under SDs (Fig. 3G; Supplemental Fig. S4, A and B). These data point to a model where, in the absence of LDs, drought stress increases the repressor activity of the FLC/SVP complex largely at the posttranscriptional level (Fig. 5). It must be noted that drought-treated wild-type plants under SDs did not phenocopy hab1-1 abi1-2 pp2ca-1 mutants undergoing normal watering in terms of the floral delay phenotype. These observations indicate that drought stress alone could not recapitulate the full effect of constitutive ABA signaling. Alternatively, the constitutive ABA activation of hab1-1 abi1-2 pp2ca-1 mutants could result in additional effects that were independent of ABA.
Different hormonal signals participate to the floral transitions by affecting florigen levels. GAs accelerate flowering through the up-regulation of FT and TSF in the leaves (Galvão et al., 2012; Porri et al., 2012). Cytokinins specifically activate TSF transcription (D’Aloia et al., 2011). However, the mode of action of GAs and cytokinins with respect to FT and TSF up-regulation appears to be independent of the photoperiod conditions. Salicylic acid application also resulted in FT up-regulation and early flowering (Martínez et al., 2004). Interestingly, this early-flowering phenotype was dependent upon GI activity, but not CO, which is reminiscent of the DE response.
Expanding sets of gene expression data indicate a positive role for ABA and drought stress in the activation of florigen-like genes, including TSF, BROTHER OF FT AND TFL1, and MOTHER OF FT AND TFL1 (Chung et al., 2010; Xi et al., 2010). In contrast, ABI3 has been proposed to negatively regulate TSF (Suzuki et al., 2003). Our data indicate an important role for ABA in the transcriptional up-regulation of FT and TSF, but limited to the LD photoperiod (Fig. 4, A and C). Moreover, increased FT levels (but not TSF) were observed in the hab1-1 abi1-2 pp2ca-1 ABA-hypersensitive mutants under LDs (Fig. 3D). Thus, TSF requires both drought- and ABA-specific components for its up-regulation. Indeed, besides the ABA-dependent activation of TSF, we found evidence for an ABA-independent mechanism of activation that could contribute to the residual DE response of aba1 mutants (Figs. 3, A and B, and 4C). Conversely, the late-flowering phenotype of hab1-1 abi1-2 pp2ca-1 mutants under SDs suggests also an inhibitory role for ABA in flowering. ABA is a mobile molecule, and its site of production and distribution are compatible with a role in the leaf vasculature (the site of florigen production) as well as the SAM (Endo et al., 2008; Seo and Koshiba, 2011). The opposing role of ABA in flowering may reflect a spatially distinct ABA signaling mechanism (the leaf and the SAM). Thus, a more precise understanding of the site of ABA action as well as the mechanism for the ABA-repressive role warrants further investigation.
CONCLUSION
Our data reveal an interaction between drought stress and photoperiod in the activation of the florigen genes, a process requiring photoperiod-activated GI protein and the phytohormone ABA. The ability to trigger a DE response allows plants to survive in ephemeral environments, characterized by sudden and unpredictable changes in water availability. As our data suggest the onset of the DE response to be tightly controlled by photoperiodic cues, drought episodes occurring in spring may be a cue for plants for yet harsher drought conditions to follow in the summertime, making a DE response advantageous. We propose that the broader significance for this photoperiod-drought stress interaction could be to allow water status signals to affect the floral transition, but limiting this to a particular temporal window (e.g. spring versus autumn).
MATERIALS AND METHODS
Plant Materials and Growing Conditions
In this study, we used wild-type Arabidopsis (Arabidopsis thaliana) plants in the Col-0 and Ler backgrounds. Mutant or transgenic lines (obtained from the Nottingham Arabidopsis Stock Centre or other laboratories) are detailed in Supplemental Table S2. Seeds were stratified in the dark at 4°C for 2 d before sowing. Seeds were germinated and plants were grown in a controlled-environment cabinet at a temperature of 20°C to 23°C, 65% relative humidity, either under LD (16 h of light/8 h of dark) or SD (8 h of light/16 h of dark) photoperiods. Light was cool-white fluorescent tubes (Osram; Sylvania) at a fluency of 120 to 150 μmol m−2 s−1 (photosynthetically active radiation).
Plants were grown in Arabasket pots plus Araflat (BETATECH) filled with a blend (4:1, v/v) of loam sandy soil and peat (Vigorplant Italia). The soil water capacity was calculated as follows: Arabasket pots were filled with soil and air dried for 72 h in an oven at 45°C and then weighed (dry weight). Arabasket pots were subsequently soaked in water and weighed (wet weight). One hundred percent relative soil water content (RSWC) was calculated with the following formula: (wet weight − dry weight)/(wet weight − dry weight) × 100. The water evaporation rate in the growth chambers was then calculated by air drying the Arabasket pots and weighing them daily until the RSWC reached the target level of 30%. At least 15 plants were tested for each genotype in two parallel experiments: normal watering (80%–90% RSWC) and low watering (30% RSWC) conditions. The RSWC was kept constant by daily application of 4 mL of water to the normally watered plants and 2 mL every 2 d to the low-watering plants. Throughout all the experiments, random Arabasket pots were weighed to monitor the RSCW. In all experiments, plants received 2 mL of 1× solution of fertilizer every 3 weeks (nitrogen:phosphorus:potassium, 7.5:3:6 + iron; COMPO).
For the SD-to-LD shift experiments, stratified seeds (20–50) were sown in Arabasket pots, and plants were grown as described above. After 3 weeks, plants were harvested at the indicated time points of the subjective day and shifted to LDs. For each time point/treatment/genotype combination, plants were harvested in two biological replicates, each one consisting of approximately 50 seedlings pooled from three different Arabaskets. Two independent shift experiments were performed.
Isolation of Double Mutants and Genotyping
Mutant combinations were generated by crossing. The agl24-2 and svp-41 mutant alleles were genotyped as detailed previously (Michaels et al., 2003; Gregis et al., 2006). gi-100 homozygous mutants were selected using the BASTA resistance carried by the T-DNA. The aba1-6 mutants were selected by genomic PCR amplification with primers flanking the aba1-6-specific polymorphism followed by BsaI restriction (Niyogi et al., 1998; Barrero et al., 2005). Genotyping primers for soc1-2 and aba1-6 and reverse transcription-PCR primers for fd-10, fkf1-10, and ztl-10 are listed in Supplemental Table S3. FD, FKF1, and ZTL transcript abundance in the fd-10, fkf1-10, and ztl-10 mutants was verified by reverse transcription-PCR (Supplemental Fig. S6).
Flowering Time Measurement and Quantification of the DE Response
Flowering time was measured by counting the number of vegetative leaves produced at bolting. Flowering time of mutant and transgenic plants used in this study is detailed in Supplemental Table S1. The DE response was calculated for each genotype as the percentage variation in the number of vegetative (rosette) leaves in plants grown under low-watering conditions (Leaves LW) relative to plants with a normal-watering regime (Leaves NW) by the following formula: (Leaves LW − Leaves NW)/Leaves NW (%). Each mutant genotype/treatment combination experiment described in this work was repeated two to four times.
RNA Extraction and Real-Time Quantitative PCR
Total RNA was extracted with TRIzol reagent (Invitrogen). A total of 1.5 µg of total RNA was used for complementary DNA synthesis with the SuperScript VILO cDNA Synthesis Kit (Invitrogen). Quantitative real-time PCR was performed with Fast SYBR Green Master Mix (Applied Biosystems), and amplification was real-time monitored on a 7900 HT Fast Real-Time PCR system (Applied Biosystems). Changes in gene expression were calculated relative to ACT2 using the ΔΔCt method (Livak and Schmittgen, 2001). Quantitative real-time PCR primers are provided in Supplemental Table S3.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers GI (AT1G22770), TSF (AT4G20370), SOC1 (AT2G45660), FT (AT1G65480), SVP (AT2G22540), CO (AT5G15840), FD (AT4G35900), FLC (AT5G10140), SMZ (AT3G54990), ABI2 (AT5G57050), RAB18 (AT5G66400), LFY (AT5G61850), AP1 (AT1G69120), LD (AT4G02560), FVE (AT2G19520), FY (AT5G13480), FCA (AT4G16280), GA1 (AT4G02780), FKF1 (AT1G68050), ZTL (AT5G57360), CRY2 (AT1G04400), PHYA (AT1G09570), FUL (AT5G60910), AGL24 (AT4G24540), FLM (AT1G77080), SNZ (AT2G39250), TOE1 (AT2G28550), TOE2 (AT5G60120), ABA1 (AT5G67030), ABA2 (AT1G52340), ABI1 (AT4G26080), ABI2 (AT5G57050), PP2CA (AT3G11410), and ACT2 (AT3G18780).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. DE response induction in Arabidopsis.
Supplemental Figure S2. Absence of the DE response in independent gi alleles.
Supplemental Figure S3. DE response in floral repressor mutants under SDs.
Supplemental Figure S4. Floral gene regulation under drought stress upon SD-to-LD shifts.
Supplemental Figure S5. Mean rosette leaf numbers in aba1 mutants.
Supplemental Figure S6. Characterization of T-DNA insertion alleles of FD, FKF1, and ZTL.
Supplemental Table S1. Flowering time of mutant and transgenic plants used in this study.
Supplemental Table S2. List of genotypes used in this study.
Supplemental Table S3. List of primers used in this study.
Supplementary Material
Acknowledgments
We thank Drs. George Coupland, Peter Huijser (Max Planck Institute for Breeding Research), Pedro Rodriguez (Consejo Superior de Investigaciones Científicas), Markus Schmid (Max Planck Institute for Developmental Biology), Richard Amasino (University of Wisconsin), Martin Kater (University of Milan), and the Nottingham Arabidopsis Stock Centre for providing seed lines. We also thank Dr. Annabel Whibley (Centre National de la Recherche Scientifique) and Dr. Desmond Bradley (John Innes Centre) for insightful comments on the manuscript.
Glossary
- DE
drought-escape
- SAM
shoot apical meristem
- LD
long-day
- LDs
long days
- SD
short-day
- SDs
short days
- ABA
abscisic acid
- Col-0
Columbia
- Ler
Landsberg erecta
- T-DNA
transfer DNA
- RSWC
relative soil water content
References
- Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, Ichinoki H, Notaguchi M, Goto K, Araki T. (2005) FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 309: 1052–1056 [DOI] [PubMed] [Google Scholar]
- Achard P, Cheng H, De Grauwe L, Decat J, Schoutteten H, Moritz T, Van Der Straeten D, Peng J, Harberd NP. (2006) Integration of plant responses to environmentally activated phytohormonal signals. Science 311: 91–94 [DOI] [PubMed] [Google Scholar]
- Amasino R. (2010) Seasonal and developmental timing of flowering. Plant J 61: 1001–1013 [DOI] [PubMed] [Google Scholar]
- An H, Roussot C, Suárez-López P, Corbesier L, Vincent C, Piñeiro M, Hepworth S, Mouradov A, Justin S, Turnbull C, et al (2004) CONSTANS acts in the phloem to regulate a systemic signal that induces photoperiodic flowering of Arabidopsis. Development 131: 3615–3626 [DOI] [PubMed] [Google Scholar]
- Andrés F, Coupland G. (2012) The genetic basis of flowering responses to seasonal cues. Nat Rev Genet 13: 627–639 [DOI] [PubMed] [Google Scholar]
- Balasubramanian S, Sureshkumar S, Lempe J, Weigel D. (2006) Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Genet 2: e106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrero JM, Piqueras P, González-Guzmán M, Serrano R, Rodríguez PL, Ponce MR, Micol JL. (2005) A mutational analysis of the ABA1 gene of Arabidopsis thaliana highlights the involvement of ABA in vegetative development. J Exp Bot 56: 2071–2083 [DOI] [PubMed] [Google Scholar]
- Bernier G, Havelange A, Houssa C, Petitjean A, Lejeune P. (1993) Physiological signals that induce flowering. Plant Cell 5: 1147–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blanvillain R, Wei S, Wei P, Kim JH, Ow DW. (2011) Stress tolerance to stress escape in plants: role of the OXS2 zinc-finger transcription factor family. EMBO J 30: 3812–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez MA, Ahn JH, Weigel D. (2003) A thermosensory pathway controlling flowering time in Arabidopsis thaliana. Nat Genet 33: 168–171 [DOI] [PubMed] [Google Scholar]
- Blazquez MA, Green R, Nilsson O, Sussman MR, Weigel D. (1998) Gibberellins promote flowering of Arabidopsis by activating the LEAFY promoter. Plant Cell 10: 791–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blázquez MA, Soowal LN, Lee I, Weigel D. (1997) LEAFY expression and flower initiation in Arabidopsis. Development 124: 3835–3844 [DOI] [PubMed] [Google Scholar]
- Cao S, Ye M, Jiang S. (2005) Involvement of GIGANTEA gene in the regulation of the cold stress response in Arabidopsis. Plant Cell Rep 24: 683–690 [DOI] [PubMed] [Google Scholar]
- Castillejo C, Pelaz S. (2008) The balance between CONSTANS and TEMPRANILLO activities determines FT expression to trigger flowering. Curr Biol 18: 1338–1343 [DOI] [PubMed] [Google Scholar]
- Cheng W-H, Endo A, Zhou L, Penney J, Chen H-C, Arroyo A, Leon P, Nambara E, Asami T, Seo M, et al (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14: 2723–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung KS, Yoo SY, Yoo SJ, Lee JS, Ahn JH. (2010) BROTHER OF FT AND TFL1 (BFT), a member of the FT/TFL1 family, shows distinct pattern of expression during the vegetative growth of Arabidopsis. Plant Signal Behav 5: 1102–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, Giakountis A, Farrona S, Gissot L, Turnbull C, et al (2007) FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science 316: 1030–1033 [DOI] [PubMed] [Google Scholar]
- D’Aloia M, Bonhomme D, Bouché F, Tamseddak K, Ormenese S, Torti S, Coupland G, Périlleux C. (2011) Cytokinin promotes flowering of Arabidopsis via transcriptional activation of the FT paralogue TSF. Plant J 65: 972–979 [DOI] [PubMed] [Google Scholar]
- Domagalska MA, Sarnowska E, Nagy F, Davis SJ. (2010) Genetic analyses of interactions among gibberellin, abscisic acid, and brassinosteroids in the control of flowering time in Arabidopsis thaliana. PLoS ONE 5: e14012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eimert K, Wang S-M, Lue WI, Chen J. (1995) Monogenic recessive mutations causing both late floral initiation and excess starch accumulation in Arabidopsis. Plant Cell 7: 1703–1712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo A, Sawada Y, Takahashi H, Okamoto M, Ikegami K, Koiwai H, Seo M, Toyomasu T, Mitsuhashi W, Shinozaki K, et al. (2008) Drought induction of Arabidopsis 9-cis-epoxycarotenoid dioxygenase occurs in vascular parenchyma cells. Plant Physiol 147: 1984–1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans LT. (1971) Flower induction and the florigen concept. Annu Rev Plant Physiol 22: 365–394 [Google Scholar]
- Fornara F, Panigrahi KCS, Gissot L, Sauerbrunn N, Rühl M, Jarillo JA, Coupland G. (2009) Arabidopsis DOF transcription factors act redundantly to reduce CONSTANS expression and are essential for a photoperiodic flowering response. Dev Cell 17: 75–86 [DOI] [PubMed] [Google Scholar]
- Fowler S, Lee K, Onouchi H, Samach A, Richardson K, Morris B, Coupland G, Putterill J. (1999) GIGANTEA: a circadian clock-controlled gene that regulates photoperiodic flowering in Arabidopsis and encodes a protein with several possible membrane-spanning domains. EMBO J 18: 4679–4688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks SJ. (2011) Plasticity and evolution in drought avoidance and escape in the annual plant Brassica rapa. New Phytol 190: 249–257 [DOI] [PubMed] [Google Scholar]
- Franks SJ, Sim S, Weis AE. (2007) Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proc Natl Acad Sci USA 104: 1278–1282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita Y, Fujita M, Shinozaki K, Yamaguchi-Shinozaki K. (2011) ABA-mediated transcriptional regulation in response to osmotic stress in plants. J Plant Res 124: 509–525 [DOI] [PubMed] [Google Scholar]
- Galvão VC, Horrer D, Küttner F, Schmid M. (2012) Spatial control of flowering by DELLA proteins in Arabidopsis thaliana. Development 139: 4072–4082 [DOI] [PubMed] [Google Scholar]
- Gregis V, Sessa A, Colombo L, Kater MM. (2006) AGL24, SHORT VEGETATIVE PHASE, and APETALA1 redundantly control AGAMOUS during early stages of flower development in Arabidopsis. Plant Cell 18: 1373–1382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Ferrándiz C, Yanofsky MF, Martienssen R. (1998) The FRUITFULL MADS-box gene mediates cell differentiation during Arabidopsis fruit development. Development 125: 1509–1517 [DOI] [PubMed] [Google Scholar]
- Harb A, Krishnan A, Ambavaram MMR, Pereira A. (2010) Molecular and physiological analysis of drought stress in Arabidopsis reveals early responses leading to acclimation in plant growth. Plant Physiol 154: 1254–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempel FD, Weigel D, Mandel MA, Ditta G, Zambryski PC, Feldman LJ, Yanofsky MF. (1997) Floral determination and expression of floral regulatory genes in Arabidopsis. Development 124: 3845–3853 [DOI] [PubMed] [Google Scholar]
- Imaizumi T, Schultz TF, Harmon FG, Ho LA, Kay SA. (2005) FKF1 F-box protein mediates cyclic degradation of a repressor of CONSTANS in Arabidopsis. Science 309: 293–297 [DOI] [PubMed] [Google Scholar]
- Ivey CT, Carr DE. (2012) Tests for the joint evolution of mating system and drought escape in Mimulus. Ann Bot (Lond) 109: 583–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger KE, Pullen N, Lamzin S, Morris RJ, Wigge PA. (2013) Interlocking feedback loops govern the dynamic behavior of the floral transition in Arabidopsis Plant Cell 25:820–833 10.1105/tpc.113.109355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeger KE, Wigge PA. (2007) FT protein acts as a long-range signal in Arabidopsis. Curr Biol 17: 1050–1054 [DOI] [PubMed] [Google Scholar]
- Jang S, Torti S, Coupland G. (2009) Genetic and spatial interactions between FT, TSF and SVP during the early stages of floral induction in Arabidopsis. Plant J 60: 614–625 [DOI] [PubMed] [Google Scholar]
- Jung J-H, Seo Y-H, Seo PJ, Reyes JL, Yun J, Chua N-H, Park C-M. (2007) The GIGANTEA-regulated microRNA172 mediates photoperiodic flowering independent of CONSTANS in Arabidopsis. Plant Cell 19: 2736–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant S, Peng M, Rothstein SJ. (2011) Genetic regulation by NLA and microRNA827 for maintaining nitrate-dependent phosphate homeostasis in Arabidopsis. PLoS Genet 7: e1002021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kardailsky I, Shukla VK, Ahn JH, Dagenais N, Christensen SK, Nguyen JT, Chory J, Harrison MJ, Weigel D. (1999) Activation tagging of the floral inducer FT. Science 286: 1962–1965 [DOI] [PubMed] [Google Scholar]
- Kim DH, Doyle MR, Sung S, Amasino RM. (2009) Vernalization: winter and the timing of flowering in plants. Annu Rev Cell Dev Biol 25: 277–299 [DOI] [PubMed] [Google Scholar]
- Kim W-Y, Ali Z, Park H-J, Park SJ, Cha J-Y, Perez-Hormaeche J, Quintero FJ, Shin G, Kim MR, Qiang Z, et al. (2013) Release of SOS2 kinase from sequestration with GIGANTEA determines salt tolerance in Arabidopsis. Nat Commun 4: 1352–1364 [DOI] [PubMed] [Google Scholar]
- Kim W-Y, Fujiwara S, Suh S-S, Kim J, Kim Y, Han L, David K, Putterill J, Nam HG, Somers DE. (2007) ZEITLUPE is a circadian photoreceptor stabilized by GIGANTEA in blue light. Nature 449: 356–360 [DOI] [PubMed] [Google Scholar]
- Kim W-Y, Hicks KA, Somers DE. (2005) Independent roles for EARLY FLOWERING 3 and ZEITLUPE in the control of circadian timing, hypocotyl length, and flowering time. Plant Physiol 139: 1557–1569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Ono N, Hayashi Y, Morimoto S, Nakamura S, Soda M, Kato Y, Ohnishi M, Nakano T, Inoue S-I, et al (2011) FLOWERING LOCUS T regulates stomatal opening. Curr Biol 21: 1232–1238 [DOI] [PubMed] [Google Scholar]
- Kobayashi Y, Kaya H, Goto K, Iwabuchi M, Araki T. (1999) A pair of related genes with antagonistic roles in mediating flowering signals. Science 286: 1960–1962 [DOI] [PubMed] [Google Scholar]
- Kumar SV, Lucyshyn D, Jaeger KE, Alós E, Alvey E, Harberd NP, Wigge PA. (2012) Transcription factor PIF4 controls the thermosensory activation of flowering. Nature 484: 242–245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurepa J, Smalle J, Van Montagu M, Inzé D. (1998) Oxidative stress tolerance and longevity in Arabidopsis: the late-flowering mutant gigantea is tolerant to paraquat. Plant J 14: 759–764 [DOI] [PubMed] [Google Scholar]
- Lafitte HR, Li ZK, Vijayakumar CHM, Gao YM, Shi Y, Xu JL, Fu BY, Yu SB, Ali AJ, Domingo J, et al. (2006) Improvement of rice drought tolerance through backcross breeding: evaluation of donors and selection in drought nurseries. Field Crops Res 97: 77–86 [Google Scholar]
- Lång V, Palva ET. (1992) The expression of a rab-related gene, rab18, is induced by abscisic acid during the cold acclimation process of Arabidopsis thaliana (L.) Heynh. Plant Mol Biol 20: 951–962 [DOI] [PubMed] [Google Scholar]
- Lee H, Suh SS, Park E, Cho E, Ahn JH, Kim SG, Lee JS, Kwon YM, Lee I. (2000) The AGAMOUS-LIKE 20 MADS domain protein integrates floral inductive pathways in Arabidopsis. Genes Dev 14: 2366–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Michaels SD, Masshardt AS, Amasino RM. (1994) The late-flowering phenotype of FRIGIDA and mutations in LUMINIDEPENDENS is suppressed in the Landsberg erecta strain of Arabidopsis. Plant J 6: 903–909 [Google Scholar]
- Lee J, Lee I. (2010) Regulation and function of SOC1, a flowering pathway integrator. J Exp Bot 61: 2247–2254 [DOI] [PubMed] [Google Scholar]
- Lee J, Oh M, Park H, Lee I. (2008) SOC1 translocated to the nucleus by interaction with AGL24 directly regulates leafy. Plant J 55: 832–843 [DOI] [PubMed] [Google Scholar]
- Leung J, Giraudat J. (1998) Abscisic acid signal transduction. Annu Rev Plant Physiol Plant Mol Biol 49: 199–222 [DOI] [PubMed] [Google Scholar]
- Li D, Liu C, Shen L, Wu Y, Chen H, Robertson M, Helliwell CA, Ito T, Meyerowitz E, Yu H. (2008) A repressor complex governs the integration of flowering signals in Arabidopsis. Dev Cell 15: 110–120 [DOI] [PubMed] [Google Scholar]
- Liu C, Chen H, Er HL, Soo HM, Kumar PP, Han J-H, Liou Y-C, Yu H. (2008a) Direct interaction of AGL24 and SOC1 integrates flowering signals in Arabidopsis. Development 135: 1481–1491 [DOI] [PubMed] [Google Scholar]
- Liu H, Yu X, Li K, Klejnot J, Yang H, Lisiero D, Lin C. (2008b) Photoexcited CRY2 interacts with CIB1 to regulate transcription and floral initiation in Arabidopsis. Science 322: 1535–1539 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Martínez C, Pons E, Prats G, León J. (2004) Salicylic acid regulates flowering time and links defence responses and reproductive development. Plant J 37: 209–217 [DOI] [PubMed] [Google Scholar]
- Mathieu J, Warthmann N, Küttner F, Schmid M. (2007) Export of FT protein from phloem companion cells is sufficient for floral induction in Arabidopsis. Curr Biol 17: 1055–1060 [DOI] [PubMed] [Google Scholar]
- Mathieu J, Yant LJ, Mürdter F, Küttner F, Schmid M. (2009) Repression of flowering by the miR172 target SMZ. PLoS Biol 7: e1000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay JK, Richards JH, Mitchell-Olds T. (2003) Genetics of drought adaptation in Arabidopsis thaliana. I. Pleiotropy contributes to genetic correlations among ecological traits. Mol Ecol 12: 1137–1151 [DOI] [PubMed] [Google Scholar]
- Melzer S, Lens F, Gennen J, Vanneste S, Rohde A, Beeckman T. (2008) Flowering-time genes modulate meristem determinacy and growth form in Arabidopsis thaliana. Nat Genet 40: 1489–1492 [DOI] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. (1999) FLOWERING LOCUS C encodes a novel MADS domain protein that acts as a repressor of flowering. Plant Cell 11: 949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM. (2001) Loss of FLOWERING LOCUS C activity eliminates the late-flowering phenotype of FRIGIDA and autonomous pathway mutations but not responsiveness to vernalization. Plant Cell 13: 935–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Ditta G, Gustafson-Brown C, Pelaz S, Yanofsky M, Amasino RM. (2003) AGL24 acts as a promoter of flowering in Arabidopsis and is positively regulated by vernalization. Plant J 33: 867–874 [DOI] [PubMed] [Google Scholar]
- Michaels SD, Himelblau E, Kim SY, Schomburg FM, Amasino RM. (2005) Integration of flowering signals in winter-annual Arabidopsis. Plant Physiol 137: 149–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizoguchi T, Wright L, Fujiwara S, Cremer F, Lee K, Onouchi H, Mouradov A, Fowler S, Kamada H, Putterill J, et al (2005) Distinct roles of GIGANTEA in promoting flowering and regulating circadian rhythms in Arabidopsis. Plant Cell 17: 2255–2270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris ER, Chevalier D, Walker JC. (2006) DAWDLE, a forkhead-associated domain gene, regulates multiple aspects of plant development. Plant Physiol 141: 932–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56: 165–185 [DOI] [PubMed] [Google Scholar]
- Nemhauser JL, Hong F, Chory J. (2006) Different plant hormones regulate similar processes through largely nonoverlapping transcriptional responses. Cell 126: 467–475 [DOI] [PubMed] [Google Scholar]
- Niyogi KK, Grossman AR, Björkman O. (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10: 1121–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DH, Somers DE, Kim YS, Choy YH, Lim HK, Soh M-S, Kim HJ, Kay SA, Nam HG. (1999) Control of circadian rhythms and photoperiodic flowering by the Arabidopsis GIGANTEA gene. Science 285: 1579–1582 [DOI] [PubMed] [Google Scholar]
- Pin PA, Nilsson O. (2012) The multifaceted roles of FLOWERING LOCUS T in plant development. Plant Cell Environ 35: 1742–1755 [DOI] [PubMed] [Google Scholar]
- Porri A, Torti S, Romera-Branchat M, Coupland G. (2012) Spatially distinct regulatory roles for gibberellins in the promotion of flowering of Arabidopsis under long photoperiods. Development 139: 2198–2209 [DOI] [PubMed] [Google Scholar]
- Putterill J, Robson F, Lee K, Simon R, Coupland G. (1995) The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80: 847–857 [DOI] [PubMed] [Google Scholar]
- Rubio S, Rodrigues A, Saez A, Dizon MB, Galle A, Kim T-H, Santiago J, Flexas J, Schroeder JI, Rodriguez PL. (2009) Triple loss of function of protein phosphatases type 2C leads to partial constitutive response to endogenous abscisic acid. Plant Physiol 150: 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samach A, Onouchi H, Gold SE, Ditta GS, Schwarz-Sommer Z, Yanofsky MF, Coupland G. (2000) Distinct roles of CONSTANS target genes in reproductive development of Arabidopsis. Science 288: 1613–1616 [DOI] [PubMed] [Google Scholar]
- Sawa M, Kay SA. (2011) GIGANTEA directly activates Flowering Locus T in Arabidopsis thaliana. Proc Natl Acad Sci USA 108: 11698–11703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa M, Nusinow DA, Kay SA, Imaizumi T. (2007) FKF1 and GIGANTEA complex formation is required for day-length measurement in Arabidopsis. Science 318: 261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Searle I, He Y, Turck F, Vincent C, Fornara F, Kröber S, Amasino RA, Coupland G. (2006) The transcription factor FLC confers a flowering response to vernalization by repressing meristem competence and systemic signaling in Arabidopsis. Genes Dev 20: 898–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo E, Lee H, Jeon J, Park H, Kim J, Noh Y-S, Lee I. (2009) Crosstalk between cold response and flowering in Arabidopsis is mediated through the flowering-time gene SOC1 and its upstream negative regulator FLC. Plant Cell 21: 3185–3197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo M, Koshiba T. (2011) Transport of ABA from the site of biosynthesis to the site of action. J Plant Res 124: 501–507 [DOI] [PubMed] [Google Scholar]
- Sheldon CC, Rouse DT, Finnegan EJ, Peacock WJ, Dennis ES. (2000) The molecular basis of vernalization: the central role of FLOWERING LOCUS C (FLC). Proc Natl Acad Sci USA 97: 3753–3758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrard ME, Maherali H. (2006) The adaptive significance of drought escape in Avena barbata, an annual grass. Evolution 60: 2478–2489 [PubMed] [Google Scholar]
- Suzuki M, Ketterling MG, Li Q-B, McCarty DR. (2003) Viviparous1 alters global gene expression patterns through regulation of abscisic acid signaling. Plant Physiol 132: 1664–1677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z, Shen L, Liu C, Liu L, Yan Y, Yu H. (2012) Genome-wide identification of SOC1 and SVP targets during the floral transition in Arabidopsis. Plant J 70: 549–561 [DOI] [PubMed] [Google Scholar]
- Valverde F, Mouradov A, Soppe W, Ravenscroft D, Samach A, Coupland G. (2004) Photoreceptor regulation of CONSTANS protein in photoperiodic flowering. Science 303: 1003–1006 [DOI] [PubMed] [Google Scholar]
- Verslues PE, Juenger TE. (2011) Drought, metabolites, and Arabidopsis natural variation: a promising combination for understanding adaptation to water-limited environments. Curr Opin Plant Biol 14: 240–245 [DOI] [PubMed] [Google Scholar]
- Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, Feil R, Lunn JE, Stitt M, Schmid M. (2013) Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science 339: 704–707 [DOI] [PubMed] [Google Scholar]
- Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, Weigel D. (2005) Integration of spatial and temporal information during floral induction in Arabidopsis. Science 309: 1056–1059 [DOI] [PubMed] [Google Scholar]
- Wilson RN, Heckman JW, Somerville CR. (1992) Gibberellin is required for flowering in Arabidopsis thaliana under short days. Plant Physiol 100: 403–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xi W, Liu C, Hou X, Yu H. (2010) MOTHER OF FT AND TFL1 regulates seed germination through a negative feedback loop modulating ABA signaling in Arabidopsis. Plant Cell 22: 1733–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JL, Lafitte HR, Gao YM, Fu BY, Torres R, Li ZK. (2005) QTLs for drought escape and tolerance identified in a set of random introgression lines of rice. Theor Appl Genet 111: 1642–1650 [DOI] [PubMed] [Google Scholar]
- Yamaguchi A, Kobayashi Y, Goto K, Abe M, Araki T. (2005) TWIN SISTER OF FT (TSF) acts as a floral pathway integrator redundantly with FT. Plant Cell Physiol 46: 1175–1189 [DOI] [PubMed] [Google Scholar]
- Yant L, Mathieu J, Dinh TT, Ott F, Lanz C, Wollmann H, Chen X, Schmid M. (2010) Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 22: 2156–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant L, Mathieu J, Schmid M. (2009) Just say no: floral repressors help Arabidopsis bide the time. Curr Opin Plant Biol 12: 580–586 [DOI] [PubMed] [Google Scholar]
- Yoo SK, Chung KS, Kim J, Lee JH, Hong SM, Yoo SJ, Yoo SY, Lee JS, Ahn JH. (2005) CONSTANS activates SUPPRESSOR OF OVEREXPRESSION OF CONSTANS 1 through FLOWERING LOCUS T to promote flowering in Arabidopsis. Plant Physiol 139: 770–778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J-W, Rubio V, Lee N-Y, Bai S, Lee S-Y, Kim S-S, Liu L, Zhang Y, Irigoyen ML, Sullivan JA, et al. (2008) COP1 and ELF3 control circadian function and photoperiodic flowering by regulating GI stability. Mol Cell 32: 617–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z, Xu X, Crosley RA, Greenwalt SA, Sun Y, Blakeslee B, Wang L, Ni W, Sopko MS, Yao C, et al. (2010) The protein kinase SnRK2.6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis. Plant Physiol 153: 99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo Z, Liu H, Liu B, Liu X, Lin C. (2011) Blue light-dependent interaction of CRY2 with SPA1 regulates COP1 activity and floral initiation in Arabidopsis. Curr Biol 21: 841–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.