Signaling through abscisic acid PYR/RCAR receptors plays a fundamental role in controlling whole-plant stomatal conductance and affects stomatal closure in response to low air humidity, darkness, O3, and elevated CO2.
Abstract
Rapid stomatal closure induced by changes in the environment, such as elevation of CO2, reduction of air humidity, darkness, and pulses of the air pollutant ozone (O3), involves the SLOW ANION CHANNEL1 (SLAC1). SLAC1 is activated by OPEN STOMATA1 (OST1) and Ca2+-dependent protein kinases. OST1 activation is controlled through abscisic acid (ABA)-induced inhibition of type 2 protein phosphatases (PP2C) by PYRABACTIN RESISTANCE/REGULATORY COMPONENTS OF ABA RECEPTOR (PYR/RCAR) receptor proteins. To address the role of signaling through PYR/RCARs for whole-plant steady-state stomatal conductance and stomatal closure induced by environmental factors, we used a set of Arabidopsis (Arabidopsis thaliana) mutants defective in ABA metabolism/signaling. The stomatal conductance values varied severalfold among the studied mutants, indicating that basal ABA signaling through PYR/RCAR receptors plays a fundamental role in controlling whole-plant water loss through stomata. PYR/RCAR-dependent inhibition of PP2Cs was clearly required for rapid stomatal regulation in response to darkness, reduced air humidity, and O3. Furthermore, PYR/RCAR proteins seem to function in a dose-dependent manner, and there is a functional diversity among them. Although a rapid stomatal response to elevated CO2 was evident in all but slac1 and ost1 mutants, the bicarbonate-induced activation of S-type anion channels was reduced in the dominant active PP2C mutants abi1-1 and abi2-1. Further experiments with a wider range of CO2 concentrations and analyses of stomatal response kinetics suggested that the ABA signalosome partially affects the CO2-induced stomatal response. Thus, we show that PYR/RCAR receptors play an important role for the whole-plant stomatal adjustments and responses to low humidity, darkness, and O3 and are involved in responses to elevated CO2.
Stomata, small pores in the leaf epidermis, are formed by a pair of guard cells that have developed mechanisms to sense and respond to various endogenous and environmental stimuli. Stomata close in response to reduction in air humidity, darkness, and CO2 enrichment. Ozone (O3), a major secondary air pollutant with adverse impacts on global vegetation (Ashmore, 2005) and climate change (Sitch et al., 2007), has also been shown to cause rapid stomatal closure (Hill and Littlefield, 1969; Vahisalu et al., 2010). The key endogenous factor triggering stomatal closure in response to drought is the plant hormone abscisic acid (ABA). Webb and Hetherington (1997) suggested that the pathways of ABA- and CO2-induced closure converge (i.e. that there is an economy in signaling pathways leading to the promotion of stomatal closure). However, the location of this convergence point is still under debate. Opening and closure of stomatal pores is achieved by the uptake and release of osmotically active ions, leading to expanding and shrinking of guard cells. Thus, the activation and inactivation of guard cell ion channels and transporters are the primary targets of signaling networks controlling stomatal movements (for review, see Kim et al., 2010; Kollist et al., 2011; Roelfsema et al., 2012).
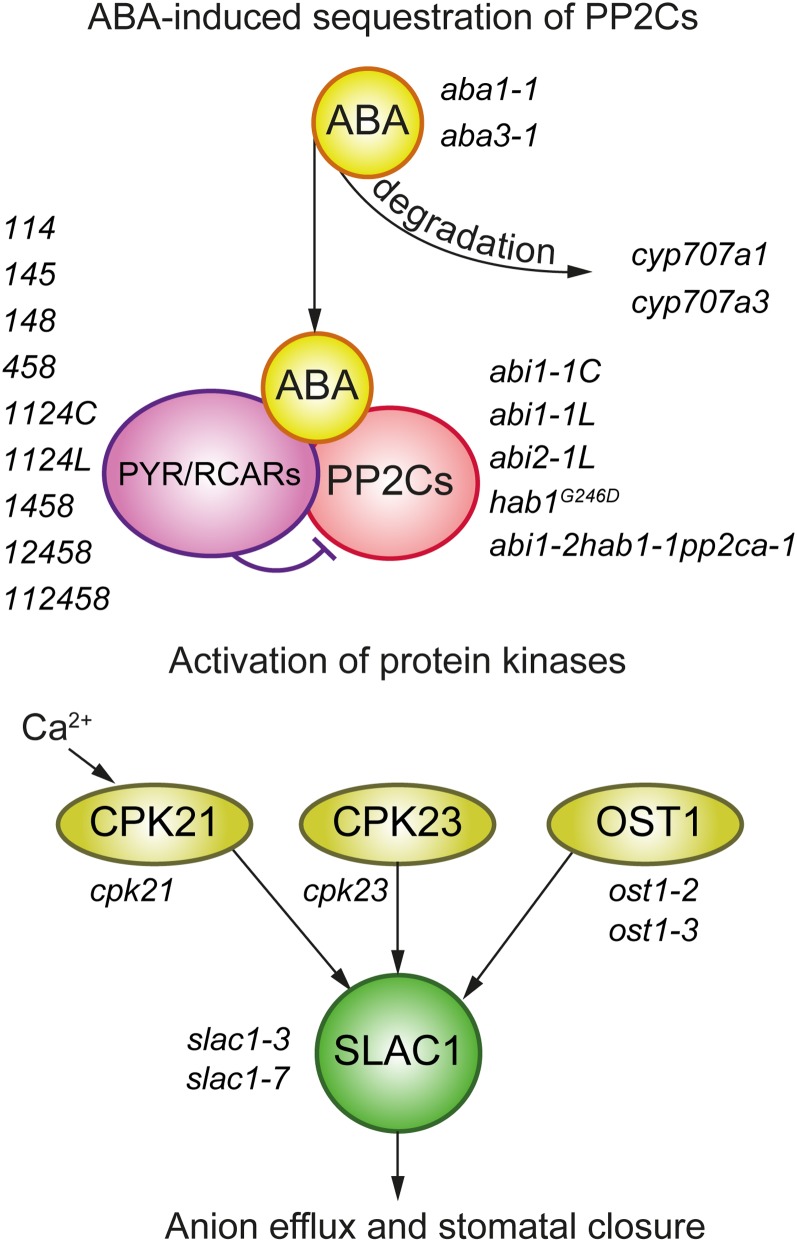
In 2009, independent groups simultaneously discovered the functional and structural mechanisms of ABA sensing by cytosolic PYRABACTIN RESISTANCE1 (PYR1)/PYR1-like (PYL)/REGULATORY COMPONENTS OF ABA RECEPTORS (RCAR) receptor proteins (Ma et al., 2009; Park et al., 2009). Identification of the guard cell slow-type anion channel gene, SLAC1, is another substantial finding in stomatal research (Negi et al., 2008; Vahisalu et al., 2008). ABA-induced stomatal closure involves the activation of SLAC1, which is controlled by PYR/RCAR-dependent sequestration of type 2 protein phosphatases (PP2Cs), such as ABI1, ABI2, HAB1, and PP2CA, and the concomitant activation of the Snf1-related subfamily 2 protein kinase SnRK2.6/OST1 (Ma et al., 2009; Nishimura et al., 2010; Park et al., 2009; Umezawa et al., 2009; Vlad et al., 2009; Weiner et al., 2010; Dupeux et al., 2011; Soon et al., 2012). Phosphorylation by OST1 activates several proteins, including SLAC1 (Geiger et al., 2009; Lee et al., 2009; Vahisalu et al., 2010). SLAC1 can also be activated by different calcium-dependent protein kinases (CPKs; Geiger et al., 2010, Brandt et al., 2012; Scherzer et al., 2012). Thus, several molecular details are known for the general activation mechanism of SLAC1 and subsequent stomatal closure. The importance of SLAC1 and OST1 in rapid stomatal responses to environmental factors such as darkness, CO2, humidity, and O3 is also established (Negi et al., 2008; Vahisalu et al., 2008; Ache et al., 2010; Xue et al., 2011). However, whether the ABA- and PYR/RCAR-dependent inhibition of PP2Cs that ultimately results in the activation of OST1 and anion channels like SLAC1 (herein defined as the ABA signalosome) is also required for rapid stomatal responses to important environmental factors is not fully resolved. One approach to address this question is to perform a side-by-side comparison of whole-plant stomatal responses of plants where various proteins of the ABA signalosome are mutated (Fig. 1; Table I).
Figure 1.
Schematic overview of ABA-induced signaling by PYR/RCAR receptors leading to sequestration of PP2Cs, activation of protein kinases OST1, CPK21, and CPK23, and subsequent phosphorylation/activation of the SLAC1 anion channel that is essential for anion efflux and stomatal closure. Mutants selected for this study are shown. Detailed descriptions of the mutants are provided in Table I. [See online article for color version of this figure.]
Table I. Descriptions of the mutants used in the study.
EMS, Ethyl methanesulfonate; T-DNA, transfer DNA.
| Genotype | Mutation | Description | Reference |
|---|---|---|---|
| ABA biosynthesis and catabolism | |||
| aba3-1 (Col-0) | EMS, G to A at position 3,707 | Defective in the conversion of ABA-aldehyde to ABA, ABA deficient | Léon-Kloosterziel et al. (1996); Nambara and Marion-Poll (2005) |
| aba1-1 (Ler) | EMS, G to A at position 2,139 (premature stop codon) | Defective in the ABA biosynthetic enzyme zeaxanthin epoxidase, strongly ABA deficient | Rock and Zeevaart (1991); Nambara and Marion-Poll (2005) |
| cyp707a1 (Col-0) | SALK_069127 | Defective in ABA 8′-hydroxylase, responsible for ABA catabolism in guard cells | Okamoto et al. (2009) |
| cyp707a3 (Col-0) | SALK_078173 | Defective in ABA 8′-hydroxylase, responsible for ABA catabolism in vascular tissues | Okamoto et al. (2009) |
| Core ABA signaling | |||
| abi1-1C (Col-0) | EMS, Gly-180 to Asp | Dominant point mutation in ABI1 resulting in the loss of PYR/RCAR binding and ABA insensitivity | Nishimura et al. (2004); Umezawa et al. (2009) |
| abi1-1L (Ler) | EMS, Gly-180 to Asp | See abi1-1C | Leung et al. (1997); Ma et al. (2009) |
| abi2-1C (Col-0) | EMS, Gly-168 to Asp | Dominant point mutation in ABI2, resulting in the loss of PYR/RCAR binding and ABA insensitivity | Nishimura et al. (2004) |
| abi2-1L (Ler) | EMS, Gly-168 to Asp | See abi2-1C | Leung et al. (1997); Ma et al. (2009) |
| abi1-2 hab1-1 pp2ca-1 (Col-0) | SALK_072009, SALK_002104, SALK_028132 | Triple knockout mutant of PP2Cs ABI1, HAB1, and PP2CA | Rubio et al. (2009) |
| hab1G246D (Col-0) | Transgenic line | Overexpression of HAB1 carrying the G246D mutation that prevents the binding to PYR/PYL and ABA insensitivity | Robert et al. (2006); Dupeux et al. (2011) |
| ost1-2 (Ler) | EMS, G to A at position 97 | Point mutation in ABA-activated protein kinase OST1 | Mustilli et al. (2002) |
| ost1-3 (Col-0) | SALK_008068 | = srk2e = snrk2.6, T-DNA knockout mutation of ABA-activated protein kinase OST1 | Yoshida et al. (2002) |
| pyr1pyl1pyl4 (114) (Col-0) | EMS + T-DNA | Triple mutant of ABA receptor proteins | Park et al. (2009) |
| pyr1pyl4pyl5 (145) (Col-0) | EMS + T-DNA + transposon | Triple mutant of ABA receptor proteins | Gonzalez-Guzman et al. (2012) |
| pyr1pyl4pyl8 (148) (Col-0) | EMS + T-DNA | Triple mutant of ABA receptor proteins | Gonzalez-Guzman et al. (2012) |
| pyl4pyl5pyl8 (458) (Col-0) | T-DNA + transposon | Triple mutant of ABA receptor proteins | Gonzalez-Guzman et al. (2012) |
| pyr1pyl1pyl2pyl4(1124C) (Col-0) | EMS + T-DNA | Quadruple mutant of ABA receptor proteins | Park et al. (2009) |
| pyr1pyl1pyl2pyl4 (1124L) (Ler) | EMS + T-DNA | Quadruple mutant of ABA receptor PYR/PYL/RCAR proteins | Park et al. (2009) |
| pyr1pyl4pyl5pyl8(1458) (Col-0) | EMS + T-DNA + transposon | Quadruple mutant of ABA receptor proteins | Gonzalez-Guzman et al. (2012) |
| pyr1pyl2pyl4pyl5pyl8(12458) (Col-0) | EMS + T-DNA + transposon | Pentuple mutant of ABA receptor proteins | Gonzalez-Guzman et al. (2012) |
| pyr1pyl1pyl2pyl4pyl5pyl8 (112458) (Col-0) | EMS + T-DNA + transposon | Sextuple mutant of ABA receptor proteins | Gonzalez-Guzman et al. (2012) |
| Other mutants | |||
| cpk21 (Col-0) | GABI_322A03 | Defective in CPK21 | This study (Supplemental Fig. S9) |
| cpk23 (Col-0) | SALK_007958 | Defective in CPK23 | Geiger et al. (2010) |
| slac1-3 (Col-0) | SALK_099139 | T-DNA insertion in SLAC1 | Vahisalu et al. (2008) |
| slac1-7 (Col-0) | C to T at position 527 | Point mutation of Ser-120 to Phe in SLAC1 | Vahisalu et al. (2010) |
Stomatal responses of several of these mutants to environmental factors have been studied previously, although often with different results. For example, initial in vitro studies indicated that OST1 was not involved in CO2-induced stomatal signaling, as the stomata of ost1-1 and ost1-2 behaved like the wild type in response to low CO2 (Mustilli et al., 2002). Similarly, mutations in OST1 did not affect stomatal regulation by light, leading to the suggestion that OST1 is specifically involved in ABA signaling (Mustilli et al., 2002). However, recently, it was shown that OST1 is a major regulator of CO2-induced stomatal closing and activation of the S-type anion channels in guard cells (Xue et al., 2011). Furthermore, dominant hypermorphic abi1-1 and abi2-1 mutations, which generate mutant PP2Cs that are refractory to inhibition by PYR/RCAR receptors (Ma et al., 2009; Park et al., 2009; Umezawa et al., 2009), have been used to address whether ABA-, CO2-, and light-induced stomatal signaling pathways converge. Stomatal opening induced by light and CO2 removal was 50% reduced in abi1-1 and abi2-1 plants; however, it was concluded that this might have been caused by constitutively more open stomata of these mutants (Leymarie et al., 1998a, 1998b). Other studies showed that light-induced stomatal opening was not disrupted in abi1-1 and abi2-1 mutants (Roelfsema and Prins, 1995; Eckert and Kaldenhoff, 2000). In contrast, Webb and Hetherington (1997) found that abi1-1 and abi2-1 did not respond clearly to elevated CO2, whereas the stomatal closure was indistinguishable from the wild type in ABA-deficient plants.
The involvement of the ABA signalosome in the regulation of the stomatal response to reduced air humidity is also disputed. During a genetic screen for mutants involved in the stomatal response to reduced air humidity, new alleles for OST1 and ABA2, an enzyme involved in ABA biosynthesis, were identified, suggesting that OST1 activity and ABA biosynthesis are essential for low-humidity-induced stomatal closure (Xie et al., 2006). Furthermore, activation of OST1 is induced by low-humidity stress (Yoshida et al., 2006). Contrarily, aba1, abi1-1, and abi2-1 mutants had wild-type stomatal responses to reduced humidity (Assmann et al., 2000). Since partial response to low humidity is observed in all studied mutants of the ABA signalosome, the existence of a separate ABA-independent pathway mediating low-humidity-induced stomatal closure has been proposed (Xie et al., 2006).
A large amount of data for stomatal signaling is collected by using isolated leaf epidermises or guard cell protoplasts, whereas the significance of these results is not always tested in intact plants. Thus, gas-exchange analysis of whole-plant and leaf stomatal responses is important in verifying the stomatal responsiveness to environmental stimuli. In this study, we used a custom-made gas-exchange device with parallel recording of stomatal responses of up to eight Arabidopsis (Arabidopsis thaliana) plants to various environmental factors (Kollist et al., 2007; Vahisalu et al., 2008, 2010). We chose mutants where different components of the ABA signalosome are affected (Fig. 1) to address the role of ABA signaling through PYR/RCAR proteins in stomatal responses to reduced air humidity, darkness, and elevated CO2 and O3 concentrations. We found that ABA signaling through PYR/RCARs is clearly required for rapid stomatal closure in response to darkness, O3, and reduced air humidity, while it is also partially involved in stomatal responses to elevated CO2. Since functional OST1 and SLAC1 were important in response to all stimuli, we discuss the possibility that other signaling elements besides ABA and its signalosome are able to activate OST1 in response to changes in CO2.
RESULTS
To study the role of the ABA signalosome in the regulation of stomatal responses to darkness, CO2, reduced humidity, and O3, we used mutants where different proteins of the ABA signalosome were affected (Fig. 1). If possible, at least two mutants for each protein were analyzed, and in many cases, mutants from different genetic backgrounds, Columbia (Col-0) and Landsberg erecta (Ler), were used in parallel (Table I). Representative photographs of plants used for gas-exchange measurements (Supplemental Fig. S1) show that even mutants with high stomatal conductance (gst) were healthy and nonwilted in our growth conditions.
ABA Signaling through PYR/RCAR Receptors Plays a Fundamental Role in Controlling Plant Steady-State gst
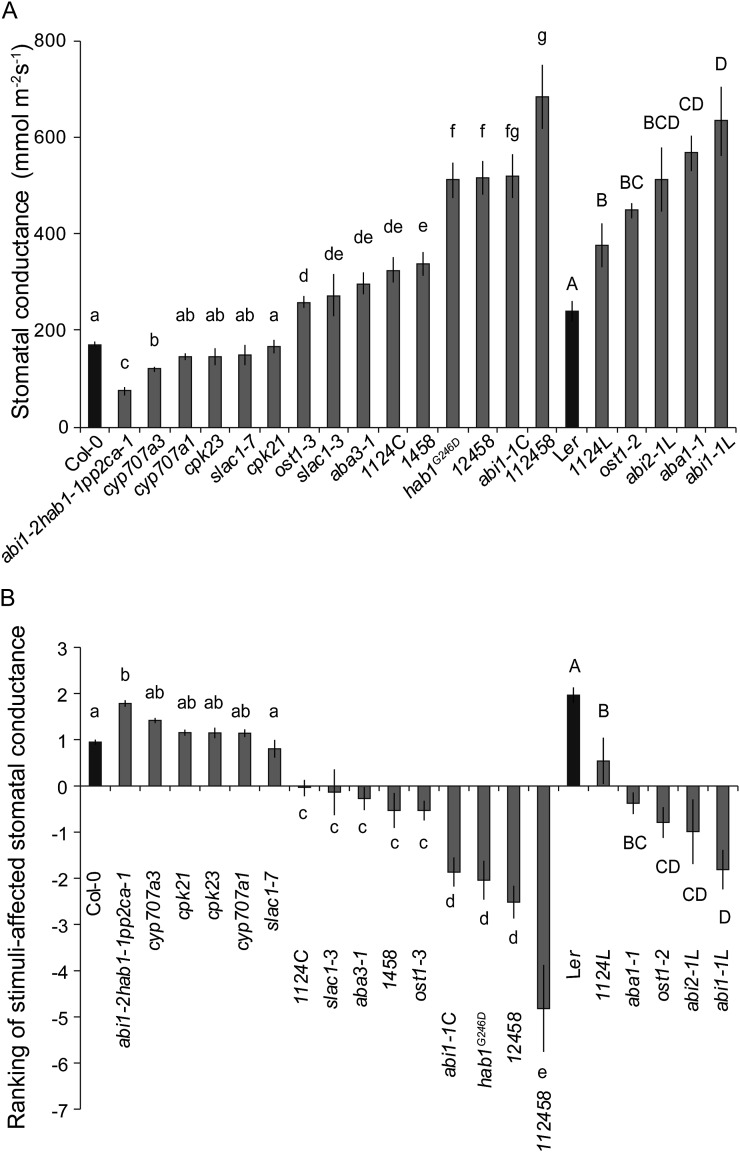
Whole-rosette gst varied severalfold among the studied mutants, ranging from 74 mmol m−2 s−1 in a triple loss-of-function mutant of ABI1, HAB1, and PP2CA, abi1-2hab1-1pp2ca-1, to 683 mmol m−2 s−1 in the sextuple PYR/RCAR loss-of-function mutant pyr1pyl1pyl2pyl4pyl5pyl8, abbreviated as 112458 (Fig. 2A). The difference in gst between two wild types, Ler and Col-0, was 1.4-fold. The gst values of 112458, the PYR/RCAR pentuple mutant pyr1pyl2pyl4pyl5pyl8 (12458), and dominant active PP2C mutants (abi1-1L and abi2-1L in Ler and abi1-1C and hab1G246D in Col-0) were highest, followed by ABA-deficient aba1-1 and aba3-1 (Fig. 2A). The hab1G246D mutation is analogous to abi1-1 and abi2-1 mutations and causes resistance to ABA-dependent inhibition by PYR/RCAR receptors, leading to strong ABA insensitivity (Robert et al., 2006; Dupeux et al., 2011). It should be noted that ABA biosynthesis mutants have reduced, but not abolished, ABA synthesis, with aba1-1 and aba3-1 still having 3% and 10% of the wild type ABA in stressed leaves, respectively (Rock and Zeevaart, 1991; Léon-Kloosterziel et al., 1996). Plants carrying loss-of-function mutations in SLAC1 and OST1 (slac1-3, ost1-2, and ost1-3) as well as in four PYR/RCAR receptors, pyr1pyl4pyl5pyl8 (1458) and pyr1pyl1pyl2pyl4 (note that we present data for this mutant in the Col-0 and Ler backgrounds, abbreviated as 1124C and 1124L, respectively), also exhibited significantly higher gst than the corresponding wild types. Gradual removal of PYR/RCAR receptor proteins had an increasing effect on gst. In two mutants with elevated ABA concentrations due to defective ABA catabolism (cyp707a1 and cyp707a3; Okamoto et al., 2009), only cyp707a1 had significantly reduced gst. OST1-dependent phosphorylation of Ser-120 in SLAC1 is critical for channel activation in Xenopus laevis oocytes (Geiger et al., 2009) and in O3-induced plant stomatal response (Vahisalu et al., 2010). However, the gst of slac1-7, with an S120F point mutation, was similar to that of Col-0. CPK21 and CPK23 are other kinases shown to activate SLAC1 in X. laevis oocytes (Geiger et al., 2010); however, gst of cpk21 and cpk23 did not differ from that of wild-type plants (Fig. 2A). Together, these results unequivocally demonstrate that mutations in the ABA signalosome have a major effect on the whole-plant stomatal conductivity and loss of water.
Figure 2.
A, There is a large variation in whole-plant steady-state stomatal conductance of plants with mutations in ABA signalosome. The average stomatal conductance values of three- to four-week-old mutants and corresponding wild types. Significant differences (P < 0.05, n = 6-46) are denoted with different small and capital letters for Col-0- and Ler-based mutants, respectively. B, Ranking of genotypes by their stomatal conductance after application of 1 h darkness, elevated CO2 and reduced humidity derived from principal component analysis. Significant differences (P < 0.05, n = 6\x{2013}46) are denoted with different small and capital letters for Col-0 and Ler-based mutants, respectively.
To address whether the differences in gst persisted after the application of 1 h of darkness, elevated CO2, and reduced humidity, we combined gst values of all mutants into one principal component analysis (PCA) axis. This PCA axis describes gst in stimuli-affected conditions. The ranking of genotypes by gst in stimuli-affected conditions (Fig. 2B) did not reveal any major differences from gst values in normal prestimuli conditions (Fig. 2A): although there were some relocations (e.g. ost1-3 and 1124C), the groups of statistical significance remained unchanged. The result that plants with more open stomata remain more open even after receiving a signal to close may indicate either that stomatal closure is impaired due to the given mutation or that the absolute extent of stomatal closure induced by these stimuli is generally limited.
Data Analysis
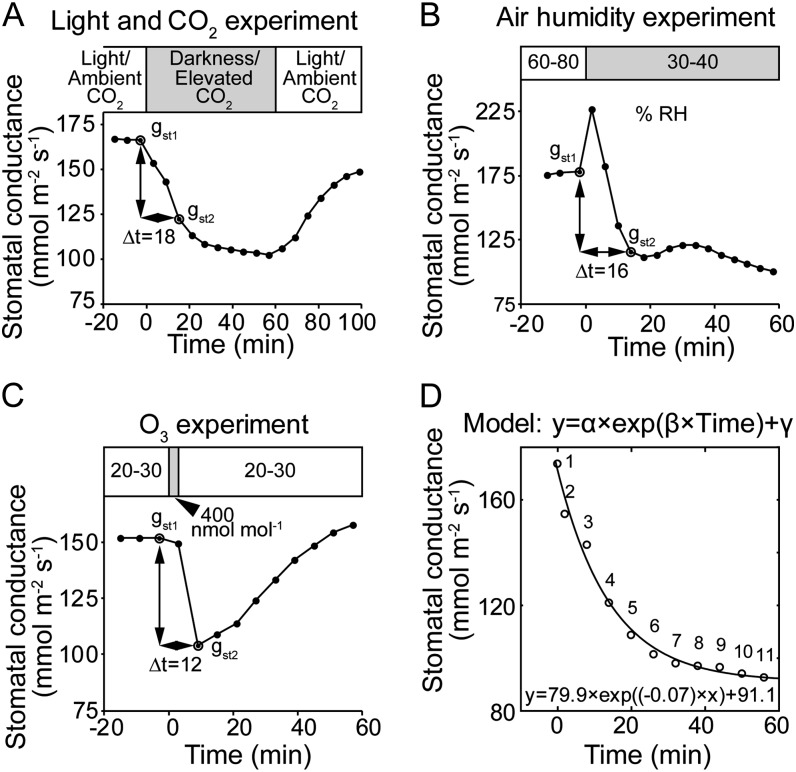
Since the number of studied mutants was high, we determined two characteristics describing stomatal closure and enabling quantitative comparisons of different genotypes. First, we calculated changes of gst as (gst2 − gst1)/(t2 − t1), as shown in Figure 3, A to C. Since t2 − t1 is a similar time interval for all mutants (Fig. 3, A–C), this number describes the initial stomatal response to the applied factor (i.e. the magnitude of change in gst). This characteristic can be effectively applied to all mutants. Stomatal closure in response to darkness, elevated CO2, and reduced air humidity of wild-type plants followed an exponential function (Fig. 3D). Thus, to provide a value describing the stomatal response kinetics of different genotypes, an exponential function was fitted to their stomatal closure responses and the maximum stomatal closure rate was calculated (Fig. 3D). However, several mutants either did not have clear stomatal responses to applied stimuli or their stomatal responses did not reach a stable phase within the time frame in which the experiments were carried out (e.g. slac1-3 and ost1-3 in Fig. 4, A and C, as examples, respectively). In such cases, fitting stomatal response to exponential function was not possible, and we interpreted this as an indication that the stomatal closure was affected due to the respective mutation. Data summarizing the results of exponential fitting are shown in Table II and Supplemental Figure S3.
Figure 3.
A to C, Representative time courses of gst in response to darkness and CO2 enrichment (A), decreased air humidity (B), and O3 pulse (C) are given to illustrate the calculation of initial changes in stomatal conductance. D, An example of fitting the kinetics of stomatal closure with exponential function, where α describes the overall stomatal response and γ is the value at which gst stabilized in response to stimuli. In case the stomatal closure significantly followed an exponential function, maximum stomatal closure rate was calculated as α × β. RH, Relative humidity.
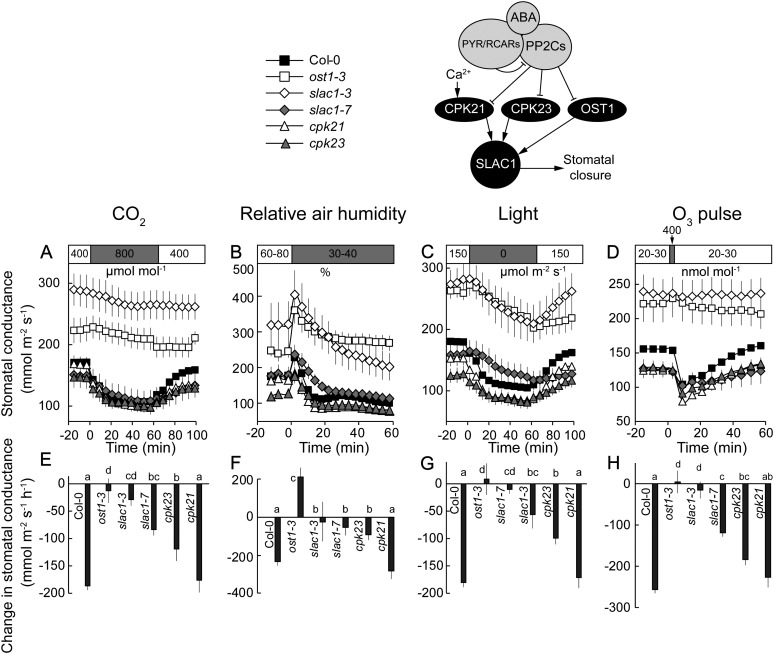
Figure 4.
Time courses of gst in response to elevated CO2 (A), reduced air humidity (B), darkness (C), and O3 pulse (D) together with corresponding changes in gst values (E–H) of the plants carrying mutations in the SLAC1 anion channel and protein kinases (OST1, CPK21, and CPK23) shown to activate SLAC1. Changes in gst (E–H) were calculated as shown in Figure 3, A to C. Significant differences (P < 0.05; n = 5–50) are denoted with different lowercase letters.
Table II. Results of fitting exponential functions to stomatal closure patterns in response to darkness, elevated CO2, and reduced air humidity.
The first number shows how many closure responses could be described with an exponential function, whereas the second number shows the total number of experiments. Boldface values indicate cases where 50% of experiments did not follow an exponential function.
| Genotype | Darkness Response | Humidity Response | CO2 Response |
|---|---|---|---|
| Col-0 | 34/34 | 21/27 | 45/46 |
| slac1-3 | 3/7 | 2/6 | 3/7 |
| ost1-3 | 1/6 | 0/6 | 2/6 |
| 112458 | 1/5 | 2/8 | 3/7 |
| abi1-1C | 3/5 | 5/7 | 1/5 |
| slac1-7 | 0/6 | 4/7 | 4/6 |
| 1458 | 3/6 | 5/5 | 6/8 |
| 12458 | 2/7 | 6/6 | 5/8 |
| 148 | 2/5 | 3/6 | 5/5 |
| 1124C | 6/6 | 4/8 | 8/8 |
| cpk23 | 5/6 | 3/7 | 6/7 |
| abi1-2hab1-1pp2c-1 | 5/5 | 2/5 | 7/7 |
| hab1G246D | 3/5 | 3/5 | 9/10 |
| 458 | 5/5 | 6/6 | 6/6 |
| 114 | 5/5 | 5/5 | 5/5 |
| 145 | 4/5 | 6/6 | 6/6 |
| aba3-1 | 4/6 | 5/6 | 6/6 |
| cpk21 | 6/6 | 5/5 | 7/7 |
| cyp707a1 | 8/8 | 5/6 | 7/7 |
| cyp707a3 | 6/6 | 5/6 | 6/6 |
| Ler | 12/12 | 9/10 | 11/12 |
| ost1-2 | 0/6 | 2/6 | 2/8 |
| abi2-1L | 2/6 | 2/6 | 2/7 |
| aba1-1 | 1/7 | 6/6 | 3/6 |
| abi1-1L | 2/6 | 2/6 | 6/8 |
| 1124L | 3/6 | 1/6 | 6/7 |
For clarity and to indicate at which step CO2-, darkness-, reduced humidity-, and O3-induced stomatal closures diverge, we present the mutants of ABA signaling and SLAC1 activation from the bottom up, starting from SLAC1 anion channel regulation and moving stepwise to mutants defective in PP2C phosphatases, PYR/PYL proteins, and, finally, ABA biosynthesis and catabolism.
SLAC1 and OST1 Are Required for Rapid Stomatal Closure in Response to All Studied Stimuli
Loss-of-function mutations in the SLAC1 anion channel and its main regulator, the protein kinase OST1, led to significantly impaired stomatal responses to darkness, high CO2, reduced air humidity, and O3, and the corresponding initial changes of gst were significantly lower than in the wild type (Fig. 4; Supplemental Fig. S2). In all genotypes, an initial sudden increase in gst was detected after the transition from humid to dry air. This is caused by a rapid increase in water evaporation from the epidermal cells and a concomitant decrease of their pressure on guard cells (Ivanoff, 1928). Due to this effect and the extremely slow closing response, the gst of OST1 loss-of-function mutants remained higher after 1 h in dry air compared with humid air (Fig. 4B; Supplemental Fig. S2B). Humidity- and darkness-induced stomatal closures were the only responses where significant differences between SLAC1 and OST1 loss-of-function plants were detected (Fig. 4, F and G).
The stomata of slac1-7 closed significantly less than those of Col-0 in response to all stimuli, confirming that the phosphorylation of Ser-120 was important for SLAC1 activation (Fig. 4; Geiger et al., 2009; Vahisalu et al., 2010). However, both the wild-type-like absolute gst (Fig. 2A) and the clearly weaker phenotype in CO2- and O3-induced stomatal responses of slac1-7 compared with those observed in slac1-3 (Fig. 4, E and H) suggest that the phosphorylation of Ser-120 does not fully explain the activation mechanism of SLAC1. The maximum stomatal closure rate of slac1-7, derived by fitting the kinetics of CO2-induced stomatal closure with exponential function, yielded values lower than in the wild type (Supplemental Fig. S3B). These results confirm that, in addition to O3-induced stomatal closure (Vahisalu et al., 2010), SLAC1 Ser-120 is the target for OST1 in CO2-, reduced humidity-, and darkness-induced responses as well, but they also suggest that for full SLAC1 activation, phosphorylation of multiple Ser residues either by OST1 or in combination with other protein kinases shown to activate SLAC1 in X. laevis oocytes (Geiger et al., 2010; Brandt et al., 2012; Hua et al., 2012; Scherzer et al., 2012) is needed.
To address the role of CPK21 and CPK23, Ca2+-related protein kinases shown to activate SLAC1 in X. laevis oocytes (Geiger et al., 2010), cpk21 and cpk23 plants were used. The stomata of cpk21 responded to the studied stimuli like the wild type, whereas the initial changes of gst of cpk23 were significantly reduced (Fig. 4, E–H), although much less than those of slac1-3 and ost1-3. Fitting the kinetics of stomatal closure with exponential function further confirmed that darkness- and humidity-induced stomatal closure is impaired in cpk23 (Table II; Supplemental Fig. S3A).
In conclusion, our results indicate that OST1-induced phosphorylation of SLAC1 is needed for rapid stomatal closure in response to all studied stimuli. Additionally, CPK23 is required, although to a minor extent.
PP2Cs Are Important for Rapid O3- and Humidity-Induced Stomatal Closure But Less Important for Darkness- and CO2-Induced Closure
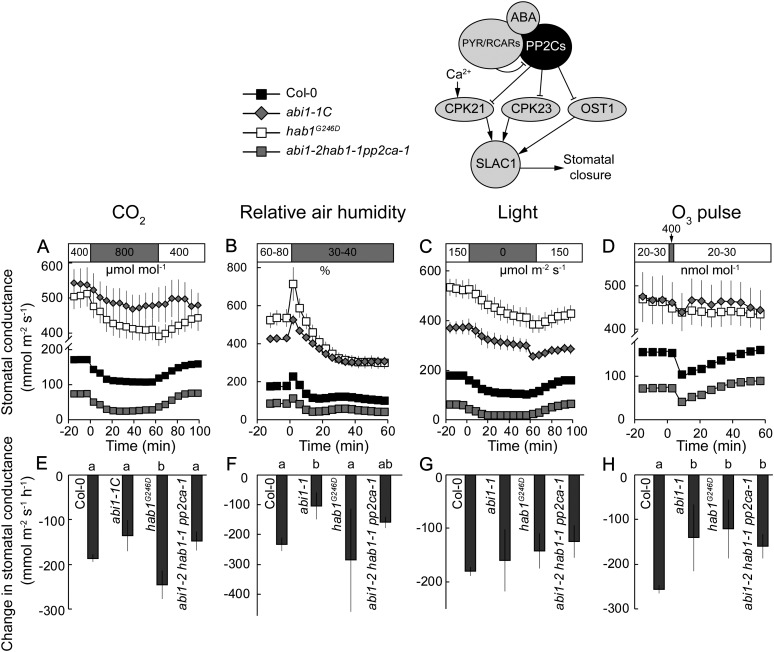
PP2Cs function as negative regulators in ABA-induced stomatal closure by inhibiting the OST1- and CPK-induced activation of SLAC1 (Geiger et al., 2009, 2010; Brandt et al., 2012). In the presence of ABA, their activity is suppressed by PYR/RCAR receptors. We used plants carrying dominant active abi1-1, abi2-1, and hab1G246D mutations that prevent ABA-dependent inhibition of PP2Cs by PYR/RCAR receptors. Humidity- and O3-induced stomatal responses and initial changes of gst were reduced in abi1-1C (Fig. 5, B, F, D, and H) and abi1-1L and abi2-1L (Supplemental Fig. S4, B, F, D, and H) as compared with their wild types, whereas in hab1G246D, only the O3 response was reduced (Fig. 5, D and H). Thus, for O3- and humidity-induced stomatal closure, the inhibition of PP2C activity is important.
Figure 5.
Time courses of gst in response to elevated CO2 (A), reduced air humidity (B), darkness (C), and O3 pulse (D) together with corresponding changes in gst values (E–H) in dominant mutants of protein phosphatases ABI1 and HAB1 (abi1-1C and hab1G246D) and in the triple knockout mutant of ABI1, HAB1, and PP2CA (abi1-2hab1-1pp2ca-1). Changes in gst values (E–H) were calculated as shown in Figure 3, A to C. Significant differences (P < 0.05; n = 5–50) are denoted with different lowercase letters.
The role of PP2Cs in CO2- and darkness-induced stomatal closure was less clear. The initial changes in gst of dominant abi1-1C and hab1G246D mutants in the Col-0 background (Fig. 5, A, E, C, and G) and similarly those of abi1-1L and abi2-1L in the Ler background (Supplemental Fig. S4, A, E, C, and G) were generally wild type like. Similar results were obtained for plants with abi1-1 and abi2-1 mutations in the Col-0 and Ler backgrounds when analyzing elevated CO2-induced stomatal closure with a leaf gas-exchange analyzer (Supplemental Fig. S5). However, obtained patterns of stomatal closure could not be described with an exponential function in several cases, such as the darkness responses of abi1-1L and abi2-1L and the CO2 responses of abi1-1C and abi2-1L (Table II), suggesting that the closure responses of these mutants were different from their wild types.
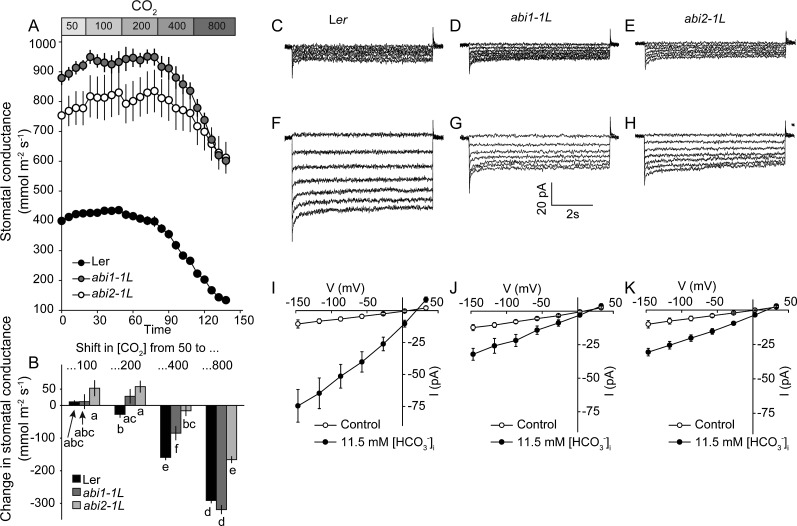
To further address the roles of ABI1 and ABI2 phosphatases for CO2-induced stomatal regulation, two additional experiments were performed. First, in a separate gas-exchange experiment, abi1-1L, abi2-1L, and Ler stomatal responses were tested within a wider range of CO2 concentrations. Plants were first acclimatized under 50 μL L−1 CO2 until stable gst values were reached (75–100 min). Thereafter, CO2 concentration was increased stepwise to 100, 200, 400, and 800 μL L−1 in 30-min intervals. Such treatment induced clear stomatal closure in all three genotypes (Fig. 6A); however, when the stomatal closures caused by each additional step in CO2 concentration were determined, differences between the genotypes emerged (Fig. 6B). There was no reduction in gst within the CO2 range from 50 to 100 μL L−1. Furthermore, from 50 to 200 μL L−1, a decrease in gst was observed only in Ler. Change from 50 to 400 μL L−1 CO2 caused a closure response in all genotypes; however, the decrease in gst was significantly smaller in abi2-1L than in abi1-1L, and this difference between abi1-1L and abi2-1L remained at 800 μL L−1, where the CO2-induced decrease in gst was similar in Ler and abi1-1L but significantly lower in abi2-1L. Second, recent research showed that β-carbonic acid anhydrases function early in CO2-induced stomatal closure (Hu et al., 2010) and that bicarbonate (HCO3−) is an important intracellular signal that triggers the activation of S-type anion channels in Arabidopsis guard cells (Xue et al., 2011). To further address the roles of ABI1 and ABI2 in CO2-induced stomatal signaling, HCO3−-induced activation of S-type anion currents was measured in abi1-1L and abi2-1L. Guard cell protoplasts from abi1-1L and abi2-1L displayed clearly reduced but still functional HCO3−-induced activation of anion currents (Fig. 6, C–K).
Figure 6.
Dominant mutations in ABI1 and particularly in ABI2 phosphatase cause partial impairment of CO2-induced stomatal responses, whereas bicarbonate-induced activation of S-type anion channels is reduced in both abi1-1L and abi2-1L guard cell protoplasts. A, Time courses of stomatal conductances in response to stepwise change of CO2 from 50 to 800 μL L-1 in abi1-1L, abi2-1L and Ler plants (n = 6). B, Changes in stomatal conductance induced by each step of [CO2]. C, D, E, Typical whole-cell recording without bicarbonate and F, G, H, with 11.5 mM free bicarbonate added to the pipette solution in the guard cell protoplasts of Ler wild type and abi1-1L and abi2-1L. Average steady-state current-voltage relationships for Ler (open circles, n = 6; filled circles, n = 7), abi1-1L (open circles, n = 6; filled circles, n = 7) and abi2-1L (open circles, n = 5; filled circles, n = 8) guard cell protoplasts are shown in I, J, and K, respectively.
In conclusion, PP2Cs are important for stomatal closure in response to reduced humidity and O3, and they also participate in darkness- and CO2-induced responses.
Removal of Six PYR/RCAR Receptor Proteins Impairs Plant Stomatal Responsiveness to O3, Reduced Humidity, Elevated CO2, and Darkness
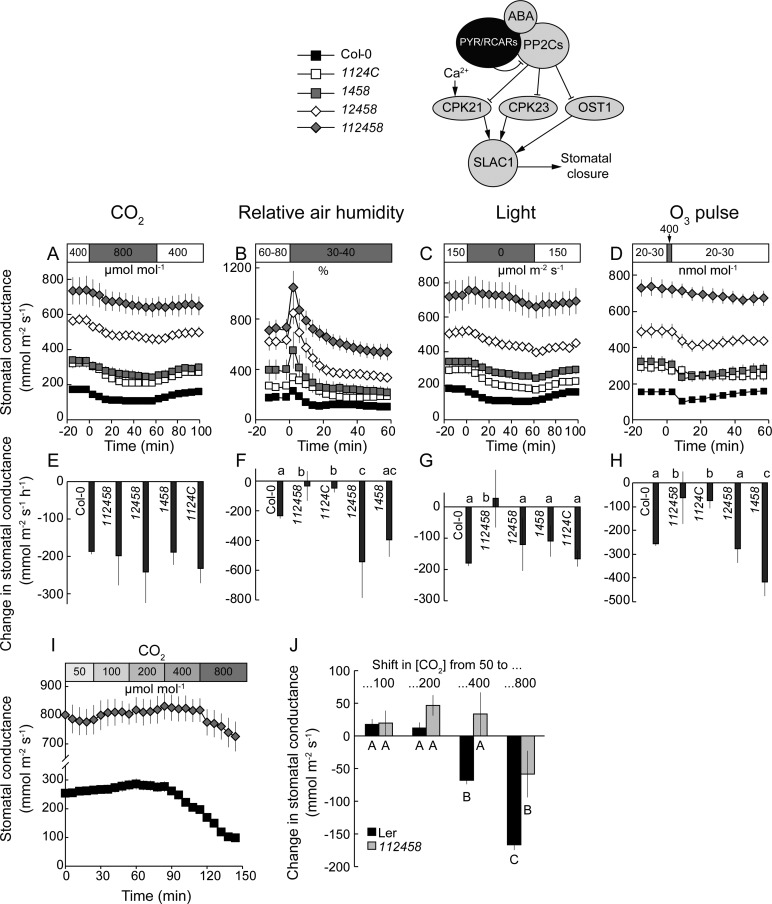
Gradual removal of PYR/RCAR receptor proteins increasingly impaired the whole-plant stomatal responsiveness to environmental factors; the sextuple 112458 PYR/RCAR mutant showed the strongest effect and displayed significantly impaired stomatal responses (Fig. 7, A–D) and reduced initial changes of gst due to all stimuli, except for elevated CO2 (Fig. 7, E–H). Additionally, patterns of 112458 stomatal closure did not follow an exponential function in darkness, CO2, and humidity experiments (Table II), suggesting that the lack of these proteins modified the fast kinetics of stomatal closure in response to these stimuli. A separate gas-exchange experiment with stepwise increases in CO2 concentration also revealed that CO2-induced stomatal closure is reduced in 112458 compared with the wild type, particularly at lower CO2 concentrations (Fig. 7, I and J). Thus, the lack of six PYR/PYL proteins significantly impaired stomatal closure due to all studied factors. Quadruple PYR/RCAR mutants displayed impaired stomatal responsiveness as well; however, here, the differences from the wild type depended on the applied stimuli and the combination of PYR/RCAR mutations. For example, O3- and humidity-induced initial changes of gst in quadruple 1124C (Fig. 7, F and H) and 1124L (Supplemental Fig. S6, F and H) mutants were significantly reduced, whereas 1458 quadruple and even 12458 pentuple mutants showed similar or even higher than wild-type closures in response to reduced humidity and O3 (Fig. 7, F and H). These data indicate a functional diversity among PYR/RCAR proteins and suggest the importance of PYL1 for stomatal functioning. Recently, it was demonstrated that PYL1 played an important role in ABA-induced transcriptional response as well (Gonzalez-Guzman et al., 2012). Various combinations of triple loss-of-function PYR/PYL mutants, including pyr1pyl1pyl4, previously found to have higher steady-state gst values than the wild type (Gonzalez-Guzman et al., 2012), generally showed initial changes of gst that were wild type like or even larger than in the wild type (Supplemental Fig. S7). This suggests that a certain threshold of PYR/RCAR receptors is required in guard cells to trigger stomatal closure in response to environmental factors. Furthermore, the compensatory changes in the concentration/activity of other PYR/RCARs in triple loss-of-function PYR/RCAR mutants can explain why their initial rates of stomatal closure were sometimes higher than in the wild type.
Figure 7.
A to H, Time courses of gst in response to elevated CO2 (A), reduced air humidity (B), darkness (C), and O3 pulse (D) together with corresponding changes in gst values (E–H) in the loss-of-function mutants of PYR/RCAR receptors. Changes in gst values (E–H) were calculated as shown in Figure 3, A to C. Significant differences (P < 0.05; n = 5–50) are denoted with different letters. I, Time courses of gst in response to stepwise changes in CO2 from 50 to 800 μL L−1 in 112458 and Col-0 plants (n = 6). J, Changes in gst induced by each step of CO2.
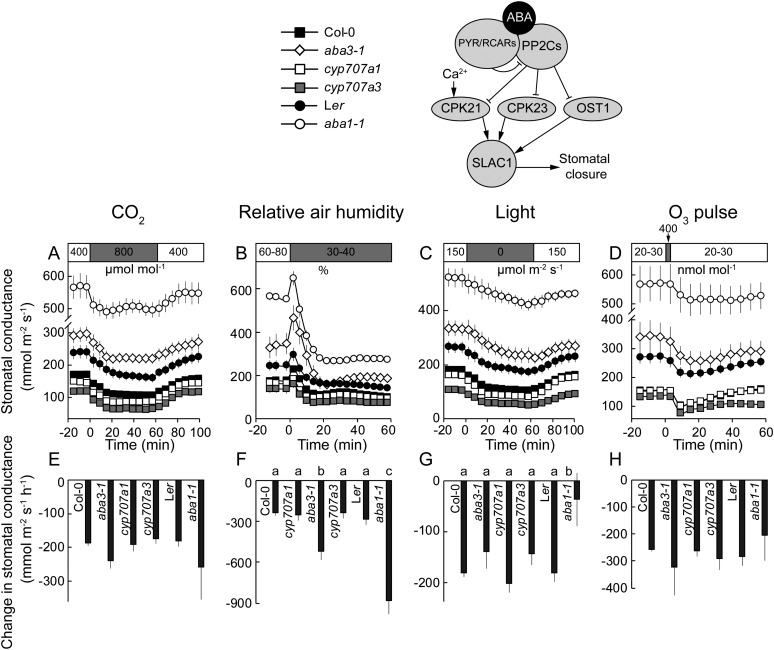
Elevated CO2, Reduced Air Humidity, Darkness, and O3-Induced Stomatal Closure of ABA Biosynthesis and Catabolism Mutants
Plants with mutations in ABA biosynthesis had weaker impairments of stomatal responses (Fig. 8) than those observed for plants impaired in ABA signaling (Figs. 4–7). The initial changes of gst in aba1-1 and aba3-1 did not differ from the Ler and Col-0 wild types, respectively (Fig. 8, E, G, and H). The only exception was significantly lower darkness-induced stomatal closure in aba1-1 than in its Ler wild type (Fig. 8G). Furthermore, the stomatal kinetics of aba1-1 did not follow an exponential function in darkness and CO2 experiments. ABA is most likely essential for proper plant development; hence, no null mutants for ABA biosynthesis have been isolated. Accordingly, residual amounts of ABA in aba1-1 and aba3-1 (Rock and Zeevaart, 1991; Léon-Kloosterziel et al., 1996; Xie et al., 2006) could be sufficient to activate ABA signaling, since half-maximal inhibition of PP2Cs takes place at nanomolar ABA concentrations in the presence of PYR/RCARs (Szostkiewicz et al., 2010).
Figure 8.
Time courses of gst in response to elevated CO2 (A), reduced air humidity (B), darkness (C), and O3 pulse (D) together with corresponding changes in gst values (E–H) in mutants of ABA biosynthesis (aba1-1 and aba3-1) and catabolism (cyp707a1 and cyp707a3). Changes in gst (E–H) were calculated as shown in Figure 3, A to C. Significant differences (P < 0.05; n = 5–50) are denoted with different lowercase letters.
Surprisingly, the initial reductions in gst due to the reduced humidity of ABA biosynthesis mutants were significantly larger than those of the respective wild types (Fig. 8F). This result is confirmed by significantly larger maximum stomatal closure rates derived from exponential curve fitting of aba1-1 and aba3-1 (Supplemental Fig. S3C). This was an unexpected but not unique result: Assmann et al. (2000) found that aba1-1 plants showed a greater than wild-type stomatal response to an increase in leaf-air vapor pressure difference from 0.4 to 0.7 kPa. Since submission of the original version of this manuscript, it was reported that guard cell-autonomous ABA biosynthesis is important for low humidity-induced stomatal responses (Bauer et al., 2013). However, our study suggests the alternative model where basal low ABA in aba3-1 and aba1-1 may enhance the low humidity-sensitivity of guard cells; possibly through increasing the sensitivity of ABA-signalosome. As compared with ABA-deficient mutants, mutants defective in ABA catabolism contain higher concentrations of ABA and could be predicted to respond more strongly to environmental stimuli. However, the initial changes in gst (Fig. 8) and maximum stomatal closure rates (Supplemental Fig. S3, A–C) of cyp707a1 and cyp707a3 were wild type like.
DISCUSSION
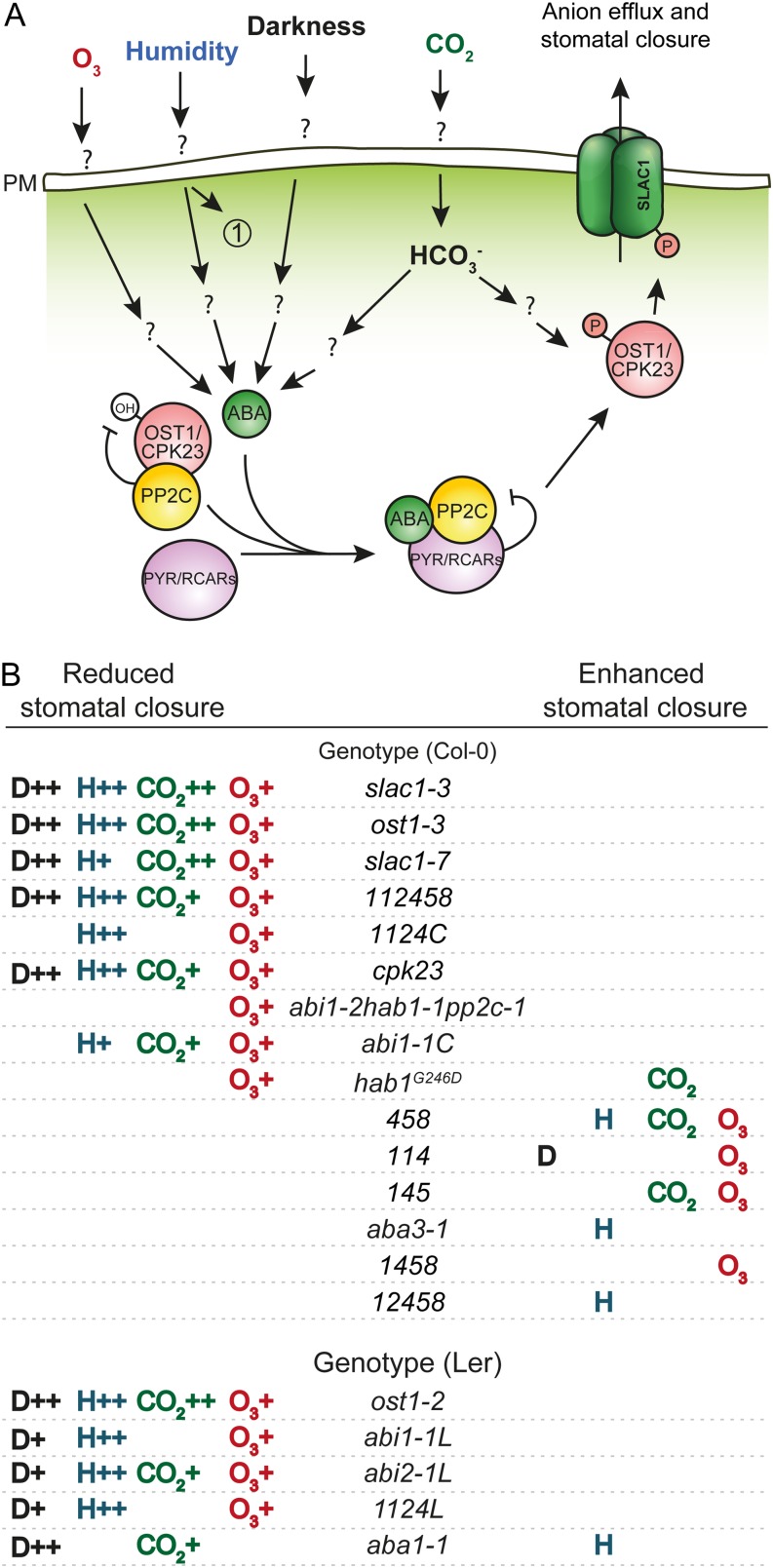
Rapid stomatal closure is one of the fastest responses in plant adaptation to sudden changes in environmental conditions. The finding that ABA perception by PYR/RCAR receptors leads to the inhibition of PP2C phosphatases has been a major breakthrough in plant science to understand ABA signaling and the regulation of stomatal aperture by ABA (Ma et al., 2009; Park et al., 2009). With these recent advances, the key question arises whether rapid changes in gst caused by physiological stimuli are affected by defined steps within the ABA signalosome in intact whole plants. Here, we have addressed the relevance of the ABA signalosome (Fig. 1) for the whole-plant steady-state gst and stomatal responses to darkness, reduced air humidity, elevated CO2, and O3 (i.e. environmental factors). As this study used a large number of mutants and four different factors, we have summarized the descriptions of mutants into one table (Table I) and their stomatal responses to studied environmental factors into a simplified figure (Fig. 9B) to help readers follow the main results of the study.
Figure 9.
Schematic model for environmental factor-induced stomatal closure (A) and summary of the stomatal responses of mutants characterized in this study (B). A, The emerging model suggests that in the case of O3, all components of the ABA signalosome are required to trigger stomatal closure. For reduced air humidity, we show that the ABA signalosome plays an important role; however, the presence of a parallel, ABA-independent pathway (marked as 1) was suggested by Assmann et al. (2000) and Xie et al. (2006). Darkness-induced stomatal closure is mediated by increased intercellular CO2 concentration that activates anion channels and by inactivation of H+-ATPase (Roelfsema et al., 2002; Shimazaki et al., 2007). CO2-induced stomatal closure involves the activation of carbonic acid anhydrases that convert CO2 to HCO3−. The results presented in this study suggest that CO2-induced stomatal closure and HCO3−-induced activation of S-type anion channels are partly controlled by the ABA signalosome. Question marks highlight that the nature of signal perception at the plasma membrane and signal transduction in the cytosol leading to the activation of the ABA signalosome remain to be addressed. B, Summary of stomatal responses to closure-inducing stimuli in the studied mutants. Mutants are presented in the order of phenotypic severity. H and D indicate air humidity and darkness, respectively. + indicates that either initial change in gst or curve fitting was different from the wild type, and ++ indicates that initial change in gst and curve fitting were both different from the wild type. The absence of a symbol indicates wild-type-like stomatal closure. Mutants that showed wild-type-like stomatal responses to all stimuli (148, cpk21, cyp707a1, and cyp707a3) are not listed here. [See online article for color version of this figure.]
Fundamental Role of the ABA Signalosome in Controlling Whole-Plant Steady-State gst
The gst values of the studied mutants varied severalfold. The whole-plant gst values were altered in accordance with the proposed functioning of the ABA signalosome: reduced ABA concentration, gradually reduced levels of functional PYR/RCAR proteins, the presence of dominant active PP2Cs, and the lack of functional OST1 and SLAC1 resulted in higher gst. Contrarily, reduced levels of functional PP2Cs in abi1-2hab1-1pp2ca-1 and higher ABA concentrations in cyp707a3 resulted in lower gst (Fig. 2). Previous gas-exchange experiments have also found that the mutants of the ABA signaling module have high gst (abi1-1 and abi1-2 [Leymarie et al., 1998a, 1998b]; aba1, abi1-1, and abi2-1 [Assmann et al., 2000]; and abi1-1, abi1-2, and ost1-3 [Xue et al., 2011]), whereas the reduced gst of triple loss-of-function PP2C mutants (hab1-1abi1-2pp2ca-1 and hab1-1abi1-2abi2-2) and ABA catabolism mutants (cyp707a1 and cyp707a3) was detected previously by Rubio et al. (2009) and Okamoto et al. (2009), respectively. However, when the stomatal apertures from extracted epidermal fragments have been measured, a larger aperture of open stomatal mutants is not always evident (ost1-1 and ost1-2 [Mustilli et al., 2002]; hab1G246D [Robert et al., 2006]; ost1-2 and abi1-1 [Siegel et al., 2009]; pyr1pyl1pyl2pyl4 [Nishimura et al., 2010]; and ost1-3 [Xue et al., 2011]). Earlier and more recent studies indicated that at least part of the stomatal responses to CO2 and light depend on signals generated by the mesophyll (Lee and Bowling, 1992; Mott et al., 2008). Thus, it is expected that mesophyll or signals from the mesophyll may also play a role in determining the plant steady-state gst.
Nevertheless, it is obvious that plant steady-state gst is not determined only by the signal flow from ABA to OST1 and the SLAC1 anion channel. For example, the gst values of dominant active PP2C mutants were almost two times higher than those of OST1 and SLAC1 loss-of-function mutants. Guard cell plasma membrane H+-ATPases, activated by phosphorylation, provide the driving force for stomatal opening (for review, see Shimazaki et al., 2007). The basal and blue light-induced phosphorylation of the guard cell H+-ATPase was higher in abi1-1 and abi2-1 than in the wild type (Hayashi et al., 2011), indicating that the higher gst of dominant active PP2C mutants could be caused by their higher H+-ATPase activity. It remains to be established whether high H+-ATPase activity also explains the highest gst of plants lacking six PYR/RCAR receptors and the mechanistic connection between the ABA signalosome and the phosphorylation of guard cell H+-ATPases.
PYR/RCARs and PP2Cs Are Important for O3-, Humidity-, and Darkness-Induced Rapid Stomatal Regulation and Are Involved in CO2-Induced Rapid Stomatal Regulation
The 112458 sextuple mutant of PYR/RCAR proteins is one of the most ABA-insensitive mutants described so far, being able to germinate and grow in the presence of 100 μm ABA (Gonzalez-Guzman et al., 2012).Strongly reduced darkness-, reduced air humidity-, and O3-induced stomatal closure of 112458 together with its altered CO2 response kinetics indicate that PYR/RCAR receptors influence the rapid initiation of stomatal closure by these stimuli. Furthermore, downstream components of the ABA signalosome (ABI2, OST1, and SLAC1) were required, since stomatal responses of these mutants were reduced (Fig. 9B). Only ABA biosynthesis mutants did not fit in, generally showing wild-type-like stomatal closure, but this can be explained by their residual ABA concentrations. Thus, rapid stomatal closure induced by reduced air humidity, darkness, elevated CO2, and O3 involves PYR/RCAR-dependent inhibition of PP2Cs, leading to the activation of OST1 and SLAC1.
For O3 and reduced humidity, the impairment of responses is clear in all key mutants (slac1-3, ost1-3, ost1-2, abi1-1C, abi1-1L, abi2-1L, 112458, 1124C, and 1124L). One possible mechanism contributing to the O3 response is the direct inhibition of ABI1 (Meinhard and Grill, 2001) and ABI2 (Meinhard et al., 2002) by hydrogen peroxide. However, in darkness and particularly CO2, stomatal closure was often evident in key mutants (except slac1-3, ost1-2, and ost1-3), and only further experiments and analysis of fast kinetics revealed that the closure response was impaired. The partial response of PYR/RCAR mutants can be explained by redundancy among 14 PYR/RCAR proteins, whereas regulation of the remaining PP2Cs by ABA and PYR/RCARs in abi1-1C, abi2-1C, hab1G246D, abi1-1L, and abi2-1L (Szostkiewicz et al., 2010) can explain partial CO2- and darkness-induced stomatal responses of dominant active PP2C mutants. An alternative explanation is that in response to CO2 and darkness, there might be an ABA-PYR/RCAR-PP2C-independent pathway for OST1 activation. In addition to ABA-dependent activation, ABA-independent activation of OST1 might be induced by osmotic and low-humidity stress (Xie et al., 2006; Yoshida et al., 2006; Boudsocq et al., 2007). Furthermore, many OST1-inducible genes are not responsive to ABA (Zheng et al., 2010).
Partial inhibition of HCO3−-induced activation of S-type anion currents in abi1-1L and abi2-1L guard cells, together with the result that the obtained CO2-induced stomatal closure patterns of abi1-1C and abi2-1L could not be described with an exponential function, indicate that these dominant active phosphatases can affect the stomatal response to CO2 (Fig. 6; Table II). It is of particular interest that the delay in CO2-induced stomatal closure was clearly stronger in abi2-1L than in abi1-1L (Fig. 6B). Recently, a new regulator, GHR1, involved in ABA- and hydrogen peroxide-induced activation of SLAC1 was identified (Hua et al., 2012). GHR1, a receptor-like kinase preferentially localized in guard cell plasma membranes, was shown to activate SLAC1 anion currents in X. laevis oocytes (Hua et al., 2012). Interestingly, GHR1 is regulated by ABI2 but not by ABI1 (Hua et al., 2012). Thus, the differential responses of abi1-1L and abi2-1L could be a result of their different roles in the regulation of GHR1.
OST1 and SLAC1 Are Important for Stomatal Closure by All Four Stimuli, But There Are Additional Components Whose Exact Roles in Plant Stomatal Regulation Remain to Be Clarified
CO2- and O3-induced stomatal closures were small, whereas darkness- and humidity-induced closures were delayed but still functional in SLAC1 loss-of-function plants (Negi et al., 2008; Vahisalu et al., 2008; Ache et al., 2010; Xue et al., 2011; this study). There are other anion channels that participate in stomatal closure together with SLAC1, including the membrane voltage-dependent rapid-type anion channel QUAC1 (Meyer et al., 2010) and SLAH3, another slow-type anion channel that is activated in X. laevis oocytes via phosphorylation by CPK21 (Geiger et al., 2011). It remains to be determined why, then, SLAC1 has a vital role in regulating CO2- and O3-induced stomatal closure (Fig. 4, A and D), whereas in humidity and darkness responses, QUAC1 or possibly SLAH3 could partly replace SLAC1 function (Fig. 4, B and C). Perhaps since darkness and drought, with accompanying decreases in air humidity, are important environmental factors that have affected plants in the evolutionary time scale, plants have developed parallel signaling pathways and ion channels mediating rapid stomatal closure in response to these factors. Furthermore, darkness-induced stomatal closure is accomplished through two different signaling pathways. First, it is mediated via phototropins and H+-ATPase (Shimazaki et al., 2007), since blue light, which activates phototropins, is part of visible light spectrum. Second, CO2 is an intermediate signal in the darkness response; photosynthesis immediately stops in darkness, resulting in increased intercellular CO2 concentration and activation of anion channels (Roelfsema et al., 2002). Thus, the partial darkness response of SLAC1 and OST1 loss-of-function mutants could be caused by signaling via phototropins and H+-ATPase, while the CO2-mediated signaling remains inactive.
It is also important to consider that in all studied ABA signalosome mutants, compensatory changes, either directly or indirectly related to the signalosome itself, could have taken place and affected the whole-plant stomatal response. For example, the regulation of PP2C activity can become less or more sensitive to ABA, depending on the PP2C:PYR/RCAR ratio (Szostkiewicz et al., 2010), representing compensation within the ABA signalosome. Besides guard cell anion channels, the ABA signalosome also regulates the activity of guard cell potassium channels (Armstrong et al., 1995; Sato et al., 2009). Recently, it was shown that plants with impaired SLAC1 have slowed stomatal opening in response to various stimuli and that this is caused by strongly reduced inward-rectifying K+ channel activity of slac1 mutants (Laanemets et al., 2013). These unexpected phenotypes of slac1 turned out to be caused by higher cytosolic pH and Ca2+ concentration (Wang et al., 2012) and increased Ca2+ sensitivity of inward-rectifying K+ channels in slac1 guard cells (Laanemets et al., 2013). These changes represent adaptive changes not directly related to the ABA signalosome and counteract the adverse effects of the slac1 mutation and allow the plant to maintain control over stomatal openness. Thus, while interpreting the results of this study, it is important to consider that some compensatory changes that also affect stomatal regulation have probably occurred in the studied mutants.
The calcium-dependent protein kinases CPK21 and CPK23 phosphorylate and activate SLAC1 similarly to OST1 (Geiger et al., 2010). The stomata of CPK23 loss-of-function plants showed slightly reduced responses to all environmental factors. Interestingly, Ma and Wu (2007) found that CPK23 acts as a positive regulator for stomatal opening: the stomatal apertures of epidermal peels were significantly decreased in the cpk23 loss-of-function mutant, resulting in enhanced drought and salt tolerance. In this study, whole-plant gst in the cpk23 mutant was similar to that in the wild type (Fig. 2A). Phosphorylation by CPK21 was found to activate both SLAC1 (Geiger et al., 2010) and SLAH3 (Geiger et al., 2011). However, no changes in stomatal responses to the studied stimuli were found in the cpk21 mutant. This may not be not surprising, considering that the family of Ca2+-dependent protein kinases is large, with possible redundancy in their function (Cheng et al., 2002). As an example, CPK3 and CPK6 were found to participate in ABA- and Ca2+-dependent regulation of guard cell S-type anion channels and stomatal closure (Mori et al., 2006). Recently, CPK6 was shown to strongly activate SLAC1-mediated anion currents in X. laevis oocytes and to allow the functional reconstitution of ABA activation of SLAC1 (Brandt et al., 2012). Interestingly, Ser-59 in the SLAC1 N terminus was phosphorylated by CPK6, and this phosphorylation is essential for SLAC1 activation (Brandt et al., 2012). Ser-59 was earlier shown to be phosphorylated also by OST1 (Vahisalu et al., 2010). The strong stomatal phenotypes of OST1 loss-of-function plants in the presence of many alternative kinases (CPK3, CPK6, CPK21, and CPK23) activating SLAC1 suggests that interaction of the Ca2+-dependent and Ca2+-independent pathways requires further investigation.
In conclusion, the signaling pathways of different stomatal closure-inducing factors converge at OST1 and SLAC1. In darkness-, O3-, and reduced air humidity-induced stomatal closure, signaling through PYR/RCAR receptors plays an important role. In response to elevated CO2, the ABA signalosome was partially involved, and the presence of a parallel, yet to be identified signaling pathway that activates OST1 is possible.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Arabidopsis (Arabidopsis thaliana) seeds were planted in soil containing 4:3 (v/v) peat:vermiculite and grown through a hole in a glass plate covering the pot as described (Kollist et al., 2007). Soil moisture was kept at 60% to 80% of maximum water capacity. Plants were grown in growth chambers (AR-66LX and AR-22L; Percival Scientific) in a 12/12-h photoperiod, 23°C/18°C temperature, 150 µmol m−2 s−1 light, and 70% to 75% relative humidity. For gas-exchange experiments, we used plants with a total rosette area between 5 and 15 cm2. This corresponds to 21- to 25-d-old plants for most mutant lines. However, some mutants (aba1-1, aba3-1, abi1-1C, and hab1G246D) had slower growth rates; thus, older plants (26–32 d old) were analyzed. The full list of the mutants used is given in Table I. Mutants were obtained from the European Arabidopsis Stock Centre (www.arabidopsis.info) and from Sean Cutler (114, 1124C, and 1124L). The mutants cpk21 (GABI_322A03), cpk23 (SALK_007958; Geiger et al., 2010), cyp707a1 (SALK_069127; Okamoto et al., 2009), and cyp707a3 (SALK_078173; Okamoto et al., 2009) were confirmed to be homozygous using PCR. The cpk21 knockout mutant was verified to lack full-length transcripts for CPK21 using reverse transcription-PCR (Supplemental Fig. S8).
Whole-Rosette gst Measurements
The Arabidopsis whole-rosette rapid-response gas-exchange measurement device was described previously (Kollist et al., 2007; Vahisalu et al., 2008). Plants were inserted into the device, and the treatments started about 2 h later, when gst had stabilized. Photographs of plants were taken before the experiment, and rosette leaf area was calculated using ImageJ 1.37v (National Institutes of Health). gst for water vapor was calculated with a custom-written program as described by Kollist et al., (2007).
In light-dark transition experiments, the gst values were first measured in light, and then darkness was applied for 60 min by covering the measuring cuvettes. In CO2 enrichment experiments, plants were kept in ambient CO2 concentration (400 μL L−1) until gst was stable, and then CO2 concentration was increased to 800 μL L−1 for 60 min. In darkness and elevated CO2 experiments, we also followed the reopening of stomata when light and ambient CO2 were restored. In O3 experiments, plants were exposed to 350 to 450 nL L−1 O3 for 3 min and kept in measuring cuvettes for 60 min after exposure. In reduced humidity experiments, plants were kept in humid air (relative humidity = 60%–80%), air humidity was abruptly reduced about two times (relative humidity = 30%–40%), and gst was followed for the next 56 min.
We present the time-resolved stomatal responses to stimuli in absolute units. Although the course of stomatal reopening in light and ambient CO2 is not discussed, it is presented in the figures for darkness and CO2 experiments. In order to provide a quantitative value for the initial stomatal responsiveness to applied stimuli, we calculated changes of gst as described in Figure 3, A–C. Furthermore, we fitted the patterns of gst values in response to darkness, elevated CO2, and reduced air humidity with exponential functions [gst = α × exp (β × time) + γ]. Fit was accepted as significant when both α and β were significantly different from 0 at P < 0.1; in this case, we calculated maximum stomatal closure rate as α × β (Fig. 3D).
Electrophysiology
Arabidopsis guard cell protoplasts were isolated as according to Siegel et al. (2009). Whole-cell patch-clamp recordings were performed as described previously (Pei et al., 1997). For S-type anion current recordings (Schroeder and Keller, 1992), the pipette solution contained 150 mm CsCl, 2 mm MgCl2, 6.7 mm EGTA, 5.86 mm CaCl2 (2 µm intracellular [Ca2+]), 5 mm Mg-ATP, and 1 mm HEPES-Tris, pH 7.1. The bath solution contained 30 mm CsCl, 2 mm MgCl2, 1 mm CaCl2, and 10 mm MES-Tris, pH 5.6. Osmolalities of all solutions were adjusted to 485 mmol kg−1 for bath solution and 500 mmol kg−1 for pipette solution by the addition of d-sorbitol. The membrane voltage was stepped from +35 mV to −145 mV with −30-mV decrements, and the holding potential was +30 mV. Liquid junction potential was determined using Clampex 10.0. No leak subtraction was applied for all current-voltage curves. Steady-state currents were the average currents during the last 500 ms of voltage pulses. For HCO3− activation of S-type anion currents, 13.5 mm total HCO3− (equivalent to 11.5 free intracellular [HCO3−] and 2 mm free intracellular [CO2]) was added freshly in the pipette solution. The details were described previously (Xue et al., 2011).
Statistical Analysis
Statistical analyses were performed with Statistica, version 7.0 (StatSoft). ANOVA (General Linear Models) was used to assess the effect of genotype on gst, initial changes in gst, and maximum stomatal closure rates, and comparisons between individual means were done using Fisher’s lsd test. Data were ln transformed when necessary. All effects were considered significant at P < 0.05. Exponential fitting of stomatal closure responses due to darkness, elevated CO2, and reduced air humidity was done with nonlinear least-squares model estimation of Statistica (Gauss-Newton estimation method). PCA was used to combine the values of gst after 1 h in darkness, elevated CO2, and reduced humidity into one PCA axis describing whole-plant gst after application of given stimuli.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Representative photographs of mutants and wild types.
Supplemental Figure S2. Time-courses of gst in response to stimuli and initial changes in gst values of ost1-2 and Ler.
Supplemental Figure S3. Maximum stomatal closure rates derived from exponential fitting of stomatal closure responses.
Supplemental Figure S4. Time-courses of gst in response to stimuli and initial changes in gst values of abi1-1L, abi2-1L and Ler.
Supplemental Figure S5. Time-courses of leaf gst in response to elevated CO2 in abi1-1 and abi2-1 in Col-0 and Ler backgrounds.
Supplemental Figure S6. Time-courses of gst in response to stimuli and initial changes in gst values of 1124L and Ler.
Supplemental Figure S7. Time-courses of gst in response to stimuli and initial changes in gst values of triple loss-of-function mutants of PYR/RCARs.
Supplemental Figure S8. The cpk21 T-DNA mutant is a transcriptional knockout.
Supplementary Material
Acknowledgments
We thank Dr. Heino Moldau for valuable scientific advice and discussions, the Arabidopsis Biological Resource Center/Nottingham Arabidopsis Stock Centre for distributing the seeds, and Sean Cutler for 114, 1124C, and 1124L seeds.
Glossary
- O3
ozone
- ABA
abscisic acid
- gst
stomatal conductance
- Col-0
Columbia
- Ler
Landsberg erecta
- PCA
principal component analysis
- HCO3−
bicarbonate
References
- Ache P, Bauer H, Kollist H, Al-Rasheid KA, Lautner S, Hartung W, Hedrich R. (2010) Stomatal action directly feeds back on leaf turgor: new insights into the regulation of the plant water status from non-invasive pressure probe measurements. Plant J 62: 1072–1082 [DOI] [PubMed] [Google Scholar]
- Armstrong F, Leung J, Grabov A, Brearley J, Giraudat J, Blatt MR. (1995) Sensitivity to abscisic acid of guard-cell K+ channels is suppressed by abi1-1, a mutant Arabidopsis gene encoding a putative protein phosphatase. Proc Natl Acad Sci USA 92: 9520–9524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore MR. (2005) Assessing the future global impacts of ozone on vegetation. Plant Cell Environ 28: 949–964 [Google Scholar]
- Assmann SM, Snyder JA, Lee Y-RJ. (2000) ABA-deficient (aba1) and ABA-insensitive (abi1-1, abi2-1) mutants of Arabidopsis have a wild-type stomatal response to humidity. Plant Cell Environ 23: 387–395 [Google Scholar]
- Bauer H, Ache P, Lautner S, Fromm J, Hartung W, Al-Rasheid KAS, Sonnewald S, Sonnewald U, Kneitz S, Lachmann N, et al (2013) The stomatal response to reduced relative humidity requires guard cell-autonomous ABA synthesis. Curr Biol 23: 53–57 [DOI] [PubMed] [Google Scholar]
- Boudsocq M, Droillard M-J, Barbier-Brygoo H, Laurière C. (2007) Different phosphorylation mechanisms are involved in the activation of sucrose non-fermenting 1 related protein kinases 2 by osmotic stresses and abscisic acid. Plant Mol Biol 63: 491–503 [DOI] [PubMed] [Google Scholar]
- Brandt B, Brodsky DE, Xue S, Negi J, Iba K, Kangasjärvi J, Ghassemian M, Stephan AB, Hu H, Schroeder JI. (2012) Reconstitution of abscisic acid activation of SLAC1 anion channel by CPK6 and OST1 kinases and branched ABI1 PP2C phosphatase action. Proc Natl Acad Sci USA 109: 10593–10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng S-H, Willmann MR, Chen H-C, Sheen J. (2002) Calcium signaling through protein kinases: the Arabidopsis calcium-dependent protein kinase gene family. Plant Physiol 129: 469–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupeux F, Antoni R, Betz K, Santiago J, Gonzalez-Guzman M, Rodriguez L, Rubio S, Park SY, Cutler SR, Rodriguez PL, et al (2011) Modulation of abscisic acid signaling in vivo by an engineered receptor-insensitive protein phosphatase type 2C allele. Plant Physiol 156: 106–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert M, Kaldenhoff R. (2000) Light-induced stomatal movement of selected Arabidopsis thaliana mutants. J Exp Bot 51: 1435–1442 [PubMed] [Google Scholar]
- Geiger D, Maierhofer T, Al-Rasheid KAS, Scherzer S, Mumm P, Liese A, Ache P, Wellmann C, Marten I, Grill E, et al (2011) Stomatal closure by fast abscisic acid signaling is mediated by the guard cell anion channel SLAH3 and the receptor RCAR1. Sci Signal 4: ra32. [DOI] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Marten I, Ache P, Matschi S, Liese A, Wellmann C, Al-Rasheid KAS, Grill E, et al (2010) Guard cell anion channel SLAC1 is regulated by CDPK protein kinases with distinct Ca2+ affinities. Proc Natl Acad Sci USA 107: 8023–8028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D, Scherzer S, Mumm P, Stange A, Marten I, Bauer H, Ache P, Matschi S, Liese A, Al-Rasheid KAS, et al (2009) Activity of guard cell anion channel SLAC1 is controlled by drought-stress signaling kinase-phosphatase pair. Proc Natl Acad Sci USA 106: 21425–21430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Guzman M, Pizzio GA, Antoni R, Vera-Sirera F, Merilo E, Bassel GW, Fernández MA, Holdsworth MJ, Perez-Amador MA, Kollist H, et al (2012) Arabidopsis PYR/PYL/RCAR receptors play a major role in quantitative regulation of stomatal aperture and transcriptional response to abscisic acid. Plant Cell 24: 2483–2496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M, Inoue S, Takahashi K, Kinoshita T. (2011) Immunohistochemical detection of blue light-induced phosphorylation of the plasma membrane H+-ATPase in stomatal guard cells. Plant Cell Physiol 52: 1238–1248 [DOI] [PubMed] [Google Scholar]
- Hill AC, Littlefield N. (1969) Ozone: effect on apparent photosynthesis rate of transpiration and stomatal closure in plants. Environ Sci Technol 3: 52–56 [Google Scholar]
- Hu H, Boisson-Dernier A, Israelsson-Nordström M, Böhmer M, Xue S, Ries A, Godoski J, Kuhn JM, Schroeder JI. (2010) Carbonic anhydrases are upstream regulators of CO2-controlled stomatal movements in guard cells. Nat Cell Biol 12: 87–93, 1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua DP, Wang C, He JN, Liao H, Duan Y, Zhu ZQ, Guo Y, Chen ZZ, Gong ZZ. (2012) A plasma membrane receptor kinase, GHR1, mediates abscisic acid- and hydrogen peroxide-regulated stomatal movement in Arabidopsis. Plant Cell 24: 2546–2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanoff L. (1928) Zur Methodik der Transpirationsbestimmung am Standort. Ber Dtsch Bot Ges 46: 306–310 [Google Scholar]
- Kim T-H, Böhmer M, Hu H, Nishimura N, Schroeder JI. (2010) Guard cell signal transduction network: advances in understanding abscisic acid, CO2, and Ca2+ signaling. Annu Rev Plant Biol 61: 561–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollist H, Jossier M, Laanemets K, Thomine S. (2011) Anion channels in plant cells. FEBS J 278: 4277–4292 [DOI] [PubMed] [Google Scholar]
- Kollist T, Moldau H, Rasulov B, Oja V, Rämma H, Hüve K, Jaspers P, Kangasjärvi J, Kollist H. (2007) A novel device detects a rapid ozone-induced transient stomatal closure in intact Arabidopsis and its absence in abi2 mutant. Physiol Plant 129: 796–803 [Google Scholar]
- Laanemets K, Wang YF, Lindgren O, Wu J, Nishimura N, Lee S, Caddell D, Merilo E, Brosche M, Kilk K, et al. (2013) Mutations in the SLAC1 anion channel slow stomatal opening and severely reduce K+ uptake channel activity via enhanced cytosolic [Ca2+] and increased Ca2+ sensitivity of K+ uptake channels. New Phytol 197: 88–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JS, Bowling DJF. (1992) Effect of the mesophyll on stomatal opening in Commelina communis. J Exp Bot 43: 951–957 [Google Scholar]
- Lee SC, Lan W, Buchanan BB, Luan S. (2009) A protein kinase-phosphatase pair interacts with an ion channel to regulate ABA signaling in plant guard cells. Proc Natl Acad Sci USA 106: 21419–21424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Léon-Kloosterziel KM, Gil MA, Ruijs GJ, Jacobsen SE, Olszewski NE, Schwartz SH, Zeevaart JAD, Koornneef M. (1996) Isolation and characterization of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J 10: 655–661 [DOI] [PubMed] [Google Scholar]
- Leung J, Merlot S, Giraudat J. (1997) The Arabidopsis ABSCISIC ACID-INSENSITIVE2 (ABI2) and ABI1 genes encode homologous protein phosphatases 2C involved in abscisic acid signal transduction. Plant Cell 9: 759–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leymarie J, Lascève G, Vavasseur A. (1998a) Interaction of stomatal responses to ABA and CO2 in Arabidopsis thaliana. Aust J Plant Physiol 25: 785–791 [Google Scholar]
- Leymarie J, Vavasseur A, Lascève G. (1998b) CO2 sensing in stomata of abi1-1 and abi2-1 mutants of Arabidopsis thaliana. Plant Physiol Biochem 36: 539–543 [Google Scholar]
- Ma S-Y, Wu W-H. (2007) AtCPK23 functions in Arabidopsis responses to drought and salt stresses. Plant Mol Biol 65: 511–518 [DOI] [PubMed] [Google Scholar]
- Ma Y, Szostkiewicz I, Korte A, Moes D, Yang Y, Christmann A, Grill E. (2009) Regulators of PP2C phosphatase activity function as abscisic acid sensors. Science 324: 1064–1068 [DOI] [PubMed] [Google Scholar]
- Meinhard M, Grill E. (2001) Hydrogen peroxide is a regulator of ABI1, a protein phosphatase 2C from Arabidopsis. FEBS Lett 508: 443–446 [DOI] [PubMed] [Google Scholar]
- Meinhard M, Rodriguez PL, Grill E. (2002) The sensitivity of ABI2 to hydrogen peroxide links the abscisic acid-response regulator to redox signalling. Planta 214: 775–782 [DOI] [PubMed] [Google Scholar]
- Meyer S, Mumm P, Imes D, Endler A, Weder B, Al-Rasheid KAS, Geiger D, Marten I, Martinoia E, Hedrich R. (2010) AtALMT12 represents an R-type anion channel required for stomatal movement in Arabidopsis guard cells. Plant J 63: 1054–1062 [DOI] [PubMed] [Google Scholar]
- Mori IC, Murata Y, Yang Y, Munemasa S, Wang Y-F, Andreoli S, Tiriac H, Alonso JM, Harper JF, Ecker JR, et al (2006) CDPKs CPK6 and CPK3 function in ABA regulation of guard cell S-type anion- and Ca2+-permeable channels and stomatal closure. PLoS Biol 4: e327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott KA, Sibbernsen ED, Shope JC. (2008) The role of the mesophyll in stomatal responses to light and CO2. Plant Cell Environ 31: 1299–1306 [DOI] [PubMed] [Google Scholar]
- Mustilli A-C, Merlot S, Vavasseur A, Fenzi F, Giraudat J. (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll A. (2005) Abscisic acid biosynthesis and catabolism. Annu Rev Plant Biol 56: 165–185 [DOI] [PubMed] [Google Scholar]
- Negi J, Matsuda O, Nagasawa T, Oba Y, Takahashi H, Kawai-Yamada M, Uchimiya H, Hashimoto M, Iba K. (2008) CO2 regulator SLAC1 and its homologues are essential for anion homeostasis in plant cells. Nature 452: 483–486 [DOI] [PubMed] [Google Scholar]
- Nishimura N, Sarkeshik A, Nito K, Park S-Y, Wang A, Carvalho PC, Lee S, Caddell DF, Cutler SR, Chory J, et al (2010) PYR/PYL/RCAR family members are major in-vivo ABI1 protein phosphatase 2C-interacting proteins in Arabidopsis. Plant J 61: 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura N, Yoshida T, Murayama M, Asami T, Shinozaki K, Hirayama T. (2004) Isolation and characterization of novel mutants affecting the abscisic acid sensitivity of Arabidopsis germination and seedling growth. Plant Cell Physiol 45: 1485–1499 [DOI] [PubMed] [Google Scholar]
- Okamoto M, Tanaka Y, Abrams SR, Kamiya Y, Seki M, Nambara E. (2009) High humidity induces abscisic acid 8′-hydroxylase in stomata and vasculature to regulate local and systemic abscisic acid responses in Arabidopsis. Plant Physiol 149: 825–834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SY, Fung P, Nishimura N, Jensen DR, Fujii H, Zhao Y, Lumba S, Santiago J, Rodrigues A, Chow TF, et al. (2009) Abscisic acid inhibits type 2C protein phosphatases via the PYR/PYL family of START proteins. Science 324: 1068–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI. (1997) Differential abscisic acid regulation of guard cell slow anion channels in Arabidopsis wild-type and abi1 and abi2 mutants. Plant Cell 9: 409–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert N, Merlot S, N’guyen V, Boisson-Dernier A, Schroeder JI. (2006) A hypermorphic mutation in the protein phosphatase 2C HAB1 strongly affects ABA signaling in Arabidopsis. FEBS Lett 580: 4691–4696 [DOI] [PubMed] [Google Scholar]
- Rock CD, Zeevaart JAD. (1991) The aba mutant of Arabidopsis thaliana is impaired in epoxy-carotenoid biosynthesis. Proc Natl Acad Sci USA 88: 7496–7499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema MRG, Hanstein S, Felle HH, Hedrich R. (2002) CO2 provides an intermediate link in the red light response of guard cells. Plant J 32: 65–75 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R. (2005) In the light of stomatal opening: new insights into ‘the Watergate.’ New Phytol 167: 665–691 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R, Geiger D. (2012) Anion channels: master switches of stress responses. Trends Plant Sci 17: 221–229 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Prins HBA. (1995) Effect of abscisic acid on stomatal opening in isolated epidermal strips of abi mutants of Arabidopsis thaliana. Physiol Plant 95: 373–378 [Google Scholar]
- Rubio S, Rodrigues A, Saez A, Dizon MB, Galle A, Kim T-H, Santiago J, Flexas J, Schroeder JI, Rodriguez PL. (2009) Triple loss of function of protein phosphatases type 2C leads to partial constitutive response to endogenous abscisic acid. Plant Physiol 150: 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato A, Sato Y, Fukao Y, Fujiwara M, Umezawa T, Shinozaki K, Hibi T, Taniguchi M, Miyake H, Goto DB, et al (2009) Threonine at position 306 of the KAT1 potassium channel is essential for channel activity and is a target site for ABA-activated SnRK2/OST1/SnRK2.6 protein kinase. Biochem J 424: 439–448 [DOI] [PubMed] [Google Scholar]
- Scherzer S, Maierhofer T, Al-Rasheid KAS, Geiger D, Hedrich R. (2012) Multiple calcium-dependent kinases modulate ABA-activated guard cell anion channels. Mol Plant 5: 1409–1412 [DOI] [PubMed] [Google Scholar]
- Schroeder JI, Keller BU. (1992) Two types of anion channel currents in guard cells with distinct voltage regulation. Proc Natl Acad Sci USA 89: 5025–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimazaki K, Doi M, Assmann SM, Kinoshita T. (2007) Light regulation of stomatal movement. Annu Rev Plant Biol 58: 219–247 [DOI] [PubMed] [Google Scholar]
- Siegel RS, Xue S, Murata Y, Yang Y, Nishimura N, Wang A, Schroeder JI. (2009) Calcium elevation-dependent and attenuated resting calcium-dependent abscisic acid induction of stomatal closure and abscisic acid-induced enhancement of calcium sensitivities of S-type anion and inward-rectifying K channels in Arabidopsis guard cells. Plant J 59: 207–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitch S, Cox PM, Collins WJ, Huntingford C. (2007) Indirect radiative forcing of climate change through ozone effects on the land-carbon sink. Nature 448: 791–794 [DOI] [PubMed] [Google Scholar]
- Soon F-F, Ng L-M, Zhou XE, West GM, Kovach A, Tan MHE, Suino-Powell KM, He Y, Xu Y, Chalmers MJ, et al. (2012) Molecular mimicry regulates ABA signaling by SnRK2 kinases and PP2C phosphatases. Science 335: 85–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostkiewicz I, Richter K, Kepka M, Demmel S, Ma Y, Korte A, Assaad FF, Christmann A, Grill E. (2010) Closely related receptor complexes differ in their ABA selectivity and sensitivity. Plant J 61: 25–35 [DOI] [PubMed] [Google Scholar]
- Umezawa T, Sugiyama N, Mizoguchi M, Hayashi S, Myouga F, Yamaguchi-Shinozaki K, Ishihama Y, Hirayama T, Shinozaki K. (2009) Type 2C protein phosphatases directly regulate abscisic acid-activated protein kinases in Arabidopsis. Proc Natl Acad Sci USA 106: 17588–17593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Kollist H, Wang Y-F, Nishimura N, Chan W-Y, Valerio G, Lamminmäki A, Brosché M, Moldau H, Desikan R, et al (2008) SLAC1 is required for plant guard cell S-type anion channel function in stomatal signalling. Nature 452: 487–491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vahisalu T, Puzõrjova I, Brosché M, Valk E, Lepiku M, Moldau H, Pechter P, Wang YS, Lindgren O, Salojärvi J, et al. (2010) Ozone-triggered rapid stomatal response involves the production of reactive oxygen species, and is controlled by SLAC1 and OST1. Plant J 62: 442–453 [DOI] [PubMed] [Google Scholar]
- Vlad F, Rubio S, Rodrigues A, Sirichandra C, Belin C, Robert N, Leung J, Rodriguez PL, Laurière C, Merlot S. (2009) Protein phosphatases 2C regulate the activation of the Snf1-related kinase OST1 by abscisic acid in Arabidopsis. Plant Cell 21: 3170–3184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Papanatsiou M, Eisenach C, Karnik R, Williams M, Hills A, Lew VL, Blatt MR. (2012) Systems dynamic modeling of a guard cell Cl− channel mutant uncovers an emergent homeostatic network regulating stomatal transpiration. Plant Physiol 160: 1956–1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AAR, Hetherington AM. (1997) Convergence of the abscisic acid, CO2, and extracellular calcium signal transduction pathways in stomatal guard cells. Plant Physiol 114: 1557–1560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner JJ, Peterson FC, Volkman BF, Cutler SR. (2010) Structural and functional insights into core ABA signaling. Curr Opin Plant Biol 13: 495–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Wang Y, Williamson L, Holroyd GH, Tagliavia C, Murchie E, Theobald J, Knight MR, Davies WJ, Leyser HMO, et al (2006) The identification of genes involved in the stomatal response to reduced atmospheric relative humidity. Curr Biol 16: 882–887 [DOI] [PubMed] [Google Scholar]
- Xue S, Hu H, Ries A, Merilo E, Kollist H, Schroeder JI. (2011) Central functions of bicarbonate in S-type anion channel activation and OST1 protein kinase in CO2 signal transduction in guard cell. EMBO J 30: 1645–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida R, Hobo T, Ichimura K, Mizoguchi T, Takahashi F, Aronso J, Ecker JR, Shinozaki K. (2002) ABA-activated SnRK2 protein kinase is required for dehydration stress signaling in Arabidopsis. Plant Cell Physiol 43: 1473–1483 [DOI] [PubMed] [Google Scholar]
- Yoshida R, Umezawa T, Mizoguchi T, Takahashi S, Takahashi F, Shinozaki K. (2006) The regulatory domain of SRK2E/OST1/SnRK2.6 interacts with ABI1 and integrates abscisic acid (ABA) and osmotic stress signals controlling stomatal closure in Arabidopsis. J Biol Chem 281: 5310–5318 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Xu X, Crosley RA, Greenwalt SA, Sun Y, Blakeslee B, Wang L, Ni W, Sopko MS, Yao C, et al. (2010) The protein kinase SnRK2.6 mediates the regulation of sucrose metabolism and plant growth in Arabidopsis. Plant Physiol 153: 99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.