Localized synthesis of an auxin transport enhances local IAA accumulation in excised Arabidopsis hypocotyls and drives the formation of adventitious roots.
Abstract
Adventitious roots emerge from aerial plant tissues, and the induction of these roots is essential for clonal propagation of agriculturally important plant species. This process has received extensive study in horticultural species but much less focus in genetically tractable model species. We have explored the role of auxin transport in this process in Arabidopsis (Arabidopsis thaliana) seedlings in which adventitious root initiation was induced by excising roots from low-light-grown hypocotyls. Inhibition of auxin transport from the shoot apex abolishes adventitious root formation under these conditions. Root excision was accompanied by a rapid increase in radioactive indole-3-acetic acid (IAA) transport and its accumulation in the hypocotyl above the point of excision where adventitious roots emerge. Local increases in auxin-responsive gene expression were also observed above the site of excision using three auxin-responsive reporters. These changes in auxin accumulation preceded cell division events, monitored by a cyclin B1 reporter (pCYCB1;1:GUS), and adventitious root initiation. We examined excision-induced adventitious root formation in auxin influx and efflux mutants, including auxin insensitive1, pin-formed1 (pin1), pin2, pin3, and pin7, with the most profound reductions observed in ATP-binding cassette B19 (ABCB19). An ABCB19 overexpression line forms more adventitious roots than the wild type in intact seedlings. Examination of transcriptional and translational fusions between ABCB19 and green fluorescent protein indicates that excision locally induced the accumulation of ABCB19 transcript and protein that is temporally and spatially linked to local IAA accumulation leading to adventitious root formation. These experiments are consistent with localized synthesis of ABCB19 protein after hypocotyl excision leads to enhanced IAA transport and local IAA accumulation driving adventitious root formation.
The root structure of plants includes a primary root from which lateral roots form and may often include adventitious roots that emerge from aerial tissues. While primary roots are formed during embryogenesis, lateral and adventitious roots are formed postembryonically (Malamy and Benfey, 1997). Both lateral and adventitious roots function to increase nutrient and water uptake and anchor plants in soil. The ability of stems to initiate adventitious root formation depends on many environmental and physiological factors (De Klerk et al., 1999; Li et al., 2009). Formation of adventitious roots on stem segments is widely exploited for clonal propagation of important horticultural, crop, and forest species. Hormonal and wounding controls of this process have been described (De Klerk et al., 1999; Abarca and Díaz-Sala, 2009; Li et al., 2009), with the role of auxin transport in adventitious rooting being described in a diversity of species, including pine (Pinus taeda; Diaz-Sala et al., 1996; Hutchison et al., 1999), carnation (Dianthus caryophyluss; Garrido et al., 2002), and mango (Mangifera indica; Li et al., 2012). The molecular mechanisms by which root excision and auxin induce adventitious root formation are not yet clear and would be facilitated by studies in a genetically tractable model species.
It is a common horticultural practice to apply auxin to stem cuttings to increase the initiation and elongation of adventitious roots (De Klerk et al., 1999; Li et al., 2009). Auxin application is also effective in enhancing adventitious root formation in intact hypocotyls of Arabidopsis (Arabidopsis thaliana; Sorin et al., 2005; Wilmoth et al., 2005). Several studies indicate that as Arabidopsis hypocotyls mature, the induction of adventitious root formation by excision or auxin is reduced, consistent with a developmental context for this process (Díaz-Sala et al., 2002; Correa et al., 2012). In addition, two Arabidopsis mutants that have endogenously high levels of indole-3-acetic acid (IAA), superroot (sur) and rooty, exhibit a proliferation of adventitious roots (Boerjan et al., 1995; Celenza et al., 1995). Protein profiles from seedlings with altered adventitious root formation, argonaute1, sur1, and sur2, identified proteins that are linked to auxin biosynthesis as candidates involved in regulation of adventitious root formation (Sorin et al., 2006).
Other studies have identified roles for auxin signaling in adventitious root formation. Plants with mutations in genes encoding auxin response factors (ARFs), which are transcription factors involved in auxin signaling, indicate that Arabidopsis ARF6, ARF8, ARF19, and ARF7 act as positive regulators (Wilmoth et al., 2005; Gutierrez et al., 2009), while ARF17 acts as a negative regulator of adventitious root development (Sorin et al., 2005). Two auxin responsive lateral organ boundary (LOB)-domain transcription factors encoded by ADVENTITIOUS ROOTLESS1 (ARL1) and CROWN ROOTLESS1 (CRL1) are also positive regulators of adventitious root formation in rice (Oryza sativa; Inukai et al., 2005; Liu et al., 2005). The gain-of-function mutant short hypocotyl2/iaa3 has decreased adventitious root formation (Tian and Reed, 1999), while auxin resistant3/iaa17 mutant alleles show increased formation of adventitious roots (Leyser et al., 1996).
Despite the evidence that auxin transport from the shoot apex is important in adventitious root formation (Visser et al., 1996; Guerrero et al., 1999; Díaz-Sala et al., 2002), the role of distinct auxin transport proteins in this process has not been reported. Auxin movement is mediated by influx proteins such as AUXIN RESISTANT1 (AUX1) and Like AUX (LAX), which facilitate auxin movement into cells (Marchant et al., 1999; Swarup et al., 2008), and efflux proteins such as PIN-FORMED (PIN) and ATP-BINDING CASSETTE TYPE B/P-GLYCOPROTEIN/MULTIDRUG RESISTANT (ABCB/PGP/MDR) proteins, which participate in auxin efflux (Gälweiler et al., 1998; Noh et al., 2001; Zazímalová et al., 2010). Defects in AUX1, LAX3, PIN1, and ABCB19/PGP19/MDR1 (from here on referred to as ABCB19) reduce initiation and/or elongation of lateral roots (Marchant et al., 2002; Benková et al., 2003; Wu et al., 2007; Swarup et al., 2008; Lewis et al., 2011). Additionally, lateral root development has been shown to depend on complex changes in expression of PIN proteins prior to lateral root initiation and in developing primordia (Benková et al., 2003; Laskowski et al., 2008; Lewis et al., 2011). A recent report describes increased auxin induction of auxin influx carriers in mango cotyledons induced to form adventitious roots (Li et al., 2012). These results indicate that a distinct set of auxin transport proteins control lateral root development and suggest that similar specificity and complexity might regulate adventitious root development.
This study utilized Arabidopsis as a model to understand the role of auxin transport in adventitious root formation and to examine the molecular mechanisms by which auxin induces adventitious root formation in shoot explants. Arabidopsis seedlings grown under low-light conditions form elongated hypocotyls in which auxin transport can be measured and are optimal for root excision to induce adventitious roots. We have examined the developmental origin and auxin response of these excision-induced roots and identified local and developmentally significant auxin-induced gene expression at the site of root excision. An array of auxin transport mutants and an ABCB19 overexpression construct were used to demonstrate the role of ABCB19 in adventitious root formation. Furthermore, we have examined expression of promoter and coding sequence fusions of ABCB19 sequence to GFP and found enhanced GFP fluorescence upon root excision. These results have uncovered an underlying mechanism by which root excision alters ABCB19-mediated auxin transport leading to the formation of adventitious roots.
RESULTS
Root Excision from Arabidopsis Hypocotyls Increases Adventitious Root Formation
We asked if Arabidopsis was a feasible model to examine the mechanisms for auxin- and excision-induced adventitious root formation. Most studies aimed at identifying the role of auxin signaling in adventitious root formation in Arabidopsis hypocotyls have used intact etiolated hypocotyls (Sorin et al., 2005, 2006; Correa et al., 2012). Basipetal IAA transport is at extremely low levels in etiolated Arabidopsis hypocotyls, but levels are more amenable to study in low-light-grown hypocotyls (Rashotte et al., 2003; Liu et al., 2011), necessitating the use of alternate growth conditions for our study. As 12- to 30-d-old light-grown Arabidopsis seedlings can be induced to form adventitious roots upon root excision (Díaz-Sala et al., 2002; Correa et al., 2012), we modified these conditions for examination of younger seedlings grown in limited light to elongate hypocotyls. This allowed us to ask if we could identify the mechanisms by which excision alters auxin transport and adventitious root formation.
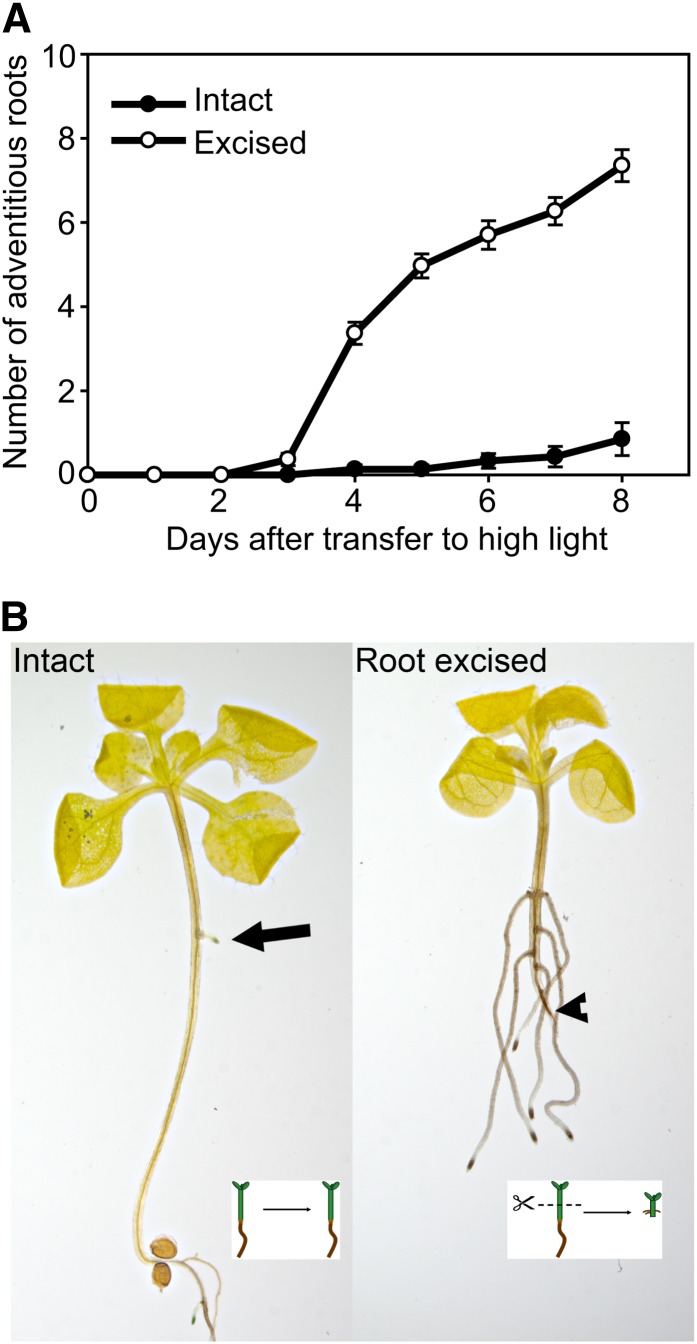
We germinated seedlings in low light (3–5 µmol m–2 s–1) to induce hypocotyl elongation. On the fifth day after sowing, seedlings were transferred to high-light conditions (85–100 µmol m–2 s–1) with and without excision of the basal half of the shoot and root system (termed root-excised hypocotyls). Intact hypocotyls form zero to one adventitious root per plant located in the middle of the hypocotyl by the eighth day after transfer to high light, while excision substantially increased the number of adventitious roots formed (Fig. 1). Excision had the advantage of reproducibly inducing adventitious roots at a position 1 to 2 mm above the site of excision, so molecular events associated with root formation could be examined at this position prior to the first detectable adventitious root.
Figure 1.
Root excision increases adventitious root formation in Arabidopsis. A, The number of adventitious roots was determined in the intact or root-excised hypocotyls (n = 20–30). B, Adventitious root formation on intact (left) and excised (right) cleared hypocotyls is shown after 7 d. Arrow points to an adventitious root in the intact hypocotyl, and arrowhead points to site of excision. Bar = 5 mm. [See online article for color version of this figure.]
Adventitious Roots Induced by Excision Emerge from the Pericycle
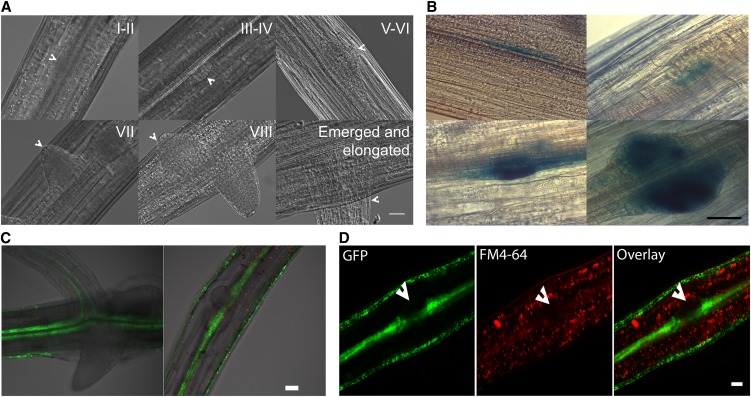
Histological analysis suggested adventitious roots forming on intact hypocotyls initiate from pericycle cells in Arabidopsis (Falasca and Altamura, 2003; Takahashi et al., 2003). A recent report indicated the developmental origin of adventitious roots may vary based on Arabidopsis tissue type and age used in the analysis (Correa et al., 2012). Therefore, we sought to identify the developmental origin of adventitious roots in our samples using differential interference contrast (DIC) imaging of the cleared wild type and two marker constructs, cyclin B1 (pCYCB1;1:GUS) and a pericycle-expressed enhancer trap line, J0121. In cleared wild-type tissues, the sequence of adventitious root development is shown in Figure 2, with the stage of root development in each image indicated in Roman numerals corresponding to the sequence of developmental stages defined for lateral roots (Malamy and Benfey, 1997). The developing adventitious roots appear to emerge from pericycle cells in these DIC images. To define early division events, we examined the pCYCB1;1:GUS reporter, which is expressed in dividing cells (DiDonato et al., 2004). In basal regions of the hypocotyl, this reporter is only expressed in adventitious root primordia (DiDonato et al., 2004), which developed close to the vascular tissue (Fig. 2B). Seedlings of the enhancer trap line J0121, which expresses GFP in pericycle cells of the hypocotyl and root (as well as epidermal cells of hypocotyls, but not roots; Laplaze et al., 2005), were examined by laser scanning confocal microscopy (LSCM). These seedlings show GFP fluorescence in pericycle cells at the base of emerged adventitious roots of root-excised hypocotyls and in early-stage adventitious root primordia (Fig. 2C). The plasma membrane dye FM4-64 is largely excluded from primordia, marking them with lower fluorescence, and in the J0121:GFP reporter line, the formation of primordia from pericycle cells is evident (Fig. 2D). Thus, in low-light-grown hypocotyls, excision-induced adventitious roots of Arabidopsis, like lateral roots and adventitious roots forming on intact hypocotyls, develop from pericycle cells.
Figure 2.
Adventitious roots emerge from pericycle tissues of the hypocotyl. DIC images of various stages of primordia development in: A, Cleared hypocotyls of the wild type at the developmental stage noted in Roman numerals; B, AtCYCB1;1:GUS transgenic hypocotyls; C, LSCM image of pericycle marker J0121:GFP in emerging adventitious roots; and D, LSCM images of pericycle marker J0121:GFP and FM4-64 staining in adventitious root primordia. The arrowhead notes a primordium from which most FM4-64 stain is excluded. Bars = 50 µm (A, C, and D) and 30 µm (B).
Transport of Auxin from the Shoot Apex Is Required for Adventitious Root Formation
We asked if Arabidopsis adventitious root formation is dependent on auxin transport under our experimental conditions, as root excision-induced adventitious roots on Arabidopsis hypocotyls show age-dependent variation in sensitivity to auxin and auxin transport inhibitors (Díaz-Sala et al., 2002; Correa et al., 2012). Treatment of root-excised hypocotyls with the IAA efflux inhibitor naphthylphthalamic acid (NPA) at 10 µm or removal of shoot apices prevented adventitious root formation 7 d after treatment. Additionally, the replacement of shoot apices of root-excised hypocotyls with localized IAA treatment restored adventitious root formation (Supplemental Table S1) but required high concentrations (100 µm) because of the localized application. By contrast, application of 100 µm NPA locally below the site of application of IAA reversed the effect of IAA. These results confirmed that auxin movement from the shoot apex is essential for adventitious rooting in our experimental system and provide background context for identifying mechanisms of auxin transport involved in excision-enhanced root formation.
In addition, we compared the effectiveness of auxin on induction of adventitious roots in intact versus root-excised hypocotyls to determine whether the response to auxin was changed by excision. Treatment of 5-d-old root-excised and intact hypocotyls with IAA increased the number of adventitious roots relative to untreated hypocotyls at concentrations ranging from 0.1 to 25 µm IAA (Supplemental Fig. S1). This result is consistent with increases in adventitious roots on intact Arabidopsis hypocotyls at IAA concentrations between 0.1 and 10 µm (Wilmoth et al., 2005) and in derooted juvenile hypocotyls (Correa et al., 2012) but differs from the report that IAA or indole-butyric acid did not stimulate adventitious rooting in mature root-excised hypocotyls (Díaz-Sala et al., 2002; Correa et al., 2012), emphasizing that developmental response to auxin varies with hypocotyl tissue age. The magnitude of induction of root formation by IAA was 1.6-fold and 13.5-fold in root-excised and intact hypocotyls, respectively. The limited response to exogenous auxin in excised hypocotyls is consistent with excision increasing auxin in the hypocotyl to a near-maximum level for root formation, resulting in limited additional response when IAA is added.
Excision Causes Local Increases in Auxin-Induced GUS Expression
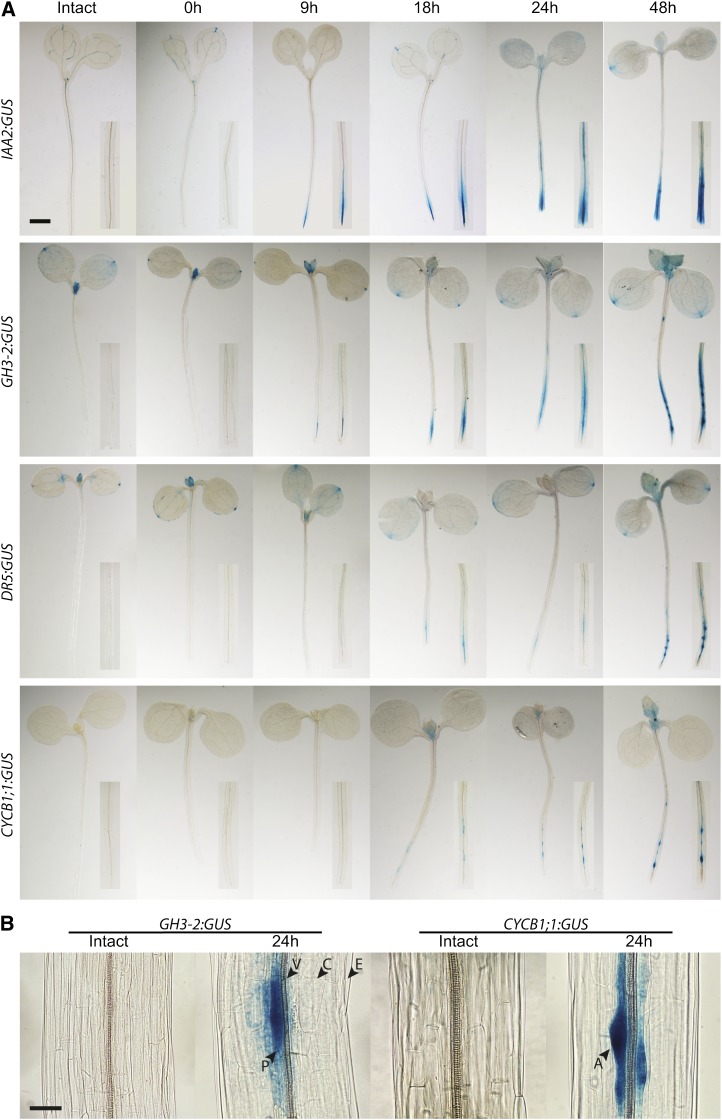
We hypothesized that the effect of excision is an increase in local auxin accumulation and signaling at the hypocotyl base, driving adventitious root formation. Hypocotyls of Arabidopsis plants transformed with the auxin responsive reporters pIAA2:GUS, pGretchen Hagen3 (GH3)-2:GUS, and pDR5:GUS were root excised, and GUS staining was initiated every hour for 9 h and at 24 and 48 h after excision (Fig. 3A). No expression was detected in these reporters in intact hypocotyls or in the hypocotyls earlier than 9 h after excision. GUS activity was detected at the base of the hypocotyl just above the site of excision and in the apices of developing adventitious roots in pIAA2:GUS and pGH3-2:GUS at 9 h after excision, while pDR5:GUS took longer to be detected because of its weaker signal in this tissue (Fig. 3A). Higher magnification images of excised hypocotyls of pGH3-2:GUS show that GUS activity is highest in pericycle cells where adventitious root primordia are forming and is much lower in surrounding cortical cells (Fig. 3B). The presence of localized increases in expression of these auxin responsive promoter constructs above the point of excision of the hypocotyl could be due to increases in auxin synthesis, signaling, or transport. To ask if this expression occurs as a result of IAA transport, we treated GH3-2:GUS hypocotyls with NPA, an auxin transport inhibitor, which prevented localized GH3-2:GUS expression (Supplemental Fig. S2), consistent with auxin transport being required for this localized auxin-induced gene expression.
Figure 3.
Excision increases local expression of auxin-responsive and cell division reporters in adventitious roots developing from pericycle cells. A, Intact or root-excised hypocotyls of pIAA2:GUS, pGH3-2:GUS, pDR5:GUS, and AtCYCB1;1:GUS were stained at the indicated time after excision. Insets show a 2.5× view of the hypocotyl base or the equivalent region in intact seedlings. Bar = 1 mm. B, Higher magnification images of intact or root-excised hypocotyls of AtGH3-2:GUS and AtCYCB1;1:GUS were stained 24 h after excision or in time-matched intact controls. Bar = 50 µm. Labels correspond to different cell files. P, Pericycle; V, vascular bundle; C, cortex; E, epidermis; A, adventitious root primordia.
We also asked if there were changes in the level of free IAA with excision. Free IAA levels were not significantly different between intact and root-excised hypocotyls at any examined time point (Supplemental Fig. S3 and Supplemental Materials and Methods S1). Although we expected elevated free IAA after root excision to parallel the local GUS reporter increases, it is likely that free IAA only changes locally, not in the entire hypocotyl. Alternatively, because GH3-2 is an enzyme that conjugates free IAA to amino acids (Staswick et al., 2005), changes in IAA levels may be masked by elevated IAA conjugation at the hypocotyl base.
Additionally, we examined the initiation of cell division for primordium development at identical time points in root-excised seedlings. pCYCB1;1:GUS expression is de-tected at 18 to 24 h after root excision (Fig. 3, A and B), much later than initial detection of either auxin reporter, suggesting that local auxin accumulation precedes cell division. Higher magnification images of excised hypocotyls in this reporter line show GUS activity only in adventitious root primordia and not in surrounding cells (Fig. 3B).
Excision Enhances Auxin Transport, and Plants with Mutations in IAA Efflux Carriers Have Reduced Adventitious Root Formation
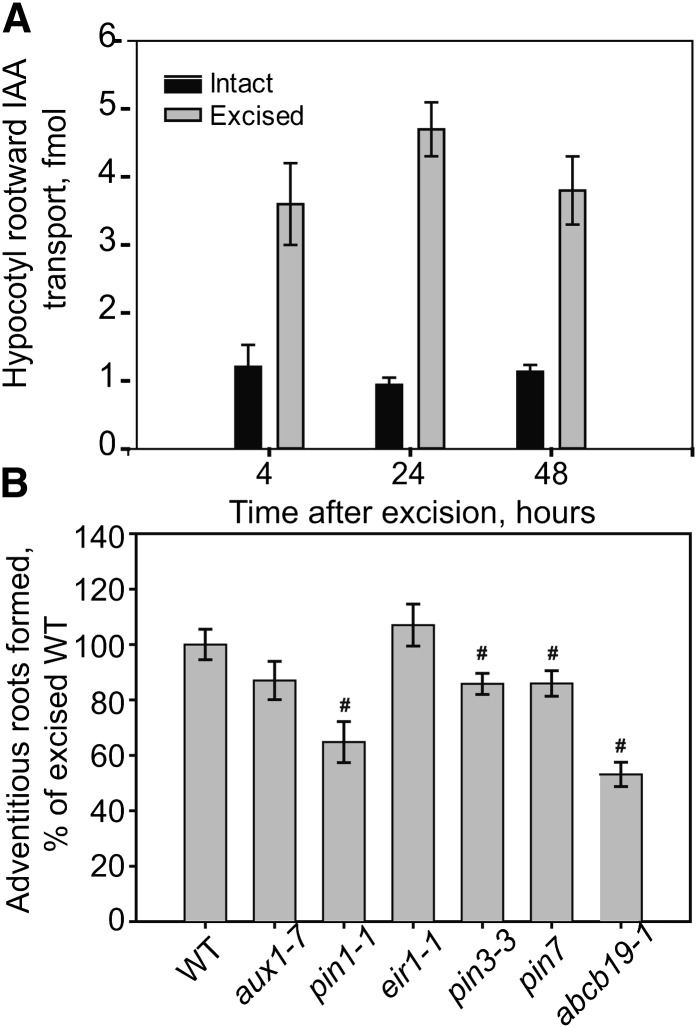
We examined rootward auxin transport in intact and root-excised hypocotyls. The amount of 3H-IAA, which is transported from the shoot apex and accumulates at the hypocotyl base, is increased within as little as 4 h after excision, with similar increases at 24 and 48 h after excision, with a maximal 400% increase (Fig. 4A). This is consistent with an increase in auxin transported into and remaining in the root-excised hypocotyl base, leading to local auxin accumulation, which drives adventitious root formation. The kinetics of this elevated IAA transport precede both the localized IAA accumulation detected with reporters and the initiation of adventitious roots, suggesting enhanced IAA delivery may drive this process.
Figure 4.
Excision induces IAA transport, and auxin transport mutants show less adventitious root formation. A, Tritiated IAA transport from the shoot apex toward the root (rootward transport) was measured in hypocotyls at the times after excision compared with intact hypocotyls. The average and se are reported, and all samples showed significant induction by excision (P < 0.001). B, The effect of mutations in genes encoding auxin transport proteins on adventitious roots 7 d after excision (n = 10–31). Number sign indicates P ≤ 0.05 between indicated genotypes compared with the wild type (WT).
As some auxin transport mutants have defects in either lateral root initiation or elongation, we asked if adventitious root formation was affected in previously characterized null alleles of auxin transport mutants aux1-7, ethylene insensitive root1 (eir1-1, a pin2 allele), pin3-3, abcb19-1 (previously called mdr1-1), pin7 (Salk 048791), and pin1-1 (Pickett et al., 1990; Okada et al., 1991; Luschnig et al., 1998; Friml et al., 2002; Laskowski et al., 2008; Lewis et al., 2009). All mutants are in ecotype Columbia (Col-0), except abcb19-1, which is in ecotype Wassilewskija (Ws; Lewis et al., 2007). The adventitious rooting for each mutant is normalized relative to the appropriate wild type (Fig. 4B). Although aux1 and pin2 showed no significant differences in adventitious root formation, pin3-3 and pin7 showed slight but significant reductions relative to the appropriate wild-type control. Of greatest significance, abcb19-1 and pin1-1 formed approximately 50% and 40% fewer adventitious roots than the wild type, respectively. This effect is at the level of initiation of adventitious roots, as all of the adventitious roots that initiated also emerged and elongated under these treatments, as judged by quantification of primordia at several time points after excision (data not shown). The important role of ABCB19 in this hypocotyl response is consistent with previously published microarray data showing high levels of transcripts of ABCB19 in hypocotyls (Van Hoewyk et al., 2008; Supplemental Fig. S4), leading us to focus on the role of ABCB19 in adventitious root development.
ABCB19 Overexpression Increases Adventitious Root Formation and Auxin Transport
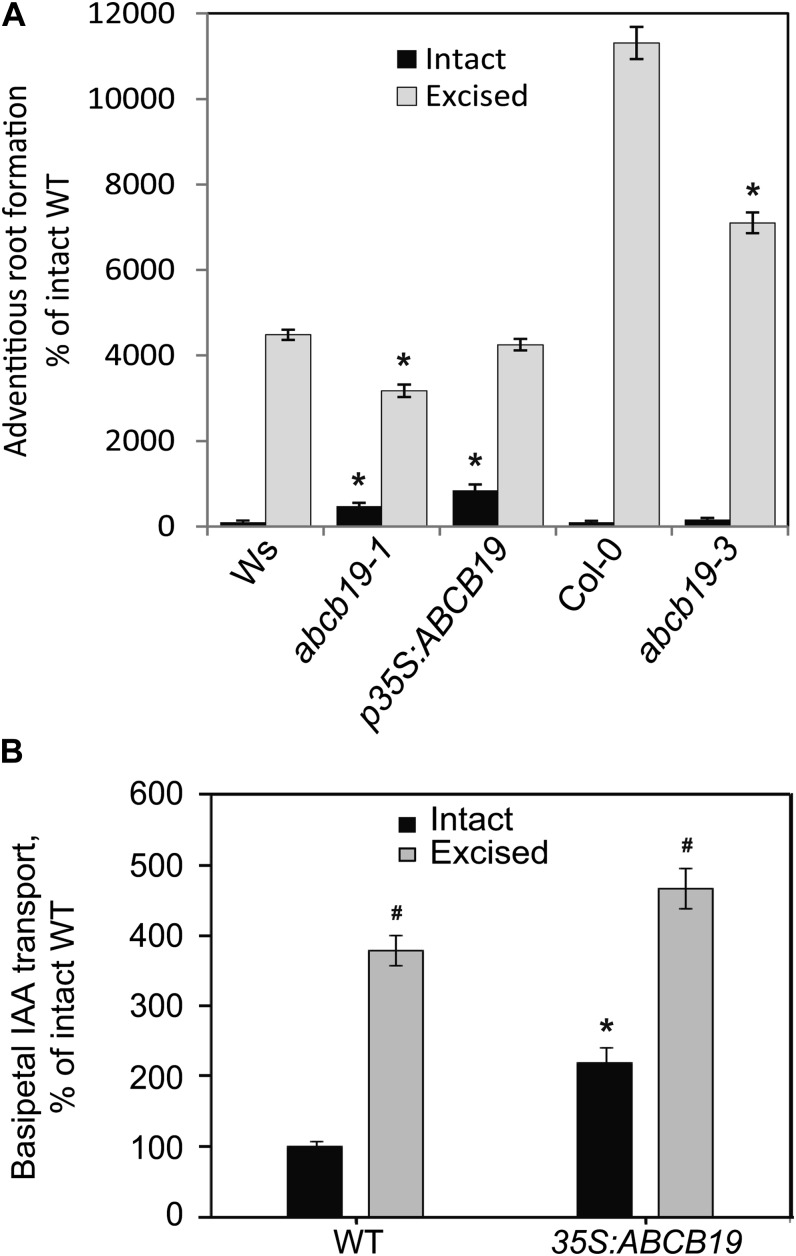
To confirm the role of ABCB19 in adventitious root formation, we quantified adventitious root numbers in intact and root-excised hypocotyls of two abcb19 null alleles, abcb19-1 and abcb19-3, in the Ws and Col-0 backgrounds, respectively (Lewis et al., 2007), and the ABCB19 overexpression line p35S:ABCB19 (in Ws; Wu et al., 2010), compared with both the Ws and Col-0 wild type (Fig. 5A). The induction of adventitious roots by root excision is greater in Col-0 than Ws, largely because of lower numbers of adventitious roots formed in intact Col-0. In both abcb19 alleles, the magnitude of the root induction by root excision is significantly reduced relative to the appropriate wild type. Intact hypocotyls of p35S-ABCB19 formed approximately 8-fold more adventitious roots than the intact wild type. By contrast, in root-excised hypocotyls, the number of adventitious roots in the overexpression line and Ws were not significantly different. These results are consistent with excision inducing ABCB19 accumulation to levels equivalent to the overexpression lines.
Figure 5.
ABCB19 expression levels are linked to excision-induced adventitious root formation and auxin transport. A, The formation of adventitious roots in intact and excised hypocotyls is reported relative to intact seedlings of the parental genotypes (Ws for abcb19-1 and 35S:ABCB19; Col for abcb19-3). n = 41\x{2013}60. Asterisk indicates P ≤ 0.05 in excised or intact hypocotyls between genotypes. In all genotypes, excision significantly increases root formation over intact samples (P ≤ 0.001), so this is not noted on the graph. B, The effect of root excision and p35S:ABCB19 on auxin transport 2 d after excision. The average and SE are reported. Asterisk indicates P ≤ 0.05 between genotypes as compared to wild type. Number sign indicates P ≤ 0.05 between intact and excised samples within genotypes. WT, Wild type.
To further test this hypothesis and the corollary that elevated ABCB19 protein synthesis increases hypocotyl auxin transport, we measured hypocotyl rootward (previously called basipetal) 3H-IAA transport in the p35S:ABCB19 line and the wild type in the presence and absence of root excision. Auxin transport is not reported for abcb19, as the mdr1-1 allele was previously reported to have reduced hypocotyl transport (Noh et al., 2001; Christie et al., 2011). In the wild type, excision causes enhancement of auxin transport and accumulation in the basal region of the hypocotyl, with approximately 400% increase relative to intact hypocotyls. In p35S:ABCB19-intact hypocotyls, transport is more than 200% of intact wild-type levels, but in excised hypocotyls, p35S:ABCB19 shows only 120% of wild-type levels (Fig. 5B), consistent with enhanced accumulation of ABCB19 in the wild type upon excision to roughly equivalent levels to the overexpression line.
Surprisingly, intact abcb19-1 and abcb19-3 hypocotyls have 4- and 1.6-fold elevated adventitious root formation relative to the wild type, respectively (Fig. 5A), contrasting with reduced formation in the root-excised mutants relative to the wild type (Fig. 4B). We hypothesize that if ABCB19 protein is synthesized at low levels in intact hypocotyls, the high levels in roots may enhance long-distance transport from the shoot apex toward the root tip, decreasing the concentration of auxin accumulating at the hypocotyl base in the wild type. In abcb19, the absence of root expression would raise the levels of auxin at the base of the hypocotyl, facilitating adventitious root formation. To explore this possibility, we examined the accumulation patterns of ABCB19 using GFP fusions.
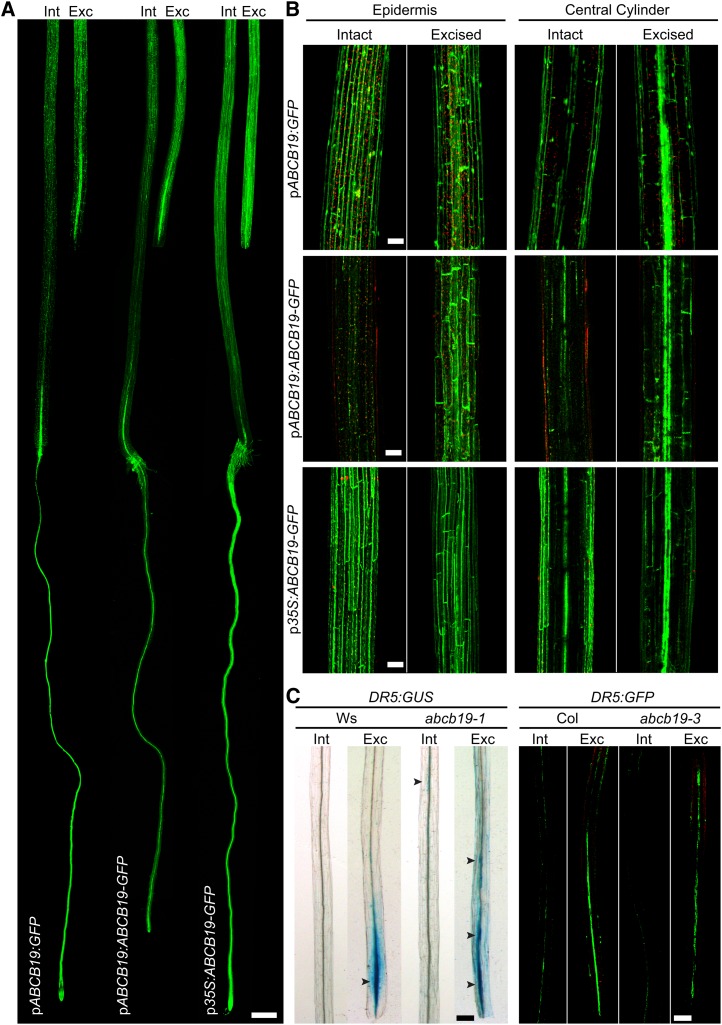
Excision and Overexpression Elevates ABCB19-GFP Fluorescence in Hypocotyls
To determine if there are changes in ABCB19 expression and protein accumulation as a result of root excision and overexpression, we examined previously published GFP reporters pABCB19-GFP, pABCB19:ABCB19-GFP, and p35S:ABCB19-GFP. The fluorescence of these reporters is localized to the plasma membrane in root cells (Wu et al., 2007, 2010) and in the epidermis in the apical hook of etiolated hypocotyls (Wu et al., 2010). This construct has not been examined along the entire length of the root or hypocotyl. Intact and excised hypocotyls of these three reporters were observed 6 h after root excision, a time point chosen to examine the changes that occur before auxin accumulation, judged by the auxin-responsive reporter lines shown in Figure 3. Images were captured using LSCM tile scans, which collect multiple adjacent images, providing information on the expression patterns throughout the whole seedling (Fig. 6A). We observed expression of these reporters in the epidermis of hypocotyls, consistent with a previous examination in the hypocotyl apex (Wu et al., 2007). We also examined expression in a broader tissue context than in the previous analysis and observed bright fluorescence in the vascular tissue (Fig. 6). We have also examined the expression in individual optical slices and see that there is membrane-associated fluorescence in the pericycle layer consistent with IAA transport capacity in those cells (but at lower intensity than the adjacent vascular signal), consistent with this protein participating in auxin transport in the cells from which adventitious roots form (Supplemental Fig. S5).
Figure 6.
Local ABCB19 transcription and pABCB19:ABCB19-GFP protein accumulations are enhanced with excision. A, LSCM tile scan images of root-excised pABCB19:ABCB19-GFP, pABCB19:GFP, and p35S:ABCB19-GFP taken 6 h post excision. Bar = 400 µm. B, Comparison of intact and root-excised hypocotyls of pABCB19:ABCB19-GFP, pABCB19:GFP, and p35S:ABCB19-GFP examined by LSCM tile scans 6 h post excision. Bars = 100 µm. C, Higher magnification LSCM images of DR5:GUS and DR5:GFP upon root excision in abcb19-1 and abcb19-3 mutant hypocotyls, respectively. Bars = 200 µm.
In intact seedlings, the expression of the two pABCB19-driven constructs is stronger in the roots than in the hypocotyls (Fig. 6A). Upon root excision, both constructs show brighter fluorescence in the region directly above the point of excision, which is even more evident at higher magnification (Fig. 6B). The one interesting difference is that the transcriptional promoter construct, pABCB19:GFP, shows increased fluorescence in only the central cylinder, while the translational fusion, pABCB19:ABCB19-GFP, shows elevated expression both in the epidermis and the central cylinder.
The effect of excision on GFP fluorescence is quantified in Table I and clearly indicates that there is elevated expression of ABCB19:GFP in vascular tissues resulting from excision, while the absence of increased epidermal expression in the transcriptional fusion hints at additional posttranslational controls of accumulation of this protein. The ABCB19-driven reporter accumulates in the hypocotyl base with similar spatial characteristics to elevated IAA accumulation, as judged by IAA2:GUS, GH3:GUS, and DR5:GUS (Fig. 3). The excision-induced elevated expression of pABCB19:ABCB19:GFP occurs much more rapidly, being strongly induced at 6 h, in contrast to the detection of the auxin-induced reporters beginning at 9 h after excision These results are consistent with ABCB19 driving auxin transport to create local auxin accumulation that induces adventitious root formation.
Table I. Excision alters GFP expression in ABCB19 transcriptional and translational fusion constructs.
Quantification of intensity was performed with 12 to 21 seedlings, and values are the average ± se. Values for pABCB19:ABCB19-GFP and 35S:ABCB19-GFP are reported as percentage of intact pABCB19:ABCB19-GFP. Values for pABCB19:GFP are reported as percentage of intact pABCB19:GFP.
| GFP Construct | Tissue | Hypocotyl Treatment | GFP Intensity |
|---|---|---|---|
| pABCB19:ABCB19-GFP | Epidermis | Intact | 100 ± 9 |
| Epidermis | Excised | 247 ± 24a | |
| Central cylinder | Intact | 100 ± 8 | |
| Central cylinder | Excised | 284 ± 46a | |
| 35S:ABCB19-GFP | Epidermis | Intact | 255 ± 4a |
| Epidermis | Excised | 268 ± 3a | |
| Central cylinder | Intact | 193 ± 13a | |
| Central cylinder | Excised | 288 ± 11a | |
| pABCB19:GFP | Epidermis | Intact | 100 ± 6 |
| Epidermis | Excised | 95 ± 7 | |
| Central cylinder | Intact | 100 ± 15 | |
| Central cylinder | Excised | 513 ± 19a |
Values significantly different from intact controls used for normalized with P < 0.05.
We also examined an ABCB19 overexpression line, 35S:ABCB19:GFP, and found that the GFP fluorescence of this construct is brighter than either endogenous promoter construct, particularly in the hypocotyl (Fig. 6, A and B). Upon excision, this expression is not substantially changed, consistent with maximal expression under the control of the 35S promoter. This effect is quantified in Table I, showing equivalent expression in the 35S promoter-driven construct to the excision-induced pABCB19-driven promoter. In intact hypocotyls of this overexpression line, the GFP signal and adventitious rooting are similarly elevated relative to the wild type.
Enhanced pABCB19-driven GFP expression after excision is consistent with the role of ABCB19 in mediating enhanced auxin transport resulting in auxin accumulation at the hypocotyl base, driving adventitious root formation. To test for the requirement of ABCB19 in localized auxin accumulation, the expression patterns of two additional auxin responsive promoter-driven reporters, pDR5:GUS and pDR5:GFP, were compared in both wild-type and abcb19 backgrounds (Fig. 6C). These reporters show enhanced expression directly above the position of root excision in wild-type seedlings. By contrast, in the abcb19-1 and abcb19-3 null mutants, the expression of both reporters is less intense at the point of excision, with more diffuse expression along the hypocotyl, consistent with the reduced induction of adventitious root formation in this mutant upon root excision relative to the wild type (Fig. 6C). The visualization of faint pDR5:GUS expression in intact abcb19-1 hypocotyls, but not wild type, is also consistent with the phenotype of this mutant, which forms more adventitious roots than the wild type in intact hypocotyls.
We have observed that root excision induces both auxin-responsive reporters and ABCB19 reporters in the hypocotyl. It is possible that either IAA accumulation results from elevated auxin transport protein synthesis or that localized elevated IAA accumulation drives auxin transport protein synthesis. To test the possibility that auxin induces ABCB19 accumulation, we applied 5µm IAA localized to the point of excision on intact and excised hypocotyls in the pABCB19-GFP line. We did not observe changes in GFP fluorescence in intact hypocotyls or 6 h after root excision between control and IAA treatment and only observed a slight increase in fluorescence in root-excised hypocotyls after 24 h with IAA treatment (Supplemental Fig. S6). Similarly, these IAA treatments do not restore wild-type adventitious root formation to excised hypocotyls of abcb19-1 (data not shown). This result suggests that excision, rather than IAA accumulation, causes the induction of ABCB19 at the hypocotyl base. It also supports our hypothesis that ABCB19 is responsible for IAA accumulation, and not vice versa, under these conditions.
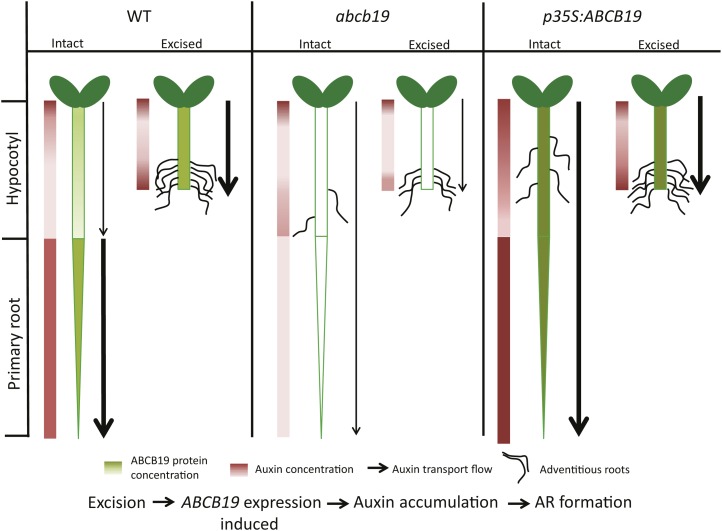
A model suggesting the mechanisms by which ABCB19 controls adventitious root formation is illustrated in Figure 7. This model summarizes ABCB19:GFP fluorescence patterns (Fig. 6), auxin accumulation patterns (Fig. 3), and auxin transport (Fig. 5). This model highlights the induction of all three processes in the wild type upon root excision, with reduced induction of all three in the abcb19 mutant. The overexpression line has elevated ABCB19 expression, auxin transport, and rooting in intact hypocotyls, but in excised hypocotyls, the root excision process elevates wild-type ABCB19 and the dependent processes to levels equivalent to the overexpression construct. This model also addresses one point of complexity in our data set, the increased adventitious rooting in intact hypocotyls of two alleles of the abcb19 mutant. Consistent with this model, the expression pattern of pABCB19:ABCB19-GFP in intact hypocotyls shows greater expression in the root than the hypocotyl (Fig. 6A). This suggests that in wild-type seedlings, high ABCB19 expression in roots may act as an auxin sink, preventing the formation of auxin maxima in the hypocotyls needed for initiation of adventitious roots. In the abcb19 mutant, the absence of this root expression may cause auxin concentration in hypocotyls to be higher than in the wild type, leading to greater numbers of adventitious roots. This model is supported by the increase in expression of the DR5:GUS reporter in abcb19-1, which suggests enhanced auxin accumulation in intact abcb19 mutant hypocotyls (Fig. 6C). Together, these results are consistent with a mechanistic model in which root excision increases the synthesis of the ABCB19 protein in the hypocotyl leading to elevated IAA transport and localized IAA accumulation that then drives the formation and elongation of adventitious roots.
Figure 7.
Model describing the proposed mechanisms by which excision enhances ABCB19 synthesis and auxin transport leading to localized auxin accumulation that drives adventitious root formation. The levels of ABCB19 protein are illustrated in green shading with darkness of shades proportional to expression levels, based on ABCB19:GFP reporter expression. The resulting levels of IAA in hypocotyls and roots are noted in shades of red to the left of each seedling, with darker red indicating higher IAA concentration. The amount of auxin transport is indicated by the width of the arrows, a thicker line denoting greater transport levels. The frequency of adventitious root formation is noted, although lateral root formation is omitted for simplicity. The flowchart at the bottom of the figure illustrates the temporal order of events occurring in the model. WT, Wild type.
DISCUSSION
Lateral and adventitious roots serve important functions in plants, providing an extensively branched network that absorbs moisture and nutrients as well as providing anchorage. Despite the apparent structural similarities between adventitious and lateral roots, much less is known about the mechanisms that drive adventitious root initiation, emergence, and elongation. The developmental sequence (Malamy and Benfey, 1997) and environmental (Malamy and Ryan, 2001) and hormonal controls (Péret et al., 2009) of lateral root formation are well studied in Arabidopsis, yet the low frequency of adventitious root formation (only zero to two per plant) in Arabidopsis has limited the studies exploring the mechanisms that drive this process (Sorin et al., 2005, 2006; Wilmoth et al., 2005; Gutierrez et al., 2009).
These experiments explored the developmental sequence and hormonal controls of excision-induced adventitious root formation in Arabidopsis. Previous groups had either used dark growth followed by light growth (Takahashi et al., 2003; Sorin et al., 2005, 2006; Gutierrez et al., 2009) or removal of the root from mature light-grown seedlings (Díaz-Sala et al., 2002; Correa et al., 2012) to induce adventitious root formation. We maximized adventitious root formation through a combination of these strategies. We grew seedlings under low light followed by excision of the lower half of the hypocotyl and transfer to high light. Adventitious roots form with 10-fold higher frequency under these treatments, compared with intact controls. Root excision also alters the position of adventitious root formation from the middle of intact hypocotyls to a single position localized 1 to 2 mm above the site of excision. This provides a position at which the molecular events that precede adventitious root formation can be examined. Enhanced root formation upon root excision facilitates the study of this process consistent with root excision used for vegetative propagation of horticultural and forest plant species (for review, see De Klerk et al., 1999; Abarca and Díaz-Sala, 2009). Examination of adventitious root developmental stages allows us to conclude that increased root formation is at the level of initiation of adventitious root primordia, rather than on emergence or elongation of adventitious roots.
We have examined adventitious root development and find that excision-induced adventitious roots emerge from Arabidopsis hypocotyl pericycle cells, like adventitious roots forming on intact hypocotyls (Falasca and Altamura, 2003; Takahashi et al., 2003). The excision-induced adventitious roots show developmental differences from those forming on month-old light-grown seedlings (Correa et al., 2012), consistent with age-dependent differences in adventitious root induction by excision and auxin (Díaz-Sala et al., 2002; Correa et al., 2012).
Although previous studies indicated that development of adventitious root formation is controlled by a complex network of auxin-signaling proteins (Sorin et al., 2005, 2006; Wilmoth et al., 2005; Gutierrez et al., 2009), our goal was to identify the mechanisms by which auxin transport and accumulation regulates adventitious root formation. The essential role of auxin transport in initiation and development of lateral roots in Arabidopsis (Péret et al., 2009) and adventitious root formation in horticultural and forest species (De Klerk et al., 1999; Abarca and Díaz-Sala, 2009) has been suggested previously. Consistent with auxin transport controlling adventitious root formation, we find that removal of the auxin source at the shoot apex or treatment with the IAA efflux inhibitor NPA blocks adventitious root formation. These effects are both reversible by IAA. This finding is consistent with inhibition of adventitious rooting after NPA or 2,3,5-triiodobenzoic acid treatments in light-grown Arabidopsis seedlings (Díaz-Sala et al., 2002), Arabidopsis inflorescence segments (Ludwig-Müller et al., 2005), etiolated carnation seedlings (Guerrero et al., 1999; Garrido et al., 2002), rice hypocotyls (Morita and Kyozuka, 2007), and pine seedlings (Diaz-Sala et al., 1996).
We find that root excision increased the flux of auxin transported from the apex to the hypocotyl base by more than 4-fold and as early as 4 h after excision, preceding any changes in adventitious root development. We detected elevated auxin-induced gene expression events directly above the point of root excision using three auxin-responsive promoter-driven GUS or GFP constructs and demonstrated that this localized gene expression was blocked by auxin transport inhibitor treatment. The increases in auxin-induced reporter expression occurred prior to the earliest cell division events leading to formation of adventitious root primordia and is blocked by treatment with auxin transport inhibitors. This finding is consistent with other auxin-dependent gene expression events that increase upon excision in tree seedlings determined by PCR and quantitative real time-PCR (Hutchison et al., 1999; Sánchez et al., 2007; Solé et al., 2008; Vielba et al., 2011) but goes beyond those prior findings to visualize the position of these localized changes using auxin-responsive reporter constructs. Together, these experiments clearly indicate an important role of auxin transport and localized auxin accumulation in excision-induced root formation.
Additionally, we explored the alternative possibility that changes in synthesis or sensitivity to auxin drives excision-induced root formation. IAA treatment stimulates adventitious root formation in intact and root-excised hypocotyls, consistent with previous reports of treatments of intact Arabidopsis hypocotyls (Wilmoth et al., 2005). Although the magnitude of the induction by IAA treatment is greater in intact than root-excised hypocotyls, the treatment of intact hypocotyls with high doses of IAA never leads to root formation that is equivalent to induction by root excision. These results suggest that auxin distribution, which is modified by excision, rather than the inherent sensitivity to auxin, drives the elevated response in excised hypocotyls. Additionally, we did not detect significant increases in free IAA levels in hypocotyls as a result of root excision (Supplemental Fig. S3), although we cannot rule out localized changes in free IAA or conjugates that may play a role in this process. Therefore, changes in auxin transport and resulting distribution of auxin is much more strongly implicated in enhanced root initiation in response to excision.
We used a genetic approach to identify auxin transport proteins required for enhanced adventitious root formation after root excision. Four mutants with defects in IAA efflux proteins, pin1, pin3, pin7, and abcb19, showed statistically significant reductions in adventitious root formation. Two abcb19 mutant alleles had the greatest reductions in excision-induced root formation, suggesting that ABCB19 may transport auxin required for adventitious root initiation. Overexpression lines of ABCB19 had enhanced auxin transport and adventitious root initiation in intact hypocotyls, consistent with this hypothesis. Although additional proteins likely participate in the regulation of adventitious root formation, this result strongly indicates a role for ABCB19 in adventitious root formation.
To further understand the mechanism of enhanced IAA transport after root excision, we examined the GFP fluorescence of ABCB19 transcriptional and translational fusions under the control of endogenous and 35S promoters. These reporters are expressed in both epidermal tissues and vascular and pericycle cells, with the later localization linked to controlling auxin delivery to developing lateral root primordia. GFP fluorescence of ABCB19 transcriptional and translational fusions increases after root excision in the region above the point of excision, with similar increases in the central cylinder in both fusions. The increases in fluorescence in the epidermal layer are restricted to the pABCB19:ABCB19-GFP construct, suggesting that root excision may also affect ABCB19 protein abundance via a posttranscriptional mechanism. This finding is intriguing in light of the recent report that ABCB19 is phosphorylated by phototropin1 as a posttranslational regulation to modulate ABCB19 auxin transport activity (Christie et al., 2011).
We also determined that localized exogenous IAA did not significantly increase pABCB19:GFP reporter levels in intact or root-excised hypocotyls. This result suggests excision induces ABCB19 expression, leading to IAA accumulation at the hypocotyl base, rather than the converse. We quantified transcript levels of ABCB19 in intact and root-excised hypocotyls using quantitative reverse transcription-PCR but found no difference in expression levels (data not shown). The lack of detectable transcript changes is likely due to the local and tissue-specific changes in ABCB19 expression that limit detection in whole seedlings. The ABCB19 overexpression construct also provides insight into this regulation. p35S:ABCB19-GFP exhibited greater fluorescence in the hypocotyl and root in intact seedlings relative to the endogenous promoter-driven reporter. By contrast, in root-excised hypocotyls, the fluorescence of this p35S-driven construct is equivalent to the endogenous promoter-driven ABCB19 construct, consistent with the induction of ABCB19 synthesis to the levels of the overexpression line, upon root excision. These expression changes are directly correlated with elevated adventitious root formation in either root-excised wild-type or overexpression lines and the absence of cumulative induction in root-excised overexpression lines.
Surprisingly, intact seedlings of two alleles of abcb19 formed more adventitious roots than the intact wild type, with either 4- or 1.6-fold increases. The detailed examination of expression of the pABCB19-driven GFP fusion constructs provided insight into the initially surprising adventitious root phenotype. pABCB19-driven transcriptional and translational fusions to GFP show strong expression in the root and weak expression in intact hypocotyls. Elevated rooting in intact abcb19 seedlings can be explained by the higher levels of expression of ABCB19 seen in wild-type roots that act as a sink, thereby reducing auxin accumulation in wild-type hypocotyl and the absence of this sink enhancing root formation in the mutant. Consistent with this hypothesis, the auxin-responsive reporter DR5:GUS was expressed in intact abcb19 hypocotyls but not in the wild type, consistent with enhanced auxin accumulation in intact hypocotyls of this mutant.
These findings suggest mechanisms by which root excision induces adventitious root formation. The model in Figure 7 summarizes the proposed mechanisms by which the events described here are linked. In wild-type plants, root excision enhances transport of auxin and localized accumulation of auxin at the site of excision driving localized initiation and emergence of adventitious roots. In abcb19 mutant plants, intact hypocotyls form more adventitious roots, likely through the reduction in auxin transport away from the hypocotyl into the root. The abcb19 mutant also shows less induction of adventitious root initiation due to reduced hypocotyl IAA transport due to a lack of ABCB19 protein. In plants in which overexpression constructs lead to higher levels of ABCB19, there are more adventitious roots formed on the hypocotyl, as the expression of this protein is most elevated in the hypocotyl. By contrast, excision does not elevate ABCB19 expression further, so the number of adventitious roots on root-excised wild-type and ABCB19 overexpression lines are similar. Together, these results provide a logical model in which excision drives synthesis of the ABCB19 auxin transport protein leading to elevated auxin transport and localized auxin levels that drive initiation of adventitious roots.
MATERIALS AND METHODS
Plant Materials and Chemicals
Col-0 and Ws ecotypes were used in this study. Seeds were provided by Edgar Spalding (abcb19 null alleles; mdr1-1 and mdr1-3 in the Ws and Col-0 backgrounds [Lewis et al., 2007]; pABCB19:GFP, pABCB19:ABCB19-GFP, and pDR5:GUS in mdr1-1 [Wu et al., 2007]; and p35S-ABCB19 and p35S:ABCB19-GFP [Wu et al., 2010]), Gretchen Hagen (AtGH3-2:GUS line 19), Marta Laskowski (pin3 and pin7; Laskowski et al., 2008), and Malcolm Bennett (IAA2:GUS and aux1; Swarup et al., 2007). All other mutants were received from the Arabidopsis Biological Resource Center, and all are null alleles.
3H-IAA (24 and 20 Ci mmol–1) was purchased from Amersham or American Radiolabeled Chemicals. NPA was purchased from Chemical Services. RNA isolation was done using Qiagen plant RNeasy kit. Reagents used for ribonuclease treatment and complementary DNA synthesis were purchased from Invitrogen. Reagents for DNase treatment were purchased from Promega. SYBR green reagent was purchased from Applied Biosystems. All other chemicals were purchased from Sigma.
Plant Growth Conditions and Quantification of Adventitious Roots
Seeds were sterilized and placed on medium containing 0.8% (w/v) Type M agar (A-4800, Sigma), 1× Murashige and Skoog nutrients (macro and micro salts), vitamins (1 µg mL–1 thiamine, 1 µg mL–1 pyridoxine HCl, and 0.5 µg mL–1 nicotinic acid), 1.5% (w/v) Suc, and 0.05% (w/v) MES, with pH adjusted to 5.8 with 1n KOH before autoclaving. Seedlings were grown in vertical orientation with constant light at 3 to 5 µmol m–2 s–2 for 5 d to induce hypocotyl elongation. These seedling were left intact or roots, and the hypocotyl base was excised using Neuro clipper scissors (Fine Science Tools) at a position 5 to 7.5 mm from the shoot apex, followed by growth for 7 d under constant 85 to 100 m–2 s–1 light (at approximately 25°C). Adventitious roots were quantified on the seventh day unless otherwise indicated using a dissecting microscope, including only roots formed on the hypocotyl above, but not including, the root-shoot junction. DIC images of developing adventitious roots were taken using a Zeiss Axio Observer inverted microscope.
GUS Staining
AtGH3-2:GUS, AtCYCB1;1:GUS, AtIAA2:GUS, and DR5:GUS transgenic seedlings were incubated in GUS substrate (100 mm sodium phosphate buffer, 0.5% (v/v) Triton X, 2 mm X-gluc salt, 0.5 mm ferricyanide, and 0.5 mm ferrocyanide) at 37°C for 24 h. Samples were washed with 100 mm sodium phosphate buffer, pH 7, and stored in 95% ethanol. The samples were fixed in 10% (v/v) formaldehyde, 5% (v/v) acetic acid, and 50% (v/v) ethanol overnight at 4°C and cleared using chloral hydrate:glycerol:water solution (8:1:2, w/v/v) at room temperature and mounted in 95% (v/v) ethanol. GUS staining was visualized by a Leica MZ16FA epifluorescent stereomicroscope.
Applications of IAA and NPA
Stocks were prepared at 10 mm in ethanol and dimethyl sulfoxide and added to cooled medium at indicated concentrations. Experiments involving IAA treatments were placed under fluorescent lights with yellow filters (Stasinopoulos and Hangarter, 1990) to prevent white light-induced degradation of IAA. For local applications, compounds were added in 1% (w/v) agar in 5 mm MES, pH 5.5, at 50°C and placed in scintillation vials. One-millimeter agar cylinders were obtained by using sterile plastic transfer pipettes to cut cores from the solidified agar. Localized application of NPA was done as a second agar cylinder below the agar cylinder containing IAA. Observations on position and number of emerged adventitious roots were performed after 7 d using a dissecting microscope.
LSCM
GFP fluorescence was observed in water-mounted samples using a Zeiss LSM710 fluorescence laser scanning confocal microscope using either the GFP channel (J0121:GFP) at 494 to 649 nm or λ scanning (ABCB19-GFP lines). GFP and chloroplast signals were separated using two channels, 493 to 556 and 637 to 721 nm, respectively. Quantification of GFP signals were performed using linear profiles through the longitudinal sides of the cells using Zeiss Zen software. Tile scanning of hypocotyls was performed using Zen software. All images for each genotype within an experiment were captured under identical laser, gain, and pinhole settings.
Auxin Transport
Hypocotyl auxin transport measurements were performed by modifying a previously published method (Lewis and Muday, 2009). Five-day-old low-light-grown seedlings, either intact or excised, were transferred to control plates, and their shoot apices were removed. Roots were excised 48 h prior to assay. An agar cylinder or agar droplet with 3H-IAA (100 nm) was applied at the shoot end and incubated in the dark for 3 h. Three-millimeter sections were removed from the basal end from the excised hypocotyls (and at a similar position from the apex in the intact hypocotyls). Transport occurred for 3 h, and radioactivity was quantified by scintillation counting.
Methods for quantification of free IAA are provided in Supplemental Materials and Methods S1.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers ABCB19 (AT3G28860), AUX1 (AT2G38120), IAA2 (AT3G23030), PIN1 (AT1G73590), PIN2 (AT5G57090), PIN3 (AT1G70940), and PIN7 (AT1G23080).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. The effect of IAA on adventitious root formation and IAA transport in hypocotyls.
Supplemental Figure S2. Hypocotyls of AtGH3-2:GUS transgenic seedlings were stained prior to excision, at the time of excision, and at 24 h after root excision with or without 10 µm NPA added.
Supplemental Figure S3. Free IAA levels were quantified using IAA extraction followed by detection using gas chromatography-mass spectrometry.
Supplemental Figure S4. Transcript abundance of genes encoding IAA signaling and IAA transport proteins from a previously published microarray data set using hypocotyl tissues (Van Hoewyk et al., 2008).
Supplemental Figure S5. pABCB19:GFP fluorescence is found in vascular and pericycle cells.
Supplemental Figure S6. The effect of IAA on GFP fluorescence in the pABCB19:GFP line.
Supplemental Table S1. The role of shoot derived auxin in adventitious root formation.
Supplemental Materials and Methods S1. The method for free IAA measurement is outlined (Barkawi et al. 2008).
Supplementary Material
Acknowledgments
We thank Sangeeta Negi, Daniel Lewis, and Hanya Chrispeels for thoughtful comments, Gretchen Hagen, Malcolm Bennett, Marta Laskowski, and Edgar Spalding for sharing mutant or transgenic Arabidopsis seeds, Anita McCauley for microscopy assistance, and Jerry Cohen and Xing Liu for assistance in performing the free IAA measurements.
Glossary
- DIC
differential interference contrast
- LSCM
laser scanning confocal microscopy
- Col-0
ecotype Columbia
- Ws
ecotype Wassilewskija
- NPA
naphthylphthalamic acid
References
- Abarca D, Díaz-Sala C. (2009) Reprogramming adult cells during organ regeneration in forest species. Plant Signal Behav 4: 793–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkawi LS, Tam YY, Tillman JA, Pederson B, Calio J, Al-Amier H, Emerick M, Normanly J, Cohen JD. (2008) A high-throughput method for the quantitative analysis of indole-3-acetic acid and other auxins from plant tissue. Anal Biochem 372: 177–188 [DOI] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J. (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115: 591–602 [DOI] [PubMed] [Google Scholar]
- Boerjan W, Cervera MT, Delarue M, Beeckman T, Dewitte W, Bellini C, Caboche M, Van Onckelen H, Van Montagu M, Inzé D. (1995) Superroot, a recessive mutation in Arabidopsis, confers auxin overproduction. Plant Cell 7: 1405–1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celenza JL, Jr, Grisafi PL, Fink GR. (1995) A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev 9: 2131–2142 [DOI] [PubMed] [Google Scholar]
- Christie JM, Yang H, Richter GL, Sullivan S, Thomson CE, Lin J, Titapiwatanakun B, Ennis M, Kaiserli E, Lee OR, et al. (2011) phot1 inhibition of ABCB19 primes lateral auxin fluxes in the shoot apex required for phototropism. PLoS Biol 9: e1001076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correa, Lda R, Troleis J, Mastroberti AA, Mariath JE, Fett-Neto AG. (2012) Distinct modes of adventitious rooting in Arabidopsis thaliana. Plant Biol (Stuttg) 14: 100–109 [DOI] [PubMed] [Google Scholar]
- De Klerk G-J, Van Der Krieken W, De Jong JC (1999) The formation of adventitious roots: new concepts, new possibilities. In Vitro Cel Dev Biol-Plant 35: 481–490 [Google Scholar]
- Díaz-Sala C, Garrido G, Sabater B. (2002) Age-related loss of rooting capability in Arabidopsis thaliana and its reversal by peptides containing the Arg-Gly-Asp (RGD) motif. Physiol Plant 114: 601–607 [DOI] [PubMed] [Google Scholar]
- Diaz-Sala C, Hutchison K, Goldfarb B, Greenwood M. (1996) Maturation-related loss in rooting competence by loblolly pine stem cutting: the role of auxin transport, metabolism and tissue sensitivity. Physiol Plant 97: 481–490 [Google Scholar]
- DiDonato RJ, Arbuckle E, Buker S, Sheets J, Tobar J, Totong R, Grisafi P, Fink GR, Celenza JL. (2004) Arabidopsis ALF4 encodes a nuclear-localized protein required for lateral root formation. Plant J 37: 340–353 [DOI] [PubMed] [Google Scholar]
- Falasca G, Altamura M. (2003) Histological analysis of adventitious rooting in Arabidopsis thaliana (L.) Heynh seedlings. Plant Biosyst 137: 265–274 [Google Scholar]
- Friml J, Wiśniewska J, Benková E, Mendgen K, Palme K. (2002) Lateral relocation of auxin efflux regulator PIN3 mediates tropism in Arabidopsis. Nature 415: 806–809 [DOI] [PubMed] [Google Scholar]
- Gälweiler L, Guan C, Müller A, Wisman E, Mendgen K, Yephremov A, Palme K. (1998) Regulation of polar auxin transport by AtPIN1 in Arabidopsis vascular tissue. Science 282: 2226–2230 [DOI] [PubMed] [Google Scholar]
- Garrido G, Ramón Guerrero J, Angel Cano E, Acosta M, Sánchez-Bravo J. (2002) Origin and basipetal transport of the IAA responsible for rooting of carnation cuttings. Physiol Plant 114: 303–312 [DOI] [PubMed] [Google Scholar]
- Guerrero JR, Garrido G, Acosta M, Sánchez-Bravo J. (1999) Influence of 2,3,5-triiodobenzoic acid and 1-N-naphthylphthalamic acid on indoleacetic acid transport in carnation cuttings: relationship with rooting. J Plant Growth Regul 18: 183–190 [DOI] [PubMed] [Google Scholar]
- Gutierrez L, Bussell JD, Pacurar DI, Schwambach J, Pacurar M, Bellini C. (2009) Phenotypic plasticity of adventitious rooting in Arabidopsis is controlled by complex regulation of AUXIN RESPONSE FACTOR transcripts and microRNA abundance. Plant Cell 21: 3119–3132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchison KW, Singer PB, McInnis S, Diaz-Sala C, Greenwood MS. (1999) Expansins are conserved in conifers and expressed in hypocotyls in response to exogenous auxin. Plant Physiol 120: 827–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inukai Y, Sakamoto T, Ueguchi-Tanaka M, Shibata Y, Gomi K, Umemura I, Hasegawa Y, Ashikari M, Kitano H, Matsuoka M. (2005) Crown rootless1, which is essential for crown root formation in rice, is a target of an AUXIN RESPONSE FACTOR in auxin signaling. Plant Cell 17: 1387–1396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplaze L, Parizot B, Baker A, Ricaud L, Martinière A, Auguy F, Franche C, Nussaume L, Bogusz D, Haseloff J. (2005) GAL4-GFP enhancer trap lines for genetic manipulation of lateral root development in Arabidopsis thaliana. J Exp Bot 56: 2433–2442 [DOI] [PubMed] [Google Scholar]
- Laskowski M, Grieneisen VA, Hofhuis H, Hove CA, Hogeweg P, Marée AF, Scheres B. (2008) Root system architecture from coupling cell shape to auxin transport. PLoS Biol 6: e307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Miller ND, Splitt BL, Wu G, Spalding EP. (2007) Separating the roles of acropetal and basipetal auxin transport on gravitropism with mutations in two Arabidopsis multidrug resistance-like ABC transporter genes. Plant Cell 19: 1838–1850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DR, Muday GK. (2009) Measurement of auxin transport in Arabidopsis thaliana. Nat Protoc 4: 437–451 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Negi S, Sukumar P, Muday GK. (2011) Ethylene inhibits lateral root development, increases IAA transport and expression of PIN3 and PIN7 auxin efflux carriers. Development 138: 3485–3495 [DOI] [PubMed] [Google Scholar]
- Lewis DR, Wu G, Ljung K, Spalding EP. (2009) Auxin transport into cotyledons and cotyledon growth depend similarly on the ABCB19 Multidrug Resistance-like transporter. Plant J 60: 91–101 [DOI] [PubMed] [Google Scholar]
- Leyser HM, Pickett FB, Dharmasiri S, Estelle M. (1996) Mutations in the AXR3 gene of Arabidopsis result in altered auxin response including ectopic expression from the SAUR-AC1 promoter. Plant J 10: 403–413 [DOI] [PubMed] [Google Scholar]
- Li S, Xue L, Xu S, Fieng H, An L. (2009) Mediators, genes, and signaling in adventitious rooting. Bot Rev 75: 230–247 [Google Scholar]
- Li Y-H, Zou MH, Feng B-H, Huang X, Zhang Z, Sun G-M. (2012) Molecular cloning and characterization of the genes encoding an auxin efflux carrier and the auxin influx carriers associated with the adventitious root formation in mango (Mangifera indica L.) cotyledon segments. Plant Physiol Biochem 55: 33–42 [DOI] [PubMed] [Google Scholar]
- Liu H, Wang S, Yu X, Yu J, He X, Zhang S, Shou H, Wu P. (2005) ARL1, a LOB-domain protein required for adventitious root formation in rice. Plant J 43: 47–56 [DOI] [PubMed] [Google Scholar]
- Liu X, Cohen JD, Gardner G. (2011) Low-fluence red light increases the transport and biosynthesis of auxin. Plant Physiol 157: 891–904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludwig-Müller J, Vertocnik A, Town CD. (2005) Analysis of indole-3-butyric acid-induced adventitious root formation on Arabidopsis stem segments. J Exp Bot 56: 2095–2105 [DOI] [PubMed] [Google Scholar]
- Luschnig C, Gaxiola RA, Grisafi P, Fink GR. (1998) EIR1, a root-specific protein involved in auxin transport, is required for gravitropism in Arabidopsis thaliana. Genes Dev 12: 2175–2187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malamy JE, Benfey PN. (1997) Organization and cell differentiation in lateral roots of Arabidopsis thaliana. Development 124: 33–44 [DOI] [PubMed] [Google Scholar]
- Malamy JE, Ryan KS. (2001) Environmental regulation of lateral root initiation in Arabidopsis. Plant Physiol 127: 899–909 [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Bhalerao R, Casimiro I, Eklöf J, Casero PJ, Bennett M, Sandberg G. (2002) AUX1 promotes lateral root formation by facilitating indole-3-acetic acid distribution between sink and source tissues in the Arabidopsis seedling. Plant Cell 14: 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ. (1999) AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J 18: 2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita Y, Kyozuka J. (2007) Characterization of OsPID, the rice ortholog of PINOID, and its possible involvement in the control of polar auxin transport. Plant Cell Physiol 48: 540–549 [DOI] [PubMed] [Google Scholar]
- Noh B, Murphy AS, Spalding EP. (2001) Multidrug resistance-like genes of Arabidopsis required for auxin transport and auxin-mediated development. Plant Cell 13: 2441–2454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada K, Ueda J, Komaki MK, Bell CJ, Shimura Y. (1991) Requirement of the auxin polar transport system in early stages of Arabidopsis floral bud formation. Plant Cell 3: 677–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, Beeckman T, Bennett MJ. (2009) Arabidopsis lateral root development: an emerging story. Trends Plant Sci 14: 399–408 [DOI] [PubMed] [Google Scholar]
- Pickett FB, Wilson AK, Estelle M. (1990) The aux1 mutation of Arabidopsis confers both auxin and ethylene resistance. Plant Physiol 94: 1462–1466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashotte AM, Poupart J, Waddell CS, Muday GK. (2003) Transport of the two natural auxins, indole-3-butyric acid and indole-3-acetic acid, in Arabidopsis. Plant Physiol 133: 761–772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez C, Vielba JM, Ferro E, Covelo G, Solé A, Abarca D, de Mier BS, Díaz-Sala C. (2007) Two SCARECROW-LIKE genes are induced in response to exogenous auxin in rooting-competent cuttings of distantly related forest species. Tree Physiol 27: 1459–1470 [DOI] [PubMed] [Google Scholar]
- Solé A, Sánchez C, Vielba JM, Valladares S, Abarca D, Díaz-Sala C. (2008) Characterization and expression of a Pinus radiata putative ortholog to the Arabidopsis SHORT-ROOT gene. Tree Physiol 28: 1629–1639 [DOI] [PubMed] [Google Scholar]
- Sorin C, Bussell JD, Camus I, Ljung K, Kowalczyk M, Geiss G, McKhann H, Garcion C, Vaucheret H, Sandberg G, et al. (2005) Auxin and light control of adventitious rooting in Arabidopsis require ARGONAUTE1. Plant Cell 17: 1343–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorin C, Negroni L, Balliau T, Corti H, Jacquemot MP, Davanture M, Sandberg G, Zivy M, Bellini C. (2006) Proteomic analysis of different mutant genotypes of Arabidopsis led to the identification of 11 proteins correlating with adventitious root development. Plant Physiol 140: 349–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasinopoulos TC, Hangarter RP. (1990) Preventing photochemistry in culture media by long-pass light filters alters growth of cultured tissues. Plant Physiol 93: 1365–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staswick PE, Serban B, Rowe M, Tiryaki I, Maldonado MT, Maldonado MC, Suza W. (2005) Characterization of an Arabidopsis enzyme family that conjugates amino acids to indole-3-acetic acid. Plant Cell 17: 616–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup K, Benková E, Swarup R, Casimiro I, Péret B, Yang Y, Parry G, Nielsen E, De Smet I, Vanneste S, et al. (2008) The auxin influx carrier LAX3 promotes lateral root emergence. Nat Cell Biol 10: 946–954 [DOI] [PubMed] [Google Scholar]
- Swarup R, Perry P, Hagenbeek D, Van Der Straeten D, Beemster GT, Sandberg G, Bhalerao R, Ljung K, Bennett MJ. (2007) Ethylene upregulates auxin biosynthesis in Arabidopsis seedlings to enhance inhibition of root cell elongation. Plant Cell 19: 2186–2196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi F, Sato-Nara K, Kobayashi K, Suzuki M, Suzuki H. (2003) Sugar-induced adventitious roots in Arabidopsis seedlings. J Plant Res 116: 83–91 [DOI] [PubMed] [Google Scholar]
- Tian Q, Reed JW. (1999) Control of auxin-regulated root development by the Arabidopsis thaliana SHY2/IAA3 gene. Development 126: 711–721 [DOI] [PubMed] [Google Scholar]
- Van Hoewyk D, Takahashi H, Inoue E, Hess A, Tamaoki M, Pilon-Smits EA. (2008) Transcriptome analyses give insights into selenium-stress responses and selenium tolerance mechanisms in Arabidopsis. Physiol Plant 132: 236–253 [DOI] [PubMed] [Google Scholar]
- Vielba JM, Díaz-Sala C, Ferro E, Rico S, Lamprecht M, Abarca D, Ballester A, Sánchez C. (2011) CsSCL1 is differentially regulated upon maturation in chestnut microshoots and is specifically expressed in rooting-competent cells. Tree Physiol 31: 1152–1160 [DOI] [PubMed] [Google Scholar]
- Visser E, Cohen JD, Barendse G, Blom C, Voesenek L. (1996) An ethylene-mediated increase in sensitivity to auxin induces adventitious root formation in flooded rumex palustris Sm. Plant Physiol 112: 1687–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilmoth JC, Wang S, Tiwari SB, Joshi AD, Hagen G, Guilfoyle TJ, Alonso JM, Ecker JR, Reed JW. (2005) NPH4/ARF7 and ARF19 promote leaf expansion and auxin-induced lateral root formation. Plant J 43: 118–130 [DOI] [PubMed] [Google Scholar]
- Wu G, Cameron JN, Ljung K, Spalding EP. (2010) A role for ABCB19-mediated polar auxin transport in seedling photomorphogenesis mediated by cryptochrome 1 and phytochrome B. Plant J 62: 179–191 [DOI] [PubMed] [Google Scholar]
- Wu G, Lewis DR, Spalding EP. (2007) Mutations in Arabidopsis multidrug resistance-like ABC transporters separate the roles of acropetal and basipetal auxin transport in lateral root development. Plant Cell 19: 1826–1837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zazímalová E, Murphy AS, Yang HB, Hoyerová K, Hosek P. (2010) Auxin transporters—why so many? Cold Spring Harb Perspect Biol 2: a001552. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.