Abstract
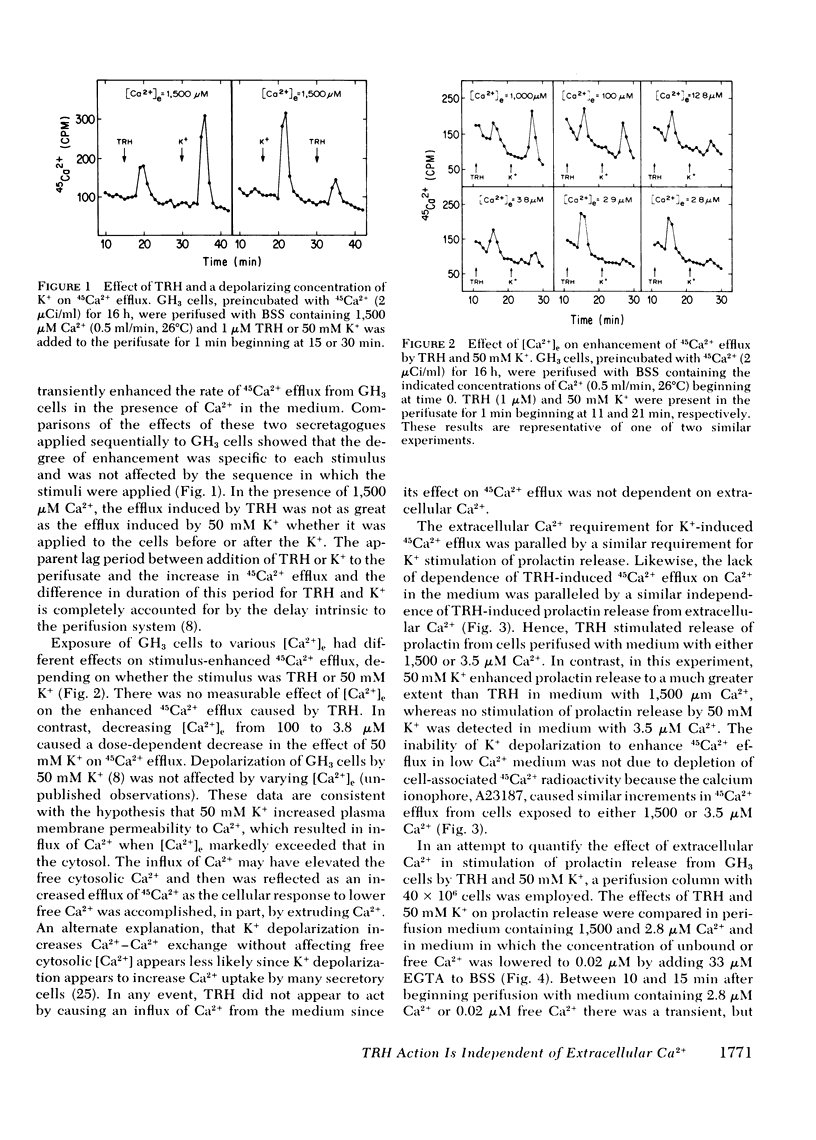
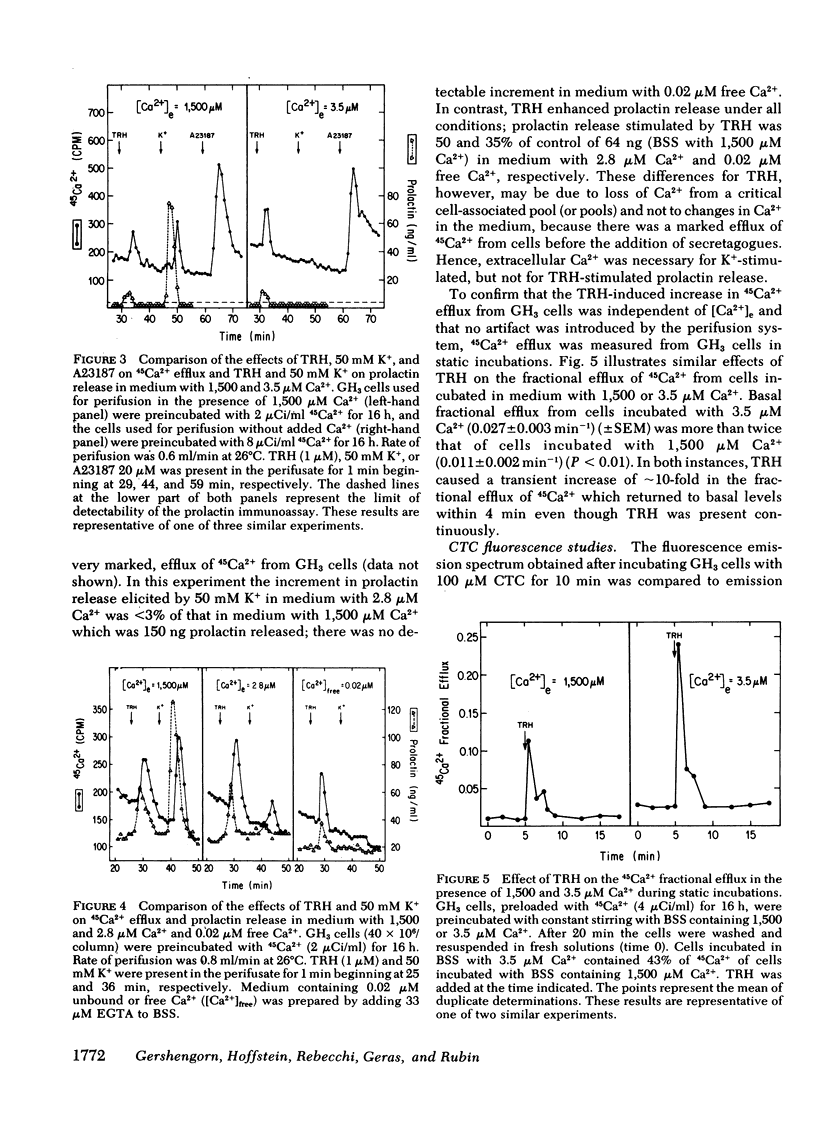
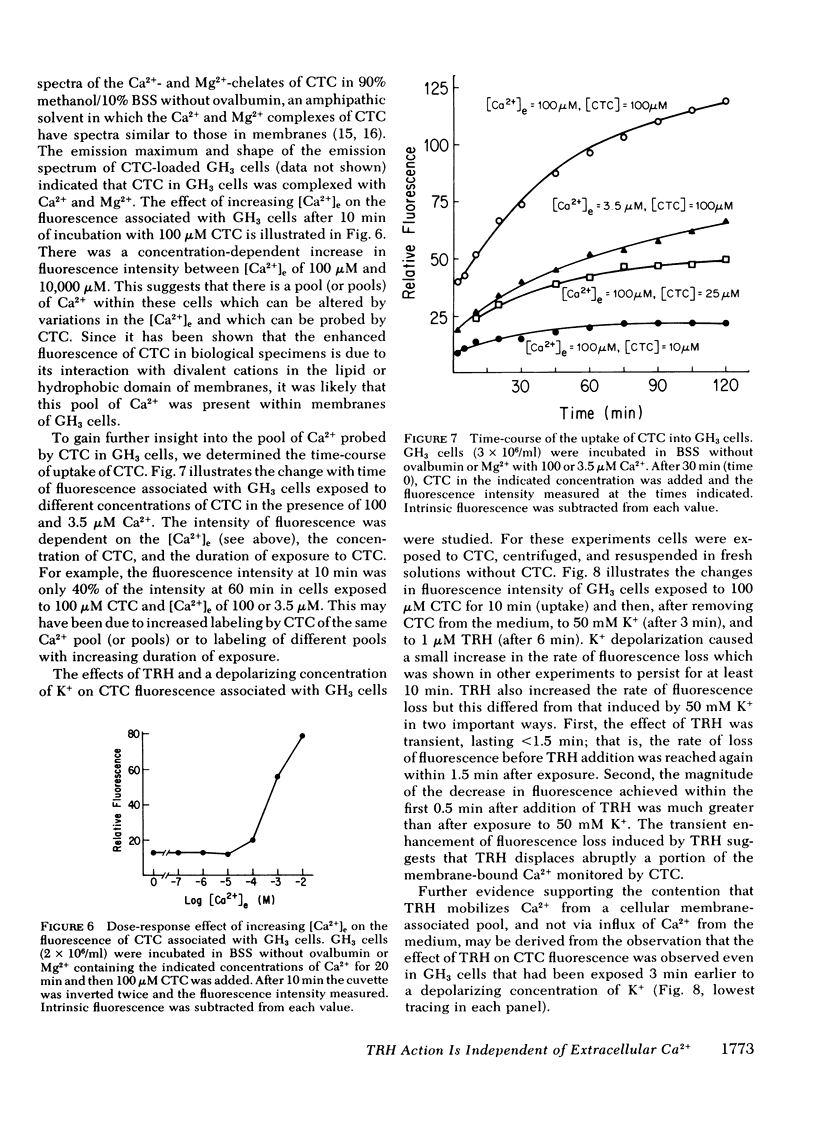
Thyrotropin-releasing hormone (TRH) stimulates prolactin release and 45Ca2+ efflux from GH3 cells, a clonal strain of rat pituitary cells. Elevation of extracellular K+ also induces prolactin release and increases 45Ca2+ efflux from these cells. In this report, we distinguish between TRH and high K+ as secretagogues and show that TRH-induced release of prolactin and 45Ca2+ is independent of the extracellular Ca2+ concentration, but the effect of high K+ on prolactin release and 45Ca2+ efflux is dependent on the concentration of Ca2+ in the medium. The increment in 45Ca2+ efflux induced by 50 mM K+ during perifusion was reduced in a concentration-dependent manner by lowering extracellular Ca2+ from 1,500 to 0.02 μM (by adding EGTA), whereas 1 μM TRH enhanced 45Ca2+ efflux similarly over the entire range of extracellular Ca2+ concentrations. Although 50 mM K+ caused release of 150 ng prolactin from 40 × 106 GH3 cells exposed to 1,500 μM Ca2+ (control), reduction of extracellular Ca2+ to 2.8 μM decreased prolactin release caused by high K+ to <3% of controls and no prolactin release was detected after exposure to 50 mM K+ in medium with 0.02 μM free Ca2+. In contrast, TRH caused release of 64 ng of prolactin from 40 × 106 GH3 cells exposed to medium with 1,500 μM Ca2+, and release caused by TRH was still 50 and 35% of control in medium with 2.8 and 0.02 μM Ca2+, respectively. Furthermore, TRH transiently increased by 10-fold the fractional efflux of 45Ca2+ from GH3 cells in static incubations with 1,500 or 3.5 μM Ca2+, hereby confirming that the enhanced 45Ca2+ efflux caused by TRH in both low and high Ca2+ medium was not an artifact of the perifusion system.
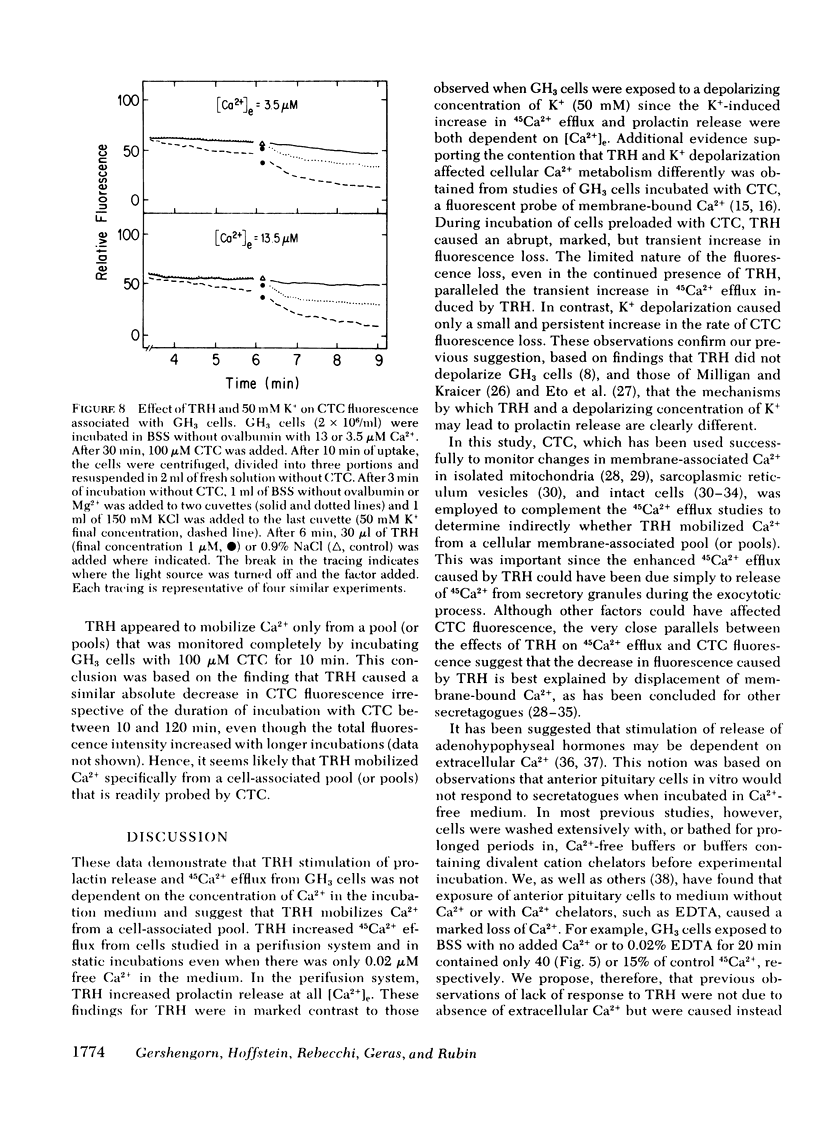
Data obtained with chlortetracycline (CTC), a probe of membrane-bound Ca2+, were concordant with those obtained by measuring 45Ca2+ efflux. Cellular fluorescence of CTC varied with the extracellular Ca2+ concentration and the duration of incubation. TRH decreased the fluorescence of cell-associated CTC in a manner strongly suggesting stimulus-induced mobilization of Ca2+, and this effect was still demonstrable in GH3 cells incubated in 50 mM K+.
These data suggest that TRH acts to mobilize sequestered cell-associated Ca2+ reflected as a 45Ca2+ efflux which is independent of the extracellular Ca2+ concentration. Mobilization of sequestered Ca2+ into the cytoplasm may elevate free intracellular Ca2+ and serve to couple stimulation by TRH to secretion of prolactin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft F. C. Measurement of growth hormone synthesis by rat pituitary cells in culture. Endocrinology. 1973 Apr;92(4):1014–1021. doi: 10.1210/endo-92-4-1014. [DOI] [PubMed] [Google Scholar]
- Biales B., Dichter M. A., Tischler A. Sodium and calcium action potential in pituitary cells. Nature. 1977 May 12;267(5607):172–174. doi: 10.1038/267172a0. [DOI] [PubMed] [Google Scholar]
- Caswell A. H., Hutchison J. D. Visualization of membrane bound cations by a fluorescent technique. Biochem Biophys Res Commun. 1971 Jan 8;42(1):43–49. doi: 10.1016/0006-291x(71)90359-7. [DOI] [PubMed] [Google Scholar]
- Caswell A. H., Warren S. Observation of calcium uptake by isolated sarcoplasmic reticulum employing a fluorescent chelate probe. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1757–1763. doi: 10.1016/0006-291x(72)90047-2. [DOI] [PubMed] [Google Scholar]
- Chandler D. E., Williams J. A. Fluorescent probe detects redistribution of cell calcium during stimulus-secretion coupling. Nature. 1977 Aug 18;268(5621):659–660. doi: 10.1038/268659a0. [DOI] [PubMed] [Google Scholar]
- Chandler D. E., Williams J. A. Intracellular divalent cation release in pancreatic acinar cells during stimulus-secretion coupling. I. Use of chlorotetracycline as fluorescent probe. J Cell Biol. 1978 Feb;76(2):371–385. doi: 10.1083/jcb.76.2.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. E., Williams J. A. Intracellular divalent cation release in pancreatic acinar cells during stimulus-secretion coupling. II. Subcellular localization of the fluorescent probe chlorotetracycline. J Cell Biol. 1978 Feb;76(2):386–399. doi: 10.1083/jcb.76.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: variations on the theme of calcium-activated exocytosis involving cellular and extracellular sources of calcium. Ciba Found Symp. 1978;(54):61–90. doi: 10.1002/9780470720356.ch4. [DOI] [PubMed] [Google Scholar]
- Dufy B., Vincent J. D., Fleury H., Du Pasquier P., Gourdji D., Tixier-Vidal A. Membrane effects of thyrotropin-releasing hormone and estrogen shown by intracellular recording from pituitary cells. Science. 1979 May 4;204(4392):509–511. doi: 10.1126/science.107590. [DOI] [PubMed] [Google Scholar]
- Eto S., Wood J. M., Hutchins M., Fleischer N. Pituitary 45 Ca ion uptake and release of ACTH, GH, and TSH: effect of verapamil. Am J Physiol. 1974 Jun;226(6):1315–1320. doi: 10.1152/ajplegacy.1974.226.6.1315. [DOI] [PubMed] [Google Scholar]
- Finkelstein M. C., Adelberg E. A. Neutral amino acid transport in an established mouse lymphocytic cell line. J Biol Chem. 1977 Oct 25;252(20):7101–7108. [PubMed] [Google Scholar]
- Gershengorn M. C. Bihormonal regulation of the thyrotropin-releasing hormone receptor in mouse pituitary thyrotropic tumor cells in culture. J Clin Invest. 1978 Nov;62(5):937–943. doi: 10.1172/JCI109222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershengorn M. C., Marcus-Samuels B. E., Geras E. Estrogens increase the number of thyrotropin-releasing hormone receptors on mammotropic cells in culture. Endocrinology. 1979 Jul;105(1):171–176. doi: 10.1210/endo-105-1-171. [DOI] [PubMed] [Google Scholar]
- Gershengorn M. C. Thyrotropin releasing hormone stimulation of prolactin release. Evidence for a membrane potential-independent, Ca2+-dependent mechanism of action. J Biol Chem. 1980 Mar 10;255(5):1801–1803. [PubMed] [Google Scholar]
- Grant G., Vale W., Guillemin R. Interaction of thyrotropin releasing factor with membrane receptors of pituitary cells. Biochem Biophys Res Commun. 1972 Jan 14;46(1):28–34. doi: 10.1016/0006-291x(72)90625-0. [DOI] [PubMed] [Google Scholar]
- Hallett M., Schneider A. S., Carbone E. Tetracycline fluorescence as calcium-probe for nerve membrane with some model studies using erythrocyte ghosts. J Membr Biol. 1972;10(1):31–44. doi: 10.1007/BF01867846. [DOI] [PubMed] [Google Scholar]
- Hinkle P. M., Tashjian A. H., Jr Receptors for thyrotropin-releasing hormone in prolactin producing rat pituitary cells in culture. J Biol Chem. 1973 Sep 10;248(17):6180–6186. [PubMed] [Google Scholar]
- Hoffstein S. T. Ultrastructural demonstration of calcium loss from local regions of the plasma membrane of surface-stimulated human granulocytes. J Immunol. 1979 Sep;123(3):1395–1402. [PubMed] [Google Scholar]
- Kidokoro Y. Spontaneous calcium action potentials in a clonal pituitary cell line and their relationship to prolactin secretion. Nature. 1975 Dec 25;258(5537):741–742. doi: 10.1038/258741a0. [DOI] [PubMed] [Google Scholar]
- Labrie F., Barden N., Poirier G., De Lean A. Binding of thyrotropin-releasing hormone to plasma membranes of bovine anterior pituitary gland (hormone receptor-adenylate cyclase-equilibrium constant-( 3 H)thyrotropin). Proc Natl Acad Sci U S A. 1972 Jan;69(1):283–287. doi: 10.1073/pnas.69.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry P. J., McMartin C. Measurement of the dynamics of stimulation and inhibition of steroidogenesis in isolated rat adrenal cells by using column perfusion. Biochem J. 1974 Aug;142(2):287–294. doi: 10.1042/bj1420287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthra R., Olson M. S. Studies of mitochondrial calcium movements using chlorotetracycline. Biochim Biophys Acta. 1976 Sep 13;440(3):744–758. doi: 10.1016/0005-2728(76)90056-6. [DOI] [PubMed] [Google Scholar]
- Martin S., York D. H., Kraicer J. Alterations in transmembrane potential of adenohypophysial cells in elevated potassium and calcium-free media. Endocrinology. 1973 Apr;92(4):1084–1088. doi: 10.1210/endo-92-4-1084. [DOI] [PubMed] [Google Scholar]
- Matthews E. K., Petersen O. H., Williams J. A. Pancreatic acinar cells: acetylcholine-induced membrane depolarization, calcium efflux and amylase release. J Physiol. 1973 Nov;234(3):689–701. doi: 10.1113/jphysiol.1973.sp010367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan J. V., Kraicer J. 45Ca uptake during the in vitro release of hormones from the rat adenohypophysis. Endocrinology. 1971 Sep;89(3):766–773. doi: 10.1210/endo-89-3-766. [DOI] [PubMed] [Google Scholar]
- Milligan J. V., Kraicer J. Physical characteristics of the Ca++ compartments associated with in vitro ACTH release. Endocrinology. 1974 Feb;94(2):435–443. doi: 10.1210/endo-94-2-435. [DOI] [PubMed] [Google Scholar]
- Naccache P. H., Showell H. J., Becker E. L., Sha'afi R. I. Involvement of membrane calcium in the response of rabbit neutrophils to chemotactic factors as evidenced by the fluorescence of chlorotetracycline. J Cell Biol. 1979 Oct;83(1):179–186. doi: 10.1083/jcb.83.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S., Kimura N. Membrane potential changes caused by thyrotropin-releasing hormone in the clonal GH3 cell and their relationship to secretion of pituitary hormone. Proc Natl Acad Sci U S A. 1979 Nov;76(11):6017–6020. doi: 10.1073/pnas.76.11.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen H., Goodman D. B. Relationships between calcium and cyclic nucleotides in cell activation. Physiol Rev. 1977 Jul;57(3):421–509. doi: 10.1152/physrev.1977.57.3.421. [DOI] [PubMed] [Google Scholar]
- Schrey M. P., Brown B. L., Ekins R. P. Studies on the role of calcium and cyclic nucleotides in the control of TSH secretion. Mol Cell Endocrinol. 1978 Sep;11(3):249–264. doi: 10.1016/0303-7207(78)90012-6. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Action potentials occur in cells of the normal anterior pituitary gland and are stimulated by the hypophysiotropic peptide thyrotropin-releasing hormone. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4064–4067. doi: 10.1073/pnas.74.9.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Lomedico M. E., Maina D. Role of calcium in the thyrotropin-releasing hormone-stimulated release of prolactin from pituitary cells in culture. Biochem Biophys Res Commun. 1978 Apr 14;81(3):798–806. doi: 10.1016/0006-291x(78)91422-5. [DOI] [PubMed] [Google Scholar]
- Tashjian A. H., Jr, Yasumura Y., Levine L., Sato G. H., Parker M. L. Establishment of clonal strains of rat pituitary tumor cells that secrete growth hormone. Endocrinology. 1968 Feb;82(2):342–352. doi: 10.1210/endo-82-2-342. [DOI] [PubMed] [Google Scholar]
- Täljedal I. B. Chlorotetracycline as a fluorescent Ca2+ probe in pancreatic islet cells. J Cell Biol. 1978 Mar;76(3):652–674. doi: 10.1083/jcb.76.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vale W., Burgus R., Guillemin R. Presence of calcium ions as a requisite for the in vitro stimulation of TSH-release by hypothalamic TRF. Experientia. 1967 Oct 15;23(10):853–855. doi: 10.1007/BF02146887. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier C., Brown M., Chan L., Ling N., Rivier J. Applications of adenohypophyseal cell cultures to neuroendocrine studies. Curr Top Mol Endocrinol. 1976;3:397–429. doi: 10.1007/978-1-4684-2598-7_23. [DOI] [PubMed] [Google Scholar]
- Vale W., Rivier C., Brown M. Regulatory peptides of the hypothalamus. Annu Rev Physiol. 1977;39:473–527. doi: 10.1146/annurev.ph.39.030177.002353. [DOI] [PubMed] [Google Scholar]
- Williams J. A. Stimulation of 45Ca2+ efflux from rat pituitary by luteinizing hormone-releasing hormone and other pituitary stimulants. J Physiol. 1976 Aug;260(1):105–115. doi: 10.1113/jphysiol.1976.sp011506. [DOI] [PMC free article] [PubMed] [Google Scholar]


