A triacylglycerol lipase knockout boosts the oil content of wild-type plants and transgenic plants genetically engineered to make more oil.
Abstract
There has been considerable interest recently in the prospect of engineering crops to produce triacylglycerol (TAG) in their vegetative tissues as a means to achieve a step change in oil yield. Here, we show that disruption of TAG hydrolysis in the Arabidopsis (Arabidopsis thaliana) lipase mutant sugar-dependent1 (sdp1) leads to a substantial accumulation of TAG in roots and stems but comparatively much lower TAG accumulation in leaves. TAG content in sdp1 roots increases with the age of the plant and can reach more than 1% of dry weight at maturity, a 50-fold increase over the wild type. TAG accumulation in sdp1 roots requires both ACYL-COENZYME A:DIACYLGLYCEROL ACYLTRANSFERASE1 (DGAT1) and PHOSPHATIDYLCHOLINE:DIACYLGLYCEROL ACYLTRANSFERASE1 and can also be strongly stimulated by the provision of exogenous sugar. In transgenic plants constitutively coexpressing WRINKLED1 and DGAT1, sdp1 also doubles the accumulation of TAG in roots, stems, and leaves, with levels ranging from 5% to 8% of dry weight. Finally, provision of 3% (w/v) exogenous Suc can further boost root TAG content in these transgenic plants to 17% of dry weight. This level of TAG is similar to seed tissues in many plant species and establishes the efficacy of an engineering strategy to produce oil in vegetative tissues that involves simultaneous manipulation of carbohydrate supply, fatty acid synthesis, TAG synthesis, and also TAG breakdown.
Vegetable oils (triacylglycerols [TAGs]) are a major global commodity. They contribute significantly to human and livestock nutrition, provide versatile feedstock for the chemical industry, and also serve as a renewable energy source in the form of biodiesel (Durrett et al., 2008; Dyer et al., 2008). World production has increased dramatically in the last decade and now exceeds 150 million metric tons per year (http://www.fas.usda.gov/oilseeds/). There is a pressing need to improve the yield of oil crops to meet the world’s growing demand for renewable oils (Lu et al., 2011). It has recently been proposed that a step change in oil yield may be achievable in terrestrial crops if they can be engineered to produce oil in vegetative tissues rather than in seeds (Durrett et al., 2008; Ohlrogge et al., 2009). It has been estimated that, if the perennial biomass crop Miscanthus giganteus produced 20% of its harvestable dry mass as oil, the total oil yield per hectare would be three times that of a conventional oilseed crop such as Brassica napus (Durrett et al., 2008).
Lipid bodies have been observed in the leaves of many plants (Lersten et al., 2006), and oil in vegetative tissues has previously been proposed to play a role in carbon storage and membrane lipid remodeling (Murphy, 2001; James et al., 2010). Nevertheless, the oil content of leaves, stems, and roots is extremely low in all but a very few plant species (Durrett et al., 2008). For example, oil accounts for much less than 0.1% of dry weight in Arabidopsis (Arabidopsis thaliana) leaves (Yang and Ohlrogge, 2009). However, a number of studies have established that the oil content can be boosted by the overexpression of individual oil biosynthetic enzymes such as ACYL-COENZYME A:DIACYLGLYCEROL ACYLTRANSFERASE1 (DGAT1; Bouvier-Navé et al., 2000) or transcriptional “master” regulators that govern the expression of multiple enzymes in the pathway, such as WRINKLED1 (WRI1), LEAFY COTYLDON1 (LEC1), and LEC2 (Cernac and Benning, 2004; Mu et al., 2008; Andrianov et al., 2010; Sanjaya et al., 2011). In addition, several mutants have been identified that exhibit ectopic oil accumulation (Ogas et al., 1997; Xu et al., 2005; Kunz et al., 2009; Slocombe et al., 2009; James et al., 2010). Among these are pxa1 (peroxisomal ABC transporter1) and cgi58 (comparative gene identification-58), which are associated with lipid catabolism. PXA1 is a peroxisomal ATP-binding cassette transporter that is required for fatty acid import for β-oxidation (Zolman et al., 2001), and CGI58 is a protein that has intrinsic lipase, phospholipase, and lysophosphatidic acid acyltransferase activities (Ghosh et al., 2009).
Oil content is controlled by the balance between synthesis and breakdown in many eukaryotes, and a deficiency in TAG hydrolysis has been shown to result in greater oil deposition (Zimmermann et al., 2004; Grönke et al., 2005; Kurat et al., 2006). We previously identified a small family of TAG lipase genes in Arabidopsis, consisting of SUGAR-DEPENDENT1 (SDP1) and SDP1-LIKE (SDP1L), which appear to be directly responsible for initiating oil breakdown in the seeds following germination (Eastmond, 2006; Kelly et al., 2011). SDP1 and SDP1L are members of an unorthodox group of lipases that are related to patatin from potato (Solanum tuberosum) but contain a divergent active site (Scherer et al., 2010). Well-characterized examples include human adipose triglyceride lipase (Zimmermann et al., 2004), Drosophila melanogaster Brummer (Grönke et al., 2005), and Saccharomyces cerevisiae TRIACYLGLYCEROL LIPASE3, TRIACYLGLYCEROL LIPASE4, and TRIACYLGLYCEROL LIPASE5 (Athenstaedt and Daum, 2005; Kurat et al., 2006).
Interestingly, although SDP1 is most strongly expressed in seeds, transcripts can also be detected in all vegetative tissues (Eastmond, 2006; Kelly et al., 2011). Likewise, genes encoding enzymes that catalyze the committed step for oil synthesis, such as DGAT1, DGAT3, and PHOSPHATIDYLCHOLINE:DIACYLGLYCEROL ACYLTRANSFERASE1 (PDAT1), are also expressed in vegetative tissues (Zhang et al., 2009; Hernández et al., 2012). Given evidence that key enzymes for both oil synthesis and breakdown are expressed in vegetative tissues, the aim of this study was to investigate whether SDP1-mediated oil turnover might limit oil accumulation in leaves, stems, and roots of wild-type Arabidopsis and also transgenic lines engineered to synthesize more oil. Our data suggest that this is the case particularly in heterotrophic tissues such as roots, where oil can accumulate to more than 1% of dry weight in sdp1, while levels as high as 17% of dry weight are achievable in sdp1 roots by providing an exogenous sugar supply and enhancing fatty acid synthesis and TAG synthesis by overexpression of WRI1 and DGAT1.
RESULTS
SDP1 Is Expressed in Vegetative Tissues of Arabidopsis
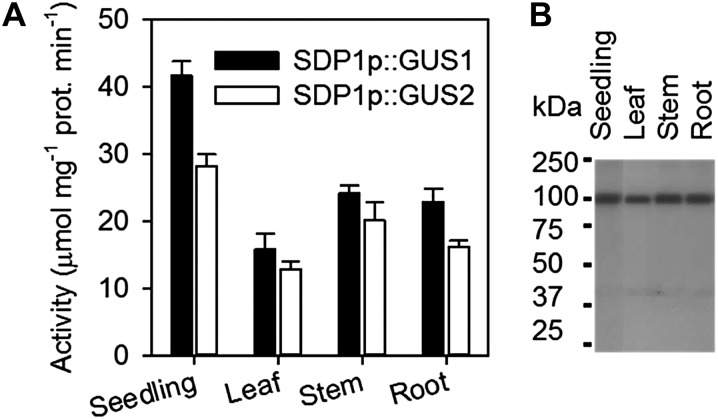
To confirm that the SDP1 promoter is active in the vegetative tissues of Arabidopsis, and to investigate the spatial distribution in more detail, we created GUS reporter lines containing GUS driven by 1.5 kb of the SDP1 promoter. Quantitative assays performed on two independent transgenic lines showed that GUS is expressed in leaves, stems, and roots of 4-week-old plants as well as in 3-d-old seedlings (Fig. 1A), where seed storage oil mobilization is occurring (Eastmond, 2006). Histochemical staining for GUS also suggested that expression was in all cells, but that it was strongest in the veins of the leaf and stele of the roots and also in the tips of main and lateral roots (Supplemental Fig. S1). To confirm that the SDP1 protein is also present in these tissues, western blotting was performed on material from a complemented sdp1-5 mutant line expressing hemagglutinin (HA)-tagged SDP1 under the control of the same SDP1 promoter (Kelly et al., 2011). SDP1-HA could clearly be detected in leaves, stems, and roots as well as in seedlings (Fig. 1B).
Figure 1.
Expression of SDP1 in vegetative tissues of Arabidopsis. A, SDP1 promoter activity in leaf, stem, and root of 4-week-old plants monitored using a transgenic line carrying an SDP1p:GUS construct. Values are means ± se of values from four separate plants. B, SDP1-HA protein content in leaf, stem, and root of 4-week-old plants monitored by western blot using a transgenic sdp1-5 line carrying an SDP1p:SDP1-HA fusion construct.
Disruption of SDP1 Leads to TAG Accumulation in Vegetative Tissues
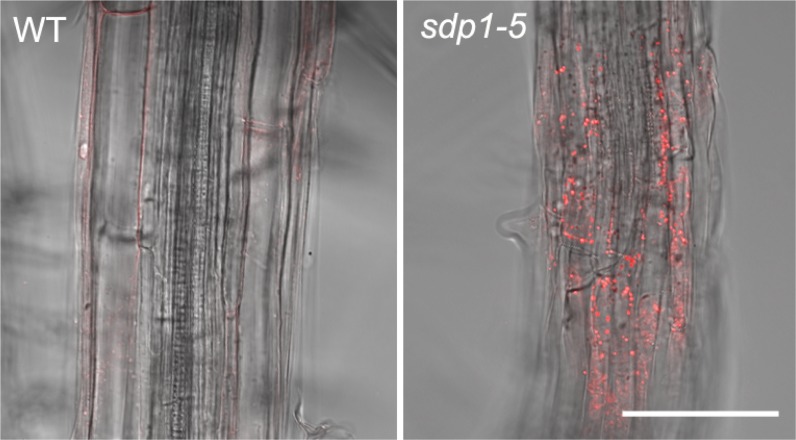
To determine whether SDP1 plays a limiting role in TAG turnover in vegetative tissues of Arabidopsis, the TAG content of leaves, stems, and roots of 4-week-old wild-type and sdp1-5 mutant plants was measured (Fig. 2). TAG was separated from total lipids by thin-layer chromatography (TLC), transmethylated, and quantified by gas chromatography of fatty acid methyl esters (Xu et al., 2005). The data showed that TAG levels are extremely low (around 0.02% of dry weight) in all three wild-type tissues, consistent with previous studies (Yang and Ohlrogge, 2009). However, in the stems and roots of sdp1-5, substantially more TAG accumulated, while the effect was much less pronounced in leaves (Fig. 2A). The highest level was found in roots, where around 20% of total fatty acids were in TAG (Fig. 2B). We decided to focus further work on roots as a convenient model heterotrophic tissue. The identity of the TAG in sdp1-5 roots was confirmed using electrospray ionization (ESI)-tandem mass spectroscopy (MS/MS; Supplemental Fig. S2A). The TAG is largely made up of C54 molecular species that are enriched in α-linolenic acid (Supplemental Fig. S2B). The accumulation of cytosolic lipid droplets in the root cells of sdp1-5 plants could also be visualized by laser scanning confocal microscopy using Nile red staining (Fig. 3).
Figure 2.
TAG accumulation in sdp1-5 mutant plants. TAG content (A) and total fatty acid content (B) are shown for root, leaf, and stem of 4-week-old wild-type (WT) and sdp1-5 plants grown on agar plates. Values are means ± se of values from four separate batches of 10 plants. Asterisks denote statistically significant differences from the wild type (WT; P < 0.05). DW, Dry weight.
Figure 3.
Lipid body accumulation in sdp1-5 roots. Laser scanning confocal images show Nile red-stained roots of 4-week-old wild-type (WT) and sdp1-5 plants grown on agar plates. Bar = 50 µm.
TAG Accumulation in sdp1 Roots Is Greater Than in Several Other Lipid Catabolism Mutants
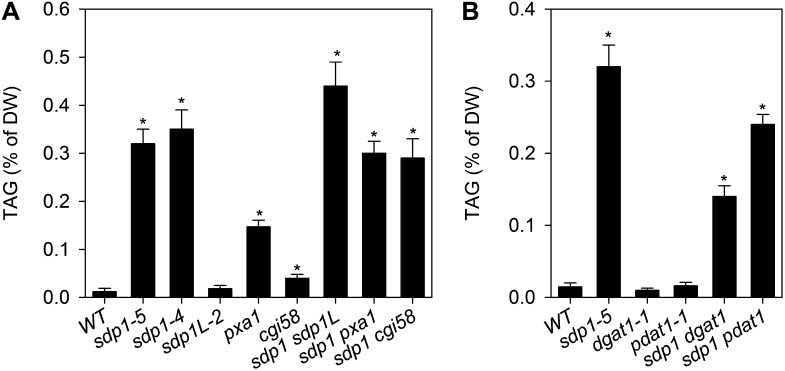
Previous studies have shown that the disruption of several other genes associated with lipid catabolism can also lead to TAG accumulation in vegetative tissues, although heterotrophic tissues have not generally been investigated (Kunz et al., 2009; Slocombe et al., 2009; James et al., 2010). PXA1 is a peroxisomal ATP-binding cassette transporter that is required for fatty acid import for β-oxidation (Zolman et al., 2001), and CGI58 is an enzyme that has been reported to have lipase, phospholipase, and lysophosphatidic acid acyltransferase activities (Ghosh et al., 2009). Analysis of TAG content in pxa1-1 and cgi58-1 roots showed that both accumulated TAG, but not as much as sdp1-5 (Fig. 4A). Furthermore, analysis of sdp1-5 pxa1-1 and sdp1-5 cgi58-1 double mutants showed no additive effect on root TAG content (Fig. 4A). SDP1 also has a single homolog in Arabidopsis called SDP1L (Kelly et al., 2011), and analysis of sdp1L-2 and sdp1-5 sdp1L-2 roots suggested that SDP1L has a comparatively minor role in TAG turnover in roots but that disruption of both genes leads to a marginally greater accumulation of TAG than sdp1-5 alone (Fig. 4A). This is consistent with gene expression data, which show that SDP1 transcripts are greater than 10-fold more abundant in vegetative tissues than SDP1L (Kelly et al., 2011).
Figure 4.
Comparison of TAG accumulation in roots of various mutants. A, TAG accumulation in lipid catabolism mutants. B, Effects of DGAT1 and PDAT1 deficiency on TAG accumulation in sdp1-5. Values are means ± se of values from four separate batches of 10 plants grown for 4 weeks on agar plates. Asterisks denote statistically significant differences from the wild type (WT; P < 0.05). DW, Dry weight.
TAG Accumulation in sdp1 Roots Is Partially Dependent on DGAT1 and PDAT1
TAG synthesis in Arabidopsis seeds relies jointly on the activities of DGAT1 (Katavic et al., 1995) and PDAT1 (Zhang et al., 2009). However, analysis of dgat1 and pdat1 mutants suggests that DGAT1 is quantitatively more important (Zhang et al., 2009). To determine whether these genes are required for TAG synthesis in sdp1-5 roots, the accumulation of TAG was measured in sdp1-5 dgat1-1 and sdp1-5 pdat1-1 double mutants. Disruption of DGAT1 resulted in a greater than 60% reduction in the accumulation of TAG in the sdp1-5 background, while disruption of PDAT1 also had a small, but statistically significant (P < 0.05), negative effect (Fig. 4B). We did not attempt to make a triple mutant because dgat1 pdat1 has been shown to be lethal (Zhang et al., 2009).
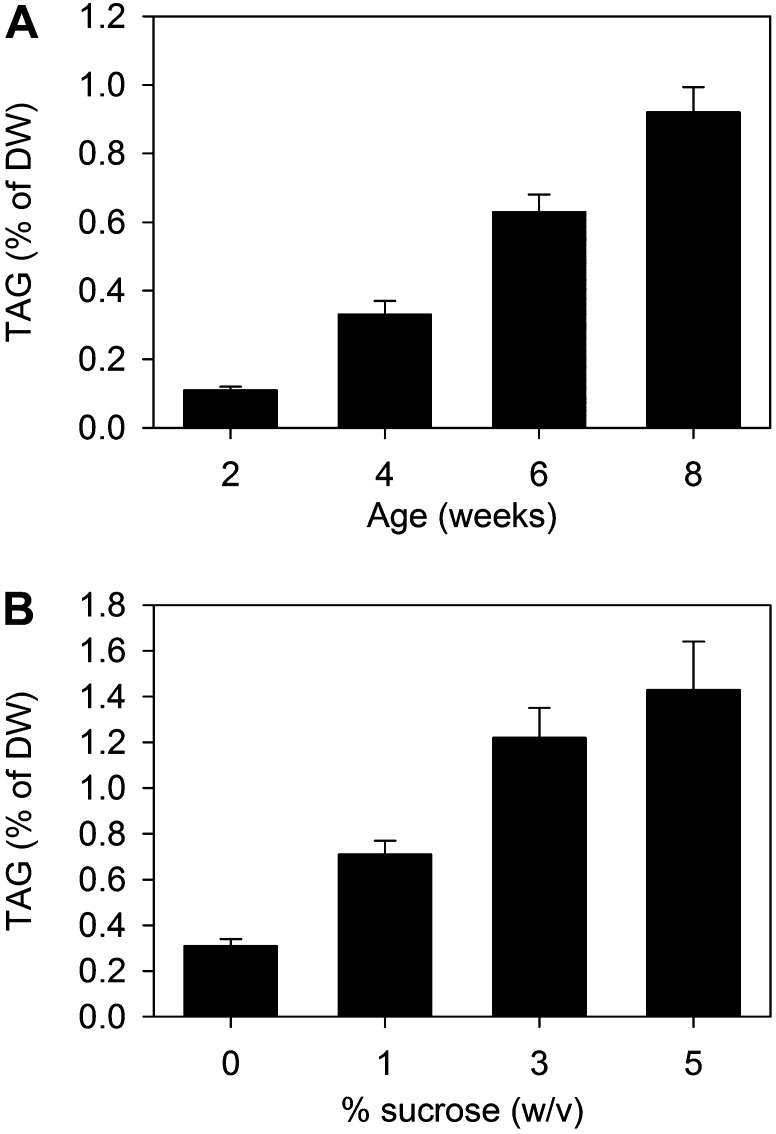
TAG Accumulates in sdp1 Roots as the Plant Ages and Is Enhanced by Exogenous Sugar
To investigate what conditions maximize TAG accumulation in sdp1-5 roots, plants were grown for increasing lengths of time and also in the presence of increasing concentrations of exogenous Suc. Both plant age and sugar supply enhanced total root TAG content (Fig. 5). TAG content increased by about 0.1% of dry weight per week when plants were grown in the absence of exogenous Suc (Fig. 5A). After 4 weeks of growth in the presence of increasing levels of Suc up to 5% (w/v), TAG content was also enhanced by up to 4-fold (Fig. 5B). Because sugar provision strongly stimulated TAG accumulation in sdp1-5 roots, we also chose to investigate whether it affected leaves, where comparatively less TAG accumulation was found under normal growth conditions (Fig. 2A). Analysis of leaves from 4-week-old sdp1-5 plants grown on medium containing 3% (w/v) Suc suggested that there is a positive effect. Many more lipid bodies were observed in leaves using Nile red staining, and more TAG was also detected by TLC (Supplemental Fig. S3).
Figure 5.
Effects of plant age and exogenous Suc on total TAG content of wild-type and sdp1-5 roots. A, Effect of plant age on root TAG content when grown in the absence of sugar. B, Effect of exogenous Suc on TAG content of roots from 4-week-old plants. Values are means ± se of values from four separate batches of 10 plants grown on agar plates. DW, Dry weight.
Disruption of SDP1 in Lines Expressing WRI1 and DGAT1 Leads to an Additive Effect on TAG Accumulation in Vegetative Tissues
Having obtained evidence that SDP1 function limits TAG accumulation in vegetative tissues of wild-type plants, we chose to test whether this is also true of lines genetically engineered to have higher oil biosynthetic capacity. Previous studies have shown that overexpression of DGAT1 and WRI1 individually leads to enhanced oil accumulation in vegetative tissues (Bouvier-Navé et al., 2000; Cernac and Benning, 2004) and also that a synergistic effect is achievable when they are expressed in combination (Vanhercke et al., 2013). WRI1 is a transcription factor that activates the expression of genes encoding multiple enzymes primarily of lower glycolysis and fatty acid synthesis (Cernac and Benning, 2004; Baud et al., 2007), and DGAT1 is the major enzyme that catalyzes the final committed step in TAG synthesis (Katavic et al., 1995; Zhang et al., 2009).
First, transfer DNA constructs containing DGAT1 or WRI1, under the control of the constitutive 35S promoter, were transformed into wild-type plants, and approximately 20 independent transformants were screened for elevated TAG content in the T2 generation using TLC. For each construct, three lines with the highest apparent TAG levels were then taken to the T3 generation, and homozygous plants were identified by segregation analysis. Enhanced expression of the transgenes was confirmed in the roots of these homozygous lines by quantitative PCR (Supplemental Table S1). The 35S:DGAT1 and 35S:WRI1 lines with the highest TAG content (D1 and W1; Supplemental Table S1) were then crossed together, and homozygous plants carrying both constructs were recovered (D1/W1). Finally, sdp1-5 was also crossed into D1 and D1/W1, and homozygous plants containing the transgenes were obtained.
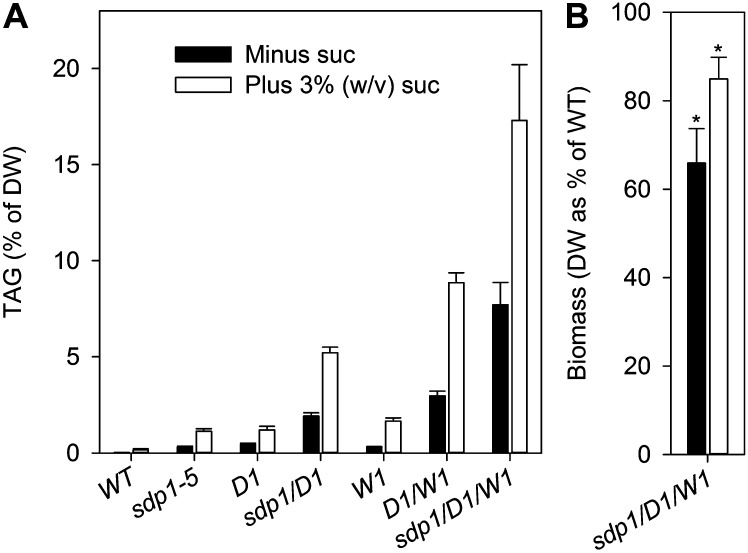
In order to compare the effects of all genotypic combinations and the provision of exogenous sugar, the TAG content was measured in roots from 4-week-old plants grown on agar medium either with or without 3% (w/v) Suc. The level of TAG increased progressively with the combination of each gene manipulation and exogenous sugar (Fig. 6A). The sum effect of DGAT1 overexpression, WRI overexpression, and SDP1 deficiency in the sdp1/D1/W1 line was approximately 8% TAG content (as a percentage of dry weight) without sugar supplementation and around 17% with Suc (Fig. 6A). Root biomass (total dry weight) was reduced by 20% to 30% in both cases (Fig. 6B). Regardless of exogenous sugar, disruption of SDP1 resulted in an approximate doubling in TAG content versus overexpression of DGAT1 and WRI1 alone (Fig. 6A). The fatty acid composition of TAG from D1/W1 and sdp1/D1/W1 roots was different from either the wild type or sdp1-5, with levels of oleic acid elevated at the expense of α-linolenic acid (Supplemental Table S2). Coexpression of WRI1 and DGAT1 in Nicotiana benthamiana leaves has previously been shown to have a similar effect on the level of oleic acid in TAG (Vanhercke et al., 2013).
Figure 6.
Combinatorial effect of Suc, DGAT1, and WRI1 overexpression and SDP1 deficiency on root TAG accumulation. TAG content (A) and total root dry weight DW (B) are shown for 4-week-old plants grown on agar plates with or without 3% (w/v) Suc. Values are means ± se of measurements on four separate batches of 10 plants. D1 and W1 are independent 35S:DGAT1 and 35S:WRI1 lines, respectively. Asterisks in B denote statistically significant differences from the wild type (WT; P < 0.05). DW, Dry weight.
Finally the TAG content of roots, stems, and leaves of 4-week-old soil-grown plants was also examined in D1/W1 and sdp1/D1/W1. In all three tissues, the sdp1 genetic background had a strong additive effect, resulting in TAG content increasing from 2% to 3% of dry weight in D1/W1 to 5% to 8% of dry weight in sdp1/D1/W1 (Fig. 7A). Soil-grown D1/W1 and sdp1/D1/W1 plants also exhibited significant reductions in both leaf and root biomass (measured as total dry weight; P < 0.05; Fig. 7B). On soil, the sdp1/D1/W1 plants also had visibly smaller rosettes but otherwise appeared to have no gross abnormalities (Supplemental Fig. S4).
Figure 7.
Effect of SDP1 deficiency on TAG content of vegetative tissues of soil-grown plants overexpressing DGAT1 and WRI1. TAG content (A) and total dry weight (DW; B) are shown for leaves, stems, and roots of 4-week-old plants grown on soil. Values are means ± se of measurements on four separate batches of 10 plants. The D1/W1 line is expressing DGAT1 and WRI1. Asterisks denote statistically significant differences from D1/W1 in A and from the wild type (WT) in B (P < 0.05).
DISCUSSION
In this study, we show that disruption of the TAG lipase SDP1 (Eastmond, 2006) leads to an accumulation of oil in vegetative tissues of Arabidopsis, suggesting that TAG turnover occurs in these tissues despite their very low steady-state TAG content (Yang and Ohlrogge, 2009). Although we show that SDP1 is expressed (and the protein is present) in all tissues of a rosette plant, the accumulation of TAG is far more pronounced in heterotrophic tissues (i.e. roots and stems) and much less substantial in leaves. TAG accumulation in whole root tissue appears to be gradual, progressing with the age of the plant, and at maturity, TAG can account for more than 1% of dry weight. This is a 50-fold increase over the wild type and boosts the total fatty acid content of the tissue by more than 50%. Heterotrophic vegetative tissues can make up a significant proportion of the harvestable biomass of many crop plants, and Arabidopsis has a comparatively short life cycle, so TAG accumulation might conceivably be even greater in these plants if SDP1 genes were disrupted. It has been suggested that even modest increases in the total lipid content of crops may be commercially useful. First, for biomass crops, the increased energy density makes electricity generation via combustion more efficient (Ohlrogge et al., 2009). Second, in forage and fodder crops, the higher calorie content is advantageous for livestock nutrition and, therefore, meat and dairy production (Hegarty et al., 2013). Disruption of SDP1 genes can be achieved by conventional mutation breeding methods as well as by transgenic approaches. In Arabidopsis, sdp1 has impaired seedling establishment (Eastmond, 2006). However, mutation of SDP1 genes might have less impact in nonoilseed species and/or species with vegetative propagation (e.g. many grasses and legumes).
We also show that TAG accumulation in all vegetative tissues of Arabidopsis plants is limited by SDP1 function when they have been genetically engineered to synthesize more oil. Coexpression of DGAT1 and WRI1 in an sdp1 background results in roots, stems, and leaves with approximately double the TAG content of wild-type plants expressing DGAT1 and WRI1. Levels of TAG range from 5% to 8% in 4-week-old soil-grown plants, and when cultured in the presence of 3% (w/v) Suc, more than 17% TAG can be made to accumulate in roots. This TAG content, as a percentage of dry weight, is very nearly equivalent to that of soybean (Glycine max) seeds. Even higher levels of TAG may be possible with further optimization of transgene expression. However, growth is also impaired as a result of these manipulations. In 4-week-old plants grown on soil, leaf and root biomass (measured as dry weight) are reduced by more than 20%. There could be many reasons for growth retardation, given the extent of the genetic manipulations. However, the shift in carbon partitioning alone might reasonably be expected to have some negative impact on plant growth. TAG has a 2-fold higher energy density than carbohydrate, and carbon conversion to fatty acids is also comparatively inefficient, although there is potential in photosynthetic tissues for refixation of the carbon released as CO2 (Durrett et al., 2008).
The fact that exogenous Suc boosts root TAG content so strongly indicates that substrate availability remains an important limiting factor for TAG accumulation in our transgenic lines. Sanjaya et al. (2011) recently showed that blocking transient starch accumulation in Arabidopsis enhances sugar levels and TAG accumulation in leaves expressing WRI1. This approach might also be effective in sdp1 plants expressing WRI1 and DGAT1. Disruption of starch synthesis has also been shown to elevate sugar levels in roots, as well as leaves, at certain points during the diurnal cycle (Bläsing et al., 2005). Numerous plant species are known to preferentially allocate carbon to stems or roots for storage (Durrett et al., 2008), and these may present more appropriate hosts than Arabidopsis for engineering TAG accumulation in heterotrophic tissues. Sugars may also exert an additional effect over substrate provision by enhancing biosynthetic capacity. Increased sugar concentration triggers wide-scale changes in gene expression and enzyme activities in Arabidopsis, many of which are associated with primary metabolism (Bläsing et al., 2005; Osuna et al., 2007). For example, there is evidence that both WRI1 and DGAT1 are induced by sugar (Lu et al., 2003; Masaki et al., 2005).
It is not clear why sdp1 leaves fail to accumulate as much TAG as stems and roots. One explanation could be that additional lipases are present in photosynthetic tissues, which allow TAG turnover to continue to occur in sdp1. Arabidopsis contains many genes that could potentially have this function (Li-Beisson et al., 2013). CGI58 is one candidate, since the protein has lipase activity (Ghosh et al., 2009) and the mutant accumulates TAG in its leaves to around 0.2% of dry weight (James et al., 2010), which is higher than we detected in sdp1. The disruption of fatty acid β-oxidation also leads to TAG accumulation in leaves (Slocombe et al., 2009; James et al., 2010), but the impact on total fatty acid content appears to be small (Yang and Ohlrogge, 2009), unless the tissue is subjected to carbohydrate starvation (Kunz et al., 2009; Slocombe et al., 2009). Therefore, TAG turnover in the cytosol may simply be less rapid in leaves than in roots and stems. Importantly, we observed that SDP1 disruption does boost TAG accumulation in leaves when exogenous sugar is applied or when DGAT1 and WRI1 are overexpressed. In both cases, TAG synthesis is artificially stimulated (Bouvier-Navé et al., 2000; Lu et al., 2003; Cernac and Benning, 2004; Masaki et al., 2005); therefore, SDP1-mediated TAG turnover must become more active under these nonphysiological conditions.
Lipid metabolism in Arabidopsis roots and stems has received rather less attention than in leaves (Li-Beisson et al., 2013). Our analysis of the pxa1 mutant, which is severely deficient in fatty acid β-oxidation (Zolman et al., 2001), showed that it also accumulates TAG in its roots. This suggests that β-oxidation does make a detectable contribution to the bulk turnover of fatty acid from membrane lipids in this tissue under normal growth conditions. Interestingly, sdp1 roots accumulate more TAG than pxa1, indicating that the rate of TAG turnover in roots might be greater than the rate of fatty acid breakdown, presuming that the rate of fatty acid synthesis is equivalent in these mutants. Analysis of an sdp1 pxa1 double mutant also shows that PXA1 is epistatic to SDP1, which implies that SDP1 and PXA1 function in the same pathway. Finally, analysis of sdp1 dgat1 and sdp1 pdat1 double mutants showed that TAG accumulation in sdp1 roots is largely dependent on DGAT1 function but that PDAT1 is also required. It has already been established that DGAT1 is necessary for normal TAG synthesis in seeds, seedlings, and leaves of Arabidopsis (Katavic et al., 1995; Lu et al., 2003; Slocombe et al., 2009), but pdat1 has not previously been shown to have a TAG phenotype. Recently, a third acyltransferase (DGAT3), with specificity toward polyunsaturated fatty acids, has also been shown to play a role in TAG synthesis in Arabidopsis seedlings (Hernández et al., 2012). Currently, we cannot discount a significant role for this gene in roots. The TAG that accumulates in sdp1 roots is enriched in polyunsaturated fatty acids, a feature that has also been reported in cgi58 leaves (James et al., 2010).
In conclusion, our data show that combined manipulation of carbohydrate supply, fatty acid synthesis, TAG synthesis, and TAG breakdown can drive substantial TAG accumulation in vegetative tissues of Arabidopsis. Further work will clearly be required to establish whether this approach is also applicable to various tissues of crop species and to optimize the technology. The accumulation of TAG in sdp1 also raises a number of questions concerning the physiological role of TAG in plant vegetative tissues. Oil bodies have been found in the leaves of many plant species (Lersten et al., 2006), and roles for cytosolic TAG in carbon storage and membrane lipid remodeling have previously been proposed in a number of studies (Murphy, 2001; James et al., 2010). However, more evidence is required to support or refute these theories. Further investigation of the role of SDP1 in roots may help to address this question.
MATERIALS AND METHODS
Plant Material and Growth Conditions
The Arabidopsis (Arabidopsis thaliana) mutants sdp1-4, sdp1-5, sdp1L-2, cgi58-1, and pxa1-1 are described by Kelly et al. (2011). The dgat1-1 and pdat1-1 mutants are described by Katavic et al. (1995) and Zhang et al. (2009), respectively. For experiments performed on medium, seeds were surface sterilized, plated on agar plates containing one-half-strength Murashige and Skoog salts (Sigma-Aldrich) with or without the addition of Suc, and imbibed in the dark for 4 d at 4°C. The plates were then placed vertically in a growth chamber set to 16 h of light (23°C)/8 h of dark (18°C); photosynthetic photon flux density was 250 μmol m−2 s−1. For some experiments, plants were also transferred to soil after 5 d on medium with 1% (w/v) Suc to rescue sdp1 (Eastmond, 2006) and grown to maturity in the same conditions as described above.
Histochemical Staining and Western Blotting
Assays for GUS were performed as described by Jefferson et al. (1987). Lipid droplets were imaged in situ by laser scanning confocal microscopy using Nile red staining (Greenspan et al., 1985). Nile red stock was made to a concentration of 1 mg mL−1 in acetone and diluted to 1 µg mL−1 in water for a working concentration. Roots were stained for 1 min and washed in deionized water. The material was mounted on a slide in water, imaged with a Zeiss LSM 780 using a 40× or a 63× objective and an excitation wavelength of 514 nm, and detected using an emission band of 539 to 648 nm. Protein extraction, quantification, SDS-PAGE, and western blotting were performed as described previously (Eastmond, 2004), except that anti-HA and anti-IgG-horseradish peroxidase (Invitrogen) were used as primary and secondary antibodies at 1:1,000 and 1:10,000 dilutions, respectively, and horseradish peroxidase was detected using an enhanced chemiluminescence kit (Perkin-Elmer).
Lipid Analysis
Total lipids were extracted from homogenized freeze-dried tissue using the method of Dörmann et al. (1995), except that tripentadecanoic acid (15:0 TAG) was added to the homogenized tissue to act as an internal standard. A proportion of the total lipid extract was subjected directly to transmethylation according to the method of Browse et al. (1986), and the fatty acid methyl esters were quantified by gas chromatography-flame ionization detection with reference to the standard (Kelly et al., 2011). The remaining lipid extract was applied to silica TLC plates, and neutral lipids were separated using a hexane:diethyether:acetic acid (70:30:1, v/v) solvent system. The lipids were visualized under UV light by staining with 0.05% (w/v) primuline in 80% (v/v) acetone, the TAG band was scraped from the plate and transmethylated, and the fatty acid methyl esters were quantified as above. ESI-MS/MS analyses were performed on a 4000 QTRAP (ABSiex) liquid chromatography-MS/MS apparatus coupled with a TriVersa NanoMate mounted with an ESI chip (Advion). For all analyses, the NanoMate was operated in positive ion mode with a spray voltage of 2 kV, vented headspace, and pressure of 0.4 p.s.i. A 50-μL aliquot of total lipid plus 15:0 TAG standard was mixed in 1 mL of 1:1 (v/v) chloroform:methanol with 10 mm ammonium acetate (James et al., 2010). A 10-μL aliquot of this mix was then loaded on the ESI chip to perform the analyses. Full scans were performed using the Q1/MS mode to acquire the spectra. Molecular species of TAG were profiled using the method described by Krank et al. (2007), except that 12 periods of 2 min were used for neutral loss scans. The data were then analyzed in Lipidview (version 1.1). The amount of fatty acid was normalized by gas chromatography-flame ionization detection.
Creation of DNA Constructs, and Arabidopsis Transformation
DGAT1, WRI1, and 1.5 kb of the SDP1 promoter were amplified by PCR from either Arabidopsis complementary DNA or genomic DNA using the following primer pairs: DGAT1 (5′-CACCATGGCGATTTTGGATTCTGCTGGCG-3′ and 5′-TCATGACATCGATCCTTTTCGGTTCATCAGG-3′), WRI1 (5′-CACCATGAAGAAGCGCTTAACCACTTCC-3′ and 5′-TTATTCAGAACCAACGAACAAGCCC-3′), and SDP1p (5′-CACCTTCGAGTTTTATTTTCGTTACTTCCA-3′ and 5′-TATTGATTCGAAGATGAATTTGGGTGTGT-3′). The products were cloned into the pENTR/D-TOPO vector and then (using the Gateway LR Clonase enzyme mix) transferred to the appropriate destination vector, according to the manufacturer’s instructions (Invitrogen). SDP1p was transferred to pBGWFS7, DGAT1 to pB2GW7, and WRI1 to pK2GW7 (Karimi et al., 2002). The constructs were transformed into Agrobacterium tumefaciens strain GV3101 by heat shock and into Arabidopsis by the floral dip method (Clough and Bent, 1998). Transformants containing transfer DNA insertions were selected via antibiotic or herbicide resistance.
Transcript Analysis
DNase-treated total RNA was isolated from Arabidopsis roots using the RNeasy kit from Qiagen. The synthesis of single-stranded complementary DNA was carried out using SuperScript II RNase H− reverse transcriptase from Invitrogen. Quantitative real-time PCR was performed as described by Rajangam et al. (2013). The primer pairs used for real-time PCR were QDGAT1 (5′-TGGATTCTGCTGGCGTTACTAC-3′ and 5′-AGCCTATCAAGATCGACGAACTCT-3′), QWRI1 (5′-AAACGAGCCAAAAGGGCTAAG-3′ and 5′-GGGCTTGTCGGGTTATGAGA-3′), and QACT2 (5′-TGTGACAATGGTACCGGTATGG-3′ and 5′-GCCCTGGGAGCATCATCTC-3′).
Statistical Analyses
Total lipid content, TAG content, and dry weight were compared among genotypes using paired Student’s t tests assuming unequal variance.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers NM_120486 (SDP1), NM_202720 (SDP1L), AF378120 (PXA1), NM_202876 (CGI58), AJ238008 (DGAT1), and AY254038 (WRI1).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. GUS staining of various tissues from an SDP1p::GUS reporter line.
Supplemental Figure S2. ESI-MS/MS analysis of total lipids from sdp1-5 roots.
Supplemental Figure S3. TAG accumulation in sdp1-5 leaves in the presence of Suc.
Supplemental Figure S4. Images of wild-type and sdp1/D1/W1 plants grown on soil.
Supplemental Table S1. Root TAG content and DGAT1 and WRI1 expression in transgenic lines.
Supplemental Table S2. Fatty acid composition of TAG from sdp1/D1/W1 roots.
Supplementary Material
Acknowledgments
We are very grateful to Dr. Richard Haslam for his invaluable assistance with mass spectrometry methods and to Prof. Ljerka Kunst for providing the dgat1-1 mutant.
Glossary
- TAG
triacylglycerol
- HA
hemagglutinin
- TLC
thin-layer chromatography
- ESI
electrospray ionization
- MS/MS
tandem mass spectroscopy
References
- Andrianov V, Borisjuk N, Pogrebnyak N, Brinker A, Dixon J, Spitsin S, Flynn J, Matyszczuk P, Andryszak K, Laurelli M, et al (2010) Tobacco as a production platform for biofuel: overexpression of Arabidopsis DGAT and LEC2 genes increases accumulation and shifts the composition of lipids in green biomass. Plant Biotechnol J 8: 277–287 [DOI] [PubMed] [Google Scholar]
- Athenstaedt K, Daum G. (2005) Tgl4p and Tgl5p, two triacylglycerol lipases of the yeast Saccharomyces cerevisiae are localized to lipid particles. J Biol Chem 280: 37301–37309 [DOI] [PubMed] [Google Scholar]
- Baud S, Mendoza MS, To A, Harscoët E, Lepiniec L, Dubreucq B. (2007) WRINKLED1 specifies the regulatory action of LEAFY COTYLEDON2 towards fatty acid metabolism during seed maturation in Arabidopsis. Plant J 50: 825–838 [DOI] [PubMed] [Google Scholar]
- Bläsing OE, Gibon Y, Günther M, Höhne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible WR, Stitt M. (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 17: 3257–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvier-Navé P, Benveniste P, Oelkers P, Sturley SL, Schaller H. (2000) Expression in yeast and tobacco of plant cDNAs encoding acyl CoA:diacylglycerol acyltransferase. Eur J Biochem 267: 85–96 [DOI] [PubMed] [Google Scholar]
- Browse J, McCourt PJ, Somerville CR. (1986) Fatty acid composition of leaf lipids determined after combined digestion and fatty acid methyl ester formation from fresh tissue. Anal Biochem 152: 141–145 [DOI] [PubMed] [Google Scholar]
- Cernac A, Benning C. (2004) WRINKLED1 encodes an AP2/EREB domain protein involved in the control of storage compound biosynthesis in Arabidopsis. Plant J 40: 575–585 [DOI] [PubMed] [Google Scholar]
- Clough SJ, Bent AF. (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Dörmann P, Hoffmann-Benning S, Balbo I, Benning C. (1995) Isolation and characterization of an Arabidopsis mutant deficient in the thylakoid lipid digalactosyl diacylglycerol. Plant Cell 7: 1801–1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durrett TP, Benning C, Ohlrogge J. (2008) Plant triacylglycerols as feedstocks for the production of biofuels. Plant J 54: 593–607 [DOI] [PubMed] [Google Scholar]
- Dyer JM, Stymne S, Green AG, Carlsson AS. (2008) High-value oils from plants. Plant J 54: 640–655 [DOI] [PubMed] [Google Scholar]
- Eastmond PJ. (2004) Cloning and characterization of the acid lipase from castor beans. J Biol Chem 279: 45540–45545 [DOI] [PubMed] [Google Scholar]
- Eastmond PJ. (2006) SUGAR-DEPENDENT1 encodes a patatin domain triacylglycerol lipase that initiates storage oil breakdown in germinating Arabidopsis seeds. Plant Cell 18: 665–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh AK, Chauhan N, Rajakumari S, Daum G, Rajasekharan R. (2009) At4g24160, a soluble acyl-coenzyme A-dependent lysophosphatidic acid acyltransferase. Plant Physiol 151: 869–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan P, Mayer EP, Fowler SD. (1985) Nile red: a selective fluorescent stain for intracellular lipid droplets. J Cell Biol 100: 965–973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grönke S, Mildner A, Fellert S, Tennagels N, Petry S, Müller G, Jäckle H, Kühnlein RP. (2005) Brummer lipase is an evolutionary conserved fat storage regulator in Drosophila. Cell Metab 1: 323–330 [DOI] [PubMed] [Google Scholar]
- Hegarty M, Yadav R, Lee M, Armstead I, Sanderson R, Scollan N, Powell W, Skøt L. (2013) Genotyping by RAD sequencing enables mapping of fatty acid composition traits in perennial ryegrass (Lolium perenne (L.)). Plant Biotechnol J 11: 572–581 [DOI] [PubMed] [Google Scholar]
- Hernández ML, Whitehead L, He Z, Gazda V, Gilday A, Kozhevnikova E, Vaistij FE, Larson TR, Graham IA. (2012) A cytosolic acyltransferase contributes to triacylglycerol synthesis in sucrose-rescued Arabidopsis seed oil catabolism mutants. Plant Physiol 160: 215–225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James CN, Horn PJ, Case CR, Gidda SK, Zhang D, Mullen RT, Dyer JM, Anderson RG, Chapman KD. (2010) Disruption of the Arabidopsis CGI-58 homologue produces Chanarin-Dorfman-like lipid droplet accumulation in plants. Proc Natl Acad Sci USA 107: 17833–17838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferson RA, Kavanagh TA, Bevan MW. (1987) GUS fusions: beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi M, Inzé D, Depicker A. (2002) GATEWAY vectors for Agrobacterium-mediated plant transformation. Trends Plant Sci 7: 193–195 [DOI] [PubMed] [Google Scholar]
- Katavic V, Reed DW, Taylor DC, Giblin EM, Barton DL, Zou JT, Mackenzie SL, Covello PS, Kunst L. (1995) Alteration of seed fatty acid composition by an ethyl methanesulfonate-induced mutation in Arabidopsis thaliana affecting diacylglycerol acyltransferase activity. Plant Physiol 108: 399–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly AA, Quettier A-L, Shaw E, Eastmond PJ. (2011) Seed storage oil mobilization is important but not essential for germination or seedling establishment in Arabidopsis. Plant Physiol 157: 866–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krank J, Murphy RC, Barkley RM, Duchoslav E, McAnoy A. (2007) Qualitative analysis and quantitative assessment of changes in neutral glycerol lipid molecular species within cells. Methods Enzymol 432: 1–20 [DOI] [PubMed] [Google Scholar]
- Kunz HH, Scharnewski M, Feussner K, Feussner I, Flügge UI, Fulda M, Gierth M. (2009) The ABC transporter PXA1 and peroxisomal β-oxidation are vital for metabolism in mature leaves of Arabidopsis during extended darkness. Plant Cell 21: 2733–2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurat CF, Natter K, Petschnigg J, Wolinski H, Scheuringer K, Scholz H, Zimmermann R, Leber R, Zechner R, Kohlwein SD. (2006) Obese yeast: triglyceride lipolysis is functionally conserved from mammals to yeast. J Biol Chem 281: 491–500 [DOI] [PubMed] [Google Scholar]
- Lersten NR, Czlapinski AR, Curtis JD, Freckmann R, Horner HT. (2006) Oil bodies in leaf mesophyll cells of angiosperms: overview and a selected survey. Am J Bot 93: 1731–1739 [DOI] [PubMed] [Google Scholar]
- Li-Beisson Y, Shorrosh B, Beisson F, Andersson M, Arondel V, Bates PD, Baud S, Bird D, DeBono A, Durrett TP, et al (2013) Acyl-lipid metabolism. The Arabidopsis Book 11: e0161, /10.1199/tab.0161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Napier JA, Clemente TE, Cahoon EB. (2011) New frontiers in oilseed biotechnology: meeting the global demand for vegetable oils for food, feed, biofuel, and industrial applications. Curr Opin Biotechnol 22: 252–259 [DOI] [PubMed] [Google Scholar]
- Lu CL, de Noyer SB, Hobbs DH, Kang J, Wen Y, Krachtus D, Hills MJ. (2003) Expression pattern of diacylglycerol acyltransferase-1, an enzyme involved in triacylglycerol biosynthesis, in Arabidopsis thaliana. Plant Mol Biol 52: 31–41 [DOI] [PubMed] [Google Scholar]
- Masaki T, Mitsui N, Tsukagoshi H, Nishii T, Morikami A, Nakamura K. (2005) ACTIVATOR OF SPOMIN:LUC1/WRINKLED1 of Arabidopsis thaliana transactivates sugar-inducible promoters. Plant Cell Physiol 46: 547–556 [DOI] [PubMed] [Google Scholar]
- Mu J, Tan H, Zheng Q, Fu F, Liang Y, Zhang J, Yang X, Wang T, Chong K, Wang XJ, et al (2008) LEAFY COTYLEDON1 is a key regulator of fatty acid biosynthesis in Arabidopsis. Plant Physiol 148: 1042–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DJ. (2001) The biogenesis and functions of lipid bodies in animals, plants and microorganisms. Prog Lipid Res 40: 325–438 [DOI] [PubMed] [Google Scholar]
- Ogas J, Cheng JC, Sung ZR, Somerville C. (1997) Cellular differentiation regulated by gibberellin in the Arabidopsis thaliana pickle mutant. Science 277: 91–94 [DOI] [PubMed] [Google Scholar]
- Ohlrogge J, Allen D, Berguson B, Dellapenna D, Shachar-Hill Y, Stymne S. (2009) Energy: driving on biomass. Science 324: 1019–1020 [DOI] [PubMed] [Google Scholar]
- Osuna D, Usadel B, Morcuende R, Gibon Y, Bläsing OE, Höhne M, Günter M, Kamlage B, Trethewey R, Scheible WR, et al (2007) Temporal responses of transcripts, enzyme activities and metabolites after adding sucrose to carbon-deprived Arabidopsis seedlings. Plant J 49: 463–491 [DOI] [PubMed] [Google Scholar]
- Rajangam AS, Gidda SK, Craddock C, Mullen RT, Dyer JM, Eastmond PJ. (2013) Molecular characterization of the fatty alcohol oxidation pathway for wax-ester mobilization in germinated jojoba seeds. Plant Physiol 161: 72–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjaya, Durrett TP, Weise SE, Benning C (2011) Increasing the energy density of vegetative tissues by diverting carbon from starch to oil biosynthesis in transgenic Arabidopsis. Plant Biotechnol J 9: 874–883 [DOI] [PubMed] [Google Scholar]
- Scherer GFE, Ryu SB, Wang X, Matos AR, Heitz T. (2010) Patatin-related phospholipase A: nomenclature, subfamilies and functions in plants. Trends Plant Sci 15: 693–700 [DOI] [PubMed] [Google Scholar]
- Slocombe SP, Cornah J, Pinfield-Wells H, Soady K, Zhang Q, Gilday A, Dyer JM, Graham IA. (2009) Oil accumulation in leaves directed by modification of fatty acid breakdown and lipid synthesis pathways. Plant Biotechnol J 7: 694–703 [DOI] [PubMed] [Google Scholar]
- Vanhercke T, El Tahchy A, Shrestha P, Zhou XR, Singh SP, Petrie JR. (2013) Synergistic effect of WRI1 and DGAT1 coexpression on triacylglycerol biosynthesis in plants. FEBS Lett 587: 364–369 [DOI] [PubMed] [Google Scholar]
- Xu C, Fan J, Froehlich JE, Awai K, Benning C. (2005) Mutation of the TGD1 chloroplast envelope protein affects phosphatidate metabolism in Arabidopsis. Plant Cell 17: 3094–3110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z, Ohlrogge JB. (2009) Turnover of fatty acids during natural senescence of Arabidopsis, Brachypodium, and switchgrass and in Arabidopsis β-oxidation mutants. Plant Physiol 150: 1981–1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, Fan J, Taylor DC, Ohlrogge JB. (2009) DGAT1 and PDAT1 acyltransferases have overlapping functions in Arabidopsis triacylglycerol biosynthesis and are essential for normal pollen and seed development. Plant Cell 21: 3885–3901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R, Strauss JG, Haemmerle G, Schoiswohl G, Birner-Gruenberger R, Riederer M, Lass A, Neuberger G, Eisenhaber F, Hermetter A, et al (2004) Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science 306: 1383–1386 [DOI] [PubMed] [Google Scholar]
- Zolman BK, Silva ID, Bartel B. (2001) The Arabidopsis pxa1 mutant is defective in an ATP-binding cassette transporter-like protein required for peroxisomal fatty acid β-oxidation. Plant Physiol 127: 1266–1278 [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.