The bark beetle-vectored fungus Ceratocystis polonica degrades stilbenoid defense compounds produced by its conifer host.
Abstract
Norway spruce (Picea abies) forests suffer periodic fatal attacks by the bark beetle Ips typographus and its fungal associate, Ceratocystis polonica. Norway spruce protects itself against fungal and bark beetle invasion by the production of terpenoid resins, but it is unclear whether resins or other defenses are effective against the fungus. We investigated stilbenes, a group of phenolic compounds found in Norway spruce bark with a diaryl-ethene skeleton with known antifungal properties. During C. polonica infection, stilbene biosynthesis was up-regulated, as evidenced by elevated transcript levels of stilbene synthase genes. However, stilbene concentrations actually declined during infection, and this was due to fungal metabolism. C. polonica converted stilbenes to ring-opened, deglycosylated, and dimeric products. Chromatographic separation of C. polonica protein extracts confirmed that these metabolites arose from specific fungal enzyme activities. Comparison of C. polonica strains showed that rapid conversion of host phenolics is associated with higher virulence. C. polonica is so well adapted to its host’s chemical defenses that it is even able to use host phenolic compounds as its sole carbon source.
Norway spruce (Picea abies), a dominant tree species in European boreal, montane, and subalpine forests, is frequently subject to fatal attacks by the bark beetle Ips typographus (Wermelinger, 2004). During attacks, these scolytine beetles introduce fungal pathogens into their hosts. One of the most virulent spruce pathogens associated with bark beetle attacks is the blue-staining ascomycete Ceratocystis polonica (Krokene and Solheim, 1998). Tree death is thought to result either from bark beetle attack alone (Six and Wingfield, 2011) or from the combined action of the bark beetle and the fungus (Franceschi et al., 2005), where the beetles damage the cambium by feeding and the fungus interrupts water flow in the xylem. Although the association between the fungus and the beetle might only be facultative (Six and Wingfield, 2011), bark beetles could potentially benefit from this necrotrophic fungus, which may help kill the host tree, unlock nutrients, and weaken host defense capacity (Paine et al., 1997).
Norway spruce trees are known to have several effective structural and chemical defense strategies against bark beetles that can ward off low-density attacks (Franceschi et al., 2005). The best known example of a chemical defense in this species is oleoresin (Keeling and Bohlmann, 2006; Schmidt et al., 2010). This viscous mixture of terpenoids stored in specialized ducts flows to the site of damage when ducts are severed and has been shown to increase in quantity after initial beetle attack. A less well-studied defense mechanism in spruce is the production of phenolic compounds in specialized cells in the bark. These substances are stored in phloem parenchyma cells (Franceschi et al., 2000; Li et al., 2012) that expand during wounding or fungal attack and show major cytological changes.
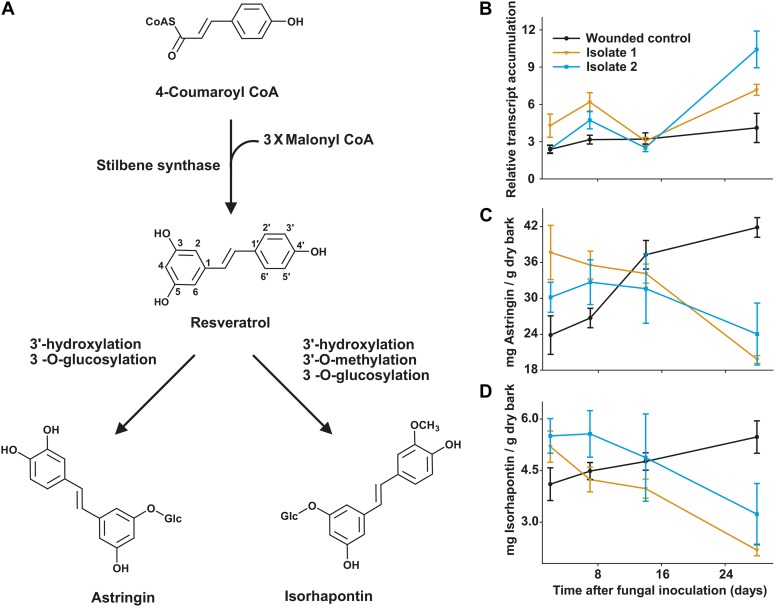
Stilbenes are a widespread group of phenolic compounds in spruce and other species of the family Pinaceae (Underwood and Pearce, 1992). They are reported to have antifungal properties and have been shown to contribute to plant disease resistance (Chong et al., 2009; Jeandet et al., 2010). In the genus Picea, the stilbene glucosides astringin and isorhapontin (Fig. 1A) occur in high concentrations in bark, roots, and foliage (Hammerbacher et al., 2011). Two stilbene synthase (STS) enzymes that contribute to the biosynthesis of these compounds have been described in Norway spruce (Hammerbacher et al., 2011). These enzymes seem to play a role in tree defense, since fungal infection induced elevated amounts of STS transcript (Hammerbacher et al., 2011) and increased enzyme levels (Brignolas et al., 1995). However, there are contradictory reports on stilbene glucoside accumulation in spruce bark during fungal infection. Significant increases in astringin were observed after inoculation of Picea glauca saplings with avirulent C. polonica (Hammerbacher et al., 2011). However, stilbene glycoside concentrations in mature Norway spruce were stable or even declined during the course of C. polonica infection (Brignolas et al., 1995; Viiri et al., 2001; Li et al., 2012).
Figure 1.
Stilbene biosynthesis and stilbene concentrations in Norway spruce bark in response to fungal infection. A, Route of stilbene biosynthesis. STS transcription (B) and concentrations of the stilbene glucosides astringin (C) and isorhapontin (D) were measured in Norway spruce saplings after inoculation and infection by two C. polonica isolates and after control wounding treatment (inoculation with sterile agar). Error bars represent se (n = 5). Significant differences were detected between 14 and 28 d after treatment for both fungus-infected and wounded control saplings (P < 0.005). [See online article for color version of this figure.]
After host infection, fungi follow different strategies to gain access to host nutrients. Biotrophic fungi acquire nutrients directly from living cells by penetrating them and causing little visible damage (Voegele and Mendgen, 2003). Necrotrophic fungi such as C. polonica lyse cells in order to release nutrients that are then assimilated by the fungi (Oliver and Solomon, 2010). During plant cell lysis, potential antifungal defense compounds may come into contact with necrotrophic fungi. However, it is not yet known how effective Norway spruce defense compounds are against C. polonica and whether this specialized, bark beetle-vectored pathogen has developed any resistance to them. This missing information may be critical in understanding how C. polonica contributes to the success of bark beetle attacks and why so many mutualistic relationships between bark beetles and blue-staining fungi have evolved.
In this work, we demonstrate that C. polonica can circumvent the antifungal activity of Norway spruce stilbenes during infection. Although high levels of STS transcripts accumulate in fungus-infected bark, a net loss of stilbene glucosides was detected at the site of infection. This reduction in stilbenes was explained by fungal biotransfomation processes, including the formation of stilbene dimers, aglycones, and ring-opened lactones, which may represent the first step of the β-ketoadipate pathway for microbial utilization of aromatic compounds as a carbon source. We could also show that different C. polonica isolates follow different stilbene biotransformation strategies and that rapid biotransformation and formation of ring-opened lactones is associated with greater levels of fungal virulence in Norway spruce bark.
RESULTS
Fungal Inoculation of Norway Spruce Bark Increases Stilbene Biosynthetic Gene Transcripts But Reduces Stilbene Accumulation
When Norway spruce saplings were inoculated with the blue-stain fungus C. polonica, the transcript levels of STS genes PaSTS1 and PaSTS2 (Hammerbacher et al., 2011) increased significantly compared with the sterile agar-inoculated control (P = 0.014). The same pattern was observed for two different C. polonica isolates (Fig. 1B). However, the levels of the stilbene glucosides, astringin and isorhapontin, declined over the 28-d time course after inoculation of the two fungal isolates (P = 0.037), while they increased in the sterile agar-inoculated control (Fig. 1, C and D).
To determine if C. polonica was the agent responsible for the reduced stilbene levels seen in infected bark, isolates 1 and 2 were grown in nutrient broth amended with 2 mg mL−1 astringin. A significant decrease in astringin was observed in the fungal culture medium compared with a sterile control medium amended with astringin (P < 0.0001) over a time course of 28 h (Table I). The rate of astringin decrease in the medium was significantly higher for isolate 2 compared with isolate 1 (P < 0.0001).
Table I. Biotransformation of the Norway spruce stilbene astringin to various metabolites by C. polonica.
Listed are the percentages ± sd of substrate recovered in individual biotransformation products 4 and 28 h after adding astringin to C. polonica isolate 1 and isolate 2 (n = 4 replicates per isolate per time point). Control indicates medium without any inoculated fungus. Cultures were amended with 2 mg mL−1 astringin. Metabolite numbers and structures are listed in Figure 2.
| Astringin Metabolite | Isolate 1 |
Isolate 2 |
Control |
|||
|---|---|---|---|---|---|---|
| 4 h | 28 h | 4 h | 28 h | 4 h | 28 h | |
| Astringin lactone (1) | 0.05 ± 0.02 | 0.13 ± 0.04 | 6.54 ± 1.47 | 9.01 ± 1.28 | 0 | 0 |
| Piceatannol (2a and 2b) | 10.3 ± 1.14 | 20.7 ± 4.94 | 3.93 ± 0.74 | 0.32 ± 0.27 | 0 | 0 |
| Astringin dimers (3a and 3b) | 3.92 ± 0.50 | 13.9 ± 2.92 | 22.3 ± 3.21 | 10.1 ± 4.36 | 0.69 ± 0.11 | 2.14 ± 0.42 |
| Piceatannol lactone (4) | 0.05 ± 0.01 | 0.08 ± 0.01 | 0.15 ± 0.04 | 0.54 ± 0.20 | 0 | 0 |
| Piceatannol dimers (5a and 5b) | 0.14 ± 0.02 | 1.12 ± 0.31 | 0.16 ± 0.01 | 0.15 ± 0.43 | 0 | 0 |
| Astringin-piceatannol dimers (6a and 6b) | 1.34 ± 0.87 | 15.0 ± 3.95 | 4.56 ± 0.87 | 3.83 ± 3.77 | 0 | 0 |
| Total identified stilbene metabolites | 15.8 ± 2.53 | 50.9 ± 12.2 | 37.6 ± 6.63 | 24.0 ± 10.3 | 0.69 ± 0.11 | 2.14 ± 0.42 |
| Remaining astringin | 77.5 ± 4.46 | 46.6 ± 11.6 | 53.7 ± 8.67 | 0.94 ± 0.27 | 96.86 ± 0.44 | 95.64 ± 0.27 |
The Stilbene Astringin Is Metabolized by the Fungus to Ring-Opened, Deglucosylated, and Dimeric Products
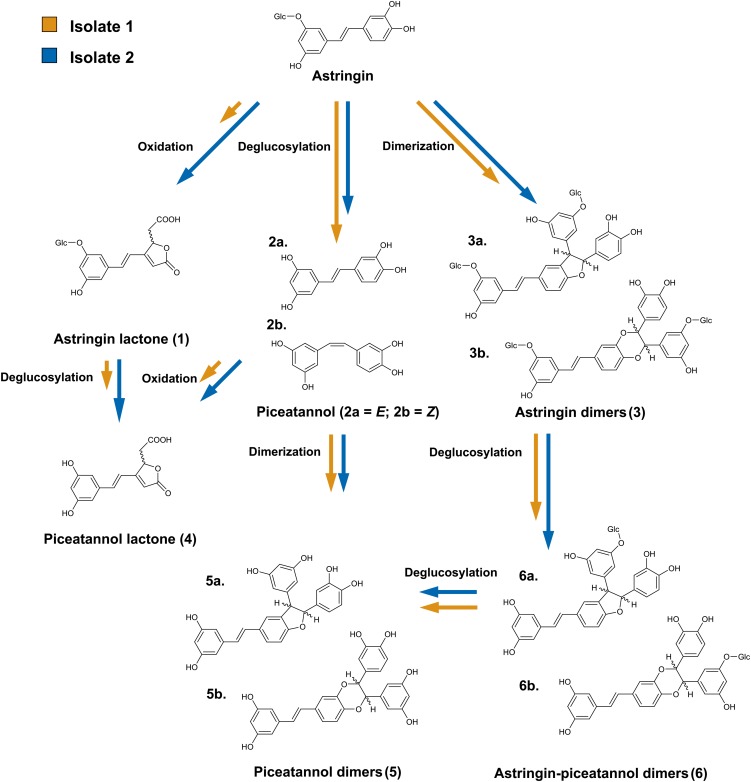
When astringin was added to C. polonica cultures, several products of fungal biotransformation were detected in culture filtrates (Fig. 2), including the ring-opened lactones 1 and 4, the deglucosylated piceatannol 2, as well as several dimeric products (3, 5, and 6). These compounds were identified after solid-phase extraction on RP-18 material followed by HPLC coupled to solid-phase extraction (SPE)-NMR spectroscopy.
Figure 2.
Biotransformation of the Norway spruce stilbene astringin by C. polonica isolate 1 and isolate 2. The lengths of the arrows denote the relative velocity of each reaction when comparing the two isolates in the first 4 h after astringin was added to the growth medium.
To our knowledge, compounds 1 and 4 are reported here for the first time, and their structural elucidation is described in Supplemental Materials and Methods S1. The structures of 2a and 2b were identified as the deglucosylated derivatives of astringin, E- and Z-piceatannol in a ratio of 2:3, based on comparison of the mass spectrometry and 1H-NMR data with reported data (Li et al., 2007). Two inseparable pairs of astringin dimers, 3a and 3b, were also isolated. Each pair comprises two diastereomers in a 1:1 ratio with R,R or S,S configurations at the indicated centers. Mass spectrometric and NMR spectroscopic analysis identified them as piceasides A and B and piceasides G and H (Li et al., 2008; Supplemental Figs. S2 and S3). Further pairs of dimers lacking one (6a and 6b) or both (5a and 5b) Glc moieties were also isolated and identified by mass spectrometry and 1H-NMR spectroscopy. Again, each pair represents a mixture of diastereomers as described above.
Separate Strains of C. polonica Metabolize Astringin at Different Rates Favoring Different Pathways
Although all 10 biotransformation products, some of which occurred as stereoisomers, could be detected in the cultures of both fungal isolates, the major pathways of stilbene breakdown differed between the two isolates. For example, the ring-opened lactone 1 (Fig. 2; Table I) was produced at significantly higher levels by isolate 2 than isolate 1 (P < 0.0001). Lactone 4, formed at lower concentrations than 1, was also detected at higher levels in cultures from isolate 2 than isolate 1 (P < 0.0001).
In contrast, E- and Z-piceatannol (2a and 2b) were produced at higher levels by isolate 1 than by isolate 2 (P < 0.0001). Moreover, a linear increase in piceatannol concentration could be observed in isolate 1 between 4 and 28 h of incubation, whereas a decrease in piceatannol content was noted in cultures colonized by isolate 2. Changes in piceatannol content in culture medium of both fungi were statistically significant between 4 and 28 h (P < 0.0001).
Two pairs of diastereomeric astringin dimers (3a and 3b) were observed in the culture medium of both fungal isolates as well as in noninoculated control medium, but the patterns of change differed. In cultures containing isolate 1 as well as in the sterile control, a gradual increase in astringin dimer concentrations was observed, with levels significantly higher in the fungal cultures than in the control (P = 0.0006). However, in cultures containing isolate 2, the highest concentration of astringin dimers was observed 4 h after the onset of the experiment, followed by a gradual decrease. Further biotransformation of astringin dimers (diglycosides, 3a and 3b) to astringin-piceatannol dimers (monoglucosides, 6a and 6b) and piceatannol dimers (aglycones, 5a and 5b) followed the same relative kinetics observed for conversion to the astringin dimers, with statistically significant differences between fungal isolates 1 and 2 (P < 0.0001).
Consistent with the greater production of astringin metabolites 1, 3a, 3b, and 4 in isolate 2 versus isolate 1, the overall rate of astringin degradation was faster in isolate 2 than isolate 1. After 28 h, less than 1% of the added astringin remained in isolate 2 cultures, while nearly 50% remained in isolate 1 cultures, and more than 95% of the astringin remained in the noninoculated medium (Table I).
C. polonica Protein Extracts Metabolize Astringin in in Vitro Assays
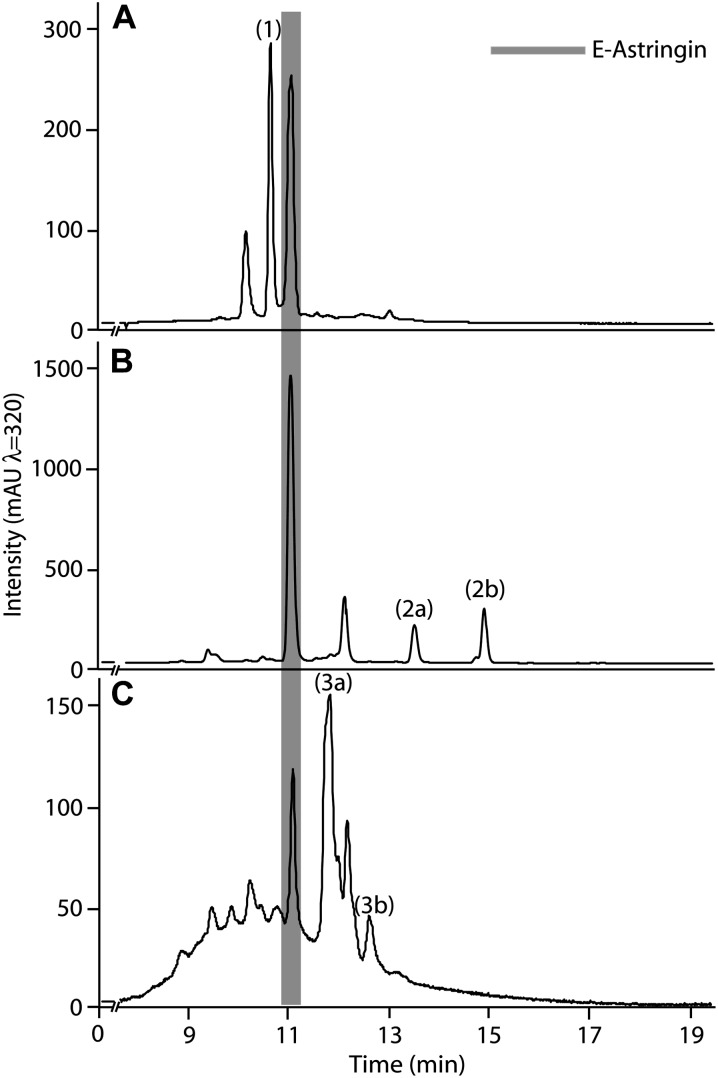
To verify that C. polonica had the capacity to biotransform stilbenes, the relevant astringin-metabolizing activities were sought in fungal enzyme extracts. After a two-step separation of a soluble protein extract from C. polonica, individual enzyme activities could be recovered for all three different types of biotransformation reactions observed in the in vivo study. After separation on the glycoprotein-binding matrix, concanavalin A-Sepharose, an unbound protein fraction hydrolyzed astringin to E- and Z-piceatannol 2 (Fig. 3B). Fractions that bound to concanavalin A-Sepharose were further purified by anion-exchange chromatography. One fraction from this second chromatographic step formed the ring-opened lactone 1 from astringin (Fig. 3A) via a putative catechol dioxygenase-like activity, while another fraction converted astringin to its dimers (3a and 3b) via a putative laccase-like activity (Fig. 3C).
Figure 3.
Stilbene biotransformation activity demonstrated in partially purified protein fractions from C. polonica. A two-step chromatography scheme (affinity separation on concanavalin A-Sepharose followed by anion exchange) separated the three astringin conversion activities indicated by the in vivo experiments: oxidation of astringin to the ring-opened lactone 1 (A); deglucosylation of astringin to form (E)- and (Z)-piceatannol (2a and 2b; B); and oxidation of astringin to form a mixture of dimeric products 3a and 3b (C). Assays for the activities in A and C were performed in 50 mm MOPSO, pH 6.8, and 10% (v/v) glycerol with 1.2 mm astringin and analyzed by LC-ESI-MS and UV-diode array detector. Assays for the activity in B were performed in 50 mm Tris, pH 7.5, and 10% (v/v) glycerol. mAU, Milliabsorbance unit.
Astringin Metabolism in C. polonica Is Induced by Contact with Stilbene-Rich Extracts of Norway Spruce
To test if C. polonica metabolism of stilbenes could be induced by contact with stilbenes, isolates 1 and 2 were grown for 4 d in medium amended with a stilbene-rich aqueous extract of Norway spruce. Contact with the spruce extract generally increased the rate of astringin degradation, as measured in vitro for all three reactions (Table II). For example, there was a 4-fold increase in the rate of formation of the ring-opened lactone by the protein fraction from isolate 2 relative to the control (P = 0.003), although no significant change in the rate of lactone formation was observed for the same fraction from isolate 1. For the deglycosylation of astringin to piceatannol, a 2-fold increase in activity was noted for the protein fraction from isolate 1 after contact with spruce extract (P < 0.0001). For isolate 2, astringin deglucosylation activity was 3-fold higher than for isolate 1 and showed a 1.5-fold increase after contact with spruce extract. The dimerization of astringin in isolate 1 was increased 2-fold by spruce extract compared with the untreated culture (P = 0.02). No changes in the rate of dimerization were observed for isolate 2 after treatment with spruce extract, but activity in both control and treated media was at a level similar to that of isolate 1 after treatment. In general, isolate 2 transformed astringin more efficiently than isolate 1 regardless of treatment with spruce extract (Table II). Conversion to the lactone 1 (P = 0.001), piceatannol 2 (P < 0.0001), as well as stilbene dimers 3 (P = 0.09) in both control and treated medium was higher in enzyme assays from isolate 2 than from isolate 1.
Table II. Rates of astringin biotransformation by C. polonica after treatment with a phenolic extract from Norway spruce.
The conversion of astringin to ring-opened lactone, aglycone, and dimeric products was measured in vitro as µg product g−1 fresh mycelium h−1 ± sd for isolates 1 and 2. Treatment consisted of incubation with 40 µg of Norway spruce methanol extract mL−1 culture for 4 d. Controls were grown in medium without spruce extract (n = 4 replicates per isolate per time point).
| Astringin Products | Rate of Conversion |
|||
|---|---|---|---|---|
| Isolate 1 |
Isolate 2 |
|||
| Control | Treated | Control | Treated | |
| Ring-opened lactone (1) | 0.021 ± 0.003 | 0.025 ± 0.004 | 0.043 ± 0.022 | 0.187 ± 0.043a |
| Aglycones (2a and 2b) | 0.112 ± 0.024 | 0.266 ± 0.027a | 0.411 ± 0.068 | 0.688 ± 0.035a |
| Dimers (3a and 3b) | 0.112 ± 0.093 | 0.263 ± 0.014a | 0.223 ± 0.056 | 0.275 ± 0.033 |
Significant differences between control and treated rates (P < 0.05). Activity for isolate 2 was always greater than for isolate 1 for each reaction (reactions 1 and 2, P < 0.05; reaction 3, P = 0.09).
Astringin Metabolism Leads to Increased Growth on Astringin-Containing Medium
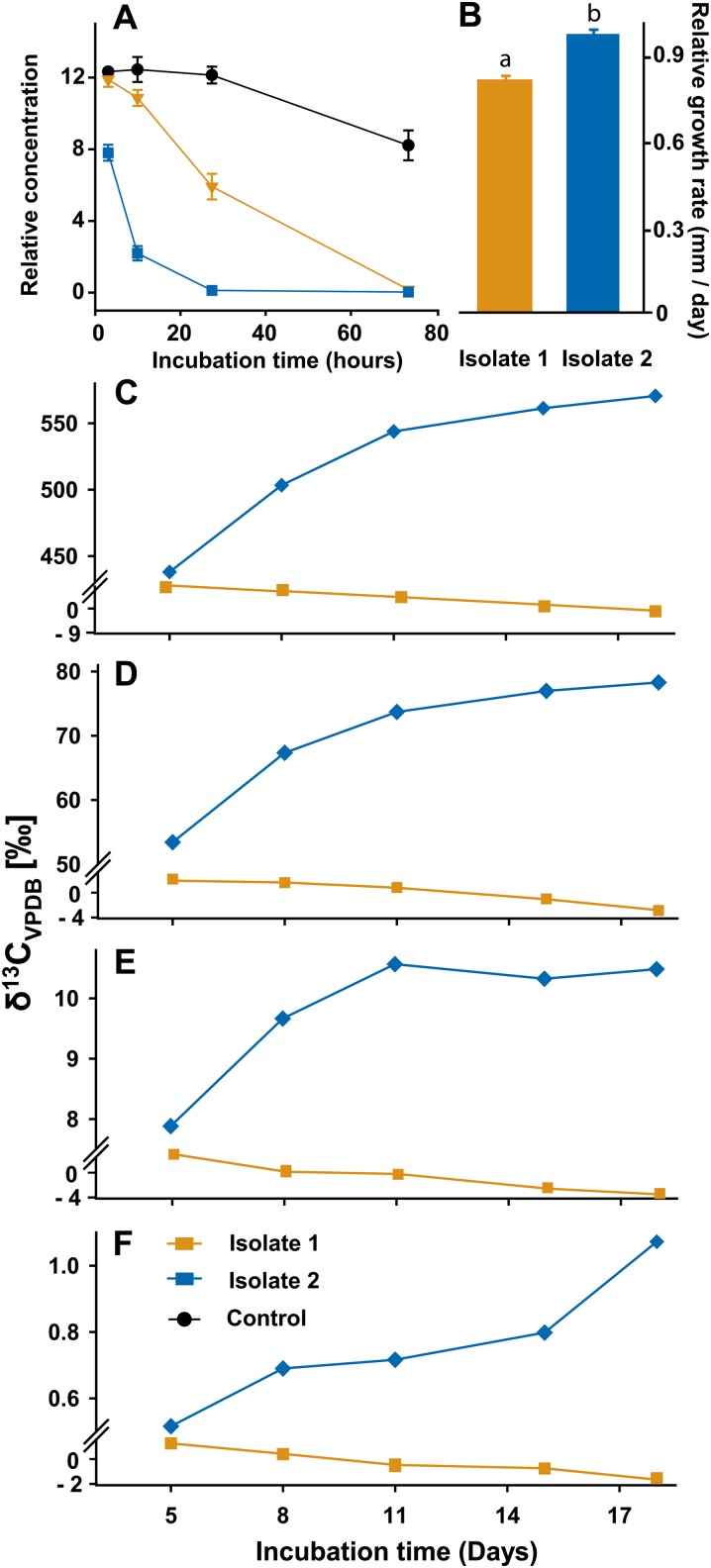
As previously shown, isolate 2 degraded astringin faster than isolate 1 both in vivo (Table I) and in vitro (Table II). To determine if a greater rate of stilbene transformation was associated with increased fungal performance in the presence of astringin, the growth rate of the two C. polonica isolates was compared on artificial medium. On solid minimal medium, the growth rate of isolate 2 was higher than the growth rate of isolate 1 (P < 0.0001), but the growth rates of both isolates declined similarly when the medium was amended with 100 µg astringin mL−1 medium (P < 0.0001). Thus, the relative growth rate of isolate 2 was higher than that of isolate 1 on artificial medium with astringin (P < 0.0001; Fig. 4B).
Figure 4.
Relative growth of C. polonica isolates 1 and 2 in artificial medium amended with astringin in relation to the rates of phenolic biotransformation and the ability to utilize phenolics as sole carbon sources. A, Biotransformation. Depletion of astringin added to artificial medium over a period of 72 h was compared with changes in astringin concentration in sterile control medium (n = 4). B, Growth. In vitro growth on solid minimal medium was determined in the presence of astringin relative to growth without astringin (n = 10). Error bars in A and B represent se. C to F. Utilization. Changes in the ratio of 13CO2 to 12CO2 were determined in fungal head space arising from respiration of [U-13C]caffeic acid. Samples were collected over 18 d of fungal growth on agar containing 1 mm caffeic acid amended with 10 µm (C), 2 µm (D), 0.4 µm (E), and 0.08 µm (F) [U-13C]caffeic acid (n = 1). VPDB, Vienna Pee Dee Belemnite. [See online article for color version of this figure.]
C. polonica Can Use Caffeic Acid as Its Sole Carbon Source
The ring-opened lactone 1 has structural similarities to muconolactone, an intermediate in the β-ketoadipate pathway employed by microbes to utilize aromatic compounds as carbon sources (Harwood and Parales, 1996). To investigate if C. polonica could utilize stilbenes as a carbon source, an experiment was conducted where both isolates 1 and 2 were grown in sealed containers on medium containing different concentrations of [U-13C]caffeic acid, a phenolic compound containing the same o-dihydroxyphenyl moiety, and conjugated C=C bond as astringin. The head space containing CO2 arising from fungal respiration was sampled at intervals and measured by isotope ratio mass spectrometry. Increases in 13CO2-12CO2 ratios were observed in the head space of cultures containing isolate 2 for concentrations of 13C-labeled caffeic acid ranging from 10 to 0.08 µm, while little or no change in 13CO2-12CO2 ratios were detected in cultures containing isolate 1 (Fig. 4, C–F). The appearance of 13CO2 upon feeding [U-13C]caffeic acid was taken as evidence of caffeic acid metabolism.
Utilization of Phenolics Is Associated with Fungal Virulence
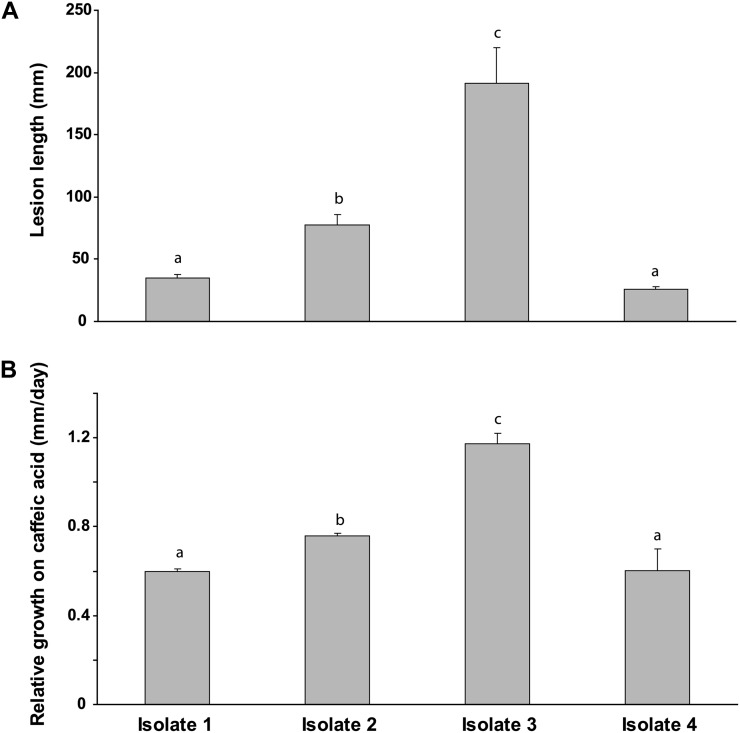
To determine if the ability of C. polonica to metabolize phenylpropanoids was associated with virulence in Norway spruce, the performance of four isolates (including the isolates 1 and 2 used previously) was assessed after inoculation in the bark of Norway spruce saplings. Virulence was assessed by measurement of lesion lengths, represented by the area of damage after vertical growth upward and downward through the phloem. More virulent fungi should produce longer lesions. The lesion lengths created by isolate 2 were more than twice as long as those created by isolates 1 and 4 (P < 0.0001), whereas isolate 3 created a lesion over 2.5 times that of isolate 2 (P < 0.0001; Fig. 5A).
Figure 5.
Relative virulence of C. polonica isolates in Norway spruce bark in relation to their ability to utilize phenolics as sole carbon sources. A, Virulence. Lesion lengths were measured in the bark of spruce saplings 28 d after inoculation (n = 5). B, Utilization. Performance was determined on solid minimal medium with caffeic acid as the sole carbon source. Growth on caffeic acid was measured relative to growth on medium amended with Glc (n = 5). Lowercase letters denote statistically significant differences in growth.
When these four isolates were grown on minimal medium containing caffeic acid as the sole carbon source, isolate 3 exhibited significantly greater mycelial growth (P < 0.0001), followed by isolate 2. The least virulent isolates, 1 and 4, exhibited the poorest growth (P < 0.0001; Fig. 5B). There is thus a correlation between fungal virulence and the utilization of aromatic compounds such as caffeic acid or astringin as a carbon source. Similar observations were made when an independent collection of C. polonica isolates, where the relative virulence is known (Krokene and Solheim, 2002), were compared for their growth rates on caffeic acid (Supplemental Fig. S5).
DISCUSSION
The attack of bark beetles on their conifer hosts is frequently associated with infection by specialized fungi that are inoculated by the beetle. In this study, we investigated the biochemical adaptations that allow one such fungus, the ascomycete C. polonica, to colonize its host tree, Norway spruce. After inoculation into Norway spruce by the Eurasian spruce bark beetle (Ips typographus), C. polonica metabolizes the major antifungal phenolic compound produced in the bark. The stilbene astringin, a diaryl-ethene derivative, is metabolized to ring-opened lactones, aglycones, and dimers by fungal enzymes whose activities are induced when fungi are in a stilbene-rich environment. The ability of C. polonica to metabolize stilbenes and structurally similar phenolic compounds is directly correlated with its virulence in spruce bark and appears to allow growth on stilbenes as a sole carbon source.
A number of previous studies had reported that the stilbene content of spruce bark declined during fungal attack (Brignolas et al., 1995; Viiri et al., 2001; Li et al., 2012). These declines were considered very puzzling because they occurred despite increases in the enzyme activity of STS (Brignolas et al., 1995), which catalyze the formation of the stilbene skeleton. Furthermore, increases in piceatannol concentrations (Viiri et al., 2001) and stilbene dimers (Li et al., 2012) were observed in spruce bark after C. polonica infection. Our results now clearly show that these metabolites originate from the fungal metabolism of host defense compounds and that C. polonica metabolism of stilbenes can override even increases in host stilbene biosynthesis.
Stilbenes have long been known as antifungal defenses in plants that inhibit fungal growth by interfering with microtubule assembly (Woods et al., 1995; Adrian et al., 1997), disrupting plasma membranes and uncoupling electron transport in fungal spores and germ tubes (Pont and Pezet, 1990; Adrian and Jeandet, 2012). It is not surprising that fungi specialized to live in stilbene-rich plant material, such as C. polonica, have developed mechanisms to circumvent the deleterious effects of these compounds. Here, we demonstrated that C. polonica not only metabolizes stilbenes but employs several different degradation routes. Using the tetrahydroxystilbene glucoside, astringin, which is produced in high amounts by Norway spruce, as a model, we identified ring-opened lactone, aglycone, and dimeric metabolites in C. polonica cultures. Some of these reaction types have been reported for other stilbenes (Breuil et al., 1998, 1999; Rodriguez-Bonilla et al., 2011). For example, stilbene dimer formation has been previously reported in the grape (Vitis vinifera) pathogen Botrytis cinerea. The dimerization of resveratrol and pterostilbenes has been shown to be an oxidative process involving the 4′-hydroxyl group of the stilbene skeleton and catalyzed by laccases and peroxidases (Breuil et al., 1998; Rodriguez-Bonilla et al., 2011).
Ring opening and lactonization has not previously been reported for stilbenes but is a logical early step of catabolism, since it results in a more polar product. Stilbenes exert their toxicity on fungi by diffusing through membranes into vegetative and reproductive structures (Adrian et al., 1998). A ring-opened and lactonized product with increased polarity should diffuse more slowly into fungal cells and, therefore, show decreased toxicity. Converting plant defense compounds to more polar products has been shown to be a successful detoxification strategy for other plant pathogens (Weltring and Barz, 1980; Esaki et al., 1998). A more recent study (Sobolev et al., 2006) reported the appearance of a novel stilbene phytoalexin in peanut (Arachis hypogaea) kernels infected by Aspergillus species. This compound has a prenylated but-2-enolide skeleton that bears a striking resemblance to the astringin lactone produced by C. polonica and so might also represent a fungal biotransformation product derived from a peanut phytoalexin.
In contrast to ring opening, deglucosylation is a seemingly disadvantageous detoxification process for the fungus, as this releases a less polar product. In fact, activation of glycoside defense compounds by deglycosylation when cell compartments are disrupted by herbivore or pathogen attack is a common feature of many plant defenses (Morant et al., 2008), and glycosylation is a known detoxifying mechanism of certain plant pathogens (Pedras et al., 2004). However, in a relatively nutrient-poor environment like Norway spruce phloem and sapwood, it might be advantageous for fungal pathogens with high tolerance to stilbenes to deglucosylate them and utilize the free Glc moieties as an energy source. Nevertheless, it is interesting that, in our study, the more virulent C. polonica strain relied less on deglycosylation and more on the other catabolic routes, ring-opened lactone formation and dimerization.
In this study, we demonstrated that metabolism of stilbenes was inducible by a crude stilbene-rich extract prepared from Norway spruce. The induction of detoxification pathways upon contact with plant antifungal defenses has been reported for other phytopathogenic fungi. Among phenolic compounds, caffeic acid induces the transcription of a gene cluster involved in the β-ketoadipate pathway in Cochliobolus heterostrophus, the causal agent of southern corn leaf blight (Shanmugam et al., 2010), while phenolic compounds and pectin induce laccases responsible for stilbene dimerization in B. cinerea (Gigi et al., 1980). However, not all detoxification pathways are inducible. In Grosmannia clavigera, a bark beetle-vectored fungal pathogen of pine (Pinus spp.), genes putatively involved in polyphenol detoxification were not observed to be induced by a pine phloem extract, which presumably would contain phenolic substances (Hesse-Orce et al., 2010).
The ability of plant pathogens to metabolize antifungal defenses has been shown to be an important virulence factor. For example, the virulence of pathogens on pea (Pisum sativum) plants depends on their ability to detoxify the pea phytoalexin pisatin (Van Etten et al., 2001), and the virulence of B. cinerea isolates on grape could be at least partially correlated with their ability to metabolize stilbenes (Sbaghi et al., 1996). Moreover, in G. clavigera, it was shown that the export of antifungal components of pine resin by an ATP-binding cassette transporter is essential for fungal growth in its host (Wang et al., 2013). In this study, we show that the virulence of C. polonica correlated with differential usage of the various pathways for stilbene biotransformation and the degradation of structurally similar compounds. Biotransformation pathways were generally induced in response to a spruce stilbene-containing extract. However, the more virulent isolate had higher constitutive enzyme activity for all three pathways, ring-opened lactone formation, deglucosylation, and dimerization, than the less virulent isolate. Moreover, application of spruce extract led to a significant increase in the rate of ring opening and deglucosylation.
The metabolism of Norway spruce stilbenes by C. polonica may serve not only to detoxify them but also to provide the fungus with nutrition. Other aromatic compounds, such as lignin breakdown products, have been shown to be metabolized by the β-ketoadipate pathway in soil bacteria and fungi, yielding energy, reducing equivalents, and releasing CO2 (Harwood and Parales, 1996). The structural similarities between the ring-opened lactone formed from astringin and an intermediate in the β-ketoadipate pathway hinted that stilbenes might also be further catabolized by C. polonica via this process. Using caffeic acid, whose structure closely matches that of astringin in the region of the molecule giving rise to the ring-opened lactone, we established that virulent C. polonica isolates did employ this aromatic compound as an energy source based on release of 13CO2 from [U-13C]caffeic acid and growth on caffeic acid as a sole carbon source. Thus, C. polonica could conceivably use the stilbenes of its host tree as nutrients, resulting in an increased growth rate and more successful colonization of the tree. The use of host defense chemicals as a carbon source to support the growth of a bark beetle-associated fungus has also previously been reported for the mountain pine beetle associate G. clavigera, which can grow on monoterpene compounds of pine resin as a sole carbon source (DiGuistini et al., 2011).
The fungus C. polonica and the bark beetle are frequent associates that are usually viewed as killing their Norway spruce host by their combined efforts (Franceschi et al., 2005), but this opinion is not universally accepted (Six and Wingfield, 2011). In any case, the exact basis for their mutualism is not fully understood (Paine et al., 1997). It has been proposed that the fungus benefits by being dispersed to new hosts and gaining entry into the tree. The beetle, on the other hand, may benefit if the fungus helps weaken or kill the tree, since tree death is essential for beetle reproduction (Franceschi et al., 2005). Our study demonstrates that fungal growth in the stilbene-rich bark of Norway spruce depends on successful metabolism of these host defense compounds, which should also benefit the bark beetle by neutralizing tree defenses.
An alternative explanation for the cooccurrence of bark beetles with C. polonica might be the nutritional benefits associated with polyphenol biotransformation by the fungus. Degradation of stilbenes may benefit bark beetles by improving the quality of substrate available for feeding their larvae, since the stilbenes remaining in the bark could be toxic. For example, the catechol groups present in the astringin core structure may be spontaneously or enzymatically rearranged to form reactive quinones (Haruta et al., 2001; Lin et al., 2010) under the semialkaline conditions prevailing in beetle guts (Balogun, 1969). These quinones can then alkylate reactive nucleophiles such as sulfhydryl and amino groups in proteins or amino acids (Felton et al., 1992; Son et al., 2010). Protein alkylation may reduce the nutritional quality of ingested food or destabilize the peritrophic membrane in the bark beetle gut (Barbehenn et al., 2008). Lactonization or dimerization of the catechol groups, as we showed for C. polonica, should prohibit quinone formation and thus protect bark beetles from the harmful effects of stilbene defense compounds.
We have shown in this study that C. polonica can transform phenolic defense compounds from Norway spruce and that it is even able to use these compounds as its sole carbon source. Further research should reveal more about how the biochemical capabilities of this fungus can contribute to its relationship with bark beetles and how its metabolism of spruce defenses modifies host tree resistance to both the fungus and the beetle.
MATERIALS AND METHODS
Inoculation of Norway Spruce Saplings with Ceratocystis polonica
Eight-year-old Norway spruce (Picea abies) saplings originating from the 3369-Schongau clone (Samenklenge and Pflanzengarten) were grown in an outdoor plot for 4 years prior to the experiment. Two C. polonica isolates (CMW 7749 = isolate 1 and CMW 7135 = isolate 2) were provided by the culture collection of the Forestry and Agricultural Biotechnology Institute (University of Pretoria). These were characterized as the least and most virulent, respectively, out of 12 isolates by measurement of lesion lengths created after inoculation of mature spruce trees (Marin, 2003). Both were grown on 2% (w/v) malt extract agar for 14 d at 25°C. Inoculations of saplings with C. polonica isolates 1 and 2 were performed 3 weeks after buds had broken during the flush of spring growth (June 10, 2008). A bark plug, 8 mm in diameter, was removed midway between the second and third branch whorl from the upper part of the sapling (2-year-old segment) with a cork borer. An 8-mm plug from one of the two C. polonica cultures was placed into the wound with the mycelium oriented toward the wood surface and sealed with Parafilm. For the control treatment, plugs of sterile malt extract agar were inserted into the wound. All treatments were applied during the same growth phase.
Bark tissue samples from five inoculated and five wounded saplings were harvested 2, 7, 14, and 28 d after the onset of the experiment. Five nonwounded control saplings were harvested at 2 d after the onset of the experiment. Fungal lesions were measured with a caliper. Bark material was flash frozen immediately after harvest in liquid nitrogen and stored at −80°C.
Inoculations of isolates 2, 3, and 4 were performed as described above using a 5-mm cork borer to wound 5-year-old clonal Picea glauca saplings. Lesion length data from this experiment were related to data from the first experiment by comparison of lesions of isolate 2 under the different conditions.
Quantitative Real-Time PCR of STS from Norway Spruce
Total RNA from inoculated treatment and wounded control bark was isolated with the Invitrap Spin Plant RNA Mini Kit (Invitek) following the protocols of the manufacturer except that an additional DNA digestion step was included (RNase Free DNase set; Qiagen). RNA was quantified by spectrophotometry. Reverse transcription of 1 µg of RNA into complementary DNA (cDNA) was achieved by using SuperScript II reverse transcriptase (Invitrogen) and 50 pmol of PolyT(12-18) primer (Invitrogen) in a reaction volume of 20 µL. cDNA was diluted to 10% (v/v) with deionized water. One microliter of diluted cDNA was used as template for quantitative real-time PCR in a reaction mixture containing Brilliant SYBR Green QPCR Master Mix (Stratagene), 10 pmol of forward primer, and 10 pmol of reverse primer. PaSTS transcripts were amplified using the forward primer 5′-GTGGCGAGCAGAACACAGACTTC-3′ and the reverse primer 5′-CAGCGATGGTACCTCCATGAACG-3′. This primer pair was designed to amplify 140 bp of both STS1 (GenBank accession no. JN400048) and STS2 (GenBank accession no. JN400047) simultaneously. PCR was performed using a Stratagene MX3000P thermocycler using the following cycling parameters: 5 min at 95°C, followed by 40 cycles of 30 s at 95°C, 30 s at 55°C, and 30 s at 72°C, and a melting curve analysis from 55°C to 95°C. Reaction controls included nontemplate controls as well as non-reverse-transcribed RNA. STS gene abundance was normalized to the abundance of the Norway spruce ubiquitin gene (Schmidt et al., 2010; GenBank accession no. EF681766) amplified with the forward primer 5′-GTTGATTTTTGCTGGCAAGC-3′ and the reverse primer 5′-CACCTCTCAGACGAAGTAC-3′. Relative transcript abundance was calculated from three technical replicates of five biological replicates and calibrated against the transcript abundance of five nonwounded control saplings (relative transcript abundance = 1).
Phenolic Extraction from Spruce to Investigate Changes after C. polonica Infection
For the extraction of phenolic compounds, spruce tissue was ground to a fine powder under liquid nitrogen and lyophilized. Approximately 40 mg of dried tissue was extracted with 2 mL of HPLC-grade methanol for 4 h at 4°C. Insoluble material was pelleted by centrifugation, and the supernatant was recovered. Insoluble material was reextracted with 1.5 mL of methanol for 16 h. Extracts were combined and evaporated to dryness under a stream of nitrogen. Dried samples were redissolved in 1 mL of methanol containing 100 µg mL−1 of the internal standard apigenin glucoside. For liquid chromatography (LC)-electrospray ionization (ESI)-mass spectrometry (MS), samples were diluted to 20% (v/v) with water.
HPLC-ESI-MS
Phenolic metabolites from spruce bark and astringin biotransformation products were separated on a Nucleodur Sphinx RP18ec column with dimensions of 250 × 4.6 mm and a particle size of 5 µm (Macherey-Nagel) using an 1100 series HPLC apparatus (Agilent Technologies). The total mobile phase flow rate for chromatographic separation was 1.0 mL min−1. The column temperature was maintained at 25°C. Phenolic compounds from spruce and fungal biotransformation were separated using 0.2% (v/v) formic acid and acetonitrile as mobile phases A and B, respectively, with the following elution profile: 0 to 1 min, 100% A; 1 to 25 min, 0% to 65% B in A; 25 to 28 min, 100% B; and 28.1 to 32 min, 100% A. Products from enzyme assays were separated with the following elution profile: 0 to 1 min, 100% A; 1 to 18 min, 0% to 100% B in A; 18 to 19 min, 100% B; and 19.1 to 22 min, 100% A.
Compound detection and quantification were accomplished with an Esquire 6000 ESI ion-trap mass spectrometer (Brucker Daltronics). Flow coming from the column was diverted in a ratio of 4:1 before entering the mass spectrometer electrospray chamber. The MS device was operated in negative mode scanning mass-to-charge ratio between 50 and 1,600 with an optimal target mass of mass-to-charge ratio 405. The mass spectrometer was operated using the following specifications: skimmer voltage, 60 V; capillary voltage, 4,200 V; nebulizer pressure, 35 p.s.i.; drying gas, 11 L min−1; gas temperature, 330°C. Capillary exit potential was kept at −121 V.
Compounds in chromatograms were identified based on retention time, their apparent molecular masses, and fragmentation spectra (Supplemental Table S3; Supplemental Fig. S4) and ultimately by NMR (see below). Brucker Daltronics Quant Analysis version 3.4 software was used for data processing and compound quantification using a standard smoothing width of 3 and Peak Detection Algorithm version 2. Linearity in ionization efficiencies was verified by analyzing serial dilutions of randomly selected samples. Ion suppression was controlled for by calculating analyte-to-internal standard ratios in serially diluted samples in which the internal standard was maintained at the same concentration. An external calibration curve for astringin was created by linear regression. Variability in the processing of individual samples was corrected by adjustment relative to the internal standard, the flavonoid apigenin glycoside.
In Vivo Biotransformation of Astringin by C. polonica in Culture
C. polonica isolate 1 and isolate 2 were grown on 2% (w/v) malt extract agar for 12 d at 25°C in darkness. Agar plugs (diameter = 4 mm) from stationary cultures were placed in 15-mL test tubes for biotransformation assays. Then, 2 mL of sterile 2% (v/v) malt extract amended with astringin to a final concentration of 2 mg mL−1 was added to the test tubes and incubated at 28°C with shaking at 220 rpm. Negative control treatments contained astringin-amended medium without fungus and fungus grown in medium without astringin. Cultures were harvested 4, 8, 24, and 72 h after the onset of the experiment. Biotransformation processes were stopped by adding 50 µL of HCl (2 n) to the test tubes. The internal standard apigenin-3-O-glucoside was added to a final concentration of 0.1 mg mL−1 prior to analysis by LC-ESI-MS. The concentrations of biotransformation products were calculated relative to the internal standard.
Preparation of Astringin Biotransformation Products for NMR Analysis
Culture medium samples from biotransformation experiments were first subjected to SPE with RP-18 as the stationary phase. After loading, the columns were washed with water, dried with nitrogen gas, and finally eluted with methanol. The methanol extracts were separated by means of HPLC, and peaks of interest were collected online by postcolumn SPE. The HPLC-SPE system consisted of an Agilent 1100 chromatography system (Agilent Technologies) and a J&M photodiode array detector (J&M Analytik) connected to a Spark Prospekt 2 SPE device (Spark Holland) equipped with HySphere resin general phase cartridges (10 × 2 mm, 10 μm). Separations were performed with linear gradient elution, with water (A) and methanol (B) as solvents, both containing 0.1% formic acid: 0 to 1 min, 100% A; 1 to 20 min, 0% to 100% B in A; 20 to 25 min, 100% B; 25 to 27 min, 100% to 0% B in A; 27 to 34 min, 100% A. The column was a Macherey-Nagel Isis RP-18e column (250 × 4 mm). The make-up flow for postcolumn SPE trapping was set to 2.5 mL min−1 using HPLC-grade water. The SPE cartridges were subsequently dried using pressurized nitrogen. HPLC-grade acetonitrile was used to extract the trapped analytes from the general phase cartridges into HPLC glass vials. After evaporation to dryness using nitrogen gas, the samples were reconstituted with 80 µL of MeOH-d4 and DMSO-d6, transferred into 2-mm (i.d.) capillary NMR tubes, and subjected to NMR measurements. Hystar 3.2 software was used to coordinate the LC-SPE experiments, and Topspin 2.1 software was used to control the NMR spectrometer and to perform data processing.
NMR and High-Resolution MS Analysis of Astringin Biotransformation Products
1H-NMR, 13C-NMR, 1H-1H-correlation spectroscopy, total correlated spectroscopy, heteronuclear multiple bond correlation, and heteronuclear single quantum coherence spectra were measured on a Bruker Avance 500 NMR spectrometer (Bruker Biospin) operating at 500.13 MHz for 1H and 125.75 MHz for 13C. An inverse triple channel cryoprobe (5 mm) was used to measure spectra at a probe temperature of 300 K. Spectra are referenced to the residual solvent signals: for methanol-d4 at δ 3.31/49.05 ppm and for DMSO-d6 at δ 2.49/39.51 ppm. Capillary tubes (2 mm i.d.) were used for all NMR measurements.
Attempts to determine the structure of compound 1 in methanol-d4 resulted in degradation during time-consuming two-dimensional heteronuclear experiments. Thus, DMSO-d6 was used.
High-resolution MS was recorded on a ultra-performance liquid chromatography-MS/MS system consisting of an Ultimate 3000 series Rapid Separation LC (Dionex) system and an Orbitrap mass spectrometer (Thermo Fisher Scientific). Ultra-performance liquid chromatography was performed using a Dionex Acclaim C18 column (150 × 2.1 mm × 2.2 μm) at a constant flow rate of 300 μL min−1. A binary solvent system of water (A) and acetonitrile (B), both containing 0.1% formic acid, was used as follows: 0 min, 20% B in A; 0 to 6 min, 20% to 95% B in A; 6 to 10 min, 95% B; 10 to 14 min, 20% B in A. Full-scan mass spectra were generated using 30,000 resolving power: the mass accuracy was better than 3 ppm.
Partial Purification of Fungal Protein Fractions Showing Stilbene Biotransformation Activity
C. polonica isolates 1 and 2 were cultured in 10% (v/v) carrot (Daucus carota) juice liquid culture for 6 d, harvested by centrifugation, and lyophilized. Dried material was finely ground using a vibrating ball mill. Powdered mycelium (0.5 g) was extracted with 10 mL of extraction buffer (50 mm Tris, pH 7.5, 5 mm ascorbic acid, 5 mm dithiothreitol, 10 mm MgCl2, 10 mm CaCl2, 10 mm MnCl2, 0.5 m NaCl, 10% glycerol, 1% polyvinylpyrrolidone [molecular weight of 360,000], 4% polyvinylpolypyrrolidone, and 0.1% Tween 20) at 4°C for 30 min with shaking. Crude fungal protein extract in extraction buffer was loaded onto an open column of concanavalin A-Sepharose (GE Healthcare) with a bed volume of 5 mL. The protein extract was incubated with the concanavalin A matrix for 30 min at 4°C before washing unbound proteins off with 5 bed volumes of washing buffer (50 mm Tris, pH 7.5, 0.5 m NaCl, and 10% glycerol). Elution of bound proteins was achieved using 2 column volumes of washing buffer amended with 500 mm α-d-methylglucopyranoside. Proteins from the column eluate were desalted at 4°C into 50 mm 3-(N-morpholino)-2-hydroxypropanesulfonic acid (MOPSO; pH 6.8) containing 10% glycerol using a HiPrep 26/10 (GE Healthcare) desalting column on an ÄKTA 900 chromatography system (GE Healthcare). The desalted protein fraction was loaded onto a 5-mL DEAE-Sepharose column and washed with 2 column volumes of DEAE washing buffer (50 mm MOPSO, pH 6.8, and 10% glycerol) at a flow rate of 5 mL min−1. Proteins were eluted from the column with DEAE wash buffer adjusted with NaCl using a step-wise gradient (100 mm, 200 mm, 300 mm, 500 mm, and 1 m NaCl). Elution steps and fraction volumes were 10 mL. Stilbene biotransformation activity was determined for crude extracts as well as for proteins that were eluted from the concanavalin A- and DEAE-Sepharose columns and for samples from the flow through of both columns. Enzyme activities were assayed in 300-µL reaction volumes containing 200 µL of enzyme from the purification steps described above and 100 µg of astringin in DEAE washing buffer. Reaction mixtures were incubated at 30°C for 4 h before stopping the reaction with 10 µL of 0.1 n HCl. After removing the protein by centrifugation, 20 µL of the reaction mixture was analyzed by LC-ESI-MS.
In Vitro Assay of Astringin Biotransformation Activity
Mycelium was cultured as above for 3 d. Each culture (10 mL) was subcultured in 50 mL of 10% (v/v) carrot medium. To determine if the biosynthesis of enzymes for stilbene biotransformation could be induced, the medium of four biological replicates was amended with 4 mg of crude spruce extract (methanol-soluble bark extract with solvent evaporated) in 2 mL of sterile water. As controls, four other replicates were subcultured after adding sterile water instead of spruce extract. Subcultured mycelium was harvested after 4 d by centrifugation and ground to a fine powder using a mortar and pestle. Then, 100 mg of ground mycelium was extracted with 5 mL of extraction buffer as above. Insoluble material was removed from extracts by centrifugation, and the resulting supernatant was filtered with a syringe filter with an exclusion size of 0.2 µm.
Astringin biotransformation activity was assayed at 30°C in 2-mL reaction volumes containing fungal protein in extraction buffer and 100 µg mL−1 astringin. A 200-µL subsample was taken from each assay reaction 45 min and 2, 4, and 8 h after the assays were initiated. The biotransformation reaction was stopped and analyzed as above.
Assessment of Fungal Growth in the Presence of Astringin
Petri dishes with synthetic nutrient agar (Nirenberg and O’Donnell, 1998) amended with astringin were prepared by cooling the autoclaved medium to 55°C and adding astringin in ethanol to a final concentration of 100 µg mL−1 medium. Medium for negative control treatments was amended with ethanol only. Medium (25 mL) was dispensed in petri dishes (diameter = 10 cm). After the medium set, agar plugs (diameter = 4 mm) from 14-d-old C. polonica stationary cultures (isolate 1 or 2) were placed in the middle of each petri dish. Cultures were sealed with Parafilm and incubated at 26°C. Diameters of the expanding fungal cultures were measured every 24 h for 5 d. Growth rates were calculated using the slope of linear growth curves. Relative growth on astringin was calibrated against the mean growth rate of each isolate on control medium (100%) by calculating the fraction of each isolate’s growth rate on astringin.
Phenolic Extraction from Spruce to Induce Fungal Biotransformation Pathways
Finely ground lyophilized spruce bark (100 g) was extracted overnight with 400 mL of methanol. The mixture was filtered and the solvent was removed under reduced pressure in a rotary evaporator (Buechi Rotavapor R-114). The dried extract was weighed and redissolved in sterile water to a concentration of 2 mg mL−1.
Measurement of Fungal Utilization of Caffeic Acid as a Carbon Source
Synthetic nutrient agar (Nirenberg and O’Donnell, 1998) was prepared. Modified synthetic nutrient agar was prepared by replacing Glc and Suc with the equivalent amount of caffeic acid. Agar plugs (5-mm diameter) from fungal cultures (isolates 1–4 and 1994-169/113, 1980-53/7/A, 1993-208/115, 1980-53/7/B, 1980-53/7/C, and 1980-53/7/A′; Krokene and Solheim, 2002) were plated on both synthetic nutrient agar and modified synthetic nutrient agar and grown at 25°C in the dark for 14 d. Radial mycelial growth was measured every 2 to 5 d, and growth rates were calculated as above. Relative growth rates were determined by normalization with growth on synthetic nutrient agar.
Water agar (2.5%, w/v) amended with 1 mm caffeic acid was prepared. Filter-sterilized caffeic acid with a natural 13C-12C carbon isotope ratio (Sigma) was mixed with [U-13C]caffeic acid (Campro Scientific) at ratios of 100:1, 100:0.2, 100:0.04, and 100:0.008 in different batches of medium. The growth medium (2 mL) was dispensed into heat-sterilized 10-mL vials (Exetainer; Labco). Mycelial plugs (length approximately 2 mm) from isolate 1 or isolate 2 stationary cultures, grown on modified synthetic nutrient agar containing 2 mm caffeic acid with a natural 13C-12C carbon isotope ratio, were placed onto the medium inside the vials and sealed with a septum under open-air conditions. Using an autosampler (CTC Combi-PAL; CTC Zwingen), 200-µL air aliquots were taken from each vial 5, 8, 11, 15, and 18 d after initiating the experiment. The aliquots were injected into a homemade, room temperature, constant pressure gas chromatograph modified from a commercial GC/general phase system (Thermo Fisher Scientific). Between the injector and a 30-m Poraplot Q capillary column, a Nafion (a sulfonated tetrafluoroethylene-based fluoropolymer; Perma Pure Products) online water-removal unit was placed. A second such unit was mounted postcolumn, followed by a third water-removal step realized by immersing the transfer line into a dry ice bath. The transfer line led to an active open splitter that was connected to an isotope ratio mass spectrometer (Delta+ XL; Thermo Fisher Scientific). The open splitter reduces the effluent such that only the eluting CO2 peak enters the mass spectrometer; other air constituents (mainly nitrogen, oxygen, and argon) are diverted to the vent and do not interfere with the isotopic analysis. Before each sample was injected, air from a continuously bleeding reference air tank, whose mixing ratio and isotopic composition were independently calibrated at the Max Planck Institute for Biogeochemistry, was analyzed. In addition, control samples were included for each 13C-12C caffeic acid ratio from medium incubated without fungus.
The actual 13CO2-12CO2 ratio values in air samples from control and sample vials were calculated from the measured δ13C values using the International Union of Pure and Applied Chemistry reference value of 0.0111802 for 13CO2:12CO2 ratio in the international Vienna Pee Dee Belemnite reference (Zhang et al., 1990; Werner and Brand, 2001). Then, isotopic enrichment of fungal samples was obtained by subtracting the measured δ13C values of the blank samples without fungus.
Statistical Analysis
The results are presented graphically as means ± se. Tabulated results are presented as means ± sd. The normality of data was tested for with the Shapiro-Wilk test. The statistical significance of differences in PaSTS transcript accumulation and stilbene concentrations in living trees was determined using linear models with tree responses as the dependent variable and time as the explanatory variable on log-transformed data. Differences between fungal biotransformations of astringin as well as differences of fungal growth on astringin-amended medium were analyzed with the nonparametric Kruskal-Wallis test, as data could not be normalized. Differences in fungal growth in spruce bark and on caffeic acid were analyzed using one-way ANOVA. Differences in reaction rates of astringin biotransformation activity by the fungus measured in vitro were analyzed using two-way ANOVA of untransformed or reciprocally (1/×) transformed data. Following ANOVA, differences in means were calculated using Tukey’s post-hoc pairwise comparisons test at a 95% confidence level. Analyses were conducted using the open-source software R (version 2.81) and the LAERCIO package for Tukey’s pairwise comparisons.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Ring-opened lactone products derived from C. polonica transformation of astringin.
Supplemental Figure S2. NMR spectrum of one set of dimeric products (3a) derived from C. polonica biotransformation of astringin in this study.
Supplemental Figure S3. NMR spectrum of another set of dimeric products (3b) derived from C. polonica biotransformation of astringin in this study.
Supplemental Figure S4. Diagnostic mass spectral fragments of astringin and its biotransformation products.
Supplemental Figure S5. Relative virulence of six C. polonica isolates first described in Krokene and Solheim (2002) is correlated with their ability to grow on caffeic acid as the sole carbon source.
Supplemental Table S1. 1H NMR (500 MHz) and 13C NMR data (125 MHz) of compounds 1 in DMSO-d6 and 4 in MeOH-d4.
Supplemental Table S2. Mass spectrometry data for astringin and identified astringin metabolites.
Supplemental Materials and Methods S1. Structural elucidation of compounds 1 and 4.
Supplementary Material
Acknowledgments
We thank Bettina Raguschke, Petra Linke, and Michael Reichelt for technical assistance and Paal Krokene and Mike Wingfield for providing C. polonica isolates.
Glossary
- STS
stilbene synthase
- SPE
solid-phase extraction
- cDNA
complementary DNA
- ESI
electrospray ionization
- LC
liquid chromatography
- MS
mass spectrometry
- DMSO
dimethyl sulfoxide
- MOPSo
3-(N-morpholino)-2-hydroxypropanesulfonic acid
References
- Adrian M, Jeandet P. (2012) Effects of resveratrol on the ultrastructure of Botrytis cinerea conidia and biological significance in plant/pathogen interactions. Fitoterapia 83: 1345–1350 [DOI] [PubMed] [Google Scholar]
- Adrian M, Jeandet P, Veneau J, Weston LA, Bessis R. (1997) Biological activity of resveratrol, a stilbenic compound from grapevines, against Botrytis cinerea, the causal agent for grey mould. J Chem Ecol 23: 1689–1702 [Google Scholar]
- Adrian M, Rajaei H, Jeandet P, Veneau J, Bessis R. (1998) Resveratrol oxidation in Botrytis cinerea conidia. Phytopathology 88: 472–476 [DOI] [PubMed] [Google Scholar]
- Balogun RA. (1969) Digestive enzymes of alimentary canal of larch bark beetle Ips cembrae (Heer). Comp Biochem Physiol 29: 1267–1270 [Google Scholar]
- Barbehenn RV, Maben RE, Knoester JJ. (2008) Linking phenolic oxidation in the midgut lumen with oxidative stress in the midgut tissues of a tree-feeding caterpillar Malacosoma disstria (Lepidoptera: Lasiocampidae). Environ Entomol 37: 1113–1118 [DOI] [PubMed] [Google Scholar]
- Breuil AC, Adrian M, Pirio N, Meunier P, Bessis R, Jeandet P. (1998) Metabolism of stilbene phytoalexins by Botrytis cinerea. 1. Characterization of a resveratrol dehydrodimer. Tetrahedron Lett 39: 537–540 [Google Scholar]
- Breuil AC, Jeandet P, Adrian M, Chopin F, Pirio N, Meunier P, Bessis R. (1999) Characterization of a pterostilbene dehydrodimer produced by laccase of Botrytis cinerea. Phytopathology 89: 298–302 [DOI] [PubMed] [Google Scholar]
- Brignolas F, Lacroix B, Lieutier F, Sauvard D, Drouet A, Claudot AC, Yart A, Berryman AA, Christiansen E. (1995) Induced responses in phenolic metabolism in two Norway spruce clones after wounding and inoculations with Ophiostoma polonicum, a bark beetle-associated fungus. Plant Physiol 109: 821–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong JL, Poutaraud A, Hugueney P. (2009) Metabolism and roles of stilbenes in plants. Plant Sci 177: 143–155 [Google Scholar]
- DiGuistini S, Wang Y, Liao NY, Taylor G, Tanguay P, Feau N, Henrissat B, Chan SK, Hesse-Orce U, Alamouti SM, et al. (2011) Genome and transcriptome analyses of the mountain pine beetle-fungal symbiont Grosmannia clavigera, a lodgepole pine pathogen. Proc Natl Acad Sci USA 108: 2504–2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esaki H, Onozaki H, Morimitsu Y, Kawakishi S, Osawa T. (1998) Potent antioxidative isoflavones isolated from soybeans fermented with Aspergillus saitoi. Biosci Biotechnol Biochem 62: 740–746 [DOI] [PubMed] [Google Scholar]
- Felton GW, Donato KK, Broadway RM, Duffey SS. (1992) Impact of oxidized plant phenolics on the nutritional quality of dietary protein to a noctuid herbivore, Spodoptera exigua. J Insect Physiol 38: 277–285 [Google Scholar]
- Franceschi VR, Krokene P, Christiansen E, Krekling T. (2005) Anatomical and chemical defenses of conifer bark against bark beetles and other pests. New Phytol 167: 353–375 [DOI] [PubMed] [Google Scholar]
- Franceschi VR, Krokene P, Krekling T, Christiansen E. (2000) Phloem parenchyma cells are involved in local and distant defense responses to fungal inoculation or bark-beetle attack in Norway spruce (Pinaceae). Am J Bot 87: 314–326 [PubMed] [Google Scholar]
- Gigi O, Marbach I, Mayer AM. (1980) Induction of laccase formation in Botrytis. Phytochemistry 19: 2273–2275 [Google Scholar]
- Hammerbacher A, Ralph SG, Bohlmann J, Fenning TM, Gershenzon J, Schmidt A. (2011) Biosynthesis of the major tetrahydroxystilbenes in spruce, astringin and isorhapontin, proceeds via resveratrol and is enhanced by fungal infection. Plant Physiol 157: 876–890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Pedersen JA, Constabel CP. (2001) Polyphenol oxidase and herbivore defense in trembling aspen (Populus tremuloides): cDNA cloning, expression, and potential substrates. Physiol Plant 112: 552–558 [DOI] [PubMed] [Google Scholar]
- Harwood CS, Parales RE. (1996) The beta-ketoadipate pathway and the biology of self-identity. Annu Rev Microbiol 50: 553–590 [DOI] [PubMed] [Google Scholar]
- Hesse-Orce U, DiGuistini S, Keeling CI, Wang Y, Li M, Henderson H, Docking TR, Liao NY, Robertson G, Holt RA, et al. (2010) Gene discovery for the bark beetle-vectored fungal tree pathogen Grosmannia clavigera. BMC Genomics 11: 536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeandet P, Delaunois B, Conreux A, Donnez D, Nuzzo V, Cordelier S, Clément C, Courot E. (2010) Biosynthesis, metabolism, molecular engineering, and biological functions of stilbene phytoalexins in plants. Biofactors 36: 331–341 [DOI] [PubMed] [Google Scholar]
- Keeling CI, Bohlmann J. (2006) Genes, enzymes and chemicals of terpenoid diversity in the constitutive and induced defence of conifers against insects and pathogens. New Phytol 170: 657–675 [DOI] [PubMed] [Google Scholar]
- Krokene P, Solheim H. (1998) Pathogenicity of four blue-stain fungi associated with aggressive and nonaggressive bark beetles. Phytopathology 88: 39–44 [DOI] [PubMed] [Google Scholar]
- Krokene P, Solheim H. (2002) Loss of pathogenicity in the blue-stain fungus Ceratocystis polonica. For Pathol 50: 497–502 [Google Scholar]
- Li SH, Nagy NE, Hammerbacher A, Krokene P, Niu XM, Gershenzon J, Schneider B. (2012) Localization of phenolics in phloem parenchyma cells of Norway spruce (Picea abies). ChemBioChem 13: 2707–2713 [DOI] [PubMed] [Google Scholar]
- Li SH, Niu XM, Zahn S, Gershenzon J, Weston J, Schneider B. (2008) Diastereomeric stilbene glucoside dimers from the bark of Norway spruce (Picea abies). Phytochemistry 69: 772–782 [DOI] [PubMed] [Google Scholar]
- Li SH, Schneider B, Gershenzon J. (2007) Microchemical analysis of laser-microdissected stone cells of Norway spruce by cryogenic nuclear magnetic resonance spectroscopy. Planta 225: 771–779 [DOI] [PubMed] [Google Scholar]
- Lin LM, Wu HY, Li WS, Chen WL, Lee YJ, Wu DC, Li P, Yeh A. (2010) Kinetic studies of the oxidation of quercetin, rutin and taxifolin in the basic medium by (ethylenediaminetetraacetato) cobalt(III) complex. Inorg Chem Commun 13: 633–635 [Google Scholar]
- Marin M (2003) Molecular taxonomy of Ceratocystis polonica sensu lato PhD dissertation. University of Pretoria, Pretoria, South Africa [Google Scholar]
- Morant AV, Jørgensen K, Jørgensen C, Paquette SM, Sánchez-Pérez R, Møller BL, Bak S. (2008) β-Glucosidases as detonators of plant chemical defense. Phytochemistry 69: 1795–1813 [DOI] [PubMed] [Google Scholar]
- Nirenberg HI, O’Donnell K. (1998) New Fusarium species and combinations within the Gibberella fujikuroi species complex. Mycologia 90: 434–458 [Google Scholar]
- Oliver RP, Solomon PS. (2010) New developments in pathogenicity and virulence of necrotrophs. Curr Opin Plant Biol 13: 415–419 [PubMed] [Google Scholar]
- Paine TD, Raffa KF, Harrington TC. (1997) Interactions among scolytid bark beetles, their associated fungi, and live host conifers. Annu Rev Entomol 42: 179–206 [DOI] [PubMed] [Google Scholar]
- Pedras MSC, Ahiahonu PWK, Hossain M. (2004) Detoxification of the cruciferous phytoalexin brassinin in Sclerotinia sclerotiorum requires an inducible glucosyltransferase. Phytochemistry 65: 2685–2694 [DOI] [PubMed] [Google Scholar]
- Pont V, Pezet R. (1990) Relation between the chemical structure and the biological activity of hydroxystilbenes against Botrytis cinerea. Phytopathol Z 130: 1–8 [Google Scholar]
- Rodríguez-Bonilla P, Méndez-Cazorla L, López-Nicolás JM, García-Carmona F. (2011) Kinetic mechanism and product characterization of the enzymatic peroxidation of pterostilbene as model of the detoxification process of stilbene-type phytoalexins. Phytochemistry 72: 100–108 [DOI] [PubMed] [Google Scholar]
- Sbaghi M, Jeandet P, Bessis R, Leroux P. (1996) Degradation of stilbene-type phytoalexins in relation to the pathogenicity of Botrytis cinerea to grapevines. Plant Pathol 45: 139–144 [Google Scholar]
- Schmidt A, Wächtler B, Temp U, Krekling T, Séguin A, Gershenzon J. (2010) A bifunctional geranyl and geranylgeranyl diphosphate synthase is involved in terpene oleoresin formation in Picea abies. Plant Physiol 152: 639–655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam V, Ronen M, Shalaby S, Larkov O, Rachamim Y, Hadar R, Rose MS, Carmeli S, Horwitz BA, Lev S. (2010) The fungal pathogen Cochliobolus heterostrophus responds to maize phenolics: novel small molecule signals in a plant-fungal interaction. Cell Microbiol 12: 1421–1434 [DOI] [PubMed] [Google Scholar]
- Six DL, Wingfield MJ (2011) The role of phytopathogenicity in bark beetle-fungus symbioses: a challenge to the classic paradigm. Annu Rev Entomol 56: 255–272 [DOI] [PubMed] [Google Scholar]
- Sobolev VS, Deyrup ST, Gloer JB. (2006) New peanut (Arachis hypogaea) phytoalexin with prenylated benzenoid and but-2-enolide moieties. J Agric Food Chem 54: 2111–2115 [DOI] [PubMed] [Google Scholar]
- Son PS, Park SA, Na HK, Jue DM, Kim S, Surh YJ. (2010) Piceatannol, a catechol-type polyphenol, inhibits phorbol ester-induced NF-kappaB activation and cyclooxygenase-2 expression in human breast epithelial cells: cysteine 179 of IKKbeta as a potential target. Carcinogenesis 31: 1442–1449 [DOI] [PubMed] [Google Scholar]
- Underwood CDT, Pearce RB. (1992) Stilbene glucoside levels and the resistance of sitka spruce (Picea sitchensis) tissues to colonization by root-rotting and butt-rotting fungi. Plant Pathol 41: 722–729 [Google Scholar]
- Van Etten H, Temporini E, Wasmann C. (2001) Phytoalexin (and phytoanticipin) tolerance as a virulence trait: why is it not required by all pathogens? Physiol Mol Plant Pathol 59: 83–93 [Google Scholar]
- Viiri H, Annila E, Kitunen V, Niemela P. (2001) Induced responses in stilbenes and terpenes in fertilized Norway spruce after inoculation with blue-stain fungus, Ceratocystis polonica. Trees Struct Funct 15: 112–122 [Google Scholar]
- Voegele RT, Mendgen K. (2003) Rust haustoria: nutrient uptake and beyond. New Phytol 159: 93–100 [DOI] [PubMed] [Google Scholar]
- Wang Y, Lim L, DiGuistini S, Robertson G, Bohlmann J, Breuil C. (2013) A specialized ABC efflux transporter GcABC-G1 confers monoterpene resistance to Grosmannia clavigera, a bark beetle-associated fungal pathogen of pine trees. New Phytol 197: 886–898 [DOI] [PubMed] [Google Scholar]
- Weltring KM, Barz W. (1980) Degradation of 3,9-dimethoxypterocarpan and medicarpin by Fusarium proliferatum. Z Naturforsch 35: 399–405 [Google Scholar]
- Wermelinger B. (2004) Ecology and management of a spruce bark beetle: a review of recent research. For Ecol Manage 202: 67–82 [Google Scholar]
- Werner RA, Brand WA. (2001) Referencing strategies and techniques in stable isotope ratio analysis. Rapid Commun Mass Spectrom 15: 501–519 [DOI] [PubMed] [Google Scholar]
- Woods JA, Hadfield JA, Pettit GR, Fox BW, McGown AT. (1995) The interaction with tubulin of a series of stilbenes based on combretastatin A-4. Br J Cancer 71: 705–711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang QL, Chang TL, Li WJ. (1990) A calibrated measurement of the atomic weight of carbon. Chin Sci Bull 35: 290–296 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.