Abstract
Background:
Determinants of Kaposi sarcoma–associated herpesvirus (KSHV) seropositivity among children living in sub-Saharan African populations where infection is endemic are not well understood. Local environmental factors, including other infectious agents, may be key.
Methods:
Within the context of a well-characterized birth cohort, we examined associations between various factors and antibodies against KSHV, measured in stored plasma samples from 1823 mother–child pairs in Entebbe, Uganda.
Results:
Seroprevalence increased with increasing age of the child (P = 0.0003) and was higher among those with KSHV seropositive mothers than in those without (12% vs 9%; odds ratio: 1.4, 95% confidence interval: 1.1 to 2.0). It was also higher among children with HIV infection (29% vs 10%; odds ratio: 3.1, 95% confidence interval: 1.2 to 8.3) or malaria parasitemia (30% vs 10%; odds ratio: 4.1, 95% confidence interval: 2.4 to 7.0) than in children without. These associations were not explained by socioeconomic status.
Conclusions:
The finding that KSHV serostatus is associated with malaria parasitemia in children is novel. In a country endemic for KSHV, malaria may be a cofactor for KSHV infection or reactivation among children.
Key Words: Kaposi sarcoma-associated herpesvirus, Sub-Saharan Africa, children, HIV, Kaposi sarcoma
BACKGROUND
Kaposi sarcoma–associated herpesvirus (KSHV) seroprevalence exhibits marked worldwide geographical variation,1,2 which may represent regional differences in modes of transmission, or point to the existence of cofactors for infection or reactivation. Studies from sub-Saharan Africa report high KSHV prevalence, with primary infection beginning in childhood and increasing with age.3,4 There is considerable evidence, too, that KSHV transmission occurs via saliva5,6 and in Africa transmission from mothers and siblings, it is likely to be an important route.4,7 Risk factors governing childhood vulnerability to infection with KSHV remain only partly elucidated. Exposures in childhood including other infectious diseases may be key. Understanding transmission dynamics is a prerequisite for the development of strategies to prevent spread and the subsequent diseases associated with this important oncogenic infection.
This study took place in Uganda, a country with a high endemic prevalence of KSHV8 and a relatively high incidence of the Kaposi sarcoma, both among HIV-infected people and among those without HIV.9,10 We hypothesize that this region has specific cofactors driving KSHV transmission. We have previously reported a high prevalence of antibodies to KSHV in pregnant women in Uganda, and risk factors for infection among these women included certain parasites.8 Few studies have so far examined parasitic infections as cofactors for seropositivity in childhood. This analysis describes the association between antibodies to KSHV in Ugandan children aged 1–5 years and common local exposures including HIV, malaria, and helminths in addition to maternal and household factors.
METHODS
Study Population
The investigation was nested within an existing study in Uganda—the Entebbe Mother and Baby Study—a large double-blind randomized placebo-controlled trial designed to determine the impact of helminth infections and their treatment on vaccine responses and infectious disease outcomes (International Standard Randomised Controlled Trials No. 32849447). The details of this study have been reported elsewhere.11 Briefly, consenting women in their second or third trimester of pregnancy, resident in Entebbe and Katabi, Uganda were recruited between April 2003 and November 2005 from the government funded antenatal clinic. The residential area for the women covers rural, urban, and fishing communities. At enrolment, sociodemographic data were collected, and blood samples were obtained by venepuncture and processed for HIV serology and CD4 count. Women found to be infected with HIV were offered single-dose nevirapine to prevent vertical HIV transmission.
Children were followed from birth and at the time of this study were between 4 and 5 years old. Children were also seen for vaccinations and if unwell, for medical treatment. At routine yearly visits, clinical data, blood for full blood count, blood slides for malaria and Mansonella perstans, and stool samples were collected from well children. Any helminth infections were treated. In addition, at approximately 6 weeks and 18 months of age, blood samples were obtained from children of HIV-infected mothers to ascertain the child’s HIV status. Blood samples from mothers and their children were processed and plasma stored in the −80°C freezer archive facility at the Uganda Virus Research Institute (UVRI), Entebbe, Uganda.
Diagnosis of Infections
Stool was examined using the Kato-Katz12,13 method for identification of hookworm, Schistosomiasis mansoni, Trichuris trichiura, Ascaris lumbricoides, and Trichostrongylus species and by charcoal culture for Strongyloides stercoralis.13 Two slides from a single stool sample were examined for each individual within 30 minutes for hookworm and the next day for other ova and parasites. Blood was examined for M. perstans by a modified Knott method.14 Plasmodium falciparum was diagnosed by examination of thick blood films and asymptomatic malaria parasitemia defined as the presence of parasites without fever. In 6-week-old children, HIV was indicated by plasma viral load.15 In mothers and children more than 18 months of age, HIV was identified by serology using a triple rapid test serial testing algorithm.16
KSHV Serological Testing
The mother’s enrolment plasma sample and the last available plasma sample for each child were selected for KSHV serological testing using enzyme-linked immunosorbent assays (ELISAs) for recombinant proteins K8.1 (a KSHV structural glycoprotein expressed during lytic infection) and ORF 73 (a nuclear antigen expressed during latency) as previously described.8,17,18 The ELISAs were performed at UVRI by the study lead and a technician, both of whom were blinded to patient details. The assays were transferred to UVRI from the Viral Oncology Section, Frederick National Laboratory for Cancer Research, United States, and have been used in more than 40 studies worldwide. Analysis of the positive and negative controls showed comparable performance at Viral Oncology Section, Frederick National Laboratory for Cancer Research, and at UVRI.8 Ten percent of samples were tested in duplicate and agreement formally tested by calculating a Kappa statistic.19 The Kappa score for agreement for the duplicate samples for K8.1 was 0.86 and for ORF 73 was 0.92. These scores represent almost perfect agreement.19
Statistical Analysis
Data were analyzed using Stata11SE (StataCorp LP, College Station, TX). As individual responses to KSHV antigens are complex and no gold standard assay exists to simplify the presentation of results, we combined the K8.1 and ORF 73 results to define evidence of KSHV infection as “positive” if either of the 2 assays were positive and “negative” if both assays were negative. The agreement between K8.1 and ORF 73 ELISA assays was assessed by calculating a Kappa statistic.19 A composite variable for household socioeconomic status was derived based on home building materials and number of rooms and items collectively owned at the mothers home.16 Children were categorized into 3 HIV status groups: HIV unexposed, HIV exposed (to maternal HIV) but not infected, and HIV exposed and infected. Because the prevalence of helminth infections in children was low, a variable for current infection with any helminth was created indicating infection with Trichostrongylus sp, A. lumbricoides, hookworm, M. perstans, S. mansoni, S. stercoralis, or T. trichiura. Other risk factors for childhood KSHV seropositivity considered were (1) childhood variables: gender, age, asymptomatic malaria parasitemia, and number of previous symptomatic malaria episodes; (2) maternal factors: KSHV serostatus, age at birth of child, educational attainment, marital status, number of babies born alive, number of children living in the home, personal monthly income, and trial treatment arm; and (3) risk factors for malaria and helminth infections: walking distance to Lake Victoria, type of toilet, use of mosquito nets, spraying home for mosquitoes, and water collection source (lake, well, bore hole, stand pipe, tap).
Potential bias in the sample of mother–child pairs was investigated by comparing the covariate distributions of mother–child pairs included in this study to the distributions of those in the cohort who had no samples available for inclusion. Further to this, mother–child pairs where the child was lost to follow-up at an early age were compared with pairs with longer child follow-up.
The outcome of interest for all analyses was KSHV serostatus of the child as defined above. The initial analysis was based on the a priori hypothesis that the age of the child would be a major determinant of risk of infection. Therefore, all odds ratios in the initial analysis (model 1) are adjusted for age of child. A multivariable logistic model (model 2) was then constructed including all risk factors that were associated with the outcome at the 5% level in model 1. Statistical significance was assessed using the likelihood ratio test. Departure from linear trend was considered for all ordered categorical exposure variables by calculation of a likelihood ratio test. To assess factors that potentially modify the risk of a KSHV seropositive mother having a KSHV seropositive child, interactions between maternal KSHV serostatus and HIV status or asymptomatic malaria parasitemia in the child were added to the regression model. No adjustment was made for multiple comparisons. All P values were 2-sided and we considered P < 0.05 to be statistically significant.
Ethical Approval
Written informed consent was obtained from each woman, for her own and her child’s participation. Ethical approval for this study was obtained from the UVRI, Science and Ethics Committee, Entebbe, Uganda, and the Uganda National Council for Science and Technology, Kampala, Uganda.
RESULTS
Plasma samples were available for KSHV serological testing from 1823 (86%) mother–child pairs. Twenty sets of twins were excluded from these analyses. Maternal age at sampling ranged from 14 to 43 years (median 23 years). The majority of women (74%) were multigravida and most were married (85%). The majority of women (85%) reported a monthly income below the World Bank poverty line of 1.25 USD per day and 25% reported that they could not read. Antenatal HIV seroprevalence among mothers was 10% with a median CD4 count for HIV seropositive women of 553 (inter-quartile range: 368–813) and 4% of women had active syphilis. Among children, the prevalence of asymptomatic malaria parasitemia was 5% and HIV seroprevalence was 1% overall. Helminth infections were rare among children: 3% prevalence of Trichostrongylus sp and 1% prevalence of A. lumbricoides infection; hookworm, M. perstans, S. mansoni, S. stercoralis, and T. trichiura were all detected in fewer than 1% of children.
Mother–child pairs with no samples available for inclusion were more likely to include a HIV seropositive mother with less education. Mothers of children whose last available sample was at 1 year were generally younger and more likely to be HIV seropositive compared with mothers of children followed up for a longer period.
Among mothers, the prevalence of antibodies to K8.1 was 41% and to ORF 73 was 53%; 32% were seropositive to both antigens and 61% had antibodies to either K8.1 or ORF 73. The seroprevalence of KSHV among children was 9% to K8.1, 6% to ORF 73, 4% to both antigens, and 11% to either ORF 73 or K8.1. There was moderate concordance between lytic K8.1 and latent ORF 73 assays in detecting KSHV seropositivity in the mothers (ΚK = 0.43) and the children (ΚK = 0.46).
Factors significantly associated with KSHV seropositivity among children are presented in Table 1. Associations for age and maternal education were consistent with linear trends. Of childhood factors, increasing age of the child was strongly associated with KSHV seropositivity in crude and adjusted analyses (model 2). Childhood infections with malaria and HIV were both associated with increased risk of being KSHV seropositive. Of maternal factors, KSHV seropositive status and low educational attainment were associated with KSHV seropositivity in the child; the effect of maternal KSHV serostatus was reduced by adjusting for other potential confounders (model 2) and the association with maternal education was not statistically significant. In an analysis restricted to HIV seropositive mothers, being a HIV seropositive child was associated with a 2-fold increase in the risk of being KSHV seropositive compared with being a HIV-negative child (odds ratio: 1.9, 95% confidence interval: 0.6 to 5.4). Use of a mosquito net in the home was marginally associated with a decreased risk of childhood KSHV seropositivity in model 1, but this association was not retained after adjusting for other potential confounders (model 2).
TABLE 1.
Factors Associated With KSHV Seropositivity in Ugandan Children Aged 1–5 Years
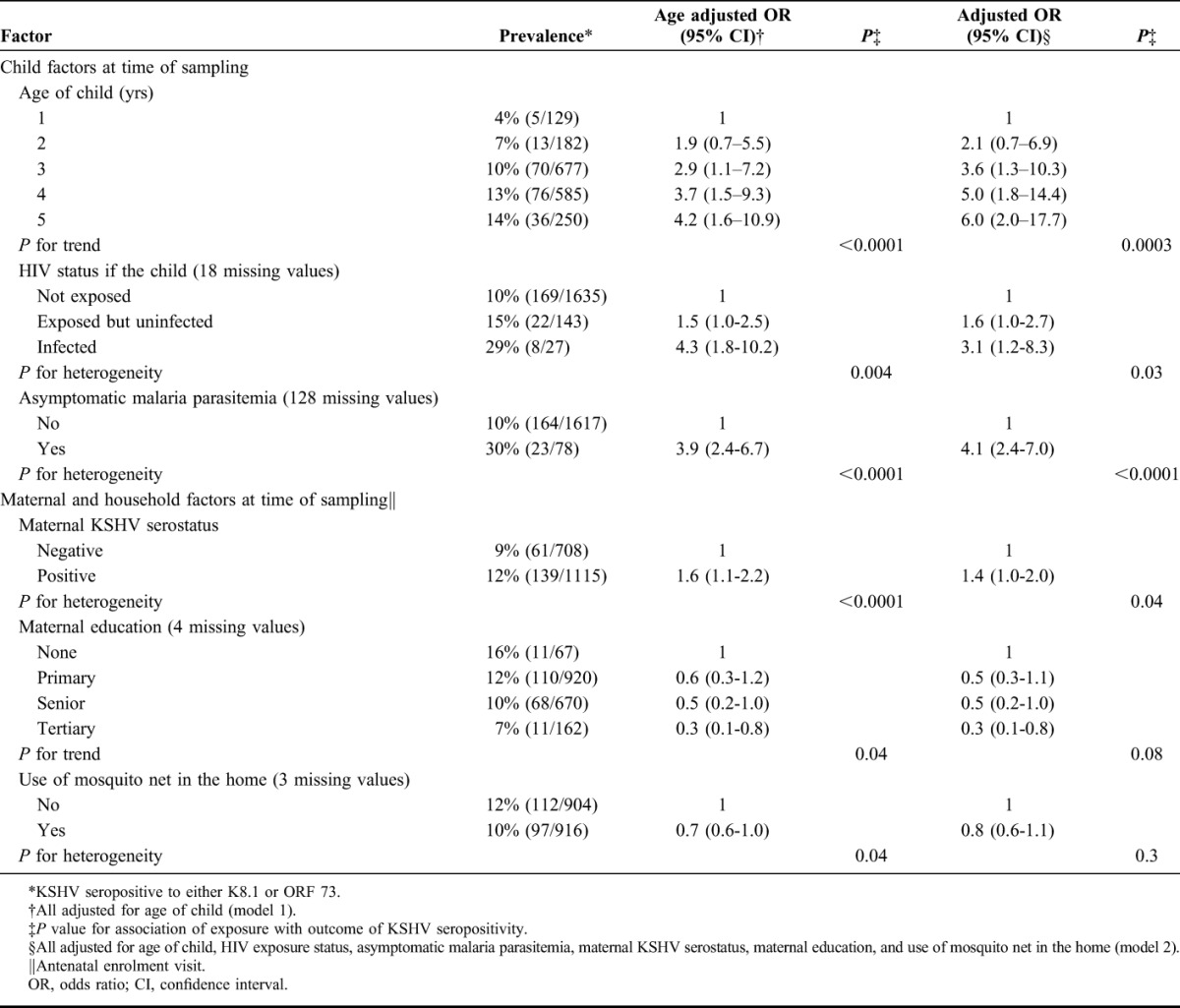
No modifiers of the relationship between KSHV serostatus of the mother and child were detected.
DISCUSSION
In this study of children aged 1–5 years in Uganda, we have demonstrated for the first time an association between KSHV seropositivity and the presence of asymptomatic malaria parasitemia. We have also confirmed associations between KSHV serostatus in Ugandan children and the following cofactors: age of the child, HIV infection in the child, and maternal KSHV serostatus.
Parasites have been hypothesized as potential cofactors for KSHV transmission. KSHV is an immune-sensitive virus,20 and the survival of parasites is dependent on their ability to interfere with host immune function.21 Ecological studies from Italy report substantial declines in KSHV seroprevalence and Kaposi sarcoma incidence in association with eradication of mosquitoes.22 It is notable that malaria is known to affect immune control of another human gamma herpesvirus, the Epstein–Barr virus. In addition, malaria interacts with the Epstein–Barr virus thereby increasing the risk of Burkitt lymphoma, the most common cancer reported in children in East Africa.23,24 Malaria may impair immune defence and therefore increase susceptibility to primary KSHV infection. In addition, malaria infection may cause KSHV reactivation increasing viral replication and shedding in saliva. Repeated malaria infections have been shown to suppress T-cell immunity,25 and this may have a detrimental effect on the control of KSHV infection. We have previously reported an association between KSHV seropositivity and malaria in women in Uganda.8 Longitudinal studies will be required to examine the nature of this association further.
HIV is an important candidate for influencing KSHV transmission rates between KSHV-infected mothers and their children. HIV-infected subjects may be more likely to shed KSHV in saliva and HIV-associated immune deficiency may increase susceptibility to KSHV infection. We found some evidence that having an HIV-infected mother was a risk factor for KSHV seropositivity if the child was HIV negative, and strong evidence that if the child was HIV positive, the odds of KSHV seropositivity were increased compared with HIV unexposed uninfected children and with HIV-exposed uninfected children. This study confirms the findings of others4,18,26 that HIV infection is associated with an increased seroprevalence of KSHV in children; only 1 study did not find such an association.27 Whether HIV is acting to increase vulnerability to infection or causing reactivation in children infected with KSHV in the past will require further longitudinal studies of KSHV infection.
In sub-Saharan Africa, KSHV is endemic and infection prevalence in children increases with age, suggesting nonsexual horizontal transmission.28 There is little evidence of vertical KSHV transmission, or that the virus can be passed through breast milk.4,29 KSHV is likely to be transmitted through saliva.5,6 The seroprevalence of KSHV in this study increased from 4% in children aged 1 year to 15% in 5-year-old children. In keeping with other studies, KSHV infection status of the mother was positively associated with infection risk in the child.4,18 However, nearly a third of KSHV seropositive children had a KSHV antibody negative mother. It is possible that these mothers became infected with KSHV after the antenatal enrolment sample or that the child was infected from another source. KSHV transmission within families in sub-Saharan Africa has been documented with infection risk for the child showing associations in decreasing order of magnitude with infection in the mother, father, and next oldest sibling.7 Childhood KSHV seropositivity was not associated with maternal age, in line with other studies in which women had a narrow age range.30,31
In this study, markers of socioeconomic status, namely household socioeconomic status, maternal education, maternal monthly income, and type of toilet, were not associated with antibodies to KSHV. The absence of an association between childhood KSHV seropositivity and maternal educational attainment is in keeping with other studies from sub-Saharan Africa.26,30 In a recent extensive report of socioeconomic factors in mother–child pairs in Uganda, the risk of being KSHV seropositive was low among mother–child pairs in the high-income group, although the trend in risk with increasing income was not linear.30 Other factors including maternal and paternal occupation, household electricity, and household density were not found to be associated. Contact with water or collecting water from ground, stream, river, lake, or wetland sources attracted particular attention in early reports of risk factors for Kaposi sarcoma. Ground water was not thought to be a marker of socioeconomic status, but rather that it may contain a potential cofactor such as a parasite.30,32,33 In Uganda, previous studies have investigated type of water supply and antibodies to KSHV. In children and their mothers, use of surface water for drinking was associated with an increased risk of KSHV seropositivity in the crude but not adjusted analysis.30 We found no association with water source nor did 2 other studies of children and adolescents attending an urban hospital in Uganda.5,34
Our study of a large mother–child population benefited from the use of robust data, collected through a well-conducted randomized controlled trial, the initial findings of which have been published elsewhere.15 The moderate concordance between lytic K8.1 and latent ORF 73 assays in detecting KSHV seropositivity is consistent with previous studies.4,18 However, there were several limitations. The mothers had relatively high CD4 counts and were receiving HIV care that may have impacted on our ability to assess the role of HIV alone. The mother’s plasma sample was collected remotely in time from the child’s sample and the overall number of HIV-infected children was small. Power to detect potential associations with childhood helminth infections was small because of the low prevalence of these infections. Selection biases may have underestimated the association with HIV and antibodies to KSHV. Finally, the cross-sectional design makes it difficult to differentiate between risk factors for primary infection and reactivation (leading to a boost in antibody titers). Further longitudinal studies are needed that collect suitable material to study KSHV viral load and KSHV-specific T-cell function.
Cofactors for KSHV transmission are incompletely understood. Fully elucidating the factors impacting on infectiousness and vulnerability will be the key to reducing KSHV transmission and thereby the incidence of HIV-associated and -unassociated Kaposi sarcoma in sub-Saharan Africa. This study raises new and interesting questions surrounding the relationship between KSHV and the malaria parasite while confirming child’s age, HIV infection, and maternal KSHV status as key cofactors.
ACKNOWLEDGMENTS
The authors thank the participants and staff of the Entebbe Mother and Baby Study and the staff of the laboratory and statistics departments of the Medical Research Council/UVRI, Uganda Research Unit on AIDS, who made this study possible.
Footnotes
K. W. conceived and coordinated the study, carried out the KSHV ELISA assays, performed the statistical analysis, and drafted the manuscript, E. L. W. performed the statistical analysis and helped to draft the manuscript, I. S. and A. N. carried out the KSHV ELISA assays, L. M. managed the study database, W. M. set up and validated the KSHV ELISA assays, and W. T. J. helped with statistical analysis and drafting the manuscript, J. N. was the head of the Entebbe Mother and Baby Study clinic, D. W. is the head of Viral Oncology Section, NCI, conceived the study and drafted the manuscript, R. N. conceived the study and drafted the manuscript, A. M. E. is the principle investigator for the Entebbe Mother and Baby Study cohort, conceived the study and helped with statistical analysis and drafting the manuscript. D. W., R. N., and A. M. E. contributed equally to this work.
Presented at the 14th International Workshop on Kaposi’s Sarcoma Herpesvirus (KSHV) and Related Agents, August 2011, Helsinki; 13th International Workshop on Kaposi’s Sarcoma Herpesvirus (KSHV) and Related Agents, August 2010, Los Angeles.
Funded by the Wellcome Trust as a PhD Training Fellowship held by K. Wakeham (grant no. 090132), a Wellcome Trust Senior Fellowships held by A. M. Elliott (grant nos. 064693 and 079110) and funded in part by the Intramural Program of the National Cancer Institute, National Institutes of Health, Department of Health and Human Services (contract HHSN261200800001E). Wellcome Trust is a UK registered charity no. 210183.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1.Dedicoat M, Newton R. Review of the distribution of Kaposi's sarcoma-associated herpesvirus (KSHV) in Africa in relation to the incidence of Kaposi's sarcoma. Br J Cancer. 2003;88:1–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dollard SC, Butler LM, Graves Jones AM, et al. Substantial regional differences in human herpesvirus 8 seroprevalence in sub-saharan Africa: insights on the origin of the "KS Belt." Int J Cancer. 2010;127:2395–2401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mbulaiteye SM, Biggar RJ, Bakaki PM, et al. Human herpesvirus 8 infection and transfusion history in children with sickle-cell disease in Uganda. J Natl Cancer Inst. 2003;95:1330–1335 [DOI] [PubMed] [Google Scholar]
- 4.Dedicoat M, Newton R, Alkharsah KR, et al. Mother-to-child transmission of human herpesvirus-8 in South Africa. J Infect Dis. 2004;190:1068–1075 [DOI] [PubMed] [Google Scholar]
- 5.Mbulaiteye SM, Pfeiffer RM, Engels EA, et al. Detection of Kaposi sarcoma-associated herpesvirus DNA in saliva and buffy-coat samples from children with sickle cell disease in Uganda. J Infect Dis. 2004;190:1382–1386 [DOI] [PubMed] [Google Scholar]
- 6.Butler LM, Dollard SC, Amin M, et al. Relationship between salivary shedding and seropositivity for Kaposi’s sarcoma-associated herpesvirus (KSHV) and Epstein-Barr virus (EBV) among South African children and adults: insights on why EBV infection is ubiquitous and KSHV is not. Paper presented at: 12th International Conference on Malignancies in AIDS and Other Acquired Immunodeficiencies (ICMAOI); April 26–27 2010; Bethesda, MD
- 7.Mbulaiteye SM, Pfeiffer RM, Whitby D, et al. Human herpesvirus 8 infection within families in rural Tanzania. J Infect Dis. 2003;187:1780–1785 [DOI] [PubMed] [Google Scholar]
- 8.Wakeham K, Webb EL, Sebina I, et al. Parasite infection is associated with Kaposi's sarcoma associated herpesvirus (KSHV) in Ugandan women. Infect Agents Cancer. 2011;6:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wabinga HR, Parkin DM, Wabwire-Mangen F, et al. Trends in cancer incidence in Kyadondo County, Uganda, 1960-1997. Br J Cancer. 2000;82:1585–1592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor JF, Templeton AC, Vogel CL, et al. Kaposi's sarcoma in Uganda: a clinico-pathological study. Int J Cancer. 1971;8:122–135 [DOI] [PubMed] [Google Scholar]
- 11.Elliott AM, Kizza M, Quigley MA, et al. The impact of helminths on the response to immunization and on the incidence of infection and disease in childhood in Uganda: design of a randomized, double-blind, placebo-controlled, factorial trial of deworming interventions delivered in pregnancy and early childhood [ISRCTN32849447]. Clin Trials. 2007;4:42–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14:397–400 [PubMed] [Google Scholar]
- 13.Bukusuba JW, Hughes P, Kizza M, et al. Screening for intestinal helminth infection in a semi-urban cohort of pregnant women in Uganda. Trop Doct. 2004;34:27–28 [DOI] [PubMed] [Google Scholar]
- 14.Melrose WD, Turner PF, Pisters P, Turner B. An improved Knott's concentration test for the detection of microfilariae. Trans R Soc Trop Med Hyg. 2000;94:176. [DOI] [PubMed] [Google Scholar]
- 15.Webb EL, Mawa PA, Ndibazza J, et al. Effect of single-dose anthelmintic treatment during pregnancy on an infant's response to immunisation and on susceptibility to infectious diseases in infancy: a randomised, double-blind, placebo-controlled trial. Lancet. 2011;377:52–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Muhangi L, Woodburn P, Omara M, et al. Associations between mild-to-moderate anaemia in pregnancy and helminth, malaria and HIV infection in Entebbe, Uganda. Trans R Soc Trop Med Hyg. 2007;101:899–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mbisa GL, Miley W, Gamache CJ, et al. Detection of antibodies to Kaposi's sarcoma-associated herpesvirus: a new approach using K8.1 ELISA and a newly developed recombinant LANA ELISA. J Immunol Methods. 2010;356:39–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Malope BI, Pfeiffer RM, Mbisa G, et al. Transmission of Kaposi sarcoma-associated herpesvirus between mothers and children in a South African population. J Acquir Immune Defic Syndr. 2007;44:351–355 [DOI] [PubMed] [Google Scholar]
- 19.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174 [PubMed] [Google Scholar]
- 20.Areste C, Blackbourn DJ. Modulation of the immune system by Kaposi's sarcoma-associated herpesvirus. Trends Microbiol. 2009;17:119–129 [DOI] [PubMed] [Google Scholar]
- 21.Maizels RM. Parasite immunomodulation and polymorphisms of the immune system. J Biol. 2009;8(7):62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ascoli V, Facchinelli L, Valerio L, et al. Distribution of mosquito species in areas with high and low incidence of classic Kaposi's sarcoma and seroprevalence for HHV-8. Med Vet Entomol. 2006;20:198–208 [DOI] [PubMed] [Google Scholar]
- 23.Moormann AM, Chelimo K, Sumba OP, et al. Exposure to holoendemic malaria results in elevated Epstein-Barr virus loads in children. J Infect Dis. 2005;191:1233–1238 [DOI] [PubMed] [Google Scholar]
- 24.Moormann AM, Chelimo K, Sumba PO, et al. Exposure to holoendemic malaria results in suppression of Epstein-Barr virus-specific T cell immunosurveillance in Kenyan children. J Infect Dis. 2007;195:799–808 [DOI] [PubMed] [Google Scholar]
- 25.Moormann AM, Heller KN, Chelimo K, et al. Children with endemic Burkitt lymphoma are deficient in EBNA1-specific IFN-gamma T cell responses. Int J Cancer. 2009;124:1721–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brayfield BP, Phiri S, Kankasa C, et al. Postnatal human herpesvirus 8 and human immunodeficiency virus type 1 infection in mothers and infants from Zambia. J Infect Dis. 2003;187:559–568 [DOI] [PubMed] [Google Scholar]
- 27.Butler LM, Dorsey G, Hladik W, et al. Kaposi sarcoma-associated herpesvirus (KSHV) seroprevalence in population-based samples of African children: evidence for at least 2 patterns of KSHV transmission. J Infect Dis. 2009;200:430–438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Malope BI, MacPhail P, Mbisa G, et al. No evidence of sexual transmission of Kaposi's sarcoma herpes virus in a heterosexual South African population. AIDS. 2008;22:519–526 [DOI] [PubMed] [Google Scholar]
- 29.Brayfield BP, Kankasa C, West JT, et al. Distribution of Kaposi sarcoma-associated herpesvirus/human herpesvirus 8 in maternal saliva and breast milk in Zambia: implications for transmission. J Infect Dis. 2004;189:2260–2270 [DOI] [PubMed] [Google Scholar]
- 30.Mbulaiteye SM, Biggar RJ, Pfeiffer RM, et al. Water, socioeconomic factors, and human herpesvirus 8 infection in Ugandan children and their mothers. J Acquir Immune Defic Syndr. 2005;38:474–479 [DOI] [PubMed] [Google Scholar]
- 31.de Sanjose S, Mbisa G, Perez-Alvarez S, et al. Geographic variation in the prevalence of Kaposi sarcoma-associated herpesvirus and risk factors for transmission. J Infect Dis. 2009;199:1449–1456 [DOI] [PubMed] [Google Scholar]
- 32.Ziegler J, Newton R, Bourboulia D, et al. Risk factors for Kaposi's sarcoma: a case-control study of HIV-seronegative people in Uganda. Int J Cancer. 2003;103:233–240 [DOI] [PubMed] [Google Scholar]
- 33.McHardy J, Williams EH, Geser A, et al. Endemic Kaposi's sarcoma: incidence and risk factors in the West Nile District of Uganda. Int J Cancer. 1984;33:203–212 [DOI] [PubMed] [Google Scholar]
- 34.Mayama S, Cuevas LE, Sheldon J, et al. Prevalence and transmission of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in Ugandan children and adolescents. Int J Cancer. 1998;77:817–820 [DOI] [PubMed] [Google Scholar]


