Abstract
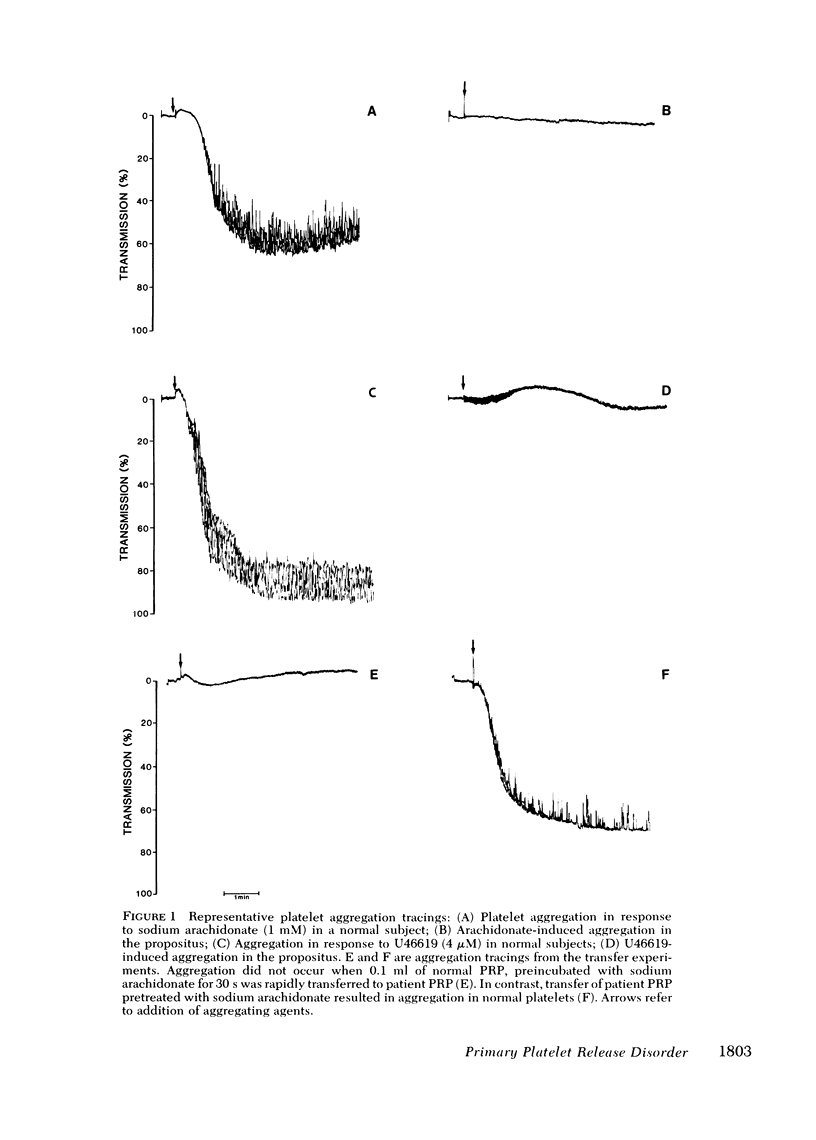
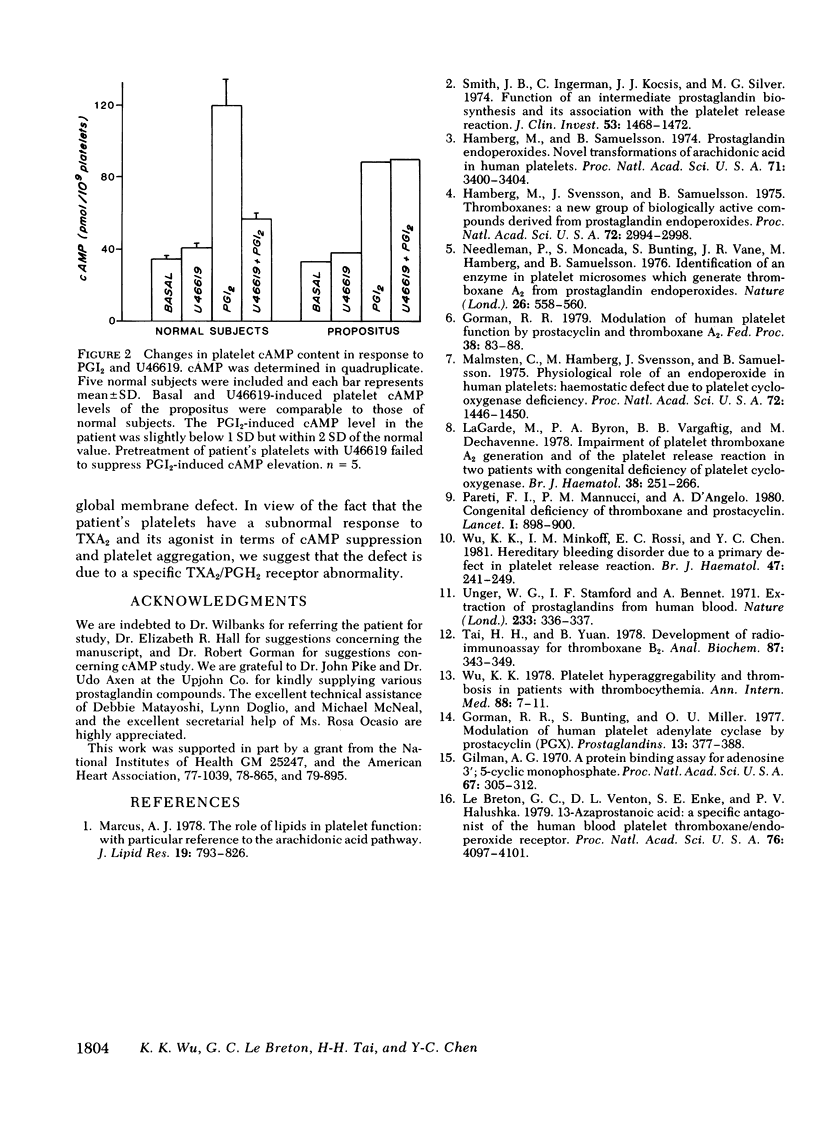
To determine the pathogenetic mechanism of a hereditary primary platelet release disorder, arachidonic acid metabolism via the cyclooxygenase pathway was investigated. The propositus' platelets exhibited defective release reaction and second-wave aggregation when stimulated by sodium arachidonate or U46619, a thromboxane A2 (TXA2) agonist. The lack of platelet response to U46619 suggested that the defect was beyond the thromboxane synthetase level. Furthermore, thromboxane B2 (TXB2) formation in the propositus' platelets (558.52 ng/10(8) platelets) was within the normal range (574.29 +/- SD 27.39 ng/10(8) platelets) and TXA2 formation appeared to be adequate for aggregating normal platelets. The results were indicative of an abnormal platelet response to TXA2. Failure of the propositus' platelets to aggregate in response to TXA2 formed in normal platelet-rich plasma induced by arachidonate confirmed this notion. To gain further insight, platelet cyclic (c) AMP content was determined. Prostacyclin induced a significant elevation of the propositus' platelet cAMP level comparable to normal values. U46619 suppressed prostaglandin I2-induced cAMP elevation in normal subjects but had no such effect in the patient. We conclude that the primary release disorder observed in this kindred is due to an abnormal platelet respnse to TXA2 possibly because of TXA2/PGH2 receptor abnormalities.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Gilman A. G. A protein binding assay for adenosine 3':5'-cyclic monophosphate. Proc Natl Acad Sci U S A. 1970 Sep;67(1):305–312. doi: 10.1073/pnas.67.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorman R. R., Bunting S., Miller O. V. Modulation of human platelet adenylate cyclase by prostacyclin (PGX). Prostaglandins. 1977 Mar;13(3):377–388. doi: 10.1016/0090-6980(77)90018-1. [DOI] [PubMed] [Google Scholar]
- Gorman R. R. Modulation of human platelet function by prostacyclin and thromboxane A2. Fed Proc. 1979 Jan;38(1):83–88. [PubMed] [Google Scholar]
- Hamberg M., Samuelsson B. Prostaglandin endoperoxides. Novel transformations of arachidonic acid in human platelets. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3400–3404. doi: 10.1073/pnas.71.9.3400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamberg M., Svensson J., Samuelsson B. Thromboxanes: a new group of biologically active compounds derived from prostaglandin endoperoxides. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2994–2998. doi: 10.1073/pnas.72.8.2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagarde M., Byron P. A., Vargaftig B. B., Dechavanne M. Impairment of platelet thromboxane A2 generation and of the platelet release reaction in two patients with congenital deficiency of platelet cyclo-oxygenase. Br J Haematol. 1978 Feb;38(2):251–266. doi: 10.1111/j.1365-2141.1978.tb01041.x. [DOI] [PubMed] [Google Scholar]
- Le Breton G. C., Venton D. L., Enke S. E., Halushka P. V. 13-Azaprostanoic acid: a specific antagonist of the human blood platelet thromboxane/endoperoxide receptor. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4097–4101. doi: 10.1073/pnas.76.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmsten C., Hamberg M., Svensson J., Samuelsson B. Physiological role of an endoperoxide in human platelets: hemostatic defect due to platelet cyclo-oxygenase deficiency. Proc Natl Acad Sci U S A. 1975 Apr;72(4):1446–1450. doi: 10.1073/pnas.72.4.1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus A. J. The role of lipids in platelet function: with particular reference to the arachidonic acid pathway. J Lipid Res. 1978 Sep;19(7):793–826. [PubMed] [Google Scholar]
- Needleman P., Moncada S., Bunting S., Vane J. R., Hamberg M., Samuelsson B. Identification of an enzyme in platelet microsomes which generates thromboxane A2 from prostaglandin endoperoxides. Nature. 1976 Jun 17;261(5561):558–560. doi: 10.1038/261558a0. [DOI] [PubMed] [Google Scholar]
- Pareti F. I., Mannucci P. M., D'Angelo A., Smith J. B., Sautebin L., Galli G. Congenital deficiency of thromboxane and prostacyclin. Lancet. 1980 Apr 26;1(8174):898–901. doi: 10.1016/s0140-6736(80)90837-5. [DOI] [PubMed] [Google Scholar]
- Smith J. B., Ingerman C., Kocsis J. J., Silver M. J. Formation of an intermediate in prostaglandin biosynthesis and its association with the platelet release reaction. J Clin Invest. 1974 May;53(5):1468–1472. doi: 10.1172/JCI107695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai H. H., Yuan B. Development of radioimmunoassay for thromboxane B2. Anal Biochem. 1978 Jul 1;87(2):343–349. doi: 10.1016/0003-2697(78)90683-8. [DOI] [PubMed] [Google Scholar]
- Unger W. G., Stamford I. F., Bennett A. Extraction of prostaglandins from human blood. Nature. 1971 Oct 1;233(5318):336–337. doi: 10.1038/233336b0. [DOI] [PubMed] [Google Scholar]
- Wu K. K., Minkoff I. M., Rossi E. C., Chen Y. C. Hereditary bleeding disorder due to a primary defect in platelet release reaction. Br J Haematol. 1981 Feb;47(2):241–249. doi: 10.1111/j.1365-2141.1981.tb02785.x. [DOI] [PubMed] [Google Scholar]
- Wu K. K. Platelet hyperaggregability and thrombosis in patients with thrombocythemia. Ann Intern Med. 1978 Jan;88(1):7–11. doi: 10.7326/0003-4819-88-1-7. [DOI] [PubMed] [Google Scholar]



