Abstract
Background
Following reforms to our city’s Safety-Net (SN) breast cancer referral process, we investigated whether factors often associated with late-stage diagnosis would differ by referral source—SN versus non-Safety-Net (NSN)—or, among SN patients, by stage at diagnosis.
Methods
From September 2008 to June 2010, SN patients with any-stage (0-IV) and NSN patients with late-stage (IIB-IV) breast cancer were identified prospectively during initial cancer-center consultations. Data were analyzed using logistic regression, chi-square, and t tests; two-tailed P < 0.05 was considered significant.
Results
Fifty-sevenwomen completed interviews (33SN, 24 NSN); 52 % of SN-referred patients were diagnosed with late-stage disease. Compared with NSN late-stage patients, SN late-stage patients were more likely to be African-American (83 % vs. 21 %, P < 0.001), to have an annual household income <$25,000 (89 % vs. 38 %, P < 0.001), and to report having a health problem in the preceding year but not being able to see a doctor because of cost (67 % vs. 25 %, P = 0.012); they were less likely to be married/partnered (22 % vs. 79 %, P < 0.001) and to have post-college education (0 % vs. 25 %, P < 0.03), any insurance (61 % vs. 96 %, P < 0.005), and to have sought medical attention within 1 week of realizing they had concerning breast findings (50 % vs. 79 %, P = 0.047). Married/ partnered patients were more likely to delay medical care by >1 week (odds ratio = 9.9, P = 0.038).
Conclusions
SN patients presented with higher-thanexpected rates of late-stage disease despite improvements in mammography rates and the referral process. Efforts to further facilitate access to care for this vulnerable SN patient population are needed.
Stage of disease at diagnosis has long been recognized as the most important prognostic indicator in breast cancer, and advanced-stage disease is associated with higher rates of recurrence and lower rates of survival.1–5 Increased utilization of mammography in the 1980s and 1990s was associated with increased diagnosis of early-stage breast cancers. These additional diagnoses inflated the overall breast cancer incidence rate because more tumors were being detected 1 to 3 years before they would have been symptomatic. By the late 1990s, incidence rates of early-stage breast cancers began to decline because a major risk factor—hormone replacement therapy—was identified, and its use among postmenopausal women was widely abandoned.5 However, even as there was fluctuation in the epidemiology of earlystage disease, incidence rates of late-stage, i.e., locally advanced and metastatic, breast cancer remained constant. Indeed, among African-American women, the incidence of late-stage disease has slightly increased since 1975.5
Fortunately, although rates of late-stage breast cancer have remained relatively stagnant, they are also relatively low. Only about 10 % of all newly diagnosed breast cancers are locally advanced or metastatic at diagnosis.5 Given the important prognostic implications of stage, many studies have been conducted to identify patient-specific and process-specific characteristics that might be associated with, predictive of, or contributors to late-stage diagnosis of breast cancer at presentation.1,2,4,6–23 In addition, when rates of late-stage diagnosis appear to differ systematically between groups of different demographic compositions, determining which factors might be associated with such disparities becomes especially important.
In 2007, we reviewed the breast cancer referral process in St. Louis’s Safety Net (SN) system, the network of community health centers that provides primary and specialty care for more than 90 % of the metropolitan area’s uninsured and underinsured population. We found that approximately 40 % of women diagnosed with breast cancer through the SN presented with stage III and IV cancers. In contrast, at the Alvin J. Siteman Cancer Center, a National Cancer Institute (NCI)-designated Comprehensive Cancer Center to which many cancer patients in the St. Louis metropolitan area are referred via the private health care system, the majority of patients (85–90 %) presented with stage I or II disease.24 In 2007, the journey toward definitive care for SN patients with abnormal breast findings typically began with evaluation at one of the city’s SN clinics, followed by referral to a specialty surgical clinic staffed mainly by residents, and concluded with definitive treatment at Siteman. Once seen at Siteman, SN patients received the standard of care afforded to similarly staged breast cancer patients who presented to Siteman through the private health care system. However, the eventual presentation of SN patients to Siteman did not mitigate the disparity in outcomes observed amongst these women.24 In addition, whereas the typical timeline from first breast abnormality to definitive treatment for privately referred patients at Siteman was approximately 2 weeks, the same timeline for SN patients ranged from 7 to 655 days, with a median of 93 days.24 This discovery raised the concern that the high incidence of late-stage breast cancer seen among SN patients might very well be related to delayed presentation for treatment.
Prompted by the findings of this review, changes were implemented to increase screening mammography rates among SN patients and to improve the efficiency with which SN patients are referred to Siteman. Specifically, the Siteman Cancer Center Mammography Van increased its hours of operation and expanded its geographic catchment area. In addition, women found to have breast abnormalities through van mammograms are now referred directly to Siteman, regardless of payor status, rather than being referred back to the SN for follow-up as had been done in the past. Finally, there has been significant expansion of Siteman Cancer Center’s Breast Cancer Navigator Program, which provides emotional support and logistical assistance to all breast cancer patients, particularly those who may not have the financial, emotional, or familial resources to facilitate cancer treatment.
In the wake of these reforms, we sought to conduct a pilot study to determine whether personal and process factors often associated with late-stage diagnosis would differ by source of referral—SN versus non-Safety-Net (NSN)—or, within the SN population, by stage at diagnosis.
METHODS
The sample size for this pilot study was determined by a desire to urgently address the knowledge gap surrounding the disparity in regional breast cancer outcomes identified in our initial retrospective study. Approximately 30 SN patients are referred for diagnosis and treatment to Siteman’s Breast Health Center each year, and our intention was to recruit 25 SN patients and a similar number of casematched NSN patients with late-stage cancer during the course of a 2-year period in order to facilitate results, program evaluation, and future planning as expeditiously as possible.
From September 2008 to June 2010, newly diagnosed SN patients with any-stage (0-IV) breast cancer and NSN patients with late-stage (IIB-IV) disease were identified prospectively during their initial consultations at Siteman Cancer Center. In recognition of the emotional distress often associated with this first appointment, we did not invite the identified women to participate in this study until at least the second visit, at which time those who chose to participate were consented in person. Women who were younger than 18 years of age, with prior history of in situ or invasive breast cancers, with cognitive impairments (e.g., dementia), or who were unwilling or unable to give consent were excluded. Once consent was obtained, each participant scheduled a time—before commencing curative therapy—during which she would be interviewed over the telephone. The study was approved by the university’s institutional review board.
Study members participated in 45- to 60-minute, semistructured interviews administered by a trained member of the research team via a Computer-assisted Telephone Interview system. Interview questions about delay in diagnosis were developed in collaboration with a health psychologist and were modified for breast cancer patients based on items from a questionnaire previously developed for colorectal cancer patients.25,26 Participants also completed the Medical Outcomes Study (MOS) Social Support Survey.27 Clinical data, including American Joint Committee on Cancer (AJCC) stage, were obtained from patients’ medical records.
The responses of SN patients with late-stage disease were compared with those of SN patients with early-stage disease (stages 0- IIA) and to those of NSN patients, all of whom (by design) had late-stage disease. Patient and process factors potentially associated with late-stage breast cancer diagnosis were analyzed using t tests (for continuous variables), chi-square tests (for categorical variables), and unadjusted as well as multivariate logistic regression models (for binary measures of stage [early vs. late stage] and timeliness of presentation [>1 week vs. ≤1 week delay]). Statistical analyses were conducted using SAS 9.2 (SAS Institute Inc., Cary, NC) and WINPEPI (JH Abramson, 2004). Two-tailed P < 0.05 was considered significant.
RESULTS
Fifty-seven women (33 SN, 24 NSN) completed interviews, with a yield of 70 % (47 SN patients invited) and 69 % (35 NSN patients invited), respectively, from the pool of potentially eligible patients. Among SN patients, two of the initially identified patients proved to be ineligible, seven declined to participate, two consented but were unable to be interviewed, and three were unable to be contacted, consented, or interviewed. Among NSN patients, one of the initially identified patients proved to be ineligible, six declined to participate, two consented but were unable to be interviewed, and two were unable to be contacted, consented, or interviewed. Selected demographic information of the 25 study-eligible nonparticipants (14 SN, 11 NSN) is reported in Table 1. Compared with participants, nonparticipants were more likely to be African-American (84 % vs. 54 %, P = 0.009) but were similar to participants with regard to referral source, marital status, and disease stage.
TABLE 1.
Study participants vs. nonparticipants
| Participants n = 57 |
Nonparticipants n = 25 |
P value | |
|---|---|---|---|
| Age (yr), mean (SD) |
53.4 (10.2) | 53.3 (12.0) | 0.968 |
| n (%) | n (%) | ||
| Race (% African American) |
31 (54.3 %) | 21 (84.0 %) | 0.009 |
| Referral source (% Safety Net) |
33 (57.9 %) | 14 (56.0 %) | 0.906 |
| Marital status (% married) |
21 (36.8 %) | 7 (28.0 %) | 0.538 |
| Stage (% late, IIB-IV) | 42 (73.7 %) | 18 (72.0 %) | 0.896 |
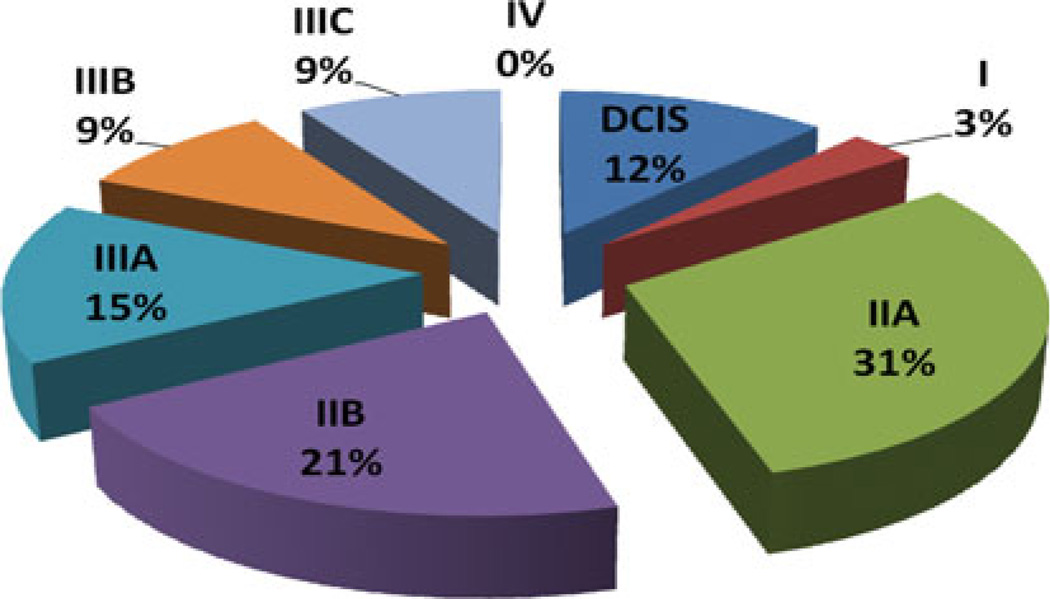
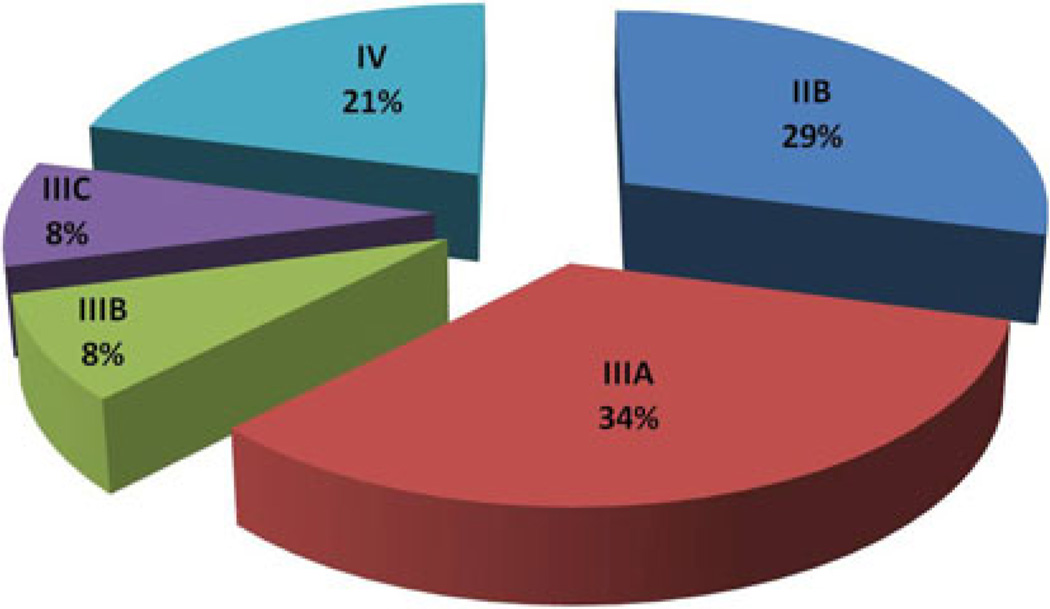
Descriptive statistics of the sample grouped by referral source (SN and NSN) are shown in Table 2. Among the 33 SN patients, 18 (52 %) were diagnosed with late-stage (IIB-IV) disease and 15 (48 %) were diagnosed with earlystage (0-IIA) cancer compared with all 24 of the NSN patients having late-stage disease (by design) (Figs. 1, 2).
TABLE 2.
Safety-Net (SN) vs. non-Safety-Net (NSN) patients
| NSN n = 24 |
SN n = 33 |
P value | |
|---|---|---|---|
| Age, mean (SD) | 53.4 (11.4) | 52.5 (9.4) | 0.753 |
| No. of children <18 years old, mean (SD) | 1.7 (0.82) | 1.6 (0.79) | 0.739 |
| Social support, mean (SD)a | 89.8 (6.9) | 80.7 (17.1) | 0.0092 |
| n (%) | n (%) | ||
| Race | <0.0001 | ||
| African American | 5 (20.8 %) | 26 (78.8 %) | |
| Native American/Alaskan Native | 0 (0.0 %) | 1 (3.0 %) | |
| Caucasian | 19 (79.2 %) | 6 (18.2 %) | |
| Hispanic ethnicity | 0.421 | ||
| Yes | 1 (4.2 %) | 0 (0.0 %) | |
| No | 23 (95.8 %) | 33 (100.0 %) | |
| Marital status | 0.00022 | ||
| Married | 16 (66.7 %) | 5 (15.2 %) | |
| In a domestic relationship | 3 (12.5 %) | 1 (3.0 %) | |
| Widowed | 1 (4.2 %) | 4 (12.1 %) | |
| Divorced | 2 (8.3 %) | 7 (21.2 %) | |
| Separated | 0 (0.0 %) | 4 (12.1 %) | |
| Single/never been married | 2 (8.3 %) | 12 (36.4 %) | |
| Education, highest level completed | 0.083 | ||
| Grade school (Kindergarten-8th) | 0 (0.0 %) | 0 (0.0 %) | |
| High school (9th–12th) | 11 (45.8 %) | 16 (48.5 %) | |
| Technical/trade school | 1 (4.2 %) | 1 (3.0 %) | |
| Some college | 4 (16.7 %) | 12 (36.4 %) | |
| College graduate: bachelor’s degree | 2 (8.3 %) | 3 (9.1 %) | |
| Graduate or professional school | 6 (25.0 %) | 1 (3.0%) | |
| Employment | 0.0034 | ||
| Employed, full-time | 13 (54.2 %) | 8 (24.2 %) | |
| Employed, part-time | 1 (4.2 %) | 7 (21.2 %) | |
| Unemployed | 1 (4.2 %) | 5 (15.2 %) | |
| Homemaker | 4 (16.7 %) | 2 (6.1 %) | |
| Retired | 3 (12.5 %) | 1 (3.0 %) | |
| Disabled | 1 (4.2 %) | 10 (30.3 %) | |
| Student | 1 (4.2 %) | 0 (0.0 %) | |
| Annual income | 0.00022 | ||
| <$10,000 | 0 (0.0 %) | 9 (27.3 %) | |
| $10,000–$14,999 | 1 (4.2 %) | 7 (21.2 %) | |
| $15,000–$19,999 | 5 (20.8 %) | 12 (36.4 %) | |
| $20,000–$24,999 | 3 (12.5 %) | 1 (3.0 %) | |
| $25,000–$34,999 | 4 (16.7 %) | 2 (6.1 %) | |
| $35,000–$49,999 | 4 (16.7 %) | 2 (6.1 %) | |
| $50,000–$74,999 | 4 (16.7 %) | 0 (0.0 %) | |
| $75,000–$99,999 | 0 (0.0 %) | 0 (0.0 %) | |
| >$100,000 | 2 (8.3 %) | 0 (0.0 %) | |
| Refused/don’t know | 1 (4.2 %) | 0 (0.0 %) | |
| Public assistance, primary | 0.023 | ||
| None | 18 (75.0 %) | 15 (45.5 %) | |
| Welfare | 0 (0.0 %) | 1 (3.0 %) | |
| Food stamps | 3 (12.5 %) | 13 (39.4 %) | |
| Unemployment | 1 (4.2 %) | 0 (0.0 %) | |
| Disability | 2 (8.3 %) | 1 (3.0 %) | |
| Other | 0 (0.0 %) | 3 (9.1 %) | |
| Health Insurance, primary | 0.00043 | ||
| Employer or spouse/partner’s employer | 13 (54.1 %) | 3 (9.1 %) | |
| Purchased by self or spouse/partner | 2 (8.3 %) | 0 (0.0 %) | |
| Medicaid | 6 (25.0 %) | 13 (39.4 %) | |
| Medicare | 2 (8.3 %) | 6 (18.2 %) | |
| Social Security | 0 (0.0 %) | 1 (3.0 %) | |
| Disability | 0 (0.0 %) | 1 (3.0 %) | |
| None | 1 (4.2 %) | 9 (27.3 %) | |
| Household size | 0.778 | ||
| Lives alone (i.e., 1 person) | 2 (8.3 %) | 4 (12.1 %) | |
| 2–4 people | 19 (79.2 %) | 27 (81.8 %) | |
| 5–7 people | 3 (12.5 %) | 2 (6.1 %) | |
| Childcare | 0.986 | ||
| Stays home with child/children | 3 (12.5 %) | 3 (9.1 %) | |
| Children attend school-based daycare | 2 (8.3 %) | 3 (9.1 %) | |
| Family and friends assist | 1 (4.2 %) | 2 (6.1 %) | |
| Children old enough to be home alone | 4 (16.7 %) | 4 (12.1 %) | |
| No children <18 years old | 11 (45.8 %) | 17 (51.5 %) | |
| Other/didn’t specify | 3 (12.5 %) | 4 (12.1 %) | |
| Neighborhood environment | <0.0001 | ||
| Urban | 6 (25.0 %) | 26 (78.8 %) | |
| Suburban | 6 (25.0 %) | 7 (21.2 %) | |
| Small town | 9 (37.5 %) | 0 (0.0 %) | |
| Rural | 3 (12.5 %) | 0 (0.0 %) | |
| Transportation, primary mode | 0.0054 | ||
| Car | 22 (91.7 %) | 15 (45.5 %) | |
| Bus | 1 (4.2 %) | 9 (27.3 %) | |
| Taxi | 0 (0.0 %) | 1 (3.0 %) | |
| Call-A-Ride | 0 (0.0 %) | 3 (9.1 %) | |
| Family and Friends | 1 (4.2 %) | 4 (12.1 %) | |
| Other/didn’t specify | 0 (0.0 %) | 1 (3.0 %) | |
| Needed to see physician in past 12 months but unable to because of cost |
6 (25 %) | 21 (63.6 %) | 0.0039 |
| How patient first discovered she might have breast cancer | 0.025 | ||
| Mammogram | 2 (8.3 %) | 12 (36.4 %) | |
| Breast exam by physician | 1 (4.2 %) | 0 (0.0 %) | |
| Incidental finding | 0 (0.0 %) | 1 (3.0 %) | |
| Mass/lump detected by sexual partner | 1 (4.2 %) | 0 (0.0 %) | |
| Symptoms | 20 (83.3 %) | 20 (60.6 %) | |
| Initial physical signs/symptoms of possible breast cancer | 0.754 | ||
| None | 1 (4.2 %) | 5 (15.2 %) | |
| Palpable mass | 18 (75 %) | 19 (57.6 %) | |
| Nipple discharge | 0 (0.0 %) | 2 (6.0 %) | |
| Inflammation/swelling | 1 (4.2 %) | 1 (3.0 %) | |
| Pain | 2 (8.3 %) | 2 (6.1 %) | |
| Nipple change/retraction | 1 (4.2 %) | 2 (6.0 %) | |
| Other | 1 (4.2 %) | 2 (6.0 %) | |
SN, n = 32 because one patient did not respond
FIG. 1.
Stage at diagnosis of Safety-Net (SN) patients
FIG. 2.
Stage at diagnosis of non-Safety-Net (NSN) patients
Both late-stage SN and NSN patients (n = 42) were more likely than early-stage SN patients (n = 15) to first discover their cancer through symptoms (33/42 [79 %] vs. 7/15 [47 %], P = 0.025) and less likely to discover their cancer through mammography (6/42 [14 %] vs. 8/15 [53 %], P = 0.025); the remaining three late-stage patients had their cancer discovered by their sexual partner (NSN patient), during a physician’s examination (NSN patient), or as an incidental finding during an unrelated medical evaluation (SN patient). Looking only at patients with late-stage disease, however, there was no statistically significant difference between SN (n = 18) and NSN (n = 24) patients with regard to how their breast cancer was first discovered (through mammography—SN: 4/18 [22 %] vs. NSN: 2/24 [8.3 %]; through symptoms—SN: 13/18 [72 %] vs. NSN: 20/24 [83 %], P = 0.3849). Likewise, among the SN patients alone, there was no statistically significant difference between early-stage (n = 15) and late-stage (n = 18) patients with regard to how their breast cancer was first discovered (through mammography—early-stage: 8/15 [53 %] vs. late-stage: 4/18 [22 %]; through symptoms— early-stage: 7/15 [47 %] vs. late-stage: 13/18 [72 %], P = 0.1451). Thus, there were no significant differences with regard to mode of disease discovery amongst study participants once stage and referral source were held constant.
Compared with NSN late-stage patients, SN late-stage patients were more likely to be African-American (83 % vs. 21 %, P < 0.001), to have an annual household income <$25,000 (89 % vs. 38 %, P < 0.001), and to report being unable to obtain medical care for a health problem in the preceding year because of cost (67 % vs. 25 %, P = 0.012); they were less likely to have attended graduate school (0 % vs. 25 %, P < 0.03), to be married/partnered (22 % vs. 79 %, P < 0.001), and to have any insurance (61 % vs. 96 %, P < 0.005). Finally, SN patients were less likely to have sought medical attention promptly (i.e., within 1 week) upon realizing they had concerning breast findings (50 % vs. 79 %, P = 0.047; Table 3). Rates of mammography utilization were not significantly different between NSN and SN late-stage patients (8 % vs. 22 %, P = 0.3849).
TABLE 3.
Selected comparisons between non-Safety-Net (NSN) and late-stage Safety-Net (SN late) patients
| NSN n = 24 | SN Late n = 18 | P value | |
|---|---|---|---|
| Age (yr), mean (SD) | 53.4 (11.4) | 52.2 (9.2) | 0.705 |
| Social support, mean (SD)a | 89.8 (6.9) | 81.8 (17.8) | 0.094 |
| n (%) | n (%) | ||
| Race (% African American) | 5 (20.8 %) | 15 (83.3 %) | <0.0001 |
| Post-college education | 6 (25.0 %) | 0 (0.0 %) | 0.029 |
| Marital status (% married/partnered) | 19 (79.2 %) | 4 (22.2 %) | 0.00024 |
| Household income (% <$25,000) | 9 (37.5 %) | 16 (88.9 %) | 0.00079 |
| Any insurance | 23 (95.8 %) | 11 (61.1 %) | 0.0046 |
| Unable to see physician in preceding 12 months because of cost |
6 (25.0 %) | 12 (66.7 %) | 0.012 |
| Waited <1 week between realizing breast findings serious and seeking medical attention |
19 (79.2 %) | 9 (50.0 %) | 0.047 |
SN late, n = 17 because one patient did not respond
There was a significant difference in social support scores between SN and NSN patients, with higher scores among NSN patients (81 [SD 17] vs. 90 [SD 7], P = 0.0092; Table 2) but not between NSN and SN late-stage patients (90 [SD 7] vs. 82 [SD 18], P = 0.094; Table 3) or between early- and late-stage SN patients (79 [SD 17] vs. 82 [SD 18], P = 0.7036). There were no significant differences in patient or process factors between SN patients with early- and late-stage disease (data not shown).
Unadjusted logistic regression was performed to determine whether any of the factors that differed significantly between SN late-stage and NSN late-stage patients were predictive of late-stage disease or of seeking medical attention within 1 week of realizing that abnormal breast findings were serious. Being married/partnered was the only factor that emerged as being significantly associated with both late-stage disease and delaying presentation to medical care by >1 week, with married/partnered patients more likely to have late-stage disease (odds ratio [OR] = 7.87; 95 % confidence interval [CI] = 1.58–39.28; P = 0.0119) and more likely to delay care by [1 week (OR = 4.08; 95 % CI = 1.14–14.64; P = 0.0308). In a multivariate model that included age, race, education, marital status, income, insurance status, primary-care access, disease stage, and social support, being married/ partnered was the only characteristic that was independently associated with delay [1 week (OR = 9.91; 95 % CI = 1.14–86.2; P = 0.0377). Married/partnered women were more likely to delay presentation than women who were not married/partnered, regardless of whether we combined all of the SN patients and all of the NSN patients together (84 % [21/25] of those partnered vs. 56 % [18/32] of those not partnered, P = 0.025) or we combined only the late-stage SN patients and the NSN patients together (83 % [19/23] of those partnered vs. 47 % [9/19] of those not partnered, P = 0.016). Stratified by referral source, the differences persisted (SN: 25 % [5/20] of those partnered vs. 7.7 % [1/13] of those not partnered, P = 0.364; NSN: 84 % [16/19] of those partnered vs. 60 % [3/5] of those not partnered, P = 0.271), but the statistical significance was attenuated, probably due to lack of power.
DISCUSSION
We found a disparity between SN and NSN patients with regard to new diagnoses of late-stage disease despite recently implemented improvements in the referral process. In addition, there is evidence of significantly increased rates of mammography utilization throughout the St. Louis metropolitan area, particularly among the medically underserved (including SN patients) (Susan Kraenzle, personal communication, June 4, 2012). However, it is difficult to estimate the size of the SN population and definitively report actual and optimal rates of mammography utilization within it. In 2010, SN institutions in the greater St. Louis region served approximately 230,000 individuals.28 It would be a mistake, however, to view this number as a true census for the region’s SN population, which is constantly in flux. Indeed, individuals served by a SN institution in a given year may go several additional years without any medical contact. Thus, it is very difficult to quantify the SN population and virtually impossible to confirm what percentage of that population consists of women for whom yearly mammograms would be recommended (i.e., aged 50–74 or younger, depending on family or personal history of breast cancer) or to calculate what percentage of these women actually end up receiving mammograms.
Nonetheless, in our study, SN and NSN patients had comparable rates of mammography use, even when stage was held constant. But while rates of new late-stage breast cancers remain approximately 10 % within the NSN population, 52 % of the SN patients in this study had late-stage disease. A number of covariates, including delayed presentation >1 week to the medical system, differed between late-stage patients from the two referral sources. It is worth exploring the extent to which these characteristics might contribute to the observed disparity in stage at diagnosis.
We had anticipated a significant association between delayed medical contact and late-stage presentation amongst our study participants. In fact, whereas our SN patients were more likely to have demographic and experiential characteristics that are often associated with both delayed presentation and late stage, including African-American race, higher rates of being uninsured or underinsured, and having lower incomes, SN patients actually had comparably similar rates of mammography, which had previously been hypothesized to be the main explanatory factor behind the disparity in stage between whites and blacks, the insured and uninsured, as well as the socioeconomically privileged and disadvantaged.5,12,13,23,29–31
We believe mitigation of this disparity in mammography utilization can, in large part, be attributed to the successful outreach efforts of Siteman’s Mammography Van and Breast Cancer Navigator programs, which have established strong ties with Federally Qualified Health Centers (FQHCs) and other primary care providers for the medically underserved throughout the region. The Mammography Van visits every FQHC and primary care clinic on its roster once each month and provides mammograms during these monthly visits. If a woman has been identified by one of these clinics as needing a screening mammogram but is not present on the day of the van’s monthly visit, she is contacted by a Breast Navigator, who schedules the appointment with her. In addition, there is a Breast Navigator hotline, the number for which is available at primary care clinics, in the mammography van, and on the web. Thus, Breast Navigators become involved in the breast care process very early on, facilitating screening as well as diagnosis and treatment for women in the SN.
Although we were encouraged by reported rates of mammography participation among SN patients in this study, we were concerned by the discovery that SN patients were more likely to delay seeking medical attention after realizing a breast symptom or mammography finding was serious and possibly indicative of cancer. The tumor doubling rate for a mammographically detectable tumor is approximately 260 days.32 A delay of 3 months has been shown to adversely affect survival, and within the breast cancer literature, 90 days has emerged as the maximum length of time that should elapse between symptom discovery and initial clinical examination.4,14,16,17,33–38 Thus, a delay >1 week may not appear to be particularly important, but we must contextualize this finding by considering the timing of our interviews relative to the timing of each participant’s cancer diagnosis. The initial weeks following a breast-cancer diagnosis are characterized by significant emotional and psychological upheaval, during which time patients might suppress (or minimize the importance of) some of the behavioral choices that could have contributed to their breast cancer diagnoses. For example, several studies have illustrated a propensity toward a minimizing form of recall bias regarding prediagnosis diet, induced abortion, alcohol consumption, and oral contraceptive use in breast cancer patients.39–42 It seems very probable that a woman who might have delayed going to a physician after realizing she had concerning breast findings would wish to minimize the extent to which her behavior might have contributed to the severity of her disease. Thus, we would argue that a statistically significant finding of disparate rates of any length of delay represents an important discovery because this disparity emerges despite the potential for recall bias that would be expected to surround this issue. Among SN patients, this delay in presentation occurred despite adequate rates of mammography. Thus, although efforts to improve mammography utilization amongst the city’s underserved have been largely successful, we may be losing women to follow-up after they receive an abnormal mammogram. Future research will need to be devoted to assisting SN patients in the critical time period between the discovery of abnormal breast findings and definitive diagnosis and treatment.
In our study, being married/partnered was more common among NSN patients and was predictive of delay >1 week, even when we controlled for several other variables, including referral source and stage of cancer. This finding appears to be at odds with much of the research on the relationship between marital status and health in general as well as breast cancer in particular. Being married or in a stable domestic relationship has been cited regularly as beneficial for the health of men and women alike, although there is some evidence that the positive health effects of marriage are greater in men.43–46 In addition, some studies have demonstrated a survival benefit associated with marriage and cohabitation amongst women with breast cancer.7,8,10,18,21,46 The benefits of being married/partnered are thought to result from the social and emotional support provided by a woman’s partner. However, at least one study notes that being older and married may be associated with delaying medical care among women with breast cancer.22 And another study looking at partner abandonment among breast cancer patients emphasized that, with regard to social support, the quality of a domestic relationship or marriage is of at least as much import as the mere existence of said relationship.47 Among our patients, it may be that the busy nature of their lives and the uncertain stability of their domestic relationships have more of an impact on the nature and temporality of their healthcare choices than the mere fact of being partnered. Thus, greater attention may need to be paid to the medical needs of married/partnered women, who may prioritize the needs of others within their households above their own.
Finally, delayed presentation has also been associated with the lack of a primary care home.28,48–51 Relative to NSN patients, a greater proportion of late-stage SN patients reported being unable to obtain medical care they needed in the preceding 12 months because of cost, a situation that serves as a significant proxy for the absence of a primary care relationship or adequate health insurance. Although SN patients are availed of free screening through various local services, we have already noted the possible gap in care that may exist between discovering an abnormal breast finding and receiving appropriate breast cancer care. Lack of a relationship with a primary care provider may be an impediment to timely follow-up, especially for women whose other life stressors might cause them to minimize their own health problems.
Our study had some limitations. First, our sample size was small because this was a pilot study conducted in one city that included patients seen first within the city’s SN system or seen first in the private sector; both groups of patients were referred to the same NCI-designated comprehensive cancer center located in St. Louis. These patients might differ from other patients in the St. Louis metropolitan area or in surrounding areas who are first seen in other medical care settings and subsequently referred elsewhere for breast cancer treatment.
Second, selection bias may limit the generalizability of our results. Nearly 30 % of potentially eligible patients were not included in the study, and among the nonparticipants were 4 people (2 SN, 2 NSN) who were consented but unable to be interviewed for logistical reasons as well as 5 patients (3 SN, 2 NSN) who were unable to be contacted. These patients may represent a segment of the population that differed from participants in important ways, including possession of fewer personal assets, including nonfinancial assets such as social support.52 Comparison of the demographic information of the 25 study-eligible nonparticipants to that of participants only revealed a significant difference with regard to race: non-participants were more likely to be African-American than participants (Table 1), reflecting a well-documented trend in medical research.53,54 Although SN late-stage patients were more likely to be African-American than NSN latestage patients, race was not found in our regression models to be independently associated with either late-stage disease or delayed presentation among our participants. Thus, it is unclear to what extent the inclusion of more African-Americans in our study might have altered some of our outcomes. In addition, with regard to referral source, rates of late-stage disease, and marital status, participants and nonparticipants were very similar, reassuring us that selection bias with regard to these factors was minimal.
Finally, as mentioned above, recall bias may have led to a minimization of personal behaviors that may have contributed to being diagnosed with late-stage cancer; thus, it may be that the magnitude of delay amongst our study participants may be greater than what they reported.
Despite rates of mammography utilization comparable to NSN patients as well as improvements in the SN referral process to Siteman, SN patients continue to present with higher-than-expected rates of late-stage disease. Our study findings raise a number of questions that warrant further investigation. First, SN patients are getting mammograms but delay seeking care for abnormal findings; thus, they may require additional education regarding the need for and assistance with obtaining timely post-mammography follow-up. In addition, women need to be continually informed about what breast signs and symptoms should prompt them to seek medical attention. Second, primary care access may be especially important for breast cancer patients with limited financial resources, minimal familial support, and other life stressors, including difficult or unstable domestic partnerships and poor health. Finally, despite the availability of free and inexpensive breast screening and treatment options as well as a cohort of invested patient guides and advocates through the Breast Cancer Navigator Program, lack of adequate insurance may still be a deterrent for many women to seeking breast cancer care or receiving it in a timely fashion. Additional community outreach and education is still needed with regard to where one can go to address medical problems even if one does not have insurance. Future studies are recommended to quantify how often and over what period of time SN breast cancer patients are following up the results of abnormal breast exams and mammograms as well as to identify ways in which follow-up care can be expedited for married/partnered women, who were unexpectedly found here to delay follow-up.
ACKNOWLEDGMENTS
This study was funded by a grant from the Harrah’s Foundation (Principal Investigator, Graham Colditz, MD, DrPH) to the Siteman Cancer Center at Barnes-Jewish Hospital and Washington University School of Medicine for its Program for the Elimination of Cancer Disparities (PECaD). The authors would like to thank Stephen Ristvedt, PhD, for his assistance in developing our questionnaire; Susan Kraenzle, BA, RN, CBCN, for providing information on the Siteman Mammography Van and Breast Cancer Navigator Programs; and Aimee James, PhD, for her assistance with earlier drafts of this manuscript. Portions of this study’s findings were presented at a PECaD Breast Cancer Community Partnership Meeting on July 14, 2011, at the 65th Annual Cancer Symposium of the Society of Surgical Oncology, March 21–24, 2012, and at the 45th Annual Professional Meeting of the Missouri Chapter of the American College of Surgeons, June 8–10, 2012.
Footnotes
CONFLICT OF INTEREST None of the authors has any financial interests or conflicts to disclose.
REFERENCES
- 1.Fitzgibbons PL, Page DL, Weaver D, et al. Prognostic factors in breast cancer. College of American Pathologists Consensus Statement 1999. Arch Pathol Lab Med. 2000;124(7):966–978. doi: 10.5858/2000-124-0966-PFIBC. [DOI] [PubMed] [Google Scholar]
- 2.Carter CL, Allen C, Henson DE. Relation of tumor size, lymph node status, and survival in 24,740 breast cancer cases. Cancer. 1989;63:181–187. doi: 10.1002/1097-0142(19890101)63:1<181::aid-cncr2820630129>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 3.Bradley KT. Prognostic and Predictive Factors in Breast Cancer. NewsPath. 2007 [Google Scholar]
- 4.Richards M, Westcombe A, Love S, Littlejohns P, Ramirez A. Influence of delay on survival in patients with breast cancer: a systematic review. Lancet. 1999;353:1119–1126. doi: 10.1016/s0140-6736(99)02143-1. [DOI] [PubMed] [Google Scholar]
- 5.American Cancer Society. Breast cancer facts & figures 2011–2012. Atlanta, GA: American Cancer SocietyI; 2011. [Google Scholar]
- 6.Wilkinson GS, Edgerton F, Wallace HJ, Reese P, Patterson J, Priore R. Delay, stage of disease and survival from breast cancer. J Chronic Dis. 1979;32(5):365–373. doi: 10.1016/0021-9681(79)90078-x. [DOI] [PubMed] [Google Scholar]
- 7.Neale AV, Tilley BC, Vernon SW. Marital status, delay in seeking treatment and survival from breast cancer. Soc Sci Med. 1986;23(3):305–312. doi: 10.1016/0277-9536(86)90352-7. [DOI] [PubMed] [Google Scholar]
- 8.Waxler-Morrison N, Hislop TG, Mears B, Kan L. Effects of social relationships on survival for women with breast cancer: a prospective study. Soc Sci Med. 1991;33(2):177–185. doi: 10.1016/0277-9536(91)90178-f. [DOI] [PubMed] [Google Scholar]
- 9.Ayanian JZ, Kohler BA, Abe T, Epstein AM. The relation between health insurance coverage and clinical outcomes among women with breast cancer. N Engl J Med. 1993;329:326–331. doi: 10.1056/NEJM199307293290507. [DOI] [PubMed] [Google Scholar]
- 10.Neale AV. Racial and marital status influences on 10 year survival from breast cancer. J Clin Epidemiol. 1994;47(5):475–483. doi: 10.1016/0895-4356(94)90294-1. [DOI] [PubMed] [Google Scholar]
- 11.Mandelblatt J, Andrews H, Kao R, Wallace R, Kerner J. Impact of access and social context on breast cancer stage at diagnosis. J Health Care Poor Underserved. 1995;6(3):342–351. doi: 10.1353/hpu.2010.0449. [DOI] [PubMed] [Google Scholar]
- 12.Lannin DR, Mathews HF, Mitchell J, Swanson MS, Swanson FH, Edwards MS. Influence of socioeconomic and cultural factors on racial differences in late-stage presentation of breast cancer. JAMA. 1998;279(22):1801–1807. doi: 10.1001/jama.279.22.1801. [DOI] [PubMed] [Google Scholar]
- 13.McCarthy EP, Burns RB, Coughlin SS, et al. Mammography Use helps to explain differences in breast cancer stage at diagnosis between older black and white women. Ann Intern Med. 1998;128(9):729–736. doi: 10.7326/0003-4819-128-9-199805010-00005. [DOI] [PubMed] [Google Scholar]
- 14.Richards M, Smith P, Ramirez A, Fentiman I, Rubens R. The influence on survival of delay in the presentation and treatment of symptomatic breast cancer. Br J Cancer. 1999;79(5):858–864. doi: 10.1038/sj.bjc.6690137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roetzheim RG, Pal N, Tennant C, et al. Effects of health insurance and race on early detection of cancer. J Natl Cancer Inst. 1999;91:1409–1415. doi: 10.1093/jnci/91.16.1409. [DOI] [PubMed] [Google Scholar]
- 16.Sainsbury JR, Johnston C, Haward B. Effect on survival of delays in referral of patients with breast-cancer symptoms: a retrospective analysis. Lancet. 1999;353(9159):1132–1135. doi: 10.1016/s0140-6736(99)02374-0. [DOI] [PubMed] [Google Scholar]
- 17.Tartter PI, Frost M, Bernstein JL. Delay in diagnosis of breast cancer. Ann Surg. 1999;229(1):91–96. doi: 10.1097/00000658-199901000-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kravdal O. The impact of marital status on cancer survival. Soc Sci Med. 2001;52:357–366. doi: 10.1016/s0277-9536(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 19.Goodson WH, Moore DH. Causes of Physician Delay in the Diagnosis of Breast Cancer. Arch Intern Med. 2002;162:1343–1348. doi: 10.1001/archinte.162.12.1343. [DOI] [PubMed] [Google Scholar]
- 20.Bish A, Ramirez A, Burgess C, et al. Understanding why women delay in seeking help for breast cancer symptoms. J Psychosom Res. 2005;58(4):321–326. doi: 10.1016/j.jpsychores.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 21.Osborne C, Ostir GV, Du X, Peek MK, Goodwin JS. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Cancer Res Treat. 2005;93(1):41–47. doi: 10.1007/s10549-005-3702-4. [DOI] [PubMed] [Google Scholar]
- 22.Facione N, Facione P. The cognitive structuring of patient delay in breast cancer. Soc Sci Med. 2006;63(12):3137–3149. doi: 10.1016/j.socscimed.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9(3):222–230. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 24.Fayanju OM, Jeffe DB, Tappenden JR, et al. Breast cancer presentation in an urban health care safety net system. Mo Med. 2012;109(5):405–411. [PMC free article] [PubMed] [Google Scholar]
- 25.Ristvedt S, Birnbaum E, Dietz D, Fleshman J, Kodner I, Read T. Delayed treatment for rectal cancer. Dis Colon Rectum. 2005;48:1736–1741. doi: 10.1007/s10350-005-0069-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ristvedt S, Trinkaus K. Psychological factors related to delay in consultation for cancer symptoms. Psycho-Oncol. 2005;14:339–350. doi: 10.1002/pon.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sherbourne C, Stewart A. The MOS social support survey. Soc Sci Med. 1991;32(6):705–714. doi: 10.1016/0277-9536(91)90150-b. 728. [DOI] [PubMed] [Google Scholar]
- 28.St. Louis Regional Health Commission. [Accessed 3 June];Progress toward building a healthier St. Louis; 2011. 2012 Access to Care Data Book http://www.stlrhc.org/LinkClick.aspx?fileticket=yvygro9%2b75k%3d&tabid=38.
- 29.Gerend MA, Pai M. Social determinants of Black-White disparities in breast cancer mortality: a review. Cancer Epidemiol Biomarkers Prev. 2008;17:2913–2923. doi: 10.1158/1055-9965.EPI-07-0633. [DOI] [PubMed] [Google Scholar]
- 30.Ward E, Jemal A, Cokkinides V, et al. Cancer disparities by race/ ethnicity and socioeconomic status. CA Cancer J Clin. 2004;54:78–93. doi: 10.3322/canjclin.54.2.78. [DOI] [PubMed] [Google Scholar]
- 31.Kerner J, Yedida M, Padgett D, et al. Realizing the promise of breast cancer screening: clinical follow-up after abnormal screening among Black women. Prev Med. 2003;37(2):92–101. doi: 10.1016/s0091-7435(03)00087-2. [DOI] [PubMed] [Google Scholar]
- 32.Kerlikowske K. Timeliness of follow-up after abnormal screening mammography. Breast Cancer Res Treat. 1996;40:53–64. doi: 10.1007/BF01806002. [DOI] [PubMed] [Google Scholar]
- 33.Caplan LS, Helzlsouer KJ. Delay in breast cancer: a review of the literature. Public Health Rev. 1992–1993;20(3–4):187–214. [PubMed] [Google Scholar]
- 34.Facione N. Delay versus help seeking for breast cancer symptoms: a critical review of the literature on patient and provider delay. Soc Sci Med. 1993;36(12):1514–1534. doi: 10.1016/0277-9536(93)90340-a. [DOI] [PubMed] [Google Scholar]
- 35.Caplan LS, Helzlsouer KJ, Shapiro S, Wesley MN, Edwards BK. Reasons for Delay in Breast Cancer Diagnosis. Prev Med. 1996;25:218–224. doi: 10.1006/pmed.1996.0049. [DOI] [PubMed] [Google Scholar]
- 36.Burgess C, Ramirez A, Love S. Who and what influences delayed presentation in breast cancer? Br J Cancer. 1998;77(8):1343–1348. doi: 10.1038/bjc.1998.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorin SS, Heck JE, Cheng B, Smith SJ. Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med. 2006;166:2244–2252. doi: 10.1001/archinte.166.20.2244. [DOI] [PubMed] [Google Scholar]
- 38.Smith ER, Adams SA, Das IP, Bottai M, Fulton J, Hebert JR. Breast cancer survival among economically disadvantaged women: the influences of delayed diagnosis and treatment on mortality. Cancer Epidemiol Biomarkers Prev. 2008;17(10):19. doi: 10.1158/1055-9965.EPI-08-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mannisto S, Pietinen P, Virtanen M, Kataja V, Uusitupa M. Diet and the risk of breast cancer in a case-control study: does the threat of disease have an influence on recall bias? J Clin Epidemiol. 1999;52(5):11. doi: 10.1016/s0895-4356(99)00010-4. [DOI] [PubMed] [Google Scholar]
- 40.Rookus MA, van Leeuwen FE. Induced abortion and risk for breast cancer: reporting (recall) bias in a dutch case-control study. J Natl Cancer Inst. 1996;88(23):1759–1764. doi: 10.1093/jnci/88.23.1759. [DOI] [PubMed] [Google Scholar]
- 41.Malone K, Daling J, Weiss N. Oral contraceptives in relation to breast cancer. Epidemiol Rev. 1993;15(1):80–97. doi: 10.1093/oxfordjournals.epirev.a036119. [DOI] [PubMed] [Google Scholar]
- 42.Giovannucci E, Stampfer MJ, Colditz GA, et al. Recall and selection bias in reporting past alcohol consumption among breast cancer cases. Cancer Causes Control. 1993;4(5):441–448. doi: 10.1007/BF00050863. [DOI] [PubMed] [Google Scholar]
- 43.Umberson D. Gender, marital status and the social control of health behavior. Soc Sci Med. 1992;34(8):907–917. doi: 10.1016/0277-9536(92)90259-s. [DOI] [PubMed] [Google Scholar]
- 44.Waldron I, Hughes ME, Brooks TL. Marriage protection and marriage selection: prospective evidence for reciprocal effects of marital status and health. Soc Sci Med. 1996;43(1):113–123. doi: 10.1016/0277-9536(95)00347-9. [DOI] [PubMed] [Google Scholar]
- 45.Johnson NJ, Backlund E, Sorlie PD, Loveless CA. Marital status and mortality: The National Longitudinal Mortality Study. Annals of Epidemiology. 2000;10:224–238. doi: 10.1016/s1047-2797(99)00052-6. [DOI] [PubMed] [Google Scholar]
- 46.Ramirez A, Westcombe A, Burgess C, Sutton S, Littlejohns P, Richards M. Factors predicting delayed presentation of symptomatic breast cancer: a systematic review. Lancet. 1999;353:1127–1131. doi: 10.1016/s0140-6736(99)02142-x. [DOI] [PubMed] [Google Scholar]
- 47.Taylor-Brown J, Kilpatrick M, Maunsell E, Dorvel M. Partner abandonment of women with breast cancer: myth or reality? Cancer Pract. 2000;8(4):160–164. doi: 10.1046/j.1523-5394.2000.84004.x. [DOI] [PubMed] [Google Scholar]
- 48.Osuch JR, Bonham VL. The timely diagnosis of breast cancer: principles of risk management for primary care providers and surgeons. Cancer. 1994;74(S1):271–278. doi: 10.1002/cncr.2820741311. [DOI] [PubMed] [Google Scholar]
- 49.Steyskal R. Minimizing the risk of delayed diagnosis of breast cancer. Medscape Women’s Health. 1996;1(7) [PubMed] [Google Scholar]
- 50.Schootman M, Myers-Geadelmann J, Fuortes L. Factors associated with adequacy of diagnostic workup after abnormal breast cancer screening results. J Am Board Fam Med. 2000;13(2):94–100. doi: 10.3122/15572625-13-2-94. [DOI] [PubMed] [Google Scholar]
- 51.Arndt V, Sturmer T, Stegmaier C, Ziegler H, Dhom G, Brenner H. Patient delay and stage of diagnosis among breast cancer patients in Germany – a population based study. Br J Cancer. 2002;86:1034–1040. doi: 10.1038/sj.bjc.6600209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jeffe D, Pe´rez M, Liu Y, Collins K, Aft R, Schootman M. Quality of life changes over time in women diagnosed with ductal carcinoma in situ, early-stage invasive breast cancer, and age-matched controls. Breast Cancer Res Treat. 2012;134(1):379–391. doi: 10.1007/s10549-012-2048-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Corbie-Smith G, Thomas SB, St, George DMM. Distrust, race, and research. Arch Intern Med. 2002;162(21):2458–2463. doi: 10.1001/archinte.162.21.2458. [DOI] [PubMed] [Google Scholar]
- 54.Shavers VL, Lynch CF, Burmeister LF. Factors that influence African-Americans’ willingness to participate in medical research studies. Cancer. 2001;91(S1):233–236. doi: 10.1002/1097-0142(20010101)91:1+<233::aid-cncr10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]