Abstract
Objective
A major goal of epilepsy research is to understand the molecular and functional basis of seizure genesis. A human GABAA γ2 gene mutation (R43Q) is associated with generalized epilepsy. Introduction of this mutation into a mouse by gene targeting recapitulates the human phenotype demonstrating a strong genotype to phenotype link. GABAA receptors play a role in the moment-to-moment control of brain function and also on the long-term wiring of the brain by directing neuronal development. Our objective was to determine whether developmental expression of the mutation alters seizure susceptibility later in life.
Methods
A tetracycline-based conditional model for activation of a hypomorphic Q43 disease allele was created and validated. Seizure susceptibility was assessed using the subcutaneous pentylenetetrazole model.
Results
Seizure susceptibility was significantly reduced in mice where the Q43 allele was suppressed during development.
Interpretation
These results demonstrate that a human epilepsy-causing mutation impacts network stability during a critical developmental period. These data suggest that identification of presymptomatic children may provide a window for therapeutic intervention before overt symptoms are observed, potentially altering the course of epileptogenesis.
Epilepsy, with a lifetime prevalence rate of 3%, is a common and serious neurological disorder. It is characterized by recurrent paroxysms resulting from hypersynchronous discharges in the brain. Mutations in more than a dozen ion channel genes have been associated with familial epilepsy syndromes providing a strong foundation on which to build an understanding of epileptogenesis. Ion channels have an acute role setting the real-time excitability of neurons and networks, and a developmental role in the coupling of neurons in networks. When mutations occur, they may influence either role to varying degrees to cause the clinical phenotype. Distinguishing acute from developmental roles has important therapeutic implications. Reversing the consequences of developmental dysfunction may not be a simple matter of compensating for the acute receptor deficit because the developmental consequences may be inherent in neural networks of patients with epilepsy.
There is no evidence that familial disease causing ion channel mutations impacts neural development. Mouse models harboring human epilepsy-causing mutations that recapitulate aspects of the human disease are particularly valuable models to test the link between gene mutation and seizures. Manipulation of the temporal expression of a single mutation may provide a means to resolving the impact of a mutation on the developing and adult brain. To this end, we engineered a mouse model in which we can manipulate the temporal expression of a GABAA γ2 mutation, previously reported in a large Australian family with febrile seizures and generalized epilepsy.1
GABAA receptors are ligand-gated chloride channels and arbiters of fast inhibitory neurotransmission in the adult brain. Perturbation of fast inhibition has been linked to seizure genesis. During brain development, GABAA receptors also play an important role in regulating neuronal differentiation, proliferation, and synaptogenesis2 that can also influence seizure genesis.
Here, we generate and characterize a conditional mouse model that allows a forebrain-specific switch of a human epilepsy GABAA γ2 (R43Q) mutation at specific times during development. Using this mouse, we demonstrate that expression of GABAA γ2 (R43Q) in the developing brain increases seizure susceptibility in adulthood.
Materials and Methods
Animals
All experiments were approved by the Animal Ethics Committee at the Howard Florey Institute (04-102). Tgα-CaMKII-tTA/LC1/Gabrg-2R/Qneo mice in the C57BL/6 background were generated by crossing Tgα-CaMKII-tTA mice3 with TgLC1 mice4 and the gene-targeted mouse line Gabrg-2R/Qneo. Gabrg-2R/Q mice were generated by crossing Gabrg-2R/Qneo to a Credeletor strain.5 Transgenic TgZ/EG mice6 were used as a Cre-reporter strain. Triple-transgenic mice, Tgα-CaMKII-tTA/LC1/Z/EG were generated by crossing Tgα-CaMKII-tTA with TgLC1 and TgZ/EG. Animals were toe-clipped at postnatal days 8 (P8) to P12 for genotyping. The Table summarizes the genotypes/alleles used in this study and gives a brief description of each.
Table. Genotypes and Alleles Used.
| Genotype | Description |
|---|---|
| Gabrg-2R/R | Wild type |
| Gabrg-2R/Q | Knock-in mouse (human genotype equivalent model) |
| Gabrg-2R/Qneo | Hypomorphic for R allele caused by Q allele suppression |
| TgZ/EG | Enhanced green fluorescent protein, LacZ conditional reporter mouse |
| Tgα-CaMKII-tTA/LC1 | Doxycycline-regulated Cre-expressing transgenic alleles |
Conditional Expression
The Tgα-CaMKII-tTA/LC1 line was used to regulate Cre-recombinase (Cre) expression using doxycycline (Dox). This line expresses the tetracycline-controlled transcriptional activator (tTA) under the control of the α-calmodulin-dependent protein kinase II (α-CaMKII) promoter. In the absence of Dox, tTA binds to the tetracycline-responsive element in the TgLC1 line and activates the bidirectional expression of luciferase and Cre. Dox binds tTA, preventing it from interacting with the tetracycline-responsive element, thus inhibiting subsequent transcription of Cre.4 Doxycycline Hyclate (Sigma-Aldrich, Castle Hill, Australia) was fed to the parental mice from conception via drinking water at a concentration of 50mg/L supplemented with 1% sucrose as described elsewhere.7
Genotyping
With the exception of the TgZ/EG mouse line, mice were genotyped using toe DNA with the following different primer sets (polymerase chain reaction [PCR] product sizes are given in brackets): Tgα-CaMKII-tTA: forward primer tTA1 (5′-GTG ATT AAC AGC GCA TTA GAG C -3′) and reverse primer tTA3 (5′-CGC CGT CTA AGT GGA GCT CGT CC-3′), (800bp); TgLC1: forward primer luc1 (5′-CTT TTA CAG ATG CAC ATA TCG AGG-3′) and reverse primer luc2 (5′-TAG GTA ACC CAG TAG ATC CAG AGG-3′), (400bp); Gabrg-2R/Qneo: forward primer CC21 (5′ -CAC TGT CAT CTT AAA CAA CCT GCT GGA A-3′) and reverse primer PGKprom2 (5′-CAG ACT GCC TTG GGA AAA GCG-3′), (364bp); Gabrg-2R/Q/Gabrg-2R/R: forward primer SP031 (5′-GTA GAA GCC AAC TCA GGA GTC ATC-3′) and reverse primer SP032 (5′-CCC TGT CTG TCC TAG AAC TCA CTC-3′) (475bp and 509bp corresponding to the Gabrg-2R and Gabrg-2Q alleles, respectively). PCR was performed using the following conditions: 96°C for 3 minutes, 35 cycles of 96°C for 20 seconds, 57°C for 30 seconds, and 72°C for 50 seconds; then a final extension at 72°C for 10 minutes using a Biometra TGradient Thermocycler (Biometra, Goettingen, Germany). TgZ/EG mice were genotyped by staining ear clippings from P15 to P21 animals with a LacZ stain using standard protocols as described elsewhere.8
Histology
Mice were killed via intraperitoneal injection of 100mg/kg sodium pentobarbital (Nembutal, Merial, Australia), then injected with heparin sodium salt in saline (100U, intraperitoneally; Sigma). This was followed by transcardial perfusion of 20ml ice-cold 0.9% NaCl, then 20ml 4% formaldehyde in 0.1M phosphate buffer. Brains were removed and postfixed in 4% formaldehyde for a further hour, washed in phosphate-buffered saline, then cryoprotected in 30% sucrose at 4°C for 24 to 48 hours. Brains were placed in a 1:1 mix of 30% sucrose and Optimal Cutting Temperature Compound (Tissue Tek; Sakura, Torrance, CA) at 4°C 24 hours before cryosectioning. Brains were mounted in OCT, and 16μm coronal sections were collected at two depths (bregma –1.70 and 0.80mm) onto SuperFrost Plus electrostatic slides (BDH Laboratory Supplies, Toronto, Ontario, Canada). Sections were dried overnight in the dark, then mounted with Cytoseal mounting solution (Fischer Scientific, Hampton, USA). Images were captured using a conventional upright confocal microscope with a standard fluorescein isothiocyanate filter, 30% laser power and a 10× objective. All cell counts were performed manually using ImageJ 1.36b (National Institutes of Health, Bethesda, MD).
Quantitative Polymerase Chain Reaction
Total RNA was extracted from whole brains (RNeasy Lipid kit; Qiagen, Chatsworth, CA) and reverse transcribed into complementary DNA (cDNA; TaqMan Reverse Transcription Kit; Applied Biosystems, Foster City, CA). TaqMan probes complementary to the genomic region containing the mutation were designed for wild-type (5′ FAM—TAT GAC AAC AAA CTT CGA CCT GAC ATC GG—TAMRA 3′) and mutant (5′ VIC—TAC GAC AAC AAG CTT CAG CCT GAC ATA GG—TAMRA 3′) alleles with differences at the R43Q site and four adjacent silent mutations engineered into the original mouse line. Nonmultiplex reactions were performed with 5ng cDNA, 1× TaqMan Universal Mastermix, 900nM of forward CC11 (5′—GTC ATC TTA AAC AAC CGC TGG AA—3′) and reverse CC 12 (5′— CCA ATG CTG TTC ACA TAC ATA TCT GT—3′) primers, and either 200nM of wild-type or mutant probes in 25μl reactions. The reactions were performed using an ABI PRISM 7700 Sequence Detection System (Applied Biosystems) at 50°C for 2 minutes, 95°C for 10 minutes, and 40 cycles of 95°C for 15 seconds and 62°C for 1 minute. Specificity of the probes to their corresponding alleles was demonstrated by performing reactions using wild-type cDNA and homozygous cDNA with the mutant and wild-type TaqMan probes, respectively. Quantitative polymerase chain reaction (qPCR) was also performed for the endogenous control 18S ribosomal RNA on the same plate as the reactions described earlier, where 1× Platinum SYBR Green qPCR Supermix (Invitrogen, La Jolla, CA) was used with the ribosomal 18S forward (5′—CGG CTA CCA CAT CCA AGG AA—3′) and reverse (5′—GCT GGA ATT ACC GCG GCT—3′) primers. Relative gene expression was quantified using standard curves. mRNA levels were normalized to the endogenous 18s ribosomal RNA. Theses normalized values were then averaged for each treatment group and relative gene expression calculated. The group with the highest expression was normalized to 100%, and other groups were scaled to this for comparison.
Pentylenetetrazol Seizure Testing
Mice between the ages of P63 and P70 were injected subcutaneously with pentylenetetrazol (PTZ; 85mg.kg−1; Sigma) and video monitored for a maximum of 1 hour. Latencies to minimal (clonic) and maximal (tonic hindlimb extension) seizures were recorded and tested for significance using Kaplan–Meier survival analysis (GraphPad Prism 4.01) using the Gehan–Breslow test. In all the survival plots, animals that did not reach the end point by 60 minutes were censored. Multiple-comparison correction was applied using Keppel's modified Bonferroni correction of the α value.
Electroencephalographic Recordings
P19 to P21 mice were anesthetized with 1 to 3% isoflurane and implanted with chronic epidural electroencephalographic electrodes. Four silver electrodes were implanted on each quadrant of the skull, and a ground electrode was placed just posterior to the olfactory bulb. Mice were allowed to recover for at least 24 hours. Signals were band-pass filtered at 0.1 to 200Hz and sampled at 1KHz using Powerlab 16/30 data acquisition system (ADInstruments, Bella Vista, Australia) with simultaneous synchronized video monitoring (Quicktime capture; ADInstruments, Bella Vista, Australia). Mice were recorded for 4-hour periods over 3 consecutive days during daylight hours. Electroencephalographic traces were screened for 6 to 7Hz spike and wave discharges and corresponding behavioral arrest, characteristic of the absence seizures these mice display. These conditions were sufficient to record spike and wave discharges in the same mice in an earlier study that first described the phenotype of the Gabrg-2R/Q knock-in mouse.9
Results
Suppression of the Q43 Allele
Intronic retention of loxP flanked Neomycin resistance (Neo) cassettes may suppress allele expression, and thereby provides the basis for a conditional gene regulated model.10 To verify the expression of the Gabrg-2Qneo allele that contains floxed Neo in intron 2 of the Gabrg-2Q allele, we performed qPCR analysis in mice before and after Neo excision (Fig 1). Comparative expression analysis of Gabrg-2R/Qneo and Gabrg-2R/Q mice demonstrated a significant hypomorphic effect of Neo within the Gabrg-2Q gene locus (see Fig 1A). Gabrg-2Q (Q43) allele expression was reduced by 91% in the Gabrg-2R/Qneo mice relative to Q43 allele expression in Gabrg-2R/Q at P15 and by 76% at P66 (see Fig 1B). This establishes the basis of an “off” state for the Q43 mutation that can be switched “on” by Neo removal using temporally and spatially restricted expression of Cre.7
Fig 1.
ffect of Neomycin resistance (Neo) cassette retention on Q43 allele transcript levels. (A) Schematic depiction of genomic structure of the Gabrg-2 alleles used to generate the conditional mouse line. Qneo = neo-retaining mutant allele; Q = neo-excised mutant allele; R = the wild-type allele. White triangles designate loxP sites; black vertical line designates the position of the R→Q mutation. (B) Normalized messenger RNA expression of the Q43 allele for each of the experimental groups. *p = 0.004; **p = 0.001. n values are shown in parentheses above each bar graph.
Reporter Mice Demonstrate Spatial and Cell-Type Extent of Cre Excision
For in vivo activation of the hypomorphic Gabrg-2Qneo allele, we chose tetracycline-controlled, Cre-mediated Neo removal.7 We first profiled the temporal and spatial characteristics of Cre expression using the TgZ/EG reporter mouse.6 Forebrain specific activation can be achieved by transgenes from the Tgα-CaMKII-tTA/LC1 mouse (Fig 2A) as demonstrated in TgZ/EG Cre-reporter mice (see Fig 2B).6 Coronal brain slices of the triple-transgenic Tgα-CaMKII-tTA/LC1/Z/EG mice showed enhanced green fluorescent protein (EGFP) expression in several regions of the forebrain including hippocampus, cortex, striatum, piriform cortex, cingulate cortices, thalamus (ventrobasal thalamus only), and amygdale (see Fig 2C). Examination of brain regions known to almost exclusively harbor inhibitory interneurons including the thalamic reticular nucleus and the molecular layer of CA1 were devoid of EGFP-positive cells, presumably as a consequence of lack of α-CaMKII promoter activation (see Fig 2D).
Fig 2.
Spatial pattern of Cre expression. Schematic depiction of the alleles used to regulate and report Cre expression. (A) Tetracycline-regulated, Cre-expressing mouse line Tgα-CaMKII-tTA/LC1 in which the α-CaMKII promoter drives the tetracycline-controlled transcription activator (tTA) and subsequently Cre. (B) Schematic depiction of the alleles in the Cre-reporter mice line TgZ/EG .In this line, the floxed βgeo (LacZ/Neo) gene is expressed before Cre excision, whereas enhanced green fluorescent protein (EGFP) is expressed after Cre excision. White triangles designate loxP sites. (C) TgZ/EG reporter mice crossed to Tgα-CaMKII-tTA/LC1 mice reported sites of Cre expression by EGFP fluorescence. Several brain regions express Cre (examined in n = 5 mice). (D) Magnification of the thalamic reticular nucleus (TRN) and the stratum radiatum of the CA1 region shown boxed in (C) highlighting the lack of interneuron expression of Cre. (E) Quantitative polymerase chain reaction (qPCR) analysis of mouse forebrains comparing allele expression. Normalized messenger RNA expression levels of the Q43 allele for the in vivo excised Tgα-CaMKII-tTA/LC1/Gabrg-2R/Qneo mouse (Q43 on always) and the mutant mouse Gabrg-2R/Q mouse (RQ) were not significantly different (p = 0.09). n values are shown in parentheses above each bar graph. Tg = transgenic; CamkII = calmodulin-dependent protein kinase II; SIBF = primary somatosensory cortex barrel field; pir = piriform cortex; cg = cingulum; RSA = retrosplenial agranular cortex; RSG = retrosplenial granular cortex; cg1-2 = cingulate cortex areas 1 and 2; CPu = caudate putamen; ec = external capsule; VB = ventral basal thalamic nucleus; nRT = reticular thalamic nucleus; ic = internal capsule; opt = optic tract; MePD = medial amygdala posterodorsal nucleus; MePV = medial amygdala posteroventral nucleus; BMA = basomedial amygdala anterior nucleus; DG = dentate gyrus; CA1 = cornu ammonis 1 region of the hippocampus. Scale bars = 100μM.
In Tgα-CaMKII-tTA/LC1/Gabrg-2R/Qneo mice, the qPCR analysis of forebrain showed that the Q43 transcript levels are similar to that of Gabrg-2R/Q mice, indicating efficient Neo removal of the Gabrg-2Qneo allele (Q43 on always; see Fig 2E) at postnatal day 66. Although there was no significant difference between these groups, there was a trend for the α-CaMKII–driven Q43 allele group to be lower, as expected given the lack of inhibitory neuron expression (see Fig 2D). This result confirms that in vivo conversion of the Gabrg-2Qneo back to Gabrg-2Q by Cre-mediated Neo excision is accompanied by a full restoration of Gabrg-2Q levels.
Conditional Activation of Q43neo Allele Maintains Subcutaneous Pentylenetetrazol Susceptibility
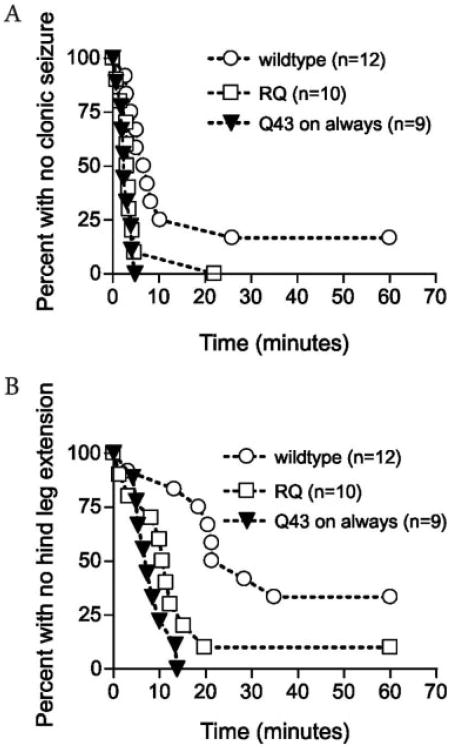
Having established the extent of activation to be expected in our conditional mouse model, it was necessary to compare the phenotypic consequences of this restricted pattern of Q43 activation with heterozygous mutant mice. Gabrg-2R/Q mice display a heightened sensitivity to subcutaneous pentylenetetrazol (scPTZ), providing a useful measure of seizure susceptibility in animals with R43Q mutation9 and, potentially, the effects of the conditional activation of the Q43neo allele. Mice were injected with scPTZ (85mg.kg−1) and monitored for two clearly defined end points: (1) time to first clonic seizure, and (2) time to first tonic hindlimb extension. Gabrg-2R/Q mice showed increased seizure susceptibility compared with Gabrg-2R/R (Fig 3) as previously reported.9 Importantly, there was no significant difference in scPTZ susceptibility between Tgα-CaMKII-tTA/LC1/Gabrg-2R/Qneo and Gabrg-2R/Q mice (see Fig 3). This demonstrates that expression of Q43 allele in the forebrain is sufficient to produce a scPTZ susceptibility phenotype.
Fig 3.
Kaplan–Meier survival plots for subcutaneous pentylenetetrazol (scPTZ) elicited seizures in the Tgα-CaMKII-tTA/LC1/Gabrg-2R/Qneo, Gabrg-2R/Q, and Gabrg-2R/R mice. In vivo activation of the hypomorphic Gabrg-2Qneo allele restores PTZ susceptibility. Survival plots illustrating (A) time to first clonic seizure and (B) time to hindlimb extension. Comparison of survival plots from Tgα-CaMKII-tTA/LC1/Gabrg-2R/Qneo (Q43 on always; n = 9; triangles) and Gabrg-2R/Q (RQ; n = 10; squares) mice showed no significant difference for either time to clonic seizure (p = 0.41) or time to first hind-limb extension (p = 0.12). Circles represent wild-type mice (n = 12).
Temporal Regulation of Q43 Expression
Q43 allele activation is triggered by absence or removal of Dox from the drinking water of the conditional mice. There is, however, a lag anticipated with Cre activation associated with epigenetic silencing of the Tet operon.11 Profiling of the Cre activation in Tgα-CaMKII-tTA/LC1/Z/EG reporter mice was performed by removal of Dox at P21 followed by comparison of EGFP-positive neurons from these mice to controls that never received Dox and hence expressed Cre from conception (Fig 4). The α-CaMKII promoter fragment of the Tgα-CaMKII-tTA/LC1/Z/EG reporter mouse used in this study has a different temporal profile of activation than the full-length promoter that switches on postnatally around day 17.12 In contrast, the promoter fragment we used switches on embryonically at around E10.13–15 Confocal imaging of the hippocampi of these mice showed the temporal profile of Cre expression (see Fig 4A). EGFP-positive cells were counted to provide a quantitative assessment of this profile and showed that the first detectable signs of Cre-recombinase activation occurred at around 9 days after Dox removal. In CA1, EGFP-positive cell counts from Dox-treated mice approached (84%) those seen in the Dox naive mice (see Fig 4B). The efficiency of reactivation appears to be correlated with the expression level of the Dox-dependent transcription factor tTA, from the time point of activation.7,11
Fig 4.
Temporal profile of Cre expression. (A) Hippocampal Cre expression as reported by Tgα-CaMKII-tTA/LC1/Z/EG mice at different time points after Dox removal. (B) Average enhanced green fluorescent protein (EGFP)–positive cell counts in CA1 region of hippocampus. (C) Quantitative polymerase chain reaction (qPCR) analysis showing percentage messenger RNA (mRNA) expression relative to the Q43 allele for the Gabrg-2R/Qneo (neo retained) and Tgα-CaMKII-tTA/LC1/Gabrg-2R/Qneo mice (conditionally suppressed). No significant difference was seen between these two groups (p = 0.897). (D) qPCR analysis showing normalized mRNA expression of the Q43 allele for the Tgα-CaMKII-tTA/LC1/Gabrg-2R/Qneo mouse subject to developmental Cre suppression by doxycycline treatment from conception to postnatal day 21 (P21; Q43 on at P21) or in vivo excision by no doxycycline treatment (Q43 on always). No significant difference was observed between these two groups (p = 0.151). n values for each experiment are shown in parentheses. CA1 = cornu ammonis 1 field of hippocampus; DG = dentate gyrus. Scale bar = 100μM.
The qPCR analysis of relative expression of Q43 alleles from the Gabrg-2R/Qneo mice and the Tgα-CaMKII-tTA/LC1/Gabrg-2R/Qneo mice kept under Dox from conception until P66 (conditionally suppressed) showed an identical poor activation of Q43 allele expression (see Fig 4C) highlighting the effectiveness of the conditional silencing of the Ptet-Bi–controlled Cre-recombinase. qPCR analysis was performed on entire forebrains of Tgα-CaMKII-tTA/LC1/Gabrg-2R/Qneo mice treated with Dox from conception to P21 (Q43 on at P21) or not treated (Q43 on always). Comparison of relative Q43 transcript levels from these groups at age P66 demonstrated an almost complete recovery of Q43 allele expression in the Dox-treated group (see Fig 4D). Collectively, these data provide the basis for examining the impact of Q43 allele expression during conception to death versus P21 to death. With this validation in mind, comparison of seizure susceptibility in these two groups will probe the developmental role of the Q43 allele.
Activation of the Hypomorphic Q43 Allele during Development Increases Seizure Susceptibility
Having established the validity of the conditional model, we next investigated the developmental impact of the Q43 mutant allele. Comparison of scPTZ susceptibility in Tgα-CaMKII-tTA/LC1/Gabrg-2R/Qneo mice treated with doxycycline from conception to P21 (Q43 on at P21) or not Dox treated (Q43 on always) demonstrated that reduced Q43 expression during development (conception to P21) significantly increased the time to first clonic seizure (Fig 5A) and to first hindlimb extension (see Fig 5B). Thus, mice that expressed the Q43 mutation throughout life had an increased susceptibility to scPTZ seizures compared with mice that expressed the mutation only after postnatal day 21.
Fig 5.
Kaplan–Meier survival plots for subcutaneous pentylenetetrazol (scPTZ) elicited seizures in the Tgα-CaMKII-tTA/LC1/Gabrg-2R/Qneo mouse subject to developmental Q43 suppression from conception to postnatal day 21 (P21) or with lifelong Q43 allele expression. Survival plots illustrating (A) time to first clonic seizure and (B) time to hindlimb extension. A significant difference was seen for both the time to first clonic seizure (p = 0.03) and time to hindlimb extension (p = 0.004). Survival plots comparing hypomorphic mice (RQneo) with conditional mice expressing the Q allele throughout life, (C) time to first clonic seizure, and (D) time to hindlimb extension. A significant difference was seen for both the time to first clonic seizure (p = 0.02) and time to hindlimb extension (p = 0.001). (E) Comparison of electroencephalographic recordings from P20 mice. Triangles represent Q43 on always (n = 9); diamonds represent Q43 on at P21 (n = 9); squares represent RQneo (n = 10).
The doxycycline-treated mice are conceived with a hypomorphic allele. The absence of full expression of both wild-type R alleles in the hypomorphic conditional mouse may heighten seizure susceptibility similar to that seen in mice expressing the mutant Q disease allele. To eliminate this possibility as a potential confound for interpretation of the earlier data (see Figs 5A, B), we compared seizure susceptibility of the Gabrg-2R/Qneo mice (hypomorphic) with conditional Tgα-CaMKII-tTA/LC1/Gabrg-2R/Qneo mice where the Q allele had been expressed in early embryonic development (Q43 on always). This comparison showed that the hypomorphic mice had significantly lower seizure susceptibility than mice expressing the Q allele (see Figs 5C, D), suggesting that mutant Q allele expression is a stronger determinant of susceptibility than hypomorphism alone. Furthermore, the increased susceptibility seen in mice with lifelong expression of the Q43 mutation is unlikely to be due to a seizure kindling effect because there were no pathological spike-wave discharges in the electroencephalographic records from either group (see Fig 5E). We also tested whether doxycycline has a protective effect on seizure susceptibility by comparing scPTZ sensitivity in wild-type mice treated with doxycycline or vehicle alone. This comparison showed no significant difference in the time to first clonic seizure (p = 0.21) or first tonic hindlimb extension (p = 0.66), suggesting that the differences in the conditional mice (see Figs 5A, B) were not due to any protective effect of doxycycline. Collectively, these results support the idea that developmental expression of the human epilepsy R43Q GABAA receptor mutation is critical to defining adult seizure susceptibility.
Discussion
Conditional gene expression in which the levels of a gene can be temporally or spatially regulated is frequently used determine gene function.16 To date, no study has attempted to determine the developmental impact of channel dysfunction caused by familial epilepsy mutation. Recently, we described a novel knock-in mouse model carrying the R43Q mutation in the γ2 subunit of the GABAA receptor. This mouse displays spontaneous 6 to 8Hz spike-wave discharges associated with behavioral arrest,9 recapitulating a major phenotype of patients harboring the mutation.1 A general hyperexcitable phenotype was also described with enhanced susceptibility to proconvulsant challenges.9 The known molecular cause of this phenotype, based on a human mutation, provides a useful framework with which to dissect out potential developmental processes involved in the generation of excitable phenotypes.
Developing methods to manipulate gene expression is critical for investigating the roles of ion channel mutations in disease genesis. Viral-mediated gene transfer and transgenic-mediated conditional expression are two broad strategies that can be used to dissect acute from developmental effects of epilepsy-causing mutations. Viral-mediated gene transfer has several drawbacks, including extent of transfer, cell-type specificity of infection, level of expression, and competition with endogenous alleles. Conditional expression of a mutant allele with the simultaneous deletion of a wild-type allele under strict temporal control may potentially be the ideal solution. A similar but simpler approach, which we use in this study, relies on the ability of the Neo selection cassette to suppress gene expression by virtue of its effect on RNA splicing.17,18
In this study, we created a mouse model in which Q43 disease allele expression is regulated using a tetracycline-controlled conditional system.19 Reduced expression of the Q43 disease allele during early development (inception to P21) significantly reduced seizure susceptibility. This suggests that mutation-mediated dysfunction in channel activity during development can be a critical determinant of seizure susceptibility in later life. Although we have not unequivocally demonstrated neuron-specific activation, our data suggest that inhibitory neurons may be spared, raising the intriguing possibility that excitatory neuron expression of the Q43 disease allele is sufficient to alter seizure susceptibility.
The association between brain development and epilepsy has long been documented.20,21 Until recently there has been little experimental evidence that mutations seen in idiopathic epilepsy are associated with demonstrable changes in brain development. A structural magnetic resonance imaging study of members of the Australian family with the GABAA γ2 (R43Q) mutation provided the first strong evidence for such an association. Family members with the GABAA γ2 (R43Q) mutation presented changes in corpus callosum volume that were not observed in family members without the mutation (S. F. Berkovic, personal communication).
GABAA receptors play a key role during brain development where they impact neuronal differentiation, proliferation, and synaptogenesis.2 Dysfunction in any of these could potentially lead to the development of an excitable phenotype. For example, the migration patterns of interneurons in the developing cortex depend on GABAA receptor function.2 Even subtle differences in interneuron placement could explain hyperexcitability as seen in the uPAR−/− knock-out mice with reduced interneurons migrating their correct region in the cortex.22 Modulating synaptic pathways is also a critical role played by GABAA receptors in early development.2 Toward the final phases of neuronal development when networks are formed, spontaneous neuronal oscillations are thought to mediate the functional and structural maturation of neuronal networks.23 Giant depolarizing potentials (GDP) are such oscillations seen in the hippocampus and are mediated by the excitatory GABAA receptor current.24,25 GABAA receptor–mediated depolarization during the giant depolarizing potential results in activation of N-methyl-d-aspartate receptors and voltage-gated calcium channels causing calcium influx26–28 that is believed to activate signaling pathways to direct the establishment and refinement of network circuitry.29 The abundant expression in most brain regions of the GABAA γ2 subunit at an early time point in development positions it as a key player in the developmental role of GABAA receptors.30 Changes in giant depolarizing potential caused by the R43Q mutation in the GABAA γ2 subunit could impact network formation and thereby contribute to the excitable phenotype seen in patients with this mutation.
In this study, we have demonstrated that a human epilepsy mutation may influence both neuronal development and the acute function of GABAA receptors. These findings may have important therapeutic implications; reversing the consequences of developmental dysfunction may not be a simple matter of compensating for the acute receptor deficit. Currently, no known antiepileptic drugs have shown the capability to prevent or alter the course of epilepsy progression.31–33 This may stem from our inability to diagnose “epilepsy” before seizure presentation. Early detection facilitated by genetic testing may provide a window for therapeutic intervention before overt symptoms are observed, potentially altering the course of epileptogenesis.
Acknowledgments
This work was supported by the National Health and Medical Research Council (400121 S.F.B. and S.P. and 454655 C.A.R. and S.P.) and the NIH NINDS (NS046378, M.V.J., S.P.).
We thank L. Bray and C. Bustos for managing the mice breeding, and Dr O. Sergeyev, K. S. Tan, and Dr K. L. Richards for performing the genotyping for this work. We thank Dr C. Lobe for the gift of the Z/EG mice and Dr P. H. Seeburg for his support in the initial phase of this project.
Footnotes
C.C. and C.A.R. contributed equally to this work.
Disclosure: S.P. and S.F.B. were paid consultants of Bionomics Limited, which holds the intellectual property surrounding this work.
References
- 1.Wallace RH, Marini C, Petrou S, et al. Mutant GABA(A) receptor gamma2-subunit in childhood absence epilepsy and febrile seizures. Nat Genet. 2001;28:49–52. doi: 10.1038/ng0501-49. [DOI] [PubMed] [Google Scholar]
- 2.Owens DF, Kriegstein AR. Is there more to GABA than synaptic inhibition? Nat Rev Neurosci. 2002;3:715–727. doi: 10.1038/nrn919. [DOI] [PubMed] [Google Scholar]
- 3.Mayford M, Bach ME, Huang YY, et al. Control of memory formation through regulated expression of a CaMKII transgene. Science. 1996;274:1678–1683. doi: 10.1126/science.274.5293.1678. [DOI] [PubMed] [Google Scholar]
- 4.Schonig K, Schwenk F, Rajewsky K, Bujard H. Stringent doxycycline dependent control of CRE recombinase in vivo. Nucleic Acids Res. 2002;30:e134. doi: 10.1093/nar/gnf134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schwenk F, Baron U, Rajewsky K. A cre-transgenic mouse strain for the ubiquitous deletion of loxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res. 1995;23:5080–5081. doi: 10.1093/nar/23.24.5080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Novak A, Guo CY, Yang WY, et al. Z/EG, a double reporter mouse line that expresses enhanced green fluorescent protein upon Cre-mediated excision. Genesis. 2000;28:147–155. [PubMed] [Google Scholar]
- 7.Krestel HE, Shimshek DR, Jensen V, et al. A genetic switch for epilepsy in adult mice. J Neurosci. 2004;24:10568–10578. doi: 10.1523/JNEUROSCI.4579-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lobe CG, Koop KE, Kreppner W, et al. Z/AP, a double reporter for Cre-mediated recombination. Dev Biol. 1999;208:281–292. doi: 10.1006/dbio.1999.9209. [DOI] [PubMed] [Google Scholar]
- 9.Tan HO, Reid CA, Single FN, et al. Reduced cortical inhibition in a mouse model of familial childhood absence epilepsy. Proc Natl Acad Sci U S A. 2007;104:17536–17541. doi: 10.1073/pnas.0708440104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nagy A. Cre recombinase: the universal reagent for genome tailoring. Genesis. 2000;26:99–109. [PubMed] [Google Scholar]
- 11.Zhu P, Aller MI, Baron U, et al. Silencing and un-silencing of tetracycline-controlled genes in neurons. PLoS ONE. 2007;2:e533. doi: 10.1371/journal.pone.0000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schweizer C, Balsiger S, Bluethmann H, et al. The gamma 2 subunit of GABA(A) receptors is required for maintenance of receptors at mature synapses. Mol Cell Neurosci. 2003;24:442–450. doi: 10.1016/s1044-7431(03)00202-1. [DOI] [PubMed] [Google Scholar]
- 13.Michalon A, Koshibu K, Baumgartel K, et al. Inducible and neuron-specific gene expression in the adult mouse brain with the rtTA2S-M2 system. Genesis. 2005;43:205–212. doi: 10.1002/gene.20175. [DOI] [PubMed] [Google Scholar]
- 14.Yamamoto A, Lucas JJ, Hen R. Reversal of neuropathology and motor dysfunction in a conditional model of Huntington's disease. Cell. 2000;101:57–66. doi: 10.1016/S0092-8674(00)80623-6. [DOI] [PubMed] [Google Scholar]
- 15.Krestel HE, Mayford M, Seeburg PH, Sprengel R. A GFP-equipped bidirectional expression module well suited for monitoring tetracycline-regulated gene expression in mouse. Nucleic Acids Res. 2001;29:E39. doi: 10.1093/nar/29.7.e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lewandoski M. Conditional control of gene expression in the mouse. Nat Rev Genet. 2001;2:743–755. doi: 10.1038/35093537. [DOI] [PubMed] [Google Scholar]
- 17.Nagy A, Moens C, Ivanyi E, et al. Dissecting the role of N-myc in development using a single targeting vector to generate a series of alleles. Curr Biol. 1998;8:661–664. doi: 10.1016/s0960-9822(98)70254-4. [DOI] [PubMed] [Google Scholar]
- 18.Meyers EN, Lewandoski M, Martin GR. An Fgf8 mutant allelic series generated by Cre- and Flp-mediated recombination. Nat Genet. 1998;18:136–141. doi: 10.1038/ng0298-136. [DOI] [PubMed] [Google Scholar]
- 19.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci U S A. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guerrini R, Canapicchi R, Dobyns WB. Epilepsy and malformations of the cerebral cortex. Neurologia. 1999;14(suppl 3):32–47. [PubMed] [Google Scholar]
- 21.Guerrini R, Marini C. Genetic malformations of cortical development. Exp Brain Res. 2006;173:322–333. doi: 10.1007/s00221-006-0501-z. [DOI] [PubMed] [Google Scholar]
- 22.Powell EM, Campbell DB, Stanwood GD, et al. Genetic disruption of cortical interneuron development causes region- and GABA cell type-specific deficits, epilepsy, and behavioral dysfunction. J Neurosci. 2003;23:622–631. doi: 10.1523/JNEUROSCI.23-02-00622.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- 24.Ben-Ari Y, Cherubini E, Corradetti R, Gaiarsa JL. Giant synaptic potentials in immature rat CA3 hippocampal neurones. J Physiol. 1989;416:303–325. doi: 10.1113/jphysiol.1989.sp017762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khalilov I, Dzhala V, Ben-Ari Y, Khazipov R. Dual role of GABA in the neonatal rat hippocampus. Dev Neurosci. 1999;21:310–319. doi: 10.1159/000017380. [DOI] [PubMed] [Google Scholar]
- 26.Garaschuk O, Hanse E, Konnerth A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol. 1998;507(pt 1):219–236. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leinekugel X, Medina I, Khalilov I, et al. Ca2+ oscillations mediated by the synergistic excitatory actions of GABA(A) and NMDA receptors in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- 28.Leinekugel X, Tseeb V, Ben-Ari Y, Bregestovski P. Synaptic GABAA activation induces Ca2+ rise in pyramidal cells and interneurons from rat neonatal hippocampal slices. J Physiol. 1995;487(pt 2):319–329. doi: 10.1113/jphysiol.1995.sp020882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang LI, Poo MM. Electrical activity and development of neural circuits. Nat Neurosci. 2001;4(suppl):1207–1214. doi: 10.1038/nn753. [DOI] [PubMed] [Google Scholar]
- 30.Laurie DJ, Wisden W, Seeburg PH. The distribution of thirteen GABAA receptor subunit mRNAs in the rat brain. III. Embryonic and postnatal development. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schmidt D, Rogawski MA. New strategies for the identification of drugs to prevent the development or progression of epilepsy. Epilepsy Res. 2002;50:71–78. doi: 10.1016/s0920-1211(02)00070-0. [DOI] [PubMed] [Google Scholar]
- 32.Temkin NR. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia. 2001;42:515–524. doi: 10.1046/j.1528-1157.2001.28900.x. [DOI] [PubMed] [Google Scholar]
- 33.White HS, Smith-Yockman M, Srivastava A, Wilcox KS. Therapeutic assays for the identification and characterization of antiepileptic and antiepileptogenic drugs. In: Pitkänen A, Schwartzkroin P, Moshé S, editors. Models of seizures and epilepsy. New York: Elsevier; 2005. pp. 539–549. [Google Scholar]