Abstract
A growing number of studies are revealing that cells can send and receive information by controlling the temporal behavior (dynamics) of their signaling molecules. In this review, we discuss what is known about the dynamics of various signaling networks and their role in controlling cellular responses. We identify general principles that are emerging in the field, focusing specifically on how the identity and quantity of a stimulus is encoded in temporal patterns, how signaling dynamics influence cellular outcomes and how specific dynamical patterns are both shaped and interpreted by the structure of molecular networks. We conclude by discussing potential functional roles for transmitting cellular information through signaling dynamics and possible applications for the treatment of disease.
A unifying theme in biology is that function is reflected in structure. Consider, for example, the highly specialized structure of a bird’s wing. The sparsely arranged bones and feather patterning create a high surface to mass ratio that enables flight. Or examine the folded conformation of an enzyme: its three-dimensional structure indicates which substrate molecules it is capable of binding and which reactions it may catalyze. Perhaps the most prevalent example of a biological structure that predicts physiological function is the genome. By knowing the sequence structure of coding DNA, one can infer whether it encodes a protein domain, a binding site, a conserved motif, or a hairpin structure. These examples demonstrate that functional information is encoded in the structural components of a cell. One may argue that all relevant information is embedded in cellular structures, if only we could measure them in sufficient detail. But is this the only way that biological information may be encoded? Are there aspects of biological function that cannot be discovered by simply looking at static structures?
In this review, we discuss an emerging trend in cell biology that suggests an additional mode for transmitting information in cells—through the dynamics of signaling molecules (Behar and Hoffmann, 2010). Here dynamics is defined as the shape of the curve describing how the concentration, activity, modification state or localization of a molecule changes over time (Figure 1A) This mode of signaling encodes information in the frequency, amplitude, duration or other features of the temporal signal (Figure 1B). It is therefore more rich and complex than transmitting information through the state of a signaling molecule at only a single point in time. We present a broad survey of what is known about the dynamics of different systems across biology, focusing on well-studied systems that have been analyzed using multiple quantitative measurement and perturbation approaches. Through these examples, we extract general principles about the role of dynamics in biology and what advantages may be conferred by transmitting information through the dynamics of signaling molecules.
Figure 1. Quantifying the dynamics of signaling molecules in living systems.

(A) Different inputs may be distinguished by differences in static quantities such as the abundance, identity (e.g. posttranslational modifications, binding of a cofactor), or location of signaling molecules. However, not only the absolute number matters (e.g. how much of a specific protein is found in a cell at a specific time), but also the temporal pattern of these variables (the shape of the curve describing changes in concentration, localization and modifications over time).
(B) Examples for measureable features of a dynamic signal including amplitude, frequency, duration, delay, and cumulative level.
(C) Cellular processes occur with characteristic time scales ranging from sub-second to several days. Taking measurements at the appropriate time scale is crucial for capturing the true dynamical behavior.
(D) Measurements of cell populations can obscure dynamics of individual cells. For example, pulses of p53 in response to DNA damage have a fixed height and width. Different number of pulses and loss of synchrony among individual cells gives the appearance of damped oscillations in the population. Similarly, the cleavage of caspase substrates during apoptosis appears to occur gradually in a population of cells. Single-cell imaging reveals that cleavage is rapid but with a variable delay from cell to cell.
Quantifying the dynamics of signaling molecules in living systems
Understanding the dynamics of biological responses requires collecting high-quality time-series data. An important consideration when measuring the dynamics of a signal is the appropriate time-scale of measurement. Some processes, such as ion transport or calcium release, occur in seconds. Others, including changes in protein levels during the cell cycle occur over minutes or hours. Changes in some observable phenotypes such as cell morphology or expression of cell surface markers can take days or longer. Thus, a good understanding of the timescale of a particular system is crucial for determining the appropriate sampling frequency to ensure that critical information is not missed (Figure 1C). For example, when the levels of the phosphorylated kinase ATM (ATM-P) were measured at high frequency during the first hour after DNA damage, the conclusion was that ATM is rapidly phosphorylated and reaches a maximal level within 5 minutes after damage, followed by a slow decrease (Jazayeri et al., 2006). When the levels of ATM-P were measured every hour for 10 hours it became clear that it shows a series of oscillations after DNA damage, an observation that led to a new model for the control of ATM and the tumor suppressor p53 in response to DNA breaks (Batchelor et al., 2008).
The dynamics of a signal can be measured across a population of cells or in individual cells. The development of fluorescent sensors that allow high-resolution time-lapse imaging in living cells has improved our ability to quantify the dynamics of biological responses in single cells. These include chemical sensors that report activation of a signaling molecule (Welch et al., 2011) as well as sensors that participate directly in the functional response such as fluorescent fusion proteins [e.g., (Albeck et al., 2008; Bakstad et al., 2012)]. A collective observation from these and additional studies is that individual cells differ widely in their dynamical responses even when challenged with the same stimulus (Cohen et al., 2008; Lee et al., 2009). As a result, the average dynamical behavior of a population often represents a distorted version of individual patterns that can lead to misinterpretations. For example, p53 dynamics in response to DNA damage were originally described as damped oscillations when measured by Western blot (Lev Bar-Or et al., 2000). Observation of single cells, however, revealed that these were actually pulses with fixed height and duration (Lahav et al., 2004). Varying number of pulses and loss of synchronization among individual cells over time led to an apparent widening and shortening of successive pulses in the population (Figure 1D). Similarly, the “switch-like” responses of individual cells to certain signals, such as the MAP kinase activity in developing oocytes (Ferrell and Machleder, 1998) or the cleavage of caspase substrates during apoptosis (Tyas et al., 2000), gave the appearance of a gradual increase in measurements of an averaged population (Figure 1D). These examples underscore the importance of tracking these responses at the single cell level.
Since tagged reporters represent significant perturbations to the cell, it is important to establish that the introduction of a reporter into a cell line does not alter its dynamical properties. This can be determined through control experiments that compare the rates of induction and degradation between the tagged and endogenous proteins using immunoblots or flow cytometry. For example, rapid accumulation of a protein in live cells often appears as distinct subpopulations in flow cytometry since the protein spends relatively little time in the intermediate state (for an example, see Albeck et al., 2008). However, over-interpretation about the underlying dynamics from flow cytometry should be avoided since simulations show that even graded individual responses can sometimes lead to bimodal populations (Birtwistle et al., 2012). When fluorescent reporters are used to study cell-to-cell variation, the use of clonal stably transfected cell lines is desirable since transiently transfected cells often express varying amounts of the effector, which may alter the dynamics and cause artificial variation between cells (Barken et al., 2005).
The identity and strength of upstream stimuli can be encoded in the dynamics of signaling molecules
One of the first concepts to emerge from studying the temporal behavior of signaling molecules is that different upstream signals can lead to different dynamical patterns of the same molecule. An early example of this behavior was found in the extracellular signal-regulated kinase (ERK) pathway (here ERK refers to the signaling module comprising both Erk1 and Erk2). It was originally observed that two separate growth factors trigger different cell fates of rat neuronal precursors; nerve growth factor (NGF) leads to differentiation whereas epidermal growth factor (EGF) leads to cell proliferation. At first glance, one might conclude that a separate signaling pathway is induced in response to each of these stimuli resulting in different fates. Closer examination, however, revealed that the two stimuli activate ERK, but with distinct dynamical patterns, (Gotoh et al., 1990; Nguyen et al., 1993; Traverse et al., 1992). Specifically, EGF triggers a transient response whereas NGF induces sustained ERK activation (Figure 2A). These observations led to the idea that PC-12 differentiation was not strictly ligand specific, but instead governed by the dynamics of ERK activity (Marshall, 1995).
Figure 2. The identity and strength of upstream stimuli can be encoded in the dynamics of signaling molecules.

(A) Dynamics of ERK activation in response to growth factors. Stimulation of mammalian cells with EGF or NGF results in transient or sustained ERK activation, respectively. Dynamics represent population responses.
(B) Dynamics of NF-κB in response to TNFα or LPS. Stimulation with TNFα results in oscillatory pattern of repeated nuclear accumulation followed by nuclear export. LPS stimulation causes a sustained level of NF-κB translocation after a short delay. Dynamics represent single-cell responses.
(C) Dynamics of yeast transcription factor Msn2. Yeast respond to glucose limitation with a coordinated burst of Msn2 translocation to the nucleus followed by a series of sporadic bursts of Msn2 activity. Increasing strength of these stresses lengthens the duration of the initial burst and increases the frequency of sporadic bursting. Oxidative stress triggers a sustained nuclear accumulation of Msn2. Increased oxidative stress intensity results in a higher amplitude and shorter delay until the signal peak. Dynamics represent single-cell responses.
(D) Dynamics of p53 in response to DNA damage. γ-radiation causes double strand DNA breaks and leads to repeated pulses of p53. Increasing damage leads to more pulses. UV radiation triggers a single pulse of p53 that increases in amplitude and duration in proportion to the UV dose. Dynamics represent single-cell responses.
Additional signaling molecules have been shown to encapsulate upstream signals in their dynamics. For example, different inflammatory stimuli induce distinct temporal profiles of the transcription factor NF-κB (Figure 2B). Under resting conditions, NF-κB is continuously shuttled between nuclear and cytosolic compartments. Activation of NF-κB by tumor necrosis factor-α (TNFα) results in prolonged occupation in the nucleus and transcription of its negative regulator IκBα. This negative feedback loop generates oscillations of transcriptionally active NF-κB (Hoffmann et al., 2002; Nelson et al., 2004; Sung et al., 2009; Tay et al., 2010). In contrast, bacterial lipopolysaccharide (LPS) leads to slower accumulation and a single prolonged wave of NF-κB activity (Covert et al., 2005; Lee et al., 2009; Werner et al., 2005).
In various systems both the identity and strength of the stimulus have been shown to alter the dynamics of the same protein. One example is the yeast transcription factor Msn2, which responds to stress by translocation to the nucleus (Figure 2C). Recent single-cell studies revealed that in response to glucose limitation or high osmolarity, nuclear Msn2 shows a transient increase with a dose-dependent duration and fixed amplitude (Hao and O’Shea, 2012). In contrast, oxidative stress leads to prolonged nuclear Msn2 accumulation with amplitude that increases with higher concentration of H2O2. Closer observation in single cells revealed that following the initial pulse, glucose limitation and osmotic stress lead to a series of Msn2 bursts. The frequency of these pulses depended on the intensity of the signal in glucose limitation but was not affected by the intensity of the osmotic stress (Hao and O’Shea, 2012).
The tumor suppressor p53 also shows both stimulus- and dose-dependent dynamics (Figure 2D). Double strand breaks (DSBs) caused by γ-radiation trigger a series of p53 pulses with fixed amplitude and duration. Higher doses of radiation increase the number of pulses without affecting their amplitude or duration (Geva-Zatorsky et al., 2010; Lahav et al., 2004). In contrast, UV triggers a single p53 pulse with a dose-dependent amplitude and duration (Batchelor et al., 2011). Lastly, stimulus strength was also shown to affect the dynamics of NF-κB activity. Increasing concentrations of TNFα led to a shortened delay in NF-κB nuclear translocation (Cheong et al., 2006; Tay et al., 2010), and increasing the frequency of TNFα stimulation led to smaller amplitude oscillations (Ashall et al., 2009). The emerging picture from these examples is that the dynamics of a signaling molecule can capture both the identity and quantity of upstream stimuli.
Dynamical patterns can also reflect a combination of two or more stimuli administered simultaneously or sequentially. For example, simultaneous treatment with multiple drugs can have an additive effect on the resulting dynamical pattern of downstream signaling proteins; that is, the individual dynamical patterns are effectively superimposed (Geva-Zatorsky et al., 2010). In other cases, different stimuli interact synergistically or antagonistically to produce a temporal profile in which certain dynamical features are either enhanced or silenced, respectively (Garmaroudi et al., 2010; Werner et al., 2008). This is often the case for sequentially administered stimuli when cells show desensitization to repeated stimulation (Ashall et al., 2009). For example, treatment of human platelets with thrombin produces a characteristic temporal pattern of intracellular calcium release. If preceded by treatment with ADP, however, the thrombin-induced pattern is attenuated (Chatterjee et al., 2010). This implies that dynamics can reflect cellular “memory” to previous stimuli and also suggests crosstalk between pathways.
The dynamics of signaling molecules are associated with specific downstream responses
Since the dynamics of various proteins vary with the stimulus, it seems plausible that downstream elements may respond to these different dynamical profiles. In fact there are a number of examples in which the dynamics of a signaling molecule were found to be associated with, or at least to precede specific cellular outcomes. As mentioned previously, the transient activation of ERK in response to EGF allows continued proliferation of neuronal precursors, whereas sustained ERK in response to NGF leads to differentiation of sympathetic-like neurons (Marshall, 1995) (Figure 3A).
Figure 3. The dynamics of signaling molecules are associated with specific downstream responses.

(A) Transient activation of ERK leads to proliferation of neuronal precursor cells. Sustained ERK levels precede differentiation into neurons.
(B) Transient nuclear accumulation of NF-κB triggers expression of nonspecific inflammatory response genes. Sustained nuclear NF-κB levels leads to expression of additional cytokines and chemokines required for adaptive immune response.
(C) p53 pulses in response to γ-irradiation are associated with cell cycle arrest. Prolonged p53 signaling, as in response to UV radiation, leads to apoptosis.
The development of highly sensitive calcium dyes in the 1980s (Grynkiewicz et al., 1985) revealed a vast variety of dynamical behaviors of calcium molecules—from oscillations induced by fertilization of mammalian eggs (Malcuit et al., 2006) to noisy spikes observed in the tiny volume of a single human platelet (Heemskerk et al., 2001). Careful study of these behaviors revealed that calcium can activate different responses based solely on its dynamical waveform. A brief spike of calcium induced prolonged activation of NF-κB and JNK that lasted well after the decay in calcium. In contrast, calcium spikes evoked only transient nuclear translocation of NFAT, whereas prolonged NFAT translocation required sustained calcium levels (Dolmetsch et al., 1997). These results suggested that NFAT may distinguish between different dynamical patterns of calcium. The possibility of such a mechanism has been revived by a recent study showing that two isoforms of NFAT, NFAT1 and NFAT4, show different nuclear localization dynamics in response to static calcium stimulation (Yissachar et al., 2013). Whether these kinetics are responsible for decoding the calcium signal, however, will require characterizing NFAT1/4 dynamics in response to different calcium dynamics.
The dynamics of NF-κB nuclear localization and DNA binding activity have been shown to control both the specificity and levels of target gene expression (Hoffmann et al., 2002; Nelson et al., 2004). Studies performed in cell populations showed that activation of NF-κB in response to TNFα (which produces NF-κB oscillations) induces expression of multiple inflammatory response genes, whereas sustained NF-κB levels induced by LPS led to similar expression patterns but also induced additional cytokine secretion as well as genes associated with the adaptive immune response (Figure 3B) (Werner et al., 2005). The dynamics of p53 have also been associated with specific cellular responses. p53 pulses following γ-irradiation were found to be associated with transient cell cycle arrest and recovery while a single prolonged pulse after UV radiation precedes apoptosis (Figure 3C) (Purvis et al., 2012).
The high-level conclusion that might arise from these studies is that cells are able to “translate” different dynamical patterns of the same signaling molecule into specific outcomes. However, an important caveat to this claim is that in addition to altering dynamics, different stimuli also affect other pathway components that may be responsible for the observed changes in downstream responses. This concern must ultimately be addressed through direct and careful perturbation of the dynamics using genetic or pharmacological strategies.
Targeted perturbations of signaling dynamics
The observation that distinct dynamical patterns are correlated with certain cellular responses does not prove that dynamics are the causal agents behind these responses. How can one test whether dynamics are actually driving cellular responses? Similar to the way researchers examine the role of a specific gene by mutating it and testing the resultant behavior, a sound approach to examining the role of dynamics is to artificially perturb the dynamics of the system and test how this affects downstream outcomes. Each method of perturbation offers varying strengths and weaknesses, with the best characterized systems using multiple approaches.
One of the first examples of controlled perturbation of dynamics was used to study the effect of calcium dynamics on gene expression. Alternating treatments of calcium-carrying ionophores and calcium-sequestering chelating agents was used to study the effect of different calcium frequencies on gene expression. This ‘patch clamp’ setup revealed that different frequencies of calcium activate none, some, or all of the transcription factors NF-κB, NFAT, and Oct/OAP (Dolmetsch et al., 1998). Similarly, the use of photoactivatable inositol 1,4,5-trisphosphate, the intracellular trigger for calcium release, led to the same striking conclusion: specific frequencies of intracellular calcium release could optimize gene expression (Li et al., 1998). Though preceded by earlier indications that the dynamics of second messengers were functional (Darmon et al., 1975), these rational perturbations of intracellular calcium dynamics provided direct evidence that specific dynamical patterns carry functional information and execute specific outcomes.
Perturbations of signaling dynamics can be achieved by inhibiting key components of the circuitry through either small molecules or genetic manipulation. All these strategies were employed in turn to study the role of NF-κB dynamics on target gene specificity. Knockout MEFS lacking NF-κB’s negative regulator, IκBα, showed sustained rather than transient NF-κB activity upon TNFα treatment (Hoffmann et al., 2002). This perturbation revealed that specific genes such as RANTES require sustained activity of nuclear NF-κB. A similar approach was used to identify a component necessary for stimulus-specific NF-κB activity under LPS stimulation. Treatment with LPS in Tnf-deficient MEFS revealed that de novo TNFα production is responsible for the sustained phase of NF-κB activity (Werner et al., 2005).
As an example of pharmacological perturbation of NF-κB dynamics, treatment of cells with leptomycin B (LMB) was used to block nuclear export, thereby trapping the inactive NF-κB-IκBα complex in the nucleus (Nelson et al., 2004) (Figure 4A). As a result, nuclear localization of the NF-κB protein is sustained but its transcriptional activity is only transient. In contrast, natural oscillations of NF-κB trigger a monotonic increase in a fluorescent reporter gene. This led to the hypothesis that NF-κB oscillations function to deliver newly activated NF-κB from the cytoplasm into the nucleus (Nelson et al., 2004). In support of this view, a more recent study employing LMB had no effect on the expression of early genes but led to inhibition of intermediate and late target genes (Sung et al., 2009).
Figure 4. Targeted perturbations of protein dynamics can help reveal the role of dynamics in cellular responses.

(A) Perturbation of NF-κB translocation dynamics alters gene expression. Stimulation with TNFα triggers IKK-dependent activation of NF-κB and targeting to the nucleus. Subsequent export leads to oscillations of NF-κB nuclear activity. Blocking nuclear export with LMB results in sustained accumulation of nuclear NF-κB. This leads, counterintuitively, to a shift from sustained to transient target gene expression, because the negative regulator IκB is also held in the nucleus.
(B and C) Altering ERK dynamics changes phenotypic responses. (B) EGF stimulation produces a transient ERK activation and allows cell proliferation of PC-12 cells. The addition of PMA, an activator of PKC, increases positive feedback from ERK to Raf and sustains the levels of activated ERK in response to EGF. The resulting profile, which resembles the dynamics of NGF stimulation, promotes differentiation. (C) NGF stimulation triggers sustained activation of ERK and leads to cellular differentiation. Inhibition of the positive feedback from ERK to Raf with the PKC inhibitor Gö7874 produces a transient-like ERK response similar to that induced by NGF. This leads to a switch from differentiation to proliferation.
(D) Artificially sustained p53 pulses promote cellular senescence. γ-irradiation induces double strand DNA breaks and activation of ATM kinase. The resulting pulses of p53 are driven in part by negative feedback from Mdm2 to p53. When the ubiquitin ligase activity of Mdm2 is blocked by the small molecule Nutlin-3, p53 levels accumulate. A sequence of Nutlin-3 doses that sustain p53 dynamics leads to cellular senescence.
A combination of theory and perturbation experiments was used to reveal the specific role of ERK dynamics in driving cell fate decisions (Santos et al., 2007). Building on previous observations (Grammer and Blenis, 1997), a pair of small molecules was then used to alter ERK dynamics and reverse the effects of EGF and NGF on PC-12 cell fate. Specifically, treatment with phorbol-12-myristate-13-acetate (PMA), which stimulates protein kinase C (PKC) activation and introduces positive feedback from ERK to Raf, resulted in sustained ERK activation and differentiation in response to EGF (Figure 4B). Conversely, treatment with the PKC inhibitor Gö7874 resulted in transient ERK activation and increased proliferation following NGF treatment (Figure 4C).
While genetic and single-treatment perturbations have proved useful in revealing the role of dynamics in these and several other contexts, another desirable way to alter protein dynamics is to deliver precise and timed perturbations to the molecule under study during the response. Our lab recently used such an approach to show that the dynamics of p53 control the selection and timing of gene expression in response to DNA damage (Purvis et al., 2012). We studied cells that naturally show pulses of p53 in response to γ-radiation. These cells typically recover from moderate doses of radiation after arresting the cell cycle and repairing their DNA. Using carefully timed doses of the small molecule Nutlin-3, which stabilizes p53 levels, we artificially switched p53 dynamics from pulsed to sustained. This switch in p53 dynamics led to activation of genes associated with irreversible cellular fates such as apoptosis and senescence, and pushed cells towards senescence. (Figure 4D). As with all pharmacological agents, cross-reactivity of the drug with other components in the cell should be carefully characterized. Although Nutlin-3 is highly selective for p53 (Tovar et al., 2006), use of more promiscuous agents should be compared with genetic perturbations to substantiate any claims about function.
It is worthwhile to note that the artificially sustained dynamics in response to γ-radiation led to a different cellular outcome (senescence) than would be predicted from the comparable dynamics produced naturally by UV treatment (apoptosis). This shows that similar dynamical patterns can have different consequences when they arise from different stimuli. It also implies that cell fate decisions are determined not only by the dynamics of the signal but by a combination of additional factors such as posttranslational modifications or spatial localization.
Another fine-grained perturbation of dynamics was used to investigate the effect of yeast Msn2 dynamics on target gene expression. A mutant isoform of protein kinase A that could be controlled by a small molecule inhibitor was used to modulate nuclear accumulation of Msn2 (Hao and O’Shea, 2012). This setup, which included a microfluidic device to dynamically administer inhibitor treatment, was used to alter the amplitude, frequency and duration of Msn2 nuclear localization. A fluorescent reporter of Msn2 transcriptional activity revealed different expression patterns correlated with different dynamical features. Specifically, gene expression showed a Hill function-like response to Msn2 amplitude, a linear relationship with the duration of Msn2 nuclear localization, and a nonlinear increase with increasing Msn2 pulse frequency. This example of perturbation has many important advantages: it directly influences the signaling molecule under question (as opposed to altering an upstream ligand); it can be continuously administered and therefore offers control over all parameters of the dynamical waveform; and it allows the ability to record the dynamics in individual cells.
These last two studies employ a similar analysis to determine whether different dynamical signals can be interpreted by cells. The analysis involves calculating the cumulative signal, or area under the curve (Figure 1B) and comparing downstream responses (e.g., gene expression) to the cumulative signal for individual cells. The level of target gene expression in response to Msn2 oscillations was lower than under sustained Msn2 even for similar levels of cumulative Msn2 (Hao and O’Shea, 2012). Similarly, sustained p53 led to greater expression of senescence genes than pulsed p53 even at the same cumulative p53 signal (Purvis et al., 2012). These findings show a non-linear relationship between the cumulative level of a transcription factor and the activation of its target genes, suggesting complex machinery for decoding protein dynamics into specific outcomes.
Linking dynamics with network structure: encoding and decoding mechanisms
The identification of network motifs in transcription networks and the comprehensive study of their dynamics in various systems have revealed a strong relationship between motif structure, dynamics and specific function (Alon, 2007; Yosef and Regev, 2011). For example feedforward loops were found to generate a pulse of activity or protect against brief fluctuations depending in the nature of their interactions. Many of the examples discussed here demonstrate that indeed dynamics play a functional role in driving cellular responses, but they do not always explain how dynamics are regulated or interpreted at the molecular level. In this section, we address two questions: what are the molecular mechanisms that give rise to specific dynamical patterns, and how can different dynamics of the same molecule be interpreted by downstream components?
Encoding dynamics
Studying the dynamics of signaling molecules in response to different stimuli can help reveal the functional feedbacks responsible for shaping the observed dynamics. For example, the differences in ERK dynamics in response to EGF or NGF were found to arise in part because of a negative feedback between ERK and SOS in the EGF pathway. In addition, NGF but not EGF signaling continues after receptor internalization, which contributes to the sustained activation of ERK (Sasagawa et al., 2005). There is also evidence for positive feedback on ERK activation through PKC (Santos et al., 2007). The implication here is that distinct responses to EGF and NGF, which are mediated by the dynamics of ERK, are brought about by differences in the identity and connectivity of various pathway components (Figure 5A).
Figure 5. Linking dynamics with network structure: encoding and decoding mechanisms.

(A and B) Differences in network architecture shape dynamical responses. (A) Transient activation of ERK in response to EGF is facilitated in part by negative feedback through SOS. Sustained ERK activation by NGF relies on positive feedback through PKC, which is not activated downstream of EGFR. (B) γ-radiation causes double-strand DNA breaks and leads to p53 pulses. Negative feedback through the phosphatase Wip1 attenuates the damage signal by dephosphorylating ATM and thereby controls the amplitude and duration of p53 pulses. UV radiation activates ATR kinase. The lack of negative feedback between Wip1 and ATR in the UV pathway is responsible for the difference in p53 dynamics.
(C and D) Network structure selectively interprets dynamics. (C) A network of early responding gene products, such as c-Fos, are induced by activated ERK. Transient ERK activation is not sufficient to productively accumulate c-Fos, whereas sustained ERK activation leads to accumulation of c-Fos. c-Fos is phosphorylated by ERK (pFos) and leads to expression of pro-differentiation genes. Thus, the accumulation of early gene products such as c-Fos serves as a persistence detector for sustained ERK activation. (D) A gene regulatory circuit discriminates transient from persistent TLR4 signals. NF-κB and C/EBPδ form a coherent feed-forward loop to stimulate maximum expression of Il6 transcription. Attenuation of transient LPS signals is mediated by inhibition through ATF3, whereas the dramatic increase in Il6 under persistent LPS stimulation is due in part to positive feedback through autoregulation of C/EBPδ.
The difference between TNFα and LPS-induced NF-κB activation dynamics (Figure 2B) is also attributed to specific network structures. The transient activation of NF-κB in response to TNFα is mediated by a negative feedback loop involving NF-κB and one of its target gene products IκB. Activation of the TNF receptor activates the IκB kinase complex, which phosphorylates IκB and triggers its subsequent degradation through ubiquitination. Degradation of IκB allows free NF-κB to bind its target genes, including IκB, resulting in subsequent inhibition of NF-κB. The long-term dynamics of NF-κB in response to persistent TNFα stimulation are controlled by another target gene product, A20. The A20 protein has a longer half-life and acts further upstream than IκB, which explains why it dampens the long-term phase of NF-κB dynamics (Basak et al., 2012; Werner et al., 2008). In contrast, sustained activation of NF-κB in response to LPS is attributed to positive feedback through an autocrine pathway that involves de novo TNFα production. Activation of the Toll-like receptor 4 (TLR4) by LPS triggers synthesis of TNFα and activation of the TNF receptor. The delay between TRL4- and TNF-dependent activation of NF-κB is proposed to stagger these responses in time and give rise to the stability of LPS-induced NF-κB activation (Covert et al., 2005).
Similarly, specific feedbacks in the DNA damage network were found to be responsible for the differential dynamics of p53 in response to γ-irradiation and UV (Batchelor et al., 2011). In both networks, PI3 kinase related kinases (ATM or ATR) relay the damage signal to p53, activating two core negative-feedback loops: one between p53 and the E3 ubiquitin ligase Mdm2, and the second between p53 and the phosphatase Wip1. An important difference, however, is that the network responding to γ-radiation includes an additional negative feedback between p53 and ATM mediated by Wip1 (Figure 5B). This feedback was shown to be essential for triggering p53 pulses in response to γ-radiation since silencing Wip1 after γ-radiation produces UV-like dynamics (Batchelor et al., 2008). In addition, the response to γ-radiation, but not UV, was found to be excitable, in which low transient inputs are sufficient for triggering a full p53 pulse. The current model only partially recapitulates the excitability observed experimentally (Batchelor et al., 2011), and additional work is required for identifying the mechanism of excitability in the response to γ-radiation.
Decoding dynamics
The second question that arises when considering the functional role of dynamics is how cells interpret different dynamical patterns. That is, what molecular mechanisms are necessary to detect time-dependent features and translate these patterns into distinct phenotypic responses? While many studies have identified functional roles for specific temporal behaviors, only a small fraction of these have determined precisely how different dynamical patterns are distinguished at the molecular level to trigger different downstream responses (Behar et al., 2007). Identifying the mechanisms that decode dynamics remains one of the most challenging goals for the field.
One of the simplest mechanisms proposed for interpreting dynamics is based on the sensitivity of downstream effectors for the molecule displaying dynamics. Under this mechanism, low-affinity effectors require sustained input levels in order to show significant activation, whereas high-affinity effectors can respond to rapidly changing input levels. There is some evidence for this mechanism in the differential activation of JNK, NF-κB, and NFAT in response to transient or sustained calcium. JNK and NF-κB, which respond to strong transient calcium bursts, have a low affinity for calcium and therefore require high concentrations for activity. This property, combined with a slow rate of degradation, allows these downstream factors to stay elevated after a brief stimulation with calcium. NFAT, in contrast, has a high affinity for calcium and a rapid rate of degradation. Thus, low and sustained calcium levels will preferentially activate NFAT over JNK and NF-κB (Dolmetsch et al., 1997). A similar mechanism was proposed to decode dynamics of the yeast stress response factor Msn2. Differences in transcription factor binding properties and the kinetics of promoter transitions were found to govern the response to different dynamical patterns of Msn2 (Hao and O’Shea, 2012). Importantly, these mechanisms do not involve additional factors but rely solely on the strengths of association between the upstream regulator and its effectors.
More complex mechanisms for decoding temporal signals are based on specific network motifs in the responding network that sense time-dependent changes in an upstream regulator. Examples of this type of decoding mechanism have been especially difficult to identify, with two notable exceptions. In the ERK pathway, transient and persistent ERK dynamics are distinguished by a set of “immediate early gene products” that accumulate in response to activated ERK (Murphy et al., 2004; Murphy et al., 2002). When ERK activation is transient, gene products such as c-Fos are induced but then undergo rapid degradation. When ERK levels are persistent, however, newly synthesized c-Fos is directly phosphorylated by the still-active kinase which stabilizes c-Fos in the nucleus. Many of these immediate early gene products are transcription factors that control cell cycle progression and other cell fate expression programs and possess ERK docking sites (Amit et al., 2007; Murphy et al., 2004). Thus, a feed-forward loop comprised of a fast arm (ERK activation) and a slow arm (c-Fos accumulation) serves as a persistence detector for the duration of ERK activation (Figure 5C). More recent work in the ERK system has shown that these two arms of the system act not only at different time scales, but also in different compartments of the cell (Nakakuki et al., 2010). Thus, ERK dynamics are decoded by a finely tuned spatiotemporal network controlling cell fate decisions.
The second example of a specific network structure that decodes dynamics was found to control the inflammatory response to TLR4 signaling (Litvak et al., 2009). Expression of key inflammatory response genes such as interleukin 6 (Il6) requires persistent TLR4 activation, whereas transient TLR4 stimulation is effectively filtered out. Using a time series of gene expression profiles in response to the TLR4-stimulating agonist LPS, two waves of transcription were identified in which a pair of gene products in the first wave, NF-κB and ATF3, were found to control expression of an inflammatory regulator in the second cluster, C/EBPδ. Persistent activation of NF-κB induced expression of C/EBPδ, which regulates itself to provide strong positive feedback. In addition, C/EBPδ synergizes with NF-κB to allow productive expression of Il6 and other inflammation genes (Figure 5D). This sophisticated decoding mechanism controls not only Il6 but tens of additional genes associated with host defense against infection. (Litvak et al., 2009).
Notably, both the ERK and TLR4 decoding networks involve some type of a feed-forward network structure. It is possible that a feed-forward loop motif may also decode p53 dynamics to control activation of senescence (Figure 4D). In this scenario, one of p53’s target genes might serve as an intermediate factor that is required for activating senescence with p53. If such a factor decays with a time scale close to the time scale of the pulses, it will not accumulate during the pulses but only during a sustained p53 response, resulting in senescence. This mechanism would explain the accelerated expression of senescence genes under sustained p53 signaling (Purvis et al., 2012). Identification of such a factor, however, will require characterizing the kinetics of p53 target gene induction in combination with knock down studies to identify which transcripts are required for expression of key senescence markers.
Decoding of temporal patterns is not limited to intracellular signals. A recent study proposes a model for how different temporal patterns of insulin are decoded by the AKT signaling network in insulin-sensing cells such as those found in the liver (Kubota et al., 2012). They first noted that intracellular AKT activation follows the same dynamical trends as external insulin levels. By subjecting cells to different dynamical patterns of insulin, they identified downstream effectors that decode different features of the temporal profile of AKT. These results are consistent with a computational model in which different kinetics and connectivity within the signaling network allow each molecule to detect specific parts of the temporal profile. Although perturbation experiments will be necessary to validate this mechanism, the study provides an attractive model in which different dynamical patterns of insulin release are translated into appropriate metabolic responses. For example, ribosomal protein S6 kinase (S6K), which is involved in protein synthesis, responded to the transient insulin response that might appear after a meal. Glucose-6-phosphatase (G6Pase), which is involved in gluconeogenesis, responds to low insulin concentrations that may be present during fasting.
The identification of molecular circuits that decode signaling dynamics remains a major challenge for the field. Decoding mechanisms promise to provide critical answers about the function of temporal signals since they represent the connection between signal patterns and functional response (Behar and Hoffmann, 2010). Computational approaches have been helpful in understanding the connection between topology, dynamics and function. For example, Ma et al. performed a computational search for all possible three-node enzyme network topologies to identify those that could achieve biochemical adaptation, a dynamical response that returns to baseline levels regardless of stimulus strength (Ma et al., 2009). A similar approach was applied to identify networks capable of achieving other emergent behaviors such as interpreting morphogen gradients (Cotterell and Sharpe, 2010). In a similar vein, Modular Response Analysis (Kholodenko et al., 2002), a method for extracting the strength and topology of dynamical subnetworks, was used to identify structural differences between NGF- and EGF-induced MAPK network topology (Santos et al., 2007). Such approaches are a valuable resource because they help narrow down the search for molecular participants that may regulate and interpret signaling dynamics.
Functions Achieved through Modulation of Signaling Dynamics
The examples presented thus far suggest that controlling the temporal behavior of signaling molecules may represent a unique signaling strategy for cells. For example, the conversion of stimulus strength to signal duration, as shown for Msn2 and p53, may be a general feature of cell signaling networks. By converting stimulus dose to signal duration, signaling networks can detect a greater range of stimulus concentrations even beyond the apparent saturation limit (Behar et al., 2008). However, there is indication that the full scope of functionality provided by signaling dynamics remains to be discovered. We now present some recent examples that illustrate the rich functional behaviors enabled by controlling signaling dynamics.
A highlighted example in the study of temporal behaviors is the manner in which transcription factor dynamics may control gene expression. In the canonical model of transcriptional activation, expression of target genes is controlled by the abundance of the transcription factor, usually with a Hill-like or linear dose-response curve (Figure 6A). For transcription factors with multiple gene targets, an increase in transcription factor levels will have different effects on each promoter since, in general, the size and shape of these response curves differs for each promoter. However, by controlling the frequency rather than the absolute level of a transcription factor, cells work within the same range of concentration and thus have a consistent effect on target promoters. This allows co-regulated genes to be expressed in the same relative proportion regardless of promoter affinities (Figure 6A). Such behavior was discovered by studying the dynamics of the yeast transcription factor Crz1, which shows bursts of nuclear localization in response to calcium (Cai et al., 2008). The concentration of calcium controls the frequency of Crz1 bursts—an analog-to-digital conversion reminiscent of the yeast response to glucose limitation (Figure 2C). Further examination showed that the frequency of Crz1 activation ensures that target genes are transcribed in the same proportion regardless of promoter affinities for Crz1 (Figure 6A).
Figure 6. Specific control mechanisms achieved through modulation of dynamics.
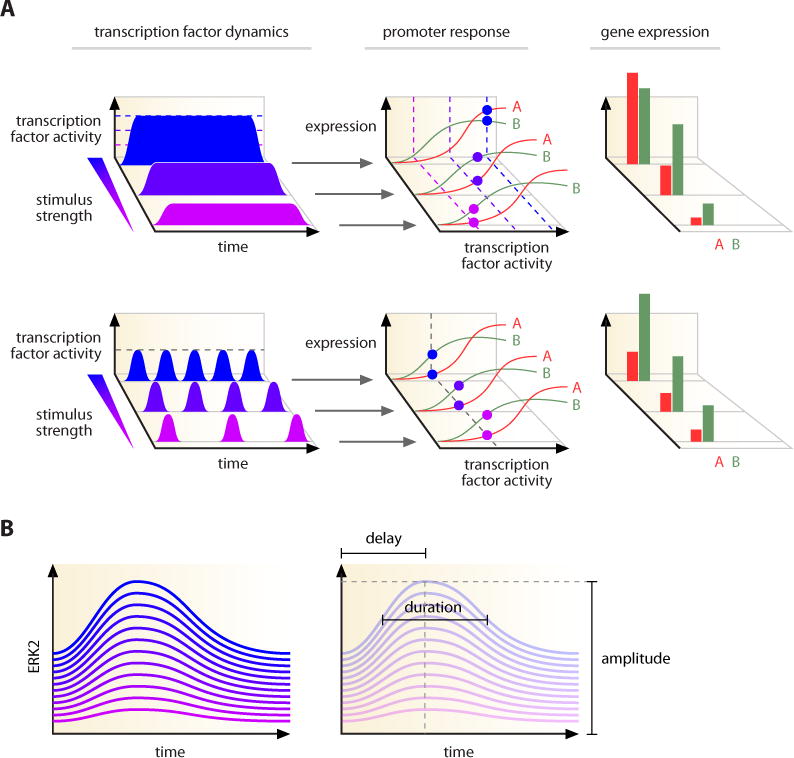
(A) (top) Different amplitudes of a transcription factor lead to different expression of target genes depending on their promoter response curves. Different amplitudes of the transcription factor are marked by purple (lowest) to blue (highest) dotted lines. Promoter response curves for two hypothetical genes, A and B, are shown as red and green lines. (bottom) Frequency modulated transcription factor dynamics maintains relative proportion of target gene expression. Regardless of stimulus strength, transcription factor activity reaches the same level (gray dotted line) and therefore activates target gene promoters at the same location in the response curves. Stimulus strength affects the frequency of the transcription factor activation; higher frequency (blue) will strike the promoters more often than lower frequency activation (purple). This leads to the accumulation of target genes at the same relative proportion (right panel). See main text and (Cai et al., 2008) for further details.
(B) Timing and fold change of ERK2 response is more conserved between individual cells than absolute levels. Individual cells vary considerably in the absolute levels of ERK2 under basal conditions as well as after stimulation with EGF. Certain parameters that describe the timing of the response, however, show less variability. The delay until peak activation, signal duration, and fold change are among the most conserved parameters. See (Cohen-Saidon et al., 2009).
The ability to measure not only the temporal features of a signal but also its precise intracellular location has shown that dynamics sometimes operate in specific parts of a cell. A prominent example of spatiotemporal signaling occurs in the Msn2 and NF-κB pathways, in which the transcription factor is regulated by shuttled in and out of the nucleus. This use of compartmentalization stands in contrast to the p53 network in which pulsatile dynamics are governed by repeated accumulation and degradation of total protein levels. Additional studies have shown how dynamics play a role in spatially distributed intracellular networks (Kholodenko et al., 2010 and references therein). In rat hippocampal neurons, for example, the long dendritic spines allow accumulation of membrane-generated signals. With appropriately tuned temporal behavior, information about the spatial structure of the spine can be transmitted to distal parts of the cell (Neves et al., 2008). Similarly, gradients in signal concentration have been shown to control tip project in mating yeast (Maeder et al., 2007) and the diffusion of Ras from the plasma membrane (Chandra et al., 2012). In each instance, the interplay between intracellular location and temporal behavior are necessary to carry out specific signaling processes.
Dynamics can also reflect information about the resting state of a cell. Studies on the dynamics of ERK2 translocation in individual human cells revealed a large variation in basal ERK2 nuclear levels (Cohen-Saidon et al., 2009). Upon EGF stimulation, cells also showed widely varying peak levels of ERK2. However, when the fold change in ERK was quantified relative to the starting levels, the dynamics of the responses were very similar (Figure 6B). This presents an elegant example for how cells can achieve a standardized response in the background on natural noise.
Pulsatile patterns can function as temporal rulers in which each pulse represents a fixed length of time. This phenomenon was demonstrated in the study of spore formation in Bacillus subtilis (Levine et al., 2012). The bacterium can defer sporulation for extended time periods by first undergoing multiple rounds of growth and proliferation. How does the bacterium measure this length of time? Time-lapse imaging of the master-regulator Spo0A in individual cells revealed that the deferral time is controlled by a positive feedback loop that allows Spo0A to accumulate to a critical level over multiple cell cycle generations. This dynamical behavior may increase the bacterium’s chance of survival, perhaps by allowing the accumulation of additional nutrients or the proliferation of additional offspring before sporulation occurs.
Conclusions and Future Directions
We have presented a thematic overview of how cells store information in temporal signaling patterns, focusing on functional outcomes connected to each dynamical pattern. In each of these cases, however, dynamics probably represent only one layer of regulation within a complex signaling response that executes different cellular outcomes. In fact, we have seen that different dynamical patterns arise because of differences in network structure or the kinetics of individual molecular interactions. Thus, changes in the identity and strength of other pathway activities, such as posttranslational modifications, are likely to work with dynamics to induce stimulus-specific responses.
A better understanding of how signaling dynamics are regulated and how they affect cellular responses may provide new insights for manipulating them in a controlled way. In turn, this may enable new pharmacological strategies for altering cell fate. Oscillations of p53, for example, have been shown to occur in mice after total body irradiation (Hamstra et al., 2006). In principle, the same perturbation of p53 pulses used to induce senescence in cell culture (Purvis et al., 2012) could be administered in vivo. This may be useful in situations in which the dynamics of healthy and diseases cells are expected to differ (e.g., (Francisco et al., 2008)). In this scenario, dynamics represent the phenotype that distinguishes cells and may be targeted by small molecules or other perturbations.
A major focus of this review was the use of perturbations to control dynamical patterns. Such strategies hold promise as an engineering tool for use in synthetic biology. There has been recent work demonstrating light-based perturbations to cellular dynamics (Levskaya et al., 2009; Toettcher et al., 2011), which could provide exceptionally noninvasive and precise control over temporal signaling.
The number of studies that are focused on the dynamics of biological responses is growing and well exceeds the number of studies we could mention in this review. As fluorescent labeling and time-lapse technology become better and cheaper, it may soon become clear that the vast majority of signals (if not all of them) are transferred through specific dynamical patterns of their components. If so, the study of signaling dynamics promises to provide rich and complex insights about circuit structure and function that could not be otherwise revealed. This is the case for calcium, p53, Msn2, NF-κB, and nearly all other systems mentioned in this review; study of their dynamical properties revealed previously unappreciated regulatory roles. The same applies to the bird’s wing: the structure of the wing may give excellent clues about its potential function, but there is no substitute for observing the fluid motion of a wing in flight.
Acknowledgments
We thank S. J. Rahi, W. Forrester, L. Murphy, R. Dolmetsch, A. Hoffmann, S. Santos, A. Aderam, V. Litvak, M. R. White, and all members of our laboratory for helpful discussions, comments and reference suggestions. We thank our colleagues and friends at Harvard Medical School and the Department of Systems Biology for creating an inspiring environment that encourages thinking about temporal aspects of cell signaling. This research was supported by the Novartis Institutes for Biomedical Research, the National Institutes of Health grant GM083303 and fellowship K99-GM102372 (J.E.P.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albeck JG, Burke JM, Spencer SL, Lauffenburger DA, Sorger PK. Modeling a snap-action, variable-delay switch controlling extrinsic cell death. PLoS Biol. 2008;6:2831–2852. doi: 10.1371/journal.pbio.0060299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alon U. Network motifs: theory and experimental approaches. Nat Rev Genet. 2007;8:450–461. doi: 10.1038/nrg2102. [DOI] [PubMed] [Google Scholar]
- Amit I, Citri A, Shay T, Lu Y, Katz M, Zhang F, Tarcic G, Siwak D, Lahad J, Jacob-Hirsch J, et al. A module of negative feedback regulators defines growth factor signaling. Nat Genet. 2007;39:503–512. doi: 10.1038/ng1987. [DOI] [PubMed] [Google Scholar]
- Ashall L, Horton CA, Nelson DE, Paszek P, Harper CV, Sillitoe K, Ryan S, Spiller DG, Unitt JF, Broomhead DS, et al. Pulsatile stimulation determines timing and specificity of NF-kappaB-dependent transcription. Science (New York, NY) 2009;324:242–246. doi: 10.1126/science.1164860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakstad D, Adamson A, Spiller DG, White MR. Quantitative measurement of single cell dynamics. Curr Opin Biotechnol. 2012;23:103–109. doi: 10.1016/j.copbio.2011.11.007. [DOI] [PubMed] [Google Scholar]
- Barken D, Wang CJ, Kearns J, Cheong R, Hoffmann A, Levchenko A. Comment on “Oscillations in NF-kappaB signaling control the dynamics of gene expression”. Science (New York, NY) 2005;308:52. doi: 10.1126/science.1107904. author reply 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basak S, Behar M, Hoffmann A. Lessons from mathematically modeling the NF-kappaB pathway. Immunol Rev. 2012;246:221–238. doi: 10.1111/j.1600-065X.2011.01092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor E, Loewer A, Mock C, Lahav G. Stimulus-dependent dynamics of p53 in single cells. Mol Syst Biol. 2011;7:488. doi: 10.1038/msb.2011.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batchelor E, Mock CS, Bhan I, Loewer A, Lahav G. Recurrent initiation: a mechanism for triggering p53 pulses in response to DNA damage. Molecular cell. 2008;30:277–289. doi: 10.1016/j.molcel.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar M, Dohlman HG, Elston TC. Kinetic insulation as an effective mechanism for achieving pathway specificity in intracellular signaling networks. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:16146–16151. doi: 10.1073/pnas.0703894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar M, Hao N, Dohlman HG, Elston TC. Dose-to-duration encoding and signaling beyond saturation in intracellular signaling networks. PLoS Comput Biol. 2008;4:e1000197. doi: 10.1371/journal.pcbi.1000197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behar M, Hoffmann A. Understanding the temporal codes of intra-cellular signals. Curr Opin Genet Dev. 2010;20:684–693. doi: 10.1016/j.gde.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birtwistle MR, Rauch J, Kiyatkin A, Aksamitiene E, Dobrzynski M, Hoek JB, Kolch W, Ogunnaike BA, Kholodenko BN. Emergence of bimodal cell population responses from the interplay between analog single-cell signaling and protein expression noise. BMC Syst Biol. 2012;6:109. doi: 10.1186/1752-0509-6-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai L, Dalal CK, Elowitz MB. Frequency-modulated nuclear localization bursts coordinate gene regulation. Nature. 2008;455:485–490. doi: 10.1038/nature07292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandra A, Grecco HE, Pisupati V, Perera D, Cassidy L, Skoulidis F, Ismail SA, Hedberg C, Hanzal-Bayer M, Venkitaraman AR, et al. The GDI-like solubilizing factor PDEdelta sustains the spatial organization and signalling of Ras family proteins. Nat Cell Biol. 2012;14:148–158. doi: 10.1038/ncb2394. [DOI] [PubMed] [Google Scholar]
- Chatterjee MS, Purvis JE, Brass LF, Diamond SL. Pairwise agonist scanning predicts cellular signaling responses to combinatorial stimuli. Nature biotechnology. 2010;28:727–732. doi: 10.1038/nbt.1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheong R, Bergmann A, Werner SL, Regal J, Hoffmann A, Levchenko A. Transient IkappaB kinase activity mediates temporal NF-kappaB dynamics in response to a wide range of tumor necrosis factor-alpha doses. J Biol Chem. 2006;281:2945–2950. doi: 10.1074/jbc.M510085200. [DOI] [PubMed] [Google Scholar]
- Cohen-Saidon C, Cohen AA, Sigal A, Liron Y, Alon U. Dynamics and variability of ERK2 response to EGF in individual living cells. Molecular cell. 2009;36:885–893. doi: 10.1016/j.molcel.2009.11.025. [DOI] [PubMed] [Google Scholar]
- Cohen AA, Geva-Zatorsky N, Eden E, Frenkel-Morgenstern M, Issaeva I, Sigal A, Milo R, Cohen-Saidon C, Liron Y, Kam Z, et al. Dynamic proteomics of individual cancer cells in response to a drug. Science (New York, NY) 2008;322:1511–1516. doi: 10.1126/science.1160165. [DOI] [PubMed] [Google Scholar]
- Cotterell J, Sharpe J. An atlas of gene regulatory networks reveals multiple three-gene mechanisms for interpreting morphogen gradients. Mol Syst Biol. 2010;6:425. doi: 10.1038/msb.2010.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covert MW, Leung TH, Gaston JE, Baltimore D. Achieving stability of lipopolysaccharide-induced NF-kappaB activation. Science (New York, NY) 2005;309:1854–1857. doi: 10.1126/science.1112304. [DOI] [PubMed] [Google Scholar]
- Darmon M, Brachet P, Da Silva LH. Chemotactic signals induce cell differentiation in Dictyostelium discoideum. Proceedings of the National Academy of Sciences of the United States of America. 1975;72:3163–3166. doi: 10.1073/pnas.72.8.3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI. Differential activation of transcription factors induced by Ca2+ response amplitude and duration. Nature. 1997;386:855–858. doi: 10.1038/386855a0. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- Ferrell JE, Jr, Machleder EM. The biochemical basis of an all-or-none cell fate switch in Xenopus oocytes. Science (New York, NY) 1998;280:895–898. doi: 10.1126/science.280.5365.895. [DOI] [PubMed] [Google Scholar]
- Francisco DC, Peddi P, Hair JM, Flood BA, Cecil AM, Kalogerinis PT, Sigounas G, Georgakilas AG. Induction and processing of complex DNA damage in human breast cancer cells MCF-7 and nonmalignant MCF-10A cells. Free radical biology & medicine. 2008;44:558–569. doi: 10.1016/j.freeradbiomed.2007.10.045. [DOI] [PubMed] [Google Scholar]
- Garmaroudi FS, Marchant D, Si X, Khalili A, Bashashati A, Wong BW, Tabet A, Ng RT, Murphy K, Luo H, et al. Pairwise network mechanisms in the host signaling response to coxsackievirus B3 infection. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:17053–17058. doi: 10.1073/pnas.1006478107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geva-Zatorsky N, Dekel E, Cohen AA, Danon T, Cohen L, Alon U. Protein dynamics in drug combinations: a linear superposition of individual-drug responses. Cell. 2010;140:643–651. doi: 10.1016/j.cell.2010.02.011. [DOI] [PubMed] [Google Scholar]
- Gotoh Y, Nishida E, Yamashita T, Hoshi M, Kawakami M, Sakai H. Microtubule-associated-protein (MAP) kinase activated by nerve growth factor and epidermal growth factor in PC12 cells. Identity with the mitogen-activated MAP kinase of fibroblastic cells. Eur J Biochem. 1990;193:661–669. doi: 10.1111/j.1432-1033.1990.tb19384.x. [DOI] [PubMed] [Google Scholar]
- Grammer TC, Blenis J. Evidence for MEK-independent pathways regulating the prolonged activation of the ERK-MAP kinases. Oncogene. 1997;14:1635–1642. doi: 10.1038/sj.onc.1201000. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985;260:3440–3450. [PubMed] [Google Scholar]
- Hamstra DA, Bhojani MS, Griffin LB, Laxman B, Ross BD, Rehemtulla A. Real-time evaluation of p53 oscillatory behavior in vivo using bioluminescent imaging. Cancer research. 2006;66:7482–7489. doi: 10.1158/0008-5472.CAN-06-1405. [DOI] [PubMed] [Google Scholar]
- Hao N, O’Shea EK. Signal-dependent dynamics of transcription factor translocation controls gene expression. Nat Struct Mol Biol. 2012;19:31–39. doi: 10.1038/nsmb.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heemskerk JW, Willems GM, Rook MB, Sage SO. Ragged spiking of free calcium in ADP-stimulated human platelets: regulation of puff-like calcium signals in vitro and ex vivo. J Physiol. 2001;535:625–635. doi: 10.1111/j.1469-7793.2001.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science (New York, NY) 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- Jazayeri A, Falck J, Lukas C, Bartek J, Smith GC, Lukas J, Jackson SP. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat Cell Biol. 2006;8:37–45. doi: 10.1038/ncb1337. [DOI] [PubMed] [Google Scholar]
- Kholodenko BN, Hancock JF, Kolch W. Signalling ballet in space and time. Nature reviews. 2010;11:414–426. doi: 10.1038/nrm2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kholodenko BN, Kiyatkin A, Bruggeman FJ, Sontag E, Westerhoff HV, Hoek JB. Untangling the wires: a strategy to trace functional interactions in signaling and gene networks. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:12841–12846. doi: 10.1073/pnas.192442699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubota H, Noguchi R, Toyoshima Y, Ozaki Y, Uda S, Watanabe K, Ogawa W, Kuroda S. Temporal Coding of Insulin Action through Multiplexing of the AKT Pathway. Molecular cell. 2012;46:820–832. doi: 10.1016/j.molcel.2012.04.018. [DOI] [PubMed] [Google Scholar]
- Lahav G, Rosenfeld N, Sigal A, Geva-Zatorsky N, Levine AJ, Elowitz MB, Alon U. Dynamics of the p53-Mdm2 feedback loop in individual cells. Nat Genet. 2004;36:147–150. doi: 10.1038/ng1293. [DOI] [PubMed] [Google Scholar]
- Lee TK, Denny EM, Sanghvi JC, Gaston JE, Maynard ND, Hughey JJ, Covert MW. A noisy paracrine signal determines the cellular NF-kappaB response to lipopolysaccharide. Sci Signal. 2009;2:ra65. doi: 10.1126/scisignal.2000599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev Bar-Or R, Maya R, Segel LA, Alon U, Levine AJ, Oren M. Generation of oscillations by the p53-Mdm2 feedback loop: a theoretical and experimental study. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11250–11255. doi: 10.1073/pnas.210171597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine JH, Fontes ME, Dworkin J, Elowitz MB. Pulsed feedback defers cellular differentiation. PLoS Biol. 2012;10:e1001252. doi: 10.1371/journal.pbio.1001252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levskaya A, Weiner OD, Lim WA, Voigt CA. Spatiotemporal control of cell signalling using a light-switchable protein interaction. Nature. 2009;461:997–1001. doi: 10.1038/nature08446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- Litvak V, Ramsey SA, Rust AG, Zak DE, Kennedy KA, Lampano AE, Nykter M, Shmulevich I, Aderem A. Function of C/EBPdelta in a regulatory circuit that discriminates between transient and persistent TLR4-induced signals. Nat Immunol. 2009;10:437–443. doi: 10.1038/ni.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Trusina A, El-Samad H, Lim WA, Tang C. Defining network topologies that can achieve biochemical adaptation. Cell. 2009;138:760–773. doi: 10.1016/j.cell.2009.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeder CI, Hink MA, Kinkhabwala A, Mayr R, Bastiaens PI, Knop M. Spatial regulation of Fus3 MAP kinase activity through a reaction-diffusion mechanism in yeast pheromone signalling. Nat Cell Biol. 2007;9:1319–1326. doi: 10.1038/ncb1652. [DOI] [PubMed] [Google Scholar]
- Malcuit C, Kurokawa M, Fissore RA. Calcium oscillations and mammalian egg activation. J Cell Physiol. 2006;206:565–573. doi: 10.1002/jcp.20471. [DOI] [PubMed] [Google Scholar]
- Marshall CJ. Specificity of receptor tyrosine kinase signaling: transient versus sustained extracellular signal-regulated kinase activation. Cell. 1995;80:179–185. doi: 10.1016/0092-8674(95)90401-8. [DOI] [PubMed] [Google Scholar]
- Murphy LO, MacKeigan JP, Blenis J. A network of immediate early gene products propagates subtle differences in mitogen-activated protein kinase signal amplitude and duration. Molecular and cellular biology. 2004;24:144–153. doi: 10.1128/MCB.24.1.144-153.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy LO, Smith S, Chen RH, Fingar DC, Blenis J. Molecular interpretation of ERK signal duration by immediate early gene products. Nat Cell Biol. 2002;4:556–564. doi: 10.1038/ncb822. [DOI] [PubMed] [Google Scholar]
- Nakakuki T, Birtwistle MR, Saeki Y, Yumoto N, Ide K, Nagashima T, Brusch L, Ogunnaike BA, Okada-Hatakeyama M, Kholodenko BN. Ligand-specific c-Fos expression emerges from the spatiotemporal control of ErbB network dynamics. Cell. 2010;141:884–896. doi: 10.1016/j.cell.2010.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson DE, Ihekwaba AE, Elliott M, Johnson JR, Gibney CA, Foreman BE, Nelson G, See V, Horton CA, Spiller DG, et al. Oscillations in NF-kappaB signaling control the dynamics of gene expression. Science (New York, NY) 2004;306:704–708. doi: 10.1126/science.1099962. [DOI] [PubMed] [Google Scholar]
- Neves SR, Tsokas P, Sarkar A, Grace EA, Rangamani P, Taubenfeld SM, Alberini CM, Schaff JC, Blitzer RD, Moraru II, et al. Cell shape and negative links in regulatory motifs together control spatial information flow in signaling networks. Cell. 2008;133:666–680. doi: 10.1016/j.cell.2008.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen TT, Scimeca JC, Filloux C, Peraldi P, Carpentier JL, Van Obberghen E. Co-regulation of the mitogen-activated protein kinase, extracellular signal-regulated kinase 1, and the 90-kDa ribosomal S6 kinase in PC12 cells. Distinct effects of the neurotrophic factor, nerve growth factor, and the mitogenic factor, epidermal growth factor. J Biol Chem. 1993;268:9803–9810. [PubMed] [Google Scholar]
- Purvis JE, Karhohs KW, Mock C, Batchelor E, Loewer A, Lahav G. p53 dynamics control cell fate. Science (New York, NY) 2012;336:1440–1444. doi: 10.1126/science.1218351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SD, Verveer PJ, Bastiaens PI. Growth factor-induced MAPK network topology shapes Erk response determining PC-12 cell fate. Nat Cell Biol. 2007;9:324–330. doi: 10.1038/ncb1543. [DOI] [PubMed] [Google Scholar]
- Sasagawa S, Ozaki Y, Fujita K, Kuroda S. Prediction and validation of the distinct dynamics of transient and sustained ERK activation. Nat Cell Biol. 2005;7:365–373. doi: 10.1038/ncb1233. [DOI] [PubMed] [Google Scholar]
- Sung MH, Salvatore L, De Lorenzi R, Indrawan A, Pasparakis M, Hager GL, Bianchi ME, Agresti A. Sustained oscillations of NF-kappaB produce distinct genome scanning and gene expression profiles. PLoS One. 2009;4:e7163. doi: 10.1371/journal.pone.0007163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay S, Hughey JJ, Lee TK, Lipniacki T, Quake SR, Covert MW. Single-cell NF-kappaB dynamics reveal digital activation and analogue information processing. Nature. 2010;466:267–271. doi: 10.1038/nature09145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toettcher JE, Gong D, Lim WA, Weiner OD. Light-based feedback for controlling intracellular signaling dynamics. Nat Methods. 2011;8:837–839. doi: 10.1038/nmeth.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar C, Rosinski J, Filipovic Z, Higgins B, Kolinsky K, Hilton H, Zhao X, Vu BT, Qing W, Packman K, et al. Small-molecule MDM2 antagonists reveal aberrant p53 signaling in cancer: implications for therapy. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:1888–1893. doi: 10.1073/pnas.0507493103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traverse S, Gomez N, Paterson H, Marshall C, Cohen P. Sustained activation of the mitogen-activated protein (MAP) kinase cascade may be required for differentiation of PC12 cells. Comparison of the effects of nerve growth factor and epidermal growth factor. Biochem J. 1992;288(Pt 2):351–355. doi: 10.1042/bj2880351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyas L, Brophy VA, Pope A, Rivett AJ, Tavare JM. Rapid caspase-3 activation during apoptosis revealed using fluorescence-resonance energy transfer. EMBO Rep. 2000;1:266–270. doi: 10.1093/embo-reports/kvd050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch CM, Elliott H, Danuser G, Hahn KM. Imaging the coordination of multiple signalling activities in living cells. Nature reviews. 2011;12:749–756. doi: 10.1038/nrm3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner SL, Barken D, Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science (New York, NY) 2005;309:1857–1861. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]
- Werner SL, Kearns JD, Zadorozhnaya V, Lynch C, O’Dea E, Boldin MP, Ma A, Baltimore D, Hoffmann A. Encoding NF-kappaB temporal control in response to TNF: distinct roles for the negative regulators IkappaBalpha and A20. Genes Dev. 2008;22:2093–2101. doi: 10.1101/gad.1680708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yissachar N, Sharar Fischler T, Cohen AA, Reich-Zeliger S, Russ D, Shifrut E, Porat Z, Friedman N. Dynamic response diversity of NFAT isoforms in individual living cells. Mol Cell. 2013;49:322–330. doi: 10.1016/j.molcel.2012.11.003. [DOI] [PubMed] [Google Scholar]
- Yosef N, Regev A. Impulse control: temporal dynamics in gene transcription. Cell. 2011;144:886–896. doi: 10.1016/j.cell.2011.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]


