Abstract
Benign breast biopsies with concurrent multiple benign lesions with different histopathologic diagnoses were termed heterogeneous benign breast disease (HBBD). Multiplicity of benign breast disease (BBD) lesions in a biopsy is a risk factor for progression to breast cancer (BC). Elucidation of the biological characteristics and clinical implications of HBBD may also be relevant to the refinement of risks for BC in women with a BBD diagnosis. In this study, we investigated the association of HBBD with histopathology, age, and ethnicity. A cohort of 4,341 women, 1,208 African Americans and 3,133 Caucasians, diagnosed with BBD, was identified after examination of an excisional breast biopsy. BBD biopsies were categorized as nonproliferative (NP, low risk or risk 1 lesions), proliferative without atypia (P, intermediate risk or risk 2 lesions), and proliferative with atypia (AH, high risk or risk 3 lesions). A BBD biopsy with only a single BBD lesion was termed simple BBD (SBBD). BBD biopsies with multiple lesions were further classified as single level HBBD (SL-HBBD) if the concurrent lesions were within the same risk level, or as multiple level HBBD (ML-HBBD) if lesions fell into more than one risk group. In this cohort, 69% of women with a BBD diagnosis fit the HBBD criteria. Among women with HBBD, ML-HBBD was almost three times more prevalent than SL-HBBD and was significantly more likely to be composed of risk 2 and risk 3 lesions. The likelihood of HBBD was 57% higher in Caucasian American women than in African American women with BBD (OR 1.57; 95% CI 1.37, 1.81). The average lesion number in HBBD was directly proportional to increasing lesion risk (p < 0.001). Compared to women with risk 1 lesions, the likelihood of HBBD was 5.59 (95% CI 4.85 to 6.44) and 17.0 (95% CI 10.2 to 28.5) times higher when risk 2 and risk 3 lesions, respectively, were present. Women in the age range of 46–55 years and >55 years had a 3.12 (95% CI 2.59, 3.75) and a 2.28 (95% CI 1.94, 2.68) fold higher likelihood of HBBD compared to those ≤45 years. Significant interaction was found between concurrent lesion levels and age (p < 0.01). The likelihood of HBBD was considerably higher across all age groups for risk 3 lesions. Compared to the reference (risk 1, age ≤45), the likelihood of HBBD for risk 2 lesions was 4.4 times greater (95% CI 3.70, 5.33) in women ≤45 years, but that likelihood increased to 17.6 (95% CI 12.8, 24.2) and 13.4 (95% CI 10.1, 17.9) times in women of 46–55 and >55 years, respectively. HBBD is more prevalent in Caucasian American women than in African American women. Women with higher risk BBD lesions are more likely to have HBBD. Lesion number and higher risk BBD lesions are significantly correlated with ML-HBBD. Additionally, the associations of HBBD and lesion risk level are modified by age.
INTRODUCTION
The breast is a preferential site for the occurrence of benign lesions with a wide range of histopathology characteristics. Epidemiological investigations indicate that more than 50% of all women will have benign breast disease (BBD) beyond age 20 (1). It has long been recognized that some BBD lesions will eventually develop into breast cancer (2–6). Dupont and Page (7) examined 10,366 breast biopsies and classified BBD lesions into three pathologic categories: nonproliferative, proliferative without atypia, and atypical hyperplasia. The risk of breast cancer has been associated with specific histopathologic characteristics of BBD (1, 4, 8–11). An increased cancer risk was found in women with proliferative lesions without atypia or with atypical hyperplasia (6, 7, 10–13). Accordingly, there is growing interest in BBD because of its prevalence and the risk of developing breast cancer.
There is an accumulating body of evidence to support multiplicity of lesions in a BBD biopsy as a relevant risk factor for subsequent progression of BC from BBD (8, 10, 14). Jacobs et al (14) reported that the risk for breast cancer from radial scars was increased when other proliferative lesions were also present, irrespective of association with atypical hyperplasia. Wang et al (8) reported that 38% of women with low risk BBD had multiple lesions, but did not take note of associated risk factors. Worsham et al (10) reported that concurrent multiple nonproliferative or proliferative BBD lesions with or without atypia in a BBD biopsy are significant predictors of risk for progression of BBD to breast cancer.
Studies on BBD have focused on mainly three broad categories of lesions: nonproliferative, proliferative, or proliferative with atypia, without addressing the issue of the contribution of concurrent multiple BBD lesions and their corresponding histopathology makeup. Thus, there is a dearth of information regarding BBD biopsy heterogeneity in the context of lesion multiplicity, race, and composition.
We describe a multi-ethnic, primary care BBD cohort, in which approximately 70% of women with a BBD excisional biopsy demonstrated multiple lesions. Based on the high prevalence of multiple BBD lesions reported here and their independent contribution as a risk factor for developing breast cancer (10), multiplicity of BBD lesions in a BBD biopsy has clinical significance and merits thorough investigation. For this study, we characterized through microscopic review, 18 distinct BBD diagnoses within three risk categories (Table 1), and assigned the term heterogeneous benign breast disease (HBBD) to an excision biopsy with multiple lesions of different histopathologic diagnoses. Thus, this study provides an in-depth portrait of the histopathologic make-up of BBD biopsies in the context of concurrent multiple BBD lesions and their resultant risk-level outcomes. The prevalence of HBBD and its association with age and race are examined. Elucidation of the biological characteristics and clinical implications of multiple BBD lesions is relevant to the refinement of risks for BC in women with a BBD diagnosis.
Table 1.
Distribution and frequency of the microscopic spectrum of benign breast lesions in the study cohort of 4,341 women
| Benign Breast Disease Risk Level Category | Lesions | Number (%) |
|---|---|---|
| Risk 1: Nonproliferative | 7680 (63.0) | |
| Simple apocrine metaplasia | 2251 (18.5) | |
| Cysts | 2231 (18.3) | |
| Periductal mastitis/duct ectasia | 437 (3.59) | |
| Mastitis | 174 (1.43) | |
| Fibrosis | 1568 (12.9) | |
| Squamous metaplasia | 15 (0.12) | |
| Fibroadenoma | 1001 (8.21) | |
| Other nonproliferative lesions | 3 (0.02) | |
| Risk 2: Proliferative without atypia | 4233 (34.7) | |
| Simple adenosis | 724 (5.94) | |
| Sclerosing adenosis | 721 (5.92) | |
| Apocrine adenosis | 29 (0.24) | |
| Hyperplasia without atypia (usual type) | 1528 (12.5) | |
| Hyperplasia without atypia (apocrine type) | 125 (1.03) | |
| Papilloma | 354 (2.90) | |
| Radial scar | 193 (1.58) | |
| Associated Fibroadenoma | 559 (4.59) | |
| hyperplasia associated | 310 | |
| adenosis associated | 173 | |
| hyperplasia & adenosis associated | 76 | |
| Risk 3: Proliferative with atypia | 273 (2.4) | |
| Atypical ductal hyperplasia (ADH) | 135 (1.11) | |
| fibroadenoma associated | 9 | |
| simple adenosis associated | 6 | |
| sclerosing adenosis associated | 3 | |
| papilloma associated | 14 | |
| other lesion associated | 2 | |
| Atypical lobular hyperplasia (ALH) | 64 | |
| simple adenosis associated | 11 | |
| sclerosing adenosis associated | 2 | |
| radial scar associated | 3 | |
| Combination of ADH and ALH (ADH & ALH) | 22 (0.18) | |
| simple adenosis associated | 2 |
Total lesion number = 12,186
MATERIALS AND METHODS
Study Population
The source population consisted of 4,970 women with BBD diagnosed by breast biopsies in the Henry Ford Health System from 1981–1994 (11). During the follow-up period ranging from 8–21 years after the first BBD diagnosis, 197 eligible women with race information and excision biopsies developed breast cancer. The median time from the original biopsy to the diagnosis of breast cancer was 7.19 years (mean 7.61 years; 95% CI for mean: 6.98, 8.24)(10). Approximately 20% of these women had stage 0 or in situ breast cancer at diagnosis, 63% had stage I or higher stage disease, and for 17%, stage could not be determined from either the HFHS tumor registry or from SEER(11).
For women with more than one BBD biopsy, only the first excision biopsy specimens were included in this study. Women with the following characteristics were excluded from our study: 1) ethnicity other than African American or Caucasian; 2) those missing data for ethnicity or age; 3) a biopsy that revealed malignancy, either in situ (LCIS, DCIS) or invasive breast cancer, prior to or within six months of a BBD biopsy, or 4) tissue sample lacking breast parenchyma or any of the BBD lesions listed in Table 1. In addition, needle biopsies were excluded because samples were presumed too small to permit accurate ascertainment of multiple lesions. The final cohort of 4,341 women, 1,208 African Americans and 3,133 Caucasians, aged 18 – 95 years, was the basis for this study.
BBD lesion histopathology criteria
Based on criteria reported elsewhere (7), BBD lesions were divided into three risk categories (10): nonproliferative (NP, low risk or risk level 1), proliferation without atypia (P, intermediate risk, or risk level 2), and proliferation with atypia (AH, high risk, or risk level 3). Microscopically, 18 distinct BBD diagnostic subtypes were identified; they were distributed as follows: 8 subtypes as NP, risk 1 lesions, 8 subtypes as P without AH, risk 2 lesions; and 2 subtypes as P with AH, risk 3 lesions (Table 1). The risk category outcome of a BBD biopsy was decided according to the highest risk lesion(s) within that specimen (hierarchical ranking).
Definition: Heterogeneous benign breast disease (HBBD)
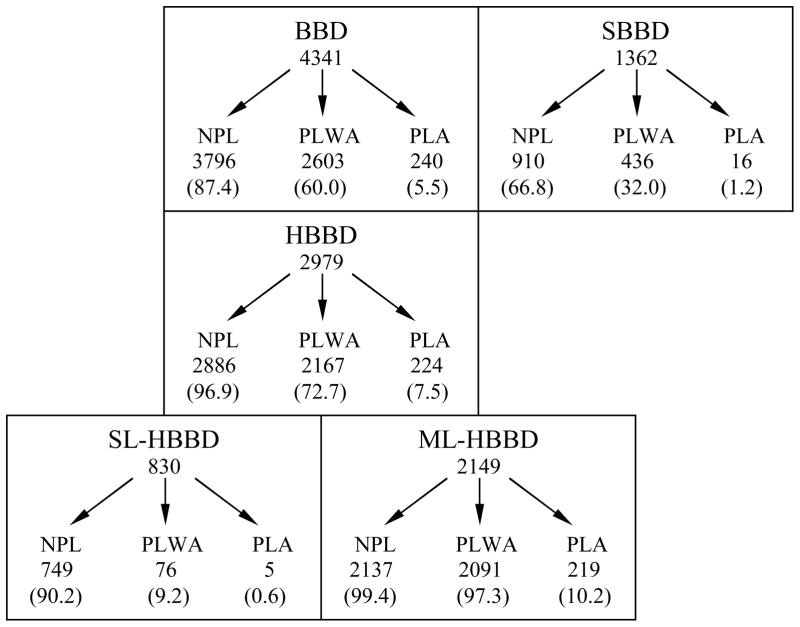
We define heterogeneous BBD (HBBD) as any BBD biopsy containing more than one BBD lesion of different histopathologic diagnoses. A BBD biopsy with only a single BBD lesion is referred to as homogeneous or simple BBD (SBBD). HBBD is further divided into single-level HBBD (SL-HBBD) and multi-level HBBD (ML-HBBD). SL-HBBD biopsies contain more-than-one BBD lesion all within the same risk level category, whereas ML-HBBD biopsies contain multiple lesions of different risk categories (Figure 1). For example, an HBBD biopsy with sclerosing adenosis (risk 2) and atypical hyperplasia (risk 3) would be classed as ML-HBBD, whereas an HBBD biopsy with a cyst and simple apocrine metaplasia, both risk 1 lesions, would be classed as SL-HBBD.
Figure 1. Classification of BBD lesions.
BBD (benign breast disease)
SBBD (simple or homogeneous benign breast disease): Only one BBD lesion is present
HBBD (heterogeneous benign breast disease): Multiple BBD lesions of different pathologic diagnoses
SL-HBBD (Single-level HBBD): Multiple BBD lesions within a single risk level
ML-HBBD (Multi-level HBBD): Multiple BBD lesions with different risk levels
NPL: Non-proliferative lesions
PLWA: Proliferative lesions without atypia
PLA: Proliferative lesions with atypia
Statistical analysis
The associations of HBBD with lesion risk, age, and ethnicity, as well as pair-wise combinations of these variables were examined using logistic-regression analysis. The main effects of each categorized variable and the corresponding interaction terms were included in each model. Age was stratified into ≤45, 46–55, and >55 years. We compared the differences in lesion number in HBBD by risk level, age, and ethnicity using Kruskal-Wallis analysis of variance on ranks and Mann-Whitney rank sum test because these data were not normally distributed. All statistical analyses were performed using the Systat 11 software package (Systat Software, Inc. Richmond, CA).
RESULTS
From the final cohort of 4,341 women, 1,208 African Americans and 3,133 Caucasians, 197 eligible women with race information and excision biopsies developed breast cancer.
There were 12,186 BBD lesions in 4,341 BBD biopsies; their distribution by risk categories is shown in Table 1. Of the individual lesions, 7,680 (62.9%) were nonproliferative, 4,233 (34.7%) were proliferative without atypia, and 273 (2.4%) were proliferative with atypia. The distribution of the BBD biopsies by highest lesion risk among the 4,341 subjects was as follows: 1,659 (38.2%) were risk 1 (nonproliferative); 2,442 (56.3%) were risk 2 (proliferative without atypia), and 240 (5.5%) were risk 3 (proliferative with atypia) (Table 2).
Table 2.
Characteristics of BBD women according to risk level
| All women (n = 4341) | Risk 1 (n = 1659) | Risk 2 (n = 2442) | Risk 3 (n = 240) | |
|---|---|---|---|---|
| Risk categories (%) | 100 | 38.2 | 56.3 | 5.5 |
| Ethnicity - no. of women (%) | ||||
| African American | 1208 (27.8) | 461 (27.8) | 699 (28.6) | 48 (20.0) |
| Caucasian | 3133 (72.2) | 1198 (72.2) | 1743 (71.4) | 192 (80.0) |
| Age - no. of women (%) | ||||
| ≤45 years | 2235 (51.5) | 965 (58.2) | 1208 (49.5) | 62 (25.8) |
| 46–55 years | 952 (21.9) | 272 (16.4) | 602 (24.7) | 78 (32.5) |
| >55 years | 1154 (26.6) | 422 (25.4) | 632 (25.9) | 100 (41.7) |
| Age - mean (SD) | 46.6 (14.3) | 44.7 (15.5) | 47.1 (13.3) | 54.1 (12.2) |
| HBBD-number of specimens (%) | 2979 (68.6) | 749 (45.1) | 2006 (82.1) | 224 (93.3) |
| Lesion Number - mean (SD) | 2.81 (1.73) | 1.66 (0.86) | 3.45 (1.72) | 4.26 (1.87) |
Of the 4,341 BBD biopsies, 1,362 were characterized as SBBD, of which 67% consisted of nonproliferative lesions, 32% of proliferative lesions without atypia, and only 1.2% of proliferative lesions with atypia. The remaining 2,979 biopsies met the criteria of HBBD each with lesions of at least 2 different histopathology diagnoses. Within the total of 10,824 individual BBD lesions diagnosed among the 2,979 HBBD biopsies, 6770 (62.5 %) were non-proliferative, 3797 (35%) were proliferative without atypia, and 257 (2. 4%) were proliferative with atypia. Almost all HBBD biopsies (97%) had at least one nonproliferative lesion, 73% had at least one proliferative lesion without atypia, and 8% contained proliferative lesions with atypia (Figure 1).
Multiple-level HBBD (ML-HBBD) was almost three times more prevalent than single level (SL-HBBD) (2,149 or 72% versus 830 or 28%, respectively, Figure 1). Of the SL- HBBD, 90% consisted of nonproliferative lesions, 9% of proliferative lesions without atypia and <1% of proliferative lesions with atypia. For ML-HBBD, 99% contained at least one nonproliferative lesion, 97% contained at least one proliferative lesion without atypia, and 10% contained proliferative lesions with atypia. The average number of lesions in ML-HBBD was twice (mean 4.31, range 2–10) that of SL-HBBD (mean 2.24, range 2–6), indicating that 90% (1,930 of 2,149) of ML-HBBD were formed from a combination of nonproliferative lesions and proliferative lesions without atypia (not shown in Figure 1).
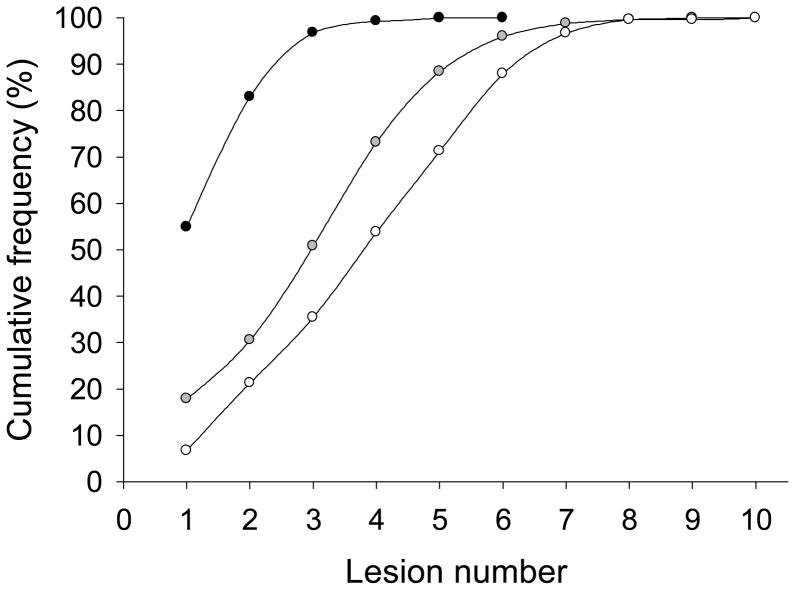
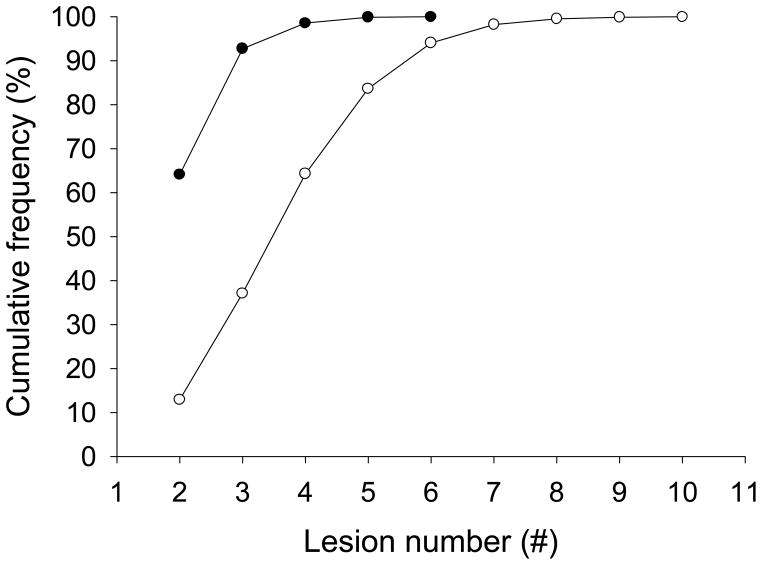
About 33% of BBD specimens contained >3 lesions. Breakdown by lesion number and risk category for HBBD overall showed a cumulative frequency of 95% for ≤ 3 lesions and a risk 1 outcome, whereas the 95th percentile for risk 2 and risk 3 outcomes in HBBD biopsies was reached with lesion numbers of 6 and higher (Figure 2). More than 90% of SL-HBBD had 2 or 3 lesions; the lesion number was increased to 7 for ML-HBBD (Figure 3).
Figure 2. Cumulative frequency of lesion risk level with lesion number.
Risk 1: Black circles
Risk 2: Gray circles
Risk 3: White circles
Figure 3. Cumulative frequency of single- and multi-level HBBD with lesion number.
SL-HBBD: Filled circles
ML-HBBD: Open circles
Characteristics of the entire BBD cohort according to histopathology findings are shown in Table 2. The proportion of HBBD and the number of BBD lesions were directly related to lesion risk level, being highest in patients with risk 3 (93%, 4.26 ± 1.87 lesions), intermediate with risk 2 (82%; 3.45 ± 1.72 lesions) and lowest with risk 1 BBD (45%; 1.66 ± 0.86 lesions).
Compared to the women with risk 1 BBD, the likelihood of HBBD was 5.59 (95% CI 4.85 to 6.44) times higher in women with risk 2 and 17.0 (95% CI 10.2 to 28.5) times higher in women with risk 3 BBD (Table 3). Among different age groups, women aged 46–55 and >55 years were 3 times (95% CI 2.59 to 3.75) and more than 2 times (95% CI 1.94 to 2.68), respectively, more likely to have a HBBD diagnosis as compared to those ≤45 years (Table 3). For ethnicity, the likelihood of HBBD was 57% (OR 1.57; 95% CI 1.37 to 1.81) higher in Caucasian American women than in African American women with BBD (Table 3).
Table 3.
Association of BBD categories with risk level, age and ethnicity
| SBBD n = 1362 | HBBD n = 2979 | Odds Ratio | 95% CI | |
|---|---|---|---|---|
| Risk Level † | ||||
| Risk 1 | 910 (66.8) | 749 (25.1) | 1 | Referent |
| Risk 2 | 436 (32.0) | 2006 (67.3) | 5.59 | 4.85 to 6.44 |
| Risk 3 | 16 (1.2) | 224 (7.5) | 17.0 | 10.2 to 28.5 |
| Age | ||||
| ≤45 years | 918 (67.4) | 1317 (44.2) | 1 | Referent |
| 46–55 years | 174 (12.8) | 778 (26.1) | 3.12 | 2.59 to 3.75 |
| >55 years | 270 (19.8) | 884 (29.7) | 2.28 | 1.94 to 2.68 |
| Ethnicity | ||||
| African American | 467 (34.3) | 741 (24.9) | 1 | Referent |
| Caucasian | 895 (65.7) | 2238 (75.1) | 1.57 | 1.37 to 1.81 |
Risk: The lesion(s) occupied the highest risk level in a BBD biopsy.
We examined possible interaction between pairs of the variables of lesion risk level, age and ethnicity. Significant interaction was found between lesion risk level and age (p < 0.01). Compared to the reference group (risk 1, age ≤45), the likelihood of HBBD for risk 2 was 4.4 times (95% CI 3.70 to 5.33) in women ≤45 years, but that likelihood increased to 17.6 (95% CI 12.8 to 24.2) and 13.4 (95% CI 10.1 to 17.9) times in women of 46–55 and >55 years, respectively (Table 4). The likelihood of HBBD was considerably higher across all age groups for risk 3 lesions (Table 4). Additionally, there was significant interaction between age and ethnicity (p < 0.01). The association between age and HBBD was more evident in Caucasian American women in the 46–55 age groups than in African American women (Table 5). The interaction between risk level and ethnicity did not show statistical significance.
Table 4.
Interaction between risk level and age
| Age | Risk Level | HBBD | |
|---|---|---|---|
| Odds ratio | 95% CI | ||
| ≤45 | |||
| Risk 1 | 1 | Referent | |
| Risk 2 | 4.42 | 3.70–5.33 | |
| Risk 3 | 14.9 | 6.35–34.9 | |
| 46–55 | |||
| Risk 1 | 2.02 | 1.54–2.65 | |
| Risk 2 | 17.6 | 12.8–24.2 | |
| Risk 3 | 29.5 | 10.7–81.2 | |
| >55 | |||
| Risk 1 | 1.82 | 1.45–2.29 | |
| Risk 2 | 13.4 | 10.1–17.9 | |
| Risk 3 | 24.9 | 10.8–57.6 | |
Interaction: p < 0.01
Table 5.
Interaction between age and ethnicity
| Ethnicity | Age | HBBD | |
|---|---|---|---|
| Odds ratio | 95% CI | ||
| African American | |||
| ≤45 | 1 | Referent | |
| 46–55 | 3.31 | 2.35–4.67 | |
| >55 | 1.83 | 1.38–2.44 | |
| Caucasian | |||
| ≤45 | 1.44 | 1.20–1.73 | |
| 46–55 | 4.31 | 3.37–5.51 | |
| >55 | 3.56 | 2.85–4.46 | |
Interaction: p < 0.01
There was no significant difference in lesion number between African American and Caucasian American HBBD biopsies (Table 6). However, significant differences were noted among women of different ages (p < 0.001) and risk categories. Women aged 46–55 years had significantly more lesions than those ≤45 and > 55 years (p < 0.05), although the age-related difference was not significant between the latter two groups (Table 6). As noted earlier, the lesion number in HBBD increased significantly with lesion risk level (p < 0.001) (Table 6).
Table 6.
Comparison of lesion number in HBBD between different groups
| Groups | median | range | p |
|---|---|---|---|
| Ethnicity | NS | ||
| African American | 2 | 2 – 9 | |
| Caucasian | 3 | 2 – 10 | |
| Age | < 0.001 | ||
| ≤45 years | 2a | 2 – 10 | |
| 46–55 years | 3b | 2 – 9 | |
| >55 years | 3 | 2 – 9 | |
| Risk Level | < 0.001 | ||
| Risk 1 | 1a | 2 – 6 | |
| Risk 2 | 3b | 2 – 10 | |
| Risk 3 | 4c | 2 – 10 |
≤45 vs 46–55 or risk 1 vs risk 2, p < 0.05
46–55 vs >55 or risk 2 vs risk 3, p < 0.05
>55 vs ≤45 or risk 3 vs risk 1, p < 0.05
DISCUSSION
Most earlier studies of BBD have focused on the risks of breast cancer associated with a few specific lesions (1, 15–18), for the most part atypical hyperplasias (2–4, 13, 19). There is very limited information with regard to the issue of lesion multiplicity in BBD biopsies. Wang et al (8) reported that 38% of women with lower grade BBD (combination of risk level 1 and risk level 2 lesions) contained multiple lesions. Jacobs et al (14) reported that coexistence of radial scar with other proliferative lesions may double the risk of breast cancer. Our group(10) utilizing multivariable regression modeling approaches for this same study cohort, showed that BBD lesion multiplicity in the categories of nonproliferative, proliferative without atypia (risk 2) or proliferative with atypia (risk 3) is a risk factor for progression to BC from BBD (10). Even when the lesions were purely nonproliferative, with one nonproliferative lesion as the reference, women in the multiple NP lesion group demonstrated increased risk [RR=1.79, 95% CI 1.0, 3.21, p=0.051](10). Proliferative lesions, single or multiple in the same biopsy, with or without AH were significant risk factors for BC (p<0.001). Women with a single P lesion without AH (P=1, n=1453) had a two fold risk for BC (RR=2.06, 95% CI 1.23, 3.43, p=0.006). Women with multiple P lesions without AH (P>1, n=1059) had a 2.87 fold risk of BC (RR=2.87, 95% CI 1.70, 4.83, p<0.0001). Women with AH as the sole lesion (n=65) had the highest risk for BC (RR=6.26, 95% CI 2.73, 14.32, p=0.0001) followed by those with AH and a concurrent P lesion (n=178) (RR=4.90, 95% CI 2.60, 9.21, p<0.0001)(10).
In the current study, over 4000 excision BBD biopsies were evaluated to tease out the extent of lesion heterogeneity regarding lesion number, lesion composition, lesion risk level, age, and ethnicity. Approximately 70% of the women in this cohort had multiple lesions. We found that HBBD biopsies are composed most often of nonproliferative lesions (62.5 %), next most often by proliferative lesions without atypia (35 %), and are least likely to contain atypical hyperplastic lesions (2.4%).
Overall, 69% of women in our BBD cohort had HBBD (multiple lesions with different histopathologic diagnoses) and 31% were single lesion BBD (SBBD). The proportions of nonproliferative lesions and those with proliferation without atypia were similar for HBBD and SBBD (62.5 versus 67% and 35% versus 32%, respectively). However, 2.4% of HBBD biopsies had lesions with atypia as compared to only 1.2% of SBBD biopsies. Furthermore, when compared to women with risk level 1 lesions, the likelihood of HBBD was 5.59 (95% CI 4.85, 6.44) times higher in women with risk level 2 and 17.0 (95% CI 10.2, 28.5) times higher in women with risk level 3 lesions (Table 3). Thus, higher risk BBD lesions are more likely to be found in HBBD.
Further dissection of HBBD indicated that multiple-level HBBD (ML-HBBD) was almost three times more prevalent than single level (SL-HBBD) and included considerably more risk 3 lesions (proliferative lesions with atypia, 10% versus <1%, respectively). The average number of lesions in SL-HBBD (mean 2.24) was considerably lower than that in ML-HBBD (mean 4.31). Approximately 90% (1,930 of 2,149) of ML-HBBD were formed from a combination of nonproliferative lesions and proliferative lesions without atypia.
Women aged 46 years and higher were significantly more likely to have a HBBD diagnosis as compared to those ≤45 years; they showed a significant interaction between risk level and age. (Table 3) Although the likelihood of HBBD was considerably higher across all age groups for risk 3 lesions, the likelihood of HBBD for risk 2 category BBD outcome was 4.4 times higher (95% CI 3.70 to 5.33) in women ≤45 years, and this likelihood increased to 17.6 (95% CI 12.8 to 24.2) and 13.4 (95% CI 10.1 to 17.9) times in women of 46–55 and >55 years, respectively (Table 4).
Hormones are thought to be the main determinant of the major benign and malignant pathologies encountered in the breast. Proliferation is negatively related to age and is influenced by the menstrual cycle with an increased rate of cell proliferation during the luteal phase of the cycle (20). The increased risk of HBBD in women 46–55 years of age may reflect a time period where the breast has reached its cumulative maximum with respect to acquisition of a cyclical proliferative environment. Conversely, as a woman becomes postmenopausal, the breast is subjected to less hormone cycling, a reduction in proliferative cells and, therefore, less symptomatology (21). A decreased risk of HBBD in women 56 and older as compared to younger women (<55, >45) may reflect a less heterogeneous breast environment with further atrophy of glandular tissue (22).
In this same cohort, we recently reported that race interaction with lesion multiplicity was not a significant predictor of progression to BC (p=0.519)(10). We showed that the effect of significant variables of age, fibrosis, and multiple lesions, whether nonproliferative, proliferative, or atypical, for breast cancer progression was not influenced by race (10). In this study, however, with regard to HBBD risk, ethnicity is an important consideration. Caucasian American women are 57% more likely to present with HBBD than African American women. Again, we found no interaction of lesion risk level and lesion number with ethnicity, supporting previous findings (10, 11), although the association between age and HBBD was more evident (p<0.01) in Caucasian American women in the 46–55 age group than in African American women.
Significant interaction was noted for lesion number, risk level, and age. Women classed as risk 3 were more likely to have a higher number of concurrent BBD lesions, and to be older (>45 years). Thus, the presence of an increased number of concurrent BBD lesions with time appears associated with an increased likelihood of higher risk lesions. The latter supports the hypothesis of a stepwise sequence of pre-malignant histological changes in which nonproliferative and proliferative forms of BBD, proliferative disease with atypia, and in situ cancer represent successive steps preceding the development of invasive breast carcinoma (23).This step-wise development of breast cancer has been demonstrated using transgenic rat (24), mouse (25) and xenograft models (23–26). Xenografts of human-origin MCF10AneoT cells have been shown to recapitulate the entire progression spectrum from nonproliferative changes such as cysts, to proliferative changes, e.g., florid hyperplasia, to lesions resembling atypical hyperplasia, carcinoma in situ, and ultimately malignant breast lesions (23–25) in transplanted murine hosts.
The most important potential clinical consequence of benign breast disease, particularly, when the epithelial proliferation is accompanied by atypia, is the development of breast cancer (7, 10, 11, 13, 27, 28). However, the risk of breast cancer after BBD by long-term follow-up, extent of cellular proliferation, and complexity of disease pattern has been studied primarily with respect to a limited number of BBD lesion histopathology subtypes (8, 21, 29–32), rarely addressing the issue of lesion multiplicity. The prevalence of HBBD as well as lesion complexity within BBD are additional factors worthy of consideration in risk estimation. Even purely nonproliferative multiple lesions appear to increase the risk for progression to BC (11). Because multiple BBD lesions comprise 70% of BBD biopsies, their number as well as the specific histopathologic diagnoses should be considered in individual risk estimates for progression to breast cancer.
Acknowledgments
Support: NIH CA 70923 (MJW), ACS EDT-116(MJW)
References
- 1.Goehring C, Morabia A. Epidemiology of benign breast disease, with special attention to histologic types. Epidemiol Rev. 1997;19:310–327. doi: 10.1093/oxfordjournals.epirev.a017960. [DOI] [PubMed] [Google Scholar]
- 2.Dupont WD, Page DL, Parl FF, Vnencak-Jones CL, Plummer WD, Jr, Rados MS, Schuyler PA. Long-term risk of breast cancer in women with fibroadenoma. N Engl J Med. 1994;331:10–15. doi: 10.1056/NEJM199407073310103. [DOI] [PubMed] [Google Scholar]
- 3.Dupont WD, Parl FF, Hartmann WH, Brinton LA, Winfield AC, Worrell JA, Schuyler PA, Plummer WD. Breast cancer risk associated with proliferative breast disease and atypical hyperplasia. Cancer. 1993;71:1258–1265. doi: 10.1002/1097-0142(19930215)71:4<1258::aid-cncr2820710415>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 4.Fitzgibbons PL, Henson DE, Hutter RV. Benign breast changes and the risk for subsequent breast cancer: an update of the 1985 consensus statement. Cancer Committee of the College of American Pathologists. Arch Pathol Lab Med. 1998;122:1053–1055. [PubMed] [Google Scholar]
- 5.Friedenreich C, Bryant H, Alexander F, Hugh J, Danyluk J, Page D. Risk factors for benign proliferative breast disease. Int J Epidemiol. 2000;29:637–644. doi: 10.1093/ije/29.4.637. [DOI] [PubMed] [Google Scholar]
- 6.Singletary SE. Rating the risk factors for breast cancer. Ann Surg. 2003;237:474–482. doi: 10.1097/01.SLA.0000059969.64262.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312:146–151. doi: 10.1056/NEJM198501173120303. [DOI] [PubMed] [Google Scholar]
- 8.Wang J, Costantino JP, Tan-Chiu E, Wickerham DL, Paik S, Wolmark N. Lower-category benign breast disease and the risk of invasive breast cancer. J Natl Cancer Inst. 2004;96:616–620. doi: 10.1093/jnci/djhs105. [DOI] [PubMed] [Google Scholar]
- 9.Jacobs TW, Byrne C, Colditz G, Connolly JL, Schnitt SJ. Pathologic features of breast cancers in women with previous benign breast disease. Am J Clin Pathol. 2001;115:362–369. doi: 10.1309/UP07-K3KD-25NL-D3M8. [DOI] [PubMed] [Google Scholar]
- 10.Worsham M, Raju U, Lu M, Kapke A, Cheng J, Wolman SR. Multiplicity of benign breast lesions is a risk factor for progression to breast cancer. Clinical Cancer Research. doi: 10.1158/1078-0432.CCR-07-0928. (In Press.) [DOI] [PubMed] [Google Scholar]
- 11.Worsham MJ, Abrams J, Raju U, Kapke A, Lu M, Cheng J, Mott D, Wolman SR. Breast cancer incidence in a cohort of women with benign breast disease from a multiethnic, primary health care population. Breast J. 2007;13:115–121. doi: 10.1111/j.1524-4741.2007.00388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Page DL, Dupont WD. Benign breast diseases and premalignant breast disease. Arch Pathol Lab Med. 1998;122:1048–1050. [PubMed] [Google Scholar]
- 13.Ghosh K, Melton LJ, 3rd, Suman VJ, Grant CS, Sterioff S, Brandt KR, Branch C, Sellers TA, Hartmann LC. Breast biopsy utilization: a population-based study. Arch Intern Med. 2005;165:1593–1598. doi: 10.1001/archinte.165.14.1593. [DOI] [PubMed] [Google Scholar]
- 14.Jacobs TW, Byrne C, Colditz G, Connolly JL, Schnitt SJ. Radial scars in benign breast-biopsy specimens and the risk of breast cancer. N Engl J Med. 1999;340:430–436. doi: 10.1056/NEJM199902113400604. [DOI] [PubMed] [Google Scholar]
- 15.Page DL, Dupont WD. Proliferative breast disease: diagnosis and implications. Science. 1991;253:915–916. doi: 10.1126/science.1876848. [DOI] [PubMed] [Google Scholar]
- 16.Kennedy M, Masterson AV, Kerin M, Flanagan F. Pathology and clinical relevance of radial scars: a review. J Clin Pathol. 2003;56:721–724. doi: 10.1136/jcp.56.10.721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu C, Ray RM, Lin MG, Gao DL, Horner NK, Nelson ZC, Lampe JW, Hu YW, Shannon J, Stalsberg H, Li W, Fitzgibbons D, Porter P, Patterson RE, Satia JA, Thomas DB. A case-control study of risk factors for fibrocystic breast conditions: Shanghai Nutrition and Breast Disease Study, China, 1995–2000. Am J Epidemiol. 2004;160:945–960. doi: 10.1093/aje/kwh318. [DOI] [PubMed] [Google Scholar]
- 18.Yeh IT, Dimitrov D, Otto P, Miller AR, Kahlenberg MS, Cruz A. Pathologic review of atypical hyperplasia identified by image-guided breast needle core biopsy. Correlation with excision specimen. Arch Pathol Lab Med. 2003;127:49–54. doi: 10.5858/2003-127-49-PROAHI. [DOI] [PubMed] [Google Scholar]
- 19.Allred DC, Mohsin SK, Fuqua SA. Histological and biological evolution of human premalignant breast disease. Endocr Relat Cancer. 2001;8:47–61. doi: 10.1677/erc.0.0080047. [DOI] [PubMed] [Google Scholar]
- 20.Potten CS, Watson RJ, Williams GT, Tickle S, Roberts SA, Harris M, Howell A. The effect of age and menstrual cycle upon proliferative activity of the normal human breast. Br J Cancer. 1988;58:163–170. doi: 10.1038/bjc.1988.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tan-Chiu E, Wang J, Costantino JP, Paik S, Butch C, Wickerham DL, Fisher B, Wolmark N. Effects of tamoxifen on benign breast disease in women at high risk for breast cancer. J Natl Cancer Inst. 2003;95:302–307. doi: 10.1093/jnci/95.4.302. [DOI] [PubMed] [Google Scholar]
- 22.Santen RJ, Mansel R. Benign breast disorders. N Engl J Med. 2005;353:275–285. doi: 10.1056/NEJMra035692. [DOI] [PubMed] [Google Scholar]
- 23.Lakhani SR. The transition from hyperplasia to invasive carcinoma of the breast. J Pathol. 1999;187:272–278. doi: 10.1002/(SICI)1096-9896(199902)187:3<272::AID-PATH265>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 24.Davies BR, Platt-Higgins AM, Schmidt G, Rudland PS. Development of hyperplasias, preneoplasias, and mammary tumors in MMTV-c-erbB-2 and MMTV-TGFalpha transgenic rats. Am J Pathol. 1999;155:303–314. doi: 10.1016/s0002-9440(10)65124-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li Y, Hively WP, Varmus HE. Use of MMTV-Wnt-1 transgenic mice for studying the genetic basis of breast cancer. Oncogene. 2000;19:1002–1009. doi: 10.1038/sj.onc.1203273. [DOI] [PubMed] [Google Scholar]
- 26.Miller FR, Soule HD, Tait L, Pauley RJ, Wolman SR, Dawson PJ, Heppner GH. Xenograft model of progressive human proliferative breast disease. J Natl Cancer Inst. 1993;85:1725–1732. doi: 10.1093/jnci/85.21.1725. [DOI] [PubMed] [Google Scholar]
- 27.Page DL, Dupont WD. Histopathologic risk factors for breast cancer in women with benign breast disease. Semin Surg Oncol. 1988;4:213–217. doi: 10.1002/ssu.2980040403. [DOI] [PubMed] [Google Scholar]
- 28.Page DL, Dupont WD. Benign breast disease: indicators of increased breast cancer risk. Cancer Detect Prev. 1992;16:93–97. [PubMed] [Google Scholar]
- 29.Page DL, Dupont WD, Rogers LW, Rados MS. Atypical hyperplastic lesions of the female breast. A long-term follow-up study. Cancer. 1985;55:2698–2708. doi: 10.1002/1097-0142(19850601)55:11<2698::aid-cncr2820551127>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 30.Shaaban AM, Sloane JP, West CR, Moore FR, Jarvis C, Williams EM, Foster CS. Histopathologic types of benign breast lesions and the risk of breast cancer: case-control study. Am J Surg Pathol. 2002;26:421–430. doi: 10.1097/00000478-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 31.Baumgartner KB, Hunt WC, Baumgartner RN, Crumley DD, Gilliland FD, McTiernan A, Bernstein L, Ballard-Barbash R. Association of body composition and weight history with breast cancer prognostic markers: divergent pattern for Hispanic and non-Hispanic White women. Am J Epidemiol. 2004;160:1087–1097. doi: 10.1093/aje/kwh313. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein L, Teal CR, Joslyn S, Wilson J. Ethnicity-related variation in breast cancer risk factors. Cancer. 2003;97:222–229. doi: 10.1002/cncr.11014. [DOI] [PubMed] [Google Scholar]