Abstract
Background and Purpose
Many patients with aneurysmal subarachnoid hemorrhage (SAH) with intraparenchymal extension develop early hematoma expansion, which is not explained by aneurysmal rerupture in half of cases. In patients with primary intracerebral hemorrhage, the computed tomography angiography (CTA) spot sign predicts hematoma expansion and poor outcome. We conducted a 2-center prospective cohort study to evaluate whether CTA spot sign predicts case fatality in aneurysmal subarachnoid hemorrhage with intraparenchymal extension.
Methods
We studied consecutive patients with aneurysmal subarachnoid hemorrhage with intraparenchymal extension. Two experienced readers, blinded to clinical data, analyzed CTAs for spot sign presence. We assessed the proportion of patients with the CTA spot sign and tested its association with in-hospital and 90-day case fatality, using univariable and multivariable logistic regression.
Results
In 32 of 236 patients (14%), we found at least 1 spot sign. Acute surgical hematoma evacuation with aneurysm occlusion occurred in 120 patients (51%). The overall in-hospital case fatality rate was 37%. The CTA spot sign was not associated with in-hospital (multivariable odds ratio, 0.51 [95% confidence interval, 0.06–3.26]) or 90-day (multivariable odds ratio, 0.59 [0.21–1.65]) case fatality.
Conclusions
The found frequency of CTA spot signs is lower after aneurysmal than primary intracerebral hemorrhage and is not associated with in-hospital or 90-day case fatality in patients with aneurysmal subarachnoid hemorrhage with intraparenchymal extension.
Keywords: CTA spot sign, in-hospital death, intracerebral hemorrhage, subarachnoid hemorrhage
A neurysmal subarachnoid hemorrhage (SAH) is associated with high morbidity and case fatality. 1 Twenty per- cent of patients develop intraparenchymal extension of the hemorrhage, which is associated with worse outcome.2 In addition, many patients develop expansion of the parenchymal hematoma in the initial 48 hours after aneurysmal rupture, but this can be attributed to rerupture of their aneurysms in only half of patients.3
The mechanism for hematoma expansion in the remaining patients is unclear but might be similar to that involved in expansion in primary intracerebral hemorrhage (ICH). In such patients, expansion of the hematoma is an important predictor of poor outcome.4,5 During recent years, contrast extravasation following computed tomography angiography (CTA), termed the spot sign, has been shown to be an important and independent predictor of both hematoma expansion and poor clinical outcome (Figure).6–9 The presence of a CTA spot sign marks those patients at highest risk for hematoma expansion, and may, therefore, provide a similar marker in patients with aneurysmal SAH with intraparenchymal extension.
Figure.
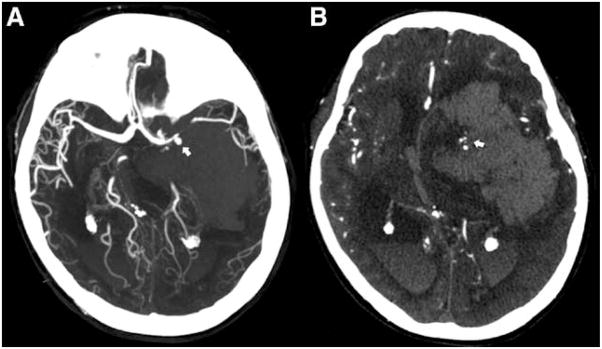
Computed tomography angiography (CTA) of aneurysmal subarachnoid hemorrhage (SAH) with intraparenchymal extension and multiple spot signs. Acute CTA of a 57-year-old patient showing an aneurysmal SAH with intraparenchymal extension. A, The reformatted CTA shows a left middle cerebral artery aneurysm, as indicated by the arrow. B, The CTA source images demonstrate a large (150 mL) left hemispheric intracerebral hemorrhage with multiple spot signs within the inferomedial aspect of the hematoma (arrow).
The aim of this study was to assess the occurrence of a CTA spot sign in patients with ICH from aneurysmal rupture and to assess whether the spot sign is a predictor of poor outcome, as it is in patients with primary ICH. We, therefore, conducted a 2-center study to assess the prevalence and predictive value of the CTA spot sign in those patients.
Methods
Study Design
We studied prospectively collected data from 2 consecutive series of patients with aneurysmal SAH and ICH: 1 at Massachusetts General Hospital Boston and 1 at University Medical Center Utrecht, Utrecht, The Netherlands. The Institutional Review Boards of both hospitals approved all parts of this study. Data sharing between the 2 centers occurred without patient identifiers, and informed consent was obtained from all study participants or their legally authorized health-care proxies.
Study Subjects
Between January 2000 (Massachusetts General Hospital) or December 2003 (University Medical Center Utrecht) and December 2011 (both centers), consecutive patients presenting with an ICH, with or without SAH, from a ruptured aneurysm were screened for eligibility. Eligibility criteria included (1) diagnosis of ICH on baseline CT; (2) diagnosis of a ruptured aneurysm on CTA, conventional angiogram, or MR angiography; and (3) availability of a baseline CTA for spot sign reading. Exclusion criteria included the presence of any other suspected cause of secondary ICH (eg, vascular malformation, traumatic ICH, brain neoplasm, or hemorrhagic transformation of an ischemic stroke). Patients with primary, nonaneurysmal ICH were also excluded from this analysis.
Clinical Data
Clinical information was collected through patient interviews (or their surrogates) and extracted from their medical records. The data collected included age, sex, previous medical history (including cardiovascular risk factors), and medications (including antiplatelet therapy and use of oral anticoagulants). Other collected variables were admission Glasgow Coma Scale (GCS) score, mean arterial blood pressure, and time from symptom onset to initial CTA. Three-month modified Rankin Scale (mRS) assessments were performed either in-person or via telephone, by trained and blinded study staff, to assess death and functional outcome. Poor functional outcome was defined as modified Rankin Scale >2.
CT Analysis
The diagnosis of a ruptured aneurysm was made based on the previously mentioned imaging modalities, by trained neuroradiologists at each center. Baseline parenchymal ICH and intraventricular hemorrhage volumes were assessed using Analyze 9.0 (Mayo Clinic, Rochester, MN) and Alice (PAREXEL International Corporation) software, according to previously published methods with high inter-rater reliability (κ=0.99).6,10,11 Follow-up hematoma volumes, and thus hematoma expansion, were not assessed in this analysis because of the high rate (51%) of hematoma evacuation before follow-up imaging. Two experienced readers (H.B.B. and D.B.), blinded to clinical data, analyzed CTAs for presence, number, size, and attenuation (in Hounsfield Units) of spot signs, according to a previously described methodology with an almost perfect inter-rater agreement (κ=0.91).10 Spot signs had to be present within the intraparenchymal hematoma but spatially distinct from the aneurysm (Figure).
Statistical Analysis
Discrete variables are presented as count and percentage (%) and continuous variables as mean and SD, or as median and interquartile range when appropriate. We tested the potential role of the CTA spot sign as predictor of poor functional outcome and in-hospital and 90-day death using univariable and multivariable logistic regression. The spot sign was analyzed as a dichotomized variable (absent or present). Multivariable models included age, sex, and variables with a P value of <0.20 in the univariable analysis. Collinear variables (measured using the variance inflation factor) were removed from the multivariable model when appropriate. Subsequently, we calculated sensitivity, specificity, positive predictive value, negative predictive value, and accuracy, as well as the C-statistic, using standard methods to assess the accuracy of the spot sign in predicting death and poor functional outcome. The threshold of significance was set at P<0.05. All statistical analyses were performed using JMP Pro version 9.0 (SAS Institute Inc, Cary, NC).
Results
Study Population
After applying the aforementioned inclusion and exclusion criteria, 236 patients remained eligible and consented for the current study. The overall consent rate for the study was >95%. Massachusetts General Hospital and University Medical Center Utrecht contributed 99 patients (42%) and 137 patients (58%), respectively. Cohort characteristics and characteristics stratified by spot sign status are shown in Table 1. In summary, mean age in the combined cohort was 56 (SD 14) years, 178 patients (75%) were females, and median GCS on presentation to the emergency department was 11 (inter-quartile range, 5–14). Acute interventions were common: 120 patients (51%) underwent surgical hematoma evacuation with aneurysm occlusion and 69 patients (29%) were treated with aneurysm coiling.
Table 1.
Cohort Characteristics
| Variable | All Patients n (%) | Spot Sign–Negative Patients n (%) | Spot Sign–Positive Patients n (%) |
|---|---|---|---|
| No. of subjects | 236 | 204 (86) | 32 (14) |
| MGH | 99 (42) | ||
| UMCU | 137 (58) | ||
| Age (mean, SD) | 56 (14) | 55 (14) | 57 (15) |
| Sex (female) | 178 (75) | 154 (75) | 24 (75) |
| Hypertension | 82 (35) | 67 (33) | 15 (47) |
| Diabetes | 6 (4) | 2 (2) | 4 (21) |
| Atrial fibrillation | 2 (1) | 1 (1) | 1 (5) |
| Congestive heart failure | 5 (4) | 3 (3) | 2 (11) |
| Antiplatelets | 28 (12) | 21 (10) | 7 (22) |
| Oral anticoagulants | 2 (2) | 2 (2) | 0 (0) |
| GCS (median, IQR) | 11 (5–14) | 10 (5–14) | 11 (5–14) |
| MABP (median, IQR) | 106 (92–118) | 103 (92–117) | 109 (96–125) |
| Time to CTA, h (median, IQR) | 10 (3–18) | 11 (4–20) | 5 (2–14) |
| CTA spot sign | 32 (14) | n/a | n/a |
| Baseline ICH volume, mL (median, IQR) | 25 (11–46) | 23 (10–43) | 36 (17–49) |
| Intraventricular extension | 55 (23) | 43 (21) | 12 (38) |
| Baseline IVH volume, mL (median, IQR) | 2 (0–19) | 1 (0–13) | 16 (7–51) |
| Intervention | |||
| Clipping | 120 (51) | 105 (51) | 15 (47) |
| Coiling | 69 (29) | 55 (27) | 14 (44) |
| In-hospital death | 87 (37) | 76 (38) | 11 (35) |
| 90-day death | 101 (61) | 86 (62) | 15 (55) |
| Poor outcome at 90 days (mRS>2) | 133 (80) | 116 (81) | 17 (71) |
CTA indicates computed tomography angiography; GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; IQR, interquartile range; IVH, intraventricular hemorrhage; MABP, mean arterial blood pressure; MGH, Massachusetts General Hospital; mRS, modified Rankin Scale; n/a, not applicable; and UMCU, Utrecht University Medical Center.
CT Imaging
At least 1 spot sign was observed in 32 patients (14%). Within the group of spot sign–positive patients, 74% had 1 spot sign, 21% had 2 spot signs, and 5% had >2 spot signs. Median ICH volume at baseline CT was 25 mL (interquartile range, 11–46), and median time to initial imaging was 10 hours (interquartile range, 3–18). Spot sign–positive patients had larger baseline ICH volumes and shorter times to imaging (P<0.05; Table 1).
Predictors of In-Hospital Death
The overall in-hospital case fatality rate was 37%. Age (P=0.0001), GCS (P<0.0001), baseline ICH volume (P=0.01), and baseline intraventricular hemorrhage volume (P=0.005) were associated with in-hospital death in univariable analysis (Table 2). Subsequently, these covariates remained independent predictors of in-hospital death in multivariable logistic regression (Table 3). Of note, GCS was not included in the multivariate analysis of in-hospital death because of strong collinearity with baseline ICH volume and better model performance with the inclusion of baseline ICH volume. The spot sign was not associated with in-hospital death in either univariable or multivariable analysis (when forced into the multivariable model).
Table 2.
Univariable Analysis of In-Hospital and 90-Day Death
| Variable | In-Hospital Death
|
90-Day Death
|
||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | 1.04 (1.02–1.06) | 0.0001 | 1.06 (1.03–1.10) | <0.0001 |
| Sex (male) | 0.77 (0.41–1.43) | 0.44 | 1.10 (0.49–2.47) | 0.84 |
| Hypertension | 1.68 (0.96–2.94) | 0.09 | 2.44 (1.17–5.09) | 0.02 |
| Antiplatelets | 1.60 (0.72–3.55) | 0.30 | 2.38 (0.74–7.71) | 0.19 |
| GCS | 1.27 (1.16–1.40) | <0.0001 | 1.07 (0.89–1.24) | 0.23 |
| MABP | 1.01 (1.00–1.02) | 0.17 | 1.01 (0.99–1.03) | 0.33 |
| Time to CTA | 1.00 (0.98–1.03) | 0.78 | 1.01 (0.98–1.04) | 0.62 |
| Baseline ICH volume | 1.02 (1.01–1.04) | 0.01 | 1.02 (0.99–1.06) | 0.23 |
| Baseline IVH volume | 1.03 (1.01–1.05) | 0.005 | 1.02 (0.98–1.06) | 0.41 |
| CTA spot sign | 0.91 (0.41–2.01) | 0.82 | 0.74 (0.30–1.85) | 0.64 |
CI indicates confidence interval; CTA, computed tomography angiography; GCS, Glasgow Coma Scale; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; MABP, mean arterial blood pressure; and OR, odds ratio.
Table 3.
Multivariable Analysis of In-Hospital and 90-Day Death
| Variable* | In-Hospital Death
|
90-Day Death
|
||
|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Age | 1.05 (1.01–1.12) | 0.049 | 1.06 (1.03–1.10) | 0.0002 |
| Sex (male) | 0.74 (0.13–3.56) | 0.71 | 1.33 (0.56–3.28) | 0.52 |
| Hypertension | 0.47 (0.11–1.86) | 0.29 | 1.77 (0.78–4.11) | 0.18 |
| Antiplatelets | n/a | … | 1.19 (0.33–5.04) | 0.80 |
| MABP | 1.02 (0.99–1.04) | 0.19 | n/a | … |
| Baseline ICH volume | 1.03 (1.01–1.06) | 0.02 | n/a | … |
| Baseline IVH volume | 1.04 (1.01–1.07) | 0.04 | n/a | … |
| CTA spot sign | 0.51 (0.06–3.26) | 0.49 | 0.59 (0.21–1.65) | 0.31 |
CI indicates confidence interval; CTA, computed tomography angiography; ICH, intracerebral hemorrhage; IVH, intraventricular hemorrhage; MABP, mean arterial blood pressure; n/a, not applicable; and OR, odds ratio.
Glasgow Coma Scale was not included in the multivariate analysis of in-hospital death because of strong collinearity with baseline ICH volume and better model performance with the inclusion of baseline ICH volume.
Predictors of 90-Day Death and Poor Functional Outcome
At 90 days, 61% of patients within the combined cohort had died. Age (P<0.0001) and a history of hypertension (P=0.02) were associated with case fatality at 90 days in univariable analysis (Table 2). In multivariable analysis, only age (P=0.0002) remained significant after adjusting for potential confounders (Table 3). As for in-hospital death, we found no association between the spot sign and the 90-day death.
Of the patients who were still alive at 3 months, 133 patients (80%) were functionally dependent. In both univariable and multivariable analyses (adjusted for the same covariables as the analysis assessing death at 90 days, plus GCS on admission), the CTA spot sign was not associated with poor functional outcome: univariable odds ratio, 0.68 (95% confidence interval [CI], 0.23–1.99) and multivariate odds ratio, 0.93 (95% CI, 0.09–7.61).
Accuracy Measures
In this 2-center cohort, the spot sign had a sensitivity of 0.13, specificity of 0.86, positive predictive value of 0.35, negative predictive value of 0.62, and an overall accuracy of 0.59 for predicting in-hospital death. For 90-day death, the spot sign had a sensitivity of 0.13, a specificity of 0.83, a positive predictive value of 0.55, a negative predictive value of 0.38, and an accuracy of 0.41.
The C-statistic for the multivariable model of in-hospital death was 0.77 without the spot sign. When introducing the spot sign, the C-statistic only marginally increased to 0.79 (nonsignificant). The multivariable model for 90-day death had a C-statistic of 0.74 without the spot sign, and the C-statistic remained the same when including the spot sign in the model.
Discussion
In this study, we show that CTA spot signs are present in patients with aneurysmal SAH with intraparenchymal extension. In our series, a spot sign was present in 1 of 7 patients, which is lower than the proportion of ≈30% in patients with primary ICH.8,9 In contrast to its strong association with poor functional outcome and death in primary ICH, spot sign presence was not associated with functional outcome or death in patients with aneurysmal SAH with intraparenchymal extension.
The only other study assessing the role of the spot sign in aneurysmal SAH included all secondary causes of ICH (not just ICH caused by ruptured aneurysms).12 In that study, the spot sign was associated with in-hospital death in univariable analysis, but not in multivariable analysis. The proportions of patients with a spot sign were similar in both studies.12 Of note, there is no overlap in patients between the current analysis and the referenced paper.
Multiple factors may contribute to the fact that the CTA spot sign predicts outcome in primary ICH, but not in aneurysmal SAH with intraparenchymal extension. Early hematoma evacuation was much more frequent (51%) in our series than it is in patients with primary ICH. Thus, hematoma enlargement and, therefore, higher risk of poor outcome might have been prevented in spot sign–positive patients who underwent early hematoma removal. It is likely that larger hematomas, which are associated with spot sign presence, have been evacuated more often than smaller ones, causing confounding by indication. Another factor is the average later scan time of patients having aneurysmal SAH with intraparenchymal extension compared with patients having primary ICH (median 10 versus 2 hours in the PREDICT [Prediction of Haematoma Growth and Outcome in Patients with Intracerebral Haemorrhage Using the CT Angiography Spot Sign] study),8 which may as well account in part for the loss in sensitivity.13
Another possibility is that the mechanisms of ongoing bleeding are so different between aneurysmal and primary ICH that the CTA spot sign marks a completely separate process in these patients. When considering Fisher’s model model of hematoma expansion in primary ICH (termed the avalanche model), expansion occurs because of the rupture of neighboring vessels around the initial hematoma, leading to additional bleeding and expansion of the hematoma.14 With the intraparenchymal hemorrhage occurring in the vicinity of the subarachnoid space rather than more deep in the brain parenchyma, the continued bleeding, as represented by the spot sign, may cause more subarachnoid blood instead of additional blood in the brain parenchyma. This increase in subarachnoid blood may not lead to worse outcome, whereas increase in ICH volume in the parenchyma does. An alternative explanation could be the state of the underlying small vessels in SAH versus primary ICH. A recent genetic association study showed the apolipoprotein E ε2 allele to be associated with hematoma expansion, probably because of small vessel fragility caused by amyloid deposition.15 Perhaps small vessels are differently affected in aneurysmal SAH compared with primary ICH, involving different pathways leading to final hematoma volumes. Further studies are warranted to answer this pathophysiological hypothesis.
A limitation of our study is the lack of follow-up CTs to assess hematoma expansion. However, because of the high rate of acute hematoma evacuation in patients with ICH from aneurysmal rupture, follow-up CTs in those patients are not usable for volumetric measurements to assess hematoma expansion. Another limitation is the relatively high missing rate of 3-month follow-up data.
Although in our study the CTA spot sign was not associated with in-hospital and 90-day case fatality, it does not mean the presence of the CTA spot sign can be ignored given the potential confounding by indication generated by early surgical interventions in this study. Further studies should focus on patients with a CTA spot sign who are currently not treated with emergency hematoma evacuation. If, in this subset of patients, hematoma enlargement occurs often and negatively influences outcome, such patients may benefit from earlier hematoma removal.
Acknowledgments
Sources of Funding
The project described was supported by grant numbers R01NS073344, R01NS059727, and 5K23NS059774 from the National Institutes of Health–National Institute of Neurological Disorders and Stroke (NIH–NINDS), American Heart Association Grant Number 0755984T, and the Andrew D. Heitman Neurovascular Research Fund. Dr Brouwers was supported by the NIH–NINDS SPOTRIAS fellowship grant P50NS051343. Dr Kimberly received a research grant from Andrew D. Heitman Neurovascular Research Fund. Dr Greenberg received a research grant from NIH. Dr Rosand received a research grant from NIH and AHA; Dr Goldstein received a research grant from NINDS.
Footnotes
Disclosures
All funding entities had no involvement in study design, data collection, analysis, and interpretation, writing of the manuscript and in the decision to submit for publication. Dr Romero is a member of Imaging Committee DIAS trial/on the advisory board of Lundbeck Pharmaceuticals. Dr Ogilvy is a consultant/on the advisory board of Edge Therapeutics. Dr Greenberg honoraria: Medtronic, Pfizer, consultant/on the advisory board of Hoffman-La Roche, Janssen Alzheimer Immunotherapy, Bristol-Myers Squibb Company. Dr Goldstein is a consultant/advisory board member CSL Behring. The other authors have no conflicts to report. The content is solely the responsibility of the authors and does not necessarily represent the official views of NIH or NINDS.
References
- 1.Rinkel GJ, Algra A. Long-term outcomes of patients with aneurysmal subarachnoid haemorrhage. Lancet Neurol. 2011;10:349–356. doi: 10.1016/S1474-4422(11)70017-5. [DOI] [PubMed] [Google Scholar]
- 2.Güresir E, Beck J, Vatter H, Setzer M, Gerlach R, Seifert V, et al. Subarachnoid hemorrhage and intracerebral hematoma: incidence, prognostic factors, and outcome. Neurosurgery. 2008;63:1088–1093. doi: 10.1227/01.NEU.0000335170.76722.B9. discussion 1093. [DOI] [PubMed] [Google Scholar]
- 3.van Asch CJ, Oudendijk JF, Rinkel GJ, Klijn CJ. Early intracerebral hematoma expansion after aneurysmal rupture. Stroke. 2010;41:2592–2595. doi: 10.1161/STROKEAHA.110.589291. [DOI] [PubMed] [Google Scholar]
- 4.Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE. VISTA Collaboration. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238–1244. doi: 10.1212/WNL.0b013e3182143317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delcourt C, Huang Y, Arima H, Chalmers J, Davis SM, Heeley EL, et al. INTERACT1 Investigators. Hematoma growth and outcomes in intracerebral hemorrhage: the INTERACT1 study. Neurology. 2012;79:314–319. doi: 10.1212/WNL.0b013e318260cbba. [DOI] [PubMed] [Google Scholar]
- 6.Goldstein JN, Fazen LE, Snider R, Schwab K, Greenberg SM, Smith EE, et al. Contrast extravasation on CT angiography predicts hematoma expansion in intracerebral hemorrhage. Neurology. 2007;68:889–894. doi: 10.1212/01.wnl.0000257087.22852.21. [DOI] [PubMed] [Google Scholar]
- 7.Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007;38:1257–1262. doi: 10.1161/01.STR.0000259633.59404.f3. [DOI] [PubMed] [Google Scholar]
- 8.Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, et al. PREDICT/Sunnybrook ICH CTA study group. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11:307–314. doi: 10.1016/S1474-4422(12)70038-8. [DOI] [PubMed] [Google Scholar]
- 9.Brouwers HB, Goldstein JN, Romero JM, Rosand J. Clinical applications of the computed tomography angiography spot sign in acute intracerebral hemorrhage: a review. Stroke. 2012;43:3427–3432. doi: 10.1161/STROKEAHA.112.664003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Goldstein JN, Rosand J, et al. Systematic characterization of the computed tomography angiography spot sign in primary intracerebral hemorrhage identifies patients at highest risk for hematoma expansion: the spot sign score. Stroke. 2009;40:2994–3000. doi: 10.1161/STROKEAHA.109.554667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Delgado Almandoz JE, Yoo AJ, Stone MJ, Schaefer PW, Oleinik A, Brouwers HB, et al. The spot sign score in primary intracerebral hemorrhage identifies patients at highest risk of in-hospital mortality and poor outcome among survivors. Stroke. 2010;41:54–60. doi: 10.1161/STROKEAHA.109.565382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delgado Almandoz JE, Kelly HR, Schaefer PW, Brouwers HB, Yoo AJ, Stone MJ, et al. CT angiography spot sign predicts in-hospital mortality in patients with secondary intracerebral hemorrhage. J Neurointerv Surg. 2012;4:442–447. doi: 10.1136/neurintsurg-2011-010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brouwers HB, Falcone GJ, McNamara KA, Ayres AM, Oleinik A, Schwab K, et al. CTA spot sign predicts hematoma expansion in patients with delayed presentation after intracerebral hemorrhage. Neurocrit Care. 2012;17:421–428. doi: 10.1007/s12028-012-9765-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fisher CM. Pathological observations in hypertensive cerebral hemorrhage. J Neuropathol Exp Neurol. 1971;30:536–550. doi: 10.1097/00005072-197107000-00015. [DOI] [PubMed] [Google Scholar]
- 15.Brouwers HB, Biffi A, Ayres AM, Schwab K, Cortellini L, Romero JM, et al. Apolipoprotein E genotype predicts hematoma expansion in lobar intracerebral hemorrhage. Stroke. 2012;43:1490–1495. doi: 10.1161/STROKEAHA.111.643262. [DOI] [PMC free article] [PubMed] [Google Scholar]


