Abstract
The antitumor activity of monoclonal antibodies is mediated by effector cells, such as natural killer (NK) cells, that express Fc receptors for immunoglobulin. Efficacy of monoclonal antibodies, including the CD20 antibody rituximab, could be improved by agents that augment the function of NK cells. Interleukin (IL)-18 is an immunostimulatory cytokine that has antitumor activity in preclinical models. The effects of IL-18 on NK cell function mediated through Fcγ receptors were examined. Human NK cells stimulated with immobilized IgG in vitro secreted IFN-γ as expected; such IFN-γ production was partially inhibited by blocking CD16 with monoclonal antibodies. IL-18 augmented IFN-γ production by NK cells stimulated with immobilized IgG or CD16 antibodies. NK cell IFN-γ production in response to immobilized IgG and/or IL-18 was inhibited by chemical inhibitors of Syk and several other kinases involved in CD16 signaling pathways. IL-18 augmented antibody-dependent cellular cytotoxicity (ADCC) of human NK cells against rituximab-coated Raji cells in vitro. IL-18 and rituximab acted synergistically to promote regression of human lymphoma xenografts in SCID mice. Inasmuch as IL-18 costimulates IFN-γ production and ADCC of NK cells activated through Fc receptors in vitro and augments antitumor activity of rituximab in vivo, it is an attractive cytokine to combine with monoclonal antibodies for treatment of human cancer.
Keywords: Cancer immunotherapy, Cytokines, Monoclonal antibodies, Lymphoma, Rituximab
Introduction
Natural killer (NK) cells are lymphocytes that participate in innate immune responses to intracellular pathogens and neoplastic cells [1, 2]. NK cells do not productively rearrange T cell receptor or immunoglobulin genes, but do express several activating and inhibitory receptors that regulate their activation and function. NK cells can spontaneously lyse certain tumor cells and pathogen-infected cells in an antibody-independent process known as natural killing or NK activity. Furthermore, NK cells can lyse antibody-coated target cells in a process known as antibody-dependent cellular cytotoxicity (ADCC). Thus, in addition to contributing to innate immunity, NK cells can participate in the elimination of infected or transformed cells during the effector phase of adaptive immune responses [1, 2].
The FcγRIIIa (CD16) complex is an Fc receptor for IgG that is expressed on approximately 90 % of human NK cells [1, 2]. Ligation of CD16 causes rapid tyrosine phosphorylation of ζ chain family members as well as ZAP-70 and Syk, with downstream activation of multiple signaling pathways, including the phospholipase C-γ/inositol-1,4,5-trisphosphate/diacylglycerol, PI3-K/ERK, and p38 MAPK pathways [3, 4]. Functional consequences of CD16-mediated stimulation of NK cells include triggering of ADCC, expression of activation antigens, and secretion of several cytokines and chemokines [1, 5].
Monoclonal antibodies are standard components of current cancer therapy. The mechanisms by which monoclonal antibodies exert antitumor activity are complex and have not been completely defined. Nevertheless, there is compelling evidence that signals mediated through Fc receptors contribute to the antitumor effects of rituximab, trastuzumab, and cetuximab [6–8]. Therefore, it is rational to combine therapeutic monoclonal antibodies with other agents (such as immunostimulatory cytokines) that can enhance the function of Fc receptor-bearing effector cells, including NK cells. IL-18 is an immunostimulatory cytokine that regulates both innate and adaptive immune responses [9]. IL-18 has antitumor activity in animal models [10, 11] and can be safely given to patients with cancer [12, 13]. We have investigated the effects of IL-18 on Fc receptor-mediated functions of NK cells in preclinical in vitro and in vivo models.
Materials and methods
Human cells and cell lines
Blood samples were obtained from patients with lymphoma who had undergone high-dose chemotherapy and autologous stem cell transplantation. Procedures for stem cell collection, administration of high-dose therapy, and autologous stem cell transplantation were as previously described [14]. Blood samples were also obtained from patients with advanced cancer enrolled on a clinical trial of recombinant human IL-18 [13]. These studies were approved by the Institutional Review Board at Indiana University Medical Center, and written informed consent was obtained from each subject prior to collection of blood samples. Peripheral blood mononuclear cells (PBMCs) were isolated on a Ficoll–diatrizoate gradient from venous blood samples. Control PBMCs were obtained from healthy volunteer donors. Freshly isolated PBMCs were used for immunofluorescence studies. Aliquots of PBMCs were cryopreserved in liquid nitrogen for subsequent in vitro studies. Enriched NK cells were obtained from PBMCs using NK cell isolation kits from Miltenyi Biotec (Aubum, CA) or Stem Cell Technologies (Vancouver, BC). The human Burkitt lymphoma cell lines Raji and Ramos were obtained from the American Type Culture Collection (Manassas, VA).
Antibodies, cytokines, and other reagents
Monoclonal antibodies specific for human CD3, CD16, CD32 (clone FL18.26), and CD56 were obtained from BD PharMingen (San Diego, CA). F(ab′)2 fragments of the 3G8 (CD16) monoclonal antibody were obtained from Ancell (Bayport, MN, USA). Neutralizing goat antihuman IFN-γ antibodies were obtained from R & D Systems (Minneapolis, MN, USA). Purified human IgG was obtained from Sigma (St. Louis, MO, USA). Rituximab, a chimeric murine/human monoclonal IgG1 antibody specific for the human CD20 antigen, was obtained from Genentech (South San Francisco, CA, USA). Recombinant human IL-12 was obtained from Genetics Institute (Cambridge, MA, USA) and IL-18 from R & D Systems (Minneapolis, MN, USA). Chemical inhibitors of extracellular signal-related kinases (ERK) (U0126), p38 mitogen-activated protein kinase (MAPK) (SB 203580), phosphatidylinositol 3-kinase (PI3-K) (Wortmannin), and Syk (piceatannol) were obtained from Calbiochem (La Jolla, CA).
Immunophenotypic analysis
PBMCs and enriched NK cells were stained directly with fluorochrome-conjugated monoclonal antibodies, washed, fixed in 1 % formaldehyde, and analyzed by flow cytometry as previously described [15] using an FACScan or FACScalibur instrument from Becton–Dickinson (San Diego, CA, USA). During analysis, forward and side-scattering properties were used to create a lymphocyte gate. Thresholds for discriminating levels of staining above background were established by analysis of cells stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)-, and allophycocyanin (APC)-conjugated control monoclonal antibodies. Arbitrary mean fluorescence intensity (MFI) units were calculated by dividing the geometric mean fluorescence of CD16 or CD32 staining on CD56 positive cells expressing these antigens at levels above background staining with control monoclonal antibodies by the geometric mean fluorescence of CD56 positive cells that did not express these antigens at levels above background staining.
For cancer patients treated with recombinant human IL-18, the absolute number of NK cells expressing CD16 was calculated by multiplying the absolute total lymphocyte count (determined by automated complete blood count with white cell differential) by the percentage of CD16 + CD56 + CD3-negative cells (determined by flow cytometry).
Analysis of IFN-γ mRNA and protein levels
PBMCs and enriched NK cells were incubated overnight at 37 °C in medium alone or medium containing IL-12, IL-18, or both cytokines as indicated and then plated at 100,000 or 200,000 cells per well in 96-well microtiter plates. After overnight incubation at 37 °C in wells containing medium alone or medium with cytokines as indicated, IFN-γ protein levels were measured using specific ELISA kit according to the manufacturer’s (R & D Systems, Minneapolis, MN) instructions. IFN-γ mRNA levels were measured by quantitative PCR as previously described [16]. For Fc receptor stimulation assays, wells of microplates were pretreated with medium alone or medium containing 100 μg/mL of human IgG, incubated overnight at 4 °C, and then washed with ice-cold PBS before adding cells. For some experiments, chemical inhibitors or F(ab′)2 fragments of CD16 monoclonal antibodies were added during the final overnight incubation with cytokines and/or immobilized IgG.
Cytotoxicity assays
Enriched NK cells were incubated overnight at 37 °C in medium with or without 100 ng/mL IL-18 or 10 U/mL IL-12 and then incubated in 96-well microtiter plates together with Raji cells that had been untreated or pretreated for 30 min with rituximab 1 μg/mL. After coculture of NK cells and Raji cells for 4 h at 37 °C, ADCC was measured using the CytoTox 96 kit from Promega (Madison, WI). For some experiments, neutralizing goat anti-human-IFN-γ antibodies were added during overnight incubation of NK cells with IL-18 and during the 4 h ADCC assay.
Human lymphoma xenograft model in vivo
All animal experiments were approved by the Institutional Animal Care and Use Committee at GlaxoSmithKline. Six- to eight-week-old immunodeficient outbred female mice (SCID on ICR background) were obtained from Charles River USA. Preparation of tumor donor animals consisted of injecting Ramos lymphoma cells (5 × 106 cells/mouse) from tissue culture to the flank of donor mice in a separate experiment prior to the therapeutic study. Ramos tumors from donor mice were harvested and passed through a sterile sieve to generate tumor cell homogenate. The tumor homogenate was diluted 1:8 in sterile PBS and immediately injected in the left flank of therapeutic study recipient mice. Tumor homogenate recipients were randomized into therapeutic groups (n = 6/group) when tumors reached mean volume 100 mm3. Daily injections of recombinant murine IL-18 (GlaxoSmithKline) at 4 mg/kg were administered subcutaneously (s.c.), and rituximab was dosed at 2, 1, and 0.5 mg/kg intravenously (i.v.) twice a week. Tumor diameters were measured using standard manual calipers, and the volumes were calculated using the following formula: V (volume) = 0.5 × [length × (width)2]. Complete regression (CR) of tumors was defined as non-palpable or <10 mm3 tumors. Percent of tumor growth inhibition was calculated at 19 days of the study (prior to euthanization of control tumors due to ethical reasons) using the following formula: 100 × (1 − [Ve19 − Ve0]/[Vc19 − Vc0], where Ve19 indicates the mean tumor volume in treatment group on day 19, Ve0 the mean tumor volume in treatment group on day 0 of therapy, Vc19 the mean tumor volume in control group on day 19, and Vc0 the mean tumor volume in control group on day 0 of therapy.
Statistical analysis
The lower limit of detection for the IFN-γ ELISA is 8 pg/mL. For data analysis, samples with IFN-γ levels <8 pg/mL were assigned a value of 8 pg/mL. Means, SE, t tests, Wilcoxon tests were calculated using PASW Statistics 17.0 software (SPSS Inc., Chicago, IL, USA). All in vivo experiments were graphed and analyzed using GraphPad Prism software. Two-way ANOVA followed by Bonferronni post-test was used to determine statistical significance between therapeutic groups in data that included two variables (time and tumor volume in the line graphs). Data packages with one variable (tumor volume) were evaluated by one-way ANOVA followed by Bonferroni post-test (comparison among >2 groups in the bar graphs).
Results
IL-18 augments IFN-γ production by human NK cells stimulated with immobilized IgG
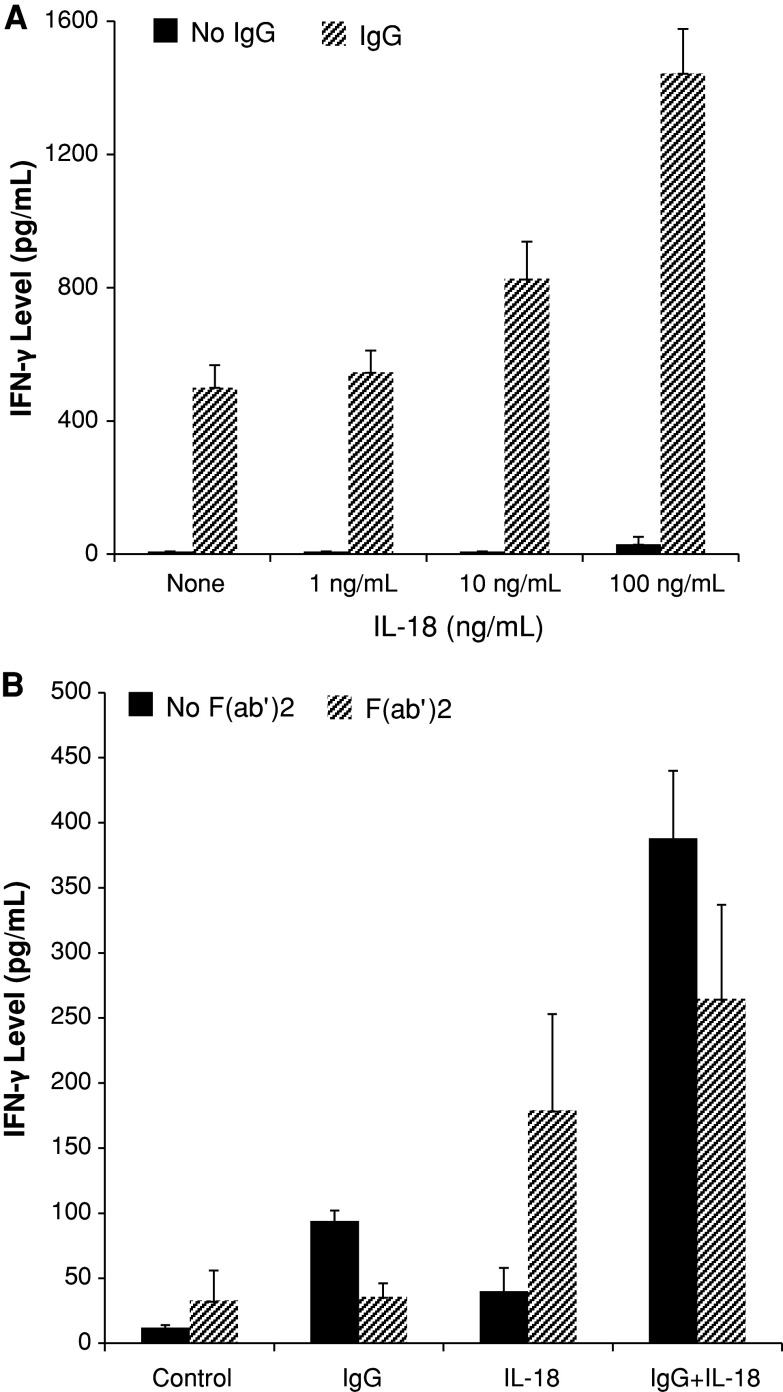
Significant amounts of IFN-γ were secreted by enriched human NK cells cultured in the presence of immobilized IgG, whereas IFN-γ was not detected in supernatants of NK cells cultured in medium alone (Fig. 1a). This result is compatible with previously published studies, showing that stimulation of NK cells via Fc receptors can promote IFN-γ production [17, 18]. We found that IL-18 enhanced, in a dose-dependent manner, the production of IFN-γ by NK cells exposed to immobilized IgG (Fig. 1a). Levels of IFN-γ mRNA were not significantly increased in NK cells stimulated with IL-18 and immobilized IgG compared to NK cells stimulated with either agent alone (data not shown). Thus, post-transcriptional mechanisms appear to promote synergistic production of IFN-γ by NK cells in response to IL-18 and immobilized IgG. In agreement with previous results [19, 20], IL-18 alone stimulated only minor NK cell production of IFN-γ (Fig. 1a) but acted synergistically with IL-12 to promote IFN-γ production (data not shown). The highest concentration (100 ng/mL) of IL-18 used in our experiments is clinically relevant. Plasma concentrations of IL-18 in excess of 100 ng/mL can be achieved in cancer patients after administration of recombinant human IL-18 in doses that are associated with acceptable toxicity and significant biological activity [12, 13].
Fig. 1.
IL-18 and immobilized IgG costimulate IFN-γ production by human NK cells. a Enriched human NK cells were incubated overnight in medium alone or medium containing 1, 10, or 100 ng/mL IL-18 as indicated and then plated overnight in the presence (striped bars) or absence (solid bars) of immobilized IgG. Supernatant IFN-γ levels were measured by ELISA. P value for comparison of medium alone versus medium plus IgG is 0.001 and for comparisons of IgG alone versus IgG plus IL-18 1, 10, or 100 ng/mL is 0.312, 0.033, 0.012, respectively. b Enriched human NK cells were incubated in wells precoated or not with immobilized IgG in medium with or without 100 ng/mL IL-18 as indicated in the presence (striped bars) or absence (solid bars) of F(ab′)2 fragments of CD16 monoclonal antibody 3G8. P value for comparison of presence versus absence of CD16 F(ab′)2 fragments for medium, IgG, IL-18, or IgG plus IL-18 is 0.023, 0.013, 0.019, 0.006, respectively. Results shown represent mean ± SE of data from 3 (a) or 5 (b) separate experiments
Production of IFN-γ in vivo is deficient in lymphoma patients treated with IL-12 after autologous stem cell transplantation [21]. A selective and profound deficiency of STAT4 in post-transplant patient PBMCs appears to be the major cause of defective IFN-γ production in this setting [16, 20]. STAT4 is not known to participate in the signaling pathways required for IFN-γ production after ligation of CD16 on NK cells [3, 22]. We therefore tested the effects of immobilized IgG on IFN-γ production by post-transplant PBMCs. Levels of IFN-γ did not differ significantly in supernatants of post-transplant versus control PBMCs after incubation in the presence of immobilized IgG (P = 0.161; data not shown). Moreover, IFN-γ production in response to IL-12 plus immobilized IgG was not significantly different (P = 0.974) comparing post-transplant patient PBMCs (819 ± 356 pg/mL; n = 4) to control PBMCs (828 ± 105 pg/mL; n = 5). As expected [20], IL-18 also synergistically enhanced IFN-γ production by post-transplant patient PBMCs stimulated with IL-12 (data not shown). Thus, stimulation with IL-18 or by ligation of CD16 can enhance IL-12-induced IFN-γ production by PBMCs obtained from lymphoma patients after autologous stem cell transplantation.
Role of CD16 in IFN-γ production by NK cells stimulated with immobilized IgG
The CD16/ζ complex appears to be the major Fc receptor that triggers ADCC and cytokine production after exposure of NK cells to antibody-coated target cells [3, 23]. Nevertheless, some isoforms of FcγRII (CD32) can participate in the activation of human NK cells [24, 25]. In the enriched NK cell preparations used for our experiments, CD16 was expressed at relatively high levels (MFI 54 ± 7; n = 10) by 88 ± 2 % of NK cells, whereas CD32 was expressed at low levels (MFI 6 ± 1) by only 3 ± 1 % of NK cells. Furthermore, IFN-γ production by enriched NK cells cultured with immobilized IgG was reduced by 77 % in the presence of CD16 F(ab′)2 fragments (Fig. 1b). CD16 F(ab′)2 fragments also had a consistent but less pronounced inhibitory effect on IFN-γ production by NK cells stimulated with IL-18 and immobilized IgG (Fig. 1b). In the absence of immobilized IgG, however, the addition of CD16 F(ab′)2 fragments augmented IFN-γ production by NK cells stimulated with IL-18 (Fig. 1b). These results are consistent with previous data showing that cross-linking of CD16 by monoclonal antibodies promotes IFN-γ production by NK cells [26]. Our results indicate that ligation of CD16 by either monoclonal antibodies or immobilized IgG can costimulate IFN-γ production by IL-18-activated human NK cells.
Effects of chemical inhibitors on IFN-γ production by human NK cells
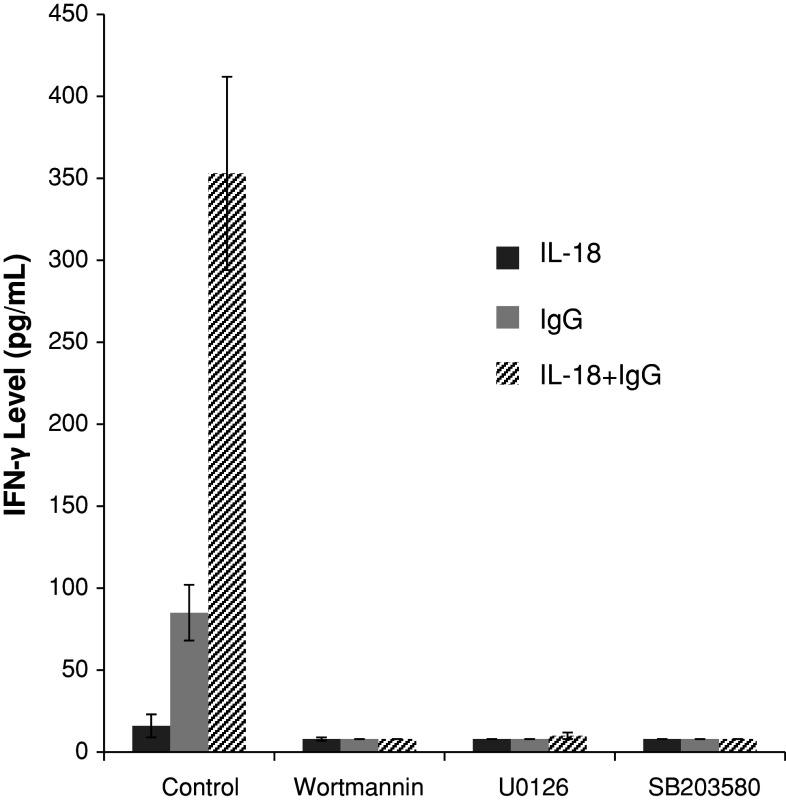
Signal transduction following ligation of CD16 on NK cells involves Syk, PI3-K, p38 MAPK, and ERK kinases [3]. Production of IFN-γ in response to immobilized IgG alone as well as immobilized IgG plus IL-18 (Fig. 2) was nearly abolished by addition of 10 μM chemical inhibitors of PI-3 K (Wortmannin), ERK (U0126), and p38 MAPK (SB203580). Furthermore, IFN-γ levels produced by enriched NK cells in response to IL-18 and immobilized IgG were reduced to undetectable levels in the presence of 10 μM chemical inhibitor of Syk (piceatannol; data not shown). As expected, NK cells stimulated with IL-18 alone produced very little IFN-γ (Fig. 2), and the levels of IFN-γ secreted by IL-18-activated NK cells were not significantly different in the presence or absence of Wortmannin (P = 0.3041), U0126 (P = 0.3075), or SB203580 (P = 0.3075).
Fig. 2.
Effect of chemical inhibitors on IFN-γ production by human NK cells. Enriched NK cells were stimulated with 100 ng/mL IL-18 alone (darker solid bars), immobilized IgG alone (lighter solid bars), or both agents (striped bars) in the absence of chemical inhibitors (“Control”) or in the presence of 10 μM Wortmannin, U0126, or SB203580 as indicated. P value for comparison of control versus inhibitor is <0.0001 for each inhibitor when comparing IFN-γ levels produced by NK cell stimulated with IgG alone or IgG plus IL-18. Results shown represent mean ± SE of data from 5 separate experiments
Effects of IL-18 on expression of Fc receptors by NK cells
It has been reported that CD16 expression is diminished on human NK cells stimulated in vitro with IL-18; the percentage of NK cells expressing CD16 decreased from >90 % before culture to approximately 60 % after 24–48 h of culture in the presence of IL-18 [27]. We did not find the percentage of NK cells expressing CD16 to be significantly different after in vitro culture for 2 or 5 days in medium containing IL-18 100 ng/mL compared to those cultured in medium alone (data not shown). However, the MFI of CD16 on NK cells cultured with IL-18 for 2 days was 24 ± 7 (mean ± SE) as compared to 41 ± 7 for NK cells cultured in medium alone (P = 0.0002). The MFI of CD16 on NK cells stimulated with IL-18 for 5 days was 12 ± 4 as compared to 22 ± 5 for NK cells cultured in medium alone (P = 0.0704). The IL-18-induced decrease in CD16 levels on NK cells was not associated with any impairment in CD16-mediated IFN-γ production (Fig. 1a) or cytolytic activity (Fig. 3).
Fig. 3.
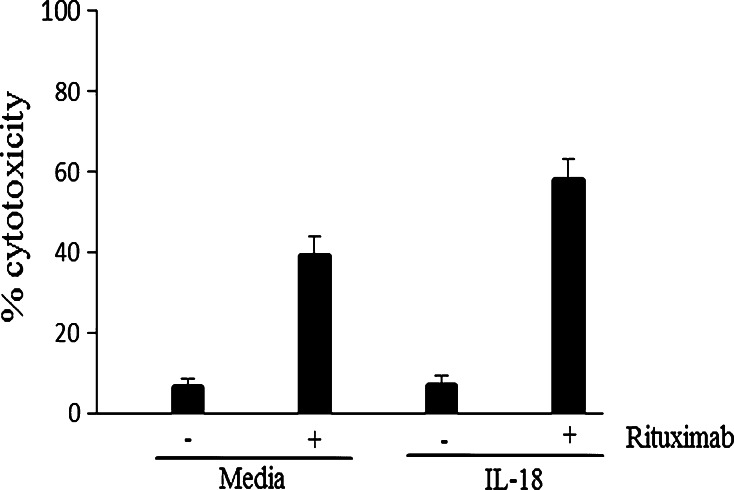
IL-18 enhances ADCC mediated by human NK cells against rituximab-sensitized lymphoma cells. Enriched human NK cells were incubated overnight in medium with or without 100 ng/mL IL-18 as indicated and then plated at an effector-to-target cell ratio of 10:1 with Raji cells that had been pre-treated or not with rituximab as indicated. P value for comparison of rituximab versus medium is 0.008, and for comparison of rituximab plus IL-18 versus rituximab alone or IL-18 alone is 0.03 and 0.003, respectively. Results shown represent mean ± SE of data from 3 separate experiments
We also examined the expression of CD16 on the NK cells of cancer patients who were given weekly intravenous infusions of recombinant human IL-18 for up to 24 consecutive weeks. At the time points that were evaluated (weeks 1–5 of study), the absolute number of CD16 + NK cells was not significantly diminished in patients receiving recombinant human IL-18 (Table 1). The MFI of CD16 expressed on NK cells was not assessed in this clinical trial. However, if the level of CD16 on the surface of NK cells did indeed decline during IL-18 therapy, the magnitude of this change was not sufficient to cause a reduction in the number of CD16 + NK cells detected in peripheral blood.
Table 1.
CD16 expression by NK cells of cancer patients receiving recombinant human IL-18
| Absolute number of CD16 + NK cells per μL | |
|---|---|
| Week 1 | 96 ± 35 |
| Week 2 | 125 ± 35 |
| Week 4 | 100 ± 35 |
| Week 5 | 105 ± 23 |
Ten patients were given weekly intravenous infusions of recombinant human IL-18 at doses of 100 (4 patients), 1,000 (3 patients), or 2,000 (3 patients) μg/kg as previously described [13]. Blood samples were obtained prior to IL-18 infusions on weeks 1, 2, 4, and 5 of study, and the absolute number of CD16 + NK cells was determined as described in Materials and Methods. Results shown are mean ± SE of data from 9 patients. One patient did not have a sample obtained at baseline (week 1) and has been excluded from the analysis
IL-18 augments ADCC mediated by human NK cells
Enriched human NK cells exhibited poor cytolytic activity against unsensitized Raji lymphoma cells, which are relatively resistant to natural killing. However, Raji cells sensitized with rituximab were efficiently killed by NK cells (Fig. 3). ADCC mediated by NK cells against rituximab-coated lymphoma cells was significantly augmented by IL-18, whereas IL-18 by itself did not promote killing of unsensitized Raji cells (Fig. 3). Lysis of rituximab-sensitized Raji cells by NK cells stimulated with IL-18 100 ng/mL was not significantly different than that by NK cells stimulated with IL-12 10 U/mL (data not shown).
Flow cytometric analysis failed to detect expression of IL-18Rα or IL-18Rβ on Raji cells. Furthermore, incubation of Raji cells for up to 48 h in the presence of IL-18 100 ng/mL did not affect their viability (data not shown). Therefore, the enhanced killing of rituximab-sensitized Raji cells detected in our ADCC assays is due to effects of IL-18 on NK cells and is not due to direct effects of residual IL-18 on Raji cells. Furthermore, expression of CD20 was not increased on Raji cells cultured in vitro with IL-18 (data not shown). As noted above, human NK cells stimulated with IL-18 in vitro actually express diminished levels of CD16. Therefore, IL-18 does not enhance ADCC by augmenting interactions between CD16 on NK cells and its ligand (Fc region of rituximab) on sensitized Raji cells. Rather, IL-18 appears to costimulate CD16-mediated cytolytic activity in a fashion similar to its costimulation of CD16-mediated IFN-γ production.
IL-18 and ligation of CD16 can synergistically augment IFN-γ production by human NK cells (Fig. 1a). IFN-γ can mediate tumor regression by direct antiproliferative and/or pro-apoptotic effects on tumor cells and by stimulating antitumor responses by effector cells [28]. We therefore investigated the potential role of IFN-γ in lysis of Raji cells by IL-18-activated NK cells. Incubation of Raji cells for up to 48 h in the presence of IFN-γ 10 ng/mL did not affect their viability or expression of CD20 (data not shown). Moreover, neutralizing anti-IFN-γ antibodies did not inhibit lysis of Raji cells by IL-18-activated NK cells (data not shown). Thus, we have no evidence that IFN-γ participates in NK cell ADCC against rituximab-sensitized target cells in vitro.
IL-18 enhances the efficacy of rituximab treatment in a murine model of lymphoma
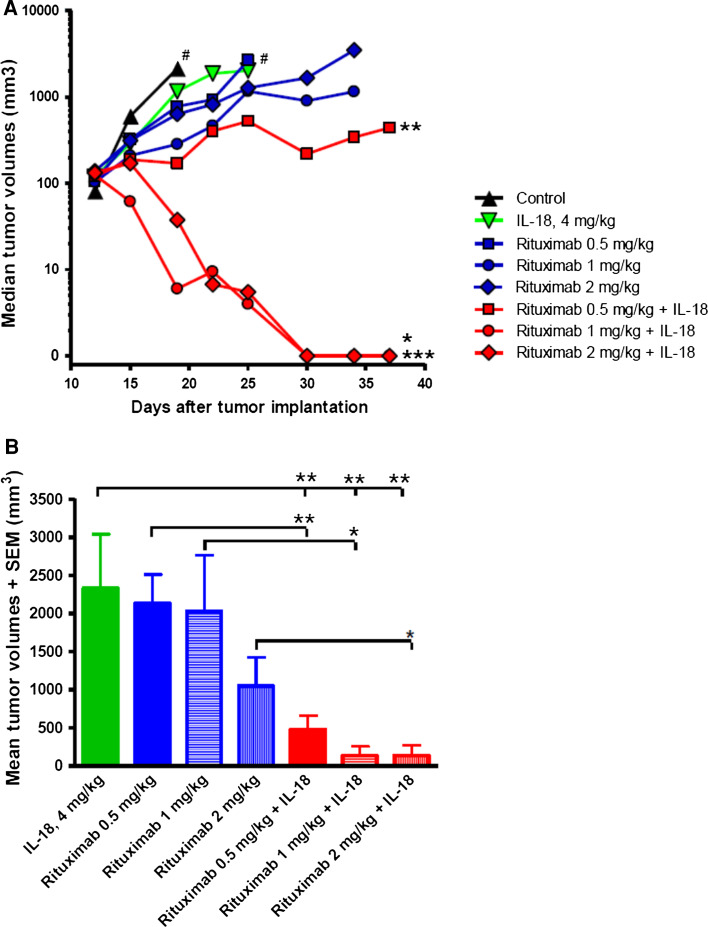
The in vivo antitumor effects of IL-18 in combination with rituximab were evaluated in human Ramos lymphoma xenograft model. IL-18 monotherapy at 4 mg/kg daily s.c., or rituximab monotherapy at doses 0.5, 1, and 2 mg/kg resulted in significant Ramos tumor inhibition as compared to the control (vehicle) group (Fig. 4a). However, none of the monotherapy groups demonstrated significant number of complete regressions (Fig. 4a; Table 2). Addition of IL-18 to rituximab led to statistically significant enhancement of antitumor effects on Ramos lymphomas in all combination therapy groups when compared to the corresponding monotherapy dose groups (Fig. 4b). Moreover, there was a remarkable improvement in complete regression rates with almost complete tumor elimination and 83 % (5/6) complete tumor regressions in the IL-18 and rituximab (2 mg/kg) dose group (Fig. 4a; Table 2). No evidence of dose-related toxicity or weight loss was observed in any of the treatment groups throughout the study.
Fig. 4.
IL-18 enhances the efficacy of rituximab in SCID mice with human B cell lymphoma xenografts. SCID–ICR mice (n = 6/group) bearing subcutaneous human Ramos lymphoma xenografts were treated with IL-18 or rituximab monotherapy, or with combination of both agents. a Tumor growth in combination therapy groups was significantly suppressed (*P < 0.05, **P < 0.01, ***P < 0.001, two-way ANOVA followed by Bonferroni post-test) as compared to the rituximab monotherapy groups. Moreover, the number of complete tumor regressions was significantly increased in combination therapy groups as compared to rituximab (Table 2) or IL-18 monotherapy (0/6). Mice in the control group in some of the monotherapy groups (#) had to be euthanized due to ethical reasons before the study ended. b Tumor volumes on day 25 show statistically significant tumor growth suppression in all combination therapy groups as compared to their respective rituximab (*P < 0.05, **P < 0.01, t test) monotherapy groups and to the IL-18 monotherapy group (**P < 0.01, one-way ANOVA followed by Bonferroni post-test)
Table 2.
Complete responses in tumor-bearing mice treated with rituximab with or without IL-18
| Rituximab dose (mg/kg) | Number of mice with complete response/total number of mice treated per group | |
|---|---|---|
| No IL-18 | IL-18 4 mg/kg | |
| 0.5 | 0/6 | 2/6 |
| 1 | 2/6 | 4/6 |
| 2 | 2/6 | 5/6 |
SCID mice (n = 6 per group) were injected with Ramos cells as described in Materials and Methods and given daily s.c. injections of recombinant murine IL-18 4 mg/kg or vehicle (“No IL-18”) as indicated together with twice weekly i.v. infusions of rituximab in doses of 0.5, 1, or 2 mg/kg as indicated
Discussion
Immunostimulatory cytokines have been shown to augment functional responses of NK cells activated through CD16 [5, 17, 18, 29]. Our results demonstrate that IL-18 can also potently costimulate NK cells activated by ligation of Fc receptors. IL-18 alone, even in concentrations as high as 100 ng/mL, did not induce lysis of unsensitized Raji cells and stimulated very low levels of IFN-γ production. However, IL-18 strongly costimulated the CD16-mediated cytolytic activity and IFN-γ production (Figs. 1a, 3). Indeed, the levels of IFN-γ secreted by NK cells stimulated with IgG and IL-18 were almost as high as those secreted by NK cells stimulated with IL-12 plus IL-18 (data not shown), a cytokine combination which has been shown to strongly costimulate IFN-γ production by human and murine NK cells [19, 20]. The very high levels of IFN-γ produced by lymphocytes stimulated with IL-12 plus IL-18 appear to be due to STAT4-dependent enhanced transcription of the IFN-γ gene as well as p38-dependent stabilization of the IFN-γ mRNA [30, 31]. The mechanisms by which IL-18 and immobilized IgG synergistically induce IFN-γ production have not been fully elucidated. Nevertheless, quantitative PCR analysis of IFN-γ mRNA levels indicates that post-transcriptional mechanisms are predominantly responsible for the synergistic production of IFN-γ in response to IL-18 and immobilized IgG. Furthermore, our data support a role for p38 in this synergy, as the p38 inhibitor SB203580 virtually abolished IFN-γ production by NK cells stimulated with IL-18 and immobilized IgG.
IL-12 can induce p38-dependent IFN-γ production by Stat4−/− murine lymphocytes [32]. Moreover, we have previously shown that IL-12 plus IL-18 can synergistically induce p38-dependent IFN-γ production by STAT4-deficient human PBMCs [20]. As signaling induced by Fc receptor ligation is not known to depend on STAT4 [3], it is not surprising that immobilized IgG and IL-12 could also strongly augment IFN-γ production by STAT4-deficient PBMCs obtained from lymphoma patients after autologous stem cell transplantation. Our data provide a good rationale to combine IL-12 therapy with either IL-18 or CD20 monoclonal antibodies in attempts to enhance IFN-γ production and antitumor immune responses after autologous transplantation for lymphoma.
CD16 is presumably the Fc receptor that mediates enhanced IFN-γ production in the presence of immobilized IgG as well as augmented NK cell ADCC against rituximab-coated target cells. CD16 F(ab′)2 fragments partially inhibited IFN-γ production by NK cells costimulated with immobilized IgG and IL-18. Complete inhibition of IFN-γ production would not be expected even if CD16 is the sole FcR mediating the response, as CD16 F(ab′)2 fragments can costimulate IL-18-induced IFN-γ production in the absence of immobilized IgG (Fig. 1b). Thus, CD16 F(ab′)2 fragments appear to block binding of a stronger agonist (immobilized IgG) to CD16, while simultaneously acting as a weaker agonist by ligating CD16 to promote IFN-γ production in concert with IL-18.
IFN-γ did not appear to enhance the lysis of rituximab-coated lymphoma cells by human NK cells in vitro. This observation does not preclude the participation of IFN-γ in NK cell-mediated antitumor immune responses in vivo. IFN-γ can augment ADCC by monocyte/macrophages, enhance presentation of antigens by dendritic cells, and promote the differentiation of Th1 helper effector cells [28]. IFN-γ also stimulates production of the CXC chemokines MIG (CXCL9) and IP-10 (CXCL-10), which can inhibit tumor angiogenesis and recruit CXCR3-bearing effector cells to tumor sites [33]. Therefore, IFN-γ produced by NK cells activated in vivo by IL-18, and monoclonal antibodies could contribute to tumor regression during cancer immunotherapy.
One strategy to improve the efficacy of monoclonal antibody-based therapies for cancer is the administration of agents that can augment the antitumor activity of Fc receptor-bearing effector cells. Our results clearly show that IL-18 can enhance ADCC mediated by NK cells against rituximab-coated lymphoma cells in vitro. We have also found that IL-18 enhances the antitumor effects of rituximab in vivo in a SCID mouse model of human B cell lymphoma. By adding IL-18 to rituximab dosed at 2 mg/kg, we were able to achieve almost complete elimination of tumor growth as well as tumor regression in 83 % (5/6) of treated mice. The SCID mice used for our lymphoma xenograft model lack mature T and B cells but have normal NK cells and macrophages. Rituximab is a chimeric monoclonal antibody consisting of murine Fab portions that specifically recognize the human CD20 antigen and human IgG1 Fc portion that binds to Fc receptors on effector cells. Human CD20 monoclonal antibodies of IgG1 isotype can trigger the in vitro lysis of human lymphoma cells by murine NK cells and phagocytosis of human lymphoma cells by murine macrophages [34]. We did not directly examine the relative contributions of NK cells and macrophages to the in vivo antitumor effects of IL-18 and rituximab. Previous studies using SCID mouse models of lymphoma have found that murine NK cells mediate antitumor activity during rituximab-based therapy [35, 36].
NK cells have also been implicated in the efficacy of IL-18-based immunotherapy against syngeneic tumors in immunocompetent mice [10, 37]. IL-18 therapy has been shown to activate NK cells in vivo, promote IFN-γ production, and cause complete tumor regression in the MOPC-315 plasmacytoma tumor model [38]. In addition to innate effector cells, CD4 and CD8 T cells can participate in antitumor activity in syngeneic mouse tumor models [10, 37]. Thus, administration of IL-18 with monoclonal antibodies to lymphoma patients could enhance innate immune responses mediated by Fc receptor-bearing NK cells and monocytes as well as promote antigen-specific antitumor T cell responses. It is therefore rational to combine IL-18 with monoclonal antibodies in the treatment of cancer. A phase I clinical trial has provided proof-of-principle that therapy with IL-18 and rituximab is feasible in patients with relapsed or refractory lymphoma [39]. Further clinical trials test the efficacy of IL-18 plus monoclonal antibodies in the treatment of cancer are warranted.
Acknowledgments
This work was supported in part by National Institutes of Health Grant RO1 CA118118 (Michael J. Robertson), Walther Scholar Grant (Shivani Srivastava) from the Indiana University Simon Cancer Center (P30 CA82709) and American Cancer Society IRG (Hua-Chen Chang and Shivani Srivastava). The authors thank Menggang Yu, Ph.D. and Sandra Althouse for statistical support and Lisa Wood, R. N. and nurses in the Indiana CTSI Clinical Research Center for assistance in collection of patient samples.
Conflict of interest
Zdenka Haskova, Margaret Whitacre, Stephen Trulli, Yi-Jiun Chen, John Toso, Zdenka L. Jonak are employees and shareholders of GlaxoSmithKline. All other authors declare that they have no conflict of interest.
References
- 1.Caligiuri MA. Human natural killer cells. Blood. 2008;112(3):461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson MJ, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–2438. [PubMed] [Google Scholar]
- 3.Leibson P. Signal transduction during natural killer cell activation: inside the mind of a killer. Immunity. 1997;6:655–661. doi: 10.1016/S1074-7613(00)80441-0. [DOI] [PubMed] [Google Scholar]
- 4.Trotta R, Fettucciari K, Azzoni L, Abebe B, Puorro KA, Eisenlohr LC, Perussia B. Differential role of p38 and c-Jun N-terminal kinase 1 mitogen-activated protein kinases in NK cell cytotoxicity. J Immunol. 2000;165:1782–1789. doi: 10.4049/jimmunol.165.4.1782. [DOI] [PubMed] [Google Scholar]
- 5.Roda JM, Parihar R, Magro C, Nuovo GJ, Tridandapani S, Carson WE. Natural killer cells produce T cell-recruiting chemokines in response to antibody-coated target cells. Cancer Res. 2006;66(1):517–526. doi: 10.1158/0008-5472.CAN-05-2429. [DOI] [PubMed] [Google Scholar]
- 6.Cartron G, Dacheux L, Salles G, Solal-Celigny P, Bardos P, Colombat P, Watier H. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood. 2002;99:754–758. doi: 10.1182/blood.V99.3.754. [DOI] [PubMed] [Google Scholar]
- 7.Musolino A, Naldi N, Bortesi B, Pezzuolo D, Capelletti M, Missale G, Laccabue D, Zerbini A, Camisa R, Bisagni G, Neri TM, Ardizzoni A. Immunoglobulin G fragment c receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J Clin Oncol. 2008;26(11):1789–1796. doi: 10.1200/JCO.2007.14.8957. [DOI] [PubMed] [Google Scholar]
- 8.Bibeau F, Lopez-Crapez E, Di Fiore F, Thezenas S, Ychou M, Blanchard F, Lamy A, Penault-Llorca F, Frebourg T, Michel P, Sabourin J-C, Boissiere-Michot F. Impact of FcγRIIa-FcγRIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J Clin Oncol. 2009;27(7):1122–1129. doi: 10.1200/JCO.2008.18.0463. [DOI] [PubMed] [Google Scholar]
- 9.Nakanishi K, Yoshimoto T, Tsutsui H, Okamura H. Interleukin-18 regulates both Th1 and Th2 responses. Ann Rev Immunol. 2001;19:423–474. doi: 10.1146/annurev.immunol.19.1.423. [DOI] [PubMed] [Google Scholar]
- 10.Osaki T, Peron J-M, Cai Q, Okamura H, Robbins PD, Kurimoto M, Lotze MT, Tahara H. IFN-γ-inducing factor/IL-18 administration mediates IFN-γ- and IL-12-independent antitumor effects. J Immunol. 1998;160:1742–1749. [PubMed] [Google Scholar]
- 11.Hashimoto W, Osaki T, Okamura H, Robbins PD, Kurimoto M, Nagata S, Peron J-M, Lotze MT, Tahara H. Differential antitumor effects of administration of recombinant IL-18 or recombinant IL-12 are mediated primarily by Fas–Fas ligand- and perforin-induced tumor apoptosis, respectively. J Immunol. 1999;163:583–589. [PubMed] [Google Scholar]
- 12.Robertson MJ, Mier JW, Logan T, Atkins M, Koon H, Koch KM, Kathman S, Pandite LN, Oei C, Kirby LC, Jewell RC, Bell WN, Thurmond LM, Weisenbach J, Roberts S, Dar MM. Clinical and biological effects of recombinant human interleukin-18 administered by intravenous infusion to patients with advanced cancer. Clin Cancer Res. 2006;12(14):4265–4273. doi: 10.1158/1078-0432.CCR-06-0121. [DOI] [PubMed] [Google Scholar]
- 13.Robertson MJ, Kirkwood JM, Logan TF, Koch KM, Kathman S, Kirby LC, Bell WN, Thurmond LM, Weisenbach J, Dar MM. A dose-escalation study of recombinant human interleukin-18 using two different schedules of administration in patients with cancer. Clin Cancer Res. 2008;14(11):3462–3469. doi: 10.1158/1078-0432.CCR-07-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Robertson MJ, Abonour R, Hromas R, Nelson RP, Fineberg NS, Cornetta K. Augmented high-dose regimen of cyclophosphamide, carmustine, and etoposide with autologous hematopoietic stem cell transplantation for relapsed and refractory aggressive non-Hodgkin’s lymphoma. Leuk Lymphoma. 2005;46:1477–1487. doi: 10.1080/10428190500158466. [DOI] [PubMed] [Google Scholar]
- 15.Robertson MJ, Caligiuri MA, Manley TJ, Levine H, Ritz J. Human natural killer cell adhesion molecules: differential expression after activation and participation in cytolysis. J Immunol. 1990;145:3194–3201. [PubMed] [Google Scholar]
- 16.Chang H-C, Han L, Goswami R, Nguyen ET, Pelloso D, Robertson MJ, Kaplan MH. Impaired development of human Th1 cells in patients with deficient expression of STAT4. Blood. 2009;113(23):5887–5890. doi: 10.1182/blood-2008-09-179820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parihar R, Dierksheide J, Hu Y, Carson WE. IL-12 enhances the natural killer cell cytokine response to Ab-coated target cells. J Clin Invest. 2002;110(7):983–992. doi: 10.1172/JCI15950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roda JM, Parihar R, Lehman A, Mani A, Tridandapani S, Carson WE. Interleukin-21 enhances NK cell activation in response to antibody-coated targets. J Immunol. 2006;177:120–129. doi: 10.4049/jimmunol.177.1.120. [DOI] [PubMed] [Google Scholar]
- 19.Fehniger TA, Shah MH, Turner MJ, VanDeusen JB, Whitman SP, Cooper MA, Suzuki K, Wechser M, Goodsaid F, Caligiuri MA. Differential cytokine and chemokine gene expression by human NK cells following activation with IL-18 or IL-15 in combination with IL-12: implications for the innate immune response. J Immunol. 1999;162:4511–4520. [PubMed] [Google Scholar]
- 20.Robertson MJ, Chang H-C, Pelloso D, Kaplan MH. Impaired interferon-γ production as a consequence of STAT4 deficiency after autologous hematopoietic stem cell transplantation for lymphoma. Blood. 2005;106:963–970. doi: 10.1182/blood-2005-01-0201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robertson MJ, Pelloso D, Abonour R, Hromas RA, Nelson RP, Jr, Wood L, Cornetta K. Interleukin-12 immunotherapy after autologous stem cell transplantation for hematologic malignancies. Clin Cancer Res. 2002;8:3383–3393. [PubMed] [Google Scholar]
- 22.Trotta R, Dal Col J, Yu J, Ciarlariello D, Thomas B, Zhang X, Allard J, Wei M, Mao H, Byrd JC, Perrotti D, Caligiuri MA. TGF-β utilizes SMAD3 to inhibit CD16-mediated IFN-γ production and antibody-dependent cellular cytotoxicity in human NK cells. J Immunol. 2008;181:3784–3792. doi: 10.4049/jimmunol.181.6.3784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Perussia B, Trinchieri G, Jackson A, Warner NL, Faust J, Rumpold H, Kraft D, Lanier LL. The Fc receptor for IgG on human natural killer cells: phenotypic, functional, and comparative studies with monoclonal antibodies. J Immunol. 1984;133(1):180–189. [PubMed] [Google Scholar]
- 24.Metes D, Ernst LK, Chambers WH, Sulica A, Herberman RB, Morel PA. Expression of functional CD32 molecules on human NK cells is determined by an allelic polymorphism of the FcγRIIC gene. Blood. 1998;91(7):2369–2380. [PubMed] [Google Scholar]
- 25.Metes D, Manciulea M, Petrusca D, Rabinowich H, Ernst LK, Popescu I, Calugaru A, Sulica A, Chambers WH, Herberman RB, Morel PA. Ligand binding specificities and signal transduction pathways of Fcγ receptor IIc isoforms: the CD32 isoforms expressed by human NK cells. Eur J Immunol. 1999;29:2842–2852. doi: 10.1002/(SICI)1521-4141(199909)29:09<2842::AID-IMMU2842>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 26.Cassatella MA, Anegon I, Cuturi MC, Griskey P, Trinchieri G, Perussia B. Fcg R (CD16) interaction with ligand induces Ca2 + mobilization and phosphoinositide turnover in human natural killer cells: role of Ca2 + in Fcγ R (CD16)-induced transcription and expression of lymphokine genes. J Exp Med. 1989;169:549–567. doi: 10.1084/jem.169.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mailliard RB, Alber SM, Shen H, Watkins SC, Kirkwood JM, Herberman RB, Kalinski P. IL-18-induced CD83 + CCR7 + NK helper cells. J Exp Med. 2005;202(7):941–953. doi: 10.1084/jem.20050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schroder K, Hertzog PJ, Ravasi T, Hume DA. Interferon-γ: an overview of signals, mechanisms and functions. J Leukoc Biol. 2004;75:163–189. doi: 10.1189/jlb.0603252. [DOI] [PubMed] [Google Scholar]
- 29.Kondadasula SV, Roda JM, Parihar R, Yu J, Lehman A, Caligiuri MA, Tridandapani S, Burry RW, Carson WE. Colocalization of the IL-12 receptor and FcγRIIIa to natural killer cell lipid rafts leads to activation of ERK and enhanced production of interferon-g. Blood. 2008;111(8):4173–4183. doi: 10.1182/blood-2007-01-068908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mavropoulos A, Sully G, Cope AP, Clark A. Stabilization of IFN-γ mRNA by MAPK p38 in IL-12- and IL-18-stimulated human NK cells. Blood. 2004;105:282–288. doi: 10.1182/blood-2004-07-2782. [DOI] [PubMed] [Google Scholar]
- 31.Nakahira M, Ahn H-J, Park W-R, Gao P, Tomura M, Park C-S, Hamaoka T, Ohta T, Kurimoto M, Fujiwara H. Synergy of IL-12 and IL-18 for IFN-γ gene expression: IL-12-induced STAT4 contributes to IFN-γ promoter activation by up-regulating the binding activity of IL-18-induced activator protein 1. J Immunol. 2002;168:1146–1153. doi: 10.4049/jimmunol.168.3.1146. [DOI] [PubMed] [Google Scholar]
- 32.Zhang S, Kaplan MH. The p38 mitogen-activated protein kinase is required for IL-12-induced IFN-γ expression. J Immunol. 2000;165:1375–1380. doi: 10.4049/jimmunol.165.3.1374. [DOI] [PubMed] [Google Scholar]
- 33.Robertson MJ. Role of chemokines in the biology of natural killer cells. J Leukoc Biol. 2002;71:173–183. [PubMed] [Google Scholar]
- 34.Overdijk MB, Verploegen S, Buijsse AO, Vink T, Leusen JHW, Bleeker WK, Parren PWHI. Crosstalk between human IgG isotypes and murine effector cells. J Immunol. 2012;189:3430–3438. doi: 10.4049/jimmunol.1200356. [DOI] [PubMed] [Google Scholar]
- 35.Daniel D, Yang B, Lawrence DA, Totpal K, Balter I, Lee WP, Gogineni A, Cole MJ, Yee SF, Ross S, Ashkenazi A. Cooperation of the proapoptotic receptor agonist rhApo2L/TRAIL with the CD20 antibody rituximab against non-Hodgkin lymphoma xenografts. Blood. 2007;110:4037–4046. doi: 10.1182/blood-2007-02-076075. [DOI] [PubMed] [Google Scholar]
- 36.Hernandez-Ilizaliturri FJ, Reddy N, Holkova B, Ottman E, Czuczman MS. Immunomodulatory drug CC-5013 or CC-4047 and rituximab enhance antitumor activity in a severe combined immunodeficient mouse lymphoma model. Clin Cancer Res. 2005;11(16):5984–5992. doi: 10.1158/1078-0432.CCR-05-0577. [DOI] [PubMed] [Google Scholar]
- 37.Wigginton JM, Lee J-K, Wiltrout TA, Alvord WG, Hixon JA, Subleski J, Back TC, Wiltrout RH. Syngergistic enhancement of ineffective endogenous antitumor immune response and induction of IFN-γ and Fas-ligand-dependent tumor eradication by combined administration of IL-18 and IL-2. J Immunol. 2002;169:4467–4474. doi: 10.4049/jimmunol.169.8.4467. [DOI] [PubMed] [Google Scholar]
- 38.Jonak ZL, Trulli S, Maier C, McCabe FL, Kirkpatrick R, Johanson K, Ho YS, Elefante L, Chen Y-J, Herzyk D, Lotze MT, Johnson RK. High-dose recombinant interleukin-18 induces an effective Th1 immune response to murine MOPC-315 plasmacytoma. J Immunother. 2002;25(Suppl. 1):S20–S27. doi: 10.1097/00002371-200203001-00004. [DOI] [PubMed] [Google Scholar]
- 39.Robertson MJ, Bauman J, Gardner O, Jonak Z, Struemper H, Germaschewski F, Koch KM, Murray S, Weisenbach J, Toso J. A phase I trial evaluating the safety and biological activity of iboctadekin (rhIL-18) in combination with rituximab in patients with CD20 + B cell non-Hodgkin’s lymphoma. Blood. 2011;118:1579–1580. doi: 10.1182/blood-2010-08-300343. [DOI] [PMC free article] [PubMed] [Google Scholar]