Abstract
The potent actions of pigment epithelium-derived factor (PEDF) on tumour-associated cells, and its extracellular localization and secretion, stimulated research on this multifunctional serpin. Such studies have identified several PEDF receptors and downstream signalling pathways. Known cellular PEDF responses have expanded from the initial discovery that PEDF induces retinoblastoma cell differentiation to its anti-angiogenic, antitumorigenic and antimetastatic properties. Although the diversity of PEDF activities seems to be complex, they are consistent with the varied mechanisms that regulate this multimodal factor. If PEDF is to be used for cancer management, a deeper appreciation of its many functions and mechanisms of action is needed.
Pigment epithelium-derived factor (PEDF; encoded by SERPINF1 and also known as EPC1 and caspin), is a serpin that has multiple biological actions. The era of PEDF research began around 1990 with the discovery that PEDF is a differentiation factor for retinoblastoma cells1,2. The PEDF protein was isolated from media that was conditioned by cultured retinal pigment epithelial cells, hence its name. Soon after, it was reported that expression of SERPINF1 is increased in quiescent young fibroblasts and is specifically associated with G0 growth arrest: PEDF expression levels are negligible in senescent fibroblasts3,4. Moreover, PEDF levels decline during ageing, and its expression is used as a marker for young cells5–7. About a decade after its discovery, PEDF was found to be a potent inhibitor of angiogenesis8. This finding, along with the fact that ageing is the major risk factor for the development of several different types of cancer — age-related changes in the tissue microenvironment facilitate tumour growth9,10 — provided the impetus to study the mechanisms of action and regulation of PEDF, and its possible applications to cancer therapy. This has led to the identification of PEDF as a major antagonist to angiogenic factors (such as vascular endothelial growth factor (VEGF)), evidence for its antitumorigenic and antimetastatic activities, and its potential use as a diagnostic and prognostic marker for cancer management.
In this Review, we discuss some of the new insights into the action of PEDF on tumours, and we focus on emerging concepts and the mechanisms of action and regulation of PEDF. We summarize the evidence that the diminishing levels of PEDF observed in various tumour types may account, at least in part, for increased malignant characteristics during tumour progression. We briefly discuss recent reports on PEDF involvement in lipid metabolism and its relevance to cancer. Finally, given that the potential use of PEDF in cancer therapeutics has generated much expectation, we also examine reports on the development of promising PEDF delivery systems.
PEDF biochemistry
As a member of the serine protease inhibitor (serpin) superfamily, PEDF belongs to a group of proteins that have a common three-dimensional structure1,11. The three-dimensional structure of human PEDF has been determined by X-ray crystallography12 (Protein Data Bank (PDB) identifier 1IMV) and shows that the protein folds like a serpin. Most serpins, such as antitrypsin, antichymotrypsin and antithrombin, are serine protease inhibitors whereas others, such as ovalbumin, angiotensinogen and maspin, do not have demonstrable protease inhibitory properties13–15. PEDF does not undergo the stressed to relaxed conformational transition that is characteristic of active serpins, and does not have demonstrable serine protease inhibitory activity16. Thus, it is a member of the subgroup of non-inhibitory serpins that are thought to have lost their protease inhibitory activity but have gained additional properties during evolution. Interestingly, like PEDF, antithrombin, angiotensinogen and maspin exhibit anti-angiogenic and antitumorigenic activities, thus suggesting that a common structural determinant among them might be crucial for their function.
SERPINF1 is localized on human chromosome 17p13.1, and it encodes a polypeptide of 418 amino acids that includes an amino-terminal secretion signal peptide, one N-glycosylation site at Asn285 (in the sequence NLT) and a serpin signature sequence YHLNQPFIFVL that ends at amino acid 3981,11,17. Most cells express PEDF transcripts, and the mature gene product is mainly secreted as a soluble monomeric glycoprotein that has an apparent molecular weight of ∼50,000 daltons, a molecular radius (Stokes radius) <3.05 nm and an amino-terminal sequence starting at amino acid position 21 of the precursor polypeptide. PEDF is biologically active at 1–100 nM, depending on the assay. It is found extracellularly in blood, the interphotoreceptor matrix (IPM), vitreous humour and aqueous humour of the eye, cerebrospinal fluid, tears and other body fluids at physiologically relevant concentrations18–22. Its biological activities are thought to depend on its interactions with cell-surface receptors: PEDF receptor (PEDFR; encoded by PNPLA2 and is also known as desnutrin, ATGL and iPLA2ζ), laminin receptor, F1 ATPase/synthase and low-density lipoprotein receptor-related protein 6 (LRP6) (FIG 1,FIG 2). It also has binding affinity for extracellular matrix (ECM) components heparin, heparan sulphate, hyaluronan and collagens23–25. The amino acids that are crucial for these interactions have been mapped on human PEDF; these are basic amino acids for heparin binding (Lys146, Lys147 and Arg149) and for hyaluronan binding (Lys189, Lys191, Arg194 and Lys197), and acidic amino acids for collagen binding (Asp256, Asp258 and Asp300)24,26.
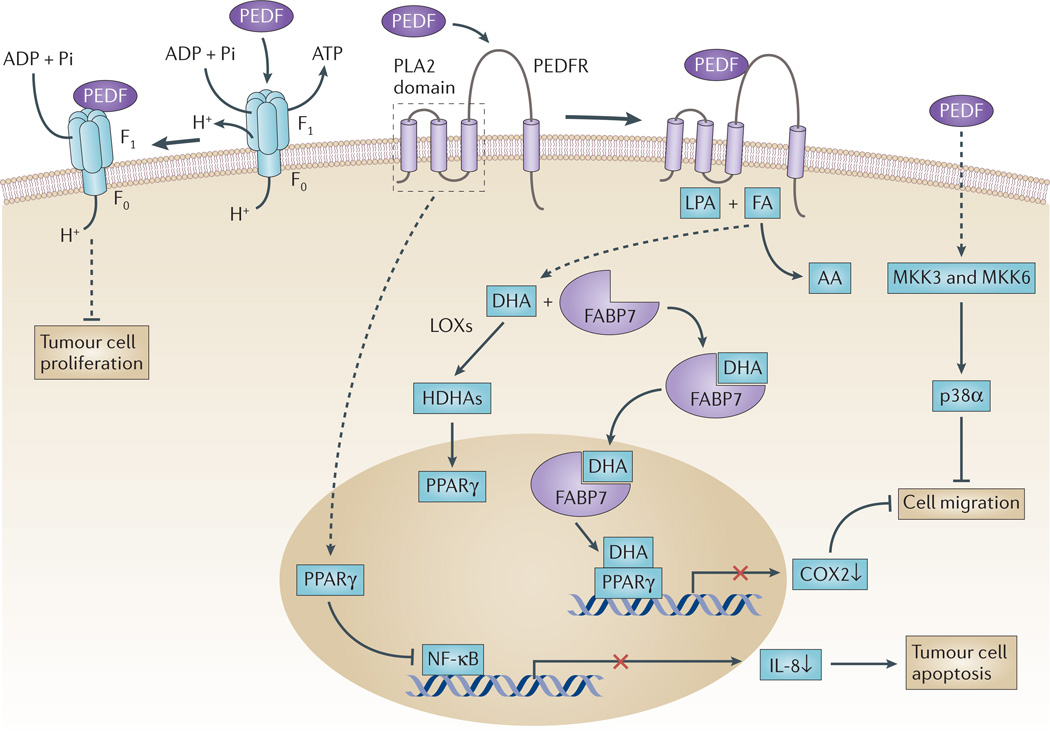
Figure 1. PEDF in tumour cells.
Pigment epithelium-derived factor (PEDF) is a ligand for several receptors, and its interaction with these receptors is thought to trigger the signalling pathways illustrated here. PEDF binds to PED F receptor (PEDFR) and stimulates its phospholipase activity91,151. When PEDFR is at the membrane, its phospholipase A2 (PLA2) active site is located close to the phospholipid bilayer where it can use phospholipids as substrates. Depending on the relative abundance of the fatty acids omega-3 docosahexaenoic acid (DHA) and omega-6 arachidonic acid (AA) in phospholipid membranes, free DHA or AA can be liberated by PEDFR. DHA is a precursor of the anti-angiogenic and neuroprotector neuroprotectin D1 (NPD1)152. Other DHA metabolites, such as hydroxy-DHAs (HDHAs), which are produced by lipoxygenases (LOXs), can act on peroxisome proliferator-activated receptor-γ (PPARγ)102,105,153. Upregulation of PPARγ leads subsequently to the suppression of nuclear factor-κB (NF-κB)-mediated transcriptional activation, reduced production of interleukin 8 (IL-8) and limited proliferation of prostate cancer cells104. Cytosolic fatty-acid-binding protein 7 (FABP7) can bind DHA with higher affinity than AA, translocate DHA to the nucleus and transfer it to PPARγ, thus resulting in the downregulation of promigratory genes, such as cyclooxygenase 2 (COX2)96. PEDF is a ligand of cell-surface F ATP synthase, and its 34-mer peptide region inhibits ATP production and reduces endothelial and tumour cell viability and angiogenesis107,108. PEDF, through interaction with a yet unknown receptor, can sequentially activate MKK3, MKK6 and p38α MAPK to inhibit cell migration77. FA, fatty acid; LPA, lysophosphatidic acid.
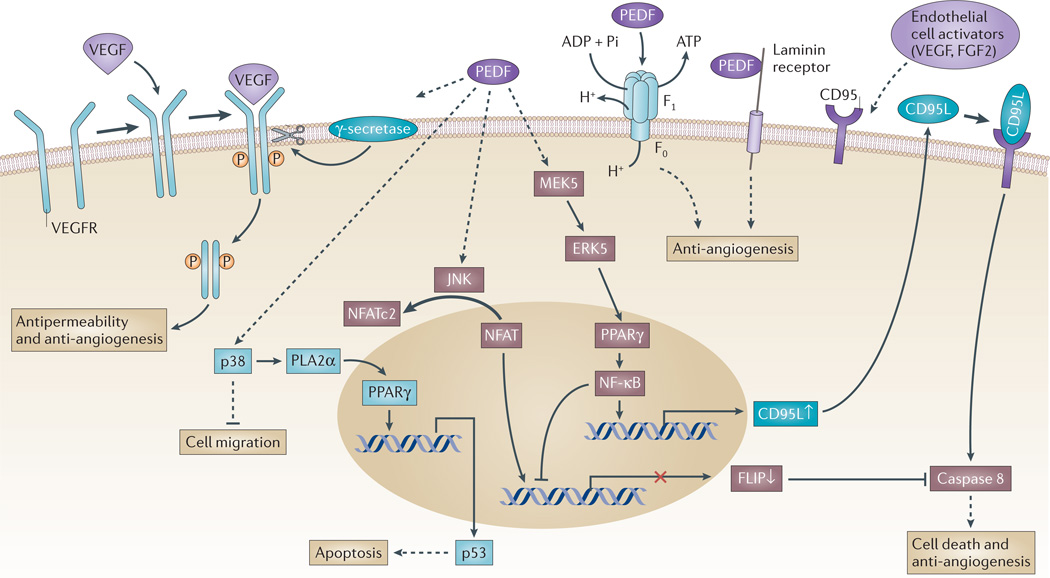
Figure 2. Signalling events of PEDF in endothelial cells.
Vascular endothelial growth factor (VEGF) binds to a homodimerized VEGF receptor (VEGFR), which becomes phosphorylated and activated. Pigment epithelium-derived factor (PEDF) can increase γ-secretase-mediated cleavage of VEGFR1 and VEGFR2 at the transmembrane region to generate an intracellular domain fragment87,154. At the same time, PEDF can inhibit VEGF-induced phosphorylation and activation of VEGFR1 (REF. 86). PEDF inhibits VEGF-driven angiogenesis and permeability through the regulated intracellular proteolysis of VEGFR. PEDF can activate the p38 MAPK pathway to inhibit endothelial cell migration77. It can also activate peroxisome proliferator-activated receptor-γ (PPARγ) through cytosolic phospholipase A2α (PLA2α) to induce the expression of TP53, which encodes the pro-apoptotic protein p53 (REF. 155). At the same time, endothelial activators such as VEGF and fibroblast growth factor 2 (FGF2) stimulate and expose CD95 (also known as FAS) on the endothelial plasma membrane. PEDF can sequentially activate MEK5 (which is a MAPK kinase), ERK5, PPARγ and nuclear factor-κB (NF-κB), which induces the expression of the pro-apoptotic gene CD95 ligand (CD95L), the protein product of which translocates to the plasma membrane. The resulting CD95L-CD95 complex induces the binding and activation of caspase 8 that under certain conditions triggers the cell death cascade66,88. At the same time, NF-κB activation has a negative impact on cellular FLICE-like inhibitory protein (FLIP) expression, which decreases the capacity of FLIP to inhibit caspase 8. Conversely, PEDF triggers JUN N-terminal kinase (JNK)-mediated phosphorylation of nuclear factor of activated T-cells, cytoplasmic 2 (NFATc2) and sequesters it in the cytoplasm, thus blocking FLIP expression. In this manner, PEDF leads to apoptosis in activated endothelial cells. PEDF is a ligand of two known proteins on endothelial cells that result in anti-angiogenic responses. PEDF binds to laminin receptor106 and cell-surface F1 ATP synthase to inhibit ATP production and inhibit angiogenesis104,107,108.
Segmentation of the PEDF polypeptide by chemical proteolysis and recombinant DNA technology provided much of the information that has been accumulated to date on the structure–function relationships of PEDF. Most proteinases cleave PEDF at its homologous serpin reactive loop, leaving a core polypeptide that retains the anti-angiogenic, differentiating and neurotrophic activities of the protein, as well as its affinity for ECM components16,23,25. More importantly, when PEDF is truncated from its carboxy-terminal end, such as bacterially expressed BH (Asp44–Pro418), BP (Asp44–Pro267), BX (Asp44–Leu228) and BA (Asp44–Thr121) fragments, it retains its neuronal-differentiating and survival activities in retinoblastoma cells, cerebellar granule cells and motor neurons16,27–31. Synthetic peptides based on the smallest BA region — a 34 amino acid peptide (Asp44–Asn77) and a 44 amino acid peptide (Val78–Thr121) — exhibit anti-angiogenic and differentiating activities, respectively32–35. A shorter peptide derived from the 34 amino acid peptide designated P18 (Asn60–Asn77) is more effective in blocking angiogenesis than the parental 34-mer peptide, and P18 inhibits the growth of prostate and renal tumour xenografts36.
PEDF and its relevance to cancer
The major biological responses to PEDF observed in vitro and in vivo are summarized in TABLE 1. These observations show that PEDF has been implicated in diverse biological processes, such as neurogenesis, neuroprotection, anti-angiogenesis, retina protection, stem cell renewal and inflammation. One prominent area of interest is the emerging anticancer role for PEDF. The strongest support for this role comes from the findings that PEDF exhibits anti-angiogenic and antimetastatic activities. Moreover, the exogenous administration of PEDF to bolster the declining intratumoural levels of PEDF during tumour progression results in the inhibition of tumour growth and prolonged survival in various animal models (TABLE 2). It is important to note that the functions and mechanisms of PEDF often act in opposition to protumorigenic processes (FIG. 3).
Table 1.
Major biological responses to PEDF
| PEDF action | Target | Refs |
|---|---|---|
| Differentiating activity | Retinoblastoma cells, macrophages,
microglia, neuroblastomas and prostate tumours |
2,35,40,150,156,157 |
| Morphogenetic | Photoreceptor cells and Muller cells | 158,159 |
| Neurite outgrowth | Retinal neurons and spinal cord motor neurons | 29,160 |
| Survival activity, anti-apoptotic | Cerebellar granule cells, motor neurons,
retinal ganglion cells, photoreceptors and retinal progenitor cells |
46,50,161–166 |
| Self-renewal factor | Neural stem cells, retinal stem cells and
human embryonic stem cells |
167–169 |
| Antipermeability | Retinal pigment epithelial cells and endothelial cells | 110,154,170 |
| Anti-inflammatory | Retina | 171 |
| Pro-inflammatory | Adipose tissue and macrophages | 172,173 |
| Anti-angiogenic | Ocular endothelial and tumour endothelial cells |
8,33,35,36,44,54,59,60,64,71, 73,77,88,106,125,126,141,142 |
| Induces cell death | Endothelial, tumour and glial cells |
35,36,44,59,60,71,73,77,88, 106,126,142 |
PEDF, pigment epithelium-derived factor.
Table 2.
Cancers that respond to PEDF
| Tumour type | PEDF action | Refs |
|---|---|---|
| Retinoblastoma |
|
1,2,39,59 |
| Neuroblastoma |
|
40,72,174 |
| Prostate |
|
35,47,68,104,157,175 |
| Melanoma |
|
56,57,80,82,176 |
| Wilms’ tumour |
|
51,52 |
| Pancreatic adenocarcinoma |
|
47,53,79,83 |
| Hepatoblastoma |
|
54 |
| Osteosarcoma |
|
55,74,75,149,177,178 |
| Chondrosarcoma |
|
76 |
| Human cervical carcinoma |
|
60 |
| Gastric carcinoma |
|
61 |
| Nasopharyngeal carcinoma |
|
62 |
| Lewis lung carcinoma |
|
63,144 |
| Colorectal peritoneal carcinoma |
|
64,71,73 |
| Glioma |
|
47,69,70,73,146 |
| Uveal melanoma |
|
81,126 |
| Breast cancer xenograft |
|
73,77 |
| Brain metastasis from
breast tumours |
|
41,78 |
HIF1α, hypoxia-inducible factor 1α; MVD, microvessel density; PEDF, pigment epithelium-derived factor; VEGF, vascular endothelial growth factor.
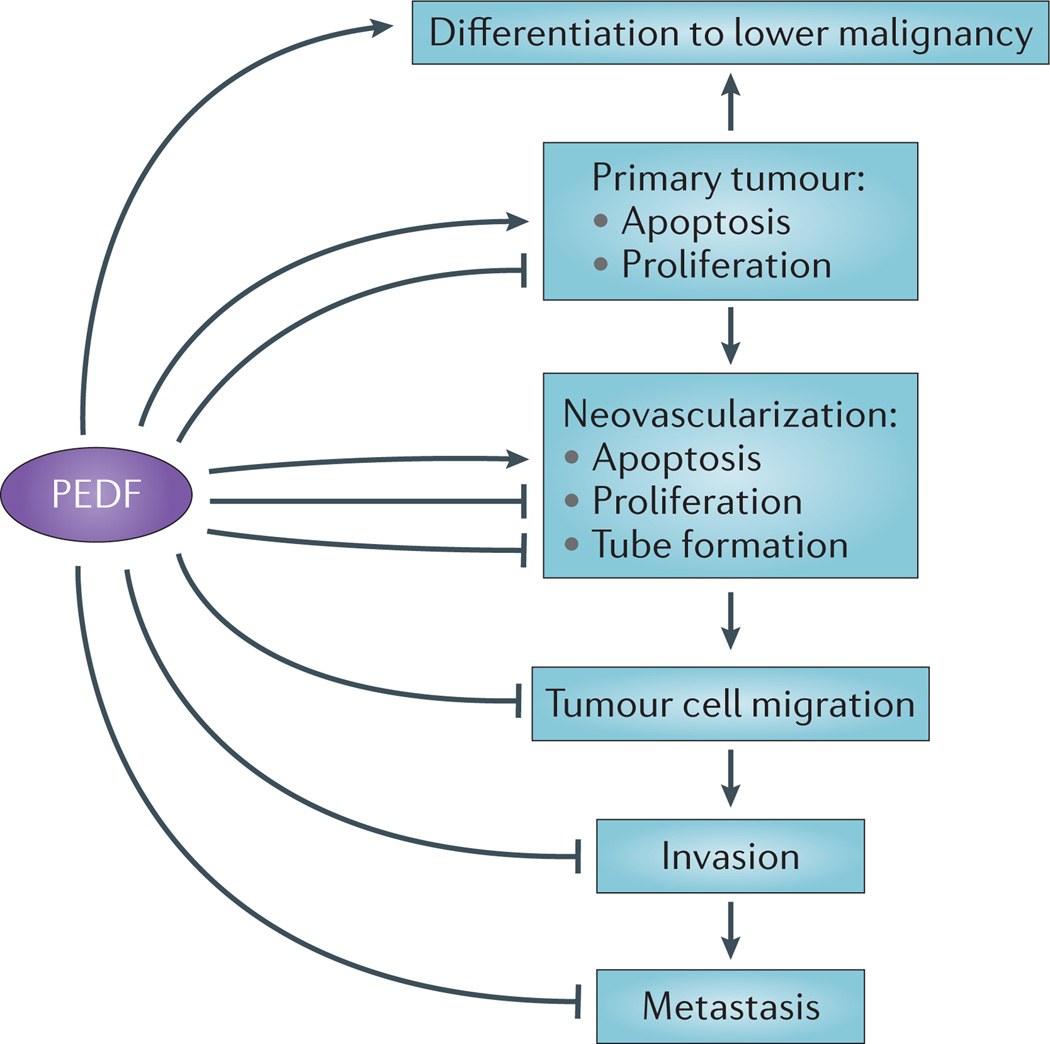
Figure 3. The effects of PEDF on tumour progression.
A linear representation of different pigment epithelium-derived factor (PEDF) targets that form a concerted set of activities to inhibit cancer progression. PEDF can differentiate tumours to a less-malignant phenotype. Tumour cells secrete angiogenic factors to activate endothelial cells. PEDF can block angiogenesis-mediated activities and neovascularization. PEDF can also block tumour migration, invasion and metastasis.
From neuronal differentiation to inhibiting tumour growth
The discovery of PEDF was based on the fact that it promotes the differentiation of human Y-79 and Weri retinoblastoma cells1,37,38. PEDF promotes neurite outgrowth from these cells with concomitant increases in the expression of neuronal markers such as neuron-specific enolase and neurofilament proteins. The ability of PEDF to promote the differentiation of retinoblastoma cells and other tumour cells of neuronal origin provided the first suggestion that PEDF could act directly on tumours and reduce their malignant phenotype. In this regard, PEDF-treated retinoblastoma cells are less tumorigenic than untreated controls, as shown by their delayed formation of tumours in rat retinas39. In the brain, Schwann cells that naturally secrete PEDF induce the differentiation of neuroblastoma cells to a less-malignant phenotype, and also inhibit tumour angiogenesis, thereby demonstrating the multifunctional antitumorigenic action of PEDF in vivo40. PEDF also causes neuroendocrine differentiation in prostate cancer cells, in addition to inhibiting tumour angiogenesis35. More recently, it was demonstrated that PEDF inhibits the number and proliferation of brain metastases of breast cancer cells and concomitantly protects neurons close to the metastases from cell death41, thus highlighting its role as a double agent in limiting brain metastases and their local consequences. The ability of PEDF to induce, perhaps simultaneously, growth arrest, tumour cell differentiation to a less-malignant phenotype and protection of normal neuronal cells requires a complex system of regulation.
From inhibition of ocular neovascularization to tumour anti-angiogenesis
In the same year that the US National Cancer Institute designated the development of anti-angiogenic therapies for cancer a national priority, Dawson and co-workers8 identified PEDF as a potent inhibitor of angiogenesis in the eye. They showed that under physiological conditions PEDF is responsible for the avascularity of the IPM and the vitreous and aqueous humour, which is crucial for visual function. Remarkably, the concentrations of PEDF in the eye are inversely correlated with ocular angiogenic development42–44, and PEDF overexpression or local protein delivery prevent ocular neovascularization and delay photo-receptor and neural retinal cell death in vivo 33,40,45–50. These findings clearly show that PEDF is an endo-genous inhibitor of angiogenesis and protects the eye. Together with the detection of PEDF in several other tissues, these observations led researchers to explore the anti-angiogenic properties of PEDF on tumours.
Numerous studies in a variety of models have shown the anti-angiogenic effects that PEDF has on tumours (TABLE 2). An increasing number of studies are confirming the initial hypothesis that decreased PEDF expression is one mechanism driving tumour growth. For example, the growth of human Wilms’ tumour xenografts in mice is suppressed by PEDF51. PEDF expression is high in normal mouse and human kidneys but is significantly decreased in Wilms’ tumours. Systemic administration of PEDF suppresses tumour growth by targeting not only the tumour-associated vasculature, but also the tumour cells52. In another example, PEDF overexpression using lentiviral vectors blocks tumour angiogenesis in an animal model of pancreatic adenocarcinoma, leading to the proposal that PEDF gene therapy may provide a new treatment approach53. Similarly, augmenting intratumoural PEDF levels using adenoviral and adeno-associated viral vectors that are engineered to express PEDF inhibits tumour growth in a hepatoblastoma xenograft model by reducing angiogenesis and decreasing VEGF expression54. PEDF overexpression also inhibits orthotopic osteo-sarcoma growth, angiogenesis and metastasis55. The negative actions of PEDF on primary melanoma tumour xenografts are associated mostly with inhibition of the angiogenic response and the subsequently decreased microvessel density (MVD) in the tumours56,57. In this model, the inhibition of melanoma growth is through the inhibition of angiogenesis58. Furthermore, in vitro, PEDF inhibits melanoma cell growth by inducing apoptotic cell death58. PEDF inhibits the growth of heterotopic SO-Rb50 retinoblastoma xenografts in mice also through its anti-angiogenic activity, whereby it decreases both MVD and VEGF expression59. Likewise, PEDF is downregulated in human cervical carcinoma nests compared with either the normal cervical epithelium or the non-neoplastic peritumoural epithelium60. The intraperitoneal injection of recombinant PEDF in mice with cervical carcinoma xenografts suppresses tumour growth. This is associated with decreases in MVD and VEGF expression levels and with the inhibition of proliferation and the induction of apoptosis in endothelial cells60. In another report, PEDF suppressed angiogenesis and the growth of gastric carcinoma in a xenograft model by downregulating hypoxia-inducible factor 1α (HIF1α) and VEGF61. It has been recently demonstrated that the combination of PEDF with radiotherapy increases the antitumour efficacy of each individual agent in an animal model of nasopharyngeal carcinoma62. In this system, the simultaneous treatment with PEDF and radiation has an additive effect on the downregulation of VEGF expression and on angio-genesis inhibition. Furthermore, PEDF overexpression by adeno-associated virus-mediated gene transfer inhibits Lewis lung carcinoma growth and inhibits metastasis in a mouse model of colorectal peritoneal carcinomatosis by decreasing MVD and increasing tumour cell apoptosis63,64.
The anti-angiogenic effect of PEDF is associated with disruption of the intratumoural vascular network. PEDF blocks the formation of endothelial capillary-like networks in culture and vessel sprouting from chicken aortic rings ex vivo 33,65. Interestingly, PEDF selectively induces endothelial cell apoptosis in actively remodelling vessels rather than mature, existing ones66,67. A remarkable characteristic of PEDF is its potent anti-angiogenic activity compared with other endogenous inhibitors of neovascularization, such as thrombospondin, angiostatin or endostatin8, which makes PEDF an excellent candidate for drug development. PEDF is thought to interact with specific receptors on the surface of endothelial cells to trigger anti-angiogenic signalling pathways that alter gene expression (FIG. 2). In summary, increasing the levels of PEDF in a number of animal models resulted in suppression of tumorigenesis, which was mediated mainly by inhibition of intratumoural neovascularization and endothelial proliferation, and by promotion of endothelial cell apoptosis and downregulation of pro-angiogenic factors.
Direct effects of PEDF on tumour cell death and proliferation
As noted in some of the studies discussed above, in addition to its differentiating and anti-angiogenic activities, PEDF exerts direct effects on tumour growth in vivo. In culture, PEDF can inhibit proliferation and promote apoptosis of tumour cells. As highlighted in a report by Doll and co-workers47, exogenous recombinant PEDF induces tumour epithelial cell apoptosis in vitro and limits the growth of prostate tumour xenografts in vivo by simultaneously causing extensive necrosis and significantly reducing the proliferation of cells in the remaining viable tumour regions. Overexpression of PEDF using adenoviral vectors can also inhibit the proliferation and augment the apoptosis of PC-3 prostate carcinoma cells, in comparison with control cells68. Similarly, a proportion of cultured glioma cells undergo cell death after exposure to PEDF in vitro, in a dose-dependent manner69. Moreover, prevention of cell growth and induction of apoptosis by overexpressing PEDF in U251 glioma cells was accompanied by thrombospondin upregulation, VEGF and fibroblast growth factor 2 (FGF2) downregulation, and a lower production of matrix metalloproteinase 9 (MMP9) relative to control cells70. Consistently, PEDF-induced apoptosis is associated with increased levels of p53 and BAX, and the concomitant inhibition of BCL-2. In vivo, xenografts of PEDF-transfected U251 glioma cells, which also undergo apoptosis, are significantly smaller than controls that do not express PEDF. These findings, along with the observed loss of PEDF expression during glioma progression, point to the potential of PEDF as a treatment for patients with malignant gliomas. Apoptosis is also detected in other xenograft models in which PEDF is overexpressed, such as mouse colorectal peritoneal carcinomatosis and Lewis lung carcinoma63,64,71. Recombinant PEDF also induced apoptosis of osteosarcoma SaOS-2 cells in vitro, and in mice it reduced the growth of SaOS-2 tibial tumours and the number of lung macrometastases72. The negative effects of PEDF on tumour cell viability have also been demonstrated using breast cancer cells in vitro and through direct intracranial implantation to model metastasis41. Konson and co-workers73 compared the rate of apoptosis in PEDF-treated MDA-MB-231, HCT116 and U87-MG tumour cells to that of PEDF-treated BAEC and HUVEC endothelial cells and observed that the apoptosis-inducing efficacy of PEDF in culture is stronger on endothelial cells relative to tumour cells.
PEDF and inhibition of tumour cell invasion and metastasis
Metastasis remains the cause of death for most cancer patients. The ability of PEDF to suppress tumour cell invasion and migration has been described in vitro and in several metastasis models in vivo. For example, it has been shown that PEDF can reduce the invasiveness of UMR 106–01 and SaOS-2 osteosarcoma cells, and can suppress the development of macroscopic pulmonary metastasis in an orthotopic human osteosarcoma model55,74. Like full length PEDF, human PEDF-derived peptides markedly increased osteosarcoma cell adhesion to type-1 collagen (collagen I) and significantly inhibited invasion75. Similarly, the addition of PEDF increased the adhesion of chondrosarcoma cells to surfaces coated with collagen I and, consistently, decreased cell invasion through collagen I gels76. The authors proposed that PEDF is a clinically appealing drug for the treatment of connective-tissue cancers, such as osteosarcoma and chondrosarcoma, and suggested the possibility of developing short PEDF peptide fragments as therapeutic tools.
Another example is in brain metastases of breast cancer origin, which are a substantial cause of morbidity and mortality for patients with breast cancer. Because PEDF is downregulated in these metastases compared with primary breast tumours, Fitzgerald and co-workers41 explored the possible benefits of restoring PEDF to higher expression levels to limit the metastatic potential of breast cancer cells. They showed that PEDF overexpression decreases the metastasis of human and mouse breast cancer cells to the brain, in a rapid and angiogenesis-independent manner. The exciting observation was that PEDF also exhibits its known survival effect on the neurons that shielded the brain from tumour-induced damage. In culture, PEDF exhibits potent antimigratory activity on human breast tumour cells77,78 and neuroprotectant activity on neurons. These observations emphasize the dual role of PEDF as both a metastatic suppressor and a neuroprotectant in the brain.
The inverse association between PEDF expression levels and metastasis has been reported in studies of the invasiveness of glioma70, metastasis to the liver from pancreatic ductal adenocarcinoma79, progression towards a metastatic phenotype in prostate tumours68, and liver and lung metastases of melanoma origin57,80. More importantly, PEDF emerged as an inhibitor of metastasis in these models both in vivo and in vitro. Lentivirus-mediated PEDF gene transfer not only decreased tumour size and intratumoural MVD, but also the number of hepatic micrometastases in a mouse model of ocular melanoma81. Consistently, silencing of PEDF expression in normal melanocytes and poorly aggressive melanoma cell lines increased their migration and invasiveness, which translated into an increased proliferative and in vivo metastatic potential80. It has been observed that metastatic melanoma cells adapt to facilitate metastasis by promoting a switch from a protease-dependent mesenchymal morphology to a protease-independent amoeboid phenotype82. Interestingly, Ladhani and co-workers82 reported that PEDF suppresses the rounded morphology of melanoma cells and inhibits the surface localization of MMP14 (also known as MT1-MMP). This shows that PEDF blocks tumour extravasation by regulating cell shape and proteolysis, and thus identified a potential mechanism for its antimetastatic activity. Overall, these findings suggest that increasing the level of endogenous PEDF may provide an effective antimetastatic approach.
Recently, Grippo and co-workers83 established a connection between tumour invasion, PEDF expression and lipid metabolism in the pancreas. PEDF levels are lowered in pancreatic cancers, and its expression is correlated with reduced hepatic metastases and an improved prognosis. These authors also reported an inverse correlation between PEDF and VEGF levels in human pancreatic cancer. Interestingly, PEDF has been implicated in regulating lipid metabolism and adipogenesis, both of which are known to influence pancreatic cancer progression83–85. In the elastase (EL)-KrasG12D mouse model of non-invasive cystic papillary neoplasms, loss of Serpinf1 results in the development of pancreatic ductal adenocarcinoma83. Moreover, these mice have increased pancreatic stromal adiposity, and the cells within the stroma express adipose markers. These findings suggest that PEDF is a crucial negative regulator of adiposity and tumour progression in the pancreas. The therapeutic implications of these findings have yet to be tested.
Molecular mechanisms of action
Although the diversity of PEDF activities may seem complex, they are consistent with the mechanisms regulating this multimodal factor. The molecular mechanisms by which PEDF functions to regulate tumour and endo-thelial cell behaviour have begun to be elucidated and are based mostly on the interactions of extracellular PEDF with different cell-surface proteins expressed by target tissues. The Boulton laboratory explored the effects of PEDF on γ-secretase, which can cleave the VEGF receptor (VEGFR)86,87. They showed that the addition of PEDF to microvascular endothelial cells significantly increases γ-secretase activity even in the absence of VEGF. This finding is associated with translocation of presenilin 1 from the perinuclear region to the cell membrane. The proposed mechanism for PEDF-mediated anti-angiogenesis involves cleavage at Val767 of the VEGFR1 transmem-brane domain and the intracellular translocation of the carboxy-terminal fragment of VEGFR1 (FIG. 2). They also showed that PEDF upregulated presenilin 1, which facilitates the association between protein tyrosine phosphatases and VEGFR1 to inhibit VEGF-induced phosphorylation of VEGFR1. Konson and co-workers77 reported that different tumour-suppressive activities of PEDF are independently regulated by two different MAPK pathways: the JUN N-terminal kinase (JNK) pathway regulates the endothelial pro-apoptotic activity and the p38 MAPK pathway regulates antimigratory activities. The contributions of the Volpert laboratory elucidated how PEDF regulates CD95 ligand (CD95L; also known as FAS ligand) and cellular FLICE-like inhibitory protein (FLIP) to inhibit neovascularization in activated endothelial cells66,88. In these studies the authors showed that PEDF activates ERK5, which activates peroxisome proliferator-activated receptor-γ (PPARγ). This results in the increased expression of CD95L on endothelial cells88 (FIG. 2). Expression of the essential partner of CD95L, CD95, is low in quiescent endothelial cells and vessels, but is increased by inducers of angiogenesis (such as VEGF and FGF2), thereby specifically sensitizing the stimulated endothelial cells to undergo apoptosis66. The anti-angiogenic activity of PEDF both in vitro and in vivo is dependent on this dual induction of CD95 and CD95L and the resulting apoptosis. Other studies have shown that VEGF and PEDF also have different effects on nuclear factor of activated T cells cytoplasmic 2 (NFATc2). NFATc2 binds to the FLIP promoter in the presence of VEGF and leads to the increased expression of FLIP and decreased caspase-8-mediated apoptosis89. Conversely, PEDF triggers JNK-mediated phosphorylation of NFATc2 and sequesters it in the cytoplasm, which prevents the NFATc2-mediated block of FLIP expression89 (FIG. 2). It has been proposed that this coordination between pro-angiogenic factors and PEDF in the regulation of angiogenesis provides one explanation for the ability of PEDF to target remodelling capillaries for destruction. These findings indicate that PEDF probably binds to a receptor or receptors that mediate these signalling pathways in endothelial cells. Indeed, initial studies showed that endothelial cells, retinoblastoma and normal retinal cells, motor neurons and prostate tumour cells expressed receptors for PEDF, as well as for the 34-mer and 44-mer peptides of PEDF32,34,35,90.
The first receptor identified for PEDF, PEDFR, was discovered in our laboratory91. PEDFR is a lipase-linked cell membrane protein that is activated on PEDF binding, and the phospholipase activity of PEDFR results in the release of free fatty acids and lysophosphatidic acids from the phospholipids in the plasma membrane91. PEDFR also has triglyceride lipase activity that is associated with lipid droplets in adipose tissues92–94. The lipid composition of the plasma membrane determines the substrate for the phospholipase, and the product determines the type of lipid mediator. For example, PEDFR activity can result in the generation of either the omega-3 fatty acid docosahexaenoic acid (DHA) or the omega-6 fatty acid arachidonic acid (AA)94,95 (FIG. 1). DHA can reduce the invasive phenotype of human melanoma, breast and renal carcinoma cells in vitro, which implies that DHA might modify the tumour cell metastatic potential and be a lipid mediator of PEDF signalling. However, the mechanisms by which DHA can directly affect the invasive phenotype of cancer cells remain unclear. AA has promigratory and tumour growth properties96, which can be antagonized by DHA97,98. It has been suggested that the relative levels of DHA and AA in the tumour environment might have a profound impact on tumour growth properties. In this manner, the composition of fatty acids in plasma membranes is likely to control the outcome of PEDF-mediated activation of PEDFR. DHA is a precursor of 10,17S-docosatriene (also known as neuroprotectin D1 (NPD1)). It is thought that an as yet unknown phospholipase acts on membrane phospholipids to free DHA that is then converted to 17S-H(p)DHA, oxidized by 15-lipoxygenase (15-LOX) to 16,17-epoxy-docosatriene, which is finally converted to NPD1 (REF. 99). NPD1 has been shown to have anti-angiogenic, neurotrophic and anti-inflammatory properties in the brain and retina99,100. Interestingly, a number of reports have shown that exogenous DHA induces cytotoxicity in a wide range of cancer cell types, which has led to the investigation of DHA in several clinical cancer trials101,102. DHA has been shown to suppress tumour invasion96,103 and retinal angiogenesis97,98, but whether this involves the PEDF pathway is not yet clear. One study using prostate cancer cells clearly shows that PEDFR is crucial for the antitumour actions of PEDF104. PEDF binding to PEDFR upregulates PPARγ, which leads to the suppression of nuclear factor-κB (NF-κB)-mediated transcriptional activation, reduced production of interleukin 8 (IL-8) and limited proliferation of prostate cancer cells (FIG. 1). Although it is not yet established how PEDF–PEDFR-mediated PPARγ induction occurs, it has been shown that 5-LOX acting on DHA to produce 4-hydroxy-DHA — or DHA binding to cytosolic fatty-acid-binding protein 7 (FABP7), which has a higher affinity for DHA than for AA — translocates DHA to the nucleus where it binds to PPARγ, thus resulting in the inactivation of NF-κB and the downregulation of promigratory genes (such as cyclooxygenase 2 (COX2))96,105 (FIG. 1).
Bernard and co-workers106 reported a second receptor for PEDF, laminin receptor, in endothelial cells. PEDF binds laminin receptor through the Asp44–Asn77 region of PEDF (the 34-mer), and this interaction is linked to the anti-angiogenic functions of PEDF. PEDF is also a ligand of a third membrane protein, F1 ATP synthase, which is expressed on the surface of endothelial and tumour cells107. The same 34 amino acid region of PEDF inhibits the formation of ATP from ADP and inorganic phosphate catalysed by the ATP synthase, and this limits angiogenesis and tumour cell viability108 (FIG 1,FIG 2). More recently, Park and co-workers109 reported that PEDF binds to LRP6, which is a WNT co-receptor, and blocks the signalling that is induced by WNT ligands in retinal pigment epithelial cells. Although WNT signalling has a role in tumorigenesis, the relevance of the PEDF–LRP6 interaction to cancer development and progression are yet to be revealed. Finally, two reports have described the inverse regulation of PEDF and the serine protease urokinase-type plasminogen activator (uPA) receptor (uPAR) in tumours, and that PEDF can downregulate uPAR expression in endothelial cells72,110. Yang and co-workers110 demonstrated that the PEDF-mediated inhibition of the VEGF-induced increase in vascular permeability involves blockade of the p38 MAPK–glycogen synthase kinase 3 (GSK3)–β-catenin signalling pathway and uPAR expression. Whether PEDF binds uPAR is not yet known.
The interactions of PEDF with ECM components also affect PEDF activity. PEDF associates with collagen and glycosaminoglycans in the ECM, with binding affinities that are sensitive to pH changes23–25,111. At the molecular level, PEDF has distinct binding sites for collagen I and for glycosaminoglycans24,26, which are separated from the homologous serpin reactive site. The ligand–receptor interactions of PEDF in retinoblastoma cells are positively modulated by glycosaminoglycans, such as heparin and heparan sulphate, but not by hyaluronan112. This suggests that glycosaminoglycans can act as cofactors of PEDF and/or its receptor to improve ligand–receptor interactions, which in turn would increase the anti-tumorigenic activity of PEDF. The involvement of the collagen-binding motif in the anti-angiogenic activity of PEDF was studied by Hosomichi and co-workers113, who showed that a recombinant PEDF variant that is unable to bind collagen I does not inhibit tumour growth unlike wild-type PEDF and a PEDF variant with an altered heparin-binding site. The findings suggest that the collagen-binding site is involved in the anti-angiogenic and antitumour activities of PEDF.
Regulation of PEDF
Given the antiproliferative and pro-apoptotic functions of PEDF, it is not surprising that its expression and levels are downregulated and/or disrupted in cancer. The downregulation of PEDF during tumour progression has created an interest in defining the mechanisms that govern PEDF regulation and turnover for translational research. PEDF abundance and activities can be regulated both by extrinsic microenvironment-altering effectors such as hormones, vitamins, oxygenation or ECM composition, as well as by molecular drivers that alter its intrinsic properties (such as post-translational modification).
Transcriptional regulation
Studies of PEDF expression using both a rat castration model and comparative immunohistochemical analyses of biopsy specimens collected from patients before and after androgen-ablation therapy demonstrated that PEDF expression is increased in the prostate after androgen withdrawal47. The relevance of these results to prostate cancer treatment has yet to be fully investigated. Similarly, treatment with 17β-estradiol results in the reduced expression of PEDF mRNA and protein in ovarian surface epithelial (OSE) cells (which are possible precursors of ovarian cancer), while simultaneously promoting OSE cell and ovarian cancer cell proliferation114.
Information on the regulation of SERPINF1 expression by retinoids originally came from studies of the retina. In addition to their involvement in vision, retinoids have many important and diverse roles, such as regulation of cell proliferation and differentiation, growth of bone tissue, immune function and activation of tumour suppressor genes. They regulate gene transcription by binding to and activating two classes of nuclear transcription factors: the retinoic acid receptors (RARs) and the retinoid X receptors (RXRs). The SERPINF1 promoter has a functional retinoic-acid-responsive element (RARE), and retinoic acid upregulates SERPINF1 expression in Y79 retino blastoma cells, endothelial and retinal pigment epithelial cells, and increases secreted PEDF protein levels, but has no effect on VEGF expression115,116. Consistently, the expression of PEDF in the retinal pigment epithelium is considerably lower in vitamin-A-deficient mice compared with normal control mice, and the VEGF levels remain the same116. These observations suggest that SERPINF1 is a transcriptional target of retinoic acid. Recently, Doyon and co-workers117 identified SERPINF1 as a novel transcriptional target for the nuclear receptor co-repressor 1 (NCOR1). Studying the transition from proliferation to differentiation in intestinal epithelial cells, they showed that silencing of NCOR1 in proliferating crypt cells results in a rapid growth arrest. They also showed that the SERPINF1 promoter is occupied by NCOR1 in proliferating epithelial cells, thereby repressing the transactivation of the SERPINF1 promoter by RAR and RXR, and increasing cell proliferation. On forced expression of PEDF there was a slower rate of proliferation, thus demonstrating that NCOR1 is required to maintain the proliferation of epithelial cells in culture and that it reduces PEDF expression at the transcriptional level.
Similarly, PEDF is a transcriptional target of dexamethasone, which is a synthetic glucocorticoid used in cancer treatment that induces terminal differentiation in specific types of cancer. In silico searches of transcriptional promoter elements yield up to six glucocorticoid receptor binding sites in the transcriptional promoter of SERPINF1. It has been shown that dexamethasone induces Serpinf1 mRNA expression and increases PEDF protein production in C6 rat glioma cells, in mouse Muller glial cells and in the trabecular meshwork from human eyes115,118,119, implying that dexamethasone may act as a potential mediator of the actions of PEDF on cancer cells.
Although PEDF expression is not regulated by p53, it has been identified as a direct transcriptional target of the p63 and p73 members of the p53 family120. In fact, there is an association between the expression of the ΔEX2p73 variant isoform of p73 and the downregulation of PEDF in human colorectal tumours121.
Hypoxia-mediated regulation
The degree of oxygenation in the tumour microenvironment participates in the regulation of tumour growth and can also regulate PEDF. Although hypoxia and hypoxia mimetics upregulate HIFα subunits, VEGF, MMPs and other pro-angiogenic factors, hypoxia is associated with reduced levels of PEDF8,65. These findings are important as they show that hypoxia increases the angiogenic potential of the tumour by raising the ratio of pro-angiogenic factors to anti-angiogenic factors. MMPs act on the ECM to liberate VEGF that is bound to the matrix, thereby increasing the levels of VEGF that are accessible to target cells. Active MMP2 and MMP9 can also proteolyze PEDF, thus abolishing its neurotrophic and anti-angiogenic activities65. These results suggest that hypoxia might decrease PEDF protein levels by stimulating the MMP-mediated proteolysis of PEDF. Interestingly, the expression and secretion of VEGF, MMP2 and MMP9 positively correlate with the progression of neovascular diseases122 and inversely correlate with PEDF levels. Several studies have explored the effects of MMP inhibitors that are used to block angiogenesis, as they should prevent the liberation of active VEGF from the ECM and maintain the levels of anti-angiogenic factors. Samtani and co-workers123 showed in rats that oral administration of doxycycline, which is a broad-spectrum antibiotic and an MMP catalytic inhibitor, increased PEDF levels in serum and inhibited neovascularization in the retina. More recently, Fernandez-Barral and co-workers124 reported that hypoxic conditions encountered during primary melanoma growth downregulate PEDF by a post-translational mechanism involving degradation by autophagy, and such a mechanism could therefore contribute to the highly metastatic characteristic of aggressive melanoma cells.
Post-translational modifications
Post-translational modifications can also regulate PEDF activities. Although PEDF glycosylation, amino-terminal modifications or unfolding of the PEDF protein are dispensable for biological activities11, the Seger laboratory has shown that different phosphorylation sites can convert PEDF from a neurotrophic to an anti-angiogenic factor125. They prepared phosphomimetic PEDF variants with increased anti-angiogenic activities that are much more efficient than wild-type PEDF at inhibiting growth and angiogenesis in breast cancer, colon cancer and glioblastoma xenograft models73. Remarkably, the antitumour activity of the phosphomimetic variants is comparable to that of the established anti-angiogenic agent bevacizumab, but they act in a VEGF-independent manner, without affecting the levels of VEGFA mRNA or VEGF receptor 2 phosphorylation. PEDF and its variants act on intratumoural endothelial apoptosis, but in contrast to results from other groups, the Seger group reported that the variant forms do not affect the survival of cancer cells in vitro, hence they concluded that the anti-angiogenic activity of these agents is the main property of the observed antitumour effect. More recently, the same group reported the molecular mechanism by which phosphomimetic PEDF exerts more-profound effects at the cellular level by inducing JNK-dependent apoptosis and p38-mediated migration arrest77. More recently, Feng and co-workers126 demonstrated that the triple phospho mimetic EEE-PEDF significantly reduced the growth and metastasis of choroidal melanoma xenografts in nude mice, and this effect was associated with inhibiting VEGF and NF-κB expression. Is has been proposed that EEE-PEDF has an increased negative charge compared with the wild-type protein. Interestingly, we have identified a natural PEDF protein variant with enhanced tumour cell antimigratory and cell-death-inducing activities that was separated from the canonical wild-type form by ion-exchange chromatography. This variant has increased negative charge compared with other forms of PEDF, but the chemistry of this molecule is unknown78. The PEDF forms with increased anticancer properties, which include the modified phosphorylated forms and the truncated peptide forms, should prove to be useful tools in the preparation of optimized PEDF molecules for therapeutic uses. Indeed, these findings encourage the development of second-generation PEDF molecules as specific, angiogenesis-targeting anticancer agents.
PEDF, cancer prognosis and therapeutic potential
In recent years it has become apparent that, in addition to the established antitumour activity of exogenously added PEDF, changes in the endogenous expression of PEDF are associated with the malignant progression of diverse tumour types. Immunohistochemical analyses of PEDF expression in a variety of human tumour specimens and normal control tissues led to an overall picture showing that increased PEDF expression is associated with a more-favourable prognosis, whereas reduced levels of PEDF are indicative of a poorer prognosis. This general assessment has been confirmed for glioma127, pancreatic adenocarcinoma79, non-small-cell lung tumours128,129, breast cancer 130,131, colorectal cancer (both in the tumours themselves121 and in the plasma of cancer patients132), invasive melanoma80, prostate cancer133, ocular melanoma81, clear-cell renal-cell carcinoma134 and ovarian cancer135. The findings that most prostatic intra-epithelial neoplasia (PIN) specimens expressed moderate to low levels of PEDF133 and that the levels in patient sera decrease from cases of benign prostatic hyperplasia (BPH) to those of increasing malignancy136 suggest that PEDF losses represent an early event in carcinogenesis that becomes more acute during malignant progression. Tissue microarray studies of matched primary and recurrent breast tumours after tamoxifen treatment showed that patients who presented with progressive disease, on average 93 months after endocrine therapy, had significantly lower PEDF levels than those who showed a complete therapeutic response137. A poor therapeutic response correlated with the low PEDF expression levels in their primary carcinomas137. The fact that these studies were also correlated with in vitro experiments showing that modulating the levels of PEDF expression is sufficient to alter the sensitivity of breast cancer cells to endocrine therapy provides strong evidence supporting the prognostic value of PEDF for disease progression and patient outcome. Most recently, PEDF has also been identified as a prognostic marker for colorectal cancer138. Analyses of PEDF levels in serum samples from normal individuals and patients with cancer demonstrate that decreased PEDF levels significantly correlate with advanced clinical stage, lymph node and distant metastasis, and poorer overall survival. In agreement with previous reports121,132, low serum PEDF levels correlate with downregulated PEDF expression in the tumours, which is also associated with disease-free survival. Nevertheless, whether changes in PEDF levels are involved in cancer onset or are a consequence of the malignant process remains to be elucidated. Although highly consistent, this picture is not perfect, and hepato-cellular carcinoma (HCC) is the most notable exception. Despite an earlier report showing that the serum concentration of PEDF was decreased in patients with cirrhosis and HCC relative to healthy individuals and to patients with chronic hepatitis139, recent results showed that PEDF levels were higher in HCC than in adjacent normal tissues and also greater in serum samples from patients with HCC than normal controls. They also showed that the effective treatment of HCC caused significant reductions in serum PEDF levels140. Although this apparent discrepancy may be due to organ- or tissue-specific differences, the overwhelming evidence supports the notion that the lower the tumour PEDF levels, the poorer the prognosis and the worse the expected outcome. It is precisely for this reason that the potential use of PEDF in cancer therapeutics has generated great expectations. Consequently, substantial efforts are currently underway to optimize the use of exogenous PEDF for cancer treatment. These strategies are primarily focused in two areas: the development of protein or peptide therapeutics with enhanced antitumorigenic activity, such as PEDF phosphomimetics73, PEDF variant forms78 or discrete PEDF-derived peptides that recapitulate the anticancer activity of full-length PEDF35,36; and the establishment of efficient methods for PEDF administration that take advantage of delivery systems using either viral vectors directly54,63,64,68,141–143, virally infected human mesenchymal stem cells144–146, microparticles or nanoparticles of various compositions71,147,148 or implanted micro-osmotic pumps149. Moreover, combination therapeutic protocols using PEDF in addition to differentiation-inducing agents such as IL-6 (REF. 150) or with radiotherapy62 are also being studied.
Concluding remarks
Ongoing studies will further clarify the details of PEDF receptor signalling cascades and their biological importance. In particular, PEDF signalling studies may uncover new surface-to-nucleus signalling cascades that are triggered by each of the PEDF receptors. Identification of the antitumorigenic activities of PEDF in vivo has only recently begun, and considerably more work is required to understand mechanistically the role of PEDF in cancer. One priority is to delineate how PEDF triggers each activity in target cells and what domain of the PEDF polypeptide is responsible for a particular effect of the entire molecule. The discovery of receptors for PEDF is an exciting development as it not only underscores the importance of PEDF in tumour inhibition but also links previously unconnected areas of cell biology. Studies on the role of these receptors on cancer development and progression and their relative quantification in tumours versus normal tissues are of interest for potential therapeutic purposes. Outstanding questions concern the multifunctionality of PEDF and its regulation by influences from various cellular contexts. Defining precisely how PEDF acts within a given set of cellular and tumoural conditions to promote its diverse actions towards preventing cancer development is of great interest. As the antitumour, anti-angiogenic and antimetastatic activities of PEDF reveal a strong potential in applying PEDF treatment in the clinic, the development of efficient, safe and cost-effective delivery systems for the use of PEDF in cancer treatment should be a high-priority research area. Interestingly, no toxicity as a result of PEDF administration has been reported in any of the animal models tested. A number of reports have provided evidence in support of the use of PEDF as a prognostic factor in cancer management. Therefore, determining tumour or serum PEDF levels at diagnosis may provide an excellent source of information towards delineating more- or less-aggressive treatment protocols in order to improve the therapeutic outcome for patients with cancer. Thus, we think that PEDF will become a valuable tool in our fight against cancer.
At a glance.
PEDF is a member of the serpin superfamily that has many functions that often act in opposition to mechanisms that drive cancer progression.
Tumour progression is associated with reduced levels of PEDF in tumours. Exogenous administration of PEDF to bolster the declining intratumoural levels of PEDF during tumour progression results in the inhibition of tumour growth and prolonged organismal survival in various animl models.
PEDF can act directly on tumours to induce differentiation to a less-malignant phenotype, promote apoptotic tumour cell death and inhibit the proliferation of tumour cells.
Numerous studies in various models have shown the anti-angiogenic effects that PEDF has on tumours. PEDF is a potent inhibitor of angiogenesis through pro-apoptotic effects on endothelial cells. It can also inhibit endothelial cell migration, endothelial tube formation, vessel sprouting and intratumoural neovascularization, and can decrease the levels of pro-angiogenic factors.
Support for its anticancer role also comes from the findings that PEDF exhibits strong antimetastatic activity by suppressing tumour cell invasion and migration; these effects have been described in vitro and in several metastasis models in vivo.
The molecular mechanisms by which PEDF functions to regulate tumour and endothelial cell behaviour are based mostly on its interactions with different cell-surface receptors and their downstream signalling pathways.
PEDF abundance and activities are regulated both by extrinsic microenvironment-altering effectors (such as hormones, vitamins, oxygenation or extracellular matrix (ECM) composition), as well as by molecular drivers that alter its intrinsic properties (such as post-translational modifications).
Numerous reports have provided evidence in support of the use of PEDF as a prognostic factor in cancer management. PEDF-positive expression is described as an independent favourable prognostic factor for cancer.
Acknowledgements
This work was supported in part by the intramural research program of the US National Institutes of Health (NIH) (Project number 1ZIA-EY000306) to S.P.B. and by grant R01-CA134727 from the US National Cancer Institute (NCI) to V.N.
Glossary
- Stokes radius
A molecular radius that consists of the radius of a hard sphere that diffuses at the same rate as a given molecule. A bigger molecule will have a larger Stokes radius compared with a more-compact molecule of the same molecular weight.
- Interphotoreceptor matrix
(IPM). This fills the part of the eye referred to by ophthalmologists as the subretinal space. It is located between the outer limiting membrane of the retina and the apical border of the retinal pigment epithelium, where it surrounds the photoreceptor inner and outer segments that project from the outer retinal surface.
- Serpin reactive loop
An exposed region in the folded protein structure of serpins that is located towards the carboxyl-terminus. It is also known as the reactive centre loop that, in inhibitory molecules, determines specificity and is involved in recognizing target proteinases.
- Neurotrophic
This term is used to describe factors that promote the initial growth and development of neurons in the central nervous system and peripheral nervous system. They can induce the regrowth of damaged neurons.
- Neovascularization
In ophthalmology, choroidal, retinal or corneal neovascularization refers to the proliferation of blood vessels and the formation of a microvasculature within the innermost layer of the choroid of the eye, inner retina or cornea, respectively.
- Carcinoma nests
Cohesive aggregates of cancer cells within predominantly normal tissues.
- Adiposity
Refers to the fat content of a given tissue
- Trabecular meshwork
An area of tissue in the eye that is located around the base of the cornea, near the ciliary body, and is responsible for draining the aqueous humour from the eye through the chamber at the front of the eye covered by the cornea.
Footnotes
Competing interests statement
The authors declare competing financial interests. See Web version for details.
DATABASES
National Cancer Institute Drug Dictionary: http://www.cancer.gov/drugdictionary bevacizumab | dexamethasone | tamoxifen
FURTHER INFORMATION
S. Patricia Becerra’s homepages: http://irp.nih.gov/pi/s-patricia-becerra; http://www.nei.nih.gov/intramural/protein_struct func.asp
ALL LINKS ARE ACTIVE IN THE ONLINE PDF
REFERENCES
- 1. Steele FR, Chader GJ, Johnson LV, Tombran-Tink J. Pigment epithelium-derived factor: neurotrophic activity and identification as a member of the serine protease inhibitor gene family. Proc. Natl Acad. Sci. USA. 1993;90:1526–1530. doi: 10.1073/pnas.90.4.1526.The first article to report the complete sequence of the human PEDF mRNA and protein, and its identification as a member of the serpin gene family with neurotrophic activity on retinoblastoma tumour cells.
- 2.Tombran-Tink J, Chader GG, Johnson LV. PEDF: a pigment epithelium-derived factor with potent neuronal differentiative activity. Exp. Res. 1991;53:411–414. doi: 10.1016/0014-4835(91)90248-d. [DOI] [PubMed] [Google Scholar]
- 3.Doggett DL, Rotenberg MO, Pignolo RJ, Phillips PD, Cristofalo VJ. Differential gene expression between young and senescent, quiescent WI-38 cells. Mech. Ageing Dev. 1992;65:239–255. doi: 10.1016/0047-6374(92)90039-g. [DOI] [PubMed] [Google Scholar]
- 4. Pignolo RJ, Cristofalo VJ, Rotenberg MO. Senescent WI-38 cells fail to express EPC-1, a gene induced in young cells upon entry into the G0 state. J. Biol. Chem. 1993;268:8949–8957.This report describes how SERPINF1 is a gene that is induced to ≥100-fold levels in young lung fibroblast cells relative to senescent cells, which fail to express it, and is a first association of PEDF with ageing.
- 5.Francis MK, et al. Loss of EPC-1/PEDF expression during skin aging in vivo. J. Invest. Dermatol. 2004;122:1096–1105. doi: 10.1111/j.0022-202X.2004.22510.x. [DOI] [PubMed] [Google Scholar]
- 6.Lanza RP, et al. Extension of cell life-span and telomere length in animals cloned from senescent somatic cells. Science. 2000;288:665–669. doi: 10.1126/science.288.5466.665. [DOI] [PubMed] [Google Scholar]
- 7.Tresini M, Pignolo RJ, Allen RG, Cristofalo VJ. Effects of donor age on the expression of a marker of replicative senescence (EPC-1) in human dermal fibroblasts. J. Cell. Physiol. 1999;179:11–17. doi: 10.1002/(SICI)1097-4652(199904)179:1<11::AID-JCP2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 8. Dawson DW, et al. Pigment epithelium-derived factor: a potent inhibitor of angiogenesis. Science. 1999;285:245–248. doi: 10.1126/science.285.5425.245.The first report on the potent anti-angiogenic activity of PEDF in the cornea and vitreous humour and on how hypoxia regulates PEDF.
- 9.McCullough KD, Coleman WB, Smith GJ, Grisham JW. Age-dependent induction of hepatic tumor regression by the tissue microenvironment after transplantation of neoplastically transformed rat liver epithelial cells into the liver. Cancer Res. 1997;57:1807–1813. [PubMed] [Google Scholar]
- 10.Sprenger CC, Plymate SR, Reed MJ. Aging-related alterations in the extracellular matrix modulate the microenvironment and influence tumor progression. Int. J. Cancer. 2010;127:2739–2748. doi: 10.1002/ijc.25615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Becerra SP. Focus on molecules: pigment epithelium-derived factor (PEDF) Exp. Res. 2006;82:739–740. doi: 10.1016/j.exer.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 12. Simonovic M, Gettins PG, Volz K. Crystal structure of human PEDF, a potent anti-angiogenic and neurite growth-promoting factor. Proc. Natl Acad. Sci. USA. 2001;98:11131–11135. doi: 10.1073/pnas.211268598.The crystal structure of glycosylated human PEDF was solved to 2.85 ansgstroms resolution, which revealed its serpin structure homology and possible receptor- and heparin-binding sites.
- 13.Filleur S, Nelius T, de Riese W, Kennedy RC. Characterization of PEDF: a multi-functional serpin family protein. J. Cell. Biochem. 2009;106:769–775. doi: 10.1002/jcb.22072. [DOI] [PubMed] [Google Scholar]
- 14.Silverman GA, et al. Serpins flex their muscle: I. Putting the clamps on proteolysis in diverse biological systems. J. Biol. Chem. 2010;285:24299–24305. doi: 10.1074/jbc.R110.112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whisstock JC, et al. Serpins flex their muscle: II. Structural insights into target peptidase recognition, polymerization, and transport functions. J. Biol. Chem. 2010;285:24307–24312. doi: 10.1074/jbc.R110.141408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Becerra SP, Sagasti A, Spinella P, Notario V. Pigment epithelium-derived factor behaves like a noninhibitory serpin. Neurotrophic activity does not require the serpin reactive loop. J. Biol. Chem. 1995;270:25992–25999. doi: 10.1074/jbc.270.43.25992.The first structure-function studies showing that the biological activity of PEDF is independent of serpin protease inhibition potential.
- 17.Tombran-Tink J, Pawar H, Swaroop A, Rodriguez I, Chader GJ. Localization of the gene for pigment epithelium-derived factor (PEDF) to chromosome 17p13.1 and expression in cultured human retinoblastoma cells. Genomics. 1994;19:266–272. doi: 10.1006/geno.1994.1057. [DOI] [PubMed] [Google Scholar]
- 18.Kuncl RW, et al. Pigment epithelium-derived factor is elevated in CSF of patients with amyotrophic lateral sclerosis. J. Neurochem. 2002;81:178–184. doi: 10.1046/j.1471-4159.2002.00813.x. [DOI] [PubMed] [Google Scholar]
- 19.Ortego J, Escribano J, Becerra SP, Coca-Prados M. Gene expression of the neurotrophic pigment epithelium-derived factor in the human ciliary epithelium. Synthesis and secretion into the aqueous humor. Invest. Ophthalmol. Vis. Sci. 1996;37:2759–2767. [PubMed] [Google Scholar]
- 20.Petersen SV, Valnickova Z, Enghild JJ. Pigment-epithelium-derived factor (PEDF) occurs at a physiologically relevant concentration in human blood: purification and characterization. Biochem. J. 2003;374:199–206. doi: 10.1042/BJ20030313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wu YQ, Becerra SP. Proteolytic activity directed toward pigment epithelium-derived factor in vitreous of bovine eyes. Implications of proteolytic processing. Invest. Ophthalmol. Vis. Sci. 1996;37:1984–1993. [PubMed] [Google Scholar]
- 22.Wu YQ, Notario V, Chader GJ, Becerra SP. Identification of pigment epithelium-derived factor in the interphotoreceptor matrix of bovine eyes. Protein Expr. Purif. 1995;6:447–456. doi: 10.1006/prep.1995.1060. [DOI] [PubMed] [Google Scholar]
- 23.Alberdi E, Hyde CC, Becerra SP. Pigment epithelium-derived factor (PEDF) binds to glycosaminoglycans: analysis of the binding site. Biochemistry. 1998;37:10643–10652. doi: 10.1021/bi9802317. [DOI] [PubMed] [Google Scholar]
- 24.Becerra SP, et al. Pigment epithelium-derived factor binds to hyaluronan. Mapping of a hyaluronan. binding site. J. Biol. Chem. 2008;283:33310–33320. doi: 10.1074/jbc.M801287200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Meyer C, Notari L, Becerra SP. Mapping the type I. collagen-binding site on pigment epithelium-derived factor. Implications for its antiangiogenic activity. J. Biol. Chem. 2002;277:45400–45407. doi: 10.1074/jbc.M208339200. [DOI] [PubMed] [Google Scholar]
- 26.Yasui N, et al. Dual-site recognition of different extracellular matrix components by anti-angiogenic/ neurotrophic serpin, PEDF. Biochemistry. 2003;42:3160–3167. doi: 10.1021/bi0206558. [DOI] [PubMed] [Google Scholar]
- 27.Araki T, Taniwaki T, Becerra SP, Chader GJ, Schwartz JP. Pigment epithelium-derived factor (PEDF) differentially protects immature but not mature cerebellar granule cells against apoptotic cell death. J. Neurosci. Res. 1998;53:7–15. doi: 10.1002/(SICI)1097-4547(19980701)53:1<7::AID-JNR2>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 28.Becerra SP, et al. Overexpression of fetal human pigment epithelium-derived factor in Escherichia coli A functionally active neurotrophic factor. J. Biol. Chem. 1993;268:23148–23156. [PubMed] [Google Scholar]
- 29.Houenou LJ, et al. Pigment epithelium-derived factor promotes the survival and differentiation of developing spinal motor neurons. J. Comp. Neurol. 1999;412:506–514. [PubMed] [Google Scholar]
- 30.Taniwaki T, Becerra SP, Chader GJ, Schwartz JP. Pigment epithelium-derived factor is a survival factor for cerebellar granule cells in culture. J. Neurochem. 1995;64:2509–2517. doi: 10.1046/j.1471-4159.1995.64062509.x. [DOI] [PubMed] [Google Scholar]
- 31.Taniwaki T, et al. Pigment epithelium-derived factor protects cultured cerebellar granule cells against glutamate-induced neurotoxicity. J. Neurochem. 1997;68:26–32. doi: 10.1046/j.1471-4159.1997.68010026.x. [DOI] [PubMed] [Google Scholar]
- 32.Alberdi E, Aymerich MS, Becerra SP. Binding of pigment epithelium-derived factor (PEDF) to retinoblastoma cells and cerebellar granule neurons. Evidence for a PEDF receptor. J. Biol. Chem. 1999;274:31605–31612. doi: 10.1074/jbc.274.44.31605. [DOI] [PubMed] [Google Scholar]
- 33.Amaral J, Becerra SP. Effects of human recombinant PEDF protein and PEDF-derived peptide 34-mer on choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2010;51:1318–1326. doi: 10.1167/iovs.09-4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bilak MM, et al. Identification of the neuroprotective molecular region of pigment epithelium-derived factor and its binding sites on motor neurons. J. Neurosci. 2002;22:9378–9386. doi: 10.1523/JNEUROSCI.22-21-09378.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Filleur S, et al. Tw o functional epitopes of pigment epithelial-derived factor block angiogenesis and induce differentiation in prostate cancer. Cancer Res. 2005;65:5144–5152. doi: 10.1158/0008-5472.CAN-04-3744.Structure-function studies that map active regions for the two PEDF functions on angiogenesis inhibition and induction of differentiation, and provide information on signalling via distinct receptors.
- 36.Mirochnik Y, et al. Short pigment epithelial-derived factor-derived peptide inhibits angiogenesis and tumor growth. Clin. Cancer Res. 2009;15:1655–1663. doi: 10.1158/1078-0432.CCR-08-2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Becerra SP. Structure-function studies on PEDF. A noninhibitory serpin with neurotrophic activity. A d v. Exp. Med. Biol. 1997;425:223–237. [PubMed] [Google Scholar]
- 38.Tombran-Tink J, Barnstable CJ. PEDF: a multifaceted neurotrophic factor. Nature Rev. Neurosci. 2003;4:628–636. doi: 10.1038/nrn1176. [DOI] [PubMed] [Google Scholar]
- 39.Seigel GM, et al. Differentiation of Y79 retinoblastoma cells with pigment epithelial-derived factor and interphotoreceptor matrix wash: effects on tumorigenicity. Growth Factors. 1994;10:289–297. doi: 10.3109/08977199409010995. [DOI] [PubMed] [Google Scholar]
- 40.Crawford SE, et al. Pigment epithelium-derived factor (PEDF) in neuroblastoma: a multifunctional mediator of Schwann cell antitumor activity. J. Cell Sci. 2001;114:4421–4428. doi: 10.1242/jcs.114.24.4421. [DOI] [PubMed] [Google Scholar]
- 41. Fitzgerald DP, et al. Opposing effects of pigment epithelium-derived factor on breast cancer cell versus neuronal survival: implication for brain metastasis and metastasis-induced brain damage. Cancer Res. 2012;72:144–153. doi: 10.1158/0008-5472.CAN-11-1904.A study that establishes PEDF as both a metastatic suppressor and a neuroprotectant in the brain. It highlights the role of PEDF as a double agent that limits brain metastases and their local consequences.
- 42.Renno RZ, Youssri AI, Michaud N, Gragoudas ES, Miller JW. Expression of pigment epithelium-derived factor in experimental choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2002;43:1574–1580. [PubMed] [Google Scholar]
- 43.Spranger J, et al. Loss of the antiangiogenic pigment epithelium-derived factor in patients with angiogenic eye disease. Diabetes. 2001;50:2641–2645. doi: 10.2337/diabetes.50.12.2641. [DOI] [PubMed] [Google Scholar]
- 44.Stellmach V, Crawford SE, Zhou W, Bouck N. Prevention of ischemia-induced retinopathy by the natural ocular antiangiogenic agent pigment epithelium-derived factor. Proc. Natl Acad. Sci. USA. 2001;98:2593–2597. doi: 10.1073/pnas.031252398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bouck N. PEDF: anti-angiogenic guardian of ocular function. Trends Mol. Med. 2002;8:330–334. doi: 10.1016/s1471-4914(02)02362-6. [DOI] [PubMed] [Google Scholar]
- 46.Cayouette M, Smith SB, Becerra SP, Gravel C. Pigment epithelium-derived factor delays the death of photoreceptors in mouse models of inherited retinal degenerations. Neurobiol. Dis. 1999;6:523–532. doi: 10.1006/nbdi.1999.0263. [DOI] [PubMed] [Google Scholar]
- 47. Doll JA, et al. Pigment epithelium-derived factor regulates the vasculature and mass of the prostate and pancreas. Nature Med. 2003;9:774–780. doi: 10.1038/nm870.This study identified PEDF as a key inhibitor of stromal vasculature and epithelial tissue growth in the prostate and pancreas using PEDF-deficient mice and human tumours.
- 48.Mori K, et al. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J. Cell. Physiol. 2001;188:253–263. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]
- 49.Mori K, et al. Regression of ocular neovascularization in response to increased expression of pigment epithelium-derived factor. Invest. Ophthalmol. Vis. Sci. 2002;43:2428–2434. [PubMed] [Google Scholar]
- 50.Takita H, et al. Retinal neuroprotection against ischemic injury mediated by intraocular gene transfer of pigment epithelium-derived factor. Invest. Ophthalmol. Vis. Sci. 2003;44:4497–4504. doi: 10.1167/iovs.03-0052. [DOI] [PubMed] [Google Scholar]
- 51.Abramson LP, et al. Wilms’ tumor growth is suppressed by antiangiogenic pigment epithelium-derived factor in a xenograft model. J. Pediatr. Surg. 2003;38:336–342. doi: 10.1053/jpsu.2003.50104. [DOI] [PubMed] [Google Scholar]
- 52.Abramson LP, et al. Pigment epithelium-derived factor targets endothelial and epithelial cells in Wilms’ tumor. J. Pediatr. Surg. 2006;41:1351–1356. doi: 10.1016/j.jpedsurg.2006.04.038. [DOI] [PubMed] [Google Scholar]
- 53.Hase R, et al. Pigment epithelium-derived factor gene therapy inhibits human pancreatic cancer in mice. Clin. Cancer Res. 2005;11:8737–8744. doi: 10.1158/1078-0432.CCR-05-1323. [DOI] [PubMed] [Google Scholar]
- 54.Browne M, et al. Gene transfer of pigment epithelium-derived factor suppresses tumor growth and angiogenesis in a hepatoblastoma xenograft model. Pediatr. Res. 2006;60:282–287. doi: 10.1203/01.pdr.0000232789.86632.91. [DOI] [PubMed] [Google Scholar]
- 55.Ek ET, Dass CR, Contreras KG, Choong PF. Pigment epithelium-derived factor overexpression inhibits orthotopic osteosarcoma growth, angiogenesis and metastasis. Cancer Gene Ther. 2007;14:616–626. doi: 10.1038/sj.cgt.7701044. [DOI] [PubMed] [Google Scholar]
- 56.Abe R, et al. Overexpression of pigment epithelium-derived factor decreases angiogenesis and inhibits the growth of human malignant melanoma cells in vivo. Am. J. Pathol. 2004;164:1225–1232. doi: 10.1016/s0002-9440(10)63210-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Garcia M, et al. Inhibition of xenografted human melanoma growth and prevention of metastasis development by dual antiangiogenic/antitumor activities of pigment epithelium-derived factor. Cancer Res. 2004;64:5632–5642. doi: 10.1158/0008-5472.CAN-04-0230.The findings in this paper establish the anti-ngiogenic and antimetastatic effects of PEDF on melanoma. This occurred through the inhibition of the angiogenic tumour response and through direct PEDF effects on both the migration and survival of melanoma cells.
- 58.Abe R, Fujita Y, Yamagishi S, Shimizu H. Pigment epithelium-derived factor prevents melanoma growth via angiogenesis inhibition. Curr. Pharm. Des. 2008;14:3802–3809. doi: 10.2174/138161208786898626. [DOI] [PubMed] [Google Scholar]
- 59.Yang H, et al. PEDF inhibits growth of retinoblastoma by anti-angiogenic activity. Cancer Sci. 2009;100:2419–2425. doi: 10.1111/j.1349-7006.2009.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang J, et al. Growth suppression of cervical carcinoma by pigment epithelium-derived factor via anti-angiogenesis. Cancer Biol. Ther. 2010;9:967–974. doi: 10.4161/cbt.9.12.11635. [DOI] [PubMed] [Google Scholar]
- 61.Zhang Y, et al. Pigment epithelium-derived factor inhibits angiogenesis and growth of gastric carcinoma by down-regulation of VEGF. Oncol. Rep. 2011;26:681–686. doi: 10.3892/or.2011.1318. [DOI] [PubMed] [Google Scholar]
- 62.Xu Z, et al. Combination of pigment epithelium-derived factor with radiotherapy enhances the antitumor effects on nasopharyngeal carcinoma by downregulating vascular endothelial growth factor expression and angiogenesis. Cancer Sci. 2011;102:1789–1798. doi: 10.1111/j.1349-7006.2011.02013.x. [DOI] [PubMed] [Google Scholar]
- 63.He SS, et al. AAV-mediated gene transfer of human pigment epithelium-derived factor inhibits Lewis lung carcinoma growth in mice. Oncol. Rep. 2012;27:1142–1148. doi: 10.3892/or.2012.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wu QJ, et al. AAV-mediated human PEDF inhibits tumor growth and metastasis in murine colorectal peritoneal carcinomatosis model. BMC Cancer. 2012;12:129. doi: 10.1186/1471-2407-12-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Notari L, et al. Pigment epithelium-derived factor is a substrate for matrix metalloproteinase type 2 and type 9: implications for downregulation in hypoxia. Invest. Ophthalmol. Vis. Sci. 2005;46:2736–2747. doi: 10.1167/iovs.04-1489. [DOI] [PubMed] [Google Scholar]
- 66. Volpert OV, et al. Inducer-stimulated Fas targets activated endothelium for destruction by anti-angiogenic thrombospondin-1 and pigment epithelium-derived factor. Nature Med. 2002;8:349–357. doi: 10.1038/nm0402-349.A report showing that the anti-ngiogenic activity of PEDF was dependent on the concomitant induction of CD95 and CD95L and the resulting apoptosis, thus exemplifying how cooperation between PEDF and pro-giogenic factors in angiogenesis inhibition may explain the ability of PEDF to target remodelling capillaries for destruction.
- 67.Fernandez-Garcia NI, Volpert OV, Jimenez B. Pigment epithelium-derived factor as a multifunctional antitumor factor. J. Mol. Med. 2007;85:15–22. doi: 10.1007/s00109-006-0111-z. [DOI] [PubMed] [Google Scholar]
- 68.Guan M, et al. Adenovirus-mediated PEDF expression inhibits prostate cancer cell growth and results in augmented expression of PAI-2. Cancer Biol. Ther. 2007;6:419–425. doi: 10.4161/cbt.6.3.3757. [DOI] [PubMed] [Google Scholar]
- 69.Zhang T, Guan M, Xu C, Chen Y, Lu Y. Pigment epithelium-derived factor inhibits glioma cell growth in vitro and in vivo . Life Sci. 2007;81:1256–1263. doi: 10.1016/j.lfs.2007.08.025. [DOI] [PubMed] [Google Scholar]
- 70.Guan M, et al. Inhibition of glioma invasion by overexpression of pigment epithelium-derived factor. Cancer Gene Ther. 2004;11:325–332. doi: 10.1038/sj.cgt.7700675. [DOI] [PubMed] [Google Scholar]
- 71.Cui FY, et al. The pigment epithelial-derived factor gene loaded in PLGA nanoparticles for therapy of colon carcinoma. Oncol. Rep. 2010;24:661–668. doi: 10.3892/or_00000905. [DOI] [PubMed] [Google Scholar]
- 72.Dass CR, Choong PF. uPAR mediates anticancer activity of PEDF. Cancer Biol. Ther. 2008;7:1262–1270. doi: 10.4161/cbt.7.8.6265. [DOI] [PubMed] [Google Scholar]
- 73.Konson A, Pradeep S, Seger R. Phosphomimetic mutants of pigment epithelium-derived factor with enhanced antiangiogenic activity as potent anticancer agents. Cancer Res. 2010;70:6247–6257. doi: 10.1158/0008-5472.CAN-10-0434. [DOI] [PubMed] [Google Scholar]
- 74.Ek ET, Dass CR, Contreras KG, Choong PF. Inhibition of orthotopic osteosarcoma growth and metastasis by multitargeted antitumor activities of pigment epithelium-derived factor. Clin. Exp. Metastasis. 2007;24:93–106. doi: 10.1007/s10585-007-9062-1. [DOI] [PubMed] [Google Scholar]
- 75.Ek ET, Dass CR, Contreras KG, Choong PF. PEDF-derived synthetic peptides exhibit antitumor activity in an orthotopic model of human osteosarcoma. J. Orthop. Res. 2007;25:1671–1680. doi: 10.1002/jor.20434. [DOI] [PubMed] [Google Scholar]
- 76.Tan ML, Choong PF, Dass CR. Anti-chondrosarcoma effects of PEDF mediated via molecules important to apoptosis, cell cycling, adhesion and invasion. Biochem. Biophys. Res. Commun. 2010;398:613–618. doi: 10.1016/j.bbrc.2010.05.098. [DOI] [PubMed] [Google Scholar]
- 77.Konson A, Pradeep S, D’Acunto CW, Seger R. Pigment epithelium-derived factor and its phosphomimetic mutant induce JNK-dependent apoptosis and p38-mediated migration arrest. J. Biol. Chem. 2011;286:3540–3551. doi: 10.1074/jbc.M110.151548. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 78.Subramanian P, et al. Identification of pigment epithelium-derived factor protein forms with distinct activities on tumor cell lines. J. Biomed. Biotechnol. 2012;2012:425907. doi: 10.1155/2012/425907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Uehara H, et al. Expression of pigment epithelium-derived factor decreases liver metastasis and correlates with favorable prognosis for patients with ductal pancreatic adenocarcinoma. Cancer Res. 2004;64:3533–3537. doi: 10.1158/0008-5472.CAN-03-3725.The first report establishing that PEDF-ositive expression was an independent favourable prognostic factor in pancreatic ductal adenocarcinoma.
- 80.Orgaz JL, et al. Loss of pigment epithelium-derived factor enables migration, invasion and metastatic spread of human melanoma. Oncogene. 2009;28:4147–4161. doi: 10.1038/onc.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang H, Grossniklaus HE. Constitutive overexpression of pigment epithelium-derived factor inhibition of ocular melanoma growth and metastasis. Invest. Ophthalmol. Vis. Sci. 2010;51:28–34. doi: 10.1167/iovs.09-4138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Ladhani O, Sanchez-Martinez C, Orgaz JL, Jimenez B, Volpert OV. Pigment epithelium-derived factor blocks tumor extravasation by suppressing amoeboid morphology and mesenchymal proteolysis. Neoplasia. 2011;13:633–642. doi: 10.1593/neo.11446.These findings reveal the coordinated regulation of cell shape and proteolysis as a plausible mechanism for the antimetastatic activity of PEDF. It involves the 34-er region of PEDF, and PEDFR.
- 83.Grippo PJ, et al. Concurrent PEDF deficiency and Kras mutation induce invasive pancreatic cancer and adipose-rich stroma in mice. Gut. 2012;61:1454–1464. doi: 10.1136/gutjnl-2011-300821. [DOI] [PubMed] [Google Scholar]
- 84.Borg ML, et al. Pigment epithelium-derived factor regulates lipid metabolism via adipose triglyceride lipase. Diabetes. 2011;60:1458–1466. doi: 10.2337/db10-0845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wang M, et al. Pigment epithelium-derived factor suppresses adipogenesis via inhibition of the MAPK/ ERK pathway in 3T3-L1 preadipocytes. Am. J. Physiol. Endocrinol. Metab. 2009;297:e1378–e1387. doi: 10.1152/ajpendo.00252.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Cai J, et al. γ-secretase and presenilin mediate cleavage and phosphorylation of vascular endothelial growth factor receptor-1. J. Biol. Chem. 2011;286:42514–42523. doi: 10.1074/jbc.M111.296590.First description that PEDF inhibits VEGF-nduced angiogenesis in microvascular endothelial cells by regulating VEGF receptor 1 (VEGFR1) cleavage and phosphorylation through a mechanism that is mediated by γ-ecretase.
- 87.Cai J, Jiang WG, Grant MB, Boulton M. Pigment epithelium-derived factor inhibits angiogenesis via regulated intracellular proteolysis of vascular endothelial growth factor receptor 1. J. Biol. Chem. 2006;281:3604–3613. doi: 10.1074/jbc.M507401200. [DOI] [PubMed] [Google Scholar]
- 88. Biyashev D, et al. Natural angiogenesis inhibitor signals through Erk5 activation of peroxisome proliferator-activated receptor γ (PPARγ) J. Biol. Chem. 2010;285:13517–13524. doi: 10.1074/jbc.M110.117374.The first evidence that ERK5 and its activating kinase MEK5 are upstream mediators of the anti-ngiogenic signalling by PEDF.
- 89.Zaichuk TA, et al. Nuclear factor of activated T cells balances angiogenesis activation and inhibition. J. Exp. Med. 2004;199:1513–1522. doi: 10.1084/jem.20040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aymerich MS, Alberdi EM, Martinez A, Becerra SP. Evidence for pigment epithelium-derived factor receptors in the neural retina. Invest. Ophthalmol. Vis. Sci. 2001;42:3287–3293. [PubMed] [Google Scholar]
- 91. Notari L, et al. Identification of a lipase-linked cell membrane receptor for pigment epithelium-derived factor. J. Biol. Chem. 2006;281:38022–38037. doi: 10.1074/jbc.M600353200.The first evidence for a cellsurface high-ffinity receptor for PEDF (PEDFR) with intrinsic phospholipase A2 activity that is stimulated on PEDF binding.
- 92.Zimmermann R, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 93.Villena JA, Roy S, Sarkadi-Nagy E, Kim KH, Sul HS. Desnutrin, an adipocyte gene encoding a novel patatin domain-containing protein, is induced by fasting and glucocorticoids: ectopic expression of desnutrin increases triglyceride hydrolysis. J. Biol. Chem. 2004;279:47066–47075. doi: 10.1074/jbc.M403855200. [DOI] [PubMed] [Google Scholar]
- 94.Jenkins CM, et al. Identification, cloning, expression, and purification of three novel human calcium-independent phospholipase A2 family members possessing triacylglycerol lipase and acylglycerol transacylase activities. J. Biol. Chem. 2004;279:48968–48975. doi: 10.1074/jbc.M407841200. [DOI] [PubMed] [Google Scholar]
- 95.Bazan NG, Calandria JM, Serhan CN. Rescue and repair during photoreceptor cell renewal mediated by docosahexaenoic acid-derived neuroprotectin D1. J. Lipid Res. 2010;51:2018–2031. doi: 10.1194/jlr.R001131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Mita R, Beaulieu MJ, Field C, Godbout R. Brain fatty acid-binding protein & omega-3/omega-6 fatty acids: mechanistic insight into malignant glioma cell migration. J. Biol. Chem. 2010;285:37005–37015. doi: 10.1074/jbc.M110.170076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Connor KM, et al. Increased dietary intake of omega-3-polyunsaturated fatty acids reduces pathological retinal angiogenesis. Nature Med. 2007;13:868–873. doi: 10.1038/nm1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moghaddam-Taaheri S, et al. Effects of docosahexaenoic acid in preventing experimental choroidal neovascularization in rodents. J. Clin. Experiment Ophthalmol. 2011;2:10. doi: 10.4172/2155-9570.1000187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mukherjee PK, Marcheselli VL, Serhan CN, Bazan NG. Neuroprotectin D1: a docosahexaenoic acid-derived docosatriene protects human retinal pigment epithelial cells from oxidative stress. Proc. Natl Acad. Sci. USA. 2004;101:8491–8496. doi: 10.1073/pnas.0402531101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mukherjee PK, et al. Neurotrophins enhance retinal pigment epithelial cell survival through neuroprotectin D1 signaling. Proc. Natl Acad. Sci. USA. 2007;104:13152–13157. doi: 10.1073/pnas.0705949104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Berquin IM, Edwards IJ, Chen YQ. Multi-targeted therapy of cancer by omega-3 fatty acids. Cancer Lett. 2008;269:363–377. doi: 10.1016/j.canlet.2008.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gleissman H, et al. Docosahexaenoic acid metabolome in neural tumors: identification of cytotoxic intermediates. FASEB J. 2010;24:906–915. doi: 10.1096/fj.09-137919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Brown MD, Hart CA, Gazi E, Bagley S, Clarke NW. Promotion of prostatic metastatic migration towards human bone marrow stoma by Omega 6 and its inhibition by Omega 3 PUFAs. Br. J. Cancer. 2006;94:842–853. doi: 10.1038/sj.bjc.6603030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Hirsch J, et al. PEDF inhibits IL8 production in prostate cancer cells through PEDF receptor/ phospholipase A2 and regulation of NFκB and PPARγ. Cytokine. 2011;55:202–210. doi: 10.1016/j.cyto.2011.04.010.This paper shows that PEDF binding to PEDFR triggers downstream signalling through PPARγ and NF-κB; this inhibits IL-8production by human hormone-refractoryprostate cancer cells and delays cell growth.
- 105.Sapieha P, et al. 5-Lipoxygenase metabolite 4-HDHA is a mediator of the antiangiogenic effect of omega-3 polyunsaturated fatty acids. Sci. Transl. Med. 2011;3:69. doi: 10.1126/scitranslmed.3001571. ra12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bernard A, et al. Laminin receptor involvement in the anti-angiogenic activity of pigment epithelium-derived factor. J. Biol. Chem. 2009;284:10480–10490. doi: 10.1074/jbc.M809259200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Notari L, et al. Pigment epithelium-derived factor binds to cell-surface F1-ATP synthase. FEBS J. 2010;277:2192–2205. doi: 10.1111/j.1742-4658.2010.07641.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Deshpande M, Notari L, Subramanian P, Notario V, Becerra SP. Inhibition of tumor cell surface ATP synthesis by pigment epithelium-derived factor: implications for antitumor activity. Int. J. Oncol. 2012;41:219–227. doi: 10.3892/ijo.2012.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Park K, et al. Identification of a novel inhibitor of the canonical Wnt pathway. Mol. Cell. Biol. 2011;31:3038–3051. doi: 10.1128/MCB.01211-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang J, Duh EJ, Caldwell RB, Behzadian MA. Antipermeability function of PEDF involves blockade of the MAP kinase/GSK/β-catenin signaling pathway and uPAR expression. Invest. Ophthalmol. Vis. Sci. 2010;51:3273–3280. doi: 10.1167/iovs.08-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.S tratikos E, Alberdi E, Gettins PG, Becerra SP. Recombinant human pigment epithelium-derived factor (PEDF): characterization of PEDF overexpressed and secreted by eukaryotic cells. Protein Sci. 1996;5:2575–2582. doi: 10.1002/pro.5560051220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alberdi EM, Weldon JE, Becerra SP. Glycosaminoglycans in human retinoblastoma cells: heparan sulfate, a modulator of the pigment epithelium-derived factor-receptor interactions. BMC Biochem. 2003;4:1. doi: 10.1186/1471-2091-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hosomichi J, Yasui N, Koide T, Soma K, Morita I. Involvement of the collagen I-binding motif in the anti-angiogenic activity of pigment epithelium-derived factor. Biochem. Biophys. Res. Commun. 2005;335:756–761. doi: 10.1016/j.bbrc.2005.07.140. [DOI] [PubMed] [Google Scholar]
- 114.Cheung LW, et al. Pigment epithelium-derived factor is estrogen sensitive and inhibits the growth of human ovarian cancer and ovarian surface epithelial cells. Endocrinology. 2006;147:4179–4191. doi: 10.1210/en.2006-0168. [DOI] [PubMed] [Google Scholar]
- 115.Tombran-Tink J, et al. Retinoic acid and dexamethasone regulate the expression of PEDF in retinal and endothelial cells. Exp. Res. 2004;78:945–955. doi: 10.1016/j.exer.2003.12.013. [DOI] [PubMed] [Google Scholar]
- 116.Uchida H, et al. Vitamin A up-regulates the expression of thrombospondin-1 and pigment epithelium-derived factor in retinal pigment epithelial cells. Exp. Res. 2005;80:23–30. doi: 10.1016/j.exer.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 117.Doyon G, St-Jean S, Darsigny M, Asselin C, Boudreau F. Nuclear receptor co-repressor is required to maintain proliferation of normal intestinal epithelial cells in culture and down-modulates the expression of pigment epithelium-derived factor. J. Biol. Chem. 2009;284:25220–25229. doi: 10.1074/jbc.M109.022632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lo WR, et al. Tissue differential microarray analysis of dexamethasone induction reveals potential mechanisms of steroid glaucoma. Invest. Ophthalmol. Vis. Sci. 2003;44:473–485. doi: 10.1167/iovs.02-0444. [DOI] [PubMed] [Google Scholar]
- 119.Perruccio EM, et al. Dexamethasone increases pigment epithelium-derived factor in perfused human eyes. Curr. Eye Res. 2008;33:507–515. doi: 10.1080/02713680802110208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Sasaki Y, et al. Identification of pigment epithelium-derived factor as a direct target of the p53 family member genes. Oncogene. 2005;24:5131–5136. doi: 10.1038/sj.onc.1208695. [DOI] [PubMed] [Google Scholar]
- 121.Diaz R, et al. p73 Isoforms affect VEGF, VEGF165b and PEDF expression in human colorectal tumors: VEGF165b downregulation as a marker of poor prognosis. Int. J. Cancer. 2008;123:1060–1067. doi: 10.1002/ijc.23619. [DOI] [PubMed] [Google Scholar]
- 122.Sivak JM, Fini ME. MMPs in the eye: emerging roles for matrix metalloproteinases in ocular physiology. Prog. Retin Eye Res. 2002;21:1–14. doi: 10.1016/s1350-9462(01)00015-5. [DOI] [PubMed] [Google Scholar]
- 123.Samtani S, Amaral J, Campos MM, Fariss RN, Becerra SP. Doxycycline-mediated inhibition of choroidal neovascularization. Invest. Ophthalmol. Vis. Sci. 2009;50:5098–5106. doi: 10.1167/iovs.08-3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fernandez-Barral A, et al. Hypoxia negatively regulates antimetastatic PEDF in melanoma cells by a hypoxia inducible factor-independent, autophagy dependent mechanism. PLoS ONE. 2012;7:e32989. doi: 10.1371/journal.pone.0032989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Maik-Rachline G, Shaltiel S, Seger R. Extracellular phosphorylation converts pigment epithelium-derived factor from a neurotrophic to an antiangiogenic factor. Blood. 2005;105:670–678. doi: 10.1182/blood-2004-04-1569. [DOI] [PubMed] [Google Scholar]
- 126.Feng Y, et al. Phosphomimetic mutants of pigment epithelium-derived factor with enhanced anti-choroidal melanoma cell activity in vitro and in vivo. Invest. Ophthalmol. Vis. Sci. 2012;53:6793–6802. doi: 10.1167/iovs.12-10326. [DOI] [PubMed] [Google Scholar]
- 127.Guan M, et al. Loss of pigment epithelium derived factor expression in glioma progression. J. Clin. Pathol. 2003;56:277–282. doi: 10.1136/jcp.56.4.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Chen J, Ye L, Zhang L, Jiang WG. The molecular impact of pigment epithelium-derived factor, PEDF, on lung cancer cells and the clinical significance. Int. J. Oncol. 2009;35:159–166. doi: 10.3892/ijo_00000324. [DOI] [PubMed] [Google Scholar]
- 129.Zhang L, Chen J, Ke Y, Mansel RE, Jiang WG. Expression of pigment epithelial derived factor is reduced in non-small cell lung cancer and is linked to clinical outcome. Int. J. Mol. Med. 2006;17:937–944. [PubMed] [Google Scholar]
- 130.Cai J, Parr C, Watkins G, Jiang WG, Boulton M. Decreased pigment epithelium-derived factor expression in human breast cancer progression. Clin. Cancer Res. 2006;12:3510–3517. doi: 10.1158/1078-0432.CCR-06-0094. [DOI] [PubMed] [Google Scholar]
- 131.Zhou D, et al. Evaluation of protein pigment epithelium-derived factor (PEDF) and microvessel density (MVD) as prognostic indicators in breast cancer. J. Cancer Res. Clin. Oncol. 2010;136:1719–1727. doi: 10.1007/s00432-010-0830-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Wagsater D, Lofgren S, Zar N, Hugander A, Dimberg J. Pigment epithelium-derived factor expression in colorectal cancer patients. Cancer Invest. 2010;28:872–877. doi: 10.3109/07357901003735675. [DOI] [PubMed] [Google Scholar]
- 133.Qingyi Z, et al. Unfavorable prognostic value of human PEDF decreased in high-grade prostatic intraepithelial neoplasia: a differential proteomics approach. Cancer Invest. 2009;27:794–801. doi: 10.1080/07357900802175617. [DOI] [PubMed] [Google Scholar]
- 134.Jiang Z, Fang Z, Ding Q. Prognostic role of pigment epithelium-derived factor in clear cell renal cell carcinoma. Urol. Int. 2010;84:334–340. doi: 10.1159/000288239. [DOI] [PubMed] [Google Scholar]
- 135.Tsuchiya T, et al. The reduction in pigment epithelium-derived factor is a sign of malignancy in ovarian cancer expressing low-level of vascular endothelial growth factor. Gynecol. Endocrinol. 2009;25:104–109. doi: 10.1080/09513590802549841. [DOI] [PubMed] [Google Scholar]
- 136.Byrne JC, et al. 2D-DIGE as a strategy to identify serum markers for the progression of prostate cancer. J. Proteome Res. 2009;8:942–957. doi: 10.1021/pr800570s. [DOI] [PubMed] [Google Scholar]
- 137.Jan R, Huang M, Lewis-Wambi J. Loss of pigment epithelium-derived factor: a novel mechanism for the development of endocrine resistance in breast cancer. Breast Cancer Res. 2012;14:R146. doi: 10.1186/bcr3356. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 138.Ji D, et al. Prognostic role of serum AZGP1, PEDF and PRDX2 in colorectal cancer patients. Carcinogenesis. 2013 Feb;7 doi: 10.1093/carcin/bgt056. [DOI] [PubMed] [Google Scholar]
- 139.Matsumoto K, et al. Antiangiogenic property of pigment epithelium-derived factor in hepatocellular carcinoma. Hepatology. 2004;40:252–259. doi: 10.1002/hep.20259. [DOI] [PubMed] [Google Scholar]
- 140.Kawaguchi T, et al. Pigment epithelium-derived factor inhibits lysosomal degradation of Bcl-xL and apoptosis in HepG2 cells. Am. J. Pathol. 2010;176:168–176. doi: 10.2353/ajpath.2010.090242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Wang L, et al. Suppression of angiogenesis and tumor growth by adenoviral-mediated gene transfer of pigment epithelium-derived factor. Mol. Ther. 2003;8:72–79. doi: 10.1016/s1525-0016(03)00128-x. [DOI] [PubMed] [Google Scholar]
- 142.Gong Q, Yang X, Cai W, Gao G, Yang Z. Expression purification of functional epitope of pigment epithelium-derived factor in E. coli with inhibiting effect on endothelial cells. Protein J. 2010;29:167–173. doi: 10.1007/s10930-010-9236-6. [DOI] [PubMed] [Google Scholar]
- 143.Mahtabifard A, Merritt RE, Yamada RE, Crystal RG, Korst RJ. In vivo gene transfer of pigment epithelium-derived factor inhibits tumor growth in syngeneic murine models of thoracic malignancies. J. Thorac. Cardiovasc. Surg. 2003;126:28–38. doi: 10.1016/s0022-5223(02)73616-7. [DOI] [PubMed] [Google Scholar]
- 144.Chen Q, et al. Therapeutic potential of bone marrow-derived mesenchymal stem cells producing pigment epithelium-derived factor in lung carcinoma. Int. J. Mol. Med. 2012;30:527–534. doi: 10.3892/ijmm.2012.1015. [DOI] [PubMed] [Google Scholar]
- 145.Gao Y, et al. Human mesenchymal stem cells overexpressing pigment epithelium-derived factor inhibit hepatocellular carcinoma in nude mice. Oncogene. 2010;29:2784–2794. doi: 10.1038/onc.2010.38. [DOI] [PubMed] [Google Scholar]
- 146.Wang Q, Zhang Z, Ding T, Chen Z, Zhang T. Mesenchymal stem cells over-expressing PEDF decreased the angiogenesis of gliomas. Biosci. Rep. 2013;33:e00019. doi: 10.1042/BSR20110124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Dass CR, Contreras KG, Dunstan DE, Choong PF. Chitosan microparticles encapsulating PEDF plasmid demonstrate efficacy in an orthotopic metastatic model of osteosarcoma. Biomaterials. 2007;28:3026–3033. doi: 10.1016/j.biomaterials.2007.03.016. [DOI] [PubMed] [Google Scholar]
- 148.Li L, et al. Pigment epithelium-derived factor gene loaded in cRGD-PEG-PEI suppresses colorectal cancer growth by targeting endothelial cells. Int. J. Pharm. 2012;438:1–10. doi: 10.1016/j.ijpharm.2012.08.043. [DOI] [PubMed] [Google Scholar]
- 149.Broadhead ML, Dass CR, Choong PF. Systemically administered PEDF against primary and secondary tumours in a clinically relevant osteosarcoma model. Br. J. Cancer. 2011;105:1503–1511. doi: 10.1038/bjc.2011.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Smith ND, et al. Pigment epithelium-derived factor and interleukin-6 control prostate neuroendocrine differentiation via feed-forward mechanism. J. Urol. 2008;179:2427–2434. doi: 10.1016/j.juro.2008.01.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Subramanian P, Notario PM, Becerra SP. Pigment epithelium-derived factor receptor (PEDF-R): a plasma membrane-linked phospholipase with PEDF binding affinity. Adv. Exp. Med. Biol. 2010;664:29–37. doi: 10.1007/978-1-4419-1399-9_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Calandria JM, et al. Selective survival rescue in 15-lipoxygenase-1-deficient retinal pigment epithelial cells by the novel docosahexaenoic acid-derived mediator, neuroprotectin D1. J. Biol. Chem. 2009;284:17877–17882. doi: 10.1074/jbc.M109.003988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Gleissman H, Johnsen JI, Kogner P. Omega-3 fatty acids in cancer, the protectors of good and the killers of evil? Exp. Cell Res. 2010;316:1365–1373. doi: 10.1016/j.yexcr.2010.02.039. [DOI] [PubMed] [Google Scholar]
- 154.Ablonczy Z, et al. Pigment epithelium-derived factor maintains retinal pigment epithelium function by inhibiting vascular endothelial growth factor-R2 signaling through γ-secretase. J. Biol. Chem. 2009;284:30177–30186. doi: 10.1074/jbc.M109.032391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Ho TC, et al. Pigment epithelium-derived factor (PEDF) promotes tumor cell death by inducing macrophage membrane tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) J. Biol. Chem. 2011;286:35943–35954. doi: 10.1074/jbc.M111.266064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sugita Y, Becerra SP, Chader GJ, Schwartz JP. Pigment epithelium-derived factor (PEDF) has direct effects on the metabolism and proliferation of microglia and indirect effects on astrocytes. J. Neurosci. Res. 1997;49:710–718. doi: 10.1002/(SICI)1097-4547(19970915)49:6<710::AID-JNR5>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 157.Nelius T, et al. Positive correlation between PEDF expression levels and macrophage density in the human prostate. Prostate. 2012 Oct;4 doi: 10.1002/pros.22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Jablonski MM, Tombran-Tink J, Mrazek DA, Iannaccone A. Pigment epithelium-derived factor supports normal development of photoreceptor neurons and opsin expression after retinal pigment epithelium removal. J. Neurosci. 2000;20:7149–7157. doi: 10.1523/JNEUROSCI.20-19-07149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jablonski MM, Tombran-Tink J, Mrazek DA, Iannaccone A. Pigment epithelium-derived factor supports normal Muller cell development and glutamine synthetase expression after removal of the retinal pigment epithelium. Glia. 2001;35:14–25. doi: 10.1002/glia.1066. [DOI] [PubMed] [Google Scholar]
- 160.Tanimoto S, Kanamoto T, Mizukami M, Aoyama H, Kiuchi Y. Pigment epithelium-derived factor promotes neurite outgrowth of retinal cells. Hiroshima J. Med. Sci. 2006;55:109–116. [PubMed] [Google Scholar]
- 161.Bilak MM, et al. Pigment epithelium-derived factor (PEDF) protects motor neurons from chronic glutamate-mediated neurodegeneration. J. Neuropathol. Exp. Neurol. 1999;58:719–728. doi: 10.1097/00005072-199907000-00006. [DOI] [PubMed] [Google Scholar]
- 162.Nomura T, et al. Survival effects of pigment epithelium-derived factor expressed by a lentiviral vector in rat cerebellar granule cells. Dev. Neurosci. 2001;23:145–152. doi: 10.1159/000048706. [DOI] [PubMed] [Google Scholar]
- 163.Zhu D, et al. Polarized secretion of PEDF from human embryonic stem cell-derived RPE promotes retinal progenitor cell survival. Invest. Ophthalmol. Vis. Sci. 2011;52:1573–1585. doi: 10.1167/iovs.10-6413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Unterlauft JD, et al. Pigment epithelium-derived factor released by Muller glial cells exerts neuroprotective effects on retinal ganglion cells. Neurochem. Res. 2012;37:1524–1533. doi: 10.1007/s11064-012-0747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Pang IH, Zeng H, Fleenor DL, Clark AF. Pigment epithelium-derived factor protects retinal ganglion cells. BMC Neurosci. 2007;8:11. doi: 10.1186/1471-2202-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cao W, et al. Pigment epithelium-derived factor protects cultured retinal neurons against hydrogen peroxide-induced cell death. J. Neurosci. Res. 1999;57:789–800. [PubMed] [Google Scholar]
- 167.Andreu-Agullo C, Morante-Redolat JM, Delgado AC, Farinas I. Vascular niche factor PEDF modulates Notch-dependent stemness in the adult subependymal zone. Nature Neurosci. 2009;12:1514–1523. doi: 10.1038/nn.2437. [DOI] [PubMed] [Google Scholar]
- 168.De Marzo A, Aruta C, Marigo V. PEDF promotes retinal neurosphere formation and expansion in vitro. Adv. Exp. Med. Biol. 2010;664:621–630. doi: 10.1007/978-1-4419-1399-9_71. [DOI] [PubMed] [Google Scholar]
- 169.Gonzalez R, et al. Screening the mammalian extracellular proteome for regulators of embryonic human stem cell pluripotency. Proc. Natl Acad. Sci. USA. 2010;107:3552–3557. doi: 10.1073/pnas.0914019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Cai J, et al. PEDF regulates vascular permeability by a γ-secretase-mediated pathway. PLoS ONE. 2011;6:e21164. doi: 10.1371/journal.pone.0021164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhang SX, et al. Pigment epithelium-derived factor (PEDF) is an endogenous antiinflammatory factor. FASEB J. 2006;20:323–325. doi: 10.1096/fj.05-4313fje. [DOI] [PubMed] [Google Scholar]
- 172.Chavan S, et al. Identification of pigment epithelium-derived factor as an adipocyte derived inflammatory factor. Mol. Med. 2012;18:1161–1168. doi: 10.2119/molmed.2012.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Famulla S, et al. Pigment epithelium-derived factor (PEDF) is one of the most abundant proteins secreted by human adipocytes and induces insulin resistance and inflammatory signaling in muscle and fat cells. Int. J. Obes. 2011;35:762–772. doi: 10.1038/ijo.2010.212. [DOI] [PubMed] [Google Scholar]
- 174.Streck CJ, et al. Adeno-associated virus vector-mediated delivery of pigment epithelium-derived factor restricts neuroblastoma angiogenesis and growth. J. Pediatr. Surg. 2005;40:236–243. doi: 10.1016/j.jpedsurg.2004.09.049. [DOI] [PubMed] [Google Scholar]
- 175.Halin S, et al. Pigment epithelium-derived factor stimulates tumor macrophage recruitment and is downregulated by the prostate tumor microenvironment. Neoplasia. 2010;12:336–345. doi: 10.1593/neo.92046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zolochevska O, Yu G, Gimble JM, Figueiredo ML. Pigment epithelial-derived factor and melanoma differentiation associated gene-7 cytokine gene therapies delivered by adipose-derived stromal/ mesenchymal stem cells are effective in reducing prostate cancer cell growth. Stem Cells Dev. 2012;21:1112–1123. doi: 10.1089/scd.2011.0247. [DOI] [PubMed] [Google Scholar]
- 177.Takenaka K, et al. Pigment epithelium-derived factor (PEDF)-induced apoptosis and inhibition of vascular endothelial growth factor (VEGF) expression in MG63 human osteosarcoma cells. Life Sci. 2005;77:3231–3241. doi: 10.1016/j.lfs.2005.05.048. [DOI] [PubMed] [Google Scholar]
- 178.Broadhead ML, Choong PF, Dass CR. Efficacy of continuously administered PEDF-derived synthetic peptides against osteosarcoma growth and metastasis. J. Biomed. Biotechnol. 2012;2012:230298. doi: 10.1155/2012/230298. [DOI] [PMC free article] [PubMed] [Google Scholar]