Abstract
Computer-assisted morphometry can provide precise measurement of hepatic fibrosis on a continuous scale. Previous morphometric studies of large cohorts of patients with treatment refractory chronic hepatitis C showed a mean increase in fibrosis of 30% to 58% in one year. The aim of the present study was to quantify fibrosis progression in biopsies obtained over 1.5 to 5 years from three groups of patients with baseline bridging fibrosis or cirrhosis (Ishak stages 3-6) enrolled in the HALT-C Trial.
Results
The main group of 346 lead-in nonresponders (viremic after 24 weeks of peginterferon-ribavirin therapy) had a mean fibrosis increase of 61% over pretreatment baseline after 2 years and 80% after 4 years. In contrast, the 78 “breakthrough/relapse” patients (undetectable serum HCV RNA after 24 weeks of peginterferon-ribavirin and receiving antiviral therapy for 48 weeks) showed a mean increase in fibrosis of 48% when biopsied 36 months from pretreatment baseline but no further increase at 60 months. Finally, the 111 “express” patients with baseline biopsies following unsuccessful peginterferon-ribavirin outside the trial, had significantly more baseline fibrosis than the others but an increase of only 21% after 21 months and a slight decrease at 45 months. Maintenance therapy with low-dose peginterferon had no effect on fibrosis changes in any of the groups.
Conclusion
Morphometry demonstrated complex, nonlinear changes in fibrosis over time in this heterogeneous cohort of patients with interferon-refractory chronic hepatitis C.
Progression of hepatic fibrosis is the mechanism by which chronic hepatitis C leads to cirrhosis and decompensated liver disease. Consequently, interest is high in developing therapeutic options for patients who are not candidates for antiviral treatment as well as those who do not achieve a sustained virological response (SVR) to antiviral therapy; these patients remain at risk for continuing fibrosis progression and the clinical consequences of hepatic decompensation and hepatocellular carcinoma (1-3). Because chronic hepatitis C takes decades to evolve into cirrhosis, and even longer to progress towards decompensation, demonstration of survival benefit resulting from antiviral therapy would require large treatment trials of very long duration; therefore, treatment benefit in many clinical trials has been demonstrated reliably by assessing fibrosis stage in paired liver biopsies obtained before and after a course of therapy.
In most studies that have relied upon liver biopsy to evaluate changes in fibrosis, semiquantitative scoring systems have been used that have a limited range of categories of severity, such as the Metavir score (4) with five or the Ishak score (5) with seven stages of fibrosis. An alternative to semiquantitative fibrosis scores is direct measurement of the amount of fibrosis in the biopsy specimen by computer-assisted morphometric image analysis. In contrast to semiquantitative fibrosis scores that rely on quantity and location of, and architectural distortion caused by fibrosis, morphometry determines the proportion of collagenous tissue in a specimen, regardless of location and provides precise measurements on a continuous scale. Within any given semiquantitative stage, a wide range of collagen may be present, and considerable overlap exists within Metavir and Ishak stages (6,7). Accurate morphometric studies, however, require good quality, unfragmented liver biopsy specimens of sufficient size, because fibrous tissue is underrepresented in small, fragmented specimens of fibrotic livers. Morphometry is also subject to sampling variability (8), but when used to show differences between treatment groups in an adequately powered study, morphometry has the potential to be the most sensitive means to show changes in the quantity of fibrosis (7).
Even though clinical and histologic progression is usually very slow, in two previous morphometric analyses of antifibrotic agents tested in randomized controlled trials, investigators found that the actual quantity of fibrous tissue increased at a rapid rate in patients with treatment-resistant chronic hepatitis C. In one study of 245 patients with advanced fibrosis and cirrhosis (Ishak stage 4-6) treated with interferon gamma-1b or placebo, the mean collagen content of the liver biopsies increased by 58% in only 48 weeks (7). Similarly, in a trial of the peroxisome proliferator-activated receptor agonist antifibrotic agent farglitazar compared to placebo in patients with moderate (Ishak stage 2-4) precirrhotic fibrosis, the mean hepatic collagen content increased by 30% in 52 weeks (9). Such changes may not have direct clinical significance, especially in patients with relatively little baseline fibrosis, but the ability to detect the change suggests that morphometry is a more useful tool than histologic staging for clinical trials of antifibrotic agents.
The Hepatitis C Antiviral Long-term Treatment against Cirrhosis (HALT-C) Trial provided a unique opportunity to evaluate morphometry for assessing changes in liver fibrosis over several years in patients with chronic hepatitis C and moderate to advanced fibrosis. In the trial, we enrolled 1,050 prior nonresponders to pegylated interferon and ribavirin with compensated liver disease and bridging fibrosis or cirrhosis. Subjects were randomized to receive either weekly injections of 90 μ g of peginterferon alfa-2a or no therapy for 3.5 years. Over the course of the trial, we found no difference between the peginterferon-treated and non-treated groups in the development of clinical outcomes or in progression from precirrhotic fibrosis to cirrhosis on liver biopsy (10). In this paper, we report results of histologic and morphometric analyses of fibrosis in liver biopsies obtained at baseline (up to 18 months before randomization), at approximately 18 months after randomization (study month 24), and at approximately 42 months after randomization (study month 48). We hypothesized that morphometric image analysis would demonstrate a linear increase in collagen content over time and might show an effect of maintenance therapy on fibrosis progression, even if standard histologic scoring did not.
Patients and Methods
The design and results of the HALT-C Trial have been reported (10,11). To be eligible for entry into the trial, patients at 10 US Medical Centers had to be nonresponders to prior interferon therapy and had to have bridging fibrosis or cirrhosis on liver biopsy. Potential participants were excluded if they had another concurrent liver disease, a history of hepatic decompensation or hepatocellular carcinoma, any contraindication to interferon therapy, or any other medical or psychiatric condition that could interfere with their completion of the trial.
Recruitment for the trial began in August 2000 and ended in August 2004. Initially, all patients underwent a 24-week lead-in phase of treatment with combination, full-dose peginterferon alfa-2a and ribavirin. Patients who were still viremic after 20 weeks of treatment (“lead-in nonresponders”) were randomized to receive no further therapy (controls) or low-dose [90 μg/week] of peginterferon (maintenance) for the next 3.5 years (Figure 1). Patients with undetectable serum HCV RNA after 20 weeks of treatment were categorized as responders and continued to receive full-dose peginterferon and ribavirin for a total of 48 weeks. Those who were found thereafter to have detectable HCV RNA while on treatment (“breakthrough”) or after completing the 48 weeks of therapy (“relapse”) were eligible for randomization for the following 3.5 years (“breakthrough/relapse patients”). After combination peginterferon and ribavirin was approved by the Food and Drug Administration, patients who had received the equivalent of lead-in treatment outside the trial were also allowed to enter the randomized phase (“express patients”). Of the 1,050 patients enrolled in the randomized phase of the study, 662 were lead-in nonresponders, 151 breakthrough/relapsers and 237 express patients.
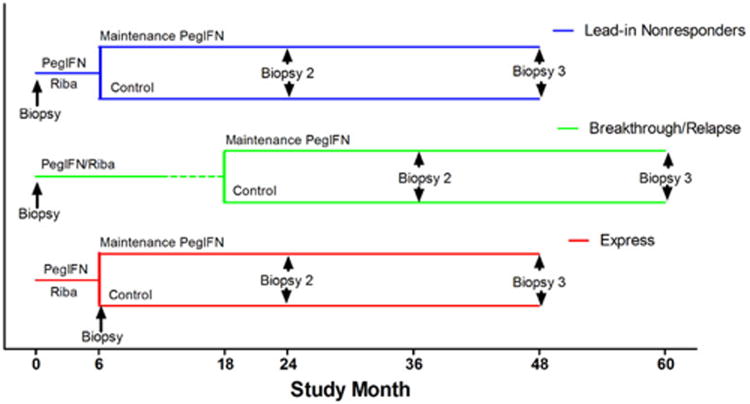
Figure 1.
Design of the HALT-C Trial, showing the source of the three cohorts of patients used in morphometric analyses of fibrosis. Lead-in nonresponder patients (blue line) were those who remained viremic the lead-in phase of Peginterferon/ribavirin for 6 months and were then randomized to maintenance peginterferon or the untreated control group. They had baseline liver biopsies before the lead-in phase and approximately 24 and 48 months later. Breakthrough/relapse patients (green line) developed undetectable HCV RNA during the lead-in phase of peginterferon/ribavirin and completed 12 months of therapy and 6 months of follow-up. Those who became viremic while still receiving therapy (breakthrough) or during follow-up (relapse) were randomized to maintenance or untreated control groups. They had baseline biopsies before the lead-in phase and approximately 36 and 60 months later. Express patients (red line) received the equivalent of the lead-in phase outside the trial but remained viremic. Most had baseline biopsies immediately before randomization to maintenance peginterferon or the control group. Subsequent biopsies were at 18 and 42 months after randomization.
Liver biopsies were evaluated for necroinflammatory changes, fibrosis, steatosis and other findings by consensus of a committee of 12 pathologists, at least 8 of whom were present at each scoring session. The Ishak fibrosis score (5) was used as a primary endpoint in the Trial. All patients were required to have a baseline liver biopsy showing bridging fibrosis (Ishak stages 3-4) or cirrhosis (Ishak stages 5-6) within 12 months of enrollment in the trial. Exceptions were allowed for patients with stage 2 (portal fibrosis) if a previous biopsy had shown unequivocal bridging fibrosis. For express subjects, baseline biopsies were obtained following unsuccessful peginterferon-ribavirin outside the trial. Repeat biopsies were performed at 18 months after entering the randomized phase (biopsy 2) and after another 24 months (biopsy 3) unless the patient refused, had a contraindication to biopsy, had a study-terminating clinical outcome, or had withdrawn from the trial.
Morphometric image analysis of hepatic fibrosis
We used liver biopsy sections stained with Sirius red for assessment of hepatic collagen content as previously described (7) with Image Pro Plus 6.0 imaging software (Media Cybernetics, Silver Spring, MD) after editing of the digitized images to remove technical artifacts and extraneous tissue, such as skin or muscle as well as Glisson's capsule or hepatic hilum if present. This method takes advantage of the fact that the degree of Sirius red staining measured by the degree of saturation of the red hue (− 0.1 to + 0.04 of the HSI [Hue, Saturation, Intensity] color model) correlates well with chemically determined collagen content and morphometrically determined estimates of the area of hepatic fibrosis. This approach relies on the analysis of identical brightfield images and images obtained with polarizing filters to subtract background mathematically at each pixel without interactive thresholding. The collagen content per unit area of the specimen is expressed in arbitrary units calculated as the sum of the pixel-wise collagen measurements divided by the number of summed pixels, corrected for the section thickness as measured by a confocal laser scanning microscope (Zeiss LSM 310) and multiplied by 5 (a constant) to yield a range of values very similar to those obtained by methods that measure the fractional area of fibrous tissue. The surface area of each specimen was computed as the number of pixels multiplied by the pixel size (8.2 × 10−6 mm2). Because the method uses the degree of saturation of color to estimate collagen content, a pixel that is darker red (i.e. more saturated), has greater weight than one that is paler red, and although the values obtained do not have a physical dimension, the relative amount of collagen can be compared between specimens and used as a continuous function in statistical analyses. All collagen in the specimen is measured by this method, including the small amount normally present in portal tracts and central veins, so biopsies with relatively little abnormal fibrous tissue are not readily distinguished by this method. Similarly, since only collagen is measured, expansion of portal tracts by inflammation but not fibrosis does not affect the result. Reproducibility, assessed by ten collagen measurements on the same slide, yielded a coefficient of variation of 7.1% for biopsies with high collagen content (>0.1 units) and 12.1% for those with low collagen content (<0.05 units).
Selection of biopsies for morphometric analysis
This data set was limited to 535 HALT-C Trial patients with at least two adequate liver specimens suitable for computerized morphometry. Fragmented specimens (19% overall, 23% of baseline biopsies) were excluded because of inherent inaccuracy, and biopsies with <10 mm2 of tissue were excluded to reduce sampling variability. 199 patients met these criteria for all three biopsies; another 135 patients had adequate specimens at baseline and biopsy 2; an additional 136 patients had adequate specimens for biopsy 2 and biopsy 3 but not at baseline; and an additional 65 had biopsies adequate for morphometry at baseline and biopsy 3 but not in biopsy 2. Overall 1,269 specimens from 535 of the 1,050 randomized patients were used in the analyses of morphometric data. These included 1,267 needle biopsies and tissue from two explanted livers. The 1,267 needle biopsies had a mean ± S.D. length of 2.0 ± 0.7 cm, median length of 1.9 cm, and mean ± S.D. area of 17.5 ± 5.8 mm2. There were 324 (31%) that were less than 15 mm long. The sections from the two explant livers had 89 and 251 mm2 of tissue.
Statistical methods
To evaluate the change in morphometric collagen over time, we used repeated measures analysis of variance assuming an autoregressive covariance structure. Time (baseline, biopsy 2, biopsy 3), randomization stratum (lead-in nonresponders, breakthrough/relapse, express) and the development of a clinical outcome (yes/no) were included as fixed effects. Correlation was measured by Spearman rank correlations. Mann-Whitney and Kruskal-Wallis tests were used for assessing differences in patient characteristics between groups. Contingency tables were analyzed with the chi-square statistic. P values were two-sided. All analyses were carried out with SAS Statistical software version 9.1 (SAS institute, Inc., Cary, NC) or Instat version 3.06 (Graphpad Software, San Diego, CA).
Results
Patient population
The baseline characteristics of the 535 patients whose biopsies were used for morphometric analysis were similar to those of the entire randomized cohort of the HALT-C Trial (Table 1). The mean age was 50, the majority were male (72%) and 95% were infected with HCV genotype 1. Baseline liver biopsy showed cirrhosis in 182 (34%) of the 535 morphometry patients compared to 41% in the overall HALT-C Trial cohort, reflecting the fact that cirrhotic patients were more likely to have fragmented or small biopsies.
Table 1.
Characteristics of patients with two consecutive unfragmented liver biopsies analyzed for morphometry compared to all randomized patients in HALT-C Trial.
| Analyzed for Morphometry (n = 535) | All HALT-C Patients (n = 1050) | |
|---|---|---|
| Age | ||
| Mean ± S.D. | 50 ±7 | 50 ± 7 |
| Range | 19 - 77 | 19 - 80 |
| Gender | ||
| Male | 72% | 71% |
| Female | 28% | 29% |
| Race | ||
| White | 71% | 72% |
| Black | 21% | 18% |
| Hispanic | 5% | 8% |
| Body Mass Index | ||
| Mean ± S.D. | 30 ±5 | 30 ±5 |
| Range | 18 – 56 | 18 - 58 |
| Diabetes | 24% | 24% |
| Genotype 1 | 95% | 94% |
| Log10 HCV RNA (mean) | 6.5 ±0.5 | 6.4 ±0.5 |
| Randomization Stratum | ||
| Lead-in nonresponder | 65% | 63% |
| Breakthrough/Relapse | 15% | 14% |
| Express | 21% | 23% |
| Treatment Group | ||
| Control | 51% | 51% |
| Long-term peginterferon | 49% | 49% |
| Baseline Liver Biopsy | n=399 | n=1050 |
| Fragmented | 0 | 24% |
| Length (mean ± S.D.) | 21 ± 7 mm | 18 ±9 mm |
| Area (mean ± S.D.) | 18 ± 6 mm2 | 14 ± 8 mm2 |
| Inflammation score (Ishak) | ||
| Mean | 7.74 ±1.91 | 7.54 ±2.06 |
| Range | 3 - 12 | 1 - 12 |
| Fibrosis score (Ishak) | ||
| Stage 2 | 11% | 7% |
| Stage 3 | 39% | 34% |
| Stage 4 | 17% | 18% |
| Stage 5 | 21% | 22% |
| Stage 6 | 13% | 19% |
| Steatosis (> 5% of tissue) | 39% | 42% |
Among the 535 patients in whom morphometric analyses were performed, 346 (65%) were randomized at the end of the lead-in phase, 78 (14%) were breakthrough/relapse patients, and 111 (21%) were express patients. A noteworthy difference among these three randomization strata was in the interval between the baseline biopsy and biopsy 2 (Table 2). The majority of the lead-in nonresponder subgroup had the baseline liver biopsy shortly before the start of the lead-in phase, with a median of 6.3 months (range 5.2 to 17.5 months) between biopsy and randomization. Most of the breakthrough/relapse patients were also biopsied just before entering the lead-in phase, but nearly all had finished 48 weeks of combination therapy and 24 weeks of follow-up before being randomized; therefore, the median time between baseline biopsy and entry into the randomized phase was 18.1 (range 0.8 to 25.9) months. Finally, most of the express patients had baseline biopsies after they had failed peginterferon-ribavirin therapy, with a median of 1.5 (range of 0.3 to 19.1) months from biopsy to randomization. For all patients, biopsy 2 was scheduled to be at 18 months after entering the randomized phase; therefore, for the lead-in nonresponder patients, biopsy 2 was a median of 25 months from baseline, while for breakthrough/relapse patients and for express patients, biopsy 2 was obtained a median of 37 months and 21 months after baseline, respectively. Biopsy 3, on the other hand, was obtained at a median of 24 months following biopsy 2 for all patients.
Table 2.
Timing of liver biopsies for the three randomization strata.
| Lead-in Nonresponder | Breakthrough/Relapse | Express | |
|---|---|---|---|
| Number of patients | 346 | 78 | 111 |
| Baseline to randomization (months) | |||
| Median | 6.3 | 18.1 | 1.5 |
| Range | 5.2 to 17.5 | 0.8 to 25.9 | 0.3 to 19.1 |
| Baseline to biopsy 2 (months) | |||
| Median | 25.3 | 36.8 | 21.4 |
| Range | 22.6 to 40.5 | 19.7 to 48.9 | 14.2 to 40.5 |
| Biopsy 2 to biopsy 3 (months) | |||
| Median | 24.2 | 23.7 | 23.9 |
| Range | 15.7 to 37.9 | 13.1 to 32.3 | 14.0 to 29.6 |
Changes in fibrosis stage
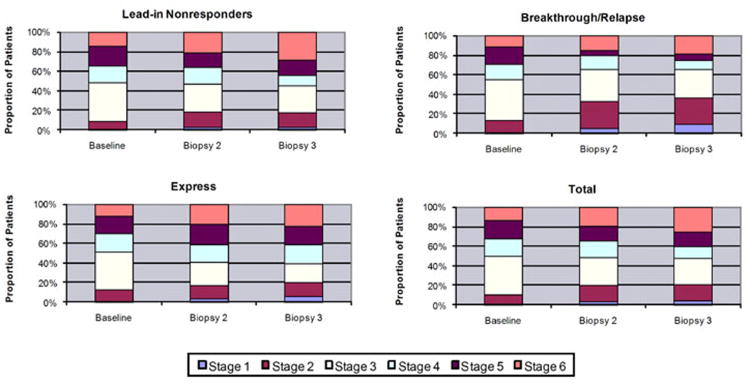
All liver specimens from the 535 morphometry patients were scored for Ishak fibrosis stage by the pathology committee in blinded review sessions. Three patients had no biopsy 2, and two were missing biopsy 3, but all others had fibrosis stages assigned at each of the three time points. Overall, between baseline and the biopsy 2, 30% of patients had an improvement (i.e., decrease) of 1 or more stages in Ishak fibrosis score, 43% remained the same, and 27% worsened, with an increase in stage (Table 3). Between biopsy 2 and biopsy 3, 26% improved, 41% remained the same and 33% worsened. From baseline to biopsy 3, 32% improved, 34% remained the same and 34% had worsening of the Ishak fibrosis score. No significant differences emerged between patients assigned to the long-term peginterferon treatment arm and those assigned to the untreated control arm, but there were differences among the three randomization strata. The distribution of fibrosis scores at the three time points for each stratum and for the entire cohort is shown in Figure 2. For the entire cohort the number of patients with stage 3 fibrosis decreased, and the number with stages 2 and 1 increased, but the total proportion of patients with relatively mild fibrosis (stages 1-3) remained constant (47 to 49%), while patients with stage 6 (established cirrhosis) increased from 14% at baseline to 20% in biopsy 2 to 26% in biopsy 3, with a corresponding decrease in stages 4 and 5 at each time point. Closer inspection of the three randomization strata revealed an increasing proportion of low scores (stages 1-3) as well as stage 6 and a decreasing proportion of stages 4 and 5 in the breakthrough/relapse group, suggesting that some patients may have benefitted from partial virologic suppression that was achieved in this group. The lead-in nonresponders and express patients tended to have worsening fibrosis scores. Differences between breakthrough/relapse patients and the other two strata were significant at biopsy 2 (p=0.01) and biopsy 3 (p=0.0004).
Table 3.
Changes in Ishak Fibrosis Scores over the course of the HALT-C Trial for the 535 patients used in the morphometric analyses. Three were missing biopsy 2 and two were missing biopsy 3. The score was considered improved if it decreased by one or more stage and worsened if it decreased by one or more stage.
| n | Improved | Same | Worsened | |
|---|---|---|---|---|
| Baseline to Biopsy 2 | 532 | 30% | 43% | 27% |
| IFN treated | 261 | 31% | 40% | 29% |
| Control | 271 | 30% | 44% | 26% |
| Month 24 to Biopsy 3 | 531 | 26% | 41% | 33% |
| IFN treated | 261 | 28% | 40% | 32% |
| Control | 270 | 24% | 42% | 34% |
| Baseline to Biopsy 3 | 533 | 32% | 34% | 34% |
| IFN treated | 262 | 33% | 34% | 33% |
| Control | 271 | 31% | 34% | 35% |
Figure 2.
Distribution of Ishak fibrosis stages at each biopsy among 535 patients used for morphometric analyses, showing an increasing proportion of patients with stage 6 cirrhosis (p<0.0001). Panels show the three randomization strata with lead-in nonresponders (n=346) in upper left, breakthrough/relapse (n=78) in upper right, express (n=111) in lower left and the total cohort (n=535) in lower right. There were no significant differences among the three strata at baseline. All three strata showed a significant increase in cirrhosis, but there were significantly fewer breakthrough/relapse patients with advanced stages at biopsy 2 (p=0.01) and biopsy 3 (p=0.004) than in the other two strata.
Morphometry
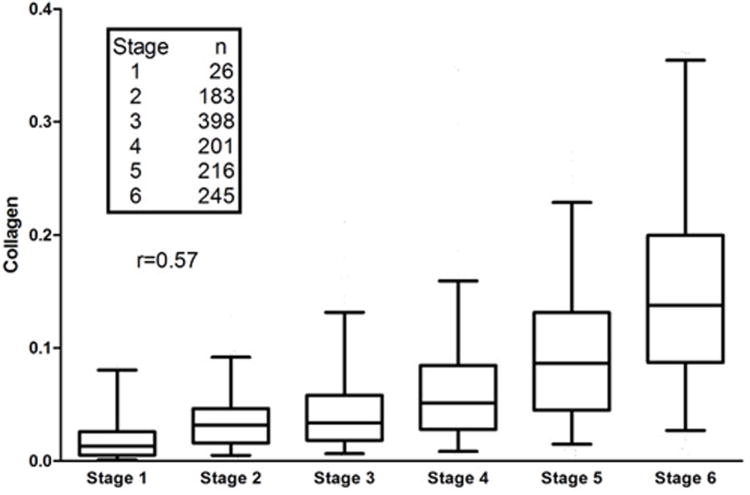
Morphometric determination of collagen content was limited to the 1,269 unfragmented biopsies with at least 10 mm2 of tissue, including 399 baseline, 470 biopsy-2 and 400 biopsy-3 specimens. Overall, a similar relationship and significant correlation occurred (Spearman r=0.57) between fibrosis stage and collagen content as reported in other studies (6,7); however, considerable overlap was observed in the amount of collagen at the various stages (Figure 3), indicating that, while correlated, morphometry, which quantifies the amount of fibrous tissue, and Ishak fibrosis score, which relies primarily on location of fibrosis and architectural distortion, measure different things.
Figure 3.
Distribution of measurements of collagen content at each Ishak fibrosis stage for the 1269 liver biopsies in the study. The boxes show the median, 25th and 75th percentiles, whereas the lines 2.5 and 97.5 percentiles (outliers not shown). The Spearman correlation coefficient is 0.57 (p<0.0001).
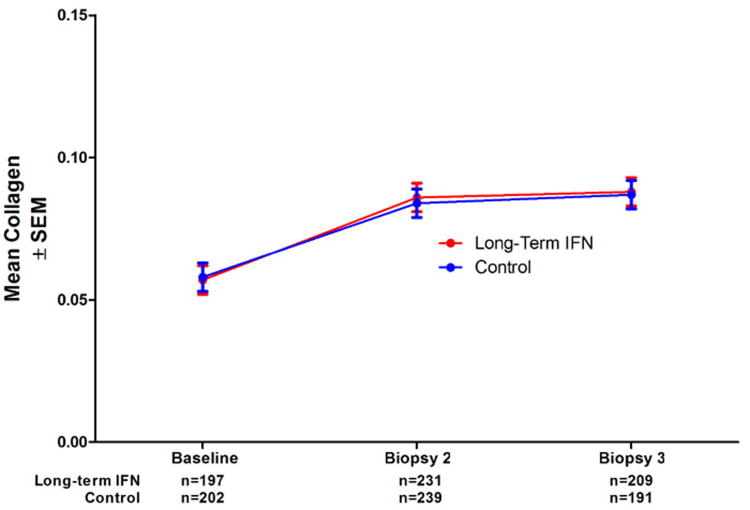
Using repeated measures analysis of variance, we found no differences in mean morphometrically determined collagen content over time between the peginterferon treated patients and the untreated controls (Figure 4). Surprisingly, however, the mean collagen content increased by 49% between baseline and biopsy 2 (95% confidence interval 35% to 63%) but then further increased only slightly to 54% above baseline at biopsy 3 (95% confidence interval 41% to 68%). Because we had anticipated that fibrosis progression would be more linear over time, we undertook additional analyses.
Figure 4.
Mean collagen content (± SEM) for the 535 patients in the study by treatment group, showing no difference between long-term interferon and untreated controls at any of the three time points.
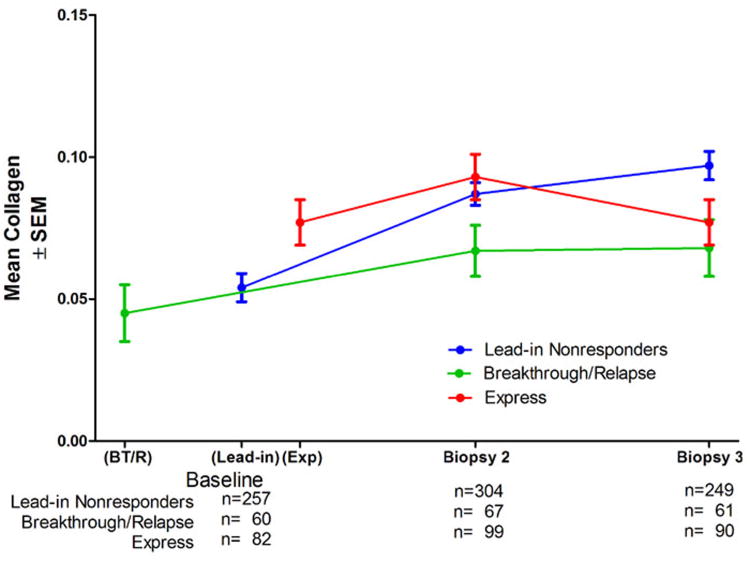
A potential explanation for the unexpected results in overall collagen change with time was found in the striking differences among the three randomization strata (Figure 5). Patients randomized at the end of the lead-in phase behaved closest to expectation, with an increase in mean collagen by 61% over baseline (95% confidence interval 47% to 76%) in biopsy 2 (median of 25.3 months after baseline) and 80% in biopsy 3 (95% confidence interval 61% to 99%; median of 49.5 months after baseline), although the difference between biopsy 2 and biopsy 3 was not statistically significant. In contrast, the breakthrough/relapse patients had slightly less mean collagen at baseline, with an increase of 48% in biopsy 2 (95% confidence interval 8% to 89%; median of 36.8 months after baseline), but no further increase in biopsy 3 (median of 60.5 months after baseline). The express patients were quite different; their baseline biopsies followed an unsuccessful course of peginterferon/ribavirin, rather than preceding the lead-in course of therapy that the other two groups received. Compared to the lead-in nonresponder patients, express patients had a mean of 47% more collagen at baseline (p<0.0001). Collagen in biopsies 2 and 3 were not statistically significantly different from baseline levels, with an increase of only 21% in biopsy 2 (95% confidence interval 1% to 40%; median of 21.4 months after baseline) and then a shift back to the baseline value in biopsy 3 (median of 45.3 months after baseline). The greater mean collagen content at baseline in the express patients was not explained by differences from the other groups in age, serum ALT, level of HCV RNA, alcohol history (12) or by inflammation or fat in the biopsy (Table 4). Within each of the three randomization strata, no significant differences occurred in mean collagen content at the various time points between those randomized to long term peginterferon and the untreated controls (data not shown). The lead-in nonresponder patients had an increase in mean collagen of 20% to 30% per year, the breakthrough/relapse patients had an increase of 10% to 16% per year, and the express patients had an increase of 0 to 13% per year.
Figure 5.
Mean collagen content (± SEM) at different time points for the 535 patients in the study by randomization strata. The median interval between baseline biopsy and biopsy 2 was 36.8 months for the breakthrough/relapse patients, 25.3 months for the end of lead-in patients and 21.4 months for the express patients. Mean collagen in biopsy 2 was significantly greater than in the baseline biopsy for lead-in nonresponders (p<0.0001) breakthrough/relapse patients (p=0.0029), but biopsy 3 was not statistically significantly greater than biopsy 2 for either group. Express patients had significantly more collagen in the baseline biopsy than lead-in and breakthrough/relapse patients (p<0.0001), but the differences between baseline biopsy, biopsy 2 and biopsy 3 for express patients were not statistically significant.
Table 4.
Baseline characteristics of the three randomization strata.
| Lead-in Nonresponder n=346 (mean ± S.D.) | Breakthrough/Relapse n =78 (mean ± S.D.) | Express n = 111 (mean ± S.D.) | p-value | |
|---|---|---|---|---|
| Age* | 50.3 ± 7.6 | 48.7 ± 4.9 | 50.2 ± 6.4 | 0.21 |
| ALT* | 110 ± 71 | 113 ± 94 | 101 ±74 | 0.51 |
| Log HCV RNA* | 6.46 ± 0.47 | 6.58 ± 0.44 | 6.38 ± 0.59 | 0.02 |
| Total lifetime alcoholic drinks (thousands)*(12) | 15.5 ± 23.3 | 22.9 ± 43.0 | 17.7 ± 24.7 | 0.09 |
| Histology at baseline Mean inflammation score (5) (± S.D.) | 7.6 ± 1.9 | 7.3 ± 2.0 | 7.6 ± 2.0 | 0.40 |
| Steatosis (>5% of tissue) | n=145 (42%) | n=22 (28%) | n=43 (57%) | 0.08 |
At randomization
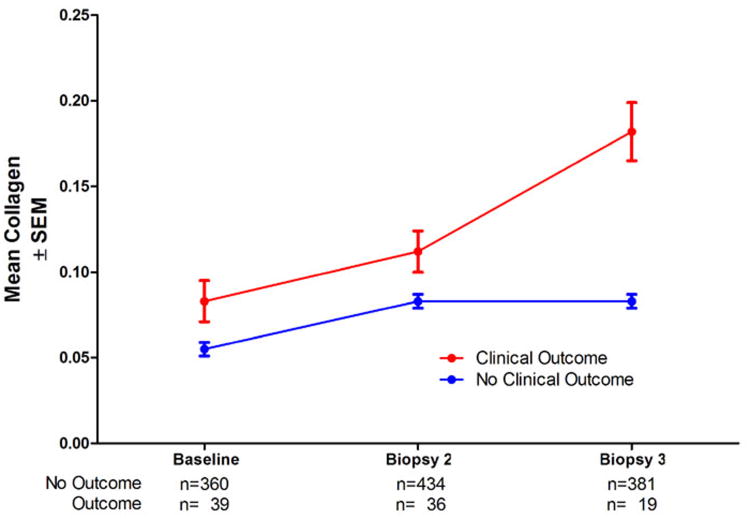
We also examined the possibility that patients with the most substantial fibrosis progression may have suffered clinical outcomes (death, ascites, variceal hemorrhage, spontaneous bacterial peritonitis, Child-Turcotte-Pugh score >7, eligibility for liver transplantation, or hepatocellular carcinoma) (11) and failed to have biopsy 3, resulting in a selection bias that could account for the apparent lack of progression between biopsy 2 and biopsy 3. In fact, however, all but one of the 535 patients in this analysis had a biopsy 3, even though only 400 were unfragmented with at least 10 mm2 of tissue, a requirement for inclusion in this analysis. Next, we asked whether fragmented, small biopsies, which were excluded in this analysis, were more likely to occur in biopsy 3, another potential source of selection bias. Patients who suffered outcomes had a 119% increase in mean collagen between baseline and biopsy 3 (95% confidence interval 79% to 159%) and significantly more mean collagen at each of the three time points (p=0.0137 at baseline; p=0.0030 for biopsy 2; p=0.0031 for biopsy 3, Figure 6) than patients without outcomes, even though only 19 patients with outcomes had biopsy 3 specimens adequate for morphometric analysis. Thus, patients with clinical outcomes had both the most fibrosis at baseline and the largest increase in fibrosis progression over time; however, because patients with outcomes had such elevated levels of fibrosis, they were less likely to have a biopsy suitable for morphometric analysis. Although the number of patients with clinical outcomes and fragmented/small biopsies is small, exclusion of their biopsy-3 specimens from morphometric analysis could have been partly responsible for the apparent deceleration in fibrosis between biopsy 2 and biopsy 3.
Figure 6.
Mean collagen content (± SEM) at different time points showing significantly more fibrosis at each time (baseline p=0.0137, biopsy 2 p=0.0030, biopsy 3 p=0.0031) in the 42 patients who had clinical outcomes than in the 493 patients who did not have an outcome.
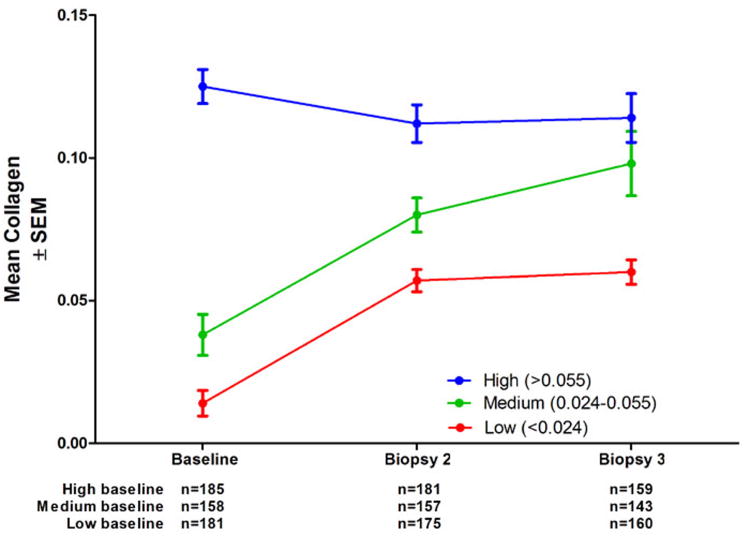
Finally, we examined the effect of baseline collagen on changes in morphometric fibrosis determinations (Figure 7). We chose ranges to approximate the highest (>0.055 units, n=181), middle (0.024-0.055 units, n=158) and lowest (<0.024 units, n=185) third of baseline values. The mean collagen of the cohort with medium baseline collagen behaved closest to expected, increasing by 110% above baseline at biopsy 2 and 158% at biopsy 3. For those with low baseline collagen the mean increased by approximately the same absolute value, but since the initial mean value was very low, the percentage increase was high (307%), but there was no further increase at biopsy 3. Finally, the mean of the cohort with high collagen actually fell by 10% at biopsy 2, but remained the same at biopsy 3. This may be explained in part by loss of patients with the most collagen to clinical outcomes, as shown in Figure 6, although a complete explanation remains elusive.
Figure 7.
Mean collagen content (± SEM) for the 535 patients in the study by baseline collagen levels (approximately one-third of patients in each group). The group with high (>.055 units) baseline collagen tended to have less in subsequent biopsies, while those with medium or low collagen increased.
Discussion
In this study, we found that with morphometric image analysis, the most sensitive histologic method for measuring fibrosis, long-term low-dose maintenance peginterferon therapy had no effect on fibrosis progression in this group of patients with chronic hepatitis C. Our data, however, did suggest that partial virologic suppression by full dose peginterferon/ribavirin during the lead-in phase may retard fibrosis progression. Moreover, our observations also raise the possibility that when full-dose peginterferon/ribavirin therapy is ineffective in producing virologic suppression, fibrosis progression may actually be accelerated.
In two previously completed short-term clinical trials in patients with chronic hepatitis C, collagen was found to increase at a relatively rapid rate that could be detected and quantified by morphometry in large cohorts. In none of these three trials did the study drugs have any effect on changes in fibrosis; therefore, data from placebo-treated controls and patients receiving study drugs were combined for analyses of fibrosis progression. In one study, 245 patients with advanced fibrosis (Ishak stage 4-6) had a 58% increase in mean collagen in 1 year (7). In another, 209 patients with moderate (stage 2-4) fibrosis had a 30% increase in mean collagen of 30% in 1 year (9).
The HALT-C Trial provided the opportunity to study longer term changes in fibrosis; however, because all patients received at least 6 months of ribavirin with full-dose peginterferon, the trial did not include a true placebo-treated control cohort. Nevertheless, this study was anticipated to provide information about the long-term progression of fibrosis, but the overall results (Figure 4) were surprising. The mean fibrosis of the cohort increased by nearly 50% between baseline and biopsy 2, similar to results reported in previous studies, but, thereafter, almost no change occurred over the next 2 years of the study. To some extent, we could explain these observations by invoking the differences in hepatic collagen results among the three randomization strata (“lead-in nonresponder,” “breakthrough/relapse,” and “express”) (Figure 5), and the inclusion of fewer biopsy-3 specimens resulting from the loss of patients with the most fibrosis to clinical outcomes (Figure 6).
The three randomization strata differed from one another in three important ways. First, a considerable difference existed in the time interval between the baseline biopsy and biopsy 2, which averaged 25.3 months for the lead-in nonresponder group, 36.8 months for the breakthrough/relapse group and 21.4 months for the express group. Second, the breakthrough/relapse patients achieved complete virologic suppression for several months, which did not occur in the other two groups. Third, the express patients underwent their biopsies after 6 months of full-dose peginterferon/ribavirin whereas the others had baseline biopsies before receiving therapy.
The cohort of patients randomized at the end of lead-in therapy had fibrosis progression closest to that reported previously, with an increase of 80% in hepatic fibrosis over four years. Although this approximately 20% per year is less than the 30 to 58% per year observed in other studies, the current study is the first to include long-term follow-up biopsies. The fibrosis progression we observed in this group was not linear, however; the increase was greater during the first two years than during the second two years. This observation may have resulted in part from the loss of patients who had outcomes (Figure 6). The breakthrough/relapse patients had documented virologic suppression for at least part of their treatment courses, which may have contributed to the accumulation of considerably less fibrosis, 50% over 5 years or 10% per year, and relatively fewer patients with cirrhosis (Figure 2). Within the breakthrough/relapse cohort, however, no significant difference emerged between patients who received long-term low-dose peginterferon and untreated controls; therefore, the retardation they had in fibrosis progression appears to have resulted from the initial viral suppression rather than from maintenance therapy. Preliminary analyses of clinical outcomes and histologic improvement suggest as well that the impact of lead-in therapy was more substantial than the impact of maintenance therapy (13). Similar to the lead-in nonresponder cohort, the most substantial increase in mean fibrosis for the breakthrough/relapse group occurred between the baseline biopsy and biopsy 2.
Fibrosis changes observed in the express patients are not readily explained but accounted for much of the apparent lack of fibrosis progression between biopsy 2 and biopsy 3 in the entire cohort (Figure 4). Even though express subjects did not differ significantly in baseline Ishak fibrosis stages from that in the lead-in nonresponder stratum, mean fibrosis as determined by morphometry was 47% higher at baseline in the express group. Measured collagen levels in the express group and the lead-in nonresponder group were nearly the same in biopsy 2 but fell inexplicably thereafter in the express group. What distinguished the express group from the lead-in nonresponder group was the fact that the express group had baseline biopsies shortly (a median of 1.5 months) after failing to respond to treatment with peginterferon/ribavirin. This observation raises the possibility that ineffective peginterferon and ribavirin therapy might accelerate hepatic fibrosis transiently and might also explain why the patients randomized at the end of the lead-in phase (lead-in nonresponders) had a greater increase in fibrosis during the first two years (during which they received ineffective full-dose peginterferon and ribavirin therapy for the initial 6 months) than during the second two year interval. Currently, however, a possible role of peginterferon and ribavirin in promoting transient fibrosis progression remains speculative.
A limitation of the current study is the fact that fewer than half of the patients enrolled in the HALT-C Trial had liver biopsies suitable for morphometric analysis, raising the possibility of selection bias. This observation demonstrates the need for a standardized biopsy approach in studying changes in hepatic fibrosis. Noninvasive techniques may become available in the future but will need to be validated against the natural history of the disease and to be correlated with liver biopsy. If biopsies are to be used as a study endpoint or as a standard for validation of noninvasive markers, then the techniques and requirements for adequacy of biopsy specimens need to be standardized. For studies of fibrosis, specimens obtained with cutting biopsy needles are superior to those obtained with suction needles (14); in addition, larger gauge needles (14 to 16 g) and biopsies at least 2 cm long are most likely to provide adequate specimens for assessing histology (15) if this is to be used as a clinical trial endpoint and to correlate with noninvasive markers. Biopsy sampling error will always be present to some extent, so it must be emphasized that morphometry is not appropriate for evaluation of changes in individual patients but should be reserved for statistical analysis and comparison of changes in large cohorts.
The availability of serial liver biopsy specimens during the HALT-C Trial provided an opportunity, for the first time, to analyze fibrosis progression by quantitative image analysis over 3.5 to 5 years in a large number of patients with advanced chronic hepatitis C. Our findings show promise for this approach in future studies and clinical trials, but at present the natural history of fibrosis progression remains an area of great uncertainty. Therefore, in the future, carefully designed prospective long-term studies such as the HALT-C Trial will be needed to define the true natural history of fibrosis in chronic hepatitis C.
Acknowledgments
This study was supported by the National Institute of Diabetes & Digestive & Kidney Diseases (contract numbers are listed below). Additional support was provided by the National Institute of Allergy and Infectious Diseases (NIAID), the National Cancer Institute, the National Center for Minority Health and Health Disparities and by General Clinical Research Center and Clinical and Translational Science Center grants from the National Center for Research Resources, National Institutes of Health (grant numbers are listed below). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Additional funding to conduct this study was supplied by Hoffmann-La Roche, Inc., through a Cooperative Research and Development Agreement (CRADA) with the National Institutes of Health.
In addition to the authors of this manuscript, the following individuals were instrumental in the planning, conduct and/or care of patients enrolled in this study at each of the participating institutions as follows:
University of Massachusetts Medical Center, Worcester, MA: (Contract N01-DK-9-2326) Gyongyi Szabo, MD, Barbara F. Banner, MD, Maureen Cormier, RN, Donna Giansiracusa, RN
University of Connecticut Health Center, Farmington, CT: (Grant M01RR-06192) Gloria Borders, RN, Michelle Kelley, RN, ANP
Saint Louis University School of Medicine, St Louis, MO: (Contract N01-DK-9-2324) Adrian M. Di Bisceglie, MD, Bruce Bacon, MD, Brent Neuschwander-Tetri, MD, Elizabeth M. Brunt, MD, Debra King, RN
Massachusetts General Hospital, Boston, MA: (Contract N01-DK-9-2319, Grant M01RR-01066; Grant 1 UL1 RR025758-01, Harvard Clinical and Translational Science Center) Raymond T. Chung, MD, Andrea E. Reid, MD, Atul K. Bhan, MD, Wallis A. Molchen, Cara C. Gooch
University of Colorado School of Medicine, Denver, CO: (Contract N01-DK-9-2327, Grant M01RR-00051, Grant 1 UL1 RR 025780-01) S. Russell Nash, MD, Jennifer DeSanto, RN, Carol McKinley, RN
University of California - Irvine, Irvine, CA: (Contract N01-DK-9-2320, Grant M01RR-00827) John C. Hoefs, MD, John R. Craig, MD, M. Mazen Jamal, MD, MPH, Muhammad Sheikh, MD, Choon Park, RN
University of Texas Southwestern Medical Center, Dallas, TX: (Contract N01-DK-9-2321, Grant M01RR-00633, Grant 1 UL1 RR024982-01, North and Central Texas Clinical and Translational Science Initiative) William M. Lee, MD, Thomas E. Rogers, MD, Peter F. Malet, MD, Janel Shelton, Nicole Crowder, LVN, Rivka Elbein, RN, BSN, Nancy Liston, MPH
University of Southern California, Los Angeles, CA: (Contract N01-DK-9-2325, Grant M01RR-00043) Sugantha Govindarajan, MD, Carol B. Jones, RN, Susan L. Milstein, RN
University of Michigan Medical Center, Ann Arbor, MI: (Contract N01-DK-9-2323, Grant M01RR-00042, Grant 1 UL1 RR024986, Michigan Center for Clinical and Health Research) Anna S. Lok, MD, Joel K. Greenson, MD, Pamela A. Richtmyer, LPN, CCRC, R. Tess Bonham, BS
Virginia Commonwealth University Health System, Richmond, VA: (Contract N01-DK-9-2322, Grant M01RR-00065) Richard K. Sterling, MD, Melissa J. Contos, MD, A. Scott Mills, MD, Charlotte Hofmann, RN, Paula Smith, RN
Liver Diseases Branch, National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Bethesda, MD: T. Jake Liang, MD, David Kleiner, MD, PhD, Yoon Park, RN, Elenita Rivera, RN, Vanessa Haynes-Williams, RN
National Institute of Diabetes and Digestive and Kidney Diseases, Division of Digestive Diseases and Nutrition, Bethesda, MD: James E. Everhart, MD, Leonard B. Seeff, MD, Patricia R. Robuck, PhD, Jay H. Hoofnagle, MD
University of Washington, Seattle, WA: (Contract N01-DK-9-2318) David R. Gretch, MD, PhD, Minjun Chung Apodaca, BS, ASCP, Rohit Shankar, BC, ASCP, Natalia Antonov, M. Ed.
New England Research Institutes, Watertown, MA: (Contract N01-DK-9-2328) Kristin K. Snow, MSc, ScD, Linda Massey, Margaret C. Bell, MS, MPH
Armed Forces Institute of Pathology, Washington, DC: Fanny Monge, Michelle Parks
Data and Safety Monitoring Board Members: (Chair) Gary L. Davis, MD, Guadalupe Garcia-Tsao, MD, Michael Kutner, PhD, Stanley M. Lemon, MD, Robert P. Perrillo, MD
Footnotes
This is publication #42 from the HALT-C Trial Group.
The HALT-C Trial was registered with clinicaltrials.gov (#NCT00006164).
Publisher's Disclaimer: Disclaimer: The opinions and assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Department of the Army or the Department of Defense.
Financial Disclosures: Financial relationships of the authors with Hoffmann-La Roche, Inc., are as follows: H.L. Bonkovsky receives research support; R.J. Fontana is on the speaker's bureau; T.R. Morgan is consultant, on the speaker's bureau and receives research support; M. L. Shiffman is a consultant, on the speaker's bureau, and receives research support; G.T. Everson is a consultant, on the speaker's bureau, and receives research support; K. L. Lindsay is a consultant and receives research support; and E. M. Brunt receives research support. Authors with no financial relationships related to this project are: Z.D. Goodman, A.M. Stoddard, M.G. Ghany, E.C. Wright, D.E. Kleiner, J. L. Dienstag, and C. Morishima.
References
- 1.Imazeki F, Yokosuka O, Fukai K, Saisho H. Favorable prognosis of chronic hepatitis C after interferon therapy by long-term cohort study. Hepatology. 2003;38:493–502. doi: 10.1053/jhep.2003.50329. [DOI] [PubMed] [Google Scholar]
- 2.Bruno S, Stroffolini T, Colombo M, Bollani S, Benvegnù L, Mazzella G, Ascione A, Santantonio T, Piccinino F, Andreone P, Mangia A, Gaeta GB, Persico M, Fagiuoli S, Almasio PL. Sustained virological response to interferon-alpha is associated with improved outcome in HCV-related cirrhosis: a retrospective study. Hepatology. 2007;45:579–587. doi: 10.1002/hep.21492. [DOI] [PubMed] [Google Scholar]
- 3.Di Marco V, Almasio PL, Ferraro D, Calvaruso V, Alaimo G, Peralta S, Di Stefano R, Craxì A. Peg-interferon alone or combined with ribavirin in HCV cirrhosis with portal hypertension: a randomized controlled trial. J Hepatol. 2007;47:484–491. doi: 10.1016/j.jhep.2007.04.020. [DOI] [PubMed] [Google Scholar]
- 4.The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;20:15–20. [PubMed] [Google Scholar]
- 5.Ishak K, Baptista A, Bianchi L, Callea F, De Groote J, Gudat F, Denk H, Desmet V, Korb G, MacSween RN, Phillips MJ, Portmann BG, Poulsen H, Scheuer PJ, Schmidt M, Thaler H. Histological grading and staging of chronic hepatitis. J Hepatol. 1995;22:696–699. doi: 10.1016/0168-8278(95)80226-6. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien MJ, Keating NM, Elderiny S, Cerda S, Keaveny AP, Afdhal NH, Nunes DP. An assessment of digital image analysis to measure fibrosis in liver biopsy specimens of patients with chronic hepatitis C. Am J Clin Pathol. 2000;114:712–718. doi: 10.1309/D7AU-EYW7-4B6C-K08Y. [DOI] [PubMed] [Google Scholar]
- 7.Goodman ZD, Becker RL, Pockros PJ, Afdhal NH. Progression of fibrosis in chronic hepatitis C: Evaluation by morphometric image analysis. Hepatology. 2007;45:886–894. doi: 10.1002/hep.21595. [DOI] [PubMed] [Google Scholar]
- 8.Bedossa P, Dargere D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449–1457. doi: 10.1016/j.hep.2003.09.022. [DOI] [PubMed] [Google Scholar]
- 9.McHutchison J, Goodman Z, et al. Double-blind, randomized, placebo-controlled, multi-center Phase II dose-ranging study to assess the antifibrotic activity of farglitazar in chronic hepatitis C infection. Hepatology. 2008;48(Supl):1139A–1140A. [Google Scholar]
- 10.Di Bisceglie AM, Schiffman ML, Everson GT, Lindsay KL, Everhardt JE, Wright EC, Lee WM, Lok AS, Bonkovsky HL, Morgan TR, Ghany MG, Morishima C, Snow KK, Dienstag JL. Prolonged therapy of advanced chronic hepatitis C with low dose peginterferon. New Engl J Med. 2008;359:2429–2441. doi: 10.1056/NEJMoa0707615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WM, Dienstag JL, Lindsay KL, Lok AS, Bonkovsky HL, Shiffman ML, Everson GT, Di Bisceglie AM, Morgan TR, Ghany MG, Morishima C, Wright EC, Everhart JE. Evolution of the HALT-C Trial: pegylated interferon as maintenance therapy for chronic hepatitis C in previous interferon nonresponders. Control Clin Trials. 2004;25:472–492. doi: 10.1016/j.cct.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 12.Skinner HA. Development and validation of a lifetime alcohol consumption assessment procedure. Toronto, Ontario, Canada: Addiciton Research Foundation; 1982. [Google Scholar]
- 13.Shiffman ML, Morishima C, Lindsay KL, Hoefs JC, Dienstag JL, Szabo G, Lee WM, Wright EC HALT-C Trial Group. Suppression of serum HCV RNA levels during maintenance peginterferon (PEGIFN) alfa-2a therapy and clinical outcomes in the HALT-C Trial. J Hepatol. 2008;48(Suppl 2):S62. [Google Scholar]
- 14.Sherman KE, Goodman ZD, Sullivan ST, Faris-Young S. Liver biopsy in cirrhotic patients. Am J Gastroenterol. 2007;102:789–793. doi: 10.1111/j.1572-0241.2007.01110.x. [DOI] [PubMed] [Google Scholar]
- 15.Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239–244. doi: 10.1016/s0168-8278(03)00191-0. [DOI] [PubMed] [Google Scholar]