Background: Androgen deprivation therapy (ADT) suppresses prostate cancer (PCa) growth, yet its effects on PCa metastasis remain unclear.
Results: ADT with MDV3100/enzalutamide or Casodex/bicalutamide versus ASC-J9® led to enhanced versus suppressed PCa metastasis.
Conclusion: Casodex/MDV3100 induces PCa metastasis via modulation of TGF-β1/Smad3/MMP9 signaling.
Significance: Targeting androgen receptor with ASC-J9® is better than targeting androgens with Casodex/MDV3100 to better battle PCa metastasis.
Keywords: Androgen, Matrix Metalloproteinase (MMP), Prostate Cancer, Transforming Growth Factor β (TGFbeta), Tumor Metastases, Anti-androgen
Abstract
Despite the fact that androgen deprivation therapy (ADT) can effectively reduce prostate cancer (PCa) size, its effect on PCa metastasis remains unclear. We examined the existing data on PCa patients treated with ADT plus anti-androgens to analyze ADT effects on primary tumor size, prostate-specific antigen (PSA) values, and metastatic incidence. We found that the current ADT with anti-androgens might lead to primary tumor reduction, with PSA decreased yet metastases increased in some PCa patients. Using in vitro and in vivo metastasis models with four human PCa cell lines, we evaluated the effects of the currently used anti-androgens, Casodex/bicalutamide and MDV3100/enzalutamide, and the newly developed anti-AR compounds, ASC-J9® and cryptotanshinone, on PCa cell growth and invasion. In vitro results showed that 10 μm Casodex or MDV3100 treatments suppressed PCa cell growth and reduced PSA level yet significantly enhanced PCa cell invasion. In vivo mice studies using an orthotopic xenograft mouse model also confirmed these results. In contrast, ASC-J9® led to suppressed PCa cell growth and cell invasion in in vitro and in vivo models. Mechanism dissection indicated these Casodex/MDV3100 treatments enhanced the TGF-β1/Smad3/MMP9 pathway, but ASC-J9® and cryptotanshinone showed promising anti-invasion effects via down-regulation of MMP9 expression. These findings suggest the potential risks of using anti-androgens and provide a potential new therapy using ASC-J9® to battle PCa metastasis at the castration-resistant stage.
Introduction
The recurrence of prostate cancer (PCa)2 with metastases after androgen deprivation therapy (ADT) is still a major concern (1). The newly developed anti-androgens were able to delay the recurrence (2, 3). Importantly, this unwanted effect was more significant when ADT was applied to patients in an earlier stage of PCa, which was correlated with a 10-year decreased survival rate (4). These unexpected findings were in agreement with early reports showing that androgen receptor (AR) might play differential roles (proliferator or suppressor) in different types of PCa cells, and the loss of AR in cytokeratin 5-positive PCa epithelial basal intermediate cells might lead to enhanced PCa metastasis (5, 6).
In this study, currently used anti-androgen Casodex (bicalutamide) (7) and the newly developed anti-androgen of MDV3100 (enzalutamide) (3) were used to evaluate their effects on PCa metastasis in various in vitro cell line experiments and in vivo mouse studies. The results showed that these anti-androgens could enhance PCa cell invasion through modulation of the TGF-β1/Smad3/MMP9 pathway. In contrast, we found that the newly developed AR degradation enhancers, ASC-J9® (8–11) and cryptotanshinone (CTS) (12), could simultaneously suppress PCa cell growth and invasion, which might help us to develop a new therapy to better battle the metastatic PCa at the castration-resistant stage.
MATERIALS AND METHODS
Human Patient Data Analysis
Patient information was collected from the Taipei Medical University (Taipei, Taiwan), the Tianjin Medical University (Tianjin, China), the First Affiliated Hospital of Medical School, Xi'an Jiaotong University (Xi'an, China), and the University Hospital in University of Occupational and Environmental Health (Kitakyushu, Japan). The samples of PCa patients before ADT were collected by transrectal ultrasonography of the prostate (TRUS)-guided prostate biopsy. After ADT, part of the specimens were collected by palliative transurethral resection of the prostate (TURP) to relieve the retention of urine. Part of samples were collected by confirming the organ metastasis with the agreement of patients. Patient inclusion criteria were as follows. All of the patients presented locally advanced or metastatic PCa and had undergone ADT therapy. The patients received the ADT combination of luteinizing hormone-releasing hormone agonist (LHRHa) with Casodex (CASO) or flutamide. The metastatic lesions were monitored before and after ADT. Bone scans and MRIs were used to examine metastatic lesions. The disease progression status was determined by the PSA level, primary tumor sizes, and metastatic foci.
Cell Culture
LNCaP, C81, C4-2, C4-2B, and CWR22Rv1 cell lines were maintained in RPMI 1640 medium containing 10% fetal bovine serum (FBS), antibiotics (100 units/ml penicillin, 100 μg/ml streptomycin), and 2 mm glutamine (Invitrogen) in 5% CO2 in a 37 °C incubator.
Cell Growth Assay
The cells were seeded in 24-well tissue culture plates in RPMI media containing 10% charcoal dextran-treated FBS (CD-FBS) for 24 h. The cells were then treated with vehicle, 10 μm Casodex, 10 μm MDV3100, 10 μm ASC-J9®, or 5 μm CTS with/without the addition of 5 μm LY294002. The cell growth was determined by a 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay (Sigma). The media containing MTT (0.5 μg/ml) were added into each well at the indicated time points. After a 2-h incubation at 37 °C, all crystals were solubilized by DMSO, and the optical density of the solution was determined spectrophotometrically at 570 nm.
Cell Invasion Assay
PCa cells were treated with anti-androgen/AR drugs and incubated for 3 days. For inhibitor studies, the appropriate inhibitors were added into the culture. Cells (1 × 105) were then placed into the upper chamber of transwell plates (8 μm) with membranes precoated with 20% Matrigel. Each sample was assayed in triplicate. The bottom chamber contained 600 μl of media supplemented with 10% FBS. The cells that invaded into the bottom were fixed and were stained using 1% toluidine blue, and the numbers were averaged after counting six randomly selected fields. Each experiment was repeated at least twice.
Orthotopic Xenograft Model
Male 6–8-week-old nude mice were purchased from NCI. The CWR22Rv1 cells incorporated with the luciferase reporter gene were obtained by transfection and stable clone selection procedures. Cells (1 × 106) mixed with Matrigel (1:1, v/v) were orthotopically injected into both anterior prostates of nude mice at 8 weeks of age. When the tumors were palpable 2 weeks after implantation, the mice were randomly assigned into five experimental groups and intraperitoneally injected with drugs as follows three times per week for 4 weeks: Group 1 (n = 20), vehicle; Group 2 (n = 13), 30 mg/kg Casodex; Group 3 (n = 12), 30 mg/kg MDV3100; Group 4 (n = 12), 75 mg/kg ASC-J9®; Group 5 (n = 12), 25 mg/kg CTS. The mouse body weights were monitored weekly during the 4 weeks of treatment. After sacrifice, the primary and metastatic tumors were evaluated by the in vivo imaging system (IVIS), and tumor tissues were removed for IHC staining.
Statistics
Data are presented as the means ± S.D. for the indicated number of separate experiments. The statistical significance of differences between two groups of data was analyzed by paired t test or Fisher's exact test, and p values of <0.05 were considered significant. In the in vivo animal experiments, measurements of tumor volume and body weights among the three groups were analyzed through one-way analysis of variance coupled with the Newman-Keuls test.
Additional details of reagents, three-dimensional invasion assay, Western blot, quantification PCR, immunohistochemistry, x-ray and CT scan, and the detection of luciferase signal by the IVIS system are described in the supplemental Materials and Methods.
RESULTS
ADT Reduced PCa Patient Primary Tumor Sizes with Decreased PSA Levels yet Increased Metastasis in Some Patients
We examined the available PCa patient information from four different hospitals in China, Taiwan, and Japan and found interesting results. We observed 11 patient cases showing reduced primary tumor size with low PSA reading yet increased metastases following the ADT treatment. For example, in the case of Patient 6 in Table 1, when treated with the ADT and anti-androgen LHRHa plus Casodex for 10 months, there was significantly reduced primary tumor size and PSA reading (from 51 to less than 0.5 ng/ml), but metastasis unexpectedly increased despite the very low PSA reading. These clinical data from some PCa patients described in Table 1 raised an interesting question and the possibility that although the current ADT with anti-androgen could reduce some PCa patients' primary tumor growth and decrease their PSA reading, it may either have little effect to prevent PCa metastases or, even worse, may promote PCa metastases in some PCa patients.
TABLE 1.
ADT reduced PCa patient primary tumors with decreased PSA yet increased metastasis
Patient data are from over 1,300 PCa patients who received ADT in four different hospitals in China, Taiwan, and Japan, as described under “Materials and Methods.” The metastatic foci are shown as the size of the largest metatumor × the number of metafoci.
| Patient | Age | ADT drug applied | PSA |
Primary tumor size |
Metastasis |
|||
|---|---|---|---|---|---|---|---|---|
| Before | After | Before | After | Before | After | |||
| years | ng/ml | mm3 | largest Φ × foci no. | |||||
| 1 | 57 | LHRHa + CASO | 2.06 | 0.01 | 30.24 | 6.75 | 2.82 × 4 | 14.0 × 7 |
| 2 | 67 | LHRHa + CASO | 2279.00 | 1.80 | 24.20 | 7.70 | 4.5 × 8 | 7.3 × 4 |
| 3 | 70 | LHRHa + CASO | 227.80 | 0.20 | 74.29 | 56.00 | 4.0 × 10 | 8.5 × 18 |
| 4 | 88 | LHRHa + CASO | 566.80 | 6.30 | 19.66 | 10.00 | 2.5 × 2 | 10.7 × 12 |
| 5 | 72 | LHRHa + CASO | 21.61 | 0.61 | 85.36 | 69.14 | 1.5 × 1 | 3.3 × 8 |
| 6 | 66 | LHRHa + CASO | 51.00 | 0.50 | 101.91 | 48.96 | 0 | 3.3 × 5 |
| 7 | 72 | LHRHa + CASO | 81.30 | 0.05 | 4.10 | 6.00 | 3.8 × 3 | 5.0 × 5 |
| 8 | 70 | LHRHa + CASO | 31.28 | 0.21 | 5.50 | 6.80 | 2.8 × 5 | 4.5 × 7 |
| 9a | 74 | LHRHa + CASO | 4.33 | 0.026 | 14.00 | 10.00 | 0 | 2 × 1 (bone); 1.3 × 7 (lung) |
| 10 | 72 | CASO | 45.00 | 14.00 | 40.60 | 30.30 | 0 | 2.0 × 1 |
| 11b | 69 | LHRHa + CASO | 0.008 | 0.008 | 0 | 2.5 × 1 (bone) | ||
| 12 | 75 | LHRHa + flutamide | 13.70 | 0.36 | 41.00 | 78.00 | 0 | 5 mm > multiple (>10): liver, lung |
a Casodex was withdrawn 9 months after complete androgen blockade initiation, because of an adverse event (liver dysfunction). Thereafter, LHRHa alone was administered. Biopsy specimens of the local recurrence lesion revealed neuroendocrine cancer (small cell carcinoma), and Gleason grade was not determined.
b Local recurrence after prostatectomy. Thus, primary tumor sizes before and after ADT were not determined, and the PSA level was low before ADT.
Anti-androgens Casodex and MDV3100 Suppressed PCa Growth yet Enhanced PCa Cell Invasion
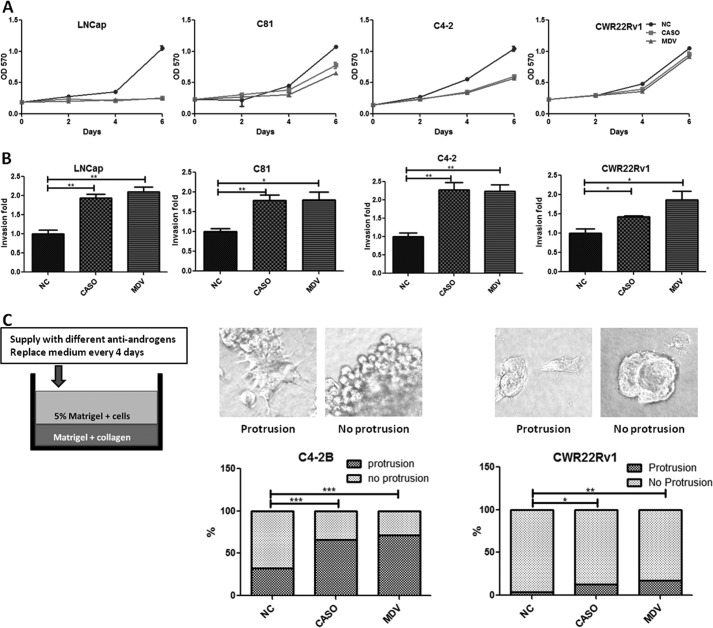
The above unexpected clinical findings in some PCa patients prompted us to apply in vitro growth and invasion assays using four different human PCa cell lines, LNCaP (representing the stage before castration resistance), and C81, C4-2, and CWR22Rv1 (representing the stage after castration resistance) to study the potential distinct ADT effects on PCa growth versus metastasis. We applied Casodex, the current most widely used anti-androgen (13), and MDV3100, a newly developed powerful anti-androgen that can delay the recurrence of increased PSA and extend PCa patient survival rate to 4.8 months (3), to test its effect on PCa cell growth, and the MTT test results showed that 10 μm Casodex and MDV3100 significantly suppressed growth of three PCa cell lines but not the CWR22Rv1 cells (Fig. 1A). However, surprisingly, we found that 10 μm Casodex or 10 μm MDV3100 promoted cell invasion of all four PCa cell lines tested (Fig. 1B).
FIGURE 1.
ADT with anti-androgens suppressed PCa cell growth but enhanced cell invasion. A, the effect of anti-androgens on PCa cell growth. The PCa cells (LNCaP, C81, C4-2, and CWR22Rv1) were treated with the anti-androgen Casodex (CASO) (10 μm), MDV3100 (10 μm) (MDV), or vehicle control and incubated in 10% CD-FBS RPMI media at 10 nm DHT, and the cell growth was analyzed by an MTT assay at the indicated time points. B, the effect of anti-androgens on PCa cell invasion. The cell lines were treated with 10 μm CASO, 10 μm MDV, or vehicle control and incubated in 10% CD-FBS RPMI medium at 10 nm DHT for 3 days. Invasion assays were then performed using Matrigel-precoated transwell pates for 48 h. The invaded cell numbers are shown as -fold change after counting six randomly selected fields. C, the three-dimensional invasion assay in C4-2B (middle) and CWR22Rv1 (right) cells. As illustrated to the left, the Matrigel/collagen mixture coated the bottom of the 48-well plates. After incubation at 37 °C for 1 h, 1 × 104 PCa cells were plated onto the plate in media containing 1% Matrigel. The media were replaced 24 h later, and the anti-androgens or vehicle control was added. After 2 weeks, the spheres with/without protrusion (top) were counted and shown as a percentage compared with the control (bottom) (magnification ×400). All of the experiments were repeated at least twice, and each experiment was performed in triplicate. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Error bars, S.D.
To confirm these anti-androgen effects on enhanced PCa cell invasion, we performed another invasion assay with the three-dimensional Matrigel/collagen-based invasion assay (14) that measures the formation of spheres with protrusions as positive indications of cell invasion. As expected, the addition of 10 μm Casodex or MDV3100 led to enhanced cell invasion of the C4-2B (Fig. 1C, left) and CWR22Rv1 cells (Fig. 1C, right). Together, results from Fig. 1 demonstrated that both Casodex and MDV3100 suppressed PCa growth yet promoted PCa cell invasion.
Combinational Therapy of Anti-androgens with Anti-Akt Led to Better Suppression of PCa Cell Growth yet Failed to Rescue PCa Invasion
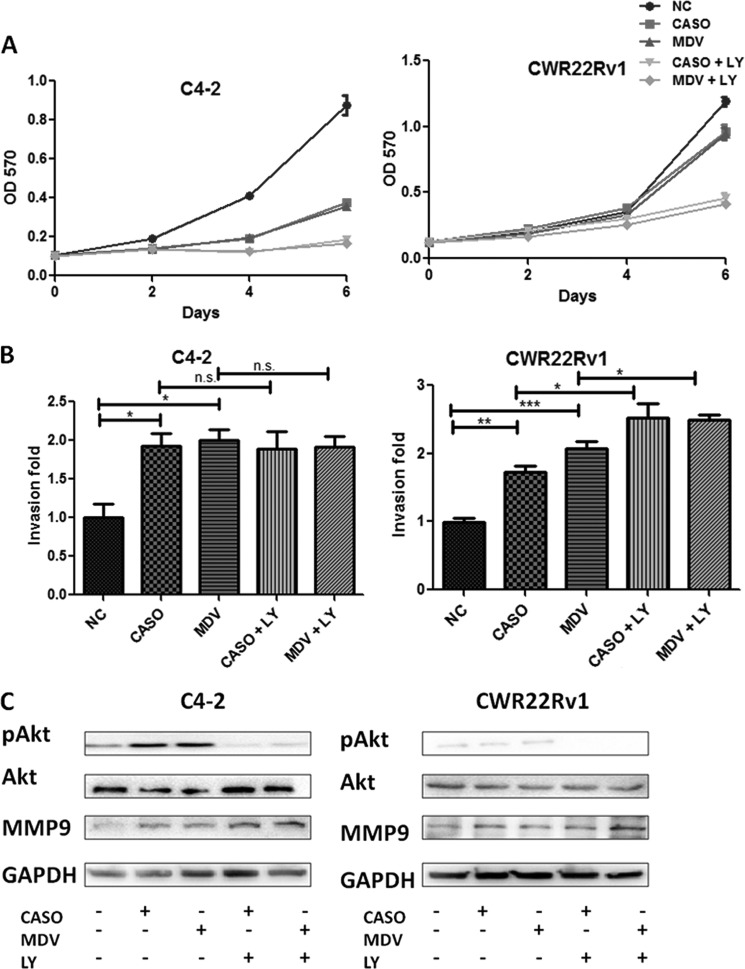
Early studies demonstrated that suppression of androgen/AR signaling might enhance the Akt phosphorylation by reducing the Akt phosphatase, PHLPP (15, 16). These findings suggested that the combinational therapy of anti-androgens with anti-Akt might result in better efficacy to suppress PCa growth. We first examined the combinational therapy effect of using PI3K-Akt inhibitor LY294002 (5 μm) with 10 μm Casodex or MDV3100 on C4-2 and CWR22Rv1 cell growth. As expected, we found that the combinational treatment further suppressed cell growth (Fig. 2A). Unfortunately, the combinational therapy still failed to prevent or reverse the cell invasion induced by anti-androgen treatment (Fig. 2B). Western blot analysis also showed that the expression of MMP-9, the cell invasion marker (17), was increased after treatment with either anti-androgens alone or combined with PI3K-Akt inhibitor LY294002 in both CWR22Rv1 and C4-2 cells (Fig. 2C), even when a very low endogenous level of phosphorylated Akt was detected in CWR22Rv1 and not C4-2 cells, which could be due to the PTEN deletion in C4-2 cells and not in CWR22Rv1 cells. Together, the results from Fig. 2 demonstrated that the combinational therapy of anti-androgens and anti-Akt might further suppress PCa growth yet still fail to prevent or reverse the cell invasion induced by anti-androgens.
FIGURE 2.
Inhibition of Akt activity failed to reverse the anti-androgen-induced PCa cell invasion and MMP9 expression. A, anti-androgen plus anti-Akt further inhibited PCa cell growth. C4-2 and CWR22Rv1 cells were treated with 10 μm CASO, 10 μm MDV3100, or vehicle control with or without 5 μm LY294002 (LY; PI3K/Akt inhibitor), incubated in 10% CD-FBS RPMI plus 10 nm DHT, and cell growth was analyzed by MTT at the indicated time points. B, anti-Akt treatments failed to reverse anti-androgen-promoted PCa invasion. C4-2 and CWR22Rv1 cells were treated with 10 μm CASO, 10 μm MDV, vehicle control with or without 5 μm LY294002 for 3 days for invasion assays. After 48 h, invaded cell numbers are shown as -fold change from six randomly selected fields (magnification, ×400). C, anti-Akt failed to reverse the anti-androgen-promoted MMP9 expression. After 3 days of inhibitor treatments, cell extracts were obtained for Western blot analysis of Akt, phospho-Akt (pAkt), MMP9, and GAPDH. All of the experiments have been repeated twice independently. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Error bars, S.D.
Anti-androgen-induced PCa Invasion Involved the Activation of TGF-β1/Smad3 Signaling and Enhanced MMP9 Expression
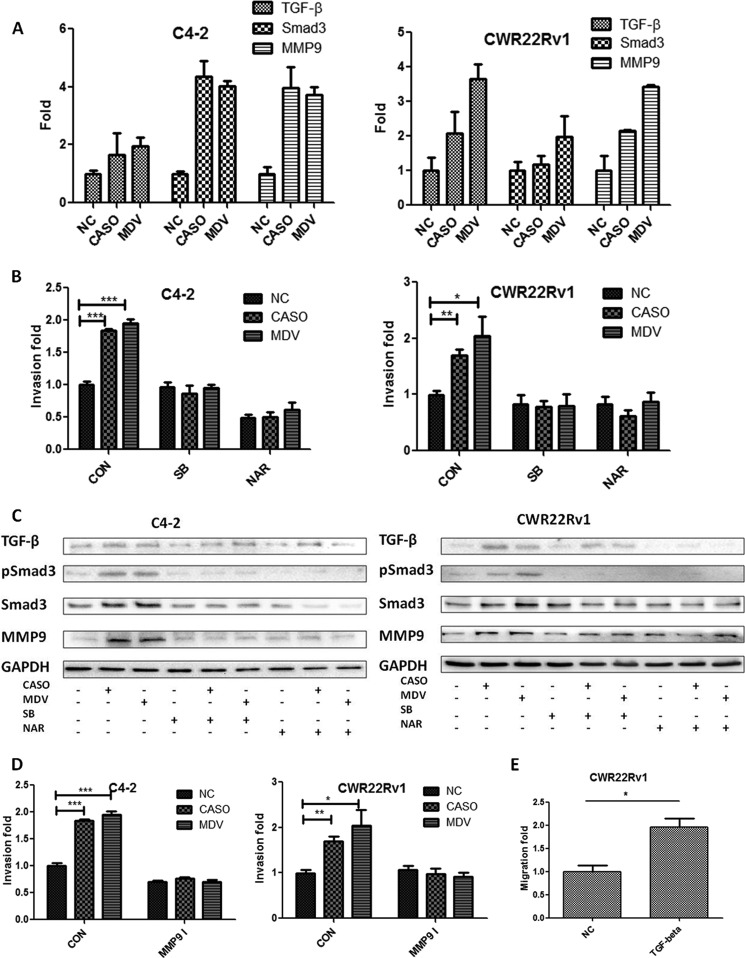
Early reports suggested that TGF-β1 signaling might have dual roles, as a suppressor of tumor growth and an enhancer for tumor metastasis at later stages (18). A recent study showed that activated AR may bind to the TGF-β1 promoter region to suppress the gene expression (19), and androgen deprivation may lead to increased expression of TGF-β1 ligand and expression of its downstream genes such as the TGF-β1 receptors and Smad3 (19–23). We investigated whether anti-androgens induce PCa cell invasion through TGF-β1 signaling and found that the addition of 10 μm Casodex or MDV3100 increased the mRNA expression of TGF-β1, Smad3, and MMP9 in C4-2 and CWR22Rv1 cells (Fig. 3A). We then examined whether the anti-androgen-induced PCa cell invasion could be altered after adding TGF-β1 receptor kinase-specific inhibitor, SB431542 (24), or the Smad3 inhibitor, naringenin (25). As expected, we found that treatment with 5 μm SB431542 or 50 μm naringenin for 3 days led to significant suppression of the C4-2 and CWR22Rv1 cell invasion induced by either Casodex or MDV3100 (Fig. 3B). In addition, expressions of p-Smad3 and MMP9, the invasion markers, were also markedly reduced by SB431542 or naringenin treatment (Fig. 3C). These results were similar to the previous study in breast cancer showing that the activated Smad3 could directly bind to the Smad response element on the MMP9 promoter to increase the MMP9 expression (17). Then MMP9-specific inhibitor (26) was used to further prove that anti-androgen-induced PCa cell invasion is through the TGF-β1/Smad3/MMP-9 pathway. The results showed that inhibition of MMP9 activity by MMP9 inhibitor interrupted C4-2 and CWR22Rv1 cell invasion (Fig. 3D). Finally, direct treatment of CWR22Rv1 cells with 5 ng/ml recombinant TGF-β1 protein also increased the cell invasion (Fig. 3E).
FIGURE 3.
Activation of TGF-β1/Smad3 signaling contributed to the anti-androgen-induced PCa cell invasion and MMP9 expression. A, anti-androgens promote TGF-β1/Smad3/MMP9 expression in PCa cells. C4-2 and CWR22Rv1 cells treated with 10 μm CASO, 10 μm MDV, or vehicle control (NC) were incubated in 10% CD-FBS RPMI at 10 nm DHT for 24 h, and then gene expression was determined by quantitative PCR. B, blocking TGF-β1/Smad3 signals reversed the CASO- or MDV-promoted PCa invasion. C4-2 and CWR22Rv1 cells were treated with 10 μm CASO, 10 μm MDV, or vehicle control and co-treated with 5 μm SB431542 (SB) or 50 μm naringenin (NAR) and incubated in 10% CD-FBS RPMI media at 10 nm DHT, and then invasion assays were performed. After 48 h, the invaded cell numbers are shown as -fold change after counting six randomly selected fields. C, blocking TGF-β1/Smad3 signals reversed the anti-androgen-increased MMP9 expression. The effect of CASO/MDV and SB431542/naringenin on the expression of TGF-β1, p-Smad3, Smad3, MMP9, and GAPDH was analyzed by Western blotting. The TGF-β1 receptor kinase inhibitor, 10 μm SB431542, and the 50 μm Smad3-specific inhibitor, naringenin, were used to block TGF-β1 receptor kinase and Smad3 signals, respectively, that were induced by anti-androgen treatment. D, blocking MMP9 activity reversed the CASO or MDV3100-promoted PCa invasion. C4-2 and CWR22Rv1 cells were treated for 3 days with the combination of MMP9 inhibitors and anti-androgens, and then invasion assays were performed. E, direct treatment of TGF-β1 recombinant protein increased PCa invasion. CWR22Rv1 cells were treated for 3 days with 5 ng/ml human recombinant TGF-β1 protein, and then an invasion assay was performed. All of the experiments were repeated twice independently. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Error bars, S.D.
Together, the results from Fig. 3 suggested that anti-androgen-induced PCa cell invasion might go through the AR → TGF-β1/Smad3/MMP-9 pathway, and interruptions of this pathway via either SB431542, naringenin, or MMP9 inhibitor all suppressed these anti-androgen-induced PCa cell invasion.
Anti-AR ASC-J9® and CTS Suppressed both PCa Growth and Invasion in the PCa Cells
All of the above results in Figs. 1–3 indicated that the currently used anti-androgens, such as Casodex or MDV3100, might suppress PCa cell growth yet promote their invasion. We investigated the possibility that any newly developed anti-androgen/AR compounds have both anti-PCa cell growth and invasion capacity so that they can simultaneously suppress PCa growth and metastasis. We first focused on two anti-AR compounds, the AR degradation enhancer ASC-J9® (8, 11, 27) and CTS (12), the tanshinone extracted from the Chinese herb danshen. Unlike the currently used anti-androgens that reduce or prevent androgen binding to AR, the newly developed anti-AR compound, ASC-J9®, has the unique capability to degrade AR protein in selective cells. On the other hand, CTS also could suppress the AR activity by inhibition of N-terminal and C-terminal interaction or change the histone methylation pattern to reduce the AR transcriptional activity (12, 28).
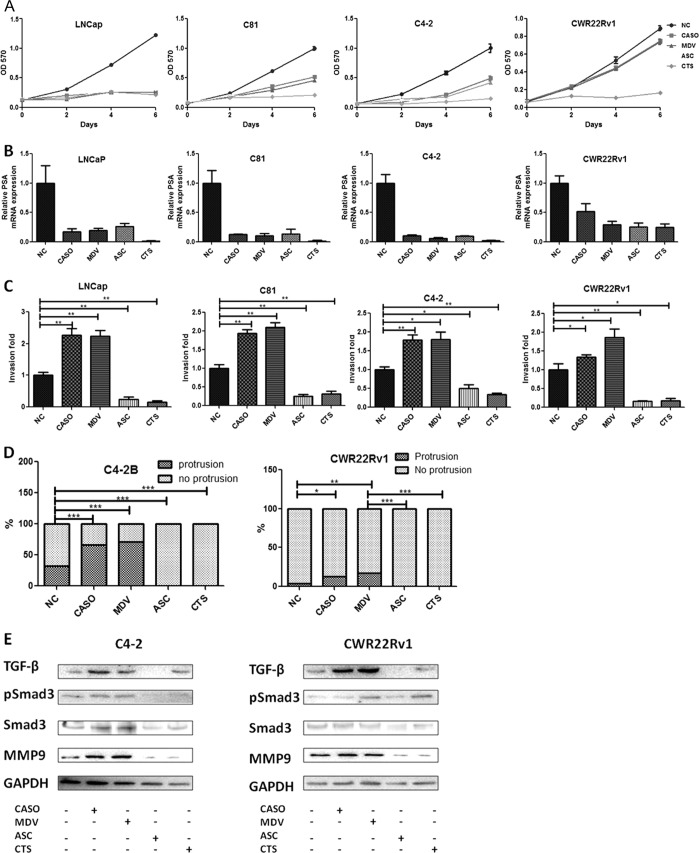
We found that 10 μm ASC-J9® and 5 μm CTS could suppress the PCa growth (Fig. 4A) and AR downstream target gene PSA expression (Fig. 4B) in all four PCa cell lines tested. Importantly, Casodex and MDV3100 induced PCa cell invasion, and both ASC-J9® and CTS significantly suppressed the PCa cell invasion in a chamber invasion assay (Fig. 4C) or in a three-dimensional Matrigel/collagen-based invasion assay (Fig. 4D). In addition, whereas Casodex and MDV3100 enhanced MMP9 expression (Fig. 4E), both ASC-J9® and CTS showed suppression of MMP9 expression in C4-2 and CWR22Rv1 cells (Fig. 4E). Furthermore, treatments with Casodex and MDV3100 led to increased expression of the Smad3 and p-Smad3 levels in C4-2 and CWR22Rv1 cells, whereas ASC-J9® and CTS treatment did not (Fig. 4E). Together, the results from Fig. 4 suggest that Casodex, MDV3100, ASC-J9®, and CTS can promote or suppress PCa cell invasion via differential regulation of the AR/TGF-β1/Smad3/MMP-9 pathway.
FIGURE 4.
Alternative anti-androgens suppressed growth and invasion of PCa cells through distinct mechanisms. A and B, the anti-androgen/AR compounds inhibited PCa cell growth (A) and PSA expression (B). LNCaP, C81, C4-2, and CWR22Rv1 cells were treated with 10 μm CASO, 10 μm MDV, 10 μm ASC-J9® (ASC), 5 μm CTS, or vehicle (NC), incubated in 10% CD-FBS RPMI at 10 nm DHT. A, cell growth was analyzed by an MTT assay at indicated time points; B, PSA expression in the PCa cells treated with various anti-androgen/AR compounds was determined by quantitative PCR after 24 h of treatment. C, ASC-J9®/CTS, but not CASO/MDV, inhibits PCa invasion. The PCa cells were treated with 10 μm CASO, 10 μm MDV, 10 μm ASC, 5 μm CTS, or vehicle control for 3 days, and then invasion assays were performed. The invaded cell numbers are shown as -fold change after counting six randomly selected fields. D, three-dimensional invasion assay in C4-2B and CWR22Rv1 cells treated with various anti-androgen/AR compounds. The three-dimensional invasion assay was performed in C4-2B and CWR22Rv1 cells treated with four different compounds or vehicle control. After 2 weeks, the spheres with/without protrusion were counted and shown as a percentage compared with the control. E, comparison of different anti-androgen/AR compounds on TGF-β1/Smad3/MMP9 signaling in PCa cells. C4-2 and CWR22Rv1 cells treated with 10 μm CASO, 10 μm MDV, 10 μm ASC, 5 μm CTS, or vehicle for 3 days were harvested. The expressions of TGF-β1, p-Smad3, Smad3, MMP9, and GAPDH were analyzed by Western blot analysis. All of the experiments have been repeated twice independently. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Error bars, S.D.
ASC-J9® and CTS, but Not Casodex or MDV3100, Suppressed PCa Metastasis in in Vivo Mouse Models
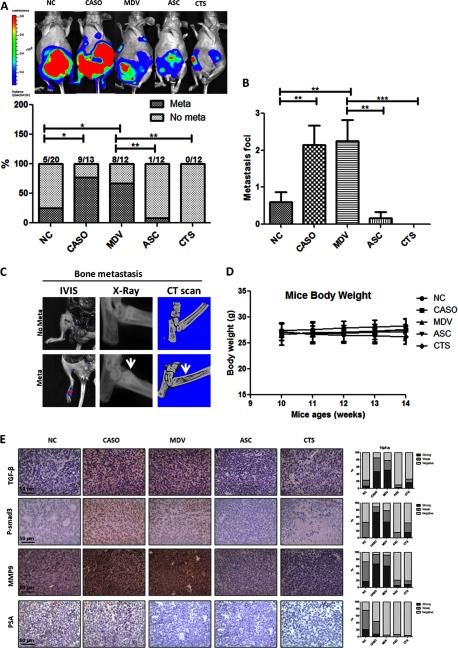
To further prove the opposite effects of these four anti-androgen/AR compounds using the in vivo mouse model, we exploited the CWR22Rv1 orthotopic xenografted mouse model. For monitoring metastases, the CWR22Rv1 cells were transfected with the firefly luciferase reporter gene, and the stable clone CWR22Rv1 (luc-CWR22RV1) was selected, expanded, and used for the injection. When the tumors were palpable 2 weeks after implantation, the mice were randomly assigned into five experimental groups and intraperitoneally injected with drugs as follows three times per week for 4 weeks: Group 1 (n = 20), vehicle; Group 2 (n = 13), Casodex; Group 3 (n = 12), MDV3100; Group 4 (n = 12), ASC-J9; Group 5 (n = 12), CTS. The metastatic lesions were evaluated by detection of the luciferase signals (Fig. 5A, top) using the IVIS system and further confirmed by tissue sections with HE staining. Most of the metastatic tumors were found in the lumbar and mesenteric lymph nodes, whereas some were located in diaphragms or bones. We found that 69.2% of Casodex-treated and 67.7% of MDV3100-treated mice had a significant increase in metastatic tumors as compared with 25% of vehicle-injected control mice. In contrast, the ASC-J9®-treated (8.3%) and CTS-treated (0%) mice had few or no metastatic tumors detected (Fig. 5A, bottom). Similar results were also obtained when the metastatic foci numbers were quantified (Fig. 5B).
FIGURE 5.
Anti-androgen/anti-AR compound treatments in CWR22Rv1 orthotopic xenograft mouse model exhibited differential effects on PCa growth and metastases. The luc-CWR22Rv1 cells were implanted into nude mice. Cancer cells (1 × 106) were mixed with Matrigel and injected into both anterior prostates of nude mice. Two weeks after inoculation, different anti-androgen/AR compounds (30 mg/kg CASO, 30 mg/kg MDV, 75 mg/kg ASC-J9® (ASC), 25 mg/kg CTS) or vehicle were intraperitoneally injected three times/week for 4 weeks. The mice were sacrificed, and the reporter gene signal was detected by the IVIS imaging system. A, metastatic tumors detected by the IVIS system. The mice were anesthetized by isoflurane and injected with d-luciferin (150 mg/kg) for 10 min before detection. The metastatic tumors were monitored by IVIS system. After sacrificing, primary tumors were removed, and the IVIS system was used again to confirm the existence of metastases. B, quantification of metastatic lesions in mice treated with different anti-androgen/anti-AR compounds. After euthanizing, metastatic tumors were confirmed by IVIS detection, and the numbers of metastatic foci in each mouse were then quantified. The numbers of mice used in each group were indicated (mice with metastasis/total mice). C, metastatic tumors in bone. Metastatic signals in the bone found in MDV3100-treated mice were confirmed by x-ray and CT scan. The white arrows indicate increased bone density and osteoblastic lesion formation. D, mouse body weight change with treatment with various compounds. Mouse body weights were checked weekly starting at the first injection of compounds. E, comparisons of TGF-β1/Smad3/MMP9 signals in in vivo xenografted PCa tissue. Tumor tissue sections were stained using IHC for TGF-β1, p-Smad3 (P-smad3), MMP9, and PSA expressions (magnification, ×400), and the quantification results are shown on the right. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Error bars, S.D.
Osteoblastic bone metastasis may represent the majority of PCa metastases seen in clinics. Interestingly, the luciferase signals were detected in two mice treated with anti-androgens (1 of 13 in the Casodex group and 1 of 12 in the MDV3100 group). Using x-ray and CT scan to examine luciferase signals in mouse feet, we found that the bone density near the luciferase signals was increased (a typical lesion of bone metastasis in clinical PCa diseases). Meanwhile, the CT scan also showed bone mass formation (Fig. 5C). The results suggested that our current in vivo mouse model could mimic the clinical conditions. Importantly, we found little change in mouse body weights among all of the mice treated with different anti-androgens (Fig. 5D), which is consistent with previous reports (19–22).
Expressions of the molecules in the TGF-β1/Smad3/MMP-9 pathway in tumor tissues of each mouse group were then evaluated by immunohistochemistry staining. Consistent with the in vitro findings, we found that expressions of TGF-β1, p-Smad3, and MMP9 were higher in Casodex- or MDV3100-treated tumor samples but lower in ASC-J9®- and CTS-treated tumor samples when compared with the tumors in the vehicle control mice (Fig. 5E). Together, the results from the in vivo mouse model studies all confirmed the above in vitro cell line studies and demonstrated that Casodex and MDV3100 treatment might promote PCa metastasis, yet ASC-J9® and CTS treatment could suppress PCa metastasis, and these differential effects involved the opposite regulation of the TGF-β1/Smad3/MMP-9 pathway.
DISCUSSION
Despite the tumor regression after ADT in advanced PCa patients, its beneficial effects on cancer-specific survival are still under debate (1, 4). Earlier reports showed that the PSA level may not reflect the pathological stage progression in some PCa patients, whose PSA levels were below 0.5 ng/ml with nodal metastasis after ADT (29, 30). Meanwhile, a recent clinical trial of abiraterone, a powerful drug that suppressed androgen biosynthesis, also found that although 79% of PCa patients have a decline in PSA level of 50% or more, 52% of PCa patients have either increased new bone lesions or increased intensity of existing bone lesions, which these investigators called “bone scan flare,” after 4 months of treatment (31). It is interesting to note that the increase of metastasis in these PCa patients was observed at the stage when the PSA level dropped to significantly low, which might be contrary to the general concept that PSA rise during ADT is the early sign before PCa progresses to enhanced metastasis and may suggest that anti-androgen/AR signaling in PCa proliferation versus metastasis could be two different pathways. We found that some PCa patients who received ADT had increased metastases although their PSA levels dropped to less than 4 ng/ml. Therefore, continual monitoring of the formation of new metastatic lesions during the ADT in PCa patients may be essential, even when the primary tumor size and the PSA levels are under control. Other studies showing the increased expression of aggressive markers after ADT in PCa patients, such as N-cadherin (32), cadherin-11 (33), and nestin (34), supported the similar conclusion that ADT might promote PCa metastases, and TRAMP mouse studies also demonstrated the increased PCa metastasis at stage when mice serum testosterone is undetectable after ADT with castration (35, 36).
A similar observation showing suppressed cancer cell growth yet promotion of cancer cell invasion upon anti-angiogenesis therapy has been reported (37). In a clinical trial of melanoma treatment, anti-angiogenesis therapy led to suppressed tumor cell growth through reduced blood vessel formation, yet it could simultaneously also promote cancer metastasis (37). Nevertheless, another study (4) also found that the primary ADT in localized PCa patients might lead to a lowering of the 10-year PCa-specific survival, suggesting that further studies may be required to determine what kind of ADT at which stages may be best for patients without leading to poor prognosis.
We noticed that although both Casodex and MDV3100 have significant anti-growth effects in LNCaP, C81, and C4-2 cells, both anti-androgen drugs showed less effect on CWR22Rv1 cell growth, which could be due to the existence of the AR variant, AR3, (27, 38). Recent reports have documented that Casodex failed to suppress AR3 transactivation, and this is probably due to the lack of an androgen-binding domain (27, 38, 39). Moreover, Hu et al. (39) also found in both in vitro and in vivo studies that MDV3100 or abiraterone treatment increased AR3 expression, which might then enhance cell cycle-related genes to promote PCa progression. In contrast, ASC-J9® could degrade both full-length AR and AR3 (27), providing another explanation of why ASC-J9® showed better therapeutic effects in battling both PCa cell growth and metastasis.
Recent reports of LNCaP cells studies have suggested that the ADT-promoted Akt signaling might explain PCa cell survival under ADT conditions (40, 41), and therefore, the ADT combined with anti-Akt could lead to better efficacy in suppressing PCa growth (Fig. 2A). However, this therapy still failed to interrupt the PCa cell invasion induced by these anti-androgen treatments, suggesting that Akt signaling might play differential roles in different types of PCa cells that might lead to differential effects on cell growth versus metastasis (42). Our finding that TGF-β1/Smad3 signaling was activated during ADT to enhance the cell invasion through activation of the MMP9 pathway might provide another new potential therapeutic approach that combines ADT with an inhibitor of TGF-β1/Smad3/MMP9 signaling to suppress both PCa cell growth and metastasis.
Early studies found that ASC-J9® could target the interaction of AR with selected co-regulators, such as ARA55 and ARA70, which resulted in degradation of the AR protein through the ubiquitination pathway in selective cells (8–11). The preclinical toxicity tests on mice also showed little toxicity with little influence of serum testosterone, and mice still had sexual activity and fertility (8–11). Because AR co-regulators can determine specific AR targets in different types of cells (43), regulation of AR co-regulator interaction may also change the gene expression profile regarding the metastasis-related genes. A previous study showed that CTS can reverse the demethylation process mediated by LSD1 and inhibit AR transactivation at the epigenetic level (28). It would be interesting to identify the potential regulation of PCa metastasis abilities via epigenetic markers.
In conclusion, the current studies point out that suppression of androgen binding to AR using Casodex and MDV3100 in PCa might carry a potential risk of increasing cancer metastasis in some PCa patients. A novel therapy via targeting AR and its downstream signaling of TGF-β1/Smad3/MMP-9 may be developed to better battle metastatic PCa at the castration-resistant stage.
Supplementary Material
Acknowledgment
We thank Karen Wolf for help with manuscript preparation.
This work was supported, in whole or in part, by National Institutes of Health Grant CA156700. This work was also supported by the George Whipple Professorship Endowment and Taiwan Department of Health Clinical Trial and Research Center of Excellence Grant DOH99-TD-B-111-004. ASC-J9® was patented by the University of Rochester, University of North Carolina, and AndroScience and then licensed to AndroScience. Both the University of Rochester and C. Chang own royalties and equity in AndroScience.

This article contains supplemental Materials and Methods.
- PCa
- prostate cancer
- ADT
- androgen deprivation therapy
- AR
- androgen receptor
- CTS
- cryptotanshinone
- LHRHa
- luteinizing hormone-releasing hormone agonist
- CASO
- Casodex
- MDV
- MDV3100
- CD-FBS
- 10% charcoal dextran-treated FBS
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- IVIS
- in vivo imaging system
- p-Smad3
- phosphorylated Smad3
- DHT
- 5α-dihydrotestosterone.
REFERENCES
- 1. Miyamoto H., Messing E. M., Chang C. (2004) Androgen deprivation therapy for prostate cancer. Current status and future prospects. Prostate 61, 332–353 [DOI] [PubMed] [Google Scholar]
- 2. de Bono J. S., Logothetis C. J., Molina A., Fizazi K., North S., Chu L., Chi K. N., Jones R. J., Goodman O. B., Jr., Saad F., Staffurth J. N., Mainwaring P., Harland S., Flaig T. W., Hutson T. E., Cheng T., Patterson H., Hainsworth J. D., Ryan C. J., Sternberg C. N., Ellard S. L., Fléchon A., Saleh M., Scholz M., Efstathiou E., Zivi A., Bianchini D., Loriot Y., Chieffo N., Kheoh T., Haqq C. M., Scher H. I., and COU-AA-301 Investigators (2011) Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Scher H. I., Beer T. M., Higano C. S., Anand A., Taplin M. E., Efstathiou E., Rathkopf D., Shelkey J., Yu E. Y., Alumkal J., Hung D., Hirmand M., Seely L., Morris M. J., Danila D. C., Humm J., Larson S., Fleisher M., Sawyers C. L. (2010) Antitumour activity of MDV3100 in castration-resistant prostate cancer. A phase 1–2 study. Lancet 375, 1437–1446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lu-Yao G. L., Albertsen P. C., Moore D. F., Shih W., Lin Y., DiPaola R. S., Yao S. L. (2008) Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA 300, 173–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niu Y., Altuwaijri S., Lai K. P., Wu C. T., Ricke W. A., Messing E. M., Yao J., Yeh S., Chang C. (2008) Androgen receptor is a tumor suppressor and proliferator in prostate cancer. Proc. Natl. Acad. Sci. U.S.A. 105, 12182–12187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Niu Y., Chang T. M., Yeh S., Ma W. L., Wang Y. Z., Chang C. (2010) Differential androgen receptor signals in different cells explain why androgen-deprivation therapy of prostate cancer fails. Oncogene 29, 3593–3604 [DOI] [PubMed] [Google Scholar]
- 7. Kolvenbag G. J., Blackledge G. R., Gotting-Smith K. (1998) Bicalutamide (Casodex) in the treatment of prostate cancer. History of clinical development. Prostate 34, 61–72 [DOI] [PubMed] [Google Scholar]
- 8. Yang Z., Chang Y. J., Yu I. C., Yeh S., Wu C. C., Miyamoto H., Merry D. E., Sobue G., Chen L. M., Chang S. S., Chang C. (2007) ASC-J9 ameliorates spinal and bulbar muscular atrophy phenotype via degradation of androgen receptor. Nat. Med. 13, 348–353 [DOI] [PubMed] [Google Scholar]
- 9. Wu M. H., Ma W. L., Hsu C. L., Chen Y. L., Ou J. H., Ryan C. K., Hung Y. C., Yeh S., Chang C. (2010) Androgen receptor promotes hepatitis B virus-induced hepatocarcinogenesis through modulation of hepatitis B virus RNA transcription. Sci. Transl. Med. 2, 32ra35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lai J. J., Lai K. P., Chuang K. H., Chang P., Yu I. C., Lin W. J., Chang C. (2009) Monocyte/macrophage androgen receptor suppresses cutaneous wound healing in mice by enhancing local TNF-α expression. J. Clin. Invest. 119, 3739–3751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lai K.-P., Huang C.-K., Chang Y.-J., Chung C.-Y., Yamashita S., Li L., Lee S., Yeh S., Chang C. (2013) New therapeutic approach to suppress castration-resistant prostate cancer using ASC-J9 via targeting androgen receptor in selective prostate cells. Am. J. Pathol. 182, 460–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu D., Lin T. H., Li S., Da J., Wen X. Q., Ding J., Chang C., Yeh S. (2012) Cryptotanshinone suppresses androgen receptor-mediated growth in androgen dependent and castration resistant prostate cancer cells. Cancer Lett. 316, 11–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Tyrrell C. J., Kaisary A. V., Iversen P., Anderson J. B., Baert L., Tammela T., Chamberlain M., Webster A., Blackledge G. (1998) A randomised comparison of “Casodex” (bicalutamide) 150 mg monotherapy versus castration in the treatment of metastatic and locally advanced prostate cancer. Eur. Urol. 33, 447–456 [DOI] [PubMed] [Google Scholar]
- 14. Xiang B., Muthuswamy S. K. (2006) Using three-dimensional acinar structures for molecular and cell biological assays. Methods Enzymol. 406, 692–701 [DOI] [PubMed] [Google Scholar]
- 15. Carver B. S., Chapinski C., Wongvipat J., Hieronymus H., Chen Y., Chandarlapaty S., Arora V. K., Le C., Koutcher J., Scher H., Scardino P. T., Rosen N., Sawyers C. L. (2011) Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19, 575–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mulholland D. J., Tran L. M., Li Y., Cai H., Morim A., Wang S., Plaisier S., Garraway I. P., Huang J., Graeber T. G., Wu H. (2011) Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell 19, 792–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chou Y. T., Wang H., Chen Y., Danielpour D., Yang Y. C. (2006) Cited2 modulates TGF-β-mediated upregulation of MMP9. Oncogene 25, 5547–5560 [DOI] [PubMed] [Google Scholar]
- 18. Padua D., Massagué J. (2009) Roles of TGFβ in metastasis. Cell Res. 19, 89–102 [DOI] [PubMed] [Google Scholar]
- 19. Qi W., Gao S., Wang Z. (2008) Transcriptional regulation of the TGF-β1 promoter by androgen receptor. Biochem. J. 416, 453–462 [DOI] [PubMed] [Google Scholar]
- 20. Kyprianou N., Isaacs J. T. (1989) Expression of transforming growth factor-β in the rat ventral prostate during castration-induced programmed cell death. Mol. Endocrinol. 3, 1515–1522 [DOI] [PubMed] [Google Scholar]
- 21. Chipuk J. E., Cornelius S. C., Pultz N. J., Jorgensen J. S., Bonham M. J., Kim S. J., Danielpour D. (2002) The androgen receptor represses transforming growth factor-β signaling through interaction with Smad3. J. Biol. Chem. 277, 1240–1248 [DOI] [PubMed] [Google Scholar]
- 22. Song K., Wang H., Krebs T. L., Wang B., Kelley T. J., Danielpour D. (2010) DHT selectively reverses Smad3-mediated/TGF-β-induced responses through transcriptional down-regulation of Smad3 in prostate epithelial cells. Mol. Endocrinol. 24, 2019–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Nishi N., Oya H., Matsumoto K., Nakamura T., Miyanaka H., Wada F. (1996) Changes in gene expression of growth factors and their receptors during castration-induced involution and androgen-induced regrowth of rat prostates. Prostate 28, 139–152 [DOI] [PubMed] [Google Scholar]
- 24. Inman G. J., Nicolás F. J., Callahan J. F., Harling J. D., Gaster L. M., Reith A. D., Laping N. J., Hill C. S. (2002) SB-431542 is a potent and specific inhibitor of transforming growth factor-β superfamily type I activin receptor-like kinase (ALK) receptors ALK4, ALK5, and ALK7. Mol. Pharmacol. 62, 65–74 [DOI] [PubMed] [Google Scholar]
- 25. Liu X., Wang W., Hu H., Tang N., Zhang C., Liang W., Wang M. (2006) Smad3 specific inhibitor, naringenin, decreases the expression of extracellular matrix induced by TGF-β1 in cultured rat hepatic stellate cells. Pharm. Res. 23, 82–89 [DOI] [PubMed] [Google Scholar]
- 26. Levin J. I., Chen J., Du M., Hogan M., Kincaid S., Nelson F. C., Venkatesan A. M., Wehr T., Zask A., DiJoseph J., Killar L. M., Skala S., Sung A., Sharr M., Roth C., Jin G., Cowling R., Mohler K. M., Black R. A., March C. J., Skotnicki J. S. (2001) The discovery of anthranilic acid-based MMP inhibitors. Part 2. SAR of the 5-position and P1(1) groups. Bioorg. Med. Chem. Lett. 11, 2189–2192 [DOI] [PubMed] [Google Scholar]
- 27. Yamashita S., Lai K. P., Chuang K. L., Xu D., Miyamoto H., Tochigi T., Pang S. T., Li L., Arai Y., Kung H. J., Yeh S., Chang C. (2012) ASC-J9 suppresses castration-resistant prostate cancer growth through degradation of full-length and splice variant androgen receptors. Neoplasia 14, 74–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wu C. Y., Hsieh C. Y., Huang K. E., Chang C., Kang H. Y. (2012) Cryptotanshinone down-regulates androgen receptor signaling by modulating lysine-specific demethylase 1 function. Int. J. Cancer 131, 1423–1434 [DOI] [PubMed] [Google Scholar]
- 29. Voges G. E., Mottrie A. M., Stöckle M., Müller S. C. (1994) Hormone therapy prior to radical prostatectomy in patients with clinical stage C prostate cancer. Prostate Suppl. 5, 4–8 [DOI] [PubMed] [Google Scholar]
- 30. Voges G. E., Mottrie A. M., Fichtner J., Mappes C., Störkel S., Stöckle M., Hohenfellner R. (1995) Maximum androgen deprivation prior to radical retropubic prostatectomy in patients with stage T3 adenocarcinoma of the prostate. Eur. Urol. 28, 209–214 [DOI] [PubMed] [Google Scholar]
- 31. Ryan C. J., Shah S., Efstathiou E., Smith M. R., Taplin M. E., Bubley G. J., Logothetis C. J., Kheoh T., Kilian C., Haqq C. M., Molina A., Small E. J. (2011) Phase II study of abiraterone acetate in chemotherapy-naive metastatic castration-resistant prostate cancer displaying bone flare discordant with serologic response. Clin. Cancer Res. 17, 4854–4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jennbacken K., Tesan T., Wang W., Gustavsson H., Damber J. E., Welén K. (2010) N-cadherin increases after androgen deprivation and is associated with metastasis in prostate cancer. Endocr. Relat. Cancer 17, 469–479 [DOI] [PubMed] [Google Scholar]
- 33. Lee Y. C., Cheng C. J., Huang M., Bilen M. A., Ye X., Navone N. M., Chu K., Kao H. H., Yu-Lee L. Y., Wang Z., Lin S. H. (2010) Androgen depletion up-regulates cadherin-11 expression in prostate cancer. J. Pathol. 221, 68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kleeberger W., Bova G. S., Nielsen M. E., Herawi M., Chuang A. Y., Epstein J. I., Berman D. M. (2007) Roles for the stem cell-associated intermediate filament Nestin in prostate cancer migration and metastasis. Cancer Res. 67, 9199–9206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gingrich J. R., Barrios R. J., Kattan M. W., Nahm H. S., Finegold M. J., Greenberg N. M. (1997) Androgen-independent prostate cancer progression in the TRAMP model. Cancer Res. 57, 4687–4691 [PubMed] [Google Scholar]
- 36. Tang Y., Wang L., Goloubeva O., Khan M. A., Zhang B., Hussain A. (2008) Divergent effects of castration on prostate cancer in TRAMP mice. Possible implications for therapy. Clin. Cancer Res. 14, 2936–2943 [DOI] [PubMed] [Google Scholar]
- 37. Pàez-Ribes M., Allen E., Hudock J., Takeda T., Okuyama H., Viñals F., Inoue M., Bergers G., Hanahan D., Casanovas O. (2009) Antiangiogenic therapy elicits malignant progression of tumors to increased local invasion and distant metastasis. Cancer Cell 15, 220–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Guo Z., Yang X., Sun F., Jiang R., Linn D. E., Chen H., Chen H., Kong X., Melamed J., Tepper C. G., Kung H. J., Brodie A. M., Edwards J., Qiu Y. (2009) A novel androgen receptor splice variant is up-regulated during prostate cancer progression and promotes androgen depletion-resistant growth. Cancer Res. 69, 2305–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hu R., Lu C., Mostaghel E. A., Yegnasubramanian S., Gurel M., Tannahill C., Edwards J., Isaacs W. B., Nelson P. S., Bluemn E., Plymate S. R., Luo J. (2012) Distinct transcriptional programs mediated by the ligand-dependent full-length androgen receptor and its splice variants in castration-resistant prostate cancer. Cancer Res. 72, 3457–3462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Murillo H., Huang H., Schmidt L. J., Smith D. I., Tindall D. J. (2001) Role of PI3K signaling in survival and progression of LNCaP prostate cancer cells to the androgen refractory state. Endocrinology 142, 4795–4805 [DOI] [PubMed] [Google Scholar]
- 41. Lin H. K., Hu Y. C., Yang L., Altuwaijri S., Chen Y. T., Kang H. Y., Chang C. (2003) Suppression versus induction of androgen receptor functions by the phosphatidylinositol 3-kinase/Akt pathway in prostate cancer LNCaP cells with different passage numbers. J. Biol. Chem. 278, 50902–50907 [DOI] [PubMed] [Google Scholar]
- 42. Virtakoivu R., Pellinen T., Rantala J. K., Perälä M., Ivaska J. (2012) Distinct roles of AKT isoforms in regulating β1-integrin activity, migration, and invasion in prostate cancer. Mol. Biol. Cell 23, 3357–3369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Lupien M., Brown M. (2009) Cistromics of hormone-dependent cancer. Endocr. Relat. Cancer 16, 381–389 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.