Background: Activation of STAT3 is the major signaling pathway of IL-23.
Results: The study provides detailed characterization of IL-23-dependent signal transduction with the focus on STAT3 phosphorylation.
Conclusion: Canonical tyrosine-dependent and non-canonical tyrosine-independent STAT3 activation sites are located within the IL-23R.
Significance: This may be the first step in elucidating the signal transduction of IL-23, an important cytokine for TH17 cell development.
Keywords: Cytokine, Interleukin, MAP Kinases (MAPKs), Signal Transduction, STAT Transcription Factor
Abstract
Signaling of interleukin 23 (IL-23) via the IL-23 receptor (IL-23R) and the shared IL-12 receptor β1 (IL-12Rβ1) controls innate and adaptive immune responses and is involved in the differentiation and expansion of IL-17-producing CD4+ T helper (TH17) cells. Activation of signal transducer and activator of transcription 3 (STAT3) appears to be the major signaling pathway of IL-23, and STAT binding sites were predicted in the IL-23R but not in the IL-12Rβ1 chain. Using site-directed mutagenesis and deletion variants of the murine and human IL-23R, we showed that the predicted STAT binding sites (pYXXQ; including Tyr-504 and Tyr-626 in murine IL-23R and Tyr-484 and Tyr-611 in human IL-23R) mediated STAT3 activation. Furthermore, we identified two uncommon STAT3 binding/activation sites within the murine IL-23R. First, the murine IL-23R carried the Y542PNFQ sequence, which acts as an unusual Src homology 2 (SH2) domain-binding protein activation site of STAT3. Second, we identified a non-canonical, phosphotyrosine-independent STAT3 activation motif within the IL-23R. A third predicted site, Tyr-416 in murine and Tyr-397 in human IL-23R, is involved in the activation of PI3K/Akt and the MAPK pathway leading to STAT3-independent proliferation of Ba/F3 cells upon stimulation with IL-23. In contrast to IL-6-induced short term STAT3 phosphorylation, cellular activation by IL-23 resulted in a slower but long term STAT3 phosphorylation, indicating that the IL-23R might not be a major target of negative feedback inhibition by suppressor of cytokine signaling (SOCS) proteins. In summary, we characterized IL-23-dependent signal transduction with a focus on STAT3 phosphorylation and identified canonical tyrosine-dependent and non-canonical tyrosine-independent STAT3 activation sites in the IL-23R.
Introduction
The interleukin (IL)-12 type cytokine family consists of the heterodimeric cytokines IL-12, IL-23, IL-27, and IL-35 (1). The α-subunits (p19, p28, and p35) share the IL-6 type cytokine fold and can pair with β-subunits (p40 and Ebi3) that are structurally similar to the membrane-bound receptor for IL-6 cytokines but lacking the transmembrane domain (2). The non-signaling α-receptor p40 subunit is covalently attached to the p35 and p19 cytokine chain to form IL-12p70 (p35/p40) (3) and IL-23 (p19/p40) (4), respectively. The β-chain Ebi3 pairs with p28 to form IL-27 (5). The newest member of the IL-12 family, IL-35, is a heterodimer composed of the non-covalently bound subunits p35 (cytokine subunit of IL-12) and Ebi3 (α-receptor subunit of IL-27) (6). Thereby, IL-35 bridges the IL-6 and the IL-12 cytokine families.
Some of the proinflammatory functions of IL-23 are related to the induction of terminal differentiation and proliferation of IL-17-producing CD4+ T helper (TH17) cells (7). TH17 cells are involved in the pathogenesis of inflammatory autoimmune diseases, including rheumatoid arthritis, multiple sclerosis, psoriasis, and inflammatory bowel disease (8). Consequently, IL-23 (p19)- or IL-23 receptor (IL-23R)-deficient mice are resistant to autoimmune and inflammatory disorders, such as experimental autoimmune encephalomyelitis (9), collagen-induced arthritis (10), and inflammatory bowel disease (11, 12). Accordingly, targeting IL-23 signal transduction pathways has become a promising therapeutic strategy. However, clinical trials with the anti-p40 antibody ustekinumab, which neutralizes both IL-23 and the related IL-12, have not been reproducibly effective in patients with Crohn disease (13, 14). IL-23 also appears to promote tumor incidence and growth (15), but at least one report indicates that IL-23 has also anti-tumorigenic activity against pediatric B-acute lymphoblastic leukemia (B-ALL) cells (16), demonstrating (e.g. for Crohn disease and opposing roles in cancer development) that the biology of IL-23 is still incompletely understood. IL-23 is mainly produced by myeloid dendritic cells after Toll-like receptor activation (17–19) and by activated proinflammatory type 1 macrophages (20). Little is known about regulation of IL-23 receptor expression, but a number of cell types have been described to express IL-23 receptor chains, including CD4+ T cells of the TH17 lineage, γδ T cells, macrophages, dendritic cells, and innate lymphoid cells, although these cells are often almost unresponsive to IL-23 due to low expression of the IL-23-specific IL-23R (21, 22).
Signaling within the IL-12 family occurs via receptor chains that are structurally homologous to the gp130 family. IL-23 induces dimerization of the IL-12Rβ1 receptor, which is also one receptor for IL-12, and the IL-23-specific IL-23R (23). Binding of IL-23 to signal-transducing type I transmembrane β-receptors induces the activation of noncovalently β-receptor-bound Janus kinases (JAKs) and subsequent signaling pathways, including signal transducers and activators of transcription (STAT) transcription factors (23), phosphoinositide 3-kinase (PI3K) (24), and NF-κB (24). So far, there are no data about the involvement of MAPK in IL-23 signal transduction. Cho et al. (24) investigated the effect of a MEK inhibitor on the induction of IL-17 but did not find any influence. STAT and SHP2 phosphorylation by Janus kinases depends on the ability of the Src homology 2 (SH2)2 domain of the STAT factors and the SHP2 interaction domain to interact with phosphorylated tyrosines embedded in factor-specific binding sites of the cytokine receptor. The IL-23R is regarded as the only signal-transducing component of the IL-23 receptor complex, and, similar to IL-12 signaling, IL-12Rβ1 is required for high affinity binding (25). The murine and human IL-23R proteins are 644- and 629-amino acid residue-long type I transmembrane proteins, respectively, with a 66% identity on the amino acid level. The intracellular domains of murine and human IL-23R comprise 247 and 252 amino acid residues, respectively. It has been suggested that IL-23 signaling is mediated through three of seven intracellular tyrosine residues of the IL-23R (Tyr-416, Tyr-504, and Tyr-626 in mice and Tyr-397, Tyr-484, and Tyr-611 in humans). The Ym416/h397EDI sequence was predicted to be a potential SHP2 binding site (23, 26), and Ym626/h611FPQ was predicted to be a potential STAT1 and STAT3 binding site (23, 27, 28). The postulated SHP2 binding site within the IL-23R might lead to the activation of the MAPK and the PI3K cascade, as is known for IL-6 signal transduction (29). The GYm504/h484KPQIS sequence has similarities in the IL-12Rβ2 known to bind to STAT4 (GY(L/V)PS (30, 31)). The STAT phosphorylation patterns (STAT1, STAT3, STAT4, and STAT5) of IL-12 and IL-23 are similar. However, IL-23-induced STAT4 phosphorylation is much weaker compared with IL-12 (23). For IL-23 signaling, STAT3 appears to be the primary mediator (23). Therefore, we focused on the analysis of STAT3 and MAPK/PI3K activation by murine and human IL-23R. Whereas the predicted MAPK/PI3K activation site was verified, STAT3 activation is far more complex than expected, with canonical tyrosine-dependent and non-canonical tyrosine-independent activation modes.
EXPERIMENTAL PROCEDURES
Cells and Reagents
Ba/F3-gp130 cells transduced with human gp130 were kindly provided by Immunex (Seattle, WA) (32), HeLa cells (ACC-57) were purchased from the Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany), and the packaging cell line Phoenix-Eco was from U. Klingmüller (DKFZ, Heidelberg, Germany) (33). All cell lines were grown in DMEM high glucose culture medium (Invitrogen) supplemented with 10% fetal calf serum (Invitrogen), 60 mg/liter penicillin, and 100 mg/liter streptomycin (Genaxxon Bioscience GmbH, Ulm, Germany) at 37 °C with 5% CO2 in a water-saturated atmosphere. For cultivation of Ba/F3-gp130 cell lines, the above-mentioned medium was supplemented with 10 ng/ml Hyper-IL-6 (HIL-6), a fusion protein of IL-6 and the soluble IL-6R, which is a mimic of IL-6 trans-signaling (34). After selection and proliferation analysis, Ba/F3-gp130 cells expressing murine IL-23R variants and murine IL-12Rβ1 were cultured in the respective medium containing 10 ng/ml recombinant mouse IL-23 as described (eBioscience, San Diego, CA) or Hyper-IL-23 (HIL-23) instead of HIL-6. HIL-23 is a fusion protein of murine p40 followed by a synthetic linker (RGGGGSGGGGSVE) and murine p19 as described previously (4) with an N-terminal FLAG and a C-terminal His tag. 0.2% of conditioned cell culture medium from a stable CHO-K1 cell line secreting HIL-23 or HIL-6 was used for cell culture, proliferation, and stimulation assays. The concentration of HIL-23 in the conditioned cell culture medium was about 5.5 μg/ml, as determined by ELISA (data not shown). Phospho-STAT3 mAb (Tyr-705) (D3A7), STAT3 mAb (124H6), phospho-STAT1 (Tyr-701) antibody, STAT1 antibody, phospho-p44/42 MAPK (Erk1/2) (Thr-202/Tyr-204) (D13.14.4E) mAb, p44/42 MAPK (Erk1/2) antibody, phospho-Akt (Ser-473) (D9E) mAb, and Akt antibody were purchased from Cell Signaling Technology (Frankfurt, Germany). Anti-c-Myc monoclonal antibody (9E10) was precipitated from hybridoma cell culture supernatant using ammonium sulfate, dissolved in PBS, and used for immunoblotting (1:50). The peroxidase-conjugated secondary antibodies were purchased from Pierce. Biotinylated anti-mouse IL-23R antibody (BAF1686) and anti-mouse IL-12Rβ1 antibody (BAF1998), streptavidin-HRP, phycoerythrin (PE)-conjugated anti-mouse IL-12Rβ1, and monoclonal anti-mouse IL-23R antibody were from R&D Systems (Minneapolis, MN). Alexa Fluor 647 mouse anti-Stat3 (Tyr(P)-705) and PE-conjugated mouse anti-Stat3 were purchased from BD Biosciences. Alexa Fluor 647-conjugated Fab goat anti-rat IgG was from Dianova (Hamburg, Germany).
Construction of Expression Plasmids
cDNAs coding for murine IL-23R (gene ID 209590) and IL-12Rβ1 (gene ID 16161) were amplified from total RNA extracts derived from mouse T cells and cloned into the pcDNA3.1 expression vector (Invitrogen). A C-terminal c-Myc tag was added for detection in cell lysates by Western blot. To create a murine/human chimeric IL-23R with human IL-23R signal transduction, named hIL-23R(mET/hC), the intracellular part of the human receptor (amino acids 377–629) was amplified by polymerase chain reaction (PCR) from a cDNA clone (IMAGE ID 4132295, Source BioScience LifeSciences (Berlin, Germany)) and inserted into pcDNA3.1-mIL-23R, where the appropriate coding sequence (amino acids 396–644) had been removed. Mutations of tyrosine to phenylalanine in murine IL-23R and the chimera were generated by PCRs using Phusion® high fidelity DNA polymerase (FINNZYMES, Thermo Scientific) followed by DpnI digestion of methylated template DNA. Deletion variants of the murine IL-23 receptor (mIL-23R) and the chimera were cloned into pcDNA3.1 using standard PCR. The sequences of the oligonucleotides used in this study will be provided upon request. Further, all cDNAs were subcloned into the eukaryotic expression vector p409 (35) for transient transfection of HeLa cells. For retroviral transduction of Ba/F3-gp130 cells, two retroviral plasmids with different resistance genes have been used. The plasmid pMOWS encodes the puromycin resistance gene (pMOWS-puro (33)), whereas pMOWS-hygromycin mediates hygromycin resistance (pMOWS-hygro (36)). Expression cassettes coding for IL-23R variants were inserted into pMOWS-puro, and those for the murine IL-12Rβ1 were inserted into the pMOWS-hygro plasmid. All generated expression plasmids have been verified by sequencing.
Transfection, Transduction, and Selection of Cells
HeLa cells (1 × 106) were transiently transfected as indicated with either p409-mIL-23R, p409-mIL-23R deletion or mutation variants, hIL-23R(mET/hC), or appropriate deletions or mutations along with p409-mIL-12Rβ1 using TurboFect transfection reagent (Fermentas, Thermo Scientific) according to the manufacturer's instructions. Ba/F3-gp130 cells were retrovirally transduced with the expression plasmid pMOWS-hygro-mIL-12Rβ1 followed by an additional transduction with pMOWS-puro plasmid derivatives coding for IL-23R variants. As a control, single transduced Ba/F3-gp130 cell lines expressing only mouse IL-12Rβ1, mouse IL-23R, or hIL-23R(mET/hC) were generated. The pMOWS expression plasmids (5 μg) were therefore transiently transfected in 8 × 105 Phoenix-Eco cells using TurboFect transfection reagent. Retroviral supernatants were produced as described (33), and 250 μl were applied to 1 × 105 Ba/F3-gp130 cells. The solution was centrifuged at 1,800 rpm for 2 h at 21 °C in the presence of 8 μg/ml Polybrene (Sigma-Aldrich). Transduced cells were grown in standard DMEM as described above supplemented with 10 ng/ml HIL-6. 48 h after transduction, transduced Ba/F3-gp130 cells were selected in 1.5 μg/ml puromycin or 1 mg/ml hygromycin B (PAA Laboratories, Marburg, Germany) or both for at least 2 weeks. After 2 weeks of antibiotic selection, HIL-6 was washed away, and the generated Ba/F3-gp130 cell lines were screened for HIL-23-dependent growth.
Stimulation Assays
For detection of phospho-STAT3 in co-transfected HeLa cells, 24 h after transfection, cells were washed with PBS and cultured for 16 h in serum-free DMEM. Afterward, cells were incubated in the absence or presence of HIL-23 as indicated and lysed in 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 2 mm EDTA, 1 mm NaF, 1 mm Na3VO4, 1% Nonidet P-40, and 1% Triton X-100, supplemented with complete protease inhibitor mixture tablets (Roche Applied Science). For detection of phospho-STAT3, phospho-STAT1, phospho-Erk1/2, and phospho-Akt, Ba/F3-gp130 cells, retrovirally transduced with cDNAs for mouse IL-12Rβ1 and IL-23R variants, were washed three times with sterile PBS and starved for at least 4 h in serum-free DMEM. Cells were stimulated with HIL-23 as indicated and harvested by centrifugation, and the pellet was directly frozen in liquid nitrogen. For analysis of phospho-Erk1/2 and phospho-Akt, cells were pretreated for 1 h with signaling pathway inhibitors, such as PD98059 (MEK inhibitor) and LY294002 (PI3K inhibitor). Cells were lysed as described above. The protein concentrations of the cell lysates were determined by a BCA protein assay (Pierce) according to the manufacturer's instructions. Activation of STAT3, Erk1/2, and Akt was determined by immunoblotting using antibodies against the phosphorylated proteins.
SDS-PAGE and Western Blot
Equal amounts of proteins from cell lysates were separated by SDS-PAGE under reducing conditions and transferred to a PVDF membrane using a Trans-Blot Turbo transfer system (Bio-Rad). The membrane was blocked in 5% fat-free dried skimmed milk in TBS-T (10 mm Tris-HCl, pH 7.6, 150 mm NaCl, 0.05% Tween 20) and probed with the primary antibody in 5% fat-free dried skimmed milk in TBS-T (STAT3, STAT1, c-Myc, Erk1/2, and Akt) or 5% BSA in TBS-T (phospho-STAT3, phospho-STAT1, phospho-Erk1/2, phospho-Akt, mIL-23R, and mIL-12Rβ1) at 4 °C overnight. The blots were washed and incubated with the secondary peroxidase-conjugated antibody or streptavidin-HRP for 1 h before applying the ECL Prime Western blotting detection reagent (GE Healthcare). The ChemoCam Imager (INTAS Science Imaging Instruments GmbH, Göttingen, Germany) was used for signal detection according to the manufacturer's instructions. Membranes were stripped in 62.5 mm Tris-HCl (pH 6.8), 2% SDS, and 0.1% β-mercaptoethanol for 30 min at 60 °C, blocked again, and reprobed with another primary antibody.
Surface and Intracellular STAT3 Staining
To detect surface expression of the IL-23R variants, cells were washed with FACS buffer (PBS containing 1% BSA) and incubated at 5 × 105 cells/100 μl of FACS buffer supplemented with 2.5 μg of monoclonal anti-mouse IL-23R antibody (R&D Systems) for 2 h on ice. After a single wash with FACS buffer, cells were incubated in FACS buffer containing a 1:100 dilution of Alexa Fluor 647-conjugated Fab goat anti-rat IgG (Dianova) for 1 h at 4 °C. Finally, cells were washed once with FACS buffer, resuspended in 500 μl of FACS buffer, and analyzed by flow cytometry (BD FACSCanto II flow cytometer, BD Biosciences). The resulting data were evaluated using the FCS Express software (De Novo Software, Los Angeles, CA). To detect surface expression of the mouse IL-12Rβ1, cells were prepared as described above and incubated with 100 μl of FACS buffer containing 10 μl of PE-conjugated anti-mouse IL-12Rβ1 (R&D Systems) for 2 h at 4 °C. For intracellular staining of phosphorylated STAT3, 1 × 106 Ba/F3 cells were washed three times with sterile PBS, starved for at least 4 h in serum-free DMEM, and stimulated with HIL-23 as indicated. Cells were harvested by centrifugation and fixed in 2% (w/v) paraformaldehyde at 37 °C for 15 min, followed by permeabilization in 90% (v/v) methanol for 30 min on ice. Cells were washed (PBS containing 0.5% BSA) and stained for phospho-STAT3 and STAT3 using Alexa Fluor 647 mouse anti-Stat3 (Tyr(P)-705) and PE-conjugated mouse anti-Stat3 antibodies (BD Biosciences) according to the manufacturer's instructions. Flow cytometry was performed using a BD FACSCanto II flow cytometer (BD Biosciences), and data were analyzed using FACS DIVA software (BD Biosciences).
Proliferation Assay
Ba/F3-gp130 cell lines were washed three times with sterile PBS and resuspended in DMEM containing 10% FCS at 5 × 104 cells/ml. The cells were cultured for 2 days in a final volume of 100 μl with or without cytokines, as indicated. The CellTiter-Blue® cell viability assay (Promega, Karlsruhe, Germany) was used to estimate the number of viable cells following the manufacturer's protocol. Fluorescence (excitation 560 nm, emission 590 nm) was recorded using the Infinite M200 PRO plate reader (Tecan, Crailsheim, Germany) immediately after the addition of the 20 μl/well CellTiter-Blue® reagent (time point 0) and up to 1 h after incubation under standard cell culture conditions. Fluorescence values were normalized by subtraction of time point 0 values. For comparison of independent Ba/F3-gp130 cell lines, the n-fold proliferation was calculated by setting the negative control (cells growing without cytokines) of each Ba/F3 cell line to a value of 1. All of the values were measured in triplicate per experiment.
RESULTS
IL-23 Induces Long Term STAT3 Activation
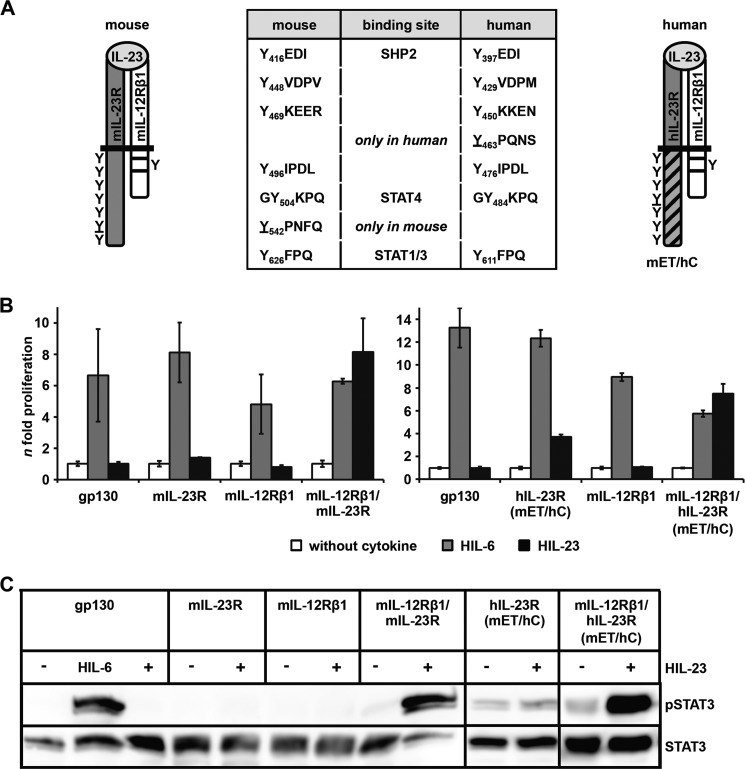
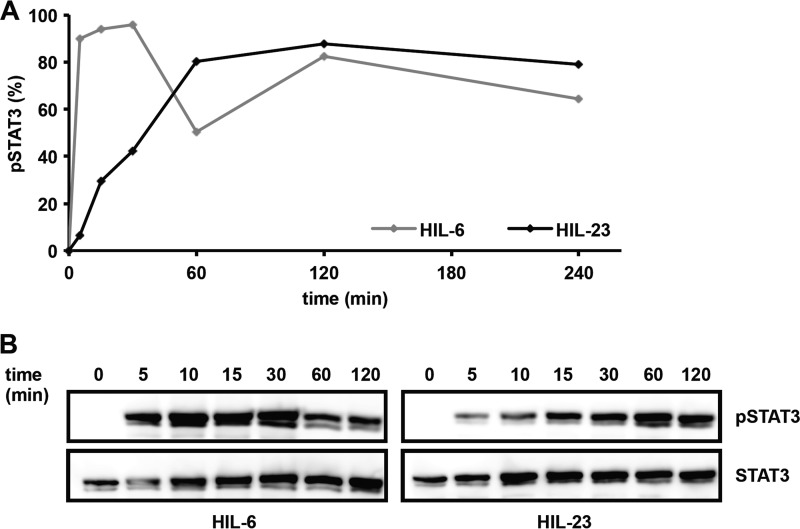
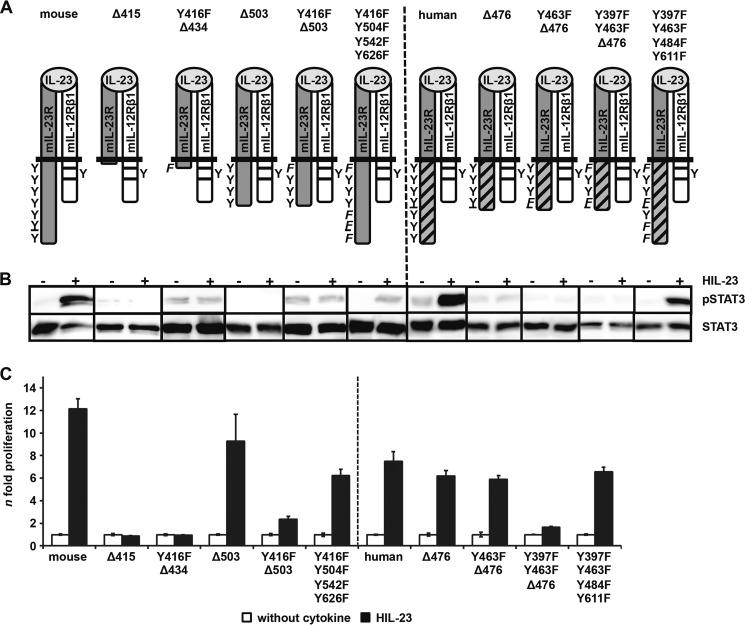
The cytoplasmic region of mIL-23R contains seven tyrosine residues; six are conserved between mIL-23R and hIL-23R. Mouse Tyr-416, Tyr-504, and Tyr-626 and human Tyr-397, Tyr-484, and Tyr-611 were defined as potential SH2 domain-binding sites for binding of SHP2, STAT4, and STAT1/3, respectively (23) (Fig. 1A). Tyr-542 is unique in mIL-23R, whereas Tyr-463 only exists in hIL-23R. Both tyrosines are not embedded in classical SH2 domain-binding sites (YXXQ) with Y542PNFQ and Y463PQ, respectively (Fig. 1A). Here, we concentrated on the activation/phosphorylation of STAT3 by the IL-23R. To study the functional role of the tyrosine residues of the murine and human IL-23R in STAT3 activation, IL-23-responsive Ba/F3-gp130 cell lines have been generated, expressing the murine receptor chains on their cell surface (supplemental Fig. 1, A and B). Further, a murine/human chimeric IL-23R with hIL-23R signal transduction has been constructed, in which the extracellular and transmembrane region (ET domain) of the mIL-23R was fused to the cytoplasmic region (C) of hIL-23R, named hIL-23R(mET/hC) (Fig. 1A). Proliferation of Ba/F3 cells depend on IL-3 and activation of STAT5. After stable transfection with a gp130 cDNA, proliferation of Ba/F3-gp130 cells depends on IL-6 and soluble IL-6R or Hyper-IL-6 and activation of STAT3 (34) (Fig. 1B). After stable transduction of the IL-23 receptor chains mIL-12Rβ1 and mIL-23R or hIL-23R(mET/hC), Ba/F3-gp130-mIL-12Rβ1-mIL-23R, and Ba/F3-gp130-mIL-12Rβ1-hIL-23R(mET/hC), cells were able to proliferate in the presence of HIL-23, whereas Ba/F3-gp130 cells expressing either mIL-23R, the hIL-23R(mET/hC), or the mIL-12Rβ1 did not grow (Fig. 1B). The dependence on IL-23 of these Ba/F3 cells was confirmed by analysis of STAT3 phosphorylation. HIL-23 induced STAT3 phosphorylation only in cells expressing mIL-23R or hIL-23R(mET/hC) plus mIL-12Rβ1 (Fig. 1C). Next, a time course experiment was carried out to investigate the dynamics of STAT3 phosphorylation in Ba/F3-gp130-mIL-12Rβ1-mIL-23R cells stimulated with HIL-23 or HIL-6 for up to 240 min. STAT3 phosphorylation was analyzed by flow cytometry and Western blotting at the indicated time points (Fig. 2, A and B). Interestingly, induction of STAT3 phosphorylation in Ba/F3-gp130-mIL-12Rβ1-mIL-23R cells upon HIL-23 stimulation is slow with a peak at 60 min, but it is prolonged in comparison with HIL-6 stimulation, which leads to a maximal induction after 5–10 min and a decline after 60 min (Fig. 2, A and B).
FIGURE 1.
The cytoplasmic domains of mouse and human IL-23R induce STAT3 activation. A, the mouse IL-23 receptor complex consists of IL-12Rβ1, containing one tyrosine residue, box 1 and box 2 motifs, and the unique IL-23R with seven tyrosine residues. Six tyrosines are conserved in mice and humans, and putative binding sites for SHP2, STAT4, and STAT1/3 have been postulated. Tyrosine residues are indicated and numbered in IL-23R. The non-conserved tyrosines Tyr-542 (mouse) and Tyr-463 (human) are underlined. B, proliferation of stably transduced Ba/F3-gp130 cells with cDNAs coding for murine IL-23R, hIL-23R(mET/hC) containing the cytoplasmic domain of the human receptor, murine IL-12Rβ1, and co-transduced Ba/F3 cells. Equal numbers of cells were cultured for 2 days in the presence of 10 ng/ml HIL-6, 0.2% HIL-6 conditioned cell culture supernatant, or 0.2% HIL-23 or without cytokine. Ba/F3-gp130 cells were used as a control. Proliferation was measured using the colorimetric CellTiter-Blue® cell viability assay. C, stably transduced Ba/F3 cells were washed three times, starved, and stimulated with 0.2% HIL-23 for 10 min. Cellular lysates were prepared, and equal amounts of proteins (50 μg/lane) were loaded onto SDS gels. Western blots were performed using antibodies specific for phospho-STAT3 and STAT3. For positive control, Ba/F3-gp130 cells were stimulated for 10 min with 0.2% HIL-6 and analyzed. Western blots are shown for one representative experiment. Error bars, S.D.
FIGURE 2.
Kinetics of IL-23-induced STAT3 activation. A, Ba/F3-gp130 cells stably transduced with cDNAs for murine IL-23R and murine IL-12Rβ1 were washed three times with PBS and starved for 2 h in serum-free DMEM. 1 × 106 cells were stimulated for the times indicated with 0.2% HIL-6 or HIL-23, harvested by centrifugation, fixed in 2% (w/v) paraformaldehyde, and permeabilized in 90% (v/v) methanol. Cells were stained for phospho-STAT3 and STAT3 overnight and analyzed by flow cytometry. B, in parallel, cellular lysates of the respective Ba/F3-gp130 cells, stimulated for the times indicated with 0.2% HIL-6 or HIL-23, were prepared and analyzed by Western blot using antibodies specific for phospho-STAT3 and STAT3.
As a second cellular model to investigate IL-23-dependent STAT3 phosphorylation, we chose HeLa cells. Recently, it was shown that STAT3 was activated in HeLa cells co-transfected with hIL-12β1 and hIL-23R (37). In co-transfection experiments, we first examined expression and signaling of mIL-12Rβ1 and mIL-23R in HeLa cells (supplemental Fig. 2A). IL-23-induced STAT3 phosphorylation was only observed in HeLa cells co-transfected with mIL-12Rβ1 and mIL-23R but not after transfection of only one receptor chain (supplemental Fig. 2A). Surface expression of mIL-23R and mIL-12Rβ1 was demonstrated by FACS analysis with the use of antibodies directed against the extracellular domains of mouse IL-12Rβ1 and mouse IL-23R (supplemental Fig. 2B). IL-23-induced STAT3 phosphorylation was also detected in HeLa cells co-transfected with mIL-12Rβ1 and hIL-23R(mET/hC) (supplemental Fig. 2C). Taken together, our results indicate that Ba/F3 and HeLa cells are good cellular systems to investigate the biological function of the mIL-23R and the hIL-23R chimera hIL-23R(mET/hC) with respect to proliferation and STAT3 phosphorylation.
IL-23-induced STAT3 Activation Is Not Restricted to the Predicted Tyr-626 SH2-binding Site Motif in Murine IL-23R and the Predicted Tyr-611 SH2-binding Site Motif in Human IL-23R
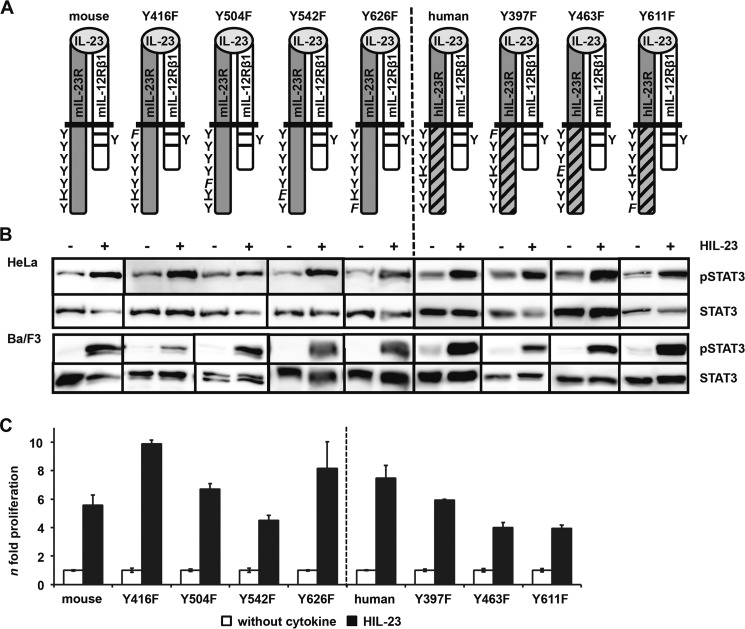
Two potential STAT binding sites (pYXXQ) in the cytoplasmic region of the IL-23R have been postulated (STAT4, mouse Tyr-504 and human Tyr-484; STAT1/3, mouse Tyr-626 and human Tyr-611 (23)). To examine the role of these individual tyrosine motifs in mIL-23R and hIL-23R(mET/hC), receptor mutants with Tyr → Phe substitutions were generated (Fig. 3A). The non-conserved tyrosine residues of mouse (Tyr-542) and human (Tyr-463) IL-23R have been included because they are part of motifs similar to the STAT binding motif (mouse Y542PNFQ, human Y463PQ) and also the potential SHP2 binding sites (mouse Y416EDI, human Y397EDI).
FIGURE 3.
Analysis of postulated STAT3 binding site within the cytoplasmic region of the murine IL-23R. A, IL-23R mutants (mouse Y426F, Y504F, Y542F, and Y626F; human Y397F, Y463F, and Y611F) were generated by site-directed mutagenesis. A scheme of the positions of the resulting tyrosine (Y) to phenylalanine (F) substitutions introduced in the wild-type IL-23R cytoplasmic domain is shown. The second receptor of the IL-23 signaling complex, IL-12Rβ1, remains unmodified. B, p409 expression vectors containing the cDNAs were co-transfected into HeLa cells as indicated. Cells were starved overnight, followed by stimulation with 0.2% HIL-23. Whole cellular lysates have been prepared, and equal amounts of proteins (50 μg/lane) were loaded onto SDS gels. Western blots were performed with antibodies specific for phospho-STAT3. Membranes were reprobed after stripping with STAT3 antibodies. Corresponding stably transduced Ba/F3-gp130 cells were washed three times with PBS and starved for 5 h, followed by stimulation with 0.2% HIL-23 for 10 min. Whole cellular lysates have been prepared and subjected to Western blot analysis as described for HeLa experiments. The presented Western blots originate from different membranes and are therefore separated by black lines. Data are representative of at least two independent experiments. C, stably transduced Ba/F3-gp130 cells were analyzed in a proliferation assay. After 48 h of stimulation with 0.2% HIL-23, proliferation of cells was measured using the colorimetric CellTiter-Blue® cell viability assay. Values represent the mean value of three repetitions and were normalized by subtraction of time point 0 values. For comparison of the independent stably transduced Ba/F3-gp130 cell lines, n-fold proliferation was calculated by setting the negative control of each Ba/F3 cell line to a value of 1. Data are representative of at least two independent experiments. Error bars, S.D.
STAT3 phosphorylation was detected for mIL-23R, the single mutant variants of mIL-23R (Y416F, Y504F, Y542F, and Y626F), the hIL-23R(mET/hC), and the mutants thereof (Y397F, Y463F, and Y611F) upon stimulation with HIL-23 in Ba/F3 and HeLa cells co-transfected with mIL-12Rβ1 (Fig. 3B). Expression of the receptor chains was verified by immunoblotting in transiently transfected HeLa cells and by flow cytometry in stably transduced Ba/F3 cells (supplemental Fig. 3, A and B). Proliferation of Ba/F3-gp130-mIL-12Rβ1-mIL-23R (and derivates thereof) and Ba/F3-gp130-mIL-12Rβ1-hIL-23R(mET/hC) (and derivates thereof) was induced by stimulation with HIL-23 (Fig. 3C). These results indicate that IL-23-mediated STAT3 activation does not rely on one single tyrosine motif within murine and human IL-23R.
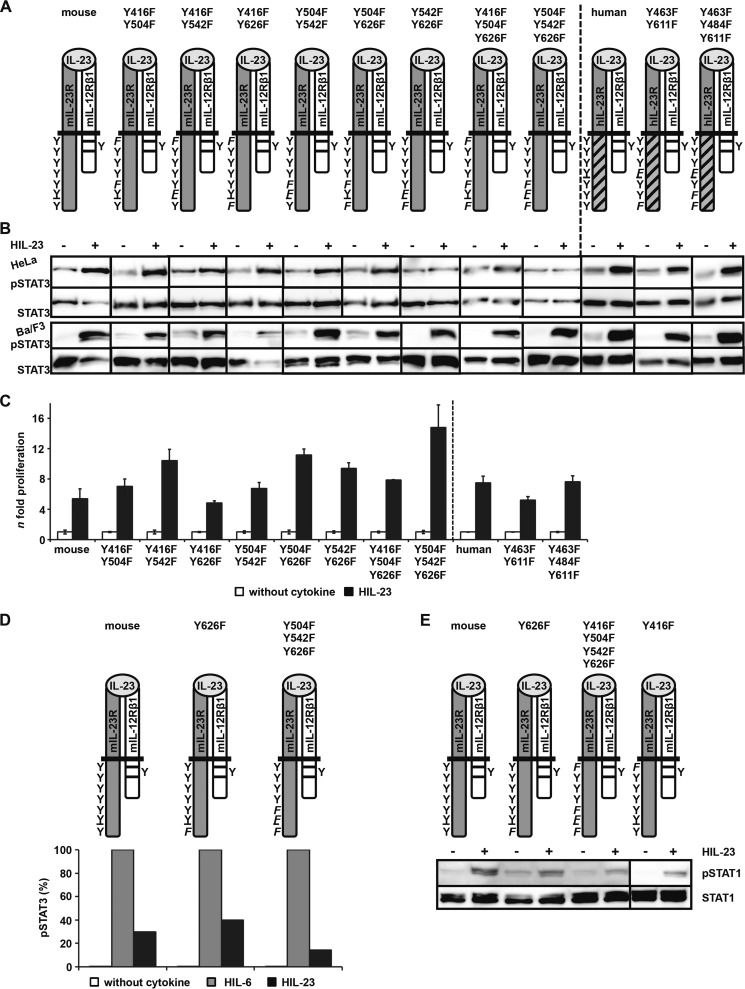
Tyr-542 and Tyr-626 in Murine IL-23R Are Important for SH2 Domain-dependent STAT3 Activation
To investigate which other Src homology 2 (SH2) domain-binding site(s) apart from Tyr-626 in murine IL-23R and the Tyr-611 motif in human IL-23R are responsible for the activation of STAT3, double and triple IL-23R Tyr → Phe mutants and combinations thereof were generated, Y416F/Y504F, Y416F/Y542F, Y416F/Y626F, Y504F/Y542F, Y504F/Y626F, Y542F/Y626F, Y416F/Y504F/Y626F, and Y504F/Y542F/Y626F for murine IL-23R and Y463F/Y611F and Y463F/Y484F/Y611F for human IL-23R in hIL-23R(mET/hC) (Fig. 4A). Expression of the receptor chains was either confirmed by immunoblotting in HeLa cells or by flow cytometry in Ba/F3 cells (supplemental Fig. 4, A and B). The mutants were analyzed after co-transfection with mIL-12Rβ1 in human HeLa cells and stimulation with HIL-23. Only the mIL-23R variants mIL-23R/Y542F/Y626F and mIL-23R/Y504F/Y542F/Y626F showed no activation of STAT3 after HIL-23 stimulation (Fig. 4B), indicating that the non-conserved and atypical SH2-binding motif surrounding tyrosine 542 in combination with the postulated STAT3 binding motif (Tyr-626) is responsible for the STAT3 phosphorylation of the murine receptor in human cells. Using murine Ba/F3 cells, analysis of the IL-23 signal transduction pathway, however, gave a different picture. After stimulation with HIL-23, phosphorylation of STAT3 and induction of cellular proliferation with apparently no differences from Ba/F3-gp130-mIL-12Rβ1-mIL-23R cells were still detected in the Ba/F3-gp130-mIL-12Rβ1-mIL-23R cell lines carrying double and triple Tyr → Phe substitutions in the intracellular domain of mIL-23R, including Ba/F3-gp130-mIL-12Rβ1-mIL-23R/Y542F/Y626F and Ba/F3-gp130-mIL-12Rβ1-mIL-23R/Y504F/Y542F/Y626F (Fig. 4, B and C). We repeated these experiments with HeLa and Ba/F3 cells several times with the same results, and we are not able to explain the differences between HeLa and Ba/F3 cells concerning STAT3 phosphorylation. One possible explanation could be that there is only a minimal phosphorylation of STAT3 in HeLa cells upon IL-23 stimulation due to the mutations of Tyr-542 and Tyr-626, which is not distinguishable from the background STAT3 activation.
FIGURE 4.
Differences in IL-23-mediated STAT3 activation in HeLa and Ba/F3-gp130 cells. A, IL-23R variants containing two or three mutations were generated by site-directed mutagenesis to define STAT3 recruitment sites within the cytoplasmic domain. B, p409 expression vectors containing the cDNAs as indicated were co-transfected into HeLa cells, and analysis was performed as described in the legend to Fig. 3B. Respective stably transduced Ba/F3-gp130 cells were generated as described and analyzed as mentioned. The presented Western blots originate from different membranes and are therefore separated by black lines. Data are representative of at least two independent experiments. C, proliferation of stably transduced Ba/F3 cells based on the colorimetric CellTiter-Blue® Cell viability assay was performed as described. Values represent the mean value of three repetitions and were normalized. For comparison of the independent stably transduced Ba/F3-gp130 cell lines, n-fold proliferation was calculated by setting the negative control of each Ba/F3 cell line to a value of 1. Data are representative of at least two independent experiments. D, IL-23R variants containing mutations of mouse Tyr-626 were washed three times with PBS and starved for 2 h in serum-free DMEM. 1 × 106 cells were stimulated for 30 min with 0.2% HIL-6 or HIL-23, harvested by centrifugation, fixed in 2% (w/v) paraformaldehyde, and permeabilized in 90% (v/v) methanol. Cells were stained for phospho-STAT3 and STAT3 overnight and analyzed by flow cytometry. E, stably transduced Ba/F3 cells were washed three times, starved for 4 h, and stimulated with 0.2% HIL-23 for 60 min. Cellular lysates were prepared, and equal amounts of proteins (50 μg/lane) were loaded onto SDS gels. Western blots were performed using antibodies specific for phospho-STAT1 and STAT1. Western blot data are shown for one representative experiment. Error bars, S.D.
To assess STAT3 activation more quantitatively, intracellular staining of STAT3 and phospho-STAT3 was performed using Ba/F3-gp130-mIL-12Rβ1-mIL-23R/Y626F, Ba/F3-gp130-mIL-12Rβ1-mIL-23R/Y504F/Y542F/Y626F, and Ba/F3-gp130-mIL-12Rβ1-mIL-23R cells (Fig. 4D). Mutation of the predicted binding site Tyr-626 did not reduce phosphorylation of STAT3, but Tyr → Phe substitutions of both potential STAT binding sites (Tyr-504 and Tyr-626) and the non-conserved tyrosine residue Tyr-542 resulted in a major reduction of STAT3 phosphorylation.
As seen for the murine IL-23R, the appropriate double and triple mutants of the hIL-23R(mET/hC) (Y463F/Y611F and Y463F/Y484F/Y611F) clearly led to phosphorylation of STAT3 and proliferation of Ba/F3-gp130-mIL-12Rβ1-hIL-23R(mET/hC) cells upon stimulation with HIL-23 (Fig. 4, A and B). Contrary to the mIL-23R, stimulation of HeLa cells co-transfected with mIL-12Rβ1 and the double or triple mutant of hIL-23R(mET/hC) with HIL-23 still led to STAT3 phosphorylation (supplemental Fig. 4B). Because IL-23R that did not carry any SH2-binding motif was still able to induce STAT3 phosphorylation, we were not able to determine which tyrosine within the human IL-23R was responsible for STAT3 phosphorylation in this experiment. We could, however, conclude that an additional non-canonical activation mechanism other than classical and atypical SH2-binding sites within the murine and human IL-23R must induce STAT3 phosphorylation after IL-23 stimulation.
Besides STAT3, IL-23 stimulation led to phosphorylation of STAT1 (23). To analyze whether the non-canonical activation of STAT3 also accounts for STAT1, we used Ba/F3-gp130-mIL-12Rβ1-mIL-23R(mutant) cells, with Y416F, Y626F, or Y416F/Y504F/Y542F/Y626F (Fig. 4E). Here, IL-23-induced phosphorylation was only detectable in Ba/F3-gp130-mIL-12Rβ1-mIL-23R and Ba/F3-gp130-mIL-12Rβ1-mIL-23R/Y416F cells and only weakly in Ba/F3-gp130-mIL-12Rβ1-IL-23R/Y626F and Ba/F3-gp130-mIL-12Rβ1-IL-23R/Y416F/Y504F/Y542F/Y626F cells, indicating that a canonical tyrosine-dependent SH2-binding site is needed for phosphorylation of STAT1 in IL-23 signal transduction.
Phospho-STAT3-independent Cellular Proliferation of IL-23-responsive Ba/F3 Cells Is Dependent on PI3K/Akt and Erk1/2 Phosphorylation
To identify the tyrosine-independent STAT3-phosphorylation site, to further identify tyrosine residues within SH2 domains needed for STAT3 phosphorylation, and to exclude the involvement of the three tyrosine residues that are not found in SH2 domain-binding sites of the IL-23R (mouse Tyr-448, Tyr-469, and Tyr-496; human Tyr-429, Tyr-450, and Tyr-476), we cloned intracellular region deletion variants of the mIL-23R, which were truncated at positions 415 (Δ415), 434 (Δ434, plus Y416F), 503 (Δ503), and 503 (Δ503, plus Y416F) (Fig. 5A). Tyr-416 was predicted to mediate SHP2-binding and MAPK and PI3K activation but might also affect the binding of JAKs, because this tyrosine is located in a putative JAK2 binding site (38). Therefore, MAPK and PI3K activation via Tyr-416 was analyzed. The smallest deletion was mIL-23R/Δ503, which included the non-SH2-binding domain tyrosines (Tyr-448, Tyr-469, and Tyr-496) but lacked the SH2-binding domain tyrosines (Tyr-504, Tyr-542, and Tyr-626). Additionally, we generated mIL-23R/Y416F/Y504F/Y542F/Y626F, with mutations in all tyrosine residues predicted to be involved in any signal transduction plus Tyr-542, found in this study to mediate STAT3 phosphorylation (Fig. 5A). Expression of the receptor chains in transiently co-transfected HeLa cells and stably transduced Ba/F3 cells was visualized by Western blot and flow cytometry, respectively (Fig. 5B and supplemental Fig. 5A). IL-23-induced STAT3 phosphorylation of all deletion variants was abrogated in HeLa and Ba/F3 cells, indicating that the non-SH2 domain tyrosines (Tyr-448, Tyr-469, and Tyr-496) were not needed for STAT3 phosphorylation and that an additional motif located C-terminally from amino acid residue 503 within the mIL-23R intracellular region apart from the SH2-binding domain tyrosines is involved in STAT3 phosphorylation (Fig. 5B). The deletion variants mIL-23R/Δ415, mIL-23R/Y416F-Δ434, and mIL-23R/Y416F-Δ503 failed to induce proliferation of the respective Ba/F3 cells dependent of HIL-23 (Fig. 5C). Furthermore, Ba/F3-gp130-mIL12Rβ1-mIL-23R/Δ503 cells did not show any obvious differences in IL-23-induced proliferation compared with Ba/F3-gp130-mIL-12Rβ1-mIL-23R cells.
FIGURE 5.
STAT3 activation is independent of tyrosine residues and not sufficient for cell proliferation. A, deletion variants of the murine IL-23R were generated by PCR. The first amino acid of the missing part of the IL-23R cytoplasmic domain is mentioned (e.g. Δ415). The postulated SHP2 binding site Tyr-416 or Tyr-397 was deleted, and a mutant variant with four Tyr → Phe substitutions was made. B, respective stably transduced Ba/F3-gp130 cells were generated as described and analyzed as mentioned. The Western blot data derived from different membranes and are therefore separated by black lines. Data are representative of at least two independent experiments. C, the colorimetric CellTiter-Blue® cell viability assay was used to determine the proliferation of the stably transduced Ba/F3-gp130 cell lines as mentioned. Values represent the mean value of three repetitions and were normalized. For comparison, n-fold proliferation was calculated by setting the negative control of each Ba/F3 cell line to a value of 1. Data are representative of at least two independent experiments. Error bars, S.D.
Next we analyzed Ba/F3-gp130-mIL-12Rβ1 cell lines stably transduced with deletion variants of the hIL-23R(mET/hC), including hIL-23R(mET/hC)-Δ476 lacking the SH2-binding site tyrosines Tyr-484 and Tyr-611 but not the Tyr-463, hIL-23R(mET/hC)/Y463F-Δ476 and hIL-23R(mET/hC)/Y397F/Y463F-Δ476. Surface expression of the IL-23 receptors was verified by flow cytometry in stably transduced Ba/F3 cells (supplemental Fig. 5B). Ba/F3-gp130-mIL-12Rβ1 cells stably transduced with any of these deleted and mutated IL-23R chains failed to induce STAT3 phosphorylation after stimulation with HIL-23, indicating that Tyr-463 and Tyr-397 were not involved in STAT3 phosphorylation and that also in the IL-23R an additional site C-terminal from amino acid residue 476 but distinct from Tyr-484 and Tyr-611 must be involved in STAT3 phosphorylation. Although Ba/F3-gp130-mIL-12Rβ1-hIL-23R(mET/hC)-Δ476 cells did not exhibit STAT3 phosphorylation, IL-23-induced proliferation was comparable with Ba/F3-gp130-mIL-12Rβ1-hIL-23R(mET/hC) cells. Additional mutation of Tyr-397 was sufficient to abrogate IL-23-induced proliferation, again indicating that MAPK/PI3K activation was needed and sufficient for cellular proliferation of Ba/F3 cells (Fig. 5C).
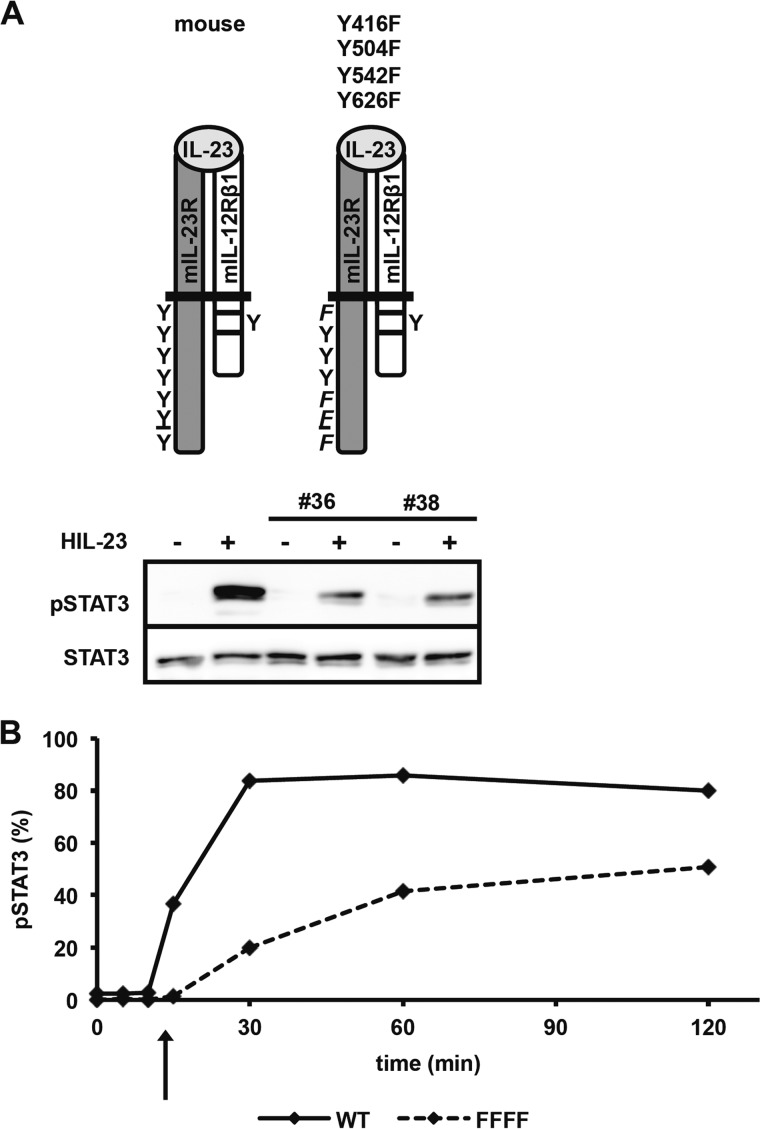
As an additional control, IL-23-induced STAT3 phosphorylation was also detected in Ba/F3-gp130 cells stably transduced with mIL-12Rβ1 and mIL-23R/Y416F/Y504F/Y542F/Y626F but with an apparently lower STAT3 phosphorylation compared with Ba/F3-gp130-mIL-12Rβ1-mIL-23R cells (Fig. 5B). To exclude a cell line-specific effect of these observations, we investigated activation of STAT3 upon HIL-23 stimulation in two independently generated stably transduced Ba/F3-gp130 cell lines (clones 36 and 38) by immunoblotting of phosphorylated and unphosphorylated STAT3 (Fig. 6A). Furthermore, differences in the kinetics of STAT3 phosphorylation in Ba/F3-gp130-mIL-12Rβ1 cells expressing the mIL-23R or the mutant mIL-23R variant (Y416F/Y504F/Y542F/Y626F) were detected by intracellular STAT3 staining, indicating that STAT3 phosphorylation is lower for the mIL-23R variant (Y416F/Y504F/Y542F/Y626F) (Fig. 6B). Because Tyr-416 is not directly involved in STAT3 phosphorylation but is located in a putative JAK2 binding site (38), this might indicate that Tyr-416 is needed for efficient binding of JAKs to the mIL-23R chain. However, the involvement of Tyr-416 in JAK binding has to be analyzed in future studies.
FIGURE 6.
Tyrosine phenylalanine substitutions resulted in altered kinetics of STAT3 activation. A, two independent stable transduced Ba/F3-gp130 cell lines (36 and 38) containing the IL-12Rβ1 and mutated IL-23R (Y426F/Y504F/Y542F/Y626F) cDNA were starved in serum-free medium and subsequently stimulated with 0.2% HIL-23 for 10 min. Cells were harvested, and whole cell lysates were prepared and analyzed by Western blot using antibodies specific against phospho-STAT3 and STAT3. B, STAT3 activation was evaluated by intracellular staining. Ba/F3-gp130 cells and the FFFF Ba/F3 cell line containing mutated IL-23R (Y426F/Y504F/Y542F/Y626F) were washed three times with PBS and starved in serum-free DMEM. 1 × 106 cells were stimulated for the times indicated with 0.2% HIL-23, harvested by centrifugation, fixed in 2% (w/v) paraformaldehyde, permeabilized in 90% (v/v) methanol, stained, and analyzed by flow cytometry.
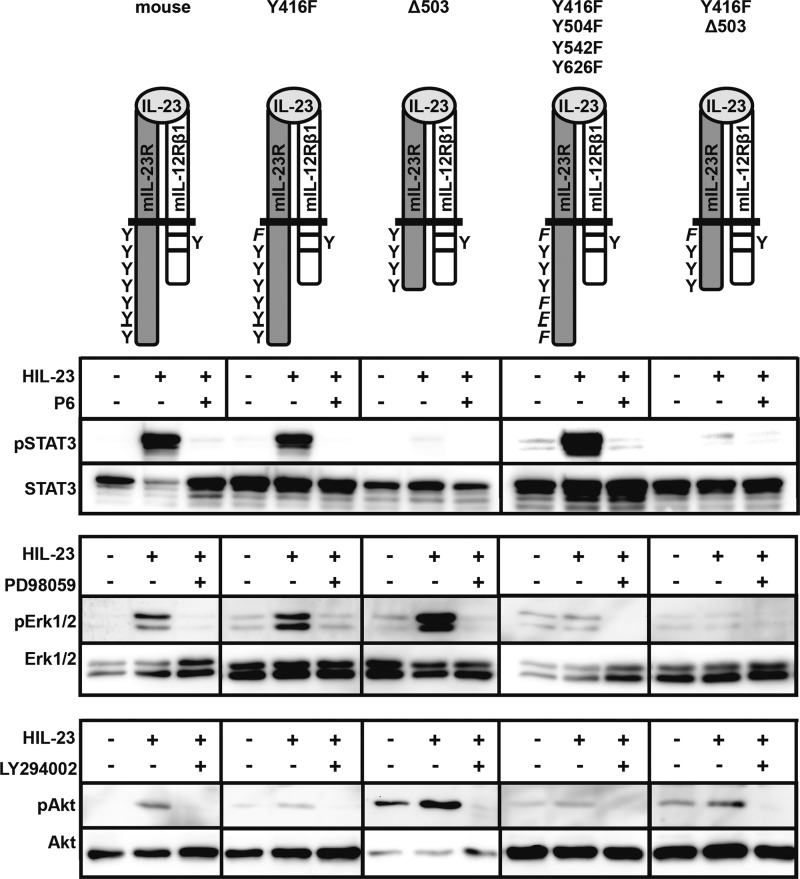
Our data indicate that STAT3 phosphorylation is not mandatory for IL-23-induced proliferation of Ba/F3 cells. Accordingly, Ba/F3-gp130-mIL-12Rβ1 cells stably transduced with mIL-23R, mIL-23R/Y416F, mIL-23R-Δ503, mIL-23R/Y416F/Y504F/Y542F/Y626F, or mIL-23R/Y416F-Δ503 were stimulated with HIL-23 and analyzed for Erk1/2 and PI3K/Akt phosphorylation. HIL-23 increases the phosphorylation of Erk1/2 and Akt in Ba/F3-gp130-IL-12Rβ1-mIL-23R and Ba/F3-gp130-IL-12Rβ1-mIL-23R-Δ503 but not in Ba/F3-gp130-IL-12Rβ1-mIL-23R/Y416F/Y504F/Y542F/Y626F cells (Fig. 7). Erk phosphorylation was also detected in Ba/F3-gp130-IL-12Rβ1-mIL-23R/Y416F but not in Ba/F3-gp130-IL-12Rβ1-mIL-23R/Y416F-Δ503 cells, indicating that the predicted SHP2 binding site Y416EDI and an additional motif within the C-terminal part of the IL-23R are needed for phosphorylation of Erk1/2. In contrast, Tyr-416 seems to be responsible for activation of PI3K/Akt because only Ba/F3-gp130-mIL-12Rβ1 cells containing non-mutated Tyr-416 showed phosphorylated Akt (Ba/F3-gp130-IL-12Rβ1-mIL-23R, Ba/F3-gp130-IL-12Rβ1-mIL-23R-Δ503). Phosphorylation of Erk1/2 and PI3K/Akt was clearly suppressed by pretreatment with MEK inhibitor PD98059 or PI3K inhibitor LY294002 (Fig. 7). For control of Erk and Akt stimulation assays, appropriate Ba/F3 cell lines were further analyzed according to STAT3 activation in the absence or presence of Jak inhibitor P6. Comparable Western blot data were obtained for mIL-23R-Δ415 containing no tyrosine residue within the IL-23R (data not shown). Our results imply that proliferation of Ba/F3-gp130-mIL-12Rβ1-mIL-23R (mutant) cells that fail to induce STAT3 phosphorylation after HIL-23 stimulation, such as mIL-23R-Δ503, is maintained by activation of Erk1/2 and PI3K/Akt.
FIGURE 7.
IL-23-mediated proliferation of stably transduced Ba/F3-gp130 cells involves PI3K/Akt activation. The indicated Ba/F3 cell lines were washed three times with PBS, starved for 4 h in serum-free medium, and pretreated for 60 min with P6 (1 μm), PD98059 (20 μm), or LY294002 (20 μm) before exposure to HIL-23 (0.2%) for 1 h. Cell lysates were analyzed for STAT3, Erk1/2, and Akt by Western blot analysis of total and phosphorylated STAT3, Erk1/2, and phosphorylated Akt using specific Abs. A representative example of two separate experiments is shown.
Identification of a Non-canonical STAT3 Activation Motif in the Intracellular Domain of the IL-23R
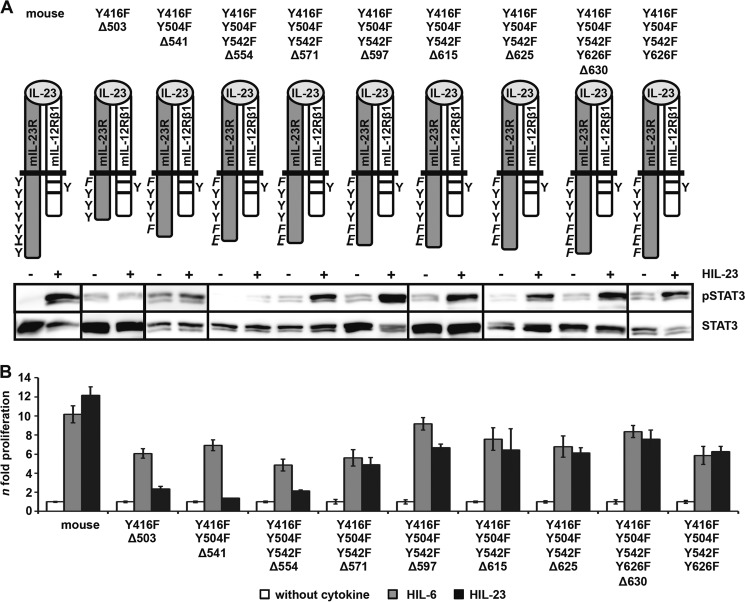
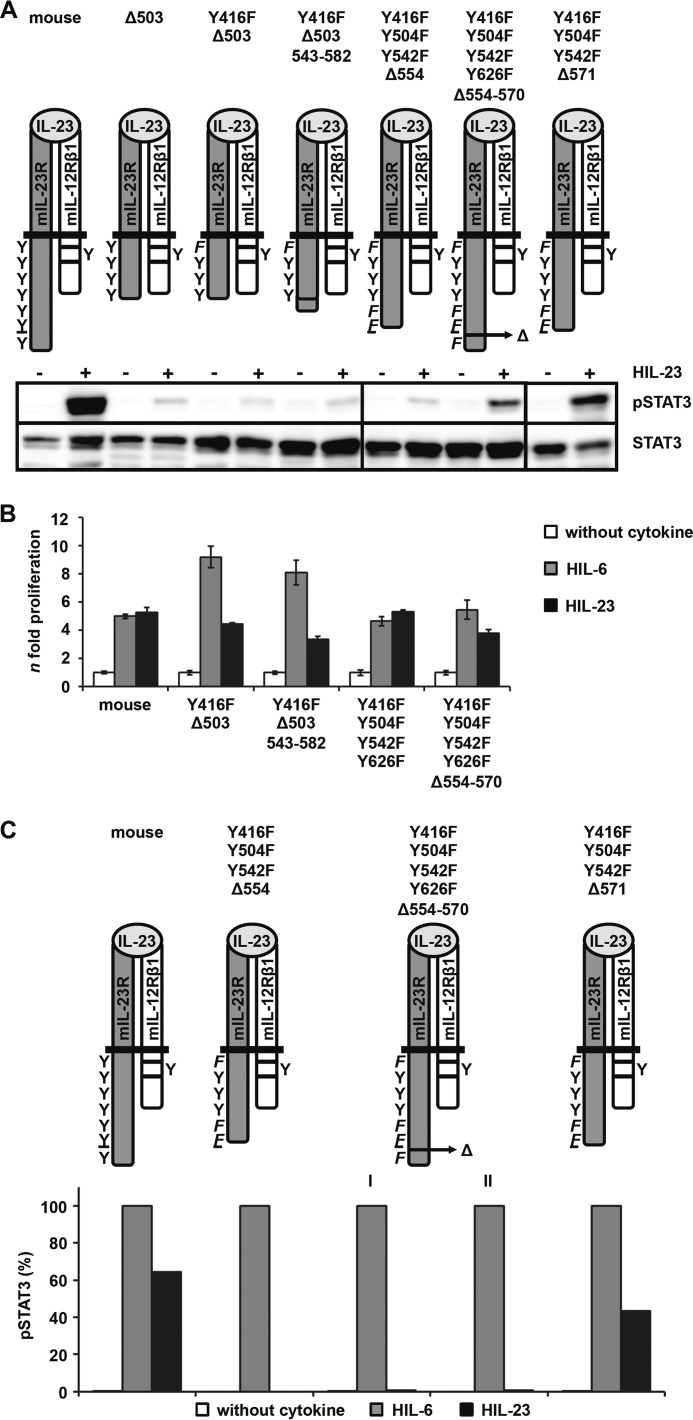
To identify the non-canonical motif within the cytoplasmic domain of the IL-23R, which is responsible for the SH2-binding site-independent STAT3 phosphorylation, we generated C-terminally truncated mIL-23R proteins with mIL-23R/Y416F/Y504F/Y542F/Y626F as backbone, resulting in the deletion variants mIL-23R/Y416F/Y504F/Y542F/Y626F-Δ630, mIL-23R/Y416F/Y504F/Y542F-Δ625, mIL-23R/Y416F/Y504F/Y542F-Δ615, mIL-23R-Y416F/Y504F/Y542F-Δ597, mIL-23R/Y416F/Y504F/Y542F-Δ571, mIL-23R/Y416F/Y504F/Y542F-Δ554, and mIL-23R/Y416F/Y504F-Δ541 (Fig. 8A). The deletion variant mIL-23R/Y416F-Δ503 was also included. All mIL-23R deletion variants were expressed on the cell surface of stably transduced Ba/F3-gp130-mIL-12Rβ1-mIL-23R cells (supplemental Fig. 6A). No IL-23-induced STAT3 phosphorylation was detected for the mIL-23R variants mIL-23R/Y416F/Y504F-Δ541 and mIL-23R/Y416F-Y504F/Y542F-Δ554, whereas phosphorylation of STAT3 was rescued for the mIL-23R variants from mIL-23R/Y416F/Y504F/Y542F-Δ571 to mIL-23R/Y416F/Y504F/Y542F/Y626F-Δ630 (Fig. 8A). To clearly detect STAT3 phosphorylation in mIL-23R variants carrying the Y416F mutation, the Western blots were overexposed compared with the mIL-23R and the STAT3 phosphorylation intensity of the mIL-23R/Y416F variant shown in Fig. 5. Furthermore, whereas Ba/F3-gp130-mIL-12Rβ1-mIL-23R/Y416F/Y504F/Y542F-Δ571 cells still proliferated dependent on HIL-23, Ba/F3-gp130-mIL-12Rβ1-mIL-23R/Y416F/Y504F/Y542F-Δ554 showed no IL-23-dependent proliferation. However, proliferation of all cell lines was inducible by HIL-6 (Fig. 8B). Analysis of STAT3 phosphorylation and cellular proliferation of the different mIL-23R deletion variants revealed a motif of 17 amino acids, which is hypothesized to be involved in non-canonical phosphorylation of STAT3 independently of SH2-binding site proteins (supplemental Fig. 6B). Interestingly, this motif is highly conserved in IL-23 receptors in mammalian species (supplemental Fig. 6B). Additional mIL-23R mutants either containing the 554–570 motif fused to non-STAT3 activating deletion variant mIL-23R/Y416F-Δ503 (Y416F-Δ503+543-582) or lacking the 17-amino acid motif (Y416F/Y504F/Y542F/Y626F-Δ554–570) were generated for further characterization (Fig. 9A). Cell surface expression of the receptor chains was verified by flow cytometry (supplemental Fig. 6C). Deletion of these 17 amino acids led to an almost complete disappearance of STAT3 phosphorylation upon IL-23 stimulation. A faint band for pSTAT3 was, however, detected by chemiluminescence in Ba/F3-gp130-mIL-12Rβ1-mIL-23R/Y416F/Y504F/Y542F/Y626F-Δ554–570 cells. Deletion variants lacking the 17 amino acids (Y416F/Y504F/Y542F-Δ554) as well as the variant containing the motif (Y416F/Y504F/Y542F-Δ571) were included as controls (Fig. 9A). Furthermore, the 17-amino acid deletion variant proliferated in the presence of IL-23 (Fig. 9B). Therefore, we independently quantified STAT3 phosphorylation by flow cytometry. Surprisingly, quantitative intracellular staining by flow cytometry revealed no activation of STAT3 upon IL-23 stimulation for Ba/F3-gp130-mIL-12Rβ1-mIL-23R/Y416F/Y504F/Y542F/Y626F-Δ554–570 cells (Fig. 9C). The deletion variants Y416F/Y504F/Y542F-Δ554 and Y416F/Y504F/Y542F-Δ571 were included as controls, and two different passages (medium containing either HIL-6 (I) or HIL-23 (II)) of Y416F/Y504F/Y542F/Y626F-Δ554–570 cells have been investigated. These data support our hypothesis that the 17-amino acid motif within the C terminus of murine IL-23R is important for STAT3 activation. However, the amino acid motif is not sufficient to mediate STAT3 activation because adding to the deletion variant Y416F-Δ503 did not induce activation of STAT3 upon IL-23 stimulation. Proliferation of Ba/F3 cell Y416F-Δ503+543-582 was comparable with the non-STAT3 activating deletion variant (Y416F-Δ503) (Fig. 9B). Proliferation of cell lines was inducible by HIL-6 but with different intensity. Values for deletion variants Y416F-Δ503 and Y416F-Δ503+543-582 were ∼4-fold higher than WT and Y416F/Y504F/Y542F/Y626F as well as Y416F/Y504F/Y542F/Y626F-Δ554–570 cells. Accordingly, direct comparison of IL-23-mediated proliferation of these cell lines is not possible.
FIGURE 8.
A part of the C-terminal domain of IL-23R is sufficient for STAT3 activation. A, a series of deletion variants starting from Y416F-Δ503 to the mutant Y426F/Y504F/Y542F/Y626F were generated and stably transduced into Ba/F3-gp130 cells containing the IL-12Rβ1. Resulting Ba/F3 cell lines were washed three times with PBS, starved, and stimulated for 10 min with 0.2% HIL-23. Equal amounts of protein were loaded onto SDS gels. Western blotting was performed using antibodies specific for phospho-STAT3 and STAT3. Data are representative for at least two experiments. Presented Western blots originate from different membranes and are therefore separated by black lines. B, equal numbers of stably transduced Ba/F3 cells were cultured for 2 days in the presence of 0.2% HIL-6 or 0.2% HIL-23 or without cytokine. Proliferation was measured with the colorimetric CellTiter-Blue® cell viability assay. Values represent the mean value of three repetitions and were normalized. For comparison, n-fold proliferation was calculated by setting the negative control of each Ba/F3 cell line to a value of 1. Data are representative of at least two independent experiments. Error bars, S.D.
FIGURE 9.
Characterization of the non-canonical STAT3 activation motif in the intracellular domain of IL-23R. A, two IL-23R variants either with a deletion of 17 amino acids (Y416F/Y504F/Y542F/Y626F-Δ554–570) or with the addition of the respective motif (Y416F-Δ503+543-582) were generated and stably transduced into Ba/F3-gp130-mIL-12Rβ1 cells. Resulting Ba/F3 cell lines were washed three times with PBS, starved, and stimulated for 10 min with IL-23. Equal amounts of protein were loaded onto SDS gels. Western blotting was performed using antibodies specific for phospho-STAT3 and STAT3. Data are representative for two experiments. The presented Western blots originate from different membranes and are therefore separated by black lines. B, equal numbers of stably transduced Ba/F3 cells were cultured for 2 days in the presence of 0.2% HIL-6 or 0.2% HIL-23 or without cytokine. Proliferation was measured with the colorimetric CellTiter-Blue® cell viability assay. Values represent the mean value of three repetitions and were normalized. For comparison, n-fold proliferation was calculated by setting the negative control of each Ba/F3 cell line to a value of 1. Data are representative of at least two independent experiments. C, IL-23R variants were washed three times with PBS and starved for 2 h in serum-free DMEM. 1 × 106 cells were stimulated for 30 min with 0.2% HIL-6 or HIL-23, harvested by centrifugation, fixed in 2% (w/v) paraformaldehyde, and permeabilized in 90% (v/v) methanol. Cells were stained for phospho-STAT3 and STAT3 overnight and analyzed by flow cytometry. Two Ba/F3-gp130-mIL-12Rβ1-mIL-23R/Y416F/Y504F/Y542F/Y626F-Δ554–570 cell passages were analyzed, cultivated in the presence of either HIL-6 (I) or HIL-23 (II). Error bars, S.D.
DISCUSSION
Receptors that activate STAT3 are characterized by one or more YXXQ motifs (39, 40). Mutation of the tyrosine or glutamine residue of this consensus sequence from receptors such as gp130 and IL-9R abrogated canonical STAT3 recruitment and phosphorylation, demonstrating that the recognition by the SH2 domain of STAT3 is critical for its activation (40, 41). Here, we have identified the tyrosine-dependent canonical and tyrosine-independent non-canonical STAT3 activation sites within the murine and human IL-23R. Our study reveals three major findings. First, the tyrosine Ym416/h397EDI motif is involved in the activation of MAPK/PI3K pathway. Second, the Ym626FPQ motif was confirmed as the canonical STAT3 binding site in murine IL-23R, and we identified an additional STAT3 binding site, which slightly deviates from the consensus SH2-binding site YXXQ, because it contains three instead of two amino acids between the tyrosine and the glutamine (YXXXQ). This motif is only found in murine IL-23R (YPNFQ) and not in the rat (YPNFT) and other organisms and accordingly also not in the human IL-23R (HPNFA) (supplemental Fig. 6B). To the best of our knowledge, it has not been described so far that an SH2-binding site can contain three amino acids between Tyr and Gln. Third, we identified an additional motif between amino acid residues 554 and 570 in the murine IL-23R that facilitates non-canonical tyrosine-independent STAT3 phosphorylation after IL-23 stimulation. Deletion of this 17-amino acid motif depleted STAT3 phosphorylation, as was demonstrated by immunoblotting and intracellular staining. However, complete abrogation of STAT3 activation was only shown by flow cytometry. Ba/F3 cells containing four Tyr → Phe mutations and the deletion of the non-canonical tyrosine-independent motif still proliferated in the presence of IL-23, perhaps indicating that IL-23 signal transduction is a complex network not only involving Jak/STAT, MAPK, and PI3K/Akt. Previous studies also revealed the involvement of NF-κB in IL-23 signaling (24). Our experiments and homology alignments indicate that this motif is conserved and accordingly also present in the human IL-23R. Protein motif analysis did not lead to the identification of a general motif between positions 553 and 571 (plus 10 amino acid residues surrounding this core sequence). Further, we failed to detect any apparent amino acid sequence identity between the identified 17-amino acid residue sequence, needed to mediate tyrosine-independent activation of STAT3, and other cytokine receptors. Two amino acid residues N-terminal of position 554 (Leu), a putative CK1 phosphorylation site (S548ASS) was identified, which is highly conserved within the IL-23Rs of all species analyzed. However, this site is N-terminal from the deletion Δ554 and therefore not likely to be involved in STAT3 activation. The related CK2 has recently been described to be mandatory for STAT signaling of oncostatin M (OSM) (42), which is an IL-6 type cytokine. A general involvement of CK2 in signaling of the IL-6/IL-12 type cytokine family cannot not be excluded at the moment. Thus far, CK1 has not been described as being involved in STAT3 signaling. Moreover, the motif for CK1 (SXX(S/T)) is frequently observed in proteins, and putative CK1 sites do not necessarily represent real target sites. Further, it was suggested that PI3K/Akt may be involved in STAT3 phosphorylation (24). However, we showed that the PI3K/Akt-mediated STAT3 activation is not related to the here described non-canonical tyrosine-independent STAT3 phosphorylation, because this also occurs in a mutant (Y416F/Y504F/Y542F/Y626F) that was not able to activate PI3K/Akt after IL-23 stimulation, and stimulation of Ba/F3-gp130-mIL12Rβ1-mIL-23R cells with IL-23 in the presence of the PI3K inhibitor LY294002 did not deplete STAT3 phosphorylation (data not shown).
Tyrosine-/SH2-independent STAT activation has been reported previously for the granulocyte colony-stimulating factor receptor (G-CSFR; STAT3) (43), IL-22 receptor (IL-22R; STAT3) (44), and interferon-α/β receptor β-chain (IFNAR2; STAT2) (45), and it was speculated that other cytokine receptors may use a similar mode of STAT3 recruitment (44). IL-23R is the first member of the gp130 type receptor family that possesses such a non-canonical STAT3 activation modus.
In the case of the G-CSFR, a mutant lacking all intracellular tyrosines was shown to recruit and activate STAT3 via an unknown mechanism, albeit at higher concentrations of G-CSF compared with tyrosine-dependent activation (43). The authors did not detect a constitutive association of STAT3 with the G-CSFR. They speculate that an intermediate molecule, interacting with the C-terminal region of the receptor, contains a phosphotyrosine binding site for the SH2 domain of STAT3 (43). In the case of IL-23R, we were also not able to co-immunoprecipitate STAT3 and IL-23R (data not shown), indicating that STAT3 and IL-23R are, as in the case of G-CSFR, not constitutively associated. Recently, Dumoutier et al. (44) demonstrated that the C-terminally located 84 amino acid residues of IL-22R contain a thus far undefined motif, which allows constitutive association with STAT3, most likely via the coiled-coil domain of STAT3. Importantly, IL-22-induced tyrosine-independent activation of STAT3 is facilitated by this 84-amino acid sequence, and mutation of all cytoplasmic tyrosine residues of the IL-22R only partially affects STAT3 activation. The authors speculated that receptor preassociation with STAT3 assures a faster response or efficient STAT3 activation in cells with lower endogenous STAT3 expression. Association of STAT3 with IL-23R to enable fast STAT3 activation is also not supported by our finding that IL-23-induced activation of STAT3 is slow compared with IL-6-induced activation of STAT3 via gp130. Therefore, we assume that binding of STAT3 to the receptor for both canonical and non-canonical STAT3 activation is cytokine-induced. However, the detailed mechanism has to be elaborated in further studies. SH2-independent recruitment of STAT3 might serve to avoid negative feedback by proteins such as suppressor of cytokine signaling 3 (SOCS3), which can compete with STAT factors for phosphotyrosines (46). IL-6-gp130-STAT3 activation is rapidly switched off by SOCS3-negative feedback regulation (47). We and others showed that IL-23-induced STAT3 activation is not switched off (48), suggesting that SOCS3 is not a negative regulator of IL-23 signaling. However, these data were obtained after overexpression of the IL-23R. Chen et al. (49) reported that SOCS3 negatively regulates IL-23 signaling during TH17 development in primary T cells. Therefore, it remains to be seen whether the non-canonical STAT3 activation plays a role in escape from negative SOCS3 feedback inhibition. STAT3 plays a major role during differentiation and proliferation of TH17 cells. The frequency of TH17 cells was reduced in experimental autoimmune encephalomyelitis (EAE)-resistant mice with a conditional deletion of STAT3 (50, 51), and deletion of SOCS3 increased the number of TH17 cells (49). It has been demonstrated that IL-6 is needed for STAT3 activation during the initial but not late phase of TH17 differentiation and not for TH17 proliferation (7). It might be speculated that the rapid SOCS3-mediated negative feedback down-regulation of IL-6-mediated STAT3 activation provokes the need for second line cytokines such as IL-23 to ensure prolonged STAT3 activation during late phase of TH17 differentiation and proliferation that is not completely negatively regulated by SOCS proteins. Sustained STAT3 activation can contribute to tumor development (52), and the IL-6/gp130 signaling pathway is a candidate for constitutive STAT3 activation in tumors (53). Therefore, STAT3 activation has to be strictly regulated, which might not be properly ensured by IL-6 signaling because the responsible receptors are more commonly expressed. IL-23R expression is very limited and not found, for example, on naive T cells. However, during differentiation of T cells to TH17 cells, these cells start to express IL-23R and become a target for IL-23 to enable target-oriented and sustained activation of STAT3. However, it remains to be seen whether the non-canonical STAT3 activation motif is needed for efficient TH17 differentiation.
Supplementary Material
Acknowledgments
We thank Meike Preuten and Ines Mancinella for excellent technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft (Bonn, Germany) Grants SCHE 907/2-1 and by Forschungskommission of Heinrich-Heine-University Düsseldorf (Medical Faculty) Grant 08/2011.

This article contains supplemental Figs. 1–6.
- SH2
- Src homology 2
- mIL
- mouse IL
- hIL
- human IL
- HIL-6 and -23
- Hyper-IL-6 and -23, respectively
- PE
- phycoerythrin
- G-CSF
- granulocyte colony-stimulating factor
- G-CSFR
- G-CSF receptor.
REFERENCES
- 1. Vignali D. A., Kuchroo V. (2012) IL-12 family cytokines. Immunological playmakers. Nat. Immunol. 13, 722–728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Jones L. L., Vignali D. A. (2011) Molecular interactions within the IL-6/IL-12 cytokine/receptor superfamily. Immunol. Res. 51, 5–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kobayashi M., Fitz L., Ryan M., Hewick R. M., Clark S. C., Chan S., Loudon R., Sherman F., Perussia B., Trinchieri G. (1989) Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 170, 827–845 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oppmann B., Lesley R., Blom B., Timans J. C., Xu Y., Hunte B., Vega F., Yu N., Wang J., Singh K., Zonin F., Vaisberg E., Churakova T., Liu M., Gorman D., Wagner J., Zurawski S., Liu Y., Abrams J. S., Moore K. W., Rennick D., de Waal-Malefyt R., Hannum C., Bazan J. F., Kastelein R. A. (2000) Novel p19 protein engages IL-12p40 to form a cytokine, IL-23, with biological activities similar as well as distinct from IL-12. Immunity 13, 715–725 [DOI] [PubMed] [Google Scholar]
- 5. Pflanz S., Timans J. C., Cheung J., Rosales R., Kanzler H., Gilbert J., Hibbert L., Churakova T., Travis M., Vaisberg E., Blumenschein W. M., Mattson J. D., Wagner J. L., To W., Zurawski S., McClanahan T. K., Gorman D. M., Bazan J. F., de Waal Malefyt R., Rennick D., Kastelein R. A. (2002) IL-27, a heterodimeric cytokine composed of EBI3 and p28 protein, induces proliferation of naive CD4+ T cells. Immunity 16, 779–790 [DOI] [PubMed] [Google Scholar]
- 6. Collison L. W., Workman C. J., Kuo T. T., Boyd K., Wang Y., Vignali K. M., Cross R., Sehy D., Blumberg R. S., Vignali D. A. (2007) The inhibitory cytokine IL-35 contributes to regulatory T-cell function. Nature 450, 566–569 [DOI] [PubMed] [Google Scholar]
- 7. McGeachy M. J., Chen Y., Tato C. M., Laurence A., Joyce-Shaikh B., Blumenschein W. M., McClanahan T. K., O'Shea J. J., Cua D. J. (2009) The interleukin 23 receptor is essential for the terminal differentiation of interleukin 17-producing effector T helper cells in vivo. Nat. Immunol. 10, 314–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bettelli E., Oukka M., Kuchroo V. (2007) TH-17 cells in the circle of immunity and autoimmunity. Nat. Immunol. 8, 345–350 [DOI] [PubMed] [Google Scholar]
- 9. Cua D. J., Sherlock J., Chen Y., Murphy C. A., Joyce B., Seymour B., Lucian L., To W., Kwan S., Churakova T., Zurawski S., Wiekowski M., Lira S. A., Gorman D., Kastelein R. A., Sedgwick J. D. (2003) Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature 421, 744–748 [DOI] [PubMed] [Google Scholar]
- 10. Murphy C. A., Langrish C. L., Chen Y., Blumenschein W., McClanahan T., Kastelein R. A., Sedgwick J. D., Cua D. J. (2003) Divergent pro- and antiinflammatory roles for IL-23 and IL-12 in joint autoimmune inflammation. J. Exp. Med. 198, 1951–1957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hue S., Ahern P., Buonocore S., Kullberg M. C., Cua D. J., McKenzie B. S., Powrie F., Maloy K. J. (2006) Interleukin-23 drives innate and T cell-mediated intestinal inflammation. J. Exp. Med. 203, 2473–2483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kullberg M. C., Jankovic D., Feng C. G., Hue S., Gorelick P. L., McKenzie B. S., Cua D. J., Powrie F., Cheever A. W., Maloy K. J., Sher A. (2006) IL-23 plays a key role in Helicobacter hepaticus-induced T cell-dependent colitis. J. Exp. Med. 203, 2485–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mannon P. J., Fuss I. J., Mayer L., Elson C. O., Sandborn W. J., Present D., Dolin B., Goodman N., Groden C., Hornung R. L., Quezado M., Yang Z., Neurath M. F., Salfeld J., Veldman G. M., Schwertschlag U., Strober W., and Anti-IL-12 Crohn's Disease Study Group (2004) Anti-interleukin-12 antibody for active Crohn's disease. N. Engl. J. Med. 351, 2069–2079 [DOI] [PubMed] [Google Scholar]
- 14. Sandborn W. J., Feagan B. G., Fedorak R. N., Scherl E., Fleisher M. R., Katz S., Johanns J., Blank M., Rutgeerts P., and Ustekinumab Crohn's Disease Study Group (2008) A randomized trial of Ustekinumab, a human interleukin-12/23 monoclonal antibody, in patients with moderate-to-severe Crohn's disease. Gastroenterology 135, 1130–1141 [DOI] [PubMed] [Google Scholar]
- 15. Langowski J. L., Zhang X., Wu L., Mattson J. D., Chen T., Smith K., Basham B., McClanahan T., Kastelein R. A., Oft M. (2006) IL-23 promotes tumour incidence and growth. Nature 442, 461–465 [DOI] [PubMed] [Google Scholar]
- 16. Cocco C., Canale S., Frasson C., Di Carlo E., Ognio E., Ribatti D., Prigione I., Basso G., Airoldi I. (2010) Interleukin-23 acts as antitumor agent on childhood B-acute lymphoblastic leukemia cells. Blood 116, 3887–3898 [DOI] [PubMed] [Google Scholar]
- 17. Re F., Strominger J. (2001) Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J. Biol. Chem. 276, 37692–37699 [DOI] [PubMed] [Google Scholar]
- 18. Napolitani G., Rinaldi A., Bertoni F., Sallusto F., Lanzavecchia A. (2005) Selected Toll-like receptor agonist combinations synergistically trigger a T helper type 1-polarizing program in dendritic cells. Nat. Immunol. 6, 769–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Carmody R. J., Ruan Q., Liou H.-C., Chen Y. H. (2007) Essential roles of c-Rel in TLR-induced IL-23 p19 gene expression in dendritic cells. J. Immunol. 178, 186–191 [DOI] [PubMed] [Google Scholar]
- 20. Verreck F. A., de Boer T., Langenberg D. M., Hoeve M. A., Kramer M., Vaisberg E., Kastelein R., Kolk A., de Waal-Malefyt R., Ottenhoff T. H. (2004) Human IL-23-producing type 1 macrophages promote but IL-10-producing type 2 macrophages subvert immunity to (myco)bacteria. Proc. Natl. Acad. Sci. U.S.A. 101, 4560–4565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Awasthi A., Riol-Blanco L., Jäger A., Korn T., Pot C., Galileos G., Bettelli E., Kuchroo V. K., Oukka M. (2009) Cutting edge. IL-23 receptor gfp reporter mice reveal distinct populations of IL-17-producing cells. J. Immunol. 182, 5904–5908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Buonocore S., Ahern P. P., Uhlig H. H., Ivanov I. I., Littman D. R., Maloy K. J., Powrie F. (2010) Innate lymphoid cells drive interleukin-23-dependent innate intestinal pathology. Nature 464, 1371–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Parham C., Chirica M., Timans J., Vaisberg E., Travis M., Cheung J., Pflanz S., Zhang R., Singh K., Vega F., To W., Wagner J., O'Farrell A.-M., McClanahan T., Zurawski S., Hannum C., Gorman D., Rennick D. M., Kastelein R. A., de Waal Malefyt R., Moore K. W. (2002) A receptor for the heterodimeric cytokine IL-23 is composed of IL-12Rβ1 and a novel cytokine receptor subunit, IL-23R. J. Immunol. 168, 5699–5708 [DOI] [PubMed] [Google Scholar]
- 24. Cho M.-L., Kang J.-W., Moon Y.-M., Nam H.-J., Jhun J.-Y., Heo S.-B., Jin H.-T., Min S.-Y., Ju J.-H., Park K.-S., Cho Y.-G., Yoon C.-H., Park S.-H., Sung Y.-C., Kim H.-Y. (2006) STAT3 and NF-κB signal pathway is required for IL-23-mediated IL-17 production in spontaneous arthritis animal model IL-1 receptor antagonist-deficient mice. J. Immunol. 176, 5652–5661 [DOI] [PubMed] [Google Scholar]
- 25. Collison L. W., Vignali D. A. (2008) Interleukin-35. Odd one out or part of the family? Immunol. Rev. 226, 248–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Case R. D., Piccione E., Wolf G., Benett A. M., Lechleider R. J., Neel B. G., Shoelson S. E. (1994) SH-PTP2/Syp SH2 domain binding specificity is defined by direct interactions with platelet-derived growth factor β-receptor, epidermal growth factor receptor, and insulin receptor substrate-1-derived phosphopeptides. J. Biol. Chem. 269, 10467–10474 [PubMed] [Google Scholar]
- 27. Heinrich P. C., Behrmann I., Müller-Newen G., Schaper F., Graeve L. (1998) Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem. J. 334, 297–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wiederkehr-Adam M., Ernst P., Müller K., Bieck E., Gombert F. O., Ottl J., Graff P., Grossmüller F., Heim M. H. (2003) Characterization of phosphopeptide motifs specific for the Src homology 2 domains of signal transducer and activator of transcription 1 (STAT1) and STAT3. J. Biol. Chem. 278, 16117–16128 [DOI] [PubMed] [Google Scholar]
- 29. Eulenfeld R., Dittrich A., Khouri C., Müller P. J., Mütze B., Wolf A., Schaper F. (2012) Interleukin-6 signalling. More than Jaks and STATs. Eur. J. Cell Biol. 91, 486–495 [DOI] [PubMed] [Google Scholar]
- 30. Naeger L. K., McKinney J., Salvekar A., Hoey T. (1999) Identification of a STAT4 binding site in the interleukin-12 receptor required for signaling. J. Biol. Chem. 274, 1875–1878 [DOI] [PubMed] [Google Scholar]
- 31. Yao B. B., Niu P., Surowy C. S., Faltynek C. R. (1999) Direct interaction of STAT4 with the IL-12 receptor. Arch. Biochem. Biophys. 368, 147–155 [DOI] [PubMed] [Google Scholar]
- 32. Gearing D. P., Ziegler S. F., Comeau M. R., Friend D., Thoma B., Cosman D., Park L., Mosley B. (1994) Proliferative responses and binding properties of hematopoietic cells transfected with low-affinity receptors for leukemia inhibitory factor, oncostatin M, and ciliary neurotrophic factor. Proc. Natl. Acad. Sci. U.S.A. 91, 1119–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ketteler R., Glaser S., Sandra O., Martens U. M., Klingmüller U. (2002) Enhanced transgene expression in primitive hematopoietic progenitor cells and embryonic stem cells efficiently transduced by optimized retroviral hybrid vectors. Gene Ther. 9, 477–487 [DOI] [PubMed] [Google Scholar]
- 34. Fischer M., Goldschmitt J., Peschel C., Brakenhoff J. P., Kallen K. J., Wollmer A., Grötzinger J., Rose-John S. (1997) I. A bioactive designer cytokine for human hematopoietic progenitor cell expansion. Nat. Biotechnol. 15, 142–145 [DOI] [PubMed] [Google Scholar]
- 35. Althoff K., Müllberg J., Aasland D., Voltz N., Kallen K., Grötzinger J., Rose-John S. (2001) Recognition sequences and structural elements contribute to shedding susceptibility of membrane proteins. Biochem. J. 353, 663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Suthaus J., Tillmann A., Lorenzen I., Bulanova E., Rose-John S., Scheller J. (2010) Forced homo- and heterodimerization of all gp130-type receptor complexes leads to constitutive ligand-independent signaling and cytokine-independent growth. Mol. Biol. Cell 21, 2797–2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sarin R., Wu X., Abraham C. (2011) Inflammatory disease protective R381Q IL23 receptor polymorphism results in decreased primary CD4+ and CD8+ human T-cell functional responses. Proc. Natl. Acad. Sci. U.S.A. 108, 9560–9565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pidasheva S., Trifari S., Phillips A., Hackney J. A., Ma Y., Smith A., Sohn S. J., Spits H., Little R. D., Behrens T. W., Honigberg L., Ghilardi N., Clark H. F. (2011) Functional studies on the IBD susceptibility gene IL23R implicate reduced receptor function in the protective genetic variant R381Q. PLoS One 6, e25038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Stahl N., Farruggella T. J., Boulton T. G., Zhong Z., Darnell J. E., Jr., Yancopoulos G. D. (1995) Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science 267, 1349–1353 [DOI] [PubMed] [Google Scholar]
- 40. Demoulin J. B., Uyttenhove C., Van Roost E., DeLestré B., Donckers D., Van Snick J., Renauld J. C. (1996) A single tyrosine of the interleukin-9 (IL-9) receptor is required for STAT activation, antiapoptotic activity, and growth regulation by IL-9. Mol. Cell Biol. 16, 4710–4716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Ohtani T., Ishihara K., Atsumi T., Nishida K., Kaneko Y., Miyata T., Itoh S., Narimatsu M., Maeda H., Fukada T., Itoh M., Okano H., Hibi M., Hirano T. (2000) Dissection of signaling cascades through gp130 in vivo. Reciprocal roles for STAT3- and SHP2-mediated signals in immune responses. Immunity 12, 95–105 [DOI] [PubMed] [Google Scholar]
- 42. Zheng Y., Qin H., Frank S. J., Deng L., Litchfield D. W., Tefferi A., Pardanani A., Lin F.-T., Li J., Sha B., Benveniste E. N. (2011) A CK2-dependent mechanism for activation of the JAK-STAT signaling pathway. Blood 118, 156–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ward A. C., Hermans M. H., Smith L., van Aesch Y. M., Schelen A. M., Antonissen C., Touw I. P. (1999) Tyrosine-dependent and -independent mechanisms of STAT3 activation by the human granulocyte colony-stimulating factor (G-CSF) receptor are differentially utilized depending on G-CSF concentration. Blood 93, 113–124 [PubMed] [Google Scholar]
- 44. Dumoutier L., de Meester C., Tavernier J., Renauld J.-C. (2009) New activation modus of STAT3. A tyrosine-less region of the interleukin-22 receptor recruits STAT3 by interacting with its coiled-coil domain. J. Biol. Chem. 284, 26377–26384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li X., Leung S., Kerr I. M., Stark G. R. (1997) Functional subdomains of STAT2 required for preassociation with the α interferon receptor and for signaling. Mol. Cell Biol. 17, 2048–2056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Palmer D. C., Restifo N. P. (2009) Suppressors of cytokine signaling (SOCS) in T cell differentiation, maturation, and function. Trends Immunol. 30, 592–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Heinrich P. C., Behrmann I., Haan S., Hermanns H. M., Müller-Newen G., Schaper F. (2003) Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 374, 1–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. de Paus R. A., van de Wetering D., van Dissel J. T., van de Vosse E. (2008) IL-23 and IL-12 responses in activated human T cells retrovirally transduced with IL-23 receptor variants. Mol. Immunol. 45, 3889–3895 [DOI] [PubMed] [Google Scholar]
- 49. Chen Z., Laurence A., Kanno Y., Pacher-Zavisin M., Zhu B.-M., Tato C., Yoshimura A., Hennighausen L., O'Shea J. J. (2006) Selective regulatory function of Socs3 in the formation of IL-17-secreting T cells. Proc. Natl. Acad. Sci. U.S.A. 103, 8137–8142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Harris T. J., Grosso J. F., Yen H.-R., Xin H., Kortylewski M., Albesiano E., Hipkiss E. L., Getnet D., Goldberg M. V., Maris C. H., Housseau F., Yu H., Pardoll D. M., Drake C. G. (2007) Cutting edge. An in vivo requirement for STAT3 signaling in TH17 development and TH17-dependent autoimmunity. J. Immunol. 179, 4313–4317 [DOI] [PubMed] [Google Scholar]
- 51. Liu X., Lee Y. S., Yu C.-R., Egwuagu C. E. (2008) Loss of STAT3 in CD4+ T cells prevents development of experimental autoimmune diseases. J. Immunol. 180, 6070–6076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Bromberg J. F., Wrzeszczynska M. H., Devgan G., Zhao Y., Pestell R. G., Albanese C., Darnell J. E., Jr. (1999) Stat3 as an oncogene. Cell 98, 295–303 [DOI] [PubMed] [Google Scholar]
- 53. Grivennikov S., Karin M. (2008) Autocrine IL-6 signaling. A key event in tumorigenesis? Cancer Cell 13, 7–9 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.