Background: Activation of integrins may improve cell retention rates in stem cell transplantation.
Results: The first small molecule agonist of integrin α4β1 is generated and enhances cell adhesion mechanisms in vitro.
Conclusion: The agonist binds at the subunit interface, inducing ligand binding with consequent displacement of compound.
Significance: The agonist may improve progenitor cell retention as an adjunct to cell-based therapy.
Keywords: Cell Adhesion, Cell Therapy, Fibronectin, Integrins, Stem Cells, Agonist, ICAM-1, Progenitor Cells, VCAM-1, VLA-4
Abstract
Activation of the integrin family of cell adhesion receptors on progenitor cells may be a viable approach to enhance the effects of stem cell-based therapies by improving cell retention and engraftment. Here, we describe the synthesis and characterization of the first small molecule agonist identified for the integrin α4β1 (also known as very late antigen-4 or VLA-4). The agonist, THI0019, was generated via two structural modifications to a previously identified α4β1 antagonist. THI0019 greatly enhanced the adhesion of cultured cell lines and primary progenitor cells to α4β1 ligands VCAM-1 and CS1 under both static and flow conditions. Furthermore, THI0019 facilitated the rolling and spreading of cells on VCAM-1 and the migration of cells toward SDF-1α. Molecular modeling predicted that the compound binds at the α/β subunit interface overlapping the ligand-binding site thus indicating that the compound must be displaced upon ligand binding. In support of this model, an analog of THI0019 modified to contain a photoreactive group was used to demonstrate that when cross-linked to the integrin, the compound behaves as an antagonist instead of an agonist. In addition, THI0019 showed cross-reactivity with the related integrin α4β7 as well as α5β1 and αLβ2. When cross-linked to αLβ2, the photoreactive analog of THI0019 remained an agonist, consistent with it binding at the α/β subunit interface and not at the ligand-binding site in the inserted (“I”) domain of the αL subunit. Co-administering progenitor cells with a compound such as THI0019 may provide a mechanism for enhancing stem cell therapy.
Introduction
Clinical trials have shown that stem/progenitor cell therapy for cardiovascular indications is well tolerated and may improve heart function (1, 2). However, low rates of cell retention and engraftment after cell delivery may be problematic in achieving maximal benefits from the therapy. Studies have shown that fewer than 10% of cells are retained in the heart within hours after intracoronary administration of bone marrow progenitor cells in patients who have had a myocardial infarction (3). Despite low retention rates, recent studies in animal models of myocardial infarction (4) and in patients with dilated cardiomyopathy (5) have indicated that the early retention of transplanted stem cells directly correlates to improved functional outcomes. Thus, finding a means to increase cell retention would lead to more robust cell therapy (6).
One approach to increase cell retention has been to target the integrin family of adhesion receptors expressed on the surface of stem and progenitor cells (6). Integrins are a family of 24 distinct heterodimeric cell surface receptors composed of an α and a β subunit (7). Integrins mediate the adhesion of cells to extracellular matrix proteins or to ligands expressed on the surface of neighboring cells. They have been validated as drug targets in humans in that inhibition of integrin function has led to the development of therapeutics for cardiovascular and autoimmune indications (8, 9). Half of the α subunits contain an extra “inserted” or “I” domain, which is responsible for ligand binding. In those integrins that lack an I domain, the binding site includes regions of both the α and β subunits (10). Integrin activity depends on the coordination of divalent cations at the metal ion-dependent adhesion site (MIDAS).2 A primary determinant of ligand binding is the coordination of the cation at the MIDAS site by an acidic residue supplied by the ligand itself (11). Thus, most peptide or small molecule competitive antagonists of integrin function contain an acidic group that interacts at the MIDAS site (10). Integrin activity also depends on conformation; integrins exist in an equilibrium between different conformational states that dictate their relative affinity for ligand (12).
The up-regulation of integrin activity or expression on the surface of progenitor cells has been used to increase the retention of progenitor cells both in vitro and in disease models in vivo. These approaches have targeted integrins either directly by using activating antibodies (13) or indirectly by genetically manipulating or preconditioning cells with recombinant proteins (14–16). Targeting key integrins directly with a small molecule agonist may be an effective means to enhance cell retention in stem cell therapy. An attractive target for such an approach is the integrin α4β1, which is expressed on the surface of several progenitor cell types, including endothelial progenitor cells (EPCs) and hematopoietic progenitor cells (HPCs) (17, 18). α4β1 has been shown to be critical for progenitor cell homing to sites of ischemia, as well as for cell fusion, in animal models of stem cell therapy (17, 19). The predominant ligands for α4β1 are vascular cell adhesion molecule-1 (VCAM-1) and the alternatively spliced connecting segment-1 (CS1) sequence of fibronectin (7). Importantly, expression of both VCAM-1 and fibronectin is up-regulated after tissue injury, including after a myocardial infarction (20, 21).
One way in which integrins are activated physiologically is through outside-in signaling via ligand binding; this was initially demonstrated when small RGD peptides that bind αIIbβ3 were found to be partial agonists, as well as competitive antagonists, of cell adhesion (22). More recently, a small molecule antagonist of αLβ2 was found to be a partial agonist of cell adhesion under low affinity conditions (23). These findings led us to hypothesize that we can convert a known integrin antagonist to a full agonist. In this study, we describe the generation and characterization of the first known small molecule agonist of α4β1, an integrin that lacks an I domain. This small molecule agonist promotes α4β1-mediated cell adhesion, rolling, spreading, and migration. As such, it has significant effects on stem/progenitor cell adhesion under both static and flow conditions and therefore may prove to be a useful adjunct to stem cell therapy.
EXPERIMENTAL PROCEDURES
Reagents and Cell Lines
The synthesis of THI0019, THI0003 (also known as TBC3567), TBC3486, and THI0455 has been previously described (24–26). For all assays described, THI0019 was dissolved in DMSO to make a 1 mm stock solution, and dilutions were made in assay buffer or media to yield the desired final working concentrations in 1% DMSO (vehicle). Synthesis of BIO5192 and its methyl ester has been previously described (27). Human collagen types II and IV, serum fibronectin, and vitronectin were purchased from Sigma. Human VCAM-1, MAdCAM-1 Fc chimera, ICAM-1, and SDF-1 were purchased from R&D Systems (Minneapolis, MN). The CS1-BSA conjugate has previously been described (28) and was synthesized at New England Peptide (Gardner, MA). Antibodies were purchased from the following: ABD Serotec (Raleigh, NC) (HP2/1 (anti-α4) and 38 (anti-αL)); R&D Systems (Minneapolis, MN) (P5D2 (anti-β1) and BBIG-I1 (anti-ICAM-1)); Invitrogen (SAM-1(anti-α5), anti-MAdCAM-1, and donkey anti-goat-647); Pharmingen (FIB27 ((anti-β7), HUTS-21 (anti-β1), WM59 (anti-CD31), 581 (anti-CD34), 89106 (anti-VEGFR2), and 9EG7 (anti-β1)); BD Biosciences (L25 (anti-α4)); Millipore (Temecula, CA) (B44 (anti-β1)); and Santa Cruz Biotechnology (Santa Cruz, CA) (WW-9 (anti-Flk-1), C-20 (goat polyclonal anti-α4)). The anti-VCAM-1 mAb P3C4 and anti-β1 mAb TS2/16 were purified from hybridomas purchased from the Developmental Studies Hybridoma Bank at University of Iowa and American Type Culture Collection, respectively. mAb 33B6 and the HPB-ALL T cell line were gifts from B. McIntyre (M. D. Anderson Cancer Center, Houston, TX). The cell lines Jurkat, K562, human umbilical vein endothelial cells, M2-10B4, HSB, and TF-1 were obtained from American Type Culture Collection (Manassas, VA) and were maintained in recommended culture media. The mutant Jurkat cell line not expressing α4 integrin (Jurkat (α4−)) (29) was a gift from Dr. David Rose, University of California at San Diego, La Jolla, CA.
Generation of EPCs
Human EPCs were generated from peripheral blood essentially as described previously (13). Buffy coats from human donors were obtained from Gulf Coast Regional Blood Center, Houston, TX, and were separated over a Ficoll gradient to isolate the mononuclear cell layer. Cells were plated in tissue culture flasks coated with human fibronectin (Sigma) in EC basal medium-2 (Clonetics, San Diego) supplemented with EGM-2 SingleQuots. After 4 days, nonadherent cells were removed by washing with phosphate-buffered saline. Fresh medium was added, and adherent cells were expanded in culture for 5 weeks. Endothelial progenitor cells were characterized by flow cytometry for surface expression of CD34, CD133, Flk-1 (KDR), CD31, and the α4 subunit of VLA-4. For confocal microscopy, cells were seeded onto a collagen-coated coverslip for 3 h at 37 °C, 5% CO2 and were incubated with 2 μg/ml Alexa Fluor 488-conjugated acetylated LDL (Invitrogen) in complete medium for 1 h at 37 °C. Cells were fixed in 2% paraformaldehyde and were stained with 4 μg/ml lectin from Ulex europaeus-Atto 594 conjugate (Sigma) for 1 h at room temperature. Images were captured on a Leica TCS SPE confocal fluorescent microscope (Mannheim, Germany) by using Leica Application Suite Advanced Fluorescence software version 2.5.2-6939.
Static Cell Adhesion Assays
Ligands (CS1-BSA, VCAM-1, MAdCAM-1, fibronectin, ICAM-1, vitronectin, and collagen I and IV) in 50 μl of 50 mm Tris-HCl (pH 7.4), 150 mm NaCl (TBS) were added to wells of a 96-well plate and allowed to coat overnight at 4 °C. To maximize the window to evaluate agonist activity, a suboptimal coating concentration of ligand was used. This ligand concentration corresponded approximately to that which would yield ≤5% adhesion as determined by dose-response curves of ligand binding to the appropriate cell type. For assays in which both antagonism and agonism effects were to be measured (e.g. Fig. 10, D, E, G, and H), the concentration of ligand corresponded to that which would yield roughly 50% adhesion. Exact ligand concentrations were as follows: Fig. 1, B, 0.03 μg/ml CS1-BSA, and C, 0.3 μg/ml CS1-BSA; Fig. 2B, 0.6 μg/ml CS1-BSA; Fig. 3, A, 0.2 μg/ml CS1-BSA, C, 0.5 μg/ml VCAM-1, and E, 0.1 μg/ml VCAM-1; Fig. 6, A and B, 1 μg/ml VCAM-1; Fig. 9, A, 1 μg/ml MAdCAM-1, C, 1 μg/ml fibronectin, and D, 5 μg/ml ICAM-1; and Fig. 10, C, 0.5 μg/ml VCAM-1, D and E, 3 μg/ml VCAM-1, F, 5 μg/ml ICAM-1, and G and H, 15 μg/ml ICAM-1. All assays were performed as described previously (30). Briefly, 2 × 106 cells were labeled for 30 min with calcein-AM (Molecular Probes), washed, resuspended in binding buffer, and added to ligand-coated plates (2 × 105 cells/well) that had been blocked with 2% BSA. After a 30-min incubation at 37 °C, the plates were washed three times with binding buffer; the adherent cells were lysed, and fluorescence was measured on a Tecan Safire2 plate reader. Because of the high background adhesion of TF-1 cells, assays with this cell line were performed at room temperature. Standard curves were run for each assay to convert fluorescence units to cell number. For each assay, the cells expressed the appropriate integrin receptor either endogenously (Jurkat/α4β1, Jurkat/α2β1, EPC/α4β1, TF-1/α4β1, K562/α5β1, K562/α1β1, human umbilical vein endothelial cells/αvβ3, Jurkat (α4−)/αLβ2, and HSB/αLβ2) or in recombinant form (K562/α4β1, K562/α4β7, and K562/α1β1). Generation of the recombinant K562 cell lines has been described previously (31). The binding buffer was TBS with 1 mm MgCl2 and 1 mm CaCl2 for low affinity α4β1 assays or TBS with 1 mm MnCl2 for high affinity α4β1 assays. For cells in which the α4β1 integrin was empirically determined to be in a very low affinity state (K562 (α4β1) and EPCs), TBS with 1 mm MnCl2 was used as the buffer. Cross-screening assays for α4β7/MAdCAM-1, α5β1/fibronectin, αvβ3/vitronectin, and α1β1/collagen IV were performed in TBS with 1 mm MnCl2. Assays for αLβ2/ICAM-1 were conducted in TBS with 2 mm MgCl2 and 5 mm EGTA. Assays for α2β1/collagen I were performed in TBS with 1 mm MgCl2.
FIGURE 10.
THI0019 overlaps with the ligand-binding site of α4 integrins but not αLβ2. A, THI0019 docks into the ligand binding pocket of integrin α4β7. Molecular surfaces of the integrin are shown in blue (α4) and light tan (β7); key residues are labeled and shown in stick form. Mg2+ bound at the MIDAS site is shown as a dark red sphere, and the Ca2+ ions bound at the SyMBS and ADMIDAS sites are shown in yellow. THI0019 is shown with a dark gray carbon backbone. As a point of reference, one of the key residues (Ser-144) for coordinating the Mg2+ cation in the MIDAS site is shown. B, structure of the cross-linking compound THI0455. The phenyl azide modification is circled. C, in the absence of UV treatment, THI0455 has similar agonist activity as THI0019 in a static adhesion assay. D, cells and compounds were treated separately with UV radiation and then mixed for the assay (UV). Irradiated cells that were mixed with compounds that had not been exposed to UV treatment were used as controls (no UV). E, cells were pre-mixed with compounds before exposure to UV treatment to induce cross-linking of the compound to the integrin (x-link). As control groups, compounds were added to the cells after the cells were exposed to UV treatment (no x-link). F–H, identical experiments were performed with αLβ2-expressing cells and ICAM-1. D and E and G and H, results are expressed as the number of cells attached ± S.D. from triplicate wells; *, p < 0.05, versus respective vehicle control (ns, not significant).
FIGURE 1.
Agonist THI0019 is generated from α4β1 antagonist TBC3486. A, two structural modifications resulted in the conversion of TBC3486 to THI0019. Compounds were evaluated for their effect on binding of Jurkat cells to CS1-BSA under high (B) and low (C) affinity conditions. Adhesion was measured by fluorescence; 100% control adhesion (dotted line) was defined as the binding of cells in the presence of vehicle alone. Results are expressed as fluorescence units ± S.D. from triplicate wells.
FIGURE 2.
Methyl ester of BIO5192 is an antagonist of α4β1. A, structure of BIO5192 and its methyl ester. B, compounds were evaluated for their ability to affect the binding of Jurkat cells to CS1-BSA under low affinity conditions, as described in Fig. 1 and under “Experimental Procedures.” Results are expressed as relative fluorescence units ± S.D. from triplicate wells. Both BIO5192 (circles, solid line) and its methyl ester form (triangles, dashed line) inhibited adhesion.
FIGURE 3.
THI0019 enhances binding of Jurkat and EPCs under both static and flow conditions. A, C, and E, dose-response curves showing the effects of THI0019 on binding of Jurkat cells to CS1-BSA containing either the wild-type LDV or a mutated LAV binding sequence (A), Jurkat cells or a mutant Jurkat line that does not express α4 integrin (α4−) to VCAM-1 (C), and EPCs to VCAM-1 (E). Results are expressed as the number of cells attached ± S.D. from triplicate wells. B, D, and F, specificity was determined by preincubating the cells with buffer (none) or antibodies (10 μg/ml) to integrin subunits or isotype control antibodies. *, p < 0.05, versus respective Ig controls. Cell detachment assays under conditions of flow were performed with Jurkat (G) or EPCs (H) and VCAM-1. Results are expressed as the mean percentage of cells attached ± S.D. from triplicate runs. *, p < 0.05, versus vehicle-treated cells.
FIGURE 6.
THI0019 promotes rolling of HPC on VCAM-1-expressing stromal cells. A, dose-response curve showing the effects of THI0019 on binding of TF-1 cells to VCAM-1 under low affinity conditions. Adhesion assays were performed as described under “Experimental Procedures.” Results are expressed as the mean number of cells attached ± S.D. from triplicate runs. B, specificity of the effect of THI0019 on cell adhesion was determined by preincubating the cells with buffer (none) or antibodies (10 μg/ml) to various integrin subunits or isotype controls. *, p < 0.05, versus respective Ig controls. C, velocity of rolling TF-1 cells on a stromal cell monolayer. The percentage of cells traveling at defined velocities is shown. D, average velocity ± S.D. from triplicate runs of the cells in C is shown. *, p < 0.05, versus vehicle-treated cells.
FIGURE 9.
THI0019 enhances α4β7, α5β1, and αLβ2-mediated cell adhesion. A, C, and D, dose-response curves of THI0019-treated cells showing the binding of K562 (α4β7) cells to MAdCAM-1 (A), K562 cells to fibronectin (C), and mutant Jurkat (α4−) cells to ICAM-1 (D). Results are expressed as the number of cells attached ± S.D. from triplicate wells. B, E, and F, specificity was determined by preincubating the cells with buffer (none) or antibodies (10 μg/ml) to integrin subunits or isotype controls. *, p < 0.05, versus respective Ig controls.
FACS Analysis
Jurkat, Jurkat (α4−), K562, K562(α4β1), or TF-1 cells (5 × 105) were suspended in 100 μl of buffer (Tyrode's containing 1 mg/ml glucose, 1 mm MgCl2, and 1 mm CaCl2). Primary mAb (10 μg/ml) was added, and cells were incubated for 1 h on ice. For LIBS experiments, vehicle or compounds were added at the same time as primary mAb. Cells were washed and resuspended in 50 μl of buffer containing FITC-conjugated GAM secondary antibody and were then incubated on ice for 30 min. After another three washes with buffer, cells were resuspended in 500 μl of buffer and were analyzed on a Beckman Coulter Epics XL-MCL.
Parallel Plate Flow Detachment Assays
Detachment assays were performed as described previously (30). Recombinant human VCAM-1 (10 μg/ml (Fig. 3, G and H) or 5 μg/ml (Fig. 5E) in 0.1 m NaHCO3 (pH 9.5)) was immobilized overnight at 4 °C onto 24 × 50-mm slides cut from 15 × 100-mm polystyrene Petri dishes. The slides were washed with PBS, blocked with 2% (w/v) BSA for 2 h at room temperature, and assembled into a parallel plate flow chamber. For detachment assays, vehicle, 10 μm THI0019, 10 μg/ml mAb TS2/16, or combinations of each were mixed with Jurkat cells in low affinity running buffer, and then 2.0 × 106 cells were injected into the flow chamber and allowed to settle on the slides for 10 min. An increasing linear gradient of shear flow was pulled over the adherent cells for 300 s with the use of a computer-controlled syringe pump (Harvard Apparatus). Shear stress calculations were determined every 50 s. The shear stress in dynes/cm2 is defined as (6 μQ)/(wh2), where μ is the viscosity of the medium (0.007); Q is the flow rate in cm3/s; w is the width of the chamber (0.3175 cm), and h is the height of the chamber (0.01524 cm). The number of cells attached was recorded by digital microscopy (VI-470 charge-coupled device video camera; Optronics Engineering) at ×20 on an inverted Nikon DIAPHOT-TMD microscope every 50 s and was plotted against time.
FIGURE 5.
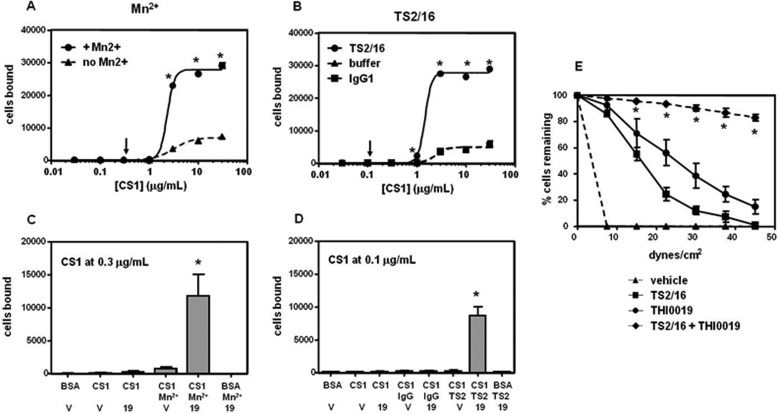
Mechanism of THI0019 is distinct from that of Mn2+ or TS2/16. Adhesion of K562 (α4β1) cells to CS1-BSA was measured in TBS containing 1 mm MgCl2, 1 mm CaCl2 with or without the addition of 1 mm MnCl2 (A) or 10 μg/ml mAb TS2/16 (TS2) (B). *, p < 0.05, versus buffer alone. B, lines for buffer and IgG1 overlap one another. C and D, CS1-BSA was coated at suboptimal concentrations as determined in A and B (arrows). Cells were treated with vehicle (v) or 10 μm compound (19) immediately before being added to the plate. Results are expressed as the number of cells bound ± S.D. from triplicate runs. E, results from parallel plate flow detachment assays are expressed as the mean percentage of cells attached ± S.D. from triplicate runs. C–E, *, p < 0.05, versus either treatment alone (see also supplemental Movies S1–S4).
Cell Rolling Assays
Stromal cells (M2-10B4) were seeded on 24 × 50-mm slides cut from 125-ml tissue culture flasks, cultured overnight under standard tissue culture conditions, and assembled to a parallel plate flow chamber. TF1 cells (2.0 × 106) were mixed with vehicle or 10 μm THI0019 in running buffer and then injected into the parallel plate flow chamber system. A constant shear flow of 0.5 dynes/cm2 was applied to the system for 300 s, and the TF1 cells rolling across the stromal cell monolayer were recorded by digital microscopy. The digital recordings were then analyzed by using the Imaris Bitplane software (version 7.6.1) to determine the velocity of individual cells moving across the monolayer. The viewing area is 500 μm, and only cells that traveled at least 400 μm were included. The velocity of a cell is defined as the distance traveled divided by the time to travel that distance.
Cell Spreading Assays
VCAM-1 (50 μl of 6 μg/ml) was coated in TBS overnight at 4 °C onto high binding 96-well plates (Costar). Plates were blocked with 2% BSA for 1 h at room temperature and were washed with complete media (RPMI 1640 supplemented with 10% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin). HPB-ALL cells (104 cells in complete media) were added to wells and incubated for indicated time points at 37 °C. Images of cells were captured at ×10 magnification on an Olympus IX71 inverted microscope (Olympus America, Inc., Center Valley, PA) equipped with an AHHO39020 CCD camera. For each treatment group, images from three separate wells were quantitated (four fields counted per well) in a blinded fashion, and the data were presented as the percentage of cells spreading. For confocal images, HPB-ALL cells were incubated on glass slides coated with VCAM-1 for 10 min. Cells were stained for actin in red (phalloidin) and α4 integrin in green (goat polyclonal anti-α4 C-20 followed by donkey anti goat-647). Nuclear staining is shown in blue (Hoechst 33342).
Migration Assays
Migration assays were performed in 3-μm pore size Transwells (24 wells, Costar, Cambridge, MA). The upper chambers were pre-coated with 3 μg/ml fibronectin or 10 μg/ml VCAM-1 in 50 μl of TBS overnight at 4 °C and were then blocked with 2% BSA for 1 h at room temperature. After washing with migration medium (RPMI 1640 medium supplemented with 1% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin), the upper chambers were loaded with Jurkat cells (2 × 105 cells) in 160 μl of migration medium. The lower chambers contained 600 μl of migration medium supplemented with 10 μg/ml SDF-1α to induce chemotaxis. Jurkat cells were mixed with vehicle (1% DMSO) or THI0019 at the indicated concentrations immediately prior to being added to the upper chamber. After a 4-h incubation at 37 °C, 5% CO2, the upper chambers were removed, and cells in the lower chamber were collected and counted on a hemocytometer. Results are expressed as the total number of cells migrated.
In Silico Docking of THI0019 in α4β7 Crystal Structure
Modeling Suite 2012 (Schrödinger LLC, New York) was used on a 16-core 2.4 GHz AMD Operon system to visualize THI0019 binding to an integrin model. A Glide (version 5.8) docking model was generated starting with a crystal structure of α4β7. The structure 3v4v was downloaded from the Protein Data Bank (32) and read into a Maestro (version 9.3). All chains except A (α4) and B (β7) were deleted. A basic protein preparation was performed using program defaults, with the addition of filling in missing side chains and deleting water molecules beyond 5 Å from heteroatoms. Missing atoms were identified in Ser-559 in chain A and Cys-455 in chain B. The complete preparation of the protein portion of the model involved the following: 1) basic protein preparation; 2) assignment of heteroatom states; 3) H-bond assignment, including PROPKA; 4) deletion of waters with less than three H-bonds to non-waters; 5) Impref “H-only” minimization; and 6) Impref “Minimize All” to root mean square deviation = 0.5. For THI0019, a Lig PREP (version 2.6) calculation was performed on the structure (imported as a SDF file) by using default criteria and Epik (version 2.3) ionization. A Glide Grid was generated using default settings based on the crystal structure ligand. In addition, a constraint was specified for metal ion. A virtual screening workflow was submitted for the crystal structure ligand and then for THI0019. The virtual screening workflow involved docking with Glide XP mode, starting with three extra conformations and rescoring with the Prime MMGBSA ΔGbind.
Cross-linking Experiments
Cells and buffers were selected to maximize the interaction of compounds with integrin. K562 (α4β1) cells or compounds in 150 μl of TBS (pH 7.4), 1 mm MnCl2 were placed in wells of a 48-well plate coated with 0.5% polyHEMA. Alternatively, cells were premixed with vehicle or 10 μm compound before being adding to wells. Plates were placed in a SpectroLinker XL-1000 (Spectronics Corp., Westbury, NY) 4.5 cm from the UV source and exposed to UV radiation at 312 nm for 3 min. Cells, compounds, or mixtures were removed from the plates and transferred to 96-well plates (5 × 104 cells/well) for the static adhesion assay. We conducted the αLβ2/ICAM-1 cross-linking studies in an identical manner, except that we used HSB cells (2 × 105 cells/well) in TBS (pH 7.4), 2 mm MgCl2, and 5 mm EGTA.
Statistical Analysis
An unpaired Student's t test was used to determine statistical significance between treatment groups. Differences were considered significant at p < 0.05.
RESULTS
Integrin Antagonist Is Converted to an Agonist
TBC3486 is a potent (IC50 = 9 nm), selective antagonist of the integrin α4β1 (31) and was used as a template for the design of an α4β1 agonist (Fig. 1A). Using cell-based adhesion assays, we measured the extent of binding of the human T cell line Jurkat to wells coated with the CS1 sequence of fibronectin conjugated to BSA. We screened compounds at concentrations from 0.1 to 30 μm, a range in which TBC3486 completely abrogated cell adhesion under both high and low affinity conditions (Fig. 1, B and C). Insertion of an oxymethylene group into the backbone of TBC3486 yielded a compound (THI0003) that was substantially less active than its parent; the reduced activity was likely due to sub-optimal alignment of the molecule upon interaction of the acid group with the MIDAS site (Fig. 1B). Under low affinity conditions, however, THI0003 showed agonist activity (Fig. 1C). Subsequent conversion of the carboxylic acid group of THI0003 to a methyl ester resulted in the formation of a compound (THI0019) that showed agonist activity under both high and low affinity conditions (Fig. 1, B and C). Thus, we chose the compound THI0019 for further evaluation. It is important to note that modification of the key carboxylic acid residue to a methyl ester does not convert all classes of α4β1 antagonists to agonists. For example, applying this same modification to the antagonist BIO5192 (KD = 10 pm) (33) resulted in the formation of a compound that retained its ability to inhibit α4β1-mediated adhesion, albeit to a weaker extent than that of the parent compound (Fig. 2).
We performed subsequent adhesion assays by using suboptimal ligand concentrations to maximize the window for observing increases in cell binding. In cell adhesion assays in which Jurkat cells bound to CS1-conjugated BSA, THI0019 showed a dose-dependent enhancement in cell binding with an effective concentration giving half-maximal binding (EC50) of 1.7 μm (Fig. 3A). In this assay format, the compound increased the number of cells bound to CS1 by 20–30-fold. However, THI0019 did not induce cell adhesion to a CS1-BSA conjugate in which the CS1 sequence had been modified by changing the key aspartic acid residue (LDV) to alanine (LAV). Furthermore, neutralizing antibodies to the α4 or β1 integrin subunit blocked the adhesion-inducing effects of THI0019, whereas antibodies to the α5, αL, β7, or β2 integrin subunits had no effect (Fig. 3B). These findings indicated that the binding of cells to CS1-BSA was integrin-dependent and that THI0019 did not induce adhesion to other regions of the CS1 sequence or to the BSA conjugate. When VCAM-1 was used as the ligand (Fig. 3C), THI0019 induced an even more pronounced 100-fold increase in Jurkat cell binding, with an EC50 of 1.2 μm. However, this effect of THI0019 was not observed when using a mutant Jurkat cell line that does not express α4 integrins (Fig. 3C) or by pretreating Jurkat cells with antibodies to VCAM-1 or the α4 or β1 integrin subunits (Fig. 3D).
To determine whether THI0019 had a similar effect on cells that are candidates for regenerative medicine, we isolated human EPCs from peripheral blood and characterized them by confocal microscopy and flow cytometry. The cells showed two hallmark characteristics of human EPCs as follows: acetylated low density lipoprotein uptake and U. europaeus lectin binding (data not shown). Furthermore, the cells showed a heterogeneous expression of the progenitor cell marker CD34 and were positive for the endothelial markers CD31 and VEGFR2, and they had little or no expression of CD133, suggesting a late EPC phenotype (data not shown). Importantly, the cells were positive for α4 integrin. Similar to our findings with Jurkat cells, THI0019 induced a dose-dependent increase in EPC binding to VCAM-1 (EC50 of 3.7 μm), increasing the number of cells bound by 10–30-fold above base line (Fig. 3E). This effect was blocked by antibodies to the α4 or β1 integrin subunits (Fig. 3F).
Cells Treated with THI0019 Are Resistant to Shear Stress
To determine whether THI0019 affects cells under conditions of shear stress, we evaluated Jurkat cells or EPCs in parallel plate flow chambers. Cells were mixed with either vehicle or 10 μm THI0019 and infused onto slides coated with VCAM-1. After allowing the cells to settle for 10 min, we determined the rate of cell detachment as the flow rate increased. Under these conditions, the calculated shear stress ranged from 0 to 45 dynes/cm2. Jurkat cells treated with vehicle gradually detached with ∼25% remaining at 15 dynes/cm2, a rate approximating arterial shear stress (Fig. 3G). In contrast, Jurkat cells treated with THI0019 detached at a much slower rate, with 63% of cells still attached at 15 dynes/cm2. The results were even more dramatic with EPCs (Fig. 3H). Vehicle-treated EPCs rapidly detached from the VCAM surface, most likely because of the low basal activity of the α4β1 integrin expressed on EPCs compared with Jurkat cells. In contrast, THI0019-treated EPCs detached at a very slow rate, and most cells remained attached even under the highest level of shear stress tested.
THI0019 Does Not Induce Ligand-induced Binding Site Epitopes
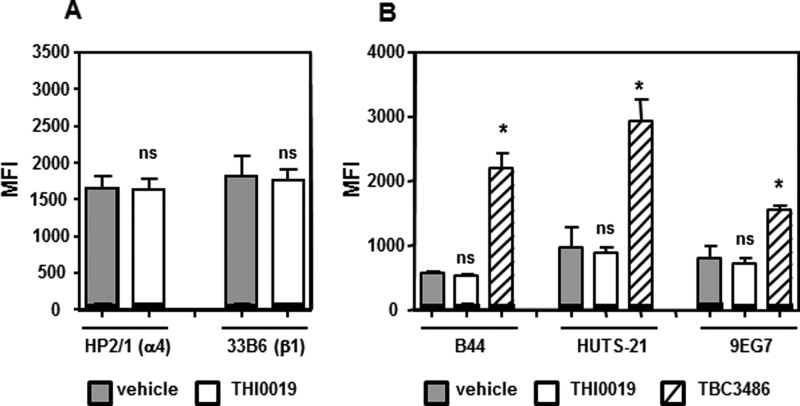
Because of the short duration of the adhesion assays, we believe the enhanced cell adhesion was probably not due to an increase in cell surface expression of the integrin. To confirm this, we used flow cytometry to show that treatment of Jurkat cells with THI0019 for 30 min had no effect on the binding of the monoclonal antibodies (mAbs) HP2/1 and 33B6, which recognize the α4 and β1 integrin subunits, respectively (Fig. 4A). Similar results were obtained after cells were incubated for 4 h with THI0019 before processing (data not shown).
FIGURE 4.
THI0019 does not induce expression of α4β1 or LIBS epitopes. Flow cytometry analysis of Jurkat cells was performed to measure the effect of THI0019 on the expression levels of α4 and β1 (A) and binding of LIBS mAbs (B). Results are expressed as mean fluorescence intensity (MFI) ± S.D. from triplicate runs. Mean fluorescence intensity values for respective IgG controls for each antibody are shown in black within the same bar. TBC3486 was used as a positive control in B. *, p < 0.05, versus vehicle-treated cells; ns, not significant.
To determine whether THI0019 alters integrin conformation, we used flow cytometry to examine the effect of the compound on the binding of a series of mAbs that recognize LIBS epitopes. Binding of ligand induces a conformational rearrangement in the β1 subunit that results in the exposure of these epitopes and increased antibody binding. In a similar fashion, the antagonist TBC3486, a ligand mimetic, has been shown to also increase binding of LIBS antibodies (31). Flow cytometry experiments (Fig. 4B) demonstrated that although TBC3486 induced the binding of the LIBS mAbs B44, HUTS-21, and 9EG7, the agonist compound THI0019 did not increase or decrease the exposure of these epitopes relative to the vehicle control.
THI0019 Is Synergistic with Mn2+ and Activating mAb TS2/16
Mn2+ cation and the mAb TS2/16 both activate integrins experimentally but by different mechanisms. For integrins such as α4β1, Mn2+ binds to the cation-binding sites in the β subunit and up-regulates integrin affinity (34). The epitope for activating TS2/16 is located in the β subunit, and upon binding, TS2/16 induces a high affinity conformation of β1 integrins (35). Because THI0019 did not induce major conformational changes in the β subunit, as shown by the LIBS analysis, we evaluated the effect of the compound when combined with these agents. For these experiments, we used a human K562 cell line engineered to express recombinant α4 because the resulting α4β1 integrin was empirically determined to be in a very low affinity state. Fig. 5, A and B, shows the dose-response curves of these cells adhering to the CS1 ligand. Cell binding was clearly enhanced in the presence of Mn2+ or TS2/16. If suboptimal concentrations of CS1 were used, either of these agents or THI0019 had little or no effect on cell adhesion (Fig. 5, C and D). However, when the cells were treated with a combination of THI0019 and Mn2+ or TS2/16, we observed a synergistic effect on cell adhesion. No increase in adhesion was seen when Mn2+ or TS2/16 was used in combination with vehicle or when BSA was used as substrate. These findings indicate that the mechanism of THI0019 agonism is different from that of Mn2+ or TS2/16.
The synergistic effect we observed under static conditions translated to increased adhesion under flow conditions. In parallel plate flow experiments using Jurkat cells, the detachment curves for cells treated with TS2/16 or THI0019 alone were shifted far to the right relative to those for vehicle-treated cells (Fig. 5E and supplemental Movies S1–S3). When THI0019 and TS2/16 were combined, few cells detached, even under very high shear stress (supplemental Movie S4).
THI0019 Facilitates Cell Rolling, Spreading, and Migration
In static adhesion assays, the binding of the hematopoietic progenitor cell line TF-1 to VCAM-1 was greatly enhanced by THI0019 in an α4β1-dependent manner (Fig. 6, A and B). To further delineate the components of cell trafficking affected by the agonist, we performed rolling assays using these cells in parallel plate flow chambers. TF-1 cells were infused across the surface of M2-10B4 stromal cells expressing VCAM-1, and a constant shear flow of 0.5 dynes/cm2 was applied. We analyzed video images to identify rolling cells (total of 200–600 events), and we determined the relative velocity of the rolling cells on the basis of the number of frames required for a cell to travel a specified distance. Of the rolling cells, a significantly higher percentage of THI0019-treated cells traveled at slower rates than vehicle-treated cells (Fig. 6C). This translated to a 27% decrease in the average velocity of the rolling cell population as a whole (Fig. 6D). As a decrease in velocity typically precedes cell arrest and firm attachment, these data indicate that the agonist is facilitating early events in the cell extravasation process, including rolling.
To determine whether THI0019 promotes cell spreading, we incubated HPB-ALL cells with vehicle or THI0019 in 96-well plates coated with BSA or VCAM-1. HPB-ALL cells were used as α4β1-dependent spreading is readily detected in this cell type (36). Images were captured and quantitated at 30, 90, and 240 min (Fig. 7A). The cells did not spread on BSA but readily spread on VCAM-1. A significantly higher percentage of THI0019-treated cells spread at 30 and 90 min than did vehicle-treated cells, and the spreading was more robust (Fig. 7B). At 240 min, the percentage of cells that spread on VCAM-1 was similar between the two groups. In a qualitative assessment of cell spreading, HPB-ALL cells were incubated on glass slides coated with VCAM-1 and evaluated by confocal microscopy. Although overall spreading was accelerated in this format relative to the 96-well plate regardless of treatment, THI0019 promoted substantially more cell spreading than did vehicle treatment (Fig. 7C). Vehicle-treated cells were generally more rounded, whereas THI0019-treated cells were more elaborately spread with α4 integrin localized in some cases to the filopodia.
FIGURE 7.
THI0019 facilitates cell spreading on VCAM-1. A, HPB-ALL cells were incubated with vehicle or THI0019 in wells coated with BSA or VCAM-1 for 30, 90, or 240 min. At each time point, images were captured, and representative images from each time point are shown. B, images were quantified, and the results are expressed as the mean percentage of cells that spread ± S.D. from triplicate wells (only the 240-min time point is shown for BSA control). *, p < 0.05, versus vehicle-treated cells. C, confocal images of vehicle-treated or THI0019-treated HPB-ALL cells incubated on glass slides coated with VCAM-1 for 10 min. Cells are stained for actin (red) and α4 integrin (green). Nuclear staining is shown in blue.
We next examined whether the THI0019-induced enhancement in cell adhesion and spreading promoted or adversely affected cell migration. Stromal cell-derived factor −1 (SDF-1) is a primary chemokine involved in progenitor cell trafficking, both in the bone marrow and in homing to sites of ischemia and inflammation (21, 37). Jurkat cells were treated with vehicle or THI0019 and placed in the upper compartment of a Transwell chamber in which the membranes were coated with either fibronectin or VCAM-1; SDF-1 was placed in the bottom chamber. The results of the assay were similar for both ligands (Fig. 8). SDF-1 induced migration of cells above background levels. When cells were treated with THI0019, the number of migrated cells increased significantly above that observed with SDF-1 alone. As typically seen in these assays, the positive effect on cell migration resulted in a bell-shaped dose-response curve. The optimal migration was reached at 0.1 μm THI0019. The extent of migration seen at the highest dose of THI0019 tested (10 μm) was not significantly different from that seen with SDF-1 alone.
FIGURE 8.
THI0019 enhances SDF-1-mediated cell migration. Results of SDF-1-induced migration of Jurkat cells in Transwell chambers coated with fibronectin (FN) or VCAM-1. Cells were treated with THI0019 or vehicle (−) as indicated. After 4 h, the number of cells in the lower chamber were counted. Results are expressed as the total number of cells migrated ± S.D. *, p < 0.05. versus SDF-1 alone.
THI0019 Cross-reacts with Other Integrins
THI0019 was derived from the antagonist TBC3486, which is highly selective for α4β1 (31). Because the agonist was active in the micromolar range, cross-reactivity with other integrins was possible. THI0019 had negligible effects on cell binding mediated by αvβ3, α1β1, and α2β1 (data not shown); however, it enhanced the binding of cells expressing the related integrin α4β7 to mucosal addressin cell adhesion molecule (MAdCAM)-1, with an EC50 similar to that previously measured for the binding of α4β1 to VCAM-1 (Fig. 9A). This effect was blocked by antibodies to α4, β7, and MAdCAM-1 but not β1 (Fig. 9B). Surprisingly, THI0019 also showed agonist activity in assays measuring α5β1- and αLβ2-mediated adhesion to fibronectin and intercellular adhesion molecule (ICAM)-1, respectively (Fig. 9, C and D). The latter was particularly unexpected because αL contains an I domain that is the primary determinant of integrin binding. Neutralizing antibodies against α4 did not inhibit these effects (Fig. 9, E and F). Using flow cytometry, we ensured that the cell lines used expressed little or no α4 integrin (data not shown) so that any effects of possible cross-talk between α4 integrins and α5β1 or αLβ2 would be minimized.
THI0019 Overlaps with the Ligand-binding Site of α4 Integrins
To further elucidate the mechanism by which THI0019 acts as an agonist of integrin activity, we docked the compound into the recently published x-ray crystal structure of α4β7 (38) because no crystal structure forms of α4β1 have been determined. The α4β7 integrin shares the same α subunit as α4β1, and, as described above, the agonist THI0019 enhanced the binding of α4β7-expressing cells to the ligand MAdCAM-1. THI0019 is a structural analog of the ligand mimetic TBC3486 and, not surprisingly, docked favorably into the ligand-binding site (ΔGbind score of −75.9) (Fig. 10A). The model predicts that THI0019 bridges both the α and β subunits, which is consistent with previous x-ray structures of integrins co-crystallized with small molecules (10, 38). Unlike most antagonists, however, there was no interaction with the MIDAS site in the β subunit, which is consistent with the LIBS antibody results. The model does predict hydrogen bonding between the amide NH group of residue Ser-238 of the β subunit with the carbamate carbonyl of the compound. In addition, the amide carbonyl and NH groups of Asn-235 are predicted to form hydrogen bonds with one of the urea NH groups and the ester carbonyl of the compound, respectively. There was clearly interaction between one of the thiophene rings and the α subunit, including π-π stacking interactions with residues Tyr-187 and Phe-214. Site-directed mutagenesis has shown that Tyr-187 is critical for α4β1 binding to VCAM-1 and CS1 (39) and for α4β7 binding to MAdCAM-1 (40). In addition, the analogous residue in the α5 subunit, Phe-187, is required for optimal binding of fibronectin to α5β1 (39).This may explain in part how THI0019 can interact with all three of these integrins. However, this binding mode results in the paradox that the binding site for the small molecule agonist overlaps with that of the ligand, at least for integrins that do not contain an I domain. As such, the compound must be displaced from the binding pocket upon ligand binding. This scenario seems plausible for α4β1 because the EC50 of THI0019 is ∼1 μm, whereas the KD of VCAM-1 binding to α4β1 has been reported as ∼10 nm (41). To test this hypothesis, we synthesized an analog of THI0019 in which one of the thiophene rings was replaced with a phenyl azide (THI0455) (Fig. 10B). Based on the model, we predicted that, similar to the thiophene, the phenyl azide would interact with the α4 subunit and thus promote adhesion. Upon exposure to ultraviolet (UV) light, the phenyl azide forms a nitrene group that can initiate reactions to cross-link the compound to the receptor. If the two binding sites overlap, cross-linking THI0455 to the integrin should inhibit rather than enhance cell adhesion. As predicted, THI0455 was as potent an agonist as THI0019 in the absence of UV treatment (Fig. 10C). When THI0455 and cells were subjected to UV light separately before mixing, THI0455 had no effect on cell adhesion, although the THI0019 control remained active (Fig. 10D). This is consistent with the hydrophobic phenyl azide being converted to a more hydrophilic photoproduct in buffer, which is not predicted to bind favorably to the integrin. For these experiments, VCAM-1 was coated at a dose that would allow detection of both agonism and antagonism. Cells treated with UV radiation in the presence of THI0455, however, showed a 50% inhibition of cell adhesion to VCAM-1 compared with cells treated with UV radiation in the presence of vehicle (Fig. 10E). When analogous experiments were performed with αLβ2-expressing cells and ICAM-1, THI0455 was again an agonist in the absence of UV treatment (Fig. 10F) and was inactivated upon exposure to UV radiation (Fig. 10G). However, in contrast to the α4β1 results, THI0455 remained an agonist after cross-linking to αLβ2 (Fig. 10H), most likely because the ligand-binding site is contained within the I domain of αLβ2 and not at the α/β subunit interface, where we predicted the compound to bind. The extent of agonism was reduced after cross-linking, most likely because any free compound was quickly inactivated by UV radiation so the effective concentration of compound is decreased (Fig. 10G).
DISCUSSION
One of the major hurdles to achieving maximal benefits from stem cell therapy is the low retention of administered cells at the target site. The integrin α4β1 plays a key role in the homing of progenitor cells to sites of injury and mediates cell adhesion to expressed ligands (17, 18). In this study, we describe the development of the first small molecule agonist of α4β1 or any integrin lacking an I domain. We generated this agonist, THI0019, by making two structural modifications to a known α4β1 antagonist. THI0019 enhanced rolling, spreading, adhesion, and migration of cells in an α4β1-dependent fashion. Although these studies were performed in vitro, our finding that THI0019 significantly increased the adhesion of EPCs and HPCs under both static and flow conditions suggests that this compound may potentially improve cell therapy by helping to home and retain cells at the site of injury.
There are similarities and key differences between THI0019 and previously described integrin agonists. Studies have shown that an RGD peptide can trigger activation of integrin αIIbβ3, which allows it to bind fibrinogen after washout of the peptide (22). Binding of the peptide induces a conformational change in the integrin that promotes binding to fibrinogen, possibly by facilitating recognition of synergy sequences present in the ligand. The RGD peptide also functions to inhibit integrin function, yet the partial agonist and antagonist effects are due to occupation of the same binding site (22). We speculate that similar events are applicable to THI0019, except that it is a full agonist. The small molecule agonist binds with relatively low affinity and induces a subtle conformational switch that facilitates ligand binding with consequent displacement of the compound. Such a conformation may promote recognition of accessory binding sites known to be present in VCAM-1 and fibronectin (42, 43).
Agonists have also been described for the β2 family of integrins (23, 44, 45), which contain I domains. A partial agonist of αLβ2 integrin has been identified that is an allosteric antagonist when screened in the presence of Mn2+ but functions as an agonist in the presence of Mg2+ and Ca2+ (23). The authors speculate that both activities are mediated by binding to the same pocket and that the interaction of the compound with the I domain is altered depending on whether Ca2+ or Mn2+ is bound. In the presence of Ca2+, the compound can bind to the I domain in a manner that induces an active conformation. By inducing sustained adhesion to substrate, the partial agonist effectively inhibited leukocyte migration by preventing detachment of the trailing edge of cells (23). In addition, two research groups have used high throughput screening to identify agonists of αMβ2 (44, 46). These compounds share common structural features and are thought to stabilize the high affinity conformation of the αMβ2 integrin (44, 45). Moreover, these compounds have been proposed for use as anti-inflammatory agents because they inhibit leukocyte migration by not allowing detachment of cells once bound firmly to their counter-receptors (44, 45). Proof-of-concept studies have demonstrated the efficacy of these compounds in animal models of peritonitis, nephritis, and coronary angioplasty (44, 45). In contrast, THI0019 promotes rather than inhibits cell migration. Therefore, THI0019 would be used clinically in a different manner. We believe it could be used as an adjunct to cell-based therapy; premixing cells with THI0019 immediately before injection may enhance the homing and engraftment of delivered cells, and potential mechanistic side effects would be minimized because the total dose injected would be low, thus limiting the circulating levels of free compound.
THI0019 is synergistic with two other known integrin activators, Mn2+ and mAb TS2/16, both of which interact with the β1 subunit. Thus, the agonist functions through a mechanism distinct from that of either Mn2+ or TS2/16. In addition, THI0019 does not induce epitopes recognized by three different LIBS mAbs, all of which bind the β subunit. This finding indicates that the compound does not engage the MIDAS site, which is consistent with the lack of a carboxylic acid group on THI0019 and THI0019's inability to induce a global conformational change in the β subunit. Unlike other known integrin activators, THI0019 may induce subtle conformational changes, and the primary effects may be mediated through the α subunit. Previous molecular docking studies of its parent antagonist molecule, TBC3486, into a model of the β1 subunit indicate that the carboxylic acid group and surrounding motifs bind to the β1 subunit, leaving the bis(arylmethyl)aminocarbonyl group available to interact with the α subunit (47). Activity data from multiple analogs indicate that the bis(arylmethyl)aminocarbonyl motif of THI0019 is much more sensitive to structural modifications than is the rest of the molecule.3 Docking of the compound into the recently published crystal structure of α4β7 predicts that although portions of THI0019 interact with the β subunit through hydrogen bonding, one of the key binding determinants is through π-π stacking of the thiophene rings with Tyr-187 and Phe-214 in the α4 subunit. These two residues have recently been shown to interact with a class of small molecule antagonists of α4β7 that were co-crystallized with the integrin (38). A precedent for the importance of the interaction with the α subunit was recently shown in studies of RUC-1, a small molecule antagonist of integrin αIIbβ3 that has a unique mode of binding (48). X-ray crystal structure data indicate that RUC-1 binds exclusively to the α subunit with only minor bridging to the β subunit through water molecules. In contrast to antagonists that are ligand mimetics that bind and induce a high affinity conformation, RUC-1 appears to bind and maintain the integrin in a closed low affinity conformation. Similar to THI0019, RUC-1 does not induce LIBS epitopes in the β subunit and has activity in the low micromolar range, probably because it does not engage in a high affinity interaction with the MIDAS site of the integrin (48). Unlike THI0019, however, RUC-1 is an antagonist of integrin function and does not induce ligand binding. Further studies, including co-crystallization of the integrin with the compound, will be required to delineate the details of the interaction between the agonist THI0019 and the integrin.
The mode of binding predicted by our docking experiments indicates that THI0019 binds at a site that overlaps the ligand binding pocket. Thus, we hypothesized that the compound would have to be displaced from this site upon ligand binding. To test this prediction, we synthesized a structural analog of THI0019 in which one of the thiophene rings was replaced with a photoreactive phenyl azide. If the sites overlapped, cross-linking of the compound to the integrin would inhibit rather than enhance cell adhesion. Mixing the cells with the analog compound followed by UV treatment converted the compound from an agonist to an antagonist, thus supporting our model. Ironically, this suggests that if a compound binds to the integrin with too high affinity, thereby preventing its displacement by ligand, it would not be able to function as an agonist. This idea is supported by the fact that additional analoging did not result in a compound that was significantly more potent than THI0019.3 This also may be the reason why the weak antagonist THI0003 (the transition compound between TBC3486 and THI0019) was a suitable intermediate to generate an agonist. In contrast, neither TBC3486 nor BIO5192, which contains a diphenyl urea motif that confers potent binding to α4β1 (49), could be converted directly to a full agonist by simply esterifying the carboxylic acid group.
THI0019 shows agonist activity against integrin α4β1 and α4β7 with an EC50 value in the 1–2 μm range. At these concentrations, it is not uncommon to detect cross-reactivity with other integrins (50, 51). In contrast to the potent parent compound, TBC3486, which was highly selective for α4β1, THI0019 also regulates adhesion mediated by α5β1 and αLβ2. Small cyclic RGD peptides have been identified previously that inhibit both α4β1 and α5β1 with IC50 values in the low micromolar range (50). Furthermore, regions of the β-propeller domain of the α5 subunit contain extensive homology to the α4 subunit, including the region surrounding residue Tyr-187. In fact, mutations to the analogous residue in α5 significantly reduce adhesion of α5β1 to fibronectin (39). Assuming a similar binding mode, any ligand (or ligand mimetic)-induced activation of the integrin by the agonist may also apply to α5β1.
The activity toward αLβ2 was somewhat surprising because this integrin contains an I domain in the α subunit. This is not without precedent because small molecule integrin antagonists have been identified that inhibit both α4β1 and αLβ2 at micromolar concentrations (51). If THI0019 occupies a site in αLβ2 that is analogous to that of α4β1, it would not be predicted to overlap with the ICAM-1-binding site, which is located in the I domain. Rather, THI0019 may bind to the β-propeller domain of the αL subunit and influence the orientation and therefore the activity of the I domain. The results of the cross-linking studies support this hypothesis. When THI0455 was cross-linked to α4β1, it competed with VCAM-1 for the ligand-binding site , behaving as an antagonist. In the case of αLβ2, however, THI0455 remained an agonist even after cross-linking, as predicted if binding occurs at the α/β interface and not within the ligand-binding site of the I domain. Previously, an epitope mapping study of antibodies that interact with the I domain of αLβ2 showed that antibodies that were antagonists of the integrin bound in proximity to the ICAM-1 contact site, whereas an antibody that had agonist activity bound distal to the ligand-binding site, closer to where the I domain contacts the β-propeller domain (52). These cross-target activities may enhance the ability of THI0019 to promote the retention of stem/progenitor cells in vivo. HPCs and EPCs have been reported to express α5β1 and αLβ2, which have been shown to be important for the homing of these cells to the bone marrow and to sites of ischemia, respectively (13, 53).
As a therapeutic agent, THI0019 has advantages over other reported activators of integrins for stem cell therapy. First, it binds the target integrin directly and does not require any extensive preconditioning or genetic manipulation of cells. Second, THI0019 does not induce the binding of any of three different LIBS mAbs. This characteristic could be advantageous in the clinical setting because the induction of LIBS neoepitopes has been associated with acute thrombocytopenia in patients treated with αIIbβ3 integrin antagonists such as tirofiban and eptifibatide (54). Third, the synergy seen with mAb TS2/16 allows for the potential of combination therapy to enhance the overall effect. Finally, large scale production of a low molecular weight small molecule such as THI0019 is likely to be less expensive than the production of previously reported biologic agents.
Supplementary Material
Acknowledgments
We thank Rebecca Bartow, Ph.D., and Nicole Stancel, Ph.D., of the Texas Heart Institute for editorial assistance and Deenadayalan Bakthavatsalam, Ph.D., for confocal microscopy advice. The Translational Chemistry Core Facility at the University of Texas M. D. Anderson Cancer Center was the recipient of National Institutes of Health Cancer Center Support Grant CA016672.
This work was supported, in whole or in part, by National Institutes of Health Grant 2 T32-CA-09598-21 (to W. S. B.). P. V., R. J. B., and C. W. G. are named on a patent application that was submitted by the Texas Heart Institute.

This article contains supplemental Movies 1–4.
R. J. Biediger, C. W. Gundlach IV, S. Khounlo, N. Warier, R. Market, P. Vanderslice, and R. A. F. Dixon, manuscript in preparation.
- MIDAS
- metal ion-dependent adhesion site
- VLA-4
- very late antigen-4
- EPC
- endothelial progenitor cell
- HPC
- hematopoietic progenitor cell
- VCAM-1
- vascular cell adhesion molecule-1
- CS1
- connecting segment-1
- LIBS
- ligand-induced binding site
- SDF-1
- stromal cell-derived factor-1
- MAdCAM-1
- mucosal addressin cell adhesion molecule-1
- ICAM-1
- intercellular adhesion molecule-1.
REFERENCES
- 1. Perin E. C., Dohmann H. F., Borojevic R., Silva S. A., Sousa A. L., Mesquita C. T., Rossi M. I., Carvalho A. C., Dutra H. S., Dohmann H. J., Silva G. V., Belém L., Vivacqua R., Rangel F. O., Esporcatte R., Geng Y. J., Vaughn W. K., Assad J. A., Mesquita E. T., Willerson J. T. (2003) Transendocardial, autologous bone marrow cell transplantation for severe, chronic ischemic heart failure. Circulation 107, 2294–2302 [DOI] [PubMed] [Google Scholar]
- 2. Schächinger V., Erbs S., Elsässer A., Haberbosch W., Hambrecht R., Hölschermann H., Yu J., Corti R., Mathey D. G., Hamm C. W., Süselbeck T., Assmus B., Tonn T., Dimmeler S., Zeiher A. M., and REPAIR-AMI Investigators (2006) Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N. Engl. J. Med. 355, 1210–1221 [DOI] [PubMed] [Google Scholar]
- 3. Schächinger V., Aicher A., Döbert N., Röver R., Diener J., Fichtlscherer S., Assmus B., Seeger F. H., Menzel C., Brenner W., Dimmeler S., Zeiher A. M. (2008) Pilot trial on determinants of progenitor cell recruitment to the infarcted human myocardium. Circulation 118, 1425–1432 [DOI] [PubMed] [Google Scholar]
- 4. Liu J., Narsinh K. H., Lan F., Wang L., Nguyen P. K., Hu S., Lee A., Han L., Gong Y., Huang M., Nag D., Rosenberg J., Chouldechova A., Robbins R. C., Wu J. C. (2012) Early stem cell engraftment predicts late cardiac functional recovery: preclinical insights from molecular imaging. Circ. Cardiovasc. Imaging 5, 481–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vrtovec B., Poglajen G., Lezaic L., Sever M., Domanovic D., Cernelc P., Socan A., Schrepfer S., Torre-Amione G., Haddad F., Wu J. C. (2013) Effects of intracoronary CD34+ stem cell transplantation in nonischemic dilated cardiomyopathy patients: 5-year follow up. Circ. Res. 112, 165–173 [DOI] [PubMed] [Google Scholar]
- 6. Chavakis E., Urbich C., Dimmeler S. (2008) Homing and engraftment of progenitor cells: a prerequisite for cell therapy. J. Mol. Cell. Cardiol. 45, 514–522 [DOI] [PubMed] [Google Scholar]
- 7. Hynes R. O. (1992) Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69, 11–25 [DOI] [PubMed] [Google Scholar]
- 8. Coller B. S. (1997) Platelet GPIIb/IIIa antagonists: the first anti-integrin receptor therapeutics. J. Clin. Invest. 100, S57–S60 [PubMed] [Google Scholar]
- 9. Polman C. H., O'Connor P. W., Havrdova E., Hutchinson M., Kappos L., Miller D. H., Phillips J. T., Lublin F. D., Giovannoni G., Wajgt A., Toal M., Lynn F., Panzara M. A., Sandrock A. W. (2006) A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354, 899–910 [DOI] [PubMed] [Google Scholar]
- 10. Xiong J. P., Stehle T., Zhang R., Joachimiak A., Frech M., Goodman S. L., Arnaout M. A. (2002) Crystal structure of the extracellular segment of integrin αVβ3 in complex with an Arg-Gly-Asp ligand. Science 296, 151–155 [DOI] [PubMed] [Google Scholar]
- 11. Lee J. O., Rieu P., Arnaout M. A., Liddington R. (1995) Crystal structure of the A domain from the α subunit of integrin CR3 (CD11b/CD18). Cell 80, 631–638 [DOI] [PubMed] [Google Scholar]
- 12. Luo B. H., Carman C. V., Springer T. A. (2007) Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 25, 619–647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chavakis E., Aicher A., Heeschen C., Sasaki K., Kaiser R., El Makhfi N., Urbich C., Peters T., Scharffetter-Kochanek K., Zeiher A. M., Chavakis T., Dimmeler S. (2005) Role of β2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J. Exp. Med. 201, 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Song H., Chang W., Lim S., Seo H. S., Shim C. Y., Park S., Yoo K. J., Kim B. S., Min B. H., Lee H., Jang Y., Chung N., Hwang K. C. (2007) Tissue transglutaminase is essential for integrin-mediated survival of bone marrow-derived mesenchymal stem cells. Stem Cells 25, 1431–1438 [DOI] [PubMed] [Google Scholar]
- 15. Chavakis E., Hain A., Vinci M., Carmona G., Bianchi M. E., Vajkoczy P., Zeiher A. M., Chavakis T., Dimmeler S. (2007) High mobility group box 1 activates integrin-dependent homing of endothelial progenitor cells. Circ. Res. 100, 204–212 [DOI] [PubMed] [Google Scholar]
- 16. Schroeter M. R., Leifheit M., Sudholt P., Heida N. M., Dellas C., Rohm I., Alves F., Zientkowska M., Rafail S., Puls M., Hasenfuss G., Konstantinides S., Schäfer K. (2008) Leptin enhances the recruitment of endothelial progenitor cells into neointimal lesions after vascular injury by promoting integrin-mediated adhesion. Circ. Res. 103, 536–544 [DOI] [PubMed] [Google Scholar]
- 17. Duan H., Cheng L., Sun X., Wu Y., Hu L., Wang J., Zhao H., Lu G. (2006) LFA-1 and VLA-4 involved in human high proliferative potential-endothelial progenitor cells homing to ischemic tissue. Thromb. Haemost. 96, 807–815 [PubMed] [Google Scholar]
- 18. Teixidó J., Hemler M. E., Greenberger J. S., Anklesaria P. (1992) Role of β1 and β2 integrins in the adhesion of human CD34hi stem cells to bone marrow stroma. J. Clin. Invest. 90, 358–367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhang S., Shpall E., Willerson J. T., Yeh E. T. (2007) Fusion of human hematopoietic progenitor cells and murine cardiomyocytes is mediated by α4β1 integrin/vascular cell adhesion molecule-1 interaction. Circ. Res. 100, 693–702 [DOI] [PubMed] [Google Scholar]
- 20. Dobaczewski M., Bujak M., Zymek P., Ren G., Entman M. L., Frangogiannis N. G. (2006) Extracellular matrix remodeling in canine and mouse myocardial infarcts. Cell Tissue Res. 324, 475–488 [DOI] [PubMed] [Google Scholar]
- 21. Abbott J. D., Huang Y., Liu D., Hickey R., Krause D. S., Giordano F. J. (2004) Stromal cell-derived factor-1α plays a critical role in stem cell recruitment to the heart after myocardial infarction but is not sufficient to induce homing in the absence of injury. Circulation 110, 3300–3305 [DOI] [PubMed] [Google Scholar]
- 22. Du X. P., Plow E. F., Frelinger A. L., 3rd, O'Toole T. E., Loftus J. C., Ginsberg M. H. (1991) Ligands “activate” integrin αIIbβ3 (platelet GPIIb–IIIa). Cell 65, 409–416 [DOI] [PubMed] [Google Scholar]
- 23. Yang W., Carman C. V., Kim M., Salas A., Shimaoka M., Springer T. A. (2006) A small molecule agonist of an integrin, αLβ2. J. Biol. Chem. 281, 37904–37912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Biediger R. J., Grabbe V. O., Holland G. W., Kassir J. M., Kogan T. P., Lin S., Market R. V., Raju B. G., Scott I. L., Wu C. (February 27, 2001) N,N-Disubstituted amides that inhibit the binding of integrins to their receptors, U. S. Patent 6,194,448
- 25. Biediger R. J., Gundlach C. W., Market R. V., Savage M. M., Vanderslice P. (May 24, 2012) Agonists That Enhance Binding of Integrin-expressing Cells to Integrin Receptors, International Patent PCT/US2011/060996
- 26. Kassir J. M., Grabbe V. O., Biediger R. J., Scott I. L., Raju B. G., Ren K., Market R. V., Keller K. M., Lin S., Kogan T. P., Krudy G. A., Chen Q., Holland G. W., Maxwell D. C., You T. J., Vanderslice P., West H., Decker E. R., Tilton R., Munsch C. L., Dixon R. A. F. (2000) Book of Abstracts 219th ACS National Meeting, San Francisco, CA, March 26–30, 2000, Abstr. MEDI284, ACS Publications, Washington, D.C [Google Scholar]
- 27. Scott D., Lee W.-C., Petter R. C., Cornebise M. (October 7, 2003) Cell adhesion inhibitors, U. S. Patent 6,630,503
- 28. Vanderslice P., Ren K., Revelle J. K., Kim D. C., Scott D., Bjercke R. J., Yeh E. T., Beck P. J., Kogan T. P. (1997) A cyclic hexapeptide is a potent antagonist of α4 integrins. J. Immunol. 158, 1710–1718 [PubMed] [Google Scholar]
- 29. Rose D. M., Liu S., Woodside D. G., Han J., Schlaepfer D. D., Ginsberg M. H. (2003) Paxillin binding to the α4 integrin subunit stimulates LFA-1 (integrin αLβ2)-dependent T cell migration by augmenting the activation of focal adhesion kinase/proline-rich tyrosine kinase-2. J. Immunol. 170, 5912–5918 [DOI] [PubMed] [Google Scholar]
- 30. Woodside D. G., Kram R. M., Mitchell J. S., Belsom T., Billard M. J., McIntyre B. W., Vanderslice P. (2006) Contrasting roles for domain 4 of VCAM-1 in the regulation of cell adhesion and soluble VCAM-1 binding to integrin α4β1. J. Immunol. 176, 5041–5049 [DOI] [PubMed] [Google Scholar]
- 31. Vanderslice P., Woodside D. G., Caivano A. R., Decker E. R., Munsch C. L., Sherwood S. J., Lejeune W. S., Miyamoto Y. J., McIntyre B. W., Tilton R. G., Dixon R. A. (2010) Potent in vivo suppression of inflammation by selectively targeting the high affinity conformation of integrin α4β1. Biochem. Biophys. Res. Commun. 400, 619–624 [DOI] [PubMed] [Google Scholar]
- 32. Berman H. M., Westbrook J., Feng Z., Gilliland G., Bhat T. N., Weissig H., Shindyalov I. N., Bourne P. E. (2000) The Protein Data Bank. Nucleic Acids Res. 28, 235–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Leone D. R., Giza K., Gill A., Dolinski B. M., Yang W., Perper S., Scott D. M., Lee W. C., Cornebise M., Wortham K., Nickerson-Nutter C., Chen L. L., LePage D., Spell J. C., Whalley E. T., Petter R. C., Adams S. P., Lobb R. R., Pepinsky R. B. (2003) An assessment of the mechanistic differences between two integrin α4β1 inhibitors, the monoclonal antibody TA-2 and the small molecule BIO5192, in rat experimental autoimmune encephalomyelitis. J. Pharmacol. Exp. Ther. 305, 1150–1162 [DOI] [PubMed] [Google Scholar]
- 34. Chen J., Salas A., Springer T. A. (2003) Bistable regulation of integrin adhesiveness by a bipolar metal ion cluster. Nat. Struct. Biol. 10, 995–1001 [DOI] [PubMed] [Google Scholar]
- 35. Arroyo A. G., García-Pardo A., Sánchez-Madrid F. (1993) A high affinity conformational state on VLA integrin heterodimers induced by an anti-β1 chain monoclonal antibody. J. Biol. Chem. 268, 9863–9868 [PubMed] [Google Scholar]
- 36. Szabo M. C., Teague T. K., McIntyre B. W. (1995) Regulation of lymphocyte pseudopodia formation by triggering the integrin α4/β1. J. Immunol. 154, 2112–2124 [PubMed] [Google Scholar]
- 37. Peled A., Petit I., Kollet O., Magid M., Ponomaryov T., Byk T., Nagler A., Ben-Hur H., Many A., Shultz L., Lider O., Alon R., Zipori D., Lapidot T. (1999) Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283, 845–848 [DOI] [PubMed] [Google Scholar]
- 38. Yu Y., Zhu J., Mi L. Z., Walz T., Sun H., Chen J., Springer T. A. (2012) Structural specializations of α(4)β(7), an integrin that mediates rolling adhesion. J. Cell Biol. 196, 131–146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Irie A., Kamata T., Puzon-McLaughlin W., Takada Y. (1995) Critical amino acid residues for ligand binding are clustered in a predicted β-turn of the third N-terminal repeat in the integrin α4 and α5 subunits. EMBO J. 14, 5550–5556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ruiz-Velasco N., Guerrero-Esteo M., Briskin M. J., Teixidó J. (2000) The α(4) integrin subunit Tyr(187) has a key role in α(4)β(7)-dependent cell adhesion. J. Biol. Chem. 275, 7052–7059 [DOI] [PubMed] [Google Scholar]
- 41. Pepinsky R. B., Mumford R. A., Chen L. L., Leone D., Amo S. E., Riper G. V., Whitty A., Dolinski B., Lobb R. R., Dean D. C., Chang L. L., Raab C. E., Si Q., Hagmann W. K., Lingham R. B. (2002) Comparative assessment of the ligand and metal ion binding properties of integrins α9β1 and α4β1. Biochemistry 41, 7125–7141 [DOI] [PubMed] [Google Scholar]
- 42. Obara M., Kang M. S., Yamada K. M. (1988) Site-directed mutagenesis of the cell-binding domain of human fibronectin: separable, synergistic sites mediate adhesive function. Cell 53, 649–657 [DOI] [PubMed] [Google Scholar]
- 43. Newham P., Craig S. E., Seddon G. N., Schofield N. R., Rees A., Edwards R. M., Jones E. Y., Humphries M. J. (1997) α4 integrin binding interfaces on VCAM-1 and MAdCAM-1. Integrin binding footprints identify accessory binding sites that play a role in integrin specificity. J. Biol. Chem. 272, 19429–19440 [DOI] [PubMed] [Google Scholar]
- 44. Björklund M., Aitio O., Stefanidakis M., Suojanen J., Salo T., Sorsa T., Koivunen E. (2006) Stabilization of the activated αMβ2 integrin by a small molecule inhibits leukocyte migration and recruitment. Biochemistry 45, 2862–2871 [DOI] [PubMed] [Google Scholar]
- 45. Maiguel D., Faridi M. H., Wei C., Kuwano Y., Balla K. M., Hernandez D., Barth C. J., Lugo G., Donnelly M., Nayer A., Moita L. F., Schürer S., Traver D., Ruiz P., Vazquez-Padron R. I., Ley K., Reiser J., Gupta V. (2011) Small molecule-mediated activation of the integrin CD11b/CD18 reduces inflammatory disease. Sci. Signal. 4, ra57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Park J. Y., Arnaout M. A., Gupta V. (2007) A simple, no-wash cell adhesion-based high throughput assay for the discovery of small molecule regulators of the integrin CD11b/CD18. J. Biomol. Screen. 12, 406–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. You T. J., Maxwell D. S., Kogan T. P., Chen Q., Li J., Kassir J., Holland G. W., Dixon R. A. (2002) A 3D structure model of integrin α4β1 complex: I. Construction of a homology model of β1 and ligand binding analysis. Biophys. J. 82, 447–457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhu J., Zhu J., Negri A., Provasi D., Filizola M., Coller B. S., Springer T. A. (2010) Closed headpiece of integrin αIIbβ3 and its complex with an αIIbβ3-specific antagonist that does not induce opening. Blood 116, 5050–5059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lin Kc., Ateeq H. S., Hsiung S. H., Chong L. T., Zimmerman C. N., Castro A., Lee W. C., Hammond C. E., Kalkunte S., Chen L. L., Pepinsky R. B., Leone D. R., Sprague A. G., Abraham W. M., Gill A., Lobb R. R., Adams S. P. (1999) Selective, tight-binding inhibitors of integrin α4β1 that inhibit allergic airway responses. J. Med. Chem. 42, 920–934 [DOI] [PubMed] [Google Scholar]
- 50. Nowlin D. M., Gorcsan F., Moscinski M., Chiang S. L., Lobl T. J., Cardarelli P. M. (1993) A novel cyclic pentapeptide inhibits α4β1 and α5β1 integrin-mediated cell adhesion. J. Biol. Chem. 268, 20352–20359 [PubMed] [Google Scholar]
- 51. Shimaoka M., Springer T. A. (2003) Therapeutic antagonists and conformational regulation of integrin function. Nat. Rev. Drug Discov. 2, 703–716 [DOI] [PubMed] [Google Scholar]
- 52. Lu C., Shimaoka M., Salas A., Springer T. A. (2004) The binding sites for competitive antagonistic, allosteric antagonistic, and agonistic antibodies to the I domain of integrin LFA-1. J. Immunol. 173, 3972–3978 [DOI] [PubMed] [Google Scholar]
- 53. Asaumi N., Omoto E., Mahmut N., Katayama Y., Takeda K., Shinagawa K., Harada M. (2001) Very late antigen-5 and leukocyte function-associated antigen-1 are critical for early stage hematopoietic progenitor cell homing. Ann. Hematol. 80, 387–392 [DOI] [PubMed] [Google Scholar]
- 54. Bougie D. W., Wilker P. R., Wuitschick E. D., Curtis B. R., Malik M., Levine S., Lind R. N., Pereira J., Aster R. H. (2002) Acute thrombocytopenia after treatment with tirofiban or eptifibatide is associated with antibodies specific for ligand-occupied GPIIb/IIIa. Blood 100, 2071–2076 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.