Background: 1,25(OH)2D3 inhibits NF-κB activation by an undefined mechanism.
Results: Vitamin D receptor protein binds to IKKβ protein, blocking TNFα-induced IKK complex formation and NF-κB activity.
Conclusion: The vitamin D receptor suppresses NF-κB activation by directly interacting with IKKβ.
Significance: This is a novel mechanism whereby 1,25(OH)2D3-VDR inhibits NF-κB.
Keywords: Inflammation, NF-κB, Nuclear Receptors, Protein-Protein Interactions, Vitamin D, IKKβ
Abstract
1,25-Dihydroxyvitamin D (1,25(OH)2D3) is known to suppress NF-κB activity, but the underlying mechanism remains poorly understood. Here we show that the vitamin D receptor (VDR) physically interacts with IκB kinase β (IKKβ) to block NF-κB activation. 1,25(OH)2D3 rapidly attenuates TNFα-induced p65 nuclear translocation and NF-κB activity in a VDR-dependent manner. VDR overexpression inhibits IKKβ-induced NF-κB activity. GST pull-down assays and coimmunoprecipitation experiments demonstrated that VDR physically interacts with IKKβ and that this interaction is enhanced by 1,25(OH)2D3. Protein mapping reveals that VDR-IKKβ interaction occurs between the C-terminal portions of the VDR and IKKβ proteins. Reconstitution of VDR−/− cells with the VDR C terminus restores the ability to block TNFα-induced NF-κB activation and IL-6 up-regulation. VDR-IKKβ interaction disrupts the formation of the IKK complex and, thus, abrogates IKKβ phosphorylation at Ser-177 and abolishes IKK activity to phosphorylate IκBα. Consequently, stabilization of IκBα arrests p65/p50 nuclear translocation. Together, these data define a novel mechanism whereby 1,25(OH)2D3-VDR inhibits NF-κB activation.
Introduction
NF-κB is a family of transcription factors consisting of five proteins: NF-κB1 (p105/p50), NF-κB2 (p100/p52), RelA (p65), RelB, and c-Rel. These proteins form homo- or heterodimers that interact with a specific cis-DNA sequence (κB element) to regulate a wide range of genes, including those involved in immunity and inflammatory responses (1, 2). NF-κB can be activated via the canonical and non-canonical pathways (3), and its activation is regulated tightly. A crucial negative regulator that controls NF-κB activation is the inhibitor of κB (IκB), which binds to p65 in the cytosol to block the nuclear translocation of the p65/p50 heterodimer. Phosphorylation of IκB by activated IκB kinase (IKK)2 initiates the ubiquitylation and eventual proteasomal degradation of IκB, and a direct consequence of IκB degradation is nuclear entry of p65/p50 to transactivate gene expression (1, 2). Thus, IKK plays an essential role in NF-κB activation. The kinase activity of IKK depends on the formation of the IKK complex by the IKKα, β, and γ subunits, which is activated upon phosphorylation by growth factors, proinflammatory cytokines (such as TNFα), and hormones through the TNF receptor or Toll-like receptor superfamily (1). IKK also phosphorylates p65 to promote its activity (3, 4).
1,25-dihydroxyvitamin D (1,25(OH)2D3), the hormonal form of vitamin D, is a pleiotropic hormone that regulates a broad range of biological activities (5). The activity of 1,25(OH)2D3 is mediated by the vitamin D receptor (VDR), a member of the nuclear receptor superfamily (6). The classic VDR action model is that, upon 1,25(OH)2D3 activation, the VDR moves into the nucleus and heterodimerizes with the retinoid X receptor, which, together, bind to the vitamin D response element (VDRE) in the target gene promoter to up-regulate gene transcription (7). However, it has been reported that the VDR can down-regulate gene transcription by directly interacting with other regulatory proteins, such as β-catenin (8) and CREB (9), through VDRE-independent mechanisms.
Our previous work has demonstrated that VDR signaling intrinsically suppresses NF-κB activation because base-line NF-κB activity is elevated in the case of genetic VDR deletion (10). Consistently, many studies have shown that 1,25(OH)2D3 down-regulates a variety of genes, including IL-12, IL-8, MCP-1, PAI-1, angiotensinogen, and microRNA-155 by blocking NF-κB activation (11–16). Therefore, 1,25(OH)2D suppression of NF-κB activation has great biological and pathological relevance. The exact molecular mechanism underlying 1,25(OH)2D3 regulation of NF-κB, however, remains to be defined. It has been reported that 1,25(OH)2D3 arrests p65 nuclear translocation, blocks NF-κB DNA binding, increases IκBα levels, or stabilizes IκBα protein (10, 12, 14, 15, 17, 18). It has also been shown that 1,25(OH)2D3 suppresses RelB transcription (19) and reduces p105/p50 and c-rel protein levels (20). Interestingly, p65 has been reported to physically interact with liganded VDR to modulate the transactivating activity of the VDR (21). Despite all these reports, a convincing mechanism to explain the relatively rapid inhibitory action of vitamin D hormone on NF-κB activity is lacking. Particularly, how vitamin D increases or stabilizes IκBα, the most critical step in NF-κB regulation, remains unexplainable. In this report we elucidate a novel molecular mechanism by which 1,25(OH)2D3-VDR attenuates NF-κB activation. Our data demonstrate that the VDR protein is able to directly interact with IKKβ protein to block the canonical NF-κB activation pathway.
EXPERIMENTAL PROCEDURES
Cell Culture and Transfection
HEK293 and RAW264.7 cells were purchased from the ATCC. Generation of VDR+/− and VDR−/− mouse embryonic fibroblasts (MEFs) were reported previously (10). All cells were cultured in DMEM supplemented with 10% FBS at 37 °C and 5% CO2. Cell transfection was carried out using Lipofectamine 2000 (Invitrogen) according to the instructions of the manufacturer. Cells were treated with 10 ng/ml recombinant mouse TNFα (Millipore) and/or 20 nm 1,25(OH)2D3 unless indicated otherwise.
Plasmids
Plasmids that express HA- or FLAG-tagged IKKβ or hVDR and its N- and C-terminal fragments (VDR-N, VDR-C, IKKβ-N, and IKKβ-C) were created in the pCI-HA or pCI-FLAG plasmids (Addgene) using a PCR-based strategy. VDR-N contains amino acids 1–119, and VDR-C contains amino acids 120–427 of human VDR (hVDR) protein. IKKβ-N contains amino acids 1–346, and IKKβ-C contains amino acids 341–756 of IKKβ protein. All plasmid constructs were confirmed by DNA sequencing. The generation of pcDNA-hVDR(R274L) and pcDNA-hVDR(R391C) was reported previously (9). The pVDRE-Luc and pNF-κB-Luc reporter plasmids were described previously (10).
Western Blot Analyses
Proteins were separated by SDS-PAGE and electroblotted onto Immobilon-P membranes. Western blot analyses were carried out as described previously (22). The following antibodies were used in this study. Anti-IKKα/β, anti-p-IKKα/β, anti-IKKα, anti-IKKβ, anti-IKKγ, and anti-HA were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-FLAG and anti-β-actin were obtained from Sigma.
Luciferase Reporter Assays
HEK293 cells or MEFs were cotransfected with pNF-κB-Luc, pCI-HA-p65, or pCI-HA-IKKβ (or its N- or C-terminal constructs) and pcDNA-VDR plasmids (or its N- or C-terminal constructs) using Lipofectamine 2000 (Invitrogen). Transfected cells were treated with TNFα in the presence or absence of 1,25(OH)2D3 as indicated in each experiment. After 24 h, the cells were lysed, and luciferase activity was determined using the Dual-luciferase reporter assay system (Promega) as reported previously (9). Luciferase activity was normalized to the Renilla luciferase activity, which served as an internal control for transfection efficiency.
GST Pull-down Assays
GST-hVDR fusion protein was generated using the pGEX-4T-1 plasmid as reported previously (9). IKKα, IKKβ, p50, and p65 proteins were synthesized in the presence of [35S]methionine using an in vitro transcription and translation system (Promega). GST or GST-hVDR beads were incubated with 35S-labeled IKKα, IKKβ, p50, or p65 overnight. In some experiments, 20 nm 1,25(OH)2D3 was included in the incubation. After being washed five times, the beads were spun down and dissolved in Laemmli sample buffer. After being boiled for 5 min, the proteins were resolved using SDS-PAGE and visualized by autoradiography.
Coimmunoprecipitation (Co-IP) Assays
Cells were rinsed twice in ice-cold PBS and lysed in cold immunoprecipitation buffer (1% Triton X-100, 150 mm NaCl, 10 mm Tris-HCl (pH 7.4), 1 mm EDTA, 1 mm EGTA (pH 8.0), 0.2 mm sodium orthovanadate) containing protease inhibitor cocktails (Roche Applied Science). Cell lysates were immunoprecipitated with immunoprecipitation antibodies (anti-VDR, anti-IKKβ, anti-FLAG, or anti-HA) according to procedures described previously (10). The precipitates were dissolved in Laemmli sample buffer and analyzed by Western blotting with immunoblotting antibodies as indicated in each experiment.
IKK Assays
IKK complexes from whole-cell extracts were precipitated with anti-IKK-γ antibodies (Santa Cruz Biotechnology) and protein A/G-Sepharose beads (Millipore). After 2 h of incubation, the beads were washed with lysis buffer and then assayed in a kinase assay mixture containing 50 mm HEPES (pH 7.4), 20 mm MgCl2, 2 mm DTT, 20 μCi [γ-32P]ATP, 10 mm unlabeled ATP, and 2 μg of GST-IκBα (amino acids 1–54) substrate (Clontech). After incubation at 30 °C for 30 min, the reaction was terminated by 5 min of boiling in loading sample buffer. Finally, the proteins were resolved by 10% SDS-PAGE, and the radiolabeled substrate bands were visualized by autoradiography. To determine the total amount of IKKβ in each sample, 50 μg of the whole-cell extracts were resolved by 10% SDS-PAGE, electrotransferred to a nitrocellulose membrane, and blotted with anti-IKKβ antibody.
Statistical Analysis
Data values were presented as mean ± S.D. Statistical comparisons were carried out using unpaired two-tailed Student's t test or one-way analysis of variance as appropriate, with p < 0.05 being considered significant.
RESULTS
Vitamin D Blocks TNFα-induced NF-κB Activation
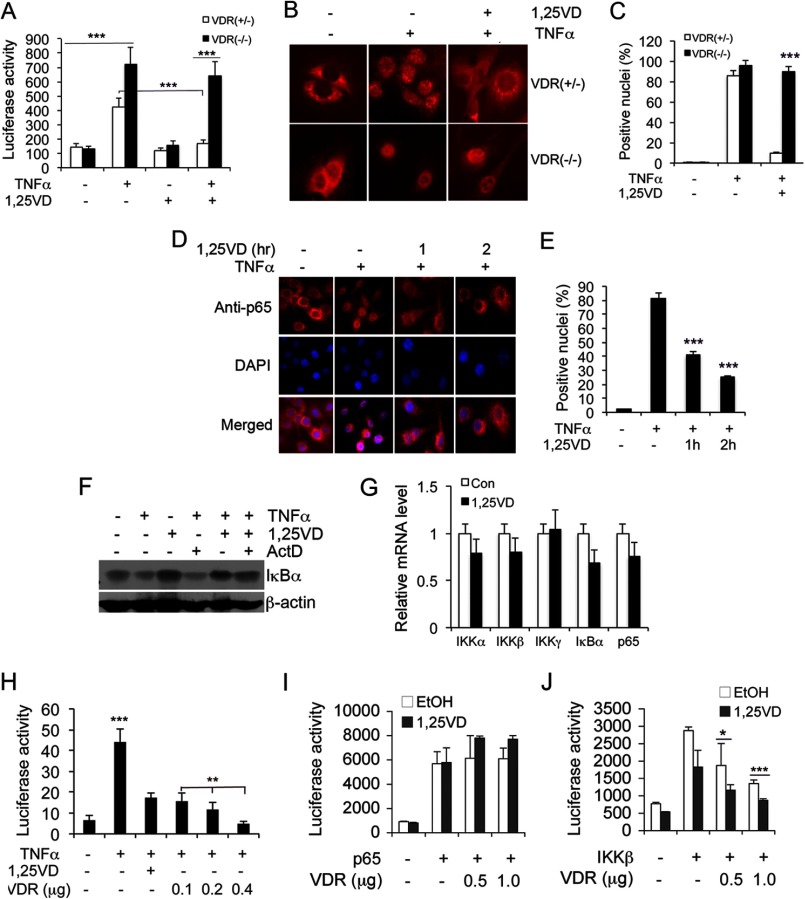
We first performed luciferase reporter assays to confirm the inhibitory effect of 1,25(OH)2D3 on NF-κB. As shown in Fig. 1, in MEF cells transfected with the pNF-κB-Luc reporter plasmid, TNFα drastically induced NF-κB luciferase activity. This induction was suppressed markedly by 1,25(OH)2D3 cotreatment in VDR+/− MEFs but not in VDR−/− MEF cells (Fig. 1A). Immunostaining showed that TNFα-induced p65 nuclear translocation was blocked by overnight 1,25(OH)2D3 pretreatment in VDR+/− MEF but not in VDR−/− MEF cells (Fig. 1, B and C), confirming the requirement of VDR for inhibition of NF-κB activation. Inhibition of TNFα-induced NF-κB activity by 1,25(OH)2D3 was also observed in HEK293 cells (not shown). Interestingly, a short exposure (1–2 h) of the VDR+/− MEF cells to 1,25(OH)2D3 was sufficient to block TNFα-induced p65 nuclear entry (Fig. 1, D and E), suggesting that it is unlikely that this inhibitory action involves a transcriptional event, which usually takes at least several hours. Indeed, 1,25(OH)2D3 blocked TNFα-induced degradation of IκBα in cells, and this activity was not affected by actinomycin D, an inhibitor of RNA synthesis (Fig. 1F). Moreover, 1,25(OH)2D3 treatment did not significantly alter the mRNA levels of NF-κB components IKKα, β, γ, IκBα, and p65 (Fig. 1G). These data confirmed that it is unlikely that 1,25(OH)2D3 suppresses NF-κB activation by a transcriptional mechanism. Interestingly, VDR overexpression in cells by transfection was sufficient to suppress TNFα-induced NF-κB activity dose-dependently in the absence of 1,25(OH)2D3 (Fig. 1H), suggesting that, at high concentrations, VDR can suppress NF-κB in a ligand-independent manner. It appears that the rapid blockade of p65 nuclear translocation can be explained by VDR interaction with p65, as reported previously.
FIGURE 1.
1,25-dihydroxyvitamin D rapidly attenuates NF-κB activation in a VDR-dependent manner. A, NF-κB luciferase reporter assays. VDR+/− and VDR−/− MEFs transfected with the pNF-κB-Luc reporter were treated with TNFα (10 ng/ml) and/or 1,25(OH)2D3 (20 nm) (1,25VD) as indicated for 24 h before measuring luciferase activity. ***, p < 0.001. B and C, effects of 1,25(OH)2D3 on p65 nuclear translocation. VDR+/− and VDR−/− MEFs were pretreated with vehicle or 1,25(OH)2D3 overnight, followed by 2 h of TNFα stimulation as indicated. The cells were immunostained with anti-p65 antibodies (B), and VDR-positive nuclei were quantified in each cell type (C). Note that p65 nuclear translocation could not be blocked by 1,25(OH)2D3 in VDR−/− MEFs. ***, p < 0.001 versus VDR+/−. D and E, rapid inhibition of p65 nuclear translocation by 1,25(OH)2D3. VDR+/− MEFs were not treated (control) or pretreated with 1,25(OH)2D3 for 0, 1, or 2 h as indicated, followed by 2 h of TNFα stimulation. Intracellular p65 location was assessed by immunostaining with anti-p65 antibodies (D), and VDR-positive nuclei were quantified in each treatment (E). Nuclei were stained with DAPI. ***, p < 0.001 versus controls. F, effect of actinomycin D on vitamin D regulation of NF-κB. MEF cells were untreated or pretreated with actinomycin D (Act D) for 30 min, followed by treatment with TNFα, 1,25(OH)2D3, or TNFα+1,25(OH)2D3, as indicated, for 15 min. Then the levels of IκBα were determined by Western blot analysis. Note that Act D has no effects on 1,25(OH)2D3 stabilization of IκBα protein. G, 1,25(OH)2D3 does not significantly alter transcription of NF-κB components. MEF cells were treated with vehicle control (Con) or 20 nm 1,25(OH)2D3 for 24 h, and the transcript levels of IKKα, IKKβ, IKKγ, IκBα, and p65 were quantified by real-time RT-PCR. H, effects of VDR overexpression on NF-κB activity. MEFs were cotransfected with pNF-κB-Luc and increasing amount of hVDR as indicated, followed by TNFα stimulation in the presence or absence of 1,25(OH)2D3. Luciferase activity was measured after 24 h. ***, p < 0.001 vs. the rest. **, p < 0.001. I and J, HEK293 cells were cotransfected with p65 (I) or IKKβ (J) and increasing amounts of VDR as indicated, followed by 24 h of treatment with ethanol or 1,25(OH)2D3 before measuring luciferase activity. *, p < 0.05; ***, p < 0.001.
It is well known that overexpression of p65 or IKKβ induces NF-κB activity in the absence of extracellular stimuli. We found that VDR cotransfection was unable to attenuate p65-induced NF-κB activity in HEK293 cells, regardless of 1,25(OH)2D3 treatment (Fig. 1I), but it markedly suppressed IKKβ-induced NF-κB activity in a VDR dose-dependent manner, even in the absence of 1,25(OH)2D3, although 1,25(OH)2D3 treatment further increased the inhibitory activity of VDR (J). Similar results were observed in MEF cells (not shown). These observations are inconsistent with the assumption that VDR-p65 interaction arrests p65 translocation, leading to inhibition of NF-κB activity, but raise a possibility of VDR-IKKβ interaction in this regulatory process.
VDR Physically Interacts with IKKβ Protein
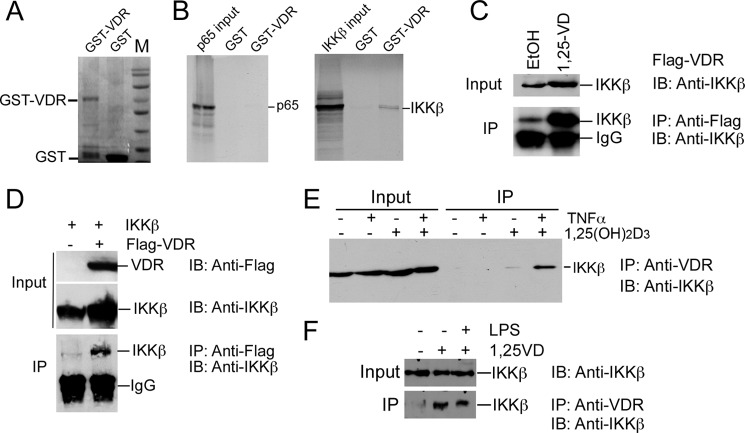
That VDR regulates biological activities by interacting with other regulatory proteins has been well documented previously. For example, our previous work showed that 1,25(OH)2D3-activated VDR binds to CREB and suppresses renin gene transcription by blocking the formation of CREB-CREB-binding protein-p300 complex on the CRE site in the renin gene promoter (9). VDR binds to β-catenin protein to inhibit its nuclear translocation in colon cancer cells, thus blocking the transduction of the oncogenic signal of β-catenin to the nuclei (8). To explore the apparently non-transcriptional mechanism whereby 1,25(OH)2D3 suppresses NF-κB activity, we performed GST pull-down assays to examine the protein-protein interaction between VDR and NF-κB components. Interestingly, purified GST-VDR fusion protein (Fig. 2A) was able to pull down 35S-labeled IKKβ protein strongly in vitro (Fig. 2B). This interaction was not altered substantially by the presence of 1,25(OH)2D3 (data not shown), consistent with the above observation that, at high concentrations, VDR suppressed NF-κB even in the absence of 1,25(OH)2D3 (Fig. 1, H–J). Surprisingly, given the previously reported VDR-p65 interaction (21), we barely detected any pull-down of 35S-labeled p65 protein by GST-VDR under the same condition (Fig. 2B). There appeared to be some weak interaction between GST-VDR and p50 or IKKα (data not shown). The latter was not unexpected, given that IKKα and IKKβ share extensive structural homology. Together, these data suggest that VDR may target IKK, not p65, to inhibit NF-κB activation.
FIGURE 2.
VDR protein directly interacts with IKKβ protein. A, Coomassie Brilliant Blue staining of purified GST-hVDR and GST. B, GST pull-down assays. Purified GST and GST-VDR were incubated with 35S-labeled p65 or 35S-labeled IKKβ as indicated. Pull-down proteins were separated by SDS-PAGE and visualized by autoradiography. C and D, co-IP assays to demonstrate VDR-IKKβ interaction in cells. C, HEK293 cells were transfected with FLAG-VDR and treated with or without 1,25(OH)2D3. Cell lysates were precipitated (IP) with anti-FLAG antibodies, and the precipitates were blotted (IB) with anti-IKKβ antibodies. D, HEK293 cells were cotransfected with IKKβ and vector or FLAG-VDR. Cell lysates were precipitated with anti-FLAG antibodies, and precipitates were blotted with anti-IKKβ antibodies. E, endogenous VDR-IKKβ interaction. HEK293 cells were treated with TNFα and/or 1,25(OH)2D3. Cell lysates were precipitated with anti-VDR antibodies, and the precipitates were blotted with anti-IKKβ antibodies. F, endogenous VDR-IKKβ interaction in a macrophage cell line stimulated with 1,25(OH)2D3. RAW264.7 cells were left untreated or treated 1,25(OH)2D3 or 1,25(OH)2D3 + LPS as indicated. Cell lysates were precipitated with anti-VDR antibodies, and the precipitates were blotted with anti-IKKβ antibodies as indicated in the co-IP experiment.
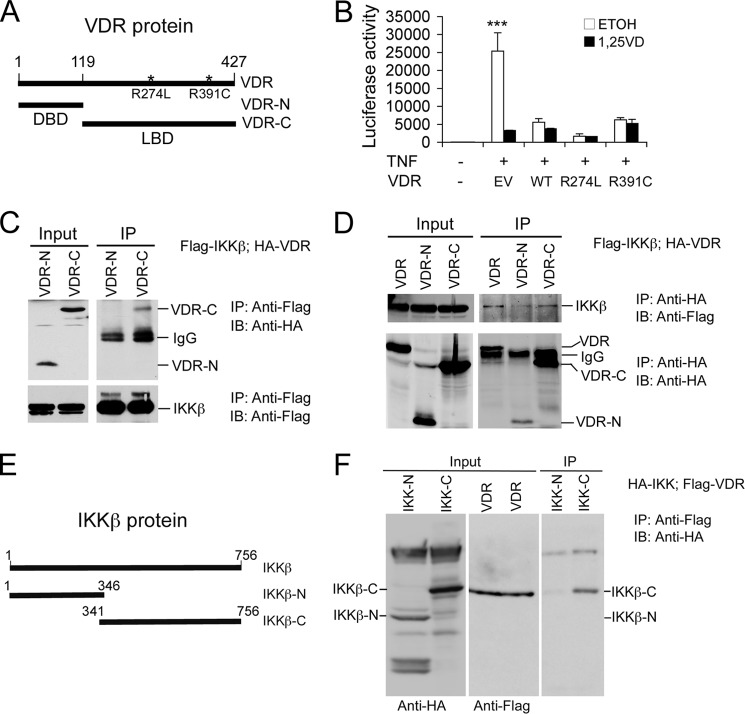
The strong association between VDR and IKKβ prompted us to focus on this interaction. Co-IP assays showed that, in HEK293 cells transfected with the FLAG-VDR plasmid, anti-FLAG antibodies were able to coprecipitate endogenous IKKβ and that this action was enhanced markedly in the presence of 1,25(OH)2D3 (Fig. 2C). When both FLAG-VDR and IKKβ were overexpressed in HEK293 cells by transfection, anti-FLAG antibodies were able to coprecipitate IKKβ without TNFα and 1,25(OH)2D3 stimulation (Fig. 2D). Furthermore, in untransfected cells, anti-VDR antibodies were able to weakly coprecipitate IKKβ in the absence of 1,25(OH)2D3. However, the VDR-IKKβ interaction was enhanced greatly in the presence of TNFα and 1,25(OH)2D3 (Fig. 2E). Through co-IP assays, we also observed 1,25(OH)2D3-induced VDR-IKKβ interaction in RAW264.7 cells, a macrophage cell line (Fig. 2F), indicating that this protein-protein interaction is not cell-specific and also occurs in immune cells. These data confirm that the physical association between VDR and IKKβ occurs within cells and that this interaction can take place independently of 1,25(OH)2D3 at high protein concentrations. Consistent with the notion that 1,25(OH)2D3 binding is not required, we observed that overexpression of hVDR mutants at R274L and R391C within the ligand-binding domain (LBD) (Fig. 3A) with extremely low 1,25(OH)2D3 affinity were still able to block TNFα-induced NF-κB activation, regardless of 1,25(OH)2D3 treatment (Fig. 3B). However, under normal physiological conditions where intracellular VDR levels are usually very low in most cell types, particularly in immune cells, VDR needs ligand activation to down-regulate NF-κB activity. This is the basis to explain why 1,25(OH)2D3 treatment suppresses NF-κB activity.
FIGURE 3.
VDR and IKKβ proteins interact through their C-terminal domains. A, schematic illustration of hVDR mutants R274L and R391C, the N-terminal portion (VDR-N) containing the DNA-binding domain (DBD), and the C-terminal portion (VDR-C) containing the LBD. B, effects of hVDR mutants on NF-κB activity. HEK293 cells were cotransfected with pNF-κB-Luc and empty vector (EV), WT hVDR, mutant hVDR(R274L), or hVDR(R391C). Luciferase activity assays were performed after TNFα stimulation in the presence of ethanol or 1,25(OH)2D3 for 24 h. ***, p < 0.001 versus the rest. C and D, HEK293 cells were cotransfected with FLAG-IKKβ and HA-VDR, HA-VDR-N, or HA-VDR-C as indicated. Cell lysates were precipitated (IP) with anti-FLAG antibodies (C) or anti-HA antibodies (D), and the precipitates were blotted (IB) with anti-HA antibodies (C) or anti-FLAG antibodies (D) as indicated. As controls, these precipitates were also blotted with the same antibodies as shown in the lower panels in C and D. Note that IKKβ interacts with VDR-C. E, schematic of IKKβ protein and its N-terminal and C-terminal constructs (IKKβ-N and IKKβ-C). F, HEK293 cells were cotransfected with FLAG-VDR and HA-IKKβ-N or HA-IKKβ-C. Cell lysates were precipitated with anti-FLAG antibodies, and the precipitates were blotted with anti-HA antibodies. The input lysates were blotted with anti-HA or anti-FLAG antibodies, respectively, as indicated at the bottom. Note that the VDR interacts with IKKβ-C and not IKKβ-N.
VDR and IKKβ Interact at Their C Terminus
VDR contains an N-terminal DNA-binding domain, a C-terminal LBD, and a hinge region between them (6) (Fig. 3A). To define which domain in the VDR molecule interacts with IKKβ, we generated plasmid constructs that express an HA-tagged N-terminal DNA binding domain (VDR-N, amino acids 1–119) and C-terminal hinge and LBD (VDR-C) of hVDR (amino acids 119–427) (Fig. 3A). Co-IP experiments showed that in HEK293 cells transfected with FLAG-IKKβ and HA-VDR, HA-VDR-N, or HA-VDR-C, anti-FLAG antibodies were able to pull down HA-VDR-C but not HA-VDR-N (Fig. 3C). Conversely, anti-HA antibodies coprecipitated FLAG-IKKβ only in cells cotransfected with HA-VDR or HA-VDR-C and not in cells transfected with HA-VDR-N (Fig. 3D). These results indicate that the C-terminal hinge and LBD fragment of VDR protein interacts with IKKβ.
To define the domain in the IKKβ molecule that interacts with the VDR, we generated plasmids expressing an HA-tagged IKKβ N-terminal fragment between amino acids 1–346 and a C-terminal fragment between amino acids 341–756, respectively (Fig. 3E). In HEK293 cells cotransfected with FLAG-hVDR and HA-IKKβ-N or HA-IKKβ-C, anti-FLAG antibodies were able to coprecipitate HA-IKKβ-C but not HA-IKKβ-N (Fig. 3F). These results indicate that the VDR interacts with the C-terminal portion of IKKβ protein in cells. Together, these data reveal that VDR and IKKβ interaction occurs at their C-terminal portions.
The C Terminus of VDR Is Functional in the Regulation of NF-κB
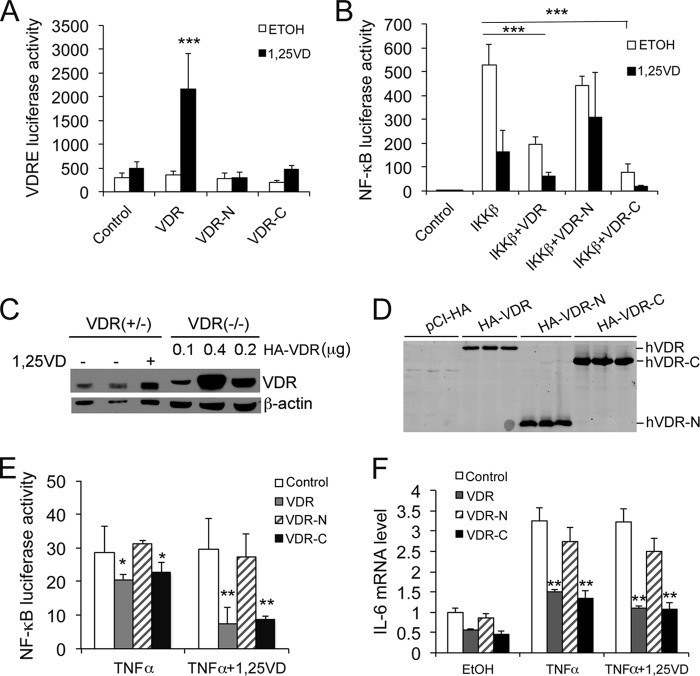
Because the VDR C terminus binds to IKKβ, a key question that needs to be addressed is whether VDR-C is able to suppress NF-κB activity. As expected, both VDR-N and VDR-C lacked transactivating activity in VDRE-Luc reporter assays (Fig. 4A). By NF-κB luciferase reporter assays, however, we observed that VDR-C, but not VDR-N, was able to attenuate IKKβ-induced NF-κB activity in HEK293 cells, similar to full-length VDR and that the inhibitory activity of both the VDR and VDR-C was enhanced in the presence of 1,25(OH)2D3 (Fig. 4B). To eliminate the potential confounding effect of the endogenous mouse VDR, we asked whether reconstitution of VDR−/− MEF cells with VDR-C would be able to restore the ability to suppress NF-κB activation. Using different plasmid doses, we observed that transfection of VDR−/− MEFs with 0.1 μg of hVDR construct/well reconstituted intracellular VDR to a level comparable with that seen in VDR+/− MEFs (Fig. 4C). Therefore, we performed VDR−/− MEF cell transfection using the same dose (0.1 μg/well) of VDR, VDR-N, VDR-C, or control empty vector to avoid overexpression (Fig. 4D). Interestingly, in VDR−/− MEFs, VDR and VDR-C, but not VDR-N, were able to attenuate TNFα-induced NF-κB activity (Fig. 4E) and IL-6 up-regulation (F), and this attenuation was enhanced when the cells were treated with 1,25(OH)2D3 (E and F). This was not surprising because VDR-C has a LBD for 1,25(OH)2D3 binding. Together, these results demonstrate that reconstitution of VDR−/− cells with the C terminus of hVDR to a physiological level is sufficient to block TNFα induction of NF-κB activity and IL-6 expression. Because VDR-C has no DNA binding domain, these observations provide very compelling evidence that VDR-IKKβ interaction can regulate biological actions independently of VDRE.
FIGURE 4.
Functional analysis of VDR protein domains in NF-κB regulation. A, VDRE luciferase reporter assays. HEK293 cells were cotransfected with p3xVDRE-Luc and VDR, VDR-N, or VDR-C followed by 24 h of 1,25(OH)2D3 stimulation. ***, p < 0.001 versus the rest. B, effects of hVDR N- and C-terminal fragments on IKKβ-induced NF-κB activity. HEK293 cells were cotransfected with pNF-κB-Luc; IKKβ; and VDR, VDR-N, or VDR-C. The transfected cells were treated with ethanol or 1,25(OH)2D3 followed by luciferase activity assays. ***, p < 0.001. C, comparison of endogenous VDR levels in VDR+/− MEFs and in VDR−/− MEFs transfected with hVDR. VDR+/− MEFs were treated with or without 1,25(OH)2D3 as indicated, and VDR−/− MEFs were transfected with different amounts of HA-hVDR (0.1, 0.2, or 0.4 μg/well) as indicated. Cell lysates were analyzed by Western blot analysis after 24 h using anti-VDR antibodies. Note the comparable VDR levels in VDR+/− MEFs and VDR−/− MEFs transfected with 0.1 μg HA-VDR plasmid/well. D, Western blot analysis with anti-HA antibodies showing that VDR−/− MEFs were reconstituted with empty vector, HA-VDR, HA-VDR-N, or HA-VDR-C by transfection at 0.1 μg plasmid DNA/well. E, VDR−/− MEFs were cotransfected with pNF-κB-Luc and control empty vector, VDR, VDR-N, or VDR-C plasmid (0.1 μg/well). The transfected cells were treated with TNFα or TNFα+1,25(OH)2D3 for 24 h followed by luciferase activity assays. *, p < 0.05; **, p < 0.01 versus corresponding control. F, VDR−/− MEFs were transfected with control empty vector, VDR, VDR-N, or VDR-C (0.1 μg/well). The transfected cells were treated with TNFα or TNFα+1,25(OH)2D3 for 6 h, and the IL-6 transcript was quantified by quantitative PCR. **, p < 0.01 versus corresponding control.
VDR-IKKβ Interaction Abolishes IKK Complex Formation and IKKβ Phosphorylation
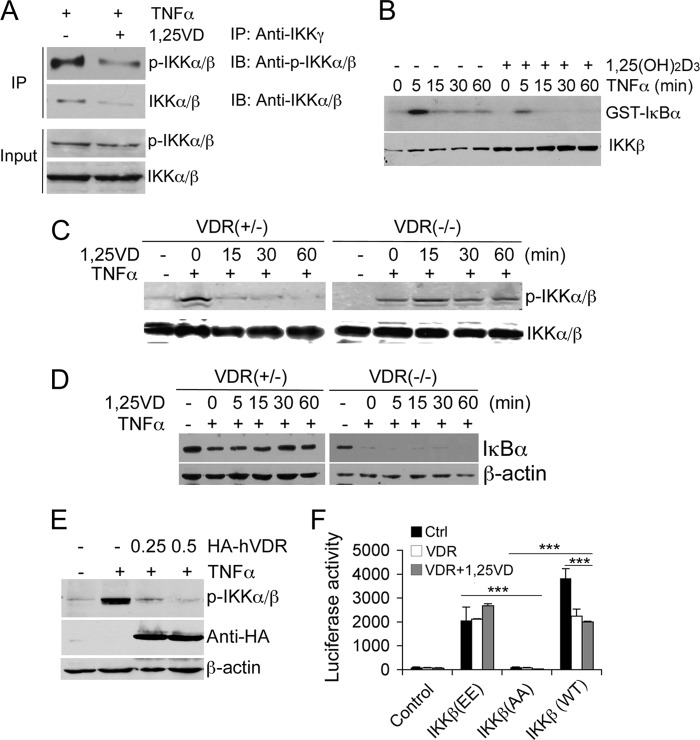
The IKK complex consists of IKKα, β, and γ, and the formation of this complex is required for IKKα/β phosphorylation and NF-κB activation because the IKK complex has kinase activity to phosphorylate IκBα. To further understand the biological consequence of VDR-IKKβ association, we investigated the effect of 1,25(OH)2D3 on IKK complex formation by co-IP assays in which anti-IKKγ antibodies were used to pull down IKKα/β. When cells were stimulated by TNFα, the amount of phospho-IKKα/β and total IKKα/β that was coprecipitated by anti-IKKγ antibodies was reduced substantially in the presence of 1,25(OH)2D3 (Fig. 5A), indicating 1,25(OH)2D3 inhibition of IKK complex formation. It is known that IKKβ accounts for nearly all of the catalytic kinase activity of the IKK holoenzyme toward IκBα (23) and that IKKβ phosphorylation at Ser-177/Ser-181 activates IKKβ. Kinase assays showed that 1,25(OH)2D3 treatment blocked TNFα-induced IκBα phosphorylation in VDR+/− MEFs (Fig. 5B). A short pretreatment (15–60 min) with 1,25(OH)2D3 also blocked TNFα-induced IKKα/β Ser-177 phosphorylation in VDR+/− MEFs but not in VDR−/− MEFs (Fig. 5C), indicating that this effect of 1,25(OH)2D3 is VDR-dependent. Consistently, a short 1,25(OH)2D3 pretreatment (5–60 min) also prevented TNFα-induced IκBα degradation in VDR+/− MEFs but not in VDR−/− MEFs (Fig. 5D). As expected, VDR−/− MEFs showed dramatic IκBα degradation in the absence of VDR protection (Fig. 5D). Moreover, when HEK293 cells were transfected with increasing amounts of HA-VDR, TNFα-induced IKKα/β Ser-177 phosphorylation was abrogated (Fig. 5E). Taken together, these data strongly suggest that 1,25(OH)2D3, by rapidly inducing VDR-IKKβ association, blocks IKK complex formation and, hence, IKKβ phosphorylation, abolishing the IKK enzymatic activity to phosphorylate IκBα.
FIGURE 5.
VDR-IKKβ interaction blocks IKK complex formation and IKKβ phosphorylation. A, effects of 1,25(OH)2D3 treatment on IKK complex formation. VDR+/− MEFs were pretreated with 1,25(OH)2D3 (30 min) followed by TNFα treatment (15 min). Cell lysates were precipitated (IP) with anti-IKKγ antibodies, and the precipitates were blotted (IB) with anti-p-IKKα/β (Ser-177) or anti-IKKα/β antibodies as indicated. B, IKK enzymatic assays. VDR+/− MEFs pretreated with or without 1,25(OH)2D3 were stimulated by TNFα for 0, 5, 15, 30, and 60 min as indicated. The IKK complex was precipitated with anti-IKKγ antibodies at the indicated time points, and kinase activity to phosphorylate IκBα was measured using GST-IκBα substrate in the presence of [γ-32P]ATP. Phosphorylated GST-IκBα was visualized by autoradiography. Levels of IKKβ were analyzed by Western blotting. C, effects of 1,25(OH)2D3 on IKKβ phosphorylation. VDR+/− or VDR−/− MEFs were pretreated with 1,25(OH)2D3 for 0, 15, 30, and 60 min as indicated, followed by 15 min of TNFα stimulation. IKKβ phosphorylation was assessed by Western blotting with anti-p-IKKα/β (Ser-177) antibodies. D, effects of 1,25(OH)2D3 on IκBα degradation. VDR+/− and VDR−/− MEFs were pretreated with 1,25(OH)2D3 for 0, 5, 15, 30, and 60 min, followed by 15 min of TNFα treatment, and IκBα protein levels were assessed by Western blotting with anti-IκBα antibodies. E, HEK293 cells were transfected with empty vector or increasing amounts of HA-hVDR (0.25 or 0.5 μg). After the transfected cells were stimulated with TNFα for 15 min, cell lysates were blotted with anti-p-IKKα/β (Ser-177), anti-HA, or anti-β-actin antibodies as indicated. F, luciferase reporter assays. HEK293 cells were cotransfected with pNF-κB-Luc and empty vector control (Ctrl); IKKβ(EE), IKKβ(AA), or IKKβ (WT); and VDR as indicated. The transfected cells were treated with ethanol or 1,25(OH)2D3 for 24 h followed by luciferase activity assays. ***, p < 0.001. Note that IKKβ(EE)-induced NF-κB activity cannot be suppressed by VDR overexpression regardless of 1,25(OH)2D3 treatment.
Finally, we used IKKβ mutants to validate the importance of blocking IKKβ phosphorylation in 1,25(OH)2D3-induced inhibitory action on NF-κB. We speculated that the blockade of IKKβ phosphorylation is likely caused by the disruption of IKK complex formation. In the IKKβ protein, Ala substitution of Ser-177 and Ser-181 (IKKβ(AA)) prevents IKK activation, whereas the phosphomimic, double Glu mutations at these Ser residues (S177E/S181E, IKKβ(EE)) render IKKβ constitutively active (23, 24). As expected, transfection of HEK293 cells with WT IKKβ or IKKβ(EE) dramatically induced NF-κB activity, but IKKβ(AA) failed to do so (Fig. 5F). Interestingly, although VDR cotransfection was able to attenuate WT IKKβ -induced NF-κB activity, it failed to reduce IKKβ(EE)-induced NF-κB activity regardless of 1,25(OH)2D3 treatment (Fig. 5F). Taken together, these observations confirm that VDR-IKKβ interaction affects IKKβ phosphorylation at Ser-177/181, leading to decreased IKK kinase activity.
DISCUSSION
Vitamin D inhibition of NF-κB has been reported frequently in the literature, but the exact molecular mechanism remains poorly understood. It has been documented previously that 1,25(OH)2D3 arrests the nuclear translocation of p65/p50 and suppresses the degradation of IκBα protein (10, 14, 25). Because the VDR can interact directly with p65 (21), the most commonly held mechanism to explain vitamin D inhibition of NF-κB is that the VDR-p65 interaction blocks the nuclear translocation of p65/p50 (10, 26). This mechanism, however, cannot explain how 1,25(OH)2D3 stabilizes IκBα, which was reported in many studies (10, 12, 14, 17, 18) and is well known as a critical step in the inhibition of NF-κB. In fact, in many studies (14, 26), including this one, VDR-p65 interaction was not detectable, suggesting that VDR-p65 interaction is weak, if there is any. Therefore, there likely exist other mechanisms to explain the stabilization of IκBα.
In this report, we present evidence that VDR binds to IKKβ to block NF-κB activation. We showed that VDR-IKKβ interaction blocks the formation of the IKK complex and, hence, reduces IKKβ phosphorylation. As a result, the IKK enzymatic activity to phosphorylate IκBα is abrogated, consequently diminishing IκBα ubiquitylation and degradation. This mechanism explains well how 1,25(OH)2D3 stabilizes IκBα. The direct consequence of reduced IκBα degradation is the retention of the p65/p50 heterodimer in the cytoplasm, leading to decreased NF-κB transcriptional activity. Thus, this model also explains the blockade of p65/p50 nuclear translocation. We conclude that this is a major mechanism whereby 1,25(OH)2D3-VDR inhibits NF-κB activation.
Our data show that VDR-IKKβ interaction occurs in the C-terminal portions of both molecules. These mapping studies provide compelling evidence that confirms the interaction between the VDR and IKKβ proteins. We demonstrated that reconstitution of VDR−/− cells with the C terminus of hVDR to a physiologically relevant level is sufficient to block the induction of NF-κB activity and IL-6 expression by TNFα. Because VDR-C has no DNA binding domain, this result confirms that VDR-IKKβ interaction can generate a biological consequence independently of the VDRE. Although VDR-IKKβ interaction does not require 1,25(OH)2D3 at high protein concentrations under some artificial conditions (e.g. in the case of cell transfection), 1,25(OH)2D3 is able to enhance this interaction in cells. The VDR C-terminal region that interacts with IKKβ remains responsive to 1,25(OH)2D3 treatment because VDR-C contains the LBD. Under normal physiological conditions, intracellular VDR levels are usually low in most cell types, particularly in immune cells. Therefore, we believe that, physiologically, VDR needs ligand activation to block NF-κB activity. This is the basis for the observation that 1,25(OH)2D3 treatment suppresses NF-κB activity.
As a ligand-activated transcription factor, the VDR usually interacts with cis-DNA elements (VDRE) in gene promoters to activate gene transcription. This mechanism is used in most stimulatory regulation of vitamin D actions. The mechanisms for negative regulation, however, are more complicated and diverse. For instance, the VDR/retinoid X receptor heterodimer and VDR homodimer inhibit the formation of the NFAT-1/AP-1 transcriptional complex in IL-2 and GM-CSF promoters to inhibit these gene expressions (27, 28). VDR binds to a negative VDRE (nVDRE) in PTH and PTHrP gene promoters and works with Ku antigen to suppress these genes (29). Ligand-activated VDR can also recruit corepressors, such as NCoR, Alien, and SMRT, to mediate transcriptional repression (30, 31), and 1,25(OH)2D3 suppresses Cyp27b1 transcription via interaction with VDIR (32). Given these diverse inhibitory mechanisms, it is not surprising that 1,25(OH)2D3-VDR down-regulates NF-κB by protein-protein interaction with IKKβ because this kind of regulatory mode has been observed in the regulation of the PKA/CREB and Wnt/β-catenin pathways. We reported previously that 1,25(OH)2D3-activated VDR binds to CREB and inhibits renin gene transcription by blocking the formation of the CREB-CBP·p300 complex on the CRE site in the renin gene promoter (9). In the case of the Wnt/β-catenin pathway, liganded VDR binds to β-catenin protein to inhibit its nuclear translocation in colon cancer cells, thus blocking the transduction of the oncogenic signal of β-catenin to the nuclei (8). Detailed mapping studies reveal that the interaction between VDR and β-catenin occurs between the VDR activator function 2 (AF-2) domain of the VDR and the β-catenin C terminus (33). Our domain mapping in this study provided evidence to confirm the interaction between the VDR and IKKβ at the C terminus of both proteins, but more detailed mapping is needed to further narrow down the interacting domains in each molecule. Given the wide spectrum of NF-κB activities that affect numerous biological processes, the inhibitory mechanism of vitamin D on NF-κB reported here could have broader implications than we now recognize, which warrants more investigation in the future. Finally, because the VDR interacts physically with different cell signaling proteins described above, the questions whether these intracellular interactions are mutually competitive in biological regulations and whether different interactions have different physiological or pathological implications also warrant further studies.
This work was supported, in whole or in part, by National Institutes of Health Grant HL085793. This work was also supported by a Foundation for Clinical Research in Inflammatory Bowel Disease (FCRIBD) grant and by a research grant from the government of Liaoning Province, China.
- IKK
- IκB kinase
- VDR
- vitamin D receptor
- 1,25(OH)2D3
- 1,25-dihydroxyvitamin D3
- VDRE
- vitamin D response element
- CREB
- cAMP response element-binding protein
- MEF
- mouse embryonic fibroblast
- hVDR
- human vitamin D receptor
- co-IP
- coimmunoprecipitation
- LBD
- ligand-binding domain.
REFERENCES
- 1. Bonizzi G., Karin M. (2004) The two NF-κB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 25, 280–288 [DOI] [PubMed] [Google Scholar]
- 2. Nakanishi C., Toi M. (2005) Nuclear factor-κB inhibitors as sensitizers to anticancer drugs. Nat. Rev. Cancer 5, 297–309 [DOI] [PubMed] [Google Scholar]
- 3. Oeckinghaus A., Hayden M. S., Ghosh S. (2011) Crosstalk in NF-κB signaling pathways. Nat. Immunol. 12, 695–708 [DOI] [PubMed] [Google Scholar]
- 4. Sakurai H., Chiba H., Miyoshi H., Sugita T., Toriumi W. (1999) IκB kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 274, 30353–30356 [DOI] [PubMed] [Google Scholar]
- 5. Bouillon R., Carmeliet G., Verlinden L., van Etten E., Verstuyf A., Luderer H. F., Lieben L., Mathieu C., Demay M. (2008) Vitamin D and human health. Lessons from vitamin D receptor null mice. Endocr. Rev. 29, 726–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Haussler M. R., Whitfield G. K., Haussler C. A., Hsieh J. C., Thompson P. D., Selznick S. H., Dominguez C. E., Jurutka P. W. (1998) The nuclear vitamin D receptor. Biological and molecular regulatory properties revealed. J. Bone Miner. Res. 13, 325–349 [DOI] [PubMed] [Google Scholar]
- 7. Sutton A. L., MacDonald P. N. (2003) Vitamin D. More than a “bone-a-fide” hormone. Mol. Endocrinol. 17, 777–791 [DOI] [PubMed] [Google Scholar]
- 8. Pálmer H. G., González-Sancho J. M., Espada J., Berciano M. T., Puig I., Baulida J., Quintanilla M., Cano A., de Herreros A. G., Lafarga M., Muñoz A. (2001) Vitamin D(3) promotes the differentiation of colon carcinoma cells by the induction of E-cadherin and the inhibition of β-catenin signaling. J. Cell Biol. 154, 369–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yuan W., Pan W., Kong J., Zheng W., Szeto F. L., Wong K. E., Cohen R., Klopot A., Zhang Z., Li Y. C. (2007) 1,25-Dihydroxyvitamin D3 suppresses renin gene transcription by blocking the activity of the cyclic AMP response element in the renin gene promoter. J. Biol. Chem. 282, 29821–29830 [DOI] [PubMed] [Google Scholar]
- 10. Sun J., Kong J., Duan Y., Szeto F. L., Liao A., Madara J. L., Li Y. C. (2006) Increased NF-κB activity in fibroblasts lacking the vitamin D receptor. Am. J. Physiol. Endocrinol. Metab. 291, E315–322 [DOI] [PubMed] [Google Scholar]
- 11. D'Ambrosio D., Cippitelli M., Cocciolo M. G., Mazzeo D., Di Lucia P., Lang R., Sinigaglia F., Panina-Bordignon P. (1998) Inhibition of IL-12 production by 1,25-dihydroxyvitamin D3. Involvement of NF-κB downregulation in transcriptional repression of the p40 gene. J. Clin. Invest. 101, 252–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Riis J. L., Johansen C., Gesser B., Møller K., Larsen C. G., Kragballe K., Iversen L. (2004) 1α,25(OH)(2)D(3) regulates NF-κB DNA binding activity in cultured normal human keratinocytes through an increase in IκBα expression. Arch. Dermatol. Res. 296, 195–202 [DOI] [PubMed] [Google Scholar]
- 13. Deb D. K., Chen Y., Zhang Z., Zhang Y., Szeto F. L., Wong K. E., Kong J., Li Y. C. (2009) 1,25-Dihydroxyvitamin D3 suppresses high glucose-induced angiotensinogen expression in kidney cells by blocking the NF-κB pathway. Am. J. Physiol. Renal Physiol. 296, F1212–1218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang Z., Yuan W., Sun L., Szeto F. L., Wong K. E., Li X., Kong J., Li Y. C. (2007) 1,25-Dihydroxyvitamin D(3) targeting of NF-κB suppresses high glucose-induced MCP-1 expression in mesangial cells. Kidney Int. 72, 193–201 [DOI] [PubMed] [Google Scholar]
- 15. Chen Y., Kong J., Sun T., Li G., Szeto F. L., Liu W., Deb D. K., Wang Y., Zhao Q., Thadhani R., Li Y. C. (2011) 1,25-Dihydroxyvitamin D(3) suppresses inflammation-induced expression of plasminogen activator inhibitor-1 by blocking nuclear factor-κB activation. Arch. Biochem. Biophys. 507, 241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Y., Liu W., Sun T., Huang Y., Wang Y., Deb D. K., Yoon D., Kong J., Thadhani R., Li Y. C. (2013) 1,25-Dihydroxyvitamin D promotes negative feedback regulation of TLR signaling via targeting microRNA-155-SOCS1 in macrophages. J. Immunol. 190, 3687–3695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Harant H., Wolff B., Lindley I. J. (1998) 1α,25-dihydroxyvitamin D3 decreases DNA binding of nuclear factor-κB in human fibroblasts. FEBS Lett. 436, 329–334 [DOI] [PubMed] [Google Scholar]
- 18. Giarratana N., Penna G., Amuchastegui S., Mariani R., Daniel K. C., Adorini L. (2004) A vitamin D analog down-regulates proinflammatory chemokine production by pancreatic islets inhibiting T cell recruitment and type 1 diabetes development. J. Immunol. 173, 2280–2287 [DOI] [PubMed] [Google Scholar]
- 19. Dong X., Craig T., Xing N., Bachman L. A., Paya C. V., Weih F., McKean D. J., Kumar R., Griffin M. D. (2003) Direct transcriptional regulation of RelB by 1α,25-dihydroxyvitamin D3 and its analogs. Physiologic and therapeutic implications for dendritic cell function. J. Biol. Chem. 278, 49378–49385 [DOI] [PubMed] [Google Scholar]
- 20. Yu X. P., Bellido T., Manolagas S. C. (1995) Down-regulation of NF-κ B protein levels in activated human lymphocytes by 1,25-dihydroxyvitamin D3 [published erratum appears in Proc. Natl. Acad. Sci. U.S.A. 1996 Jan 9;93(1):524]. Proc. Natl. Acad. Sci. U.S.A. 92, 10990–10994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lu X., Farmer P., Rubin J., Nanes M. S. (2004) Integration of the NFκB p65 subunit into the vitamin D receptor transcriptional complex. Identification of p65 domains that inhibit 1,25-dihydroxyvitamin D3-stimulated transcription. J. Cell Biochem. 92, 833–848 [DOI] [PubMed] [Google Scholar]
- 22. Li Y. C., Bolt M. J. G., Cao L.-P., Sitrin M. D. (2001) Effects of vitamin D receptor inactivation on the expression of calbindins and calcium metabolism. Am. J. Physiol. Endocrinol. Metab. 281, E558-E564 [DOI] [PubMed] [Google Scholar]
- 23. Zandi E., Chen Y., Karin M. (1998) Direct phosphorylation of IκB by IKKα and IKKβ. Discrimination between free and NF-κB-bound substrate. Science 281, 1360–1363 [DOI] [PubMed] [Google Scholar]
- 24. Mercurio F., Zhu H., Murray B. W., Shevchenko A., Bennett B. L., Li J., Young D. B., Barbosa M., Mann M., Manning A., Rao A. (1997) IKK-1 and IKK-2. Cytokine-activated IκB kinases essential for NF-κB activation. Science 278, 860–866 [DOI] [PubMed] [Google Scholar]
- 25. Chen S., Ni X. P., Humphreys M. H., Gardner D. G. (2005) 1,25 dihydroxyvitamin D amplifies type a natriuretic peptide receptor expression and activity in target cells. J. Am. Soc. Nephrol. 16, 329–339 [DOI] [PubMed] [Google Scholar]
- 26. Tan X., Wen X., Liu Y. (2008) Paricalcitol inhibits renal inflammation by promoting vitamin D receptor-mediated sequestration of NF-κB signaling. J. Am. Soc. Nephrol. 19, 1741–1752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alroy I., Towers T. L., Freedman L. P. (1995) Transcriptional repression of the interleukin-2 gene by vitamin D3. Direct inhibition of NFATp/AP-1 complex formation by a nuclear hormone receptor. Mol. Cell Biol. 15, 5789–5799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Towers T. L., Freedman L. P. (1998) Granulocyte-macrophage colony-stimulating factor gene transcription is directly repressed by the vitamin D3 receptor. Implications for allosteric influences on nuclear receptor structure and function by a DNA element. J. Biol. Chem. 273, 10338–10348 [DOI] [PubMed] [Google Scholar]
- 29. Nishishita T., Okazaki T., Ishikawa T., Igarashi T., Hata K., Ogata E., Fujita T. (1998) A negative vitamin D response DNA element in the human parathyroid hormone-related peptide gene binds to vitamin D receptor along with Ku antigen to mediate negative gene regulation by vitamin D. J. Biol. Chem. 273, 10901–10907 [DOI] [PubMed] [Google Scholar]
- 30. Polly P., Herdick M., Moehren U., Baniahmad A., Heinzel T., Carlberg C. (2000) VDR-Alien. A novel, DNA-selective vitamin D(3) receptor-corepressor partnership. FASEB J. 14, 1455–1463 [DOI] [PubMed] [Google Scholar]
- 31. Sánchez-Martínez R., Zambrano A., Castillo A. I., Aranda A. (2008) Vitamin D-dependent recruitment of corepressors to vitamin D/retinoid X receptor heterodimers. Mol. Cell Biol. 28, 3817–3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Murayama A., Kim M.-S., Yanagisawa J., Takeyama K., Kato S. (2004) Transrepression by a liganded nuclear receptor via a bHLH activator through co-regulator switching. EMBO J. 23, 1598–1608 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33. Shah S., Islam M. N., Dakshanamurthy S., Rizvi I., Rao M., Herrell R., Zinser G., Valrance M., Aranda A., Moras D., Norman A., Welsh J., Byers S. W. (2006) The molecular basis of vitamin D receptor and β-catenin crossregulation. Mol. Cell 21, 799–809 [DOI] [PubMed] [Google Scholar]