Background: A subset of juvenile myelomonocytic leukemia (JMML) patients harbor mutations in the E3 ubiquitin ligase CBL.
Results: CBL mutations result in increased GM-CSFR phosphorylation, elevated JAK2 and LYN levels, and enhanced survival.
Conclusion: CBL JMML mutants display hypersensitive GM-CSF signaling that can be modulated via inhibition of JAK2 and/or SRC kinases.
Significance: Mutation of CBL in JMML is associated with altered GM-CSF function.
Keywords: Cytokine, E3 Ubiquitin Ligase, JAK Kinase, Signal Transduction, SRC, CBL, GM-CSF, Juvenile Myelomonocytic Leukemia
Abstract
Juvenile myelomonocytic leukemia (JMML) is characterized by hypersensitivity to granulocyte-macrophage colony-stimulating factor (GM-CSF). SHP2, NF-1, KRAS, and NRAS are mutated in JMML patients, leading to aberrant regulation of RAS signaling. A subset of JMML patients harbor CBL mutations associated with 11q acquired uniparental disomy. Many of these mutations are in the linker region and the RING finger of CBL, leading to a loss of E3 ligase activity. We investigated the mechanism by which CBL-Y371H, a linker region mutant, and CBL-C384R, a RING finger mutant, lead to enhanced GM-CSF signaling. Expression of CBL mutants in the TF-1 cell line resulted in enhanced survival in the absence of GM-CSF. Cells expressing CBL mutations displayed increased phosphorylation of GM-CSF receptor βc subunit in response to stimulation, although expression of total GM-CSFR βc was lower. This suggested enhanced kinase activity downstream of GM-CSFR. JAK2 and LYN kinase expression is elevated in CBL-Y371H and CBL-C384R mutant cells, resulting in enhanced phosphorylation of CBL and S6 in response to GM-CSF stimulation. Incubation with the JAK2 inhibitor, TG101348, abolished the increased phosphorylation of GM-CSFR βc in cells expressing CBL mutants, whereas treatment with the SRC kinase inhibitor dasatinib resulted in equalization of GM-CSFR βc phosphorylation signal between wild type CBL and CBL mutant samples. Dasatinib treatment inhibited the elevated phosphorylation of CBL-Y371H and CBL-C384R mutants. Our study indicates that CBL linker and RING finger mutants lead to enhanced GM-CSF signaling due to elevated kinase expression, which can be blocked using small molecule inhibitors targeting specific downstream pathways.
Introduction
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a cytokine that regulates the differentiation, survival, proliferation, and functional activation of granulocytes and monocytes in the myeloid lineage (1). In order to mediate its functional activity, GM-CSF binds to its receptor, the GM-CSF receptor (GM-CSFR),2 consisting of GM-CSR α and GM-CSFR β common (βc) subunits. The βc subunit of the receptor is shared by the IL-3 and IL-5 receptors. βc is the major signaling subunit and is tyrosine-phosphorylated in response to cytokine stimulation. βc phosphorylation results in the recruitment of various effectors leading to activation of downstream signaling (2–4). GM-CSFR α is the major binding subunit, which dictates the binding specificity of the receptor complex for GM-CSF (2, 4, 5). The engagement of GM-CSFR by GM-CSF results in the formation of a dodecameric complex of GM-CSF·GM-CSFR α·GM-CSFR βc, which leads to the trans-phosphorylation of GM-CSFR βc-associated JAK2 kinases (6). The activated JAK2 kinases phosphorylate GM-CSFR βc tyrosine residues, allowing for the recruitment of various effectors and the activation of downstream signaling. GM-CSF stimulation results in the activation of the JAK-STAT, PI3K, and the RAS/MAPK pathways, contributing to GM-CSF-mediated differentiation, proliferation, and survival (reviewed in Refs. 1 and 4).
GM-CSF hypersensitivity is one of the defining characteristics of juvenile myelomonocytic leukemia (JMML). This disease is classified by the World Health Organization as a mixed myelodysplastic/myeloproliferative disease (7). JMML patients must show persistent monocytosis in the absence of the BCR-ABL oncogene (8). Genetic lesions deregulating the RAS signaling pathway have been identified to lead to JMML pathogenesis. 10–15% of JMML patients harbor mutations in neurofibromin protein (NF1) (9, 10), a GTPase-activating protein, which negatively regulates RAS by enhancing the hydrolysis of the active GTP-bound conformation of RAS to the inactive GDP-bound form (11). 35% of JMML patients have mutations in SHP2 (12–14), a protein-tyrosine phosphatase that positively regulates the RAS signaling pathway (15, 16). RAS-activating mutations account for another 20–25% of JMML-associated mutations (17–19). Several groups determined that a proportion of JMML patients have 11q uniparental disomy. CBL mutations were identified upon further analysis of the 11q uniparental disomy samples (20–22). The presence of NF1, SHP2, RAS, and CBL mutations are mutually exclusive, raising the question of how CBL mutations can lead to disease development.
CBL mutations have been identified in numerous other myeloid malignancies, including acute myeloid leukemia and myelodysplastic, myeloproliferative, and mixed myelodysplastic/myeloproliferative disease (23–28). The majority of these mutations localize to the CBL linker region or the RING finger. CBL is a ubiquitin E3 ligase, which specifies target proteins for ubiquitination. The linker region and RING finger of CBL play very important roles in its E3 ligase functionality. The linker region contains two conserved tyrosine residues, Tyr-368 and Tyr-371, whose phosphorylation activates and positively regulates E3 ligase activity of CBL (29–31). The CBL RING finger is responsible for recruiting active E2s carrying an ubiquitin moiety, allowing for the transfer of ubiquitin to the target substrate (29, 32). Therefore, it is not surprising that mutations in the linker region and RING finger of CBL identified in myeloid malignancies result in a loss of E3 ligase activity.
JMML is associated with GM-CSF hypersensitivity; however, there is a lack of evidence that specifically examines the role of CBL downstream of the GM-CSF receptor and how JMML-associated CBL mutations may affect GM-CSF signaling. CBL is known to become phosphorylated downstream of the βc in response to stimulation by IL-3 (33, 34) and GM-CSF (35, 36). Furthermore, CBL associates with the βc subunit upon IL-5 stimulation (37). However, these studies do not directly address the role of CBL in GM-CSF signaling.
The objective of this study is to investigate how JMML-associated CBL mutations modulate GM-CSF signaling and lead to GM-CSF hypersensitivity. We expressed wild type CBL, CBL-Y371H, and CBL-C384R in the human TF-1 hematopoietic cell line, which expresses the endogenous GM-CSF receptor at physiological levels (38). Upon GM-CSF stimulation, elevated phosphorylation of GM-CSF βc was observed, resulting from elevated JAK2 and LYN phosphorylation. Expression of CBL JMML mutants resulted in enhanced S6 phosphorylation and increased cell survival. Treatment with pharmacologic inhibitors delineated which proximal GM-CSF-dependent pathways are dependent on JAK2 and LYN activation. In summary, our data show that CBL JMML mutants result in enhanced GM-CSF signaling via modulation of JAK2 and LYN tyrosine kinases.
EXPERIMENTAL PROCEDURES
Constructs
pMSCV-HA-CBL retroviral vectors were used to create JMML-associated CBL mutants. HA-tagged CBL-Y371H and CBL-C384R were constructed using QuikChange XL site-directed mutagenesis kits (Stratagene) according to the manufacturer's protocol.
Retrovirus Production
HEK 293T cells were plated onto 10-cm dishes (Sarstedt) and grown to around 75% confluence. The 293T cells were transiently transfected with pSV (Gag and Pol proteins) and pVSV-G (envelope protein) and pMSCV-GFP retroviral vectors expressing HA-wild type CBL, HA-CBL-Y371H, or HA-CBL-C384R using Lipofectamine 2000 (Invitrogen) as directed in the manufacturer's protocol. Briefly, 4.5 μg of pSV, 1.5 μg of VSV-G, and 4.5 μg of pMSCV-IRES-GFP-HA-CBL constructs were mixed with Lipofectamine 2000 in serum- and antibiotic-free DMEM H-21 for 20 min at room temperature. 293T cells were washed with serum-free DMEM H-21, and 4 ml of serum- and antibiotic-free DMEM H-21 was added to the cells. DNA-Lipofectamine 2000 mixture was added to the plated cells and incubated for 6 h at 37 °C. Transfection medium was removed from the cells, and 10 ml of RPMI 1640 medium with 10% (v/v) fetal calf serum was added. Transfected 293T supernatant containing VSV-G pseudotyped virus carrying pMSCV-HA-CBL was collected at 48 and 72 h post-transfection.
Cell Lines and Culture
TF-1 cells were maintained in RPMI 1640 medium, 10% (v/v) fetal calf serum, 100 units of penicillin/ml, 100 μg of streptomycin/ml, and 2 ng/ml recombinant human GM-CSF. In order to stably express wild type and mutant CBL, TF-1 cells were infected with VSV-G pseudotyped retrovirus carrying wild type CBL, CBL-Y371H, and CBL-C384R constructs. TF-1 cells were suspended in retroviral supernatant supplemented with 8 ng/ml Polybrene and centrifuged in 50-ml tubes at 12,000 rpm for 75 min. TF-1 cells were resuspended in fresh retroviral Polybrene solution and incubated overnight in 6-well dishes at 37 °C. A final round of spinoculation with fresh retroviral supernatant was performed on day 2. TF-1 cells were allowed to expand for 48 h. GFP-positive infected TF-1 cells expressing HA-CBL constructs were purified using fluorescence-activated cell sorting (FACS).
XTT Assay
TF-1 cells were cytokine-depleted overnight, as described below. 2000 cells were transferred to each well of a 96-well plate in a final volume of 100 μl of RPMI, 10% FCS with the indicated concentrations of GM-CSF. Plates were incubated at 37 °C for 48 h prior to the addition of 3 μm phenazine methosulfate (Sigma) and 2 mg/ml XTT (Diagnostic Chemicals). The cells were incubated with the XTT/phenazine methosulfate solution for 6 h at 37 °C. The absorption of the reduction product at 450 nm was measured using a spectrophotometer plate reader.
Annexin V Flow Cytometric Analysis
TF-1 cells were cytokine-depleted and maintained in cytokine-free media for 72 h. Cells were stained with annexin V-phycoerythrin (PE) according to the manufacturer's protocol (BD Pharmingen). Stained cells were analyzed using a BD Biosciences FACSCalibur flow cytometer.
Cytokine Deprivation, Stimulation, and Lysis
TF-1 cells were washed three times in 10 mm HEPES (pH 7.4), Hanks' balanced salts; depleted in RPMI 1640 medium supplemented with 10% fetal calf serum overnight at 37 °C; and then stimulated with 0.5 ng/ml recombinant human GM-CSF. Proteasomal and lysosomal degradation was inhibited by incubation of cells with 30 μm MG132 (Calbiochem/Millipore) and 100 μm chloroquine (Sigma), respectively, for 2 h prior to stimulation. JAK2 and LYN kinase activity was inhibited by treatment with 2 m TG101348 (generously provided by Dr. R. Levine) for 4 h or with 0.1 m dasatinib (gift from Dr. D. Hedley) for 2 h, respectively. TF-1 cells were stimulated with 0.5 ng/ml recombinant human GM-CSF for the indicated times at 37 °C.
The cells were washed once in 10 mm HEPES (pH 7.4), Hanks' balanced salts containing 10 mm sodium pyrophosphate, 10 mm sodium fluoride, 10 mm EDTA, and 1 mm sodium orthovanadate lysed in ice-cold lysis buffer containing 1% Triton X-100, 50 mm Tris-HCl (pH 8.0), 150 mm NaCl, 10 mm sodium pyrophosphate, 10 mm sodium fluoride, 10 mm EDTA, 1 mm sodium orthovanadate, 1 mm phenylmethylsulfonyl fluoride and supplemented with “cOmplete” protease inhibitor mixture tablets (Roche Applied Science). After 5 min on ice, the lysates were centrifuged at 10,000 × g for 5 min at 4 °C.
Antibodies
The 4G10 phosphotyrosine-specific monoclonal antibody and anti-ERK1/2 were purchased from Upstate Biotechnology/Millipore. Anti-IL-3/IL-5/GM-CSFR βc N-20 (used for immunoblotting) and K-17 (used for immunoprecipitations)), anti-Shp2, and anti-phospho-ERK1/2 antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Antibodies specific for phospho-JAK2 (Tyr-1007/1008), JAK2, phospho-SRC family (Tyr-416), phospho-Ser-235/236 S6, and Lynp56 were acquired from Cell Signaling Technologies. An anti-phospho STAT5a/b antibodies were purchased from Zymed Laboratories Inc./Invitrogen. Anti-STAT5 antibody was purchased from BD Biosciences. The monoclonal 12CA5 anti-HA antibody was acquired from Roche Applied Science.
Immunoprecipitations
Antibodies along with a 50-μl volume of protein A or protein G-Sepharose 4B beads (Amersham Biosciences) were added to 2 mg of lysates for an overnight incubation at 4 °C. The beads were washed three times in ice-cold lysis buffer. The immune complexes were eluted by boiling in Laemmli sample buffer containing 100 mm DTT. Samples were resolved by SDS-PAGE and transferred to a polyvinylidene fluoride (PVDF) membrane for Western blotting.
Western Blotting
Following the electrophoretic transfer of proteins to PVDF membrane (PerkinElmer Life Sciences), the membranes were blocked at room temperature with 2.5% BSA (w/v) or 5% nonfat dry milk (w/v) in Tris-buffered saline (50 mm Tris (pH 8.0) and 150 mm NaCl) with Tween 20 for 1 h. Membranes were then incubated with an optimal concentration of the primary antibody in Tris-buffered saline containing Tween 20 (TBST) for 1 h at room temperature or overnight at 4 °C. Membranes were washed four times in TBST and incubated with the relevant HRP-conjugated secondary antibody for 30–60 min. Membranes were washed four times in TBST and visualized by enhanced chemiluminescence with autoradiographic film (ECL, Amersham Biosciences). For reprobing, membranes were stripped in 62.5 mm Tris-HCl (pH 6.8), 2% SDS, and 0.1 m β-mercaptoethanol for 30 min at 50 °C; rinsed twice in TBST; and blocked in 2.5% BSA in Tris-buffered saline prior to primary antibody incubation. Western blots were scanned and quantified using ImageJ software. Quantified Western blots are presented as mean ± S.E. Statistical analysis was performed using Student's t test.
RESULTS
Enhanced and Prolonged Phosphorylation of the GM-CSFR βc in CBL Mutant-expressing Cells
GM-CSF hypersensitivity is one of the hallmark features of JMML (39). We utilized the TF-1 hematopoietic cell line to investigate the role of JMML-associated CBL mutations in vitro. TF-1 cells endogenously express the GM-CSF receptor α (GM-CSFRα) and the IL-3/IL-5/GM-CSF receptor β common chain (GM-CSFR βc), and are responsive to GM-CSF. HA-tagged wild type CBL, CBL-Y371H, and CBL-C384R mutants were stably expressed in TF-1 cells (Fig. 1A). All exogenous constructs showed high expression of CBL when compared with vector-infected cells (Fig. 1A). This is especially advantageous because studies have shown that expression of wild type CBL can rescue or mask the effects of CBL mutants (23, 40).
FIGURE 1.
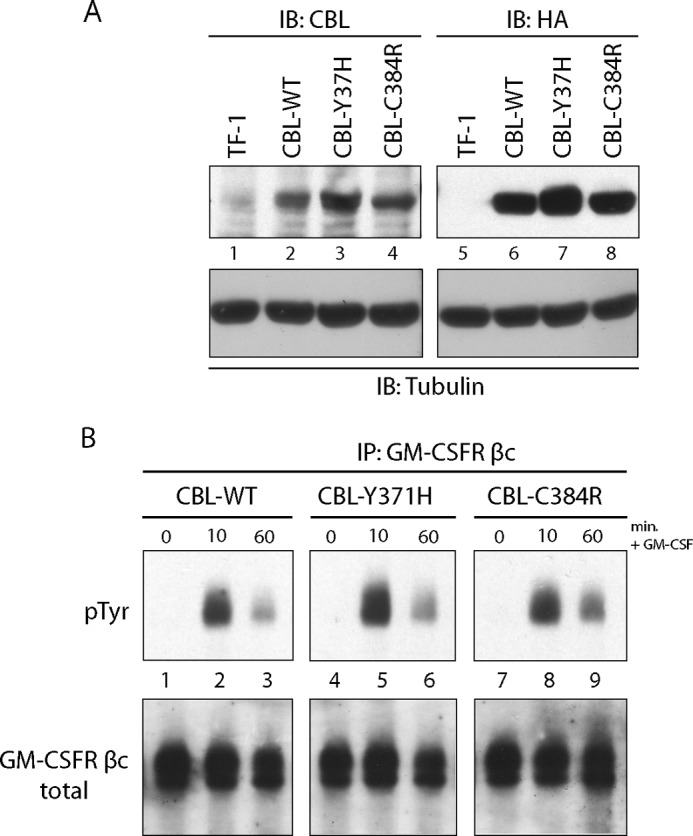
Enhanced phosphorylation of GM-CSF receptor βc upon expression of CBL mutants. A, lysates from parental TF-1 cells as well as from TF-1 cells expressing wild type CBL, CBL-Y371H, and CBL-C384R were probed for CBL expression. IB, immunoblot. B, GM-CSFR βc immunoprecipitations (IP) were performed on lysates collected from TF-1 cells expressing CBL wild type, CBL-Y371H, and CBL-C384R mutants. The cells were stimulated with 0.5 ng/ml GM-CSF for the indicated times. Blots were probed with Tyr(P)- and GM-CSFR βc-specific antibodies.
Binding of GM-CSF to the GM-CSF receptor complex activates JAK2, leading to the tyrosine phosphorylation of GM-CSFR βc. In order to investigate the effects of CBL-Y371H and CBL-C384R mutants on GM-CSF signaling, we examined the phosphorylation of the GM-CSFR βc after stimulation. TF-1 cells expressing wild type CBL, CBL-Y371H, and CBL-C384R were cytokine-depleted and stimulated with GM-CSF. GM-CSFR βc was immunoprecipitated from lysates and immunoblotted with the 4G10 anti-phosphotyrosine antibody. Upon GM-CSF stimulation, cells expressing CBL-Y371H and CBL-C384R mutants (Fig. 1B, lanes 5 and 6 and lanes 8 and 9) show enhanced phosphorylation of GM-CSFR βc compared with the wild type CBL controls (lanes 2 and 3). Furthermore, prolonged phosphorylation of GM-CSFR βc was observed in CBL mutant samples.
CBL plays an important role in stimulation-induced endocytosis, trafficking, and degradation of numerous tyrosine kinase (29, 32) and cytokine receptors (41, 42). To determine whether expression of CBL-Y371H and CBL-C384R mutants has an effect on the expression of the GM-CSFR βc, as well as its GM-CSF induced degradation, lysates collected from GM-CSF-stimulated TF-1 cells were probed for GM-CSFR βc. Because it is likely that CBL-Y371H (23, 30) and CBL-C384R (43) mutations result in a loss of CBL E3 ligase activity, GM-CSFR βc levels were expected to be higher in mutant CBL-expressing cells. Surprisingly, expression of CBL-Y371H and CBL-C384R (Fig. 2A, lanes 4–9) results in a decrease in GM-CSFR βc compared with wild type CBL (lanes 1–3)-expressing cells at 0, 10, and 60 min (Fig. 2B). However, it is important to note that GM-CSFR βc expression decreased after GM-CSF stimulation in wild type and mutant CBL-expressing cells at comparable rates. These results indicate that although expression of CBL-Y371H and CBL-C384R results in an overall decreased expression of GM-CSFR βc, the degradation of the receptor poststimulation appears not to be disrupted.
FIGURE 2.
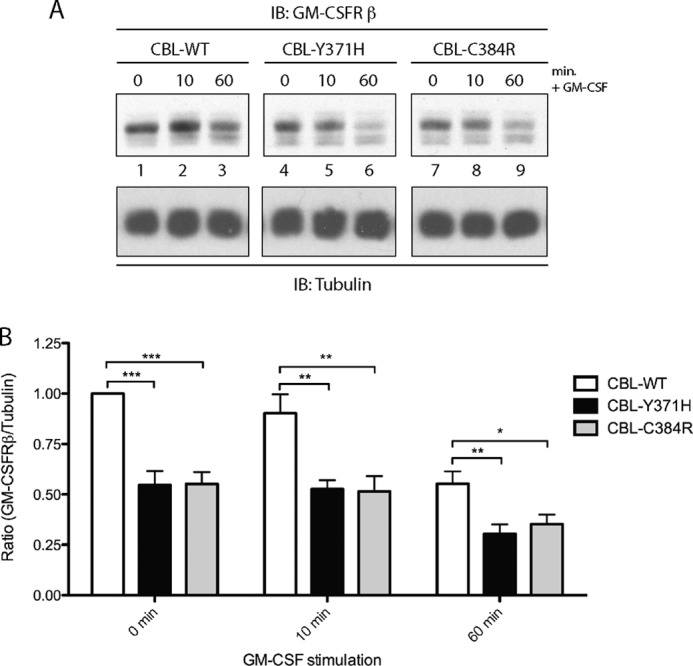
TF-1 cells expressing CBL mutants have lower expression levels of GM-CSFR βc. Lysates were collected as described in the legend to Fig. 1. A, immunoblotting (IB) was performed with GM-CSFR βc-specific antibody. Blots were reprobed for tubulin as a loading control. B, blots were quantified using ImageJ software. Values provided are ratios of GM-CSFR βc to the tubulin loading control, normalized to wild type values at time point 0 (mean ± S.E. (error bars), n = 8; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001).
Elevated Levels of JAK2 Kinase in CBL-Y371H- and CBL-C384R-expressing TF-1 Cells
Upon binding of GM-CSF to GM-CSFRα, a GM-CSFR βc homodimer is recruited (6), bringing the βc-associated JAK2 kinase (44, 45) into close proximity, allowing transphosphorylation and subsequent activation of the kinase. Activation of JAK2 is essential for downstream GM-CSF signaling (46, 47). The enhanced and prolonged phosphorylation of GM-CSFR βc, along with a decrease in the overall expression of the receptor in CBL mutant-expressing cells may be indicative of elevated kinase activity downstream of GM-CSFR βc. Because JAK2 is the main tyrosine kinase downstream of GM-CSFR, the GM-CSF-induced activation of JAK2 was assessed. TF-1 cells expressing CBL-Y371H and CBL-C384R displayed elevated levels of phosphorylated JAK2 upon GM-CSF stimulation (Fig. 3A, top, lanes 5 and 6 and lanes 8 and 9), compared with the wild type CBL controls (lanes 2 and 3). Reprobing for total JAK2, revealed that the observed enhancement of the phospho-JAK2 signal may be due to an increase in the expression of JAK2 in CBL mutant-expressing cells (Fig. 3, A (middle, lanes 4–9) and B).
FIGURE 3.
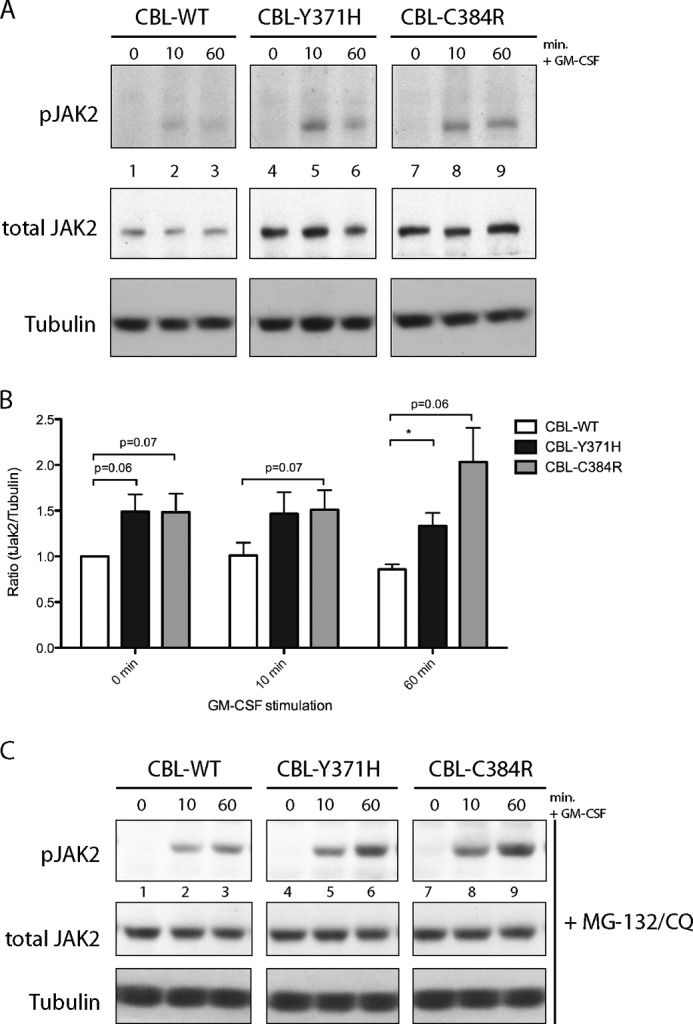
Expression of CBL-Y371H and CBL-C384R mutants results in elevated JAK2. A, lysates collected from TF-1 cells expressing wild type and CBL mutants were probed for phospho-Tyr-1007/1008 JAK2 and reprobed for total JAK2 and tubulin. B, blots were quantified using ImageJ software. Values presented are ratios of total JAK2 to tubulin, normalized to wild type CBL at time 0 (mean ± S.E. (error bars), n = 4; *, p ≤ 0.05). C, TF-1 cells were treated with proteasomal and lysosomal inhibitors, MG-132 and chloroquine (CQ), prior to stimulation (n = 4).
Linker and RING finger mutations disrupt the E3 ligase activity of CBL (29, 31, 32). Such loss of E3 ligase activity has been shown to result in a significant decrease in the EPO-induced ubiquitination of JAK2 (23). In order to determine whether the increase in total JAK2 in CBL mutant expressing cell lines was due to compromised ubiquitination and degradation of the kinase, TF-1 cells expressing wild type CBL, CBL-Y371H, and CBL-C384R were treated with proteasomal (MG-132) and lysosomal (chloroquine) inhibitors prior to stimulation. Inhibition of proteasomal and lysosomal degradation stabilized JAK2 to comparable levels in all three cell lines (Fig. 3C, middle). These results indicate that the expression of CBL-Y371H and CBL-C384R mutants affects the degradation of JAK2, leading to elevated levels, which in turn contribute to increased JAK2 phosphorylation and potentially signaling in response to GM-CSF stimulation.
Increased Expression of LYN Kinase in CBL Mutant Cells
Although JAK2 is the primary kinase activated downstream of GM-CSF receptor, members of the SRC family kinase are stimulated in response to GM-CSF (48–50). Specifically, LYN has been shown to directly associate with GM-CSFR βc (51) and to play an important role in mediating the anti-apoptotic effects of GM-CSF in polymorphonuclear leukocytes (49, 52). LYN also associates with GM-CSFRα and is involved in the survival signal required for factor-independent growth of cells expressing the FIΔ GM-CSFR βc mutant (48). Considering that TF-1 cells expressing CBL-Y371H and CBL-C384R show enhanced survival while exhibiting lower expression of GM-CSFR βc, we wanted to determine whether LYN phosphorylation and/or expression was altered in CBL mutant-expressing cells. Lysates collected from GM-CSF-stimulated cells were probed with an antibody specific for pY396-LYN, residing in a phosphorylated motif within the LYN activation loop. Increased LYN phosphorylation was observed in cells expressing CBL-Y371H and CBL-C384R mutants (Fig. 4A, top, lanes 4–9), compared with those expressing wild type CBL (lanes 1–3). Reprobing for total LYN revealed that CBL mutant cells have increased levels of LYN (p56) (Fig. 4, A (middle, lanes 4–9) and B). This observed increase in LYN expression leads to elevated LYN phosphorylation, which may contribute to the enhanced survival of CBL mutant TF-1 cells.
FIGURE 4.
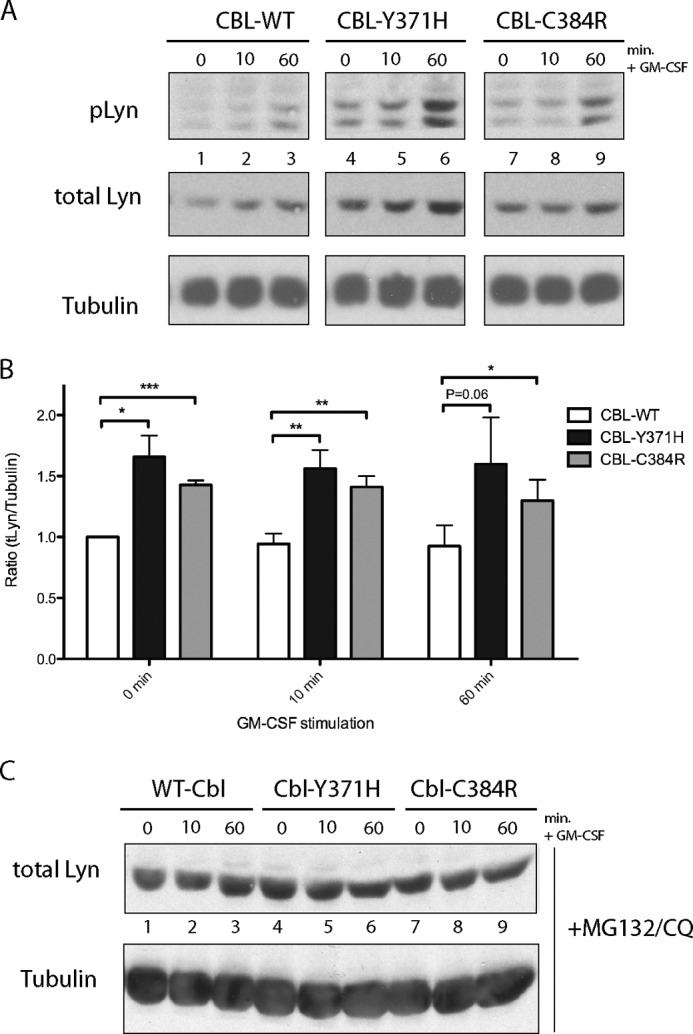
Increased expression of LYN kinase in CBL-Y371H and CBL-C384R mutant TF-1 cells. A, whole cell lysates collected from TF-1 cells expressing CBL-WT and CBL mutants were immunoblotted with antibodies specific for Tyr(P)-396-LYN. Blots were reprobed for total LYN (p56) and tubulin. B, blots were quantified using ImageJ software. Values presented are ratios of total LYN to tubulin, normalized to CBL-WT at time 0 (mean ± S.E. (error bars), n = 6; *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001).
Similar to JAK2, treatment with MG-132 and chloroquine stabilized total LYN levels to comparable levels in wild type and mutant cells, indicating that the increase in LYN levels is probably due to the loss of E3 ubiquitin ligase activity of the CBL mutants (Fig. 4C).
Expression of CBL Mutants Results in Constitutive S6 Phosphorylation and Enhanced Factor-free Survival
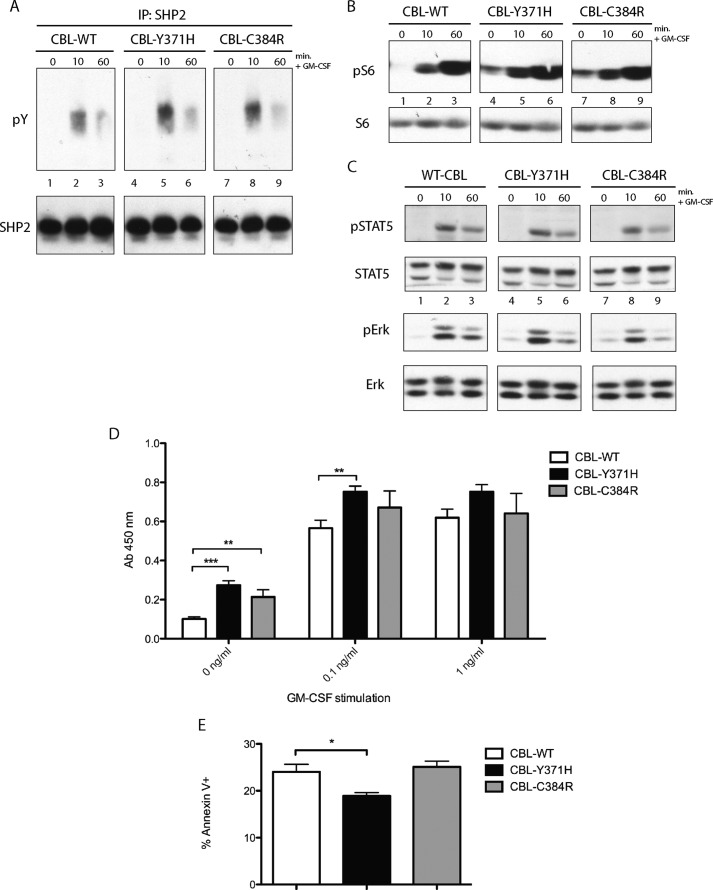
Investigation of signaling effectors further downstream of the GM-CSF receptor revealed enhanced phosphorylation of SHP2 in CBL mutant-expressing cells relative to wild type CBL controls (Fig. 5A). Constitutive phosphorylation of S6 in TF-1 cells expressing CBL-Y371H and CBL-C384R, compared with wild type CBL-expressing controls (Fig. 5B), was also observed. No significant differences were observed in the phosphorylation of STAT5 or ERK1/2 (Fig. 5C) between wild type CBL- and mutant CBL-expressing TF-1 cells after GM-CSF stimulation.
FIGURE 5.
Expression of CBL mutants results in constitutive phosphorylation of S6 and enhanced survival of TF-1 cells. A, SHP2 immunoprecipitations (IP) were performed on lysates collected from TF-1 cells expressing CBL-WT, CBL-Y371H, and CBL-C384R, which were depleted of cytokines overnight and stimulated with GM-CSF. B, lysates collected were also probed with phospho-Ser-325/326 S6 and total S6. C, TF-1 cells expressing wild type CBL, CBL-Y371H, and CBL-C384R were depleted of cytokine overnight and stimulated with GM-CSF. Lysates collected were probed with phospho-STAT (pSTAT5) or phospho-ERK (pErk) and reprobed for total STAT5 or ERK. D, TF-1 cells expressing WT-CBL or CBL mutants Y371H or C384R were depleted of cytokine overnight. Starved cells were plated in 96-well plates and incubated for 48 h, prior to the addition of XTT reagent. Reduction of XTT reagent was measured using a spectrometer (n = 4). **, p ≤ 0.001; ***, p ≤ 0.0001. E, TF-1 cells expressing wild type or CBL mutants were maintained in cytokine-free media for 72 h. The cells were then stained with annexin V and analyzed via flow cytometry (n = 3; *, p ≤ 0.05). Error bars, S.E.
XTT assays were performed to determine whether the expression of the CBL linker and RING finger mutants results in enhanced survival and growth. TF-1 cells expressing CBL-Y371H and CBL-C384R mutants displayed enhanced survival in the absence of GM-CSF relative to cells expressing wild type CBL (Fig. 5D). The increase in the survival of the mutant-expressing cells decreases with increasing concentration of GM-CSF. Cell counting assays were also performed to confirm these results (data not shown). To determine whether the increase in survival was due to a decrease in apoptosis, annexin V staining was performed on cytokine-deprived wild type and mutant CBL-expressing cells. There was a decrease in annexin V-positive TF-1 cells expressing CBL-Y371H relative to wild type CBL-expressing controls, whereas the proportion of annexin V-positive CBL-C384R-expressing cells was comparable with wild type CBL controls (Fig. 5E). These results indicate that JMML-associated CBL mutations result in enhanced survival of TF-1 cells at low doses of GM-CSF.
Constitutive and Enhanced Phosphorylation of CBL Mutants Is Dependent on an SRC Family Kinase
CBL is phosphorylated downstream of numerous oncogenic protein-tyrosine kinases (BCR/ABL and v-SRC), receptor tyrosine kinases (PDGF-R, FLT-3, and c-KIT), and cytokine receptors (TPO and EPO) (reviewed in Ref. 53). For example, CBL is tyrosine-phosphorylated downstream of the βc chain in response to IL-3 (33, 34) and GM-CSF (35, 36).
The carboxyl-terminal region of CBL contains three major tyrosine phosphorylation sites, Tyr-700, Tyr-731, and Tyr-774 (54). Phosphorylation of these tyrosines leads to the recruitment of SRC homology 2 domain-containing effectors. Phosphorylated Tyr-700 of CBL binds the guanine nucleotide exchange factor, VAV (55). Phosphorylated Tyr-731 acts as the binding site for the p85 regulatory subunit of PI3K (56, 57), and members of the CRK adaptor protein family have been shown to bind both phosphorylated Tyr-700 and Tyr-774 (58–60). Downstream pathways mediated by CBL tyrosine phosphorylation sites play an important role in CBL-mediated signaling. Linker region mutations of CBL have been shown to lead to enhanced tyrosine phosphorylation of CBL in Ba/F3-FLT3 cells (24).
We were interested in determining whether expression of the linker region Y371H and the RING finger C384R mutations would result in altered CBL tyrosine phosphorylation. CBL was immunoprecipitated from wild type and mutant CBL TF-1 lysates, and phosphorylation was assessed by Tyr(P) immunoblotting (Fig. 6). CBL-Y371H (lanes 4–6) and CBL-C384R (lanes 7–9) were highly phosphorylated compared with CBL wild type controls (lanes 1–3). Both CBL mutants showed constitutive phosphorylation in the absence of GM-CSF stimulation (lanes 4 and 7).
FIGURE 6.
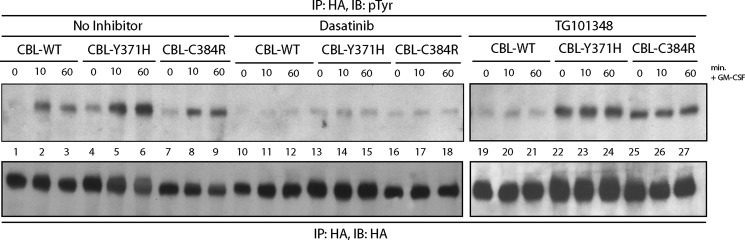
Dasatinib treatment abolishes the enhanced phosphorylation of CBL-Y371H and CBL-C384R. TF-1 cells expressing WT and mutant CBL were incubated with dasatinib and TG101348 for 2 and 4 h, respectively, prior to GM-CSF stimulation. Cells were lysed, and HA immunoprecipitations were performed. Blots were probed with Tyr(P) antibodies and reprobed with HA-specific antibodies. IP, immunoprecipitation; IB, immunoblot.
Although numerous members of the SRC family kinases, including LYN (61–63), are capable of phosphorylating CBL, there is evidence that Janus kinase family members may also target CBL for tyrosine phosphorylation (64). To determine whether inhibition of JAK2 or SRC family kinases could abolish the enhanced and constitutive phosphorylation of CBL, TF-1 cells expressing wild type and mutant CBL were treated with either the JAK2-specific inhibitor TG101348 or the SRC family kinase inhibitor dasatinib prior to stimulation. The constitutive and enhanced phosphorylation of CBL-Y371H and CBL-C384R mutants (lanes 22–27) persisted in TG101348-treated samples (Fig. 6), whereas pretreatment with dasatinib abolished the elevated phosphorylation of CBL mutants (lanes 13–18) to levels comparable with wild type CBL controls (lanes 10–12). These results indicate that downstream of the GM-CSF receptor, CBL is phosphorylated by a SRC family kinase, potentially LYN, and mutations in the linker region or the RING finger domain of CBL lead to not only enhanced but also constitutive phosphorylation of CBL.
TG101348-mediated Inhibition of Enhanced Phosphorylation of GM-CSFR βc in CBL Mutant-expressing Cells
We were interested in determining how the inhibition of JAK2 or SRC family kinases downstream of GM-CSFR would modulate the elevated phosphorylation of GM-CSFR βc observed in CBL mutant-expressing cells (Figs. 1B and 7, lanes 1–9). TF-1 cells were treated with TG101348 and dasatinib, alone or in combination, prior to stimulation and subsequent GM-CSFR βc immunoprecipitation. TG101348 treatment resulted in a significant inhibition of GM-CSF-induced phosphorylation of GM-CSFR βc in both wild type CBL- and CBL mutant-expressing cells, resulting in an equally down-modulated level of the phosphorylated receptor (Fig. 7, lanes 10–18). This was expected, because JAK2 is known to be the kinase responsible for tyrosine phosphorylation of the GM-CSFR βc in response to stimulation (47, 65, 66).
FIGURE 7.
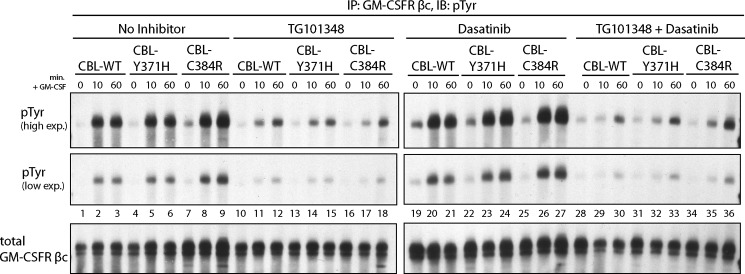
Inhibition of JAK2 activity via treatment with TG101348 abolishes the enhanced phosphorylation of GM-CSFR β in CBL mutant-expressing cells. TF-1 cells expressing WT-CBL, CBL-Y371H, and CBL-C384R were cytokine-depleted and treated with 2 mm TG101348 for 4 h or 0.1 mm dasatinib for 2 h prior to GM-CSF stimulation. GM-CSFRb-specific immunoprecipitations were performed on collected lysates. Immunoblotting was performed using Tyr(P)- and GM-CSFR βc-specific antibodies. IP, immunoprecipitation; IB, immunoblot.
Interestingly, inhibition of SRC family kinases did not result in the inhibition of GM-CSFR βc phosphorylation, but it did result in an equivalent level of GM-CSFR βc phosphorylation in wild type CBL and CBL mutant samples (Fig. 7, lanes 19–27). Treatment with both TG101348 and dasatinib inhibited GM-CSFR βc phosphorylation (Fig. 7, lanes 28–36) to similar levels observed in samples treated with only TG101348.
DISCUSSION
Hypersensitivity to GM-CSF is one of the defining characteristics of juvenile myelomonocytic leukemia. Until recently, genes mutated in JMML patients (NF1, SHP2, NRAS, and KRAS) were directly involved in the RAS signaling pathway. The Loh and Maciejewski groups (20–22) found that 10–15% of JMML patients have mutations in CBL and that these mutations are associated with acquired uniparental disomy. The majority of CBL mutations identified clustered in the linker region and RING finger, both of which play an important role in the E3 ligase activity of CBL. The identification of these JMML-associated CBL mutations raised a number of questions about the role of CBL downstream of GM-CSF receptor, whether CBL is involved in RAS signaling downstream of GM-CSFR, and how mutations in CBL can lead to hypersensitivity to GM-CSF. In order to address these questions, we utilized the TF-1 hematopoietic cell line, which is GM-CSF-responsive (38) and has a low endogenous CBL expression. To examine the functional consequences of CBL linker region mutations, we expressed CBL-Y371H linker and CBL-C384R RING finger mutants, because these are two of the most common CBL mutations observed in JMML patients (21, 22).
We found that expression of these CBL mutants in GM-CSF-stimulated TF-1 cells leads to enhanced and prolonged phosphorylation of GM-CSFR βc, which occurred concurrently with elevated expression of both JAK2 and LYN. Assessment of downstream signaling revealed enhanced phosphorylation of S6 in CBL-Y371H- and CBL-C384R-expressing cells relative to wild type CBL controls. TF-1 cells expressing CBL mutations showed enhanced survival in the absence of GM-CSF, and specifically in the case of CBL-Y371H-expressing cells, this is probably due to a decrease in induction of apoptosis. We also observed elevated and constitutive phosphorylation of CBL-Y371H and CBL-C384R mutants, which was inhibited upon treatment with dasatinib, an SRC family kinase inhibitor. Interestingly, treatment with dasatinib also led to equalization of GM-CSFR βc phosphorylation between wild type CBL- and CBL mutant-expressing cells. However, inhibition of JAK2 activity by TG101348 resulted in complete inhibition of GM-CSFR βc phosphorylation in CBL mutant and wild type CBL control cells.
Expression of CBL-Y371H and CBL-C384R mutants results in prolonged and elevated receptor tyrosine phosphorylation, which supports the observed enhancement in survival (67). Martinez-Moczygemba and Huston (37, 68), using the IL-5 receptor as a model for βc subunit-sharing cytokine receptors (IL-3/IL-5/GM-CSF), demonstrated that increased βc subunit ubiquitination is observed after stimulation. This ubiquitination event leads to the proteasomal degradation of the cytoplasmic tail of the βc subunit, which attenuates downstream signaling (37, 68). They also found that the ubiquitination of the βc subunit may be mediated by CBL, because the E3 ligase was co-immunoprecipitated with the βc subunit. The loss of E3 ligase activity of CBL-Y371H and CBL-C384R mutants may prevent GM-CSFR βc ubiquitination and proteasomal degradation of the cytoplasmic region of the receptor and culminate in the observed enhancement of GM-CSFR βc phosphorylation and elevated levels of JAK2. However, this is unlikely, because we have observed total levels of GM-CSFR βc to be lower in CBL mutant cells. Alternatively, increased JAK2 and LYN expression could be correlated with a negative feedback loop that down-regulates GM-CSFR βc levels in TF-1 cells. The ubiquitination of βc in response to IL-5 stimulation is dependent on JAK2 activity (68, 69). In CBL-Y371H- and CBL-C384R-expressing cells, the enhanced JAK2 activity can potentially lead to increased ubiquitination of the βc by other ubiquitin ligases, resulting in decreased overall expression of the receptor subunit.
JAK2 is also regulated via CBL-mediated ubiquitination (23, 70). The expression of CBL mutants Y371H and C384R may result in decreased ubiquitination of JAK2, culminating in elevated levels of JAK2 as well as enhanced JAK2-mediated phosphorylation of GM-CSFR βc. We found that in CBL mutant cells, there was elevated expression of JAK2 kinase, which was abolished upon treatment with proteasomal and lysosomal inhibitors.
Similar to JAK2, several members of the SRC family kinases (71), including LYN, have been shown to be ubiquitinated (72) by CBL (73, 74). The enhanced expression of LYN in TF-1 cells expressing CBL-Y371H and CBL-C384R indicates that, downstream of GM-CSFR, CBL is responsible for LYN ubiquitination. LYN mediates the tyrosine phosphorylation of CBL downstream of β1 integrin (61), granulocyte-colony stimulating factor (G-CSF) (62, 75), and the B-cell antigen receptor (63, 76). We have observed enhanced tyrosine phosphorylation of CBL-Y371H and CBL-C384R mutants, which is abolished upon treatment with the SRC family kinase inhibitor dasatinib. This confirms that LYN is a kinase responsible for tyrosine phosphorylation of CBL downstream of the GM-CSFR, and the enhanced phosphorylation of CBL mutants may be a functional consequence of the elevated levels of LYN in CBL mutant-expressing cells.
Phosphorylation of CBL Tyr-731 leads to the activation of the PI3K pathway via recruitment of the p85 regulatory subunit of PI3K (54, 56). The Corey group (62, 77) has shown that LYN couples to the PI3K pathway in a CBL-dependent manner, whereby activated LYN binds and phosphorylates CBL, allowing for the recruitment of p85 to the complex. LYN also activates the PI3K pathway downstream of GM-CSFR α (48), promoting cell survival. We have shown that expression of CBL JMML mutants defective in E3 ubiquitin ligase activity leads to aberrant regulation of LYN downstream of GM-CSFR, which ultimately leads to constitutive and enhanced phosphorylation of S6, suggesting elevated PI3K pathway activity. Interestingly, basal activation of S6 has been observed in mononuclear cells isolated from JMML patients (78). Furthermore, in the absence of LYN, the antiapoptotic effects of GM-CSF are abolished (49, 52). At low concentrations of GM-CSF, the signaling pathways activated downstream of the receptor lead to cell survival only, whereas stimulation with higher doses results in cell proliferation and cell survival (79). We have shown that expression of CBL mutants results in enhanced survival, especially at low doses, or in the complete absence of GM-CSF in TF-1 cells. These data suggest that CBL linker and RING finger mutants lower the threshold concentration of GM-CSF required to induce cell survival. Together, these results suggest that in CBL-Y371H- and CBL-C384R-expressing cells, the modulation of the PI3-K pathway due to the increase in LYN levels may be contributing to the observed enhancement of survival in the absence of GM-CSF.
The mechanism by which these mutations lead to elevated GM-CSF signaling and enhanced survival depends on the role of CBL as both an E3 ligase and an adaptor protein. CBL-Y371H and CBL-C384R mutants compromise the E3 ligase activity of CBL. We have shown that these loss-of-function mutations lead to increased levels of JAK2 and LYN kinases downstream of the GM-CSFR, potentially due to loss of ubiquitination of the kinases or to the inhibition of GM-CSFR βc ubiquitination and cytoplasmic domain degradation. These events contribute to the elevated levels of GM-CSF signaling observed in CBL mutant-expressing TF-1 cells. Sanada et al. (23) have shown that Cbl−/− LSK cells show a mild cytokine hypersensitivity, but transformation of Cbl−/− LSK cells with Cbl linker region mutants significantly enhances the cytokine hypersensitivity, indicating a gain-of-function of the mutants that cannot be ascribed to a simple loss of CBL E3 ligase activity. Inhibition of Cbl-b function by mutant Cbl has been proposed as a possible mechanism (80, 81). However, it is possible that functionality of CBL as an adaptor protein may be contributing to the gain of function of the mutants. Our group, as well as others, has shown that expression of CBL linker and RING finger mutants results in enhanced tyrosine phosphorylation of CBL (24, 81, 82). This increase in CBL phosphorylation culminates in enhanced PI3K and RAS pathway activation. We found that treatment with dasatinib results in loss of CBL phosphorylation. Interestingly, dasatinib treatment equalized GM-CSFR βc phosphorylation in wild type- and CBL mutant-expressing TF-1 cells. Therefore, dasatinib treatment may decouple the gain-of-function capacity of CBL mutants from the loss of E3 ligase activity. Considering that treatment with JAK2 inhibitor, TG101348, results in complete inhibition of GM-CSFR βc phosphorylation, dasatinib may provide a treatment option for the enhanced signaling driven by CBL gain-of-function mutations.
Prior studies have shown that expression of CBL linker region and RING finger mutants inhibits stimulation-induced ubiquitination of the EGF-R (23, 29), FLT3, c-KIT, and JAK2 (downstream of EPO-R) (23). Niemeyer et al. (40) have shown that expression of CBL linker region mutants in Ba/F3-EPO-R cells results in an enhanced phosphorylation of ERK1/2, AKT, and S6. In Ba/F3 cells expressing the FLT3 receptor tyrosine kinase, expression of CBL-Y371 mutants leads to elevated FLT3 phosphorylation as well as AKT and STAT5 (24). Although these studies are useful to probe how CBL mutations perturb FLT3 signaling in AML, no studies have examined the role of CBL JMML mutations in GM-CSF-dependent signaling pathways to date. Our analysis reveals that expression of CBL-Y371H or CBL-C384R in TF-1 cells generates GM-CSF hypersensitivity that affects proximal steps in receptor activation, including GM-CSFR βc, JAK2, and LYN coupling to PI3K activation. Unlike with the Ba/F3 results, we failed to observe effects on ERK1/2 or STAT5 activation. Whether this is a function of the unique receptor systems or distinct cell lines remains to be determined.
In conclusion, these studies have confirmed that CBL plays a negative regulatory role downstream of GM-CSFR. We have shown that the enhancement of GM-CSF signaling observed upon loss of CBL E3 ligase activity is due to increased stability of JAK2 and LYN. These results provide in vitro support for further investigation into the applicability of JAK2 and SRC family kinase inhibitors for use in treatment of myeloid malignancies associated with CBL linker region and RING finger mutations.
Acknowledgments
We thank Ben Neel and members of the Neel and Barber laboratories for helpful discussions throughout this work.
This work was supported by Canadian Institutes of Health Research Grant FRN#42428.
- GM-CSFR
- GM-CSF receptor
- XTT
- sodium 3,3′-(1-((phenylamino)carbonyl)-3,4-tetrazolium)bis(4-mehoxy-6-nitro)benzene sulfonic acid hydrate)
- JMML
- juvenile myelomonocytic leukemia
- VSV
- vesicular stomatitis virus.
REFERENCES
- 1. Hercus T. R., Broughton S. E., Ekert P. G., Ramshaw H. S., Perugini M., Grimbaldeston M., Woodcock J. M., Thomas D., Pitson S., Hughes T., D'Andrea R. J., Parker M. W., Lopez A. F. (2012) The GM-CSF receptor family. Mechanism of activation and implications for disease. Growth Factors 30, 63–75 [DOI] [PubMed] [Google Scholar]
- 2. Park L. S., Martin U., Sorensen R., Luhr S., Morrissey P. J., Cosman D., Larsen A. (1992) Cloning of the low-affinity murine granulocyte-macrophage colony-stimulating factor receptor and reconstitution of a high-affinity receptor complex. Proc. Natl. Acad. Sci. U.S.A. 89, 4295–4299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hayashida K., Kitamura T., Gorman D. M., Arai K., Yokota T., Miyajima A. (1990) Molecular cloning of a second subunit of the receptor for human granulocyte-macrophage colony-stimulating factor (GM-CSF). Reconstitution of a high-affinity GM-CSF receptor. Proc. Natl. Acad. Sci. U.S.A. 87, 9655–9659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barreda D. R., Hanington P. C., Belosevic M. (2004) Regulation of myeloid development and function by colony stimulating factors. Dev. Comp. Immunol. 28, 509–554 [DOI] [PubMed] [Google Scholar]
- 5. Gearing D. P., King J. A., Gough N. M., Nicola N. A. (1989) Expression cloning of a receptor for human granulocyte-macrophage colony-stimulating factor. EMBO J. 8, 3667–3676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hansen G., Hercus T. R., McClure B. J., Stomski F. C., Dottore M., Powell J., Ramshaw H., Woodcock J. M., Xu Y., Guthridge M., McKinstry W. J., Lopez A. F., Parker M. W. (2008) The structure of the GM-CSF receptor complex reveals a distinct mode of cytokine receptor activation. Cell 134, 496–507 [DOI] [PubMed] [Google Scholar]
- 7. Emanuel P. D. (2008) Juvenile myelomonocytic leukemia and chronic myelomonocytic leukemia. Leukemia 22, 1335–1342 [DOI] [PubMed] [Google Scholar]
- 8. Chan R. J., Cooper T., Kratz C. P., Weiss B., Loh M. L. (2009) Juvenile myelomonocytic leukemia. A report from the 2nd International JMML Symposium. Leuk. Res. 33, 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Side L. E., Emanuel P. D., Taylor B., Franklin J., Thompson P., Castleberry R. P., Shannon K. M. (1998) Mutations of the NF1 gene in children with juvenile myelomonocytic leukemia without clinical evidence of neurofibromatosis, type 1. Blood 92, 267–272 [PubMed] [Google Scholar]
- 10. Side L., Taylor B., Cayouette M., Conner E., Thompson P., Luce M., Shannon K. (1997) Homozygous inactivation of the NF1 gene in bone marrow cells from children with neurofibromatosis type 1 and malignant myeloid disorders. N. Engl. J. Med. 336, 1713–1720 [DOI] [PubMed] [Google Scholar]
- 11. Xu G. F., Lin B., Tanaka K., Dunn D., Wood D., Gesteland R., White R., Weiss R., Tamanoi F. (1990) The catalytic domain of the neurofibromatosis type 1 gene product stimulates ras GTPase and complements ira mutants of S. cerevisiae. Cell 63, 835–841 [DOI] [PubMed] [Google Scholar]
- 12. Tartaglia M., Niemeyer C. M., Fragale A., Song X., Buechner J., Jung A., Hählen K., Hasle H., Licht J. D., Gelb B. D. (2003) Somatic mutations in PTPN11 in juvenile myelomonocytic leukemia, myelodysplastic syndromes and acute myeloid leukemia. Nat. Genet. 34, 148–150 [DOI] [PubMed] [Google Scholar]
- 13. Loh M. L., Vattikuti S., Schubbert S., Reynolds M. G., Carlson E., Lieuw K. H., Cheng J. W., Lee C. M., Stokoe D., Bonifas J. M., Curtiss N. P., Gotlib J., Meshinchi S., Le Beau M. M., Emanuel P. D., Shannon K. M. (2004) Mutations in PTPN11 implicate the SHP-2 phosphatase in leukemogenesis. Blood 103, 2325–2331 [DOI] [PubMed] [Google Scholar]
- 14. Kratz C. P., Niemeyer C. M., Castleberry R. P., Cetin M., Bergsträsser E., Emanuel P. D., Hasle H., Kardos G., Klein C., Kojima S., Stary J., Trebo M., Zecca M., Gelb B. D., Tartaglia M., Loh M. L. (2005) The mutational spectrum of PTPN11 in juvenile myelomonocytic leukemia and Noonan syndrome/myeloproliferative disease. Blood 106, 2183–2185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Noguchi T., Matozaki T., Horita K., Fujioka Y., Kasuga M. (1994) Role of SH-PTP2, a protein-tyrosine phosphatase with Src homology 2 domains, in insulin-stimulated Ras activation. Mol. Cell Biol. 14, 6674–6682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shi Z. Q., Yu D. H., Park M., Marshall M., Feng G. S. (2000) Molecular mechanism for the Shp-2 tyrosine phosphatase function in promoting growth factor stimulation of Erk activity. Mol. Cell Biol. 20, 1526–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kalra R., Paderanga D. C., Olson K., Shannon K. M. (1994) Genetic analysis is consistent with the hypothesis that NF1 limits myeloid cell growth through p21ras. Blood 84, 3435–3439 [PubMed] [Google Scholar]
- 18. Miyauchi J., Asada M., Sasaki M., Tsunematsu Y., Kojima S., Mizutani S. (1994) Mutations of the N-ras gene in juvenile chronic myelogenous leukemia. Blood 83, 2248–2254 [PubMed] [Google Scholar]
- 19. Flotho C., Valcamonica S., Mach-Pascual S., Schmahl G., Corral L., Ritterbach J., Hasle H., Aricò M., Biondi A., Niemeyer C. M. (1999) RAS mutations and clonality analysis in children with juvenile myelomonocytic leukemia (JMML). Leukemia 13, 32–37 [DOI] [PubMed] [Google Scholar]
- 20. Muramatsu H., Makishima H., Jankowska A. M., Cazzolli H., O'Keefe C., Yoshida N., Xu Y., Nishio N., Hama A., Yagasaki H., Takahashi Y., Kato K., Manabe A., Kojima S., Maciejewski J. P. (2010) Mutations of an E3 ubiquitin ligase c-Cbl but not TET2 mutations are pathogenic in juvenile myelomonocytic leukemia. Blood 115, 1969–1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Loh M. L., Sakai D. S., Flotho C., Kang M., Fliegauf M., Archambeault S., Mullighan C. G., Chen L., Bergstraesser E., Bueso-Ramos C. E., Emanuel P. D., Hasle H., Issa J. P., van den Heuvel-Eibrink M. M., Locatelli F., Stary J., Trebo M., Wlodarski M., Zecca M., Shannon K. M., Niemeyer C. M. (2009) Mutations in CBL occur frequently in juvenile myelomonocytic leukemia. Blood 114, 1859–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Makishima H., Cazzolli H., Szpurka H., Dunbar A., Tiu R., Huh J., Muramatsu H., O'Keefe C., Hsi E., Paquette R. L., Kojima S., List A. F., Sekeres M. A., McDevitt M. A., Maciejewski J. P. (2009) Mutations of e3 ubiquitin ligase cbl family members constitute a novel common pathogenic lesion in myeloid malignancies. J. Clin. Oncol. 27, 6109–6116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sanada M., Suzuki T., Shih L. Y., Otsu M., Kato M., Yamazaki S., Tamura A., Honda H., Sakata-Yanagimoto M., Kumano K., Oda H., Yamagata T., Takita J., Gotoh N., Nakazaki K., Kawamata N., Onodera M., Nobuyoshi M., Hayashi Y., Harada H., Kurokawa M., Chiba S., Mori H., Ozawa K., Omine M., Hirai H., Nakauchi H., Koeffler H. P., Ogawa S. (2009) Gain-of-function of mutated C-CBL tumour suppressor in myeloid neoplasms. Nature 460, 904–908 [DOI] [PubMed] [Google Scholar]
- 24. Fernandes M. S., Reddy M. M., Croteau N. J., Walz C., Weisbach H., Podar K., Band H., Carroll M., Reiter A., Larson R. A., Salgia R., Griffin J. D., Sattler M. (2010) Novel oncogenic mutations of CBL in human acute myeloid leukemia that activate growth and survival pathways depend on increased metabolism. J. Biol. Chem. 285, 32596–32605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Sargin B., Choudhary C., Crosetto N., Schmidt M. H., Grundler R., Rensinghoff M., Thiessen C., Tickenbrock L., Schwäble J., Brandts C., August B., Koschmieder S., Bandi S. R., Duyster J., Berdel W. E., Müller-Tidow C., Dikic I., Serve H. (2007) Flt3-dependent transformation by inactivating c-Cbl mutations in AML. Blood 110, 1004–1012 [DOI] [PubMed] [Google Scholar]
- 26. Dunbar A. J., Gondek L. P., O'Keefe C. L., Makishima H., Rataul M. S., Szpurka H., Sekeres M. A., Wang X. F., McDevitt M. A., Maciejewski J. P. (2008) 250K single nucleotide polymorphism array karyotyping identifies acquired uniparental disomy and homozygous mutations, including novel missense substitutions of c-Cbl, in myeloid malignancies. Cancer Res. 68, 10349–10357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grand F. H., Hidalgo-Curtis C. E., Ernst T., Zoi K., Zoi C., McGuire C., Kreil S., Jones A., Score J., Metzgeroth G., Oscier D., Hall A., Brandts C., Serve H., Reiter A., Chase A. J., Cross N. C. (2009) Frequent CBL mutations associated with 11q acquired uniparental disomy in myeloproliferative neoplasms. Blood 113, 6182–6192 [DOI] [PubMed] [Google Scholar]
- 28. Reindl C., Quentmeier H., Petropoulos K., Greif P. A., Benthaus T., Argiropoulos B., Mellert G., Vempati S., Duyster J., Buske C., Bohlander S. K., Humphries K. R., Hiddemann W., Spiekermann K. (2009) CBL exon 8/9 mutants activate the FLT3 pathway and cluster in core binding factor/11q deletion acute myeloid leukemia/myelodysplastic syndrome subtypes. Clin. Cancer Res. 15, 2238–2247 [DOI] [PubMed] [Google Scholar]
- 29. Levkowitz G., Waterman H., Ettenberg S. A., Katz M., Tsygankov A. Y., Alroy I., Lavi S., Iwai K., Reiss Y., Ciechanover A., Lipkowitz S., Yarden Y. (1999) Ubiquitin ligase activity and tyrosine phosphorylation underlie suppression of growth factor signaling by c-Cbl/Sli-1. Mol. Cell 4, 1029–1040 [DOI] [PubMed] [Google Scholar]
- 30. Dou H., Buetow L., Hock A., Sibbet G. J., Vousden K. H., Huang D. T. (2012) Structural basis for autoinhibition and phosphorylation-dependent activation of c-Cbl. Nat. Struct. Mol. Biol. 19, 184–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kassenbrock C. K., Anderson S. M. (2004) Regulation of ubiquitin protein ligase activity in c-Cbl by phosphorylation-induced conformational change and constitutive activation by tyrosine to glutamate point mutations. J. Biol. Chem. 279, 28017–28027 [DOI] [PubMed] [Google Scholar]
- 32. Joazeiro C. A., Wing S. S., Huang H., Leverson J. D., Hunter T., Liu Y. C. (1999) The tyrosine kinase negative regulator c-Cbl as a RING-type, E2-dependent ubiquitin-protein ligase. Science 286, 309–312 [DOI] [PubMed] [Google Scholar]
- 33. Anderson S. M., Burton E. A., Koch B. L. (1997) Phosphorylation of Cbl following stimulation with interleukin-3 and its association with Grb2, Fyn, and phosphatidylinositol 3-kinase. J. Biol. Chem. 272, 739–745 [DOI] [PubMed] [Google Scholar]
- 34. Barber D. L., Mason J. M., Fukazawa T., Reedquist K. A., Druker B. J., Band H., D'Andrea A. D. (1997) Erythropoietin and interleukin-3 activate tyrosine phosphorylation of CBL and association with CRK adaptor proteins. Blood 89, 3166–3174 [PubMed] [Google Scholar]
- 35. Naccache P. H., Gilbert C., Barabé F., Al-Shami A., Mahana W., Bourgoin S. G. (1997) Agonist-specific tyrosine phosphorylation of Cbl in human neutrophils. J. Leukoc. Biol. 62, 901–910 [DOI] [PubMed] [Google Scholar]
- 36. Odai H., Sasaki K., Iwamatsu A., Hanazono Y., Tanaka T., Mitani K., Yazaki Y., Hirai H. (1995) The proto-oncogene product c-Cbl becomes tyrosine phosphorylated by stimulation with GM-CSF or Epo and constitutively binds to the SH3 domain of Grb2/Ash in human hematopoietic cells. J. Biol. Chem. 270, 10800–10805 [DOI] [PubMed] [Google Scholar]
- 37. Martinez-Moczygemba M., Huston D. P. (2001) Proteasomal regulation of βc signaling reveals a novel mechanism for cytokine receptor heterotypic desensitization. J. Clin. Invest. 108, 1797–1806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kitamura T., Tange T., Terasawa T., Chiba S., Kuwaki T., Miyagawa K., Piao Y. F., Miyazono K., Urabe A., Takaku F. (1989) Establishment and characterization of a unique human cell line that proliferates dependently on GM-CSF, IL-3, or erythropoietin. J. Cell Physiol. 140, 323–334 [DOI] [PubMed] [Google Scholar]
- 39. Emanuel P. D., Bates L. J., Castleberry R. P., Gualtieri R. J., Zuckerman K. S. (1991) Selective hypersensitivity to granulocyte-macrophage colony-stimulating factor by juvenile chronic myeloid leukemia hematopoietic progenitors. Blood 77, 925–929 [PubMed] [Google Scholar]
- 40. Niemeyer C. M., Kang M. W., Shin D. H., Furlan I., Erlacher M., Bunin N. J., Bunda S., Finklestein J. Z., Sakamoto K. M., Gorr T. A., Mehta P., Schmid I., Kropshofer G., Corbacioglu S., Lang P. J., Klein C., Schlegel P. G., Heinzmann A., Schneider M., Starý J., van den Heuvel-Eibrink M. M., Hasle H., Locatelli F., Sakai D., Archambeault S., Chen L., Russell R. C., Sybingco S. S., Ohh M., Braun B. S., Flotho C., Loh M. L. (2010) Germline CBL mutations cause developmental abnormalities and predispose to juvenile myelomonocytic leukemia. Nat. Genet. 42, 794–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Saur S. J., Sangkhae V., Geddis A. E., Kaushansky K., Hitchcock I. S. (2010) Ubiquitination and degradation of the thrombopoietin receptor c-Mpl. Blood 115, 1254–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tanaka Y., Tanaka N., Saeki Y., Tanaka K., Murakami M., Hirano T., Ishii N., Sugamura K. (2008) c-Cbl-dependent monoubiquitination and lysosomal degradation of gp130. Mol. Cell Biol. 28, 4805–4818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Thien C. B., Walker F., Langdon W. Y. (2001) RING finger mutations that abolish c-Cbl-directed polyubiquitination and downregulation of the EGF receptor are insufficient for cell transformation. Mol. Cell 7, 355–365 [DOI] [PubMed] [Google Scholar]
- 44. Quelle F. W., Sato N., Witthuhn B. A., Inhorn R. C., Eder M., Miyajima A., Griffin J. D., Ihle J. N. (1994) JAK2 associates with the β c chain of the receptor for granulocyte-macrophage colony-stimulating factor, and its activation requires the membrane-proximal region. Mol. Cell Biol. 14, 4335–4341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Brizzi M. F., Zini M. G., Aronica M. G., Blechman J. M., Yarden Y., Pegoraro L. (1994) Convergence of signaling by interleukin-3, granulocyte-macrophage colony-stimulating factor, and mast cell growth factor on JAK2 tyrosine kinase. J. Biol. Chem. 269, 31680–31684 [PubMed] [Google Scholar]
- 46. Parganas E., Wang D., Stravopodis D., Topham D. J., Marine J. C., Teglund S., Vanin E. F., Bodner S., Colamonici O. R., van Deursen J. M., Grosveld G., Ihle J. N. (1998) Jak2 is essential for signaling through a variety of cytokine receptors. Cell 93, 385–395 [DOI] [PubMed] [Google Scholar]
- 47. Watanabe S., Itoh T., Arai K. (1996) Roles of JAK kinases in human GM-CSF receptor signal transduction. J. Allergy Clin. Immunol. 98, S183–S191 [DOI] [PubMed] [Google Scholar]
- 48. Perugini M., Brown A. L., Salerno D. G., Booker G. W., Stojkoski C., Hercus T. R., Lopez A. F., Hibbs M. L., Gonda T. J., D'Andrea R. J. (2010) Alternative modes of GM-CSF receptor activation revealed using activated mutants of the common β-subunit. Blood 115, 3346–3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wei S., Liu J. H., Epling-Burnette P. K., Gamero A. M., Ussery D., Pearson E. W., Elkabani M. E., Diaz J. I., Djeu J. Y. (1996) Critical role of Lyn kinase in inhibition of neutrophil apoptosis by granulocyte-macrophage colony-stimulating factor. J. Immunol. 157, 5155–5162 [PubMed] [Google Scholar]
- 50. Corey S., Eguinoa A., Puyana-Theall K., Bolen J. B., Cantley L., Mollinedo F., Jackson T. R., Hawkins P. T., Stephens L. R. (1993) Granulocyte macrophage-colony stimulating factor stimulates both association and activation of phosphoinositide 3OH-kinase and src-related tyrosine kinase(s) in human myeloid derived cells. EMBO J. 12, 2681–2690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Li Y., Shen B. F., Karanes C., Sensenbrenner L., Chen B. (1995) Association between Lyn protein tyrosine kinase (p53/56lyn) and the β subunit of the granulocyte-macrophage colony-stimulating factor (GM-CSF) receptors in a GM-CSF-dependent human megakaryocytic leukemia cell line (M-07e). J. Immunol. 155, 2165–2174 [PubMed] [Google Scholar]
- 52. Yousefi S., Hoessli D. C., Blaser K., Mills G. B., Simon H. U. (1996) Requirement of Lyn and Syk tyrosine kinases for the prevention of apoptosis by cytokines in human eosinophils. J. Exp. Med. 183, 1407–1414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Tsygankov A. Y., Teckchandani A. M., Feshchenko E. A., Swaminathan G. (2001) Beyond the RING. CBL proteins as multivalent adapters. Oncogene 20, 6382–6402 [DOI] [PubMed] [Google Scholar]
- 54. Feshchenko E. A., Langdon W. Y., Tsygankov A. Y. (1998) Fyn, Yes, and Syk phosphorylation sites in c-Cbl map to the same tyrosine residues that become phosphorylated in activated T cells. J. Biol. Chem. 273, 8323–8331 [DOI] [PubMed] [Google Scholar]
- 55. Marengère L. E., Mirtsos C., Kozieradzki I., Veillette A., Mak T. W., Penninger J. M. (1997) Proto-oncoprotein Vav interacts with c-Cbl in activated thymocytes and peripheral T cells. J. Immunol. 159, 70–76 [PubMed] [Google Scholar]
- 56. Liu Y. C., Elly C., Langdon W. Y., Altman A. (1997) Ras-dependent, Ca2+-stimulated activation of nuclear factor of activated T cells by a constitutively active Cbl mutant in T cells. J. Biol. Chem. 272, 168–173 [DOI] [PubMed] [Google Scholar]
- 57. Fukazawa T., Reedquist K. A., Trub T., Soltoff S., Panchamoorthy G., Druker B., Cantley L., Shoelson S. E., Band H. (1995) The SH3 domain-binding T cell tyrosyl phosphoprotein p120. Demonstration of its identity with the c-cbl protooncogene product and in vivo complexes with Fyn, Grb2, and phosphatidylinositol 3-kinase. J. Biol. Chem. 270, 19141–19150 [DOI] [PubMed] [Google Scholar]
- 58. Reedquist K. A., Fukazawa T., Panchamoorthy G., Langdon W. Y., Shoelson S. E., Druker B. J., Band H. (1996) Stimulation through the T cell receptor induces Cbl association with Crk proteins and the guanine nucleotide exchange protein C3G. J. Biol. Chem. 271, 8435–8442 [DOI] [PubMed] [Google Scholar]
- 59. Sawasdikosol S., Chang J. H., Pratt J. C., Wolf G., Shoelson S. E., Burakoff S. J. (1996) Tyrosine-phosphorylated Cbl binds to Crk after T cell activation. J. Immunol. 157, 110–116 [PubMed] [Google Scholar]
- 60. Andoniou C. E., Thien C. B., Langdon W. Y. (1996) The two major sites of cbl tyrosine phosphorylation in abl-transformed cells select the crkL SH2 domain. Oncogene 12, 1981–1989 [PubMed] [Google Scholar]
- 61. Meng F., Lowell C. A. (1998) A β 1 integrin signaling pathway involving Src-family kinases, Cbl and PI-3 kinase is required for macrophage spreading and migration. EMBO J. 17, 4391–4403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Grishin A., Sinha S., Roginskaya V., Boyer M. J., Gomez-Cambronero J., Zuo S., Kurosaki T., Romero G., Corey S. J. (2000) Involvement of Shc and Cbl-PI 3-kinase in Lyn-dependent proliferative signaling pathways for G-CSF. Oncogene 19, 97–105 [DOI] [PubMed] [Google Scholar]
- 63. Tezuka T., Umemori H., Fusaki N., Yagi T., Takata M., Kurosaki T., Yamamoto T. (1996) Physical and functional association of the cbl protooncogen product with an src-family protein tyrosine kinase, p53/56lyn, in the B cell antigen receptor-mediated signaling. J. Exp. Med. 183, 675–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Uddin S., Gardziola C., Dangat A., Yi T., Platanias L. C. (1996) Interaction of the c-cbl proto-oncogene product with the Tyk-2 protein tyrosine kinase. Biochem. Biophys. Res. Commun. 225, 833–838 [DOI] [PubMed] [Google Scholar]
- 65. Muto A., Watanabe S., Itoh T., Miyajima A., Yokota T., Arai K. (1995) Roles of the cytoplasmic domains of the alpha and β subunits of human granulocyte-macrophage colony-stimulating factor receptor. J. Allergy Clin. Immunol. 96, 1100–1114 [DOI] [PubMed] [Google Scholar]
- 66. Sakamaki K., Miyajima I., Kitamura T., Miyajima A. (1992) Critical cytoplasmic domains of the common β subunit of the human GM-CSF, IL-3 and IL-5 receptors for growth signal transduction and tyrosine phosphorylation. EMBO J. 11, 3541–3549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Itoh T., Liu R., Yokota T., Arai K. I., Watanabe S. (1998) Definition of the role of tyrosine residues of the common β subunit regulating multiple signaling pathways of granulocyte-macrophage colony-stimulating factor receptor. Mol. Cell Biol. 18, 742–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Martinez-Moczygemba M., Huston D. P., Lei J. T. (2007) JAK kinases control IL-5 receptor ubiquitination, degradation, and internalization. J. Leukoc. Biol. 81, 1137–1148 [DOI] [PubMed] [Google Scholar]
- 69. Lei J. T., Mazumdar T., Martinez-Moczygemba M. (2011) Three lysine residues in the common β chain of the interleukin-5 receptor are required for Janus kinase (JAK)-dependent receptor ubiquitination, endocytosis, and signaling. J. Biol. Chem. 286, 40091–40103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Nagao T., Oshikawa G., Wu N., Kurosu T., Miura O. (2011) DNA damage stress and inhibition of Jak2-V617F cause its degradation and synergistically induce apoptosis through activation of GSK3β. PLoS One 6, e27397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Andoniou C. E., Lill N. L., Thien C. B., Lupher M. L., Jr., Ota S., Bowtell D. D., Scaife R. M., Langdon W. Y., Band H. (2000) The Cbl proto-oncogene product negatively regulates the Src-family tyrosine kinase Fyn by enhancing its degradation. Mol. Cell Biol. 20, 851–867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bhattacharyya S. P. (2001) Ubiquitination of Lyn-kinase in rat basophilic leukemia RBL-2H3 cells. Immunol. Lett. 75, 131–136 [DOI] [PubMed] [Google Scholar]
- 73. Kaabeche K., Lemonnier J., Le Mée S., Caverzasio J., Marie P. J. (2004) Cbl-mediated degradation of Lyn and Fyn induced by constitutive fibroblast growth factor receptor-2 activation supports osteoblast differentiation. J. Biol. Chem. 279, 36259–36267 [DOI] [PubMed] [Google Scholar]
- 74. Kyo S., Sada K., Qu X., Maeno K., Miah S. M., Kawauchi-Kamata K., Yamamura H. (2003) Negative regulation of Lyn protein-tyrosine kinase by c-Cbl ubiquitin-protein ligase in Fc ϵ RI-mediated mast cell activation. Genes Cells 8, 825–836 [DOI] [PubMed] [Google Scholar]
- 75. Wang L., Rudert W. A., Loutaev I., Roginskaya V., Corey S. J. (2002) Repression of c-Cbl leads to enhanced G-CSF Jak-STAT signaling without increased cell proliferation. Oncogene 21, 5346–5355 [DOI] [PubMed] [Google Scholar]
- 76. Nishizumi H., Horikawa K., Mlinaric-Rascan I., Yamamoto T. (1998) A double-edged kinase Lyn. A positive and negative regulator for antigen receptor-mediated signals. J. Exp. Med. 187, 1343–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Dombrosky-Ferlan P. M., Corey S. J. (1997) Yeast two-hybrid in vivo association of the Src kinase Lyn with the proto-oncogene product Cbl but not with the p85 subunit of PI 3-kinase. Oncogene 14, 2019–2024 [DOI] [PubMed] [Google Scholar]
- 78. Kotecha N., Flores N. J., Irish J. M., Simonds E. F., Sakai D. S., Archambeault S., Diaz-Flores E., Coram M., Shannon K. M., Nolan G. P., Loh M. L. (2008) Single-cell profiling identifies aberrant STAT5 activation in myeloid malignancies with specific clinical and biologic correlates. Cancer Cell 14, 335–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Guthridge M. A., Powell J. A., Barry E. F., Stomski F. C., McClure B. J., Ramshaw H., Felquer F. A., Dottore M., Thomas D. T., To B., Begley C. G., Lopez A. F. (2006) Growth factor pleiotropy is controlled by a receptor Tyr/Ser motif that acts as a binary switch. EMBO J. 25, 479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Naramura M., Nandwani N., Gu H., Band V., Band H. (2010) Rapidly fatal myeloproliferative disorders in mice with deletion of Casitas B-cell lymphoma (Cbl) and Cbl-b in hematopoietic stem cells. Proc. Natl. Acad. Sci. U.S.A. 107, 16274–16279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rathinam C., Thien C. B., Flavell R. A., Langdon W. Y. (2010) Myeloid leukemia development in c-Cbl RING finger mutant mice is dependent on FLT3 signaling. Cancer Cell 18, 341–352 [DOI] [PubMed] [Google Scholar]
- 82. Thien C. B., Blystad F. D., Zhan Y., Lew A. M., Voigt V., Andoniou C. E., Langdon W. Y. (2005) Loss of c-Cbl RING finger function results in high-intensity TCR signaling and thymic deletion. EMBO J. 24, 3807–3819 [DOI] [PMC free article] [PubMed] [Google Scholar]