Background: A potential GTPase-activating protein (GAP) regulates the activity of Rab27B in pancreatic acinar cells.
Results: Overexpression of EPI64B specifically decreases the level of active Rab27B and enhances secretion.
Conclusion: EPI64B acts as a Rab27B GAP in pancreatic acinar cells and regulates secretion.
Significance: Deciphering functions of EPI64B can deepen our understanding in how Rab27B is regulated.
Keywords: Adenovirus, Pancreas, Rab Proteins, Secretion, Small GTPases, Rab27B, GTPase-activating protein, EPI64B, Secretion
Abstract
The small GTPase Rab27B localizes to the zymogen granule membranes and plays an important role in regulating protein secretion by pancreatic acinar cells, as does Rab3D. A common guanine nucleotide exchange factor (GEF) for Rab3 and Rab27 has been reported; however, the GTPase-activating protein (GAP) specific for Rab27B has not been identified. In this study, the expression in mouse pancreatic acini of two candidate Tre-2/Bub2/Cdc16 (TBC) domain-containing proteins, EPI64 (TBC1D10A) and EPI64B (TBC1D10B), was first demonstrated. Their GAP activity on digestive enzyme secretion was examined by adenovirus-mediated overexpression of EPI64 and EPI64B in isolated pancreatic acini. EPI64B almost completely abolished the GTP-bound form of Rab27B, without affecting GTP-Rab3D. Overexpression of EPI64B also enhanced amylase release. This enhanced release was independent of Rab27A, but dependent on Rab27B, as shown using acini from genetically modified mice. EPI64 had a mild effect on both GTP-Rab27B and amylase release. Co-overexpression of EPI64B with Rab27B can reverse the inhibitory effect of Rab27B on amylase release. Mutations that block the GAP activity decreased the inhibitory effect of EPI64B on the GTP-bound state of Rab27B and abolished the enhancing effect of EPI64B on the amylase release. These data suggest that EPI64B can serve as a potential physiological GAP for Rab27B and thereby participate in the regulation of exocytosis in pancreatic acinar cells.
Introduction
Pancreatic acinar cells produce a wide array of digestive enzymes, of which the most abundant is amylase. Digestive enzymes are stored in a specialized organelle, the zymogen granule. Stimulation of acinar cells by secretagogues triggers transport of zymogen granules to the plasma membrane, fusion of zymogen granule membrane with the apical membrane, and the subsequent release of digestive enzymes (1, 2). A number of Rab proteins have been identified in pancreatic acinar cells and play important roles in intracellular membrane trafficking (3). Rab proteins form the largest group of Ras-related small GTPase superfamily and cycle between active GTP-bound and inactive GDP-bound conformations. In the GTP-bound state, Rabs can bind to a variety of downstream effector proteins and perform specific functions related to vesicular transport. This cyclic process is mediated by guanine nucleotide exchange factors (GEFs),4 which activate the Rabs by catalyzing the exchange of GDP to GTP binding of G protein, and GTPase-activating proteins (GAPs), which can induce the GTP hydrolysis by facilitating the intrinsic GTPase activity and thereby turn off signaling (4, 5). In our previous studies, Rab27B, in addition to Rab3D, was shown to be associated with the zymogen granule membrane and play an important role in regulating exocytosis in pancreatic acinar cells (6, 7). Rab27B was originally purified and characterized as a GTP-binding protein in human platelets (8) and then identified as a Rab27 isoform in human melanocytes (9). The other Rab27 isoform, Rab27A, is a key regulator in dense core granule secretion, and mutations in the Rab27A gene cause defects in granule exocytosis in cytotoxic T lymphocytes and melanocytes in hemophagocytic syndrome (Griscelli syndrome) (10) and in ashen mice (11). Rab27B was found to be expressed in a wide range of exocytic tissues and localized to secretory granules in the cell periphery close to the plasma membrane (12). A number of studies have demonstrated that Rab27B plays a critical role in the secretion in melanocytes (13, 14), platelets (15), urothelial umbrella cells (16), mast cells (17), pituitary endocrine cells (18), parietal cells (19), neutrophils (20), parotid (21, 22) and lacrimal (23) acinar cells. In addition, together with Rab27A, Rab27B was shown to control different steps of the exosome secretion pathway (24).
The regulation of Rab27 activity has been studied by several laboratories. Rab3GEP/MADD was shown to be a nonredundant GEF for Rab27A activation in melanocytes (25) as well as a GEF for all four Rab3 isoforms (26). The Tre-2/Bub2/Cdc16 (TBC) domain has been a hallmark for Rab GAPs (27). Two TBC proteins, EPI64/TBC1D10A and EPI64B/TBC1D10B/FLJ13130, have been identified as GAPs for Rab27A in melanocytes (28). The third member of EPI64 subfamily is EPI64C/TBC1D10C/mFLJ00332, which together with EPI64 and EPI64B was proved to function as a GAP for Rab35 (29, 30). EPI64C shows lower similarity to EPI64 and EPI64B in the amino acid sequence (28). Therefore, we hypothesized that EPI64 subfamily protein(s) would possess potential Rab27B-GAP activity in pancreatic acinar cells.
In the present study, we report the identification of EPI64B as a GAP for Rab27B in mouse pancreatic acinar cells. Overexpression of EPI64B in isolated mouse acini specifically inhibited the activation of exogenous and endogenous Rab27B, but not Rab3D or other closely related small G proteins involved in secretion. CCK- and carbachol-stimulated amylase release were enhanced by EPI64B overexpression, and this effect was dependent on Rab27B but independent of Rab27A. EPI64B can reverse the effect of Rab27B in CCK-stimulated amylase release. The Rab27B-GAP activity and secretion-enhancing effect of EPI64 were dependent on the TBC domain. These findings indicate that EPI64B plays a role in regulating secretion in pancreatic acinar cells and that Rab27B turnover plays an important role in acinar cell secretion.
EXPERIMENTAL PROCEDURES
Materials
An RNeasy Mini Kit was purchased from Qiagen. The plasmid expressing GST (glutathione S-transferase)-Rim (Rab3-interacting molecule) (amino acids 1–399) fusion protein was obtained from Dr. Ronald W. Holz (University of Michigan). The plasmid encoding GST-SHD (synaptotagmin-like protein (Slp) homology domain) fusion protein was provided by Dr. Hisanori Horiuchi (8). Glutathione-Sepharose 4B beads were purchased from GE Healthcare. Rabbit polyclonal anti-Rab27B antibody was from Synaptic Systems (Goettingen, Germany). Anti-Rab3D antiserum was a gift from Dr. Mark McNiven (Mayo Clinic, Rochester, MN). Rabbit polyclonal anti-TBC1D10B antibody was purchased from Sigma (AV34521). Collagenase NB8 was from SERVA (Heidelberg, Germany). All other reagents were from Sigma.
Mice
Pancreas tissues from ashen mice (C3H/HeSnJ background) were kindly provided by Dr. Edward Stuenkel (University of Michigan) (31). Rab27A/Rab27B double knock-out mice (C57BL/6J background) were obtained from Dr. Miguel Seabra (Imperial College London) (32). All aforementioned mice were used between 8 and 12 weeks of age.
Isolation, Viral Infection of Pancreatic Acini, and Analysis of Amylase Secretion
Pancreatic acini were isolated from 5–7-week-old male ICR mice by collagenase digestion as described previously (33, 34). Isolated acini were resuspended in Dulbecco's modified Eagle's medium (DMEM) containing 0.1% BSA, 0.02% soybean trypsin inhibitor, and antibiotics and incubated at 37 °C in a tissue culture incubator with 5% CO2 for 16 h. The adenoviruses were constructed and used as described previously (33, 35). Briefly, cDNA clones of mouse EPI64 and EPI64B genes purchased from Open Biosystems (Lafayette, CO) were subcloned into adenoviral shuttle vector pAdTrack-CMV with either an HA or Myc tag at the N terminus and then recombined with adenoviral backbone vector pAdEasy-1. Mature adenoviral particles were obtained and titered as described previously (33, 35). Adenovirus encoding cyan fluorescent protein (cerulean)-tagged Rab27A was constructed as described above from a plasmid provided by Dr. Edward Stuenkel (36). Adenovirus encoding Xpress-tagged Rab27B wild type (WT) was a kind gift from Dr. Tetsuro Izumi (18). An adenovirus (GFP) expressing both bacterial β-galactosidase and enhanced GFP was used as control as described previously (33, 35). Control GFP, Xpress-Rab27B WT adenovirus (3 × 106 pfu/ml), or HA- or Myc-tagged EPI64 or EPI64B adenoviruses (2 × 107 pfu/ml) alone, or the combination of two adenoviruses as indicated was added to the culture medium at the beginning of the incubation. Under this condition, >95% of acini were infected as indicated by their enhanced GFP expression. Overnight cultured acini were harvested in Hepes-Ringer buffer and then incubated with secretagogues. After the specified time the acinar suspension was centrifuged for 20 s in a microcentrifuge, and the supernatant was assayed for amylase activity using Phadebas reagent (Amersham Biosciences and Upjohn) as described previously (34, 37). Secretion was expressed as a percentage of initial acinar amylase total content or -fold increase, compared with control basal value. The data presented are means ± S.E. Statistical significance was calculated by one-way analysis of variance with Newman-Keuls post-test, with p < 0.05 representing statistical significance.
Pulldown and Western Blotting
Expression of GST fusion proteins was induced in BL21 (DE3) Escherichia coli (Novagen) at room temperature with 0.1 mm isopropyl 1-thio-β-d-galactopyranoside (IPTG) (GST-Rab27B) or at 37 °C with 0.2 mm IPTG (GST-SHD and -Rim). Fusion proteins were purified using glutathione-Sepharose beads. For GST-SHD and GST-Rim pulldown, overnight cultured pancreatic acini were washed with ice-cold PBS and lysed in buffer containing 50 mm Tris, pH 7.4, 150 mm NaCl, 10 mm MgCl2, 1 mm DTT, 0.2% Triton X-100, 2% glycerol supplemented with Complete EDTA-free protease inhibitors mixture tablets (Roche Applied Science). Cell lysates were clarified by centrifugation at 13,000 rpm at 4 °C for 10 min, and protein concentration was determined using protein assay reagent (Bio-Rad). In most experiments, 1 ml (1 mg/ml) of acinar lysate was incubated with 10 μg of GST-SHD or GST-Rim attached to glutathione-Sepharose 4B beads for 1 h at 4 °C. Beads were washed three times, and the remaining bound proteins were eluted with 2×SDS sample buffer and separated on SDS-PAGE. Bound Rab27B or Rab3D proteins were detected by Western blotting using antibodies as indicated in the figure legends and visualized with ECL reagent and recorded by Alpha Innotech FluorChem IS-8900 imager (Santa Clara, CA). Results were quantitated using FluorChem IS-8900 software (Alpha Innotech). Data are presented as means ± S.E. from multiple independent experiments. Statistical significance was calculated by one-way analysis of variance with the Newman-Keuls post test, with p < 0.05 representing statistical significance.
For GST-Rab27B pulldown, experiments were performed as described previously (38). When indicated, 30 μm AlCl3 and 10 mm NaF were included in the GDP loading buffer to induce GDP/AlFx-bound small GTPases. 1 ml (2.5 mg/ml) of acinar lysate was incubated with 20 μg of GST-Rab27B or GST only attached to glutathione-Sepharose 4B beads for 2 h at 4 °C. The bound proteins were eluted with 2×SDS sample buffer and separated on SDS-PAGE.
Immunocytochemistry
Immunocytochemistry on the pancreatic acini was performed as described previously (35). Briefly, adenovirus-infected mouse pancreatic acini were collected after overnight culture and allowed to settle in test tubes. After being fixed with 4% formaldehyde in PBS buffer, acinar preparations were rinsed, frozen, and cryosectioned. Primary antibody was mouse anti-HA monoclonal antibody (diluted 1:100). Secondary antibody was Alexa Fluor 594-conjugated rabbit anti-mouse IgG (1:200). Epifluorescence images were captured using an Olympus BX-51 upright light microscope equipped with an Olympus DP-70 camera and processed in Photoshop. Confocal images were obtained using an Olympus FluoView 500 confocal microscope.
RESULTS
Expression of EPI64 and EPI64B in Isolated Mouse Pancreatic Acini
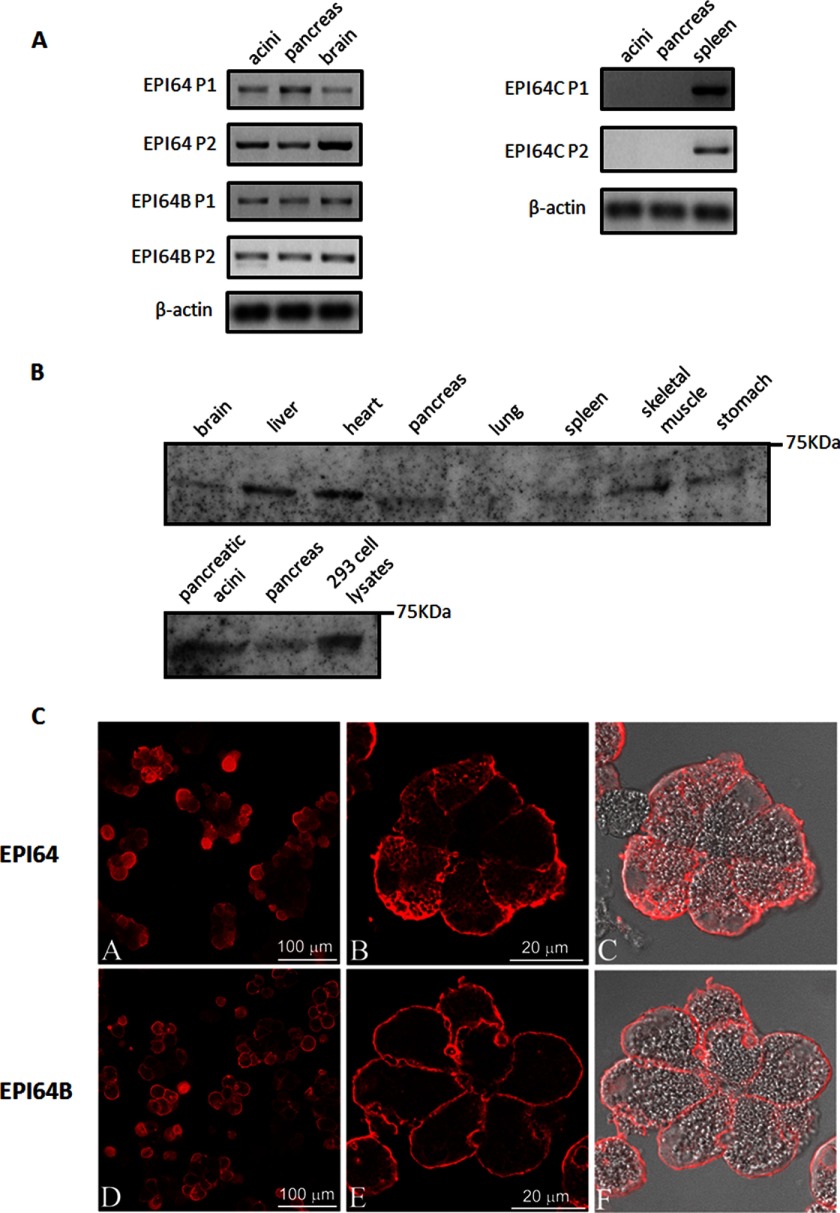
To detect the mRNA expression of EPI64 subfamily members in different mouse tissues, total RNA from isolated mouse pancreatic acini, mouse pancreas, brain, and spleen were extracted, and RT-PCR was performed using two pairs of DNA primers covering different coding sequence regions of each gene (primer sequences available upon request). Both EPI64 and EPI64B mRNA were detected in mouse pancreas and isolated mouse pancreatic acini (Fig. 1A). Expression of the other EPI64 subfamily member, EPI64C/TBC1D10C, could not be detected in either mouse pancreas or isolated acini (Fig. 1A). To further confirm the PCR results, we examined the expression of EPI64B protein in different mouse tissues using specific antibody. EPI64B was most abundant in liver and heart, but could be observed in almost all tissues examined; EPI64B was detected in both pancreas and isolated pancreatic acini (Fig. 1B). These findings indicate that EPI64 and EPI64B could serve as a potential physiological GAP for Rab27B in pancreatic acini. When overexpressed in isolated acini, both EPI64 and EPI64B showed typical membrane localization, not only to the apical membrane, but also to the basolateral membrane (Fig. 1C), which is similar to what has been demonstrated in melanocytes (28). CCK treatment had no effect on this cellular localization pattern (data not shown), indicating that apical localization or translocation is not a requirement that needs to be met for EPI64 and EPI64B to be functional.
FIGURE 1.
Detection of EPI64 subfamily members in mouse pancreas and isolated pancreatic acini by RT-PCR and Western blotting. A, RNA was extracted, and the mRNA levels of EPI64, 64B, and 64C were examined by RT-PCR for intact pancreas and acini. Two pairs of primers (P1 and P2) covering different regions of the mRNA were used for each gene. cDNA from mouse brain or spleen was used as positive control; β-actin serves as internal control. B, total lysates obtained from different mouse tissues and anti-TBC1D10B antibody were used for Western blotting. 293 cell lysates were used as a positive control. C, EPI64 and EPI64B immunofluorescence was localized in isolated mouse pancreatic acini. Mouse pancreatic acini were cultured with adenoviruses encoding HA-tagged EPI64 (A, C, and E) or EPI64B (B, D, and F) for 16 h after isolation. Acini were fixed and cryosectioned. Immunofluorescence was performed using anti-HA antibody.
Effects of EPI64 and EPI64B Overexpression on the Active State of Rab27B and Rab3D
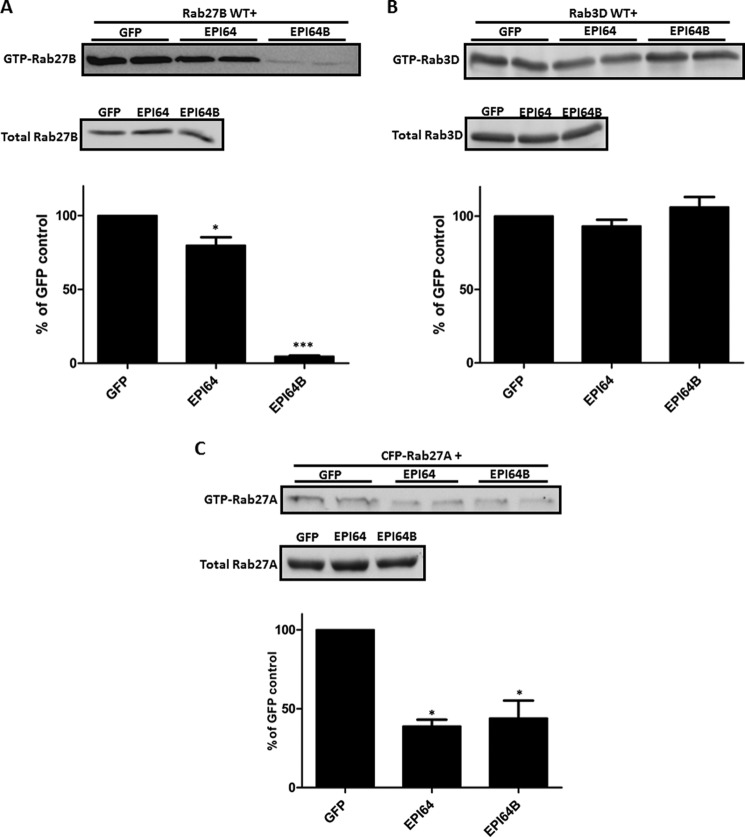
To determine whether EPI64 and 64B have GAP activities on the GTP-bound state of Rab27B and Rab3D in acinar cells, we constructed adenoviruses expressing either HA- or Myc-tagged EPI64 and EPI64B proteins. After also confirming the expression of tagged EPI64 or EPI64B in isolated pancreatic acini by immunoblotting (data not shown), we examined the effects of their overexpression on the GTP binding state of exogenous Rab27B or Rab3D by GST-SHD pulldown and GST-Rim pulldown, respectively, following procedures published previously (8, 33). Isolated pancreatic acini were co-infected with Xpress-tagged Rab27B WT (7) or HA-tagged Rab3D WT (35) adenovirus along with EPI64, EPI64B, or GFP adenoviruses. As shown in Fig. 2A, EPI64B dramatically decreased the exogenous GTP-Rab27B levels by 95.3 ± 0.6%, whereas EPI64 had a mild inhibitory effect, only reducing active Rab27B by 20.1 ± 5.5%. In contrast, overexpression of EPI64 and EPI64B had no effect on the GTP-Rab3D levels (Fig. 2B). As a comparison, EPI64 and EPI64B reduced the exogenous GTP-Rab27A by 61.1% ± 5.5% and 56.1% ± 11.2% (Fig. 2C).
FIGURE 2.
EPI64B acts as a GAP for Rab27B in mouse pancreatic acini. Acini were isolated as described under “Experimental Procedures” and co-infected with 3 × 106 pfu/ml Xpress-tagged Rab27B WT, HA-tagged Rab3D WT, or CFP-tagged Rab27A adenovirus along with 2 × 107 pfu/ml EPI64, EPI64B, or GFP adenovirus and then incubated for 16 h. A, GTP-Rab27B levels were examined by pulldown assay from pancreatic acinar cell lysates with GST-SHD followed by Western blotting with anti-Xpress antibody. Bars indicate means ± S.E. (error bars) from five independent experiments. B, GTP-Rab3D levels were examined similarly by pulldown assay with GST-Rim followed by Western blotting with anti-HA antibody. C, GTP-Rab27A levels were examined by GST-SHD pulldown, followed by Western blotting with anti-GFP antibody. Bars indicate means ± S.E. (error bars) from four independent experiments. *, p < 0.05; ***, p < 0.001.
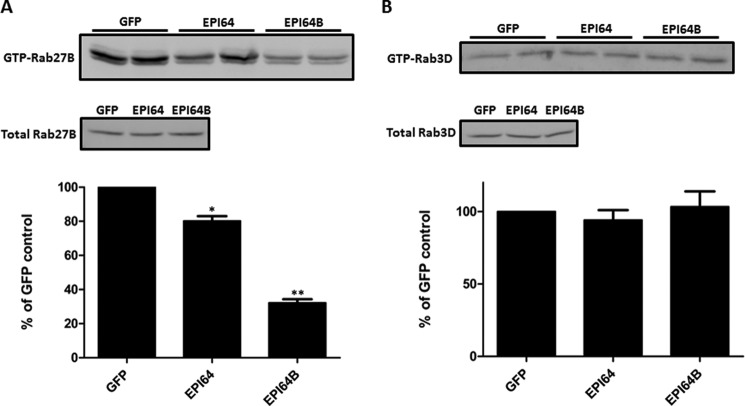
To further confirm the GAP activities of EPI64 and EPI64B on the endogenous small G proteins, EPI64 or EPI64B was overexpressed in isolated pancreatic acini, and endogenous GTP-Rab27B and GTP-Rab3D levels were examined by GST-SHD and GST-Rim pulldown. Compared with GFP control, overexpression of EPI64B decreased the endogenous GTP-bound Rab27B by 67.8 ± 5.5%, and EPI64 expression decreased the active Rab27B level by 19.8 ± 7% (Fig. 3A). By contrast, overexpression of EPI64 (94.1 ± 15.4% versus GFP control) and EPI64B (103.4 ± 25.7% versus GFP control) failed to affect the endogenous GTP-bound Rab3D levels (Fig. 3B). We concluded from these results that EPI64B had GAP activity specific for Rab27B, but not Rab3D.
FIGURE 3.
Effects of EPI64 protein overexpression on the active state of endogenous Rab27B and Rab3D. Isolated mouse pancreatic acini were incubated with 2 × 107 pfu/ml adenoviruses coding for EPI64, EPI64B, or GFP, respectively, for 16 h. Acini were then lysed, and GST-SHD pulldown and GST-Rim pulldown were performed to examine the endogenous GTP-Rab27B and GTP-Rab3D levels. Anti-Rab27B and anti-Rab3D antibodies were used for Western blotting. Bars indicate means ± S.E. (error bars) from multiple independent experiments (A, n = 7; B, n = 4). *, p < 0.05; **, p < 0.01.
Overexpression of EPI64B Enhanced CCK-induced Amylase Release
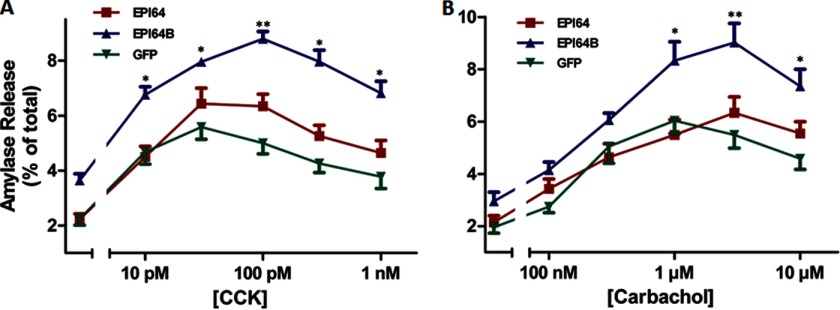
The ability of EPI64 and EPI64B on the active state of Rab27B implied that it may play a role in regulating acinar exocytosis. To determine the function of EPI64 and EPI64B in acinar secretion, we overexpressed HA-tagged EPI64 and EPI64B in pancreatic acini from ICR mice using adenoviruses at the titer of 2 × 107 pfu/ml. As a function of the viral titer and culture time, both HA-tagged proteins were expressed in acinar cells in equivalent amounts as confirmed by anti-HA Western blotting (data not shown). CCK and carbachol were used to induce the secretion from acini. In the cultured acini with control and EPI64 viral infection, amylase release was stimulated by 10 pm CCK, reached a maximum at 30 pm, and showed reduced release (supramaximal inhibition) thereafter (Fig. 4A). Basal amylase release was enhanced in acini expressing EPI64B, although it was not statistically significant. The full dose-response curve was elevated by EPI64B expression compared with GFP control and reached peak value at 100 pm CCK, whereas EPI64 had little effect compared with control, in acini stimulated with 30 pm and higher concentrations of CCK. Similarly, when carbachol stimulation was studied, EPI64B-infected acini had slightly higher basal level of amylase release and exhibited stronger enhancing effect of carbachol at concentrations of 1 μm and higher. EPI64 overexpression had little effect on carbachol-induced amylase release (Fig. 4B). A lactate dehydrogenase leakage test demonstrated that this enhancing effect was not due to the damage to the plasma membrane integrity caused by EPI64B overexpression or adenoviral infection (data not shown). EPI64B overexpression also had no effect on the ability of acini to synthesize protein from amino acids (data not shown).
FIGURE 4.
Overexpression of EPI64B enhances CCK and carbachol-stimulated amylase release in isolated ICR mouse pancreatic acini. Isolated acini were incubated with 2 × 107 pfu/ml adenoviruses coding for EPI64, EPI64B, or GFP alone for 16 h. Acini were then resuspended and incubated with various concentrations of CCK (A) or carbachol (B) for 30 min, and amylase release was determined. Results are mean ± S.E. (error bars) from multiple independent experiments (A, n = 6; B, n = 5). *, p < 0.05; **, p < 0.01.
Enhancing Effect of EPI64B on Amylase Release Is Independent of Rab27A and Dependent on Rab27B
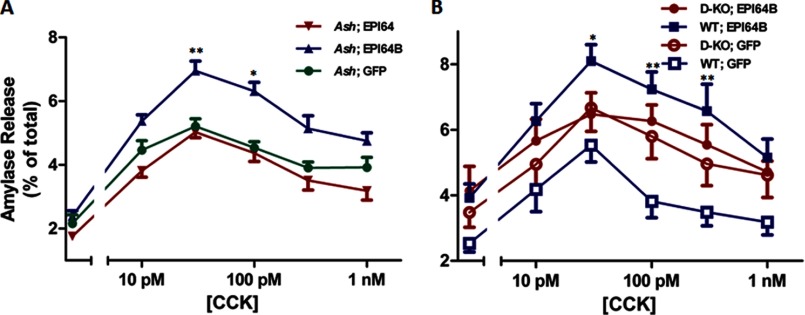
Previous studies have shown that EPI64B has Rab27A-GAP activity in melanocytes (28). Although the expression and function of Rab27A in pancreatic acinar cells are poorly understood, we investigated whether the enhancing effect of EPI64B on amylase release could be dependent on Rab27A. We overexpressed EPI64 and EPI64B in isolated pancreatic acini from ashen mice, which are deficient of Rab27A, and then examined the effects of the two proteins on amylase release. All three curves peaked at 30 pm CCK; expression of EPI64B still enhanced amylase release, and EPI64 had little effect on the amylase secretion (Fig. 5A). Thus, we obtained consistent results of EPI64B on CCK-induced amylase release in both ICR and ashen mouse acini. To further confirm the enhancing effect is through Rab27B, we overexpressed EPI64B or GFP in isolated acini from either Rab27A/Rab27B double knock-out mice or control C57BL/6J mice. EPI64B failed to show obvious effects on the amylase release in the double KO mouse acini, while still enhancing the amylase release in WT mouse acini (Fig. 5B). These data indicate that EPI64B has an enhancing effect on the amylase release, and this effect is dependent on Rab27B and independent of Rab27A.
FIGURE 5.
EPI64B enhances amylase secretion in the absence of Rab27A, but not when Rab27A and Rab27B are both missing. A, isolated pancreatic acini from ashen mouse pancreas were incubated with 2 × 107 pfu/ml adenoviruses coding for EPI64, EPI64B, or GFP for 16 h. B, isolated pancreatic acini from Rab27A/27B double KO or WT mouse pancreas were incubated with 2 × 107 pfu/ml adenoviruses coding for EPI64B or GFP for 16 h. Acini were then resuspended and incubated with various concentrations of CCK for 30 min, and amylase release was determined. Results are mean ± S.E. (error bars) from five (A) and four (B) independent experiments. *, p < 0.05; **, p < 0.01, comparing Ash; EPI64B with Ash; GFP (A) or WT; EPI64B with WT; GFP (B).
EPI64B Can Reverse the Effect of Rab27B on the Amylase Release
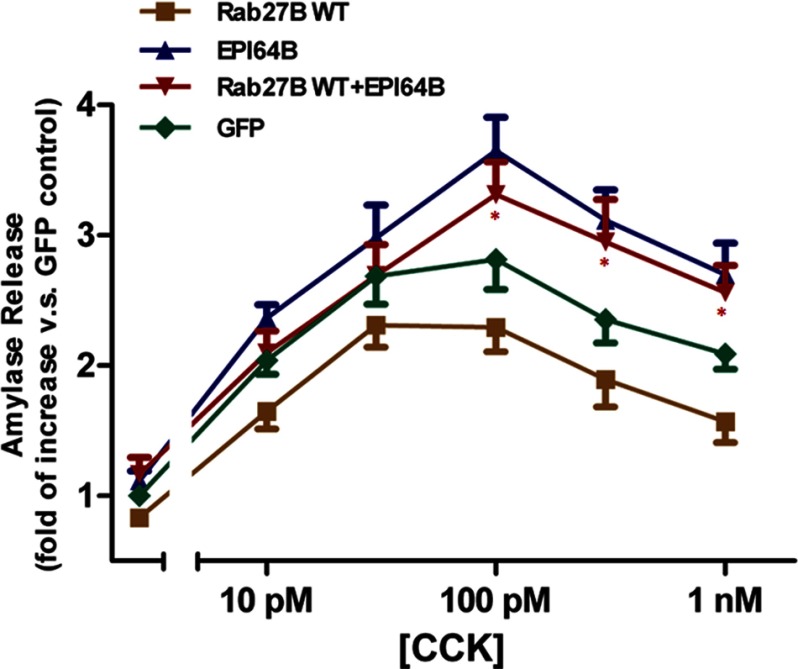
To further confirm that the enhancing effect of EPI64B is specifically through Rab27B, we co-infected the ICR mouse acini with Xpress-tagged Rab27B WT and EPI64B and compared the amylase release with that of EPI64B or Rab27B WT infected acini. The expression of EPI64B had an enhancing effect, whereas Rab27B WT inhibited amylase release. Co-expression of EPI64B and Rab27B WT reversed the inhibitory effect of Rab27B WT alone. The difference between co-expression and Rab27B WT alone is statistically significant at concentrations of 100 pm, 300 pm, and 1 nm CCK (Fig. 6). Taken together, these data demonstrate that EPI64B had an enhancing effect on the CCK-induced amylase release, and this effect is specific through Rab27B.
FIGURE 6.
EPI64B reverses the inhibition by overexpression of Rab27B. Isolated ICR mouse pancreatic acini were incubated with 3 × 106 pfu/ml Xpress-tagged Rab27B WT, 2 × 107 pfu/ml EPI64B, or GFP adenovirus alone, or co-incubated with Rab27B WT and EPI64B adenoviruses for 16 h. Acini were then resuspended and incubated with various concentrations of CCK for 30 min. Results are mean ± S.E. (error bars) from five independent experiments. Each value was compared with the basal level of control (GFP) acini. *, p < 0.05, comparing Rab27B WT + EPI64B with Rab27B WT alone at each indicated concentration.
Inhibiting the GAP Activity of EPI64B Attenuates Its Effects on the Active State of Rab27B and CCK-induced Amylase Release
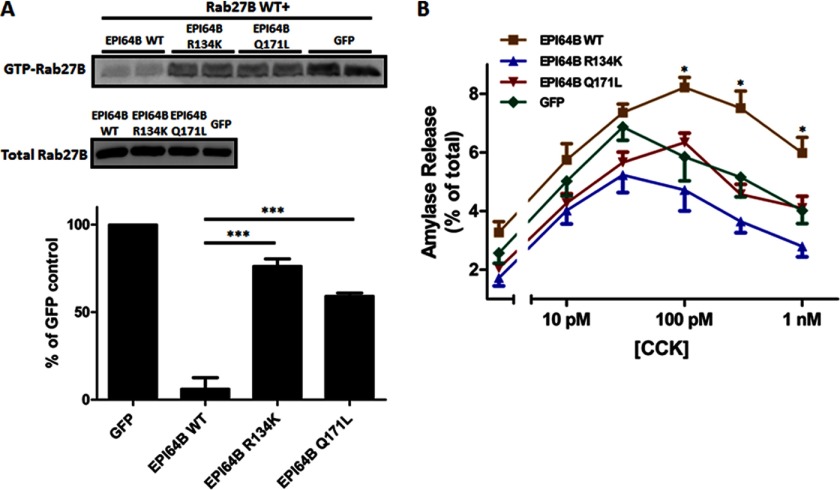
To further confirm that EPI64B possesses Rab27B-GAP activity and exerts its secretion-enhancing function through the GAP activity, we constructed mutant forms of EPI64B by replacing the conserved arginine and glutamine required for GAP activity with lysine and leucine (referred to as R134K and Q171L). After co-overexpression of Xpress-Rab27B WT with EPI64B WT, EPI64B R134K, or EPI64B Q171L in the isolated acini, the GTP-binding states of exogenous Rab27B were examined. As clearly shown in the GTP-Rab27B pulldown assays, compared with wild-type EPI64B, overexpression of EPI64B R134K and EPI64B Q171L adenoviruses demonstrated much less inhibitory effects on the GTP-bound level of exogenous Rab27B (Fig. 7A). EPI64B R134K and EPI64B Q171L expression also failed to exhibit enhancing effects on the CCK-induced amylase release (Fig. 7B). These data indicate that the TBC domain of EPI64B is responsible for the Rab27B-GAP activity and that the secretion-enhancing effect of EPI64B is dependent on the normal function of the TBC domain.
FIGURE 7.
Mutating the arginine and glutamine fingers of EPI64B important for Rab GAP activity reduces the ability of EPI64B. A, isolated mouse pancreatic acini were co-infected with 3 × 106 pfu/ml Xpress-tagged Rab27B WT and 2 × 107 pfu/ml EPI64B WT, EPI64B R134K, EPI64B Q171L, or GFP adenovirus, respectively, and then incubated for 16 h. GTP-Rab27B levels were examined by pulldown assay from pancreatic acinar cell lysates with the GST-SHD followed by Western blotting with anti-Xpress antibody. Bars indicate means ± S.E. (error bars) from three independent experiments. ***, p < 0.001 for mutants compared with EPI64B WT. B, isolated ICR mouse pancreatic acini were incubated 2 × 107 pfu/ml EPI64B WT, EPI64B R134K, EPI64B Q171L, or GFP adenovirus, respectively, for 16 h. Acini were then resuspended and incubated with various concentrations of CCK for 30 min, and amylase release was determined by amylase assay. Results are mean ± S.E. (error bars) from three independent experiments. *, p < 0.05 (EPI64B WT versus GFP).
EPI64B Can Interact with Rab27B Only during Transition State
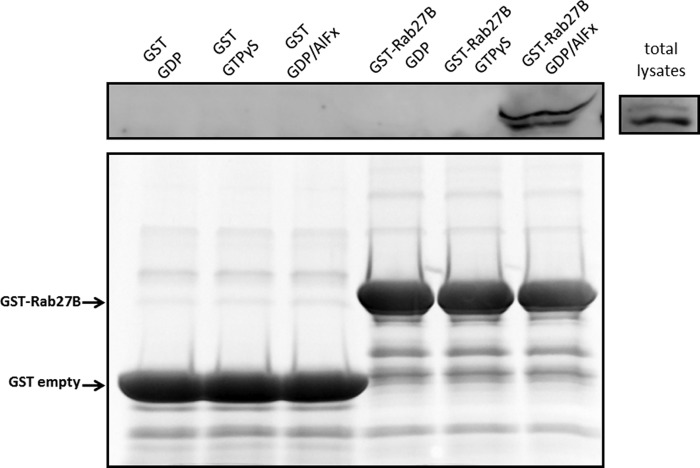
Previous studies have shown that GAPs preferentially interact with their target GTPases during the transition state of GTP hydrolysis (38–40). This interaction is transient and occurs with lower affinity when wild-type GTPases are locked in either GDP- or GTP-bound states. However, aluminum fluoride can bind to GDP-bound small GTPases and form a ternary complex that is believed to mimic the transition state of the GTPase reaction (39, 40). To examine the physical interaction between EPI64B and Rab27B, we overexpressed HA-tagged EPI64B in mouse acini and pulled down the total lysates with differently preloaded GST-Rab27B WT beads. As shown in Fig. 8, HA-EPI64B specifically interacted with GDP/AlFx-loaded GST-Rab27B, but not GST-Rab27B bound to GDP or GTPγS, or GST beads alone. These data also support a role for EPI64B as a potential GAP of Rab27B.
FIGURE 8.
EPI64B physically interacts with Rab27B in vitro only when Rab27B is in the transition state. Isolated mouse pancreatic acini were incubated with 2 × 107 pfu/ml adenovirus coding for HA-tagged EPI64B for 16 h. GST only or GST-Rab27B WT (Rab27B) beads were pretreated with GDP, GTPγS, or GDP/AlFx, respectively. The beads were then incubated with total lysates from acini overexpressing HA-tagged EPI64B. Western blotting of protein absorbed to the beads was performed using anti-HA antibody. The bottom panel shows the Coomassie staining of GST and GST-Rab27B proteins used in the pulldown.
DISCUSSION
In this study, we have presented evidence that the TBC domain-containing protein, EPI64B/TBC1D10B/FLJ13130, is a potential physiological GAP for Rab27B and plays an important role in regulating secretion in pancreatic acinar cells. Previous studies have shown that Rab3D and Rab27B are both present on the zymogen granule membranes (6, 42) and regulate exocytosis in pancreatic acinar cells (7, 33, 35). Rab3 and Rab27 subfamilies are closest to each other in the Rab phylogenetic trees and in functions; Rab3A/B/C/D and Rab27A/B share downstream effectors in some cell types (43). It has been shown that Rab3GEP, a GEF for Rab3A, exhibits GEF activity on Rab27A and Rab27B, indicating that these structurally similar small G proteins can be regulated by common activators (25). However, Rab3GAP was identified as specific for Rab3 subfamily members (44). A recent study has demonstrated that EPI64/TBC1D10A can serve as a physiological GAP for Rab27A in parotid acinar cells and regulate amylase release (45). We verified the expression of EPI64 and EPI64B in isolated pancreatic acini by RT-PCR and EPI64B by Western blotting and then made epitope-tagged EPI64 and EPI64B adenoviruses. Overexpression of both EPI64 and EPI64B can inhibit GTP-binding levels of exogenous and endogenous Rab27B, but EPI64B showed much more potent effects, whereas neither EPI64 nor EPI64B had detectable GAP activities on either exogenous or endogenous Rab3D. We next examined the effects of EPI64 and EPI64B on the secretion. Surprisingly, overexpression of EPI64B enhanced both CCK- and carbachol-induced amylase releases from the isolated pancreatic acini, whereas EPI64 showed little effect. Although its function is unclear, Rab27A can be detected in pancreatic acinar cells by RT-PCR and Western blotting (data not shown). The overexpression of EPI64B decreased the level of GTP-bound Rab27A to an extent similar to that with the overexpression of EPI64 (Fig. 2C), suggesting the specificity of EPI64B GAP activity on Rab27B in pancreatic acinar cells. By testing the effects of EPI64 and EPI64B in isolated ashen mouse acini, which are deficient in Rab27A, we confirmed that Rab27A is not involved in the secretion enhanced by EPI64B. In contrast, overexpression of EPI64B in acini from Rab27A/27B double KO mouse had no effect on the amylase release, indicating that the enhancing effect of EPI64B was dependent on Rab27B. In addition to Rab27A, Rab35 has also been shown to be a target small G protein of EPI64B (46). Due to different functions and limited involvement of Rab35 in secretion in acinar cells (45), EPI64B is likely to mainly act as Rab27B GAP at the final secretory steps in the pancreatic acinar cells. Taken together, due to the abundance on the zymogen granules and involvement in the exocytosis, Rab27B should be the primary target of EPI64B in pancreatic acinar cells.
The conserved arginine and glutamine finger structures in the TBC domain were shown to be required for the GAP activity of the TBC domain-containing proteins, and the mutations of the two amino acids can abolish the GAP activity (28, 47, 48). We examined the effects of the corresponding mutant forms of EPI64B that bear the two respective point mutations. Although the overexpression of EPI64B R134K and EPI64B Q171L both showed modest inhibitory effects on the GTP binding of Rab27B, the GTP-Rab27B levels were dramatically increased compared with the overexpression of wild-type EPI64B. The point mutations also totally abolished the enhancing effect of EPI64B on the CCK-stimulated amylase release, indicating that the enhancing effect is dependent on the TBC domain and GAP activity.
Although the activation of some small GTPases, such as RhoA, leads to the translocation of the GTPase from cytosol to membrane fraction (49), treatment of CCK did not cause the redistribution of Rab27B from cytosol to the membrane. Consistently, the reduction of GTP-Rab27B level by EPI64B failed to change the localization of Rab27B, either (data not shown).
The enhancing effect of EPI64B on the amylase release in isolated acini contradicts the widely held view that inhibition of active secretory Rabs will decrease exocytosis. A recently published study showed that inhibition of EPI64, either by antibody or antisense locked nucleic acid, reduced amylase release from parotid acinar cells (45). It has also been reported that in pancreatic acini, knockout of Slp1, a downstream effector of Rab27B, increased both the numbers of zymogen granules and amylase secretion in fasted mice (50). We have also found that GTPγS-loaded GST-Rab27B had lower affinity than GDP-loaded GST-Rab27B for the endogenous myosin Vc, which is a motor protein that regulates trafficking.5 These results suggest that ablation of the Rab27B ability to bind to its effectors may not lead to the blockade of secretion. In addition, GAP-stimulated GTP-GDP exchange may be required for Rab27 recycling and sustained amylase release as indicated in parotid acinar cells (45).
The present data are not consistent with the previous finding that constitutively active Rab27B can stimulate secretion, whereas the dominant negative form inhibits secretion in rat acinar cells (7). It needs to be noted that the current study was performed in mouse acini. We have found that the overexpression of constitutively active Rab27B had mild inhibitory effect on the amylase release from mouse acini, similar to that of overexpressed Rab27B WT, shown in Fig. 6. Thus, we are not sure whether this difference reflects the species or the specific conditions applied in each study. In any case, we speculate that in mouse acini, Rab27B needs to undergo a proper GTP-GDP cycle to mediate amylase release. The overexpression of GAP might accelerate this cycle by stimulating GTP-GDP exchange, whereas overexpression of the mutant form sequesters the cycle and has inhibitory effect on sustained amylase release. The study of the Rab27A/27B double knock-out mice also fails to support a model in which the GTP-liganded Rab27B acts as a stimulator as secretion was slightly enhanced in the double-KO acini across the CCK full dose-response curve (Fig. 5B), although this was not significant by analysis of variance. This comparison will be tested more directly in the future by evaluating freshly prepared acini from double and single knock-out mice. A recent study showed that EPI64 needs to bind to Slp1 to exert its Rab8a GAP activity (41), suggesting that in some cases, in some cell types, EPI64B may have other functions in addition to its role as a Rab27B GAP.
In conclusion, in the current study, we identified EPI64B as a physiological GAP for Rab27B in pancreatic acinar cells. EPI64B can specifically inhibit the GTP-binding state of Rab27B, but not Rab3D. EPI64B enhanced amylase secretion in a Rab27A-independent way and reversed the effect of overexpressed Rab27B. EPI64B can also interact with Rab27B during the transition state. All of these data indicate that EPI64B is involved in the regulation of secretion. Future work should be focused on unveiling the mechanism of action and regulation of EPI64B.
Acknowledgments
We thank Brad Nelson and Nancy Vogel for technical assistance in immunohistochemistry and mouse maintenance; Dr. Tetsuro Izumi for Xpress-tagged Rab27B wild-type adenovirus; Dr. Hisanori Horiuchi for the plasmids encoding GST-SHD; Dr. Ronald Holz for the plasmids encoding GST-Rim1; Dr. Mark McNiven for antiserum to Rab3D; Dr. Miguel Seabra for providing the Rab27A/27B double knock-out mice; Dr. Sarah Hamm-Alvarez for helping obtain Rab27A/27B knock-out mice; and Dr. Xiaowei Chen and Dr. Edward Stuenkel for stimulating discussions and valuable suggestions.
This work was supported, in whole or in part, by National Institutes of Health Grant R37DK041122 (to J. A. W.).
Y. Hou, X. Chen, and J. A. Williams, unpublished results.
- GEF
- guanine nucleotide exchange factor
- CCK
- cholecystokinin
- GAP
- GTPase-activating protein
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- Rim
- Rab3-interacting molecule
- SHD
- synaptotagmin-like protein (Slp) homology domain
- TBC
- Tre-2/Bub2/Cdc16.
REFERENCES
- 1. Williams J. A., Yule D. I. (2012) in Physiology of the Gastrointestinal Tract (Johnson L. R., Ghishan F. K., Merchant J. L., Said H. M., Wood J. D., eds) 5th Ed., pp. 1361–1398, Academic Press, Orlando, FL [Google Scholar]
- 2. Petersen O. H., Tepikin A. V. (2008) Polarized calcium signaling in exocrine gland cells. Annu. Rev. Physiol. 70, 273–299 [DOI] [PubMed] [Google Scholar]
- 3. Williams J. A., Chen X., Sabbatini M. E. (2009) Small G proteins as key regulators of pancreatic digestive enzyme secretion. Am. J. Physiol. Endocrinol. Metab. 296, E405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bos J. L., Rehmann H., Wittinghofer A. (2007) GEFs and GAPs: critical elements in the control of small G proteins. Cell 129, 865–877 [DOI] [PubMed] [Google Scholar]
- 5. Barr F., Lambright D. G. (2010) Rab GEFs and GAPs. Curr. Opin. Cell Biol. 22, 461–470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chen X., Walker A. K., Strahler J. R., Simon E. S., Tomanicek-Volk S. L., Nelson B. B., Hurley M. C., Ernst S. A., Williams J. A., Andrews P. C. (2006) Organellar proteomics: analysis of pancreatic zymogen granule membranes. Mol. Cell. Proteomics 5, 306–312 [DOI] [PubMed] [Google Scholar]
- 7. Chen X., Li C., Izumi T., Ernst S. A., Andrews P. C., Williams J. A. (2004) Rab27b localizes to zymogen granules and regulates pancreatic acinar exocytosis. Biochem. Biophys. Res. Commun. 323, 1157–1162 [DOI] [PubMed] [Google Scholar]
- 8. Kondo H., Shirakawa R., Higashi T., Kawato M., Fukuda M., Kita T., Horiuchi H. (2006) Constitutive GDP/GTP exchange and secretion-dependent GTP hydrolysis activity for Rab27 in platelets. J. Biol. Chem. 281, 28657–28665 [DOI] [PubMed] [Google Scholar]
- 9. Chen D., Guo J., Miki T., Tachibana M., Gahl W. A. (1997) Molecular cloning and characterization of Rab27a and Rab27b, novel human Rab proteins shared by melanocytes and platelets. Biochem. Mol. Med. 60, 27–37 [DOI] [PubMed] [Google Scholar]
- 10. Ménasché G., Pastural E., Feldmann J., Certain S., Ersoy F., Dupuis S., Wulffraat N., Bianchi D., Fischer A., Le Deist F., de Saint Basile G. (2000) Mutations in RAB27A cause Griscelli syndrome associated with haemophagocytic syndrome. Nat. Genet. 25, 173–176 [DOI] [PubMed] [Google Scholar]
- 11. Wilson S. M., Yip R., Swing D. A., O'Sullivan T. N., Zhang Y., Novak E. K., Swank R. T., Russell L. B., Copeland N. G., Jenkins N. A. (2000) A mutation in Rab27a causes the vesicle transport defects observed in ashen mice. Proc. Natl. Acad. Sci. U.S.A. 97, 7933–7938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gomi H., Mori K., Itohara S., Izumi T. (2007) Rab27b is expressed in a wide range of exocytic cells and involved in the delivery of secretory granules near the plasma membrane. Mol. Biol. Cell 18, 4377–4386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen Y., Samaraweera P., Sun T. T., Kreibich G., Orlow S. J. (2002) Rab27b association with melanosomes: dominant negative mutants disrupt melanosomal movement. J. Invest. Dermatol. 118, 933–940 [DOI] [PubMed] [Google Scholar]
- 14. Westbroek W., Lambert J., De Schepper S., Kleta R., Van Den Bossche K., Seabra M. C., Huizing M., Mommaas M., Naeyaert J. M. (2004) Rab27b is up-regulated in human Griscelli syndrome type II melanocytes and linked to the actin cytoskeleton via exon F-myosin Va transcripts. Pigment Cell Res. 17, 498–505 [DOI] [PubMed] [Google Scholar]
- 15. Tolmachova T., Abrink M., Futter C. E., Authi K. S., Seabra M. C. (2007) Rab27b regulates number and secretion of platelet dense granules. Proc. Natl. Acad. Sci. U.S.A. 104, 5872–5877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen Y., Guo X., Deng F. M., Liang F. X., Sun W., Ren M., Izumi T., Sabatini D. D., Sun T. T., Kreibich G. (2003) Rab27b is associated with fusiform vesicles and may be involved in targeting uroplakins to urothelial apical membranes. Proc. Natl. Acad. Sci. U.S.A. 100, 14012–14017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mizuno K., Tolmachova T., Ushakov D. S., Romao M., Abrink M., Ferenczi M. A., Raposo G., Seabra M. C. (2007) Rab27b regulates mast cell granule dynamics and secretion. Traffic 8, 883–892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Zhao S., Torii S., Yokota-Hashimoto H., Takeuchi T., Izumi T. (2002) Involvement of Rab27b in the regulated secretion of pituitary hormones. Endocrinology 143, 1817–1824 [DOI] [PubMed] [Google Scholar]
- 19. Suda J., Zhu L., Okamoto C. T., Karvar S. (2011) Rab27b localizes to the tubulovesicle membranes of gastric parietal cells and regulates acid secretion. Gastroenterology 140, 868–878 [DOI] [PubMed] [Google Scholar]
- 20. Johnson J. L., Brzezinska A. A., Tolmachova T., Munafo D. B., Ellis B. A., Seabra M. C., Hong H., Catz S. D. (2010) Rab27a and Rab27b regulate neutrophil azurophilic granule exocytosis and NADPH oxidase activity by independent mechanisms. Traffic 11, 533–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Imai A., Yoshie S., Nashida T., Fukuda M., Shimomura H. (2009) Redistribution of small GTP-binding protein, Rab27B, in rat parotid acinar cells after stimulation with isoproterenol. Eur. J. Oral Sci. 117, 224–230 [DOI] [PubMed] [Google Scholar]
- 22. Imai A., Yoshie S., Nashida T., Shimomura H., Fukuda M. (2004) The small GTPase Rab27B regulates amylase release from rat parotid acinar cells. J. Cell Sci. 117, 1945–1953 [DOI] [PubMed] [Google Scholar]
- 23. Chiang L., Ngo J., Schechter J. E., Karvar S., Tolmachova T., Seabra M. C., Hume A. N., Hamm-Alvarez S. F. (2011) Rab27b regulates exocytosis of secretory vesicles in acinar epithelial cells from the lacrimal gland. Am. J. Physiol. Cell Physiol. 301, C507–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ostrowski M., Carmo N. B., Krumeich S., Fanget I., Raposo G., Savina A., Moita C. F., Schauer K., Hume A. N., Freitas R. P., Goud B., Benaroch P., Hacohen N., Fukuda M., Desnos C., Seabra M. C., Darchen F., Amigorena S., Moita L. F., Thery C. (2010) Rab27a and Rab27b control different steps of the exosome secretion pathway. Nat. Cell Biol. 12, 19–30 [DOI] [PubMed] [Google Scholar]
- 25. Figueiredo A. C., Wasmeier C., Tarafder A. K., Ramalho J. S., Baron R. A., Seabra M. C. (2008) Rab3GEP is the nonredundant guanine nucleotide exchange factor for Rab27a in melanocytes. J. Biol. Chem. 283, 23209–23216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Wada M., Nakanishi H., Satoh A., Hirano H., Obaishi H., Matsuura Y., Takai Y. (1997) Isolation and characterization of a GDP/GTP exchange protein specific for the Rab3 subfamily small G proteins. J. Biol. Chem. 272, 3875–3878 [DOI] [PubMed] [Google Scholar]
- 27. Bernards A. (2003) GAPs galore! A survey of putative Ras superfamily GTPase activating proteins in man and Drosophila. Biochim. Biophys. Acta 1603, 47–82 [DOI] [PubMed] [Google Scholar]
- 28. Itoh T., Fukuda M. (2006) Identification of EPI64 as a GTPase-activating protein specific for Rab27A. J. Biol. Chem. 281, 31823–31831 [DOI] [PubMed] [Google Scholar]
- 29. Patino-Lopez G., Dong X., Ben-Aissa K., Bernot K. M., Itoh T., Fukuda M., Kruhlak M. J., Samelson L. E., Shaw S. (2008) Rab35 and its GAP EPI64C in T cells regulate receptor recycling and immunological synapse formation. J. Biol. Chem. 283, 18323–18330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Hsu C., Morohashi Y., Yoshimura S., Manrique-Hoyos N., Jung S., Lauterbach M. A., Bakhti M., Grønborg M., Möbius W., Rhee J., Barr F. A., Simons M. (2010) Regulation of exosome secretion by Rab35 and its GTPase-activating proteins TBC1D10A-C. J. Cell Biol. 189, 223–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Merrins M. J., Stuenkel E. L. (2008) Kinetics of Rab27a-dependent actions on vesicle docking and priming in pancreatic beta-cells. J. Physiol. 586, 5367–5381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Barral D. C., Ramalho J. S., Anders R., Hume A. N., Knapton H. J., Tolmachova T., Collinson L. M., Goulding D., Authi K. S., Seabra M. C. (2002) Functional redundancy of Rab27 proteins and the pathogenesis of Griscelli syndrome. J. Clin. Invest. 110, 247–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chen X., Ernst S. A., Williams J. A. (2003) Dominant negative Rab3D mutants reduce GTP-bound endogenous Rab3D in pancreatic acini. J. Biol. Chem. 278, 50053–50060 [DOI] [PubMed] [Google Scholar]
- 34. Williams J. A. (2010) Isolation of rodent pancreatic acinar cells and acini by collagenase digestion. The Pancreapedia: Exocrine Pancreas Knowledge Base, 10.3998/panc.2010.18 [DOI] [Google Scholar]
- 35. Chen X., Edwards J. A., Logsdon C. D., Ernst S. A., Williams J. A. (2002) Dominant negative Rab3D inhibits amylase release from mouse pancreatic acini. J. Biol. Chem. 277, 18002–18009 [DOI] [PubMed] [Google Scholar]
- 36. Lam A. D., Ismail S., Wu R., Yizhar O., Passmore D. R., Ernst S. A., Stuenkel E. L. (2010) Mapping dynamic protein interactions to insulin secretory granule behavior with TIRF-FRET. Biophys. J. 99, 1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Williams J. A., Korc M., Dormer R. L. (1978) Action of secretagogues on a new preparation of functionally intact, isolated pancreatic acini. Am. J. Physiol. 235, E517–E524 [DOI] [PubMed] [Google Scholar]
- 38. Chen X. W., Leto D., Xiong T., Yu G., Cheng A., Decker S., Saltiel A. R. (2011) A Ral GAP complex links PI3-kinase/Akt signaling to RalA activation in insulin action. Mol. Biol. Cell 22, 141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Scheffzek K., Ahmadian M. R., Kabsch W., Wiesmüller L., Lautwein A., Schmitz F., Wittinghofer A. (1997) The Ras-RasGAP complex: structural basis for GTPase activation and its loss in oncogenic Ras mutants. Science 277, 333–338 [DOI] [PubMed] [Google Scholar]
- 40. Vincent S., Brouns M., Hart M. J., Settleman J. (1998) Evidence for distinct mechanisms of transition state stabilization of GTPases by fluoride. Proc. Natl. Acad. Sci. U.S.A. 95, 2210–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hokanson D. E., Bretscher A. P. (2012) EPI64 interacts with Slp1/JFC1 to coordinate Rab8a and Arf6 membrane trafficking. Mol. Biol. Cell 23, 701–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chen X., Ulintz P. J., Simon E. S., Williams J. A., Andrews P. C. (2008) Global topology analysis of pancreatic zymogen granule membrane proteins. Mol. Cell. Proteomics 7, 2323–2336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fukuda M. (2008) Regulation of secretory vesicle traffic by Rab small GTPases. Cell Mol. Life Sci. 65, 2801–2813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Fukui K., Sasaki T., Imazumi K., Matsuura Y., Nakanishi H., Takai Y. (1997) Isolation and characterization of a GTPase-activating protein specific for the Rab3 subfamily of small G proteins. J. Biol. Chem. 272, 4655–4658 [DOI] [PubMed] [Google Scholar]
- 45. Imai A., Yoshie S., Ishibashi K., Haga-Tsujimura M., Nashida T., Shimomura H., Fukuda M. (2011) EPI64 protein functions as a physiological GTPase-activating protein for Rab27 protein and regulates amylase release in rat parotid acinar cells. J. Biol. Chem. 286, 33854–33862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Fuchs E., Haas A. K., Spooner R. A., Yoshimura S., Lord J. M., Barr F. A. (2007) Specific Rab GTPase-activating proteins define the Shiga toxin and epidermal growth factor uptake pathways. J. Cell Biol. 177, 1133–1143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Frasa M. A., Koessmeier K. T., Ahmadian M. R., Braga V. M. (2012) Illuminating the functional and structural repertoire of human TBC/RabGAPs. Nat. Rev. Mol. Cell Biol. 13, 67–73 [DOI] [PubMed] [Google Scholar]
- 48. Albert S., Will E., Gallwitz D. (1999) Identification of the catalytic domains and their functionally critical arginine residues of two yeast GTPase-activating proteins specific for Ypt/Rab transport GTPases. EMBO J. 18, 5216–5225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bi Y., Page S. L., Williams J. A. (2005) Rho and Rac promote acinar morphological changes, actin reorganization, and amylase secretion. Am. J. Physiol. Gastrointest. Liver Physiol. 289, G561–570 [DOI] [PubMed] [Google Scholar]
- 50. Saegusa C., Kanno E., Itohara S., Fukuda M. (2008) Expression of Rab27B-binding protein Slp1 in pancreatic acinar cells and its involvement in amylase secretion. Arch. Biochem. Biophys. 475, 87–92 [DOI] [PubMed] [Google Scholar]