Background: Extracellular ceramide 1-phosphate is presumed to interact with extracellular proteins to mediate cellular invasion. These proteins are unidentified.
Results: C-1-P interacts with both annexin a2 and p11 proteins. C-1-P-mediated vascular endothelial cell invasion requires expression of these proteins.
Conclusion: Extracellular C-1-P mediates invasion via an interaction with the annexin a2-p11 heterotetramer.
Significance: Gradients of C-1-P may guide vascular endothelial cell invasion during wound healing.
Keywords: Annexin, Cell Invasion, Chemotaxis, Endothelial Cell, Sphingolipid, S100A10, Annexin a2, Ceramide 1-Phosphate, p11, Receptor
Abstract
The bioactive sphingolipid, ceramide 1-phosphate (C-1-P), has been implicated as an extracellular chemotactic agent directing cellular migration in hematopoietic stem/progenitor cells and macrophages. However, interacting proteins that could mediate these actions of C-1-P have, thus far, eluded identification. We have now identified and characterized interactions between ceramide 1-phosphate and the annexin a2-p11 heterotetramer constituents. This C-1-P-receptor complex is capable of facilitating cellular invasion. Herein, we demonstrate in both coronary artery macrovascular endothelial cells and retinal microvascular endothelial cells that C-1-P induces invasion through an extracellular matrix barrier. By employing surface plasmon resonance, lipid-binding ELISA, and mass spectrometry technologies, we have demonstrated that the heterotetramer constituents bind to C-1-P. Although the annexin a2-p11 heterotetramer constituents do not bind the lipid C-1-P exclusively, other structurally similar lipids, such as phosphatidylserine, sphingosine 1-phosphate, and phosphatidic acid, could not elicit the potent chemotactic stimulation observed with C-1-P. Further, we show that siRNA-mediated knockdown of either annexin a2 or p11 protein significantly inhibits C-1-P-directed invasion, indicating that the heterotetrameric complex is required for C-1-P-mediated chemotaxis. These results imply that extracellular C-1-P, acting through the extracellular annexin a2-p11 heterotetrameric protein, can mediate vascular endothelial cell invasion.
Introduction
The anionic sphingolipid ceramide 1-phosphate (C-1-P),3 and the anabolic enzyme ceramide kinase, which generates C-1-P, enhance cellular migration and chemotaxis of circulating hematopoietic stem/progenitor cells and macrophages (1–4). C-1-P is readily secreted from cells (5) and can elicit selective actions dependent on localization within an intra- or extracellular compartment. Although there is evidence that extracellular C-1-P mediates macrophage migration through a pertussis toxin-sensitive G-protein-coupled receptor (1), the identity of an extracellular receptor for C-1-P is unknown. It is also unclear what effect circulating, extracellular C-1-P might have on the vascular endothelium.
The concept of immunomodulation by C-1-P has become increasingly nuanced. It is firmly established that C-1-P activates cytosolic phospholipase A2 through an allosteric site, leading to the production of proinflammatory arachidonic acid metabolites (6–9). However, ample evidence now indicates that C-1-P negatively regulates inflammatory cytokine production. We have shown, for example, that exogenous C-1-P abrogates LPS-mediated production of TNF-α, IL-6, IL-8, and IL-1β in human peripheral blood mononuclear cells (10). Additionally, recent evidence documents the direct inhibition of TNF-α-converting enzyme by C-1-P binding (11). These data supported previous research demonstrating that C-1-P, produced via ceramide generated from acid sphingomyelinase activity, could negatively modulate TNF-α release (12, 13).
The evidence indicating that C-1-P might differentially regulate autocrine and paracrine inflammatory mediators led us to this study. Our goal was to investigate the mechanism(s) by which extracellular C-1-P might interact with vascular endothelial cells to regulate wound healing processes. Vascular wound healing requires recruitment of circulating immune cells and spatiotemporal production of chemokines, cytokines, and coagulation/fibrinolytic factors to initiate repair (14). However, wound healing processes after vascular stenting can go awry and contribute to prolonged inflammation and neointimal hyperplasia (15). We have now examined the role of C-1-P in endothelial cell invasion through a newly characterized mechanism and comment on its potential contribution to vascular wound healing.
We began this work after conducting a screen for proteins that interacted with sucrose-loaded vesicles of C-1-P.4 After identifying the interacting proteins by mass spectrometry, our results suggested that the annexin a2 protein bound to C-1-P. The annexin family of proteins shares a structural motif of four annexin repeats, folded into a domain that allows transient alignment with membrane lipids (16). Annexin a2 shares common features with other annexin family members, such as binding to anionic phospholipids in a calcium-dependent manner (17, 18). However, annexin a2 is distinguished from other annexins by its regulation of (19, 20) and interaction with the p11 (S100A10) protein. Annexin a2 and p11 form an extracellular heterotetrameric complex (hereafter referred to as A2t), composed of two molecules of each protein on the plasma membrane (21, 22). This complex, expressed on vascular endothelial cells, serves as a receptor platform for multiple proteins that differentially regulate wound healing processes, such as fibrinolysis and vascular invasion through the extracellular matrix (23–32).
For A2t-mediated vascular fibrinolysis and endothelial cell invasion, it is generally accepted that both the p11 and annexin a2 proteins are required, although evidence for a direct role of annexin a2 to activate these processes has been contested (33–36). Supporting the requirement for both proteins, mice deficient in either annexin a2 or p11 protein expression have defective fibrin clearance and angiogenesis (29, 37). However, it is important to note that annexin a2 protein expression has been shown to regulate the stability of p11 protein in vitro (20, 38, 39) and in vivo (19), therefore making it difficult to interpret which protein deficit was responsible for the observed phenotype. Subsequently, the A2t ligand, plasminogen, was found to bind to monomeric p11 protein but not monomeric annexin, bolstering evidence that p11 might be the effector protein for A2t-mediated fibrinolysis (23). Regardless, annexin a2 appears to be required to stabilize and localize p11 to the plasma membrane (40), where the heterotetramer contributes to remodeling of the extracellular matrix in the vasculature.
Membrane phospholipids may differentially regulate A2t function, stability, or localization (41). In previous reports, the heterotetramer demonstrated limited interaction with phosphatidylcholine but associated readily with a mixture of anionic lipids containing phosphatidylserine (23) or phosphoinositides (42). In contrast, monomeric p11 (not the heterotetrameric form associated with annexin a2) did not interact with phospholipids (23). Both electron microscopy and scanning force microscopy have revealed images of annexin a2 positioned as a bridge between p11 and the plasma membrane (43, 44), arguing that the annexin moieties may function to localize p11 to the plasma membrane. It is unknown how the interaction of A2t with membrane phospholipids might affect protein function. Our goal was to elucidate whether the lipid microenvironment, and C-1-P enrichment in particular, would alter A2t activity. Additionally, we sought to identify effector proteins that mediated chemotaxis by C-1-P. Because annexin a2 has an affinity for anionic phospholipids (like C-1-P), we investigated the role of sphingolipids in the A2t-mediated vascular wound healing response of endothelial cell invasion.
EXPERIMENTAL PROCEDURES
Materials
All lipids were purchased from Avanti Polar Lipids (Alabaster, AL) and were as follows: ceramide, N-palmitoyl-d-erythro-sphingosine; PA, 1-palmitoyl-2-oleoyl-sn-glycero-3-phosphate; C-1-P, N-palmitoyl-ceramide 1-phosphate; S-1-P, d-erythro-sphingosine 1-phosphate; DOPS, 1,2-dioleoyl-sn-glycero-3-phospho-l-serine; DOPC, 1,2-dioleoyl-sn-glycero-3-phosphocholine; DOPE, 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine. BSA was purchased from EMD Millipore (Rockland, MA). Other chemicals were purchased from Sigma, unless indicated otherwise. For Western blotting, p11 (ab89438) and annexin a2 (ab41803) antibodies were purchased from Abcam (Cambridge, MA), and β-actin antibody was purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). siRNAs were purchased from Invitrogen. The ceramide kinase gene, prepared from HUVEC cDNA, was cloned into the pcDNA 3.1 vector, from Invitrogen. HPA sensor chips were from GE Healthcare.
Cell Culture
Primary human retinal microvascular endothelial cells (HREC) and primary human coronary artery endothelial cells (HCAEC) were maintained, according to instructions provided by the supplier, in their specified media (Cell Systems Corp. (Kirkland, WA) and Cell Applications, Inc. (San Diego, CA), respectively). HREC were maintained in phenol red-free medium with 10% serum and CultureBoostTM (Cell Systems Corp.). When conducting serum starvation, HREC were maintained in basal medium without serum, CultureBoostTM, or other supplementation, and HCAEC were serum-starved in basal medium without growth supplementation. All cells were incubated at 37 °C in 5% CO2. For all experiments, HREC did not exceed passage 9, and HCAEC did not exceed passage 15.
Preparation of Lipids in BSA Vehicle
Lipids were dried under a stream of nitrogen gas and then resuspended in DMSO. The lipid/DMSO solution was then added to a fatty acid-free BSA solution to achieve a final concentration of 1 mm lipid, 1 mm BSA in 20 mm HEPES with 10% DMSO. Complexes were allowed to form by rocking at room temperature for 30 min, followed by sonication until clarity improved. Lipids in BSA vehicle were stored at −20 °C, and multiple freeze/thaw cycles were avoided.
BrdU Cell Proliferation Assay
Cells were seeded at a density of 20,000 cells/well into 96-well plates, followed by serum starvation overnight prior to treatment. Cells were treated with drug or lipid for 24 h. BrdU labeling lasted for 24 h concurrent with the treatment regimen. BrdU incorporation into DNA was measured with the colorimetric BrdU cell proliferation ELISA (Roche Applied Science) and was performed according to the manufacturer's instructions.
Migration Assay
Cell migration was measured with the collagen-coated OrisTM cell migration assay (Platypus Technologies, Madison, WI), according to the manufacturer's instructions. 50,000 cells/well were seeded in serum-free medium into the stoppered, collagen-coated 96-well plates and allowed to adhere for at least 4 h. Stoppers were then removed, and cell treatments were applied for 18–24 h. For detection of migrated cells, cells were washed with Hanks' buffered salt solution with calcium and magnesium, followed by calcein AM fluorescence staining for 30 min.
Transfection
HREC and HCAEC were electroporated with the Amaxa Nucleofector II instrument, following Amaxa HCAEC kit instructions, using protocol S-005, with 0.5 × 106 cells and either 2 μg of plasmid DNA or 25 pmol of siRNA. Cells were allowed to recover for 48–72 h prior to further experimentation.
Western Blotting
Cells were rinsed with ice-cold PBS and scraped into lysis buffer composed of 50 mm HEPES, pH 7.5, 137 mm NaCl, 2 mm NaVO4, 10 mm Na4P2O7, 1% Nonidet P-40, 10% glycerol, 50 mm NaF, 1 mm EGTA, 2 mm EDTA, 2 mm β-glycerophosphate, 10 mm benzamidine, 2 mm PMSF, and protease inhibitor mixture (Roche Applied Science). Lysates were incubated on ice for 20 min and centrifuged at 14,000 × g for 10 min, and supernatant was retained. Samples were loaded in 4–12% gradient NuPAGE precast gels (Invitrogen), and proteins were transferred to nitrocellulose (GE Healthcare). Blots were blocked for 1 h at room temperature in 5% nonfat milk in TBS-T, followed by incubation with primary antibody overnight at 4 °C. Following three washes with TBS-T, the secondary antibody was added for 2 h at room temperature. Blots were then washed three times with TBS-T, and proteins were detected by enhanced chemiluminescence (GE Healthcare). Quantification of blot density was measured by ImageJ software.
Annexin a2-Lipid Interactions by Surface Plasmon Resonance
The Biacore X system (GE Healthcare) was used to conduct surface plasmon resonance analysis of recombinant human annexin a2 protein (U.S. Biological, Swampscott, MA) binding to vesicles harboring 10 mol % C-1-P, PS, or PA in 10 mm HEPES, 160 mm KCl, 1 mm CaCl2, pH 7.4. Briefly, an HPA monolayer chip was coated with DOPC/DOPE/x (70:20:10), where x represents C-1-P, DOPS, or PA, on flow channel 2 and with DOPC/DOPE (80:20) on flow channel 1 as a control. Prior to lipid coating, 20 μl of 40 mm octyl glucoside (Fisher) was injected at a flow rate of 20 μl/min to activate the hydrophobic surface of the HPA chip. The respective lipid mixtures were injected independently at 1 μl/min and set to a 4-h delay to allow the lipid monolayer to properly form. Following coating with lipid vesicles, the supported bilayer surfaces were stabilized with three 10-μl injections of NaOH at 100 μl/min. A 20-μl injection of 5 μm BSA was injected at 20 μl/min over both flow channels to assess the coating of the HPA surface and to reduce nonspecific interactions of annexin a2 with the hydrophobic chip. Lipid coating of 1500 response units (RU) was achieved for C-1-P and DOPS surfaces, whereas coating of 1100 RU was achieved for the PA surface. Control surfaces were matched in density to the surfaces within 15% of RU signal. To assess annexin a2 lipid binding affinity and specificity, 50-μl protein injections were completed at a flow rate of 10 μl/min from 1 to 800 nm, depending on the Kd. The surface was regenerated by injecting 10 μl of 50 mm NaOH at 100 μl/min. All equilibrium binding sensorgrams were subtracted from control surfaces to obtain the maximum binding response at each protein concentration. RU signals at saturation were then plotted versus annexin a2 concentration and fit with a nonlinear least squares analysis of the binding isotherm (Req = Rmax/(1 + Kd/C) to determine the Kd. Each annexin a2 Kd was solved using more than five different protein concentrations, and experiments were performed in triplicate on the respective supported bilayer surfaces to calculate an S.D. value.
Immunoprecipitation of p11
For most immunoprecipitation samples, normal mouse IgG (Santa Cruz Biotechnology, Inc.) or p11 antibody (ab89438, Abcam) was rotated with GammaBindTM G beads (GE Healthcare) at 4 °C for 6–18 h, and then beads were washed three times with PBS. However, high background binding of C-1-P to these beads precluded their use for C-1-P measurements, so we instead coupled normal mouse IgG and p11 antibodies to Dynabeads (Invitrogen), following the manufacturer's instructions, to measure p11-associated C-1-P. Cells were lysed by sonication in a detergent-free buffer that consisted of 50 mm Tris-Cl, pH 7.5, 280 mm NaCl, 0.5 mm EDTA, 1 mm PMSF, and protease inhibitor mixture (Roche Applied Science). Cell lysate was added to antibody/bead slurry, rotated overnight at 4 °C, and then washed five times with PBS. Immunoprecipitation of p11 was verified by silver staining, following the kit instructions (Pierce), or Western blotting.
Lipid Extraction and Measurement by Mass Spectrometry
Sphingolipids from cell lysates and immunoprecipitations were extracted and measured by mass spectrometry on an AB Sciex 4000 Q Trap® LC/MS/MS (Framingham, MA) instrument as described previously (10). Phosphoglycerolipids and sphingolipids from conditioned medium were extracted as described previously (45) by methyl-tert-butyl ether extraction and analyzed on an AB Sciex TripleTOF® 5600 instrument. Peak areas for lipids measured were compared with those of internal standards. All data reported are based on monoisotopic mass and are represented as pmol/mg protein. For lipid extractions following immunoprecipitation of p11, lipid mass was normalized to the total mass of protein immunoprecipitated.
Lipid Binding A2t ELISA
Lipids were bound to Polysorp Immunoplates (Nunc, Rochester, NY) overnight at 25 °C, and 1.67 nmol of lipid was added to each well in 100 μl of TBS. After five washes with TBS, wells were blocked with 1% BSA in TBS for 1 h. Purified bovine A2t (GenWay Biotech, San Diego, CA), diluted in TBS with 10 mm CaCl2, was incubated in the lipid-coated wells for 2 h, followed by three washes of TBS or of TBS + 10 mm CaCl2, as indicated (for calcium-dependent binding, all subsequent washes and incubations were performed with the addition of 10 mm CaCl2). Antibodies for p11 or annexin a2 were added to wells at a 1:5000 dilution for 1 h. After three washes, HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, Inc.) were added to each well at a 1:2000 dilution. Bound antibody was detected with o-phenylenediamine dihydrochloride (Sigma) at 492 nm.
Invasion Assay
Cell invasion through BD MatrigelTM was measured using the BD Biosciences endothelial cell invasion kit with a 3-μm FluoroblokTM membrane 24-well plate, following instructions from the manufacturer. Alternatively, the FluoroblokTM plates were purchased and coated with 100 μl of 300 μg/ml BD MatrigelTM diluted and prepared according to the manufacturer's instructions. For HREC, 125,000–250,000 cells were seeded in each well; for HCAEC, 50,000 cells were seeded per well. Calcein AM labeling in Hanks' buffered salt solution was used to detect cells that had invaded through the MatrigelTM after 24 h on a Molecular Devices Flex Station 3 plate reader (Sunnyvale, CA).
Statistical Analyses
Data are presented as means ± S.E. Statistics were performed using GraphPad Prism version 5.01 software utilizing one-way analyses of variance with the Tukey post-test or Student's t test as appropriate.
RESULTS
To examine the role of exogenous C-1-P to mediate annexin a2-p11 heterotetramer function in the vasculature, all studies were conducted in HREC and HCAEC. Because C-1-P is considered a promigratory sphingolipid (1–3), we initially hypothesized that C-1-P would mediate cellular invasion and migration through an interaction with the annexin a2-p11 heterotetramer. We first assessed whether C-1-P could initiate chemotaxis of endothelial cells through MatrigelTM, a basement membrane matrix preparation. Indeed, we found that exogenous C-1-P dramatically enhanced the capacity of both cell lines to invade through MatrigelTM in a dose-dependent manner, up to 10 μm in HREC and 3 μm in HCAEC (Fig. 1, A and B). Further, in HCAEC, the number of cells induced to invade the barrier by exogenous 3 μm C-1-P exceeded the number of cells induced to invade by complete medium containing serum, which contains multiple growth factors (Fig. 1B).
FIGURE 1.
C-1-P selectively mediates endothelial cell invasion in a manner independent of prostanoid production. To measure invasion, cells were seeded on top of 0.3-μm transwell inserts coated with MatrigelTM. Lipids or an equivalent volume of BSA vehicle solution were added to the bottom of the transwells to measure chemotaxis. After 24 h, cells that had invaded through the MatrigelTM and transwell inserts were stained with calcein AM, and relative fluorescence units (RFU) per well were quantified. C-1-P induced invasion dose-dependently in HREC cells (A) and in HCAEC cells (B), up to 3 μm lipid. Error bars, S.E. of at least three replicates. no Tx, no treatment. *, p < 0.05; **, p < 0.01; ***, p < 0.001 when compared with BSA vehicle. #, p < 0.05; ##, p < 0.01; ###, p < 0.001 when compared with serum-supplemented wells. Endothelial cell invasion was tested with a variety of lipids at 3 μm to determine specificity. In HREC, C-1-P and PA induced invasion (C), whereas only C-1-P induced invasion significantly in HCAEC (D). Cer, ceramide. C-1-P-mediated endothelial cell invasion was not dependent on prostanoid production. Invasion assays were performed as described previously with 3 μm C-1-P but with the addition of 100 μm acetylsalicylic acid or 10 μm celecoxib for HREC (E) and HCAEC (F). One representative experiment is shown of at least two independent experiments. Error bars, S.E. of at least three replicates. *, p < 0.05; **, p < 0.01; ***, p < 0.001 when compared with BSA vehicle equivalent or as indicated. cel, celecoxib.
To explore the specificity of C-1-P-mediated invasion, we tested whether other lipids, such as the structurally similar sphingolipids ceramide and sphingosine 1-phosphate (S-1-P), or the anionic phosphoglycerolipids PA and DOPS, which are known to interact with A2t (17, 23), could direct invasion in endothelial cells. We found that in HREC, 3 μm C-1-P dramatically promoted invasion compared with a BSA vehicle control, whereas 3 μm PA produced comparatively moderate chemotaxis (Fig. 1C). In HCAEC, only 3 μm C-1-P induced statistically significant chemotaxis (Fig. 1D). Overall, these data suggest that 3 μm C-1-P selectively stimulates macrovascular and microvascular endothelial cell invasion through MatrigelTM.
We also investigated the contribution of prostanoid production to the induction of endothelial invasion by C-1-P. C-1-P is a positive regulator of cytosolic phospholipase A2 (cPLA2), enhancing the membrane localization and activity of this rate-limiting enzyme in prostanoid production (7–9). To elucidate a contribution by prostanoids, we monitored C-1-P-mediated invasion in the presence of two cyclooxygenase inhibitors, acetylsalicylic acid (ASA) or celecoxib, which limit prostanoid production downstream of cPLA2. In HREC, neither ASA nor celecoxib could inhibit C-1-P-mediated chemotaxis (Fig. 1E). Instead, in HREC, celecoxib augmented C-1-P-mediated invasion. In HCAEC, ASA had no effect on C-1-P-mediated invasion (Fig. 1F). We did detect a decrease in C-1-P-mediated invasion with celecoxib (Fig. 1F), indicating that prostanoids may play a minor role in C-1-P-mediated invasion in HCAEC. In total, however, these data suggest that C-1-P-induced endothelial cell invasion is not primarily mediated via prostanoid synthesis.
We next measured the contribution of migration and proliferation in C-1-P-mediated endothelial cell invasion. Because C-1-P substantially promoted invasion through an extracellular matrix-like barrier, it was consistent that 3 μm C-1-P promoted cellular migration over a collagen substrate in HREC and HCAEC when compared with a BSA vehicle control (Fig. 2, A and B). When we assessed the capacity of C-1-P to induce endothelial cell proliferation, we found that, in agreement with previous work from our laboratory (46), 3 μm C-1-P did not increase endothelial cell proliferation as measured by BrdU incorporation (Fig. 2, C and D). Although C-1-P treatment induces cell proliferation and migration in many cell types (1, 47–52), cell proliferation did not contribute to C-1-P-mediated invasion or migration in HREC or HCAEC.
FIGURE 2.
C-1-P mediates endothelial cell migration but not proliferation. To measure migration, cells were treated with 3 μm lipid or BSA vehicle and allowed to migrate over a collagen-coated surface for 18–24 h, after which cells were stained with calcein AM, and migration was measured by fluorescence. In HREC (A) and HCAEC (B) cell lines, 3 μm C-1-P induced migration. Error bars represent the S.E. of at least four replicates. *, p < 0.05; **, p < 0.01; ***, p < 0.001 when compared with no treatment. #, p < 0.05; ##, p < 0.01; ###, p < 0.001 when compared with equivalent volume of BSA vehicle. To measure proliferation, cell lines were serum-starved and then treated with BrdU and either 3 μm lipid or complete medium containing 10% serum for 24 h. Proliferation was detected with a BrdU ELISA for HREC (C) and HCAEC (D). One representative experiment of at least three independent experiments is shown. Error bars, S.E. of at least six replicates. *, p < 0.05; **, p < 0.01; ***, p < 0.001 when compared with no treatment (no Tx).
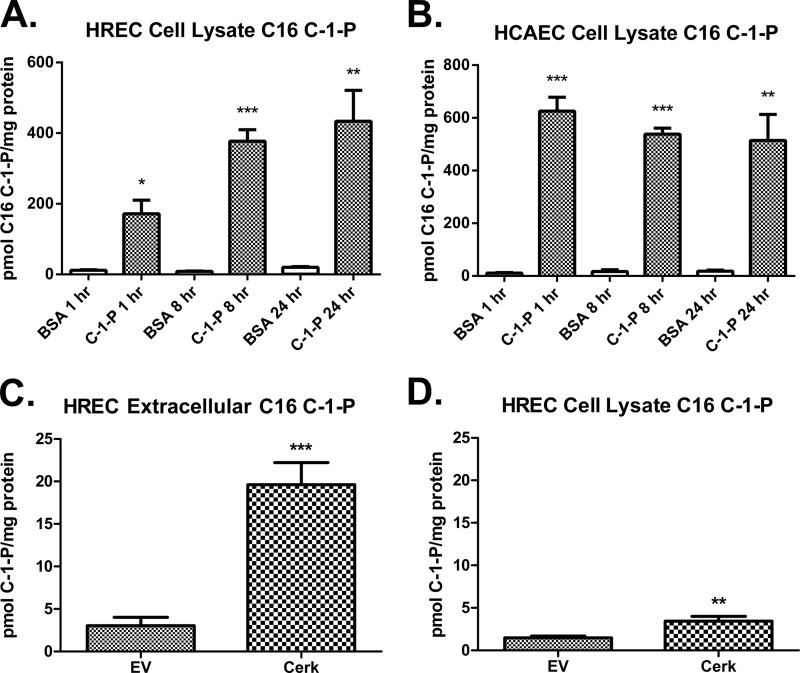
To monitor sphingolipid and phospholipid metabolism following exogenous delivery of C16 C-1-P, we extracted sphingolipids from cell lysates at various time points and quantified them by mass spectrometry. In HREC and HCAEC, treatment with exogenous C16 C-1-P resulted in a dramatic increase in C16 C-1-P mass in cell lysates after 1 h of treatment (Fig. 3, A and B). Further increase in C-1-P mass was detected at 8 and 24 h in HREC, whereas C16 C-1-P mass in HCAEC was stable over time, alluding to a difference in the delivery kinetics between these two cell lines. In support of C16 C-1-P as the effector lipid throughout this report, the mass of other C-1-P species did not significantly change after exogenous treatment of C16 C-1-P; nor was there any significant change in phosphatidylserine mass or of phosphatidylserine fatty acid composition (data not shown).
FIGURE 3.
Exogenous delivery of C-1-P dramatically increases cellular C-1-P mass over 24 h. C-1-P is available to extracellular receptors. HREC (A) and HCAEC (B) were treated with 3 μm C16 C-1-P or BSA vehicle for 1, 8, or 24 h. Lipids were extracted from cell lysates and measured by mass spectrometry to monitor metabolism. HREC were transfected with pcDNA3 as an empty vector (EV) control or ceramide kinase pcDNA3 (Cerk). Extracellular (C) and intracellular (D) C-1-P mass was measured by mass spectroscopy following sphingolipid extraction from 48-h conditioned medium (spun at 3000 × g for 10 min to remove cellular debris) and cell lysates. Error bars, S.E. of at least 3–5 replicates. *, p < 0.05; **, p < 0.01; ***, p < 0.001 when compared with BSA vehicle equivalent or EV-transfected cells.
All experiments in this work utilized C16 chain length C-1-P. To demonstrate the physiological relevance of our experimental design, we monitored the contribution of C16 C-1-P to total C-1-P mass, endogenous C-1-P production, and extracellular C-1-P. As measured by mass spectrometry, total C-1-P mass from untreated HREC cell lysates consisted of 55% C16 C-1-P, 28% C18 C-1-P, 11% C24:0 C-1-P, and 6% C24:1 C-1-P (data not shown). Likewise, in HCAEC cell lysates, C-1-P species consisted of 89% C16 C-1-P, 3% C18 C-1-P, 7% C24:0 C-1-P, and 1% C24:1 C-1-P (data not shown). Thus, the most prevalent endogenous species in both HREC and HCAEC is C16 C-1-P.
To confirm that ceramide kinase-derived C-1-P is indeed found in extracellular space and available to act on extracellular receptors, we transfected HREC with an empty vector or ceramide kinase expression vector to measure intracellular and extracellular C-1-P (Fig. 3, C and D). To monitor extracellular C-1-P, we measured C-1-P mass in conditioned medium (collected and spun at 3000 × g for 10 min to remove cellular debris) and compared that with C-1-P mass from cell lysates 48 h following transfection. We found that empty vector- and ceramide kinase-transfected HREC were exposed to 2- and 5-fold more extracellular C-1-P mass, respectively, than the mass of C-1-P contained within the cell lysate fraction. This demonstrated that C-1-P was readily available to act on extracellular receptors on endothelial cells. Ceramide kinase transfection led to a significant increase in both extracellular and intracellular C-1-P in HREC when compared with a basal level in empty vector-transfected cells, demonstrating that ceramide kinase overexpression leads to an increase in total C-1-P mass and the amount of C-1-P mass found extracellularly.
To demonstrate that extracellular C-1-P could bind to A2t and its protein constituents, we employed surface plasmon resonance, immunoprecipitation, and lipid-binding ELISA strategies. Specifically, surface plasmon resonance was employed to measure the lipid binding affinity and selectivity of annexin a2 for anionic lipid-containing membranes. All measurements were conducted in the presence of calcium. Using the HPA sensor chip, an adsorbed monolayer of PC/PE (80:20) was used as a control surface on flow channel 1, whereas flow channel 2 was used for the active surface, which consisted of PC/PE/x (70:20:10), where x represents C-1-P, PS, or PA. Five or more concentrations of annexin a2 (from 1 to 800 nm) were injected to obtain RU saturation values at each respective concentration of annexin a2. Saturating RU signals were then plotted versus annexin a2 concentration to obtain an equilibrium binding curve, which was fit with a nonlinear least squares analysis of the binding isotherm to determine the Kd. The results (Fig. 4) demonstrate that the annexin a2 protein binds to 10 mol % C-1-P membranes with the highest affinity, 11 nm (Fig. 4E), which was 17- and 14-fold stronger than binding to PS and PA vesicles, respectively.
FIGURE 4.
Annexin A2 selectively binds to 10 mol % C-1-P-containing membranes as compared with other anionic lipids. By implementing surface plasmon resonance, equilibrium binding constants were determined in triplicate on a supported monolayer surface containing PC/PE/x (70:20:10), where x represents C-1-P, PS, or PA, and subtracted from a control surface composed of PC/PE (80:20). Each replicate was determined by injecting increasing concentrations of annexin a2 over the supported monolayer, where each point represents a saturation point on the sensogram. Kd values for each lipid at 1 mm Ca2+ were determined by fitting ≥5 points with a nonlinear least squares analysis of the binding isotherm (Req = Rmax/(1 + Kd/C). A, representative sensograms from the data showing PC/PE/C-1-P binding responses at the indicated concentrations of annexin a2. Equilibrium binding curves for PC/PE/C-1-P (B), PC/PE/PS (C), and PC/PE/PA (D). E, Kd values determined from an average of three separate experiments ± S.D.
Next, we tested the hypothesis that C-1-P interacted with the p11 protein component of A2t. We treated HREC or HCAEC with 3 μm lipids or a BSA vehicle control for 10 min, immunoprecipitated p11 (Fig. 5, A and D), and then quantified the associated phosphoglycero- and sphingolipids by mass spectrometry. Exogenous C-1-P treatment dramatically increased the amount of C16 C-1-P associated with p11 protein when compared with a C-1-P-treated sample immunoprecipitated with a nonspecific IgG control or a BSA vehicle-treated sample immunoprecipitated with p11 (Fig. 5, B and E). We found that exogenous DOPS treatment did not alter p11-associated DOPS mass when compared with a PA-treated control (Fig. 5, C and F), although it should be noted that DOPS did bind annexin a2 in surface plasmon resonance experiments (Fig. 4), albeit with lower affinity than C-1-P. We also detected strong binding of PA to p11 in HCAEC (Fig. 5G). Despite readily detecting a PA internal standard in HREC lipid extracts, demonstrating that our method was robust, we did not detect any p11-associated PA in HREC (data not shown). Overall, these data support a C-1-P/p11 interaction. The data further indicate that exogenously delivered C-1-P was enriched in p11 protein complexes in two independent cell lines. Although we were able to successfully immunoprecipitate p11 protein (Fig. 5, A and D), repeated attempts to immunoprecipitate annexin a2 were confounded by strong binding of annexin a2 protein to normal, nonspecific IgG antibody controls (data not shown). This may be because annexin a2 is posited to bind to and function as an IgG transporter in the placenta (53).
FIGURE 5.
C-1-P interacts with p11 in endothelial cells. Cell lysates were immunoprecipitated with a nonspecific normal mouse IgG control or p11 antibody, separated by PAGE, and silver-stained to verify successful immunoprecipitation of p11 in HREC (A). M, marker; IgG, nonspecific IgG control immunoprecipitation (IP); p11, p11 antibody immunoprecipitation; T, total cell lysate. For HCAEC, immunoprecipitation of p11 was confirmed by Western blot (D). HREC or HCAEC were treated for 10 min with a 3 μm concentration of the indicated lipid or equivalent volume of BSA vehicle, and cells were then collected in a detergent-free lysis buffer and immunoprecipitated with normal mouse IgG or p11 antibody. After washing, the immunoprecipitation beads were added to 2:1 methanol/chloroform, followed by sphingolipid extraction and mass spectroscopy quantification to measure associated C-1-P in HREC (B) and HCAEC (E). For measurement of DOPS (C and F) and PA (G) by mass spectroscopy, beads were added to 1:3.3 water/methyl-tert-butyl ether, followed by phospholipid extraction. Note that we did not detect any PA associated with p11 in HREC cells. Shown is pmol of lipid/mg of protein input/immunoprecipitation, and, as such, data should be interpreted as -fold increase as compared with IgG control rather than as absolute values. *, p < 0.05; **, p < 0.01; ***, p < 0.001. Error bars, S.E.
After measuring binding of annexin a2 to lipids via surface plasmon resonance (Fig. 4) and measuring the p11 interactions with lipids by immunoprecipitation and mass spectrometry (Fig. 5), we wanted to provide confirmation that C-1-P could directly associate with the A2t heterotetrameric protein complex. To accomplish this, we measured the relative binding of lipids to purified bovine A2t by utilizing a lipid-binding ELISA strategy (Fig. 6). Results were verified by independently testing both a p11 and annexin a2 antibody for detection of a positive interaction. Detection with the p11 antibody (Fig. 6A) and the annexin a2 antibody (Fig. 6B) generated similar results and served to confirm that the A2t heterotetrameric protein complex remained stable during the experiment. As expected, A2t demonstrated strong binding to DOPS and PA, because these interactions have been previously characterized in cell-free systems (17, 23) and in our surface plasmon resonance experiment (Fig. 4). We also detected binding of A2t to C-1-P. To confirm that this assay detected C-1-P binding to A2t with high specificity, we performed all steps of the ELISA, with the exclusion of A2t protein, in C-1-P-coated wells. No measurable signal was detected in these wells, eliminating any concern that C-1-P interacted nonspecifically with any of the ELISA antibodies or reagents. No significant binding of A2t to ceramide or DOPC was detected, suggesting a requirement for negatively charged lipids. Surprisingly, no measurable interaction between A2t and S-1-P was detected, suggesting that two hydrophobic chains may be required for a lipid interaction with A2t. In the next series of experiments, we explored the role of calcium in lipid binding to A2t. Because the binding of A2t to PA and DOPS is calcium-dependent (17), we measured a requirement for calcium in the interaction of C-1-P and A2t. C-1-P, like PA and DOPS, required calcium to bind A2t (Fig. 6C).
FIGURE 6.
C-1-P interacts with A2t proteins. C-1-P binds the heterotetrameric protein complex in a cell-free assay. ELISA plates were coated with 1.67 nmol of the indicated lipid and incubated with purified bovine A2t with buffer containing 10 mm CaCl2, followed by detection of a lipid-protein interaction with p11 (A) or annexin a2 (B) antibodies. Binding of A2t to C-1-P was calcium-dependent. When the assay was performed in the absence of CaCl2, little binding to any lipid was detectable (C). Shown are relative binding data, represented by absorbance, with background subtracted. No A2t, no heterotetramer added to wells. Cer, ceramide. *, p < 0.05; **, p < 0.01; ***, p < 0.001 when compared with no A2t in A and B or when compared with no CaCl2 in C. One representative experiment, of at least two independent experiments, is shown. Error bars, S.E.
Next, we sought evidence that C-1-P-mediated invasion in endothelial cells required A2t. To interfere with A2t expression, we disrupted p11 or annexin a2 protein expression through siRNA-mediated knockdown (Fig. 7, A and B). In HREC, knockdown of annexin a2 led to decreased p11 protein expression (Fig. 7A), consistent with reports in the literature that annexin a2 regulates p11 expression (19, 20, 38, 39). In contrast, the modest knockdown of annexin a2 achieved in HCAEC (38% knockdown when compared with negative control siRNA) did not decrease p11 protein expression in this endothelial cell line (Fig. 7B), suggesting cell-specific differences in A2t regulation. Regardless, we assessed the ability of annexin a2- or p11-depleted cells to invade across a MatrigelTM barrier. HREC with decreased p11 protein expression were significantly less responsive to C-1-P-mediated invasion when compared with the negative control siRNA-transfected HREC (Fig. 7C). Likewise, transfection of HREC with annexin a2 siRNA, which decreased both annexin a2 and p11 expression, significantly reduced C-1-P-mediated invasion (Fig. 7C). In HCAEC, knockdown of either p11 or annexin a2 significantly decreased C-1-P-mediated invasion through MatrigelTM (Fig. 7D). In sum, C-1-P-mediated invasion in vascular endothelial cells is dependent on expression of either p11 or annexin a2 proteins, implicating a dependence on the A2t heterotetrameric protein.
FIGURE 7.
Each constituent of the annexin a2-p11 heterotetramer is required for endothelial cell invasion mediated by C-1-P. HREC or HCAEC were transfected with negative control, p11, or annexin a2 (ANX A2) siRNAs. Cells were collected 48–72 h later for protein isolation or invasion assays. Protein knockdown in HREC (A) or HCAEC (B) was confirmed by Western blotting with p11 and ANX A2 antibodies. To monitor A2t-dependent invasion, siRNA-transfected HREC (C) or HCAEC (D) were treated with 10 or 3 μm C-1-P, respectively, and monitored for invasion through MatrigelTM. Error bars, S.E. of at least three replicates. *, p < 0.05; **, p < 0.01; ***, p < 0.001 when compared with the negative control siRNA-transfected cells. #, p < 0.05; ##, p < 0.01; ###, p < 0.001 when compared with an equivalent volume of BSA vehicle per siRNA transfected.
DISCUSSION
We have now demonstrated that C-1-P interacts directly with A2t in both macro- and microvascular endothelial cells to mediate invasion across an extracellular matrix barrier. Through surface plasmon resonance, we determined that 10 mol % C-1-P-containing membranes selectively bind annexin a2 protein at Kd = 11 nm (Fig. 4). By immunoprecipitating p11 protein and identifying associated lipids, we also demonstrated an interaction between C-1-P and p11 in endothelial cells (Fig. 5). Because this finding implied an interaction with A2t but did not rule out direct binding to monomeric p11 or another p11-protein complex interaction, we sought additional confirmation of an interaction between C-1-P and the A2t heterotetramer. Therefore, binding to the A2t complex was confirmed with a lipid-binding ELISA utilizing the purified annexin a2-p11 heterotetrameric protein complex (Fig. 6). Additionally, we demonstrated a requirement for both A2t constituents, the p11 and annexin proteins, in mediating chemotaxis by exogenous C-1-P through an siRNA-mediated knockdown strategy (Fig. 7).
In support of C16 C-1-P as the causative lipid in inducing endothelial cell invasion, we found that exogenous administration of C16 C-1-P led to a dramatic and prolonged increase in cellular C16 C-1-P mass (Fig. 3). Further, in these experiments, no significant changes in the cellular mass of other sphingolipids or phospholipids were detected (data not shown).
Our finding that A2t mediates cellular invasion by C-1-P (Figs. 1 and 7) is the first report to link binding of extracellular C-1-P with an identifiable cell surface protein receptor. Although A2t does not exclusively bind to C-1-P (Fig. 6), C-1-P significantly stimulated A2t-mediated cell invasion in HREC and HCAEC endothelial cell lines (Fig. 7). In contrast, of the panel of lipids with similar structural characteristics that we tested, PA, which also bound A2t (Figs. 4 and 6), only induced limited invasion in HREC relative to C-1-P (Fig. 1C). DOPS, which also bound A2t (Figs. 4 and 6), had no effect on endothelial cell invasion (Fig. 1, C and D). We did note a slight induction of invasion by 3 μm S-1-P in HCAEC (Fig. 1D), and although it did not achieve statistical significance, further examination with reduced concentrations of S-1-P may reveal bioactivity to induce endothelial cell invasion. It has been reported previously that S-1-P utilized annexin a2 protein as an effector of endothelial cell invasion in human umbilical vein endothelial cells (54). Our data do not, however, implicate S-1-P as a high affinity interactor with the heterotetrameric form of annexin a2-p11, because we found no binding of S-1-P to the A2t protein in the lipid-binding ELISA (Fig. 6) and no significant changes in S-1-P associated with p11 following immunoprecipitation (data not shown).
Induction of C-1-P-mediated invasion was dramatically inhibited when expression of p11 or annexin a2 was knocked down by siRNA technology (Fig. 7), indicating involvement of A2t. Because C-1-P potentiates cPLA2 activity (6–9), we examined the contribution of cPLA2 activation to C-1-P-mediated invasion. We found that cyclooxygenase inhibition by acetylsalicylic acid or celecoxib, which blocks prostanoid production, did not dramatically limit chemotaxis induced by C-1-P (Fig. 1, E and F). These data do not support a prominent role for cPLA2 in C-1-P-mediated endothelial cell invasion.
This report documents the binding of C-1-P to annexin a2, one of a few newly discovered C-1-P binding proteins (such as cPLA2 and TNF-α-converting enzyme) (6–9, 11). Unlike the binding of cPLA2 to C-1-P, which has been described as a coincidence detector for C-1-P and zwitterionic membranes (55), annexin a2 binds with very strong affinity (in the low nanomolar range) to membranes containing 10% C-1-P, further supporting the hypothesis that the annexin a2-p11 heterotetrameric protein complex may function as an extracellular C-1-P “receptor.” Further supporting this possibility, the affinity of annexin a2 for C-1-P was 14-fold greater than that of PA. One explanation for this difference could be the physical state of the membrane. C-1-P, for example, has been shown to induce domain formation in vesicles (56). C-1-P and PA also have different biophysical properties in membranes that may explain the difference in selectivity. First, C-1-P and PA have different pKa values of the phosphomonoester headgroup (57, 58) that may elicit a larger anionic charge on C-1-P (−2) in DOPE and DOPC membranes. Additionally, 0.5–1 Ca2+ ions have been found to bind to each C-1-P molecule in a model membrane (59), which could suggest a Ca2+-bridging model between annexin a2 and C-1-P in membranes.
We have presented evidence that C-1-P potently induced macro- and microvascular endothelial cell invasion and migration but not proliferation (Fig. 1 and 2), implying that C-1-P may promote positive remodeling during vascular wound healing. During arterial wound healing following angioplasty, differential ceramide kinase activity in endothelial cells and smooth muscle cells may have enabled ceramide-coated balloon catheters to promote re-endothelialization while preventing restenosis due to undesirable smooth muscle cell proliferation (46). We hypothesize that C-1-P-mediated suppression of proinflammatory cytokine release (10–12) during endothelial cell invasion may also control inflammation and limit restenosis during vascular wound healing, and this is an area of future investigation.
Although it is possible that intracellular accumulation of C-1-P after exogenous administration plays a role in C-1-P-stimulated invasion, we believe that extracellular C-1-P (Fig. 3), acting via extracellular A2t, fits into an accumulating body of work indicating that extracellular C-1-P gradients create spatial maps guiding cellular navigation. In RAW 264.7 cells, a mouse macrophage-like cell line, exogenous C-1-P acted as a chemoattractant, inducing cell migration through a G-protein-coupled receptor (1). In Drosophila, C-1-P gradients in the developing embryo regulate dorsal/ventral patterning by counteracting Wnt inhibitor of dorsal (WntD) signaling (3). Further, Drosophila double mutants with disrupted ceramide kinase and multisubstrate lipid kinase activities (the only enzymes known to phosphorylate ceramide) displayed defective migration of primordial germ cells to the gonads (3). S-1-P morphogenic gradients (generated by endothelial cell efflux (60)) regulate the egress of lymphocytes from lymphoid tissues to peripheral blood (61). In like fashion, extracellular C-1-P can direct the navigation of circulating cells, such as hematopoietic stem/progenitor cells (HSPC). HSPC are guided to mobilize from or home to the bone marrow and circulatory system through peptides, such as stromal derived factor-1 (SDF-1), and sphingolipids such, as S-1-P and C-1-P (4). Specifically, S-1-P opposes the action of SDF-1, the prototypical HSPC retention signal, to facilitate HSPC egress from the bone marrow (4, 62–64). In contrast, C-1-P, in combination with SDF-1, acts as a potent homing factor for HSPC to return to the bone marrow from circulation (2). Notably, C-1-P mass increases in the bone marrow following irradiative myeloablation and recruits transplanted hematopoietic stem cells to the bone marrow for engraftment in a protease-rich environment hostile to proteins and peptides, such as SDF-1 (2). Lipid phosphate phosphatases, promiscuous enzymes that can dephosphorylate C-1-P, S-1-P, phosphatidic acid, and lysophosphatidic acid, terminate bioactive lipid gradient signals (65). This termination mechanism further supports the concept that bioactive sphingolipid gradients are true signals that can direct cells to navigate or home to different environments. Extracellular C-1-P released after vascular wounding may be a non-inflammatory mechanism for vascular progenitor cell recruitment to the wounded region and is an area for future investigation. Identification of A2t as an extracellular C-1-P-interacting protein further supports the possibility that sphingolipid gradients guide cellular migration and invasion.
This work was supported, in whole or in part, by National Institutes of Health Grants R01HL076789 and EY018336 (to M. K.). This work has also been supported by an American Diabetes Association Research Award (to M. K.). Core Facility services and instruments (ABI 4000 QTrap, 5600 TripleTOF) used in this project were funded, in part, under a grant with the Pennsylvania Department of Health using Tobacco Settlement Funds. Additional support for these studies came from American Heart Association Grant GRNT12080254 (to R. V. S.). Penn State Research Foundation has previously licensed ceramide-based delivery systems and nanotechnologies to Keystone Nano Inc. (State College, PA). M. Kester is Chief Medical Officer of Keystone Nano Inc.
J. L. Hankins, T. E. Fox, and M. Kester, manuscript in preparation.
- C-1-P
- ceramide 1-phosphate
- A2t
- annexin a2-p11 heterotetramer
- ASA
- acetylsalicylic acid
- cPLA2
- cytosolic phospholipase A2
- DOPC
- phosphatidylcholine
- DOPS
- phosphatidylserine
- HCAEC
- human coronary artery endothelial cell(s)
- HREC
- human retinal microvascular endothelial cell(s)
- HSPC
- hematopoietic stem/progenitor cell(s)
- PA
- phosphatidic acid
- RU
- response unit(s)
- S-1-P
- sphingosine 1-phosphate
- SDF-1
- stromal derived factor-1
- DOPE
- 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine
- PC
- phosphatidylcholine
- PE
- phosphatidylethanolamine
- PS
- phosphatidylserine.
REFERENCES
- 1. Granado M. H., Gangoiti P., Ouro A., Arana L., González M., Trueba M., Gómez-Muñoz A. (2009) Ceramide 1-phosphate (C1P) promotes cell migration involvement of a specific C1P receptor. Cell. Signal. 21, 405–412 [DOI] [PubMed] [Google Scholar]
- 2. Kim C. H., Wu W., Wysoczynski M., Abdel-Latif A., Sunkara M., Morris A., Kucia M., Ratajczak J., Ratajczak M. Z. (2012) Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow. A novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia 26, 106–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McElwain M. A., Ko D. C., Gordon M. D., Fyrst H., Saba J. D., Nusse R. (2011) A suppressor/enhancer screen in Drosophila reveals a role for wnt-mediated lipid metabolism in primordial germ cell migration. PLoS One 6, e26993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ratajczak M. Z., Kim C., Wu W., Shin D. M., Bryndza E., Kucia M., Ratajczak J. (2012) The role of innate immunity in trafficking of hematopoietic stem cells. An emerging link between activation of complement cascade and chemotactic gradients of bioactive sphingolipids. Adv. Exp. Med. Biol. 946, 37–54 [DOI] [PubMed] [Google Scholar]
- 5. Boath A., Graf C., Lidome E., Ullrich T., Nussbaumer P., Bornancin F. (2008) Regulation and traffic of ceramide 1-phosphate produced by ceramide kinase. Comparative analysis to glucosylceramide and sphingomyelin. J. Biol. Chem. 283, 8517–8526 [DOI] [PubMed] [Google Scholar]
- 6. Lamour N. F., Subramanian P., Wijesinghe D. S., Stahelin R. V., Bonventre J. V., Chalfant C. E. (2009) Ceramide 1-phosphate is required for the translocation of group IVA cytosolic phospholipase A2 and prostaglandin synthesis. J. Biol. Chem. 284, 26897–26907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Subramanian P., Stahelin R. V., Szulc Z., Bielawska A., Cho W., Chalfant C. E. (2005) Ceramide 1-phosphate acts as a positive allosteric activator of group IVA cytosolic phospholipase A2 α and enhances the interaction of the enzyme with phosphatidylcholine. J. Biol. Chem. 280, 17601–17607 [DOI] [PubMed] [Google Scholar]
- 8. Subramanian P., Vora M., Gentile L. B., Stahelin R. V., Chalfant C. E. (2007) Anionic lipids activate group IVA cytosolic phospholipase A2 via distinct and separate mechanisms. J. Lipid Res. 48, 2701–2708 [DOI] [PubMed] [Google Scholar]
- 9. Stahelin R. V., Subramanian P., Vora M., Cho W., Chalfant C. E. (2007) Ceramide-1-phosphate binds group IVA cytosolic phospholipase a2 via a novel site in the C2 domain. J. Biol. Chem. 282, 20467–20474 [DOI] [PubMed] [Google Scholar]
- 10. Hankins J. L., Fox T. E., Barth B. M., Unrath K. A., Kester M. (2011) Exogenous ceramide-1-phosphate reduces lipopolysaccharide (LPS)-mediated cytokine expression. J. Biol. Chem. 286, 44357–44366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lamour N. F., Wijesinghe D. S., Mietla J. A., Ward K. E., Stahelin R. V., Chalfant C. E. (2011) Ceramide kinase regulates the production of tumor necrosis factor α (TNFα) via inhibition of TNFα-converting enzyme. J. Biol. Chem. 286, 42808–42817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Józefowski S., Czerkies M., Łukasik A., Bielawska A., Bielawski J., Kwiatkowska K., Sobota A. (2010) Ceramide and ceramide 1-phosphate are negative regulators of TNF-α production induced by lipopolysaccharide. J. Immunol. 185, 6960–6973 [DOI] [PubMed] [Google Scholar]
- 13. Rozenova K. A., Deevska G. M., Karakashian A. A., Nikolova-Karakashian M. N. (2010) Studies on the role of acid sphingomyelinase and ceramide in the regulation of tumor necrosis factor α (TNFα)-converting enzyme activity and TNFα secretion in macrophages. J. Biol. Chem. 285, 21103–21113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brancato S. K., Albina J. E. (2011) Wound macrophages as key regulators of repair. Origin, phenotype, and function. Am. J. Pathol. 178, 19–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mitra A. K., Agrawal D. K. (2006) In stent restenosis. Bane of the stent era. J. Clin. Pathol. 59, 232–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gerke V., Moss S. E. (2002) Annexins. From structure to function. Physiol. Rev. 82, 331–371 [DOI] [PubMed] [Google Scholar]
- 17. Blackwood R. A., Ernst J. D. (1990) Characterization of Ca2+-dependent phospholipid binding, vesicle aggregation and membrane fusion by annexins. Biochem. J. 266, 195–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ross M., Gerke V., Steinem C. (2003) Membrane composition affects the reversibility of annexin A2t binding to solid supported membranes. A QCM study. Biochemistry 42, 3131–3141 [DOI] [PubMed] [Google Scholar]
- 19. He K. L., Deora A. B., Xiong H., Ling Q., Weksler B. B., Niesvizky R., Hajjar K. A. (2008) Endothelial cell annexin A2 regulates polyubiquitination and degradation of its binding partner S100A10/p11. J. Biol. Chem. 283, 19192–19200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Puisieux A., Ji J., Ozturk M. (1996) Annexin II up-regulates cellular levels of p11 protein by a post-translational mechanisms. Biochem. J. 313, 51–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Waisman D. M. (1995) Annexin II tetramer. Structure and function. Mol. Cell. Biochem. 149, 301–322 [DOI] [PubMed] [Google Scholar]
- 22. Gerke V., Weber K. (1985) The regulatory chain in the p36-kd substrate complex of viral tyrosine-specific protein kinases is related in sequence to the S-100 protein of glial cells. EMBO J. 4, 2917–2920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. MacLeod T. J., Kwon M., Filipenko N. R., Waisman D. M. (2003) Phospholipid-associated annexin A2-S100A10 heterotetramer and its subunits. Characterization of the interaction with tissue plasminogen activator, plasminogen, and plasmin. J. Biol. Chem. 278, 25577–25584 [DOI] [PubMed] [Google Scholar]
- 24. Hajjar K. A., Jacovina A. T., Chacko J. (1994) An endothelial cell receptor for plasminogen/tissue plasminogen activator. I. Identity with annexin II. J. Biol. Chem. 269, 21191–21197 [PubMed] [Google Scholar]
- 25. Werb Z., Mainardi C. L., Vater C. A., Harris E. D., Jr. (1977) Endogenous activation of latent collagenase by rheumatoid synovial cells. Evidence for a role of plasminogen activator. N. Engl. J. Med. 296, 1017–1023 [DOI] [PubMed] [Google Scholar]
- 26. He C. S., Wilhelm S. M., Pentland A. P., Marmer B. L., Grant G. A., Eisen A. Z., Goldberg G. I. (1989) Tissue cooperation in a proteolytic cascade activating human interstitial collagenase. Proc. Natl. Acad. Sci. U.S.A. 86, 2632–2636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mazzieri R., Masiero L., Zanetta L., Monea S., Onisto M., Garbisa S., Mignatti P. (1997) Control of type IV collagenase activity by components of the urokinase-plasmin system. A regulatory mechanism with cell-bound reactants. EMBO J. 16, 2319–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lijnen H. R., Silence J., Lemmens G., Frederix L., Collen D. (1998) Regulation of gelatinase activity in mice with targeted inactivation of components of the plasminogen/plasmin system. Thromb. Haemost. 79, 1171–1176 [PubMed] [Google Scholar]
- 29. Ling Q., Jacovina A. T., Deora A., Febbraio M., Simantov R., Silverstein R. L., Hempstead B., Mark W. H., Hajjar K. A. (2004) Annexin II regulates fibrin homeostasis and neoangiogenesis in vivo. J. Clin. Invest. 113, 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kassam G., Le B.-H., Choi K.-S., Kang H.-M., Fitzpatrick S. L., Louie P., Waisman D. M. (1998) The p11 subunit of the annexin II tetramer plays a key role in the stimulation of t-PA-dependent plasminogen activation. Biochemistry 37, 16958–16966 [DOI] [PubMed] [Google Scholar]
- 31. Laumonnier Y., Syrovets T., Burysek L., Simmet T. (2006) Identification of the annexin A2 heterotetramer as a receptor for the plasmin-induced signaling in human peripheral monocytes. Blood 107, 3342–3349 [DOI] [PubMed] [Google Scholar]
- 32. Sato Y., Okamura K., Morimoto A., Hamanaka R., Hamaguchi K., Shimada T., Ono M., Kohno K., Sakata T., Kuwano M. (1993) Indispensable role of tissue-type plasminogen activator in growth factor-dependent tube formation of human microvascular endothelial cells in vitro. Exp. Cell. Res. 204, 223–229 [DOI] [PubMed] [Google Scholar]
- 33. Kwon M., MacLeod T. J., Zhang Y., Waisman D. M. (2005) S100A10, annexin A2, and annexin a2 heterotetramer as candidate plasminogen receptors. Front. Biosci. 10, 300–325 [DOI] [PubMed] [Google Scholar]
- 34. Cesarman-Maus G., Hajjar K. A. (2005) Annexin checks in. Response to Waisman. Br. J. Haematol. 131, 554–556 [Google Scholar]
- 35. Waisman D. M. (2005) Annexin A2 may not play a role as a plasminogen receptor. Br. J. Haematol. 131, 553–554; author reply 554–556 [DOI] [PubMed] [Google Scholar]
- 36. Cesarman-Maus G., Hajjar K. A. (2005) Molecular mechanisms of fibrinolysis. Br. J. Haematol. 129, 307–321 [DOI] [PubMed] [Google Scholar]
- 37. Surette A. P., Madureira P. A., Phipps K. D., Miller V. A., Svenningsson P., Waisman D. M. (2011) Regulation of fibrinolysis by S100A10 in vivo. Blood 118, 3172–3181 [DOI] [PubMed] [Google Scholar]
- 38. Hou Y., Yang L., Mou M., Hou Y., Zhang A., Pan N., Qiang R., Wei L., Zhang N. (2008) Annexin A2 regulates the levels of plasmin, S100A10 and Fascin in L5178Y cells. Cancer Invest. 26, 809–815 [DOI] [PubMed] [Google Scholar]
- 39. Zobiack N., Rescher U., Ludwig C., Zeuschner D., Gerke V. (2003) The annexin 2/S100A10 complex controls the distribution of transferrin receptor-containing recycling endosomes. Mol. Biol. Cell 14, 4896–4908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Deora A. B., Kreitzer G., Jacovina A. T., Hajjar K. A. (2004) An annexin 2 phosphorylation switch mediates p11-dependent translocation of annexin 2 to the cell surface. J. Biol. Chem. 279, 43411–43418 [DOI] [PubMed] [Google Scholar]
- 41. Hajjar K. A., Guevara C. A., Lev E., Dowling K., Chacko J. (1996) Interaction of the fibrinolytic receptor, annexin II, with the endothelial cell surface. Essential role of endonexin repeat 2. J. Biol. Chem. 271, 21652–21659 [DOI] [PubMed] [Google Scholar]
- 42. Gokhale N. A., Abraham A., Digman M. A., Gratton E., Cho W. (2005) Phosphoinositide specificity of and mechanism of lipid domain formation by annexin A2-p11 heterotetramer. J. Biol. Chem. 280, 42831–42840 [DOI] [PubMed] [Google Scholar]
- 43. Lambert O., Gerke V., Bader M. F., Porte F., Brisson A. (1997) Structural analysis of junctions formed between lipid membranes and several annexins by cryo-electron microscopy. J. Mol. Biol. 272, 42–55 [DOI] [PubMed] [Google Scholar]
- 44. Menke M., Ross M., Gerke V., Steinem C. (2004) The molecular arrangement of membrane-bound annexin A2-S100A10 tetramer as revealed by scanning force microscopy. Chembiochem 5, 1003–1006 [DOI] [PubMed] [Google Scholar]
- 45. Matyash V., Liebisch G., Kurzchalia T. V., Shevchenko A., Schwudke D. (2008) Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 49, 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. O'Neill S. M., Olympia D. K., Fox T. E., Brown J. T., Stover T. C., Houck K. L., Wilson R., Waybill P., Kozak M., Levison S. W., Weber N., Karavodin L. M., Kester M. (2008) C(6)-ceramide-coated catheters promote re-endothelialization of stretch-injured arteries. Vasc. Dis. Prev. 5, 200–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gomez-Muñoz A., Frago L. M., Alvarez L., Varela-Nieto I. (1997) Stimulation of DNA synthesis by natural ceramide 1-phosphate. Biochem. J. 325, 435–440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Kim T. J., Kang Y. J., Lim Y., Lee H. W., Bae K., Lee Y. S., Yoo J. M., Yoo H. S., Yun Y. P. (2011) Ceramide 1-phosphate induces neointimal formation via cell proliferation and cell cycle progression upstream of ERK1/2 in vascular smooth muscle cells. Exp. Cell. Res. 317, 2041–2051 [DOI] [PubMed] [Google Scholar]
- 49. Gangoiti P., Bernacchioni C., Donati C., Cencetti F., Ouro A., Gómez-Muñoz A., Bruni P. (2012) Ceramide 1-phosphate stimulates proliferation of C2C12 myoblasts. Biochimie 94, 597–607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gangoiti P., Granado M. H., Wang S. W., Kong J. Y., Steinbrecher U. P., Gómez-Muñoz A. (2008) Ceramide 1-phosphate stimulates macrophage proliferation through activation of the PI3-kinase/PKB, JNK and ERK1/2 pathways. Cell. Signal. 20, 726–736 [DOI] [PubMed] [Google Scholar]
- 51. Gomez-Muñoz A., Duffy P. A., Martin A., O'Brien L., Byun H. S., Bittman R., Brindley D. N. (1995) Short-chain ceramide-1-phosphates are novel stimulators of DNA synthesis and cell division. Antagonism by cell-permeable ceramides. Mol. Pharmacol. 47, 833–839 [PubMed] [Google Scholar]
- 52. Mitra P., Maceyka M., Payne S. G., Lamour N., Milstien S., Chalfant C. E., Spiegel S. (2007) Ceramide kinase regulates growth and survival of A549 human lung adenocarcinoma cells. FEBS Lett. 581, 735–740 [DOI] [PubMed] [Google Scholar]
- 53. Kristoffersen E. K., Ulvestad E., Bjørge L., Aarli A., Matre R. (1994) Fc gamma-receptor activity of placental annexin II. Scand. J. Immunol. 40, 237–242 [DOI] [PubMed] [Google Scholar]
- 54. Su S. C., Maxwell S. A., Bayless K. J. (2010) Annexin 2 regulates endothelial morphogenesis by controlling AKT activation and junctional integrity. J. Biol. Chem. 285, 40624–40634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ward K. E., Bhardwaj N., Vora M., Chalfant C. E., Lu H., Stahelin R. V. (2013) The molecular basis of ceramide-1-phosphate recognition by C2 domains. J. Lipid Res. 54, 636–648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stock R. P., Brewer J., Wagner K., Ramos-Cerrillo B., Duelund L., Jernshøj K. D., Olsen L. F., Bagatolli L. A. (2012) Sphingomyelinase D activity in model membranes. Structural effects of in situ generation of ceramide-1-phosphate. PLoS One 7, e36003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kooijman E. E., Burger K. N. (2009) Biophysics and function of phosphatidic acid. A molecular perspective. Biochim. Biophys. Acta 1791, 881–888 [DOI] [PubMed] [Google Scholar]
- 58. Kooijman E. E., Sot J., Montes L. R., Alonso A., Gericke A., de Kruijff B., Kumar S., Goñi F. M. (2008) Membrane organization and ionization behavior of the minor but crucial lipid ceramide-1-phosphate. Biophys. J. 94, 4320–4330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Kooijman E. E., Vaknin D., Bu W., Joshi L., Kang S. W., Gericke A., Mann E. K., Kumar S. (2009) Structure of ceramide-1-phosphate at the air-water solution interface in the absence and presence of Ca2+. Biophys. J. 96, 2204–2215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Fukuhara S., Simmons S., Kawamura S., Inoue A., Orba Y., Tokudome T., Sunden Y., Arai Y., Moriwaki K., Ishida J., Uemura A., Kiyonari H., Abe T., Fukamizu A., Hirashima M., Sawa H., Aoki J., Ishii M., Mochizuki N. (2012) The sphingosine-1-phosphate transporter Spns2 expressed on endothelial cells regulates lymphocyte trafficking in mice. J. Clin. Invest. 122, 1416–1426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Eichner A., Sixt M. (2011) Setting the clock for recirculating lymphocytes. Sci. Signal. 4, pe43. [DOI] [PubMed] [Google Scholar]
- 62. Golan K., Vagima Y., Ludin A., Itkin T., Cohen-Gur S., Kalinkovich A., Kollet O., Kim C., Schajnovitz A., Ovadya Y., Lapid K., Shivtiel S., Morris A. J., Ratajczak M. Z., Lapidot T. (2012) S1P promotes murine progenitor cell egress and mobilization via S1P1-mediated ROS signaling and SDF-1 release. Blood 119, 2478–2488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Juarez J. G., Harun N., Thien M., Welschinger R., Baraz R., Pena A. D., Pitson S. M., Rettig M., DiPersio J. F., Bradstock K. F., Bendall L. J. (2012) Sphingosine-1-phosphate facilitates trafficking of hematopoietic stem cells and their mobilization by CXCR4 antagonists in mice. Blood 119, 707–716 [DOI] [PubMed] [Google Scholar]
- 64. Ratajczak M. Z., Kim C. H., Abdel-Latif A., Schneider G., Kucia M., Morris A. J., Laughlin M. J., Ratajczak J. (2012) A novel perspective on stem cell homing and mobilization. Review on bioactive lipids as potent chemoattractants and cationic peptides as underappreciated modulators of responsiveness to SDF-1 gradients. Leukemia 26, 63–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Brindley D. N., English D., Pilquil C., Buri K., Ling Z. C. (2002) Lipid phosphate phosphatases regulate signal transduction through glycerolipids and sphingolipids. Biochim. Biophys. Acta 1582, 33–44 [DOI] [PubMed] [Google Scholar]