Background: Ca2+ moves across the outer mitochondrial membrane (OMM) through the voltage-dependent anion channel (VDAC).
Results: Disrupting the interaction between VDAC and the antiapoptotic protein Bcl-xL reduces mitochondrial Ca2+ uptake.
Conclusion: Bcl-xL/VDAC interactions promote Ca2+ uptake by increasing transfer across the OMM.
Significance: Mitochondrial matrix Ca2+ is tightly regulated at the OMM by the modulation of VDAC.
Keywords: Bcl-2 Proteins, Calcium Signaling, Imaging, Mitochondria, Protein-Protein Interactions, Voltage-dependent Anion Channel
Abstract
The role of the antiapoptotic protein Bcl-xL in regulating mitochondrial Ca2+ ([Ca2+]mito) handling was examined in wild-type (WT) and Bcl-xL knock-out (Bcl-xL-KO) mouse embryonic fibroblast cells. Inositol 1,4,5-trisphosphate-generating agonist evoked cytosolic Ca2+ transients that produced a larger [Ca2+]mito uptake in WT cells compared with Bcl-xL-KO. In permeabilized cells, stepping external [Ca2+] from 0 to 3 μm also produced a larger [Ca2+]mito uptake in WT; moreover, the [Ca2+]mito uptake capacity of Bcl-xL-KO cells was restored by re-expression of mitochondrially targeted Bcl-xL. Bcl-xL enhancement of [Ca2+]mito uptake persisted after dissipation of the mitochondrial membrane potential but was absent in mitoplasts lacking an outer mitochondrial membrane. The outer membrane-localized voltage-dependent anion channel (VDAC) is a known Ca2+ permeability pathway that directly interacts with Bcl-xL. Bcl-xL interacted with VDAC1 and -3 isoforms, and peptides based on the VDAC sequence disrupted Bcl-xL binding. Peptides reduced [Ca2+]mito uptake in WT but were without effect in Bcl-xL-KO cells. In addition, peptides reduced [Ca2+]mito uptake in VDAC1 and VDAC3 knock-out but not VDAC1 and -3 double knock-out mouse embryonic fibroblast cells, confirming that Bcl-xL interacts functionally with VDAC1 and -3 but not VDAC2. Thus, an interaction between Bcl-xL and VDAC promotes matrix Ca2+ accumulation by increasing Ca2+ transfer across the outer mitochondrial membrane.
Introduction
The Bcl-2 proteins are a family of apoptosis regulators that contain both pro- and antiapoptotic members. Apoptotic stimuli cause activation of proapoptotic members that converge on the mitochondria to increase their membrane permeability. The subsequent release of apoptogenic factors, such as cytochrome c, represents a critical step in the cell death signaling cascade. This process is held in check by the antiapoptotic Bcl-2 proteins, which preserve mitochondrial membrane integrity and inhibit cell death by directly binding and sequestering the proapoptotic members (1).
In addition to influencing mitochondrial permeability directly, Bcl-2 proteins also impinge on apoptotic signaling through their ability to regulate Ca2+ homeostasis (2). We and others have shown that this can be mediated through direct interactions between the endoplasmic reticulum (ER)3-localized inositol 1,4,5-trisphosphate receptor (InsP3R) Ca2+ release channel and antiapoptotic Bcl-2 members, including Bcl-2 itself, Bcl-xL, and Mcl-1 (3–7). The functional consequences of these interactions are complex with both inhibition and stimulation of Ca2+ signals being reported. This apparent paradox likely reflects differences among Bcl-2 family members in their binding determinants and abilities to allosterically modulate the InsP3R (8). Collectively, these data have given rise to a generalized model in which antiapoptotic Bcl-2 proteins confer protection either by limiting Ca2+ overload during apoptotic stimuli or by priming cellular resistance through enhanced mitochondrial metabolism (2).
Our previous studies have demonstrated that Bcl-xL binds to all three InsP3R isoforms to increase their sensitivity to low levels of InsP3 stimulation (4–6). This results in increased Ca2+ signaling and bioenergetics, a requirement for Bcl-xL to be maximally effective as an antiapoptotic mediator. More recently, we used mouse embryonic fibroblast (MEF) cell lines derived from Bcl-xL knock-out (Bcl-xL-KO) embryos to examine the effect of targeting Bcl-xL to specific organelles (9). When expressed exclusively at the ER, Bcl-xL had predicable effects on Ca2+ handling but surprisingly did not confer any apoptosis protection. In contrast, targeting to the mitochondria alone was protective; however, the maximal antiapoptotic activity of Bcl-xL was only seen when it was present at both the ER and mitochondria. One interpretation of these data would be that localization of Bcl-xL at the mitochondria is required to sense Bcl-xL-regulated InsP3R-dependent Ca2+ signals.
Uptake of Ca2+ into the mitochondrial matrix requires transport across two membranes. Across the inner membrane, this is governed by the calcium uniporter, a channel whose properties have been characterized (10) but whose molecular identity has only recently been described (11, 12). Movement across the outer membrane is mediated largely by the voltage-dependent anion channel (VDAC), a porin channel that serves as the major diffusion pathway for ions and metabolites (13). Of the three VDAC isoforms, VDAC1 is the most abundantly expressed (14) and extensively characterized (13). Indeed, due to the abundance and large pore size of VDAC1, the outer membrane was originally thought to be freely permeable to Ca2+, although recent evidence suggests that it may function as a more regulated Ca2+ permeability (15–17).
Intriguingly, Bcl-xL binds directly to VDAC1; however, there is a lack of consensus as to whether it serves to promote (18) or inhibit (19) the function of the channel as a permeability pathway for metabolites and large anions. Similarly, in electrophysiological studies, Bcl-xL has been reported to both increase (18) and decrease (20) conductance of VDAC1 reconstituted in artificial membranes. It is not known whether Bcl-xL regulates mitochondrial Ca2+ uptake by interacting with VDAC1 or other VDAC isoforms. The purpose of the current study was to define how the interaction between Bcl-xL and VDAC shapes mitochondrial Ca2+ signaling.
EXPERIMENTAL PROCEDURES
Cell Culture
The generation of MEF cells from wild-type and Bcl-xL-KO embryos and selection of cell lines stably expressing Bcl-xL targeted to the ER or mitochondria in the KO background have been described previously (9). MEF cells were maintained in Dulbecco's modified Eagle's medium (DMEM) (Mediatech, Manassas, VA) supplemented with 10% fetal bovine serum (FBS) (Gemini, West Sacramento, CA), 100 units ml−1 penicillin (Mediatech), and 100 μg ml−1 streptomycin (Mediatech) in an incubator with 95% humidity and 5% CO2 at 37 °C.
Solutions and Reagents
Hanks' balanced salt solution contained 137.9 mm NaCl, 5.33 mm KCl, 0.44 mm KH2PO4, 0.34 mm Na2HPO4, 5.56 mm glucose, 4.17 mm NaHCO3, 1.8 mm CaCl2, 0.49 mm MgCl2, 0.41 mm MgSO4, 10 mm HEPES, pH 7.4 with NaOH. To prepare Ca2+-free Hanks' balanced salt solution, CaCl2 was substituted with MgCl2, and 1 mm EGTA was added. Intracellular-like medium (ICM) contained 120 mm KCl, 10 mm NaCl, 1 mm KH2PO4, 20 mm HEPES, 2 mm sodium succinate, 1 mm EGTA, pH 7.1 with KOH. The free [Ca2+] was adjusted to the desired level by varying the ratio of Ca2+/HEDTA, calculated using Maxchelator (C. Patton, Stanford University, CA). The following pharmacological reagents were obtained from the indicated sources: digitonin, oligomycin, and FCCP (Sigma-Aldrich); rotenone (MP Biomedicals, Santa Ana, CA); cyclosporin A (Enzo Life Sciences Inc., Farmingdale, NY); dithiothreitol (DTT) (Fisher Scientific); Sarkosyl (IBI Scientific, Peosta, IA); CHAPS (Calbiochem); ruthenium red and valinomycin (EMD Millipore, Billerica, MA); Rhod-2 AM and tetramethylrhodamine (TMRE) (Invitrogen), and Fluo-2 AM (TEFLabs, Inc., Austin, TX). The following peptides based on the human VDAC1 sequence were synthesized by Biomatik (Wilmington, DE): control (LVLGYEGWLA), N-terminal (GLGKSARDVFTKGYGFG), and L14-15 (LAWTAGNSNTR). Cell-permeant versions were tagged with antennapedia homeodomain-derived antennapedia (Antp; RQIKIWFQNRRMKWKK) at the C terminus of each peptide. Anti-VDAC1 mAb was purchased from EMD Millipore.
GST Pulldown Assay and Western Blot
Mouse VDAC1, -2, and -3 cDNAs were subcloned into pGEX-6P-1 (GE Healthcare). Recombinant GST fusion proteins were expressed in Escherichia coli and purified using glutathione-Sepharose beads (GE Healthcare) as described (6). Beads were then incubated with cell lysates or recombinant Bcl-xL (2 μg) as described (6). Samples were analyzed by Western blot using antibodies against Bcl-xL (BD Biosciences) and GST (ViroGen Corp., Watertown, MA) with anti-β-actin (Sigma-Aldrich) as a loading control.
Isolation of Mitochondrial and Mitoplast Preparations
Mitochondria were isolated by following established protocols (21). Briefly, MEF cells were harvested and homogenized in mitochondrial isolation buffer (200 mm mannitol, 70 mm sucrose, 1 mm EGTA, 10 mm HEPES, 0.05% BSA, protease inhibitor mixture, pH 7.4 with KOH). Following centrifugation, the mitochondrial fraction was resuspended in ICM buffer prior to experimentation. Mitoplasts were generated by incubating isolated mitochondria in 4 volumes of hypotonic solution (5 mm sucrose, 1 mm EGTA, 5 mm HEPES, pH 7.4 with KOH) for 20 min and then equilibrated on ice with 1 volume of hypertonic solution (750 mm KCl, 80 mm HEPES, 1 mm EGTA, pH 7.4 with KOH) before centrifugation. For Western blot analysis of isolated mitochondria and mitoplasts, samples were lysed and analyzed using standard approaches and blotted with anti-uncoupling protein 3 (Alpha Diagnostic International Inc., San Antonio, TX) and anti-cytochrome c (Santa Cruz Biotechnology Inc., Santa Cruz, CA).
Bcl-xL Knockdown Mediated by Lentivirally Encoded shRNA
Lentiviral transduction particles (Sigma-Aldrich) carrying Bcl-xL shRNA or empty vector controls were added to cells in culture, and selection antibiotic (puromycin; 2 μg ml−1) was added after 3 days. Single colonies were then isolated by limited dilution, and Bcl-xL knockdown was confirmed by Western blot.
Cytoplasmic and Mitochondrial [Ca2+] Measurement
For simultaneous measurement of cytoplasmic Ca2+ ([Ca2+]cyto) and mitochondrial Ca2+ ([Ca2+]mito), cells cultured on glass coverslips were loaded with 3 μm Rhod-2 AM by incubation at 37 °C for 30 min followed by 10 μm Fluo-2 AM at room temperature for a further 40 min. In experiments requiring the flash photolysis of caged Ca2+, the membrane-permeable caged EGTA compound o-nitrophenyl EGTA AM (5 μm) was added along with Fluo-2 AM, and Ca2+ was photoreleased by brief pulses (150–550 ms) of UV light delivered uniformly throughout the image field. Coverslips were then mounted in a recording chamber positioned on the stage of an inverted microscope (IX71, Olympus America Inc., Center Valley, PA). Cells were visualized using a PlanApo 60×, 1.42 numerical aperture oil immersion objective, and confocal images were acquired using a VT-Infinity 3 (VisiTech International, Sunderland, UK). Fluo-2 and Rhod-2 were alternately excited using the 488- and 568-nm lines, respectively, of a krypton-argon laser. The emitted fluorescence was filtered using a dual bandpass filter set (VisiTech International) and collected and analyzed using HCImage software (Hamamatsu Corp., Sewickley, PA). The chamber was continuously perfused with Hanks' balanced salt solution at room temperature, and a rapid solution changer was used to switch the composition of the solution bathing the cells under study. For isolated mitochondria and mitoplasts, samples were prepared from MEF cells, loaded with Rhod-2, and imaged as described above. Wide field fluorescence microscopy was used to measure [Ca2+]mito in plasma membrane-permeabilized preparations. Rhod-2-loaded cells were mounted in a recording chamber on the stage of an inverted IX71 microscope (Olympus America Inc.) and excited at 548 nm. Emitted fluorescence was filtered at 605 nm and collected using a charge-coupled device-based imaging system running SimplePCI software (Hamamatsu Corp.). The solution perfusion and exchange system was similar to that described above. Cells were permeabilized by 3–4-min exposure to digitonin (25 μg ml−1) applied in Ca2+-free ICM. The permeabilized preparation was then allowed to equilibrate in regular Ca2+-free ICM for 15 min prior to experimental recording.
Confocal and Electron Microscopy
To image ER and mitochondria confocally, cells were first transfected with eYFP-ER plasmid (Clontech) using an Amaxa Nucleofector device (Lonza, Allendale, NJ) and seeded onto 35-mm glass coverslips. After 16–24 h, the cells were labeled with CellLight® Mitochondria-RFP (Invitrogen) according to the manufacturer's instructions. After a further 24 h in culture, coverslips were mounted in a chamber, and confocal images were acquired using a VT-Infinity 3 system as described above. To image ER and mitochondria using electron microscopy, cells were plated onto ACLAR film disks (Jed Pella, Redding, CA) in a 12-well tissue culture plate with 20,000 cells in each well. After incubation for 24 h, cells were fixed with 3% glutaraldehyde in cacodylate buffer (0.1 m cacodylate in 1× PBS) at 4 °C overnight. Prior to embedding, cells were treated with 2% osmium tetroxide followed by dehydration in graded ethanol (60, 90, and 100%). Cells were then embedded in LX-112 epoxy plastic (Ladd Research, Williston, VT). Ultrathin sections of 80 nm were cut, mounted on uncoated copper grids, and stained with lead citrate and saturated aqueous uranyl acetate. Images were obtained with a Philips CM12 transmission electron microscope (Philips, Andover, MA) at 80 kV.
Mitochondrial Membrane Potential (ΔΨm) Measurement
Cells were permeabilized as described above and perfused with ICM containing TMRE (20 nm) for 15 min. Images were acquired using wide field fluorescence microscopy, and TMRE was present throughout the recording period. To monitor ΔΨm in isolated mitochondria and mitoplasts, 30 μl of the mitochondria or the mitoplast preparation was added to the recording chamber with 20 nm TMRE, and measurements were made using confocal microscopy as described above.
Data Collection and Analysis
In confocal microscopy experiments using a 60× objective, data from three to six intact cells or ∼100 isolated mitochondria/mitoplasts were acquired per image field. When using wide field fluorescence microscopy, data were collected using a 20× objective, enabling capture of ∼30 cells per image field. For all imaging experiments, multiple fields were acquired from each coverslip, and the data pooled from three to four independent coverslips were acquired on at least two different days. When comparison of different cell lines was required, the cells were cultured at the same density and passaged in parallel, and data were acquired on the same day. Fluorescence intensity changes were background-subtracted and normalized to the initial fluorescence value F0 and expressed as F/F0. Data were summarized as mean ± S.E., and differences between means were assessed using the Student's t test for unpaired comparisons. A one-way ANOVA with Fisher's least significant difference post hoc analysis was used for multiple comparisons. For all tests, the differences between means were accepted as statistically significant at the 95% level (p < 0.05).
RESULTS
Mitochondrially Localized Bcl-xL Enhances Mitochondrial Ca2+ Uptake in Intact and Permeabilized MEF Cells
To investigate the role of Bcl-xL in regulating [Ca2+]mito uptake in response to ER Ca2+ release, wild-type (WT) and Bcl-xL-KO cells were co-loaded with Rhod-2 and Fluo-2 to simultaneously monitor [Ca2+]mito and [Ca2+]cyto, respectively, and imaged using confocal microscopy (Fig. 1A). To evoke an InsP3R-dependent Ca2+ release, cells were challenged with 1 mm ATP applied in zero Ca2+-containing buffer. Consistent with our previous observations (9), a larger amplitude InsP3-dependent ER Ca2+ release was observed in Bcl-xL-KO cells compared with WT (Fig. 1, A and B). A concomitant rise in [Ca2+]mito was observed in both cell types, but surprisingly the magnitude of the [Ca2+]mito uptake in Bcl-xL-KO cells was smaller than that in WT despite the larger ER Ca2+ release (Fig. 1B). These data suggest that deletion of Bcl-xL impinges on the ability of mitochondria to accumulate Ca2+. However, this was not due to structural or morphological changes because neither the mitochondrial biomass nor the architecture of ER-mitochondrial contact sites was different in Bcl-xL-KO cells (Fig. 1, C–F).
FIGURE 1.

Bcl-xL promotes ER to mitochondrial Ca2+ transfer without affecting ER or mitochondrial morphology. A, representative images showing intact WT and Bcl-xL-KO cells co-loaded with Rhod-2 and Fluo-2 during stimulation with 1 mm ATP to evoke InsP3-dependent ER Ca2+ release. B, representative time course plots of [Ca2+]cyto and [Ca2+]mito are shown. The peak amplitude (mean ± S.E.) of the [Ca2+]cyto response was significantly larger (p < 0.05; Student's t test) in Bcl-xL-KO (3.20 ± 0.36) compared with WT (1.93 ± 0.41), whereas the peak amplitude of the [Ca2+]mito transient was smaller in Bcl-xL-KO (1.83 ± 0.20) compared with WT (2.84 ± 0.38). C, WT and Bcl-xL-KO MEFs were labeled with enhanced YFP and RFP targeted to the ER and mitochondria, respectively. Representative confocal sections show mitochondria (red), ER (green), and regions of colocalization (white). The degree of ER-mitochondrial colocalization was assessed using Manders' coefficient, and no significant difference was observed between the coefficients calculated for WT (0.49 ± 0.01) and Bcl-xL-KO (0.48 ± 0.01) cells (p > 0.05; Student's t test). D, electron micrographs of WT and Bcl-xL-KO cells (19,500×). Arrows indicate points of close ER-mitochondrial apposition. E, the ER-mitochondrial contacts identified that were less than or equal to 100 nm were sorted into five groups (0–20, 21–40, 41–60, 61–80, and 81–100 nm) and graphed based on the number of contacts in each group normalized to the total number of contacts less than or equal to 100 nm (wild type, 88 contacts; Bcl-xL-KO, 92 contacts). F, the expression levels of several mitochondrial proteins were detected by Western blot. COX-IV, cytochrome c oxidase subunit IV.
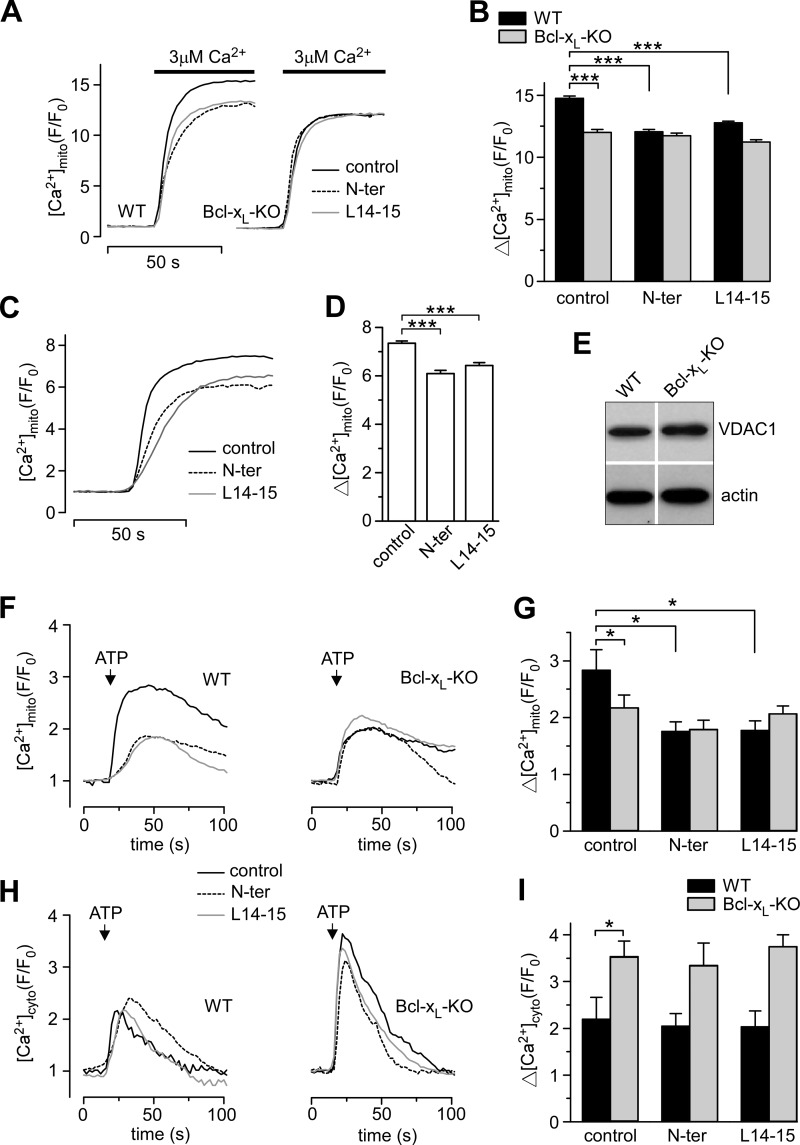
To examine [Ca2+]mito uptake independently of ER Ca2+ release, Rhod-2-loaded WT and Bcl-xL-KO cells were digitonin-permeabilized and bathed in Ca2+-free ICM containing mitochondrial substrates. After equilibration, cells were exposed to Ca2+-containing medium, and [Ca2+]mito uptake was monitored in response to a range of physiologically relevant Ca2+ concentrations (Fig. 2A). As expected, addition of the [Ca2+]mito uptake blocker ruthenium red (10 μm) completely inhibited [Ca2+]mito uptake (22). Compared with WT, the magnitude of [Ca2+]mito uptake was markedly smaller in Bcl-xL-KO cells (Fig. 2B), suggesting that enhanced [Ca2+]mito uptake in Bcl-xL-expressing cells is independent of physiological coupling between the ER and mitochondria. A similar conclusion was drawn from a second series of experiments in intact cells. Here, a step increase in [Ca2+]cyto evoked by photoreleasing caged Ca2+ resulted in more [Ca2+]mito uptake in WT compared with Bcl-xL-KO cells (Fig. 2, C and D).
FIGURE 2.
Bcl-xL promotes mitochondrial Ca2+ uptake in permeabilized cells and under different [Ca2+]cyto. A, representative traces showing [Ca2+]mito in permeabilized WT and Bcl-xL-KO cells during step increases in external [Ca2+] from 0 to 1.25, 3.4, or 10.2 μm in the presence or absence of ruthenium red (RR; 10 μm). B, summary of Δ[Ca2+]mito in response to physiological changes in external [Ca2+]mito (mean ± S.E.; *, p < 0.05; ANOVA). C, intact WT and Bcl-XL-KO cells were co-loaded with Rhod-2, Fluo-2, and o-nitrophenyl EGTA, and Ca2+ was uncaged by pulses of UV light ranging from 150 to 550 ms. Representative time course plots of [Ca2+]cyto and [Ca2+]mito are shown. D, the amplitude of the change in [Ca2+]mito with respect to the amplitude of the evoked [Ca2+]cyto change in WT and Bcl-xL-KO cells. Data from individual cells were pooled and binned according to the amplitude of [Ca2+]cyto transients. The corresponding mean ± S.E. [Ca2+]mito response was then plotted against the mean ± S.E. [Ca2+]cyto response for each bin. Differences between means of [Ca2+]mito for each bin were assessed using ANOVA (*, p < 0.05). E, typical recordings of [Ca2+]mito in permeabilized cells stably expressing ER (Bcl-xL-ER) or mitochondrially targeted (Bcl-xL-mito) in the Bcl-xL-KO background during steps from 0 to 3 μm external [Ca2+]. F, summary bar graphs of the peak [Ca2+]mito amplitude and the maximal rate of mitochondrial Ca2+ uptake (mean ± S.E.; ***, p < 0.001; ANOVA). Error bars represent S.E.
We next asked whether re-expressing Bcl-xL could restore [Ca2+]mito uptake to Bcl-xL-KO cells. Cell lines were generated in the Bcl-xL-KO background that stably expressed Bcl-xL specifically targeted to either ER (Bcl-xL-ER) or mitochondria (Bcl-xL-mito). These cells were expanded from single clones expressing Bcl-xL at levels similar to that detected in WT and extensively characterized in our previous study (9). Permeabilized cells loaded with Rhod-2 and equilibrated in zero Ca2+ medium were exposed to 3 μm Ca2+ to stimulate [Ca2+]mito uptake (Fig. 2E). The peak amplitude of the [Ca2+]mito response and the maximum Ca2+ uptake rate were measured (Fig. 2F). Targeting Bcl-xL to ER in Bcl-xL-KO cells had no effect on [Ca2+]mito uptake; in contrast, uptake was promoted by re-expressing Bcl-xL at the mitochondria (Fig. 2F). These findings demonstrate that localization of Bcl-xL to the mitochondria, but not ER, restores [Ca2+]mito uptake, recapitulating the WT.
Modulation of Mitochondrial Membrane Potential Is Not a Requirement for Bcl-xL to Promote Mitochondrial Ca2+ Uptake
The electrochemical driving force for [Ca2+]mito uptake depends on both the ΔΨm and the cytosolic-mitochondrial [Ca2+] gradient. Interestingly, antiapoptotic Bcl-2 proteins, including Bcl-xL, have been shown to help maintain a hyperpolarized potential during cellular stress (23, 24). Given that [Ca2+]mito uptake will drive depolarization and thus limit further Ca2+ accumulation (25), it is possible that Bcl-xL promotes [Ca2+]mito uptake by stabilizing ΔΨm and maintaining the driving force for Ca2+.
Resting ΔΨm was measured in WT and Bcl-xL-KO cells after permeabilization and equilibration in ICM containing mitochondrial substrates and the membrane potential probe TMRE (20 nm). TMRE fluorescence was normalized to the fluorescence acquired after complete ΔΨm dissipation by the protonophore FCCP (10 μm). There was no significant difference in resting ΔΨm between WT and Bcl-xL-KO cells (Fig. 3A). We next measured dynamic changes in ΔΨm by monitoring TMRE fluorescence in permeabilized WT and Bcl-xL-KO cells. As expected, switching the external bathing medium from 0 to 3 μm Ca2+ evoked a depolarization that was reversed upon Ca2+ removal (Fig. 3B). The magnitude of depolarization evoked by 3 μm Ca2+, however, was larger in WT, presumably due to more Ca2+ accumulation in these cells. Thus, Bcl-xL does not promote [Ca2+]mito uptake by maintaining a hyperpolarized ΔΨm and strong Ca2+ driving force. Interestingly, upon Ca2+ removal, ΔΨm recovered much faster in WT compared with Bcl-xL-KO when quantified as the halftime for recovery to base line (Fig. 3C), consistent with the hypothesis that Bcl-xL stabilizes ΔΨm (see “Discussion”).
FIGURE 3.
Bcl-xL promotes mitochondrial Ca2+ uptake independently of mitochondrial membrane potential. A, ΔΨm in WT and Bcl-xL-KO cells assessed by TMRE fluorescence normalized to fluorescence after addition of FCCP (10 μm). Data represent mean ± S.E. (p > 0.05; Student's t test). B, representative traces of ΔΨm in response to the addition and removal of 3 μm [Ca2+]. The amplitude of the Ca2+-induced depolarization (ΔF/F0 TMRE) in WT and Bcl-xL-KO cells was 0.80 ± 0.01 and 0.75 ± 0.01, respectively (p < 0.001; Student's t test). C, the half-times for the ΔΨm recovery upon Ca2+ removal are summarized. Data represent mean ± S.E. (***p < 0.001; Student's t test). D, permeabilized cells were treated with FCCP, oligomycin, rotenone, and valinomycin to collapse ΔΨm. Traces are depicted showing the Ca2+ gradient-driven [Ca2+]mito uptake and efflux during application and removal of 3 μm Ca2+. E, the summary bar graphs show the peak [Ca2+]mito amplitude and the maximal rate of [Ca2+]mito uptake in 0 ΔΨm (mean ± S.E.; **, p < 0.01; ***, p < 0.001; Student's t test). F, typical records showing ΔΨm hyperpolarization measured with TMRE in response to stepping [K+] from 140 to 0.1 mm in permeabilized WT and Bcl-xL-KO cells incubated without mitochondria substrates in the presence of FCCP, oligomycin, rotenone, and valinomycin. The amplitude of the hyperpolarization (ΔF/F0 TMRE) in WT and Bcl-xL-KO cells was 2.61 ± 0.06 and 2.71 ± 0.04, respectively (mean ± S.E.; p > 0.05; Student's t test). G, representative traces showing [Ca2+]mito during a step increase in bathing [Ca2+] from 0 to 3 μm when ΔΨm was [K+] gradient-driven. Under these conditions, the mean ± S.E. amplitude (ΔF/F0) recorded in WT and Bcl-xL-KO cells was 10.96 ± 0.19 and 8.20 ± 0.16 (p < 0.001; Student's t test), and the maximum uptake rate ((ΔF/F0)/Δt) was 1.12 ± 0.02 in WT compared with 0.89 ± 0.02 in Bcl-xL-KO (p < 0.001; Student's t test). Error bars represent S.E.
To further confirm that Bcl-xL does not affect [Ca2+]mito uptake by influencing ΔΨm, [Ca2+]mito uptake was measured under conditions where the driving force was exclusively due to the Ca2+ gradient. Cells loaded with Rhod-2 were permeabilized and perfused in Ca2+-free ICM. To completely dissipate ΔΨm, the solution was switched to ICM without mitochondrial substrates and containing 5 μm FCCP, 8 μg ml−1 oligomycin, 10 μm rotenone (26). After 2 min, the [Ca2+] in the bathing medium was increased to 3 μm (Fig. 3D). The amplitude and maximum rate of Ca2+ uptake, however, were larger in the WT group (Fig. 3E), indicating that Bcl-xL enhances Ca2+ uptake when the driving force is only due to the [Ca2+] gradient. In addition, [Ca2+]mito uptake was monitored under conditions in which ΔΨm was generated artificially in WT and Bcl-xL-KO cells. Here, WT and Bcl-xL-KO cells were permeabilized, and the ΔΨm was dissipated as described. The bathing [K+] was then decreased to 0.1 mm (KCl was substituted with choline chloride) in the presence of the K+ ionophore valinomycin (50 ng ml−1). The ensuing outward K+ current rapidly hyperpolarized the ΔΨm as measured with TMRE, and the K+-driven ΔΨm attained a new steady state in about 50 s and remained stable for ∼30 s (Fig. 3F). Under these conditions, it would be extremely unlikely for Bcl-xL to influence ΔΨm; however, the effect of Bcl-xL on [Ca2+]mito uptake persisted (Fig. 3G). These data strongly support a model in which Bcl-xL regulation of Ca2+ uptake into the matrix is largely independent of any influence it may have on ΔΨm.
Promotion of [Ca2+]mito Uptake by Bcl-xL Is Dependent on the Integrity of the Outer Mitochondrial Membrane
The mitochondrial permeability transition pore (mPTP) is a molecular complex spanning the outer and inner membranes whose formation collapses ΔΨm (27). The mPTP is normally associated with pathological states, but transient mPTP formation under physiological conditions can function as a Ca2+ efflux pathway. Importantly, inhibiting this efflux pathway by blocking mPTP has been shown to promote [Ca2+]mito uptake (28). Because Bcl-xL also has an inhibitory effect on mPTP (29), we tested the hypothesis that Bcl-xL mediates its effect on [Ca2+]mito uptake through mPTP inhibition. Isolated mitochondria were prepared from WT and Bcl-xL-KO cells, suspended in zero Ca2+ medium, loaded with Rhod-2, and imaged confocally. Consistent with the data collected using intact and permeabilized cells, the peak amplitude of the [Ca2+]mito response and the maximum [Ca2+]mito uptake rate were significantly larger in WT compared with Bcl-xL-KO (Fig. 4A, control traces). Treatment with the mPTP inhibitor cyclosporine A, however, had no effect on Ca2+ uptake in either WT or Bcl-xL-KO mitochondria (Fig. 4, A and B), indicating that mPTP modulation cannot account for the effect of Bcl-xL on [Ca2+]mito handling.
FIGURE 4.
Promotion of [Ca2+]mito uptake by Bcl-xL is dependent on the integrity of the outer mitochondrial membrane but not on mPTP opening. A, typical traces showing [Ca2+]mito uptake monitored confocally in Rhod-2-loaded mitochondria isolated from WT and Bcl-xL-KO cells. Extracellular [Ca2+] was stepped from 0 to 3 μm in the absence or presence of cyclosporine A (CsA; 1 μm). B, bar graphs summarizing (mean ± S.E.) the peak [Ca2+]mito uptake and maximal uptake rate (***, p < 0.001; ANOVA). C, representative Western blot detecting uncoupling protein 3 (UCP3) and cytochrome c (cyto c) in isolated mitochondrial and mitoplast preparations. D, representative confocal sections of mitoplasts in the presence of TMRE (20 nm) before and after the addition of FCCP (10 μm). Scale bar, 2 μm. E, summary data (mean ± S.E.) depicting peak [Ca2+]mito uptake and uptake rate monitored in response to a step increase in extracellular [Ca2+] from 0 to 3 μm in mitoplasts prepared from WT and Bcl-xL-KO cells (p > 0.05; Student's t test). Error bars represent S.E.
It is widely accepted that Bcl-xL resides primarily on the outer mitochondrial membrane; however, an important role for its localization at the inner membrane has recently been identified (30, 31). We therefore asked whether the effect of Bcl-xL on matrix Ca2+ accumulation persisted after removal of the outer membrane. Mitoplasts were prepared, and disruption of the outer mitochondrial membrane was confirmed by the loss of the intermembrane protein cytochrome c (Fig. 4C). The inner mitochondrial membrane remained viable as indicated by the presence of the inner membrane protein uncoupling protein 3 along with an intact ΔΨm measured with TMRE (Fig. 4, C and D). With respect to the magnitude and maximum rate of Ca2+ uptake, there was no statistically significant difference between mitoplasts prepared from WT or Bcl-xL-KO cells (Fig. 4E). These data indicate that localization to the outer mitochondrial membrane is required for Bcl-xL to promote [Ca2+]mito uptake.
The Effect of Bcl-xL on [Ca2+]mito Uptake Is Dependent on an Interaction with VDAC
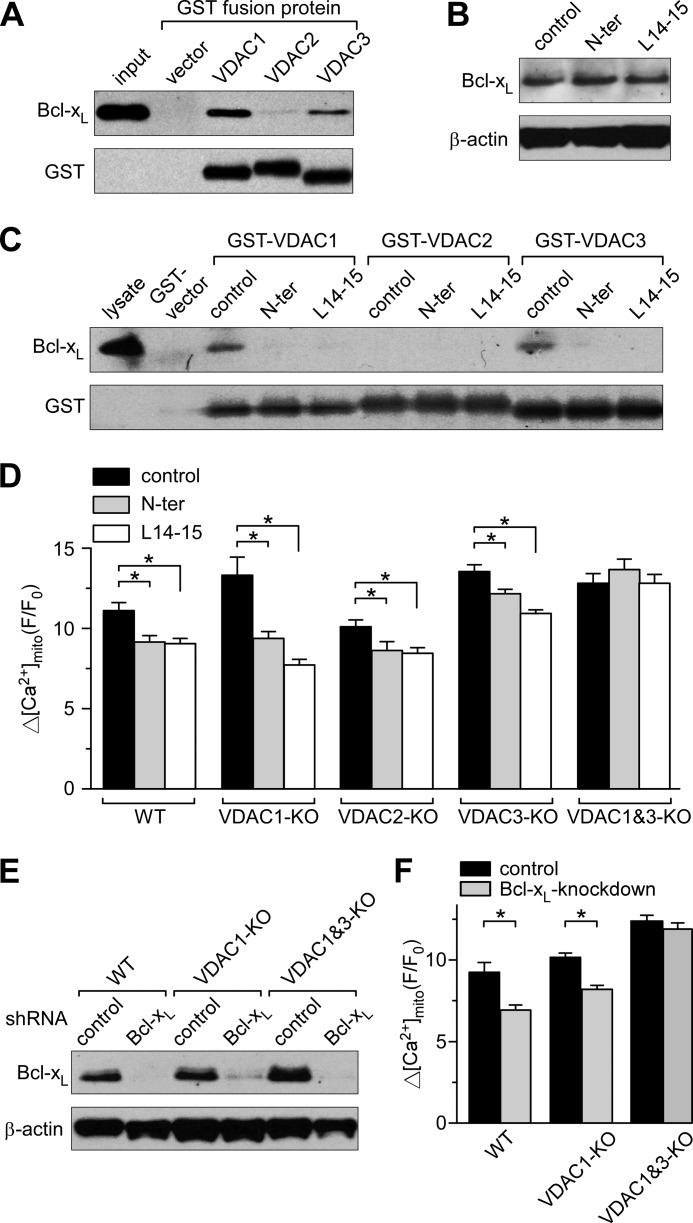
The VDAC is the major permeability pathway for Ca2+ transfer across the outer mitochondrial membrane (27). This channel is known to directly interact with several members of the Bcl-2 family, including Bcl-xL (19, 32–34). Based on an analysis of the binding determinants, peptides representing sections of the VDAC1 sequence were shown previously to be highly effective in disrupting the interaction between either Bcl-2 or Bcl-xL and VDAC1 (20, 35). We adopted this approach to study the effect of VDAC1/Bcl-xL interactions on [Ca2+]mito uptake. Two peptides (N-terminal (N-ter) and L14-15) were synthesized based on previously published sequences shown to block binding between VDAC1 and Bcl-2 (35). In lysates from WT MEF cells, the interaction between VDAC1 and Bcl-xL (detected using a GST pulldown assay) was eliminated by pretreating cells with cell-permeant N-ter and L14-15 peptides but not by pretreatment with control peptide (see Fig. 6C). In imaging studies, permeabilized cells were incubated with peptides (2 μm for 2 min) prior to stimulating [Ca2+]mito uptake and remained present throughout the experiment. This effectively reduced the amplitude of [Ca2+]mito uptake in WT to levels normally observed in Bcl-xL-KO cells but had no effect on Bcl-xL-KO cells (Fig. 5, A and B). These results strongly indicate that Bcl-xL facilitates [Ca2+]mito uptake by interacting with VDAC. To support the hypothesis that this interaction is likely to be ubiquitous, the effect of cell-permeant peptides on [Ca2+]mito uptake were assessed in HeLa cells and found to have qualitatively similar effects (Fig. 5, C and D). The fact that peptides are effective in other cell types also increases confidence that observations made in the Bcl-xL-KO MEF cells are not due to artifacts or adaptations to gene knock-out. In our previous study, we performed microarray analysis of WT and Bcl-xL-KO cell lines and failed to find any difference in expression of calcium signaling genes, including InsP3R isoforms (9). These microarray data were re-examined in the current study to assess VDAC expression levels. Although there was no difference between cell types in the levels of VDAC2 and -3, the ratio of WT to Bcl-xL-KO VDAC1 mRNA was slightly decreased (0.904; p = 0.01); however, this did not translate into detectable reductions in VDAC1 protein assessed using Western blot (Fig. 5E). To further probe the effects of VDAC1-based peptides, intact cells were incubated with cell-permeant peptides (20 μm for 1 h prior to recording and present throughout), and [Ca2+]mito uptake in response to InsP3R-dependent Ca2+ release was recorded. [Ca2+]mito uptake was decreased in WT but remained unchanged in Bcl-xL-KO cells (Fig. 5, F and G). Peptide incubation had no effect on [Ca2+]cyto in the same cells (Fig. 5, H and I).
FIGURE 6.
Bcl-xL interacts with VDAC1 and VDAC3 to promote [Ca2+]mito uptake. A, Western blot of recombinant purified Bcl-xL pulled down by GST fusion proteins of VDAC1, -2, and -3 is shown in the upper lanes with the loading control blots of GST depicted below. B, Western blot detecting Bcl-xL expression levels in WT MEF cells after incubation with cell-permeant control peptide or peptides based on the VDAC1 sequence (N-ter and L14-15; 20 μm for 1 h). C, Western blot detection of Bcl-xL pulled down by GST fusion proteins of VDAC isoforms from WT MEF cell lysates pretreated with control or VDAC1-based peptides. D, summary bar graphs showing the peak [Ca2+]mito uptake in permeabilized VDAC knock-out cells stepped from 0 to 3 μm [Ca2+] in the absence or presence of 2 μm VDAC1 peptides (mean ± S.E.; *, p < 0.05; ANOVA). Cell lines included VDAC single knockouts (VDAC1-KO, VDAC2-KO, and VDAC3-KO) and VDAC1 and -3 double knock-out (VDAC1&3-KO). E, Western blot showing Bcl-xL shRNA knockdown in WT, VDAC1 knock-out, and VDAC1 and -3 double knock-out MEF cells. F, summary bar graphs showing the effect of Bcl-xL knockdown on the peak [Ca2+]mito uptake in permeabilized WT and VDAC knock-out cells upon stepping from 0 to 3 μm [Ca2+] (mean ± S.E.; *, p < 0.05; Student's t test). Error bars represent S.E.
FIGURE 5.
Peptides based on the VDAC1 sequence inhibit Bcl-xL-dependent [Ca2+]mito uptake. A and B, representative traces of [Ca2+]mito in permeabilized cells stepped from 0 to 3 μm [Ca2+] in the absence or presence of 2 μm VDAC1 control peptide (ctrl), N-ter, or L14-15 peptide. Summary bar graphs of the peak [Ca2+]mito amplitudes are shown (mean ± S.E.; ***, p < 0.001; ANOVA). C and D, representative traces of [Ca2+]mito in permeabilized HeLa cells in which the bathing medium was stepped from 0 to 3 μm [Ca2+] in the presence of VDAC1-based peptides (2 μm) are shown in C, and summary bar graphs of the peak [Ca2+]mito amplitudes are shown in D (mean ± S.E.; ***, p < 0.001; ANOVA). E, Western blot detection of VDAC1 in cell lysates of WT and Bcl-xL-KO MEF cells. F and G, intact WT and Bcl-xL-KO cells were incubated with 20 μm cell-permeant VDAC1 peptides, and InsP3-dependent ER Ca2+ release was evoked by 1 mm ATP. Representative traces of [Ca2+]mito and summary bar graphs of the peak amplitudes are shown (mean ± S.E.; *, p < 0.05; ANOVA). The respective mean ± S.E. maximum uptake rates ((ΔF/F0)/Δt) for WT and Bcl-xL-KO under control conditions were 0.21 ± 0.04 and 0.22 ± 0.03 (not significant; ANOVA); 0.14 ± 0.01 and 0.17 ± 0.02 in the presence of N-ter (not significant; ANOVA), and 0.13 ± 0.02 and 0.16 ± 0.02 (not significant; ANOVA) in the presence of L14-15. H and I, traces depicting the [Ca2+]cyto signals measured in the same cells shown in F along with summary bar graphs of the peak [Ca2+]cyto amplitudes (mean ± S.E.; *, p < 0.05; ANOVA). Error bars represent S.E.
Bcl-xL Interacts with VDAC1 and VDAC3 to Regulate [Ca2+]mito Uptake
To determine whether Bcl-xL interacts with all three VDAC isoforms, GST fusion proteins of VDAC1, -2, and -3 were generated, and the binding of purified recombinant Bcl-xL was assessed. GST-VDAC1 and -3 bound robustly to purified recombinant Bcl-xL (Fig. 6A). In addition, GST-VDAC1 and -3 effectively pulled down endogenous Bcl-xL from WT MEF cell lysates (Fig. 6C). In contrast, binding of purified recombinant Bcl-xL to VDAC2 was barely detectable (Fig. 6A), and GST-VDAC2 was unable to pull down detectable quantities of Bcl-xL from cell lysates (Fig. 6C). Pretreating WT MEF cells with cell-permeant N-ter and L14-15 peptides had no effect on Bcl-xL expression levels (Fig. 6B) but completely inhibited the pulldown of Bcl-xL by GST-VDAC1 and -3 (Fig. 6C). Collectively, these biochemical data suggest that Bcl-xL interacts with VDAC1 and -3 and that binding is effectively disrupted by peptides based on the VDAC1 sequence.
Next we investigated the effect of Bcl-xL and VDAC isoform interactions on [Ca2+]mito uptake. WT and VDAC knock-out MEF cells, including VDAC1, VDAC2, VDAC3 single knockouts and a VDAC1 and -3 double knock-out cell line, were loaded with Rhod-2 and permeabilized, and [Ca2+]mito uptake was measured as described earlier. The presence of N-ter and L14-15 peptides decreased the [Ca2+]mito uptake in all cell lines except the VDAC1 and -3 double KO in which only VDAC2 was expressed. Consistent with the binding studies, these data suggest that Bcl-xL interacts with both VDAC1 and -3 to promote [Ca2+]mito uptake and that this is inhibited by disrupting the interaction using peptides based on the VDAC1 sequence. Of note, deletion of VDAC isoforms either singly or in combination did not appear to reduce [Ca2+]mito uptake. This was surprising and suggests some form of adaptation (see “Discussion”).
To further verify the effect of Bcl-xL on VDAC isoforms, particularly VDAC2, we generated stable Bcl-xL knockdown cell lines in the MEF WT, VDAC1, and VDAC1 and -3 double KO background. Bcl-xL knockdown was achieved by infecting cells with lentiviral particles carrying Bcl-xL shRNA and verified by Western blot (Fig. 6E). When compared with cells generated using control shRNA, WT and VDAC1 KO cells generated with Bcl-xL shRNA showed less [Ca2+]mito uptake when permeabilized and challenged with 3 μm Ca2+ (Fig. 6F). As expected, knocking down Bcl-xL had no effect on [Ca2+]mito uptake in VDAC1 and -3 double KO cells, consistent with binding data showing that Bcl-xL does not interact with the VDAC2 isoform. Taken together, these results indicate that Bcl-xL interacts with VDAC1 and VDAC3, but not VDAC2, to regulate [Ca2+]mito uptake.
DISCUSSION
It is well established that VDAC is a major permeability pathway for the transfer of Ca2+ across the outer mitochondrial membrane (15–17). The major finding of the current study is that this permeability pathway is tightly regulated by a protein/protein interaction between Bcl-xL and VDAC isoforms 1 and 3. Moreover, endogenous levels of mitochondrially localized Bcl-xL under basal conditions in the absence of apoptotic stimuli facilitates Ca2+ uptake into the mitochondrial matrix in response to physiological Ca2+ elevations.
Morphological and Structural Properties of Mitochondria Are Unaffected by the Loss of Bcl-xL
There are several scenarios in which previously reported functions of Bcl-xL could be invoked to explain the observed data. Bcl-xL has been shown to regulate mitochondrial fusion, fission, and biomass (36), and mitochondrial morphology can be an important factor in shaping the Ca2+ signal (37). Could Bcl-xL-dependent structural changes in mitochondria be at play in the current study? This does not seem to be the case because imaging experiments at both confocal and electron microscopic resolutions did not reveal any apparent difference between mitochondria in WT and Bcl-xL-KO cells (Fig. 1, C–F). Moreover, in both cell types, the expression levels of key mitochondrial proteins were the same, indicating that there is no difference in the total number of mitochondria.
Regions of close contact between mitochondria and ER represent specific microdomains that enable Ca2+ released from ER channels to be effectively sensed by closely apposed mitochondria either by virtue of proximity or through protein/protein interactions that bridge the organelles (15, 38). Considering that Bcl-xL interacts with both VDAC and InsP3R, it is possible that all three proteins exist in a tertiary complex at the ER-mitochondria interface to facilitate ER-mitochondrial Ca2+ transfer. We failed to find any difference in the number or distance between ER-mitochondrial contact sites in WT and Bcl-xL-KO cells, arguing against such a model; however, redistribution of proteins to the contact sites remains a possibility. Nevertheless, increased Bcl-xL-dependent [Ca2+]mito uptake persisted in permeabilized cells and isolated mitochondria but not in mitoplasts, strongly implicating a mechanism intrinsic to the outer mitochondrial membrane.
Bcl-xL Does Not Promote Mitochondrial Ca2+ Uptake by Influencing mPTP or ΔΨm
The observations that antiapoptotic Bcl-2 members regulate the sensitivity to mPTP opening (24, 39, 40) and that physiological mPTP activation may be important in mitochondrial Ca2+ homeostasis (28) raise the possibility that Bcl-xL increases [Ca2+]mito uptake by inhibiting mPTP activity. In this model, [Ca2+]mito uptake transiently induces the formation of the mPTP, which functions as a Ca2+ removal pathway; Bcl-xL would be expected to inhibit mPTP and hence removal, thereby promoting greater matrix Ca2+ accumulation. However, this was not the case in the current study because pharmacological inhibition of mPTP with cyclosporin A had no effect on [Ca2+]mito uptake in mitochondria isolated from either WT or Bcl-xL-KO cells (Fig. 4).
Both Bcl-2 and Bcl-xL have long been known to regulate ΔΨm by helping to maintain hyperpolarization during mitochondrial stress (39, 41, 42). Presumably, this enables a greater, more tolerable [Ca2+]mito load and would be antiapoptotic because ΔΨm loss is a prerequisite for cytochrome c release. More recently, a series of elegant studies in neurons demonstrated that Bcl-xL when localized to the inner mitochondrial membrane functions to inhibit an ion leak pathway that is essential to its role in ΔΨm stabilization (30, 31). Interestingly, Bcl-xL knockdown in these cells was associated with increased ΔΨm (31). In our study, resting ΔΨm was similar in WT and Bcl-xL-KO cells, although faster repolarization was observed in WT after a Ca2+-dependent depolarization, consistent with the role of Bcl-xL role in ΔΨm stabilization. The function of Bcl-xL as a ΔΨm modulator, however, cannot account for the majority of its effects on [Ca2+]mito because increased [Ca2+]mito uptake persisted under conditions where Bcl-xL cannot impinge on ΔΨm: either when ΔΨm was completely collapsed (Fig. 3D) or when ΔΨm was generated artificially by a K+ gradient (Fig. 3G).
Outer Membrane-localized Bcl-xL Facilitates Ca2+ Uptake into the Mitochondrial Matrix
There are two lines of evidence to support the idea that Bcl-xL exerts the majority of its effects on [Ca2+]mito uptake by acting at the outer, rather than the inner, mitochondrial membrane. First, expressing mitochondrially targeted Bcl-xL in the Bcl-xL-KO background recapitulated the [Ca2+]mito uptake phenotype of WT cells (Fig. 2E). In these studies, Bcl-xL was tagged with the membrane localization sequence of the listerial protein ActA, a well established approach that targets proteins to the outer membrane with cytoplasmic orientation (9, 43). Second, the increase in [Ca2+]mito uptake conferred by Bcl-xL was lost in mitoplasts prepared from mitochondria isolated from Bcl-xL-expressing cells (Fig. 4E), effectively ruling out the possibility that Bcl-xL functions by interacting with inner membrane proteins.
The Effect of Bcl-xL on Mitochondrial Ca2+ Uptake Requires an Interaction with VDAC1 or VDAC3
Traditionally, the outer mitochondrial membrane was not thought to offer a significant permeability barrier to Ca2+; however, this paradigm has been challenged by studies demonstrating that it not only limits Ca2+ delivery to the uniporter (44) but can do so in a regulated fashion (16, 17, 26). Our study provides a novel physiological mechanism whereby the magnitude of Ca2+ transfer to the mitochondrial matrix is regulated by protein/protein interactions between Bcl-xL and VDAC isoforms 1 and 3 (Figs. 5 and 6). To our knowledge, ours is also the first study to demonstrate that Bcl-xL binds to VDAC1 and -3 but not to VDAC2. The lack of binding to VDAC2 is particularly interesting because the proapoptotic Bcl-2 protein BAK is known to interact only with VDAC2 and functions to recruit BAK to the outer mitochondrial membrane (45, 46). Our data therefore raise the possibility that the binding exclusivity and functional outcomes of the Bak/VDAC2 interaction could be facilitated by the inability of VDAC2 to interact with Bcl-xL.
We have focused exclusively on Bcl-xL; however, Bcl-2 is also known to bind VDAC1 (35). Despite a large degree of structural and functional homology between these two antiapoptotic proteins, it cannot be assumed that they both affect VDAC function in the same way. In discussion of the current data, therefore, it is only meaningful to consider the literature regarding the Bcl-xL/VDAC interaction. VDAC gating involves voltage-dependent transitions between high and low conductance states that are associated with dramatic changes in selectivity. Open conformations are high conductance, anion-selective states permissive to the transfer of metabolites; closed conformations are lower conductance states favoring cation selectivity (13). Biochemical and structural studies have defined the Bcl-xL/VDAC1 interaction (33, 34, 47); however, the functional implications are still unclear. Initial studies suggested that Bcl-xL closed the channel as evidenced by the inhibition of radiolabeled sucrose uptake into VDAC1-containing liposomes (47). More recent electrophysiological experiments, however, have not produced a consistent set of observations with Bcl-xL being shown to promote both the open (18) and closed (20) conformations across similar voltage ranges. Because the closed state is cation-selective and more Ca2+-permeable (17), our observation that Bcl-xL facilitated [Ca2+]mito uptake is consistent with a model in which Bcl-xL promotes VDAC closing. On the other hand, Bcl-xL-induced VDAC opening cannot be ruled out because a large conductance, cation-selective open state has been described (17, 48), and it is possible that Bcl-xL could increase the frequency of transitions to this state. Future studies will address exactly how Bcl-xL binding affects VDAC1 Ca2+ permeability.
In addition to direct effects on VDAC cation permeability, there are several other ways in which Bcl-xL could affect [Ca2+]mito uptake. The interaction between VDAC and the adenine nucleotide translocase of the inner membrane (49) provides precedent that VDAC exists in complexes spanning the outer and inner membranes. It remains to be determined whether or not VDAC directly interacts with the Ca2+ transport machinery on the inner membrane in a similar way, but it is an intriguing possibility. Another factor to consider is that Bcl-xL regulates the oligomeric state of the channel. In the absence of Bcl-xL, VDAC self-associates to form dimers, trimers, and tetramers (50), whereas binding of Bcl-xL promotes the formation and stabilization of VDAC dimers (34). The potential physiological importance of VDAC self-association was illustrated by bilayer experiments showing that VDAC cross-linking had profound effects on channel conductance (50). Intriguingly, when compared with WT controls, we observed increased [Ca2+]mito uptake in VDAC1 and VDAC3 knockouts (Fig. 6D). This is somewhat counterintuitive because it is already known that deletion of any given VDAC isoform does not result in compensatory up-regulation of the remaining isoforms in these knock-out MEF cells (51). This opens up the possibility that knocking down a specific isoform alters Ca2+ permeation through the remaining isoforms. Perhaps such functional compensation is driven by changes in the hetero-oligomeric makeup of VDAC multimers. However, this is pure speculation, and additional studies will be required to define intermembrane complexes and assess the effect of VDAC oligomerization and the role of individual isoforms on the Ca2+ permeability of the outer membrane.
The Physiological Implications for Bcl-xL-regulated [Ca2+]mito
We showed previously that Bcl-xL functions at the ER by allosterically modulating the InsP3R to increase the frequency of oscillating Ca2+ signals under resting conditions (5, 6). We now demonstrate that through its interactions with VDAC1 and VDAC3 Bcl-xL also enables the ER-released Ca2+ to be more effectively taken up by mitochondria. This is physiologically significant because constitutive Ca2+ signaling to the mitochondrial matrix is essential for the maintenance of cellular bioenergetics (52). Moreover, the ability to maintain bioenergetic competence in the face of apoptotic stimuli is strongly antiapoptotic, and this is now recognized to be one of the mechanisms by which Bcl-xL is protective (18, 31, 53, 54). Thus, our data suggest a role for mitochondrial Ca2+ signaling in bioenergetics regulated by outer membrane-localized Bcl-xL.
Acknowledgments
Confocal imaging was carried out at the Calcium Imaging Research Support Laboratory, Department of Physiology and Biophysics, Rosalind Franklin University. We thank William J. Craigen, Baylor College of Medicine, for kindly providing VDAC1, -2, and -3 knock-out and VDAC1 and -3 double knock-out MEF cell lines and Christopher P. Baines, University of Missouri, for providing VDAC1, -2, and -3 cDNAs. We also thank Kristen E. Olberding for technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants CA106599 and RR018733 (to C. L.). This work was also supported by an American Heart Association scientist development grant and Rosalind Franklin University (to C. W.) and funding from the Kentucky Lung Cancer Research Program (to C. L.).
- ER
- endoplasmic reticulum
- [Ca2+]mito
- mitochondrial Ca2+
- MEF
- mouse embryonic fibroblast
- InsP3
- inositol 1,4,5-trisphosphate
- InsP3R
- inositol 1,4,5-trisphosphate receptor
- [Ca2+]cyto
- cytosolic Ca2+
- VDAC
- voltage-dependent anion channel
- ICM
- intracellular-like medium
- HEDTA
- N-(2-hydroxyethyl)ethylenediaminetriacetic acid
- FCCP
- carbonyl cyanide p-trifluoromethoxyphenylhydrazone
- TMRE
- tetramethylrhodamine
- N-ter
- N-terminal
- ΔΨm
- mitochondrial membrane potential
- ANOVA
- analysis of variance
- mPTP
- mitochondrial permeability transition pore.
REFERENCES
- 1. Youle R. J., Strasser A. (2008) The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9, 47–59 [DOI] [PubMed] [Google Scholar]
- 2. Distelhorst C. W., Bootman M. D. (2011) Bcl-2 interaction with the inositol 1,4,5-trisphosphate receptor: role in Ca2+ signaling and disease. Cell Calcium 50, 234–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Chen R., Valencia I., Zhong F., McColl K. S., Roderick H. L., Bootman M. D., Berridge M. J., Conway S. J., Holmes A. B., Mignery G. A., Velez P., Distelhorst C. W. (2004) Bcl-2 functionally interacts with inositol 1,4,5-trisphosphate receptors to regulate calcium release from the ER in response to inositol 1,4,5-trisphosphate. J. Cell Biol. 166, 193–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eckenrode E. F., Yang J., Velmurugan G. V., Foskett J. K., White C. (2010) Apoptosis protection by Mcl-1 and Bcl-2 modulation of inositol 1,4,5-trisphosphate receptor-dependent Ca2+ signaling. J. Biol. Chem. 285, 13678–13684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li C., Wang X., Vais H., Thompson C. B., Foskett J. K., White C. (2007) Apoptosis regulation by Bcl-xL modulation of mammalian inositol 1,4,5-trisphosphate receptor channel isoform gating. Proc. Natl. Acad. Sci. U.S.A. 104, 12565–12570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. White C., Li C., Yang J., Petrenko N. B., Madesh M., Thompson C. B., Foskett J. K. (2005) The endoplasmic reticulum gateway to apoptosis by Bcl-xL modulation of the InsP3R. Nat. Cell Biol. 7, 1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zhong F., Davis M. C., McColl K. S., Distelhorst C. W. (2006) Bcl-2 differentially regulates Ca2+ signals according to the strength of T cell receptor activation. J. Cell Biol. 172, 127–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Monaco G., Decrock E., Akl H., Ponsaerts R., Vervliet T., Luyten T., De Maeyer M., Missiaen L., Distelhorst C. W., De Smedt H., Parys J. B., Leybaert L., Bultynck G. (2012) Selective regulation of IP3-receptor-mediated Ca2+ signaling and apoptosis by the BH4 domain of Bcl-2 versus Bcl-xL. Cell Death Differ. 19, 295–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eno C. O., Eckenrode E. F., Olberding K. E., Zhao G., White C., Li C. (2012) Distinct roles of mitochondria- and ER-localized Bcl-xL in apoptosis resistance and Ca2+ homeostasis. Mol. Biol. Cell 23, 2605–2618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kirichok Y., Krapivinsky G., Clapham D. E. (2004) The mitochondrial calcium uniporter is a highly selective ion channel. Nature 427, 360–364 [DOI] [PubMed] [Google Scholar]
- 11. De Stefani D., Raffaello A., Teardo E., Szabò I., Rizzuto R. (2011) A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature 476, 336–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Baughman J. M., Perocchi F., Girgis H. S., Plovanich M., Belcher-Timme C. A., Sancak Y., Bao X. R., Strittmatter L., Goldberger O., Bogorad R. L., Koteliansky V., Mootha V. K. (2011) Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature 476, 341–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Colombini M. (2012) VDAC structure, selectivity, and dynamics. Biochim. Biophys. Acta 1818, 1457–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Huizing M., Ruitenbeek W., van den Heuvel L. P., Dolce V., Iacobazzi V., Smeitink J. A., Palmieri F., Trijbels J. M. (1998) Human mitochondrial transmembrane metabolite carriers: tissue distribution and its implication for mitochondrial disorders. J. Bioenerg. Biomembr. 30, 277–284 [DOI] [PubMed] [Google Scholar]
- 15. Rapizzi E., Pinton P., Szabadkai G., Wieckowski M. R., Vandecasteele G., Baird G., Tuft R. A., Fogarty K. E., Rizzuto R. (2002) Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J. Cell Biol. 159, 613–624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Báthori G., Csordás G., Garcia-Perez C., Davies E., Hajnóczky G. (2006) Ca2+-dependent control of the permeability properties of the mitochondrial outer membrane and voltage-dependent anion-selective channel (VDAC). J. Biol. Chem. 281, 17347–17358 [DOI] [PubMed] [Google Scholar]
- 17. Tan W., Colombini M. (2007) VDAC closure increases calcium ion flux. Biochim. Biophys. Acta 1768, 2510–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Vander Heiden M. G., Li X. X., Gottleib E., Hill R. B., Thompson C. B., Colombini M. (2001) Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J. Biol. Chem. 276, 19414–19419 [DOI] [PubMed] [Google Scholar]
- 19. Shimizu S., Shinohara Y., Tsujimoto Y. (2000) Bax and Bcl-xL independently regulate apoptotic changes of yeast mitochondria that require VDAC but not adenine nucleotide translocator. Oncogene 19, 4309–4318 [DOI] [PubMed] [Google Scholar]
- 20. Arbel N., Ben-Hail D., Shoshan-Barmatz V. (2012) Mediation of the anti-apoptotic activity of BCL-XL protein upon interaction with VDAC1 protein. J. Biol. Chem. 287, 23152–23161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Frezza C., Cipolat S., Scorrano L. (2007) Organelle isolation: functional mitochondria from mouse liver, muscle and cultured fibroblasts. Nat. Protoc. 2, 287–295 [DOI] [PubMed] [Google Scholar]
- 22. Szabadkai G., Duchen M. R. (2008) Mitochondria: the hub of cellular Ca2+ signaling. Physiology 23, 84–94 [DOI] [PubMed] [Google Scholar]
- 23. Vander Heiden M. G., Chandel N. S., Williamson E. K., Schumacker P. T., Thompson C. B. (1997) Bcl-xL regulates the membrane potential and volume homeostasis of mitochondria. Cell 91, 627–637 [DOI] [PubMed] [Google Scholar]
- 24. Shimizu S., Eguchi Y., Kamiike W., Funahashi Y., Mignon A., Lacronique V., Matsuda H., Tsujimoto Y. (1998) Bcl-2 prevents apoptotic mitochondrial dysfunction by regulating proton flux. Proc. Natl. Acad. Sci. U.S.A. 95, 1455–1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Duchen M. R. (2000) Mitochondria and calcium: from cell signalling to cell death. J. Physiol. 529, 57–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Szanda G., Halász E., Spät A. (2010) Protein kinases reduce mitochondrial Ca2+ uptake through an action on the outer mitochondrial membrane. Cell Calcium 48, 168–175 [DOI] [PubMed] [Google Scholar]
- 27. Duchen M. R., Verkhratsky A., Muallem S. (2008) Mitochondria and calcium in health and disease. Cell Calcium 44, 1–5 [DOI] [PubMed] [Google Scholar]
- 28. Elrod J. W., Wong R., Mishra S., Vagnozzi R. J., Sakthievel B., Goonasekera S. A., Karch J., Gabel S., Farber J., Force T., Brown J. H., Murphy E., Molkentin J. D. (2010) Cyclophilin D controls mitochondrial pore-dependent Ca2+ exchange, metabolic flexibility, and propensity for heart failure in mice. J. Clin. Investig. 120, 3680–3687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Marzo I., Brenner C., Zamzami N., Susin S. A., Beutner G., Brdiczka D., Rémy R., Xie Z. H., Reed J. C., Kroemer G. (1998) The permeability transition pore complex: a target for apoptosis regulation by caspases and bcl-2-related proteins. J. Exp. Med. 187, 1261–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Alavian K. N., Li H., Collis L., Bonanni L., Zeng L., Sacchetti S., Lazrove E., Nabili P., Flaherty B., Graham M., Chen Y., Messerli S. M., Mariggio M. A., Rahner C., McNay E., Shore G. C., Smith P. J., Hardwick J. M., Jonas E. A. (2011) Bcl-xL regulates metabolic efficiency of neurons through interaction with the mitochondrial F1FO ATP synthase. Nat. Cell Biol. 13, 1224–1233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chen Y.-B., Aon M. A., Hsu Y.-T., Soane L., Teng X., McCaffery J. M., Cheng W.-C., Qi B., Li H., Alavian K. N., Dayhoff-Brannigan M., Zou S., Pineda F. J., O'Rourke B., Ko Y. H., Pedersen P. L., Kaczmarek L. K., Jonas E. A., Hardwick J. M. (2011) Bcl-xL regulates mitochondrial energetics by stabilizing the inner membrane potential. J. Cell Biol. 195, 263–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rostovtseva T. K., Antonsson B., Suzuki M., Youle R. J., Colombini M., Bezrukov S. M. (2004) Bid, but not Bax, regulates VDAC channels. J. Biol. Chem. 279, 13575–13583 [DOI] [PubMed] [Google Scholar]
- 33. Shi Y., Chen J., Weng C., Chen R., Zheng Y., Chen Q., Tang H. (2003) Identification of the protein-protein contact site and interaction mode of human VDAC1 with Bcl-2 family proteins. Biochem. Biophys. Res. Commun. 305, 989–996 [DOI] [PubMed] [Google Scholar]
- 34. Malia T. J., Wagner G. (2007) NMR structural investigation of the mitochondrial outer membrane protein VDAC and its interaction with antiapoptotic Bcl-xL. Biochemistry 46, 514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Arbel N., Shoshan-Barmatz V. (2010) Voltage-dependent anion channel 1-based peptides interact with Bcl-2 to prevent antiapoptotic activity. J. Biol. Chem. 285, 6053–6062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Berman S. B., Chen Y. B., Qi B., McCaffery J. M., Rucker E. B., 3rd, Goebbels S., Nave K.-A., Arnold B. A., Jonas E. A., Pineda F. J., Hardwick J. M. (2009) Bcl-xL increases mitochondrial fission, fusion, and biomass in neurons. J. Cell Biol. 184, 707–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Szabadkai G., Simoni A. M., Chami M., Wieckowski M. R., Youle R. J., Rizzuto R. (2004) Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol. Cell 16, 59–68 [DOI] [PubMed] [Google Scholar]
- 38. Szabadkai G., Bianchi K., Várnai P., De Stefani D., Wieckowski M. R., Cavagna D., Nagy A. I., Balla T., Rizzuto R. (2006) Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 175, 901–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Decaudin D., Geley S., Hirsch T., Castedo M., Marchetti P., Macho A., Kofler R., Kroemer G. (1997) Bcl-2 and Bcl-XL antagonize the mitochondrial dysfunction preceding nuclear apoptosis induced by chemotherapeutic agents. Cancer Res. 57, 62–67 [PubMed] [Google Scholar]
- 40. Murphy A. N., Bredesen D. E., Cortopassi G., Wang E., Fiskum G. (1996) Bcl-2 potentiates the maximal calcium uptake capacity of neural cell mitochondria. Proc. Natl. Acad. Sci. U.S.A. 93, 9893–9898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kowaltowski A. J., Smaili S. S., Russell J. T., Fiskum G. (2000) Elevation of resting mitochondrial membrane potential of neural cells by cyclosporin A, BAPTA-AM, and bcl-2. Am .J. Physiol. Cell Physiol. 279, C852–C859 [DOI] [PubMed] [Google Scholar]
- 42. Gottlieb E., Vander Heiden M. G., Thompson C. B. (2000) Bcl-xL prevents the initial decrease in mitochondrial membrane potential and subsequent reactive oxygen species production during tumor necrosis factor α-induced apoptosis. Mol. Cell. Biol. 20, 5680–5689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fiebig A. A., Zhu W., Hollerbach C., Leber B., Andrews D. W. (2006) Bcl-XL is qualitatively different from and ten times more effective than Bcl-2 when expressed in a breast cancer cell line. BMC Cancer 6, 213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Csordás G., Madesh M., Antonsson B., Hajnóczky G. (2002) tcBid promotes Ca2+ signal propagation to the mitochondria: control of Ca2+ permeation through the outer mitochondrial membrane. EMBO J. 21, 2198–2206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cheng E. H., Sheiko T. V., Fisher J. K., Craigen W. J., Korsmeyer S. J. (2003) VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301, 513–517 [DOI] [PubMed] [Google Scholar]
- 46. Roy S. S., Ehrlich A. M., Craigen W. J., Hajnóczky G. (2009) VDAC2 is required for truncated BID-induced mitochondrial apoptosis by recruiting BAK to the mitochondria. EMBO Rep. 10, 1341–1347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Shimizu S., Narita M., Tsujimoto Y. (1999) Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399, 483–487 [DOI] [PubMed] [Google Scholar]
- 48. Pavlov E., Grigoriev S. M., Dejean L. M., Zweihorn C. L., Mannella C. A., Kinnally K. W. (2005) The mitochondrial channel VDAC has a cation-selective open state. Biochim. Biophys. Acta 1710, 96–102 [DOI] [PubMed] [Google Scholar]
- 49. Crompton M., Virji S., Ward J. M. (1998) Cyclophilin-D binds strongly to complexes of the voltage-dependent anion channel and the adenine nucleotide translocase to form the permeability transition pore. Eur. J. Biochem. 258, 729–735 [DOI] [PubMed] [Google Scholar]
- 50. Zalk R., Israelson A., Garty E. S., Azoulay-Zohar H., Shoshan-Barmatz V. (2005) Oligomeric states of the voltage-dependent anion channel and cytochrome c release from mitochondria. Biochem. J. 386, 73–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Baines C. P., Kaiser R. A., Sheiko T., Craigen W. J., Molkentin J. D. (2007) Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat. Cell Biol. 9, 550–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cárdenas C., Miller R. A., Smith I., Bui T., Molgó J., Müller M., Vais H., Cheung K.-H., Yang J., Parker I., Thompson C. B., Birnbaum M. J., Hallows K. R., Foskett J. K. (2010) Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 142, 270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Vander Heiden M. G., Chandel N. S., Schumacker P. T., Thompson C. B. (1999) Bcl-xL prevents cell death following growth factor withdrawal by facilitating mitochondrial ATP/ADP exchange. Mol. Cell 3, 159–167 [DOI] [PubMed] [Google Scholar]
- 54. Yi C. H., Pan H., Seebacher J., Jang I.-H., Hyberts S. G., Heffron G. J., Vander Heiden M. G., Yang R., Li F., Locasale J. W., Sharfi H., Zhai B., Rodriguez-Mias R., Luithardt H., Cantley L. C., Daley G. Q., Asara J. M., Gygi S. P., Wagner G., Liu C.-F., Yuan J. (2011) Metabolic regulation of protein N-α-acetylation by Bcl-xL promotes cell survival. Cell 146, 607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]