Background: Dock4, a guanine nucleotide exchange factor for Rac1, is associated with neuropsychiatric diseases.
Results: Dock4 regulates neurite differentiation in neuroblastoma cells and hippocampal neurons.
Conclusion: Dock4 is an important regulator during neural differentiation.
Significance: This study contributes to a better understanding of the molecular and cellular events during neural differentiation and may provide new insights into the molecular pathophysiology of neuropsychiatric diseases.
Keywords: Actin, Dendrite, Guanine Nucleotide Exchange Factor (GEF), Neurite Outgrowth, Neurodifferentiation, Rac1, Rho GTPases, Dedicator of Cytokinesis, ELMO, Retinoic Acid
Abstract
Precise regulation of neurite growth and differentiation determines accurate formation of synaptic connections, whose disruptions are frequently associated with neurological disorders. Dedicator of cytokinesis 4 (Dock4), an atypical guanine nucleotide exchange factor for Rac1, is found to be associated with neuropsychiatric diseases, including autism and schizophrenia. Nonetheless, the neuronal function of Dock4 is only beginning to be understood. Using mouse neuroblastoma (Neuro-2a) cells as a model, this study identifies that Dock4 is critical for neurite differentiation and extension. This regulation is through activation of Rac1 and modulation of the dynamics of actin-enriched protrusions on the neurites. In cultured hippocampal neurons, Dock4 regulates the establishment of the axon-dendrite polarity and the arborization of dendrites, two critical processes during neural differentiation. Importantly, a microdeletion Dock4 mutant linked to autism and dyslexia that lacks the GEF domain leads to defective neurite outgrowth and neuronal polarization. Further analysis reveals that the SH3 domain-mediated interaction of Dock4 is required for its activity toward neurite differentiation, whereas its proline-rich C terminus is not essential for this regulation. Together, our findings reveal an important role of Dock4 for neurite differentiation during early neuronal development.
Introduction
Rho family GTPases, a protein family that regulates actin cytoskeleton dynamics, are crucial for various stages of neuronal development and synaptic plasticity (1). Mutations of Rho GTPases, their regulators, and their downstream signaling molecules have been found in various neurological diseases (2).
The family of dedicator of cytokinesis (Dock),3 or the Dock180-related family, is a protein family that belongs to the atypical guanine nucleotide exchange factors (GEFs) for Rac and/or Cdc42 GTPase (3, 4). In comparison with the typical Dbl family of GEFs that possesses a pleckstrin homology-Dbl homology module, the Dock family instead contains a Dock homology region (DHR) 1-DHR2 module, of which DHR2 is responsible for its GEF activity. To date, 11 members of Dock, namely Dock1–11, have been identified in the mammalian system. Emerging evidence suggests that the Dock family is involved in various processes during neural development (5). For instance, Dock1 (Dock180) regulates axon pathfinding and spine morphogenesis (6, 7), Dock3 is important for axonal growth and survival (8), and Dock7 regulates neuronal polarization and cortical neurogenesis (9, 10). Moreover, members of Dock, including Dock3, Dock8, and Dock9, have been found to be associated with neurodegenerative and neuropsychiatric diseases (11–15). These lines of evidence highlight a pivotal role of the Dock family during normal neuronal development and brain functioning.
It is noteworthy that Dock4 has been found recently to be associated with several neuropsychiatric diseases, including autism, dyslexia, and schizophrenia (16–19). Dock4 was originally identified as a gene disrupted during tumorigenesis (20). Multiple studies in fibroblasts then confirmed that Dock4 is capable of controlling cell migration by transducing several upstream signals, such as Wnt, platelet-derived growth factor, and RhoG, toward activation of Rac1 (21–24). However, the functional roles of Dock4 in the central nervous system are only beginning to be understood (25–27). The detailed molecular mechanism on how Dock4 controls neuronal development and whether the disruption of Dock4 activity is associated with brain dysfunction still remain unclear.
Dock4 contains an N-terminal Src-homology 3 (SH3) domain followed by a common DHR1-DHR2 module and a proline-rich C terminus. The SH3 domain and the C terminus of Dock4 are known to mediate inter- or intramolecular interactions, whereas the DHR1 domain is capable of binding to phospholipids (28–31). It was found that a rare heterozygous microdeletion associated with autism and dyslexia leads to a fusion transcript between the DOCK4 gene and the IMMP2L gene, whose chromosomal locus is located downstream of DOCK4 (18, 19). Intriguingly, this fusion transcript generates a shorter Dock4 protein product that lacks the complete DHR2 domain and the C terminus. This suggests that the GEF activity of Dock4 may be important for normal brain function.
In this study, we identify a crucial role of Dock4 in neurite differentiation. Dock4 regulates neurite growth of Neuro-2a cells through a mechanism that depends on Rac1 activity and actin dynamics. Such regulation requires the SH3 domain and the DHR2 domain but not the C terminus of Dock4. Importantly, we report that the disease-related Dock4 truncated mutant fails to promote neurite outgrowth. We further show that Dock4 is important for both neuronal polarization and dendrite arborization of cultured hippocampal neurons. Collectively, our findings suggest that Dock4 may be important for normal brain wiring through acting on neurite differentiation.
EXPERIMENTAL PROCEDURES
Constructs, Antibodies, and Reagents
Two shRNAs of Dock4 (D4-shRNA1 and D4-shRNA-2), which target two common sequences of mouse and rat Dock4 (5′-GAAGTTGTTCGGTTTCTCT-3′ and 5′-TGGTGATATGCTTGATCTT-3′), and their corresponding scramble shRNAs (D4-scr-1, 5′-GTGCATTGTACTGGTCTTT-3′ and D4-scr-2, 5′-GGTATCTGCGTTATGATTT-3′) were synthesized by Invitrogen and subcloned to the pSUPER vector as described previously (32). Dock4 scramble shRNAs or shRNAs were subcloned to the lentiviral vector pFUGW, which contains a GFP coding sequence separated by an internal ribosome entry site. pFUGW shRNAs were transfected into HEK293T cells together with an HIV-1 packing vector Δ8.9 and a vesicular stomatitis virus glycoprotein (VSVg) envelope plasmid to generate lentiviral particles as described previously (33).
Human Dock4 cDNA (GenBank accession number BC117688) was purchased from Thermo Scientific (Rockford, IL), and the coding sequence of human Dock4 was subcloned into the pcDNA3.0 vector with an N-terminal HA tag. Dock4 mutants, including 945VS (amino acids 1–945 + Val + Ser), ΔSH3 (amino acids 81–1966), ΔC (amino acids 1–1592), SH3 (amino acids 1–161), and SH3-L (amino acids 1–417) were generated by PCR and subcloned into pcDNA3.0 vector with N-terminal HA tags. Rac1 WT and its dominant negative mutant (DN, T17N) were described previously (34). GFP-UtrCH was purchased from Addgene (Cambridge, MA). Mouse ELMO2 cDNA was purchased from Origene Technologies (Rockville, MD).
Primary antibodies against Dock4, ELMO2, and GAPDH were purchased from Abcam (Cambridge, UK); α-tubulin and β-tubulin III were from Sigma; Tau1, MAP2, and NSC23766 were from Millipore (Darmstadt, Germany); Rac1 was from BD Biosciences; HA was from Santa Cruz Biotechnology (Santa Cruz, CA); GFP and rhodamine phalloidin were from Invitrogen; and RA was from Sigma.
Cell Culture and Transfection
Neuro-2a cells (ATCC) were cultured in minimum Eagle's medium (Invitrogen) supplemented with 10% FBS (Invitrogen). For RA-induced differentiation, the culture medium was switched into minimum Eagle's medium supplied with 0.5% FBS in the presence of 20 μm RA. HEK293T cells (ATCC) were cultured in DMEM (Invitrogen) supplemented with 10% FBS. Plasmids were transfected into Neuro-2a or HEK293T cells using Lipofectamine LTX with Plus reagent (Invitrogen).
Primary cortical or hippocampal neurons were prepared and cultured from E18 Sprague-Dawley rat embryos as described previously (35). Transfection of dissociated neurons was performed using Lipofectamine 2000 (Invitrogen). Briefly, 3 μg of D4-scr-1 or D4-shRNA-1 and 1 μg of GFP were cotransfected into 5 × 105 dissociated hippocampal neurons using 4 μl of Lipofectamine 2000. Then, neurons were plated on 18-mm coverslips (0.5 × 105 each) coated with poly-d-lysine (1 mg/ml, Sigma) and fed with neurobasal medium (Invitrogen) supplemented with 2% B27 (Invitrogen). Neuronal morphology was analyzed after 3 days in vitro (DIV). For examination of dendrite morphology, 0.8 × 105 hippocampal neurons were plated on 18-mm coverslips. Lentiviral shRNAs were added to infect the neurons at 3 or 7 DIV, and dendrite morphology was analyzed at 10 or 14 DIV, respectively.
Immunocytochemistry
To visualize neurites in differentiated Neuro-2a cells, cells were fixed in 4% paraformaldehyde (Sigma) for 20–30 min and permeabilized with 0.4% Triton X-100 (Sigma). Immunostaining with mouse anti-β-tubulin III antibody was followed by incubation with Alexa Fluor 546 goat anti-mouse IgG antibody (Invitrogen). Alternatively, F-actin was detected using rhodamine phalloidin. The cells were then mounted with Mowiol (BD Biosciences). Cell morphology was photographed with a Zeiss Axio Imager A2 microscope (Carl Zeiss AG, Oberkochen, Germany). Quantifications of the neurites (≥ 20 μm) and protrusions were made using ImageJ software (National Institutes of Health, Bethesda, MD).
To determine neuronal polarization, 3 DIV hippocampal neurons were fixed with 4% paraformaldehyde and subjected to immunostaining with Tau1 and MAP2 antibodies. Neurites with strong Tau1 signal at proximal ends were counted as axons. Neuron morphology was photographed using a Zeiss LSM 510 confocal microscope (Carl Zeiss AG). Neurite length was measured using ImageJ software.
For analysis of dendrite development, 10 or 14 DIV hippocampal neurons were fixed and subjected to immunostaining with GFP antibody to enhance the GFP signal in infected neurons. Neurons were then mounted with ProLong Gold antifade reagent (Invitrogen). The neurons were photographed with a Zeiss LSM 510 confocal microscope. Sholl analysis and dendritic length quantification were performed using ImageJ software.
Live-cell Imaging
Neuro-2a cells were transfected with GFP-UtrCH together with D4-scr-1 or D4-shRNA-1. 24 h after transfection, cells were treated with RA for an additional 24 h and then placed in an environmentally controlled chamber with 5% CO2 at 37 °C and coupled with a Zeiss LSM 510 confocal microscope. A ×63 oil immersion objective and the 488/561-nm laser lines were used for acquisition of GFP-UtrCH. Images were collected every 20 s for a period of 5–10 min, and the still images of each recording session were analyzed for protrusion dynamics using ImageJ software.
Rac1 Activity Assay
A Rac1 activity assay was performed as described previously (32). Briefly, 1 day after transfection, Neuro-2a cells were treated with RA for 2 days. Cells were lysed with a buffer containing 50 mm Tris (pH 7.2), 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS, 500 mm NaCl, 10 mm MgCl2 and protease inhibitors. Lysates were incubated with agarose beads conjugated with the p21 Rac/Cdc42 binding domain fused to GST (GST-PBD), which specifically binds to GTP-bound Rac1, at 4 °C for 60 min. The beads were washed four times with Tris buffer containing 1% Triton X-100, 150 mm NaCl, 10 mm MgCl2, and protease inhibitors. Bound Rac1-GTP proteins were then resuspended with sample buffer and subjected to Western blot analysis. Signal intensity was quantified by densitometry using ImageJ software.
RESULTS
Expression Pattern of Dock4 in the Brain
To gain insights into the neuronal function of Dock4, we first examined the expression pattern of Dock4 in the brain. Dock4 protein expression in rat whole brain was relatively low in the embryonic stage and was up-regulated in the postnatal stage and adulthood (Fig. 1A). Examination in different brain regions of adult rats revealed that Dock4 was expressed at the highest level in the hippocampus and was also enriched in the cortex and cerebellum (Fig. 1B). Interestingly, although Dock4 expression in the cortex was higher at the embryonic and early postnatal stages (Fig. 1C), Dock4 in the hippocampus was up-regulated during postnatal development (D). We further confirmed that Dock4 expression was relatively low at the early developmental stages of primary hippocampal neurons cultured from E18 rat embryos and was increased robustly starting from 7 DIV (Fig. 1E).
FIGURE 1.
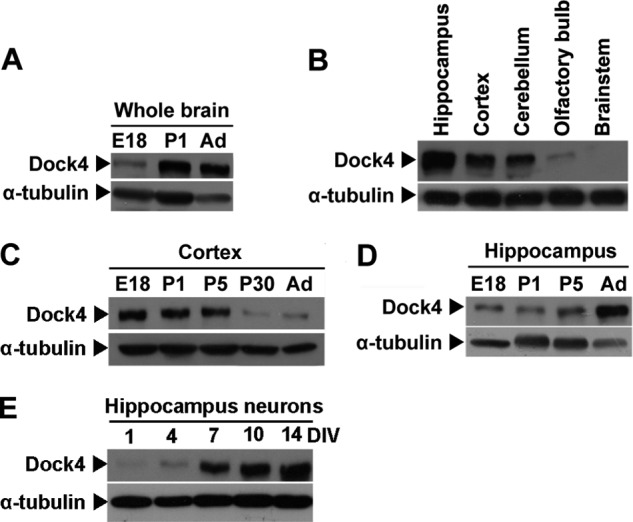
Expression pattern of Dock4 in rat brain. A, lysates from rat whole brain at E18, postnatal day 1 (P1) and adult (Ad) were analyzed by SDS-PAGE and immunoblotting with Dock4 antibody. B, lysates from various rat brain regions, as indicated, were analyzed by SDS-PAGE and immunoblotting with anti-Dock4 antibody. Lysates from rat cortex (C) or hippocampus (D) at different developmental stages (E18-Ad) were analyzed by SDS-PAGE and immunoblotting with Dock4. E, lysates from cultured hippocampal neurons at 1–14 DIV were analyzed by SDS-PAGE and immunoblotting with anti-Dock4 antibody. α-tubulin served as a loading control in A–E.
Dock4 Is Important for Neurite Differentiation of Neuro-2a Cells
To explore the functional roles of Dock4 in neuronal differentiation, we examined whether Dock4 regulates neurite outgrowth of Neuro-2a cells. Neuro-2a cells have been studied widely as a model system for neuronal differentiation. They exhibit a prominent extension of neurites that are positive for neuronal markers, such as microtubule-associated protein 2 (MAP2) or class III β-tubulin (β-tubulin III), upon the treatment of all-trans RA (36). We found that Dock4 protein is highly expressed in both parental and RA-stimulated Neuro-2a cells (Fig. 2A). Two shRNAs (D4-shRNA-1 and D4-shRNA-2), which target two different common sequences of mouse and rat Dock4, and their corresponding scramble shRNAs (D4-scr-1 and D4-scr-2) were constructed. Examination in Neuro-2a cells (Fig. 2B) or rat cortical neurons (C) confirmed that both shRNAs exhibited high potencies to silence endogenous Dock4 expression in either mouse or rat cells.
FIGURE 2.
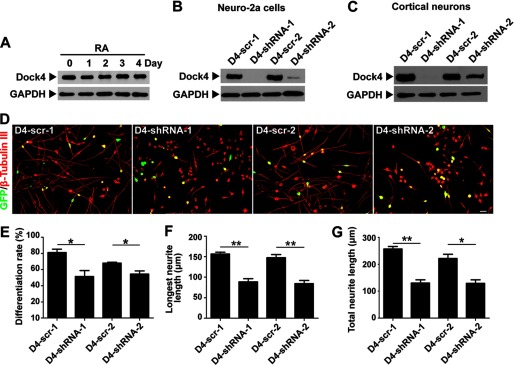
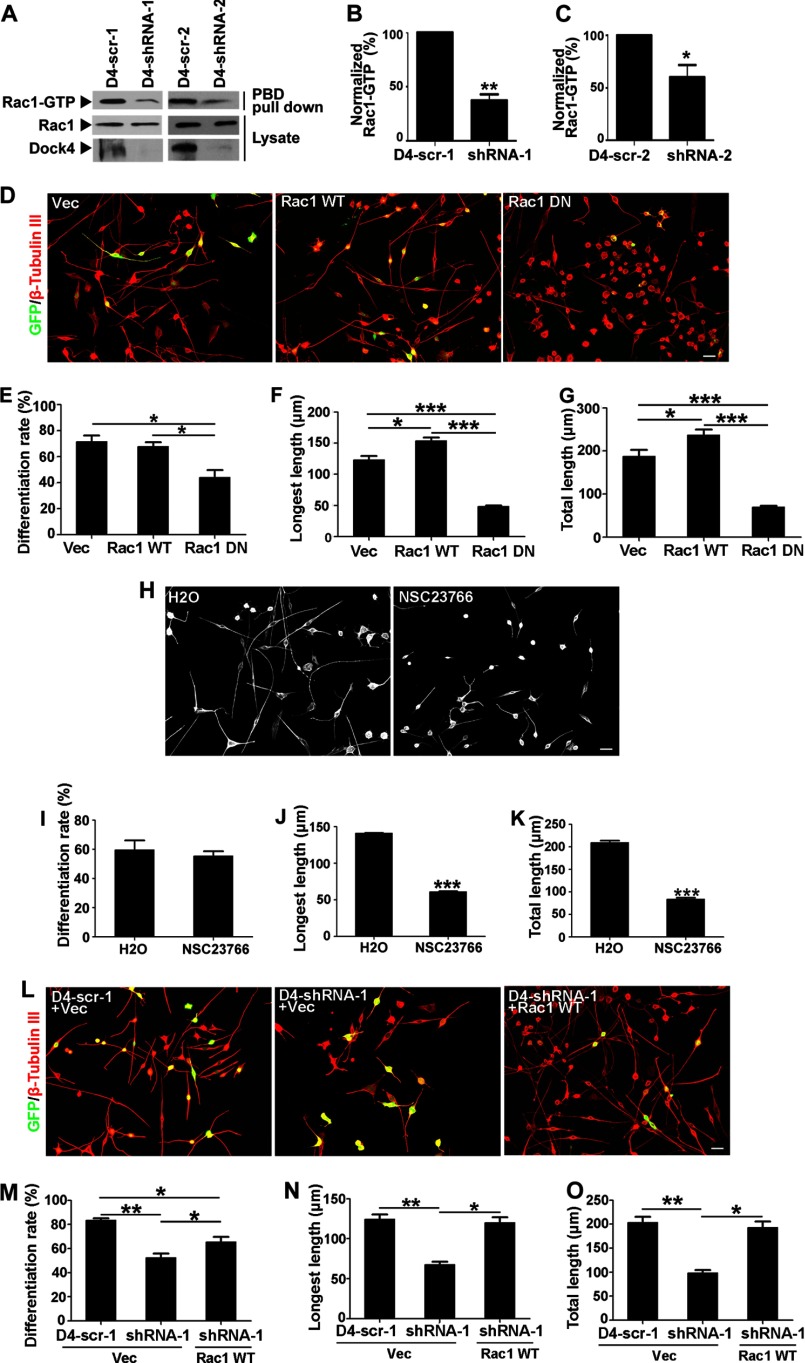
Dock4 is important for RA-induced neurite differentiation of Neuro-2a cells. A, Dock4 protein is highly expressed in both parental and RA-stimulated Neuro-2a cells. Neuro-2a cells were treated with RA (20 μm) for 1–4 days as indicated, and cell lysates were subjected to Western blot analysis using Dock4 antibody. B, Neuro-2a cells were transfected with Dock4 shRNAs (D4-shRNA-1 and D4-shRNA-2) and their scramble shRNAs (D4-scr-1 and D4-scr-2, respectively), and cell lysates were analyzed by immunoblotting with Dock4 antibody. C, rat cortical neurons were infected by lentiviral Dock4 shRNAs at 3 DIV, and cell lysates were collected at 7 DIV. Dock4 expression was detected by Western blot analysis. GAPDH served as a loading control for A–C. D–G, Dock4 is important for RA-induced neurite differentiation. D, Neuro-2a cells were cotransfected with GFP together with D4-scr-1, D4-shRNA-1, D4-scr-2, or D4-shRNA-2, followed by treatment with RA for 2 days. Cells were immunostained using β-tubulin III antibody for visualization of neurites. Scale bar = 50 μm. Cell differentiation rate (% of cells that bear neurites, E), average length of the longest neurite (F), and average length of total neurites (G) were measured. *, p < 0.05; **, p < 0.01, Student's t test. At least 40 cells/group were analyzed in each experiment. n = 3. Error bars depict mean ± S.E.
The RA-induced differentiated Neuro-2a cells exhibited a characteristic bipolar-like shape and normally bear two long neurites extending in opposing directions from the cell body. Notably, suppression of Dock4 by either D4-shRNA-1 or D4-shRNA-2 remarkably inhibited neurite outgrowth induced by RA (Fig. 2D). Although 70–80% of D4-scr-1 or D4-scr-2 transfected Neuro-2a cells extended neurites after treatment of RA for 2 days, only half of the Dock4-suppressed cells bore neurites (Fig. 2E). The average neurite number of those differentiated Dock4 knockdown cells did not change (data not shown). Nonetheless, the length of the longest neurite and the total neurites were both reduced significantly in Dock4 knockdown cells when compared with those expressing scramble shRNAs (Fig. 2, F and G). Together, these findings suggest that Dock4 is essential for RA-induced neurite differentiation and extension of Neuro-2a cells.
Dock4-regulated Neurite Outgrowth Is through Activation of Rac1
As Dock4 is an atypical GEF for Rac1, we investigated whether Dock4 regulates Rac1 activity in Neuro-2a cells. Indeed, knockdown of Dock4 by either D4-shRNA-1 or D4-shRNA-2 substantially attenuated Rac1 activity in RA-treated Neuro-2a cells (Fig. 3, A–C). To examine whether Rac1 activity is important for RA-induced neurite outgrowth, we overexpressed Rac1 WT or its DN mutant in Neuro-2a cells. Interestingly, although elevation of Rac1 activity by overexpressing Rac1 WT promoted growth of neurites, attenuation of Rac1 activity by overexpressing Rac1 DN drastically inhibited differentiation (Fig. 3, D–G). The requirement of Rac1 activity is further confirmed by an inhibition of neurite outgrowth when Rac1 activity was blocked by NSC23766, a Rac-specific inhibitor (Fig. 3, H–K) (37).
FIGURE 3.
Dock4-mediated Rac1 activation is essential for RA-induced neurite differentiation of Neuro-2a cells. A, Dock4 is important for Rac1 activation in Neuro-2a cells. Neuro-2a cells were transfected with D4-scr-1, D4-shRNA-1, D4-scr-2, or D4-shRNA-2, followed by treatment with RA for 2 days. Cell lysates were then subjected to a Rac1 activity assay for detection of GTP-bound Rac1 (Rac1-GTP). PBD, the p21 Rac/Cdc42 binding domain which only binds to Rac1-GTP. B and C, Rac1-GTP levels were quantified and normalized. *, p < 0.05; **, p < 0.01, Student's t test from three independent experiments. D–K, Rac1 is important for RA-induced neurite outgrowth. D, Neuro-2a cells were transfected with plasmids expressing GFP (Vec), GFP-Rac1 wild-type (Rac1 WT), or GFP-Rac1 dominant negative mutant (Rac1 DN) followed by treatment with RA for 2 days. Cells were immunostained using β-tubulin III antibody for visualization of neurites. Cell differentiation rate (E), average length of the longest neurite (F), and average length of total neurites (G) were measured. H, Neuro-2a cells were treated with RA (20 μm) in the presence of NSC23766 (50 μm), a Rac1 inhibitor, or its vehicle H2O for 2 days. Cells were immunostained using β-tubulin III antibody for visualization of neurites. Cell differentiation rate (I), average length of the longest neurite (J), and average length of total neurites (K) were measured. L–O, activation of Rac1 restores neurite differentiation in Dock4-deficient Neuro-2a cells. L, Neuro-2a cells were cotransfected with D4-scr-1 and Vec, D4-shRNA-1 and Vec, or D4-shRNA-1 and Rac1 WT, followed by treatment with RA for 2 days. Cells were immunostained using β-tubulin III antibody for visualization of neurites. Cell differentiation rate (M), average length of the longest neurite (N), and average length of total neurites (O) were measured. Scale bars = 50 μm. For all statistical analyses on differentiation rate or neurite length, at least 40 cells/group were analyzed in each experiment. n = 3. *, p < 0.05; **, p < 0.01; ***, p < 0.001; Student's t test. Error bars depict mean ± S.E.
We then investigated whether restoration of Rac1 activity rescues neurite outgrowth in Dock4-deficient cells. Indeed, overexpression of Rac1 WT in Dock4-deficient cells substantially restored normal neurite outgrowth (Fig. 3, L–O). As Dock4 is the only Dock that has also been shown to activate Rap1, another small GTPase of the Ras family (20), we examined whether Rap1 is important for Dock4 mediated neurite differentiation. However, Rap1 overexpression failed to rescue the defect in Dock4-deficient cells (data not shown). Together, these findings suggest that activation of Rac1 is a critical downstream molecular event of Dock4-regulated neurite outgrowth.
Dock4 Regulates F-actin Organization of Neurites
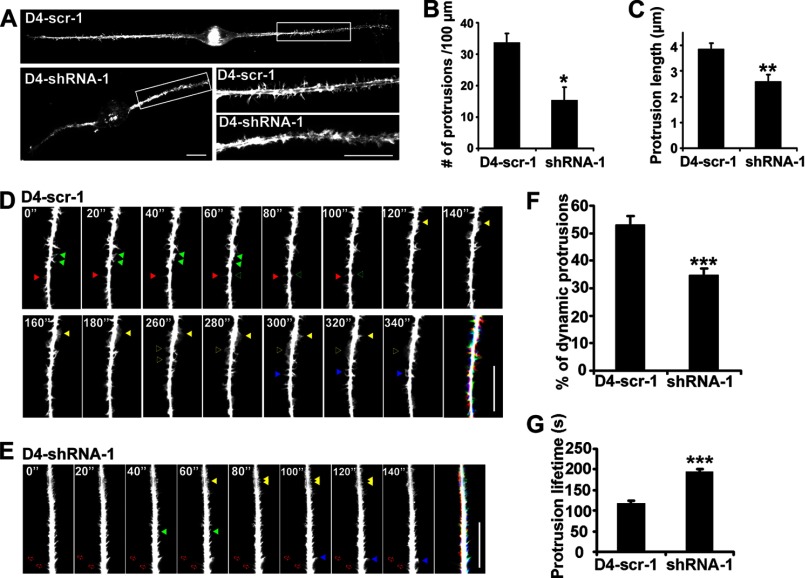
Given that Rac1 is a critical actin organizer for neuronal morphogenesis, we then examined whether Dock4-regulated neurite outgrowth is dependent on a reorganization of the actin cytoskeleton. Interestingly, extensive short F-actin-enriched protrusions extended from the neurites of control Neuro-2a cells when stimulated by RA (Fig. 4A). By contrast, Dock4 knockdown neurites extended significantly fewer protrusions (Fig. 4, A and B). Moreover, these Dock4-deficient protrusions were significantly shorter than D4-scr-1-expressed ones (Fig. 4C). To access the dynamics of these protrusions, we took advantage of an actin probe, the calponin homology domain of utrophin fused to GFP (GFP-UtrCH), to visualize the distribution and motility of F-actin in live cells. Neuro-2a cells were transfected with GFP-UtrCH together with D4-scr-1 or D4-shRNA-1, and were exposed to RA for 1 day. Time-lapse images were taken to monitor the protrusions. Notably, the control protrusions exhibited rapid remodeling, such as elongation and retraction, elimination, initiation, and turning (Fig. 4D and supplemental Movie 1). Moreover, transient sheet-like protrusions (lamellipodia) were observed frequently, which rapidly transformed from or into spike-like protrusions or fused with other lamellipodia (Fig. 4D and supplemental Movie 1). By contrast, much fewer morphological changes were observed in Dock4-deficient protrusions, some of which even remained static (Fig. 4E and supplemental Movie 2). Quantification analysis confirmed that Dock4-deficient protrusions were less dynamic (Fig. 4G) and had a longer lifetime (H). Consistent with the observations in fixed cells, Dock4-deficient protrusions were much shorter than control ones (Fig. 4, D and E). These findings uncover an essential role of Dock4 in regulating the actin reorganization of neurite protrusions.
FIGURE 4.
Dock4 regulates actin dynamics of Neuro-2a cells. A–C, Dock4 knockdown leads to reduced extension of actin-rich protrusions. A, Neuro-2a cells were cotransfected with GFP together with D4-scr-1 or D4-shRNA-1, followed by treatment with RA for 2 days. F-actin was labeled with rhodamine phalloidin. The insets are shown at higher magnifications. Scale bars = 20 μm. Average number of protrusions/100 μm of neurites (B) and average length of protrusions (C) were quantified. *, p < 0.05; **, p < 0.01; Student's t test. D–F, Dock4 regulates motility of the actin-rich protrusions. D and E, Neuro-2a cells were cotransfected with the actin probe GFP-UtrCH together with D4-scr-1 (D) or D4-shRNA-1 (E), followed by treatment with RA for 1 day. Representative time-lapse images of GFP-UtrCH signals in live neurites were taken at 20-s intervals. A merged image from images taken at 0, 2, and 4 min (red, green, and blue, respectively; pseudo-colors) is shown for each group. Filled red arrowheads, a protrusion undergoing extending and retracting; filled green arrowheads, a protrusion being eliminated; empty green arrowheads, a newly initiated protrusion; filled yellow arrowheads, protrusions that were interchanging between filopodia-like and lamellipodia-like morphologies; empty yellow arrowheads, two lamellipodia-like protrusions fused into one; filled blue arrowheads, a protrusion changing orientation; empty red arrows, static protrusions. Scale bars = 20 μm. F, percentage of dynamic protrusions. Protrusions that disappeared, were newly initiated, or exhibited altered positions were counted as dynamic protrusions. Images taken at 0, 1, 2, 3, 4, and 5 min of each cell were analyzed. G, lifetime of the protrusions. At least six protrusions randomly selected from each neurite were analyzed. ***, p < 0.001; Student's t test. 15 cells from two independent experiments were analyzed in each group. Error bars depict mean ± S.E.
The SH3 Domain and DHR2 Domain Are Important for Dock4-regulated Neurite Outgrowth
It was found that a rare heterozygous microdeletion associated with autism and dyslexia leads to a fusion transcript between the DOCK4 gene and the IMMP2L gene (18, 19). Intriguingly, this fusion transcript generates a novel protein product that contains only the N-terminal half of Dock4 (amino acids 1–945), followed by two novel amino acids, Val and Ser, and a premature stop codon. Moreover, patients with this fusion transcript express lower levels of the normal DOCK4 transcript. Given that this protein product lacks the whole DHR2 domain and the C terminus, it is tempting to hypothesize that the GEF activity of Dock4 is essential for normal brain function.
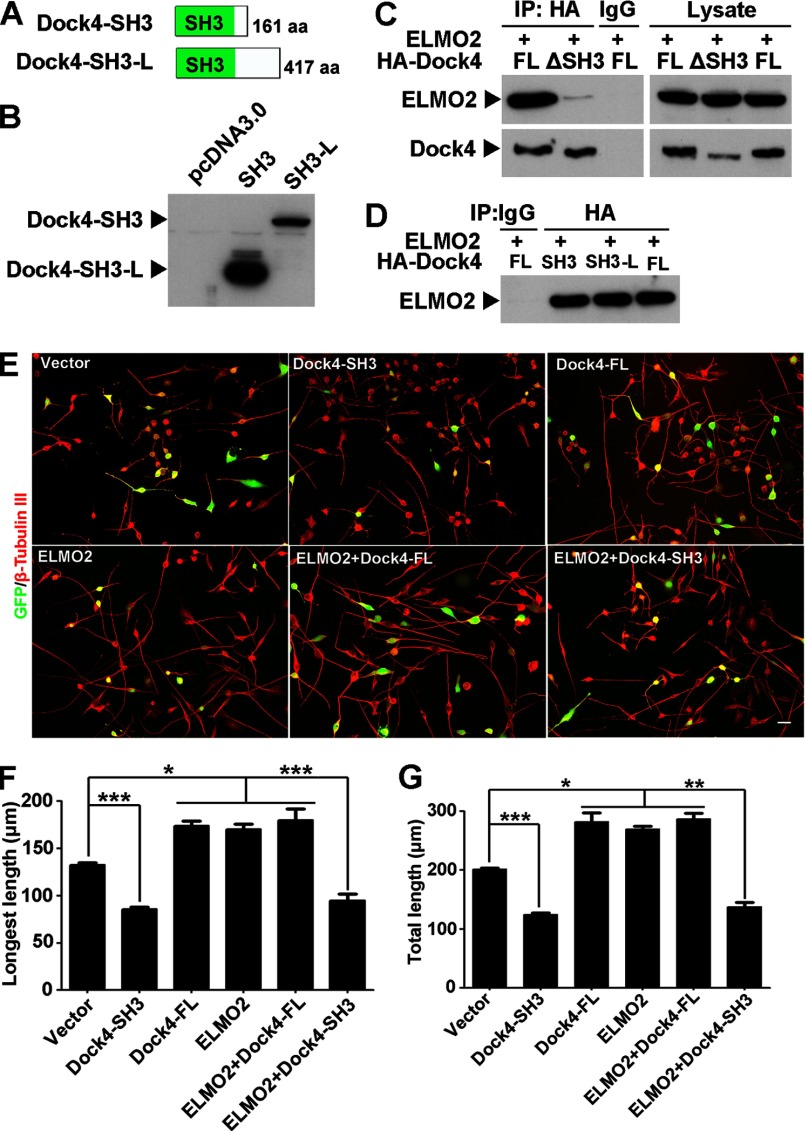
To study whether the disease-linked shorter Dock4 product (945VS) exhibits a functional deficit in neurite differentiation, we constructed plasmids expressing full-length (FL) human Dock4 or 945VS. Moreover, to dissect the roles of different domains of Dock4, we also constructed two mutants of Dock4, one with the SH3 domain deleted (ΔSH3) and the other with the proline-rich C terminus deleted (ΔC, Fig. 5A). Interestingly, Dock4-FL and ΔC had a similar ability to promote Rac1 activation, whereas ΔSH3 had no effect on Rac1 activity and 945VS exhibited a strong inhibition on Rac1 activity (Fig. 5, B and C). Consistent with the ability toward Rac1 activation, Dock4-FL and ΔC significantly promoted neurite growth by ∼30% when compared with the vector control, which is indicative of a nonessential role of the Dock4 C terminus in this process (Fig. 5, D–G). By contrast, either Dock4-945VS or ΔSH3 failed to enhance neurite length (Fig. 5, D–G). Thus, both the SH3 domain and the DHR2 domain, but not the C terminus, are required for Dock4 GEF activity and neurite outgrowth.
FIGURE 5.
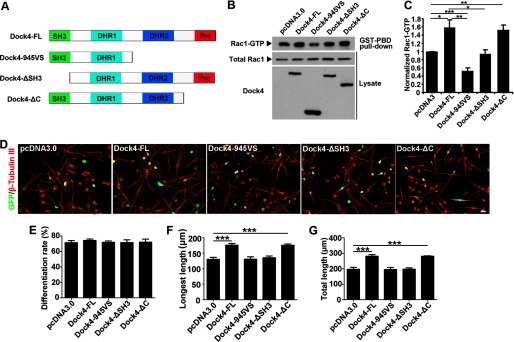
The SH3 domain and DHR2 domain are important for Dock4-regulated neurite outgrowth of Neuro-2a cells. A, schematics showing domains and deletion mutants of Dock4. Pro, proline-rich region; 945VS, the mutant contains amino acids 1–945 followed by a Val and a Ser residue; ΔSH3 or ΔC, the SH3 domain or the C terminus is deleted. B, Dock4 FL or various deletion mutants were expressed in HEK293T cells, and the levels of Rac1-GTP (Rac1 activity) were analyzed. C, Rac1-GTP levels were quantified and normalized. *, p < 0.05; **, p < 0.01; ***p < 0.001; Student's t test from three independent experiments. D, Neuro-2a cells were cotransfected with GFP together with Dock4-FL and its deletion mutants, followed by treatment with RA for 2 days. Scale bar = 50 μm. Cells were immunostained using β-tubulin III antibody for visualization of neurites. Cell differentiation rate (E), average length of the longest neurite (F), and average length of total neurites (G) were measured. ***, p < 0.001, Student's t test. At least 40 cells/group were analyzed in each experiment. n = 3. Error bars depict mean ± S.E.
To further verify the role of the Dock4 SH3 domain, we constructed two N-terminal fragments of Dock4 that contained the SH3 domain, Dock4-SH3 (amino acids 1–161) and SH3-L (amino acids 1–417, Fig. 6, A and B). Engulfment and cell motility (ELMO), a family of adaptor proteins, has been shown to couple to the Dock family and promotes the GEF activity of Docks in diverse systems. Similar to Dock180, Dock4 interacts with ELMO2 through its SH3 domain (Fig. 6C) (31). Dock4-SH3 and SH3-L showed a similar binding ability to ELMO2 as Dock4-FL, suggesting that these fragments act as competitive binding partners of Dock4 (Fig. 6D). Indeed, expression of Dock4-SH3 in Neuro-2a cells exhibited an inhibitory effect not only on normal neurite outgrowth but also on ELMO2-promoted neurite outgrowth (Fig. 6, E–G). This dominant negative effect of Dock4-SH3 provides further evidence that the SH3 domain, probably through interaction with ELMO2, is critical for Dock4-mediated neurite outgrowth.
FIGURE 6.
The SH3-mediated interaction is important for Dock4-regulated neurite outgrowth. A, two N-terminal fragments of Dock4, SH3 (amino acids (aa) 1–161) and SH3-L (amino acids 1–417), were constructed as depicted. B, the expression of Dock4-SH3 and SH3-L were confirmed in HEK293T cells by overexpression. C, ELMO2 and HA-tagged Dock4-FL or ΔSH3 were cotransfected into HEK293T cells. The cell lysates were immunoprecipitated (IP) with HA antibody, followed by immunoblotting using ELMO2 antibody. D, ELMO2 and HA-tagged Dock4-SH3 and SH3-L were cotransfected into HEK293T cells. The cell lysates were immunoprecipitated with HA antibody, followed by immunoblotting using ELMO2 antibody. E, Neuro-2a cells were cotransfected with GFP and various plasmids as indicated, followed by treatment with RA for 2 days. Cells were immunostained using β-tubulin III antibody for visualization of neurites. Scale bar = 50 μm. Average length of the longest neurite (F) and average length of total neurites (G) were measured. *, p < 0.05; **, p < 0.01; ***, p < 0.001; Student's t test. At least 40 cells/group were analyzed in each experiment. n = 3. Error bars depict mean ± S.E.
Dock4 Regulates Neurite Outgrowth and Axon-Dendrite Polarization of Hippocampal Neurons
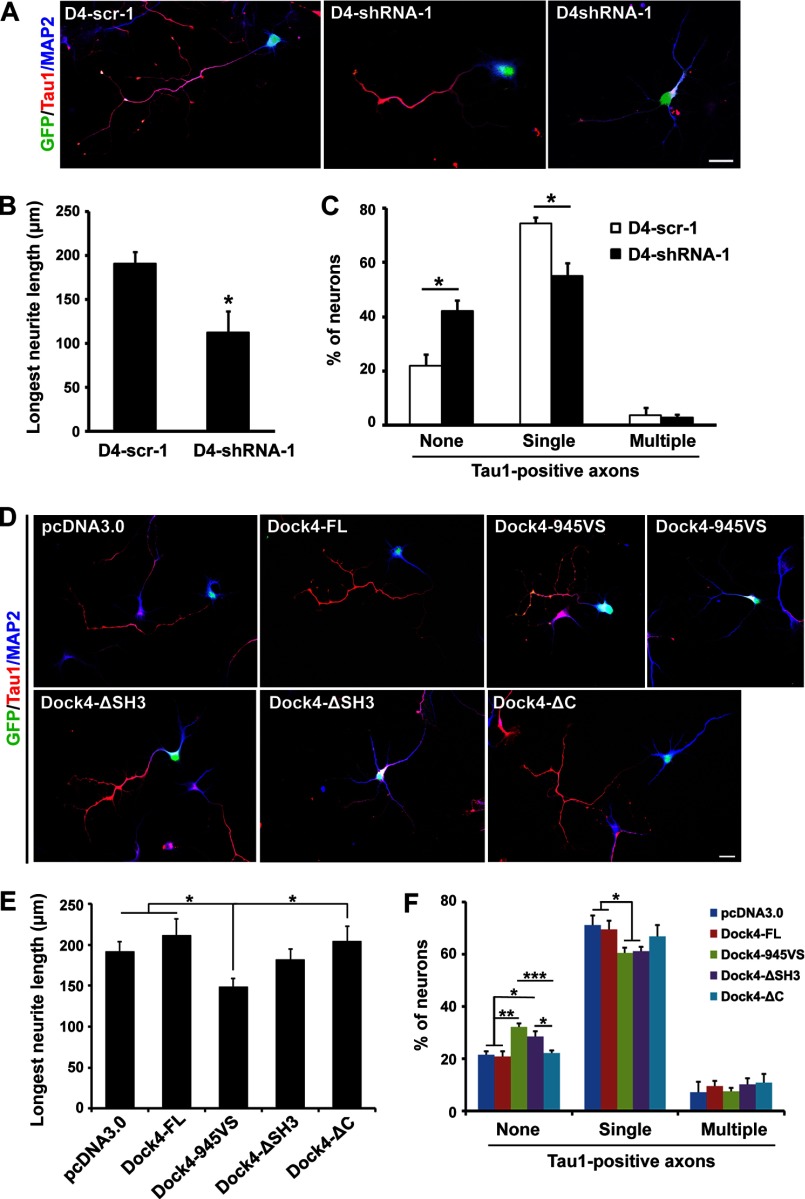
During the early development of cultured hippocampal neurons, numerous short neurites first extended from the cell bodies. One of these neurites is later differentiated into an axon, whereas all the other neurites are differentiated into dendrites (38). Establishment of such axon-dendrite polarity is a key determinant of accurate wiring of the neuronal network. To investigate whether Dock4 is important for neurite differentiation and neuronal polarization of neurons, we introduced D4-scr-1 or D4-shRNA-1 into dissociated hippocampal neurons before plating and examined neuronal morphology at 3 DIV (Fig. 7A). Intriguingly, knockdown of Dock4 led to significantly reduced neurite length and a decreased percentage of neurons with single Tau1-positive axons, suggesting an important role of Dock4 in neurite growth and polarity establishment (Fig. 7, B and C). However, Tau1 staining was grossly normal when Dock4 was silenced after polarity had been established, and the protein expression of Tau1 was not altered by Dock4 knockdown (data not shown).
FIGURE 7.
Dock4 regulates neurite growth and neuronal polarization of hippocampal neurons. A, dissociated E18 hippocampal neurons were cotransfected with GFP and D4-scr-1 or D4-shRNA-1 and cultured for 3 days. Axon-like or dendrite-like processes were immunostained with Tau1 (red) or MAP2 (blue) antibodies, respectively. B, knockdown of Dock4 leads to reduced neurite length. *, p < 0.05; Student's t test. C, knockdown of Dock4 impairs the establishment of neuronal polarity. The percentages of neurons with no, single, or multiple (≥ 2) Tau1-positive axons were quantified. *, p < 0.05; Student's t test. D, Dock4 FL or various deletion mutants together with GFP were transfected into dissociated E18 hippocampal neurons and immunostaining against Tau1 and MAP2 was performed at 3 DIV. E, the average length of the longest neurite was quantified. *, p < 0.05; Student's t test. F, the percentages of neurons with no, single, or multiple Tau1-positive axons were quantified. *p < 0.05; **p < 0.01; ***p < 0.001; Student's t test. For quantifications of the longest neurite length, at least 20 neurons/group were measured in each experiment, n = 4. For quantifications of neuronal polarity, 40–80 neurons/group were analyzed in each experiment, n = 4. Scale bars = 20 μm. Error bars depict mean ± S.E.
To determine the roles of different domains of Dock4 in neurite growth and polarization, Dock4-FL and its mutants were overexpressed in hippocampal neurons (Fig. 7D). Consistent with what was observed in Neuro-2a cells, the disease-linked mutant Dock4-945VS markedly reduced the neurite length and the percentage of neurons with single axons, suggesting that GEF activity is essential for neurite outgrowth and neuronal polarization (Fig. 7, E and F). Interestingly, although the neurite length in Dock4-ΔSH3-expressing neurons was not shortened significantly, an increased percentage of these neurons failed to extend single axons (Fig. 7, E and F). Thus, both the GEF activity and the SH3 domain of Dock4 are important for neuronal polarization during early development of hippocampal neurons.
Dock4 Is Important for Dendrite Arborization of Hippocampal Neurons
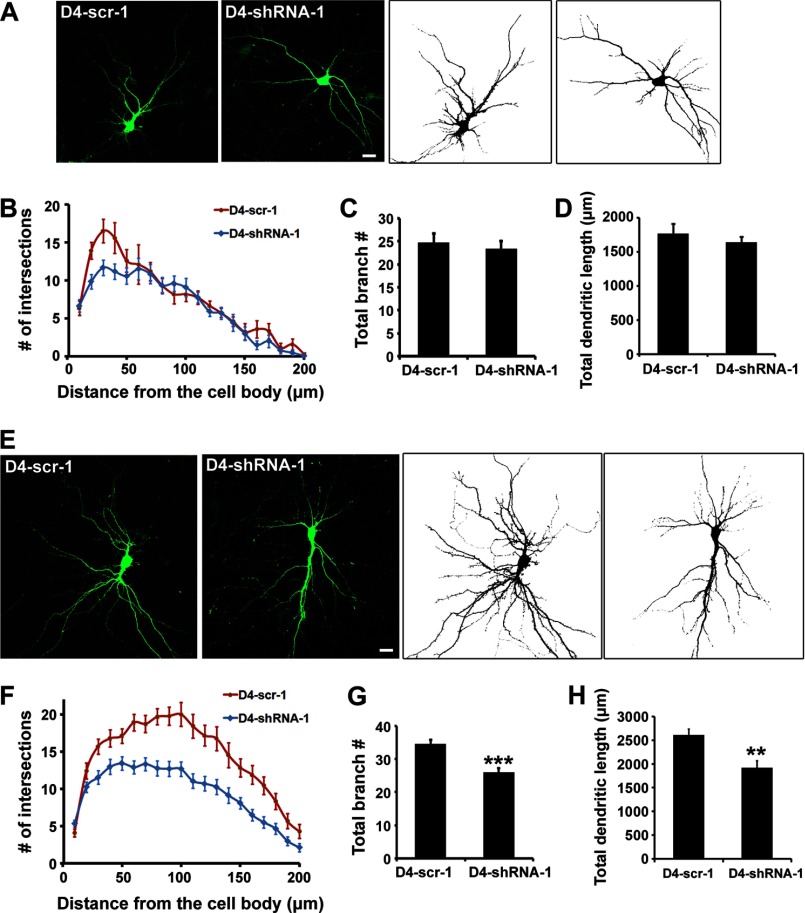
A previous study reported that Dock4 is involved in dendrite development (25). To verify the specific stages upon which Dock4 acts during dendrite development, we acutely silenced Dock4 expression by lentiviral Dock4 shRNA at two stages: 3 DIV, when minor neurites start to grow into dendrites, or 7 DIV, when dendrite arborization occurs extensively (Fig. 8, A and E). Dendrite morphology of the infected neurons was then examined at 10 DIV or 14 DIV, respectively. Interestingly, the effect of Dock4 on dendritic complexity was distinct at these two stages. At 10 DIV, the dendritic intersections at 20–40 μm from the cell body were fewer in Dock4-deficient neurons than in control ones, as assessed by Sholl analysis (Fig. 8B). Despite this abnormality, the intersection numbers at further distances from the cell body, the total branch points, and the total dendritic length were all similar in control or Dock4 knockdown neurons (Fig. 8, B–D). At 14 DIV, however, the intersection numbers at 30–200 μm from the cell body in Dock4-deficient neurons were all much smaller when compared with control ones, which is indicative of a globally reduced dendritic complexity by Dock4 knockdown (Fig. 8F). Moreover, the total branch points and the total dendritic length were also decreased significantly in Dock4-deficient neurons (Fig. 8, G and H). These findings collectively suggest that Dock4 may not be critical for initial dendrite elongation but plays more important roles on dendritic branching and arborization.
FIGURE 8.
Dock4 is important for dendrite arborization of hippocampal neurons. A, hippocampal neurons were infected with lentiviral scramble shRNA or shRNA of Dock4 (D4-scr-1 or D4-shRNA-1) at 3 DIV, and dendrite morphology was examined at 10 DIV. B, numbers of dendritic intersections at 0–200 μm from the cell body were accessed by Sholl analysis. Total dendritic branch number (C) and total dendrite length (D) were quantified. E, hippocampal neurons were infected with D4-scr-1 or D4-shRNA-1 lentivirus at 7 DIV, and dendrite morphology was examined at 14 DIV. F, Sholl analysis of neurons. Total dendritic branch number (G) and total dendrite length (H) were quantified. **, p < 0.01; ***, p < 0.001; Student's t test. Scale bars = 20 μm. 30 neurons from three independent experiments in each group were measured. Error bars depict mean ± S.E.
DISCUSSION
This study identifies a crucial role of the atypical RacGEF Dock4 in neurite differentiation. Dock4 regulates neurite growth of Neuro-2a cells through a mechanism that depends on Rac1 activity and actin dynamics. Such regulation requires the SH3 domain and DHR2 domain but not the proline-rich C terminus. We further show that Dock4 is important for both neuronal polarization and dendrite arborization of cultured hippocampal neurons. Importantly, the disease-related Dock4 truncated mutant (945VS) fails to promote neurite outgrowth and impairs neuronal polarization. Because neurite differentiation and dendrite development are critical early developmental events for accurate formation of neural circuits, our findings suggest that Dock4 may be important for normal brain wiring through acting on early neuronal development.
We showed that numerous short, actin-rich protrusions extended from the neurites of Neuro-2a cells after exposure to RA. These protrusions exhibited various morphologies and were highly dynamic. Interestingly, Dock4 silencing led to a remarkable reduction in protrusion number and length, which was associated with decreased protrusion dynamics. Thus, Dock4 plays important roles in regulating actin organization of the neurites. It has been suggested that RA-induced neurites in Neuro-2a cells exhibit several properties that are similar to dendrites, such as immunochemical positivity for MAP2 and negativity for neurofilament-H (NF-H) and synaptic vesicle 2 (SV2) (39). Indeed, we identify that Dock4 is important for dendrite development of the hippocampal neurons. Specifically, Dock4 is not required for the initial dendrite growth but is essential for the subsequent dendrite arborization. Because normal Rac1 activity and actin cytoskeleton are critical for dendrite branching, the Dock4-dependent dendrite regulation may be through a similar modulation of Rac1 and actin dynamics as in Neuro-2a cells.
Inhibition of Rac1 activity by Rac1-DN or NSC23766 resulted in a more dramatic inhibitory effect on neurite elongation when compared with Dock4 knockdown (Fig. 3). This suggests a possible involvement of other Rac GEFs in RA-induced neurite growth. Indeed, interference of Dock3 and Dock7, two Docks that play important roles in neuronal polarization and axon formation, also led to impairment of neurite growth (data not shown). Thus, Dock4 and these two Docks may act in concert to control RA-induced neurite growth. Interestingly, given that the Rac1 inhibitor did not affect the differentiation rate and that Rac1 activation fully restores neurite length but not the differentiation rate in Dock4 knockdown cells, the differentiation may be regulated by a more complex way than neurite elongation. Indeed, a number of downstream molecules other than Rac1 have been identified as Dock4 effector proteins. A recent finding suggests that the actin-binding protein cortactin interacts with Dock4 and participates in dendritic spine morphogenesis (27). Moreover, Dock3 controls axonal outgrowth through a dual modulation, Rac1 and Wiskott-Aldrich syndrome protein family verprolin-homologous protein (WAVE)-dependent actin reorganization, and GSK3β-mediated microtubule assembly (40, 41). Dock7-mediated neuronal polarization is through inactivation of the microtubule destabilizing protein stathmin/Op18 by Rac1 (10). Thus, it will be of interest to explore whether Dock4-regulated neurite differentiation is through cortactin or similar mechanisms as those mediated by Dock3 or Dock7.
During neuronal development, GEFs for Rho GTPases normally couple to membrane receptors or signaling molecules to transduce extracellular stimuli toward activation of Rho GTPases (1). This study shows that the SH3 domain of Dock4 is important for its GEF activity and RA-induced neurite outgrowth. ELMO2 is a possible SH3-interacting protein that promotes Dock4-mediated neurite growth. A detailed analysis on how SH3-ELMO2 interaction is regulated by RA and how ELMO2 modulates Dock4 activity awaits further investigation. Moreover, it has been shown that activation of the PI3K (3, 4, 5) is involved in RA-induced neurite outgrowth in neuroblastoma SH-SY5Y cells (42). Given that the DHR1 domain of Dock4 is capable of binding to phosphatidylinositol trisphosphate (3, 4, 5), a lipid product of PI3K (30), it is possible that Dock4 is recruited and activated by the increased production of phosphatidylinositol trisphosphate resulting from RA-dependent PI3K activation. On the other hand, Dock4 is involved in signaling pathways mediated by several extracellular or upstream factors, including Wnts and RhoG, which are important factors for neuronal development (21, 23, 43). Thus, it will be important to examine whether transcription or activity of these molecules are regulated by RA.
The association of Dock4 with several neuropsychiatric diseases suggests that Dock4 is a critical regulator during brain development. This study reveals a role of Dock4 in neurite differentiation and dendrite arborization. Whether Dock4 is involved in other neuronal function awaits further investigation. A recent study revealed a role of Dock4 in dendritic spine morphogenesis of hippocampal neurons (27). Interestingly, Dock4 expression during development was regulated differentially in the cortex and hippocampus (Fig. 1, C and D). Thus, Dock4 may exert diverse functional roles in multiple neuronal developmental stages, such as neuronal migration, synapse formation, and plasticity. Notably, emerging evidence reveals that RA signaling is pivotal in both neural differentiation and synaptic homeostasis, and RA deficiency has been suggested to be linked with various neuropsychiatric and neurodegenerative diseases (44). Thus, the findings in this study may uncover Dock4 as a mediator of RA signaling during normal or diseased brain development.
Supplementary Material
Acknowledgments
We are grateful to Dr. J. Xia (Hong Kong University of Science and Technology) and Dr. J. Xu (Zhejiang University) for providing the pFUGW vector, HIV-1 packing vector Δ8.9, and the VSVg envelope plasmid.
This work was supported by National Natural Science Foundation of China for Young Scholars Grant 81101015; by Research Fund for the Doctoral Program of Higher Education of China Grant 20110001120103; by Fundamental Research Funds for the Central Universities of China Grant 21612205; by Bureau of Science and Information Technology of Guangzhou Municipality for Young Scholars Grant 2011J2200048; by Science, Industry, Trade and Information Technology Commission of Shenzhen Municipality for Distinguished Young Scholars Grant JC201005260217A; by Program of Introducing Talents of Discipline to Universities of China (111 Project) Grant B13038; and by National Program on Key Basic Research Project (973 Program) Grant 2013CB530900.

This article contains supplemental Movies 1 and 2.
- Dock
- dedicator of cytokinesis
- GEF
- guanine nucleotide exchange factor
- DHR
- Dock homology region
- DN
- dominant negative
- RA
- retinoic acid
- DIV
- day(s) in vitro
- E18
- embryonic day 18
- FL
- full-length
- ELMO
- engulfment and cell motility
- UtrCH
- the calponin homology domain of utrophin.
REFERENCES
- 1. Govek E. E., Newey S. E., Van Aelst L. (2005) The role of the Rho GTPases in neuronal development. Genes Dev. 19, 1–49 [DOI] [PubMed] [Google Scholar]
- 2. Nadif Kasri N., Van Aelst L. (2008) Rho-linked genes and neurological disorders. Pflugers Arch. 455, 787–797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Côté J. F., Vuori K. (2002) Identification of an evolutionarily conserved superfamily of DOCK180-related proteins with guanine nucleotide exchange activity. J. Cell Sci. 115, 4901–4913 [DOI] [PubMed] [Google Scholar]
- 4. Meller N., Merlot S., Guda C. (2005) CZH proteins. A new family of Rho-GEFs. J. Cell Sci. 118, 4937–4946 [DOI] [PubMed] [Google Scholar]
- 5. Miyamoto Y., Yamauchi J. (2010) Cellular signaling of Dock family proteins in neural function. Cell. Signal. 22, 175–182 [DOI] [PubMed] [Google Scholar]
- 6. Li X., Gao X., Liu G., Xiong W., Wu J., Rao Y. (2008) Netrin signal transduction and the guanine nucleotide exchange factor DOCK180 in attractive signaling. Nat. Neurosci. 11, 28–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kim J. Y., Oh M. H., Bernard L. P., Macara I. G., Zhang H. (2011) The RhoG/ELMO1/Dock180 signaling module is required for spine morphogenesis in hippocampal neurons. J. Biol. Chem. 286, 37615–37624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Chen Q., Peto C. A., Shelton G. D., Mizisin A., Sawchenko P. E., Schubert D. (2009) Loss of modifier of cell adhesion reveals a pathway leading to axonal degeneration. J. Neurosci. 29, 118–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang Y. T., Wang C. L., Van Aelst L. (2012) DOCK7 interacts with TACC3 to regulate interkinetic nuclear migration and cortical neurogenesis. Nat. Neurosci. 15, 1201–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Watabe-Uchida M., John K. A., Janas J. A., Newey S. E., Van Aelst L. (2006) The Rac activator DOCK7 regulates neuronal polarity through local phosphorylation of stathmin/Op18. Neuron 51, 727–739 [DOI] [PubMed] [Google Scholar]
- 11. de Silva M. G., Elliott K., Dahl H. H., Fitzpatrick E., Wilcox S., Delatycki M., Williamson R., Efron D., Lynch M., Forrest S. (2003) Disruption of a novel member of a sodium/hydrogen exchanger family and DOCK3 is associated with an attention deficit hyperactivity disorder-like phenotype. J. Med. Genet. 40, 733–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen Q., Yoshida H., Schubert D., Maher P., Mallory M., Masliah E. (2001) Presenilin binding protein is associated with neurofibrillary alterations in Alzheimer's disease and stimulates tau phosphorylation. Am. J. Pathol. 159, 1597–1602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Griggs B. L., Ladd S., Saul R. A., DuPont B. R., Srivastava A. K. (2008) Dedicator of cytokinesis 8 is disrupted in two patients with mental retardation and developmental disabilities. Genomics 91, 195–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Vinci G., Chantot-Bastaraud S., El Houate B., Lortat-Jacob S., Brauner R., McElreavey K. (2007) Association of deletion 9p, 46,XY gonadal dysgenesis and autistic spectrum disorder. Mol. Hum. Reprod. 13, 685–689 [DOI] [PubMed] [Google Scholar]
- 15. Detera-Wadleigh S. D., Liu C. Y., Maheshwari M., Cardona I., Corona W., Akula N., Steele C. J., Badner J. A., Kundu M., Kassem L., Potash J. B., Gibbs R., Gershon E. S., McMahon F. J. (2007) Sequence variation in DOCK9 and heterogeneity in bipolar disorder. Psychiatr. Genet. 17, 274–286 [DOI] [PubMed] [Google Scholar]
- 16. Alkelai A., Lupoli S., Greenbaum L., Kohn Y., Kanyas-Sarner K., Ben-Asher E., Lancet D., Macciardi F., Lerer B. (2012) DOCK4 and CEACAM21 as novel schizophrenia candidate genes in the Jewish population. Int. J. Neuropsychopharmacol. 15, 459–469 [DOI] [PubMed] [Google Scholar]
- 17. Poelmans G., Buitelaar J. K., Pauls D. L., Franke B. (2011) A theoretical molecular network for dyslexia. Integrating available genetic findings. Mol. Psychiatry 16, 365–382 [DOI] [PubMed] [Google Scholar]
- 18. Pagnamenta A. T., Bacchelli E., de Jonge M. V., Mirza G., Scerri T. S., Minopoli F., Chiocchetti A., Ludwig K. U., Hoffmann P., Paracchini S., Lowy E., Harold D. H., Chapman J. A., Klauck S. M., Poustka F., Houben R. H., Staal W. G., Ophoff R. A., O'Donovan M. C., Williams J., Nöthen M. M., Schulte-Körne G., Deloukas P., Ragoussis J., Bailey A. J., Maestrini E., Monaco A. P., and International Molecular Genetic Study of Autism Consortium (2010) Characterization of a family with rare deletions in CNTNAP5 and DOCK4 suggests novel risk loci for autism and dyslexia. Biol. Psychiatry 68, 320–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Maestrini E., Pagnamenta A. T., Lamb J. A., Bacchelli E., Sykes N. H., Sousa I., Toma C., Barnby G., Butler H., Winchester L., Scerri T. S., Minopoli F., Reichert J., Cai G., Buxbaum J. D., Korvatska O., Schellenberg G. D., Dawson G., de Bildt A., Minderaa R. B., Mulder E. J., Morris A. P., Bailey A. J., Monaco A. P. (2010) High-density SNP association study and copy number variation analysis of the AUTS1 and AUTS5 loci implicate the IMMP2L-DOCK4 gene region in autism susceptibility. Mol. Psychiatry 15, 954–968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yajnik V., Paulding C., Sordella R., McClatchey A. I., Saito M., Wahrer D. C., Reynolds P., Bell D. W., Lake R., van den Heuvel S., Settleman J., Haber D. A. (2003) DOCK4, a GTPase activator, is disrupted during tumorigenesis. Cell 112, 673–684 [DOI] [PubMed] [Google Scholar]
- 21. Upadhyay G., Goessling W., North T. E., Xavier R., Zon L. I., Yajnik V. (2008) Molecular association between β-catenin degradation complex and Rac guanine exchange factor DOCK4 is essential for Wnt/β-catenin signaling. Oncogene 27, 5845–5855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hiramoto K., Negishi M., Katoh H. (2006) Dock4 is regulated by RhoG and promotes Rac-dependent cell migration. Exp. Cell Res. 312, 4205–4216 [DOI] [PubMed] [Google Scholar]
- 23. Hiramoto-Yamaki N., Takeuchi S., Ueda S., Harada K., Fujimoto S., Negishi M., Katoh H. (2010) Ephexin4 and EphA2 mediate cell migration through a RhoG-dependent mechanism. J. Cell Biol. 190, 461–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kawada K., Upadhyay G., Ferandon S., Janarthanan S., Hall M., Vilardaga J. P., Yajnik V. (2009) Cell migration is regulated by platelet-derived growth factor receptor endocytosis. Mol. Cell. Biol. 29, 4508–4518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ueda S., Fujimoto S., Hiramoto K., Negishi M., Katoh H. (2008) Dock4 regulates dendritic development in hippocampal neurons. J. Neurosci. Res. 86, 3052–3061 [DOI] [PubMed] [Google Scholar]
- 26. Biersmith B., Liu Z. C., Bauman K., Geisbrecht E. R. (2011) The DOCK protein sponge binds to ELMO and functions in Drosophila embryonic CNS development. PloS ONE 6, e16120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ueda S., Negishi M., Katoh H. (2013) Rac GEF Dock4 interacts with cortactin to regulate dendritic spine formation. Mol. Biol. Cell 24, 1602–1613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Côté J. F., Vuori K. (2007) GEF what? Dock180 and related proteins help Rac to polarize cells in new ways. Trends Cell Biol. 17, 383–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Premkumar L., Bobkov A. A., Patel M., Jaroszewski L., Bankston L. A., Stec B., Vuori K., Côté J. F., Liddington R. C. (2010) Structural basis of membrane targeting by the Dock180 family of Rho family guanine exchange factors (Rho-GEFs). J. Biol. Chem. 285, 13211–13222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kanai A., Ihara S., Ohdaira T., Shinohara-Kanda A., Iwamatsu A., Fukui Y. (2008) Identification of DOCK4 and its splicing variant as PIP3 binding proteins. IUBMB Life 60, 467–472 [DOI] [PubMed] [Google Scholar]
- 31. Lu M., Kinchen J. M., Rossman K. L., Grimsley C., Hall M., Sondek J., Hengartner M. O., Yajnik V., Ravichandran K. S. (2005) A steric-inhibition model for regulation of nucleotide exchange via the Dock180 family of GEFs. Curr. Biol. 15, 371–377 [DOI] [PubMed] [Google Scholar]
- 32. Shi L., Fu W. Y., Hung K. W., Porchetta C., Hall C., Fu A. K., Ip N. Y. (2007) α2-Chimaerin interacts with EphA4 and regulates EphA4-dependent growth cone collapse. Proc. Natl. Acad. Sci. U.S.A. 104, 16347–16352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Xu J., Xiao N., Xia J. (2010) Thrombospondin 1 accelerates synaptogenesis in hippocampal neurons through neuroligin 1. Nat. Neurosci. 13, 22–24 [DOI] [PubMed] [Google Scholar]
- 34. Ip J. P., Shi L., Chen Y., Itoh Y., Fu W. Y., Betz A., Yung W. H., Gotoh Y., Fu A. K., Ip N. Y. (2012) α2-Chimaerin controls neuronal migration and functioning of the cerebral cortex through CRMP-2. Nat. Neurosci. 15, 39–47 [DOI] [PubMed] [Google Scholar]
- 35. Fu W. Y., Chen Y., Sahin M., Zhao X. S., Shi L., Bikoff J. B., Lai K. O., Yung W. H., Fu A. K., Greenberg M. E., Ip N. Y. (2007) Cdk5 regulates EphA4-mediated dendritic spine retraction through an ephexin1-dependent mechanism. Nat. Neurosci. 10, 67–76 [DOI] [PubMed] [Google Scholar]
- 36. Shea T. B., Fischer I., Sapirstein V. S. (1985) Effect of retinoic acid on growth and morphological differentiation of mouse NB2a neuroblastoma cells in culture. Brain Res. 353, 307–314 [DOI] [PubMed] [Google Scholar]
- 37. Gao Y., Dickerson J. B., Guo F., Zheng J., Zheng Y. (2004) Rational design and characterization of a Rac GTPase-specific small molecule inhibitor. Proc. Natl. Acad. Sci. U.S.A. 101, 7618–7623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yoshimura T., Arimura N., Kaibuchi K. (2006) Signaling networks in neuronal polarization. J. Neurosci. 26, 10626–10630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wu G., Fang Y., Lu Z. H., Ledeen R. W. (1998) Induction of axon-like and dendrite-like processes in neuroblastoma cells. J. Neurocytol. 27, 1–14 [DOI] [PubMed] [Google Scholar]
- 40. Namekata K., Harada C., Guo X., Kimura A., Kittaka D., Watanabe H., Harada T. (2012) Dock3 stimulates axonal outgrowth via GSK-3β-mediated microtubule assembly. J. Neurosci. 32, 264–274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Namekata K., Harada C., Taya C., Guo X., Kimura H., Parada L. F., Harada T. (2010) Dock3 induces axonal outgrowth by stimulating membrane recruitment of the WAVE complex. Proc. Natl. Acad. Sci. U.S.A. 107, 7586–7591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Pan J., Kao Y. L., Joshi S., Jeetendran S., Dipette D., Singh U. S. (2005) Activation of Rac1 by phosphatidylinositol 3-kinase in vivo. Role in activation of mitogen-activated protein kinase (MAPK) pathways and retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J. Neurochem. 93, 571–583 [DOI] [PubMed] [Google Scholar]
- 43. Kalkman H. O. (2012) A review of the evidence for the canonical Wnt pathway in autism spectrum disorders. Mol. Autism 3, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Shearer K. D., Stoney P. N., Morgan P. J., McCaffery P. J. (2012) A vitamin for the brain. Trends Neurosci. 35, 733–741 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.