Abstract
♦ Background: Intraperitoneal (IP) administration of antibiotics is a mainstay of therapy in the treatment of peritoneal dialysis-related peritonitis. The therapeutic options against gram-positive organisms in patients intolerant to vancomycin are limited.
♦ Methods: This case report and review of the literature used a search of PubMed with the terms “daptomycin,” “intraperitoneal,” and “peritoneal” for 2004 through 7 February 2013 to find relevant publications.
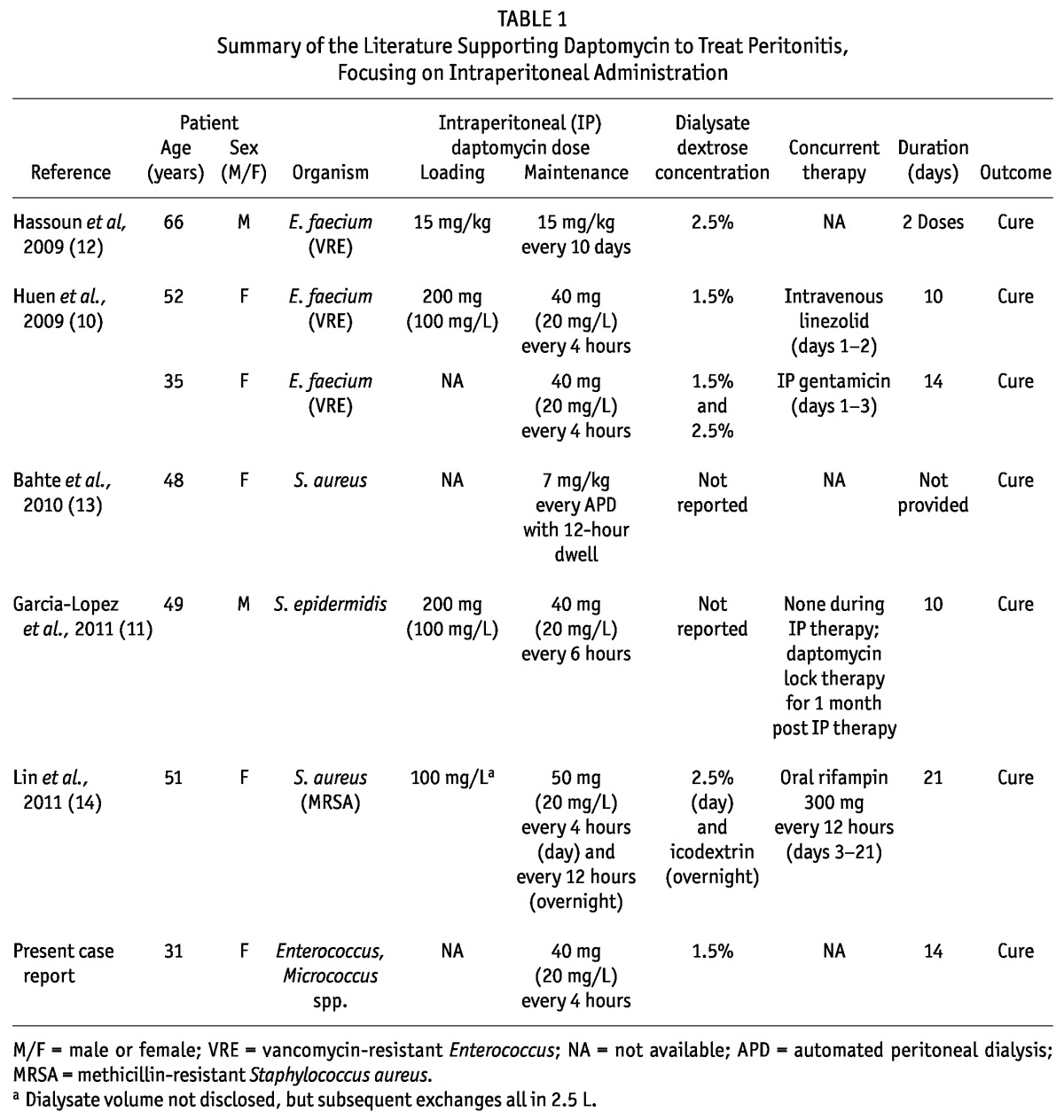
♦ Results: In addition to our patient, we identified 6 case reports of IP daptomycin for the treatment of peritonitis. Our patient was treated with a 14-day course of IP daptomycin, with resolution of signs and symptoms of peritonitis. She presented again 7 weeks later with signs and symptoms of peritonitis and was treated with a repeat course of IP daptomycin. Among the 6 patients reported in the literature, 4 received loading doses of daptomycin. Daptomycin 20 mg per liter of dialysate was administered in 4 patients, and the other 2 patients received higher doses based on body weight (milligrams per kilogram). Treatment duration averaged 10 or 14 days. In all 6 cases, clinical cure was reported.
♦ Conclusions: Although limited to case reports, the available literature suggests that IP daptomycin is a viable alternative for peritoneal dialysis-related peritonitis. However, routine use of this agent must be cautioned, because further prospective studies are required.
Key words: Daptomycin, intraperitoneal, peritonitis
Intraperitoneal (IP) vancomycin is a mainstay of therapy in treating peritoneal dialysis (PD)-related peritonitis. The therapeutic options for targeting gram-positive organisms are limited in patients intolerant to vancomycin. We describe our experience with IP daptomycin in a patient with a severe vancomycin allergy, and we review the available literature. A search of PubMed using the terms “daptomycin,” “intraperitoneal,” and “peritoneal” was conducted for 2004 through 7 February 2013.
Case Report
A 31-year-old woman presented to the emergency department with nausea, vomiting, abdominal pain, and chills. The patient’s history was significant for type 1 diabetes mellitus, with end-stage renal disease managed using automated PD (APD). She had a documented allergy to vancomycin of wheezing, rash, and pruritus.
On presentation, the patient was afebrile, with a blood pressure of 185/94 mmHg and a heart rate of 95 bpm. Her labs revealed a white blood cell (WBC) count of 11 500/mm3 with 90.3% neutrophils. Blood and peritoneal effluent were obtained for Gram stain and culture.
She experienced an episode of coffee-grounds emesis, later attributed to a Mallory-Weiss tear, and was given a one-time dose of intravenous (IV) ceftriaxone. A pantoprazole infusion was also started. She was admitted for further infection work-up and management of her gastrointestinal bleed. On arrival to the medical floor, single IV doses of cefazolin and ciprofloxacin were administered. At that time, peritonitis was not suspected.
Chest and abdominal radiography were unrevealing. Peritoneal effluent from the emergency department revealed a WBC count of 115/mm3, with 8% polymorphonuclear leukocytes. Gram stain of the peritoneal fluid was negative, but the culture preliminarily grew gram-positive cocci. On hospital day 3, given symptoms of abdominal pain, nausea, and chills, and an elevated effluent WBC count, her presentation was felt to be consistent with PD-related peritonitis.
In light of multiple recent hospitalizations, coverage against methicillin-resistant Staphylococcus aureus (MRSA) was sought, avoiding vancomycin because of the patient’s history of allergy. Linezolid and daptomycin were considered for empiric therapy, and PubMed was searched for “intraperitoneal linezolid” and “intraperitoneal daptomycin.” No case reports of IP linezolid were available for review. Ultimately, IP daptomycin was administered in the hope of treating the infection and salvaging the PD catheter. No systemic therapy was given in addition to the IP daptomycin.
The IP daptomycin was administered at a concentration of 40 mg in 2 L of 1.5% dextrose-containing replacement fluid (20 mg/L) in each dwell every 4 hours; no loading dose was used. The pharmacy reconstituted each daptomycin vial with 10 mL 0.9% sodium chloride per manufacturer recommendation (1), for a resulting concentration of 50 mg/mL, and dispensed 0.8 mL (40 mg) in a syringe for each PD exchange. The daptomycin was added to the dialysate immediately before the start of each dwell, a regimen that required a switch from APD to continuous ambulatory PD (CAPD).
Notably, this patient had residual renal function, reporting approximately 500 mL urine daily. The initial peritoneal fluid culture eventually grew sparse Micrococcus [daptomycin minimum inhibitory concentration (MIC) ≤ 0.25 μg/mL) and Enterococcus (ampicillin MIC ≤ 2 μg/mL, vancomycin MIC 1 μg/mL, daptomycin not tested). The patient was discharged on hospital day 6 and completed a 14-day course of IP daptomycin with rapid resolution of her symptoms. She transitioned back to APD at the end of IP daptomycin therapy. The patient tolerated IP daptomycin well, although creatinine phosphokinase levels were not measured.
Seven weeks after completion of therapy, the patient was readmitted with similar symptoms. Effluent analysis revealed a WBC count of 425/mm3 with 31% polymorphonuclear leukocytes. Blood and peritoneal fluid cultures yielded no growth. Given clinical concern for recurrent peritonitis, the woman was treated empirically with 14 days of IP daptomycin (40 mg in 2 L replacement solution, exchanged every 4 hours) and IP ceftazidime (1000 mg once daily). Additionally, a stool DNA amplification assay returned positive for Clostridium difficile, and oral vancomycin was given. Despite this patient’s allergy to IV vancomycin, she tolerated oral vancomycin well, probably because of its negligible systemic absorption. Upon completion of antibiotics, the patient’s symptoms resolved without further recurrence.
Discussion
Catheter-related peritonitis is a major cause of hospitalization and mortality in patients undergoing PD. Appropriate empiric antimicrobial management is key to preventing complications related to treatment failure. Gram-positive and gram-negative organisms can both cause PD-related peritonitis. Coagulase-negative staphylococci and S. aureus are frequently the causative organisms in infections thought to be acquired by the pericatheter route and contact contamination. Vancomycin-resistant Enterococcus (VRE), MRSA, and fungal organisms should be considered in nosocomial infections (2).
Daptomycin is a lipopeptide antibiotic indicated for the treatment of bacteremia, right-sided endocarditis, and complicated skin and skin-structure infections. It has concentration-dependent bactericidal activity against gram-positive pathogens (1). The MIC for susceptible organisms is 1 μg/mL or less for staphylococci and 4 μg/mL or less for vancomycin-susceptible enterococci (3).
Tobudic et al. studied daptomycin, among other antibiotics, in four different PD fluids and at increasing drug concentrations in an in vitro model of MRSA peritonitis (4). Two dextrose-containing dialysate fluids (Dianeal PD4, 1.36% dextrose, and Physioneal 40, 1.36% dextrose: Baxter Healthcare Corporation, Deerfield, IL, USA), one amino acid-containing fluid (Nutrineal PD4: Baxter Healthcare Corporation), and one icodextrin-containing fluid (Extraneal: Baxter Healthcare Corporation) were studied. Daptomycin doses representing 1×MIC, 4×MIC, and 8×MIC (MRSA, MIC 0.25 μg/mL) were allowed to dwell for up to 24 hours. Overall, compared with the other antibiotics, daptomycin demonstrated better efficacy in decreasing bacterial counts. At 8×MIC, daptomycin demonstrated bactericidal activity in all fluids with the exception of one 1.36% dextrose-containing fluid (Physioneal 40). The authors did not investigate drug stability in the dialysate.
In vivo, daptomycin-treated peritonitis has been described in multiple case studies at various doses, routes, and frequencies. One potential advantage in peritonitis treatment may be the ability of daptomycin to penetrate biofilm, which can accumulate on PD catheters and lead to recurrent episodes of peritonitis and, ultimately, to PD failure (5). An early pharmacokinetic study in 1 patient showed that dialysate concentrations after a single dose of IV daptomycin were above the MIC of the organisms studied (6), which led to several case reports of IV daptomycin being used to treat peritonitis (7,8). However, a recent commentary suggests avoiding the IV route because of limited penetration of daptomycin into the peritoneal cavity (9). Furthermore, the International Society for Peritoneal Dialysis guidelines recommend IP therapy in preference to the IV route because of its superiority in peritonitis treatment (2). For those reasons, the present article focuses on IP administration of daptomycin.
Administration of IP daptomycin was first described in 2 cases of vancomycin-resistant E. faecium peritonitis (10). The first patient received a loading dose of 200 mg daptomycin diluted in 2 L PD solution for the first 6-hour dwell, followed by 40 mg daptomycin in each subsequent exchange (20 mg/L of replacement fluid) every 4 hours for 10 days. The second patient received daptomycin at 20 mg/L of replacement fluid in each PD exchange every 4 hours for 14 days, without a loading dose. The doses for these patients were chosen to achieve a peritoneal daptomycin concentration 5 times the MIC of the isolated Enterococcus (patient 1: MIC not reported; patient 2: 4 μg/mL). Both patients were also empirically started on second agents—IV linezolid and IP gentamicin respectively—before therapy was narrowed to daptomycin monotherapy. Both patients had residual renal function, approximately 1000 mL and 2300 mL daily respectively. The PD catheters were not removed, and both patients achieved clinical resolution.
Similarly, García-López et al. (11) reported the successful treatment of S. epidermidis peritonitis using IP daptomycin. A loading dose of 200 mg daptomycin diluted in 2 L PD solution for 1 exchange was followed by 40 mg daptomycin in each subsequent exchange (20 mg/L of replacement fluid) 4 times daily for 10 days. Upon completion of IP therapy, the patient received daptomycin lock therapy (350 mg in 7 mL for 12 hours) weekly for 1 month. The patient achieved clinical cure with no recurrences.
Interestingly, Hassoun et al. (12) described a case of high-dose, less-frequent IP daptomycin to treat VRE peritonitis in a patient undergoing CAPD. Secondary to noncompliance, the patient received 15 mg/kg IP daptomycin for just 2 doses spaced 10 days apart. Cultures obtained 17 days after the second dose of daptomycin showed no signs of infection.
Bahte et al. (13) presented a patient with recurrent S. aureus peritonitis. The IP daptomycin administered was weight-based at 7 mg/kg and given at the completion of APD, remaining in the peritoneum for 12 hours. Pharmacokinetic data revealed that serum levels of the drug rose to more than 10 μg/mL after IP administration—well above the desired MIC for staphylococcal infections. Although not explicitly stated, it was implied that the patient’s peritonitis resolved. The authors concluded that weight-based IP daptomycin might be plausible for the treatment of systemic gram-positive infections.
Lastly, Lin et al. (14) presented a patient with persistent MRSA peritonitis despite high-dose IP vancomycin therapy who was switched to IP daptomycin plus oral rifampin. After a loading dose of daptomycin 100 mg/L of replacement fluid, the patient received daptomycin 20 mg/L of replacement fluid per exchange. The loading dose and the overnight exchanges dwelled for 12 hours, and during the day, exchanges were performed every 4 hours. Oral rifampin was added on day 3 of daptomycin therapy. The patient received a total of 21 days of IP daptomycin and 18 days of oral rifampin with a resultant cure.
Potential limitations of the approaches used by the Hassoun (12), Bahte (13), and Lin (14) groups is the unknown stability of daptomycin in extended dwells. Daptomycin undergoes 15% - 20% degradation in 5% dextrose at room temperature in 24 hours, causing concerns with respect to stability when left to dwell (10). Peyro Saint Paul and colleagues (15) investigated the compatibility of varying concentrations of daptomycin (50 mg/L to 200 mg/L) in three dialysates (Physioneal 40, 1.36% dextrose; Nutrineal, amino acids; and Extraneal, icodextrin). Samples obtained from the icodextrin-containing solution showed more than 10% variance among samples, which was deemed unreliable, and no further testing for stability was performed. However, samples from the 1.36% dextrose and amino-acid solutions showed daptomycin at all concentrations studied was stable for at least 6 hours at 25°C and 37°C.
Peyro Saint Paul and colleagues therefore recommended that daptomycin be extemporaneously added to dialysate solutions and that dwell times be limited to 6 hours (15). Huen et al. (10) injected daptomycin into the dialysate bag immediately before exchanges and limited dwells to 4 hours. Our team took a similar approach. Syringes containing 40 mg daptomycin were dispensed from the pharmacy, and the nurses and patient were educated about injecting into the replacement solution immediately before the exchange. The decision to forego a loading dose in our patient was related to her clinical stability and inconsistent use of loading doses in the published case reports retrieved.
Our case contributes to the small body of literature that supports the use of IP daptomycin to treat peritonitis (summarized in Table 1). Intraperitoneal administration offers several advantages over systemic therapy: It delivers a higher drug concentration at the site of infection, with a smaller dose of the required drug, which may potentially lessen the likelihood of toxic side effects. Further data focusing on extended stability across dialysis solutions may expand the role of daptomycin in the treatment of PD-related peritonitis. Additionally, prospective randomized trials evaluating the optimal dose of daptomycin need to be performed before this regimen can routinely be recommended.
TABLE 1.
Summary of the Literature Supporting Daptomycin to Treat Peritonitis, Focusing on Intraperitoneal Administration
Conclusions
The available literature suggests that IP daptomycin is a viable alternative for the treatment of PD-related peritonitis; however, its routine use must be cautioned, because further prospective studies are required.
Disclosures
JFG, MK, and MTL declare no financial conflicts of interest. MVGM reports paid consultation services in October 2012 for Cubist Pharmaceuticals, Inc., the manufacturers of daptomycin.
References
- 1. Cubicin [package insert]. Lexington, MA: Cubist Pharmaceuticals; 2010. [Google Scholar]
- 2. Li PKT, Szeto CC, Piraino B, Bernardini J, Figueiredo AE, Gupta A, et al. Peritoneal dialysis-related infections recommendations: 2010 update. Perit Dial Int 2010; 30:393–423 [Erratum in: Perit Dial Int 2011; 31:512] [DOI] [PubMed] [Google Scholar]
- 3. Rybak MJ. The efficacy and safety of daptomycin: first in a new class of antibiotics for gram-positive bacteria. Clin Microbiol Infect 2006; 12(Suppl 1):24–32 [DOI] [PubMed] [Google Scholar]
- 4. Tobudic S, Poeppl W, Kratzer C, Vychytil A, Burgmann H. Comparative in vitro antimicrobial activity of vancomycin, teicoplanin, daptomycin and ceftobiprole in four different peritoneal dialysis fluids. Eur J Clin Microbiol Infect Dis 2012; 31:1327–34 [DOI] [PubMed] [Google Scholar]
- 5. García-López L, Fernández-Reyes Luis MJ, Criado-Illana MT, Gómez-Sayago L, Heras-Benito M. Intraperitoneal administration of daptomycin in recurrent peritonitis with suspected biofilm [English, Spanish]. Nefrologia 2012; 32:139–42 [DOI] [PubMed] [Google Scholar]
- 6. Goedecke VA, Clajus C, Burkhardt O, Martens-Lobenhoffer J, Bode-Boger SM, Kielstein JT, et al. Pharmacokinetics and dialysate levels of daptomycin given intravenously in a peritoneal dialysis patient. Scand J Infect Dis 2009; 41:155–7 [DOI] [PubMed] [Google Scholar]
- 7. Burklein D, Heyn J, Kirchhoff C, Ozimek A, Traunmuller F, Joukhadar C, et al. Analysis of plasma and peritoneal fluid concentrations of daptomycin in a patient with Enterococcus faecium peritonitis. Int J Antimicrob Agents 2008; 32:369–71 [DOI] [PubMed] [Google Scholar]
- 8. Levy F, Camarero Temiño V, Blasco Mollá A, Ortega Lafont MP, Abaigar Luquin P, Izquierdo Ortiz MJ, et al. Treatment with intravenous daptomycin for a peritonitis relapse caused by Staphylococcus epidermidis [English, Spanish]. Nefrologia 2011; 31:374–5 [DOI] [PubMed] [Google Scholar]
- 9. Pérez-Fontán M, Rodríguez-Carmona A, Rodríguez-Mayo M. Enterococcal peritonitis in peritoneal dialysis patients: last name matters. Perit Dial Int 2011; 31:513–17 [DOI] [PubMed] [Google Scholar]
- 10. Huen SC, Hall I, Topal J, Mahnensmith RL, Brewster UC, Abu-Alfa AK. Successful use of intraperitoneal daptomycin in the treatment of vancomycin-resistant Enterococcus peritonitis. Am J Kidney Dis 2009; 54:538–41 [DOI] [PubMed] [Google Scholar]
- 11. García-López L, Gómez Sayago L, Fernández-Reyes Luis MJ. Intraperitoneal daptomycin [English, Spanish]. Nefrologia 2011; 31:375–6 [DOI] [PubMed] [Google Scholar]
- 12. Hassoun AA, Coomer RW, Mendez-Vigo L. Intraperitoneal daptomycin used to successfully treat vancomycin-resistant Enterococcus peritonitis. Perit Dial Int 2009; 29:671–3 [PubMed] [Google Scholar]
- 13. Bahte SK, Bertram A, Burkhardt O, Martens-Lobenhoffer J, Goedecke V, Bode-Böger SM, et al. Therapeutic serum concentrations of daptomycin after intraperitoneal administration in a patient with peritoneal dialysis-associated peritonitis. J Antimicrob Chemother 2010; 65:1312–14 [DOI] [PubMed] [Google Scholar]
- 14. Lin SY, Ho MW, Liu JH, Liu YL, Yeh HC, Hsieh TL, et al. Successful salvage of peritoneal catheter in unresolved methicillin-resistant Staphylococcus aureus peritonitis by combination treatment with daptomycin and rifampin. Blood Purif 2011; 32:249–52 [DOI] [PubMed] [Google Scholar]
- 15. Peyro Saint Paul L, Albessard F, Gaillard C, Debruyne D, Ryckelynck JP, Coquerel A, et al. Daptomycin compatibility in peritoneal dialysis solutions. Perit Dial Int. 2011; 31:492–5 [DOI] [PubMed] [Google Scholar]


