Abstract
♦ Background: Insulin resistance is associated with multiple risk factors for cardiovascular (CV) disease in the general population. Patients on peritoneal dialysis (PD) are more likely to develop insulin resistance. However, no evaluation of the impact of insulin resistance on CV disease morbidity or mortality in patients on PD has been performed.
♦ Methods: Our prospective cohort study included all non-diabetic patients on PD at our center (n = 66). Insulin resistance was evaluated at baseline by the homeostasis model assessment method (HOMA-IR) using fasting glucose and insulin levels. The cohort was followed for up to 58 months (median: 41.3 months; interquartile range: 34.3 months). A multivariate Cox model was used to analyze the impact of insulin resistance on CV disease mortality.
♦ Results: Fourteen CV events occurred in the higher HOMA-IR group [IR-H (HOMA-IR values in the range 2.85 - 19.5), n = 33], but only one event occurred in the lower HOMA-IR group (IR-L (HOMA-IR values in the range 0.83 - 2.71), n = 33) during the follow-up period. Level of HOMA-IR was a significant predictor of CV events [risk ratio: 17.7; 95% confidence interval (CI): 2.10 to 149.5; p = 0.008]. In the IR-H group, 10 patients died (8 CV events), but in the IR-L group, only 4 patients died (1 CV event). Patients in the IR-H group experienced significantly higher CV mortality (hazard ratio: 9.02; 95% CI: 1.13 to 72.2; p = 0.04). Even after adjustments for age, systolic blood pressure, body mass index, C-reactive protein, triglycerides, resistin, and leptin, HOMA-IR remained an independent predictor of CV mortality (hazard ratio: 14.8; 95% CI: 1.22 to 179.1; p = 0.03).
♦ Conclusions: Insulin resistance assessed using HOMA-IR was an independent predictor of CV morbidity and mortality in a cohort of nondiabetic patients on PD. Insulin resistance is a modifiable risk factor; the reduction of insulin resistance may reduce CV risk and improve survival in this group of patients.
Key words: Cardiovascular mortality, cardiovascular morbidity, insulin resistance, chronic kidney disease, homeostatic model assessment
In the general population, insulin resistance is associated with multiple risk factors for cardiovascular disease, including abdominal obesity, lipid abnormalities, and type 2 diabetes mellitus. The clustering of these abnormalities is called “metabolic syndrome” or “insulin resistance syndrome” (1). Insulin resistance may play a central role in the pathogenesis of metabolic syndrome (2,3). Furthermore, hyperinsulinemia has been found an independent predictor of ischemic heart disease in the general population (4).
Peritoneal dialysis (PD) exposes patients to a glucose load that may worsen the state of insulin resistance and metabolic disturbance in patients with chronic kidney disease. Patients on PD are therefore more prone to insulin resistance, dyslipidemia, and obesity (5). The elucidation of the relationship between insulin resistance and cardiovascular morbidity and mortality in this group of patients is particularly important because cardiovascular disease is the major cause of mortality in patients on PD.
Some studies have documented risk factors for cardiovascular disease in the general population that are also predictive of cardiovascular disease in patients with chronic kidney disease, including insulin resistance, diabetes, waist circumference, and total cholesterol (6-8). Recently, two studies investigated the association between insulin resistance and cardiovascular morbidity in PD patients (9,10). Tatar et al. showed that insulin resistance was an independent risk factor for arterial stiffness as indicated by carotid-femoral pulse wave velocity (9). Sevinc Ok et al. suggested that high glucose exposure from dialysis solution was a risk factor for vascular calcification (10). However, epidemiologic studies and clinical trials have fostered uncertainty about the impact of metabolic disorders on mortality in patients with chronic kidney disease. Some studies have demonstrated no relationship or have described a “reverse epidemiologic phenomenon” in patients on dialysis and in chronic kidney disease patients not on dialysis (11-17). A low body mass index (BMI) is associated with hospitalization and mortality in hemodialysis (HD) patients (14), and hypocholesterolemia is a significant predictor of death in patients on HD (17). Moreover, no prospective study has evaluated the effects of insulin resistance level on cardiovascular mortality in patients on PD. Only one study has used the homeostatic model assessment of insulin resistance (HOMA-IR) to predict cardiovascular mortality in patients on HD (6).
We performed a prospective observational cohort study in nondiabetic patients on PD to evaluate the effect of insulin resistance on cardiovascular morbidity and mortality.
Methods
Participants
The local Ethics Committee on Human Studies at the Huashan Hospital of Fudan University, China, approved the protocol. Informed consent was obtained from each patient. Patients were recruited from the outpatient unit from November 2006 to March 2009. All patients on continuous ambulatory PD (CAPD) without a past history of diabetes mellitus and with a fasting serum glucose of 7.0 mmol/L or less were eligible for inclusion in the study. Patients received regular CAPD for at least 3 months (median: 15.1 months; interquartile range: 15.4). For regular CAPD, 2 L of a glucose-based solution (Baxter Healthcare, Guangzhou, China) was exchanged 3 - 5 times daily. Patients exhibited no acute infection, obvious inflammation, neoplasia, or unstable cardiovascular disease for 1 month before enrollment. All 66 patients identified agreed to participate in the study.
Study Design
We used the HOMA formula to determine insulin resistance at baseline (18,19). Using the median value as the cutoff point for insulin resistance, patients were divided in two groups: a high HOMA-IR (IR-H) group, with HOMA-IR values at or greater than the median; and a low HOMA-IR (IR-L) group, with HOMA-IR values less than the median. The differences in baseline characteristics, including age, sex, cause of renal failure, CAPD duration, daily glucose absorption from dialysate, dialysis adequacy, residual renal function, and cardiovascular episodes before CAPD, were investigated. Additionally, differences in baseline dysmetabolism parameters, including BMI, blood pressure (BP), serum cholesterol, triglycerides, low-density lipoprotein (LDL), high-density lipoprotein (HDL), C-reactive protein (CRP), ferritin, albumin, total adiponectin, leptin, and resistin were investigated.
Patients were followed from November 2006 until death, a switch to HD, renal transplantation, or August 2011. The median follow-up was 41.3 months (interquartile range: 34.3 months). The primary endpoint was cardiovascular mortality. The secondary endpoint was a cardiovascular event.
Assessment of Insulin Resistance
Insulin resistance was assessed using the HOMA-IR equation:
 |
Definition of Cardiovascular Events
Coronary artery disease was diagnosed when a participant met one or more of these criteria:
Percutaneous coronary intervention or coronary artery bypass grafting
Presence of significant stenosis on coronary angiography
Presence of ST-T abnormalities on electrocardiography in association with typical symptoms attributable to angina pectoris
Acute decompensated heart failure was diagnosed primarily by clinical signs and symptoms such as dyspnea, cough, fatigue, hypertension, tachycardia, crackles indicative of interstitial pulmonary edema, and wheezing.
Cerebrovascular disease was diagnosed using clinical history, confirmed by positive findings of infarction or bleeding on computed tomography or magnetic resonance imaging.
Peripheral artery disease was diagnosed in the presence of intermittent claudication or leg pain at rest (or both) and significant arterial stenosis on angiography.
Definition of Cardiovascular Death
All fatal events were reviewed and classified by the study group using information from death certificates, hospital records, and interviews with attending physicians, next of kin, and witnesses. Deaths were classified as cardiovascular or non-cardiovascular. Cardiovascular death was defined as mortality caused by cardiovascular events, specified as already described. The outcome of this analysis was time to cardiovascular death. Patients who left the study or died of a non-cardiovascular cause were censored for the survival analysis.
Measurements and Assays
At baseline, patients arrived between 0800 h and 0900 h after an overnight fast of at least 8 hours. Weight was measured after the peritoneal cavity was emptied. Blood pressure was measured using a standard mercury sphygmomanometer after the patient had rested in a supine position for at least 5 minutes. The average of 3 measurements was used for the analysis. Fasting serum samples were drawn before the first daily dialysate dwell for measurement of serum cholesterol, triglycerides, LDL, HDL, glucose, insulin, albumin, ferritin, CRP, adiponectin, leptin, and resistin.
Insulin was measured using a chemiluminescence assay (ADVIA Centaur XP: Siemens, New York, NY, USA). Adiponectin, leptin, and resistin were measured using ELISA kits (ABC-ELISA: R&D Systems, Minneapolis, MN, USA). Other measurements were performed using standard clinical laboratory methods.
Statistical Analysis
All variables were evaluated for normal distribution using the Kolmogorov-Smirnov one-sample test for goodness of fit. Normally distributed data are presented as mean ± standard deviation, and non-Gaussian data are presented as median and interquartile range. The Student unpaired t-test and the nonparametric Mann-Whitney U-test were used to investigate differences between groups. The Pearson chi-square test was used to evaluate associations between categorical variables. Binary logistic regression was used to derive estimates of the odds ratios for cardiovascular events. Survival curves were estimated using the Kaplan-Meier method, followed by a log-rank test. Prognostic variables for survival were first examined using a univariate Cox proportional hazards regression model, and then significant variables were forced into a multivariate Cox model. A p value less than 0.05 (2-tailed) was considered statistically significant. All analyses were performed using the SPSS software application (version 11.5: SPSS, Chicago, IL, USA) for Windows.
Results
General Features of the Study Population
Of the 66 patients included in the study, none was lost to follow-up. Mean age was 62.1 ± 16.3 years, and 31 patients (43.9%) were male. The HOMA-IR exhibited a skewed distribution, with a median of 2.78 (3.81). Each study group included 33 patients.
Table 1 presents the differences in baseline characteristics between the IR-H and IR-L groups. No differences in age, sex, CAPD duration, cause of renal failure, daily transperitoneal glucose absorption, adequacy of dialysis (total Kt/V), residual renal function, or average BP were observed between the groups. Moreover, no significant differences in the proportion of patients receiving angiotensin-converting enzyme inhibitors, angiotensin II type 1 receptor blockers, or lipid-lowering agents, including statins and fibrates, were observed. No significant differences in the past history of cardiovascular events before CAPD therapy were observed. A higher average BMI in the IR-H group was the only difference observed.
TABLE 1.
Baseline Characteristicsa of the High and Low Insulin Resistance Groups
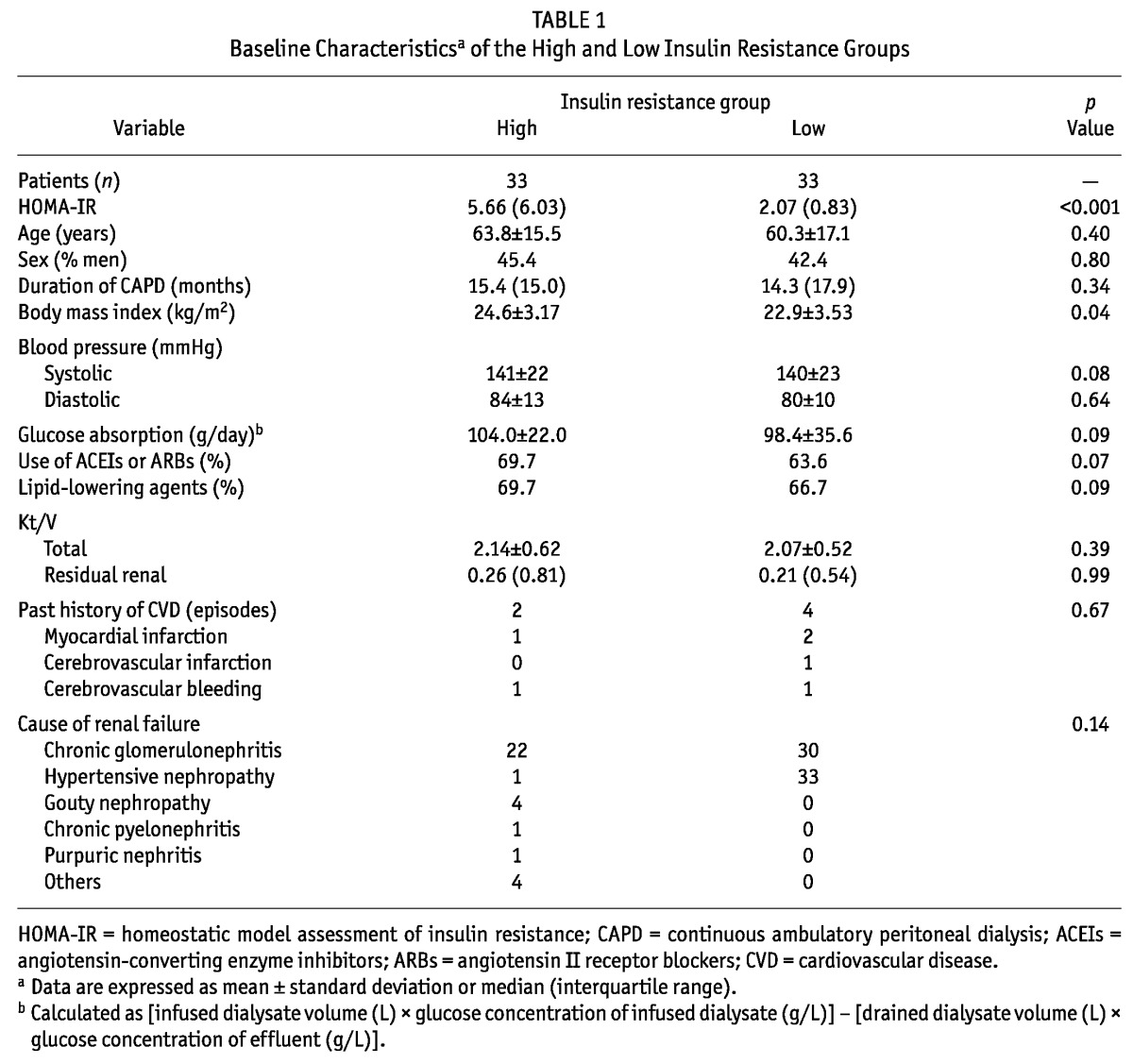
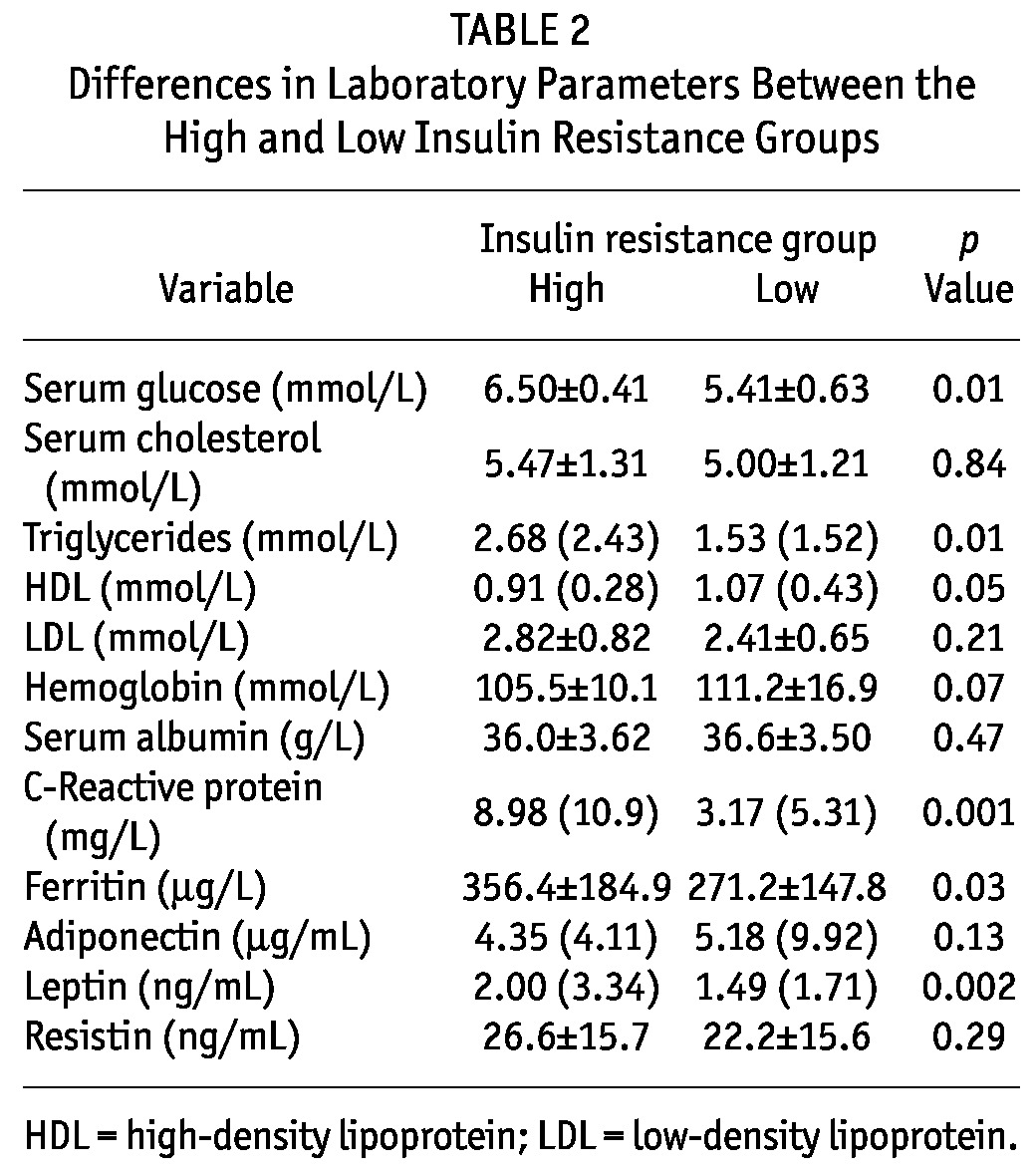
Table 2 presents the differences in laboratory parameters between the IR-H and IR-L groups. Serum glucose was higher in the IR-H group than in the IR-L group. Serum cholesterol and LDL were similar in both groups, but serum triglycerides were higher and HDL levels were relatively lower in the IR-H group. The IR-H group had significantly higher CRP and ferritin levels than did the IR-L group. No significant differences in hemoglobin or albumin levels were observed between the groups. The IR-H group had relatively higher leptin levels, but adiponectin and resistin levels were similar in both groups.
TABLE 2.
Differences in Laboratory Parameters Between the High and Low Insulin Resistance Groups
Outcome Data
In the IR-H group, 13 patients experienced 14 cardiovascular events (2 acute myocardial infarctions, 1 angina pectoris, 1 acute decompensated heart failure, 7 cerebrovascular infarctions, 2 cerebrovascular bleeds, and 1 phlebothrombosis in a lower limb) during the follow-up period. In the IR-L group, only 1 patient experienced a single cardiovascular event (acute myocardial infarction).
During the follow-up period, 2 patients underwent renal transplantation, and 11 switched to HD. In the IR-H group, 10 patients died (8 from fatal cardiovascular events, 1 from a serious peritonitis, and 1 for unknown reasons), but in the IR-L group, only 4 patients died (1 from a fatal cardiovascular event, 1 from a serious pneumonia, 1 because of intestinal perforation, and 1 from a serious peritonitis). At the end of the follow-up period, 39 patients were alive on PD.
Association Between Cardiovascular Morbidity and HOMA-IR
Binary logistic regression analysis revealed that, compared with the IR-L group, the IR-H group had a significantly higher risk of cardiovascular morbidity, even after adjustment for sex, age, and dialysis duration [relative risk: 17.7; 95% confidence interval (CI): 2.10 to 149.5; p = 0.008].
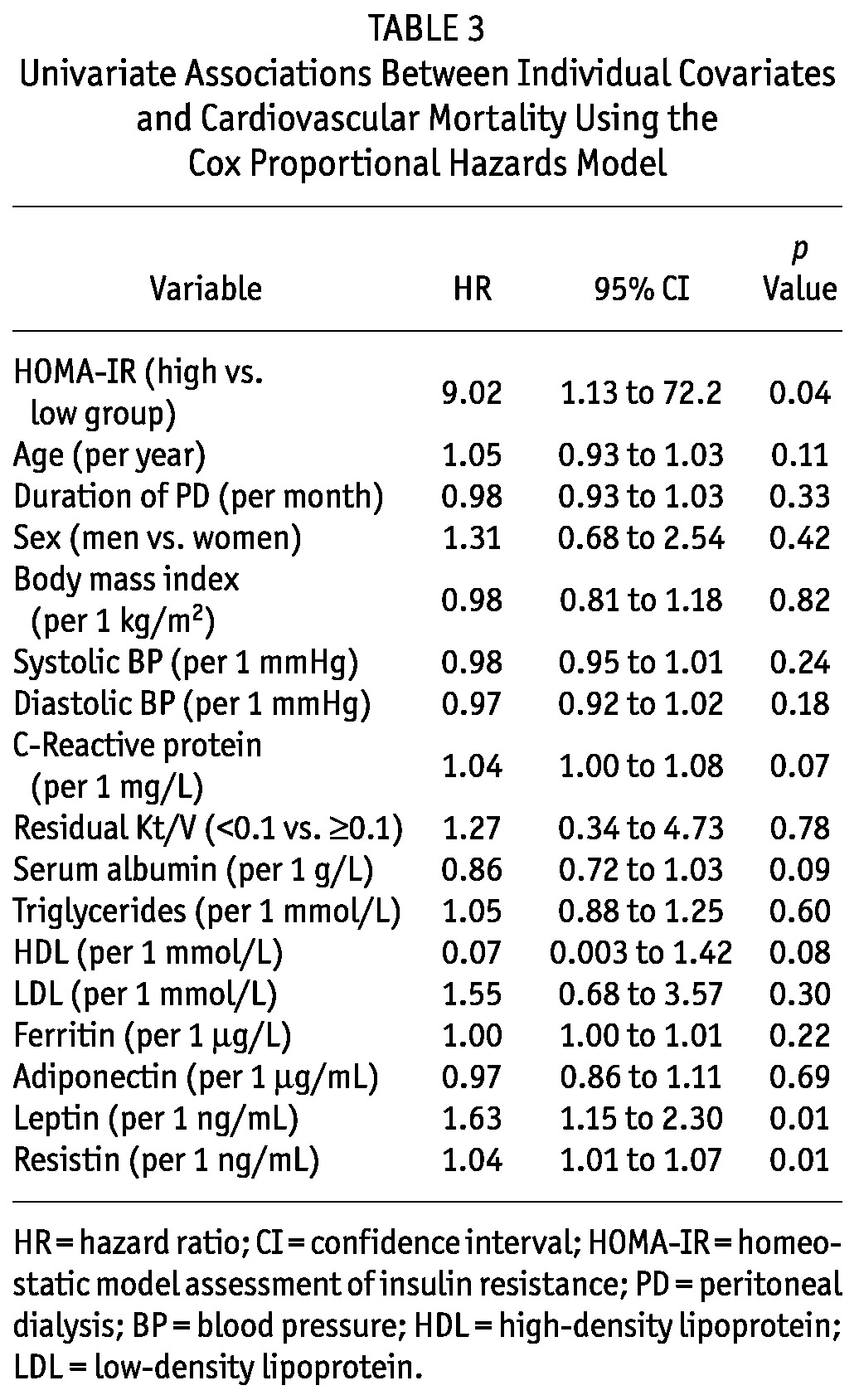
Univariate Association Between Cardiovascular Mortality and Covariates
Patients in the IR-H group had a significantly higher risk of cardiovascular mortality [hazard ratio (HR): 9.02; 95% CI: 1.13 to 72.2; p = 0.04]. Figure 1 shows the survival curves estimated by the Kaplan-Meier method. Table 3 presents the univariate associations between mortality and other covariates. Elevated leptin and resistin were significant univariate predictors of cardiovascular mortality.
Figure 1.
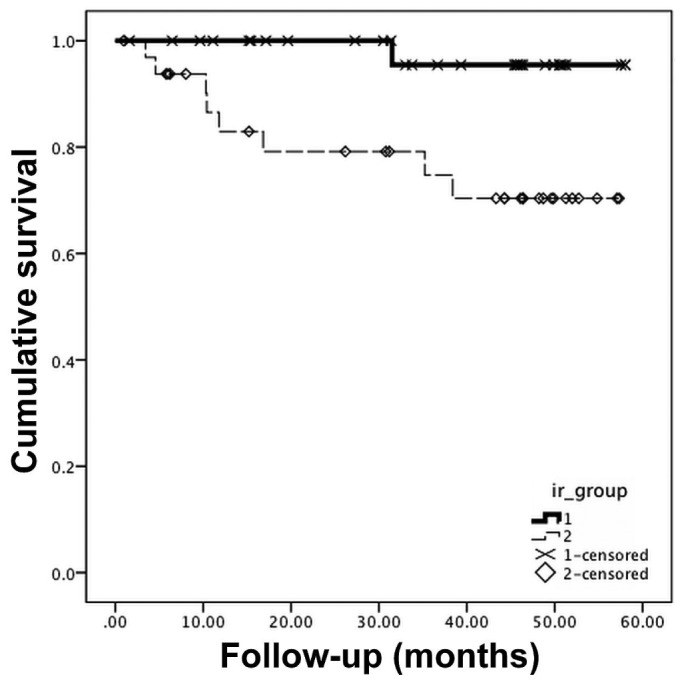
— Kaplan-Meier survival curves demonstrating the effects of insulin resistance (IR) evaluated using the homeostasis model assessment method on cardiovascular mortality in patients under treatment with peritoneal dialysis. 1 = low IR group; 2 = high IR group.
TABLE 3.
Univariate Associations Between Individual Covariates and Cardiovascular Mortality Using the Cox Proportional Hazards Model
Multivariate Analysis for Cardiovascular Mortality and HOMA-IR
In the multivariate Cox model, HOMA-IR remained an independent predictor of cardiovascular mortality after adjustment for resistin and leptin (HR: 11.02; 95% CI: 1.15 to 105.4; p = 0.04). Age, BMI, systolic blood pressure, CRP, and triglycerides were added to the model to examine whether the effect of HOMA-IR on cardiovascular mortality was independent of those factors. Even after adjustment for all those factors, HOMA-IR remained an independent predictor of cardiovascular mortality (HR: 14.80; 95% CI: 1.22 to 179.1; p = 0.03).
Discussion
Peritoneal dialysis maintains hemodynamic stability because drainage occurs slowly and continuously. Medically, PD should therefore be effective for preventing cardiovascular disease. However, PD is not superior to HD (20,21). A study including adult Australian and New Zealand patients commencing dialysis between 1997 and 2007 (21) demonstrated that, compared with HD, PD is associated with an increased risk of cardiovascular mortality after the first year (incidence rate, PD versus HD: 1.25; 95% CI: 1.12 to 1.32).
The risk factors for cardiovascular morbidity and mortality in PD patients require close examination. Our study revealed that, as an index of insulin resistance, HOMA-IR is an independent predictor of cardiovascular mortality in nondiabetic patients on PD. In a prospective observational cohort study of 183 nondiabetic patients on HD, Shinohara et al. found that the effect of HOMA-IR on cardiovascular mortality was independent of age, CRP, presence of preexisting vascular complications, BMI, hypertension, and dyslipidemia (6), which is consistent with our results. However, no similar studies in patients on PD have been reported in the literature. This information is of potential clinical value because it might encourage the use of therapeutic options—including glucose-sparing dialysate, drugs, and physical activity— to improve insulin sensitivity in patients on PD and to reduce cardiovascular risk.
Numerous factors have been implicated in the pathogenesis of the insulin resistance that occurs before the initiation of dialysis therapy, including anemia, dyslipidemia, uremia, malnutrition, parathyroid hormone excess, vitamin D deficiency, and metabolic acidosis. This situation is partially corrected after dialysis is initiated (5). Thrice-weekly HD for 10 weeks improved insulin resistance in end-stage renal disease patients (22). However, the development of insulin resistance in PD patients after an initial improvement is generally attributed to the high glucose load that is absorbed from dialysate, which contributes to a wide spectrum of metabolic abnormalities, including hypertriglyceridemia, poor glycemic control, new-onset diabetes, hypertension, and central obesity.
Insulin resistance in the general population is related to several cardiovascular risk factors, including hyperglycemia, dyslipidemia, obesity, and inflammation, and it is the central pathophysiologic process of the metabolic syndrome (2,3). We hypothesized that the association between HOMA-IR and cardiovascular mortality would depend on those risk factors. Although the patients in the IR-H group had higher BMI levels, more lipid disturbances, and higher inflammation levels, HOMA-IR was an independent predictor of cardiovascular mortality after adjustment for those factors in this cohort of patients. Survival studies in patients on HD suggest that a high BMI is associated with improved survival (14,23,24). That finding can be explained by considering that underweight patients are more likely to fall ill and that, because of nutrition and energy deficits, tend to recover more slowly than normal-weight and overweight patients.
The effect of HOMA-IR on cardiovascular mortality was independent of BMI in our study. Several explanations for that observation are possible. First, our patients were relatively stable and in good health at the time of enrollment. Average serum albumin in our study group was in the normal range. Underweight patients who lost weight because of acute illness or wasting at baseline were therefore not included in the study. Short-term mortality because of a negative nitrogen balance, inflammatory disorders, or other causes is strongly associated with a lower BMI in dialysis patients. Second, BMI does not differentiate between muscle and fat, and visceral fat plays an important role in the development of insulin resistance and atherosclerosis (25). The protective effect of increased BMI has been limited to patients on HD with normal or high muscle mass (26). Low-grade systemic inflammation is an important potential factor in the pathogenesis of insulin resistance in end-stage renal disease. We therefore expected that the association between HOMA-IR and cardiovascular mortality would be dependent on inflammation and that the association would be insignificant when CRP or inflammation-associated adipokines such as resistin and leptin were included in the multivariate Cox model. However, the effect of HOMA-IR on cardiovascular mortality was independent of CRP in the present study. Resistin was also an independent predictor of cardiovascular mortality in our study, which is consistent with reports that resistin is correlated with cardiovascular risk factors (27,28) and is a predictor of cardiovascular events (29,30) in the general population. Those results suggest that insulin resistance and inflammation might independently affect cardiovascular mortality in patients on PD. In addition, several of the insulin resistance-associated risk factors for chronic kidney disease were not included in our model. Those risk factors include the small dense LDL phenotype (31), increased levels of plasminogen activator inhibitor 1 (32), and hyperhomocysteinemia (33).
Several limitations of the present study should be acknowledged. First, the sample size—66 PD patients— is smaller than that of large registry analyses. The point estimates (HRs) of HOMA-IR therefore have wide 95% confidence intervals. Second, the duration of PD in the study population varied widely, and a mix of early and long-term patients may not be adequate to study correlations and metabolic links that likely change with time. Third, insulin resistance can best be evaluated using the hyperinsulinemic euglycemic clamp technique; however, in this study, we use HOMA-IR to estimate the insulin resistance level of the subjects at baseline. The HOMA-IR technique is easy to perform, and results using HOMA-IR are consistent with results obtained using the euglycemic clamp (18,19). The HOMA-IR index can also be applied to subjects with renal failure and to nondiabetic PD patients (34-37).
Conclusions
Our study revealed that insulin resistance as assessed by HOMA-IR was an independent predictor of cardiovascular mortality in a cohort of nondiabetic patients on PD. Insulin resistance is a modifiable risk factor; reduction of insulin resistance may lower cardiovascular risk and improve survival in this group of patients.
Disclosures
The authors have no financial conflicts of interest to disclose.
Acknowledgments
We thank Dr. Zhijie Zhang from the Department of Public Health, Fudan University, and Dr. Bobin Chen from the Department of Hematology, Huashan Hospital, for their assistance with the statistical analyses. This project was supported by the National Science and Technology Ministry (ID: 2011BAI10B05).
References
- 1. DeFronzo RA, Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care 1991; 14:173–94 [DOI] [PubMed] [Google Scholar]
- 2. Reaven GM. Banting lecture 1988. Role of insulin resistance in human disease. Diabetes 1988; 37:1595–607 [DOI] [PubMed] [Google Scholar]
- 3. Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet 2005; 365:1415–28 [DOI] [PubMed] [Google Scholar]
- 4. Després JP, Lamarche B, Mauriège P, Cantin B, Dagenais GR, Moorjani S, et al. Hyperinsulinemia as an independent risk factor for ischemic heart disease. N Engl J Med 1996; 334:952–7 [DOI] [PubMed] [Google Scholar]
- 5. Fortes PC, de Moraes TP, Mendes JG, Stinghen AE, Ribeiro SC, Pecoits-Filho R. Insulin resistance and glucose homeostasis in peritoneal dialysis. Perit Dial Int 2009; 29(Suppl 2):S145–8 [PubMed] [Google Scholar]
- 6. Shinohara K, Shoji T, Emoto M, Tahara H, Koyama H, Ishimura E, et al. Insulin resistance as an independent predictor of cardiovascular mortality in patients with end-stage renal disease. J Am Soc Nephrol 2002; 13:1894–900 [DOI] [PubMed] [Google Scholar]
- 7. Muntner P, He J, Astor BC, Folsom AR, Coresh J. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol 2005; 16:529–38 [DOI] [PubMed] [Google Scholar]
- 8. Nishizawa Y, Shoji T, Kakiya R, Tsujimoto Y, Tabata T, Ishimura E, et al. Non-high-density lipoprotein cholesterol (non-HDL-C) as a predictor of cardiovascular mortality in patients with end-stage renal disease. Kidney Int Suppl 2003; (84):S117–20 [DOI] [PubMed] [Google Scholar]
- 9. Tatar E, Demirci MS, Kircelli F, Gungor O, Turan MN, Sevinc Ok E, et al. Association of insulin resistance with arterial stiffness in nondiabetic peritoneal dialysis patients. Int Urol Nephrol 2012; 44:255–62 [DOI] [PubMed] [Google Scholar]
- 10. Sevinc Ok E, Asci G, Kircelli F, Duman S, Dheir H, Sezis Demirci M, et al. Relationship between glucose exposure via peritoneal dialysis solutions and coronary artery calcification in non-diabetic peritoneal dialysis patients. Int Urol Nephrol 2012; 44:1847–53 [DOI] [PubMed] [Google Scholar]
- 11. Shlipak MG, Fried LF, Cushman M, Manolio TA, Peterson D, Stehman-Breen C, et al. Cardiovascular mortality risk in chronic kidney disease: comparison of traditional and novel risk factors. JAMA 2005; 293:1737–45 [DOI] [PubMed] [Google Scholar]
- 12. Kovesdy CP, Anderson JE, Kalantar-Zadeh K. Inverse association between lipid levels and mortality in men with chronic kidney disease who are not yet on dialysis: effects of case mix and the malnutrition-inflammation-cachexia syndrome. J Am Soc Nephrol 2007; 18:304–11 [DOI] [PubMed] [Google Scholar]
- 13. Chmielewski M, Carrero JJ, Nordfors L, Lindholm B, Stenvinkel P. Lipid disorders in chronic kidney disease: reverse epidemiology and therapeutic approach. J Nephrol 2008; 21:635–44 [PubMed] [Google Scholar]
- 14. Fleischmann E, Teal N, Dudley J, May W, Bower JD, Salahudeen AK. Influence of excess weight on mortality and hospital stay in 1346 hemodialysis patients. Kidney Int 1999; 55:1560–7 [DOI] [PubMed] [Google Scholar]
- 15. Hakim RM, Lowrie E. Obesity and mortality in ESRD: is it good to be fat? Kidney Int 1999; 55:1580–1 [DOI] [PubMed] [Google Scholar]
- 16. Ikizler TA. Resolved: being fat is good for dialysis patients: the Godzilla effect: pro. J Am Soc Nephrol 2008; 19:1059–62 [DOI] [PubMed] [Google Scholar]
- 17. Iseki K, Yamazato M, Tozawa M, Takishita S. Hypocholesterolemia is a significant predictor of death in a cohort of chronic hemodialysis patients. Kidney Int 2002; 61:1887–93 [DOI] [PubMed] [Google Scholar]
- 18. Emoto M, Nishizawa Y, Maekawa K, Hiura Y, Kanda H, Kawagishi T, et al. Homeostasis model assessment as a clinical index of insulin resistance in type 2 diabetic patients treated with sulfonylureas. Diabetes Care 1999; 22:818–22 [DOI] [PubMed] [Google Scholar]
- 19. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985; 28:412–19 [DOI] [PubMed] [Google Scholar]
- 20. Jaar BG, Coresh J, Plantinga LC, Fink NE, Klag MJ, Levey AS, et al. Comparing the risk for death with peritoneal dialysis and hemodialysis in a national cohort of patients with chronic kidney disease. Ann Intern Med 2005; 143:174–83 [DOI] [PubMed] [Google Scholar]
- 21. Johnson DW, Dent H, Hawley CM, McDonald SP, Rosman JB, Brown FG, et al. Association of dialysis modality and cardiovascular mortality in incident dialysis patients. Clin J Am Soc Nephrol 2009; 4:1620–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DeFronzo RA, Tobin JD, Rowe JW, Andres R. Glucose intolerance in uremia. Quantification of pancreatic beta cell sensitivity to glucose and tissue sensitivity to insulin. J Clin Invest 1978; 62:425–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Glanton CW, Hypolite IO, Hshieh PB, Agodoa LY, Yuan CM, Abbott KC. Factors associated with improved short term survival in obese end stage renal disease patients. Ann Epidemiol 2003; 13:136–43 [DOI] [PubMed] [Google Scholar]
- 24. Johansen KL, Young B, Kaysen GA, Chertow GM. Association of body size with outcomes among patients beginning dialysis. Am J Clin Nutr 2004; 80:324–32 [DOI] [PubMed] [Google Scholar]
- 25. Axelsson J, Rashid Qureshi A, Suliman ME, Honda H, Pecoits-Filho R, Heimbürger O, et al. Truncal fat mass as a contributor to inflammation in end-stage renal disease. Am J Clin Nutr 2004; 80:1222–9 [DOI] [PubMed] [Google Scholar]
- 26. Beddhu S, Pappas LM, Ramkumar N, Samore M. Effects of body size and body composition on survival in hemodialysis patients. J Am Soc Nephrol 2003; 14:2366–72 [DOI] [PubMed] [Google Scholar]
- 27. de Luis DA, Gonzalez Sagrado M, Conde R, Aller R, Izaola O, Perez Castrillon JL, et al. Relation of resistin levels with cardiovascular risk factors and insulin resistance in non-diabetes obese patients. Diabetes Res Clin Pract 2009; 84:174–8 [DOI] [PubMed] [Google Scholar]
- 28. de Luis DA, Sagrado MG, Conde R, Aller R, Izaola O, de la Fuente B, et al. Relation of resistin levels with cardiovascular risk factors, insulin resistance and inflammation in naive diabetes obese patients. Diabetes Res Clin Pract 2010; 89:110–14 [DOI] [PubMed] [Google Scholar]
- 29. Momiyama Y, Ohmori R, Uto-Kondo H, Tanaka N, Kato R, Taniguchi H, et al. Serum resistin levels and cardiovascular events in patients undergoing percutaneous coronary intervention. J Atheroscler Thromb 2011; 18:108–14 [DOI] [PubMed] [Google Scholar]
- 30. Lim S, Koo BK, Cho SW, Kihara S, Funahashi T, Cho YM, et al. Association of adiponectin and resistin with cardiovascular events in Korean patients with type 2 diabetes: the Korean atherosclerosis study (KAS): a 42-month prospective study. Atherosclerosis 2008; 196:398–404 [DOI] [PubMed] [Google Scholar]
- 31. Reaven GM, Chen YD, Jeppesen J, Maheux P, Krauss RM. Insulin resistance and hyperinsulinemia in individuals with small, dense low density lipoprotein particles. J Clin Invest 1993; 92:141–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Potter van Loon BJ, Kluft C, Radder JK, Blankenstein MA, Meinders AE. The cardiovascular risk factor plasminogen activator inhibitor type 1 is related to insulin resistance. Metabolism 1993; 42:945–9 [DOI] [PubMed] [Google Scholar]
- 33. Tamura T, Johnston KE, Bergman SM. Homocysteine and folate concentrations in blood from patients treated with hemodialysis. J Am Soc Nephrol 1996; 7:2414–18 [DOI] [PubMed] [Google Scholar]
- 34. Cioni A, Sordini C, Cavallini I, Bigazzi R, Campese VM. Angiotensin receptor blocker telmisartan improves insulin sensitivity in peritoneal dialysis patients. Perit Dial Int 2010; 30:66–71 [DOI] [PubMed] [Google Scholar]
- 35. Furuya R, Odamaki M, Kumagai H, Hishida A. Beneficial effects of icodextrin on plasma level of adipocytokines in peritoneal dialysis patients. Nephrol Dial Transplant 2006; 21:494–8 [DOI] [PubMed] [Google Scholar]
- 36. Shoji T, Emoto M, Nishizawa Y. HOMA index to assess insulin resistance in renal failure patients. Nephron 2001; 89:348–9 [DOI] [PubMed] [Google Scholar]
- 37. Lee SW, Park GH, Lee SW, Song JH, Hong KC, Kim MJ. Insulin resistance and muscle wasting in non-diabetic end-stage renal disease patients. Nephrol Dial Transplant 2007; 22:2554–62 [DOI] [PubMed] [Google Scholar]


