Editor:
Recent advances in clinical practice and improved biocompatibility of dialysate might reduce the risk of peritoneal damage in peritoneal dialysis (PD) patients.
In long-term PD patients, morphology changes of peritoneum include denudation of peritoneal mesothelial cells, interstitial sclerosis, and hyalinizing vasculopathy (1). Almost all long-term PD patients show abnormal peritoneal histology (2,3). Introduction of biocompatible fluids with a neutral pH and lesser concentrations of glucose degradation products (GDPs), combined with recent Japanese PD practice, might exert a favorable effect on peritoneum (4,5).
Here, we report the case of a 62-year-old woman whose peritoneal morphology was preserved during 10 years of PD under recent Japanese clinical practice.
Case Description
A 62-year-old Japanese woman with end-stage renal disease as a result of autosomal dominant polycystic kidney disease was initiated on PD in 2001. Daily PD combined with once-weekly hemodialysis (HD) was started when she became anuric in 2006. She had been treated with Dianeal PD-4 (Baxter Healthcare Corporation, Deerfield, IL, USA) for the first 6 years. The concentrations of electrolytes and buffer in that solution were 132 mEq/L Na+, 2.5 mEq/L Ca2+, 0.5 mEq/L Mg2+, 95.0 mEq/L Cl-, and 40.0 mEq/L lactate, with a pH between 4.5 and 5.5.
After transfer to our hospital, this patient’s dialysate was changed to Midpeliq L (Terumo Medical Corporation, Tokyo, Japan), with which she was treated for the subsequent 4 years. Midpeliq L comes in a double-chambered solution bag and, compared with Dianeal PD-4, has about one fifth the concentration of GDPs (4,6). The final concentrations of electrolytes and buffer in Midpeliq L are 135 mEq/L Na+, 2.5 mEq/L Ca2+, 0.5 mEq/L Mg2+, 98.0 mEq/L Cl-, and 40.0 mEq/L lactate, with a pH between 6.3 and 7.3. Osmolarity of the solution is 350 mOsm/L (1.35% glucose) or 414 mOsm/L (2.5% glucose).
Our patient experienced no episodes of peritonitis during PD therapy. Her peritoneal function remained stable, with constant dialysate-to-plasma creatinine values in peritoneal equilibration tests of about 0.4 - 0.5 throughout her PD therapy. Her PD regimen before transfer to HD was automated PD with 5 L 1.35% glucose dialysate and 5 L 2.5% glucose dialysate (mixed during automated PD to yield a glucose concentration of about 1.9%), with three 4-hour exchanges using Midpeliq L. No change was made in the regimen throughout the treatment period.
Our patient was transferred to HD at the time of her retirement. Her PD catheter was removed in April 2011. Microscopic examination of parietal peritoneum obtained at the time of catheter removal revealed almost normal peritoneal structure (Figure 1).
Figure 1.
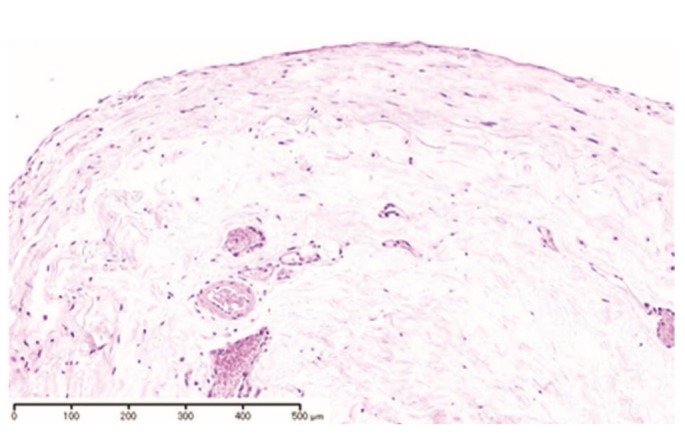
— Microscopic examination revealed unremarkable changes in parietal peritoneum (hematoxylin and eosin stain).
Discussion and Conclusions
It is well documented that, after several years of PD, the peritoneum is damaged in almost all PD patients and that the damage progresses with time on PD (1,3). Accumulating evidence suggests that several factors contribute to peritoneal damage in PD patients, including chronic uremia, peritonitis, and long-term use of bioincompatible fluids (1-3). Recent approaches to improve PD technique survival in Japan involve the introduction of combination therapy with HD, especially for anuric patients; prevention of peritonitis through patient education; and use of biocompatible dialysis solutions (lower concentrations of GDPs and neutral pH).
Recently, Tejde et al. reported 2 patients who developed encapsulating peritoneal sclerosis, a severe form of peritoneal damage, in spite of using neutral-pH dialysate (7). However, in both cases, the patients had experienced repeated peritonitis episodes and were rapid peritoneal transporters who required icodextrin.
Our patient was treated for the first 6 years with conventional glucose solution having a significant GDP content and low pH. She had achieved good fluid management and did not develop peritonitis, and her peritoneal function remained stable throughout PD therapy (0.4 - 0.5 by peritoneal equilibration test). Those factors might collectively exert a favorable effect on peritoneal morphology despite long-term PD.
Disclosures
The authors have no financial conflicts of interest to declare.
References
- 1. Williams JD, Craig KJ, Topley N, Von Ruhland C, Fallon M, Newman GR, et al. on behalf of the Peritoneal Biopsy Study Group. Morphologic changes in the peritoneal membrane of patients with renal disease. J Am Soc Nephrol 2002; 13:470–9 [DOI] [PubMed] [Google Scholar]
- 2. Hendriks PM, Ho-dac-Pannekeet MM, van Gulik TM, Struijk DG, Phoa SS, Sie L, et al. Peritoneal sclerosis in chronic peritoneal dialysis patients: analysis of clinical presentation, risk factors, and peritoneal transport kinetics. Perit Dial Int 1997; 17:136–43 [PubMed] [Google Scholar]
- 3. Honda K, Hamada C, Nakayama M, Miyazaki M, Sherif AM, Harada T, et al. Impact of uremia, diabetes, and peritoneal dialysis itself on the pathogenesis of peritoneal sclerosis: a quantitative study of peritoneal membrane morphology. Clin J Am Soc Nephrol 2008; 3:720–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Choi HY, Kim DK, Lee TH, Moon SJ, Han SH, Lee JE, et al. The clinical usefulness of peritoneal dialysis fluids with neutral pH and low glucose degradation product concentration: an open randomized prospective trial. Perit Dial Int 2008; 28:174–82 [PubMed] [Google Scholar]
- 5. Ayuzawa N, Ishibashi Y, Takazawa Y, Kume H, Fujita T. Peritoneal morphology after long-term peritoneal dialysis with biocompatible fluid: recent clinical practice in Japan. Perit Dial Int 2012; 32:159–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Itami N, Tsuji Y, Katsuki Y, Ohira S. Midpeliq reduces glucose load. Adv Perit Dial 2003; 19:236–9 [PubMed] [Google Scholar]
- 7. Tejde M, Linder M, Karsberg M, Ekspong A. The use of biocompatible solutions does not prevent development of encapsulating peritoneal sclerosis. Perit Dial Int 2010; 30:112–21 [DOI] [PubMed] [Google Scholar]


