Abstract
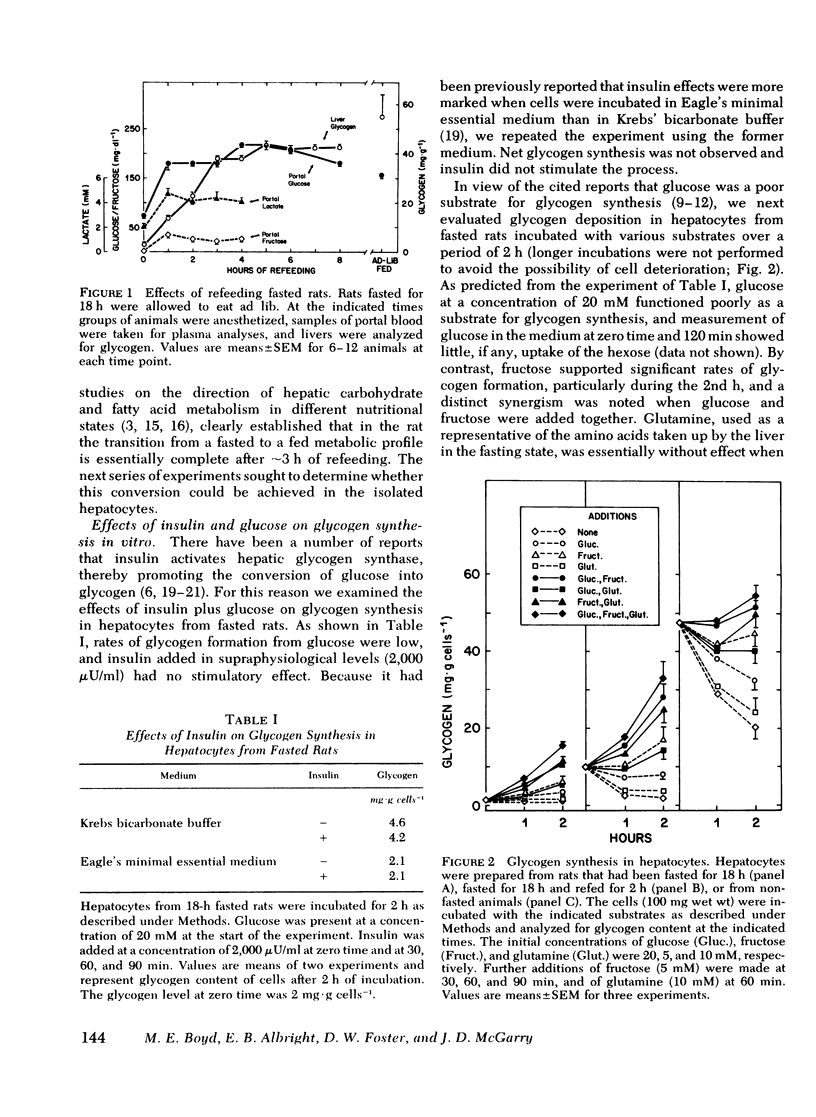
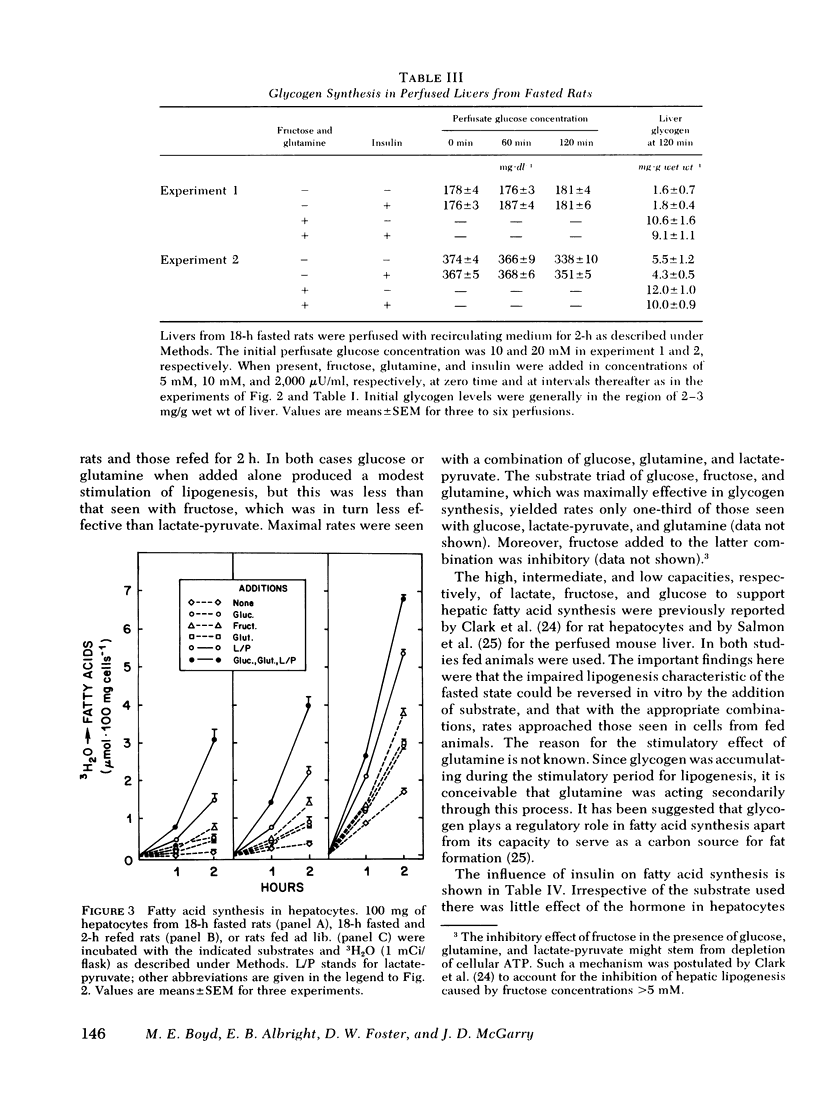
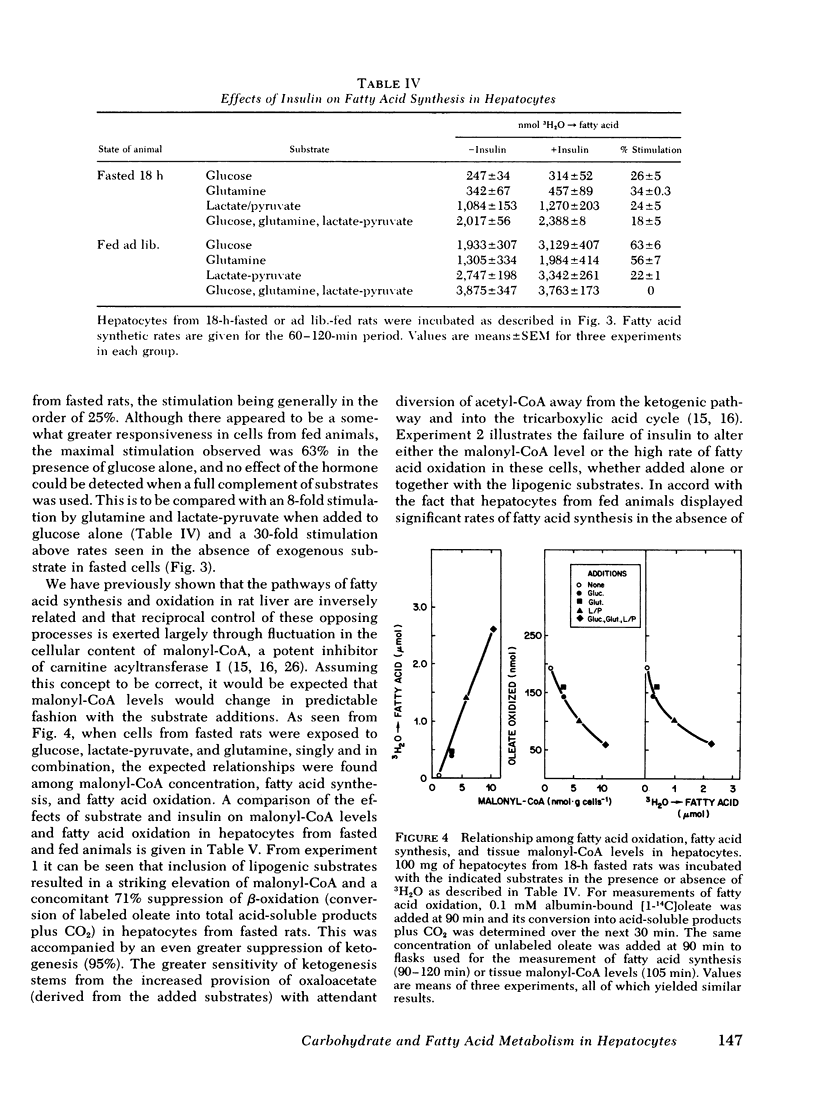
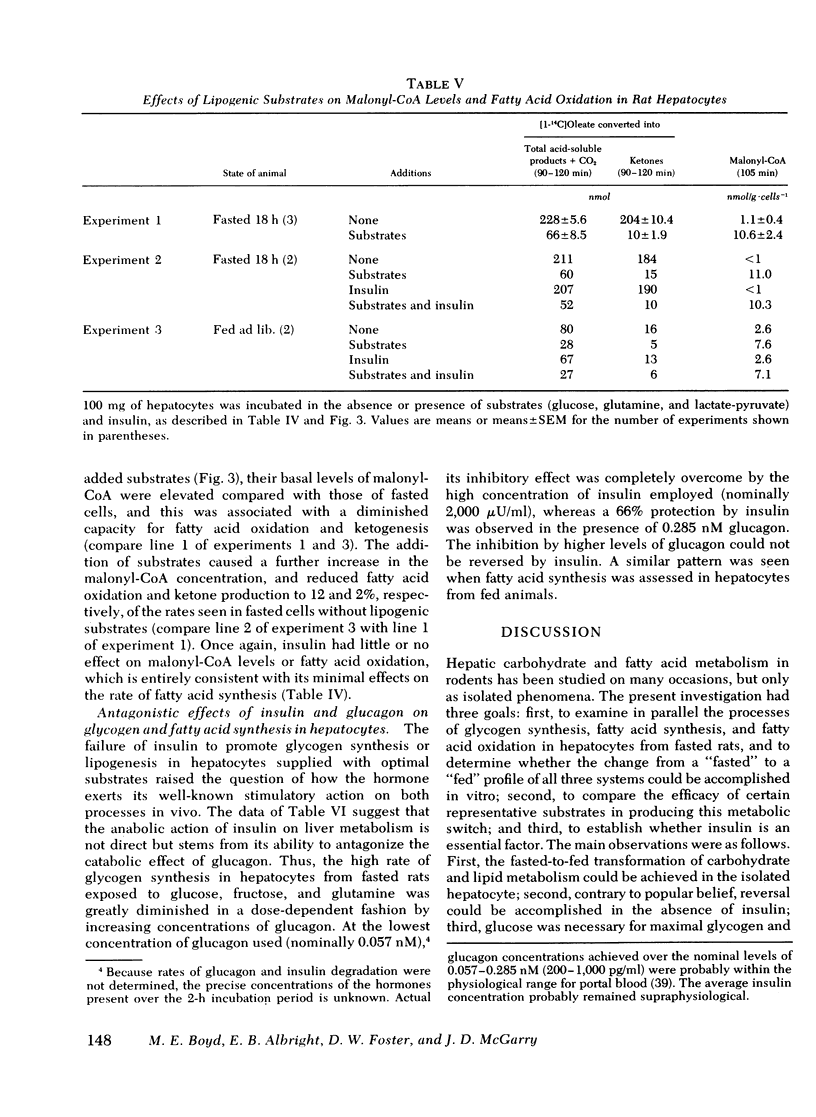
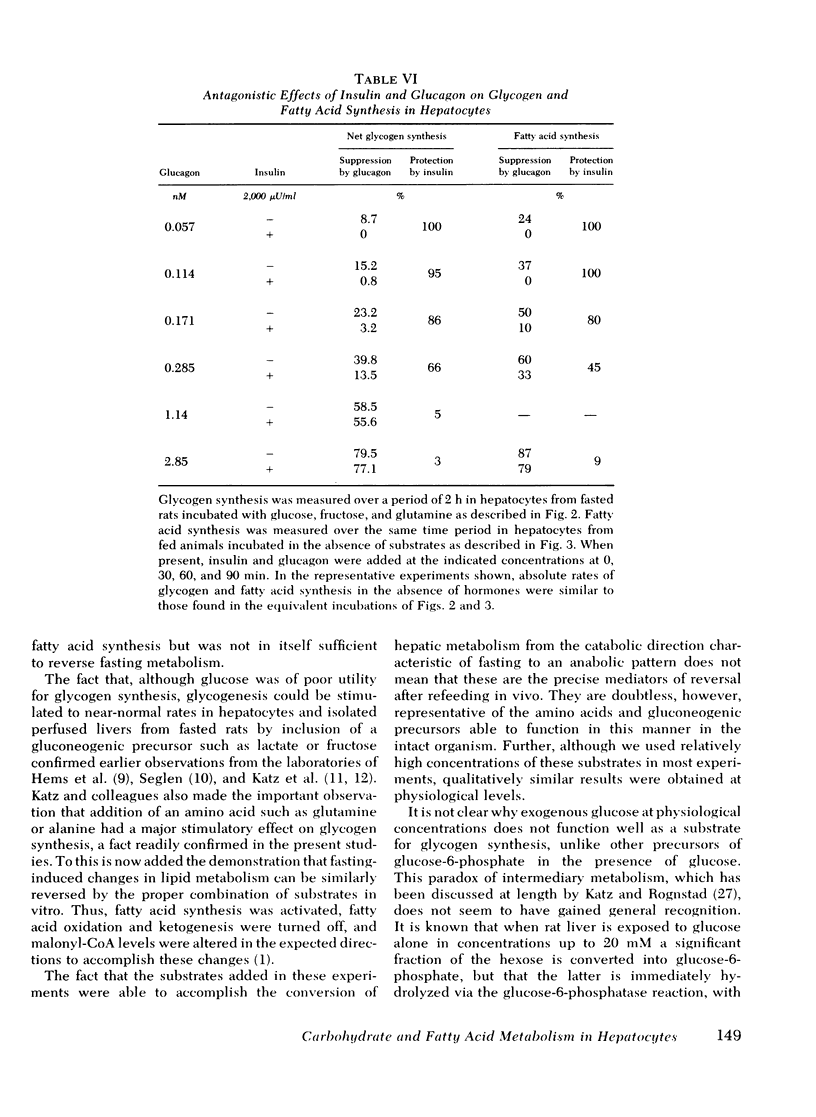
Studies were conducted to determine whether the direction of hepatic carbohydrate and lipid metabolism in the rat could be switched simultaneously from a "fasted" to a "fed" profile in vitro. When incubated for 2 h under appropriate conditions hepatocytes from fasted animals could be induced to synthesize glycogen at in vivo rates. There was concomitant marked elevation of the tissue malonyl-coenzyme A level, acceleration of fatty acid synthesis, and suppression of fatty acid oxidation and ketogenesis. In agreement with reports from some laboratories, but contrary to popular belief, glucose was not taken up efficiently by the cells and was thus a poor substrate for eigher glycogen synthesis or lipogenesis. The best precursor for glycogen formation was fructose, whereas lactate (pyruvate) was most efficient in lipogenesis. In both case the addition of glucose to the gluconeogenic substrates was stimulatory, the highest rates being obtained with the further inclusion of glutamine. Insulin was neither necessary for, nor did it stimulate, glycogen deposition or fatty acid synthesis under favorable substrate conditions. Glucagon at physiological concentrations inhibited both glycogen formation and fatty acid synthesis. Insulin readily reversed the effects of glucagon in the submaximal range of its concentration curve. The following conclusions were drawn. First, the fasted-to-fed transition of hepatic carbohydrate and lipid metabolism can be accomplished in vitro over a time frame similar to that operative in vivo. Second, reversal appears to be a substrate-driven phenomenon, in that insulin is not required. Third, unless an unidentified factor (present in protal blood during feeding) facilitates the uptake of glucose by liver it seems unlikely that glucose is the immediate precursor for liver glycogen or fat synthesis in vivo. A likely candidate for the primary substrate in both processes is lactate, which is rapidly formed from glucose by the small intestine and peripheral tissues. Fructose and amino acids may also contribute. Fourth, the requirement for insulin in the reversal of the fasting state of liver metabolism in vivo can best be explained by its ability to offset the catabolic actions of glucagon.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Assal J. P., Levrat R., Stauffacher W., Renold A. E. Metabolic consequences of portacaval shunting in the rat: effects on glucose tolerance and serum immunoreactive insulin response. Metabolism. 1971 Sep;20(9):850–858. doi: 10.1016/0026-0495(71)90047-3. [DOI] [PubMed] [Google Scholar]
- Avruch J., Witters L. A., Alexander M. C., Bush M. A. Effects of glucagon and insulin on cytoplasmic protein phosphorylation in hepatocytes. J Biol Chem. 1978 Jul 10;253(13):4754–4761. [PubMed] [Google Scholar]
- Baldwin D., Jr, Terris S., Steiner D. F. Characterization of insulin-like actions of anti-insulin receptor antibodies. Effects on insulin binding, insulin degradation, and glycogen synthesis in isolated rat hepatocytes. J Biol Chem. 1980 May 10;255(9):4028–4034. [PubMed] [Google Scholar]
- Beynen A. C., Vaartjes W. J., Geelen M. J. Acute effects of insulin on fatty acid metabolism in isolated rat hepatocytes. Horm Metab Res. 1980 Sep;12(9):425–430. doi: 10.1055/s-2007-999166. [DOI] [PubMed] [Google Scholar]
- Beynen A. C., Vaartjes W. J., Geelen M. J. Opposite effects of insulin and glucagon in acute hormonal control of hepatic lipogenesis. Diabetes. 1979 Sep;28(9):828–835. doi: 10.2337/diab.28.9.828. [DOI] [PubMed] [Google Scholar]
- Brunengraber H., Boutry M., Lowenstein J. M. Fatty acid and 3- -hydroxysterol synthesis in the perfused rat liver. Including measurements on the production of lactate, pyruvate, -hydroxy-butyrate, and acetoacetate by the fed liver. J Biol Chem. 1973 Apr 25;248(8):2656–2669. [PubMed] [Google Scholar]
- Chan T. M., Exton J. H. A rapid method for the determination of glycogen content and radioactivity in small quantities of tissue or isolated hepatocytes. Anal Biochem. 1976 Mar;71(1):96–105. doi: 10.1016/0003-2697(76)90014-2. [DOI] [PubMed] [Google Scholar]
- Clark D. G., Rognstad R., Katz J. Lipogenesis in rat hepatocytes. J Biol Chem. 1974 Apr 10;249(7):2028–2036. [PubMed] [Google Scholar]
- Davidson M. B., Berliner J. A. Acute effects of insulin on carbohydrate metabolism in rat liver slices: independence from glucagon. Am J Physiol. 1974 Jul;227(1):79–87. doi: 10.1152/ajplegacy.1974.227.1.79. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E., Hendler R., Wahren J., Felig P. Influence of hyperinsulinemia, hyperglycemia, and the route of glucose administration on splanchnic glucose exchange. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5173–5177. doi: 10.1073/pnas.75.10.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Exton J. H., Mallette L. E., Jefferson L. S., Wong E. H., Friedmann N., Miller T. B., Jr, Park C. R. The hormonal control of hepatic gluconeogenesis. Recent Prog Horm Res. 1970;26:411–461. doi: 10.1016/b978-0-12-571126-5.50014-5. [DOI] [PubMed] [Google Scholar]
- Exton J. H., Park C. R. Control of gluconeogenesis in liver. I. General features of gluconeogenesis in the perfused livers of rats. J Biol Chem. 1967 Jun 10;242(11):2622–2636. [PubMed] [Google Scholar]
- Felig P., Wahren J., Hendler R. Influence of oral glucose ingestion on splanchnic glucose and gluconeogenic substrate metabolism in man. Diabetes. 1975 May;24(5):468–475. doi: 10.2337/diab.24.5.468. [DOI] [PubMed] [Google Scholar]
- Golden S., Wals P. A., Okajima F., Katz J. Glycogen synthesis by hepatocytes from diabetic rats. Biochem J. 1979 Sep 15;182(3):727–734. doi: 10.1042/bj1820727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hems D. A. Short-term hormonal control of hepatic carbohydrate and lipid catabolism. FEBS Lett. 1977 Aug 15;80(2):237–245. doi: 10.1016/0014-5793(77)80449-3. [DOI] [PubMed] [Google Scholar]
- Hems D. A., Whitton P. D., Taylor E. A. Glycogen synthesis in the perfused liver of the starved rat. Biochem J. 1972 Sep;129(3):529–538. doi: 10.1042/bj1290529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hers H. G. The control of glycogen metabolism in the liver. Annu Rev Biochem. 1976;45:167–189. doi: 10.1146/annurev.bi.45.070176.001123. [DOI] [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Glycogen synthesis by rat hepatocytes. Biochem J. 1979 May 15;180(2):389–402. doi: 10.1042/bj1800389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Golden S., Wals P. A. Stimulation of hepatic glycogen synthesis by amino acids. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3433–3437. doi: 10.1073/pnas.73.10.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J., Rognstad R. Futile cycles in the metabolism of glucose. Curr Top Cell Regul. 1976;10:237–289. doi: 10.1016/b978-0-12-152810-2.50013-9. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. In support of the roles of malonyl-CoA and carnitine acyltransferase I in the regulation of hepatic fatty acid oxidation and ketogenesis. J Biol Chem. 1979 Sep 10;254(17):8163–8168. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. Regulation of hepatic fatty acid oxidation and ketone body production. Annu Rev Biochem. 1980;49:395–420. doi: 10.1146/annurev.bi.49.070180.002143. [DOI] [PubMed] [Google Scholar]
- McGarry J. D., Leatherman G. F., Foster D. W. Carnitine palmitoyltransferase I. The site of inhibition of hepatic fatty acid oxidation by malonyl-CoA. J Biol Chem. 1978 Jun 25;253(12):4128–4136. [PubMed] [Google Scholar]
- McGarry J. D., Meier J. M., Foster D. W. The effects of starvation and refeeding on carbohydrate and lipid metabolism in vivo and in the perfused rat liver. The relationship between fatty acid oxidation and esterification in the regulation of ketogenesis. J Biol Chem. 1973 Jan 10;248(1):270–278. [PubMed] [Google Scholar]
- McGarry J. D., Stark M. J., Foster D. W. Hepatic malonyl-CoA levels of fed, fasted and diabetic rats as measured using a simple radioisotopic assay. J Biol Chem. 1978 Nov 25;253(22):8291–8293. [PubMed] [Google Scholar]
- McGarry J. D., Takabayashi Y., Foster D. W. The role of malonyl-coa in the coordination of fatty acid synthesis and oxidation in isolated rat hepatocytes. J Biol Chem. 1978 Nov 25;253(22):8294–8300. [PubMed] [Google Scholar]
- McGarry J., Wright P. H., Foster D. W. Hormonal control of ketogenesis. Rapid activation of hepatic ketogenic capacity in fed rats by anti-insulin serum and glucagon. J Clin Invest. 1975 Jun;55(6):1202–1209. doi: 10.1172/JCI108038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. B., Jr, Larner J. Mechanism of control of hepatic glycogenesis by insulin. J Biol Chem. 1973 May 25;248(10):3483–3488. [PubMed] [Google Scholar]
- Rémésey C., Demigné C., Aufrère J. Inter-organ relationships between glucose, lactate and amino acids in rats fed on high-carbohydrate or high-protein diets. Biochem J. 1978 Feb 15;170(2):321–329. doi: 10.1042/bj1700321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon D. M., Bowen N. L., Hems D. A. Synthesis of fatty acids in the perused mouse liver. Biochem J. 1974 Sep;142(3):611–618. doi: 10.1042/bj1420611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland W. G., Blackmore P. F., Exton J. H. The role of calcium in alpha-adrenergic inactivation of glycogen synthase in rat hepatocytes and its inhibition by insulin. Diabetes. 1980 Aug;29(8):617–622. doi: 10.2337/diab.29.8.617. [DOI] [PubMed] [Google Scholar]
- Tolman E. L., Schworer C. M., Jefferson L. S. Effects of hypophysectomy on amino acid metabolism and gluconeogenesis in the perfused rat liver. J Biol Chem. 1973 Jul 10;248(13):4552–4560. [PubMed] [Google Scholar]
- Wahren J., Felig P., Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976 Apr;57(4):987–999. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witters L. A., Alberico L., Avruch J. Insulin regulation of glycogen synthase in the isolated rat hepatocyte. Biochem Biophys Res Commun. 1976 Apr 19;69(4):997–1003. doi: 10.1016/0006-291x(76)90471-x. [DOI] [PubMed] [Google Scholar]
- Witters L. A., Avruch J. Insulin regulation of hepatic glycogen synthase and phosphorylase. Biochemistry. 1978 Feb 7;17(3):406–410. doi: 10.1021/bi00596a004. [DOI] [PubMed] [Google Scholar]
- Witters L. A., Moriarity D., Martin D. B. Regulation of hepatic acetyl coenzyme A carboxylase by insulin and glucagon. J Biol Chem. 1979 Jul 25;254(14):6644–6649. [PubMed] [Google Scholar]
- Zahlten R. N., Stratman F. W., Lardy H. A. Regulation of glucose synthesis in hormone-sensitive isolated rat hepatocytes. Proc Natl Acad Sci U S A. 1973 Nov;70(11):3213–3218. doi: 10.1073/pnas.70.11.3213. [DOI] [PMC free article] [PubMed] [Google Scholar]



