Abstract
There is evidence that the right dorsolateral prefrontal cortex (DLPFC) may play a certain role in decision making related to reward value and time perception and, in particular, in the inhibitory control of impulsive decision making. Using the theta burst stimulation (TBS) and a delay discounting (DD) task, we investigated the potential role of right DLPFC in impulsive decision making defined by the rate of discounting delayed reward. Healthy right-handed volunteers underwent three stimulation sessions, intermittent TBS (iTBS), continuous TBS (cTBS), and sham. The steepness of the discount function (k-value), reaction time for choice and consistency were measured for each subjects. cTBS of the DLPFC reduced by 36.88 % the k-value of the DD task compared to sham condition. In contrast, iTBS did not affect impulsivity level. There were no changes neither in reaction time for choice nor consistency after either the iTBS or cTBS compared with the sham stimulation. These results demonstrate that cTBS-induced modulation of cortical excitability of the right DLPFC may affect and reduce impulsive decision making. These observations may provide some insights into the role of the right DLPFC in modulating impulsivity level and calculating reward value at different time scales under less ambiguous circumstances.
Keywords: rTMS, theta burst stimulation, dorsolateral prefrontal cortex, decision making, impulsivity, delay discounting task
In everyday life, individuals tend to compare potential benefit versus cost and choose the most valuable option under a given situation. During this decision-making process, time is an important factor in calculating the value of a reward. Patients with impulsive behavior such as drug abuse, pathologic gambling, obsessive-compulsive disorder, and attention deficit hyperactivity disorder1–3 may assign different weights to rewards in relation to time.
To measure impulsivity in several decision-making or response choice circumstances, several tasks, such as the go/no-go task, the stop task, as well as the gambling task, have been used. In the case of the go/no-go and stop paradigms, these tasks focus on motor impulsivity and are not designed to assess how people solve complex decision problems by controlling their impulsivity. The gambling task is a task that mimics a real life situation, but this task was originally designed to measure the risk taking based on uncertainty of premises and outcomes.4 Delay discounting (DD) is a behavioral analytic approach to understand how each individual makes a choice between a smaller reward given immediately and a larger reward given after a time delay, thus assessing the degree of cognitive impulsivity or self-control.5,6 In the DD task, subjects have the opportunity to choose between different amounts of monetary reward available with varying time delays without taking risks.
The involvement of prefrontal cortex (PFC) in decision making related to reward value and time perception has been already documented. Electrophysiologic studies in experimental animals have documented the activation of PFC neurons in response to time delay and expected reward amount with a neural activation pattern reflecting reward preference.7 There is evidence that the right hemisphere plays an important role in inhibiting impulsive behavior and, in particular, the inferior frontal cortex, orbitofrontal cortex, and dorsolateral prefrontal cortex (DLPFC) are critical for the control of this inhibition.8–10 The right DLPFC holds a certain role in the process of general decision making.11 Recently, some studies have shown how right DLPFC may affect decision-making processes12–15 by modulating its neuronal excitability using noninvasive stimulation tools such as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS). However, although these studies focused on the risk-taking aspect of decision making under ambiguous situation, the role of right DLPFC on the choice of reward over various time domain under calculated risk and nonambiguous situation has not been addressed. We investigated whether individual choices related to the reward value and time delay can be modulated by changes in PFC excitability using noninvasive focal stimulation in normal healthy subjects. We used the DD task to test changes in level of impulsivity after theta burst stimulation (TBS) of the right DLPFC. Subjects underwent three different stimulation paradigms including continuous TBS (cTBS), intermittent TBS (iTBS), and sham stimulation. These offline TBS paradigms have two main advantages: they produce a long-lasting inhibitory (cTBS) and excitatory (iTBS) effects limited to the underlying cortex16–19 and prevent any exogenous influence of the sound and proprioceptive sensation (given by the TMS) during the task performance.20 We reasoned that TBS-induced changes in excitability level of the DLPFC in right hemisphere would affect decision making by either reducing (cTBS-induced cortical depression) or increasing (iTBS-induced cortical activation) impulsivity level and influencing reward value at different time scales.
Methods
Subjects and design
Seven right-handed young healthy subjects (mean age: 22.4 ± 4.3 years; age range: 18–29 years; four women) were enrolled in this study. Handedness was assessed using the Edinburgh handedness inventory.21 Baseline impulsivity scores were collected before the TBS experiment using Barratt Impulsivity Scale-11 (BIS). Exclusion criteria included history of psychiatric and/or neurologic disorder (particularly epilepsy), any previous exposure to stimulant drugs, pregnancy, migraine, or previous experience of enrollment in rTMS experiments. Subjects were screened for depression with the use of the Beck Depression Inventory (BDI) with an exclusion criterion of a score of >10. To rule out structural lesions in the brain and to provide anatomic reference for the analysis, a T1-weighted magnetic resonance image (MRI) was obtained for each subject. Written informed consent was obtained in all cases. The study protocols were approved by the Ethical Committee of the Center for Addiction and Mental Health Research, University of Toronto.
All subjects underwent three different stimulations (cTBS, iTBS, and sham) over the right DLPFC and conducted the behavioral task 3 minutes after the completion of TBS in each stimulation session. Stimulation conditions were counterbalanced across the subjects. There was at least a 45- minute interval between each TBS condition (Figure 1) to minimize the carry-over effect of the prior TBS.
Figure 1.
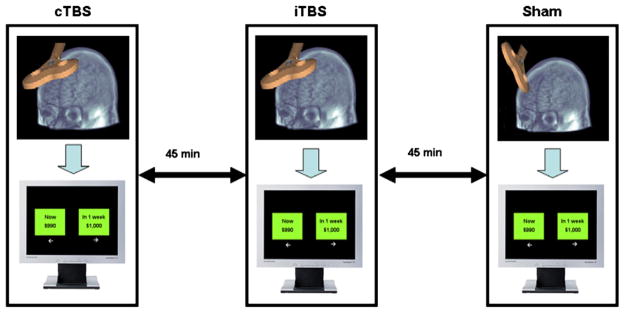
Display of the research procedure.
TBS
TBS was carried out with the biphasic Magstim Rapid2 magnetic stimulator (The Magstim Company Ltd, Whitland, UK), using a figure-of-eight focal coil (70 mm diameter). The coil was held in a fixed position by a mechanical arm over the target area and was oriented so that the induced electric current flowed in a posterior-anterior direction. Stimulus intensities, expressed as a percentage of the maximum stimulator output, were set at 80% of the active motor threshold (AMT). AMT was defined from the contra-lateral first dorsal interosseus (FDI) muscle with AgCl surface electrodes fixed on the skin with a belly-tendon montage as the lowest stimulus intensity able to elicit five motor-evoked potentials (MEPs) of at least 200 μV averaged over 10 consecutive stimuli delivered over the motor cortex at intervals longer than 5 seconds. During the determination of AMT, subjects were instructed to maintain a steady muscle contraction of 20% of maximum voluntary contraction. Detailed descriptions of TBS paradigms have been reported elsewhere.17
Each burst consists of three stimuli pulses at 50 Hz, with each train being repeated every 200 milliseconds (5 Hz). During the iTBS, trains of bursts were repeated for 2 seconds every 10 seconds for 192 seconds (600 pulses) and cTBS consisted of continuous repetition of trains for 40 seconds (600 pulses). It has been shown that these two stimulation paradigms have an opposite effect in the underlying cortex, as cTBS depresses cortical excitability and iTBS enhances neural excitability.17 The sham stimulation was delivered with the coil positioned at a perpendicular angle to the target area using either the iTBS or cTBS protocol, in a counterbalanced manner, across the subjects.
Location of the target site
To target the right DLPFC, we used a procedure that takes advantage of the standardized stereotaxic space of Talairach and Tournoux22 and frameless stereotaxy.23,24 A high-resolution MRI (GE Signa 1.5 T, T1-weighted images, 1-mm slice thickness) of every subject’s brain was acquired and transformed into standardized stereotaxic space using the algorithm of Collins et al.25 The coordinates selected for right DLPFC (x = 40, y = 32, and z = 30) were similar to those used in our previous studies.24
The Talairach coordinates were converted into each subject’s native MRI space using the reverse native-to-Talairach transformation.23 The positioning of the TMS coil over these locations, marked on the native MRI, was performed with the aid of a frameless stereotaxic system (Rogue Research, Montreal, Canada).
Behavioral task
A computerized DD task was used for data acquisition (Figure 2). The task was based on the Kirby’s DD inventory.26 The task was composed of 120 trials; in each trial the amounts of monetary reward for immediate and delay options were decided by the fixed k value and the delay time based on the hyperbolic function of DD, V = A/(1 + kD), where V is the value of the delayed outcome (i.e., the indifference value), A is the delayed reward, D is the length of the delay, and k expresses the steepness of the discount function.27–29 Based on this function, higher k-values are associated with preference for immediate small-size reward and lower k-values are expression of delayed large-size reward. Thus, low k-values are an index of minor impulsivity. Subjects were instructed that they had to make preference judgments about hypothetical rewards shown on a computer screen.
Figure 2.
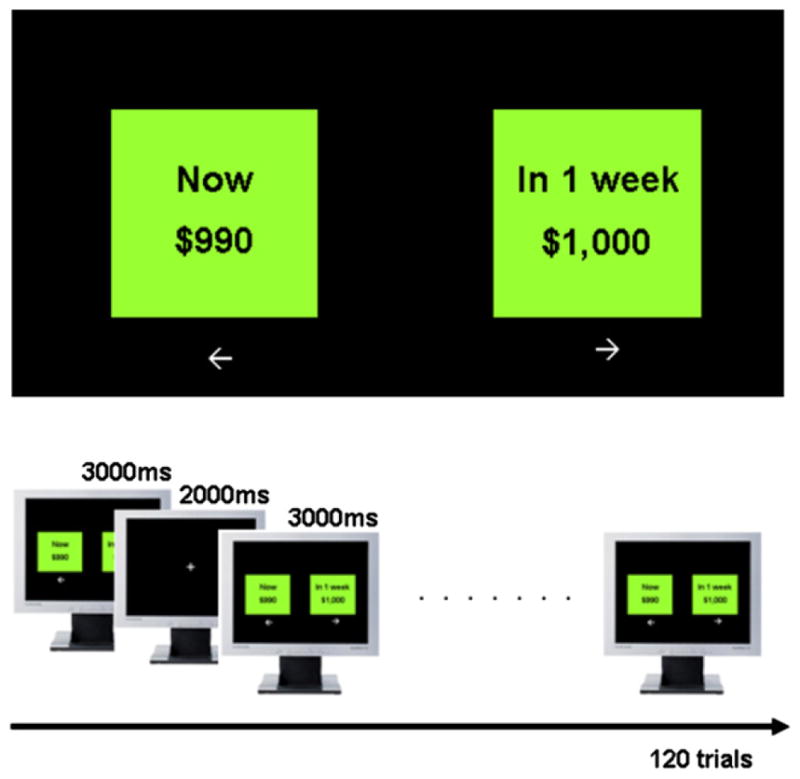
Visual display and timing of trials for delay discounting (DD) task.
All reward choices were made by pressing either the ← or → key on keyboard with the subject’s dominant hand (right hand for all subjects). The available time delays were 12 in total (1 week, 2 weeks, 3 weeks, 1 month, 3 months, 6 months, 1 year, 2 years, 3 years, 4 years, 5 years, and 10 years) and there were two categories of delayed reward magnitude: small (1–500 CAD) and large (600–1000 CAD). The predefined k-values were 0.0005, 0.0028, 0.0050, 0.0275, 0.05, 0.075, 0.1, 0.3, 0.5, and 0.7 for the 120 trials; the same number of trials was assigned for each k-value and reward magnitude. The examples of choice trial are presented on Table 1. The choice stimulations were presented on the screen for 3 seconds and the interstimulation interval was 2 seconds.
Table 1.
Example of trials in the two reward sizes with an associated discount rate (k) of 0.005
| Choice option
|
Delay | k | Reward sizea | |
|---|---|---|---|---|
| SIR | LDR | |||
| 44 | 55 | 1 y | 0.005 | Small |
| 820 | 828 | 2 wk | 0.005 | Large |
SIR, smaller-immediate reward; LDR, larger-delayed reward.
Categories of delayed reward size: small (1–500 CAD) and large (600–1000 CAD).
The following instruction was read to all subjects before the experiment and a sample behavioral test was conducted to confirm that the subjects properly understood the instruction: “After every rTMS stimulation you will be shown two options on the computer screen and asked to decide between a gain NOW and a gain sometime in the FUTURE. The future gain may be several days from now or as remote as 10 years from now. For example, you may see: ‘a gain of $1000 in 1 year’ in your right-hand side and ‘a gain of $990 now’ in your left-hand side. If you prefer the option in the left side, please press the LEFT key. If you prefer the right-side option, please press the RIGHT key.” The trial order was randomized for each session across the subjects.
Analysis
The outcome measures of this study were as follows: (1) individual discounting value k, (2) reaction time for choice decision, and (3) consistency of choice, a percentage of the same option choice for a given k-value. The k-values were estimated separately in small and large reward magnitude as the geometric mean between the lowest implied indifference k-value in which subjects chose the delayed option, and the highest implied indifference k-value in which subjects chose the immediate option.26,30 The geometric mean is a type of mean that indicates the central tendency of a set of numbers and is used because the task required subjects to express preferences.30 We expected that changes in the individual k-value would vary in a relative fashion of reward amount and delay period. If the response consistence was more than 66% for one response (small, immediate, or large delay option) within the given k-value, then that k-value was assigned to immediate choice preference or delay choice preference.
To compare the effect of TBS each subject was analyzed with a nonparametric Friedman test corrected by exact test, using SPSS (version 13.0 for Windows) software (SPSS, Chicago, IL) to test the effect of the TBS protocol used. As a post hoc analysis, we also conducted nonparametric Wilcoxon signed rank test to test the TBS effect using the sham condition as a baseline. The significance level for all statistical analysis was set at P < .05.
Results
The Figure 3 and Table 2 show the total k-value across the stimulation conditions. There were significant differences among the three stimulation conditions (Friedman test, χ2 = 7.71, df = 2, P < .05) in total k-value of the DD task. The post hoc analysis revealed that cTBS-induced inhibition of the right DLPFC reduced individual k-value of the DD task compared with the sham condition (cTBS: 0.0085 ± 0.011 versus sham: 0.0149 ± 0.014, Wilcoxon test, Z = 2.37, P < .05). This reduction in k-value indicated a decreased impulsivity and the mean magnitude of change in k-value after the cTBS was −36.88% ± 29.84%. In contrast, iTBS had no effect on k-value when compared with the sham condition (iTBS: 0.0155 ± 0.019 versus sham: 0.0149 ± 0.014, Wilcoxon test, Z = 0.34, P = .74) (Figure 3). The decrease in k-value did not change with the increase in reward magnitude (Wilcoxon test, Z = −0.51, P = .61) implying that cTBS-induced reduction in impulsivity was unrelated to the magnitude of reward. Additional analysis using repeated measure of analysis of variance (ANOVA) (3 [stimulation condition] × 2 [reward delay]) revealed that subjects showed no differences in k-value between “near-future (<1 year)” and “far-future (>1 year)” regardless stimulation condition (F1,6 = 3.07, P = .13) with no interaction between delay time and stimulation condition (F2,12 = 1.91, P = .19).
Figure 3.
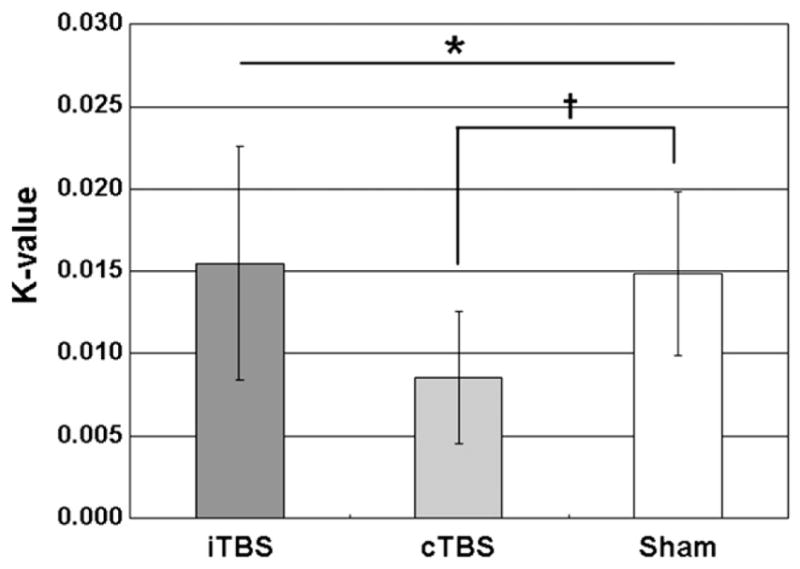
Mean total k-value for the each theta burst stimulation (TBS) conditions. Error bars represent the standard errors of the mean. *P < .05 using a nonparametric Freedman test. †P < .05 using a nonparametric Wilcoxon signed rank test.
Table 2.
Mean and standard deviation of BIS score and k-value in each TBS session
| BIS |
k-Value
|
|||
|---|---|---|---|---|
| Sham | iTBS | cTBS | ||
| Mean | 59.6 | 0.0149 | 0.0155 | 0.0086 |
| SD | 7.7 | 0.0135 | 0.0189 | 0.0107 |
BIS = Barratt Impulsivity Scale-11; TBS = theta burst stimulation; iTBS = intermittent TBS; cTBS = continuous TBS; SD = standard deviation.
The correlation analysis did not reveal any relationship between baseline BIS scores (mean ± SD: 59.6 ± 7.7) and cTBS-induced percentage changes in k-values (Pearson correlation, r = 0.63, P = .13), suggesting that individual impulsivity baseline did not contribute and influence k-value changes.
The specific cTBS-induced reduction in impulsivity k-value was not an expression of changes in motor performance and response in choice consistency. In fact, there were no effects of TBS stimulation on reaction time (Friedman test, χ2 = 0.00, df = 2, P > .99; Figure 4A) and on consistency in response choice (Friedman test, χ2 = 3.42, df = 2, P = .18; Figure 4B).
Figure 4.
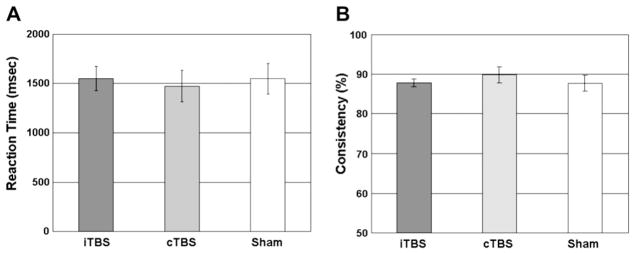
Mean reaction time (A) and consistency for response choice (B) in each theta burst stimulation (TBS) condition. Error bars represent the standard errors of the mean.
Discussion
The pattern of DD observed in our study indicated that cTBS of the right DLPFC induced healthy subjects to trade immediate reward for delayed larger amount of reward. In other word, cTBS-induced inhibition of the right DLPFC influenced decision making by reducing impulsive choices and favoring delayed-large rewards instead of immediate-small rewards. There were no changes on reaction time and response consistency after TBS indicating that the changes of k-value after cTBS were not the result of different time consuming or changes of consistency in decision making.
DD and risk taking require the same decision-making process but DD is closely related with the subjective value of delayed reward, as there is no punishment and no unpredictable state. Mental demands are more dependent on the calculation of time and reward size than on the calculation of the reward gaining probability during the task. Although the rewards in a DD task were hypothetical, several studies have shown that hypothetical and actual rewards produce similar patterns of discounting.31,32
There are two possible mechanisms of impulsive response that can modulate the DD value. The first mechanism relates to the inhibitory control exerted by the PFC shown to be impaired particularly by lesion of the right PFC.33,34 It should be acknowledged, however, that the ventromedial PFC, involving medial orbitofrontal cortex and anterior cingulate cortex (encompassing BA 25, lower BA 24/32, and medial BA 11/12/10) also plays an important inhibitory role on behavior and decision making.35,36 Thus, it is not unlikely, given the strong anatomic connections37 and coactivation38 between DLPFC and orbitofrontal cortex, that our observations may have been mediated by functional interactions between these two prefrontal areas.
The second mechanism may envisage changes in impulsivity level as consequence of alteration in time perception. Wittmann and Paulus39 demonstrated that the subjective experience of time is associated with a higher level of impulsivity. Interestingly, right DLPFC has been indicated as a critical region for time processing.40–43 In particular, Koch et al.42 showed that lesion in the right PFC caused an underestimation of time duration compared with normal subjects. The same observation was replicated by rTMS-induced inhibition over the right DLPFC with selective underestimation of perception of time intervals.43 Based on these premises, we should also consider the alternative that the “virtual lesion-like effects” of the DLPFC induced by our cTBS paradigm may have increased the selection of delay options (reduced k-value) as a result of underestimation of time delay. In this regard, a number of studies have suggested an alteration in time perception associated with impulsivity. In fact, patients with psychiatric disorders, including substance abuse44–46 and neurodegenerative diseases,47 who have experienced impulsive behavior and have demonstrated an impairment in time perception. In our study, the decrease in k-value did not change with the increase in reward magnitude, implying that cTBS-induced reduction in impulsivity was unrelated to the magnitude of reward. Some studies support this observation; the increase of k-values in the drug abuser group was preserved and showed the same pattern of k-value changes regardless of reward magnitude.1,26
Our study was not designed to investigate the underlying neurochemical changes associated with decreased impulsivity; however, we could speculate that changes in the dopaminergic system may well be responsible for these observations. In fact, the acute administration in healthy humans of d-amphetamine, a prototypical stimulant that increases synaptic level of dopamine, decreases several forms of impulsive behavior, including the DD measures with decreased k-values.29 As suggested by animal and human studies, the prefrontal-basal ganglia network is very likely the underlying neural circuit engaged in the reward choice at different time scales. In rats, lesioning of the nucleus accumbens resulted in a tendency to choose small immediate rewards over large future rewards.48 Similarly, functional imaging studies have demonstrated the engagement of different corticostriatal neural loops (i.e., orbitofrontal cortex-ventral striatum versus DLPFC-dorsal striatum) in relation to time-related decision making.49,50
There was no significant modulation of DD value after the iTBS. Although iTBS has been shown to have an excitatory effect over the motor cortex,17 it is not surprising that lack of a significant influence on the DD task. In fact, in the literature, there are several examples showing that although cTBS has a consistent effect on different complex behaviors, the same does not apply to iTBS. For instance, Franca et al.51 found that although cTBS applied over the occipital cortex affected phosphene threshold, no effect was observed with iTBS. Similarly, Wilkinson et al.52 showed that although cTBS over M1 affected probabilistic sequence learning, iTBS did not produce any consistent effect. On the same line, Voss et al.53 provided evidence that cTBS again over M1 while affecting performance in a force-matching task, iTBS did not produce any effect. Thus, whereas the reasons for this lack of iTBS effect on different behavioral performances are not immediately obvious, all those studies have claimed that differences in the physiology and functional state of the underlying cortex during the task at hand may be responsible for these observations. In fact, the modulatory effects of different rTMS interventions are critically dependent on the functional state of the stimulated cortex.17,54–57
In conclusion, we demonstrated that modulation of cortical excitability over the DLPFC induces changes in impulsivity level. The inhibition of the right DLPFC reduces the level of impulsive decision making. The current data provide insight into the role of the right DLPFC in decision making and in calculating the reward value at different time delays under less risky and nonambiguous circumstances in humans.
Acknowledgments
This work was funded by the Canadian Institutes of Health Research to A.P.S. (MOP-64423). S.S.C. was supported by the Korea Research Foundation Grant funded by the Korean Government (MOEHRD, Basic Research Promotion Fund) (KRF-2007-359-E00014).
References
- 1.Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004;99(4):461–471. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- 2.Barkley R, Edwards G, Laneri M, Fletcher K, Metevia L. Executive functioning, temporal discounting, and sense of time in adolescents with attention deWcit hyperactivity disorder (ADHD) and oppositional deWant disorder (ODD) J Abnorm Child Psychol. 2001;29:541–556. doi: 10.1023/a:1012233310098. [DOI] [PubMed] [Google Scholar]
- 3.Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behav Processes. 2003;64(3):345–354. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 4.Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–12. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 5.Ainslie G. Specious reward: a behavioral theory of impulsiveness and impulse control. Psychol Bull. 1975;82:463–494. doi: 10.1037/h0076860. [DOI] [PubMed] [Google Scholar]
- 6.Dixon MR, Jacobs EA, Sanders S, et al. Impulsivity, self-control, and delay discounting in persons with acquired brain injury. Behav Intervent. 2005;20:101–120. [Google Scholar]
- 7.Kalenscher T, Windmann S, Diekamp B, et al. Single units in the pigeon brain integrate reward amount and time-to-reward in an impulsive choice task. Curr Biol. 2005;15(7):594–602. doi: 10.1016/j.cub.2005.02.052. [DOI] [PubMed] [Google Scholar]
- 8.Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003;6:115–116. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 10.Conway MA, Fthenaki A. Disruption of inhibitory control of memory following lesions to the frontal and temporal lobes. Cortex. 2003;39:667–686. doi: 10.1016/s0010-9452(08)70859-1. [DOI] [PubMed] [Google Scholar]
- 11.Fleck MS, Daselaar SM, Dobbins IG, Cabeza R. Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and nonmemory tasks. Cereb Cortex. 2006;6(11):1623–1630. doi: 10.1093/cercor/bhj097. [DOI] [PubMed] [Google Scholar]
- 12.van’t Wout M, Kahn RS, Sanfey AG, Aleman A. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex affects strategic decision-making. Neuroreport. 2005;16(16):1849–1852. doi: 10.1097/01.wnr.0000183907.08149.14. [DOI] [PubMed] [Google Scholar]
- 13.Fecteau S, Knoch D, Fregni F, et al. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J Neurosci. 2007;727(46):12500–12505. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Knoch D, Gianotti LR, Pascual-Leone A, et al. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci. 2006;26:6469–6472. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006;314:829–832. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- 16.Di Lazzaro V, Pilato F, Saturno E, et al. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2005;565(Pt 3):945–950. doi: 10.1113/jphysiol.2005.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45(2):201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 18.Hubl D, Nyffeler T, Wurtz P, et al. Time course of blood oxygenation level-dependent signal response after theta burst transcranial magnetic stimulation of the frontal eye field. Neuroscience. 2008;151(3):921–928. doi: 10.1016/j.neuroscience.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 19.Ko JH, Monchi O, Ptito A, et al. Theta burst stimulation-induced inhibition of dorsolateral prefrontal cortex reveals hemispheric asymmetry in striatal dopamine release during a set-shifting task—a TMS–[11C]raclopride PET study. Eur J Neurosci. 2008;28:2147–2155. doi: 10.1111/j.1460-9568.2008.06501.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vallesi A, Shallice T, Walsh V. Role of the prefrontal cortex in the foreperiod effect: TMS evidence for dual mechanisms in temporal preparation. Cereb Cortex. 2007;17:466–474. doi: 10.1093/cercor/bhj163. [DOI] [PubMed] [Google Scholar]
- 21.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 22.Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. New York: Thieme; 1988. [Google Scholar]
- 23.Paus T. Imaging the brain before, during, and after transcranial magnetic stimulation. Neuropsychologia. 1999;37(2):219–224. doi: 10.1016/s0028-3932(98)00096-7. [DOI] [PubMed] [Google Scholar]
- 24.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001;21(15):1–4. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18(2):192–205. [PubMed] [Google Scholar]
- 26.Kirby K, Petry N, Bickel W. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128:78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology. 1999;146:455–464. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- 28.Richards JB, Zhang L, Mitchell SH, De Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999;71:121–143. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002;27(5):813–825. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 30.Monterosso JR, Ainslie G, Xu J, et al. Frontoparietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapp. 2007;28(5):383–393. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnson MW, Bickel WK. Within-subject comparison of real and hypothetical money rewards in delay discounting. J Exp Anal Behav. 2002;77:129–146. doi: 10.1901/jeab.2002.77-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Madden GJ, Begotka AM, Raiff BR, Kastern LL. Delay discounting of real and hypothetical rewards. Exp Clin Psychopharmacol. 2003;11:139–145. doi: 10.1037/1064-1297.11.2.139. [DOI] [PubMed] [Google Scholar]
- 33.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004;8(4):170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 34.Conway MA, Fthenaki A. Disruption of inhibitory control of memory following lesions to the frontal and temporal lobes. Cortex. 2003;39(4–5):667–686. doi: 10.1016/s0010-9452(08)70859-1. [DOI] [PubMed] [Google Scholar]
- 35.Damasio AR. The Somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996;351:1413–1420. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 36.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005;6:691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 37.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999;11(3):1011–1036. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 38.Knoch D, Treyer V, Regard M, et al. Lateralized and frequency-dependent effects of prefrontal rTMS on regional cerebral blood flow. Neuroimage. 2006;31(2):641–648. doi: 10.1016/j.neuroimage.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 39.Wittmann M, Paulus MP. Decision making, impulsivity and time perception. Trends Cogn Sci. 2008;12(1):7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Harrington DL, Haaland KY, Knight RT. Cortical networks underlying mechanisms of time perception. J Neurosci. 1998;18(3):1085–1095. doi: 10.1523/JNEUROSCI.18-03-01085.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nat Neurosci. 2001;4:317–323. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- 42.Koch G, Oliveri M, Carlesimo GA, Caltagirone C. Selective deficit of time perception in a patient with right prefrontal cortex lesion. Neurology. 2002;59(10):1658–1659. doi: 10.1212/01.wnl.0000032504.45792.8f. [DOI] [PubMed] [Google Scholar]
- 43.Koch G, Oliveri M, Torriero S, Caltagirone C. Underestimation of time perception after repetitive transcranial magnetic stimulation. Neurology. 2003;60(11):1844–1846. doi: 10.1212/wnl.60.11.1844. [DOI] [PubMed] [Google Scholar]
- 44.Reynolds B, Schiffbauer R. Measuring state changes in human delay discounting: an experiential discounting task. Behav Processes. 2004;67:343–356. doi: 10.1016/j.beproc.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 45.Berlin HA, Rolls ET. Time perception, impulsivity, emotionality, and personality in self-harming borderline personality disorder patients. J Personal Disord. 2004;18:358–378. doi: 10.1521/pedi.18.4.358.40349. [DOI] [PubMed] [Google Scholar]
- 46.Wittmann M, Leland DS, Paulus MP. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res. 2007;179:643–653. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- 47.Koch G, Costa A, Brusa L, et al. Impaired reproduction of second but not millisecond time intervals in Parkinson’s disease. Neuropsychologia. 2008;46(5):1305–1313. doi: 10.1016/j.neuropsychologia.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Cardinal RN, Robbins TW, Everitt BJ. The effects of d-amphetamine, chlordiazepoxide, alpha-flupenthixol and behavioral manipulations on choice of signaled and unsignaled delayed reinforcement in rats. Psychopharmacology. 2000;152:362–375. doi: 10.1007/s002130000536. [DOI] [PubMed] [Google Scholar]
- 49.Tanaka SC, Doya K, Okada G, et al. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004;7(8):887–893. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka SC, Samejima K, Okada G, et al. Brain mechanism of reward prediction under predictable and unpredictable environmental dynamics. Neural Netw. 2006;19(8):1233–1241. doi: 10.1016/j.neunet.2006.05.039. [DOI] [PubMed] [Google Scholar]
- 51.Franca M, Koch G, Mochizuki H, et al. Effects of theta burst stimulation protocols on phosphene threshold. Clin Neurophysiol. 2006;117(8):1808–1813. doi: 10.1016/j.clinph.2006.03.019. [DOI] [PubMed] [Google Scholar]
- 52.Wilkinson L, Teo JT, Obeso I, et al. The contribution of primary motor cortex is essential for probabilistic implicit sequence learning: evidence from theta burst magnetic stimulation [published ahead of print March 20 2009] J Cogn Neurosci. doi: 10.1162/jocn.2009.21208. doi: 10.1162. [DOI] [PubMed] [Google Scholar]
- 53.Voss M, Bays PM, Rothwell JC, et al. An improvement in perception of self-generated tactile stimuli following theta-burst stimulation of primary motor cortex. Neuropsychologia. 2007;45(12):2712–2717. doi: 10.1016/j.neuropsychologia.2007.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fierro B, Brighina F, Vitello G, et al. Modulatory effects of low- and high-frequency repetitive transcranial magnetic stimulation on visual cortex of healthy subjects undergoing light deprivation. J Physiol. 2005;565(Pt2):659–665. doi: 10.1113/jphysiol.2004.080184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci. 1998;18(3):1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Enomoto H, Ugawa Y, Hanajima R, et al. Decreased sensory cortical excitability after 1 Hz rTMS over the ipsilateral primary motor cortex. Clin Neurophysiol. 2001;112:2154–2158. doi: 10.1016/s1388-2457(01)00667-8. [DOI] [PubMed] [Google Scholar]
- 57.Pascual-Leone A, Valls-Sole J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]


