Abstract
ErbB4has emerged as a leading susceptibility gene for schizophrenia but the function of the ErbB4 receptor in the adult brain is unknown. Here we show in the adult hippocampus that long-term potentiation (LTP) of transmission at Schaffer-collateral CA1 synapses was markedly enhanced in mutant mice lacking ErbB4. Concordantly, LTP was enhanced by acutely blocking ErbB4 in wild type animals, indicating that ErbB4 activity constitutively suppresses LTP. Moreover, increasing ErbB4 signaling further suppressed LTP. By contrast, altering ErbB4 activity did not affect basal synaptic transmission or short-term facilitation. Our findings suggest that cognitive deficits in schizophrenia may be a consequence of hyperfunction of ErbB4 signaling leading to suppressed glutamatergic synaptic plasticity, thus opening new approaches for treatment of this disorder.
Keywords: ErbB4, transgenic mouse, neuregulin, Schaffer collateral-CA1 synapses, long-term potentiation, theta burst stimulation, synaptic plasticity, paired-pulse facilitation
Introduction
A growing body of linkage and association evidence in humans implicates ErbB4 as a leading susceptibility gene for schizophrenia [10,21], but a critical unresolved issue is defining the essential functions of ErbB4 in the brain and the link to schizophrenia [22]. Because deficits in cognition are a core feature of schizophrenia [12] and because a critical cellular substrate for normal cognitive functioning is synaptic plasticity at glutamatergic synapses [13], we used a loss-of-function approach to investigate the hypothesis that ErbB4 may function to regulate glutamatergic synaptic transmission or plasticity.
ErbB4 is expressed in adult CNS [5], and in the adult hippocampus, a brain region increasingly implicated in schizophrenia [3,10,20], ErbB4 is abundantly expressed by pyramidal cells (eg. [4,7,14] and Fig. 1a, b, c) and is enriched at glutamatergic synapses in the post synaptic density [7]. ErbB4 associates with the scaffolding protein PSD-95 [4,6,7] and through this with the NMDA receptor [4,6,7], a subtype of glutamate receptor having a key role in synaptic plasticity. Our evidence indicates that tonic activity of ErbB4 constitutively suppresses long-term potentiation (LTP) of glutamatergic synaptic transmission in the adult hippocampus. Our findings reveal a previously unknown essential function of ErbB4 in the brain, suggesting that cognitive deficits in schizophrenia may be a consequence of hyperfunction of ErbB4 signaling thereby suppressing plasticity at glutamatergic synapses.
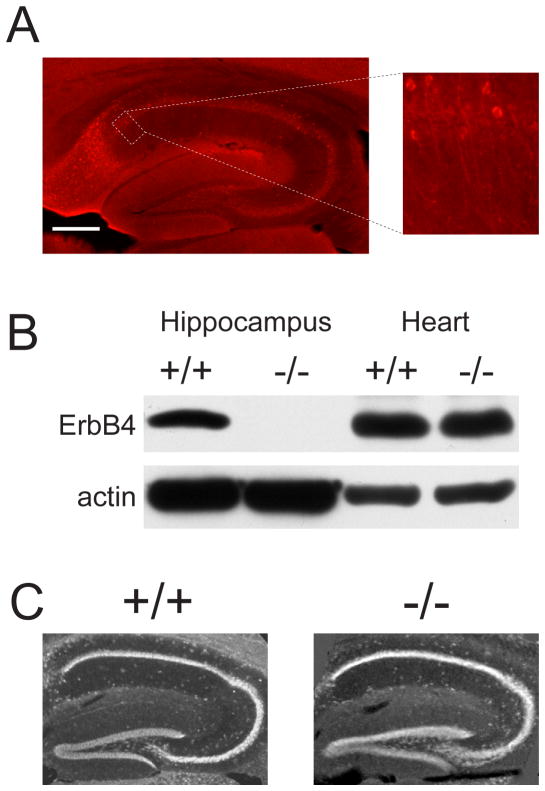
Fig. 1.
Hippocampal ErbB4 expression and morphology in ErbB4+/+HER4heart and ErbB4−/−HER4heart mice. (a) ErbB4 immunoreactivity (shown in red) in a parasagittal hippocampal section from an adult ErbB4+/+HER4heart mouse. Scale bar, 200 μm. Right: Higher magnification fluorescent micrograph representing CA1 region indicated by the dotted line. Scale bar, 150 μm. (b) Adjacent ErbB4+/+HER4heart hippocampal coronal sections incubated with anti-ErbB4 antibody (left) or with anti-ErbB4 antibody pre-incubated with its antigenic peptide (right). ErbB4 immunoreactivity in red and NeuN immunoreactivity is shown in green. Scale bar, 100 μm. (c) Photomicrograph of hippocampal CA1 from an adult ErbB4+/+HER4heart mouse showing distribution of immunoreactivity for ErbB4 (red), MAP2 (blue) and NeuN (green). Scale bar, 25 μm (d) Western blot analysis of ErbB4 protein expression in ErbB4+/+HER4heart (+/+) and ErbB4−/−HER4heart (−/−) mice. (e) Nissl staining in parasagittal hippocampal sections from ErbB4+/+HER4heart (+/+) and ErbB4−/−HER4heart (−/−) mice. Scale bar, 200 μm.
Methods
ErbB4 null mutant mice
ErbB4 null mice were kindly provided by Martin Gassmann [23]. Embryonic lethality of ErbB4−/− mice was genetically rescued by expressing ErbB4 under a cardiac-specific myosin promoter (ErbB4−/−HER4heart). These mice do not express ErbB4 in the brain or non-cardiac tissues.
Immunocytochemistry
Anesthetized adult male ErbB4+/+HER4heart and ErbB4−/−HER4heart mice were perfused transcardially with 4% paraformaldehyde. Brains were removed, post-fixed, and placed in 30% sucrose. Para-sagittal 50 μm frozen sections were collected in cold 0.1 M phosphate-buffered saline and stained with Nissl or processed for immunohistochemistry using anti-NeuN antibody (1:2000, Chemicon) or anti-ErbB4 antibody (SC-283; 1:500, Santa Cruz) with or without its immunogenic peptide. Immunostaining was revealed using fluorescently-conjugated secondary antibodies.
Immunoblotting
Hippocampal or heart tissue from ErbB4+/+HER4heart and ErbB4−/−HER4heart mice was homogenized in buffer (in mM): 20 Tris-HCl, pH 8, 137 NaCl, 1% NP-40, 10% glycerol, and the protease inhibitor cocktail (Sigma). Proteins were resolved in SDS-PAGE, transferred onto a nitrocellulose membrane, and visualized using enhanced chemiluminescence. Antibodies used were ErbB4 (Cell Signaling Technology) and β-actin (Sigma).
Electrophysiology
Hippocampal slices (300 μm) prepared from anesthetized (20% urethane, i.p.) ~ 20 week-old ErbB4+/+HER4heart (n = 4 mice) or ErbB4−/−HER4heart (n = 4 mice) mice or 21 day old male Sprague Dawley rats (Charles River) were placed in a holding chamber for ≥1 hr prior to recording. A single slice was then transferred to a recording chamber and superfused with artificial cerebral spinal fluid (ACSF; at 2 ml/min) composed of (in mM): 132 NaCl, 3 KCl, 1.25 NaH2PO4, 2 MgCl, 11 D-glucose, 20 NaH2CO3 and 2 CaCl2 saturated with 95% O2 (balance 5% CO2) at 28 ± 2 °C (pH 7.40; 315–325 mOsm). ACSF was supplemented with bicuculline methiodide (5 μM). Field excitatory postsynaptic potentials (fEPSPs) were evoked using bipolar tungsten electrodes located ~50 μm from the CA1 cell-body layer and were recorded using ACSF-filled glass micropipettes placed in the stratum radiatum 60–80 μm from the cell body layer. Stimulation of Schaffer collateral afferents consisted of single pulses (0.08 ms duration) at 0.1 Hz; intensity was set to 30–35% of that which produced maximum synaptic responses. Theta burst stimulation consisted of 15 bursts of 4 pulses at 100 Hz, delivered at an interstimulus interval of 200 ms. ACSF was supplemented as indicated with neuregulin-1β, NRG-1β, (2 nM), which was stored as single-use aliquots in aqueous solution at −80 °C. ACSF was also supplemented as indicated with PD158780 (10 μM; dissolved in DMSO), which was made fresh immediately before the experiment. fEPSP slope was calculated as the slope of the 10–60% rising phase. Raw data were amplified using a MultiClamp 700A amplifier and a Digidata 1322A acquisition system sampled at 10 KHz, and analyzed with Clampfit 9.0 and Sigmaplot 7 software. Recordings were done with the experimenter blind to mouse genotype. Statistical comparison of data, presented as mean (± SEM), was done using two-way analysis of variance (ANOVA) with the Tukey Test. Experiments were in accordance with policies of The Hospital for Sick Children Animal Care Committee and the Canadian Council on Animal Care.
Results
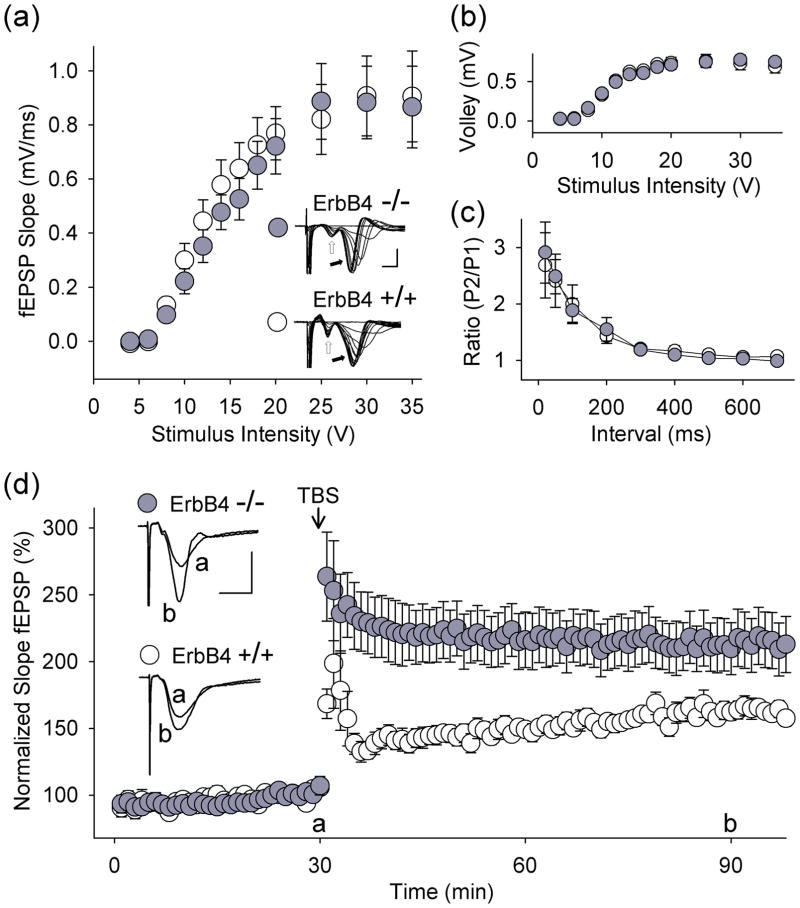
In our experiments we used mutant mice [23] in which ErbB4 had been deleted but in which expression of the human ErbB4 was driven in the heart by the α-myosin heavy chain promoter (ErbB4−/−HER4heart) which rescued an otherwise lethal cardiac defect (Fig. 1d). In adult ErbB4−/−HER4heart mice, we found that the size, anatomy and gross morphology of the hippocampus were not different from wild-type littermate controls (ErbB4+/+HER4heart; Fig. 1e). Moreover, in electrophysiological recordings using adult ErbB4−/−HER4heart mice, we found that at Schaffer collateral-CA1 synapses in acute hippocampal slices, the basal stimulus-response relationships for afferent fiber volleys and fEPSPs (Fig. 2a,b) and for paired-pulse facilitation, a measure of presynaptic function (Fig. 2c), were not different from wild-type littermates. We thus next investigated synaptic plasticity at these synapses by studying LTP induced by theta-burst stimulation (TBS), a stimulation paradigm mimicking the endogenous theta rhythm that is critical for normal cognitive processing [8]. We found that theta-burst-induced LTP (tbLTP) was dramatically increased in the ErbB4−/−HER4heart mice (Fig. 2d): in ErbB4 mutant animals fEPSP slope was 210 ± 20 % (n = 14) 60 min after TBS compared with 162 ± 6 % (n = 14) in wild-type mice (p < 0.01). Thus, while ErbB4−/−HER4heart mice had no abnormality of basal synaptic transmission or pre-synaptic function, they showed greatly increased tbLTP. Therefore, these findings indicate that ErbB4 genetically suppresses a prominent form of synaptic plasticity at Schaffer collateral-CA1 synapses.
Fig. 2.
Loss of function of ErbB4 enhances tbLTP in CA1 hippocampus. (a) fEPSP slope and (b) fiber volley amplitude plotted as a function of stimulus intensity (ErbB4+/+HER4heart (ErbB4+/+), open circles; ErbB4−/−HER4heart (ErbB4−/−), filled circles). Strength of Schaffer collateral stimulation is indicated on the horizontal axis. Representative traces show fiber volley (open arrow) and fEPSPs (filled arrow; scale bars: 2 ms, 1 mV). In all panels, data are shown as mean ± SEM. (c) Paired-pulse facilitation of fEPSPs in slices from ErbB4+/+HER4heart (open circles) and ErbB4−/−HER4heart(filled circles) mice. Interstimulus interval is indicated on the horizontal axis. P1, first response; P2, second response. (d) Summary scatter plot shows grouped normalized fEPSP slope every 1 min in slices from ErbB4+/+HER4heart (open circles, n = 14 slices) and from ErbB4−/−HER4heart (filled circles, n = 14 slices) mice. Theta-burst stimulation was delivered to Schaffer collateral-CA1 synapses at the 30 min time point. fEPSP slope was normalized with respect to the mean slope of fEPSPs recorded during the 10 min period immediately before TBS. Inset: average of six consecutive fEPSPs recorded before or after TBS (‘a’ or ‘b’, respectively; scale bars: 10 ms, 0.5 mV). p < 0.01, ErbB4+/+HER4heart vs. ErbB4−/−HER4heart, 60 min after TBS.
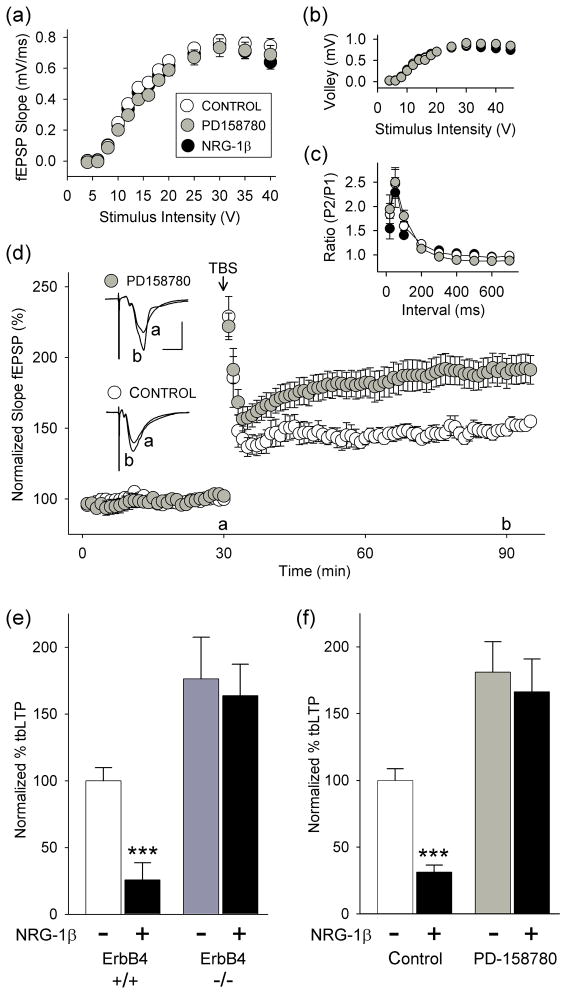
To determine whether the enhancement of tbLTP seen in the ErbB4−/−HER4heart mice was due to loss of ErbB4 signaling in the adult or to an abnormality due to lack of ErbB4 during development, we examined the effect of acutely inhibiting ErbB4 receptors on tbLTP in normal adult animals. For this purpose we used the potent and membrane-permeable ErbB kinase inhibitor, PD158780, which is known to prevent receptor autophosphorylation and signaling [19]. We found that bath-applied PD158780 (10 μM) had no effect on volley amplitude, basal fEPSPs or on paired-pulse facilitation (Fig. 3a,b,c). However, tbLTP was greatly enhanced in slices treated with PD158780 (Fig. 3d): with PD158780 application fEPSP slope was 189 ± 11 % (n = 21) 60 min after TBS compared with 149 ± 4 % (n = 13) in control, untreated slices (p < 0.001). As PD158780 may act on ErbB receptors in addition to ErbB4, we tested it in slices from ErbB4−/−HER4heart mice and found that it had no effect on tbLTP (data not illustrated), indicating that the enhancement of tbLTP by PD158780 is dependent upon ErbB4. Thus, in the adult hippocampus ErbB4 signaling constitutively suppresses synaptic plasticity.
Fig 3.
Pharmacological inhibition of ErbB4 increases tbLTP in CA1 hippocampus and prevents the suppression of tbLTP by NRG-1β. (a) fEPSP slope and (b) fiber volley amplitude plotted as a function of stimulus intensity at rat Schaffer collateral-CA1 synapses in control slices (open circles) and in slices treated with NRG-1β (2 nM; black circles) or PD158780 (10 μM; gray circles). Strength of Schaffer collateral stimulation is indicated on the horizontal axis. In this and the subsequent panel, data are shown as mean ± SEM. (c) Paired-pulse facilitation of fEPSPs in control slices and in slices treated with NRG-1β (black circles) or PD158780 (gray circles). Interstimulus interval is indicated on the horizontal axis. P1, first response; P2, second response. (d) Summary scatter plot shows grouped normalized fEPSP slope plotted every 1 min in control (open circles, n = 13) and PD158780-treated (filled circles, n = 21; in ACSF beginning 25 min before TBS with final concentration of 10 μM) slices from rats. Inset: average of six consecutive fEPSPs recorded before or after TBS (‘a’ or ‘b’, respectively; scale bars: 10 ms, 0.6 mV). p < 0.001, control vs. PD158780, 60 min after TBS. (e) Histogram shows TBS-induced increase in fEPSP slope 60 min after TBS in slices from ErbB4+/+HER4heart (white bar) and ErbB4−/−HER4heart (dark gray bar) mice without (−) and with (+; black bars) NRG-1β treatment (in ACSF beginning 20 min before TBS with final concentration of 2 nM). Results are expressed as a percentage of TBS-induced increase in fEPSP slope (% tbLTP) with tbLTP in ErbB4+/+HER4heart slices (white bar) normalized to 100 %. *** p < 0.001 vs. ErbB4+/+HER4heart (white bar); p < 0.05, ErbB4−/−HER4heart/NRG-1β (black bar) vs. ErbB4+/+HER4heart (white bar). (f) Histogram shows normalized TBS-induced mean increase in fEPSP slope in control (white bar) and PD158780-treated slices (light gray bar) without (−) and with (+; black bars) NRG-1β (2 nM administered as above). *** p < 0.001 vs. control (white bar); p < 0.01, PD158780/NRG-1β (black bar) vs. control (white bar). Data are taken 60 min after TBS.
To determine whether increasing ErbB4 signaling beyond the constitutive level may further suppress tbLTP we compared the effect of neuregulin, NRG-1β, an agonist for receptors of the ErbB family, in ErbB4−/−HER4heart versus wild-type mice. We and others have shown that NRG-1β impairs synaptic plasticity [1,7,9]. We found here that bath-applying NRG-1β (2 nM) nearly abolished tbLTP in slices from wild-type mice but had no effect on tbLTP in those from ErbB4−/−HER4heart mice (Fig. 3e), indicating that NRG-1β inhibits tbLTP through activating ErbB4. Importantly, NRG-1β had no effect on basal synaptic transmission or paired-pulse facilitation (Fig. 3a,b,c) and PD158780 prevented the suppression of tbLTP by applying NRG-1β in slices from normal animals (Fig. 3f). Thus, under basal conditions the effect of ErbB4 signaling on tbLTP is not maximal in normal animals, and causing hyperfunction in this signaling pathway further suppresses synaptic plasticity.
Discussion
To study the non-redundant functions of ErbB4 in the hippocampus we used ErbB4−/−HER4heart mice. We found that basal synaptic transmission and paired-pulse facilitation were not different from wild type whereas LTP was increased in the mice lacking ErbB4. There were no detectable developmental abnormalities in the hippocampus of the ErbB4 mutant mice that might have accounted for this enhanced LTP, and moreover, acutely administering PD158780 to adult hippocampal slices from wild type animals was without effect on basal synaptic transmission but caused an increase in LTP. We note that the increase in LTP by PD15870 appears be mediated by blockade of ErbB4 as this compound had no effect on LTP in the ErbB4 mutant mice. The cognate ligand for ErbB4 receptors is NRG1 [18] and we found that the suppression of LTP by administering NRG-1β [1,7,9] was prevented in the ErbB4 mutant mice and also by PD158780. The most parsimonious explanation for these findings taken together is that a non-redundant function of NRG1-ErbB4 signaling in the hippocampus is to constitutively suppress LTP at Schaffer collateral-CA1 synapses.
The enhancement of LTP in the ErbB4 null mutant mice, and the enhancement by acutely administering PD158780, is consistent with predictions from the suppression of LTP produced by administering exogenous NRG-1β [1,7,9]. Recently, it has been reported in hippocampal slice cultures that synaptic spine density and pairing-induced LTP are reduced by decreasing the expression of ErbB4 or by overexpressing a kinase dead ErbB4 mutant [11]. While it is conceivable that the difference between this study and those in which NRG-1β was administered as possibly due to exogenous NRG-1β not mimicking the endogenous ligand [11], this explanation cannot account for the enhancement of LTP seen presently in the mice lacking ErbB4 nor the enhancement by applying PD158780. Rather, the apparently divergent results may reflect differences between acute slices and cultures, or between NRG1-ErbB4 signaling during development as opposed to in the adult.
ErbB4 receptors are known to be expressed by GABAergic neurons [25] and activation of ErbB4 enhances presynaptic release of GABA in prefrontal cortex [24]. In present study, recordings were carried out in the presence of the GABAA receptor antagonist, bicuculline. Thus, our findings are independent of GABAA-mediated inhibitory mechanisms.
Both ErbB4 and NRG1 have emerged as leading susceptibility genes for schizophrenia [15,16,21]. One prominent hypothesis regarding the pathogenesis of schizophrenia is NMDA receptor hypofunction [2,17]. Recently, it has been found in post-mortem prefrontal cortex in schizophrenia that NRG1-ErbB4 signaling is enhanced and that there is increased NRG1-induced attenuation of NMDA receptor signaling [6]. Here we find in mice and rats that a constitutive function of ErbB4 is to suppress activity-dependent glutamatergic synaptic plasticity without altering synaptic transmission per se and that this plasticity, which is NMDA receptor-dependent, can be further suppressed by enhancing NRG1-ErbB4 signaling. Taken together, we propose that a cellular substrate for the cognitive dysfunction in schizophrenia is suppression of the plasticity of glutamatergic synaptic transmission by a gain-of-function of NRG1-ErbB4 signaling.
Conclusion
We find that NRG1-ErbB4 signaling constitutively suppresses LTP at Schaffer collateral synapses in CA1 hippocampus without affecting basal synaptic transmission. This provides a unifying concept for understanding the mechanistic basis of schizophrenia risk pathways in that cognitive deficits may result from excessive function of NRG1-ErbB4 signaling further suppressing synaptic plasticity. Interfering with the suppression of synaptic plasticity caused by NRG1-ErbB4 signaling thus represents a novel strategy for treatment of this prevalent and disabling disorder.
Acknowledgments
Supported by grants from the Canadian Institutes of Health Research (CIHR) to MWS and the NIH/NINDS to LM. MWS is an International Research Scholar of the Howard Hughes Medical Institute and holds a Canada Research Chair (Tier I) in Neuroplasticity and Pain. We thank Dr. Martin Gassmann for kindly providing ErbB4−/−HER4heart mice.
Footnotes
Disclaimer: The authors have no conflicts of interest to declare.
References
- 1.Bjarnadottir M, Misner DL, Haverfield-Gross S, Bruun S, Helgason VG, Stefansson H, et al. Neuregulin1 (NRG1) signaling through Fyn modulates NMDA receptor phosphorylation: differential synaptic function in NRG1+/− knock-outs compared with wild-type mice. J Neurosci. 2007;27:4519–4529. doi: 10.1523/JNEUROSCI.4314-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coyle JT, Tsai G, Goff D. Converging evidence of NMDA receptor hypofunction in the pathophysiology of schizophrenia. Ann N Y Acad Sci. 2003;1003:318–327. doi: 10.1196/annals.1300.020. [DOI] [PubMed] [Google Scholar]
- 3.Gao XM, Sakai K, Roberts RC, Conley RR, Dean B, Tamminga CA. Ionotropic glutamate receptors and expression of N-methyl-D-aspartate receptor subunits in subregions of human hippocampus: effects of schizophrenia. Am J Psychiatry. 2000;157:1141–1149. doi: 10.1176/appi.ajp.157.7.1141. [DOI] [PubMed] [Google Scholar]
- 4.Garcia RA, Vasudevan K, Buonanno A. The neuregulin receptor ErbB-4 interacts with PDZ-containing proteins at neuronal synapses. Proc Natl Acad Sci U S A. 2000;97:3596–3601. doi: 10.1073/pnas.070042497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gerecke KM, Wyss JM, Karavanova I, Buonanno A, Carroll SL. ErbB transmembrane tyrosine kinase receptors are differentially expressed throughout the adult rat central nervous system. J Comp Neurol. 2001;433:86–100. doi: 10.1002/cne.1127. [DOI] [PubMed] [Google Scholar]
- 6.Hahn CG, Wang HY, Cho DS, Talbot K, Gur RE, Berrettini WH, et al. Altered neuregulin 1-erbB4 signaling contributes to NMDA receptor hypofunction in schizophrenia. Nat Med. 2006;12:824–828. doi: 10.1038/nm1418. [DOI] [PubMed] [Google Scholar]
- 7.Huang YZ, Won S, Ali DW, Wang Q, Tanowitz M, Du QS, et al. Regulation of neuregulin signaling by PSD-95 interacting with ErbB4 at CNS synapses. Neuron. 2000;26:443–455. doi: 10.1016/s0896-6273(00)81176-9. [DOI] [PubMed] [Google Scholar]
- 8.Kahana MJ, Seelig D, Madsen JR. Theta returns. Curr Opin Neurobiol. 2001;11:739–744. doi: 10.1016/s0959-4388(01)00278-1. [DOI] [PubMed] [Google Scholar]
- 9.Kwon OB, Longart M, Vullhorst D, Hoffman DA, Buonanno A. Neuregulin-1 reverses long-term potentiation at CA1 hippocampal synapses. J Neurosci. 2005;25:9378–9383. doi: 10.1523/JNEUROSCI.2100-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Law AJ, Kleinman JE, Weinberger DR, Weickert CS. Disease-associated intronic variants in the ErbB4 gene are related to altered ErbB4 splice-variant expression in the brain in schizophrenia. Hum Mol Genet. 2007;16:129–141. doi: 10.1093/hmg/ddl449. [DOI] [PubMed] [Google Scholar]
- 11.Li B, Woo RS, Mei L, Malinow R. The neuregulin-1 receptor erbB4 controls glutamatergic synapse maturation and plasticity. Neuron. 2007;54:583–597. doi: 10.1016/j.neuron.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald AW, III, Carter CS. Cognitive experimental approaches to investigating impaired cognition in schizophrenia: a paradigm shift. J Clin Exp Neuropsychol. 2002;24:873–882. doi: 10.1076/jcen.24.7.873.8386. [DOI] [PubMed] [Google Scholar]
- 13.MacDonald AW, III, Chafee MV. Translational and developmental perspective on N-methyl-D-aspartate synaptic deficits in schizophrenia. Dev Psychopathol. 2006;18:853–876. [PubMed] [Google Scholar]
- 14.Mechawar N, Lacoste B, Yu WF, Srivastava LK, Quirion R. Developmental profile of neuregulin receptor ErbB4 in postnatal rat cerebral cortex and hippocampus. Neuroscience. 2007;148:126–139. doi: 10.1016/j.neuroscience.2007.04.066. [DOI] [PubMed] [Google Scholar]
- 15.Nicodemus KK, Luna A, Vakkalanka R, Goldberg T, Egan M, Straub RE, et al. Further evidence for association between ErbB4 and schizophrenia and influence on cognitive intermediate phenotypes in healthy controls. Mol Psychiatry. 2006;11:1062–1065. doi: 10.1038/sj.mp.4001878. [DOI] [PubMed] [Google Scholar]
- 16.Norton N, Moskvina V, Morris DW, Bray NJ, Zammit S, Williams NM, et al. Evidence that interaction between neuregulin 1 and its receptor erbB4 increases susceptibility to schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2006;141:96–101. doi: 10.1002/ajmg.b.30236. [DOI] [PubMed] [Google Scholar]
- 17.Pilowsky LS, Bressan RA, Stone JM, Erlandsson K, Mulligan RS, Krystal JH, et al. First in vivo evidence of an NMDA receptor deficit in medication-free schizophrenic patients. Mol Psychiatry. 2006;11:118–119. doi: 10.1038/sj.mp.4001751. [DOI] [PubMed] [Google Scholar]
- 18.Plowman GD, Green JM, Culouscou JM, Carlton GW, Rothwell VM, Buckley S. Heregulin induces tyrosine phosphorylation of HER4/p180erbB4. Nature. 1993;366:473–475. doi: 10.1038/366473a0. [DOI] [PubMed] [Google Scholar]
- 19.Rewcastle GW, Murray DK, Elliott WL, Fry DW, Howard CT, Nelson JM, et al. Tyrosine kinase inhibitors. 14. Structure-activity relationships for methylamino-substituted derivatives of 4-[(3-bromophenyl)amino]-6-(methylamino)-pyrido[3,4-d]pyrimidine (PD 158780), a potent and specific inhibitor of the tyrosine kinase activity of receptors for the EGF family of growth factors. J Med Chem. 1998;41:742–751. doi: 10.1021/jm970641d. [DOI] [PubMed] [Google Scholar]
- 20.Siekmeier PJ, Hasselmo ME, Howard MW, Coyle J. Modeling of context-dependent retrieval in hippocampal region CA1: implications for cognitive function in schizophrenia. Schizophr Res. 2007;89 :177–190. doi: 10.1016/j.schres.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 21.Silberberg G, Darvasi A, Pinkas-Kramarski R, Navon R. The involvement of ErbB4 with schizophrenia: association and expression studies. Am J Med Genet B Neuropsychiatr Genet. 2006;141:142–148. doi: 10.1002/ajmg.b.30275. [DOI] [PubMed] [Google Scholar]
- 22.Straub RE, Weinberger DR. Schizophrenia genes - famine to feast. Biol Psychiatry. 2006;60:81–83. doi: 10.1016/j.biopsych.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 23.Tidcombe H, Jackson-Fisher A, Mathers K, Stern DF, Gassmann M, Golding JP. Neural and mammary gland defects in ErbB4 knockout mice genetically rescued from embryonic lethality. Proc Natl Acad Sci U S A. 2003;100:8281–8286. doi: 10.1073/pnas.1436402100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woo RS, Li XM, Tao Y, Carpenter-Hyland E, Huang YZ, Weber J, et al. Neuregulin-1 enhances depolarization-induced GABA release. Neuron. 2007;54:599–610. doi: 10.1016/j.neuron.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 25.Yau HJ, Wang HF, Lai C, Liu FC. Neural development of the neuregulin receptor ErbB4 in the cerebral cortex and the hippocampus: preferential expression by interneurons tangentially migrating from the ganglionic eminences. Cereb Cortex. 2003;13:252–264. doi: 10.1093/cercor/13.3.252. [DOI] [PubMed] [Google Scholar]