Abstract
Decision making is a cognitive function relaying on a complex neural network. In particular, the right dorsolateral prefrontal cortex (DLPFC) plays a key role within this network. We used positron emission tomography (PET) combined with continuous theta burst transcranial magnetic stimulation (cTBS) to investigate neuronal and behavioral changes in normal volunteers while performing a delay discounting (DD) task. We aimed to test whether stimulation of right DLPFC would modify the activation pattern of the neural circuit underlying decision making during the DD task and influence discounting behavior.
We found that cTBS of the right DLPFC influenced decision making by reducing impulsivity and inducing participants to favor large but delayed rewards instead of immediate but small rewards. Stimulation also affected activation in several prefrontal areas associated with DD. In particular, we observed a reduced regional cerebral blood flow (rCBF) in the ipsilateral DLPFC (BA 46) extending into the rostral part of the prefrontal cortex (BA 10) as well as a disrupted relationship between impulsivity (k-value) and rCBF in these and other prefrontal areas.
These findings suggest that transcranial magnetic stimulation of the DLPFC influences the neural network underlying impulsive decision making behavior.
Keywords: rTMS, Theta burst stimulation, Dorsolateral prefrontal cortex, Decision making, Impulsivity, Delay discounting task
Introduction
It has been demonstrated that both humans and experimental animals generally value immediate reward more than delayed reward. In everyday life, individuals tend to compare potential benefit versus cost and choose the most valuable option under a given situation. During this decision making process, people may assign different weights to rewards, with time playing an important factor in calculating the value of that reward.
Frequently, individual characteristics such as personality and personal preferences guide this decision making process. Impulsivity is a personality trait that is considered to play an important role in decision making. This behavior is associated with actions that are poorly conceived, prematurely expressed, unduly risky, or inappropriate to the situation and that often result in undesirable outcomes [1]. As a measure of impulsivity, delay discounting (DD) is a behavioral analytic approach utilized to understand how each individual makes a choice between a smaller reward given immediately and a larger reward given after a time delay. This metric can therefore be used to assess impulsivity [2]. Significantly elevated discounting tendencies are observed in various patient groups with impulsive behavior. For example, drug abusers or pathological gambler, obsessive compulsive disorder and attention deficit hyperactivity disorder patients show larger preference for immediate but smaller reward then delayed but larger reward in a DD task [3–5].
Thus far, there have been a number of studies that have tried to investigate the neural substrates underlying the decision making process associated with the DD paradigm. These reports have documented the involvement of different areas in the prefrontal cortex (PFC) including the dorsolateral prefrontal cortex (DLPFC), medial prefrontal cortex (MePFC) with the anterior cingulated cortex (ACC) and the orbitofrontal cortex (OFC), along with the inferior parietal region and ventral striatum (i.e. nucleus accumbens) [6–17].
Despite functional neuroimaging studies providing some insight on the neural control of DD decisions, imaging alone suffers from the limitation that it can only provide neuronal correlates of cognitive performance and often cannot determine a causal relation between observed brain activity and behavioral performance [18,19]. Thus, the specific functional relevance (active role vs simple epiphenomenon) of those structures during DD remains to be established.
There is evidence that the right hemisphere plays an important role in inhibiting impulsive behavior. In particular, the DLPFC along with the inferior frontal cortex and OFC are critical for response inhibition [20–23]. Recently, using non-invasive stimulation tools such as repetitive transcranial magnetic stimulation (rTMS) and transcranial direct current stimulation (tDCS), some studies have suggested that the right DLPFC may affect decision making [24–27] by modulating its neuronal excitability. In particular, we were able to show that cTBS of the right DLPFC reduced the discounting rate (k-value) during the DD task compared to sham stimulation providing evidence that cTBS-induced modulation of cortical excitability of the right DLPFC may reduce impulsive decision making [28].
The aim of the present study was to extend our previous observations [28] to investigate, during positron emission tomography (PET), whether TBS-induced changes in the level of excitability of the DLPFC in the right hemisphere would affect the activations elsewhere associated with decision making during DD and interfere with impulsivity level.
Methods
Subjects
Eight right-handed young healthy subjects (mean age: 22.6 ± 2.7 y; age range: 18–27 y; 4 women) were enrolled in this study. Exclusion criteria included history of psychiatric and/or neurological disorder, including epilepsy, any previous exposure to stimulant drugs, head injury, pregnancy, and migraine. Handedness was assess using the Edinburgh Handedness Inventory [29]. Applicants with a laterality index lower than 40 for Edinburgh Handedness Inventory were excluded from the study. Subjects were screened as well for depression using the Beck Depression Inventory (BDI) with an exclusion criterion of a score of >10. A trait measure of impulsivity was collected using the Barratt Impulsivity Scale-11 (BIS). The BIS is a self-reported questionnaire containing 30 questions on a 4-point Likert scale reflecting frequency of occurrence. Scoring yields a total score and three subscale scores: attention (rapid shifts and impatience with complexity), motor (impetuous action) and non-planning (lack of future orientation) [30] scores. Higher scores indicate higher impulsivity. The mean values for our subjects are presented in Table 1. Written informed consent was obtained in all cases before the study enrollment; the study protocol was approved by the Ethical Committee of the Center for Addiction and Mental Health Research, University of Toronto.
Table 1.
Characteristics of the participants.
| Characteristic | |
|---|---|
| Age | 22.6 y (S.D = 2.7) |
| Gender | |
| Female | 50% |
| Male | 50% |
| Education | 14.5 y (1.3) |
| BDI | 2.0 (2.1) |
| BIS | |
| Total | 58.0 (10.0) |
| Motor | 21.6 (4.1) |
| Attention | 14.9 (3.6) |
| Non-planning | 21.6 (4.6) |
BDI = Beck Depression Inventory; BIS = Barratt Impulsivity Scale-11.
Behavioral task
This study used three behavioral tasks: delay discounting (DD), magnitude discrimination and a physical discrimination task (Fig. 1). The magnitude discrimination and physical discrimination tasks were used as control tasks. These control tasks were designed to be identical to the DD task with regards to visual perception, motor effects, number of trials and number of choice options in each trial. The choice stimuli were presented on the screen for 3 s and the inter-stimulus interval was 2 s.
Figure 1.
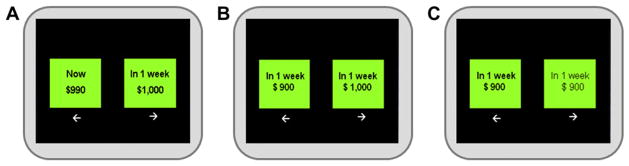
Examples of each behavioral task. (A) Delay discounting task, (B) Magnitude discrimination task, (C) Physical discrimination task.
In each trial of the DD task, the amounts of monetary reward for immediate and delay options were decided by the fixed k-value, and the delay time were based on the hyperbolic function of delay discount, V = A/(1 + kD), where V is the value of the delayed outcome (i.e., the indifference value), A is the delayed reward, D is the length of the delay, and k expresses the steepness of the discount function [31–33]. Based on this function, higher k-values are associated with preference for immediate small-size reward and lower k-values show a preference for delayed large-size reward. Thus, low k-values are an index of minor impulsivity. Subjects were instructed to express a preference judgment between two hypothetical rewards shown on a computer screen. All reward choices were made by pressing either the ←or → key on keyboard with the subject’s dominant hand (right hand for all subjects). The available time delays were 6 in total (1 week, 3 weeks, 3 months, 6 months, 1 year, and 3 years). The predefined k-values were 0.0005, 0.0028, 0.0050, 0.0275, 0.05, 0.075, 0.1, 0.3, 0.5 and 0.7; the same number of trials was assigned for each k-value.
In the magnitude preference task (i.e. control task), subjects had to express a preference between two hypothetical rewards shown on a computer screen just like in the DD task but there was no temporal delay component. In the physical discrimination task (i.e. control task), subjects had to select the option that was presented in a bold font. Before the experiment, each task was introduced to all subjects and sample behavioral tests were conducted to confirm that the subjects properly understood the instructions for each task.
Theta burst stimulation
TBS was performed as described in Cho et al. [28] and carried out with the biphasic Magstim Rapid2 magnetic stimulator (Magstim, UK), using a figure-of-eight focal coil (70 mm diameter). The coil was held in a fixed position by a mechanical arm over the target area and was oriented so that the induced electric current flowed in a posterior-anterior direction. Stimulus intensities, expressed as a percentage of the maximum stimulator output, were set at 80% of the active motor threshold (AMT).
AMT was defined as the lowest stimulus intensity able to elicit 5 motor evoked potentials (MEPs) of at least 200 uV of the contra-lateral first dorsal interosseus (FDI) muscle, monitored with AgCl surface electrodes fixed on the skin with a belly-tendon montage, averaged over 10 consecutive stimuli delivered over the motor cortex at intervals longer than 5 s. During the determination of the AMT, subjects were instructed to maintain a steady muscle contraction of 20% of maximum voluntary contraction. Each TBS burst consisted of three stimuli pulses at 50 Hz, with each train being repeated every 200 ms (5 Hz). cTBS consisted of continues repetition of trains for 40 s (600 pulses). The sham stimulation was delivered with the coil positioned at a perpendicular angle to the target area using same protocol of cTBS. These off-line TBS paradigms have two main advantages: they produce a long-lasting inhibitory effect with comparably short stimulation duration [34–36] and they prevent any exogenous influence of the sound and proprioceptive sensation (given by the stimulation) during the task performance [37].
Location of the target site
In order to target the right DLPFC, we used a procedure that takes advantage of the standardized stereotaxic space of Talairach and Tournoux [38] and frameless stereotaxy [39]. A high-resolution MRI (GE Signa 1.5 T, T1-weighted images, FSPGR with repletion time = 11.9 ms, echo time = 5 ms, flip angle = 40 mm, slice thickness = 1 mm, NEX = 1, matrix size = 256 × 192) of every subject’s brain was acquired and transformed into standardized stereotaxic space using the algorithm of Collins et al. [40]. The coordinates selected for right DLPFC (x = 40, y = 32, z = 30) were similar to those used in our previous studies [39]. The Talairach coordinates were converted into each subject’s native MRI space using the reverse native-to-Talairach transformation. The positioning of the TMS coil over these locations, marked on the native MRI, was performed with the aid of a frameless stereotaxic system (Rogue Research, Montreal, Canada).
PET scan
PET scans were obtained using a whole body PET camera system, Siemens-Biograph HiRez XVI (Siemens Molecular Imaging, Knoxville, TN) operating in 3D mode with an in-plane resolution of ~4.6 mm full width at half-maximum (FWHM), using oxygen 15-labeled water ( ) as a radioactive tracer to measure changes in regional cerebral blood flow (rCBF). Three minutes after the completion of the TBS, 10 mCi was injected into the antecubital vein over 50 s and dynamic scanning was performed for 90 s starting 10 s after injection. The interval between successive administrations was 11 min to allow for adequate decay of radioactivity to background levels. In total, twelve emission scans were obtained for each subject. Before the first emission scan, a scout view was obtained to determine accurate positioning of the subject, and a low dose (0.2 mSv) CT scan was acquired for attenuation purpose. Images were reconstructed with a two-dimensional filtered back-projection, resulting in 81 slices with a 256 × 256 pixel-matrix (pixel size, 2 mm). To minimize head movements during the scanning sessions, participants were restrained using a thermoformed custom head mask and positioned in the scanner using identical laser landmarks.
During the PET acquisition, subjects underwent four different conditions (Sham stimulation - delay discounting task [S-DD]; Sham stimulation - magnitude discrimination task [S-MD]; Sham stimulation - physical discrimination task, [S-PD]; cTBS -delay discounting task [T-DD]) with three acquisition scans per condition. Stimulation conditions were pseudo-counterbalanced except for the T-DD condition, to exclude carry-over effect of TBS (Fig. 2). Subjects completed a behavioral questionnaire before and after TBS in which they rated the level of their comfort, fatigue, anxiety, mood, irritation and pain before and after each condition sessions. Ratings were made on a seven-point Likert scale.
Figure 2.
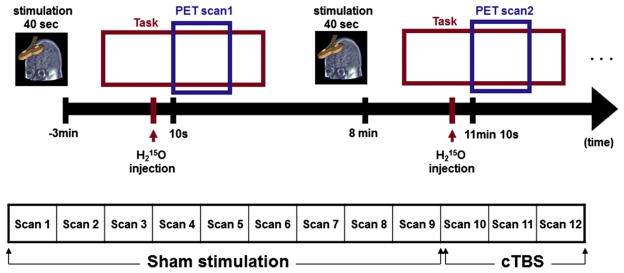
Experimental design: timelines of the experiment. A total 12 scans were acquired for each subject. To exclude carry-over effects of cTBS, cTBS scans were followed by sham stimulation scans.
Imaging and behavioral analysis
K-values were estimated as the geometric mean between the lowest implied indifference k-value in which subjects chose the delayed option, and the highest implied indifference k-value in which subjects chose the immediate option. The delayed rewards in the questionnaire were constructed in three magnitude categories: small delayed rewards ($1–200), medium delayed rewards ($201–600) and large delayed rewards ($601–1001). We estimated the discount rate separately within each category for each participant as described in a previous paper [2,3]. The final individual k-value was calculated as the geometric mean of the three rates for the small, medium and large reward magnitudes for each participant. Only subjects who made consistent choices for an assigned k-value were included. Over all consistency was computed based on the percentage of participant’s choices that were consistent with their assigned k-value. For all analyses the distributions of k-values were normalized using the natural-log transformation (ln(k)) [2,14].
All PET images were preprocessed and analyzed using Statistical Parametric Mapping 2 (Wellcome Department of Imaging Neuroscience, London, UK) implemented in the Matlab (Mathworks Inc., USA) environment. After the realignment procedure for motion correction among the scans, realigned images were transformed into standardized stereotaxic space using a standardized PET template image. The resultant normalized images were then smoothed with a Gaussian function at 12 mm full width half-maximum. Variations in global flow across subjects and scans were removed by proportionally scaling each image to have an arbitrary level of 50 mL/100 mL/min. A group analysis using a general linear model with fixed effects was conducted to investigate across the four conditions the main effect of task and cTBS. Effects on rCBF were evaluated at the voxel level by examining statistically significant changes (P < 0.05) corrected for multiple comparisons using the false discovery rate (FDR) [41]. In the results tables (Tables 3 and 4), the local maxima were reported for clusters that were composed of more than 50 contiguous significant voxels. Anatomical localization was guided by the Talairach atlas [38]. All behavioral and ROI data analysis were done using SPSS (SPSS Inc., Chicago, Illinois) software. The acceptable P values for all statistical analysis were set at P < 0.05.
Table 3.
Effect of cTBS: brain areas showing decrease in the rCBF after cTBS during performing DD task.
| Region | BA | Coordinatesa
|
T-score | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R inferior frontal gyrus | BA 10 | 41 | 58 | 1 | 4.87 |
| L lingual gyrus | BA 18 | −18 | −78 | −10 | 4.71 |
| L middle temporal gyrus (temporal pole) | BA 38 | −32 | 16 | −31 | 5.38 |
| L angular gyrus | BA 39 | −65 | −49 | 24 | 4.98 |
| L fusiform gyrus | BA 20 | −61 | −25 | −24 | 4.21 |
| L cerebellum | −38 | −38 | −25 | 6.52 | |
L = left; R = right; BA = Brodmann’s area.
Reporting criterion: P < 0.05 with false discovery rate correction for multiple comparison.
Talairach coordinate (mm).
Table 4.
Behavioral ratings before and after PET scan.
| Pre scan | Post scan | Za | P | |
|---|---|---|---|---|
| Discomfort–comfort | 2.63 (0.52) | 1.88 (1.25) | −1.86 | N.S |
| Fatigued–rested | 2.00 (0.96) | 1.63 (1.69) | −0.75 | N.S |
| Anxious–calm | 2.50 (0.76) | 2.63 (0.51) | −0.38 | N.S |
| Sad–happy | 2.50 (1.41) | 2.50 (0.93) | 0.00 | N.S |
| Irritated–soothed | 2.25 (1.39) | 2.63 (0.74) | −0.81 | N.S |
| Feel pain–do not feel pain | 3.00 (0.00) | 2.63 (0.74) | −1.34 | N.S |
Wilcoxon Signed Ranks Test.
Results
A one-way repeated measures ANOVA for whole brain group analysis across all conditions showed changes in rCBF in the bilateral lateral prefrontal cortex (BA 10/46: [x, y, z = 42, 55, 5], F = 11.54; [x, y, z = −38, 38, 15], F = 8.65), medial prefrontal areas (BA 10: [x, y, z = 2, 59, 12], F = 10.53), posterior cingulate (BA 23: [x, y, z = −6, −39, 37], F = 11.69), supramarginal gyri (BA 40: [x, y, z = −63, −43, 32], F = 9.84), precuneus (BA 7: [x, y, z = 8, −42, 48], F = 11.22; [x, y, z = −6, −42, 50], F = 9.84), inferior posterior parietal lobule (IPL, BA 40: [x, y, z = 38, −58, 47], F = 14.50; [x, y, z = −40, −50, 56], F = 8.49), inferior temporal gyri (BA 20: [x, y, z = 55, −55, −12], F = 10.88; [x, y, z = −55, −13, −31], F = 14.23) including fusiform, temporal pole, and cerebellum ([x, y, z = 12, −73, −20], F = 9.84; [x, y, z = −42, −71, −18], F = 10.27). When we tested the main effect of the task (Table 2; Fig. 3), the DD task (S-DD), compared to control tasks (i.e. S-MD and S-PD), recruited frontal regions including the right DLPFC (BA 46) extending into the rostral part of the PFC (rPFC) encompassing the inferior frontal gyrus (BA 10) along with the bilateral IPL (BA 40), precuneus (BA 7), fusiform (BA 19) and inferior temporal gyri (BA 37). Activation in the cerebellum was also found to be significant.
Table 2.
Effect of DD task: brain areas showing increase in the rCBF during DD task relative to the control conditions.
| Region | BA | Coordinatesa
|
T-score | ||
|---|---|---|---|---|---|
| x | y | z | |||
| R middle/inferior frontal gyrus | BA 10/46 | 41 | 53 | 5 | 4.98 |
| R inferior parietal lobule | BA 7/40 | 38 | −58 | 47 | 6.43 |
| L inferior parietal lobule | BA 7 | −36 | −62 | 51 | 4.27 |
| R inferior temporal gyrus | BA 37 | 55 | −55 | −14 | 5.21 |
| L precuneus | BA 7 | −2 | −73 | 50 | 3.90 |
| R fusiform gyrus | BA 19 | 38 | −60 | −5 | 4.90 |
| L fusiform gyrus | BA 19 | −38 | −58 | −9 | 3.83 |
| R cerebellum | 38 | −70 | −34 | 4.43 | |
| L cerebellum | −42 | −71 | −20 | 5.02 | |
L = left; R = right; BA = Brodmann’s area.
Reporting criterion: P < 0.05 with false discovery rate correction for multiple comparison.
Talairach coordinate (mm).
Figure 3.
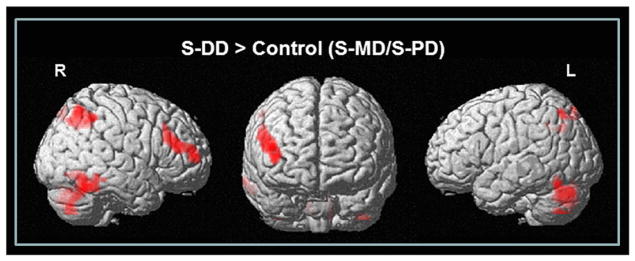
Brain regions showing increased rCBF during S-DD task compared to control tasks (S-MD/S-PD) (P < 0.05 FDR corrected for multiple comparisons with 50 voxels of spatial extended threshold).
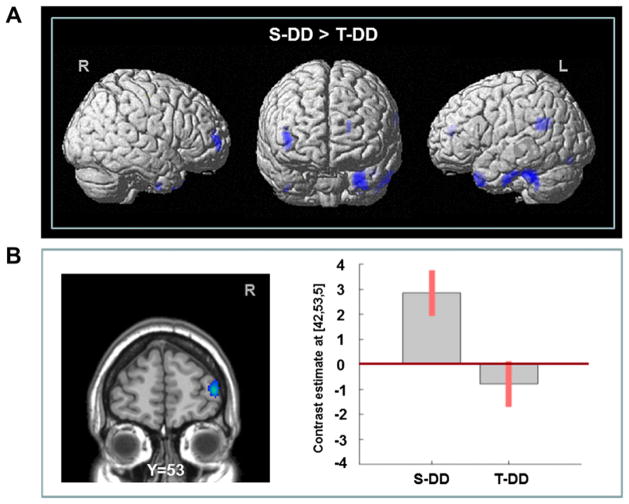
Interestingly, the recruitment of several of these areas was compromised (Table 3; Fig. 4) by active stimulation of DLPFC during DD (i.e. T-DD) which compared to sham stimulation (i.e. S-DD) revealed reduced rCBF in the right DLPFC (BA 9/46), ipsilateral rPFC (BA 10), lingual (BA 18), angular (BA 39) gyri and inferior temporal area (BA 37) including the fusiform (BA 20) and temporal pole (BA 38), along with cerebellum in the left. No brain areas showed increased activation following cTBS compared with sham stimulation during the DD task. At the behavioral level, active stimulation of DLPFC during DD induced a reduction in ln(k) value as compared to sham stimulation (i.e. S-DD) (paired t-test: t = 2.09, P < 0.05) (Fig. 5).
Figure 4.
(A) Brain regions showing decreased activation following right DLPFC-cTBS while performing DD task. (B) Specific cTBS-induced rCBF response from right rPFC during S-DD and T-DD. Values were extracted from a cluster mean from the area shown in the left.
Figure 5.
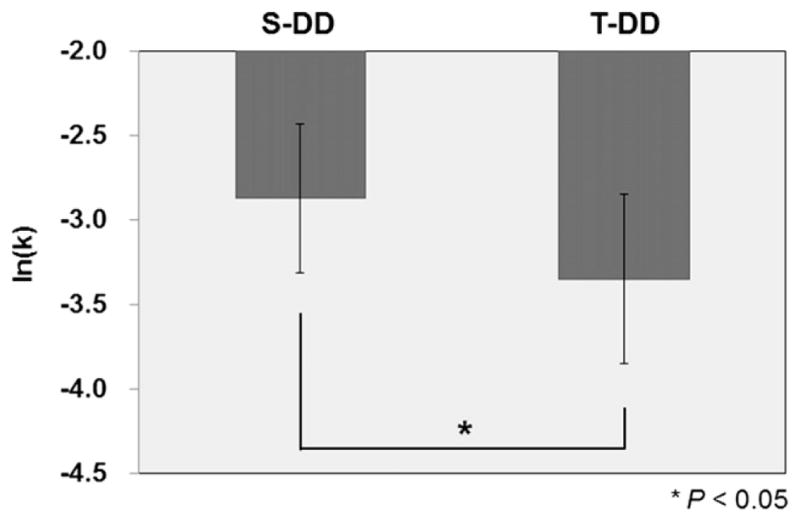
The mean of natural-log transformed k-value (ln(k)) for the S-DD and T-DD conditions. Error bars represent the standard errors of the mean. *P < 0.05, paired-sample t-test (two-tailed).
To investigate whether any relationship existed between task performance and TMS-induced changes in blood flow, we conducted a voxel-wise regression analysis between ln(k) value and blood flow during both S-DD and T-DD conditions. We found that during S-DD condition there was a significant positive correlation between impulsivity ln(k) value and rCBF in several prefrontal areas including DLPFC (9/46), rPFC (BA 10) and OFC (BA 11) (Fig. 6A). Particularly in the rPFC, lower activation was associated with lower ln(k) value (i.e. lower impulsivity) (Spearman rho = 0.74, P < 0.05). This correlation was disrupted by active stimulation with cTBS (T-DD) (Spearman rho = 0.43, NS) (Fig. 6B). All together these observations suggest that cTBS, while reducing the activation in those prefrontal areas involved in decision making, decreased impulsivity associated with DD task.
Figure 6.
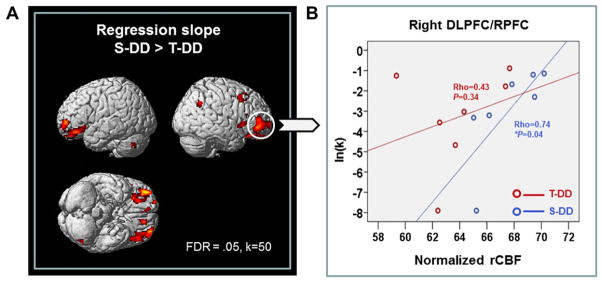
(A) Neurobehavioral correlation between ln(k) of DD tasks and rCBF: brain regions that showed steeper regression slope in S-DD than T-DD (B) Regression plots between normalized rCBF of the right rPFC and impulsive scale ln(k) during cTBS (red circle) and sham stimulation (blue circle) condition. *P < 0.05, Spearman’s correlation (two-tailed).
To rule out a potential contribution of comfort, fatigue, anxiety, mood, irritation and pain to these findings, these behavioral measures before and after DLPFC-cTBS were measured and the statistical analysis revealed no significant cTBS effect (Table 4).
Discussion
The aim of the present study was to investigate the underlying neural substrate of DD, and whether this neural network was modulated by rTMS. As already noted in our previous studies [28], the observed pattern of delay discounting indicated that cTBS of the right DLPFC induced healthy subjects to trade immediate reward for delayed larger amount of reward. In other words, cTBS of the right DLPFC influenced decision making by reducing impulsive choices and causing the favoring of delayed-large rewards instead of immediate-small rewards. While influencing impulsivity in this way, we also found that cTBS of the right DLPFC affected the activation in those prefrontal areas associated with the DD task. In particular, we observed that cTBS of the right DLPFC reduced rCBF activation in the ipsilateral DLPFC/rPFC and at the same time disrupted the relationship between impulsivity (k-value) and rCBF in these and other prefrontal areas.
There are two possible mechanisms by which TMS may modulate the delay discounting value. The first mechanism relates to the inhibitory control exerted by the PFC [22,42]. The observed TMS effects are consistent with previous data showing that cTBS may inhibit neuronal excitability in cortical target regions [35], similarly to low frequency rTMS [43,44]. The cTBS-induced inhibition of these prefrontal areas or the ‘noise’ into the midst of organized networks [45] may have created a “lesion-like effect” which disrupted the relationship between impulsivity and rCBF and increased the selection of delay options (reduced k-value).
The rPFC (BA 10) is located dorsal to the DLPFC and both are closely interconnected [46,47]. The dorsomedial-limbic and ventral-limbic streams from rPFC targeting the cingulate cortex and amygdala, respectively [48] implicate the involvement of rPFC in decision making and memory processes. A number of reports have shown that activation of this brain region is correlated with tasks that required selection, comparison and judgment in mnemonic functions (see Ref. [49] for review). More recently, a specific role for this region was suggested in gain-based decision making [50,51] and in integrating the outcome of two or more separate cognitive operations in the pursuit of a higher behavioral goal [52]. The significant correlation between impulsivity level and rCBF in rPFC (BA 10) during the S-DD condition (and its disruption by cTBS) observed in the present study support the involvement of this area in the calculation of a reward value. The cTBS-impaired activation of the right DLPFC is also consistent with the specific role that this area plays in the process of general decision making [23]. Recently, some studies have shown how the right DLPFC may affect decision making processes [24–27] by modulating its neuronal excitability using non-invasive stimulation tools such as rTMS and tDCS.
It should be acknowledged that the medial OFC also plays an important inhibitory role in reward based behavior and decision making [53,54]. The significant correlation between impulsivity level and rCBF in this area (i.e. medial OFC) during the S-DD condition (and its disruption by cTBS) support the involvement of this area in decision making processes and the calculation of reward value. Thus, given the strong anatomical connections [47] between DLPFC and OFC, it is not unlikely that our observations may have been mediated by functional interactions between these two prefrontal areas.
The second mechanism by which TMS may have incurred changes in impulsivity level is via alterations in time perception. Wittmann and Paulus [55] demonstrated that the increases in the subjective experience of time is associated with a higher level of impulsivity. Interestingly, right DLPFC has been indicated as a critical region for time processing [56–59]. In particular, Koch et al. (2002) showed that lesions in the right PFC caused an underestimation of time duration compared with normal subjects. The same observation was replicated by rTMS-induced inhibition over the right DLPFC with selective under-estimation of perception of time intervals [59]. All together these observations seem to suggest that the “lesion-like effect” of the DLPFC induced by our cTBS paradigm may have increased the selection of delay options (reduced k-value) as a result of an under-estimation of time delay.
Brain stimulation also modulated the brain-behavior relationship in posterior cortical areas such as IPL. Considering that an inhibitory control is active during delay discounting, the involvement of IPL is not surprising. DLPFC activation, coupled with IPL activation, has been observed during decision making procedures in previous studies. For example, Tanaka et al. [60] found that when subjects learned to obtain large delayed rewards while incurring small immediate losses, the DLPFC, inferior parietal cortex and cerebellum were activated. Similarly, McClure et al. [12,13] also showed activation of DLPFC-parietal neural circuitry when decisions involving delayed but larger options were presented to the subjects. More recently, a similar activation of DLPFC-parietal areas during the same decision making task has been reported [6,12,14,17]. Thus, it seems that both DLPFC and IPL are critical for certain cognitive and behavioral functions involving hierarchical decisions [61,62]. Moreover, IPL has also been noted for its role in maintaining information during the delay phase of working memory [63,64], in arithmetic processing [65–67] and time estimation/time-related decision making, engaging a fronto-parietal network along with other subcortical areas (i.e. cerebellum and basal ganglia) (see Ref. [68] for review). Taken together, these observations account for the bilateral activation of the IPL we observed during S-DD and its disruption following cTBS of the DLPFC. We also found changes in rCBF at the level of the ventral visual stream (fusiform and inferior temporal gyrus) and precuneus. All these areas may be associated with visual word processing [69,70] and memory retrieval [71].
Our study was not designed to investigate the underlying neurochemical changes associated with decreased impulsivity; however, we could speculate that changes in the dopaminergic system may well be responsible for these observations. In fact, acute administration in healthy humans of D-amphetamine decreases several forms of impulsive behavior including the delay discounting measures with decreased k-values [31].
In conclusions, our study provided evidence of TMS-induced cortical modulation during cognitive processing by influencing the neural network underlying impulsive decision making behavior. These observations support a role for TMS in testing the specific functional relevance of neural correlates associated with cognitive tasks.
Acknowledgments
A.P.S. is supported by a grant from the Canadian Institutes of Health Research (MOP 110962), Canada Research Chair Program and Edmond J. Safra Philanthropic Foundation.
Footnotes
The authors report no financial or other conflict of interest relevant to the subject of this article.
References
- 1.Evenden JL. Varieties of impulsivity. Psychopharmacology (Berl) 1999 Oct;146(4):348–61. doi: 10.1007/pl00005481. [DOI] [PubMed] [Google Scholar]
- 2.Kirby KN, Petry NM. Heroin and cocaine abusers have higher discount rates for delayed rewards than alcoholics or non-drug-using controls. Addiction. 2004 Apr;99(4):461–71. doi: 10.1111/j.1360-0443.2003.00669.x. [DOI] [PubMed] [Google Scholar]
- 3.Kirby KN, Petry NM, Bickel WK. Heroin addicts have higher discount rates for delayed rewards than non-drug-using controls. J Exp Psychol Gen. 1999;128(1):78–87. doi: 10.1037//0096-3445.128.1.78. [DOI] [PubMed] [Google Scholar]
- 4.Barkley RA, Edwards G, Laneri M, Fletcher K, Metevia L. Executive functioning, temporal discounting, and sense of time in adolescents with attention deficit hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD) J Abnorm Child Psychol. 2001 Dec;29(6):541–56. doi: 10.1023/a:1012233310098. [DOI] [PubMed] [Google Scholar]
- 5.Alessi SM, Petry NM. Pathological gambling severity is associated with impulsivity in a delay discounting procedure. Behav Processes. 2003 Oct 31;64(3):345–54. doi: 10.1016/s0376-6357(03)00150-5. [DOI] [PubMed] [Google Scholar]
- 6.Ballard K, Knutson B. Dissociable neural representations of future reward magnitude and delay during temporal discounting. NeuroImage. 2009 Mar 1;45(1):143–50. doi: 10.1016/j.neuroimage.2008.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bickel WK, Pitcock JA, Yi R, Angtuaco EJ. Congruence of BOLD response across intertemporal choice conditions: fictive and real money gains and losses. J Neurosci. 2009 Jul 8;29(27):8839–46. doi: 10.1523/JNEUROSCI.5319-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hariri AR, Brown SM, Williamson DE, Flory JD, de Wit H, Manuck SB. Preference for immediate over delayed rewards is associated with magnitude of ventral striatal activity. J Neurosci. 2006 Dec 20;26(51):13213–7. doi: 10.1523/JNEUROSCI.3446-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoffman WF, Schwartz DL, Huckans MS, McFarland BH, Meiri G, Stevens AA, et al. Cortical activation during delay discounting in abstinent methamphetamine dependent individuals. Psychopharmacology (Berl) 2008 Dec;201(2):183–93. doi: 10.1007/s00213-008-1261-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kable JW, Glimcher PW. The neural correlates of subjective value during intertemporal choice. Nat Neurosci. 2007 Dec;10(12):1625–33. doi: 10.1038/nn2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marco-Pallares J, Mohammadi B, Samii A, Munte TF. Brain activations reflect individual discount rates in intertemporal choice. Brain Res. 2010 Jan 18; doi: 10.1016/j.brainres.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 12.McClure SM, Ericson KM, Laibson DI, Loewenstein G, Cohen JD. Time discounting for primary rewards. J Neurosci. 2007 May 23;27(21):5796–804. doi: 10.1523/JNEUROSCI.4246-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClure SM, Laibson DI, Loewenstein G, Cohen JD. Separate neural systems value immediate and delayed monetary rewards. Science. 2004 Oct 15;306(5695):503–7. doi: 10.1126/science.1100907. [DOI] [PubMed] [Google Scholar]
- 14.Monterosso JR, Ainslie G, Xu J, Cordova X, Domier CP, London ED. Fronto-parietal cortical activity of methamphetamine-dependent and comparison subjects performing a delay discounting task. Hum Brain Mapping. 2007 May;28(5):383–93. doi: 10.1002/hbm.20281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shamosh NA, Deyoung CG, Green AE, Reis DL, Johnson MR, Conway AR, et al. Individual differences in delay discounting: relation to intelligence, working memory, and anterior prefrontal cortex. Psychol Sci. 2008 Sep;19(9):904–11. doi: 10.1111/j.1467-9280.2008.02175.x. [DOI] [PubMed] [Google Scholar]
- 16.Wittmann M, Leland DS, Paulus MP. Time and decision making: differential contribution of the posterior insular cortex and the striatum during a delay discounting task. Exp Brain Res. 2007 Jun;179(4):643–53. doi: 10.1007/s00221-006-0822-y. [DOI] [PubMed] [Google Scholar]
- 17.Xu L, Liang ZY, Wang K, Li S, Jiang T. Neural mechanism of intertemporal choice: from discounting future gains to future losses. Brain Res. 2009 Mar 19;1261:65–74. doi: 10.1016/j.brainres.2008.12.061. [DOI] [PubMed] [Google Scholar]
- 18.Rushworth MF, Hadland KA, Paus T, Sipila PK. Role of the human medial frontal cortex in task switching: a combined fMRI and TMS study. J Neurophysiol. 2002 May;87(5):2577–92. doi: 10.1152/jn.2002.87.5.2577. [DOI] [PubMed] [Google Scholar]
- 19.Johnson JA, Strafella AP, Zatorre RJ. The role of the dorsolateral prefrontal cortex in bimodal divided attention: two transcranial magnetic stimulation studies. J Cogn Neurosci. 2007;19(6):907–20. doi: 10.1162/jocn.2007.19.6.907. [DOI] [PubMed] [Google Scholar]
- 20.Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999 Jul 6;96(14):8301–6. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aron AR, Fletcher PC, Bullmore ET, Sahakian BJ, Robbins TW. Stop-signal inhibition disrupted by damage to right inferior frontal gyrus in humans. Nat Neurosci. 2003 Feb;6(2):115–6. doi: 10.1038/nn1003. [DOI] [PubMed] [Google Scholar]
- 22.Conway MA, Fthenaki A. Disruption of inhibitory control of memory following lesions to the frontal and temporal lobes. Cortex. 2003 Sep-Dec;39(4–5):667–86. doi: 10.1016/s0010-9452(08)70859-1. [DOI] [PubMed] [Google Scholar]
- 23.Fleck MS, Daselaar SM, Dobbins IG, Cabeza R. Role of prefrontal and anterior cingulate regions in decision-making processes shared by memory and non-memory tasks. Cereb Cortex. 2006 Nov;16(11):1623–30. doi: 10.1093/cercor/bhj097. [DOI] [PubMed] [Google Scholar]
- 24.van ’t Wout M, Kahn RS, Sanfey AG, Aleman A. Repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex affects strategic decision-making. Neuroreport. 2005 Nov 7;16(16):1849–52. doi: 10.1097/01.wnr.0000183907.08149.14. [DOI] [PubMed] [Google Scholar]
- 25.Fecteau S, Knoch D, Fregni F, Sultani N, Boggio P, Pascual-Leone A. Diminishing risk-taking behavior by modulating activity in the prefrontal cortex: a direct current stimulation study. J Neurosci. 2007 Nov 14;27(46):12500–5. doi: 10.1523/JNEUROSCI.3283-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knoch D, Gianotti LR, Pascual-Leone A, Treyer V, Regard M, Hohmann M, et al. Disruption of right prefrontal cortex by low-frequency repetitive transcranial magnetic stimulation induces risk-taking behavior. J Neurosci. 2006 Jun 14;26(24):6469–72. doi: 10.1523/JNEUROSCI.0804-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knoch D, Pascual-Leone A, Meyer K, Treyer V, Fehr E. Diminishing reciprocal fairness by disrupting the right prefrontal cortex. Science. 2006 Nov 3;314(5800):829–32. doi: 10.1126/science.1129156. [DOI] [PubMed] [Google Scholar]
- 28.Cho SS, Ko JH, Pellecchia G, Van Eimeren T, Cilia R, Strafella AP. Continuous theta burst stimulation of right dorsolateral prefrontal cortex induces changes in impulsivity level. Brain Stimul. 2010 Jul;3(3):170–6. doi: 10.1016/j.brs.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971 Mar;9(1):97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 30.Patton JH, Stanford MS, Barratt ES. Factor structure of the Barratt impulsiveness scale. J Clinical Psychology. 1995 Nov;51(6):768–74. doi: 10.1002/1097-4679(199511)51:6<768::aid-jclp2270510607>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 31.de Wit H, Enggasser JL, Richards JB. Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology. 2002 Nov;27(5):813–25. doi: 10.1016/S0893-133X(02)00343-3. [DOI] [PubMed] [Google Scholar]
- 32.Mitchell SH. Measures of impulsivity in cigarette smokers and non-smokers. Psychopharmacology (Berl) 1999 Oct;146(4):455–64. doi: 10.1007/pl00005491. [DOI] [PubMed] [Google Scholar]
- 33.Richards JB, Zhang L, Mitchell SH, de Wit H. Delay or probability discounting in a model of impulsive behavior: effect of alcohol. J Exp Anal Behav. 1999 Mar;71(2):121–43. doi: 10.1901/jeab.1999.71-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Lazzaro V, Pilato F, Saturno E, Oliviero A, Dileone M, Mazzone P, et al. Theta-burst repetitive transcranial magnetic stimulation suppresses specific excitatory circuits in the human motor cortex. J Physiol. 2005 Jun 15;565(Pt 3):945–50. doi: 10.1113/jphysiol.2005.087288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005 Jan 20;45(2):201–6. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- 36.Hubl D, Nyffeler T, Wurtz P, Chaves S, Pflugshaupt T, Lüthi M, et al. Time course of blood oxygenation level-dependent signal response after theta burst transcranial magnetic stimulation of the frontal eye field. Neuroscience. 2008;151(3):921–8. doi: 10.1016/j.neuroscience.2007.10.049. [DOI] [PubMed] [Google Scholar]
- 37.Vallesi A, Shallice T, Walsh V. Role of the prefrontal cortex in the foreperiod effect: TMS evidence for dual mechanisms in temporal preparation. Cereb Cortex. 2007 Feb;17(2):466–74. doi: 10.1093/cercor/bhj163. [DOI] [PubMed] [Google Scholar]
- 38.Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain: 3-dimensional proportional system: an approach to cerebral imaging. Stuttgart; Thieme: 1988. [Google Scholar]
- 39.Strafella AP, Paus T, Barrett J, Dagher A. Repetitive transcranial magnetic stimulation of the human prefrontal cortex induces dopamine release in the caudate nucleus. J Neurosci. 2001 Aug 1;21(15):RC157. doi: 10.1523/JNEUROSCI.21-15-j0003.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994 Mar-Apr;18(2):192–205. [PubMed] [Google Scholar]
- 41.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002 Apr;15(4):870–8. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 42.Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends Cogn Sci. 2004 Apr;8(4):170–7. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 43.Siebner HR, Rothwell J. Transcranial magnetic stimulation: new insights into representational cortical plasticity. Exp Brain Res. 2003 Jan;148(1):1–16. doi: 10.1007/s00221-002-1234-2. [DOI] [PubMed] [Google Scholar]
- 44.Di Lazzaro V, Oliviero A, Berardelli A, Mazzone P, Insola A, Pilato F, et al. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002 Jun;144(4):549–53. doi: 10.1007/s00221-002-1106-9. [DOI] [PubMed] [Google Scholar]
- 45.Walsh V, Rushworth M. A primer of magnetic stimulation as a tool for neuropsychology. Neuropsychologia. 1999 Feb;37(2):125–35. [PubMed] [Google Scholar]
- 46.Yeterian EH, Pandya DN, Tomaiuolo F, Petrides M. The cortical connectivity of the prefrontal cortex in the monkey brain. Cortex. 2012 Jan;48(1):58–81. doi: 10.1016/j.cortex.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petrides M, Pandya DN. Dorsolateral prefrontal cortex: comparative cytoarchitectonic analysis in the human and the macaque brain and corticocortical connection patterns. Eur J Neurosci. 1999 Mar;11(3):1011–36. doi: 10.1046/j.1460-9568.1999.00518.x. [DOI] [PubMed] [Google Scholar]
- 48.Petrides M, Pandya DN. Efferent association pathways from the rostral prefrontal cortex in the macaque monkey. J Neurosci. 2007 Oct 24;27(43):11573–86. doi: 10.1523/JNEUROSCI.2419-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramnani N, Owen AM. Anterior prefrontal cortex: insights into function from anatomy and neuroimaging. Nat Rev Neurosci. 2004 Mar;5(3):184–94. doi: 10.1038/nrn1343. [DOI] [PubMed] [Google Scholar]
- 50.Roiser JP, Stephan KE, den Ouden HE, Friston KJ, Joyce EM. Adaptive and aberrant reward prediction signals in the human brain. NeuroImage. 2010 Apr 1;50(2):657–64. doi: 10.1016/j.neuroimage.2009.11.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Eimeren T, Ko JH, Pellechia G, Cho SS, Houle S, Strafella AP. Prefrontal D2-receptor stimulation mediates flexible adaptation of economic preference hierarchies. Hum Brain Mapp. 2011 doi: 10.1002/hbm.21425. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koechlin E, Basso G, Pietrini P, Panzer S, Grafman J. The role of the anterior prefrontal cortex in human cognition. Nature. 1999 May 13;399(6732):148–51. doi: 10.1038/20178. [DOI] [PubMed] [Google Scholar]
- 53.Kringelbach ML. The human orbitofrontal cortex: linking reward to hedonic experience. Nat Rev Neurosci. 2005 Sep;6(9):691–702. doi: 10.1038/nrn1747. [DOI] [PubMed] [Google Scholar]
- 54.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans R Soc Lond B Biol Sci. 1996 Oct 29;351(1346):1413–20. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 55.Wittmann M, Paulus MP. Decision making, impulsivity and time perception. Trends Cogn Sci. 2008 Jan;12(1):7–12. doi: 10.1016/j.tics.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 56.Harrington DL, Haaland KY, Knight RT. Cortical networks underlying mechanisms of time perception. J Neurosci. 1998 Feb 1;18(3):1085–95. doi: 10.1523/JNEUROSCI.18-03-01085.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rao SM, Mayer AR, Harrington DL. The evolution of brain activation during temporal processing. Nat Neurosci. 2001 Mar;4(3):317–23. doi: 10.1038/85191. [DOI] [PubMed] [Google Scholar]
- 58.Koch G, Oliveri M, Carlesimo GA, Caltagirone C. Selective deficit of time perception in a patient with right prefrontal cortex lesion. Neurology. 2002 Nov 26;59(10):1658–9. doi: 10.1212/01.wnl.0000032504.45792.8f. [DOI] [PubMed] [Google Scholar]
- 59.Koch G, Oliveri M, Torriero S, Caltagirone C. Underestimation of time perception after repetitive transcranial magnetic stimulation. Neurology. 2003 Jun 10;60(11):1844–6. doi: 10.1212/wnl.60.11.1844. [DOI] [PubMed] [Google Scholar]
- 60.Tanaka SC, Doya K, Okada G, Ueda K, Okamoto Y, Yamawaki S. Prediction of immediate and future rewards differentially recruits cortico-basal ganglia loops. Nat Neurosci. 2004 Aug;7(8):887–93. doi: 10.1038/nn1279. [DOI] [PubMed] [Google Scholar]
- 61.Rushworth MF, Passingham RE, Nobre AC. Components of switching intentional set. J Cogn Neurosci. 2002 Nov 15;14(8):1139–50. doi: 10.1162/089892902760807159. [DOI] [PubMed] [Google Scholar]
- 62.Brass M, Ullsperger M, Knoesche TR, von Cramon DY, Phillips NA. Who comes first? The role of the prefrontal and parietal cortex in cognitive control. J Cogn Neurosci. 2005 Sep;17(9):1367–75. doi: 10.1162/0898929054985400. [DOI] [PubMed] [Google Scholar]
- 63.Smith EE, Jonides J. Neuroimaging analyses of human working memory. Proc Natl Acad Sci U S A. 1998 Sep 29;95(20):12061–8. doi: 10.1073/pnas.95.20.12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koch G, Oliveri M, Torriero S, Carlesimo GA, Turriziani P, Caltagirone C. rTMS evidence of different delay and decision processes in a fronto-parietal neuronal network activated during spatial working memory. NeuroImage. 2005;24(1):34–9. doi: 10.1016/j.neuroimage.2004.09.042. [DOI] [PubMed] [Google Scholar]
- 65.Menon V, Rivera SM, White CD, Glover GH, Reiss AL. Dissociating prefrontal and parietal cortex activation during arithmetic processing. NeuroImage. 2000;12(4):357–65. doi: 10.1006/nimg.2000.0613. [DOI] [PubMed] [Google Scholar]
- 66.Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science. 1999 May 7;284(5416):970–4. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- 67.Kong J, Wang C, Kwong K, Vangel M, Chua E, Gollub R. The neural substrate of arithmetic operations and procedure complexity. Cog Brain Res. 2005;22(3):397–405. doi: 10.1016/j.cogbrainres.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 68.Rubia K, Smith A. The neural correlates of cognitive time management: a review. Acta Neurobiol Exp (Wars) 2004;64(3):329–40. doi: 10.55782/ane-2004-1517. [DOI] [PubMed] [Google Scholar]
- 69.Vinckier F, Dehaene S, Jobert A, Dubus JP, Sigman M, Cohen L. Hierarchical coding of letter strings in the ventral stream: dissecting the inner organization of the visual word-form system. Neuron. 2007 Jul 5;55(1):143–56. doi: 10.1016/j.neuron.2007.05.031. [DOI] [PubMed] [Google Scholar]
- 70.Cohen L, Dehaene S, Naccache L, Lehericy S, Dehaene-Lambertz G, Henaff MA, et al. The visual word form area: spatial and temporal characterization of an initial stage of reading in normal subjects and posterior split-brain patients. Brain. 2000 Feb;123(Pt 2):291–307. doi: 10.1093/brain/123.2.291. [DOI] [PubMed] [Google Scholar]
- 71.Wheeler ME, Buckner RL. Functional dissociation among components of remembering: control, perceived oldness, and content. J Neurosci. 2003 May 1;23(9):3869–80. doi: 10.1523/JNEUROSCI.23-09-03869.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]