Abstract
Restricted feeding (RF) schedules are potent zeitgebers capable of entraining metabolic and hormonal rhythms in peripheral oscillators in anticipation of food. Behaviorally, this manifests in the form of food anticipatory activity (FAA) in the hours preceding food availability. Circadian rhythms of FAA are thought to be controlled by a food-entrainable oscillator (FEO) outside of the suprachiasmatic nucleus (SCN), the central circadian pacemaker in mammals. Although evidence suggests that the FEO and the SCN are capable of interacting functionally under RF conditions, the genetic basis of these interactions remains to be defined. In this study, using dexras1-deficient (dexras1−/−) mice, the authors examined whether Dexras1, a modulator of multiple inputs to the SCN, plays a role in regulating the effects of RF on activity rhythms and gene expression in the SCN. Daytime RF under 12L:12D or constant darkness (DD) resulted in potentiated (but less stable) FAA expression in dexras1−/− mice compared with wild-type (WT) controls. Under these conditions, the magnitude and phase of the SCN-driven activity component were greatly perturbed in the mutants. Restoration to ad libitum (AL) feeding revealed a stable phase displacement of the SCN-driven activity component of dexras1−/− mice by ~2 h in advance of the expected time. RF in the late night/early morning induced a long-lasting increase in the period of the SCN-driven activity component in the mutants but not the WT. At the molecular level, daytime RF advanced the rhythm of PER1, PER2, and pERK expression in the mutant SCN without having any effect in the WT. Collectively, these results indicate that the absence of Dexras1 sensitizes the SCN to perturbations resulting from restricted feeding.
Keywords: Circadian rhythms, Coupling, Dexras1, MAPK signaling, Period genes, Suprachiasmatic nucleus, Temporal restricted feeding
INTRODUCTION
In their natural habitat, most animals are subjected to the constant pressures of limited food availability, which is constrained in both time and space. Behavioral and physiological adaptations that allow animals to predict the timing of food availability are, therefore, essential for their survival (reviewed in Mistlberger, 2011). Evolved as an adaptive mechanism, the circadian timing system organizes behavior and physiology in anticipation of predictable cycles of events in the environment that are critical to the organism’s well-being (reviewed in Pittendrigh, 1993). In mammals, the suprachiasmatic nucleus (SCN) of the hypothalamus constitutes the master circadian pacemaker (Moore & Eichler, 1972; Stephan & Zucker, 1972). Light is the primary stimulus that entrains (resets) the phase of the SCN pacemaker (otherwise known as the light-entrainable oscillator or LEO), which receives photic information via retinal inputs from the eye (Castel et al., 1993; Hannibal et al., 1997; Lee et al., 2003). The SCN, in turn, synchronizes other circadian oscillators throughout the organism (Pando et al., 2002). When food is freely available, behavioral activity is dictated primarily by the timing of the SCN and is synchronized to the external light (L)-dark (D) cycle (Boulos et al., 1980; Honma et al., 1983). Scheduled restricted food access, on the other hand, triggers emergence of a second activity component, or food anticipatory activity (FAA), that precedes the expected mealtime (Boulos et al., 1980; Honma et al., 1983). FAA, which exhibits many properties characteristic of a clock-controlled process (reviewed in Mistlberger, 1994), is thought to be driven by one or several food-entrainable oscillators (FEOs) outside of the SCN, but their exact identity remains contentious (Fuller et al., 2008; Gooley et al., 2006; Landry et al., 2006, 2007; LeSauter et al., 2009).
Regardless of the precise anatomical location of the FEO, the relationship between the SCN (LEO) and FEO has been a topic of considerable research interest. Early studies showed that arrhythmic SCN-lesioned rats and mice expressed FAA under temporal restricted feeding (RF), suggesting the existence of a separate and independent FEO outside of the SCN (Marchant & Mistlberger, 1997; Stephan et al., 1979a, 1979b). Subsequent studies challenged this notion that the two-oscillator systems were entirely independent of one another. Stephan (1986a, 1986b, 1986c) was one of the first to suggest that the LEO and FEO of the rat were weakly coupled, with one affecting the phase and period of the other in an asymmetric fashion. Similar evidence for coupling interactions between the LEO and FEO was found in other species, including rabbit (Jilge & Stahle, 1993) and pigeons (Rashotte & Stephan, 1996). In one of the most extreme cases to date, SCN-driven activity rhythms of the CS mouse strain (established from a hybrid of the NBC and SII strains at Nagoya University in 1956) were shown to entrain perfectly to a daily restricted feeding (RF) schedule (Abe et al., 1989). Other inbred (Balb/c) and outbred (Mus domesticus) mouse strains have shown a variable degree of entrainment to RF (Castillo et al., 2004; Holmes & Mistlberger, 2000), whereas SCN-driven activity rhythms in the C57Bl/6J strain are somewhat more resilient to perturbations by RF (Abe et al., 1989; Marchant & Mistlberger, 1997; Pendergast et al., 2009).
Our previous work identified Dexras1 as a regulator of SCN clock entrainment to various zeitgebers (Cheng et al., 2004, 2006; Koletar et al., 2011). Genetic ablation of dexras1 results in complex changes in the phase response curve (PRC) to light (Cheng et al., 2006), and a potentiation in arousal-induced nonphotic phase shifts (Koletar et al., 2011). Dexras1 is expressed in the murine SCN in a rhythmic fashion (Takahashi et al., 2003). As a guanine nucleotide exchange factor for G protein α subunits (Cismowski et al., 1999; Fang et al., 2000), Dexras1 can couple to and modulate the activity of the mitogen-activated protein kinase (MAPK)/extra-cellular signal-regulated kinase (ERK) pathway within the SCN in response to light (Cheng et al., 2004, 2006). However, in addition to those that are light responsive, numerous other cell-surface receptor systems, including receptors for neuropeptide Y (a mediator of nonphotic signals) (Harrington et al., 1985; Huhman & Albers, 1994; Yanielli & Harrington, 2001) and ghrelin (a putative candidate for food-related signaling) (Blum et al., 2009; Yanielli et al., 2007; Zigman et al., 2006), also engage G-protein signaling within the SCN, and may, therefore, be co-regulated by Dexras1. This led us to suggest that Dexras1 functions within the SCN to integrate multiple incoming signals from the environment or elsewhere within the organism (Cheng et al., 2004). Therefore, in order to more fully define the role of Dexras1 in clock physiology, we used scheduled feeding as another zeitgeber to challenge the circadian organization of dexras1-deficient (dexras1−/−) mice. Here, we provide evidence indicating that loss of dexras1 has a profound effect on light-entrainable rhythms and timing of the SCN clock.
MATERIALS AND METHODS
Animals and Housing
Generation of dexras1 knockout mice has been previously described (Cheng et al., 2004). All experimental procedures were performed on male dexras1−/− mice, 6 to 8 wks of age at the start of the experiment, backcrossed for at least 13 generations onto a C57BL/6J inbred background. Age-matched male C57BL/6J mice (Jackson Laboratories, Bar Harbor, ME, USA) were used as wild-type (WT) controls. For behavioral experiments, mice were individually housed in polycarbonate cages (33 × 15 × 13 cm) equipped with running wheels (10.8 cm diameter) placed into a ventilated cabinet with computer-controlled light schedules (Phenome Technologies, Chicago, IL, USA). The light source was white light-emitting diodes (LEDs), which provided a light intensity of ~80 lux at cage bottom level. For protein expression studies, mice were group-housed in polycarbonate cages (29 × 19 × 13 cm) without running wheels, and placed into the aforementioned cabinets. Mice were given ad libitum access to water throughout the experiment. Rodent chow (2019 Teklad Global 19% Protein Extruded Rodent Diet; Harlan Laboratories Canada, Mississauga, ON, Canada) was provided ad libitum unless otherwise indicated. All animal handling and experimental procedures were approved by the Animal Welfare Committees of the University of Ottawa and the University of Toronto, in accordance with guidelines by the Canadian Council on Animal Care and international ethical standards for chronobiological experimentation (Portaluppi et al., 2010).
Scheduled Feeding Paradigms
Daytime Restricted Feeding Under an LD cycle
In Experiment 1, dexras1−/− (n = 15) and WT (n = 15) mice were entrained to a 12-h:12-h light-dark (LD) cycle for at least 2 wks under ad libitum (AL) feeding conditions (LD-AL). Zeitgeber times (ZT) 0 and 12 were defined as onset of light and darkness, respectively. On the last day of AL, food was removed from the hopper and cage bottom at ZT 11. During the RF schedule, mice were provided with food from ZT 6 to ZT 11. For ease of access, four pellets of rodent chow were placed at the cage bottom rather than in the food hopper during RF. The RF schedule lasted for 11 or 20 consecutive days under the same (fixed) LD cycle (LD-RF). On the 11th day of RF, a subset of animals (dexras1−/− [n = 8] and WT [n = 8] mice) was put back in AL conditions (the food hopper was filled with rodent chow at ZT 11), and released into constant dark (DD) conditions for 2 wks (DD-AL) to assess the phase of the SCN-driven rhythms.
Daytime Restricted Feeding Under a DD cycle
In Experiment 2, a separate cohort of dexras1−/− (n = 15) and WT (n = 15) mice under AL conditions was entrained to a 12L:12D cycle for at least 2 wks (LD1-AL) and released into DD for 14 or 22 d (DD1-AL). They were returned to a 12L:12D cycle until stably entrained (LD2-AL). On the last day of AL, food was removed from the hopper at ZT 11, and mice were released into DD. The RF schedule lasted for six consecutive days starting from the first day of DD (DD2-RF). During the RF schedule, mice were provided with food from ZT 6 to ZT 11 (relative to the previous LD cycle), as described above. On the last day of RF, mice were returned to AL conditions (the food hopper was filled with rodent chow at ZT 11) and maintained in DD for an additional 2 wks (DD2-AL).
mPERIOD1 and mPERIOD2 Protein Expression Studies
In Experiment 3, dexras1−/− and WT mice under AL conditions were stably entrained to a fixed 12L:12D cycle for at least 2 wks. For the RF-treated groups, on the last LD cycle, food was removed from the hopper and cage bottom at ZT 11 prior to release of mice into DD. The AL control groups were released into DD at the same time but were maintained on ad libitum feeding throughout. The RF schedule lasted for six consecutive days starting from the first day of DD. During the RF schedule, mice were provided with food from ZT 6 to ZT 11 (relative to the previous LD cycle) by placing four pellets of rodent chow per animal at the cage bottom. On the last day of RF, mice were returned to AL conditions (the food hopper was filled with rodent chow at ZT 11). The next day, mice were killed at ZT 2, 8, and 14 (relative to the previous LD cycle), and SCN tissues were harvested for immunohistochemistry of mPERIOD1 and mPERIOD2. Four mice were used for each condition (genotype × feeding condition × time of killing).
In a separate experiment, C57Bl/6J mice that had been stably entrained to a fixed 12L:12D cycle were dark adapted for two consecutive days prior to killing at ZT 2, 6, 10, 14, 18, and 22 (relative to the previous LD cycle). Three mice were used for each time point. This experiment was performed to determine the circadian expression profile of mPERIOD1 and mPERIOD2, and was used for comparative purposes for the interpretation of the results of Experiment 3.
pERK Protein Expression Studies
In Experiment 4, mice were treated exactly as stated in Experiment 3 except that the RF schedule lasted for four consecutive days, and mice were killed on the fourth day of RF. Mice were killed at ZT 6 (immediately prior to food presentation), ZT 10 (during food presentation), and ZT 14 (after food presentation), and SCN tissues were harvested for immunohistochemistry of pERK. AL controls that were maintained on ad libitum feeding throughout were released into DD at the same time and killed on the fourth day of DD. Four mice were used for each condition (genotype × feeding condition × time of killing).
In a separate experiment, C57Bl/6J mice that had been stably entrained to a fixed 12L:12D cycle were dark adapted for two consecutive days prior to killing at ZT 2, 6, 10, 14, 18, and 22 (relative to the previous LD cycle). Three mice were used for each time point. This experiment was performed to determine the circadian expression profile of pERK, and was used for comparative purposes during the interpretation of the results of Experiment 4.
Late-Night Restricted Feeding Under a DD Cycle
In Experiment 5, a separate cohort of dexras1−/− (n = 6) and WT (n = 6) mice under AL conditions was entrained to a 12L:12D cycle for at least 2 wks (LD1-AL) prior to release into DD for 11 d (DD1-AL). They were returned to a 12L:12D cycle until stably entrained (LD2-AL). On the last day of AL, mice were released into DD and food was removed from the hopper at ZT 4. The RF schedule started on the first cycle of DD and lasted for six consecutive days (DD2-RF). During the RF schedule, mice were provided with food from ZT 23 to ZT 4 (relative to the previous LD cycle), as described above. On the last day of RF, mice were returned to AL conditions (the food hopper was filled with rodent chow at ZT 4) and maintained in DD for an additional 8 wks (DD2-AL).
In a separate experiment, a different cohort of dexras1−/− (n = 7) and WT (n = 7) mice, age-matched to the animals used for Experiment 5, was entrained to a 12L:12D cycle for at least 2 wks prior to release into DD for 8 wks. Mice were ad libitum–fed throughout the experiment. This experiment was performed as a control to examine potential spontaneous changes in period under long-term DD conditions.
Circadian Behavior Analysis
Wheel revolutions were acquired in 5-min bins using the ClockLab software (version 7.6.0.324; Actimetrics, Evanston, IL, USA). ClockLab and MatLab (The MathWorks, Natick, MA, USA) software were used to analyze activity data and to produce double-plotted actograms and periodograms. FAA onset was defined as the first bin in which activity was ≥50% of the FAA acrophase activity per day (peak activity level for that day), according to the method of Landry et al. (2007). FAA offset was defined as the last bin where activity was ≥10% of the FAA acrophase activity per day preceded by three of six bins having at least 10% of the FAA acrophase activity, similar to the definition of activity offset by Valentinuzzi et al. (1997). FAA was determined as raw values (wheel revolutions) and as the percent of total daily wheel-running activity during the 24-h cycle. Duration of FAA was defined as the interval between the daily onset and offset of FAA. The precision of the duration, onset, or offset of FAA was calculated as the mean of the standard error of the respective variables (Landry et al., 2007). To calculate the aforementioned FAA parameters, data from d 2 to 11 of RF (Experiment 1) or the entire 6-d RF schedule (Experiments 2 and 3) were used. Nocturnality score (nocturnal activity) was calculated as the total activity during lights-off, expressed as raw values (wheel revolutions) and percent of total daily wheel-running activity (Landry et al., 2007). Unless otherwise specified, nocturnal activity and total daily activity were calculated using 10-d blocks in the indicated interval. Duration of nocturnal activity (α) was determined according to the method of Valentinuzzi et al. (1997). Period (τ) under DD, i.e., τDD, was determined with a computer-fitted regression line to activity onsets over 10 consecutive days within the specified interval, and independently verified with the χ2 periodogram. The phase angle of LD entrainment was calculated as the difference in hours between the onset of nocturnal activity and dark onset. Phase shifts were determined using the linear regression method (Daan & Pittendrigh, 1976). For Experiment 1, a regression line was fitted to the activity onsets between d 4 and 14 after return to AL under DD (DD-AL), and compared with a second regression line fitted to the activity onsets in the last 7 d prior to RF under LD (LD-AL). Both lines were extrapolated to the last day of RF (which coincides with the last LD cycle prior to DD), and the phase shift was defined as the difference between the projected onset times on this day. For Experiment 2, regression lines were fitted to the activity onsets under LD1-AL and DD1-AL, and the apparent phase shift resulting from release into DD was measured as the displacement between these two lines on the last day of the LD cycle. A second pair of regression lines was fitted, one to the activity onsets between d 4 and 14 after return to AL under DD (DD2-AL), and the other to the activity onsets in the last 7 d prior to RF under DD (LD2-AL). Both lines were extrapolated to the last day immediately prior to RF (which coincides with the last LD2 cycle prior to release to DD2), and the phase shift was calculated as the difference between the projected onset times on this day. Daily onset times were determined by ClockLab and, if necessary, corrected by an experienced observer (H. Y. Cheng) blind to the genotype of the animal; the best-fit line was then drawn by ClockLab.
Tissue Harvest, Processing, and Immunohistochemistry
Mice were killed by cervical dislocation under dim red light, and eyes were covered with black electrical tape. Brains were rapidly dissected and cut into 800-μm-thick coronal sections containing the SCN in cooled oxygenated media using an oscillating tissue slicer (Electron Microscopy Sciences, Hatfield, PA, USA). Tissues were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 6 h at room temperature, cryoprotected in 30% sucrose in PBS at 4°C, and cut into thin (30-μm) sections using a freezing microtome (Leica Microsystems, Wetzlar, Germany). Tissues were stored in 30% sucrose in PBS at 4°C until use.
Immunohistochemistry (IHC) was conducted as described previously (Alvarez-Saavedra et al., 2011). Briefly, sections were washed in PBS with .1% Triton X-100 (PBST), treated with .3% H2O2 in PBS for 20 min, washed again in PBST, blocked (1 h, room temperature) in 10% horse serum/PBST, and incubated (overnight, 4°C) in the following primary antibodies: rabbit anti-Per1 (1:4000; gift of S. Reppert); rabbit anti-Per2 (1:6000; gift of D. Weaver); and rabbit anti-phospho-p44/42 MAP kinase (Thr202/Tyr204) (1:4000; Cell Signaling Technology, Beverly, MA, USA). The next day, sections were washed five times in PBST, and incubated (2 h, room temperature) with biotinylated anti-rabbit IgG(H + L) (immunoglobulin G heavy + light chain) secondary antibodies (1:300; Vector Laboratories, Burlingame, CA, USA). Immunodetection was accomplished using the Vectastain Elite ABC Kit (Vector Laboratories) and Per-oxidase DAB Substrate Kit (Vector Laboratories) according to the manufacturer’s instructions. Sections were mounted on gelatin-coated microscope slides, dried, washed briefly in water, dehydrated in an ascending series of alcohol (70%, 95%, and 100% ethanol), cleared with xylene, and cover-slipped with Permount Mounting Media (Fisher Scientific, Ottawa, ON, Canada).
Image Acquisition and Analysis
IHC images were captured using the 10× and 20× objectives of a Zeiss Axio Observer Z1 epifluorescent/light microscope equipped with an AxioCam MRm Rev.3 monochromatic digital camera (Zeiss, Oberkochen, Germany). Quantification of SCN photomicrographs was done using ImageJ (http://rsbweb.nih.gov/ij) as previously described (Alvarez-Saavedra et al., 2011). Each unilateral SCN was digitally outlined and measured individually by the sum of all grayscale values from 0 to 255 divided by the total number of pixels within the region of interest to generate the mean pixel value. Background values of nonimmunoreactive tissue were obtained by placing a digital oval (150 × 200 pixels) on the adjacent lateral hypothalamus, and subtracting this mean value from the SCN mean gray values. For all experiments, data were averaged from two central SCN sections per animal, and these values were pooled to generate mean values (reported at relative arbitrary units) for each treatment group.
Statistical Analysis
Paired Student’s t tests, and two- (genotype × feeding condition) and three- (genotype × feeding condition × time point) way analyses of variance (ANOVA) of independent and repeated measures were used to analyze behavioral and protein expression data, followed by post hoc Fisher’s least significant difference (LSD) tests with alpha set at <.05. Statistical analyses were performed with statistical tools from Statistics Online Computational Resource (SOCR) (http://www.socr.ucla.edu/htmls/SOCR_Analyses.html) and SPSS software (IBM Corporation, Armonk, NY, USA). Values are reported as mean ± SEM.
RESULTS
Behavioral Effects of Daytime RF Under LD Conditions in dexras1−/− Mice
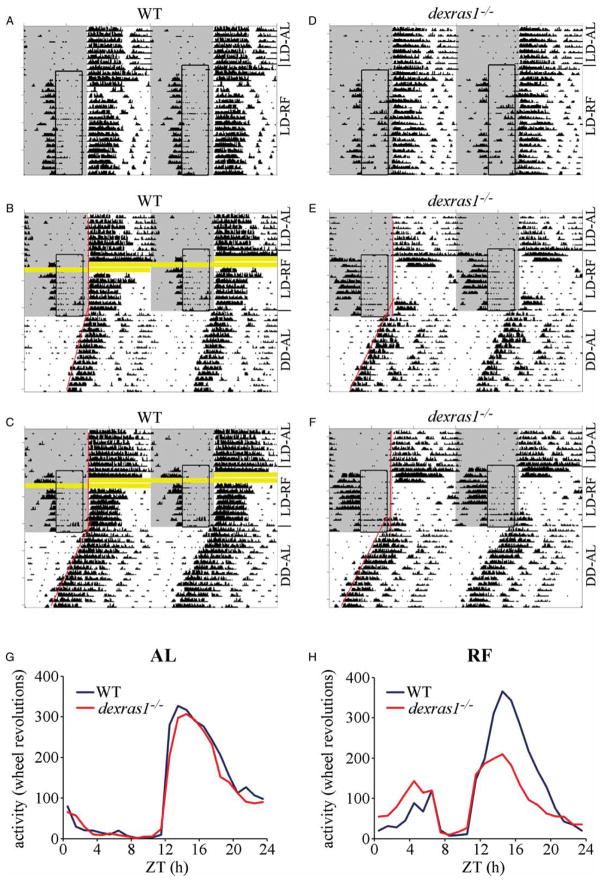
As a starting point to examine the effects of dexras1 ablation on the circadian behavior of food-restricted mice, we analyzed the behavior of dexras1−/− (n = 15) and WT (n = 15) mice that received daily 5-h access to food during their “resting” phase (ZT 6 to ZT 11) under LD (Figure 1 and Table 1). All animals expressed FAA by d 3 of RF. The magnitude of FAA, whether expressed as wheel revolutions or as % of total daily activity (% FAA), was ~2-fold greater in dexras1−/− mice than in WT (Table 1). In addition, mean duration of FAA was significantly prolonged in the mutant mice compared with the WT, as a result of a markedly earlier onset of FAA (Table 1). Yet, in contrast with WT controls, there was significantly more variability in the expression of FAA within individual dexras1−/− mice as a function of day during the RF schedule, and this was reflected in our measurements of the precision of FAA duration and onset (Table 1). These data indicate that the daytime RF under LD elicits augmented expression of FAA in the absence of dexras1, but the FAA is much less stable in the mutant mice than in WT controls as reflected in the greater day-to-day variation in the magnitude, duration, onset, and/or offset of FAA.
FIGURE 1.
Dexras1 ablation perturbs the behavioral effects of daytime RF under LD. A–F, Representative double-plotted actograms of wheel-running activity of (A–C) WT and (D–F) dexras1−/− mice subjected to a RF schedule for (B, C, E, F) 11 or (A, D) 20 consecutive days, between ZT 6 and ZT 11, under 12L:12D conditions. Areas shaded in gray indicate periods of light. Boxed regions indicate periods of food availability. Red lines denote regression lines fitted to daily activity onsets. Yellow bars indicate missing data resulting from faulty magnetic switch closures. LD-AL = ad libitum feeding under 12L:12D; LD-RF = restricted feeding under LD; DD-AL = ad libitum feeding under DD. G, H, Aggregate activity profiles of WT (blue) and dexras1−/− (red) mice under (G) AL feeding and (H) RF conditions under 12L:12D. For each animal, wheel-running activity was pooled in 1-h bins for 10 consecutive days before and after the start of the RF schedule and averaged to yield one 24-h profile. Data indicate mean ± SEM wheel turns (y-axis) per 1-h bin for each genotype as a function of ZT (x-axis) (n = 15/genotype).
TABLE 1.
Statistical comparison behavior of the circadian of WT and dexras1−/− mice following daytime restricted feeding under LD (Experiment 1)
| WT (n = 15) | dexras1−/− (n = 15) | F value | p value | |
|---|---|---|---|---|
| Ad libitum (pre-RF) | ||||
| Total daily activity [wheel revolutions] | 2729 ±101 | 2694 ± 219 | F(1,245) = .1 | .81 |
| Nocturnal activity [wheel revolutions] | 2587 ±118 | 2448 ± 196 | F(1,244) = 2.2 | .14 |
| Phase angle of LD entrainment [h] | −.03 ± .03 | −.03 ± .06 (n = 7) | F(1,198) =.0 | .99 |
| α [h] | 12.3 ± .3 | 12.6 ± .3 | F(1,220) = 4.8 | .03 |
| Restricted feeding | ||||
| Total daily activity [wheel revolutions] | 2777 ±135 | 2303 ±192 | F(1,247) = 18.0 | 3.5e-5 |
| Nocturnal activity [wheel revolutions] | 2323 ±179 | 1621 ± 209 | F(1,244) = 26.6 | 5.2e-7 |
| % nocturnal activity [%] | 80.3 ± 3.0 | 64.4 ± 7.0 | F(1,245) = 23.8 | 1.9e-6 |
| Food anticipatory activity (FAA) [wheel revolutions] | 336 ± 34 | 629 ±121 | F(1,245) = 12.7 | 4.3e-4 |
| % FAA [%] | 13.7 ± 1.6 | 30.3 ± 5.7 | F(1,244) = 25.2 | 9.9e-7 |
| Duration of FAA [h] | 1.83 ± .35 | 2.86 ± .40 | F(1,290) = 12.3 | 5.3e-4 |
| Onset of FAA [ZT] | 4.53 ± .10 | 4.00 ± .34 | F(1,286) = 20.2 | 1.0e-5 |
| Offset of FAA [ZT] | 6.00 ± .07 | 6.11 ± .07 | F(1,290) = 3.0 | .08 |
| Precision of duration of FAA* | .24 ± .13 | .70 ± .15 | F(1,247) = 8.7 | 6.5e-3 |
| Precision of onset of FAA* | .27 ± .03 | .43 ± .06 | F(1,28) = 5.6 | .03 |
| Precision of offset of FAA* | .11 ± .01 | .14 ± .03 | F(1,28) = 1.1 | .31 |
| Phase angle of LD entrainment [h] | .02 ± .02 | −.68 ± .03 (n = 7) | F(1,166) = 64.6 | 1.7e-13 |
| α [h] | 7.95 ± .27 | 6.50 ± .47 | F(1,282) = 11.7 | 7.3e-4 |
|
| ||||
| Post-RF | WT (n = 8) | dexras1 −/− (n = 8) | F value | p value |
|
| ||||
| τDD [h] | 23.61 ± .07 | 23.53 ± .09 | F(1,14) = .4 | .53 |
| phase shift [h] | .36 ± .18 | 2.04 ± .42 | F(1,14) = 13.4 | 2.0e-3 |
Data are mean ± SEM.
Precision is measured as the mean of the SE for each parameter.
Arguably, the more noteworthy phenotype pertained to the effects of RF on nocturnal activity. Under AL conditions in LD, dexras1−/− mice were very similar to WT controls in terms of the magnitude of nocturnal activity (expressed as wheel revolutions) and the phase angle (ψ) of LD entrainment; there was a very subtle, but significant, increase in the duration of nocturnal activity (α) in the mutants relative to the WT (Table 1). Daytime RF suppressed nocturnal activity in dexras1−/− mice (Figure 1E and F) to a much greater extent than in WT (Figure 1A–C), and this effect was significant between the genotypes when nocturnal activity was compared either as raw values (wheel revolutions) or as % of total daily activity (% nocturnal activity) (Figure 1G and H, Table 1). In 8 out of 15 dexras1−/− mice, this suppression was so pronounced that virtually all nocturnal activity was lost between d 3 and d 8 of the RF regimen, with some nocturnal activity reemerging on d 9 of RF (Figure 1E and F). In the remaining 7 out of 15 dexras1−/− mice (Figure 1D), nocturnal activity was suppressed but not abolished during the RF regimen: their phase angle of LD entrainment (~.68 h prior to dark onset) was significantly advanced relative to (i) WT controls under food restriction (Table 1) as well as (ii) phase angle measurements taken in the same dexras1−/− subjects prior to RF (F(1,150) = 35.2, p = 3.9e-8). RF had no significant effect on the phase angle of LD entrainment in WT mice relative to AL conditions (F(1,215) = 1.68, p = .2). Although the duration of nocturnal activity (α) was reduced in both WT (F(1,257) = 281, p < 1e-15) and dexras1−/− mice (F(1,245) = 413, p < 1e-15) during the RF schedule, there was greater compression of α in the mutants than in the WT, by ~1.5 h (Table 1). On d 11 of RF, the dexras1−/−mice (n = 8) that had lost their nocturnal activity during the RF regimen, along with an equal number of WT controls, were released into DD under AL conditions for 2 wks in order to assess the effects of RF on the phase of light-entrainable activity (LEA) rhythms. Normal LEA was reestablished in dexras1−/− mice following return to AL feeding conditions, and this activity component free-ran with a stable period (τDD) that did not differ significantly from that of the WT (Table 1). More importantly, RF induced a phase displacement of ~2 h in advance of the expected time in dexras1−/− mice, but had only a modest effect (~.36 h) in the WT (Figure 1B, C, E, and F and Table 1). This pattern of behavior—advanced phase angle of LD entrainment, compression of α, and a phase displacement upon return to AL conditions under DD—is consistent with the interpretation that the phase of SCN-driven activity rhythms becomes more sensitive to the effects of daytime food restriction in the absence of dexras1.
Behavioral Effects of Daytime RF Under DD Conditions in dexras1−/− Mice
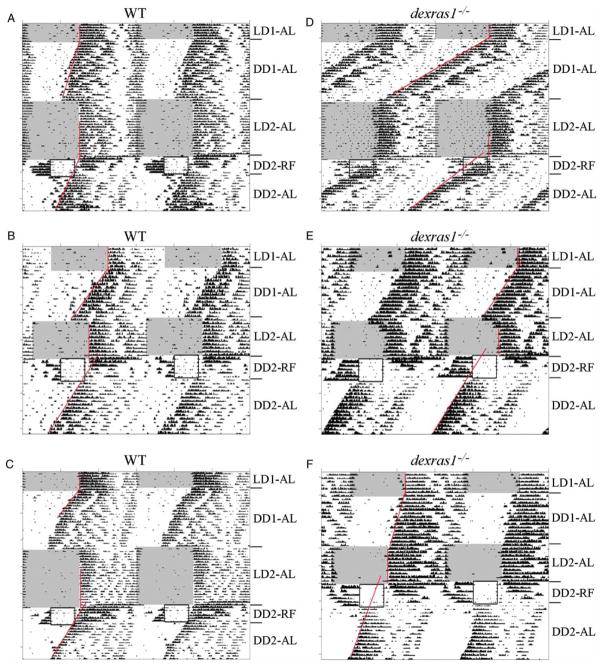
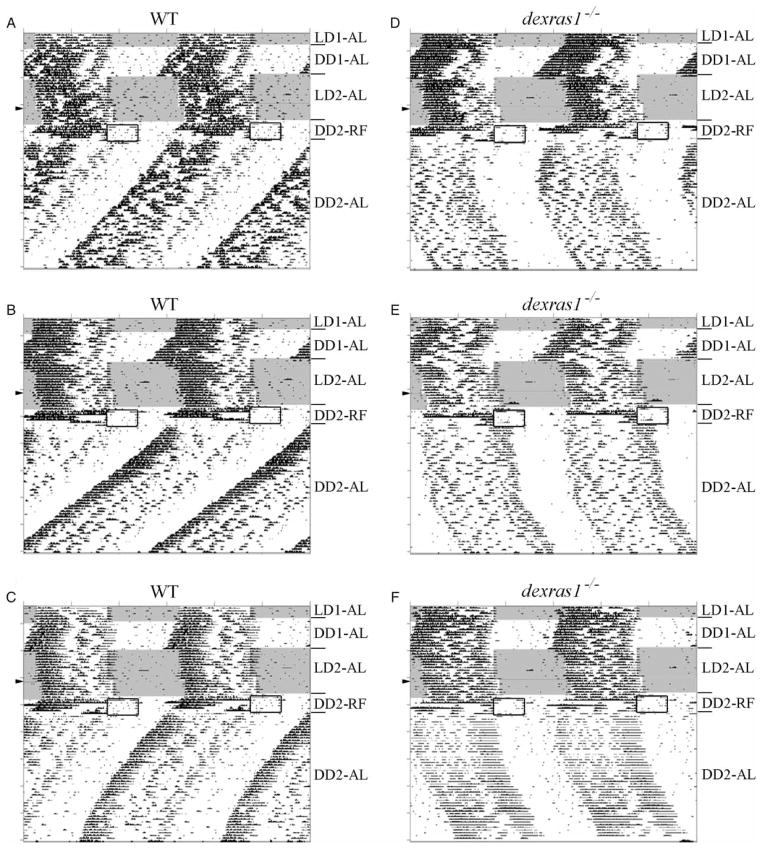
Based on the previous experiment, daytime food restriction and the LD cycle appeared to have strong and opposing effects on the LEA component of dexras1−/− mice. To isolate the effects of daytime RF from the competing influence of the LD cycle, we examined the behavior of dexras1−/− (n = 15) and WT (n = 15) mice that were subjected to a 6-d RF regimen under DD, with food made available between ZT 6 and ZT 11 (Figure 2 and Table 2). The magnitude of FAA, whether expressed as wheel revolutions or as % of total daily activity (% FAA), was significantly greater in dexras1−/− mice than in WT controls (Table 2). During the RF schedule, all WT mice exhibited concurrently two distinct activity components, the FAA and the LEA (Figure 2A–C). Closer visual inspection of the WT actograms revealed that the onsets of the LEA for the first 2–3 d of RF appeared to be phase-locked to the 24-h feeding cycle before free-running with a period <24 h (Figure 2A–C). This phenotype was observed in only 2 out of 15 dexras1−/− mice. The remaining mutant subjects were quite aberrant in their LEA component (Figure 2D–F and Figure S1). In 8 out of 15 dexras1−/− mice, onsets of the LEA during the first 4 d of RF appeared to delay by irregular, and sometimes large, increments; at the same time, there was a gradual reduction in the magnitude of the LEA until virtually all night-time activity was lost in the final 2 d of RF (Figure 2E and F). In 5 out of 15 dexras1−/− mice, LEA onsets appeared to advance in an irregular fashion until the LEA and FAA merged with one another by d 4 of RF (Figure 2D). Regarding the FAA, its duration and offset were significantly more precise in WT animals relative to dexras1−/− mice (Table 2). Additionally, the duration of FAA was significantly longer, and the offset of FAA significantly later (by ~1.5 h each), in the dexras1−/− mice relative to the WT, owing, in part, to the consolidation of the FAA with the LEA in 5 of our mutants (Table 2).
FIGURE 2.
The behavioral effects of daytime RF under DD are altered in the absence of Dexras1. A–F, Representative double-plotted actograms of wheel-running activity of (A–C) WT and (D–F) dexras1−/− mice subjected to RF for six consecutive days under DD, between ZT 6 and ZT 11 (relative to the previous 12L:12D cycle). Subsequently, mice were returned to ad libitum (AL) feeding conditions and maintained in DD for an additional 2 wks. Areas shaded in gray indicate periods of light. Boxed regions indicate periods of food availability. Red lines denote regression lines fitted to daily activity onsets. LD1-AL, ad libitum feeding under the first 12L:12D schedule. DD1-AL = ad libitum feeding under the first DD schedule; LD2-AL = ad libitum feeding under the second 12L:12D schedule; DD2-RF = restricted feeding under the second DD schedule; DD2-AL = return to ad libitum feeding under the second DD schedule.
TABLE 2.
Statistical comparison of the circadian behavior of WT and dexras1−/− mice following daytime restricted feeding under DD (Experiment 2).
| WT (n = 15) | dexras1−/− (n = 15) | F value | p value | |
|---|---|---|---|---|
| Pre-RF (ad libitum)* | ||||
| Total daily activity [wheel revolutions] | 2069 ± 66 | 2343 ± 74 | F(1,178) = 77 | 6.0e-3 |
| Phase of activity onset on day 7 of DD (ψ ) [h]¶ | 2.65 ± .41 | 3.91 ± .51 | F(1,27) = 4.4 | .05 |
| Period before RF (τpre) [h] | 23.62 ± .06 | 23.59 ±.09 | F(1,28) = .09 | .77 |
| Phase shift [h]** | .11 ± .09 | .18 ±.10 | F(1,28) = .24 | .63 |
| During daytime RF | ||||
| Total daily activity [wheel revolutions] | 1777 ± 210 | 1494 ±167 | F(1,175) = 7.2 | 7.0e-3 |
| Food anticipatory activity (FAA) [wheel revolutions] | 704 ± 79 | 996 ± 88 | F(1,175) = 14.6 | 1.8e-4 |
| % FAA [%] | 43.2 ± 4.2 | 62.9 ± 3.5 | F(1,175) = 21.6 | 6.3e-6 |
| Duration of FAA [h] | 2.98 ± .16 | 4.41 ± .27 | F(1,173) = 29.3 | 2.0e-7 |
| Onset of FAA [ZT]# | 3.48 ± .25 | 3.50 ±.31 | F(1,171) = .05 | .83 |
| Offset of FAA [ZT]# | 6.12 ± .06 | 7.60 ±.60 | F(1,173) = 14.8 | 1.7e-4 |
| Precision of duration of FAA## | .34 ± .03 | .96 ±.17 | F(1,28) = 13.4 | 1.0e-3 |
| Precision of onset of FAA## | .34 ± .03 | .80 ±.25 | F(1,28) = 3.2 | .08 |
| Precision of offset of FAA## | .15 ± .02 | .87 ±.25 | F(1,28) = 8.3 | 7.4e-3 |
| Post-RF (ad libitum) | ||||
| Period after RF (τpo) [h] | 23.57 ± .03 | 23.74 ±.11 | F(1,28) = 2.1 | .16 |
| Δτ (τpo − τpre) [h] | −.05 ± .08 | .15 ±.08 | F(1,28) = 3.0 | .09 |
| Phase shift [h]*** | −.76 ± .36 | 2.08 ±.27 | F(1,28) = 39.3 | 9.0e-7 |
| Phase of activity onset on the first day post-RF (ψ1) [h]¶ | 2.00 ± .31 | 5.70 ±.33 | F(1,27) = 42.1 | 5.9e-7 |
| Δψ (ψ1 −ψ ) [h] | −.65 ± .61 | 1.79 ±.47 | F(1,27) = 6.31 | .02 |
Data are mean ± SEM.
Measured during DD1-AL.
Expressed in hours before dark onset of the previous LD cycle.
Expressed as ZT of the previous LD cycle.
Precision is measured as the mean of the SE for each parameter.
Measured as the displacement of the regression lines drawn through the activity onsets of LD1-AL and DD1-AL.
Measured as the displacement of the regression lines drawn through the activity onsets of LD2-AL and DD2-AL.
At the end of the RF regimen, mice were returned to AL feeding conditions under DD for an additional 2 wks to assess the phase of the LEA component. AL restored the LEA component in the 8 mutant mice that had lost this activity during the final days of RF (Figure 2E and F). Stable free-running rhythms were observed in all WT and dexras1−/− mice during this 2-wk interval, with no significant change in period length compared with pre-RF values determined during DD1-AL (WT τpre vs. τpo: F(1,28) = .52, p = .48; knockout [KO] τpre vs. τpo: F(1,28) = 1.1, p = .31) (Figure 2 and Table 2). For all WT and mutant mice, the onset of the LEA component on the first day post-RF (corresponding to d 7 in DD) commenced several hours after the usual onset of FAA (Figure 2). In the case of dexras1−/− mice, the mean onset of LEA (on d 1 post-RF) coincided with the time of food presentation during the RF regimen (~.3 h after usual food presentation), whereas mean LEA onset was significantly later in the WT controls, ~2 h before the usual time of dark onset (of the previous 12L:12D cycle) (Figure 2, Table 2, and Figure S1). Importantly, this advanced phase of LEA onset on the first day post-RF in dexras1−/−mice cannot be predicted from the activity onset measured in the same individuals prior to RF treatment on d 7 of DD1-AL (5.70 ± .33 vs. 3.91 ± .51 h; F(1,26) = 8.61, p = 2.2e-3). In contrast, there was no significant difference in the mean onset of activity on the first day post-RF vs. d 7 of DD1-AL in the WT controls (2.00 ± .31 vs. 2.65 ± .41; F(1,28) = 1.56, p = .31). Finally, phase-shift determinations revealed that the phase of the stable free-running rhythm had advanced by ~2 h in RF-treated dexras1−/− mice, whereas it appeared to have delayed by ~.76 h in RF-treated WT mice (Figure 2 and Table 2). This apparent phase delay in the WT can be accounted for by the fact that the LEA component phase-locked for 2–3 d before starting to free-run on d 4 of RF. Collectively, these data suggest that in the absence of light and dexras1 expression, light-entrainable activity rhythms are exquisitely sensitive to the phase-modulating effects of daytime food restriction.
Daytime Restricted Feeding Alters Rhythmic Expression of PER1 and PER2 in the SCN of dexras1−/− Mice
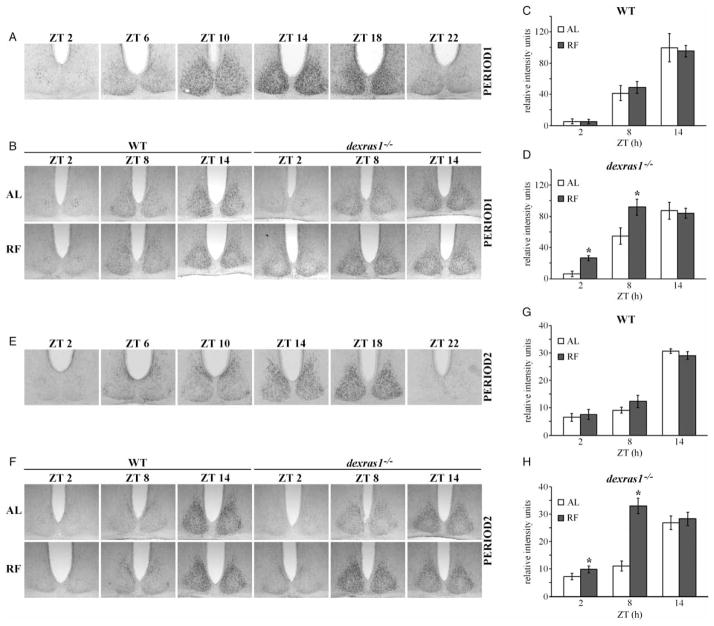
Given that the phase of behavioral activity rhythms in dexras1−/− mice had advanced following RF, we investigated whether molecular rhythms of clock gene expression within the SCN were also affected by RF in these mutant animals. After 6 d of daytime RF (ZT 6–11) under DD conditions, WT mice exhibited a profile of PER1 (Figure 3B [left] and C) and PER2 (Figure 3F [left] and G) expression within the SCN that was comparable to ad libitum–fed WT controls. In contrast, the expression of both PER1 (Figure 3B [right] and D) and PER2 (Figure 3F [right] and H) at ZT 2 and ZT 8 were significantly elevated in the SCN of RF-treated dexras1−/− mice relative to AL-fed dexras1−/− controls (PER1 ZT 2: F(1,14) = 22.6, p = 3.1e-4; PER1 ZT 8: F(1,13) = 8.4, p = .01; PER1 ZT 14: F(1,13) = .04, p = .83; PER2 ZT 2: F(1,16) = 6.2, p = .03; PER2 ZT 8: F(1,13) = 38.3, p = 3.3e-5; PER2 ZT 14: F(1,14) = .8, p = .39). Statistical analyses revealed an effect of ZT on PER1 (F(2,90) = 47.3, p < .001) and PER2 (F(2,90) = 127.8, p < .001) expression in the WT mice, but no effect of feeding condition (PER1: F(1,90) = .02, p = .9; PER2: F(1,90) = .5, p = .48), and no interaction between feeding condition and ZT (PER1: F(2,90) = .2, p = .82; PER2: F(2,90) = 1.2, p = .31). For dexras1−/− mice, there was an effect of feeding condition (PER1: F(1,90) = 7.34, p = .01; PER2: F(1,90) = 27.2, p = 6.3e-6), as well as ZT (PER1: F(2,90) = 35.6, p < .001; PER2: F(2,90) = 40.7, p < .001) on PER1 and PER2 expression, and an interaction between the two on PER2 expression (PER1: F(2,90) = 2.7, p = .08; PER2: F(2,90) = 1.2, p = 3.5e-5). When compared with typical PER1 (Figure 3A) and PER2 (Figure 3E) rhythms in the SCN of C57BL/6J mice, PER1 and PER2 expression in the RF-treated dexras1−/− mice appeared to be phase-advanced relative to AL-treated mutants. The distinctive expression at ZT 2 (a cluster of PER1- and PER2-IR nuclei in the center of the SCN) was present in the AL-fed dexras1−/− mice but was notably absent in the RF-treated dexras1−/− mice (Figure 3B and F). Instead, PER1 and PER2 expression at ZT 2 in these mice was found more peripherally in the dorsomedial SCN (Figure 3B and F). Increased expression at ZT 8 suggests that the actual phase of the SCN was closer to the zenith of PER1 and PER2 expression in the RF-treated dexras1−/− mice than in AL-fed controls. From these data, we conclude that daytime food restriction can alter the phase of clock gene expression in the absence of dexras1.
FIGURE 3.
Daytime food restriction alters the rhythms of PER1 and PER2 expression in the SCN of dexras1−/− mice. A, E, Representative photomicrographs of (A) PER1 and (E) PER2 expression in the SCN of C57BL/6J mice across the circadian cycle. Mice were dark-adapted for two consecutive days and killed at 4-h intervals at the indicated ZT relative to the previous 12L:12D cycle (n = 3/time point). B, F, Representative photomicrographs of (B) PER1 and (F) PER2 expression in the SCN of ad libitum–fed (AL) and food-restricted (RF) WT and dexras1−/− mice. Mice were subjected to RF from ZT 6 to ZT 11 under DD conditions for six consecutive days and then returned to ad libitum feeding conditions for 1 d. Mice were killed on d 7 at the indicated ZT relative to the previous 12L:12D cycle. AL controls were exposed to DD for the same length of time (7 d) but were provided food ad libitum throughout the experiment (n = 4/group). C, D, G, H, Quantification of (C, D) PER1 and (G, H) PER2 expression in the SCN of (C, G) WT and (D, H) dexras1−/− mice. Data are presented as mean ± SEM gray scale intensity, in relative arbitrary units, normalized to background staining (n = 4/group). *p < .05 vs. AL controls.
Daytime Restricted Feeding Alters Rhythmic Activation of the MAPK-ERK Pathway in the SCN of dexras1−/− Mice
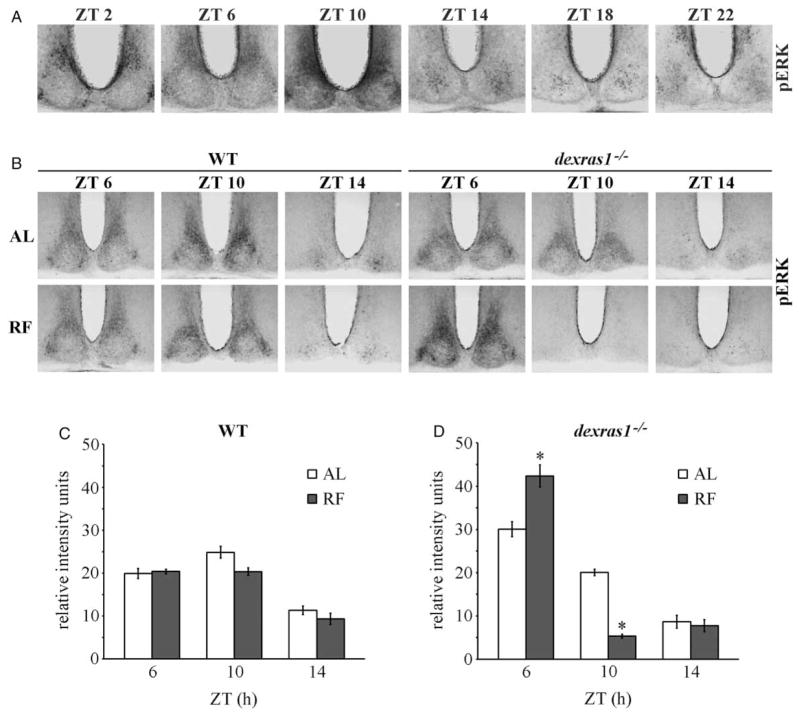
Our previous studies showed that light-induced phase shifts are altered in dexras1−/− mice as a result of aberrant coupling between light and ERK activation in the mutant SCN (Cheng et al., 2004, 2006). A number of biochemical studies have implicated Dexras1 in the regulation of the MAPK/ERK pathway (Cismowski et al., 1999; Fang et al., 2000), whose activity within the SCN is both rhythmic and light inducible (Butcher et al., 2002; Obrietan et al., 1998). The effects of daytime RF in dexras1−/−mice were next examined in the context of MAPK/ERK activation. A 4-d RF regimen (ZT 6–11) under DD had no effect on the expression of phosphoactive ERK1/2 (pERK) in the SCN of WT mice at the ZTs examined (Figure 4B [left] and C). Statistical analyses revealed an effect of ZT (F(2,90) = 78.7, p < .001), but no effect of feeding condition (F(1,90 = 1.5, p = .22) and no interaction between feeding condition and ZT (F(2,90) = 1.1, p = .35) in the WT controls. In contrast, pERK expression was dramatically altered in food-restricted dexras1−/− mice compared with AL controls (Figure 4B [left] and D). There was significant increase in pERK levels at ZT 6 (F(1,30) = 23.2, p = 9.6e-6) and sharp decline in expression at ZT 10 (F(1,30) = 55.8, p = 2.5e-8). In the mutant mice, there was significant effect of ZT (F(2,90) = 199.9, p < .001) and interaction between ZT and feeding condition (F(2,90) = 40.3, p = 3.2e-13) on pERK expression, but no overall effect of feeding condition (F(1,90 = 1.4, p = .237). Compared with pERK rhythms in the SCN of C57BL/6J mice (Figure 4A), daytime RF appeared to have advanced the phase of pERK expression in the SCN of dexras1−/−mice relative to AL controls. Collectively, the data show that the phase of MAPK/ERK rhythms is sensitive to daytime food restriction upon genetic ablation of dexras1.
FIGURE 4.
pERK rhythms are altered in the SCN of dexras1−/− mice during daytime restricted feeding. A, Representative photomicrographs of pERK expression in the SCN of C57BL/6J mice across the circadian cycle. Mice were dark-adapted for two consecutive days and killed at 4-h intervals at the indicated ZT relative to the previous 12L:12D cycle. B, Representative photomicrographs of pERK expression in the SCN of ad libitum–fed (AL) and food-restricted (RF) WT and dexras1−/− mice. Mice were subjected to RF from ZT 6 to ZT 11 under DD conditions for four consecutive days, and killed on the fourth day at the indicated ZT relative to the previous 12L:12D cycle. AL controls were exposed to DD for the same length of time but were provided food ad libitum throughout the experiment (n = 4/group). C, D, Quantification of pERK expression in the SCN of (C) WT and (D) dexras1−/− mice. Data are presented as mean ± SEM gray scale intensity, in relative arbitrary units, normalized to background staining (n = 4/group). *p < .05 vs. AL controls.
Behavioral Effects of Late-Night/Early-Morning RF Under DD Conditions in dexras1−/− Mice
To further characterize the effects of RF on dexras1−/−mice, we examined the behavior of mutant (n = 6) and WT (n = 6) mice that were restriction-fed during the late night/early morning (ZT 23 to ZT 4) under DD for six consecutive days (Figure 5 and Table 3). All animals displayed FAA during the RF regimen (Figure 5), although we chose not to quantitatively analyze the parameters of FAA (e.g., duration, onset, precision) because of its superposition with the LEA component. Instead, we calculated total daily activity on d 2 (WT vs. dexras1−/− mice [wheel revolutions]: 4256 ± 162 vs. 3535 ± 356; F(1,11) = 3.7, p = .08) and d 6 (WT vs. dexras1−/− mice [wheel revolutions]: 201 ± 53 vs. 292 ± 48; F(1,11) = 1.3, p = .26) of food restriction and found no significant difference between the genotypes. However, there was significant decline in total daily activity on d 6 of RF compared with d 2 in both genotypes, due to the near absence of activity during the first half of the animal’s subjective night (Figure 5).
FIGURE 5.
Short-term RF in the late night/early morning has long-lasting period effects in dexras1−/− mice. A–F, Representative double-plotted actograms of wheel-running activity of (A–C) WT and (D–F) dexras1−/− mice subjected to RF for six consecutive days under DD, between ZT 23 and ZT 4 (relative to the previous 12L:12D cycle). Subsequently, mice were returned to ad libitum (AL) feeding conditions and maintained in DD for an additional 8 wks. Areas shaded in gray indicate periods of light. Boxed regions indicate periods of food availability. Arrowhead denotes a 1-h shift in the LD schedule due to the transition from Daylight Savings Time to Standard Time that was not compensated for by the computer software. LD1-AL = ad libitum feeding under the first 12L:12D schedule; DD1-AL = ad libitum feeding under the first DD schedule; LD2-AL = ad libitum feeding under the second 12L:12D schedule; DD2-RF = restricted feeding under the second DD schedule; DD2-AL = return to ad libitum feeding under the second DD schedule.
TABLE 3.
Statistical analysis of the circadian period of WT and dexras1−/− mice before and after late-night/early-morning RF under DD (Experiment 5).
| RF-naíve* | WT (n = 7) | dexras1−/− (n = 7) | F value | p value |
|---|---|---|---|---|
| Period in week 1 of DD (τ1) [h] | 23.76 ± .01 | 23.67 ± .10 | F(1,13) = .7 | .43 |
| Period in week 3 of DD (τ3) [h] | 23.78 ± .01 | 23.71 ± .12 | F(1,13) = .4 | .56 |
| Δτ1 (τ3 − τ1) [h] | .03 ± .01 | .04 ± .04 | F(1,13)= .1 | .78 |
| Period in week 5 of DD (τ5) [h] | 23.77 ± .02 | 23.71 ± .08 | F(1,13)= .1 | .79 |
| Δτ2 (τ5 − τ1) [h] | .01 ± .01 | .08 ± .03 | F(1,13) = 3.2 | .10 |
|
| ||||
| Night-time RF | WT (n = 6) | dexras1−/− (n = 6) | F value | p value |
|
| ||||
| Period before RF (τpre) [h] | 23.66 ± .07 | 23.76 ± .10 | F(1,10)= .6 | .47 |
| Period in week 2 post-RF (τpo1) [h] | 23.62 ± .04 | 23.95 ± .08 | F(1,10) = 12.7 | 5.1e-3 |
| Δτ3 (τpo1 − τpre) [h] | −.04 ± .06 | .19 ± .04 | F(1,10) = 8.8 | .01 |
| Period in week 4 post-RF (τpo2) [h] | 23.63 ± .05 | 24.14 ± .11 | F(1,10) = 16.6 | 2.2e-3 |
| Δτ4 (τpo2 − τpre) [h] | −.02 ± .06 | .38 ± .07 | F(1,10) = 19.0 | 1.4e-3 |
Data are mean ± SEM.
A separate cohort of age-matched mice was maintained on ad libitum feeding under DD for 5 weeks for τ measurements.
After 6 d of RF, mice were returned to AL feeding conditions under DD for an additional 8 wks to assess the period of the LEA component (Figure 5 and Table 3). For each animal, period was measured at three different intervals: (i) pre-RF (first week of DD1-AL; τpre); (ii) second week post-RF (in DD2-AL; τpo1); and (iii) fourth week post-RF (in DD2-AL; τpo2). WT mice exhibited a stable free-running rhythm with a constant period throughout the 8-wk interval that did not differ significantly from pre-RF τ values (τpre vs. τpo1: F(1,10) = .2, p = .56; τpre vs. τpo2: F(1,10) = .1, p = .72) (Figure 5A–C and Table 3). In contrast, in all dexras1−/− mice examined, the free-running rhythm that emerged after return to AL showed a gradual and constant lengthening during the first 3 wks post-RF before reaching a stable period of >24 h (Figure 5D–F and Table 3). This is reflected in the mean period change (Δτ3 and Δτ4) within individual subjects for both post-RF intervals relative to the pre-RF state (Table 3). The mutant actograms during this interval are reminiscent of the “Eskin’s knee” (Eskin, 1971) that is commonly observed in mice under LL conditions. Overall, there was significant increase in the mean period of the mutant mice at both post-RF intervals compared with pre-RF τ values (τpre vs. τpo1: F(1,10) = 10.4, p = 7.9e-3; τpre vs. τpo2: F(1,10) = 16.3, p = 2.7e-3). Even though WT and dexras1−/− mice had a comparable free-running period prior to RF, τ values were significantly longer for the mutant mice than for the WT at both post-RF intervals (Table 3). To rule out the possibility that the observed period lengthening was a consequence of prolonged DD rather than an effect of the RF treatment, we subjected a separate cohort of RF-naïve dexras1−/− (n = 7) and WT (n = 7) mice to 8 wks of DD under AL feeding conditions, and measured the period in the first (τ1), third (τ3), and fifth (τ5) weeks of DD (corresponding to pre-RF, second week post-RF, and fourth week post-RF, respectively, in terms of length of time in DD). The fact that extended DD had no significant impact on the free-running period in either genotype (WT τ1 vs. τ3: F(1,12) = 2.4, p = .14; WT τ1 vs. τ5: F(1,12) = .4, p = .52; KO τ1 vs. τ3: F(1,14) = .8, p =.35; KO τ1 vs. τ5: F(1,14) = 1.2, p = .08) suggests that the period lengthening in the dexras1−/− mice was an effect of the RF treatment (Table 3). Last but not least, analysis of daily wheel-running activity revealed significant effect of genotype (F(1,235) = 23.4, p = 2.4e-6) and feeding condition (F(1,235) = 161, p < .001), and interaction between the two (F(1,235) = 1101, p < .001). Post hoc analysis showed that WT and dexras1−/− mice exhibited comparable levels of nocturnal activity during DD1-AL (WT vs. dexras1−/− mice [wheel revolutions]: 2630 ± 272 vs. 2459 ± 251; F(1,117) = .56, p = .46), and that the RF treatment evoked a long-lasting, significant reduction in daily activity in both the mutants (1207 ± 301; F(1,117) = 118, p < .001 vs. DD1-AL) and the WT (1911 ± 135; F(1,117) = 47.2, p = 3.3e-10 vs. DD1-AL) in the weeks following RF (during DD2-AL) (Figure 5). However, this reduction in daily activity was more profound in dexras1−/− mice relative to the WT (F(1,117) = 42.1, p = 2.1e-9), resulting in a 50.2% ± 14.4% suppression of activity in the mutants compared with only 23.2% ± 9.3% in the WT. Collectively, these data indicate that short-term food restriction during the late night/early morning has long-lasting effects on the period of free-running rhythms in the absence of dexras1.
DISCUSSION
The present study examined the potential involvement of Dexras1, a regulator of photic and nonphotic responses of the circadian clock (Cheng et al., 2004, 2006; Koletar et al., 2011), in the behavioral and physiological consequences of temporal restricted feeding. Using dexras1 gene–ablated mice, we showed that lack of dexras1 expression had profound effect on the behavior of food-restricted mice. Compared with WT, dexras1−/− mice exhibited greater, but less stable, FAA under daytime RF conditions (whether LD or DD). Absence of dexras1 also resulted in greater perturbations in the LEA component during the daytime RF regimen, in terms of reductions in activity level and changes in the phase of onset. Return to AL feeding conditions revealed that daytime RF had advanced (displaced) the phase of the LEA component in the mutant but not the WT mice. RF in the late night/early morning elicited long-lasting increase in the period of free-running activity, but had no effect on the WT. At the molecular level, daytime RF appeared to advance the phase of PER1, PER2, and pERK expression in the SCN of dexras1−/− mice, while having no influence on the WT. Collectively, our data suggest that Dexras1 plays an important role in regulating the behavioral outcome of temporal restricted feeding and the response of the SCN to RF.
The effects of daytime RF are well documented in the literature in a number of species, including nocturnal rodents, such as Mus musculus and Rattus norvegicus (Abe et al., 1989, 2007; Boulos et al., 1980; Castillo et al., 2004; Challet et al., 1997; Marchant & Mistlberger, 1997; Rosenwasser et al., 1981). Animals adapt to conditions of limited food availability by increasing food-seeking behavior, or FAA, in the hours preceding food presentation. FAA is accompanied by other physiological adaptations, e.g., increased body temperature, adrenal corticosterone secretion, and gastrointestinal motility, that prepare the animal for food intake (Challet et al., 2007; Comperatore & Stephan, 1987; Krieger, 1974). Electrolytic lesions of the SCN do not abolish FAA (Stephan et al., 1979a), and, in fact, have recently been shown to increase the magnitude of FAA and advance its onset (Angeles-Castellanos et al., 2010). The latter observation is in line with the possibility, first proposed by Mistlberger (2006), that expression of the behavioral outputs of the FEO requires that SCN outputs must first be suppressed or at least dissociated from their normal inhibitory control of daytime activity (masking). The augmented expression of FAA in our mutant mice may, therefore, be explained by greater reduction in the suppressive effects of the SCN on FEO outputs under food-restricted conditions. This may be due to loss of dexras1 expression within the SCN, itself, or alternatively within the FEO (independent of any effects of the SCN). Although we cannot rule out the latter possibility, at present we do not know whether Dexras1 is in fact expressed in the purported sites of the FEO, a topic of continued debate (Fuller et al., 2008; Gooley et al., 2006; Landry et al., 2006, 2007; LeSauter et al., 2009).
There is general consensus that the FEO and the SCN comprise two distinct oscillator systems that can mutually influence one another, although probably with unequal strengths (Stephan, 1986a, 1986b, 1986c; reviewed in Blum et al., 2011). Several studies have shown that under certain conditions RF can influence the expression of SCN-driven rhythms of behavior and gene expression. For instance, Castillo et al. (2004) showed that scheduled feeding under DD was able to entrain to varying extents the LEA component of house mice (Mus domesticus). Similar observations were made in Balb/c mice by Holmes and Mistlberger (2000) and in the CS strain by Abe et al. (1989). Challet’s group has demonstrated that metabolic cues associated with the nutritional content of food, e.g., high-fat or hypocaloric, rather than the time of food availability, are important factors in determining the resetting properties of the SCN in response to food or light (Challet et al., 1998; Mendoza et al., 2008a, 2008b). More recently, Mendoza et al. (2010) showed that the SCN of Per2Brdm1;Cry2−/−mutant mice are more sensitive to the entraining effects of scheduled feeding than WT. In our present study, there was some evidence that even in WT mice, SCN-driven activity may be somewhat, albeit mildly, sensitive to daytime RF, inasmuch as the LEO- and FEO-driven activity components showed weak relative coordination as indicated by the transient phase-locking of LEA onsets during the first few days of RF under DD (Figure 2).
Our study shows that genetic ablation of Dexras1 heightens the sensitivity of SCN-driven rhythms to the synchronizing effects of daytime RF. This was apparent in the 7 dexras1−/− mice that retained nocturnal activity throughout the RF schedule under LD but showed an advanced phase angle of LD entrainment. For the mutant mice that lost night-time activity during a portion of the RF schedule (whether under LD or DD), it was not possible to determine the effects of RF on the phase of the LEA component until the mice were returned to AL feeding in DD. In these animals, the SCN clock seemed to have effectively uncoupled from the activity rhythms during the RF regimen. This suggestion of decoupling is further supported by the fact that even though the LEA onsets were highly variable during the first 4 d of RF in DD in the dexras1−/− mice (8 showed irregular delays and 5 showed irregular advances), tissues harvested from all dexras1−/− mice (n = 4) at each time point on d 4 of RF exhibited consistent levels and distribution of pERK in the SCN that were in keeping with our interpretation of advanced pERK rhythms. In other words, irrespective of the behavioral status of the animal, pERK rhythms in the SCN had phase-advanced. In Experiment 2, upon return to AL feeding, not only did dexras1−/− mice start to free-run from a significantly advanced phase (~2 h earlier than predicted) on the first day post-RF, but phase-shift determinations also revealed an ~2 h advance once the animals had established stable free-running rhythms. Similar results were observed in Experiment 1 following return to AL feeding. This behavioral advance is also reflected in the state of the molecular clock within the SCN of the mutants: 1 d after return to AL (following 6 d of RF in DD), levels and distribution of PER1 and PER2 were altered in the mutant SCN in a manner consistent with a phase advance.
Perhaps the most pressing question is, what is the mechanism underlying this increased sensitivity to the synchronizing effects of RF in the mutants? One possibility is that the SCN of dexras1−/− mice is hyperresponsive to the behavioral arousal (FAA) elicited by temporal restricted feeding. This interpretation would be in keeping with our previous studies showing that dexras1−/− mice phase-advance in response to novel wheel exposure and neuropeptide Y application to the SCN (Cheng et al., 2004; Koletar et al., 2011). However, two observations reduce the likelihood of this interpretation. First, if arousal is causal to the phase shift, one might expect a positive correlation between the magnitude of FAA and the magnitude of the phase advance in the mutant animals; there was no evidence of this in our correlative analysis (Experiment 1 [LD-RF]: R2 = .0092 [n = 8]; Experiment 2 [DD-RF]: R2 = .0007 [n = 15]). Second, based on the nonphotic phase response curve (PRC) for novel wheel exposure for dexras1−/−mice (Koletar et al., 2011), one might expect the late-night/early-morning RF regimen also to produce large phase advances, since the PRC shows a large advance zone from ZT 18 to 23, which coincides with the timing of FAA in Experiment 5, and another from ZT 2 to 8. The most profound phenotype following late-night/early-morning RF under DD was the gradual lengthening of the free-running period. As a result, within the same animal, the calculated phase shift would vary greatly depending on the post-RF interval chosen to draw the regression line. In light of this caveat, even though all RF-treated dexras1−/− mice appeared to show a phase advance (when the regression line was drawn through d 4 to d 14 post-RF), there was positive correlation between the magnitude of the phase shift and length of the circadian period during this interval (R2 = .72), suggesting the apparent phase advance is merely a by-product of the longer period.
The second possibility is that loss of dexras1 alters the sensitivity of the SCN to light under RF conditions. We have previously shown that the photic PRC of dexras1−/−mice differs dramatically from that of the WT, with a small delay zone in the early night and large advance zones in the mid-day and late night (Cheng et al., 2004, 2006). Under this scenario, RF would further alter the PRC of the mutants in such a way that the LEA component cannot remain perfectly synchronized with the LD cycle. Although this interpretation can explain the results from Experiment 1, it cannot account for the phenotype of the mutant mice when RF was administered under DD.
The third possibility, and one alluded to throughout this Discussion, is that dexras1 ablation alters the coupling between the SCN and the FEO (and their respective outputs) upon temporal restricted feeding. Presumably, in both WT and mutant mice, RF engages the FEO from its original dampened state (Blum et al., 2011). In dexras1−/− mice, this is followed by efficient decoupling of the SCN from behavioral activity, which explains both the increase in FAA and the disappearance of nocturnal activity. From there, differences in the relative coupling strengths of the SCN and FEO in WT vs. dexras1−/− mice may influence the degree to which FEO-derived signals exert a synchronizing effect on the SCN. For instance, it may be that the coupling strength of the SCN to the FEO is much stronger in the mutant than in WT mice (and the reverse is presumably true for the coupling strength of the FEO to the SCN). This can lead to a stronger effect of the FEO on the phase and/or period of the SCN oscillator in the mutants but not the WT, either in the form of stronger relative coordination or (if the coupling strength is sufficiently great) entrainment of the SCN. Indeed, we feel that aberrations in coupling offer the single, most unifying explanation to account for the constellation of phenotypes exhibited by dexras1−/− mice under food restriction.
The mutant phenotype following late-night/early-morning RF is perhaps the most difficult to interpret, although that, too, might be explained within the context of coupling. This RF schedule had no long-term effect on the period of the LEA component of the WT animals after return to AL feeding conditions, whereas the period of the free-running rhythm of dexras1−/−mice was markedly lengthened, persisting for many weeks. This post-RF pattern of activity, characterized by a knee-shaped change in period and a reduction in total activity, mirrors constant light (LL) behavior of WT mice (Aschoff, 1960). LL-induced effects are usually attributed to weakened coupling between constituent circadian oscillators (Aschoff & Wever, 1976), either within the SCN or between the SCN and extra-SCN oscillators (Granados-Fuentes et al., 2004; Ohta et al., 2005). One possibility is that, in WT mice, return to AL feeding results in strong recoupling within the network of oscillators that controls behavioral output (including the SCN and FEO), with the SCN reestablishing dominant control of activity. In contrast, these oscillators may remain loosely coupled in the dexras1−/− mice in the post-RF period. Another possibility is that coupling within the SCN is compromised in the mutants following food restriction. Several studies have shown that G-protein signaling within the SCN is crucial for intercellular synchronization between SCN neurons (Aton et al., 2005, 2006), and have suggested a role of Dexras1, a G-protein regulator, in the synchronization process (Aton et al., 2006). This latter suggestion was based on our previous finding that dexras1−/− mice exhibited desynchronous behavior, including a hamster-like “splitting,” under LL (Cheng et al., 2004).
The molecular effects of daytime RF on the expression of pERK and PER1/2 in the SCN of dexras1−/− mice are interesting and, from a certain perspective, at odds with the general notion that RF suppresses the expression of LEO-driven outputs (discussed in Blum et al., 2012). Previous studies have shown that RF blunts the expression and secretion of arginine vasopressin (AVP) and vasoactive intestinal peptide (VIP) in and from the SCN without affecting the timing of their rhythmic expression (Andrade et al., 2004; Gooley et al., 2006; Kalsbeek et al., 1998). (Consistent with these studies, other “nonphotic” manipulations, such as dark pulses or novel wheel-induced arousal in the subjective day, have been shown to diminish the expression of pERK [Antle et al., 2008; Coogan & Piggins, 2005] and the period genes in the SCN [Maywood et al., 1999; Mendoza et al., 2004]). The mechanisms underlying this dampening of neuropeptide expression following RF are unclear, but do not appear to involve changes in clock gene expression in the SCN (Damiola et al., 2000; Girotti et al., 2009; Mendoza et al., 2005). Indeed, these latter studies demonstrating unperturbed clock gene expression in restriction-fed animals are in keeping with our observations that RF does not alter the expression of PER1/2 in the SCN of wild-type mice relative to ad libitum–fed controls. Finally, although our data (based on three time points) are consistent with the interpretation that daytime RF advances the phase of pERK and PER1/2 rhythms in the SCN of dexras1−/− mice, additional time points would be needed to strengthen this conclusion.
Taken together, our results indicate that genetic ablation of dexras1 has a profound effect on the behavior of mice under temporal restricted feeding, perturbing the phase and/or period of SCN-driven rhythms. Although the exact mechanisms underlying the mutant phenotype are presently unclear, alterations in oscillator coupling in these animals may be responsible for the observed effects. Future work on dexras1−/− mice will hopefully shed more light on the molecular and cellular bases underlying the heightened sensitivity of the mutant SCN to feeding cues.
Acknowledgments
The authors wish to thank Drs. Christian Beaulé and Sean W. Cain for helpful discussion, and Drs. Steven Reppert and David Weaver for providing the PER1 and PER2 antibodies, respectively.
Footnotes
Declaration of Interest: This work was supported by operating grants to H.-Y.M.C. from the Canadian Institute of Health Research (CIHR) and the National Sciences and Engineering Research Council (NSERC) of Canada. H.-Y. M.C. is the recipient on an Ontario Early Researcher Award. P.B.-C. is supported by a graduate scholarship from the Fonds de Recherche du Québec Nature et Technologies (FQRNT).
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Abe H, Kida M, Tsuji K, Mano T. Feeding cycles entrain circadian rhythms of locomotor activity in CS mice but not in C57BL/6J mice. Physiol Behav. 1989;45:397–401. doi: 10.1016/0031-9384(89)90146-7. [DOI] [PubMed] [Google Scholar]
- Abe H, Honma S, Honma K. Daily restricted feeding resets the circadian clock in the suprachiasmatic nucleus of CS mice. Am J Physiol Regul Integr Comp Physiol. 2007;292:R607–R615. doi: 10.1152/ajpregu.00331.2006. [DOI] [PubMed] [Google Scholar]
- Alvarez-Saavedra M, Antoun G, Yanagiya A, Oliva-Hernandez R, Cornejo-Palma D, Perez-Iratxeta C, Sonenberg N, Cheng HY. miRNA-132 orchestrates chromatin remodeling and translational control of the circadian clock. Hum Mol Genet. 2011;20:731–751. doi: 10.1093/hmg/ddq519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade JP, Pereira PA, Silva SM, Sá SI, Lukoyanov NV. Timed hypocaloric food restriction alters the synthesis and expression of vasopressin and vasoactive intestinal peptide in the suprachiasmatic nucleus. Brain Res. 2004;1022:226–233. doi: 10.1016/j.brainres.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Angeles-Castellanos M, Salgado-Delgado R, Rodriguez K, Buijs RM, Escobar C. The suprachiasmatic nucleus participates in food entrainment: a lesion study. Neuroscience. 2010;165:1115–1126. doi: 10.1016/j.neuroscience.2009.11.061. [DOI] [PubMed] [Google Scholar]
- Antle MC, Tse F, Koke SJ, Sterniczuk R, Hagel K. Non-photic phase shifting of the circadian clock: role of the extracellular signal-responsive kinases I/II/mitogen-activated protein kinase pathway. Eur J Neurosci. 2008;28:2511–2518. doi: 10.1111/j.1460-9568.2008.06533.x. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Biological clocks. Cold Spring Harbor Symp Quant Biol. 1960;25:11–28. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- Aschoff J, Wever R. Human circadian rhythms: a multioscillatory system. Fed Proc. 1976;35:2326–2332. [PubMed] [Google Scholar]
- Aton SJ, Colwell CS, Harmar AJ, Waschek J, Herzog ED. Vaso-active intestinal polypeptide mediates circadian rhythmicity and synchrony in mammalian clock neurons. Nat Neurosci. 2005;8:476–483. doi: 10.1038/nn1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aton SJ, Huettner JE, Straume M, Herzog ED. GABA and Gi/o differentially control circadian rhythms and synchrony in clock neurons. Proc Natl Acad Sci U S A. 2006;103:19188–19193. doi: 10.1073/pnas.0607466103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum ID, Patterson Z, Khazall R, Lamont EW, Sleeman MW, Horvath TL, Abizaid A. Reduced anticipatory locomotor responses to scheduled meals in ghrelin receptor deficient mice. Neuroscience. 2009;164:351–359. doi: 10.1016/j.neuroscience.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum ID, Lamont EW, Abizaid A. Competing clocks: metabolic status moderates signals from the master circadian pacemaker. Neurosci Biobehav Rev. 2011;36:254–270. doi: 10.1016/j.neubiorev.2011.06.003. [DOI] [PubMed] [Google Scholar]
- Boulos Z, Rosenwasser AM, Terman M. Feeding schedules and the circadian organization of behavior in the rat. Behav Brain Res. 1980;1:39–65. doi: 10.1016/0166-4328(80)90045-5. [DOI] [PubMed] [Google Scholar]
- Butcher GQ, Doner J, Dziema H, Collamore M, Burgoon PW, Obrietan K. The p42/44 mitogen-activated protein kinase pathway couples photic input to circadian clock entrainment. J Biol Chem. 2002;277:29519–29525. doi: 10.1074/jbc.M203301200. [DOI] [PubMed] [Google Scholar]
- Castel M, Belenky M, Cohen S, Ottersen OP, Storm-Mathisen J. Glutamate-like immunoreactivity in retinal terminals of the mouse suprachiasmatic nucleus. Eur J Neurosci. 1993;5:368–381. doi: 10.1111/j.1460-9568.1993.tb00504.x. [DOI] [PubMed] [Google Scholar]
- Castillo MR, Hochstetler KJ, Tavernier RJ, Greene DM, Bult-Ito A. Entrainment of the master circadian clock by scheduled feeding. Am J Physiol Regul Integr Comp Physiol. 2004;287:R551–R555. doi: 10.1152/ajpregu.00247.2004. [DOI] [PubMed] [Google Scholar]
- Challet E, Pévet P, Vivien-Roels B, Malan A. Phase-advanced daily rhythms of melatonin, body temperature, and locomotor activity in food-restricted rats fed during daytime. J Biol Rhythms. 1997;12:65–79. doi: 10.1177/074873049701200108. [DOI] [PubMed] [Google Scholar]
- Challet E, Solberg LC, Turek FW. Entrainment in calorie-restricted mice: conflicting zeitgebers and free-running conditions. Am J Physiol. 1998;274:R1751–R1761. doi: 10.1152/ajpregu.1998.274.6.R1751. [DOI] [PubMed] [Google Scholar]
- Cheng HYM, Obrietan K, Cain SW, Lee BY, Agostino PV, Joza NA, Harrington ME, Ralph MR, Penninger JM. Dexras1 potentiates photic and suppresses nonphotic responses of the circadian clock. Neuron. 2004;43:715–728. doi: 10.1016/j.neuron.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Cheng HY, Dziema H, Papp J, Mathur DP, Koletar M, Ralph MR, Penninger JM, Obrietan K. The molecular gatekeeper Dexras1 sculpts the photic responsiveness of the mammalian circadian clock. J Neurosci. 2006;26:12984–12995. doi: 10.1523/JNEUROSCI.4253-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cismowski MJ, Takesono A, Ma C, Lizano JS, Xie X, Fuernkranz H, Lanier SM, Duzic E. Genetic screens in yeast to identify mammalian nonreceptor modulators of G-protein signaling. Nat Biotechnol. 1999;17:878–883. doi: 10.1038/12867. [DOI] [PubMed] [Google Scholar]
- Comperatore CA, Stephan FK. Entrainment of duodenal activity to periodic feeding. J Biol Rhythms. 1987;2:227–242. doi: 10.1177/074873048700200306. [DOI] [PubMed] [Google Scholar]
- Coogan AN, Piggins HD. Dark pulse suppression of p-ERK and c-Fos in the hamster suprachiasmatic nuclei. Eur J Neurosci. 2005;22:158–168. doi: 10.1111/j.1460-9568.2005.04193.x. [DOI] [PubMed] [Google Scholar]
- Daan S, Pittendrigh CS. A functional analysis of circadian pacemakers in nocturnal rodents, II. The variability of phase response curves. J Comp Physiol. 1976;106:253–266. [Google Scholar]
- Damiola F, Le Minh N, Preitner N, Kornmann B, Fleury-Olela F, Schibler U. Restricted feeding uncouples circadian oscillators in peripheral tissues from the central pacemaker in the suprachiasmatic nucleus. Genes Dev. 2000;14:2950–2961. doi: 10.1101/gad.183500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskin A. Some properties of the system controlling the circadian activity of sparrows. In: Menaker M, editor. Biochronmetry. Washington, DC: National Academy of Sciences; 1971. pp. 55–80. [Google Scholar]
- Fang M, Jaffrey SR, Sawa A, Ye K, Luo X, Snyder SH. Dexras1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- Fuller PM, Lu J, Saper CB. Differential rescue of light- and food-entrainable circadian rhythms. Science. 2008;320:1074–1077. doi: 10.1126/science.1153277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girotti M, Weinberg MS, Spencer RL. Diurnal expression of functional and clock-related genes throughout the rat HPA axis: system-wide shifts in response to a restricted feeding schedule. Am J Physiol Endocrinol Metab. 2009;296:E888–E897. doi: 10.1152/ajpendo.90946.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooley JJ, Schomer A, Saper CB. The dorsomedial hypothalamic nucleus is critical for the expression of food-entrainable circadian rhythms. Nat Neurosci. 2006;9:398–407. doi: 10.1038/nn1651. [DOI] [PubMed] [Google Scholar]
- Granados-Fuentes D, Prolo LM, Abraham U, Herzog ED. The suprachiasmatic nucleus entrains, but does not sustain, circadian rhythmicity in the olfactory bulb. J Neurosci. 2004;24:615–619. doi: 10.1523/JNEUROSCI.4002-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hannibal J, Ding JM, Chen D, Fahrenkrug J, Larsen PJ, Gillette MU, Mikkelsen JD. Pituitary adenylate cyclase-activating peptide (PACAP) in the retinohypothalamic tract: a potential daytime regulator of the biological clock. J Neurosci. 1997;17:2637–2644. doi: 10.1523/JNEUROSCI.17-07-02637.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrington ME, Nance DM, Rusak B. Neuropeptide Y immunoreactivity in the hamster geniculo-suprachiasmatic tract. Brain Res Bull. 1985;15:465–472. doi: 10.1016/0361-9230(85)90037-1. [DOI] [PubMed] [Google Scholar]
- Holmes MM, Mistlberger RE. Food anticipatory activity and photic entrainment in food-restricted BALB/c mice. Physiol Behav. 2000;68:655–666. doi: 10.1016/s0031-9384(99)00231-0. [DOI] [PubMed] [Google Scholar]
- Honma K, von Goetz C, Aschoff J. Effects of restricted daily feeding on freerunning circadian rhythms in rats. Physiol Behav. 1983;30:905–913. doi: 10.1016/0031-9384(83)90256-1. [DOI] [PubMed] [Google Scholar]
- Huhman KL, Albers HE. Neuropeptide Y microinjected into the suprachiasmatic region phase shifts circadian rhythms in constant darkness. Peptides. 1994;15:1475–1478. doi: 10.1016/0196-9781(94)90126-0. [DOI] [PubMed] [Google Scholar]
- Jilge B, Stähle H. Restricted food access and light-dark: impact of conflicting zeitgebers on circadian rhythms of the rabbit. Am J Physiol. 1993;264:R708–R715. doi: 10.1152/ajpregu.1993.264.4.R708. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A, van Heerikhuize JJ, Wortel J, Buijs RM. Restricted daytime feeding modifies suprachiasmatic nucleus vasopressin release in rats. J Biol Rhythms. 1998;13:18–29. doi: 10.1177/074873098128999880. [DOI] [PubMed] [Google Scholar]
- Koletar MM, Cheng HY, Penninger JM, Ralph MR. Loss of dexras1 alters nonphotic circadian phase shifts and reveals a role for the intergeniculate leaflet (IGL) in gene-targeted mice. Chronobiol Int. 2011;28:553–562. doi: 10.3109/07420528.2011.592235. [DOI] [PubMed] [Google Scholar]
- Krieger DT. Food and water restriction shifts corticosterone, temperature, activity and brain amine periodicity. Endocrinology. 1974;95:1195–1201. doi: 10.1210/endo-95-5-1195. [DOI] [PubMed] [Google Scholar]
- Landry GJ, Simon MM, Webb IC, Mistlberger RE. Persistence of a behavioral food-anticipatory circadian rhythm following dorsomedial hypothalamic ablation in rats. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1527–R1534. doi: 10.1152/ajpregu.00874.2005. [DOI] [PubMed] [Google Scholar]
- Landry GJ, Yamakawa GR, Webb IC, Mear RJ, Mistlberger RE. The dorsomedial hypothalamic nucleus is not necessary for the expression of circadian food-anticipatory activity in rats. J Biol Rhythms. 2007;22:467–478. doi: 10.1177/0748730407307804. [DOI] [PubMed] [Google Scholar]
- Lee HS, Nelms JL, Nguyen M, Silver R, Lehman MN. The eye is necessary for a circadian rhythm in the suprachiasmatic nucleus. Nat Neurosci. 2003;6:111–112. doi: 10.1038/nn1006. [DOI] [PubMed] [Google Scholar]
- LeSauter J, Hoque N, Weintraub M, Pfaff DW, Silver R. Stomach ghrelin-secreting cells as food-entrainable circadian clocks. Proc Natl Acad Sci U S A. 2009;106:13582–13587. doi: 10.1073/pnas.0906426106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant EG, Mistlberger RE. Anticipation and entrainment to feeding time in intact and SCN-ablated C57BL/6j mice. Brain Res. 1997;765:273–282. doi: 10.1016/s0006-8993(97)00571-4. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Mrosovsky N, Field MD, Hastings MH. Rapid down-regulation of mammalian period genes during behavioral resetting of the circadian clock. Proc Natl Acad Sci U S A. 1999;96:15211–15216. doi: 10.1073/pnas.96.26.15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza JY, Dardente H, Escobar C, Pevet P, Challet E. Dark pulse resetting of the suprachiasmatic clock in Syrian hamsters: behavioral phase-shifts and clock gene expression. Neuroscience. 2004;127:529–537. doi: 10.1016/j.neuroscience.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Graff C, Dardente H, Pevet P, Challet E. Feeding cues alter clock gene oscillations and photic responses in the suprachiasmatic nuclei of mice exposed to a light/dark cycle. J Neurosci. 2005;25:1514–1522. doi: 10.1523/JNEUROSCI.4397-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J, Pévet P, Challet E. High-fat feeding alters the clock synchronization to light. J Physiol. 2008a;586:5901–5910. doi: 10.1113/jphysiol.2008.159566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendoza J, Drevet K, Pévet P, Challet E. Daily meal timing is not necessary for resetting the main circadian clock by calorie restriction. J Neuroendocrinol. 2008b;20:251–260. doi: 10.1111/j.1365-2826.2007.01636.x. [DOI] [PubMed] [Google Scholar]
- Mendoza J, Albrecht U, Challet E. Behavioural food anticipation in clock genes deficient mice: confirming old phenotypes, describing new phenotypes. Genes Brain Behav. 2010;9:467–477. doi: 10.1111/j.1601-183X.2010.00576.x. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian food anticipatory activity: formal models and physiological mechanisms. Neurosci Biobehav Rev. 1994;18:171–195. doi: 10.1016/0149-7634(94)90023-x. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Circadian rhythms: perturbing a food-entrained clock. Curr Biol. 2006;16:R968–R969. doi: 10.1016/j.cub.2006.10.020. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE. Neurobiology of food anticipatory circadian rhythms. Physiol Behav. 2011;104:535–545. doi: 10.1016/j.physbeh.2011.04.015. [DOI] [PubMed] [Google Scholar]
- Moore RY, Eichler VB. Loss of a circadian adrenal corticosterone rhythm following suprachiasmatic lesions in the rat. Brain Res. 1972;42:201–206. doi: 10.1016/0006-8993(72)90054-6. [DOI] [PubMed] [Google Scholar]
- Obrietan K, Impey S, Storm DR. Light and circadian rhythmicity regulate MAP kinase activation in the suprachiasmatic nuclei. Nat Neurosci. 1998;1:693–700. doi: 10.1038/3695. [DOI] [PubMed] [Google Scholar]
- Ohta H, Yamazaki S, McMahon DG. Constant light desynchronizes mammalian clock neurons. Nat Neurosci. 2005;8:267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- Pando MP, Morse D, Cermakian N, Sassone-Corsi P. Phenotypic rescue of a peripheral clock genetic defect via SCN hierarchical dominance. Cell. 2002;110:107–117. doi: 10.1016/s0092-8674(02)00803-6. [DOI] [PubMed] [Google Scholar]
- Pendergast JS, Nakamura W, Friday RC, Hatanaka F, Takumi T, Yamazaki S. Robust food anticipatory activity in BMAL1-deficient mice. PLoS ONE. 2009;4:e4860. doi: 10.1371/journal.pone.0004860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS. Temporal organization: reflections of a Darwinian clock-watcher. Annu Rev Physiol. 1993;55:16–54. doi: 10.1146/annurev.ph.55.030193.000313. [DOI] [PubMed] [Google Scholar]
- Portaluppi F, Smolensky MH, Touitou Y. Ethics and methods for biological rhythm research on animals and human beings. Chronobiol Int. 2010;27:1911–1929. doi: 10.3109/07420528.2010.516381. [DOI] [PubMed] [Google Scholar]
- Rashotte ME, Stephan FK. Coupling between light- and food-entrainable circadian oscillators in pigeons. Physiol Behav. 1996;59:1005–1010. doi: 10.1016/0031-9384(95)02125-6. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Boulos Z, Terman M. Circadian organization of food intake and meal patterns in the rat. Physiol Behav. 1981;27:33–39. doi: 10.1016/0031-9384(81)90296-1. [DOI] [PubMed] [Google Scholar]
- Stephan FK. The role of period and phase in interactions between feeding- and light-entrainable circadian rhythms. Physiol Behav. 1986a;36:151–158. doi: 10.1016/0031-9384(86)90089-2. [DOI] [PubMed] [Google Scholar]
- Stephan FK. Coupling between feeding- and light-entrainable circadian pacemakers in the rat. Physiol Behav. 1986b;38:537–544. doi: 10.1016/0031-9384(86)90422-1. [DOI] [PubMed] [Google Scholar]
- Stephan FK. Interaction between light- and feeding-entrainable circadian rhythms in the rat. Physiol Behav. 1986c;38:127–133. doi: 10.1016/0031-9384(86)90142-3. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Zucker I. Circadian rhythms in drinking behavior and locomotor activity of rats are eliminated by hypothalamic lesions. Proc Natl Acad Sci U S A. 1972;69:1583–1586. doi: 10.1073/pnas.69.6.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephan FK, Swann JM, Sisk CL. Anticipation of 24-hr feeding schedules in rats with lesions of the suprachiasmatic nucleus. Behav Neural Biol. 1979a;25:346–363. doi: 10.1016/s0163-1047(79)90415-1. [DOI] [PubMed] [Google Scholar]
- Stephan FK, Swann JM, Sisk CL. Entrainment of circadian rhythms by feeding schedules in rats with suprachiasmatic lesions. Behav Neural Biol. 1979b;25:545–554. doi: 10.1016/s0163-1047(79)90332-7. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Umeda N, Tsutsumi Y, Fukumura R, Ohkaze H, Sujino M, van der Horst G, Yasui A, Inouye ST, Fujimori A, Ohhata T, Araki R, Abe M. Mouse dexamethasone-induced RAS protein 1 gene is expressed in a circadian rhythmic manner in the suprachiasmatic nucleus. Brain Res Mol Brain Res. 2003;110:1–6. doi: 10.1016/s0169-328x(02)00543-0. [DOI] [PubMed] [Google Scholar]
- Valentinuzzi VS, Scarbrough K, Takahashi JS, Turek FW. Effects of aging on the circadian rhythm of wheel-running activity in C57BL/6 mice. Am J Physiol. 1997;273:R1957–R1964. doi: 10.1152/ajpregu.1997.273.6.R1957. [DOI] [PubMed] [Google Scholar]
- Yannielli PC, Harrington ME. The neuropeptide Y Y5 receptor mediates the blockade of “photic-like” NMDA-induced phase shifts in the golden hamster. J Neurosci. 2001;21:5367–5373. doi: 10.1523/JNEUROSCI.21-14-05367.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannielli PC, Molyneux PC, Harrington ME, Golombek DA. Ghrelin effects on the circadian system of mice. J Neurosci. 2007;27:2890–2895. doi: 10.1523/JNEUROSCI.3913-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494:528–548. doi: 10.1002/cne.20823. [DOI] [PMC free article] [PubMed] [Google Scholar]