Abstract
Six transmembrane protein of prostate (Stamp) proteins play an important role in prostate cancer cell growth. Recently, we found that Stamp2 has a critical role in the integration of inflammatory and metabolic signals in adipose tissue where it is highly expressed and regulated by nutritional and metabolic cues. In this study, we show that all Stamp family members are differentially regulated during adipogenesis: whereas Stamp1 expression is significantly decreased upon differentiation, Stamp2 expression is increased. In contrast, Stamp3 expression is modestly changed in adipocytes compared to preadipocytes, and has a biphasic expression pattern during the course of differentiation. Suppression of Stamp1 or Stamp2 expression both led to inhibition of 3T3-L1 differentiation in concert with diminished expression of the key regulators of adipogenesis - CCAAT/enhancer binding protein alpha (C/ebpα) and peroxisome proliferator-activated receptor gamma (Pparγ). Upon Stamp1 knockdown, mitotic clonal expansion was also inhibited. In contrast, Stamp2 knockdown did not affect mitotic clonal expansion, but resulted in a marked decrease in superoxide production that is known to affect adipogenesis. These results suggest that Stamp1 and Stamp2 play critical roles in adipogenesis, but through different mechanisms.
Introduction
Over the last decades, there has been a dramatic increase in the prevalence of obesity. A recent estimate indicated that more than 1.5 billion people world-wide are overweight or obese [1]. This is a consequence of imbalances in expenditure and intake of energy along with changes in nutrition sources [2]. Obesity is linked to an increased risk of developing various diseases such as type 2 diabetes, cardiovascular disease, hepatic steatosis, airway disease, neurodegeneration, biliary disease, and certain cancers [3]. These maladies are now among the leading causes of death worldwide [4].
The increase in obesity has focused attention on adipose tissue function and development. Adipogenesis, the process by which fibroblastic precursor cells or preadipocytes are converted into mature adipocytes, has been one of the most intensively studied model systems for cellular differentiation [5]. Most of the adipogenesis research has utilized pre-adipocyte cell culture models (e.g. the murine cell lines 3T3-L1 and 3T3-F442A) [6]. For 3T3-L1 cells, a hormonal mixture commonly containing dexamethasone, isobutylmethylxanthine and insulin is used to activate signaling pathways which initiate a cascade of transcription factors that drive the adipogenic program through the stages of mitotic clonal expansion, growth arrest, and terminal differentiation [7], [8]. The nuclear receptor Pparγ and members of the C/ebp family are critical determinants of this process together with an assembly of transcriptional co-regulators. More recently, new mechanisms and cellular processes regulating the adipogenic conversion have been reported (for a brief overview, see [9]). Of these, oxidative stress and reactive oxygen species (ROS) have been implicated in pre-adipocyte differentiation [10]. ROS can affect the preadipocytes as both an external or internal signal, and depending on the source and localization, it may either promote or inhibit differentiation in a given model system [11]–[16].
The Stamp family of proteins (also known as STEAPs) consists of three members (Stamp1-3) that share high sequence similarity in the putative six-transmembrane domain; a region homologous to F(420)H(2):NADP(+) oxidoreductases found in archaea and bacteria, as well as to the yeast FRE family of metalloreductases [17]. All Stamps have metalloreductase activity in HEK293T cells [18]. Furthermore, Stamp3 has been shown to be essential for normal iron metabolism in mice [19]. Stamp2 expression is induced by tumor necrosis factor alpha (TNFα) in 3T3-L1 cells (thus also called TNFα-induced adipose-related protein (Tiarp)) and its expression is increased during differentiation [20]. In addition, studies in knockout mice showed that Stamp2 integrates inflammatory and nutritional signaling in mice on a regular diet [21]. More recently, we have found that Stamp2 controls intermediary metabolites to regulate inflammatory responses and atherosclerosis in mice [22]. Human STAMP2 expression in human adipocytes is stimulated by TNFα and interleukin 6, and STAMP2 levels positively correlate with insulin sensitivity [23], [24]. Furthermore, recent human studies found STAMP2 expression decreased in obese and/or insulin resistant individuals [25]–[27]. These findings point to a protective role of Stamp2 in adipose tissue function in both human and mice. However, a recent report found that STAMP2 expression was increased in obese patients and this was linked to reduced insulin response in isolated adipocytes [28].
Here, we investigated the expression of the Stamp family during adipogenic conversion of 3T3-L1 cells, and show that they are differentially regulated during adipogenesis with distinct profiles. We also show that both Stamp1 and Stamp2 affect 3T3-L1 adipogenesis. Herein we explore the molecular details of this process.
Materials and Methods
Cell Lines and Cell Culture
3T3-L1 cell line (a generous gift from the lab of Gökhan S. Hotamisligil, ATCC, CL-173) was maintained in Dulbecco’s modified Eagle’s medium (DMEM) (Lonza) supplemented with 10% fetal bovine serum (FBS) (Saveen Werner), 50 U/ml penicillin-streptomycin (Lonza) and 2 mM L-Glutamine (Lonza) in a 5% CO2 humidified atmosphere. 3T3-L1 cells were differentiated to adipocytes by adding standard adipogenic cocktail (0.5 mM methylisobutylxanthine [Sigma-Aldrich], 5 µg/ml insulin [Sigma-Aldrich], and 1 µM dexamethasone [Sigma-Aldrich]) to post-confluent cells. 48 h later, cells were re-fed with normal growth medium containing 5 µg/ml insulin every second day until day 8. When indicated, 1 µM pioglitazone (Pio) (Sigma-Aldrich) was added to the adipogenic cocktail.
Lentivirus Production and Establishment of Stable 3T3-L1 Cell Lines
pLKO.1 plasmids (Sigma-Aldrich) containing shRNA against Stamp1 (sh-St1_1 [TRCN0000253445] and sh-St1_2 [TRCN0000253448]), Stamp2 (sh-St2_1 [TRCN0000249066] and sh-St2_2 [TRCN0000249065]) or green fluorescent protein (GFP) (shGFP) were transfected together with a packaging plasmid (pCMV-ΔR8.2) and an envelope plasmid (pCMV-VGS-G) into HEK293T cells using Fugene 6 (Invitrogen). 48 h post transfection conditioned medium was harvested, filtered through a 0.45 µm filter (Millipore) and added to 3T3-L1 fibroblasts. 36 h post infection the 3T3-L1 cells were subjected to selection with 2 µg/ml puromycin for 7 days after which the cells were maintained in medium with 1 µg/ml puromycin. Unless specified, sh-St1_1 and sh-St2_1 cells were used in the experiments presented.
Immunofluoresence Microscopy
3T3-L1 cells were plated on cover slips, grown to post confluency and treated with adipogenic cocktail for 16 h. Cells were washed briefly with phosphate buffered saline (PBS) and fixed in methanol at −20°C for 5 min. Cells were then blocked with 1% BSA (Sigma-Aldrich) for 30 min before incubation with C/ebpβ antiserum (1∶100) (Abcam, ab32358) at 4°C overnight and incubated with Alexa Fluor 488 goat anti-rabbit secondary antibodies (1∶500) (Invitrogen) for 1 h at room temperature. DAPI (Sigma-Aldrich) staining was used for visualizing the nuclei. Images were acquired with an Olympus FlowView FV1000.
Oil Red O Staining
The cells were washed briefly with PBS and then fixed with 0.5% gluteraldehyde in PBS followed by washes with PBS and 60% isopropanol (Arcus) in PBS. The cells were then stained in Oil Red O solution (3 parts Oil Red O [0.5 g [Sigma-Aldrich] in 200 ml isopropanol] and 2 parts MQ water) for 15 min and washed with 60% isopropanol followed by a final wash in PBS. Images were taken with an AxioCam HRc (Zeiss). For quantification, the Oil Red O was extracted from the cells with 100% isopropanol for 5 min. The extracts were clarified by centrifugation at 10,000 g for 2 min and absorbance at 460 nm was determined with a multiplate reader (Victor2, PerkinElmer).
Cell Counting
At indicated stages of differentiation, the cells were washed with PBS, dissociated with Trypsin EDTA (Lonza), diluted in DMEM and counted using a haemocytometer.
NBT Assay
The cells were washed briefly with PBS and then incubated with 0.1 mg/ml Nitro Blue Tetrazolium (NBT) (Sigma-Aldrich) in PBS at 37°C for 90 min to allow blue formazan crystals to form. The cells were then washed with PBS and images were acquired with an AxioCam HRc (Zeiss). To quantify the formazan produced, the cells were lysed with a 2 M KOH/DMSO (Sigma-Aldrich) (1/1.17, v/v) solution for 15 min. The lysate was clarified by centrifugation at 10,000 g for 2 min and absorbance at 570 nm was determined with a multiplate reader (Victor2, PerkinElmer).
Western Analysis
The cells were washed with PBS at the indicated time points and protein extracts were made in lysis buffer (20 mM HEPES [pH7.7], 0.3 M, NaCl 0.2 mM EDTA, 1.5 mM MgCl2, 1% Triton X-100, 0.1% SDS with 1X Protease inhibitor cocktail [Roche] and Phosphatase inhibitor cocktail [Roche]) for 1 h. 50–100 µg of protein extract was resolved on a 10% polyacrylamide-SDS gel, blotted to a PVDF membrane and incubated with antisera against C/ebpβ (1∶500) (Abcam, ab32358), C/ebpα (1∶500) (Santa Cruz, sc-61), Pparγ (1∶1000) (a generous gift from Professor H.I. Nebb, Santa Cruz, sc-7273), STEAP4 (1∶500) (Proteintech, 11944-1-AP) or β-actin (Sigma-Aldrich) (1∶10000) in 5% BSA (1% for STEAP4 antibody) in Tris buffered saline (TBS)-0.1% Tween. Images were obtained with a Kodak imaging station 4000R and the band intensities were determined using Carestream Imaging Software.
Quantitative Reverse-transcription PCR (qRT-PCR)
Total RNA was extracted from cells using the Trizol reagent (Invitrogen). mRNA transcripts were converted to cDNA by the Superscript II (Invitrogen) reverse transcriptase using oligo(dT) primers (Sigma-Aldrich). cDNA was quantified by the Lightycler480 system using the SYBR Green dye (Roche). For each primer pair the crossing point (CP) values of a given PCR for a sample were set relative to the CP value of the wild type control group, while also correcting for primer specific reaction efficiency with an internal standard curve. The values were then normalized to the expression of the ribosomal gene 36B4. All PCR products were analyzed by melting curve analysis. qRT-PCR primer sequences (all from Sigma-Aldrich) used in this study are as follows: Stamp1, forward 5′-ATA GGA AGT GGG GAT TTT GC-3′, reverse 5′-AGA TGT CTC AGG TCC CAC AA-3′; Stamp2, forward 5′- TCA CTT CCT TGC CAT CAG-3′, reverse 5′- GCT CCA CCT TAG AAT CGA AG-3′; Stamp3, forward 5′- CCG TCC ATT GCT AAT TCC CTC-3′, reverse 5′- CGG CAG GTA GAA CTT GTA GTG-3′; aP2: forward 5′-GTC ACC ATC CGG TCA GAG AG-3′, reverse 5′-TCG ACT TTC CAT CCC ACT TC-3′; Pparγ, forward 5′-GCC CTT TGG TGA CTT TAT GG-3′, reverse 5′-GGC GGT CTC CAC TGA GAA TG-3′; C/ebpα, forward 5′-GCG GCA AAG CCA AGA AGT C-3′, reverse 5′-GCG GTC ATT GTC ACT GGT CA-3′; 36B4, forward 5′-AAG CGC GCG TCC TGG CAT TGT CT-3′, reverse 5′-CCG CAG GGG CAG CAG TGG T-3′.
Statistics
Statistical analyses were performed using the Student’s t-test. Data are presented as means and error bars represent standard deviation. Significance was defined as p<0.05. All analyses were repeated with the Mann-Whitney-Wilcoxon (MWW) test, which resulted in the same outcome as obtained with the Student’s t-test.
Results
Regulation of Stamp Expression during 3T3-L1 Adipogenesis
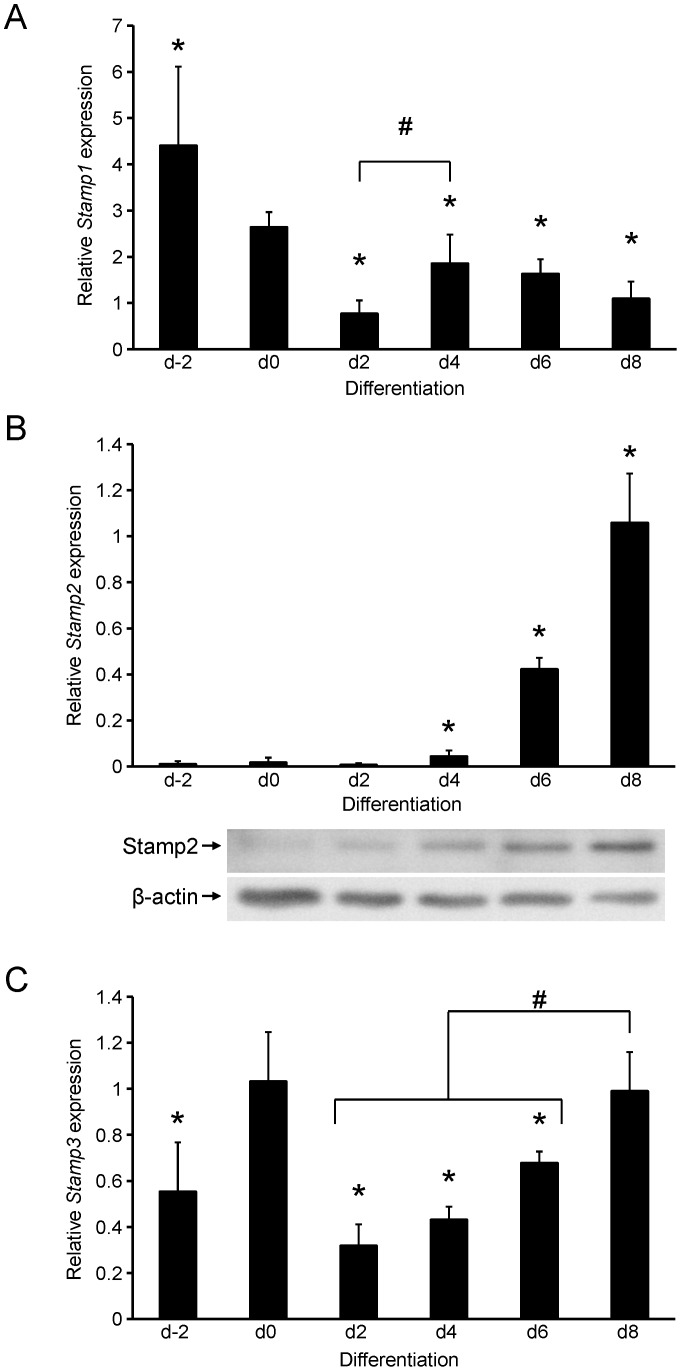
The increase in Stamp2 expression during 3T3-L1 differentiation into adipocytes has been reported previously [20], [21]. In addition, Stamp3 protein level was slightly increased upon 3T3-L1 differentiation and decreased in the adipose tissue of rats fed a high fat diet after a treatment with an anti-obesity drug [29], [30]. To investigate the coordinated expression of all Stamp family members in the same system, we differentiated 3T3-L1 fibroblasts into mature adipocytes and harvested proliferating cells (d-2), postconfluent cells (d0), and cells differentiated for 2, 4, 6 and 8 days (d2-d8), isolated RNA, and determined Stamp mRNA expression by qRT-PCR analysis (Figure 1). Stamp1 mRNA expression was highest in proliferating 3T3-L1 fibroblasts (Figure 1A). Upon confluency, there was an approximately 40% reduction in Stamp1 expression which decreased a further 70% at 48 h. Interestingly, the Stamp1 expression rebounded at day 4 to about twice that of day 2 and then declined again during the rest of differentiation. Consistent with previous reports, Stamp2 mRNA expression was low until day 4 when its expression increased to about 4-fold higher than in proliferating cells (Figure 1B, top). Stamp2 levels increased dramatically after that reaching 60-fold higher levels by day 8 compared with what observed at day 0. Consistently, Stamp2 protein levels were barely detectable at day 0 and increased by approximately 30-fold by day 8 of differentiation (Figure 1B, bottom). In contrast to Stamp1 and Stamp2, Stamp3 expression showed a biphasic pattern rather than a distinct directional regulation pattern as it increased approximately 2-fold upon confluency, then dropped significantly to about 30% of this level by day 2, and then continued to rise again reaching similar levels of expression seen at confluency by day 8. Available antisera for Stamp1 and Stamp3 did not function in Western analysis and thus we were unable to explore relative changes to the protein levels for these proteins (data not shown). These data show that all Stamp family members are expressed in 3T3-L1 cells and are differentially regulated during adipogenesis.
Figure 1. Regulation of Stamp family expression during 3T3-L1 adipogenesis.
(A–C) qRT-PCR analysis of 3T3-L1 cells harvested at the indicated time points (days) of differentiation. The figures show the mRNA expression of Stamp1 (A), Stamp2 (B, top), and Stamp3 (C) normalized to the reference gene 36B4. The results are from three independent experiments, n = 9. *p<0.05 compared to d0; #p<0.05 between brackets. (B, bottom) Western analysis showing Stamp2 and β-actin protein levels from day 0 to day 8 of differentiation in 3T3-L1 cells harvested in parallel to those used for qRT-PCR analysis. The figure presented is representative of two independent experiments.
Knockdown of Stamp1 and Stamp2 Expression Suppresses 3T3-L1 Adipogenesis
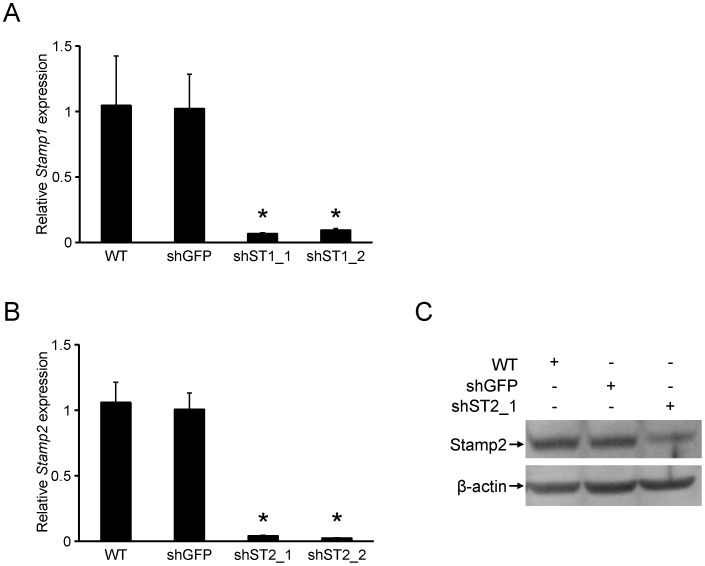
Since Stamp1 and Stamp2 expression were most significantly affected during adipogenesis, we investigated the possible consequence of their knockdown on 3T3-L1 differentiation. To that end, we generated 3T3-L1 cell lines stably expressing short hairpin RNAs (shRNAs) against either Stamp1 (sh-St1 cells) or Stamp2 (sh-St2 cells), as well as Green Fluorescent Protein (GFP) (sh-GFP cells). There was an approximately 90% and 95% knockdown, respectively, of Stamp1 and Stamp2 expression, in these cell lines compared to sh-GFP cells (Figures 2A and 2B). Moreover, Western analysis showed that Stamp2 protein levels were reduced by about 70% in sh-St2 adipocytes compared to that of sh-GFP expressing or WT cells (Figure 2C).
Figure 2. Stamp1 or Stamp2 knockdown in 3T3-L1 cells.
(A–B) qRT-PCR analysis of WT, sh-GFP, sh-St1 and sh-St2 cells. The figures show relative mRNA expression of Stamp1 (A) and Stamp2 (B) normalized to the reference gene 36B4 from one experiment, n = 3. *p<0.05 compared to sh-GFP cells. The data presented are representative of three independent experiments. (C) Western analysis showing Stamp2 and β-actin protein levels at day 8 of differentiation in WT, sh-GFP and sh-St2 cells. The data presented are representative of two independent experiments.
To assess the possible functional effect of Stamp1 and Stamp2 knockdown on 3T3-L1 cells, we differentiated sh-St1, sh-St2, sh-GFP and the wild type (WT) cells into adipocytes in the presence or absence of pioglitazone (Pio), an agonist of Pparγ and a known promoter of adipogenesis [31]. The cells were stained for lipid accumulation with Oil Red O to determine the extent of differentiation. The WT and sh-GFP cells showed similar levels of adipogenic conversion, distinctly increased compared to undifferentiated WT cells (Figures 3A and 3B). Differentiation in the presence of Pio further increased adipogenesis for WT and sh-GFP cells with 30% and 35%, respectively, consistent with previous findings [32]. sh-St1 and sh-St2 cells had significantly lower levels of differentiation (70% and 40%, respectively) in regular differentiation medium compared with the WT and sh-GFP control cells (Figures 3A and 3B). However, in the presence of Pio, this defect was rescued and the differentiation of sh-St1 and sh-St2 cells was comparable to that of WT and sh-GFP cells. These data show that Stamp1 or Stamp2 knockdown impairs adipogenesis and that this may be due to disrupted Pparγ signaling.
Figure 3. Stamp1 or Stamp2 knockdown reduces 3T3-L1 adipogenesis.
(A–B) Oil Red O staining of WT, sh-GFP, sh-St1 and sh-St2 cells differentiated with pioglitazone (Pio) or vehicle (Ctrl). (A) Representative images of the staining. (B) Quantification from three independent experiments, n = 9. *p<0.05 compared to sh-GFP cells; #p<0.05 between Ctrl and Pio groups. (C) Relative cell number of the same cells as in (A). The results are from three independent experiments, n = 9. *p<0.05 compared to sh-GFP cells; #p<0.05 between Ctrl and Pio groups. (D) Relative cell number of sh-St1 cells differentiated with increasing concentrations of Pio. The results are from one experiment, n = 3. *p<0.05 compared to shGFP; #p<0.05 between brackets.
We have previously shown that STAMP1 and STAMP2 both increase human prostate cancer cell proliferation [33]–[35]. Since both Stamp1 and Stamp2 are differentially regulated during 3T3-L1 adipogenesis (Figures 1A and 1B), we examined if silenced expression of either would 3T3-L1 cell proliferation. To that end, equal numbers of WT, sh-GFP, sh-St1 and sh-St2 cells were cultured, induced with an adipogenic cocktail with or without Pio and cell numbers determined after 8 days of differentiation (Figure 3C). Without Pio, there was no difference in cell number of the WT, sh-GFP and sh-St2 adipocytes. However, the sh-St1 cell growth was retarded by 40–50% compared with sh-GFP cells, suggesting that the mitotic clonal expansion phase of 3T3-L1 adipogenesis is blocked upon Stamp1 loss [7]. Differentiation in the presence of Pio did not affect cell growth at day 8 of differentiation for WT, sh-GFP and sh-St2 cells. In contrast, Pio partially rescued the cell number defect in sh-St1 cells with an increase from 40–50% to 65% compared to that of sh-GFP cells. This partial rescue was already maximal with 1 µM Pio and did not increase further up to 8 µM (Figure 3D). Taken together, these data show that Stamp1 or Stamp2 knockdown inhibits adipogenesis, and in the case of Stamp1, this may be, at least in part, through disruption of the mitotic clonal expansion in 3T3L1 cells.
Stamp1 and Stamp2 Knockdown Affects Stamp Expression in 3T3-L1 Adipocytes
As presented above, all Stamps are expressed and regulated during 3T3-L1 adipogenesis (Figure 1). To assess whether Stamp1 or Stamp2 knockdown influences expression of other Stamps, we determined Stamp expression in WT, sh-GFP, sh-St1 and sh-St2 cells at different time points during adipogenesis. As previously observed, Stamp1 expression was decreased by 85% in sh-St1 cells, compared to WT and sh-GFP cells at day 0 (Figure 4A). At day 8, Stamp1 levels were 60% lower in WT and sh-GFP cells compared with the same cells at day 0, consistent with Figure 1A, and remained low in sh-St1 cells. Interestingly, in sh-St2 cells, Stamp1 expression increased by about 2-fold at day 8 of differentiation compared to WT and sh-GFP cells, but this was lost upon Pio treatment suggesting that Pparγ may inhibit Stamp1 expression in these cells.
Figure 4. Stamp1 or Stamp2 knockdown reduces Pparγ and aP2, but not C/ebpα, mRNA expression in 3T3-L1 adipocytes.
(A–F) qRT-PCR analysis of WT, sh-GFP, sh-St1 and sh-St2 cells harvested at day 0 (d0) and day 8 of differentiation with (d8+Pio) or without (d8) Pio. The figures show the relative mRNA expression of Stamp1 (A), Stamp2 (B), Stamp3 (C), aP2 (D), Pparγ (E), and C/ebpα (F) normalized to the reference gene 36B4 from three independent experiments, n = 9. *p<0.05 compared to sh-GFP cells; #p<0.05 between brackets.
Stamp2 expression was low at day 0 and increased dramatically in WT and sh-GFP cells at day 8 of differentiation (Figure 4B). Consistent with Figure 2B, Stamp2 expression was decreased by 90% in sh-St2 cells. Interestingly, at day 8, Stamp2 expression in sh-St1 cells was reduced by 60% compared to WT and sh-GFP cells. In the presence of Pio, Stamp2 expression in WT, sh-GFP and sh-St2 cells increased by about 2.5-fold compared to levels at day 8 in the absence of Pio. In contrast, Stamp2 levels in the sh-St1 cells rose by nearly 5-fold to reach comparable levels of WT and sh-GFP cells in response to Pio. These data suggest that Pparγ activation in increases Stamp2 expression.
We also assessed Stamp3 levels and observed no change in expression under similar conditions (Figure 4C), except for in sh-St1 cells where Stamp3 expression was 2-fold higher at day 8 and 65% higher at day 8+ Pio compared with at day 0. These data suggest that Stamp1 and Stamp3 may have overlapping roles in 3T3-L1 adipocytes.
Stamp1 or Stamp2 Knockdown Interferes with Adipogenic Gene Expression
The data presented above showed that Stamp1 or Stamp2 knockdown suppresses 3T3-L1 differentiation (Figures 3A and 3B). We thus investigated whether the expression of a common adipogenic marker, aP2, and the two main transcription factors regulating adipogenesis, Pparγ and C/ebpα [36], were affected by Stamp knockdown. We first determined mRNA expression of aP2, Pparγ and C/ebpα in WT, sh-GFP, sh-St1 and sh-St2 cells at different time points during adipogenesis. aP2 expression was not detected at day 0 of differentiation (Figure 4D), but was present at day 8 for all cell lines. Consistent with the observed reduction in differentiation of sh-St1 and sh-St2 cells presented above (Figures 3A and 3B), aP2 expression was 50% lower in these cells compared to WT and sh-GFP cells. At day 8+ Pio, aP2 expression increased significantly in all cell types, and its levels in sh-St1 cells were now similar to that found in the WT or sh-GFP cells, in agreement with the rescue effect of Pio on the differentiation of sh-St1 cells (Figures 3A and 3B). Surprisingly, although aP2 expression in sh-St2 cells was 2-fold upregulated at day 8+ Pio compared to day 8, the aP2 mRNA levels were still 40% lower at day 8+ Pio compared to sh-GFP cells. This suggests that aP2 levels generally correlated with differentiation properties of the different cells lines, except for in sh-St2 cells.
There was no significant difference in the Pparγ expression at day 0 of differentiation among the different cell lines (Figure 4E). At day 8, Pparγ expression in WT and sh-GFP cells increased by about 20-fold compared with that observed at day 0, consistent with previous findings [37]. In agreement with the reduction in adipogenesis seen in Figures 3A and 3B, the sh-St1 and sh-St2 cells had 40% less Pparγ expression compared to sh-GFP cells. Furthermore, consistent with the rescue effect on 3T3-L1 differentiation with Pio presented above, there was an overall upregulation in Pparγ expression at day 8+ Pio, where Pparγ expression in sh-St1 and sh-St2 cells was comparable to that in sh-GFP cells. These data show that Pparγ levels correlated well with the differentiation properties of the different cell lines.
Similar to Pparγ, C/ebpα expression was low for all cell lines at day 0 of differentiation and increased by about 20-fold by day 8 (Figure 4F). However, unlike Pparγ expression, all cell lines displayed comparable C/ebpα expression at day 8. At day 8+ Pio, there was a trend towards increased C/ebpα expression for all cell lines. These data suggest that Stamp1 or Stamp2 knockdown do not significantly affect C/ebpα expression.
We next investigated Pparγ and C/ebpα protein expression in the sh-GFP, sh-St1 and sh-St2 cells. These were harvested and cell extracts were made at different time points during adipogenesis and subjected to Western analysis. As shown in Figures 5A and 5B, Pparγ expression was not detectable at day 0, consistent with Figure 4E and previous reports [37]. By day 4 of differentiation, expression of both isoforms 1 and 2 of Pparγ were detected. In agreement with the observed mRNA expression (Figure 4E), Pparγ protein was expressed at 70% and 50% lower levels (for both isoforms) in the sh-St1 and sh-St2 cells, respectively, compared with sh-GFP cells. This decrease in expression was also present at day 8 of differentiation as both sh-St1 and sh-St2 cells had a 60% reduction in Pparγ expression compared to sh-GFP cells. Consistent with the mRNA levels presented in Figure 4E, differentiation with Pio resulted in similar Pparγ levels in both the control and knockdown cells (Figures 5A and 5B).
Figure 5. Stamp1 or Stamp2 knockdown reduces Pparγ and C/ebpα protein expression in 3T3-L1 adipocytes.
(A) Western analysis showing Pparγ, C/ebpα and β-actin protein levels at day 0 (d0), day 4 (d4) and day 8 (d8) of differentiation in WT, sh-GFP, sh-St1 and sh-St2 cells, plus cells at day 8 differentiated with Pio (d8+ Pio). The data presented are representative of two independent experiments. (B–C) Quantification of Westerns in (A) with relative Pparγ (B) and C/ebpα (C) protein levels normalized to β-actin from two independent experiments, n = 6. *p<0.05.
Similarly, C/ebpα expression was low at day 0 and increased 40-fold when the sh-GFP cells reached day 4 of differentiation, consistent with the data in Figure 4F. However, at day 4, the C/ebpα protein levels were 70% and 60% lower in the sh-St1 and sh-St2 cells, respectively, compared with sh-GFP cells. While C/ebpα expression continued to increase by 2.5-fold from day 4 to day 8 of differentiation in sh-GFP adipocytes, the expression in sh-St1 and sh-St2 cells remained 60% and 70% lower, respectively. Treatment with Pio rescued the loss in C/ebpα expression similar to what was seen for Pparγ (Figures 5A and 5C), but in contrast to the lack of changes observed at its mRNA level (Figure 3F). These data show that suppression of adipogenesis upon Stamp1 or Stamp2 knockdown is correlated to downregulation of Pparγ and C/ebpα protein expression.
Stamp1 or Stamp2 Knockdown do not Affect C/ebpβ Expression and Nuclear Localization at the Early Stages of 3T3-L1 Adipogenesis
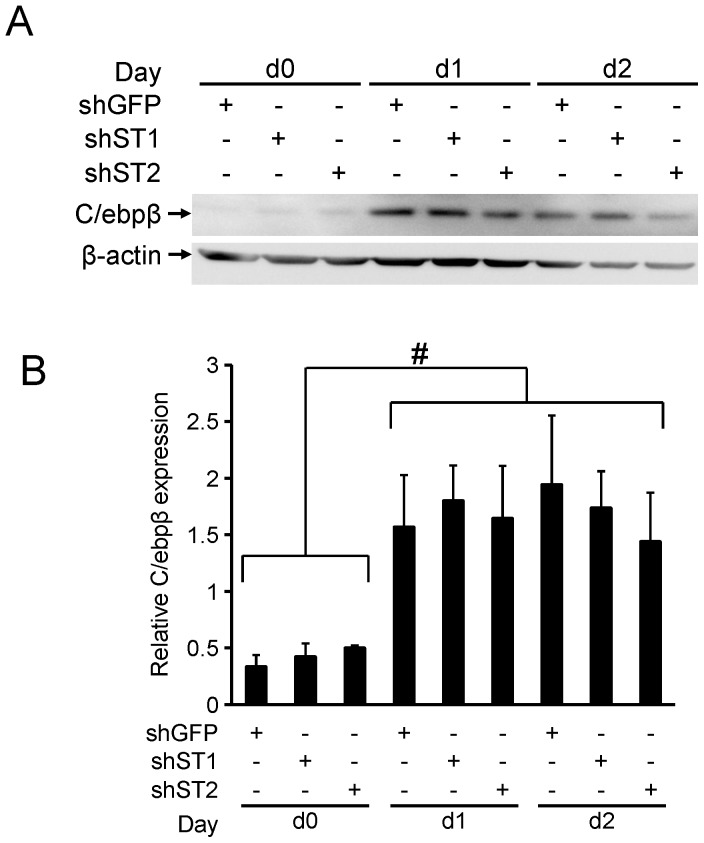
The transcription factor C/ebpβ is required for both Pparγ and C/ebpα expression early in adipogenesis [38]. We therefore examined whether there were alterations in C/ebpβ expression in early 3T3-L1 differentiation upon Stamp1 or Stamp2 knockdown. To that end, sh-GFP, sh-St1 and sh-St2 cells were harvested at days 0, 1 and 2 of differentiation and C/ebpβ expression was determined (Figure 6). Consistent with previous findings [39], C/ebpβ was expressed at low levels in sh-GFP cells at day 0, increased by approximately 3-fold by day 1, and remained unchanged at day 2. The sh-St1 and sh-St2 cells expressed C/ebpβ at comparable levels to the sh-GFP cells at all time points suggesting that C/ebpβ expression is not affected by Stamp1 or Stamp2.
Figure 6. Stamp1 or Stamp2 knockdown does not affect C/ebpβ protein expression in early 3T3-L1 adipogenesis.
(A) Western analysis showing C/ebpβ protein levels at day 0 (d0), day 1 (d1) and day 2 (d2) of differentiation with the same cells as in (5A). (B) Quantification of Western analysis results in (A) with relative C/ebpβ protein levels normalized to β-actin from two independent experiments, n = 6. #p<0.05 between brackets.
C/ebpβ phosphorylation has been reported to induce its translocation to the centromeres of chromosomes, which has been linked to regulation of mitotic clonal expansion in 3T3-L1 adipogenesis [39]. We examined if this process was affected by Stamp1 or Stamp2 knockdown. WT, sh-GFP,sh-St1 and sh-St2 cells were differentiated and C/ebpβ localization was assessed with immunofluorescence confocal microscopy (Figure S1). In all cell lines, we observed the characteristic punctate localization of C/ebpβ [39]. These data suggest that C/ebpβ expression and localization are not affected by Stamp1 and Stamp2 knockdown.
Stamp2 Knockdown Reduces Superoxide Production in 3T3-L1 Cells Independent of Adipogenesis
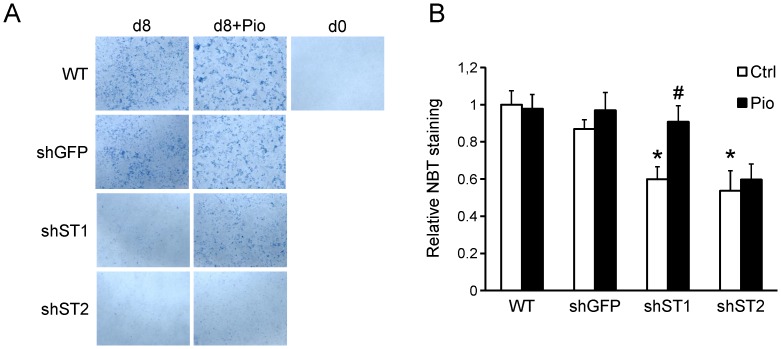
When ectopically expressed in HEK293T cells, all Stamps demonstrate metalloreductase activity [18] which is predicted to be driven by NADPH oxidation. A byproduct of NADPH oxidation is generation of superoxide which has been implicated in adipogenesis [40]. To assess whether Stamp1 or Stamp2 knockdown affect superoxide production in adipocytes, we used the nitroblue tetrazolium (NBT) assay on WT, sh-GFP, sh-St1 and sh-St2 adipocytes [41]. There was a 40% and 50% reduction in the generation of superoxide in the sh-St1 and sh-St2 cells, respectively, compared to WT and sh-GFP cells (Figure 7). When the cells were differentiated with Pio, the superoxide production was unchanged for the WT, sh-GFP or sh-St2 cells. Interestingly, Pio significantly increased superoxide levels in sh-St1 cells which reached comparable levels to those in WT and sh-GFP cells. These data indicate that both Stamp1 and Stamp2 play a role in superoxide production in 3T3-L1 adipocytes and that the loss of superoxide levels upon Stamp1 knockdown can be rescued by Pparγ activation.
Figure 7. Stamp2 knockdown reduces superoxide production in 3T3-L1 cells independent of adipocyte differentiation.
(A–B) NBT assay with WT, sh-GFP, sh-St1 and sh-St2 cells differentiated with (Pio) or vehicle (Ctrl). (A) Representative images of the staining. (B) Quantification from three independent experiments, n = 9. *p<0.05 compared to sh-GFP cells; #p<0.05 between Ctrl and Pio groups.
Discussion
In this study, we examined the expression and possible function of Stamp family members during adipogenic differentiation. Previous work has shown that Stamp2 mRNA and protein expression is upregulated during adipogenesis [20], [21]. Stamp3 protein expression was also found upregulated by 75% in 3T3-L1 adipocytes compared to confluent fibroblasts [30]. Here we show that Stamp1 and Stamp3 mRNA expression are also differentially regulated during adipogenic conversion. These data, along with the effects of Stamp1 and Stamp2 knockdown on adipogenic conversion that we present, suggest that the Stamp family has a significant role in regulating adipogenesis.
Stamp1 knockdown significantly reduced 3T3-L1 differentiation (Figures 3A and 3B) which was unexpected as Stamp1 was clearly downregulated upon adipogenic conversion (Figure 1A). Assessment of the number of adipocytes revealed that the sh-St1 cells displayed stunted proliferation upon induction of differentiation compared to the WT and sh-GFP cells suggesting that Stamp1 knockdown interfered with the mitotic clonal expansion (Figures 3C and 3D). The mechanism(s) through which Stamp1 may regulate mitotic clonal expansion is currently not known.
In contrast to sh-St1 cells, sh-St2 cells proliferated at the same rate as the WT and sh-GFP cells during differentiation (Figure 3C) indicating that the mechanisms through which Stamp1 and Stamp2 regulate adipogenesis is different. Furthermore, there may be differences in the functioning and regulation of the mouse and human STAMP2 proteins. Inhibition of STAMP2 in human preadipocytes using an antibody, or STAMP2 knockdown prior to differentiation, did not affect adipogenesis [24], [42]. In contrast, there were clear inhibitory effects of Stamp2 knockdown in 3T3-L1 cells (Figure 3A and 3B). However, it is important to note that in the studies concerning Stamp2 in human preadipocytes, the thiazolidinedione (TZD) rosiglitazone (Rosi), a Pparγ activator, was used to induce adipogenesis, rather than the regular differentiation cocktail [31]. This could be, at least in part, the reason for these differential findings.
Here, we also show that both TZDs, Rosi and Pio, counteracted the inhibitory effect of Stamp2 knockdown on 3T3-L1 cell differentiation (Figures 3A, 3B and S2). These data support the notion that Pparγ activation can circumvent Stamp2 knockdown-mediated deficiency in adipogenesis in both mouse and human cells. In agreement with the inhibitory effects of Stamp1 and Stamp2 knockdown on 3T3-L1 adipogenesis, Pparγ expression was inhibited in both the sh-St1 and sh-St2 cells (Figures 4E, 5A and 5B). Interestingly, C/ebpα mRNA expression was similar in sh-St1 and sh-St2 cells compared to WT and sh-GFP cells, but C/ebpα protein levels were downregulated (Figures 4F, 5A and 5C); this suggests that there may be posttranscriptional control mechanisms that regulate C/ebpα expression. Consistent with the effect of Pio on 3T3-L1 differentiation addressed above, the overall Pparγ and C/ebpα expression increased and the protein levels were restored in sh-St1 and sh-St2 cells at day 8+Pio.
Functional C/ebpβ expression and activity was previously shown to be essential for inducing Pparγ expression and for the mitotic clonal expansion during adipogenesis [39]. However, there was no difference in C/ebpβ expression and localization between sh-GFP, sh-St1 and sh-St2 cells (Figures 6 and S1). Consistent with the stunted growth of the sh-St1 cells, we have previously shown that STAMP1 knockdown in human prostate cancer cells decreases proliferation by deregulating cell cycle related protein expression and activation of the mitogen-activated protein kinase (MAPK) pathway [35]. Whether Stamp1 could be involved in regulation of similar events during 3T3-L1 adipogenesis would need to be addressed in future studies.
Increased superoxide production was previously reported during 3T3-L1 adipocyte maturation [40]. Consistently, sh-St1 and sh-St2 cells showed lower superoxide production than WT and sh-GFP cells (Figure 7), and the generation of superoxide was restored in sh-St1 cells when differentiated with Pio. However, in sh-St2 cells superoxide levels remained low even with Pio. This again supports the notion that Stamp1 and Stamp2 affect adipogenesis through different mechanisms. These data are also consistent with the function of the metalloreductase domain of Stamp2 that can reduce iron with NADPH as an electron donor generating superoxide in the process [18]. Recently, an increase in mammalian target of rapamycin complex 1 (mTORC-1) dependent mitochondrial complex III superoxide production has been found to be required for Pparγ expression during adipocyte differentiation [11]. It was also found that Forkhead box O 1 (FOXO1) contributes to regulating endogenous antioxidants along with the increased ROS production during adipogenesis as FOXO1 knockdown led to downregulation of antioxidants and decreased 3T3-L1 differentiation [43]. Interestingly, in Stamp2 knockout mice several endogenous antioxidants are also downregulated [21]. Together with our findings, these point to a role of Stamp2 as a direct activator of 3T3-L1 adipogenesis through production of pro-adipogenic ROS signals. Another possibility is that Stamp2 may have a more indirect role in this process by contributing to antioxidant production that protects adipocytes from increasing ROS levels during adipogenesis. If Stamp2 levels are decreased, this will result in lower antioxidant production during differentiation, which could force the cells to adapt to lower ROS levels that can in turn retard the adipogenic conversion. In support of the latter, increased ROS and decreased insulin response was seen when STAMP2 was inhibited with an antibody in mature human adipocytes [44].
In summary, our data suggest that the Stamp family may have important roles in 3T3-L1 adipogenesis, which may involve distinct pathways for the different Stamps. These data pave the way to further explore the functions of the Stamp family in adipogenesis.
Supporting Information
Stamp1 or Stamp2 knockdown does not affect C/ebpβ nuclear distribution in 3T3-L1 cells after induction of differentiation. (A–B) Immunofluorescence confocal microscopy analysis of expressed C/ebpβ (green) in the nuclei (blue) of WT, sh-GFP, sh-St1 and sh-St2 cells 16 h after induction of differentiation. (A) Representative images of the staining from two independent experiments. (B) Quantification from one experiment, n = 100.
(TIF)
Rosiglitazone reverses Stamp1 or Stamp2 knockdown-induced decrease in adipogenesis. Oil Red O staining of WT, sh-GFP, sh-St1 and sh-St2 cells differentiated with rosiglitazone (Rosi) or vehicle (Ctrl). Figure shows quantification from one experiment, n = 3. *p<0.05 compared to sh-GFP cells; #p<0.05 between Ctrl and Rosi groups.
(TIF)
Acknowledgments
We thank Gökhan S. Hotamisligil for scientific input, helpful discussions, critical reading of the manuscript and providing the 3T3-L1 cell line. We thank Torstein Lindstad and Kathryn E. Wellen for sharing unpublished data. We thank Hilde I. Nebb for the gift of Pparγ antibody.
Funding Statement
This work was supported by funds from the Norwegian Research Council (www.forskningsradet.no), Norwegian Cancer Society (www.kreftforeningen.no), and University of Oslo (www.uio.no). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, et al. (2011) National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet 377: 557–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Popkin BM, Adair LS, Ng SW (2012) Global nutrition transition and the pandemic of obesity in developing countries. Nutr Rev 70: 3–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hotamisligil GS (2006) Inflammation and metabolic disorders. Nature 444: 860–867. [DOI] [PubMed] [Google Scholar]
- 4.WHO (2012) Obesity and overweight.
- 5. Park KW, Halperin DS, Tontonoz P (2008) Before they were fat: adipocyte progenitors. Cell Metab 8: 454–457. [DOI] [PubMed] [Google Scholar]
- 6. Lafontan M (2012) Historical perspectives in fat cell biology: the fat cell as a model for the investigation of hormonal and metabolic pathways. Am J Physiol Cell Physiol 302: C327–359. [DOI] [PubMed] [Google Scholar]
- 7. Otto TC, Lane MD (2005) Adipose development: from stem cell to adipocyte. Crit Rev Biochem Mol Biol 40: 229–242. [DOI] [PubMed] [Google Scholar]
- 8. Rosen ED, MacDougald OA (2006) Adipocyte differentiation from the inside out. Nat Rev Mol Cell Biol 7: 885–896. [DOI] [PubMed] [Google Scholar]
- 9. Lowe CE, O’Rahilly S, Rochford JJ (2011) Adipogenesis at a glance. J Cell Sci 124: 2681–2686. [DOI] [PubMed] [Google Scholar]
- 10. Gummersbach C, Hemmrich K, Kroncke KD, Suschek CV, Fehsel K, et al. (2009) New aspects of adipogenesis: radicals and oxidative stress. Differentiation 77: 115–120. [DOI] [PubMed] [Google Scholar]
- 11. Tormos KV, Anso E, Hamanaka RB, Eisenbart J, Joseph J, et al. (2011) Mitochondrial complex III ROS regulate adipocyte differentiation. Cell Metab 14: 537–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lee H, Lee YJ, Choi H, Ko EH, Kim JW (2009) Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. J Biol Chem 284: 10601–10609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pessler-Cohen D, Pekala PH, Kovsan J, Bloch-Damti A, Rudich A, et al. (2006) GLUT4 repression in response to oxidative stress is associated with reciprocal alterations in C/EBP alpha and delta isoforms in 3T3-L1 adipocytes. Arch Physiol Biochem 112: 3–12. [DOI] [PubMed] [Google Scholar]
- 14. Carriere A, Carmona MC, Fernandez Y, Rigoulet M, Wenger RH, et al. (2004) Mitochondrial reactive oxygen species control the transcription factor CHOP-10/GADD153 and adipocyte differentiation: a mechanism for hypoxia-dependent effect. J Biol Chem 279: 40462–40469. [DOI] [PubMed] [Google Scholar]
- 15. Findeisen HM, Pearson KJ, Gizard F, Zhao Y, Qing H, et al. (2011) Oxidative stress accumulates in adipose tissue during aging and inhibits adipogenesis. PLoS One 6: e18532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu D, Lin Y, Kang T, Huang B, Xu W, et al. (2012) Mitochondrial dysfunction and adipogenic reduction by prohibitin silencing in 3T3-L1 cells. PLoS One 7: e34315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ohgami RS, Campagna DR, Greer EL, Antiochos B, McDonald A, et al. (2005) Identification of a ferrireductase required for efficient transferrin-dependent iron uptake in erythroid cells. Nat Genet 37: 1264–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ohgami RS, Campagna DR, McDonald A, Fleming MD (2006) The Steap proteins are metalloreductases. Blood 108: 1388–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lambe T, Simpson RJ, Dawson S, Bouriez-Jones T, Crockford TL, et al. (2009) Identification of a Steap3 endosomal targeting motif essential for normal iron metabolism. Blood 113: 1805–1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moldes M, Lasnier F, Gauthereau X, Klein C, Pairault J, et al. (2001) Tumor necrosis factor-alpha-induced adipose-related protein (TIARP), a cell-surface protein that is highly induced by tumor necrosis factor-alpha and adipose conversion. J Biol Chem 276: 33938–33946. [DOI] [PubMed] [Google Scholar]
- 21. Wellen KE, Fucho R, Gregor MF, Furuhashi M, Morgan C, et al. (2007) Coordinated regulation of nutrient and inflammatory responses by STAMP2 is essential for metabolic homeostasis. Cell 129: 537–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ten Freyhaus H, Calay ES, Yalcin A, Vallerie SN, Yang L, et al. (2012) Stamp2 Controls Macrophage Inflammation through Nicotinamide Adenine Dinucleotide Phosphate Homeostasis and Protects against Atherosclerosis. Cell Metab 16: 81–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen X, Zhu C, Ji C, Zhao Y, Zhang C, et al. (2010) STEAP4, a gene associated with insulin sensitivity, is regulated by several adipokines in human adipocytes. Int J Mol Med 25: 361–367. [DOI] [PubMed] [Google Scholar]
- 24. Cheng R, Qiu J, Zhou XY, Chen XH, Zhu C, et al. (2011) Knockdown of STEAP4 inhibits insulin-stimulated glucose transport and GLUT4 translocation via attenuated phosphorylation of Akt, independent of the effects of EEA1. Mol Med Report 4: 519–523. [DOI] [PubMed] [Google Scholar]
- 25. Zhang CM, Chi X, Wang B, Zhang M, Ni YH, et al. (2008) Downregulation of STEAP4, a highly-expressed TNF-alpha-inducible gene in adipose tissue, is associated with obesity in humans. Acta Pharmacol Sin 29: 587–592. [DOI] [PubMed] [Google Scholar]
- 26. Elbein SC, Kern PA, Rasouli N, Yao-Borengasser A, Sharma NK, et al. (2011) Global gene expression profiles of subcutaneous adipose and muscle from glucose-tolerant, insulin-sensitive, and insulin-resistant individuals matched for BMI. Diabetes 60: 1019–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Arner P, Stenson BM, Dungner E, Naslund E, Hoffstedt J, et al. (2008) Expression of six transmembrane protein of prostate 2 in human adipose tissue associates with adiposity and insulin resistance. J Clin Endocrinol Metab 93: 2249–2254. [DOI] [PubMed] [Google Scholar]
- 29. Joo JI, Kim DH, Choi JW, Yun JW (2010) Proteomic analysis for antiobesity potential of capsaicin on white adipose tissue in rats fed with a high fat diet. J Proteome Res 9: 2977–2987. [DOI] [PubMed] [Google Scholar]
- 30. Ye F, Zhang H, Yang YX, Hu HD, Sze SK, et al. (2011) Comparative proteome analysis of 3T3-L1 adipocyte differentiation using iTRAQ-coupled 2D LC-MS/MS. J Cell Biochem 112: 3002–3014. [DOI] [PubMed] [Google Scholar]
- 31. Hausman GJ, Poulos SP, Pringle TD, Azain MJ (2008) The influence of thiazolidinediones on adipogenesis in vitro and in vivo: potential modifiers of intramuscular adipose tissue deposition in meat animals. J Anim Sci 86: E236–243. [DOI] [PubMed] [Google Scholar]
- 32. Kletzien RF, Clarke SD, Ulrich RG (1992) Enhancement of adipocyte differentiation by an insulin-sensitizing agent. Mol Pharmacol 41: 393–398. [PubMed] [Google Scholar]
- 33. Korkmaz KS, Elbi C, Korkmaz CG, Loda M, Hager GL, et al. (2002) Molecular cloning and characterization of STAMP1, a highly prostate-specific six transmembrane protein that is overexpressed in prostate cancer. J Biol Chem 277: 36689–36696. [DOI] [PubMed] [Google Scholar]
- 34. Korkmaz CG, Korkmaz KS, Kurys P, Elbi C, Wang L, et al. (2005) Molecular cloning and characterization of STAMP2, an androgen-regulated six transmembrane protein that is overexpressed in prostate cancer. Oncogene 24: 4934–4945. [DOI] [PubMed] [Google Scholar]
- 35. Wang L, Jin Y, Arnoldussen YJ, Jonson I, Qu S, et al. (2010) STAMP1 is both a proliferative and an antiapoptotic factor in prostate cancer. Cancer Res 70: 5818–5828. [DOI] [PubMed] [Google Scholar]
- 36. Rosen ED, Spiegelman BM (2000) Molecular regulation of adipogenesis. Annu Rev Cell Dev Biol 16: 145–171. [DOI] [PubMed] [Google Scholar]
- 37. MacDougald OA, Lane MD (1995) Transcriptional regulation of gene expression during adipocyte differentiation. Annu Rev Biochem 64: 345–373. [DOI] [PubMed] [Google Scholar]
- 38. Tanaka T, Yoshida N, Kishimoto T, Akira S (1997) Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J 16: 7432–7443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Tang QQ, Otto TC, Lane MD (2003) CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc Natl Acad Sci U S A 100: 850–855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Furukawa S, Fujita T, Shimabukuro M, Iwaki M, Yamada Y, et al. (2004) Increased oxidative stress in obesity and its impact on metabolic syndrome. J Clin Invest 114: 1752–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Choi HS, Kim JW, Cha YN, Kim C (2006) A quantitative nitroblue tetrazolium assay for determining intracellular superoxide anion production in phagocytic cells. J Immunoassay Immunochem 27: 31–44. [DOI] [PubMed] [Google Scholar]
- 42. Qin DN, Kou CZ, Ni YH, Zhang CM, Zhu JG, et al. (2010) Monoclonal antibody to the six-transmembrane epithelial antigen of prostate 4 promotes apoptosis and inhibits proliferation and glucose uptake in human adipocytes. Int J Mol Med 26: 803–811. [DOI] [PubMed] [Google Scholar]
- 43. Higuchi M, Dusting GJ, Peshavariya H, Jiang F, Hsiao ST, et al. (2013) Differentiation of human adipose-derived stem cells into fat involves reactive oxygen species and Forkhead box O1 mediated upregulation of antioxidant enzymes. Stem Cells Dev 22: 878–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Qin DN, Zhu JG, Ji CB, Chunmei S, Kou CZ, et al. (2011) Monoclonal antibody to six transmembrane epithelial antigen of prostate-4 influences insulin sensitivity by attenuating phosphorylation of P13K (P85) and Akt: possible mitochondrial mechanism. J Bioenerg Biomembr 43: 247–255. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Stamp1 or Stamp2 knockdown does not affect C/ebpβ nuclear distribution in 3T3-L1 cells after induction of differentiation. (A–B) Immunofluorescence confocal microscopy analysis of expressed C/ebpβ (green) in the nuclei (blue) of WT, sh-GFP, sh-St1 and sh-St2 cells 16 h after induction of differentiation. (A) Representative images of the staining from two independent experiments. (B) Quantification from one experiment, n = 100.
(TIF)
Rosiglitazone reverses Stamp1 or Stamp2 knockdown-induced decrease in adipogenesis. Oil Red O staining of WT, sh-GFP, sh-St1 and sh-St2 cells differentiated with rosiglitazone (Rosi) or vehicle (Ctrl). Figure shows quantification from one experiment, n = 3. *p<0.05 compared to sh-GFP cells; #p<0.05 between Ctrl and Rosi groups.
(TIF)