Abstract
Purpose
Research on medication safety in pregnancy often utilizes health plan and birth certificate records. This study discusses methods used to link mothers with infants, a crucial step in such research.
Methods
We describe how 8 sites participating in the Medication Exposure in Pregnancy Risk Evaluation Program created linkages between deliveries, infants and birth certificates for the 2001–2007 birth cohorts. We describe linkage rates across sites and, for two sites, we compare the characteristics of populations linked using different methods.
Results
Of 299,260 deliveries, 256,563 (86%; range by site, 74–99%) could be linked to infants using a deterministic algorithm. At two sites, using birth certificate data to augment mother-infant linkage increased the representation of mothers who were Hispanic or non-white, younger, Medicaid recipients, or had low educational level. A total of 236,460 (92%; range by site, 82–100%) deliveries could be linked to a birth certificate.
Conclusions
Tailored approaches enabled linking most deliveries to infants and to birth certificates, even when data systems differed. The methods used may affect the composition of the population identified. Linkages established with such methods can support sound pharmacoepidemiology studies of maternal drug exposure outside the context of a formal registry.
Keywords: Birth Certificates, Medicaid, Pregnancy Outcome/epidemiology, Medical Record Linkage
Introduction
There is little information about medication safety during pregnancy. Medication use during pregnancy is common (in one study, 64% of deliveries 1). It often occurs before a woman knows she is pregnant and may also occur as a result of new medical conditions that arise during pregnancy 2. For most medications, little is known about their effect on congenital anomalies and other birth outcomes. Evidence from randomized clinical trials is lacking due to the ethical challenges of conducting human trials for medications with unknown effects on birth outcomes. For many medications, the only available data come from animal studies and sources with limited generalizability such as case reports and pregnancy registries. Consequently, postmarketing research and surveillance are important strategies to study the effects of medication exposure during pregnancy on maternal and neonatal outcomes 3.
In response to the need for better information about medication safety in pregnancy, the U.S. Food and Drug Administration (FDA) developed the Medication Exposure in Pregnancy Risk Evaluation Program (MEPREP). As detailed elsewhere 4, MEPREP is a collaborative research program between the FDA and researchers at 11 health plans (Table 1) with 12 million enrollees (about 4% of the U.S. population). Health plan data offer opportunities for studying medication safety in pregnancy because they provide comprehensive demographic, medication use, diagnosis, and procedure information for a defined population. They can provide large and diverse populations that are more representative of the general population than drug- or disease- specific registries. These data can be enriched by linkage to state birth certificates, which contain information that may not be readily available from health plan data, such as gestational age; parental race, ethnicity, and education; birth weight; and maternal tobacco use.
Table 1.
MEPREP sites, 2001–2007
| Number of infants (%) | |
|---|---|
| HMO Research Network sites using the MEPREP hierarchical algorithm Fallon Community Health Plan (Worcester, MA) Group Health Cooperative (Seattle, WA) Harvard Pilgrim Health Care (Boston, MA) HealthPartners (Minneapolis, MN) Kaiser Permanente Colorado (Denver, CO) Kaiser Permanente Georgia (Atlanta, GA) Kaiser Permanente Northwest (Portland, OR) LCF Research (Albuquerque, NM) |
257,268 (24.2%) |
| Vanderbilt University/Tennessee State Medicaid | 350,522 (33.0%) |
| Kaiser Permanente Northern California and Southern California | 453,369 (42.7%) |
A crucial step in utilizing health plan data to study medication safety in pregnancy is to identify valid linkages between mothers and their infants. Approaches to linking individuals across health care datasets in the United States vary widely and include use of a single reliable key (e.g., social security number) or the incorporation of several characteristics using deterministic or probabilistic matching 5, 6. In deterministic matching, logical rules are applied that define whether a match exists. For example, if an individual has the same name and birth date in two files, the link would be considered true. In probabilistic matching, researchers apply algorithms that assign a probability score to a match; matches that exceed a predefined threshold are considered true. In either case, the linking process can affect the final population available for study. For example, the rate of linkage error may differ according to race and ethnicity 7, 8. This has implications for study interpretation, including internal validity and generalizability.
There has been little published description of approaches to link mothers to infants using health plan data or other data sources for pregnancy outcome studies. While individual studies have briefly described their own methods, to our knowledge no prior publication has provided a comprehensive algorithm that can be tailored to specific data sources or health plans. This paper describes an approach that 8 MEPREP health plans used to link mothers to infants, including establishing linkages to birth certificates. We also explored how using different linking methods affected the composition of the final study population at two plans, particularly related to race, ethnicity and socioeconomic status.
Methods
Overview of MEPREP
The goal of MEPREP is to facilitate and conduct studies of medication safety in pregnancy. A description of MEPREP has been previously published 4. In brief, 11 sites identified cohorts of mother-infant pairs and then created standardized datasets formatted according to shared standards. Variables include health plan data (e.g., enrollment, utilization, diagnoses, and prescription dispensing) as well as birth certificate variables. To protect patient privacy, all individual-level data remain within the health plan, using a distributed data model 4, 9, 10. Because of standardized data formatting, a computer program written at one site can be run at other sites, facilitating cross-site analyses. MEPREP currently includes 1.2 million infants delivered to 933,917 mothers from 2001 through 2008. The current analyses focus on the 2001–2007 birth cohorts, which include about 1 million infants born to about 830,000 mothers, because 2008 linkages had not been completed at the time this work was conducted. IRB approval was obtained at all participating sites and from state departments of public health where required.
Three of the 11 MEPREP sites already had well-established procedures in place for linking mothers to infants, and so their procedures are not described in detail in this paper. Kaiser Permanente Northern and Southern California have established birth registries through their health plan affiliated hospitals. Since nearly all of their enrollees deliver at a hospital owned and operated by the plan, these registries are believed to capture over 99% of their deliveries, and thus these plans did not need to use any additional methods to identify deliveries or create mother-infant linkages. Vanderbilt University researchers working with Tennessee Medicaid data use a previously described probabilistic algorithm 6 to link newly enrolled infants with both birth certificates and Medicaid data for women delivering infants. For the other 8 sites (Table 1), MEPREP needed to develop an algorithm that applied standard procedures and definitions, to ensure cross-site consistency, but also recognized and took advantage of the sites’ varying data resources. This paper describes our experience with linkage at those 8 sites, all members of the HMO Research Network. Overall, these sites provided about one-fourth of the final MEPREP cohort.
Linkage of mothers and infants using health plan data
Each of the 8 sites started by identifying deliveries from women’s health plan utilization data. These were linked to infant enrollees using a deterministic algorithm (referred to in the rest of the paper as the “MEPREP hierarchical algorithm”) (Figure 1). To identify deliveries, the algorithm selected female members aged 10–55 years with least one International Classification of Diseases, 9th revision, Clinical Modification (ICD-9-CM) diagnosis code or an ICD-9 CM or Current Procedural Terminologies (CPT) procedure code indicating infant delivery (Appendix). This list was originally developed by manual review of ICD-9-CM and CPT code books to identify all codes that indicated delivery. Although the MEPREP cohort is currently limited to live births, codes for stillbirths were included to maximize capture of deliveries, for instance to capture multiple gestations where a stillbirth occurred but one or more infants survived. The investigators used these codes in prior studies (e.g., 1, 11–15). One participating site found that, compared to a perinatal database, the diagnosis and procedure codes had sensitivity and positive predictive value (PPV) of 98% for identifying deliveries 16. For the present study, investigators updated the list of codes to include a few (<20) that did not exist originally.
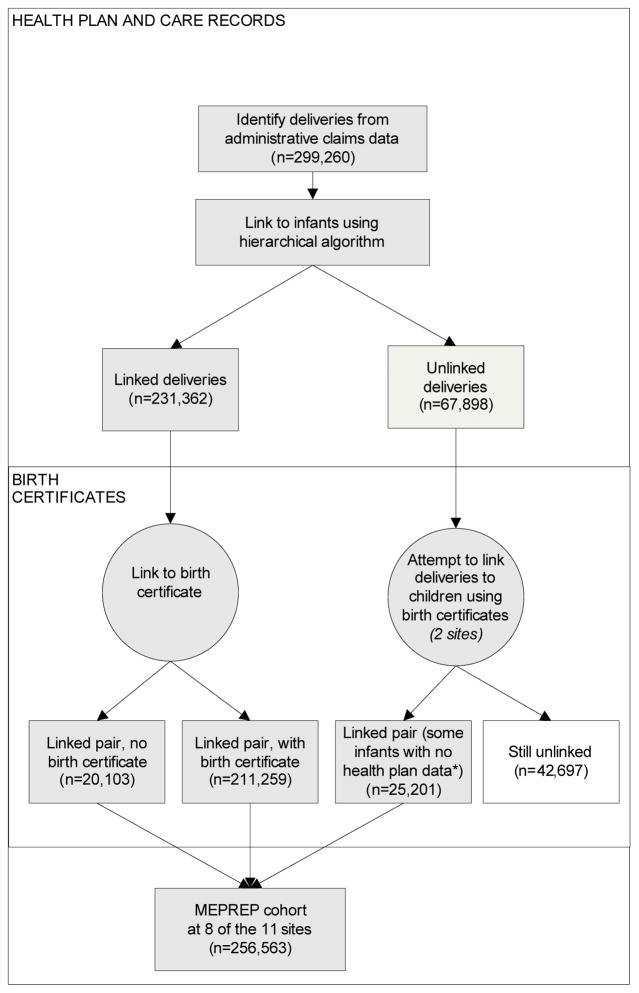
Fig. 1.
Process used to link deliveries to infants within health plans and to link health plan data to birth certificates at 8 sites, 2001–2007
*Includes 6,018 infants not linked to health plan records. Because birth certificates include data on some outcomes identified around the time of birth, this subset is still useful for some MEPREP activities but may not be included in all studies.
After a delivery-related diagnosis or procedure code was identified, any additional codes in the following 120 days were considered to represent the same delivery since it is not plausible that a woman could have more than one delivery in this time period. To identify infants, the algorithm selected all members born within the study time period who had at least one day of health plan enrollment. The delivery and infant datasets were linked using the following steps, in order of preference:
A birth registry, if available;
Health plan subscriber number;
Last name and address of the mother and infant; or
Other resources, as available.
Two sites were able to use state birth records to identify additional pairs. To do so, these sites linked deliveries to birth certificates and infants to birth certificates independently, based on first and last names, dates of birth for the woman and infant, and date of delivery (from the woman’s records). Then they identified as new matches those women and infants who were previously unmatched who linked to the same birth certificate. Some of the infants identified in this process had not been previously identified in the health plan data and were added to the MEPREP cohort.
In order to increase linkage rates and accuracy, sites could implement additional provisions to address site-specific nuances. When linking is done using resources other than a birth registry, the exact date of delivery is not available; instead, plans have information about the admission and discharge dates for the delivery hospitalization. To improve standardization, it was recommended that the infant date of birth fall in the time period between three days prior to the mother’s admission date and her discharge date for the delivery hospitalization. The three-day window before admission allows for out-of-hospital births. Sites using name/address matching double-checked potential linkages by checking whether the infant birth fell within the specified time period. They also used computerized or manual review to allow for misspellings and variability in address formatting. We excluded uncertain matches made using this method (for example, 501 First Street vs. 501 First Avenue in a city where both addresses exist).
Linkage of health plan records to birth certificates
Each site worked with their state department of health to develop procedures to link health plan records to birth certificates for this study. At one site, the health plan catchment area spans two states, so the site worked with two departments of health. In all, the 8 sites worked with 8 state departments of health.
Four sites sent information about the delivery to the state health department, who performed the linking to birth certificates and returned birth certificate data to the researcher. At the other four sites, MEPREP researchers did the linking. The keys used to establish linkages to birth certificates included all or several of the following: parent names, infant name and sex, mother’s birth date, delivery date, infant birth date, ZIP code and hospital code. Social security number was used as a key in only one state. In two states, the matching process included variations on the mother’s name (alternate spellings or names that sound alike). One state maintains a data enclave, which allows the researchers to access relevant information directly via controlled on-site access.
Statistical analysis
We calculated the proportion of deliveries identified from health plan data that could be linked to an infant using the MEPREP hierarchical algorithm, overall and for individual sites and calendar years. We also calculated the proportion of linked pairs that could be linked to a birth certificate. Finally, at two sites that used both birth certificate and health plan records to establish mother-infant linkages, we examined whether characteristics of the population differed by linking method within each site. Statistical significance was assessed using the chi-square test. All tests were two-tailed, with alpha of 0.05.
Results
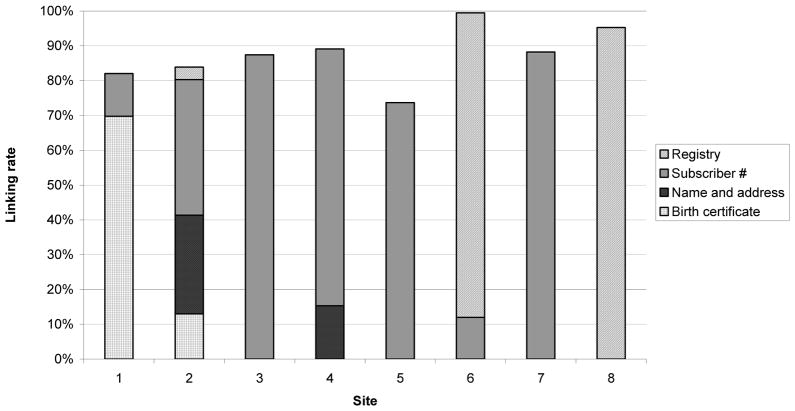
We identified 299,260 deliveries at 8 health plans from 2001–2007. Of these, 256,563 (86%) could be linked to an infant (range by site, 74% to 99%; Figure 3). This proportion declined from 87% in 2001 to 82% in 2007. Seven of eight sites primarily used one data source to link deliveries to infants. However, the primary key varied by site. About half of linkages were made using subscriber number or family unit identifier. Birth registry information was available at three sites and captured 4% to 95% of identified deliveries at these sites. Two sites identified additional matches using name and address matching.
At the two sites that used birth certificates to enhance linkages, incorporating this additional step increased the linkage rate for deliveries from 12 to 82% at one site and from 71 to 84% at the other (Figure 2). This process identified an additional 25,201 mother-infant pairs, beyond those identified using other methods. There were 6,018 infants matched to deliveries in this process who were not identified as having any health plan enrollment, accounting for about 10% of deliveries at each site.
Fig. 2.
Proportion of deliveries linked to an infant, by linkage method and site, 2001–2007
We were able to link 236,460 (92%) of the 256,563 linked pairs to birth certificates. This proportion ranged from 82% to 100% by site and was similar across years. We did not detect any pattern in terms of which procedures produced a higher linking rate (e.g., which key was used or which entity did the linking).
At the two sites that augmented linkages using birth certificates, we analyzed characteristics of mothers who were linked to infants via birth certificates versus health plan records alone (Table 2). Compared to women linked to infants by health plan records, women linked only through birth certificate data were more likely to be Hispanic (64% vs. 42%) at one site and more likely to be nonwhite at the other (28% vs. 19%). At both sites, these women were younger and more likely to have Medicaid insurance and not to have completed high school.
Table 2.
Comparison of women linked to infants using different methods at two sites which augmented linkage using birth certificate data*
| SITE 1 | SITE 2 | |||
|---|---|---|---|---|
| Links from birth certificates (60% of pairs) |
Links from health plan records alone (40% of pairs) |
Links from birth certificates (13% of pairs) |
Links from health plan records alone (87% of pairs) |
|
| Hispanic (%) | 64.3 | 41.5 | 7.4 | 5.6 |
| Race (%) | ||||
| White | 91.7 | 92.1 | 72.3 | 81.1 |
| Black | 2.3 | 1.2 | 11.0 | 4.6 |
| Asian American | 0.1 | 0.5 | 10.6 | 9.1 |
| Pacific Islander | 1.1 | 3.2 | 2.3 | 2.6 |
| Native American | 3.9 | 3.0 | 2.0 | 1.1 |
| Education: < high school graduate (%) |
30.3 | 4.1 | 16.5 | 3.7 |
| Age (%) | ||||
| <18 | 8.1 | 0.7 | 4.0 | 1.0 |
| 18–39 | 91.0 | 95.4 | 83.3 | 76.8 |
| 40+ | 0.9 | 3.9 | 2.3 | 4.1 |
| Medicaid insurance (%) | 73.8 | 6.6 | 32.0 | 6.0 |
| Smoking status (%) | ||||
| Smoker | 12.5 | 4.1 | 4.0 | 1.0 |
| Nonsmoker | 85.9 | 94.2 | 18.0 | 20.0 |
| Unknown | 1.6 | 1.7 | 78.0 | 79.0 |
| Nulliparous (%) | 71.4 | 73.9 | 48.0 | 42.0 |
Limited to the two sites which identified additional linked mother-infant pairs from the public record portion of state birth certificate files. Characteristics included in the table are derived from birth certificate data. All comparisons between groups linked using different methods within each site are statistically significant (p<0.01).
Discussion
Health plan data offer great potential for supporting research on medication safety in pregnancy because of the large and diverse populations included, but such research requires the ability to link health records of mothers with those of their infants. These linkages are not readily available for most health plans. Using the hierarchical algorithm described above, we were able to create a large cohort of mothers and infants for future studies. Additionally, the sites were able to obtain birth certificate records for the majority of these linked pairs. The MEPREP experience illustrates the potential for sound pharmacoepidemiologic studies of maternal drug exposure to be conducted outside the context of a formal registry. The algorithm we describe supports standardization, while also providing flexibility which allows tailoring to the needs and data resources of individual health plans.
Multiple linking approaches were needed because different data are available at different sites, partly due to the fact that participating health plans vary in terms of clinical integration. Some plans are part of systems that provide both care and coverage to all subscribers. In such a system, a birth registry can be created by recording data uniformly at the point of care. In contrast, other plans primarily provide health insurance to members who receive most or all care from external providers. When many different external providers participate, it is not feasible to create a birth registry. Even within plans that provide both care and coverage, some have their own hospitals while others have few or no hospitals. In our experience, linkages are the most straightforward to establish in systems where a birth registry exists and the research group has access to that data. However, even in less integrated systems, high linkage rates can be achieved using the algorithms and approaches described here. Our success at identifying linkages at 8 health plans–which differ considerably in terms of their degree of integration and the data resources available– indirectly supports the generalizability and relevance of the algorithm beyond these 8 plans.
The MEPREP cohort is designed to maximize capture of information about medication exposure and outcomes and to minimize the biases (e.g., recall bias, referral bias, self-selection in studies of volunteers) that can arise in studies of medication safety in pregnancy. Previous studies have suggested that data quality improves when health plan and birth certificate data are used in combination, rather than alone 17, 18. At the two sites that used birth certificates to enhance linkages, incorporating the additional data source increased the linkage rate for deliveries considerably at one site (from 12 to 82%) and moderately at the other (from 71 to 84%). Furthermore, adding this data source substantially increased the diversity of the cohort at those sites. This should enhance generalizability of findings from future studies. The infants that were linked to birth certificate but not health plan data were retained in the final cohort because their data can support studies of certain outcomes, particularly those that are readily identified during the birth hospitalization (e.g., preterm birth, low birth weight, neonatal intensive care unit admission or certain major congenital anomalies). Because all of these infants have birth certificate data available, some outcomes of interest can be identified from the birth certificate. Alternatively, for some states, ICD-9 codes for the hospital stay are available linked to birth certificate data, and these can allow the identification of certain conditions noted during the birth hospitalization.
We were not able to match all deliveries to an infant. This may arise when an infant is adopted or is not enrolled in the health plan after the birth, e.g., if a parent’s healthcare coverage changes or if the infant is covered under the insurance plan of a family member other than the mother. A particular challenge arises when linking mothers and infants insured by Medicaid. This difficulty is illustrated by our experience at the MEPREP site that could only link 12% of deliveries using health plan records but increased the linkage rate to 82% using birth certificates. The low initial number arose because 80% of mothers and infants receiving care at that site are insured by Medicaid. After birth, if the mother does not specifically enroll her infant in that site’s Medicaid plan, the infant is automatically enrolled in the state’s default Medicaid administering plan – in which case the MEPREP health plan does not receive utilization information. Thus, at this site, linked pairs could only be identified from name and address matching using birth certificate information. In our experience, when we conducted name and address matching using birth certificates, linkage rates were lower if the mother and infant had different last names or if there had been frequent address changes around the time of the birth. Additional possible reasons for missing linkages to birth certificates include a child being born out of state or having a name change after birth 6.
Several additional limitations should be noted. First, we do not know whether MEPREP algorithms capture all deliveries. As noted above, a previous study at one site found high sensitivity of claims data for identifying deliveries 16. Our linkage rates compare favorably with one previously published approach: in a Georgia study of 89 hospitals, 85% of hospital discharge records could be linked to birth certificates 19. Second, we favored accuracy of matches rather than completeness because of the need to measure medication exposure accurately in future studies. Future studies with validation components could further refine the matching criteria. Third, we did not have resources to validate linkages through other means, such as chart review. These limitations are likely to omit only a small percentage of the population; however, future analyses using this cohort should consider potential selection biases.
We focused on health plans in the United States, but the challenges we faced and the procedures we developed may be relevant in other settings. Conducting linkages across multiple health plans or data sets requires local knowledge about each system’s data resources, as well as careful consideration of optimal matching algorithms to achieve both a high linkage rate and high accuracy while minimizing the potential for systematic biases. Representation from each data partner is important to ensure that data linkages are maximized, limitations are adequately expressed, and interpretations are sound.
In conclusion, linking mother-infant pairs within multiple health systems provides a mechanism for establishing large and diverse birth cohorts, which is especially valuable for studies of rare exposures or outcomes. This work demonstrates the potential for sound pharmacoepidemiologic studies of maternal drug exposures to be conducted using electronic health data. Such data offer expanding opportunities to improve knowledge about medication safety and ultimately the health of women and infants.
Take-home messages.
Health plan data offer great potential for studying medication safety in pregnancy because they include large, diverse, and defined populations. A crucial step is identifying linkages between mothers and infants.
The Medication Exposure in Pregnancy Risk Evaluation Program (MEPREP) developed a hierarchical linking algorithm used at 8 different health plans. This algorithm supports standardization yet allows tailoring to each plan’s data resources, such as birth registries and individual or family subscriber numbers.
With this approach, 8 MEPREP sites were able to link 86% of 299,260 deliveries to infants (range, 74–99% across plans) for the years 2001–2007.
Using birth certificates to identify additional mother-infant pairs substantially increased linkage rates at 2 sites and identified a more diverse population, including more Hispanic or non-white individuals.
Linkages created using these tools can support sound pharmacoepidemiologic studies of maternal drug exposures outside the context of a formal registry.
Acknowledgments
Research sponsor: U.S. Food and Drug Administration, Office of Surveillance and Epidemiology, Center for Drug Evaluation and Research, contracts HHSF223200510012C, HHSF223200510009C, and HHSF223200510008C. U.S. National Institute on Aging, grant K23AG028954.
We gratefully acknowledge the participating state departments of health and each site’s programmers, project managers, and other project staff. Rod Walker helped calculate statistics for the within-site comparison. We would also like to thank the peer reviewers whose helpful comments improved this manuscript. This study was supported through contracts HHSF223200510012C, HHSF223200510009C, and HHSF223200510008C (U.S. Food and Drug Administration, Office of Surveillance and Epidemiology, Center for Drug Evaluation and Research). Dr. Dublin was supported by National Institute on Aging grant K23AG028954. The views expressed in this paper are those of the authors and are not intended to convey official US Food and Drug Administration (FDA) policy or guidance. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health.
Appendix: Codes used to identify deliveries at the 8 MEPREP sites included in these analyses*
| ICD-9-CM diagnosis codes | Abbreviated Description |
|---|---|
| 641.01 | PLACENTA PREVIA WITHOUT HEMORRHAGE, DELIVERED |
| 641.11 | HEMORRHAGE FROM PLACENTA PREVIA, DELIVERED |
| 641.21 | PREMATURE SEPARATION OF PLACENTA, DELIVERED |
| 641.31 | ANTEPARTUM HEMORRHAGE ASSOC WITH COAG DEFECT, DELIVERED |
| 641.81 | OTHER ANTEPARTUM HEMORRHAGE, DELIVERED |
| 641.91 | UNSPECIFIED ANTEPARTUM HEMORRHAGE, DELIVERED |
| 642.01 | BENIGN ESSENTIAL HYPERTENSION WITH DELIVERY |
| 642.02 | BENIGN ESSENTIAL HYPERTENSION, WITH DELIVERY WITH POSTPARTUM COMPLICATION |
| 642.11 | HYPERTENSION SECONDARY TO RENAL DISEASE, WITH DELIVERY |
| 642.12 | HYPERTENSION SECONDARY TO RENAL DISEASE, WITH DELIVERY WITH POSTPARTUM COMPLICATION |
| 642.21 | OTHER PRE-EXISTING HYPERTENSION, WITH DELIVERY |
| 642.22 | OTHER PRE-EXISTING HYPERTENSION, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 642.31 | TRANSIENT HYPERTENSION OF PREGNANCY, WITH DELIVERY |
| 642.32 | TRANSIENT HYPERTENSION OF PREGNANCY, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 642.41 | MILD OR UNSPECIFIED PRE-ECLAMPSIA, WITH DELIVERY |
| 642.42 | MILD OR UNSPECIFIED PRE-ECLAMPSIA, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 642.51 | SEVERE PRE-ECLAMPSIA, WITH DELIVERY |
| 642.52 | SEVERE PRE-ECLAMPSIA, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 642.61 | ECLAMPSIA, WITH DELIVERY |
| 642.62 | ECLAMPSIA, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 642.71 | PRE-ECLAMPSIA OR ECLAMPSIA SUPERIMPOSED ON PRE-EXISTING HYPERTENSION, WITH DELIVERY |
| 642.72 | PRE-ECLAMPSIA OR ECLAMPSIA SUPERIMPOSED ON PRE-EXISTING HYPERTENSION, WITH DELIVERY, WITH CURRENT POSTPARTUM COMPLICATION |
| 642.91 | UNSPECIFIED HYPERTENSION, WITH DELIVERY |
| 642.92 | UNSPECIFIED HYPERTENSION, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 643.01 | MILD HYPEREMESIS GRAVIDARUM, DELIVERED |
| 643.11 | HYPEREMESIS GRAVIDARUM WITH METABOLIC DISTURBANCE, DELIVERED |
| 643.21 | LATE VOMITING OF PREGNANCY, DELIVERED |
| 643.81 | OTHER VOMITING COMPLICATING PREGNANCY, DELIVERED |
| 643.91 | UNSPECIFIED VOMITING OF PREGNANCY, DELIVERED |
| 644.2 | EARLY ONSET OF DELIVERY |
| 644.20 | EARLY ONSET OF DELIVERY, UNSPECIFIED AS TO EPISODE OF CARE |
| 644.21 | EARLY ONSET OF DELIVERY, DELIVERED |
| 645.01 | PROLONGED PREGNANCY, WITH DELIVERY |
| 645.11 | POST TERM PREGNANCY, DELIVERED |
| 645.21 | PROLONGED PREGNANCY, DELIVERED |
| 645.22 | PROLONGED PREGNANCY, DELIVERED, ANTEPARTUM CONDITION OR COMPLICATION |
| 646.01 | PAPYRACEOUS FETUS, DELIVERED |
| 646.11 | EDEMA OR EXCESSIVE WEIGHT GAIN IN PREGNANCY, DELIVERED |
| 646.12 | EDEMA OR EXCESSIVE WEIGHT GAIN IN PREGNANCY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 646.21 | UNSPECIFIED RENAL DISEASE IN PREGNANCY, WITH DELIVERY |
| 646.22 | UNSPECIFIED RENAL DISEASE IN PREGNANCY, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 646.31 | HABITUAL ABORTER, DELIVERED |
| 646.41 | PERIPHERAL NEURITIS IN PREGNANCY, WITH DELIVERY |
| 646.42 | PERIPHERAL NEURITIS IN PREGNANCY, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 646.51 | ASYMPTOMATIC BACTERIURIA IN PREGNANCY, WITH DELIVERY |
| 646.52 | ASYMPTOMATIC BACTERIURIA IN PREGNANCY, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 646.61 | INFECTIONS OF GENITOURINARY TRACT IN PREGNANCY, WITH DELIVERY |
| 646.62 | INFECTIONS OF GENITOURINARY TRACT IN PREGNANCY, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 646.71 | LIVER DISORDERS IN PREGNANCY, WITH DELIVERY |
| 646.81 | OTHER SPECIFIED COMPLICATIONS OF PREGNANCY, WITH DELIVERY |
| 646.82 | OTHER SPECIFIED COMPLICATIONS OF PREGNANCY, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 646.91 | UNSPECIFIED COMPLICATION OF PREGNANCY, WITH DELIVERY |
| 647.01 | SYPHILIS OF MOTHER, COMPLICATING PREGNANCY, WITH DELIVERY |
| 647.02 | SYPHILIS OF MOTHER, COMPLICATING PREGNANCY, WITH DELIVERY WITH MENTION OF POSTPARTUM COMPLICATION |
| 647.11 | GONORRHEA OF MOTHER, WITH DELIVERY |
| 647.12 | GONORRHEA OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 647.21 | OTHER VENEREAL DISEASES OF MOTHER, WITH DELIVERY |
| 647.22 | OTHER VENEREAL DISEASES OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 647.31 | TUBERCULOSIS OF MOTHER, WITH DELIVERY |
| 647.32 | TUBERCULOSIS OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 647.41 | MALARIA OF MOTHER, WITH DELIVERY |
| 647.42 | MALARIA OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 647.51 | RUBELLA OF MOTHER, WITH DELIVERY |
| 647.52 | RUBELLA OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 647.61 | OTHER VIRAL DISEASES OF MOTHER, WITH DELIVERY |
| 647.62 | OTHER VIRAL DISEASES OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 647.81 | OTHER SPECIFIED INFECTIOUS AND PARASITIC DISEASES OF MOTHER, DELIVERED |
| 647.82 | OTHER SPECIFIED INFECTIOUS AND PARASITIC DISEASES OF MOTHER, DELIVERED, WITH MENTION OF POSTPARTUM COMPLICATION |
| 647.91 | UNSPECIFIED INFECTION OR INFESTATION OF MOTHER, WITH DELIVERY |
| 647.92 | UNSPECIFIED INFECTION OR INFESTATION OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 648.01 | DIABETES MELLITUS OF MOTHER, WITH DELIVERY |
| 648.02 | DIABETES MELLITUS OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 648.11 | THYROID DYSFUNCTION OF MOTHER, WITH DELIVERY |
| 648.12 | THYROID DYSFUNCTION OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 648.21 | ANEMIA OF MOTHER, WITH DELIVERY |
| 648.22 | ANEMIA OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 648.31 | DRUG DEPENDENCE OF MOTHER, WITH DELIVERY |
| 648.32 | DRUG DEPENDENCE OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 648.41 | MENTAL DISORDERS OF MOTHER, WITH DELIVERY |
| 648.42 | MENTAL DISORDERS OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 648.51 | CONGENITAL CARDIOVASCULAR DISORDERS OF MOTHER, WITH DELIVERY |
| 648.52 | CONGENITAL CARDIOVASCULAR DISORDERS OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 648.61 | OTHER CARDIOVASCULAR DISEASES OF MOTHER, WITH DELIVERY |
| 648.62 | OTHER CARDIOVASCULAR DISEASES OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 648.71 | BONE AND JOINT DISORDERS OF BACK, PELVIS, AND LOWER LIMBS, DELIVERED |
| 648.72 | BONE AND JOINT DISORDERS OF BACK, PELVIS, AND LOWER LIMBS, DELIVERED, WITH MENTION OF POSTPARTUM COMPLICATION |
| 648.81 | ABNORMAL GLUCOSE TOLERANCE OF MOTHER, WITH DELIVERY |
| 648.82 | ABNORMAL GLUCOSE TOLERANCE OF MOTHER, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 648.91 | OTHER CURRENT CONDITIONS CLASSIFIABLE ELSEWHERE OF MOTHER, DELIVERED |
| 648.92 | OTHER CURRENT CONDITIONS CLASSIFIABLE ELSEWHERE OF MOTHER, DELIVERED, WITH MENTION OF POSTPARTUM COMPLICATION |
| 650 | NORMAL DELIVERY |
| 651.01 | TWIN PREGNANCY, DELIVERED |
| 651.11 | TRIPLET PREGNANCY, DELIVERED |
| 651.21 | QUADRUPLET PREGNANCY, DELIVERED |
| 651.31 | TWIN PREGNANCY WITH FETAL LOSS AND RETENTION OF ONE FETUS |
| 651.41 | TRIPLET PREGNANCY WITH FETAL LOSS AND RETENTION OF ONE OR MORE FETUS(ES) |
| 651.51 | QUADRUPLET PREGNANCY WITH FETAL LOSS AND RETENTION OF ONE OR MORE FETUS(ES) |
| 651.61 | OTHER MULTIPLE PREGNANCY WITH FETAL LOSS AND RETENTION OF ONE OR MORE FETUS(ES) |
| 651.81 | OTHER SPECIFIED MULTIPLE GESTATION, DELIVERED |
| 651.91 | UNSPECIFIED MULTIPLE GESTATION, DELIVERED |
| 652.01 | UNSTABLE LIE, DELIVERED |
| 652.11 | BREECH OR OTHER MALPRESENTATION SUCCESSFULLY CONVERTED TO CEPHALIC PRESENTATION |
| 652.21 | BREECH PRESENTATION WITHOUT MENTION OF VERSION, DELIVERED |
| 652.31 | TRANSVERSE OR OBLIQUE PRESENTATION, DELIVERED |
| 652.41 | FACE OR BROW PRESENTATION, DELIVERED |
| 652.51 | HIGH HEAD AT TERM, DELIVERED |
| 652.61 | MULTIPLE GESTATION WITH MALPRESENTATION OF ONE FETUS OR MORE, DELIVERED |
| 652.71 | PROLAPSED ARM OF FETUS, DELIVERED |
| 652.81 | OTHER SPECIFIED MALPOSITION OR MALPRESENTATION, DELIVERED |
| 652.91 | UNSPECIFIED MALPOSITION OR MALPRESENTATION, DELIVERED |
| 653.01 | MAJOR ABNORMALITY OF BONY PELVIS, NOT FURTHER SPECIFIED, DELIVERED |
| 653.11 | GENERALLY CONTRACTED PELVIS, DELIVERED |
| 653.21 | INLET CONTRACTION OF PELVIS, DELIVERED |
| 653.31 | OUTLET CONTRACTION OF PELVIS, DELIVERED |
| 653.41 | FETOPELVIC DISPROPORTION, DELIVERED |
| 653.51 | UNUSUALLY LARGE FETUS CAUSING DISPROPORTION, DELIVERED |
| 653.61 | HYDROCEPHALIC FETUS CAUSING DISPROPORTION, DELIVERED |
| 653.71 | OTHER FETAL ABNORMALITY CAUSING DISPROPORTION, DELIVERED |
| 653.81 | DISPROPORTION OF OTHER ORIGIN, DELIVERED |
| 653.91 | UNSPECIFIED DISPROPORTION, DELIVERED |
| 654.01 | CONGENITAL ABNORMALITIES OF UTERUS, WITH DELIVERY |
| 654.02 | CONGENITAL ABNORMALITIES OF UTERUS, DELIVERED, WITH MENTION OF POSTPARTUM COMPLICATION |
| 654.11 | TUMORS OF BODY OF UTERUS, WITH DELIVERY |
| 654.12 | TUMORS OF BODY OF UTERUS, DELIVERED, WITH MENTION OF POSTPARTUM COMPLICATION |
| 654.21 | PREVIOUS CESAREAN DELIVERY, DELIVERED |
| 654.31 | RETROVERTED AND INCARCERATED GRAVID UTERUS, DELIVERED |
| 654.32 | RETROVERTED AND INCARCERATED GRAVID UTERUS, DELIVERED, WITH MENTION OF POSTPARTUM COMPLICATION |
| 654.41 | OTHER ABNORMALITIES IN SHAPE OR POSITION OF GRAVID UTERUS |
| 654.42 | OTHER ABNORMALITIES IN SHAPE OR POSITION OF GRAVID UTERUS, WITH MENTION OF POSTPARTUM COMPLICATION |
| 654.51 | CERVICAL INCOMPETENCE, WITH DELIVERY |
| 654.52 | CERVICAL INCOMPETENCE, DELIVERED, WITH MENTION OF POSTPARTUM COMPLICATION |
| 654.61 | OTHER CONGENITAL OR ACQUIRED ABNORMALITY OF CERVIX, DELIVERED |
| 654.62 | OTHER CONGENITAL OR ACQUIRED ABNORMALITY OF CERVIX, |
| DELIVERED, WITH MENTION OF POSTPARTUM COMPLICATION | |
| 654.71 | CONGENITAL OR ACQUIRED ABNORMALITY OF VAGINA, DELIVERED |
| 654.72 | CONGENITAL OR ACQUIRED ABNORMALITY OF VAGINA, DELIVERED, WITH MENTION OF POSTPARTUM COMPLICATION |
| 654.81 | CONGENITAL OR ACQUIRED ABNORMALITY OF VULVA, DELIVERED |
| 654.82 | CONGENITAL OR ACQUIRED ABNORMALITY OF VULVA, DELIVERED, WITH MENTION OF POSTPARTUM COMPLICATION |
| 654.91 | OTHER AND UNSPECIFIED ABNORMALITY OF ORGANS AND SOFT TISSUE WITH DELIVERY |
| 654.92 | OTHER AND UNSPECIFIED ABNORMALITY OF ORGANS AND SOFT TISSUE WITH DELIVERY WITH MENTION OF POSTPARTUM COMPLICATION |
| 655.01 | CENTRAL NERVOUS SYSTEM MALFORMATION IN FETUS, DELIVERED |
| 655.11 | CHROMOSOMAL ABNORMALITY IN FETUS, AFFECTING MANAGEMENT OF MOTHER |
| 655.21 | HEREDITARY DISEASE IN FAMILY POSSIBLY AFFECTING FETUS, AFFECTING MANAGEMENT OF MOTHER, DELIVERED |
| 655.31 | SUSPECTED DAMAGE TO FETUS FROM VIRAL DISEASE IN THE MOTHER |
| 655.41 | SUSPECTED DAMAGE TO FETUS FROM OTHER DISEASE IN THE MOTHER |
| 655.51 | SUSPECTED DAMAGE TO FETUS FROM DRUGS, AFFECTING MANAGEMENT OF MOTHER |
| 655.61 | SUSPECTED DAMAGE TO FETUS FROM RADIATION, AFFECTING MANAGEMENT OF MOTHER |
| 655.71 | DECREASED FETAL MOVEMENTS, DELIVERED |
| 655.81 | OTHER KNOWN OR SUSPECTED FETAL ABNORMALITY |
| 655.91 | UNSPECIFIED SUSPECTED FETAL ABNORMALITY, AFFECTING MANAGENT OF MOTHER |
| 656.01 | FETAL-MATERNAL HEMORRHAGE, WITH DELIVERY |
| 656.11 | RHESUS ISOIMMUNIZATION, AFFECTING MANAGEMENT OF MOTHER |
| 656.21 | ISOIMMUNIZATION FROM OTHER AND UNSPECIFIED BLOOD-GROUP |
| 656.31 | FETAL DISTRESS, AFFECTING MANAGEMENT OF MOTHER, DELIVERED |
| 656.41 | INTRAUTERINE DEATH, AFFECTING MANAGEMENT OF MOTHER, DELIVED |
| 656.51 | POOR FETAL GROWTH, AFFECTING MANAGEMENT OF MOTHER, DELIVE |
| 656.61 | EXCESSIVE FETAL GROWTH, AFFECTING MANAGEMENT OF MOTHER, DELIVERED |
| 656.71 | OTHER PLACENTAL CONDITIONS, AFFECTING MANAGEMENT OF MOTHER |
| 656.81 | OTHER SPECIFIED FETAL AND PLACENTAL PROBLEMS, AFFECTING MANAGEMENT OF MOTHER |
| 656.91 | UNSPECIFIED FETAL AND PLACENTAL PROBLEM, AFFECTING MANAGENT OF MOTHER |
| 657.01 | POLYHYDRAMNIOS, WITH DELIVERY |
| 658.01 | OLIGOHYDRAMNIOS, DELIVERED |
| 658.11 | PREMATURE RUPTURE OF MEMBRANES, DELIVERED |
| 658.2 | DELAYED DELIVERY AFTER SPONTANEOUS OR UNSPECIFIED RUPTURE |
| 658.20 | DELAYED DELIVERY AFTER SPONTANEOUS OR UNSPECIFIED RUPTURE |
| 658.21 | DELAYED DELIVERY AFTER SPONTANEOUS OR UNSPECIFIED RUPTURE |
| 658.23 | DELAYED DELIVERY AFTER SPONTANEOUS OR UNSPECIFIED RUPTURE |
| 658.3 | DELAYED DELIVERY AFTER ARTIFICIAL RUPTURE OF MEMBRANES |
| 658.30 | DELAYED DELIVERY AFTER ARTIFICIAL RUPTURE OF MEMBRANES |
| 658.31 | DELAYED DELIVERY AFTER ARTIFICIAL RUPTURE OF MEMBRANES |
| 658.33 | DELAYED DELIVERY AFTER ARTIFICIAL RUPTURE OF MEMBRANES |
| 658.41 | INFECTION OF AMNIOTIC CAVITY, DELIVERED |
| 658.81 | OTHER PROBLEMS ASSOCIATED WITH AMNIOTIC CAVITY AND MEMBRANES |
| 658.91 | UNSPECIFIED PROBLEM ASSOCIATED WITH AMNIOTIC CAVITY AND MEMBRANES |
| 659.01 | FAILED MECHANICAL INDUCTION OF LABOR, DELIVERED |
| 659.11 | FAILED MEDICAL OR UNSPECIFIED INDUCTION OF LABOR, DELIVERED |
| 659.21 | UNSPECIFIED TYPE MATERNAL PYREXIA DURING LABOR, DELIVERED |
| 659.31 | GENERALIZED INFECTION DURING LABOR, DELIVERED |
| 659.41 | GRAND MULTIPARITY, WITH CURRENT PREGNANCY, DELIVERED |
| 659.51 | ELDERLY PRIMIGRAVIDA, DELIVERED |
| 659.61 | ELDERLY MULTIGRAVIDA, DELIVERED |
| 659.71 | ABNORMALITY IN FETAL HEART RATE/RHYTHM, DELIVERED |
| 659.81 | OTHER SPECIFIED INDICATIONS FOR CARE OR INTERVENTION RELATED TO LABOR AND DELIVERY, DELIVERED |
| 659.91 | UNSPECIFIED INDICATION FOR CARE OR INTERVENTION RELATED TO LABOR AND DELIVERY, DELIVERED |
| 660.01 | OBSTRUCTION CAUSED BY MALPOSITION OF FETUS AT ONSET OF LABOR |
| 660.11 | OBSTRUCTION BY BONY PELVIS DURING LABOR, WITH DELIVERY |
| 660.21 | OBSTRUCTION BY ABNORMAL PELVIC SOFT TISSUES DURING LABOR |
| 660.31 | DEEP TRANSVERSE ARREST AND PERSISTENT OCCIPITOPOSTERIOR POSTERIOR |
| 660.41 | SHOULDER (GIRDLE) DYSTOCIA, WITH DELIVERY |
| 660.51 | LOCKED TWINS, WITH DELIVERY |
| 660.61 | FAILED TRIAL OF LABOR, UNSPECIFIED, WITH DELIVERY |
| 660.71 | FAILED FORCEPS OR VACUUM EXTRACTOR, UNSPECIFIED, WITH DELIVERY |
| 660.81 | OTHER CAUSES OF OBSTRUCTED LABOR, WITH DELIVERY |
| 660.91 | UNSPECIFIED OBSTRUCTED LABOR, WITH DELIVERY |
| 661.01 | PRIMARY UTERINE INERTIA, WITH DELIVERY |
| 661.11 | SECONDARY UTERINE INERTIA, WITH DELIVERY |
| 661.21 | OTHER AND UNSPECIFIED UTERINE INERTIA, WITH DELIVERY |
| 661.31 | PRECIPITATE LABOR, WITH DELIVERY |
| 661.41 | HYPERTONIC, INCOORDINATE, OR PROLONGED UTERINE CONTRACTIONS, DELIVERED |
| 661.91 | UNSPECIFIED ABNORMALITY OF LABOR, WITH DELIVERY |
| 662.01 | PROLONGED FIRST STAGE OF LABOR, DELIVERED |
| 662.11 | PROLONGED LABOR, UNSPECIFIED TYPE, DELIVERED |
| 662.21 | PROLONGED SECOND STAGE OF LABOR, DELIVERED |
| 662.31 | DELAYED DELIVERY OF SECOND TWIN, TRIPLET, ETC., DELIVERED |
| 663.01 | PROLAPSE OF CORD COMPLICATING LABOR AND DELIVERY, DELIVERERED |
| 663.11 | CORD AROUND NECK, WITH COMPRESSION, COMPLICATING LABOR AND DELIVERY, DELIVERED |
| 663.21 | OTHER AND UNSPECIFIED CORD ENTANGLEMENT, WITH COMPRESSION |
| 663.31 | OTHER AND UNSPECIFIED CORD ENTANGLEMENT |
| 663.41 | SHORT CORD COMPLICATING LABOR AND DELIVERY, DELIVERED |
| 663.51 | VASA PREVIA COMPLICATING LABOR AND DELIVERY, DELIVERED |
| 663.61 | VASCULAR LESIONS OF CORD COMPLICATING LABOR AND DELIVERY |
| 663.81 | OTHER UMBILICAL CORD COMPLICATIONS DURING LABOR AND DELIVERY |
| 663.91 | UNSPECIFIED UMBILICAL CORD COMPLICATION DURING LABOR AND DELIVERY |
| 664.01 | FIRST-DEGREE PERINEAL LACERATION, WITH DELIVERY |
| 664.11 | SECOND-DEGREE PERINEAL LACERATION, WITH DELIVERY |
| 664.21 | THIRD-DEGREE PERINEAL LACERATION, WITH DELIVERY |
| 664.31 | FOURTH-DEGREE PERINEAL LACERATION, WITH DELIVERY |
| 664.41 | UNSPECIFIED PERINEAL LACERATION, WITH DELIVERY |
| 664.51 | VULVAR AND PERINEAL HEMATOMA, WITH DELIVERY |
| 664.81 | OTHER SPECIFIED TRAUMA TO PERINEUM AND VULVA, WITH DELIVERY |
| 664.91 | UNSPECIFIED TRAUMA TO PERINEUM AND VULVA, WITH DELIVERY |
| 665.01 | RUPTURE OF UTERUS BEFORE ONSET OF LABOR, WITH DELIVERY |
| 665.11 | RUPTURE OF UTERUS DURING LABOR, DELIVERED |
| 665.22 | INVERSION OF UTERUS, DELIVERED WITH POSTPARTUM COMPLICATIONS |
| 665.31 | LACERATION OF CERVIX, WITH DELIVERY |
| 665.41 | HIGH VAGINAL LACERATION, WITH DELIVERY |
| 665.51 | OTHER INJURY TO PELVIC ORGANS, WITH DELIVERY |
| 665.61 | DAMAGE TO PELVIC JOINTS AND LIGAMENTS, WITH DELIVERY |
| 665.71 | PELVIC HEMATOMA, WITH DELIVERY |
| 665.72 | PELVIC HEMATOMA, DELIVERED WITH POSTPARTUM COMPLICATION |
| 665.81 | OTHER SPECIFIED OBSTETRICAL TRAUMA, WITH DELIVERY |
| 665.82 | OTHER SPECIFIED OBSTETRICAL TRAUMA, DELIVERED, WITH MENTION OF POSTPARTUM COMPLICATION |
| 665.91 | UNSPECIFIED OBSTETRICAL TRAUMA, WITH DELIVERY |
| 665.92 | UNSPECIFIED OBSTETRICAL TRAUMA, DELIVERED, WITH MENTION OF POSTPARTUM COMPLICATION |
| 666.02 | THIRD-STAGE POSTPARTUM HEMORRHAGE, WITH DELIVERY |
| 666.12 | OTHER IMMEDIATE POSTPARTUM HEMORRHAGE, WITH DELIVERY |
| 666.22 | DELAYED AND SECONDARY POSTPARTUM HEMORRHAGE, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 666.32 | POSTPARTUM COAGULATION DEFECTS, WITH DELIVERY |
| 667.02 | RETAINED PLACENTA WITHOUT HEMORRHAGE, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 667.12 | RETAINED PORTIONS OF PLACENTA OR MEMBRANES, WITHOUT HEMORRHAGE, DELIVERED, WITH MENTION OF POSTPARTUM COMPLICATION |
| 668.01 | PULMONARY COMPLICATIONS OF ANESTHESIA OR OTHER SEDATION IN LABOR AND DELIVERY |
| 668.02 | PULMONARY COMPLICATIONS OF ANESTHESIA OR OTHER SEDATION IN LABOR AND DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 668.11 | CARDIAC COMPLICATIONS OF ANESTHESIA OR OTHER SEDATION IN LABOR AND DELIVERY |
| 668.12 | CARDIAC COMPLICATIONS OF ANESTHESIA OR OTHER SEDATION IN LABOR AND DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 668.21 | CENTRAL NERVOUS SYSTEM COMPLICATIONS OF ANESTHESIA OR OTHER SEDATION IN LABOR AND DELIVERY |
| 668.22 | CENTRAL NERVOUS SYSTEM COMPLICATIONS OF ANESTHESIA OR OTHER SEDATION IN LABOR AND DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 668.81 | OTHER COMPLICATIONS OF ANESTHESIA OR OTHER SEDATION IN LABOR AND DELIVERY |
| 668.82 | OTHER COMPLICATIONS OF ANESTHESIA OR OTHER SEDATION IN LABOR AND DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 668.91 | UNSPECIFIED COMPLICATION OF ANESTHESIA OR OTHER SEDATION |
| 668.92 | UNSPECIFIED COMPLICATION OF ANESTHESIA OR OTHER SEDATION, WITH MENTION OF POSTPARTUM COMPLICATION |
| 669.01 | MATERNAL DISTRESS, WITH DELIVERY |
| 669.02 | MATERNAL DISTRESS, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 669.11 | SHOCK DURING OR FOLLOWING LABOR AND DELIVERY |
| 669.12 | SHOCK DURING OR FOLLOWING LABOR AND DELIVERY WITH MENTION OF POSTPARTUM COMPLICATION |
| 669.21 | MATERNAL HYPOTENSION SYNDROME, WITH DELIVERY |
| 669.22 | MATERNAL HYPOTENSION SYNDROME, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 669.32 | ACUTE RENAL FAILURE WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 669.41 | OTHER COMPLICATIONS OF OBSTETRICAL SURGERY AND PROCEDURES |
| 669.42 | OTHER COMPLICATIONS OF OBSTETRICAL SURGERY AND PROCEDURES |
| 669.5 | FORCEPS OR VACUUM EXTRACTOR DELIVERY |
| 669.50 | FORCEPS OR VACUUM EXTRACTOR DELIVERY |
| 669.51 | FORCEPS OR VACUUM EXTRACTOR DELIVERY |
| 669.6 | FORCEPS OR VACUUM EXTRACTOR DELIVERY |
| 669.60 | BREECH EXTRACTION, WITHOUT MENTION OF INDICATION |
| 669.61 | BREECH EXTRACTION, WITHOUT MENTION OF INDICATION |
| 669.7 | FORCEPS OR VACUUM EXTRACTOR DELIVERY WITHOUT MENTION OF INDICATION |
| 669.70 | CESAREAN DELIVERY, WITHOUT MENTION OF INDICATION |
| 669.71 | CESAREAN DELIVERY, WITHOUT MENTION OF INDICATION |
| 669.81 | OTHER COMPLICATIONS OF LABOR AND DELIVERY, DELIVERED |
| 669.82 | OTHER COMPLICATION OF LABOR AND DELIVERY, DELIVERED, WITH MENTION OF POSTPARTUM COMPLICATION |
| 669.91 | UNSPECIFIED COMPLICATION OF LABOR AND DELIVERY |
| 669.92 | UNSPECIFIED COMPLICATION OF LABOR AND DELIVERY |
| 670.02 | MAJOR PUERPERAL INFECTION, DELIVERED |
| 671.01 | VARICOSE VEINS OF LEGS, WITH DELIVERY |
| 671.02 | VARICOSE VEINS OF LEGS, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 671.11 | VARICOSE VEINS OF VULVA AND PERINEUM, WITH DELIVERY |
| 671.12 | VARICOSE VEINS OF VULVA AND PERINEUM, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 671.21 | SUPERFICIAL THROMBOPHLEBITIS WITH DELIVERY |
| 671.22 | SUPERFICIAL THROMBOPHLEBITIS WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 671.31 | DEEP PHLEBOTHROMBOSIS, ANTEPARTUM, WITH DELIVERY |
| 671.42 | DEEP PHLEBOTHROMBOSIS, POSTPARTUM, WITH DELIVERY |
| 671.51 | OTHER PHLEBITIS AND THROMBOSIS WITH DELIVERY |
| 671.52 | OTHER PHLEBITIS AND THROMBOSIS WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 671.81 | OTHER VENOUS COMPLICATIONS, WITH DELIVERY |
| 671.82 | OTHER VENOUS COMPLICATIONS, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 671.91 | UNSPECIFIED VENOUS COMPLICATION, WITH DELIVERY |
| 671.92 | UNSPECIFIED VENOUS COMPLICATION, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 672.02 | PYREXIA OF UNKNOWN ORIGIN, WITH DELIVERY |
| 673.01 | OBSTETRICAL AIR EMBOLISM, WITH DELIVERY |
| 673.02 | OBSTETRICAL AIR EMBOLISM, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 673.11 | AMNIOTIC FLUID EMBOLISM, WITH DELIVERY |
| 673.12 | AMNIOTIC FLUID EMBOLISM, WITH DELIVERY WITH MENTION OF POSTPARTUM COMPLICATION |
| 673.21 | OBSTETRICAL BLOOD-CLOT EMBOLISM, WITH DELIVERY |
| 673.31 | OBSTETRICAL PYEMIC AND SEPTIC EMBOLISM, WITH DELIVERY |
| 673.32 | OBSTETRICAL PYEMIC AND SEPTIC EMBOLISM, WITH DELIVERY WITH MENTION OF POSTPARTUM COMPLICATION |
| 673.81 | OTHER OBSTETRICAL PULMONARY EMBOLISM, WITH DELIVERY |
| 673.82 | OTHER OBSTETRICAL PULMONARY EMBOLISM, WITH DELIVERY WITH MENTION OF POSTPARTUM COMPLICATION |
| 674.01 | CEREBROVASCULAR DISORDERS, WITH DELIVERY |
| 674.02 | CEREBROVASCULAR DISORDERS, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 674.12 | DISRUPTION OF CESAREAN WOUND, WITH DELIVERY |
| 674.22 | DISRUPTION OF PERINEAL WOUND, WITH DELIVERY |
| 674.32 | OTHER COMPLIC OF OBSTET SURG WOUNDS, WITH DELIVERY |
| 674.42 | PLACENTAL POLYP, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 674.51 | PERIPARTUM CARDIOMYOPATHY, DELIVERED |
| 674.52 | PERIPARTUM CARDIOMYOPATHY, DELIVERED, WITH MENTION OF POSTPARTUM CONDITION |
| 674.82 | OTHER COMPLICATIONS OF PUERPERIUM, WITH DELIVERY |
| 674.92 | UNSPECIFIED COMPLICATIONS OF PUERPERIUM, WITH DELIVERY |
| 675.01 | INFECTION OF NIPPLE, WITH DELIVERY |
| 675.02 | INFECTION OF NIPPLE, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 675.11 | ABSCESS OF BREAST, WITH DELIVERY |
| 675.12 | ABSCESS OF BREAST, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 675.21 | NONPURULENT MASTITIS, WITH DELIVERY |
| 675.22 | NONPURULENT MASTITIS, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 675.81 | OTHER SPEC INFECT OF BREAST, WITH DELIVERY |
| 675.82 | OTHER SPEC INFECT OF BREAST, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 675.91 | UNSPEC INFECT OF BREAST, WITH DELIVERY |
| 675.92 | UNSPEC INFECT OF BREAST, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 676.01 | RETRACTED NIPPLE, WITH DELIVERY |
| 676.02 | RETRACTED NIPPLE, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 676.11 | CRACKED NIPPLE, WITH DELIVERY |
| 676.12 | CRACKED NIPPLE, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 676.21 | ENGORGEMENT OF BREASTS, WITH DELIVERY |
| 676.22 | ENGORGEMENT OF BREASTS, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 676.31 | OTHER DISORDER OF BREASTS, WITH DELIVERY |
| 676.32 | OTHER DISORDER OF BREASTS, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 676.41 | FAILURE OF LACTATION, WITH DELIVERY |
| 676.42 | FAILURE OF LACTATION, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 676.51 | SUPPRESSED LACTATION, WITH DELIVERY |
| 676.52 | SUPPRESSED LACTATION, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 676.61 | GALACTORRHEA, WITH DELIVERY |
| 676.62 | GALACTORRHEA, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 676.81 | OTHER DISORDERS OF LACTATION, WITH DELIVERY |
| 676.82 | OTHER DISORDERS OF LACTATION, WITH DELIVERY, WITH |
| MENTION OF POSTPARTUM COMPLICATION | |
| 676.91 | UNSPECIFIED DISORDER OF LACTATION, WITH DELIVERY |
| 676.92 | UNSPECIFIED DISORDER OF LACTATION, WITH DELIVERY, WITH MENTION OF POSTPARTUM COMPLICATION |
| 763 | BREECH DELIVERY AND EXTRACTION AFFECTING FETUS OR NEWBORN |
| 763.2 | FORCEPS DELIVERY AFFECTING FETUS OR NEWBORN |
| 763.3 | DELIVERY BY VACUUM EXTRACTOR AFFECTING FETUS OR NEWBORN |
| 763.4 | CESAREAN DELIVERY AFFECTING FETUS OR NEWBORN |
| 763.6 | PRECIPITATE DELIVERY AFFECTING FETUS OR NEWBORN |
| V27 | OUTCOME OF DELIVERY |
| V27.0 | SINGLE NEWBORN |
| V27.1 | SINGLE STILLBORN |
| V27.2 | TWINS, BOTH LIVEBORN |
| V27.3 | TWINS, ONE LIVEBORN |
| V27.4 | TWINS, STILLBORN |
| V27.5 | OTHER MULTIPLE BIRTH, ALL LIVEBORN |
| V27.6 | OTHER MULTIPLE BIRTH, SOME LIVEBORN |
| V27.7 | OTHER MULTIPLE BIRTH, ALL STILLBORN |
| V27.9 | MOTHER WITH UNSPECIFIED OUTCOME OF DELIVERY |
| V30.0 | SINGLE NEWBORN, BORN IN HOSPITAL |
| V30.00 | SINGLE NEWBORN, BORN IN HOSPITAL |
| V30.01 | SINGLE NEWBORN, BORN IN HOSPITAL, CESAREAN |
| V30.1 | SINGLE LIVEBORN, BORN BEFORE ADMISSION TO HOSPITAL |
| V31.0 | TWIN, BORN IN HOSPITAL |
| V31.00 | TWIN, BORN IN HOSPITAL |
| V31.01 | TWIN, BORN IN HOSPITAL, CESAREAN |
| V31.1 | TWIN BIRTH, MATE LIVEBORN, BORN BEFORE ADMISSION TO HOSPITAL |
| V31.2 | TWIN BIRTH, MATE LIVEBORN, BORN OUTSIDE HOSPITAL AND NOT HOSPITALIZED |
| V32.0 | TWIN, BORN IN HOSPITAL, MATE STILLBORN |
| V32.01 | TWIN, BORN IN HOSPITAL, CESAREAN, MATE STILLBORN |
| V32.1 | TWIN BIRTH, MATE STILLBORN, BORN BEFORE ADMISSION TO HOSPITAL |
| V32.2 | TWIN BIRTH, MATE STILLBORN, BORN OUTSIDE HOSPITAL AND NOT HOSPITALIZED |
| V33.0 | TWIN, BORN IN HOSPITAL |
| V33.01 | TWIN, BORN IN HOSPITAL, CESAREAN |
| V33.1 | TWIN BIRTH, UNSPECIFIED WHETHER MATE LIVEBORN OR STILLBORN, BORN BEFORE ADMISSION TO HOSPITAL |
| V34.0 | OTHER MULTIPLE, BORN IN HOSPITAL |
| V34.00 | OTHER MULTIPLE, BORN IN HOSPITAL |
| V34.01 | OTHER MULTIPLE, BORN IN HOSPITAL, CESAREAN |
| V34.1 | OTHER MULTIPLE BIRTH (THREE OR MORE), MATES ALL LIVEBORN, BORN BEFORE ADMISSION TO HOSPITAL |
| V35.0 | OTHER MULTIPLE, BORN IN HOSPITAL |
| V35.00 | OTHER MULTIPLE, BORN IN HOSPITAL |
| V35.01 | OTHER MULTIPLE, BORN IN HOSPITAL, CESAREAN |
| V35.1 | OTHER MULTIPLE BIRTH (THREE OR MORE), MATES ALL STILLBORN, BORN BEFORE ADMISSION TO HOSPITAL |
| V36.0 | OTHER MULTIPLE, BORN IN HOSPITAL |
| V36.00 | OTHER MULTIPLE, BORN IN HOSPITAL |
| V36.01 | OTHER MULTIPLE, BORN IN HOSPITAL, CESAREAN |
| V36.1 | OTHER MULTIPLE BIRTH (THREE OR MORE), MATES LIVEBORN AND STILLBORN, BORN BEFORE ADMISSION TO HOSPITAL |
| V37.0 | OTHER MULTIPLE, BORN IN HOSPITAL |
| V37.00 | OTHER MULTIPLE, BORN IN HOSPITAL |
| V37.01 | OTHER MULTIPLE, BORN IN HOSPITAL, CESAREAN |
| V37.1 | OTHER MULTIPLE BIRTH (THREE OR MORE), UNSPECIFIED WHETHER MATES LIVEBORN OR STILLBORN, BORN BEFORE ADMISSION TO HOSPITAL |
| V39.0 | UNSPEC, BORN IN HOSPITAL |
| V39.00 | UNSPEC, BORN IN HOSPITAL |
| V39.01 | UNSPEC, BORN IN HOSPITAL, CESAREAN |
| V39.1 | LIVEBORN, UNSPECIFIED WHETHER SINGLE, TWIN OR MULTIPLE, BORN BEFORE ADMISSION TO HOSPITAL |
| ICD-9-CM Procedure Codes | Description |
|---|---|
| 72 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.0 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.1 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.2 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.21 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.29 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.3 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.31 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.39 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.4 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.5 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.51 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.52 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.53 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.54 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.6 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.7 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.71 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.79 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.8 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 72.9 | FORCEPS, VACUUM, AND BREECH DELIVERY |
| 73 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.0 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.01 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.09 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.1 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.2 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.21 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.22 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.3 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.4 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.5 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.51 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.59 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.6 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.8 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.9 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.91 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.92 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.93 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.94 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 73.99 | OTHER PROCEDURES INDUCING OR ASSISTING DELIVERY |
| 74 | CESAREAN SECTION AND REMOVAL OF FETUS |
| 74.1 | CESAREAN SECTION AND REMOVAL OF FETUS |
| 74.2 | CESAREAN SECTION AND REMOVAL OF FETUS |
| 74.4 | CESAREAN SECTION AND REMOVAL OF FETUS |
| 74.9 | CESAREAN SECTION AND REMOVAL OF FETUS |
| 74.99 | CESAREAN SECTION AND REMOVAL OF FETUS |
| CPT Procedure Codes | Description |
|---|---|
| 59400 | ROUTINE OB CARE INCL ANTEPARTUM CAR, VAGINAL DELIVER, POSTPARTUM CARE |
| 59409 | VAGINAL DELIVERY ONLY |
| 59410 | VAGINAL DELIVERY INCL POSTPARTUM CARE |
| 59510 | ROUTINE OB CARE INCL ANTEPARTUM CAR, CESAREAN DELIVER, POSTPARTUM CARE |
| 59514 | CESAREAN DELIVERY ONLY |
| 59515 | CESAREAN DELIVERY, INCL POSTPARTUM CARE |
| 59610 | OB CARE INCL ANTEPARTUM CAR, VAG DELIVER, POSTPART CARE. AFTER PREV C-SECT |
| 59612 | VAG DELIVERY ONLY AFTER PREV C-SECT |
| 59614 | VAG DELIVERY AFTER PREV C-SECT, INCL POSTPARTUM CARE |
| 59618 | OB CARE INCL ANTEPARTUM CAR, CES DELIVER, POSTPART CARE. AFTER PREV C-SECT |
| 59620 | CESAREAN DELIVERY ONLY AFTER PREV C-SECT |
| 59622 | CESAREAN DELIVERY AFTER PREV C-SECT, INCL POSTPARTUM CARE |
Footnotes
Conflict of Interest statement: The corresponding author reported no possible study interpretation conflicts. No author reported a potential financial conflict. SD received a Merck/American Geriatrics Society New Investigator Award.
Prior presentation: The content of this paper was presented as an oral presentation at the 2012 International Conference on Pharmacoepidemiology in Barcelona, Spain, August 23–26.
References
- 1.Andrade SE, Gurwitz JH, Davis RL, et al. Prescription drug use in pregnancy. Am J Obstet Gynecol. 2004 Aug;191(2):398–407. doi: 10.1016/j.ajog.2004.04.025S000293780400420X. [pii] [DOI] [PubMed] [Google Scholar]
- 2.U.S. Food and Drug Administration. Evaluating the risks of drug exposure in human pregnancies. 2005. Reviewer Guidance. [Google Scholar]
- 3.Adam MP, Polifka JE, Friedman JM. Evolving knowledge of the teratogenicity of medications in human pregnancy. Am J Med Genet C Semin Med Genet. 2011 Aug 15;157(3):175–182. doi: 10.1002/ajmg.c.30313. [DOI] [PubMed] [Google Scholar]
- 4.Andrade SE, Davis RL, Cheetham TC, et al. Medication Exposure in Pregnancy Risk Evaluation Program. Matern Child Health J. 2011 Oct 15; doi: 10.1007/s10995-011-0902-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley CJ, Penberthy L, Devers KJ, Holden DJ. Health services research and data linkages: issues, methods, and directions for the future. Health Services Research. 2010;45(5 Pt 2):1468–1488. doi: 10.1111/j.1475-6773.2010.01142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Piper JM, Ray WA, Griffin MR, Fought R, Daughtery JR, Mitchel E., Jr Methodological issues in evaluating expanded Medicaid coverage for pregnant women. Am J Epidemiol. 1990 Sep;132(3):561–571. doi: 10.1093/oxfordjournals.aje.a115692. [DOI] [PubMed] [Google Scholar]
- 7.Ford JB, Roberts CL, Taylor LK. Characteristics of unmatched maternal and baby records in linked birth records and hospital discharge data. Paediatr Perinat Epidemiol. 2006 Jul;20(4):329–337. doi: 10.1111/j.1365-3016.2006.00715.x. PPE715 [pii] [DOI] [PubMed] [Google Scholar]
- 8.Leiss JK, Giles D, Sullivan KM, Mathews R, Sentelle G, Tomashek KM. U.S. Maternally linked birth records may be biased for Hispanics and other population groups. Ann Epidemiol. 2010 Jan;20(1):23–31. doi: 10.1016/j.annepidem.2009.09.003. S1047-2797(09)00307-X [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maro JC, Platt R, Holmes JH, et al. Design of a national distributed health data network. Ann Intern Med. 2009 Sep 1;151(5):341–344. doi: 10.7326/0003-4819-151-5-200909010-00139. 0000605-200909010-00140 [pii] [DOI] [PubMed] [Google Scholar]
- 10.Brown JS, Holmes JH, Shah K, Hall K, Lazarus R, Platt R. Distributed health data networks: a practical and preferred approach to multi-institutional evaluations of comparative effectiveness, safety, and quality of care. Med Care. 2010 Jun;48(6 Suppl):S45–51. doi: 10.1097/MLR.0b013e3181d9919f. [DOI] [PubMed] [Google Scholar]
- 11.Davis RL, Rubanowice D, McPhillips H, et al. Risks of congenital malformations and perinatal events among infants exposed to antidepressant medications during pregnancy. Pharmacoepidemiol Drug Saf. 2007 Oct;16(10):1086–1094. doi: 10.1002/pds.1462. [DOI] [PubMed] [Google Scholar]
- 12.Andrade SE, Raebel MA, Brown J, et al. Outpatient use of cardiovascular drugs during pregnancy. Pharmacoepidemiol Drug Saf. 2008 Mar;17(3):240–247. doi: 10.1002/pds.1550. [DOI] [PubMed] [Google Scholar]
- 13.Andrade SE, Raebel MA, Brown J, et al. Use of antidepressant medications during pregnancy: a multisite study. Am J Obstet Gynecol. 2008 Feb;198(2):194, e191–195. doi: 10.1016/j.ajog.2007.07.036. S0002-9378(07)00915-5 [pii] [DOI] [PubMed] [Google Scholar]
- 14.Andrade SE, McPhillips H, Loren D, et al. Antidepressant medication use and risk of persistent pulmonary hypertension of the newborn. Pharmacoepidemiol Drug Saf. 2009 Mar;18(3):246–252. doi: 10.1002/pds.1710. [DOI] [PubMed] [Google Scholar]
- 15.Davis RL, Eastman D, McPhillips H, et al. Risks of congenital malformations and perinatal events among infants exposed to calcium channel and beta-blockers during pregnancy. Pharmacoepidemiol Drug Saf. 2011 Feb;20(2):138–145. doi: 10.1002/pds.2068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Raebel MA, Ellis JL, Andrade SE. Evaluation of gestational age and admission date assumptions used to determine prenatal drug exposure from administrative data. Pharmacoepidemiol Drug Saf. 2005 Dec;14(12):829–836. doi: 10.1002/pds.1100. [DOI] [PubMed] [Google Scholar]
- 17.Lydon-Rochelle MT, Holt VL, Cardenas V, et al. The reporting of pre-existing maternal medical conditions and complications of pregnancy on birth certificates and in hospital discharge data. Am J Obstet Gynecol. 2005 Jul;193(1):125–134. doi: 10.1016/j.ajog.2005.02.096. S000293780500342X [pii] [DOI] [PubMed] [Google Scholar]
- 18.Reichman NE, Hade EM. Validation of birth certificate data. A study of women in New Jersey’s HealthStart program. Ann Epidemiol. 2001 Apr;11(3):186–193. doi: 10.1016/s1047-2797(00)00209-x. S1047-2797(00)00209-X [pii] [DOI] [PubMed] [Google Scholar]
- 19.Kahn EB, Berg CJ, Callaghan WM. Cesarean Delivery Among Women With Low-Risk Pregnancies. Obstet Gynecol. 2009;113(1) doi: 10.1097/AOG.0b013e318190bb33. [DOI] [PubMed] [Google Scholar]